Introduction
Nicotine, or 3-(1-Methylpyrrolidin-2-yl) pyridine,
is an alkaloid that is found in the tobacco plant (1,2).
Nicotine use can lead to a number of health complications,
including heart and lung diseases, and increases the risk of cancer
occurrence (3) and the
susceptibility to several infectious diseases, including
tuberculosis, pneumonia and sexually transmitted diseases such as
chlamydia (4). However, increasing
evidence suggests that nicotine also has beneficial health effects,
particularly in terms cognitive function.
Nicotine acts as an agonist of nicotinic cholinergic
receptors (nAChRs), which are found in both the central nervous
system (CNS) and the peripheral nervous system (2,5,6). Each
nAChR comprises five α or β subunits (7). There are nine potential α subunits and
three β subunits, and different nAChR receptor subtypes possess
varying compositions of these subunits (8,9). The
most abundant receptor subtypes present in the human brain are
α4β2, α3β4
(heterogenic) and α7 (homomeric) (10). The α3β4 nAChR
is known to mediate the cardiovascular effects of nicotine
(11), while the homomeric
α7 nAChR is speculated to be involved in synaptic
transmission, as well as in learning and sensory gating (12,13).
Stimulation of nAChRs in the CNS by nicotine or acetylcholine
regulates the release of a variety of neurotransmitters, such as
dopamine, glutamate, serotonin, norepinephrine and γ-aminobutyric
acid (14,15). Therefore, alterations in the
expression or function of nAChRs, as a result of a disease, may
alter the release of other neurotransmitters and, thus, affect
brain function.
It is commonly known that long-term exposure to
nicotine causes nAChR desensitization (16), leading to memory impairment in
otherwise healthy individuals (17). Such nicotine-induced cognitive
dysfunction is associated with several mechanisms, including
activation of the phosphodiesterase-5 (PDE-5) signaling pathway and
inhibition of estrogen biosynthesis (18,19).
In particular, nicotine stimulates the expression of PDE-5
(19,20), which plays a role in cleaving cyclic
guanosine monophosphate and cyclic adenosine monophosphate that
activate downstream signaling pathways contributing to memory
impairment (21–23). Nicotine also blocks estrogen
synthase (aromatase) in the brain, which is important for estrogen
biosynthesis (18,24). Estrogen activates estrogen receptors
in the brain, which function as transcriptional factors and enhance
the expression of several neurotransmitters (including glutamate,
acetylcholine, serotonin and noradrenaline), and thus stimulate the
neuronal circuits required for memory encoding (25). Therefore, alterations in estrogen
biosynthesis due to nicotine (20,26),
as well as the nicotine-induced elevation of PDE-5 levels, can lead
to cognitive impairment in healthy individuals.
In contrast to these detrimental effects of nicotine
on cognitive function, some studies report that nicotine also has
beneficial effects on memory and learning processes. Thus, the
present review summarizes the potential benefits of nicotine on
cognition (Fig. 1).
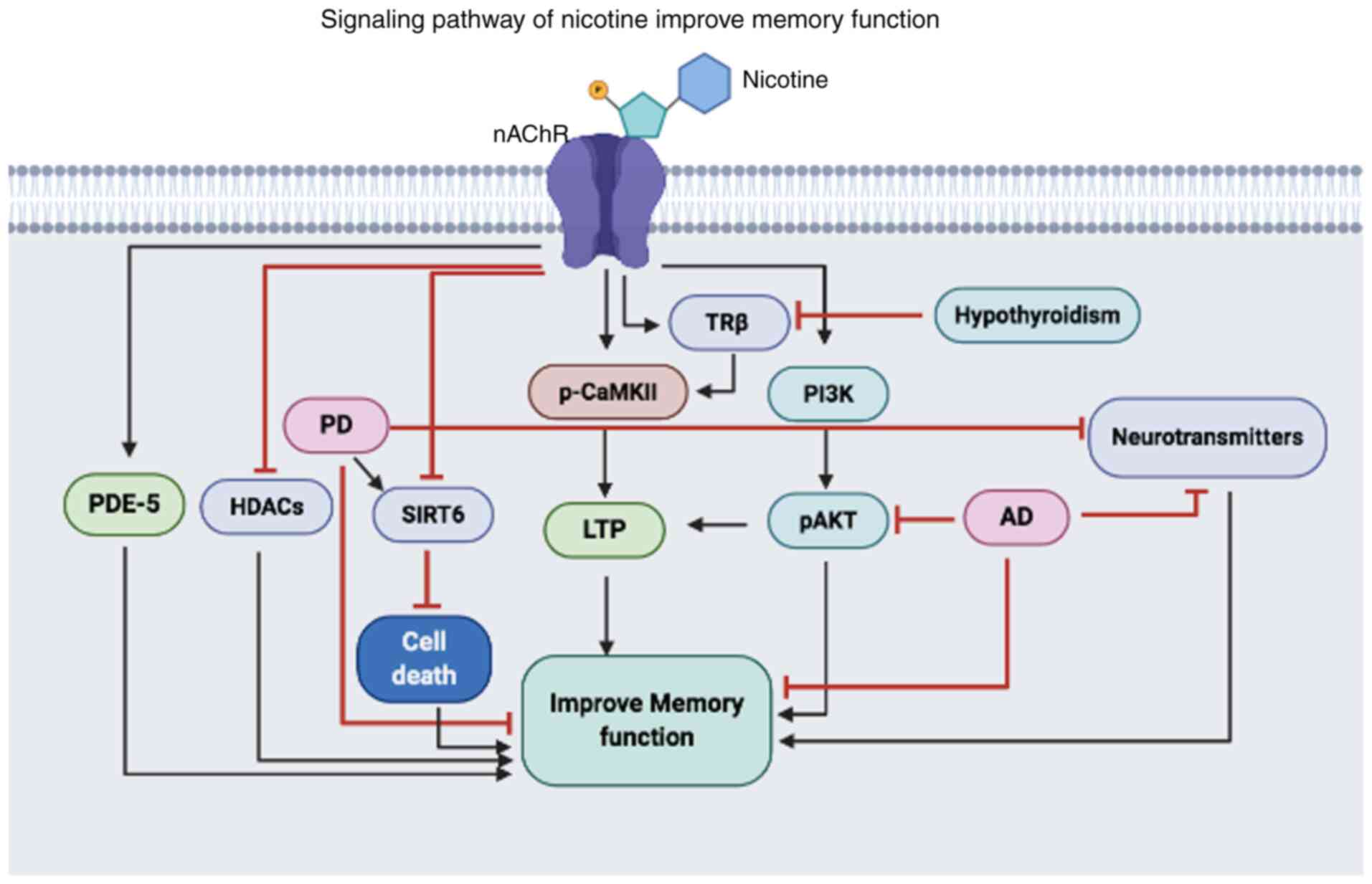 | Figure 1.Illustration of the proposed
mechanisms of nicotine in improving memory dysfunction. Nicotine
activates nAChR, which can activate or inhibit the expression and
functions of various proteins. Nicotine can activate PDE-5, TRβ and
CaMKII, and activation of these proteins can lead to increased
neuronal communication that ultimately improves memory function. In
addition, nicotine activates the pro-survival PI3K/AKT pathway that
increases LTP and improves memory dysfunction caused by AD. Also,
nicotine can inhibit HDACs and SIRT6, which are increased in PD,
thus reducing the activity of these proteins reduces neural
apoptosis and improves memory dysfunction. PDE-5,
phosphodiesterase-5; HDAC, histone deacetylases; PD, Parkinson's
disease; SIRT6, Sirtuin 6; LTP, long-term potentiation; p-,
phosphorylated; CAMKII, calmodulin-dependent protein kinase II;
TRβ, thyroid receptor subunit β; PI3K, phosphoinositide 3-kinase;
AD, Alzheimer's disease; nAChR, nicotinic cholinergic
receptors. |
Benefits of nicotine in Alzheimer's disease
(AD)
AD is a neurodegenerative disease that primarily
affects older adults and causes dementia (27). AD is characterized by the deposition
of toxic amyloid-β (Aβ) and tau proteins in the brain (28,29).
In particular, the accumulation of Aβ has been demonstrated to
inhibit mitochondrial function, leading to increased reactive
oxygen species formation and the stimulation of inflammatory
processes (30). Indeed, several
studies have revealed that Aβ deposition alters the physiological
function of the brain and causes neuronal dysfunction (31,32).
Unfortunately, there is still no cure for AD, and the disease is
currently managed by slowing its progression with the
administration of antioxidants and drugs such as cholinesterase
inhibitors (33).
According to the cholinergic hypothesis, the
cognitive decline in AD arises from deficiencies in central
cholinergic neurotransmission due to the loss of acetylcholine
(34). Therefore, cholinesterase
inhibitors (such as donepezil and galantamine), which block the
degradation of acetylcholine, remain the first-line approach to
restore central cholinergic function in AD. Moreover, changes in
the expression and density of α7 nAChRs in the
hippocampus have been observed in AD and appear to have the most
impact on cognitive function (35).
Such α7 nAChRs have also been found to be co-localized
with plaques in AD (36).
Therefore, agonists of α7 nAChRs, including nicotine,
may be useful for treating AD.
The stimulation of nAChRs by nicotine also likely
affects downstream signaling molecules, including protein kinases,
which are important regulators of synaptic plasticity and memory
(37). In particular, protein
kinase B (also referred to as Akt) is a central molecule of the
phosphoinositide 3-kinase (PI3K)/Akt signaling pathway, which plays
a vital role in the regulatory functions of neurons in the CNS,
including neuronal survival (38–42),
and learning and memory encoding (38,43,44).
Therefore, it is hypothesized that the stimulation of nAChRs by
nicotine or its analogs activates the PI3K/Akt signaling pathway,
which, in turn, regulates learning and memory processes (42,45).
Indeed, acute and chronic administration of nicotine was reported
to improve cognitive impairment in patients with AD (46–48).
Moreover, acute nicotine administration during
electroencephalography (EEG) performed in patients with AD who
received cholinesterase inhibitors was found to shift the EEG
readings towards normal levels (49). Thus, nicotine administration may
have a beneficial effect on the cognitive decline observed in
AD.
Benefits of nicotine in Parkinson's disease
(PD)
PD is the second most common neurodegenerative
disorder after AD that affects older individuals (50). Although the exact cause of PD is
still not fully understood, its pathogenesis involves the loss or
degeneration of the dopaminergic neurons (dopamine-producing
neurons) in the substantia nigra of the midbrain (51). This loss of dopaminergic neurons
causes impairment of motor control, tremors, rigidity and
bradykinesia, and cognitive impairment (52,53).
Studies in animal models of PD have revealed that nicotine can
protect the brain cells from damage (54,55).
Smoking cigarettes is also reported to reduce the risk of PD
occurrence (53), and nicotine may
help improve some symptoms of PD, such as dyskinesia and memory
impairments (55). Indeed, the
neuroprotective effects of nicotine in PD have been examined in
vitro and in vivo, and are hypothesized to be primarily
due to its pro-survival effects on dopaminergic neurons (56).
In addition to activating pro-survival signaling
pathways in the brain, such as the aforementioned PI3K/Akt pathway,
nicotine may also slow the progression of PD by inhibiting Sirtuin
6 (SIRT6), an NAD+-dependent class III deacetylase
(57). This suppression of SIRT6
was found to reduce apoptosis and increase neuron survival
(57). Consistently, several
studies reported that the overexpression of SIRT6 impairs
contextual fear memory formation (58,59).
Despite this, another study found that loss of SIRT6 in the brain
also causes memory impairment (60). Therefore, the downstream effects of
nicotine on SIRT6 in PD require further investigation.
Benefits of nicotine on memory processes in
patients with thyroid disease
Studies have revealed that thyroid hormones
(61), including thyroxine (T4) and
triiodothyronine (T3), regulate brain development, neurogenesis,
synaptogenesis and myelination (62,63).
T3 and T4 are synthesized in the thymus (64,65),
released into the bloodstream, and eventually exert their effects
by binding to a nuclear receptor termed the thyroid hormone
receptor (TR), which is present in two different isoforms, α and β
(66). The expression levels of
these isoforms differ among tissues: The α1 receptor is primarily
expressed in the heart and the skeletal muscle (67), whereas β1 is mainly expressed in the
liver, kidney and brain (68).
TRs are also abundantly expressed in the
hippocampus, which is the part of the brain that is responsible for
memory formation (63). Therefore,
in diseases such as hyperthyroidism, hypothyroidism and cretinism,
in which abnormal thyroid hormone levels are present (69,70),
hippocampal function may be affected, thus resulting in cognitive
impairment (71). Indeed,
neuroimaging studies have demonstrated that the structure and
function of the hippocampus are altered in patients with
hypothyroidism (72–74).
Of note, acute nicotine administration has been
reported to activate TRs (particularly TRβ in the brain) and, thus,
may enhance learning and memory processes in certain individuals
(66). Furthermore, TRβ knockout in
mice did not affect memory function following nicotine
administration, confirming the role of TRβ in memory processes
(75). In addition, memory
impairment caused by hypothyroidism was revealed to be improved by
nicotine via the modulation of calcineurin, which regulates the
function of calmodulin-dependent protein kinase II (CaMKII) to
improve synaptic plasticity (76).
However, the precise underlying mechanisms of nicotine
administration in improving cognitive impairments in patients with
thyroid diseases require further investigation.
Effects of nicotine on cognitive function in
healthy individuals
There is mounting evidence that nicotine
administration may improve memory in otherwise healthy individuals.
For example, research revealed that sleep deprivation causes memory
impairment by downregulating the phosphorylation of CaMKII, which
is an essential regulator of cell proliferation and synaptic
plasticity (77–79). CaMKII was previously found to
regulate the expression of glutamate receptor subunit-1 and its
trafficking to the synaptic surface, which is necessary for normal
brain function and memory formation (80). Consistently, acute nicotine
administration was found to improve memory impairments caused by
sleep deprivation by enhancing the phosphorylation of CaMKII
(81). Therefore, nicotine may
improve memory impairments caused by a lack of sleep in otherwise
healthy individuals.
Nicotine-induced chromatin modifications may
improve memory and learning
Some studies have indicated that nicotine affects
chromatin in the cell nucleus (82–84).
Chromatin is composed of four subunits, called histones, which can
be modified via acetylation, methylation or phosphorylation
(85), thereby regulating gene
transcription (86,87). In particular, histone
acetyltransferases and histone deacetylases (HDACs) play essential
roles in the chromatin modifications involved in various cellular
functions, including memory and synaptic plasticity (88,89).
For example, inhibition of HDACs can increase the expression of key
genes involved in memory processes, which are regulated by the cAMP
response element-binding protein (CREB)-CREB-binding protein
transcriptional complex (89). In
particular, HDAC4 has been demonstrated to be crucial for learning
and memory processes (89,90). As cigarette smoking has been
reported to modulate the regulation of chromatin by altering the
functionality of HDACs, such as HDAC6, in the lungs (83), it may also have a similar effect in
the CNS. Indeed, it has been revealed that nicotine can inhibit
HDACs in the brain, and, thus, improve memory function (84). However, further study is required to
investigate the effect of nicotine on cognitive function through
chromatin modulation.
Electrophysiological effects of nicotine:
Strengthening synapses
The neurons in the brain interconnect to form
networks, which are organized according to function (91). Therefore, understanding these
connections allows certain areas to be stimulated and recorded, to
monitor neurotransmitter release and receptor response in
particular regions of the brain. Long-term potentiation (LTP) is
used to measure synaptic plasticity, and can provide a cellular
model of learning and memory encoding. For example, an increase in
the level of glutamate released from the presynaptic to the
postsynaptic neurons was found to enhance excitatory postsynaptic
potential in the hippocampus during spatial learning tasks
(92). Previously, studies have
reported that acute nicotine exposure rescues LTP in individuals
with sleep deprivation (81). In
addition, chronic administration of nicotine has been revealed to
improve LTP in AD, chronic stress models and hypothyroidism models
(74,93,94).
There is also mounting evidence that the restoration of LTP due to
nicotine exposure is related to the normalization of the
phosphorylation of essential kinases, such as CREB and CaMKIV
(48,78,95).
Therefore, nicotine administration may strengthen synapses between
two neurons, leading to improved memory in both healthy individuals
and those with diseases such as AD or hypothyroidism.
Conclusions
The findings reported in the studies included in the
present review article indicate that nicotine can stimulate memory
function. Therefore, although nicotine is similar to other
psychoactive substances, in that it can induce dependence or abuse,
it also has certain beneficial effects, including enhancing
cognitive function in healthy individuals and restoring memory
function in patients with diseases, such as AD, PD and
hypothyroidism.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
Not applicable.
Author's contributions
AA designed the review paper, performed the
literature search and wrote the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Benowitz NL, Hukkanen J and Jacob P III:
Nicotine chemistry, metabolism, kinetics and biomarkers. Handb Exp
Pharmacol. 192:29–60. 2009.doi: 10.1007/978-3-540-69248-5_2.
View Article : Google Scholar
|
|
2
|
Broide RS, Winzer-Serhan UH, Chen Y and
Leslie FM: Distribution of alpha7 nicotinic acetylcholine receptor
subunit mRNA in the developing mouse. Front Neuroanat. 13:762019.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mishra A, Chaturvedi P, Datta S, Sinukumar
S, Joshi P and Garg A: Harmful effects of nicotine. Indian J Med
Paediatr Oncol. 36:24–31. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bagaitkar J, Demuth DR and Scott DA:
Tobacco use increases susceptibility to bacterial infection. Tob
Induc Dis. 4:122008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Unwin N: Nicotinic acetylcholine receptor
and the structural basis of neuromuscular transmission: Insights
from Torpedo postsynaptic membranes. Q Rev Biophys. 46:283–322.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Skok VI: Nicotinic acetylcholine receptors
in autonomic ganglia. Auton Neurosci. 97:1–11. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gotti C, Zoli M and Clementi F: Brain
nicotinic acetylcholine receptors: Native subtypes and their
relevance. Trends Pharmacol Sci. 27:482–491. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dani JA: Neuronal nicotinic acetylcholine
receptor structure and function and response to nicotine. Int Rev
Neurobiol. 124:3–19. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hone AJ and McIntosh JM: Nicotinic
acetylcholine receptors in neuropathic and inflammatory pain. FEBS
Lett. 592:1045–1062. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zaveri N, Jiang F, Olsen C, Polgar W and
Toll L: Novel α3β4 nicotinic acetylcholine receptor-selective
ligands. Discovery, structure-activity studies, and pharmacological
evaluation. J Med Chem. 53:8187–8191. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Aberger K, Chitravanshi VC and Sapru HN:
Cardiovascular responses to microinjections of nicotine into the
caudal ventrolateral medulla of the rat. Brain Res. 892:138–146.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Levin ED, Bettegowda C, Blosser J and
Gordon J: AR-R17779, and alpha7 nicotinic agonist, improves
learning and memory in rats. Behav Pharmacol. 10:675–680. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hajos M, Hurst RS, Hoffmann WE, Krause M,
Wall TM, Higdon NR and Groppi VE: The selective alpha7 nicotinic
acetylcholine receptor agonist PNU-282987 [N-[(3R)-
1-Azabicyclo[2.2.2]oct-3-yl]-4-chlorobenzamide hydrochloride]
enhances GABAergic synaptic activity in brain slices and restores
auditory gating deficits in anesthetized rats. J Pharmacol Exp
Ther. 312:1213–1222. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Benowitz NL: Pharmacology of nicotine:
Addiction, smoking-induced disease, and therapeutics. Annu Rev
Pharmacol Toxicol. 49:57–71. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
D'Souza MS and Markou A: Neuronal
mechanisms underlying development of nicotine dependence:
Implications for novel smoking-cessation treatments. Addict Sci
Clin Pract. 6:4–16. 2011.PubMed/NCBI
|
|
16
|
Picciotto MR, Addy NA, Mineur YS and
Brunzell DH: It is not ‘either/or’: Activation and desensitization
of nicotinic acetylcholine receptors both contribute to behaviors
related to nicotine addiction and mood. Prog Neurobiol. 84:329–342.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sun Z, Smyth K, Garcia K, Mattson E, Li L
and Xiao Z: Nicotine inhibits memory CTL programming. PLoS One.
8:e681832013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Echeverria Moran V: Brain effects of
nicotine and derived compounds. Front Pharmacol. 4:602013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hotston MR, Jeremy JY, Bloor J, Koupparis
A, Persad R and Shukla N: Sildenafil inhibits the up-regulation of
phosphodiesterase type 5 elicited with nicotine and tumour necrosis
factor-alpha in cavernosal vascular smooth muscle cells: Mediation
by superoxide. BJU Int. 99:612–618. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Henderson VW: Cognitive changes after
menopause: Influence of estrogen. Clin Obstet Gynecol. 51:618–626.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Domek-Łopacińska K and Strosznajder JB:
Cyclic GMP metabolism and its role in brain physiology. J Physiol
Pharmacol. 56 (Suppl 2):S15–S34. 2005.
|
|
22
|
Cui Q and So KF: Involvement of cAMP in
neuronal survival and axonal regeneration. Anat Sci Int.
79:209–212. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Peixoto CA, Nunes AK and Garcia-Osta A:
Phosphodiesterase-5 inhibitors: Action on the signaling pathways of
neuroinflammation, neurodegeneration, and cognition. Mediators
Inflamm. 2015:9402072015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Biegon A, Kim SW, Logan J, Hooker JM,
Muench L and Fowler JS: Nicotine blocks brain estrogen synthase
(aromatase): In vivo positron emission tomography studies in female
baboons. Biol Psychiatry. 67:774–777. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bean LA, Ianov L and Foster TC: Estrogen
receptors, the hippocampus, and memory. Neuroscientist. 20:534–545.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Luine VN: Estradiol and cognitive
function: Past, present and future. Horm Behav. 66:602–618. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Neugroschl J and Wang S: Alzheimer's
disease: Diagnosis and treatment across the spectrum of disease
severity. Mt Sinai J Med. 78:596–612. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Murphy MP and LeVine H III: Alzheimer's
disease and the amyloid-beta peptide. J Alzheimers Dis. 19:311–323.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Deshpande A, Mina E, Glabe C and Busciglio
J: Different conformations of amyloid beta induce neurotoxicity by
distinct mechanisms in human cortical neurons. J Neurosci.
26:6011–6018. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Schilling T and Eder C: Amyloid-β-induced
reactive oxygen species production and priming are differentially
regulated by ion channels in microglia. J Cell Physiol.
226:3295–3302. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Palop JJ and Mucke L: Amyloid-beta-induced
neuronal dysfunction in Alzheimer's disease: From synapses toward
neural networks. Nat Neurosci. 13:812–818. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jagust W: Is amyloid-β harmful to the
brain? Insights from human imaging studies. Brain. 139:23–30. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mendiola-Precoma J, Berumen LC, Padilla K
and Garcia-Alcocer G: Therapies for prevention and treatment of
Alzheimer's disease. Biomed Res Int. 2016:25892762016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Grossberg GT: Cholinesterase inhibitors
for the treatment of Alzheimer's disease: Getting on and staying
on. Curr Ther Res Clin Exp. 64:216–235. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cheng Q and Yakel JL: The effect of α7
nicotinic receptor activation on glutamatergic transmission in the
hippocampus. Biochem Pharmacol. 97:439–444. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Buckingham SD, Jones AK, Brown LA and
Sattelle DB: Nicotinic acetylcholine receptor signalling: Roles in
Alzheimer's disease and amyloid neuroprotection. Pharmacol Rev.
61:39–61. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Giese KP and Mizuno K: The roles of
protein kinases in learning and memory. Learn Mem. 20:540–552.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Diez H, Garrido JJ and Wandosell F:
Specific roles of Akt iso forms in apoptosis and axon growth
regulation in neurons. PLoS One. 7:e327152012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Huang EJ and Reichardt LF: Neurotrophins:
Roles in neuronal development and function. Annu Rev Neurosci.
24:677–736. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Del Puerto A, Wandosell F and Garrido JJ:
Neuronal and glial purinergic receptors functions in neuron
development and brain disease. Front Cell Neurosci.
7:1972013.PubMed/NCBI
|
|
41
|
Brunet A, Datta SR and Greenberg ME:
Transcription-dependent and -independent control of neuronal
survival by the PI3K-Akt signaling pathway. Curr Opin Neurobiol.
11:297–305. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Shu Y, Zhang H, Kang T, Zhang JJ, Yang Y,
Liu H and Zhang L: PI3K/Akt signal pathway involved in the
cognitive impairment caused by chronic cerebral hypoperfusion in
rats. PLoS One. 8:e819012013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Horwood JM, Dufour F, Laroche S and Davis
S: Signalling mechanisms mediated by the phosphoinositide
3-kinase/Akt cascade in synaptic plasticity and memory in the rat.
Eur J Neurosci. 23:3375–3384. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chiang HC, Wang L, Xie ZL, Yau A and Zhong
Y: PI3 kinase signaling is involved in A beta-induced memory loss
in Drosophila. Proc Natl Acad Sci USA. 107:7060–7065. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yi JH, Baek SJ, Heo S, Park HJ, Kwon H,
Lee S, Jung J, Park SJ, Kim BC, Lee YC, et al: Direct
pharmacological Akt activation rescues Alzheimer's disease like
memory impairments and aberrant synaptic plasticity.
Neuropharmacology. 128:282–292. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Newhouse P, Kellar K, Aisen P, White H,
Wesnes K, Coderre E, Pfaff A, Wilkins H, Howard D and Levin ED:
Nicotine treatment of mild cognitive impairment: A 6-month
double-blind pilot clinical trial. Neurology. 78:91–101. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Majdi A, Kamari F, Sadigh-Eteghad S and
Gjedde A: Molecular insights into memory-enhancing metabolites of
nicotine in brain: A systematic review. Front Neurosci.
12:10022018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Srivareerat M, Tran TT, Salim S, Aleisa AM
and Alkadhi KA: Chronic nicotine restores normal Aβ levels and
prevents short-term memory and E-LTP impairment in Aβ rat model of
Alzheimer's disease. Neurobiol Aging. 32:834–844. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Knott V, Engeland C, Mohr E, Mahoney C and
Ilivitsky V: Acute nicotine administration in Alzheimer's disease:
An exploratory EEG study. Neuropsychobiology. 41:210–220. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Sherer TB, Chowdhury S, Peabody K and
Brooks DW: Overcoming obstacles in Parkinson's disease. Mov Disord.
27:1606–1611. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Barber M, Stewart D, Grosset D and MacPhee
G: Patient and carer perception of the management of Parkinson's
disease after surgery. Age Ageing. 30:171–172. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kinoshita KI, Tada Y, Muroi Y, Unno T and
Ishii T: Selective loss of dopaminergic neurons in the substantia
nigra pars compacta after systemic administration of MPTP
facilitates extinction learning. Life Sci. 137:28–36. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ma C, Liu Y, Neumann S and Gao X: Nicotine
from cigarette smoking and diet and Parkinson disease: A review.
Transl Neurodegener. 6:182017. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Lu JYD, Su P, Barber JEM, Nash JE, Le AD,
Liu F and Wong AHC: The neuroprotective effect of nicotine in
Parkinson's disease models is associated with inhibiting PARP-1 and
caspase-3 cleavage. PeerJ. 5:e39332017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Quik M, O'Leary K and Tanner CM: Nicotine
and Parkinson's disease: Implications for therapy. Mov Disord.
23:1641–1652. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Barreto GE, Iarkov A and Moran VE:
Beneficial effects of nicotine, cotinine and its metabolites as
potential agents for Parkinson's disease. Front Aging Neurosci.
6:340. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Nicholatos JW, Francisco AB, Bender CA,
Yeh T, Lugay FJ, Salazar JE, Glorioso C and Libert S: Nicotine
promotes neuron survival and partially protects from Parkinson's
disease by suppressing SIRT6. Acta Neuropathol Commun. 6:1202018.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Kim H, Kim HS and Kaang BK: Elevated
contextual fear memory by SIRT6 depletion in excitatory neurons of
mouse forebrain. Mol Brain. 11:492018. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Yin X, Gao Y, Shi HS, Song L, Wang JC,
Shao J, Geng XH, Xue G, Li JL and Hou YN: Overexpression of SIRT6
in the hippocampal CA1 impairs the formation of long-term
contextual fear memory. Sci Rep. 6:189822016. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Kaluski S, Portillo M, Besnard A, Stein D,
Einav M, Zhong L, Ueberham U, Arendt T, Mostoslavsky R, Sahay A and
Toiber D: Neuroprotective functions for the histone deacetylase
SIRT6. Cell Rep. 18:3052–3062. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Rousset B, Dupuy C, Miot F and Dumont J:
Chapter 2 Thyroid Hormone Synthesis and Secretion. Endotext.
Feingold KR, Anawalt B, Boyce A, et al: MDText.com, Inc.; South
Dartmouth, MA: 2000, https://www.ncbi.nlm.nih.gov/books/NBK285550/September
2–2015
|
|
62
|
Diez D, Grijota-Martinez C, Agretti P, De
Marco G, Tonacchera M, Pinchera A, de Escobar GM, Bernal J and
Morte B: Thyroid hormone action in the adult brain: Gene expression
profiling of the effects of single and multiple doses of
triiodo-L-thyronine in the rat striatum. Endocrinology.
149:3989–4000. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Desouza LA, Ladiwala U, Daniel SM, Agashe
S, Vaidya RA and Vaidya VA: Thyroid hormone regulates hippocampal
neurogenesis in the adult rat brain. Mol Cell Neurosci. 29:414–426.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Fekete C and Lechan RM: Central regulation
of hypothalamic-pituitary-thyroid axis under physiological and
pathophysiological conditions. Endocr Rev. 35:159–194. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Mariotti S and Beck-Peccoz P: Physiology
of the Hypothalamic-Pituitary Thyroidal System. Endotext. De Groot
LJ, Beck-Peccoz P, Chrousos G, et al: MDText.com, Inc.; South
Dartmouth, MA: 2000, https://www.ncbi.nlm.nih.gov/books/NBK278958August
14–2016
|
|
66
|
Cheng SY: Multiple mechanisms for
regulation of the transcriptional activity of thyroid hormone
receptors. Rev Endocr Metab Disord. 1:9–18. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Bradley DJ, Towle HC and Young WS III:
Spatial and temporal expression of alpha- and beta-thyroid hormone
receptor mRNAs, including the beta 2-subtype, in the developing
mammalian nervous system. J Neurosci. 12:2288–2302. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Williams GR: Cloning and characterization
of two novel thyroid hormone receptor beta isoforms. Mol Cell Biol.
20:8329–8342. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Brent GA: Mechanisms of thyroid hormone
action. J Clin Invest. 122:3035–3043. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Yen PM: Physiological and molecular basis
of thyroid hormone action. Physiol Rev. 81:1097–1142. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Ge JF, Peng L, Hu CM and Wu TN: Impaired
learning and memory performance in a subclinical hypothyroidism rat
model induced by hemi-thyroid electrocauterisation. J
Neuroendocrinol. 24:953–961. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Cooke GE, Mullally S, Correia N, O'Mara SM
and Gibney J: Hippocampal volume is decreased in adults with
hypothyroidism. Thyroid. 24:433–440. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Singh S, Rana P, Kumar P, Shankar LR and
Khushu S: Hippocampal neurometabolite changes in hypothyroidism: An
in vivo (1) H magnetic resonance spectroscopy study before and
after thyroxine treatment. J Neuroendocrinol. 282016.doi:
10.1111/jne.12399.
|
|
74
|
Alzoubi KH, Aleisa AM, Gerges NZ and
Alkadhi KA: Nicotine reverses adult-onset hypothyroidism-induced
impairment of learning and memory: Behavioral and
electrophysiological studies. J Neurosci Res. 84:944–953. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Leach PT, Kenney JW, Connor DA and Gould
TJ: Thyroid receptor β involvement in the effects of acute nicotine
on hippocampus-dependent memory. Neuropharmacology. 93:155–163.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Alzoubi KH, Aleisa AM and Alkadhi KA:
Molecular studies on the protective effect of nicotine in
adult-onset hypothyroidism-induced impairment of long-term
potentiation. Hippocampus. 16:861–874. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Pi HJ, Otmakhov N, El Gaamouch F, Lemelin
D, De Koninck P and Lisman J: CaMKII control of spine size and
synaptic strength: Role of phosphorylation states and nonenzymatic
action. Proc Natl Acad Sci USA. 107:14437–14442. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Aleisa AM, Alzoubi KH, Gerges NZ and
Alkadhi KA: Chronic psychosocial stress-induced impairment of
hippocampal LTP: Possible role of BDNF. Neurobiol Dis. 22:453–462.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Misrani A, Tabassum S, Wang M, Chen J,
Yang L and Long C: Citalopram prevents sleep-deprivation-induced
reduction in CaMKII-CREB-BDNF signaling in mouse prefrontal cortex.
Brain Res Bull. 155:11–18. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Mao LM, Jin DZ, Xue B, Chu XP and Wang JQ:
Phosphorylation and regulation of glutamate receptors by CaMKII.
Sheng Li Xue Bao. 66:365–372. 2014.PubMed/NCBI
|
|
81
|
Aleisa AM, Helal G, Alhaider IA, Alzoubi
KH, Srivareerat M, Tran TT, Al-Rejaie SS and Alkadhi KA: Acute
nicotine treatment prevents REM sleep deprivation-induced learning
and memory impairment in rat. Hippocampus. 21:899–909.
2011.PubMed/NCBI
|
|
82
|
Shilatifard A: Chromatin modifications by
methylation and ubiquitination: Implications in the regulation of
gene expression. Annual Rev Biochem. 75:243–269. 2006. View Article : Google Scholar
|
|
83
|
Marwick JA, Kirkham PA, Stevenson CS,
Danahay H, Giddings J, Butler K, Donaldson K, Macnee W and Rahman
I: Cigarette smoke alters chromatin remodeling and induces
proinflammatory genes in rat lungs. Am J Respir Cell Mol Biol.
31:633–642. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Volkow ND: Epigenetics of nicotine:
Another nail in the coughing. Sci Transl Med. 3:107ps1432011.
View Article : Google Scholar
|
|
85
|
Kouzarides T: Chromatin modifications and
their function. Cell. 128:693–705. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Brehove M, Wang T, North J, Luo Y, Dreher
SJ, Shimko JC, Ottesen JJ, Luger K and Poirier MG: Histone core
phosphorylation regulates DNA accessibility. J Biol Chem.
290:22612–22621. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Zhang Y, Griffin K, Mondal N and Parvin
JD: Phosphorylation of histone H2A inhibits transcription on
chromatin templates. J Biol Chem. 279:21866–21872. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Legube G and Trouche D: Regulating histone
acetyltransferases and deacetylases. EMBO Rep. 4:944–947. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Vecsey CG, Hawk JD, Lattal KM, Stein JM,
Fabian SA, Attner MA, Cabrera SM, McDonough CB, Brindle PK, Abel T
and Wood MA: Histone deacetylase inhibitors enhance memory and
synaptic plasticity via CREB: CBP-dependent transcriptional
activation. J Neurosci. 27:6128–6140. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Kim MS, Akhtar MW, Adachi M, Mahgoub M,
Bassel-Duby R, Kavalali ET, Olson EN and Monteggia LM: An essential
role for histone deacetylase 4 in synaptic plasticity and memory
formation. J Neurosci. 32:10879–10886. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Pulvermuller F, Garagnani M and Wennekers
T: Thinking in circuits: Toward neurobiological explanation in
cognitive neuroscience. Biol Cybern. 108:573–593. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Richter-Levin G, Canevari L and Bliss TV:
Long-term potentiation and glutamate release in the dentate gyrus:
Links to spatial learning. Behav Brain Res. 66:37–40. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Aleisa AM, Alzoubi KH and Alkadhi KA:
Nicotine prevents stress-induced enhancement of long-term
depression in hippocampal area CA1: Electrophysiological and
molecular studies. J Neurosci Res. 83:309–317. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Alkadhi KA: Chronic stress and Alzheimer's
disease-like pathogenesis in a rat model: Prevention by nicotine.
Curr Neuropharmacol. 9:587–597. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Alzoubi KH and Alkadhi KA: Chronic
nicotine treatment reverses hypothyroidism-induced impairment of
L-LTP induction phase: Critical role of CREB. Mol Neurobiol.
49:1245–1255. 2014. View Article : Google Scholar : PubMed/NCBI
|















