Introduction
Atopic dermatitis (AD) is a chronic inflammatory
disease characterized by severe itching and is caused by genetic
and environmental factors, including microbial infection (1). Keratinocyte-derived cytokines and
chemokines, such as interleukin-6 (IL-6), IL-8, thymus and
activation-regulated chemokine (TARC), macrophage-derived chemokine
(MDC), regulated upon activation, normal T cell expressed, and
secreted (RANTES) and monocyte chemoattractant protein-1 (MCP-1),
are closely related to the pathophysiology of AD (2–4). It is
known that the combination of tumor necrosis factor-α (TNF-α) and
interferon-γ (IFN-γ) induces the expression of inflammatory
cytokines and chemokines in keratinocytes (5–7). The
activation of AKT/MAPK/NF-κB is known to be associated with the
production of inflammatory molecules, including IL-6, IL-8, TARC,
MDC, RANTES and MCP-1, in TNF-α/IFN-γ (TI)-stimulated HaCaT cells
(4,5,8,9). A
previous study reported that antioxidants exert protective effects
in AD by regulating the activation of mitogen-activated protein
kinase (MAPK) and nuclear factor-κB (NF-κB) (10).
The anti-inflammatory properties of caffeic acid
derivatives have been demonstrated in various studies (11,12).
3,4,5-Trihydroxycinnamic acid (THCA), a derivative of caffeic acid,
exerts anti-inflammatory activity in activated BV2 microglia,
RAW264.7 macrophages and A549 airway epithelial cells (13–16).
In these studies, THCA also exerted protective effects in
experimental animal models of sepsis, acute lung injury and
allergic asthma. However, its effect and molecular mechanisms have
not been examined in activated keratinocytes. Therefore, in the
present study, we examined whether THCA regulates the TI
mixture-induced inflammatory response in HaCaT cells.
Materials and methods
Cell culture
HaCaT cells, the human keratinocyte cell line, were
purchased from CLS Cell Lines Service and cultured in DMEM
containing 10% fetal bovine serum (HyClone; GE Healthcare Life
Sciences), 100 U/ml penicillin and 100 µg/ml streptomycin at 37°C
in a 5% CO2 atmosphere. HaCaT cells were incubated with
3,4,5-trihydroxycinnamic acid (AApin Chemicals Limited) (2.5, 5,
10, 25, 50 and 100 µM) for 24 h. An MTT assay was used to determine
cell viability based on a previous study (1).
Reverse transcription-quantitative PCR
(RT-qPCR)
Cells were plated in 12-well plates
(1×104 cells/per well), pretreated with THCA (5, 10, 25
and 50 µg/ml) for 30 min, and subsequently administered TNF-α (10
ng/ml, Invitrogen; Thermo Fisher Scientific, Inc.) and IFN-γ (10
ng/ml, Merck KGaA) and maintained for 6 h at 37°C. The extraction
of total RNA, synthesis of cDNA and relative mRNA levels of
cytokines and chemokines were determined as described previously
(1). Primer sequences are listed in
Table I. GAPDH was used as the
housekeeping gene.
 | Table I.Primer sequences used for reverse
transcription-quantitative PCR. |
Table I.
Primer sequences used for reverse
transcription-quantitative PCR.
| Gene | Forward primer
sequence (5′→3′) | Reverse primer
sequence (5′→3′) |
|---|
| IL-6 |
GACAGCCACTCACCTCTTCA |
AGTGCCTCTTTGCTGCTTTC |
| IL-8 |
ATGACTTCCAAGCTGGCCGTGGCT |
TTATGAATTCTCAGCCCTCTTCAAAAA |
| TARC |
CACGCAGCTCGAGGGACCAATGTG |
TCAAGACCTCTCAAGGCTTTGCAGG |
| MDC |
AGGACAGAGCATGGCTCGCCTACAGA |
TAATGGCAGGGAGGTAGGGCTCCTGA |
| RANTES |
CTGCCTCCCCATATTCCTCGG |
GAGTTGATGTACTCCCGAACCC |
| MCP-1 |
TCTGTGCCTGCTGCTCATAG |
CAGATCTCCTTGGCCACAAT |
| GAPDH |
CCTCCAAAATCAAGTGG |
CCATCCACAGTCTTCTGG |
Enzyme-linked immunosorbent (ELISA)
assay
Cells were plated in 96-well plates
(5×105 cells/per well), pretreated with THCA (5, 10, 25
and 50 µg/ml) for 30 min, subsequently treated with 10 ng/ml
TNF-α/IFN-γ (TI) and incubated for 24 h at 37°C. The secretion
levels of IL-6, IL-8, TARC, RANTES, MDC and MCP-1 in culture media
were detected using commercial ELISA kits.
Isolation of nuclear and cytoplasmic
proteins
Cells were seeded in growth medium at a density of
1×105 cells/per well in 60-mm cell culture dishes and
incubated with THCA for 30 min. Then, TI mixture was administered
to each well, and the plates were maintained for 1 h. Nuclear and
cytoplasmic proteins were isolated using a protein extraction kit
(cat. no. 71183, Merck) according to the manufacturer's
instructions.
Western blotting
To determine the phosphorylation of AKT, MAPK and
NF-κB, cells were plated in 60-mm cell culture dishes
(1×105 cells/well), pretreated with THCA for 30 min, and
subsequently maintained with TI mixture for 1 h. To detect the
phosphorylation of nuclear factor erythroid 2-related factor 2
(Nrf2) and the expression of HO-1/NQO1, cells were plated in 60-mm
cell culture dishes (1×105 cells/well) and maintained
with THCA for 1 or 16 h. Cell lysates were prepared using lysis
buffer (C-3228; Sigma-Aldrich; Merck KGaA). The protein equivalents
of samples were separated by 10–12% SDS polyacrylamide gels and
transferred to polyvinylidene fluoride (PVDF) membranes. Five
percent skim milk was used as a blocking solution for each
membrane. Then, membranes were probed with primary antibodies as
follows: Anti-phosphorylated (p)-AKT (4060S), anti-p-ERK (9101S),
anti-p-JNK (4668S), anti-p-p38 (9211S), anti-p-NF-κB p65 (3033S),
AKT (4691S), ERK (9102S), JNK (9252S), p38 (9212S), NF-κB p65
(8242S), anti-β-actin (4967S; all, 1:1,000; Cell Signaling
Technology, Inc.), anti-HO-1 (27338), anti-p-IκB (1;1,000; 15087;
Invitrogen; Thermo Fisher Scientific, Inc.), anti-LaminA/C
(1;1,000; sc-376248; Santa Cruz Biotechnology, Inc.), anti-NQO1,
(1;1,000; N5288; Sigma-Aldrich), anti-p-Nrf2 (1;1,000; NBP2-67465;
Novus Biologicals) and anti-Nrf2 (1:1,000; 137550; Abcam).
Subsequently, each membrane was exposed to HRP-conjugated secondary
antibodies and developed with an ECL solution (Thermo Fisher
Scientific, Inc.) The visualization of all bands was performed
using the image analyzer, and the density of each band was
determined using ImageJ.
Immunocytochemistry
The conditions of cell seeding and fixation were
based on a previous study (1).
Cells were washed with PBS, blocked in 3% BSA for 1 h at RT and
then maintained with the anti-NF-κB p65 subunit (1:250; Cell
Signaling Technology, Inc.) or anti-Nrf2 (1:250; Abcam) for 24 h at
4°C. Then, the cells were washed with PBS and maintained with Alexa
Fluor 488-conjugated goat anti-rabbit IgG (1:250; Invitrogen;
Thermo Fisher Scientific, Inc.) for 1 h at RT. Finally, cells were
stained with Gold Antifade reagent containing DAPI (Invitrogen;
Thermo Fisher Scientific, Inc.) for 5 min and were subsequently
visualized using a confocal microscope.
Statistical analysis
One-way analysis of variance followed by Tukey's
post hoc test was used to determine significant differences between
the TI group and the THCA treated groups (SPSS Statistics 20; IBM
Corp.). Data are expressed as the means ± standard deviation
(SD).
Results
Effect of THCA on IL-6 and IL-8 in
activated HaCaT cells
To examine the inhibitory effect of THCA treatment
on IL-6 and IL-8, MTT assays were first conducted. As presented in
Fig. 1A, no noticeable reduction in
cell viability was observed following treatment with 2.5–50 µg/ml
THCA. The secretion levels of IL-6 and IL-8 were evaluated with an
ELISA. As presented in Fig. 1B and
C, the production of these molecules was notably increased by
TI mixture administration in HaCaT cells, whereas THCA pretreatment
exerted inhibitory activity on TI mixture-induced upregulation of
IL-6 and IL-8. As shown in Fig. 1D and
E, RT-qPCR revealed that the administration of TI resulted in
significant upregulation of IL-6 and IL-8 mRNA expression in HaCaT
cells. However, THCA pretreatment reduced this upregulation.
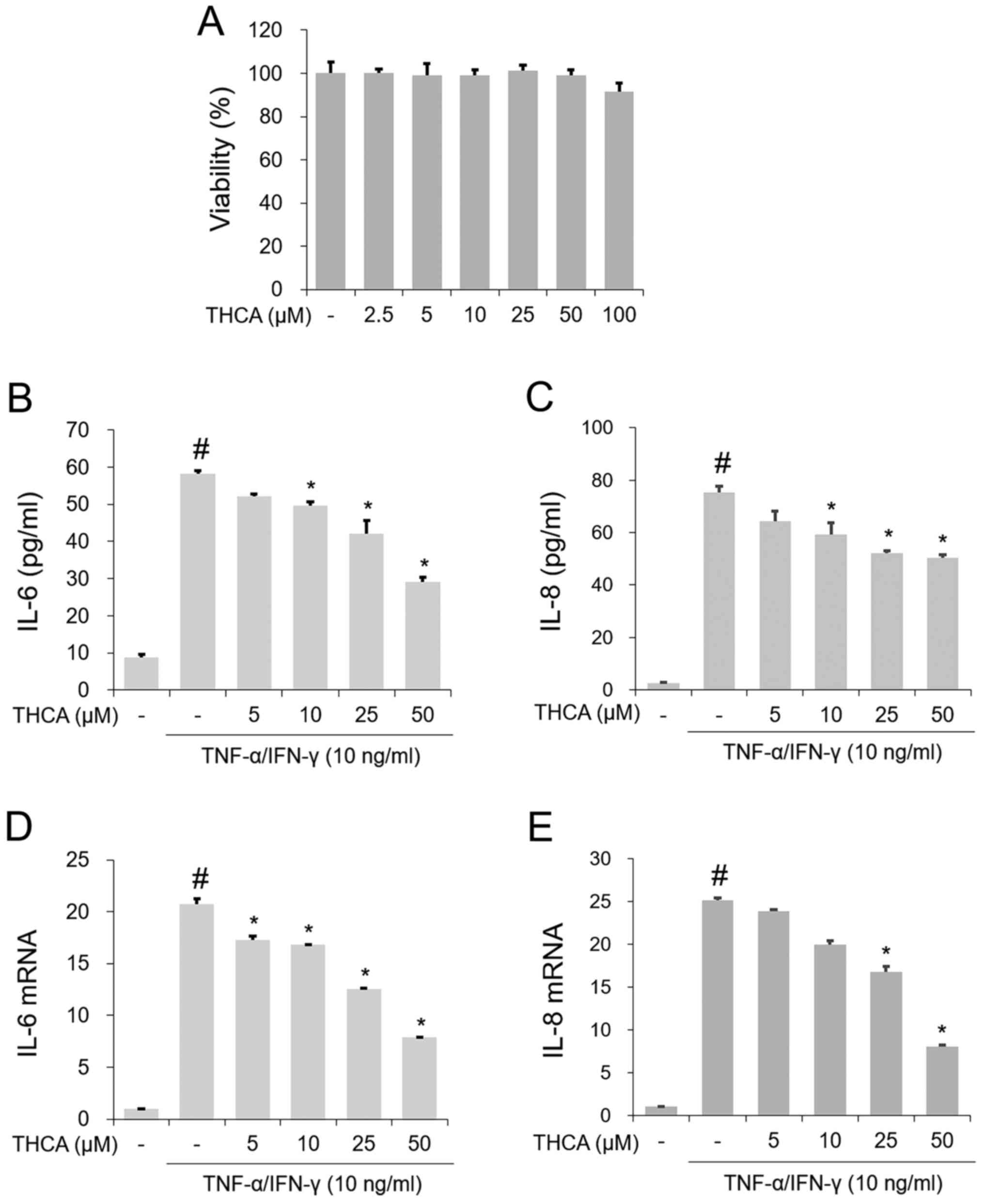 | Figure 1.Effect of THCA on TI-induced IL-6 and
IL-8 in HaCaT cells. (A) Cell viability was determined with the MTT
assay. HaCaT cells were treated with THCA (2.5, 5, 10, 25, 50 and
100 µg/ml) for 24 h. The secretion levels of (B) IL-6 and (C) IL-8
were determined using ELISAs. HaCaT cells were pretreated with THCA
(5, 10, 25 and 50 µg/ml) 30 min prior to incubation with 10 ng/ml
TI for 24 h. The mRNA levels of (D) IL-6 and (E) IL-8 were
determined using reverse transcription-quantitative PCR assays.
HaCaT cells were pretreated with THCA 30 min prior to incubation
with 10 ng/ml TI. Data are expressed as the mean ± standard
deviation. #P<0.05 vs. negative control group;
*P<0.05 vs. TI only group. TI, TNF-α/IFN-γ; TNF-α, tumor
necrosis factor-α; IFN-γ, interferon-γ; THCA,
3,4,5-trihydroxycinnamic acid; IL, interleukin-6. |
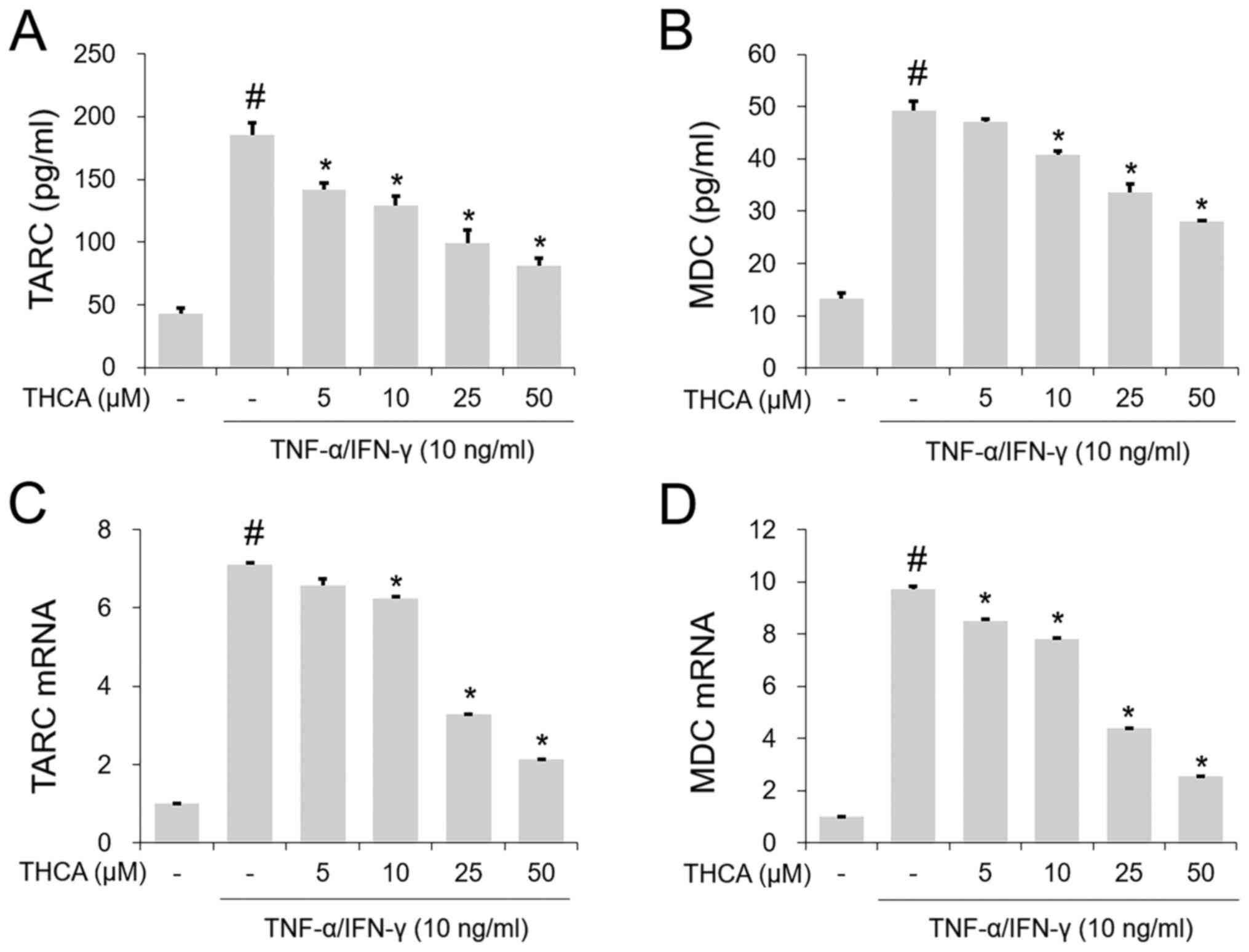
Effects of THCA on TARC and MDC in
activated HaCaT cells
The TI mixture markedly increased the secretion of
TARC and MDC, whereas this increase was reduced by THCA
pretreatment (Fig. 2A and B). Next,
the mRNA levels of these molecules were determined using RT-qPCR.
As shown in Fig. 2C and D, the
increases in the mRNA levels of each molecule were confirmed
following administration of the TI mixture. THCA pretreatment
decreased this increase (Fig. 2C and
D).
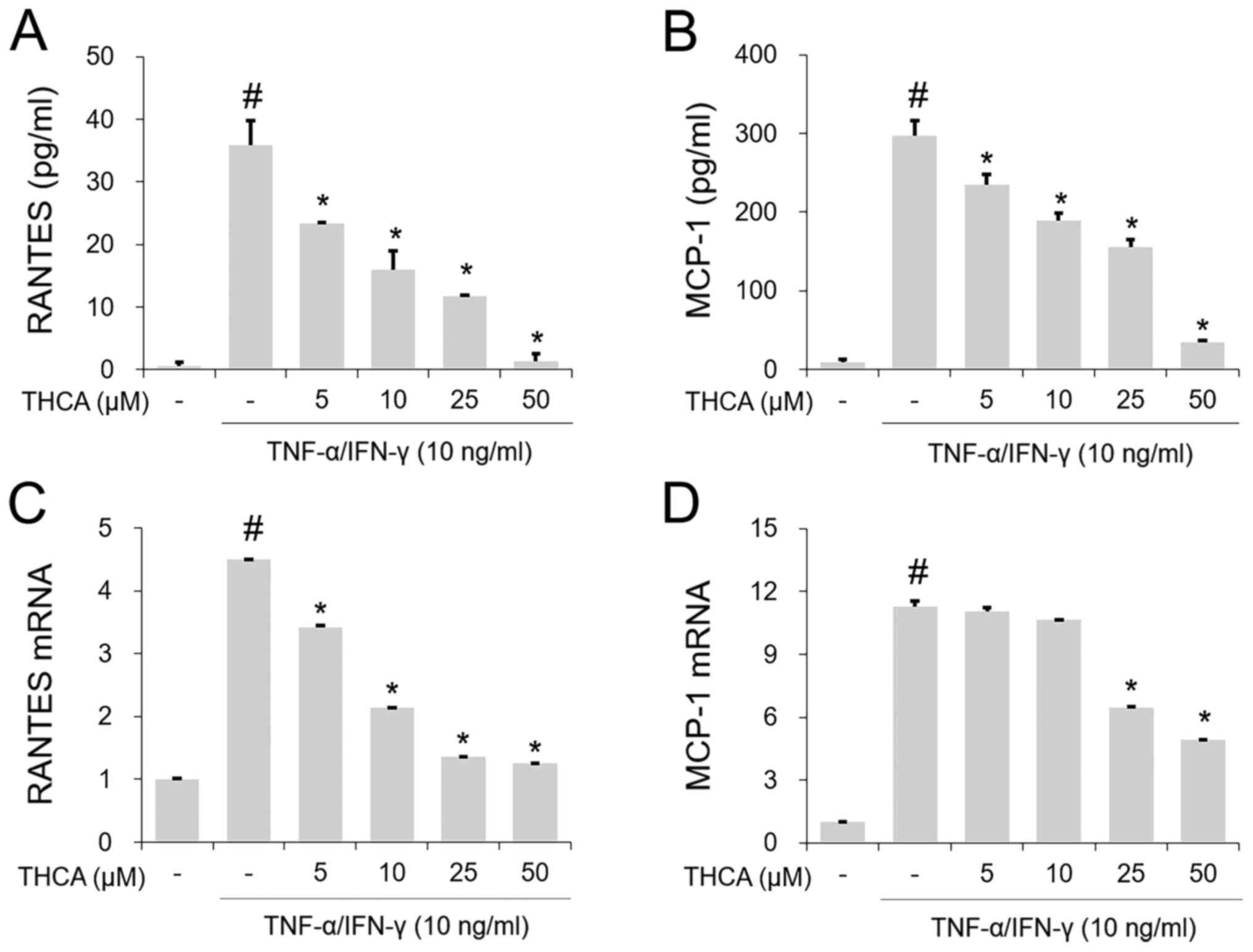
Effects of THCA on RANTES and MCP-1 in
activated HaCaT cells
The TI mixture-induced notable increases in RANETS
and MCP-1 secretion were significantly blocked by THCA pretreatment
(Fig. 3A and B). In addition, THCA
inhibited the increased mRNA expression levels of RANETS and MCP-1
in TI mixture-stimulated HaCaT cells (Fig. 3C and D).
Effects of THCA on AKT and MAPK
phosphorylation in activated HaCaT cells
Western blotting results showed that AKT and MAPK
(ERK, JNK and p38) phosphorylation levels were increased by
administration of the TI mixture in HaCaT cells (Fig. 4A and B). Pretreatment with THCA
resulted in suppression of AKT and ERK phosphorylation in
TI-stimulated HaCaT cells. However, the phosphorylation levels of
JNK and p38 were not affected by THCA pretreatment.
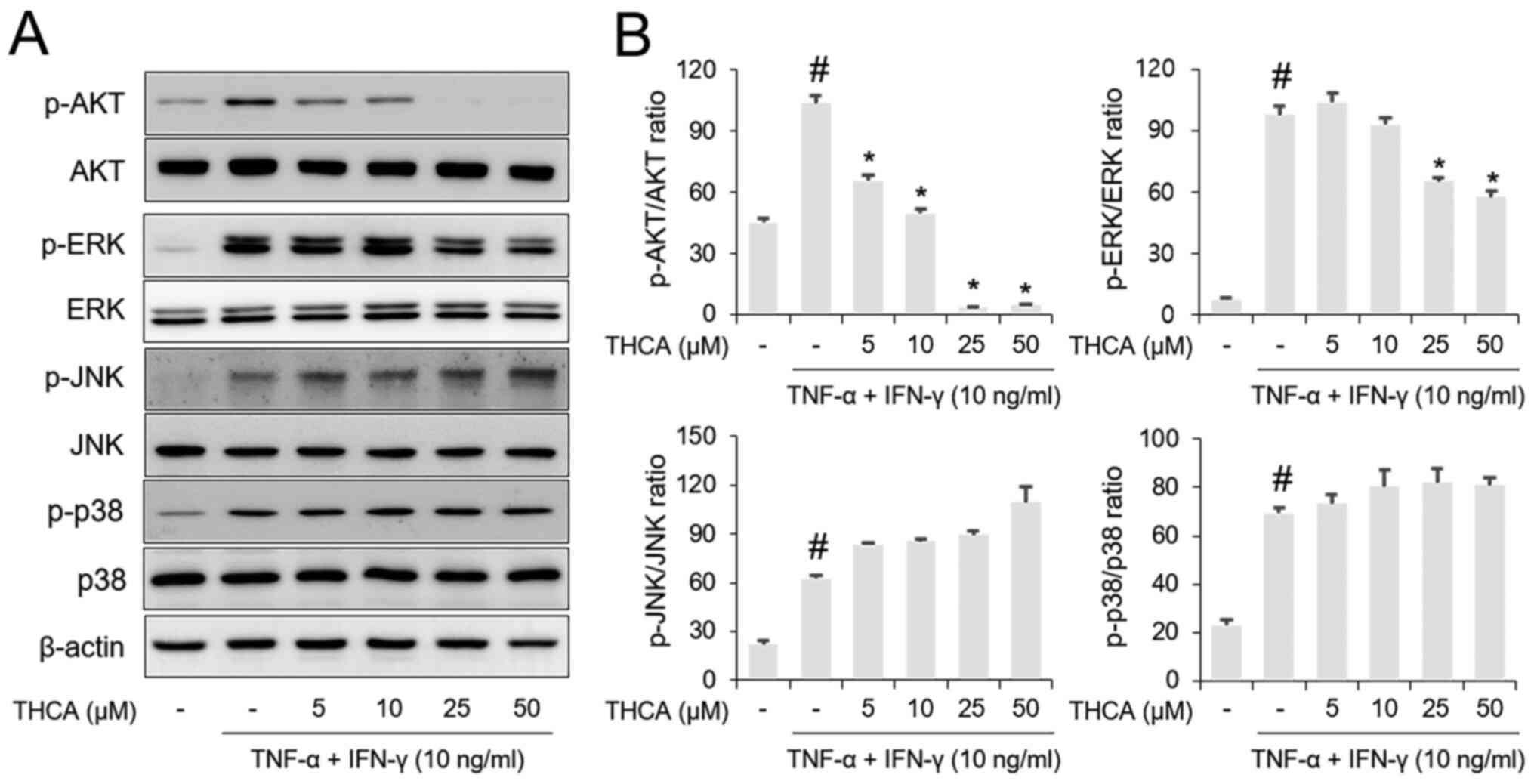 | Figure 4.Effects of THCA on TI-induced AKT and
MAPK activation in HaCaT cells. (A) The phosphorylation levels of
AKT, ERK, JNK and p38 were determined using western blot analysis.
HaCaT cells were pretreated with THCA 30 min prior to incubation
with 10 ng/ml TI for 1 h. (B) Quantitative analysis of p-AKT,
p-ERK, p-JNK and p-p38 was performed using ImageJ. Data are
expressed as the means ± standard deviation. #P<0.05
vs. negative control group; *P<0.05 vs. TI only group. TI,
TNF-α/IFN-γ; TNF-α, tumor necrosis factor-α; IFN-γ, interferon-γ;
THCA, 3,4,5-trihydroxycinnamic acid; p, phosphorylated. |
Effect of THCA on NF-κB p65
phosphorylation in activated HaCaT cells
Next, the effects of THCA on NF-κB p65 and IκBα
activation were examined by western blotting. As shown in Fig. 5A and B, THCA treatment attenuated
the phosphorylation of NF-κB p65/IκBα in TI mixture-stimulated
HaCaT cells. THCA exerted a suppressive effect on the nuclear
translocation of NF-κB p65 (Fig. 5C and
D). This effect of THCA on nuclear translocation was confirmed
using immunocytochemistry (ICC) (Fig.
5E).
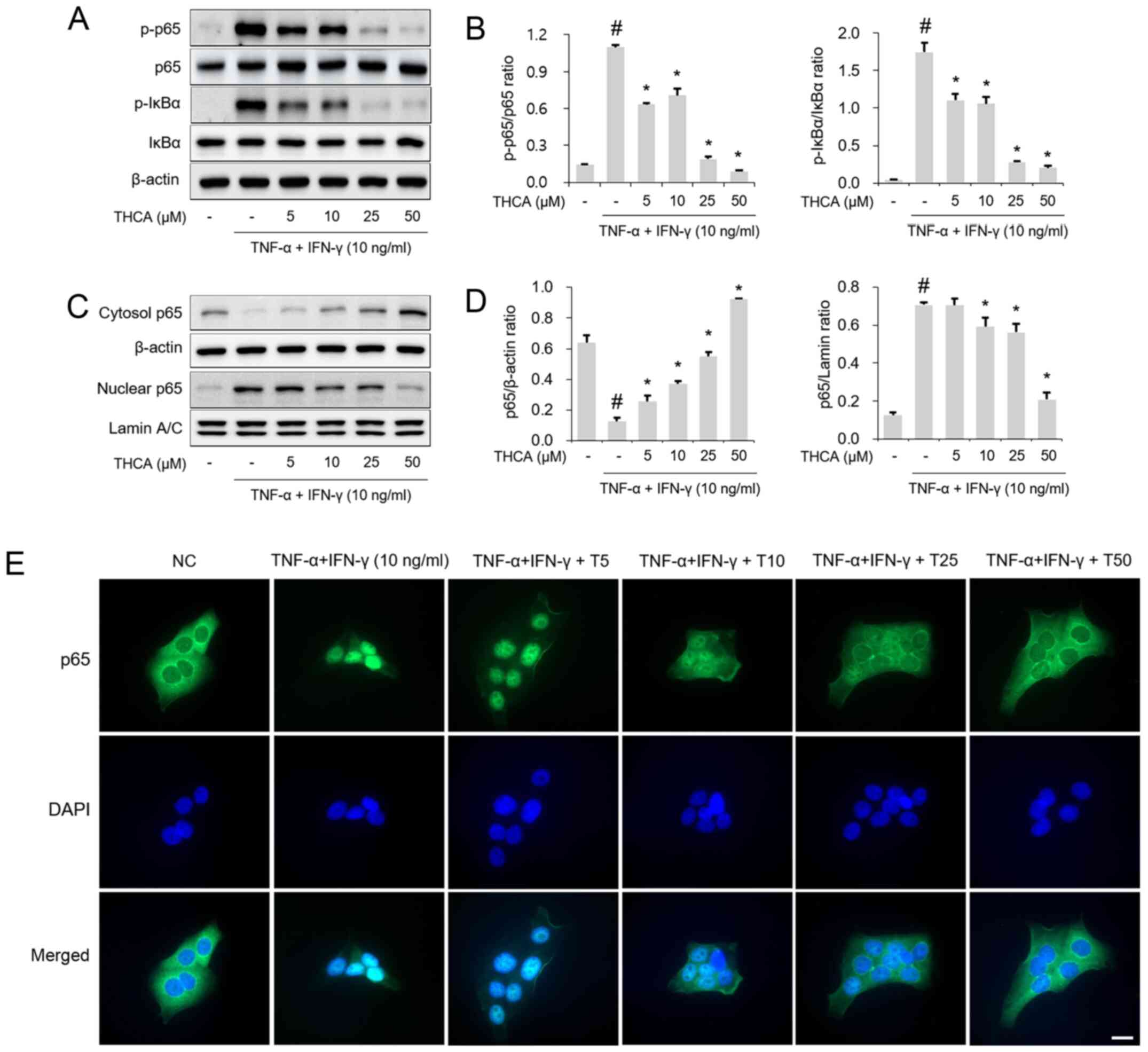 | Figure 5.Effect of THCA on TI-induced NF-κB
activation in HaCaT cells. (A) Levels of NF-κB p65 and IκBα
phosphorylation were determined using western blot analysis. HaCaT
cells were pretreated with THCA 30 min prior to incubation with 10
ng/ml TI for 1 h. (B) Quantitative analysis of p-NF-κB p65 and
p-IκB was performed using ImageJ. (C) Levels of NF-κB p65 nuclear
translocation were determined using western blot analysis. HaCaT
cells were pretreated with THCA 1 h prior to incubation with 10
ng/ml TI for 1 h. Then, nuclear and cytosolic fractions were
obtained. (D) Quantitative analysis of NF-κB p65 expression in the
nucleus and cytosol was performed using ImageJ. (E) Levels of NF-κB
p65 nuclear translocation were determined using
immunocytochemistry. Scale bar, 20 µm. Data are expressed as the
means ± standard deviation. #P<0.05 vs. negative
control group; *P<0.05 vs. TI only group. TI, TNF-α/IFN-γ;
TNF-α, tumor necrosis factor-α; IFN-γ, interferon-γ; THCA,
3,4,5-trihydroxycinnamic acid; p, phosphorylated; DAPI,
4′,6-diamidino-2-phenylindole. |
Effects of THCA on HO-1 and NQO1
induction in HaCaT cells
As presented in Fig.
6, THCA treatment significantly upregulated the expression of
heme oxygenase-1 (HO-1) in HaCaT cells (Fig. 6A and B). THCA treatment also
resulted in upregulation of NAD(P)H:quinone oxidoreductase 1 (NQO1)
expression in HaCaT cells. Furthermore, Nrf2 activation and nuclear
translocation were also increased by THCA treatment (Fig. 6A-D). This ability of THCA on nuclear
translocation was confirmed using ICC (Fig. 6E).
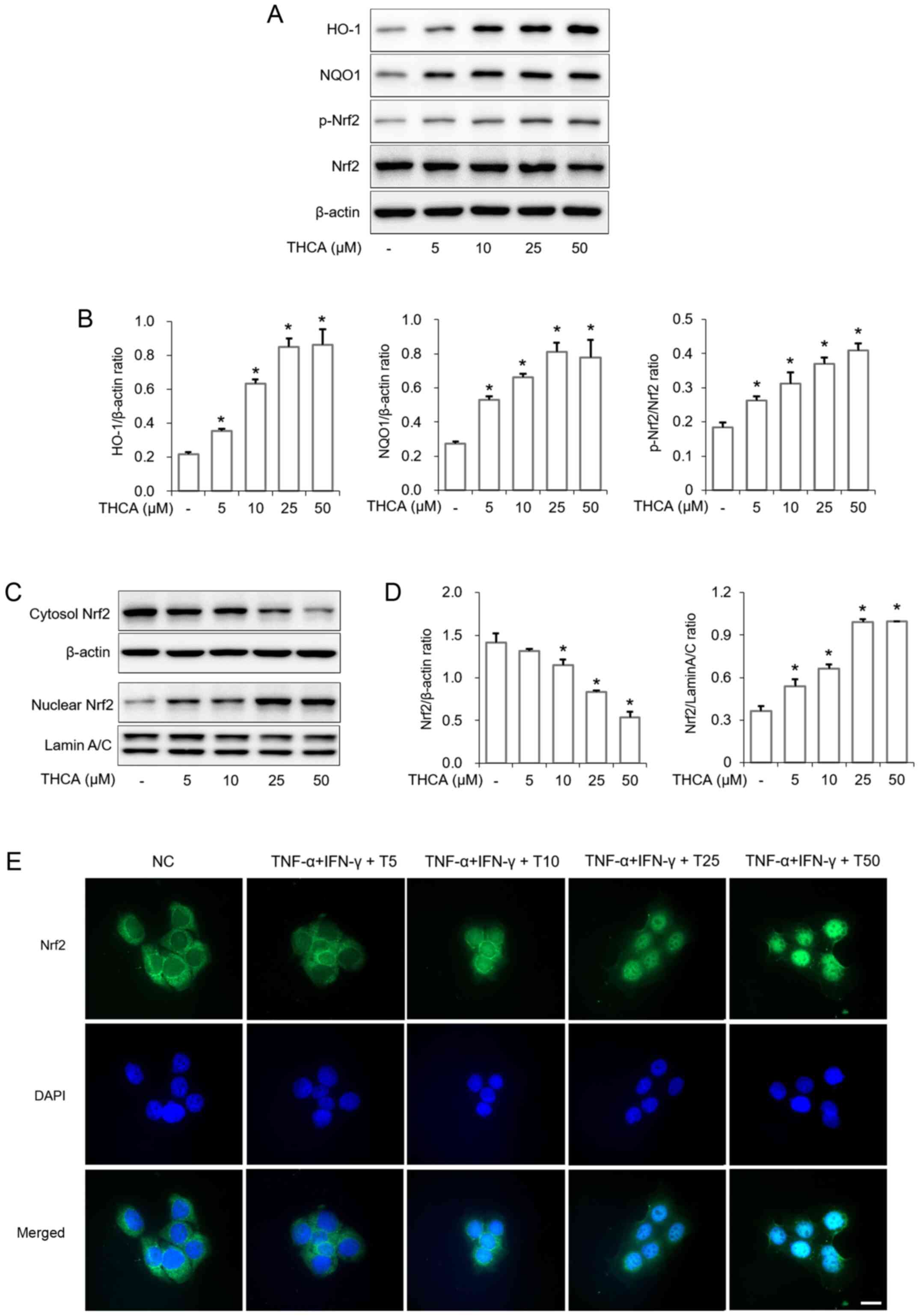 | Figure 6.Effects of THCA on the expression of
HO-1/NQO1 and the activation of Nrf2 in HaCaT cells. (A) Levels of
HO-1 and NQO1 expression and Nrf2 phosphorylation were determined
using western blot analysis. HaCaT cells were treated with THCA for
16 h to detect the levels of HO-1 and NQO1 expression. Cells were
also treated with THCA for 1 h to detect the levels of Nrf2
activation. (B) Quantitative analysis of HO-1, NQO1 and p-Nrf2 was
performed using ImageJ. (C) The level of Nrf2 nuclear translocation
was determined using western blot analysis. HaCaT cells were
treated with THCA for 1 h. Then, nuclear and cytosolic fractions
were obtained. (D) Quantitative analysis of Nrf2 expression was
performed using ImageJ. Data are expressed as the means ± standard
deviation. (E) Levels of Nr2 nuclear translocation were determined
using immunocytochemistry. Scale bar, 20 µm. *P<0.05 vs.
negative control group. THCA, 3,4,5-trihydroxycinnamic acid; HO-1,
heme oxygenase-1; NQO1, NAD(P)H quinone dehydrogenase 1; Nrf2,
nuclear factor erythroid 2-related factor 2; DAPI,
4′,6-diamidino-2-phenylindole. |
Discussion
In the present study, the inhibitory effect of THCA
on AD-like markers and its molecular mechanism were studied in
vitro using HaCaT human keratinocytes. Accumulating evidence
has reported that an increased levels of IL-6 and IL-8 are involved
in the pathogenesis of AD and is closely associated with the
promotion of keratinocyte proliferation and migration (3,17,18).
Inflammatory chemokines have been reported to control the migration
of inflammatory cells to sites of infection and inflammation;
however, sustained levels of chemokines, such as TARC, MDC, RANTES
and MCP-1, are associated with the initiation and disease severity
of AD (5,19–22).
Considering all these reports, the suppression of inflammatory
cytokines and chemokines could lead to the amelioration of AD
symptoms. As mentioned earlier, the mixture of TI has been used to
induce an inflammatory response in human keratinocytes, such as
HaCaT cells (5,6); therefore, we selected the TI mixture
as an inducer and evaluated the anti-inflammatory effect of THCA.
In the present study, the experimental results showed that THCA
ameliorates the production of IL-6, IL-8, TARC, MDC, RANTES and
MCP-1 in activated HaCaT cells (Figs.
1–3). These results indicate
that THCA exerts anti-inflammatory activity in activated HaCaT
cells.
Accumulated evidence has shown that the production
of inflammatory cytokines and chemokines is associated with the
AKT/MAPK/NF-κB signaling pathways in experimental atopic models
(1,4,7). Thus,
these signaling pathways represent therapeutic targets for the
improvement of AD. Previous studies have shown the inhibitory
activity of THCA on NF-κB in lipopolysaccharide-stimulated BV2
microglia and RAW264.7 cells (13,14).
Recently, its inhibitory effect was confirmed on AKT/MAPK/NF-κB in
activated A549 cells (15). In this
study, THCA effectively decreased the activation of AKT, ERK, JNK,
p38 and NF-κB in stimulated-A549 cells and exerted inhibitory
effects on AKT, ERK, JNK and NF-κB in lung of asthmatic mice
(15). THCA also significantly
reduced the activation of ERK and p38 in lung of COPD-like mice
(16). In these studies, the
regulatory effects of THCA on AKT and MAPK was excellent.
Furthermore, the regulatory effects of THCA on MAPK activation were
confirmed in both in vitro and in vivo. Thus, we
expected this effect of THCA on AKT, MAPK and NF-κB to be confirmed
in this study. However, no modulatory effects were found on p-JNK
and p-38. Considering that THCA did not totally exert the
regulatory effect in both JNK (16)
and p38 (15) activation in
previous studies, it was not surprising that THCA only exerts an
inhibitory effect on ERK activation in the present study. Thus,
this result indicated that THCA have inhibitory effect on AKT, ERK
and NF-κB activation in TNF-α/IFN-γ stimulated HaCaT cells.
Collectively, regulatory ability of THCA on AKT/ERK/NF-κB may
contribute the amelioration of inflammatory response induced by TI
stimulation in the present study (Figs.
4 and 5).
Antioxidant proteins, such as HO-1 and NQO1, exert
anti-inflammatory properties in HaCaT cells (23,24).
Researchers have reported that the induction of HO-1 ameliorates
the inflammatory response in activated HaCaT cells by reducing TARC
and MDC expression levels and regulating NF-κB nuclear
translocation (25,26). NQO1 was shown to be related to
antimelanogenic efficacy in UVA-irradiated keratinocytes (27). Thus, HO-1 and NQO1 are recognized as
protective molecules in AD (28).
Nrf2 has an important role in HO-1 and NQO1 expression (29). In this study, THC showed inhibitory
effects on inflammatory molecules and AKT/ERK/NF-κB phosphorylation
in activated HaCaT cells (Figs.
1–5). Based on these results,
we studied whether THCA leads to the induction of HO-1/NQO1 and the
activation of Nrf2. We confirmed that THCA induces HO-1/NQO1
expression and Nrf2 activation in HaCaT cells (Fig. 6). Thus, this ability of THCA may be
associated with amelioration of the inflammatory response in
activated HaCaT cells. However, it is uncertain whether THCA
directly affect NF-κB nuclear translocation.
In the previous studies, THCA exerted strong
anti-inflammatory properties in LPS-stimulated RAW264.7 macrophages
(14) and this study also showed
the protective effect of THC on LPS-induced endotoxemia mice. In
addition, THCA had anti-inflammatory properties in PMA-stimulated
airway epithelial cells (15) and
protective effects on COPD like mice and allergic asthma mice
(15,16). In the present study, we confirmed
that THCA has anti-inflammatory effects in TNF-α/IFN-γ-stimulated
HaCaT cells. Collectively, these results indicated that THCA could
ameliorate the inflammatory response in various inflammatory
diseases. Thus, this experimental approach will support the
validity that THCA has a variety of anti-inflammatory actions.
In summary, THCA exerted anti-inflammatory
activities on TI-stimulated HaCaT cells (Fig. 7). In particular, its inhibitory
effects on important AD markers were excellent, and its regulatory
effects on AKT/ERK/NF-κB activation were also remarkable.
Furthermore, THCA upregulated the activation of Nrf2 and the
expression of antioxidant proteins. Therefore, our results suggest
that THCA can be used as an adjuvant in AD. However, further
experiments are needed to prove the ameliorative effect in AD
animal models.
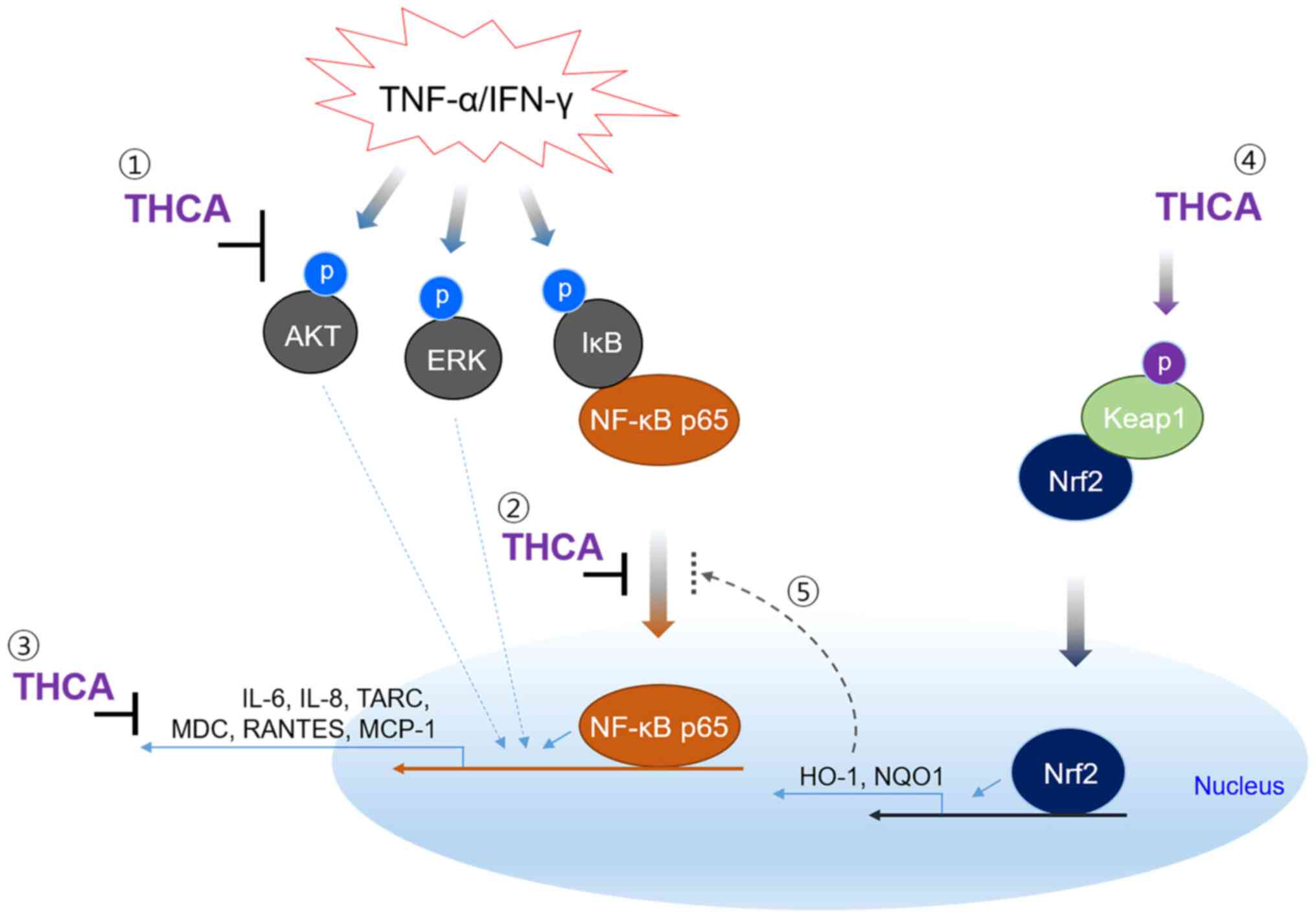 | Figure 7.The mechanism for suppression of
inflammatory cytokine and chemokines in human keratinocyte cell
line by THCA. TNF-α/IFN-γ induces AKT and MAPK activation, and
results in IκB activation and NF-κB nuclear translocation leading
to inflammatory responses. THCA suppresses the generation of
inflammatory cytokine and chemokines, such as IL-6, IL-8, TARC,
MDC, RANTES and MCP-1. THCA also induces the expression of HO-1 and
NQO1 by stimulating Nrf2 activation and nuclear translocation.
These results indicate that the inhibitory ability of THCA on
inflammatory molecules may be mediated by regulating the activation
of AKT, ERK and NF-κB. The result also suggest that THCA-induced
antioxidant protein, HO-1 may affect the nuclear translocation of
NF-κB. THCA, 3,4,5-trihydroxycinnamic acid; TNF-α, tumor necrosis
factor-α; IFN-γ, interferon-γ; AKT, protein kinase B; MAPK,
mitogen-activated protein kinase; NF-κB, nuclear factor-kappa B;
IL-6, interleukin-6; TARC, thymus and activation-regulated
chemokine; MDC, macrophage-derived chemokine; RANTES, regulated
upon activation, normal T cell expressed and secreted; MCP-1,
monocyte chemoattractant protein-1; HO-1, heme oxygenase-1; NQO1,
NAD(P)H quinone dehydrogenase 1; Nrf2, nuclear factor erythroid
2-related factor 2. |
Acknowledgements
Not applicable.
Funding
The present study was supported by a grant from the
Korea Research Institute of Bioscience and Biotechnology Research
Initiative Program (grant no. KGM5522113) and the Bio & Medical
Technology Development Program of the National Research Foundation
(NRF) and the Korean government (MSIT) (grant. no.
NRF2020R1A2C2101228).
Availability of data and materials
All data generated and/or analyzed during the
present study are included in this published article.
Authors' contributions
JWP designed the present study, performed the in
vitro experiments, and made substantial contributions to the
analysis and interpretation of data. JHO and DH performed the in
vitro experiments and made substantial contributions to the
analysis and interpretation of data. SMK, JHM and JYS contributed
to data analysis. WC, HJL and SRO made substantial contributions to
the conception and design of the present study. JWL and KSA
designed the present study, wrote the manuscript and was involved
in revising it critically for important intellectual content. All
authors discussed the results, and read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Park JW, Lee HS, Lim Y, Paik JH, Kwon OK,
Kim JH, Paryanto I, Yunianto P, Choi S, Oh SR and Ahn KS:
Rhododendron album Blume extract inhibits TNF-α/IFN-γ-induced
chemokine production via blockade of NF-κB and JAK/STAT activation
in human epidermal keratinocytes. Int J Mol Med. 41:3642–3652.
2018.PubMed/NCBI
|
|
2
|
Huang WC, Dai YW, Peng HL, Kang CW, Kuo CY
and Liou CJ: Phloretin ameliorates chemokines and ICAM-1 expression
via blocking of the NF-κB pathway in the TNF-α-induced HaCaT human
keratinocytes. Int Immunopharmacol. 27:32–37. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kwon DJ, Bae YS, Ju SM, Goh AR, Choi SY
and Park J: Casuarinin suppresses TNF-α-induced ICAM-1 expression
via blockade of NF-κB activation in HaCaT cells. Biochem Biophys
Res Commun. 409:780–785. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xiong X, Huang C, Wang F, Dong J, Zhang D,
Jiang J, Feng Y, Wu B, Xie T and Cheng L: Qingxue jiedu formulation
ameliorated DNFB-induced atopic dermatitis by inhibiting
STAT3/MAPK/NF-κB signaling pathways. J Ethnopharmacol.
270:1137732020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kong L, Liu J, Wang J, Luo Q, Zhang H, Liu
B, Xu F, Pang Q, Liu Y and Dong J: Icariin inhibits TNF-α/IFN-γ
induced inflammatory response via inhibition of the substance P and
p38-MAPK signaling pathway in human keratinocytes. Int
Immunopharmacol. 29:401–407. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee HS, Park JW, Kwon OK, Lim Y, Kim JH,
Kim SY, Zamora N, Rosales K, Choi S, Oh SR and Ahn KS:
Anti-inflammatory effects of ethanol extract from the leaves and
shoots of Cedrela odorata L. in cytokine-stimulated keratinocytes.
Exp Ther Med. 18:833–840. 2019.PubMed/NCBI
|
|
7
|
Lee da H and Lee CS: Flavonoid myricetin
inhibits TNF-α-stimulated production of inflammatory mediators by
suppressing the Akt, mTOR and NF-κB pathways in human
keratinocytes. Eur J Pharmacol. 784:164–172. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang JH, Yoo JM, Lee E, Lee B, Cho WK,
Park KI and Yeul Ma J: Anti-inflammatory effects of Perillae herba
ethanolic extract against TNF-α/IFN-γ-stimulated human keratinocyte
HaCaT cells. J Ethnopharmacol. 211:217–223. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee KS, Chun SY, Lee MG, Kim S, Jang TJ
and Nam KS: The prevention of TNF-α/IFN-γ mixture-induced
inflammation in human keratinocyte and atopic dermatitis-like skin
lesions in Nc/Nga mice by mineral-balanced deep sea water. Biomed
Pharmacother. 97:1331–1340. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Aye A, Song YJ, Jeon YD and Jin JS:
Xanthone suppresses allergic contact dermatitis in vitro and in
vivo. Int Immunopharmacol. 78:1060612020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Armutcu F, Akyol S, Ustunsoy S and Turan
FF: Therapeutic potential of caffeic acid phenethyl ester and its
anti-inflammatory and immunomodulatory effects (Review). Exp Ther
Med. 9:1582–1588. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Choi HG, Tran PT, Lee JH, Min BS and Kim
JA: Anti-inflammatory activity of caffeic acid derivatives isolated
from the roots of Salvia miltiorrhiza bunge. Arch Pharm Res.
41:64–70. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee JW, Choi YJ, Park JH, Sim JY, Kwon YS,
Lee HJ, Kim SS and Chun W: 3,4,5-Trihydroxycinnamic acid inhibits
lipopolysaccharide-induced inflammatory response through the
activation of Nrf2 pathway in BV2 microglial cells. Biomol Ther
(Seoul). 21:60–65. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee JW, Bae CJ, Choi YJ, Kim SI, Kwon YS,
Lee HJ, Kim SS and Chun W: 3,4,5-Trihydroxycinnamic acid inhibits
lipopolysaccharide (LPS)-induced inflammation by Nrf2 activation in
vitro and improves survival of mice in LPS-induced endotoxemia
model in vivo. Mol Cell Biochem. 390:143–153. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Park JW, Kim SM, Min JH, Kim MG, Kwon OK,
Hwang D, Oh JH, Park MW, Chun W, Lee HJ, et al:
3,4,5-Trihydroxycinnamic acid exerts anti-asthmatic effects in
vitro and in vivo. Int Immunopharmacol. 88:1070022020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Min JH, Kim MG, Kim SM, Park JW, Chun W,
Lee HJ, Oh SR, Ahn KS and Lee JW: 3,4,5-Trihydroxycinnamic acid
exerts a protective effect on pulmonary inflammation in an
experimental animal model of COPD. Int Immunopharmacol.
85:1066562020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sawamura D, Meng X, Ina S, Sato M, Tamai
K, Hanada K and Hashimoto I: Induction of keratinocyte
proliferation and lymphocytic infiltration by in vivo introduction
of the IL-6 gene into keratinocytes and possibility of keratinocyte
gene therapy for inflammatory skin diseases using IL-6 mutant
genes. J Immunol. 161:5633–5639. 1998.PubMed/NCBI
|
|
18
|
Gallucci RM, Sloan DK, Heck JM, Murray AR
and O'Dell SJ: Interleukin 6 indirectly induces keratinocyte
migration. J Invest Dermatol. 122:764–772. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lim HS, Jin SE, Kim OS, Shin HK and Jeong
SJ: Alantolactone from saussurea lappa exerts antiinflammatory
effects by inhibiting chemokine production and STAT1
phosphorylation in TNF-α and IFN-γ-induced in HaCaT cells.
Phytother Res. 29:1088–1096. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gros E, Bussmann C, Bieber T, Förster I
and Novak N: Expression of chemokines and chemokine receptors in
lesional and nonlesional upper skin of patients with atopic
dermatitis. J Allergy Clin Immunol. 124:753–760.e1. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim HH, Bae Y and Kim SH: Galangin
attenuates mast cell-mediated allergic inflammation. Food Chem
Toxicol. 57:209–216. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pivarcsi A and Homey B: Chemokine networks
in atopic dermatitis: Traffic signals of disease. Curr Allergy
Asthma Rep. 5:284–290. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang CH, Chang LC, Hu S, Hsiao CY and Wu
SJ: Spilanthol inhibits TNF-α-induced ICAM-1 expression and
pro-inflammatory responses by inducing heme oxygenase-1 expression
and suppressing pJNK in HaCaT keratinocytes. Mol Med Rep.
18:2987–2994. 2018.PubMed/NCBI
|
|
24
|
Kleszczyński K, Ernst IMA, Wagner AE,
Kruse N, Zillikens D, Rimbach G and Fischer TW: Sulforaphane and
phenylethyl isothiocyanate protect human skin against UVR-induced
oxidative stress and apoptosis: Role of Nrf2-dependent gene
expression and antioxidant enzymes. Pharmacol Res. 78:28–40. 2013.
View Article : Google Scholar
|
|
25
|
Kim H, Youn GS, An SY, Kwon HY, Choi SY
and Park J: 2,3-Dimethoxy-2′-hydroxychalcone ameliorates
TNF-α-induced ICAM-1 expression and subsequent monocyte
adhesiveness via NF-kappaB inhibition and HO-1 induction in HaCaT
cells. BMB Rep. 49:57–62. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jeong SI, Choi BM and Jang SI:
Sulforaphane suppresses TARC/CCL17 and MDC/CCL22 expression through
heme oxygenase-1 and NF-κB in human keratinocytes. Arch Pharm Res.
33:1867–1876. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hseu YC, Chen XZ, Vudhya Gowrisankar Y,
Yen HR, Chuang JY and Yang HL: The skin-whitening effects of
Ectoine via the suppression of α-MSH-stimulated melanogenesis and
the activation of antioxidant Nrf2 pathways in UVA-irradiated
keratinocytes. Antioxidants (Basel). 9:632020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen L and Zhong JL: MicroRNA and heme
oxygenase-1 in allergic disease. Int Immunopharmacol.
80:1061322020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Choi M, Park M, Lee S, Lee JW, Choi WJ and
Lee C: Establishment of Nrf2-deficient HaCaT and immortalized
primary human foreskin keratinocytes and characterization of their
responses to ROS-induced cytotoxicity. Toxicol In Vitro.
61:1046022019. View Article : Google Scholar : PubMed/NCBI
|