Introduction
Alzheimer's disease (AD), the most common
neurodegenerative disease, is clinically characterized by
progressive memory impairment and cognitive dysfunction (1). Pathologically, the disease is
characterized by the presence of extracellular plaques of amyloid-β
(Aβ), intracellular tangles of hyperphosphorylated tau protein
(2) and loss of forebrain
cholinergic neurons (3). As
reported, the average course of disease from AD to death is ~10
years. In addition, early symptoms of the disease are not obvious,
and the majority of patients with AD do not know when they develop
the disease. Therefore, as time passes, the condition of patients
with AD becomes more serious, eventually leading to the complete
loss of learning, cognition and language abilities (4).
There are numerous factors that contribute to the
development of AD, and various pathogenesis mechanisms have been
reported. In the Aβ theory, Aβ-protein is considered to be the
central link to the incidence of AD. A previous study has shown
that the concentration of Aβ in the brain tissue of patients with
early AD is significantly higher compared with that in the healthy
population, which has a toxic effect on brain neurons and leads to
the loss or death of neurons (5).
In the Tau protein abnormal modification theory, abnormal
modification of Tau protein can cause the Tau protein to lose the
compositional function of tubulin, triggering the disintegration of
microtubules, thereby affecting the normal transport function of
neuronal cells and nerve endings, and ultimately leading to neuron
degeneration and synaptic degeneration (6). As presented in the cholinergic system
damage theory, the central cholinergic system of patients with AD
has obvious damage and defects, which are consistent with the
clinical symptoms of patients with AD (7). Oxidative stress refers to the
imbalance between the peroxide action and antioxidant mechanisms in
the body, which leads to excessive production of oxygen-free
radicals and peroxides, causing damage to cells (8). In the glutamate receptor theory,
glutamate and its receptors play an important role in the function
of the body's nervous system. Its receptors are involved in the
growth and development of neurons, the performance of learning and
memory capabilities and the maintenance of synaptic plasticity
(9). Apoptosis of brain neuronal
cells in patients with AD is the main cause of nerve damage, and it
is also the final pathway of various theories on the pathogenesis
of AD. Based on relevant literature studies, Aβ aggregation,
oxidative stress and mitochondrial dysfunction in patients with AD
are all important causes of neuronal cell apoptosis (10). Due to the diversity and complexity
of its mechanisms, there is not yet a cure or efficient form of
treatment for AD. Therefore, the development of anti-AD drugs has
become an important area of research.
Natural compounds and their derivatives have
attracted increasing attention due to their inherent chemical
diversity, and a number of them have become potential drug
candidates (11). Drynaria
rhizome, also known as Gu-Sui-Bu, is a common plant widely
distributed throughout southern China (12). The roots of Drynaria rhizome
were conventionally regarded as a medicine against osteoporosis and
bone resorption, while recently it has increasingly been used to
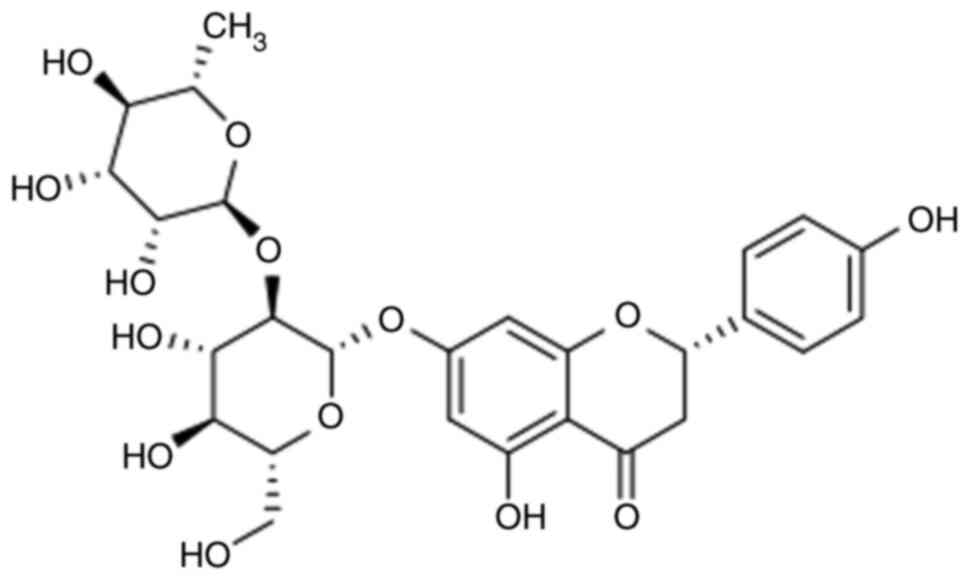
treat neurodegenerative diseases, such as AD (13). Naringin (Fig. 1), one of the main active substances
extracted from Drynaria rhizome has attracted increased
attention due to its extensive antioxidant, anti-inflammatory,
anti-apoptotic, anti-ulcer, anti-osteoporosis and anticancer
effects (14,15). According to previous reports,
naringin can attenuate the behavioral changes and cognitive
impairment in the epilepsy model induced by haiendic acid (16) and the Huntington model induced by
3-nitropropionic acid (17). In
addition, naringin treatment can decrease oxidative damage and
reverse histopathological changes in the cortex, striatum and
hippocampus caused by ischemia-reperfusion (18). Furthermore, previous experiments
have shown that it can also improve the long-term memory of
transgenic AD mice, and also play a neuroprotective role (19). However, to the best of our
knowledge, there is currently little information available
regarding the effects of naringin on long-term cognitive impairment
in patients with AD, and the mechanisms that may be involved are
not yet fully understood.
The present study isolated naringin from
Drynaria rhizome and studied the effects of naringin on
cognitive impairment and neuropathology in an AD mouse model. The
results of the present study suggested that naringin can improve
the ability of learning and memory in AD model mice through a
variety of ways, thus exerting neuroprotective effects.
Materials and methods
Groups and treatments
A total of 75 male mice (age, 8 weeks; weight, 25±5
g), provided by Liaoning Changsheng Biotechnology Co., Ltd., were
included in the present study [SCXK (Liaoning) 2015-0001]. All
animals were raised in an environment where the ambient temperature
and relative humidity were 21±2°C and 60±10%, respectively. The
present study was approved by the ethics committee of Clinical
Medical College of Jiamusi University (approval no. 201803). All
procedures were in strict accordance with the recommendations in
the Guide for the Care and Use of Laboratory Animals of the
National Institutes of Health.
The mice were randomly divided into five groups:
Sham group (distilled water), hydrocortisone model group (25
mg/kg/day; Chengdu Mansitebio-Technology Co, Ltd.), KN15112502
group (585 mg/kg/day; Shijiazhuang No. 4, Pharmaceutical), naringin
group (100 mg/kg/day) and naringin + estrogen receptor (ER)
inhibitor group (ICI182780, 0.072 mg/kg/day; Tocris Bioscience).
The Sham group was used as the control group to verify the success
of the model construction. KN15112502 is Kang Nao Shuai Jiao Nang,
which served as a positive control in the study to testify the
effect of naringin on memory impairment mice. The naringin group is
a single-drug group to verify its neuroprotective effect on mice
with memory impairment. ICI182780 is an ER inhibitor that can block
the binding to the receptor. By adding this inhibitor, it is
determined whether naringin binds to ER and mediates the
neuroprotective effect of associated pathways. The Sham group and
the hydrocortisone model group were given intragastrically with
distilled water; the KN15112502 group and the naringin group were
gavaged with the corresponding drugs; the Naringin + ER inhibitor
group was intraperitoneally injected with ER inhibitor, and
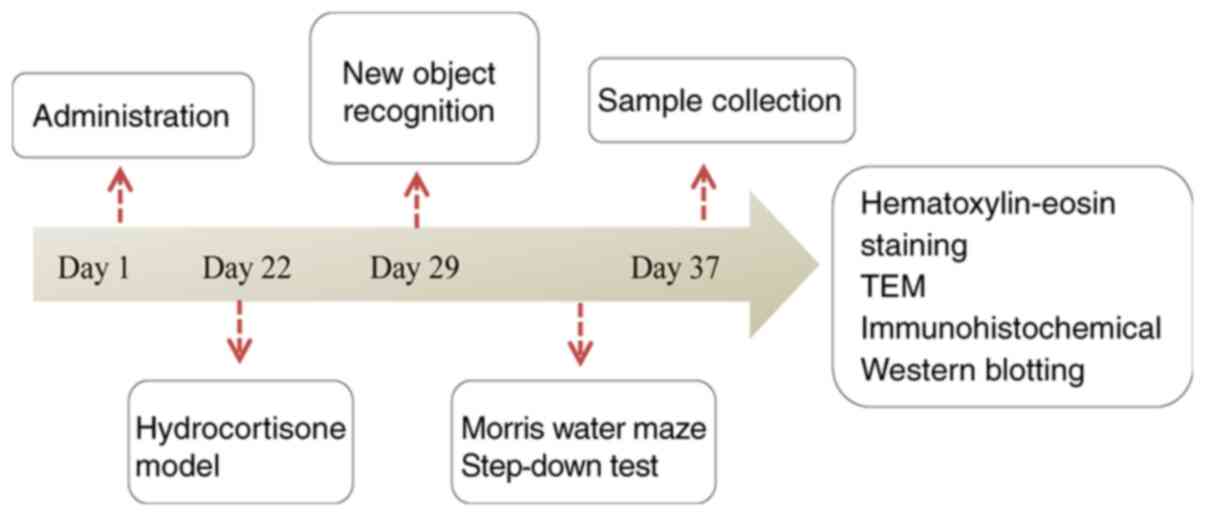
naringin was given by gavage after 15 min. After 3 weeks of
continuous gavage, except for the control group, hydrocortisone was
administered to the other four groups for modeling. After the model
was established (20), the drug was
continuously administered for 1 week, and then behavioral
experiments were conducted on the mice in each group. The flowchart
of animal experimental procedures is shown in Fig. 2.
New object recognition (NOR)
Briefly, the procedure is divided into two phases:
Training phase and testing phase. In the training phase, two
identical objects were prepared at one end of the test box, and the
mice were placed with their backs to the objects in the box and
left to explore freely for 5 min. After 1 h of training, any of the
two objects in the test box was replaced with another completely
different new object, and the other object remains unchanged. The
mice were placed in the same place and allowed to explore freely
for 5 min. The recognition time of the new and old objects by the
mice were represented by Tn and Tf, respectively, and the
recognition index was calculated as (TN-TF)/(Tn+Tf).
Morris water maze (MWM)
During the 5 consecutive days of training, each rat
was placed in water to search for the platform situated in the
water. From the time the mice entered the water, the time it took
them to locate the platform within 90 sec was recorded, which was
the escape latency. If the mice found the platform within 90 sec,
they were allowed to stay on it for 30 sec. If the mice could not
find the platform within 90 sec, they were artificially placed on
the platform for 30 sec to enhance their memory. At the 6th day,
the platform was removed and the same water inlet point was
selected. Within 90 sec, the traveled distance, time and number of
crossing the original platform were recorded.
Step-down test
The mice were placed on the platform jumper for 3
min. The copper gate was electrified and the mice were trained for
5 min. During this period of time, the mice would experience the
repeated process of jumping onto the platform after being shocked,
from the platform to the copper grid, and then jumping back to the
platform after being shocked. After 24 h, the mice were tested and
the method was consistent with the training. Briefly, the mice were
first placed on the platform, and the number of jumps from the
platform within 5 min was recorded as the number of errors. The
incubation period was defined as the time the mouse first jumped
off the platform.
Hematoxylin and eosin (H&E)
staining
After the behavioral experiment, the mice were
anesthetized (1% pentobarbital sodium, 35 mg/kg, intraperitoneal
injection; Dainippon Sumitomo Pharma) and decapitated. The brain
was quickly removed, and the hypothalamus and hippocampus were
stripped. Briefly, the hippocampal and hypothalamic tissues were
fixed in 10% formalin (cat. no. 0-10-02; Beijing Yili Fine
Chemicals Co., Ltd.) for 24 h at 37°C. Subsequently, the fixed
tissues were embedded in paraffin and cut into 5-µm sections with a
microtome. After regular dewaxing using 50% xylene for 20 min and
dehydration with 80% ethanol for 20 min at 37°C, the sections were
stained with hematoxylin for 5 min at 37°C, and eosin for 4 min at
37°C. Finally, histopathological changes in the hippocampus and
hypothalamus were observed under a light microscope (magnification,
х200).
Transmission electron microscopy
(TEM)
The hippocampus tissues (1 mm3) were
fixed in 2.5% phosphate-buffered glutaraldehyde for 20 min at 37°C
and post-fixed in 1% osmium tetroxide in water at 37°C for 30 min.
This was repeated three times, for 15 min each time. Following
gradient dehydration for each time 15 min with ethanol and acetone
(50% ethanol; 70% ethanol; 90% ethanol; 90% acetone; 100% acetone)
at 37°C, the cells were embedded and sectioned. The samples were
double stained with 50% uranyl acetate and lead citrate for 20 min
at 37°C, and the ultrastructure of the hippocampus tissues were
observed with a JEM-1200EX TEM (magnification, ×200; JEOL,
Ltd.).
Immunohistochemical analysis
Briefly, endogenous peroxidase activity within the
sections was quenched by incubating the sections with 3%
H2O2 for 15 min at 37°C after regular
dewaxing and dehydration. According to the aforementioned method,
the tissues were incubated in a humidified chamber with primary
antibodies: ERα (1:1,000; cat. no. 8644T; Cell Signaling
Technology, Inc.), ERβ (1:1,000; cat. no. kl437Hu22-KALANG;
http://www.biomart.cn/infosupply/31407572.htm)
overnight at 4°C. On the following day, the tissues were washed
with PBS and incubated with secondary antibody (1:1,000; cat. no.
bs-0295G-H; BIOSS) for 1 h at 37°C. For the negative controls, the
primary antibodies were replaced with PBS. Stained sections were
counterstained with DAB for 5 min at 37°C and observed using a
light microscope (magnification, х200; Olympus Corporation).
Western blotting
According to the manufacturer's instructions, the
proteins were extracted using RIPA lysate with proteinase inhibitor
and phosphatase inhibitor (Roche Diagnostics) and the concentration
was measured using a bicinchoninic acid assay. Protein samples (35
µg) were then separated via SDS-PAGE gels (12%) and transferred to
a PVDF membrane (EMD Millipore). After blocking with 5% non-fat
dried milk for 2.5 h at 37°C, the PVDF membrane was incubated with
primary antibodies against: APP (1:1,000; cat. no. bs-12503R;
BIOSS), BACE1 (1:1,000; cat. no. 5606T; Cell Signaling Technology,
Inc.), CDK5 (1:1,000; cat. no. bs-10258Rm; BIOSS),
p-Tau396 (1:1,000; cat. no. bs-3446R; BIOSS), glutamate
receptor subunit 1 (NMDAR1) (1:1,000; cat. no. bs-23343R; BIOSS),
glutamate receptor 2 (GluR2; 1:1,000; cat. no. bs-13385R; BIOSS),
calcium/calmodulin-dependent protein kinase type II (CAMKII;
1:1,000; Boster), Bad (1:500; cat. no. A00183; Boster), caspase-3
(1:500; cat. no. bs-0081R; BIOSS), Bcl-2 (1:500; cat. no. bs-0032R;
BIOSS), ERβ (1:1,000; cat. no. kl437Hu22; KALANG; http://www.biomart.cn/infosupply/31407572.htm), p-P38
(1:1,000; cat. no. bs-2210R; BIOSS) and β-actin (1:1,000; cat. no.
bs-0061R; BIOSS) overnight. On the following day, the protein
samples were incubated with the secondary antibody (1:1,000; cat.
no. bs-0295G-H; BIOSS) at room temperature for 45 min. The western
blotting images were observed with ECL reagent (Beyotime Institute
of Biotechnology), and the optical density of the target band was
measured using a gel image processing system (Gel-Pro-Analyzer
software).
Measurement of superoxide dismutase
(SOD), malondialdehyde (MDA), nitric oxide (NO), choline acetyl
transferase (ChAT), acetylcholinesterase (AchE) and acetylcholine
(Ach) levels
Concentrations of SOD, MDA, NO and Ach, and the
activity of ChAT and AchE in the tissues were assessed using a
commercially available SOD kit (Nanjing Jiancheng Bioengineering
Institute), MDA kit (Nanjing Jiancheng Bioengineering Institute),
NO kit (Nanjing Jiancheng Bioengineering Institute), Ach kit
(Nanjing Jiancheng Bioengineering Institute), ChAT kit (Nanjing
Jiancheng Bioengineering Institute) and AchE kit (Nanjing Jiancheng
Bioengineering Institute), respectively.
Statistical analysis
SPSS software (version 18.0; SPSS, Inc.) was used to
analyze the data. Differences among multiple groups were
statistically analyzed using one-way ANOVA and post hoc comparisons
(Bonferroni test). P<0.05 was considered to indicate a
statistically significant difference. Each experiment was repeated
three times.
Results
Naringin improved memory and cognition
in mice with memory impairment
To investigate the potential of naringin as a
therapeutic option for AD, the present study assessed the memory
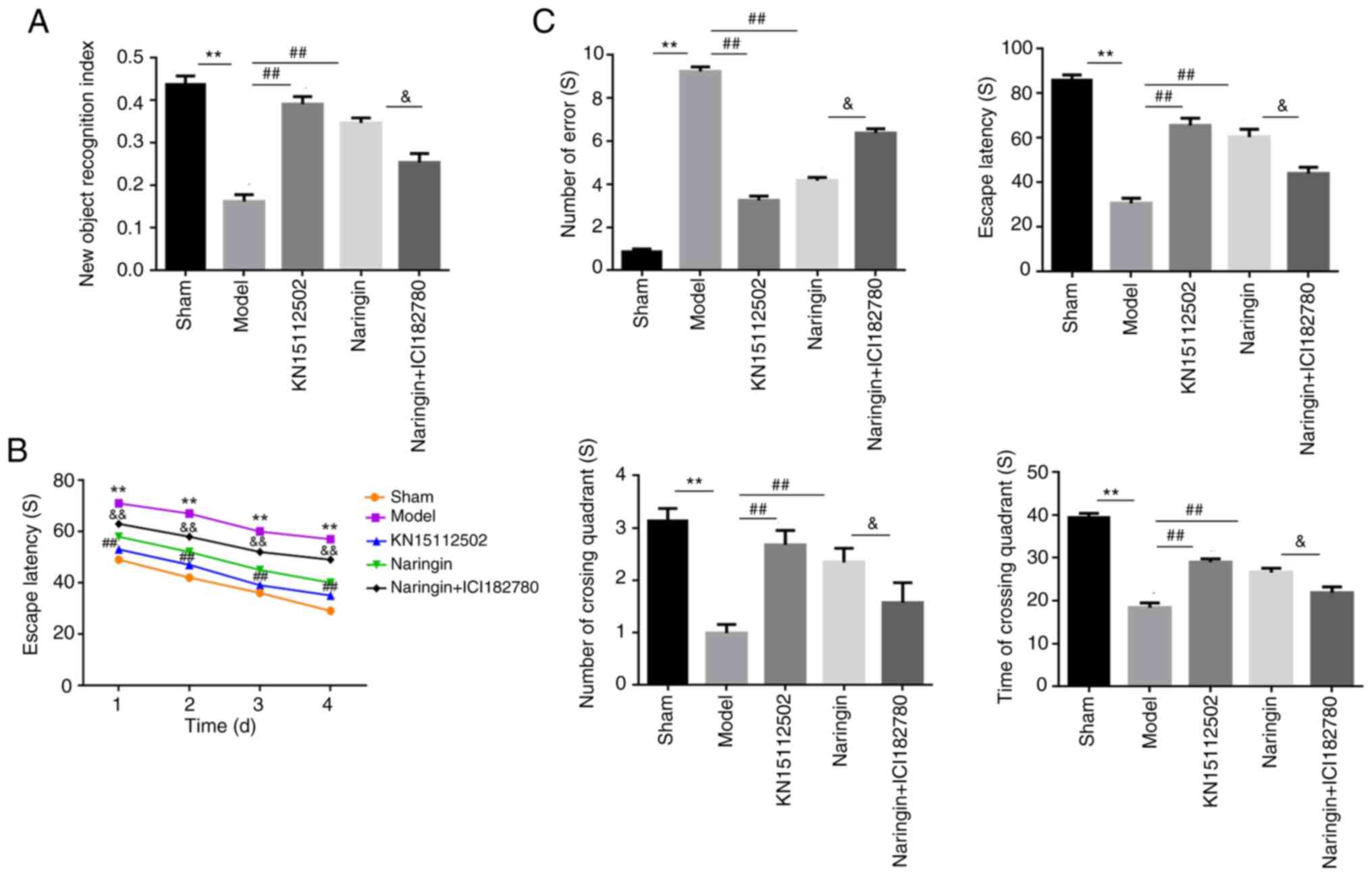
and cognitive abilities of the mice. As presented in Fig. 3A, the NOR index in the model group
was significantly decreased when compared with the sham group
(P<0.01). However, compared with the model group, the NOR index
in the naringin and KN15112502 groups were increased significantly
(P<0.01). Furthermore, compared with the Naringin group, the NOR
index of mice was significantly decreased following the addition of
ER inhibitor (P<0.05). In addition, there was no significant
difference in the NOR index between the Naringin group and the
KN15112502 group.
To further evaluate the memory and cognition in mice
with memory impairment, the present study performed a MWM. The
results revealed that, compared with the mice in the sham group,
the mice with memory impairment spent more time locating the
platform (P<0.01), and there was no significant difference
between naringin-treated mice and control mice. When the ER
inhibitor was added, the escape latency of the naringin + ER
inhibitor group was significantly increased (P<0.01; Fig. 3B). On day 5, probe trials were
performed to assess the maintenance of spatial memory. Compared
with the sham group, mice with memory impairment crossed the
platform position less frequently and spent less time on the
platform (P<0.01), and this was significantly reversed with the
addition of naringin and KN15112502. However, the ER inhibitor
significantly decreased the number of times crossing the platform
and the staying time in the naringin + ER inhibitor group of mice
(P<0.05; Fig. 3B).
In Fig. 3C, a
significant decrease in the escape latency and a significant
increase in the number of mistakes in the model group is observed
when compared with the sham group (P<0.01). However, when
Naringin or KN15112502 was added, the escape latency of the mice
increased significantly, and the number of mistakes also decreased
significantly (P<0.01). Furthermore, after treatment with the ER
inhibitor, the escape latency of the naringin + ER inhibitor group
was significantly decreased, and the number of mistakes was
significantly increased (P<0.05).
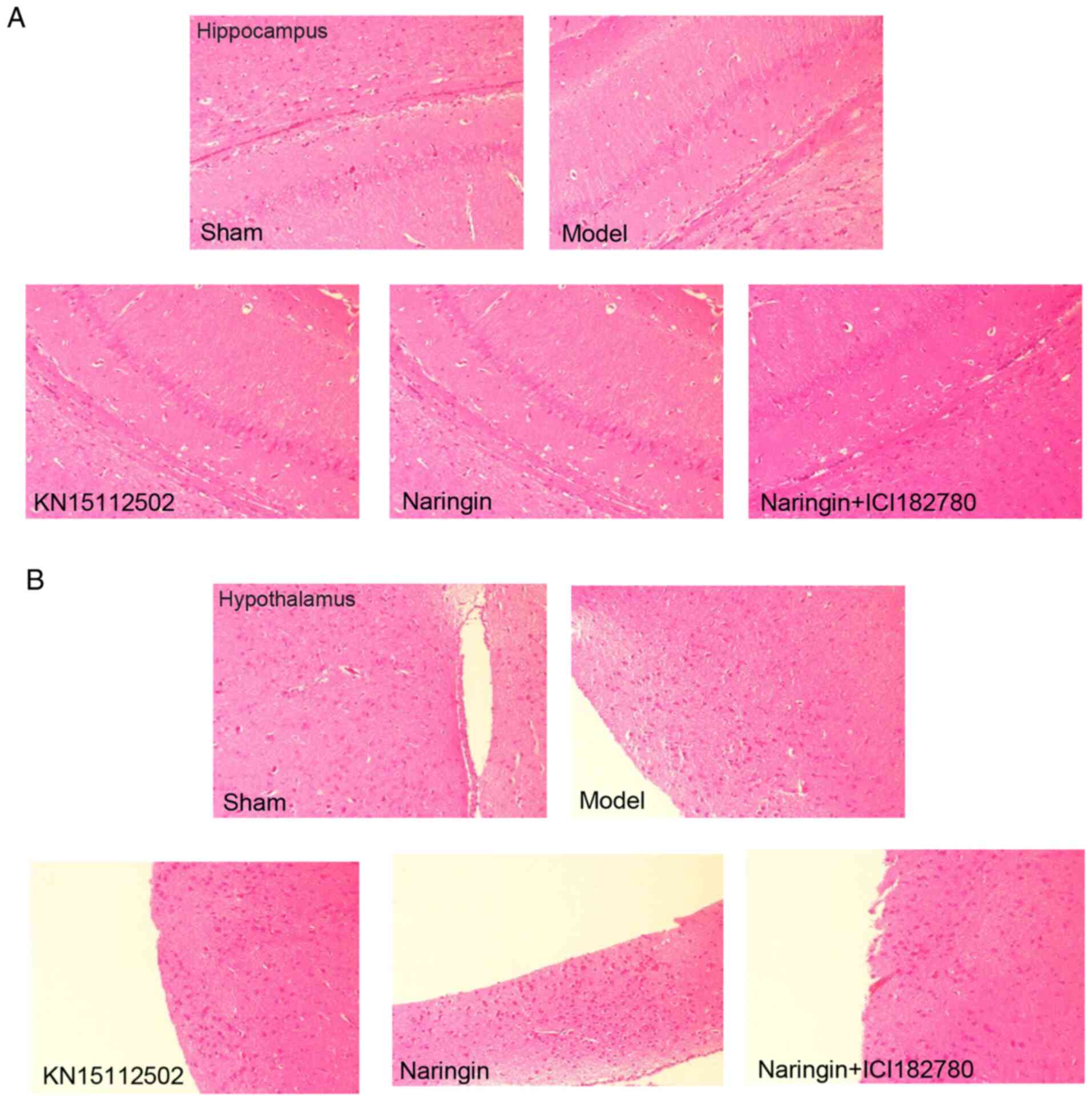
Pathological observation with H&E
staining
As presented in Fig. 4A
and B, the nuclei of the hippocampus and hypothalamus in the
sham group were closely and uniformly arranged. The neuron cells
were evenly distributed with an intact structure and no nuclear
shrinkage was observed. The cells were stained uniformly, with
clear and obvious nuclear membranes and nucleoli, and abundant
cytoplasm. In the model group, the nuclei of the hippocampal and
hypothalamus were arranged loosely and unevenly. The distribution
of neuron cells was disordered, the structure was destroyed, the
morphology was changed and the number of neuron cells was
significantly decreased. In addition, the nuclei were shrunk and
the intercellular space was abnormally enlarged.
In the naringin and KN15112502 groups, the nuclei in
the hippocampus and hypothalamus of the mice were evenly and
tightly arranged. The number of neurons was high, the distribution
was relatively uniform and compact, the structure was complete, and
there was no obvious nuclear pyknosis. The cells were stained more
evenly, and the nuclear membrane and nucleolus were obvious and
clear. In the naringin + ER inhibitor group, the arrangement of
nuclei in the hippocampus and hypothalamus of the mice was loose
and uneven. The structure of neurons was incomplete, the morphology
changed, the number of neurons was decreased significantly, and the
phenomenon of nuclear pyknosis was common (Fig. 4A and B).
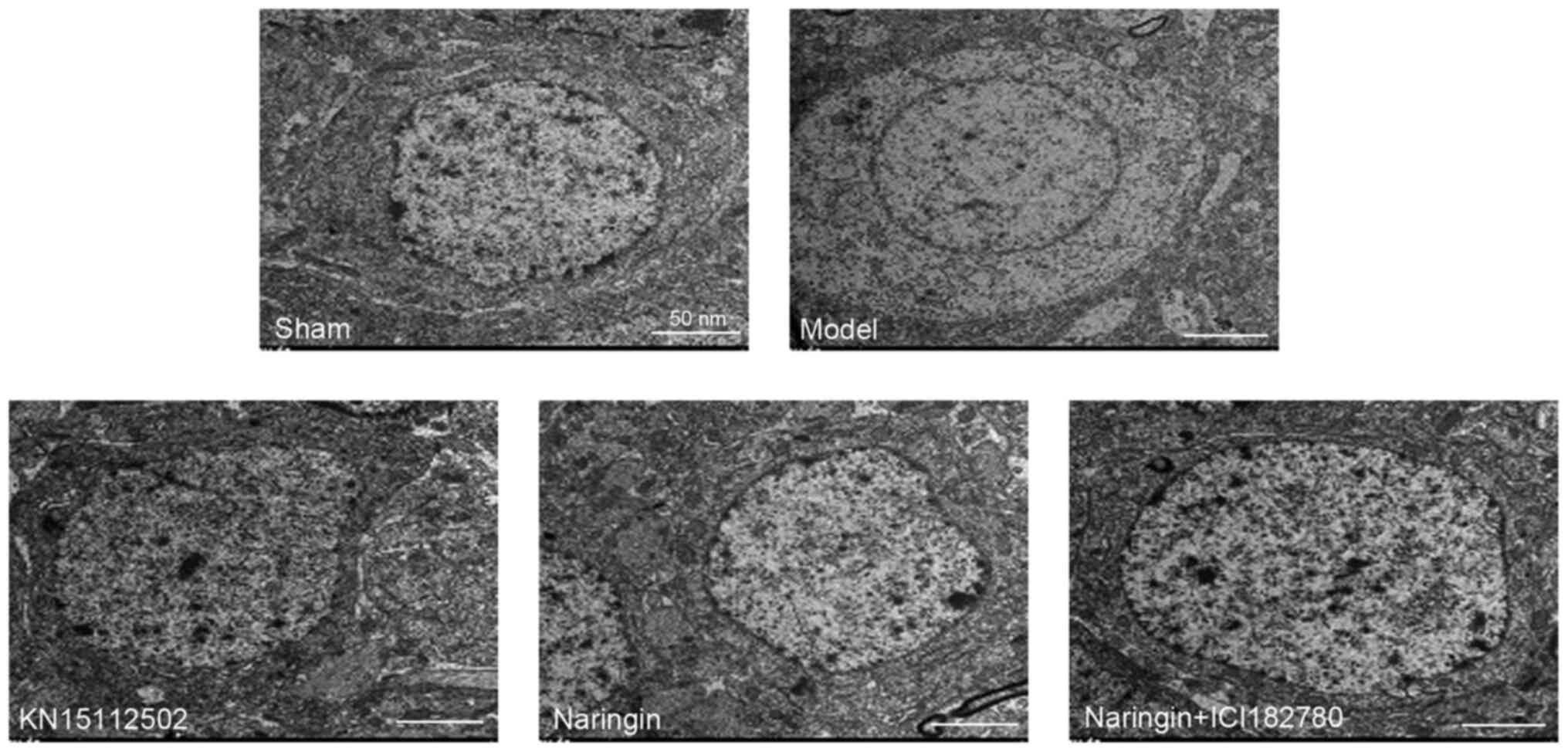
Microstructure observation with
TEM
In the sham group, the endoplasmic reticulum and
Golgi bodies were widely distributed in the cytoplasm of neurons in
the hippocampus, with intact mitochondrial structures and obvious
mitochondrial cristae. The nuclear membrane was clear and distinct,
the nucleus was normal in size and the chromatin was evenly
distributed with a shape of fine sand. The neuropil area was rich
in synapses and vesicles with normal structure. There were no
lipofuscin particles, and the number of free ribosomes was large.
In the model group, the structures of neuron endoplasmic reticulum,
mitochondrial cristae and Golgi bodies in the hippocampus of mice
were changed, and threshing and swelling occurred. The synapse
structure was destroyed and the number was decreased. The
lipofuscin particles are generated in large quantities, chromatin
is gathered at the edge of the cell. The nucleus of the neuron was
significantly contracted and the nuclear membrane was thickened
(Fig. 5).
In the naringin and KN15112502 groups, the nuclear
membrane and nucleoli of neurons were clearly visible in the
hippocampus of mice, and the nucleoli were normal in size. The
number of organelles, synapses and vesicles increased, and the
structure of mitochondria did not change significantly. In
addition, there were fewer lipofuscin particles. In the naringin +
ER inhibitor group, the number of lipofuscin particles in the
hippocampus increased, the nucleus of the neuron contracted, the
nuclear membrane thickened, and chromatin gathered at the edge of
the cell. The number of synapses and vesicles decreased, the
mitochondrial structure was destroyed, and the endoplasmic
reticulum was shelled and expanded (Fig. 5).
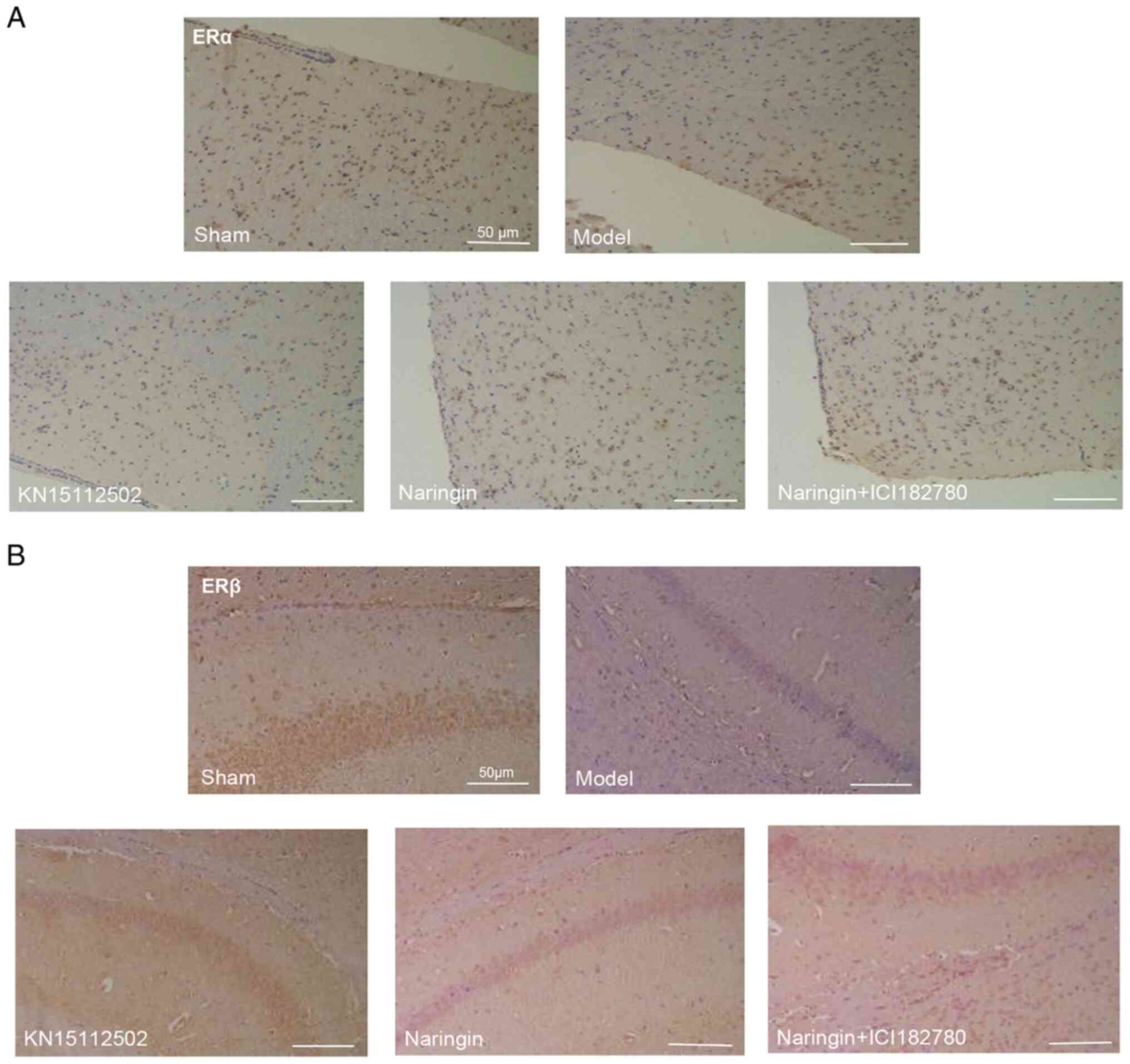
Naringin upregulates the expression of
ERβ and ERα
To investigate whether the neuroprotective effect of
naringin was ER-dependent, immunohistochemical staining was
performed in the present study. As presented in Fig. 6A and B, compared with the sham
group, the expression levels of ERβ in the hippocampus and the ERα
in the hypothalamus were significantly decreased. However,
following naringin or KN15112502 treatment, all protein expression
levels were significantly increased. More notably, when ER
inhibitor was added, the expression of the aforementioned proteins
was significantly downregulated. In addition, there were no
significant differences in the expression of ERβ in the hippocampus
or the ERα in the hypothalamus between the naringin group and the
KN15112502 group. Accordingly, the mean density of ERβ and ERα
showed a consistent trend (Fig. 6A and
B).
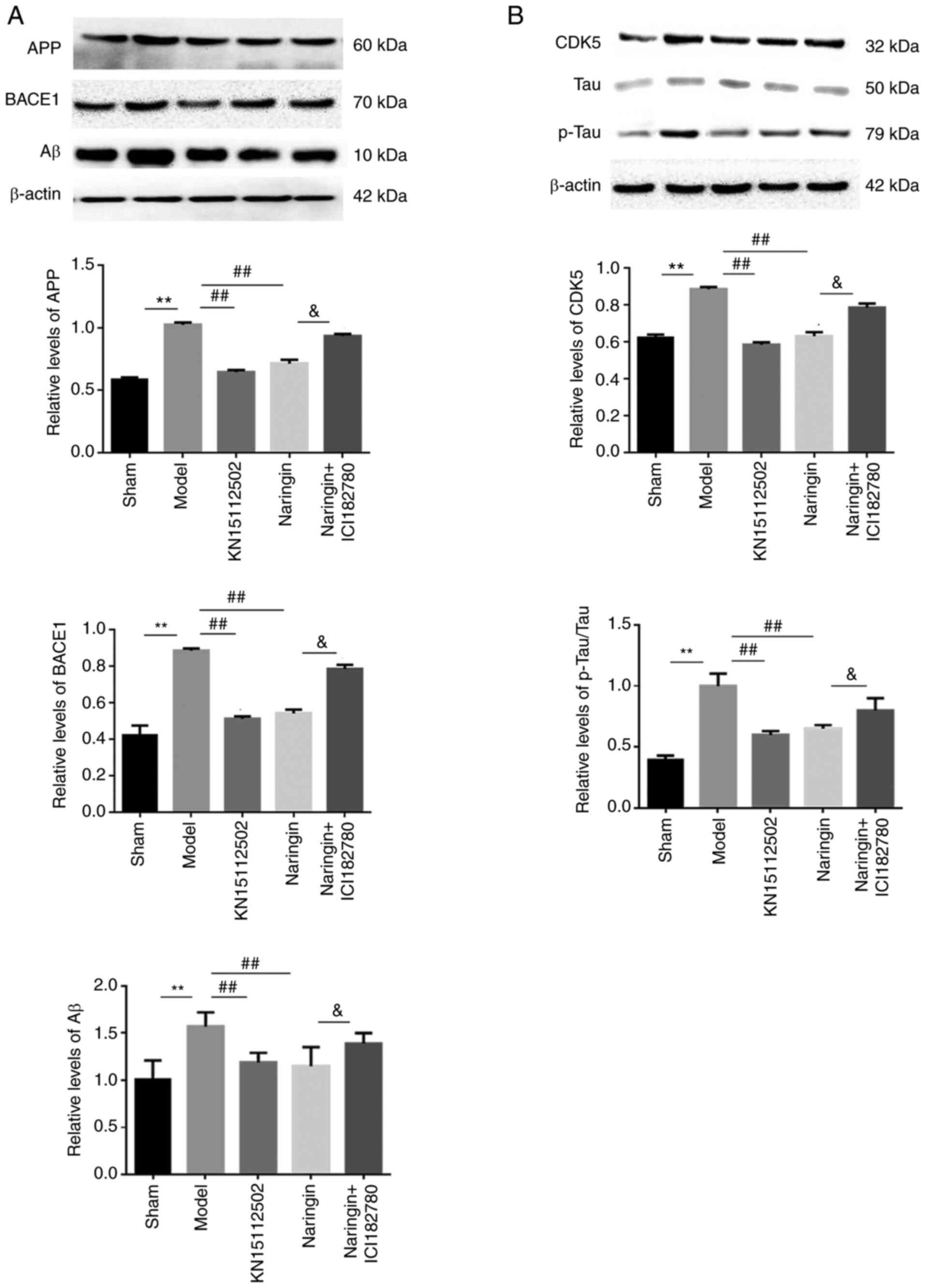
Naringin exerts neuroprotective
effects by affecting Aβ metabolism
To determine whether the neuroprotective effects of
naringin affected the metabolic pathways of Aβ, the protein
expression levels of Aβ, APP and BACE1 were detected. As presented
in Fig. 7A, it was observed that
the expression of Aβ, APP and BACE1 proteins were evidently
increased in the hippocampus of model mice, while naringin or
KN15112502 significantly inhibited this elevation (P<0.01).
Further, after the addition of ER inhibitor, Aβ, APP and BACE1
proteins in the hippocampus of mice in the naringin + ER inhibitor
group were significantly increased compared with those in the
naringin group (P<0.05).
Naringin exerts neuroprotective
effects by inhibiting the hyperphosphorylation of Tau
To assess whether the neuroprotective effect of
naringin was involved in the phosphorylation of Tau, the protein
expression levels of p-Tau and CDK5 were detected. Compared with
the sham group, the expression levels of p-Tau and CDK5 proteins in
the hippocampus of the model group mice were significantly
increased. Conversely, when Naringin or KN15112502 was added, the
expression of both proteins was decreased when compared with the
model group (P<0.01). Furthermore, the addition of ER inhibitor
slowed down the inhibitory effect of naringin on p-Tau and CDK5
(P<0.05). In addition, there were no significant differences in
p-Tau and CDK5 protein expression in the hippocampus between the
naringin group and the KN15112502 group (Fig. 7B).
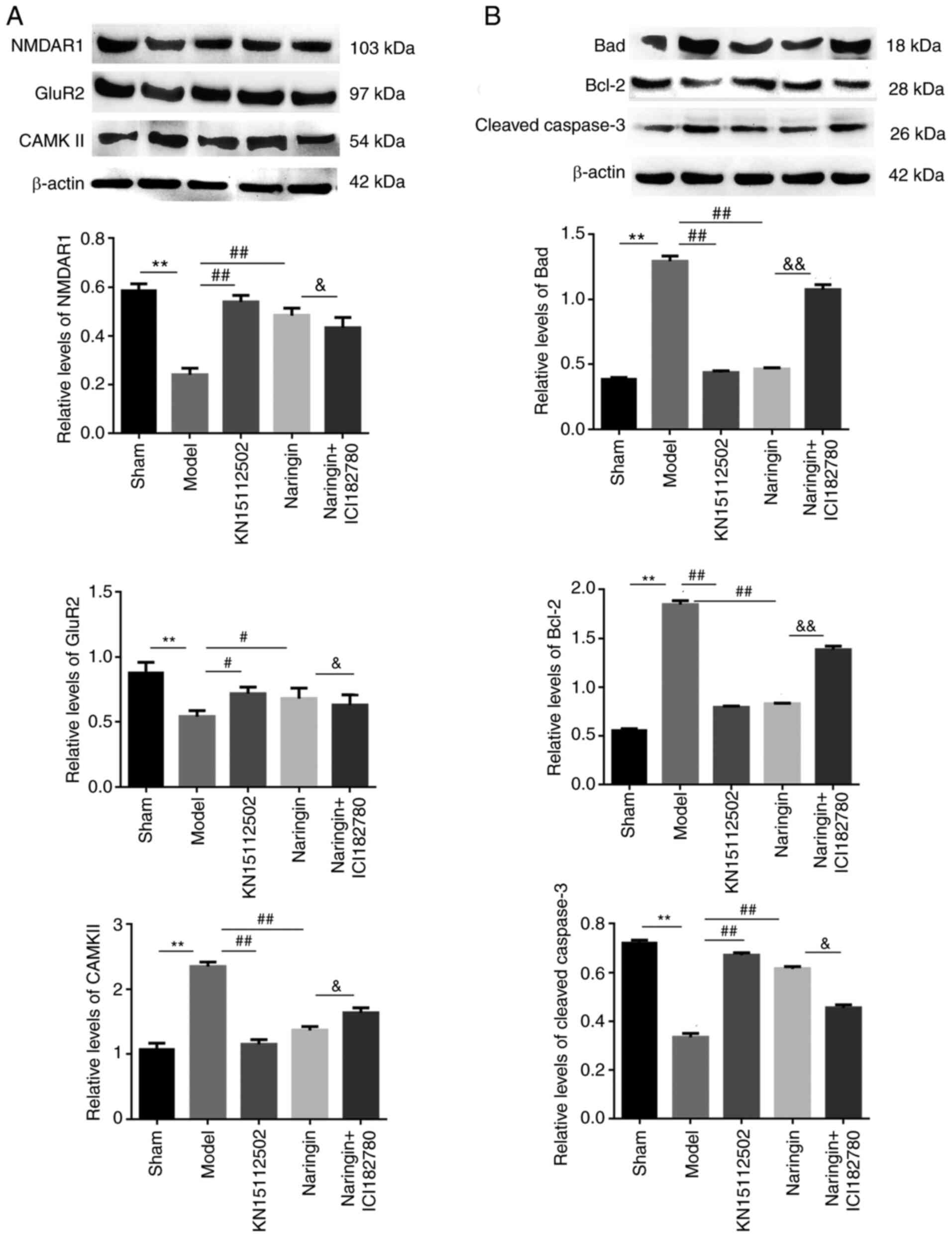
Naringin exerts neuroprotective
effects by regulating the glutamate receptor system
To assess whether the neuroprotective effects of
naringin affected the glutamate receptor system, the protein
expression levels of NMDAR1, GluR2 and CAMKII were detected. As
depicted in Fig. 8A, hydrocortisone
increased the expression of CAMKII protein and decreased the
expression of NMDAR1 and GluR2 proteins (P<0.01). Furthermore,
treatment with naringin or KN15112502 reversed the expression of
the aforementioned proteins when compared with the model group.
Compared with the drug-only group, the addition of ER inhibitors
significantly increased the expression of CAMKII protein in the
hippocampus. On the contrary, the expression of NMDAR1 and GluR2
proteins were significantly decreased (P<0.05).
Naringin exerts neuroprotective
effects by inhibiting apoptosis
In order to assess whether the neuroprotective
effect of Naringin affected cell apoptosis, the present study
detected apoptosis-associated protein expression (caspase-3, Bad
and Bcl-2). As demonstrated by the western blotting data,
hydrocortisone treatment increased the expression of
cleaved-caspase-3 and Bad protein, and decreased the expression of
Bcl-2 protein (P<0.01). However, the intervention of Naringin or
KN15112502 decreased the expression of cleaved-caspase-3 and Bad
protein, but increased the expression of Bcl-2 protein.
Furthermore, the addition of ER inhibitor reversed the effect of
naringin on the expression of the aforementioned proteins
(P<0.05; Fig. 8B).
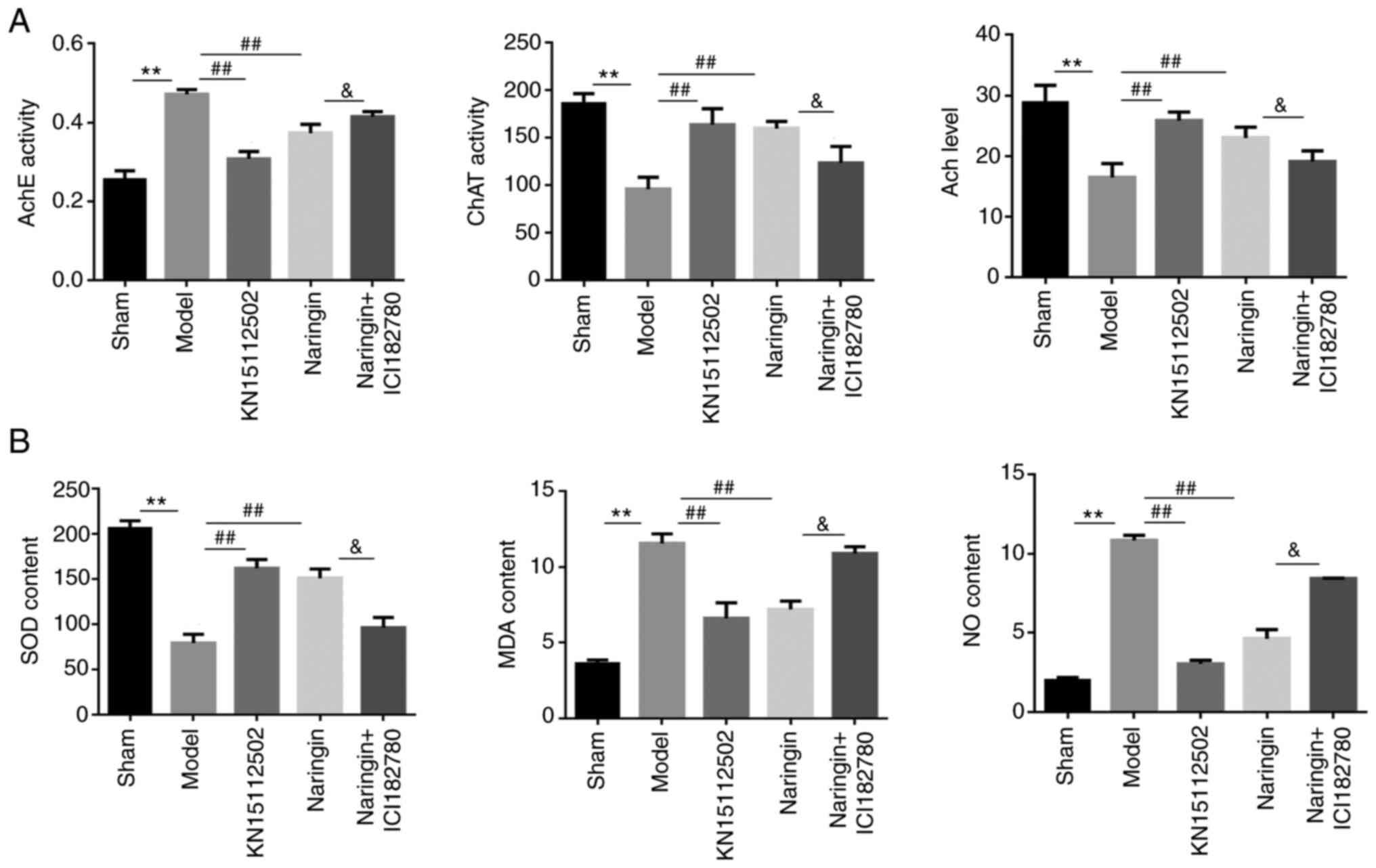
Naringin exerts neuroprotective
effects by regulating the acetylcholinergic system
To assess whether the neuroprotective effects of
naringin affect the acetylcholinergic system, the present study
detected the Ach content and the ChAT and AchE activity in the
hippocampus of each group. In the model group, the Ach content and
the ChAT activity in mice were significantly decreased, while AchE
activity was significantly increased (P<0.01). In the naringin
or KN15112502 groups, the expression patterns of the aforementioned
three proteins were in contrary to that of the model group, that
is, the Ach content and ChAT activity were significantly increased,
while AchE activity decreased significantly (P<0.01). Notably,
after the addition of ER inhibitor, the Ach content and the ChAT
activity in the in the naringin + ER inhibitor group decreased
significantly, while the AchE activity increased significantly
(P<0.05; Fig. 9A).
Naringin exerts neuroprotective
effects by inhibiting oxidative stress
To assess whether the neuroprotective effect of
naringin affected oxidative stress, the content of SOD, MDA and NO
were measured in the hippocampus of mice. As presented in Fig. 9B, hydrocortisone increased MDA and
NO content and decreased SOD content compared with the Sham group
(P<0.01). In naringin or KN15112502 group, when the
corresponding drug intervention was added, the content of MDA and
NO in the hippocampus was significantly decreased, while the
content of SOD significantly increased (P<0.01). Notably, when
ER inhibitor was added, the MDA and NO contents in the naringin +
ER inhibitor group were significantly increased, and SOD contents
were significantly decreased when compared with naringin
(P<0.05).
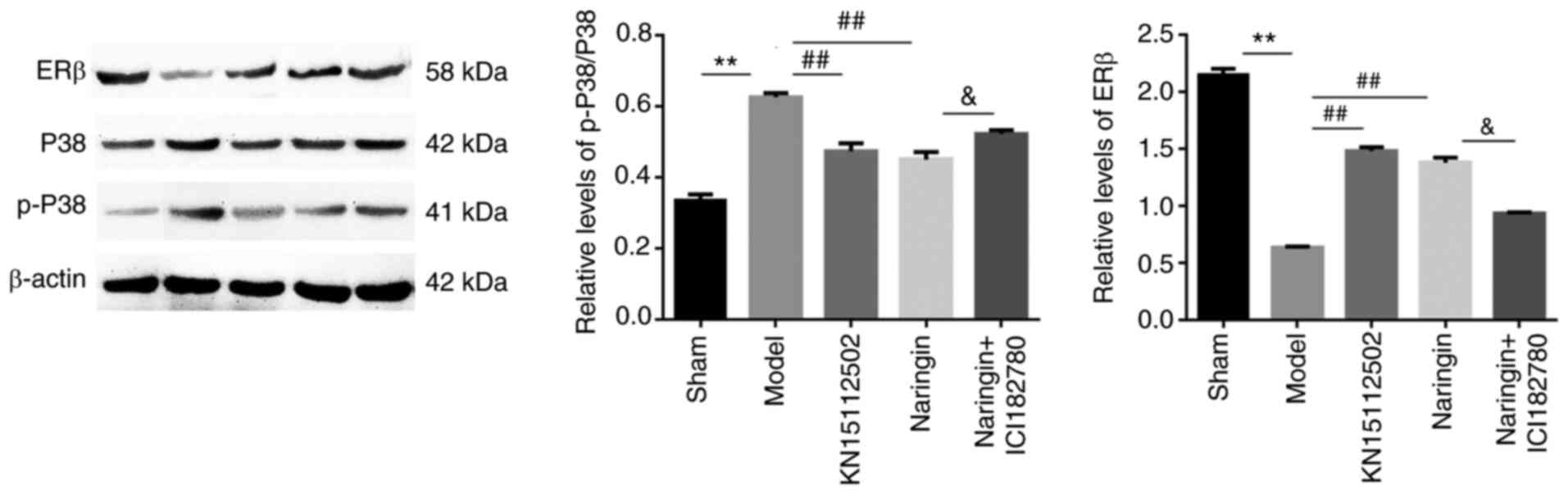
Naringin upregulates the expression of
ERβ and downregulates the expression of P38/P-P38 in the
hippocampus
In order to assess whether the MAPK/P38 signaling
pathway was involved in the neuroprotective effect of naringin, the
changes in protein expression of P38/p-P38 and ERβ were detected.
As presented in Fig. 10, it was
observed that the expression of ERβ protein in the model group was
significantly decreased, and the expression of P38/p-P38 protein
was significantly increased. However, following Naringin or
KN15112502 treatment, the expression of P38/P-P38 protein was
significantly decreased, while the opposite was observed for ERβ
(P<0.01). Notably, when the ER inhibitor was added, the ER
inhibitor decreased the effect of naringin on the expression of the
aforementioned proteins. In addition, there was no significant
difference in ERβ and P38/p-P38 protein expression between the
naringin group and KN15112502 groups.
Discussion
The obvious clinical manifestation of AD is the
decline in learning and memory ability (19). Rodents have an instinctive habit of
exploring strange objects, and their learning and cognitive
abilities can be reflected by the amount of time spent exploring
new and old objects (21). In the
present study, NOR results showed that naringin significantly
increased the recognition index of the model group mice and
improved their memory and learning disabilities. MWM is commonly
used to measure spatial memory and learning ability (22). The data in the present study
indicated that naringin sharply decreased the latency of mice in
the model group, and significantly increased the number of times
the mice crossed the platform, as well as the residence time in the
target quadrant, indicating that naringin could improve their
spatial learning and memory ability. The step-down test is designed
by using the animals' habits of being active, exploring space,
seeking advantages and avoiding disadvantages, and is usually used
to test learning and memory abilities (23). When the mice received multiple
electric shocks, they will have a memory of this and avoid jumping
in again. In the present study, the frequency of receiving electric
shock of mice was significantly decreased, and the number of
jumping mistakes was also decreased in the naringin group,
indicating that naringin could enhance the memory ability of mice.
Collectively, behavioral studies indicated that naringin evidently
improved the learning and memory disorders of mice with memory
impairment, and played a neuroprotective effect.
APP is catalyzed by α, β and γ-type starch
secretases, and its products can be divided into soluble amyloid
protein (sAPP) and insoluble Aβ. β-site APP cleaving enzyme 1
(BACE1) can catalyze the hydrolysis of APP, which in turn leads to
the increase in Aβ production (24). Excessive deposition of Aβ is toxic
to nerve cells and can cause nerve cell damage (25). Previously it has been reported that
glycyrrhizin decreased the production of Aβ, weakened the toxicity
to nerve cells and significantly improved memory and cognitive
performance in ovariectomized AD model mice (26). The results of the present study
demonstrated that naringin inhibited the expression of BACE1,
decreased the content of APP and the production of Aβ, which had a
neuroprotective effect on model mice. Tau protein is a phosphorous
microtubule protein, which can stabilize microtubule structure
(27). However, its abnormal
modification can cause the Tau protein to lose the compositional
function of tubulin and trigger the disintegration of microtubules,
which affects the normal transport function of neuronal cells and
nerve endings (6). Besides,
hyperphosphorylated Tau protein has a double-helical structural
change, which promotes the formation of NFTs and induces AD.
Cyclin-dependent kinase 5 (CDK5) is a major regulatory enzyme for
Tau phosphorylation, which can affect the phosphorylation of Tau
(28). The inhibition of CDK5 can
decrease the production of hyperphosphorylated Tau and thus
decrease the damage to nerve cells. For instance, curcumins are
suggested to have therapeutic potential for AD by inhibiting Tau
phosphorylation (29). In the
present study, it was demonstrated that naringin decreased the
expression levels of CDK-5 and p-Tau, indicating that naringin
played neuroprotective effect by inhibiting the
hyperphosphorylation of Tau.
Glutamate (Glu) is an excitatory neurotransmitter
that plays a variety of functions by binding to receptors and is an
important participant in cognitive and memory functions (30). NMDAR1 and GluR2 are two important
receptors in the glutamate system. Excessive Glu activates NMDA
excessively, causing continuous large influx of Ca2+,
leading to cell damage, and CaMKII is activated and phosphorylated,
which intensifies NMDA-mediated Ca2+ influx, causing
more serious damage to nerve cells (31,32).
In the present study, it was demonstrated that naringin
intervention increased the expression of NMDAR1 and GluR2 and
inhibited the expression of CaMKII, suggesting that naringin can
exert neuroprotective effects by affecting the glutamate receptor
system. Neuron loss is an obvious pathological feature of AD, while
neuron apoptosis is the main cause of neuron loss (33). In previous molecular research on
neuronal cell apoptosis, Bad has been shown to be a pro-apoptotic
protein, and Bcl-2 is an anti-apoptotic protein (34). In addition, caspase-3 is the major
executor of apoptosis (35). Gu
et al (36) showed that
FC101 downregulates the expression of Bcl-2, increased the
expression of Bad and cleaved caspase-3. In the present study, it
was revealed that Naringin inhibited cleaved caspase-3 and Bad,
increased the Bcl-2 expression, and thereby decreased the apoptosis
of cells and played a neuroprotective role, which is consistent
with previous reports.
Currently, a previous study has shown that the
central cholinergic system in patients with AD is obviously damaged
and defective, and is consistent with the clinical symptoms of
these patients (7). Ach can
regulate the plasticity of synapses in nerve endings for memory and
learning (7). ChaT is a key enzyme
synthesized by Ach (37), while the
main role of AchE is to degrade Ach. In patients with AD, the
content of AchE was significantly increased, and the level of ChaT
was significantly decreased, resulting in dysfunctions in the
synthesis, release and degradation of Ach, and ultimately resulting
in a significant decline in brain learning and memory functions
(38). In the present study,
naringin markedly increased the activity of ChAT in the
hippocampus, decreased the activity of AchE, increased the content
of Ach and increased the activity and function of cholinergic
neurons, thereby exerting neuroprotective effects. Oxidative stress
can lead to peroxidation effects on nerve cells, resulting in their
damage (39). MDA and excessive NO
produced by lipid peroxidation are neurotoxic, and their content
can reflect the degree of damage to nerve cells. SOD has a strong
antioxidant ability, which can effectively remove excess superoxide
anions and decrease the oxidation of cells to achieve the
protection of cells (40). Wang
et al (41) revealed that
Scutellarin can decrease MDA content, enhance SOD activity and
decrease cell damage caused by oxidative stress. Consistently, in
the present study, Naringin intervention increased the content of
SOD and decreased the production of MDA and NO in the hippocampus
of mice, indicating that naringin could decrease its toxicity to
nerve cells and play a neuroprotective role by inhibiting oxidative
stress.
Phytoestrogen-derived plants have similar effects to
estrogen and can bind with ER to regulate the gene transcription
process of certain substances in neurons so as to control their
expression. In addition, it can also mediate multiple signaling
pathways to protect nerve cells and improve learning and memory
capabilities (42). MAPK is a
silk/threonine protein kinase that exerts a series of physiological
effects by binding to membrane ERs (43). P38 is one of the main proteins in
the MAPK family, and p38 MAPK is the main way for MAPK to regulate
the physiological response of cells. When p38 MAPK is stimulated,
it forms a stress MAPK signaling pathway, accelerates cell
apoptosis and promotes inflammation (44). In the present study, it was
demonstrated that naringin significantly increased the protein
expression of ERβ and ERα. However, following the addition of ER
inhibitor, the results of the naringin+ER inhibitor group in the
aforementioned tests were significantly different from those of the
naringin group, but almost the same as the results of the model
group, suggesting that naringin can play a neuroprotective role in
memory-impaired mice through the ER pathway. In addition, it was
also revealed that naringin could decrease the p-P38/P38 content,
indicating that naringin may activate the P38 MAPK pathway after
binding with ER and plays a neuroprotective role. However, this
mechanism needs to be verified by further experimental studies.
The study concluded that naringin may interact with
ERs in the hippocampus of mice to mediate Aβ metabolism, Tau
protein hyperphosphorylation, acetylcholinergic system, glutamate
receptor system, oxidative stress and cell apoptosis, thereby
playing neuroprotective effects. Furthermore, naringin can also
affect the expression of p-P38/P38. However, there are some
limitations in the present study. Firstly, the direct interaction
between naringin and ERs to regulate metabolic pathways associated
with AD was not demonstrated; therefore, future studies will prove
through further experiments, such as by co-immunoprecipitation
studies. Secondly, it was found that Naringin affected the
expression of p-P38/P38, but whether it plays a role through the
P38 MAPK pathway requires further verification.
Acknowledgements
Not applicable.
Funding
This study was supported by National Natural Science
Foundation of China (grant no. 81673581), Heilongjiang University
of Chinese Medicine Excellent Innovation Talents (grant no.
2018RCD19), Jiamusi University Provincial Universities Fundamental
Research Business Expenses Research Project (grant no.
2019-KYYWF-1349), Heilongjiang Postdoctoral Fund (grant no.
LBH-Z16252) and Research and development and cultivation projects
of scientific and technological achievements of universities in
Heilongjiang Province (grant no. TSTAU-C2018020).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JW and NZ conceived and designed the study. XM, MF
and SW provided the materials, and samples and analyzed the data.
WC and JW were involved in the acquisition of data. JW and NZ
evaluated the authenticity of all the raw data and confirmed its
legitimacy. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Clinical Medical College of Jiamusi University. All procedures are
in strict accordance with the recommendations in the Guide for the
Care and Use of Laboratory Animals of the National Institutes of
Health.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sachdeva AK, Kuhad A and Chopra K:
Naringin ameliorates memory deficits in experimental paradigm of
Alzheimer's disease by attenuating mitochondrial dysfunction.
Pharmacol Biochem Behav. 127:101–110. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ballatore C, Lee VM and Trojanowski JQ:
Tau-mediated neurodegeneration in Alzheimer's disease and related
disorders. Nat Rev Neurosci. 8:663–672. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Auld DS, Kornecook TJ, Bastianetto S and
Quirion R: Alzheimer's disease and the basal forebrain cholinergic
system: Relations to beta-amyloid peptides, cognition, and
treatment strategies. Prog Neurobiol. 68:209–245. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Honjo H, Iwasa K, Kawata M, Fushiki S,
Hosoda T, Tatsumi H, Oida N, Mihara M, Hirasugi Y, Yamamoto H, et
al: Progestins and estrogens and Alzheimer's disease. J Steroid
Biochem Mol Biol. 93:305–308. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gao Y, Li C, Yin J, Shen J, Wang H, Wu Y
and Jin H: Fucoidan, a sulfated polysaccharide from brown algae,
improves cognitive impairment induced by infusion of Aβ peptide in
rats. Environ Toxicol Pharmacol. 33:304–311. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hampel H, Blennow K, Shaw LM, Hoessler YC,
Zetterberg H and Trojanowski JQ: Total and phosphorylated tau
protein as biological markers of Alzheimer's disease. Exp Gerontol.
45:30–40. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ferreira-Vieira TH, Guimaraes IM, Silva FR
and Ribeiro FM: Alzheimer's disease: Targeting the cholinergic
system. Curr Neuropharmacol. 14:101–115. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Medina M: Recent developments in tau-based
therapeutics for neurodegenerative diseases. Recent Pat CNS Drug
Discov. 6:20–30. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
He LY, He XH, Pang GF, Liang QH, Yang Z
and Hu CY: The pathogenesis and treatment of Alzheimer's disease.
Chin Geriatric Care Med. 15:12–14. 2017.(In Chinese).
|
|
10
|
Chen L and DiWu YC: Research progress on
the relationship between hippocampal nerve cell senescence and
neurogenesis and Alzheimer's disease. Hebei J Tradit Chin Med.
39:1583–1587. 2017.(In Chinese).
|
|
11
|
Guzior N, Wieckowska A, Panek D and
Malawska B: Recent development of multifunctional agents as
potential drug candidates for the treatment of Alzheimer's disease.
Curr Med Chem. 22:373–404. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu ZL, Xu MY, Wang HT, Xu QX, Liu MY, Jia
CP, Geng F and Zhang N: Pharmacokinetics of eight flavonoids in
rats assayed by UPLC-MS/MS after oral administration of
Drynariae rhizoma extract. J Anal Methods Chem.
2018:47891962018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang Z, Kuboyama T and Tohda C: A
Systematic strategy for discovering a therapeutic drug for
Alzheimer's disease and its target molecule. Front Pharmacol.
8:3402017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen R, Qi QL, Wang MT and Li QY:
Therapeutic potential of naringin: An overview. Pharm Biol.
54:3203–3210. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Golechha M, Sarangal V, Bhatia J, Chaudhry
U, Saluja D and Arya DS: Naringin ameliorates
pentylenetetrazol-induced seizures and associated oxidative stress,
inflammation, and cognitive impairment in rats: Possible mechanisms
of neuroprotection. Epilepsy Behav. 41:98–102. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Golechha M, Chaudhry U, Bhatia J, Saluja D
and Arya DS: Naringin protects against kainic acid-induced status
epilepticus in rats: Evidence for an antioxidant, anti-inflammatory
and neuroprotective intervention. Biol Pharm Bull. 34:360–365.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kumar P and Kumar A: Protective effect of
hesperidin and naringin against 3-nitropropionic acid induced
Huntington's like symptoms in rats: Possible role of nitric oxide.
Behav Brain Res. 206:38–46. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cao W, Feng SJ and Kan MC: Naringin
targets NFKB1 to alleviate oxygen-glucose
deprivation/reoxygenation-induced injury in PC12 cells via
modulating HIF-1α/AKT/mTOR-signaling pathway. J Mol Neurosci.
71:101–111. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang DM, Yang YJ, Zhang L, Zhang X, Guan
FF and Zhang LF: Naringin enhances CaMKII activity and improves
long-term memory in a mouse model of Alzheimer's disease. Int J Mol
Sci. 14:5576–5586. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li C, Wang H and Wang L: Replication of a
rat model of renal insufficiency Alzheimer's disease. J Anhui Coll
Tradit Chin Med. 32:62–67. 2013.(In Chinese).
|
|
21
|
Mathiasen JR and DiCamillo A: Novel object
recognition in the rat: A facile assay for cognitive function. Curr
Protoc Pharmacol Chapter. 5:Unit 5.59. 2010.
|
|
22
|
Gao Y, Yin H, Zhang Y, Dong Y, Yang F, Wu
X and Liu H: Dexmedetomidine protects hippocampal neurons against
hypoxia/reoxygenation-induced apoptosis through activation
HIF-1α/p53 signaling. Life Sci. 232:1166112019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bolt D, Giger R, Wirth S and Swanenburg J:
Step-down test assessment of postural stability in patients with
chronic ankle instability. J Sport Rehabil. 27:2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kimura A, Hata S and Suzuki T: Alternative
selection of β-site APP-cleaving enzyme 1 (BACE1) cleavage sites in
amyloid β-protein precursor (APP) harboring protective and
pathogenic mutations within the Aβ sequence. J Biol Chem.
291:24041–24053. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Holtzman DM, Morris JC and Goate AM:
Alzheimer's disease: The challenge of the second century. Sci
Transl Med. 3:77sr12011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ali MY, Jannat S, Edraki N, Das S, Chang
WK, Kim HC, Park SK and Chang MS: Flavanone glycosides inhibit
β-site amyloid precursor protein cleaving enzyme 1 and
cholinesterase and reduce Aβ aggregation in the amyloidogenic
pathway. Chem Biol Interact. 309:1087072019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lopes S, Lopes A, Pinto V, Guimarães MR,
Sardinha VM, Duarte-Silva S, Pinheiro S, Pizarro J, Oliveira JF,
Sousa N, et al: Absence of Tau triggers age-dependent sciatic nerve
morphofunctional deficits and motor impairment. Aging Cell.
15:208–216. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Saito T, Oba T, Shimizu S, Asada A, Iijima
KM and Ando K: Cdk5 increases MARK4 activity and augments
pathological tau accumulation and toxicity through tau
phosphorylation at Ser262. Hum Mol Genet. 28:3062–3071. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ma QL, Yang F, Rosario ER, Ubeda OJ, Beech
W, Gant DJ, Chen PP, Hudspeth B, Chen C, Zhao Y, et al:
Beta-amyloid oligomers induce phosphorylation of tau and
inactivation of insulin receptor substrate via c-Jun N-terminal
kinase signaling: Suppression by omega-3 fatty acids and curcumin.
J Neurosci. 29:9078–9089. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fan S, Xian X, Li L, Yao X, Hu Y, Zhang M
and Li W: Ceftriaxone improves cognitive function and upregulates
GLT-1-related glutamate-glutamine cycle in APP/PS1 mice. J
Alzheimers Dis. 66:1731–1743. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ashpole NM and Hudmon A: Excitotoxic
neuroprotection and vulnerability with CaMKII inhibition. Mol Cell
Neurosci. 46:720–730. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pellegrini-Giampietro DE, Gorter JA,
Bennett MV and Zukin RS: The GluR2 (GluR-B) hypothesis:
Ca(2+)-permeable AMPA receptors in neurological disorders. Trends
Neurosci. 20:464–470. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Caricasole A, Copani A, Caraci F, Aronica
E, Rozemuller AJ, Caruso A, Storto M, Gaviraghi G, Terstappen GC
and Nicoletti F: Induction of Dickkopf-1, a negative modulator of
the Wnt pathway, is associated with neuronal degeneration in
Alzheimer's brain. J Neurosci. 24:6021–6027. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jarskog LF, Selinger ES, Lieberman JA and
Gilmore JH: Apoptotic proteins in the temporal cortex in
schizophrenia: High Bax/Bcl-2 ratio without caspase-3 activation.
Am J Psychiatry. 161:109–115. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lok J and Martin LJ: Rapid subcellular
redistribution of Bax precedes caspase-3 and endonuclease
activation during excitotoxic neuronal apoptosis in rat brain. J
Neurotrauma. 19:815–825. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gu Y, Chen X, Shang C, Singh K, Barzegar
M, Mahdavian E, Salvatore BA, Jiang S and Huang S: Fusarochromanone
induces G1 cell cycle arrest and apoptosis in COS7 and HEK293
cells. PLoS One. 11:e1126412014. View Article : Google Scholar
|
|
37
|
Goodenough S, Schäfer M and Behl C:
Estrogen-induced cell signalling in a cellular model of Alzheimer's
disease. J Steroid Biochem Mol Biol. 84:301–305. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang H, Muiznieks LD, Ghosh P, Williams D,
Solarski M, Fang A, Ruiz-Riquelme A, Pomès R, Watts JC,
Chakrabartty A, et al: Somatostatin binds to the human amyloid β
peptide and favors the formation of distinct oligomers. Elife.
6:e284012017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Torres-Cuevas I, Parra-Llorca A,
Sánchez-Illana A, Nuñez-Ramiro A, Kuligowski J, Cháfer-Pericás C,
Cernada M, Escobar J and Vento M: Oxygen and oxidative stress in
the perinatal period. Redox Biol. 12:674–681. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Amin MM, Rafiei N, Poursafa P, Ebrahimpour
K, Mozafarian N, Shoshtari-Yeganeh B, Hashemi M and Kelishadi R:
Association of benzene exposure with insulin resistance, SOD, and
MDA as markers of oxidative stress in children and adolescents.
Environ Sci Pollut Res Int. 25:34046–34052. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang Z, Yu J, Wu J, Qi F, Wang H, Wang Z
and Xu Z: Scutellarin protects cardiomyocyte ischemia-reperfusion
injury by reducing apoptosis and oxidative stress. Life Sci.
157:200–207. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang YX, Tian K, He CC, Ma XL, Zhang F,
Wang HG, An D, Heng B, Jiang YG and Liu YQ: Genistein inhibits
hypoxia, ischemic-induced death, and apoptosis in PC12 cells.
Environ Toxicol Pharmacol. 50:227–233. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Xu XF, Liu F, Xin JQ, Fan JW, Wu N, Zhu
LJ, Duan LF, Li YY and Zhang H: Respective roles of the
mitogen-activated protein kinase (MAPK) family members in
pancreatic stellate cell activation induced by transforming growth
factor-β1 (TGF-β1). Biochem Biophys Res Commun. 501:365–373. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sui X, Kong N, Ye L, Han W, Zhou J, Zhang
Q, He C and Pan H: p38 and JNK MAPK pathways control the balance of
apoptosis and autophagy in response to chemotherapeutic agents.
Cancer Lett. 344:174–179. 2014. View Article : Google Scholar : PubMed/NCBI
|