Introduction
Hepatoblastoma is one of the most common primary
malignant liver tumour types in children, predominantly occurring
in the first 2 years of life (1).
The incidence rate is ~1.2/1,000,000 individuals, and it has been
increasing in recent years, possibly due to the improved survival
of premature and low-birth-weight infants (2,3). The
current treatment strategy for hepatoblastoma includes
chemotherapy, surgical resection and liver transplantation.
Moreover, the application of platinum-based neoadjuvant
chemotherapy has significantly improved the clinical outcome of
hepatoblastoma (4). In the past
three decades, the overall survival has increased from 30 to 70%
(1). However, 25% of cases develop
metastasis, and the prognosis remains unsatisfactory (5). To date, little is known regarding the
molecular basis of the development of hepatoblastoma. Hence,
further understanding of the underlying mechanisms is of great
significance to identify novel diagnostic biomarkers and to enhance
the therapeutic efficacy for patients with hepatoblastoma.
Long non-coding RNAs (lncRNAs) are a class of
transcripts with lengths >200 nucleotides that have no or very
limited potential to encode proteins (6). It has been reported that lncRNAs are
widely involved in the development of a variety of diseases,
especially cancer (6). Recently,
studies have suggested that lncRNAs are often dysregulated, and may
serve important roles in the pathogenesis of hepatoblastoma
(7,8).
Taurine upregulated gene 1 (TUG1), a 7.1-kb lncRNA
located at chromosome 22q12, has been shown to be upregulated and
to contribute to the cell proliferation, migration and angiogenesis
of hepatoblastoma, as well as negatively regulate its apoptosis
(9,10), which provides a novel potential
therapeutic marker for hepatoblastoma treatment. However, how TUG1
acts at the molecular level requires further investigation. In
recent years, accumulating experimental evidence has indicated that
TUG1 acts as a ‘molecular sponge’ of microRNAs (miRNAs/miRs) to
indirectly participate in post-transcriptional processing and
promote tumour development in certain types of cancer (11,12).
In a study by He et al (13), TUG1 accelerated zinc finger E-box
binding homeobox (ZEB) 1-mediated epithelial-mesenchymal transition
(EMT) acquisition by functioning as a competitive endogenous RNA
(ceRNA) for miR-142-3p. Moreover, Yu et al (14) reported that TUG1 acted as a sponge
of miR-204-5p by upregulating RUNX family transcription factor 2 in
calcific aortic valve disease.
miRNAs represent a class of small non-coding RNA
molecules that function in RNA silencing and gene expression
regulation at the post-transcriptional level (3). miRNAs have emerged as novel players in
different diseases, especially cancer (6). It has been revealed that miRNAs, such
as the miR-100/let-7a-2/miR-125b-1 and miR-371-3 clusters, mediate
hepatoblastoma pathogenesis, at least partially, by controlling the
abnormally activated Myc or Wnt pathway (3). In addition, miR-204-5p is reported to
be a tumour suppressor and is able to regulate cell proliferation
and invasion in multiple cancer types, including breast cancer
(15) and oesophageal squamous cell
carcinoma (16). miR-204 was also
found to be dysregulated in lung adenocarcinoma and control the
biological behaviours of endothelial cells by targeting the Janus
kinase 2 (JAK2)/STAT3 pathway (17). The JAK2/STAT3 pathway regulates the
expression of genes associated with proliferation, migration,
survival, invasion and angiogenesis (18). STAT3 is required for cancer
initiation, development and progression by modulating oncogenes
such as cyclin D2 and c-Myc (18).
Moreover, Dong et al (19)
reported that TUG1 expression was significantly upregulated in
human hepatoblastoma samples and cell lines, and its upregulation
caused VEGFA induction via miR-204-5p, thereby contributing to the
hypervascularity of hepatoblastoma. Based on these previous
studies, we hypothesized that high expression of TUG1 in
hepatoblastoma cells possibly controls angiogenesis via the
miR-204-5p/JAK2/STAT3 network.
In the present study, the molecular mechanism
underlying TUG1 in hepatoblastoma was investigated and the effect
of miR-204-5p was clarified. The present findings provided novel
insight into the molecular basis of angiogenesis in hepatoblastoma
and shed light on lncRNA-directed therapeutics.
Materials and methods
Tissue collection
All human specimens were collected from patients
with diagnosed hepatoblastoma (n=10; 6 male and 4 female; age
range, 4 months-4 years) at Hunan Children's Hospital (Changsha,
China) between October 2018 and November 2019 with informed consent
obtained from every patient's legal guardian. Hepatoblastoma
tissues and non-cancerous adjacent tissues (the adjacent tumour
tissues (normal controls) were defined as tissues resected ≥3 cm
from the tumour margin that tested as normal by histopathologic
assessments.) were obtained from the patients during biopsy and/or
surgery. The present study was approved by the Hunan Children's
Hospital Ethics Committee.
Cell culture
Human hepatic tumour cell lines, HuH-6 and HepG2,
and the non-malignant liver cell line THLE-3 were obtained from the
American Type Culture Collection, and human umbilical vein
endothelial cells (HUVECs) were purchased from the Chinese Academy
of Sciences Cell Bank. Cells were cultured in DMEM (Gibco; Thermo
Fisher Scientific, Inc.) supplemented with 10% FBS (Biological
Industries), 100 IU/ml penicillin and 100 IU/ml streptomycin
(Tianjin Hao Yang Biological Products Technology Co., Ltd.) in a
humidified incubator with 5% CO2 at 37°C.
Cell transfection
To silence the expression of TUG1, short hairpin
RNAs (shRNAs) against TUG1 (shRNA1, shRNA2, shRNA3) and negative
control shRNA (shNC) were synthesized by GeneCopoeia, Inc. It was
found that shRNA2 had the best silencing effect, and thus, shRNA2
was used for the subsequent experiments. The shRNA sequences used
in the study are listed in Table
SI. The miR-204-5p mimics (5′-UUCCCUUUGUCAUCCUAUGCCU-3′),
inhibitor (5′-AGGCAUAGGAUGACAAAGGGAA-3′), NC (mimics NC,
5′-UUUGUACUACACAAAAGUACUG-3′; inhibitor NC,
5′-CAACGCUGCAUGGUACCAUGCU-3′) and siNC
(5′-GCGGUUAGCGUCUAUCUGAGU-3′), siJAK2 (5′-UUAAAGAGGAAGAUUUUUCUG-3′)
were purchased from Sangon Biotech Co., Ltd.
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) was used for shRNA and shNC (0.5 µg)
transfection, while Lipofectamine® RNAiMAX (Invitrogen;
Thermo Fisher Scientific, Inc.) was used for miR-204-5p (mimics,
inhibitor and NC, 50 nM) and siJAK2/siNC (50 nM) transfection at
37°C for 24 h. Cells were harvested after 48 h of transfection.
Preparation of conditioned media
(CM)
CM were collected after 3 days of cells being
transfected with shNC or shRNA, centrifuged at 1,500 × g at 4°C for
5 min and then concentrated using a 10-kDa ultrafiltration
centrifuge tube (EMD Millipore) at 8,000 × g (4°C) for 1 h.
Subsequently, the CM were either used or stored at −80°C for
subsequent experiments.
RNA extraction and reverse
transcription-quantitative (RT-q)PCR
Total RNA from cells or human samples was harvested
using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) on ice according to the manufacturer's
instructions, and then reverse-transcribed into cDNA using
PrimeScript RT reagent kit (Takara Bio, Inc.). RT was performed in
accordance with the Applied Biosystems TaqMan miRNA assay protocol
(Applied Biosystems; Thermo Fisher Scientific, Inc.) at 85°C for 5
sec, 37°C for 10 min and 4°C for 15 min, with U6 as the normalizer.
qPCR and data collection were conducted with SYBR Green buffer
(Takara Bio, Inc.) on an ABI 7500 Fast instrument (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The following
thermocycling conditions were used for qPCR: 95°C for 5 min;
followed by 40 cycles at 95°C for 15 sec, 60°C for 30 sec and 72°C
for 30 sec. The relative RNA expression levels were calculated with
the 2−ΔΔCq (20) method
and normalized to that of GAPDH (internal control). The primer
sequences used in the study were as follows: TUG1 forward (F),
5′-ACGACTGAGCAAGCACTACC-3′ and reverse (R),
5′-CTCAGCAATCAGGAGGCACA-3′; miR-204-5p F,
5′-GCCAGATCTGGAAGAAGATGGTGGTTAGT-3′ and R,
5′-GGCGAATTCACAGTTGCCTACAGTATTCA-3′; JAK2 F,
5′-GGGAGGTGGTCGCTGTAAAA-3′ and R, 5′-ACCAGCACTGTAGCACACTC-3′; GAPDH
F, 5′-TGTGTCCGTCGTGGATCTGA-3′ and R, 5′-CCTGCTTCACCACCTTCTTGA-3′;
and U6 F, 5′-GCTTCGGCAGCACATATACTAA-3′ and R,
5′-AACGCTTCACGAATTTGCGT-3′.
ELISA for VEGFA detection
A total of 5 days after culture of HuH-6 cells with
shNC and shTUG1, the release of pro-angiogenic VEGFA from the
respective medium samples (CM) was quantitatively detected using a
human VEGFA ELISA kit (Invitrogen; Thermo Fisher Scientific, Inc.;
cat. no. BMS277-2) in accordance with the manufacturer's protocols.
Recombinant VEGFA was used as the standard. The optical density was
measured using a microplate reader (Molecular Devices LLC) at a
wavelength of 450 nm. The experiments were performed independently
three times with three replicates each.
Tube formation assay
CM were collected from HuH-6 cells transfected with
shTUG1 or shNC. A total of 8×104 HUVECs were seeded in
96-well plates precoated with 50 µl per well Matrigel Basement
Membrane Matrix (BD Bioscience; 45 min at 37°C), and incubated with
100 µl per well of the indicated CM for 8 h at 37°C. Images of
tubular structures were captured using an inverted light microscope
at ×100 magnification. The cumulative tube length and total
branching length were analysed and quantified using ImageJ software
(National Institutes of Health; v1.8.0).
Plasmid construction
To knock down the expression of TUG1, shRNA1,
shRNA2, shRNA3 and negative control shRNA (shNC) were constructed
by GeneCopoeia, Inc. Packaging and amplification of lentiviruses
were conducted in 293T cells (American Type Culture Collection)
according to standard protocols. HuH-6 cells were infected with
lentivirus carrying shRNA or shNC (0.5 µg) for 24 h at 37°C and
subsequently cultured under selection with 1 µg/ml puromycin
(Invitrogen; Thermo Fisher Scientific, Inc.) for 3 weeks.
Luciferase reporter assay
StarBase V2.0 (http://starbase.sysu.edu.cn) analysis was performed to
predict the binding site of miR-204-5p with lncRNA TUG1 and JAK2.
HuH-6 cells were co-transfected with pGL3 luciferase reporter
(Promega Corporation) plasmids (2 µg) containing the human JAK2 (or
TUG1) 3′-untranslated region (3′-UTR) with putative miR-204-5p
binding sites or the corresponding mutant (MUT), and miR-204-5p
mimics or the NC mimics (50 nM) using Lipofectamine®
2000 (Invitrogen; Thermo Fisher Scientific, Inc.) at 37°C for 6 h.
After 48 h, cells were collected and lysed with Passive Lysis
Buffer. The levels of firefly luciferase were consecutively
measured using the Dual Glo Luciferase Assay system (Promega
Corporation) on the MultiScan MCC/340 system (Thermo Fisher
Scientific, Inc.) following the manufacturer's manual, with
Renilla luciferase activity as the internal control to
eliminate the variability resulting from transfection
efficiency.
Protein extraction and western
blotting
Cells were collected and lysed on ice in RIPA buffer
(Santa Cruz Biotechnology, Inc.) in the presence of 0.1% protease
inhibitor PMSF (Thermo Fisher Scientific, Inc.). Total protein was
quantified using Bradford assays (Bio-Rad Laboratories, Inc.).
Equal amounts of protein samples (50 µg) were loaded and separated
by denaturing 10% SDS-PAGE gels and then transferred to PVDF
membranes (Bio-Rad Laboratories, Inc.). After blocking with 5%
non-fat milk for 4 h at 37°C, the membranes were incubated with
various primary antibodies at 4°C overnight, and this was followed
by incubation with HRP-conjugated secondary antibody for 1 h at
room temperature. Protein bands were visualized with Tanon 5200
using a ECL kit (Thermo Fisher Scientific, Inc.). Antibodies were
diluted as follows: Anti-VEGFA (Invitrogen; Thermo Fisher
Scientific, Inc.; 1:1,000; cat. no. MA5-12184), anti-phosphorylated
(p)-JAK2 [Cell Signaling Technology, Inc. (CST); 1:1,000; cat. no.
3771S], anti-JAK2 (Santa Cruz Biotechnology, Inc.; 1:1,000; cat.
no. sc-390539), anti-p-STAT3 (CST; 1:1,000; cat. no. 9145T),
anti-STAT3 (Abcam; 1:1,000; cat. no. ab68153), anti-GAPDH (HUABIO;
1:5,000; cat. no. ER1706-83) and goat anti-rabbit or mouse
secondary antibody (HUABIO; 1:5,000; cat. no. HA1019/HA1020).
Protein bands were quantified using ImageJ software (v1.8.0;
National Institutes of Health) with GAPDH as the loading
control.
Statistical analysis
All statistical analyses were performed using SPSS
16.0 software (SPSS, Inc.). Data are presented as the mean ± SD.
Statistical analyses were performed with unpaired Student's t-test
(two-tailed) and one-way ANOVA following by Tukey's post hoc test.
Pearson's analysis was used to analysis the corelation of TUG1 and
miR-204-5p. P<0.05 was considered to indicate a statistically
significant difference. All experiments were performed three times
independently.
Results
TUG1 is upregulated, while miR-204-5p
is downregulated in hepatoblastoma tissues and cell lines
It has been previously reported that TUG1 is
abnormally expressed in hepatoblastoma cell lines (17). The present study further collected
10 paired hepatoblastoma specimens and their paracancerous tissue
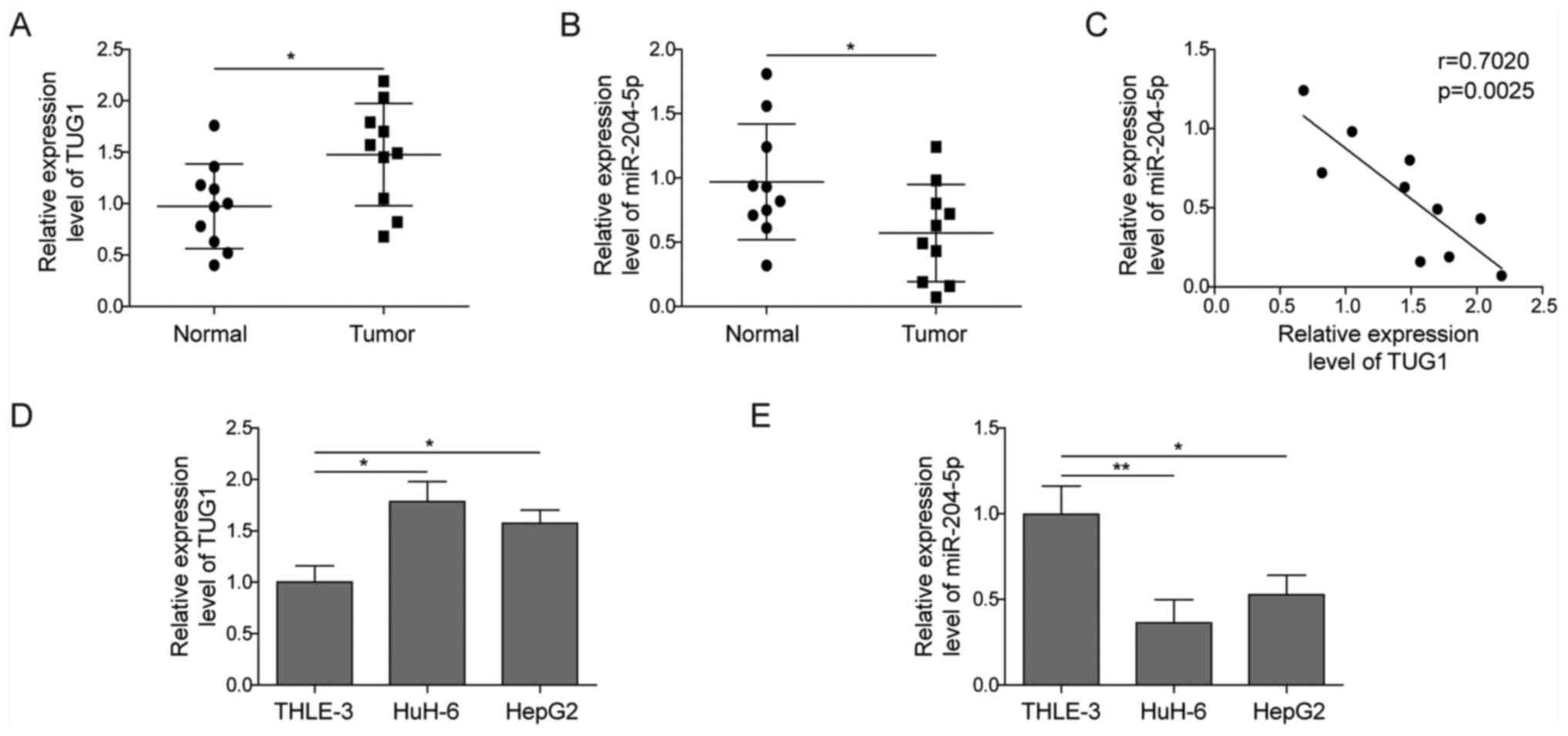
specimens to detect the expression level of TUG1. As shown in
Fig. 1A, TUG1 expression was
significantly higher in tumour samples compared with in normal
controls.
miR-204-5p was reported to participate in
angiogenesis in ovarian cancer (21). The present study aimed to determine
its role in hepatoblastoma. In contrast to TUG1 expression,
miR-204-5p expression was found to be ~50% downregulated in
hepatoblastoma tissues (Fig. 1B).
Using Pearson's analysis, TUG1 was found to be negatively
correlated with miR-204-5p (Fig.
1C). Consistent with the results in the tumour specimens,
upregulation of TUG1 and downregulation of miR-204-5p were also
observed in the human hepatoblastoma cell lines HuH-6 and HepG2
compared with the non-malignant liver cell line THLE-3 (Fig. 1D and E). Taken together, these data
indicated the potential relationship between TUG1 and miR-204-5p in
hepatoblastoma.
Knockdown of TUG1 suppressed
angiogenesis in vitro
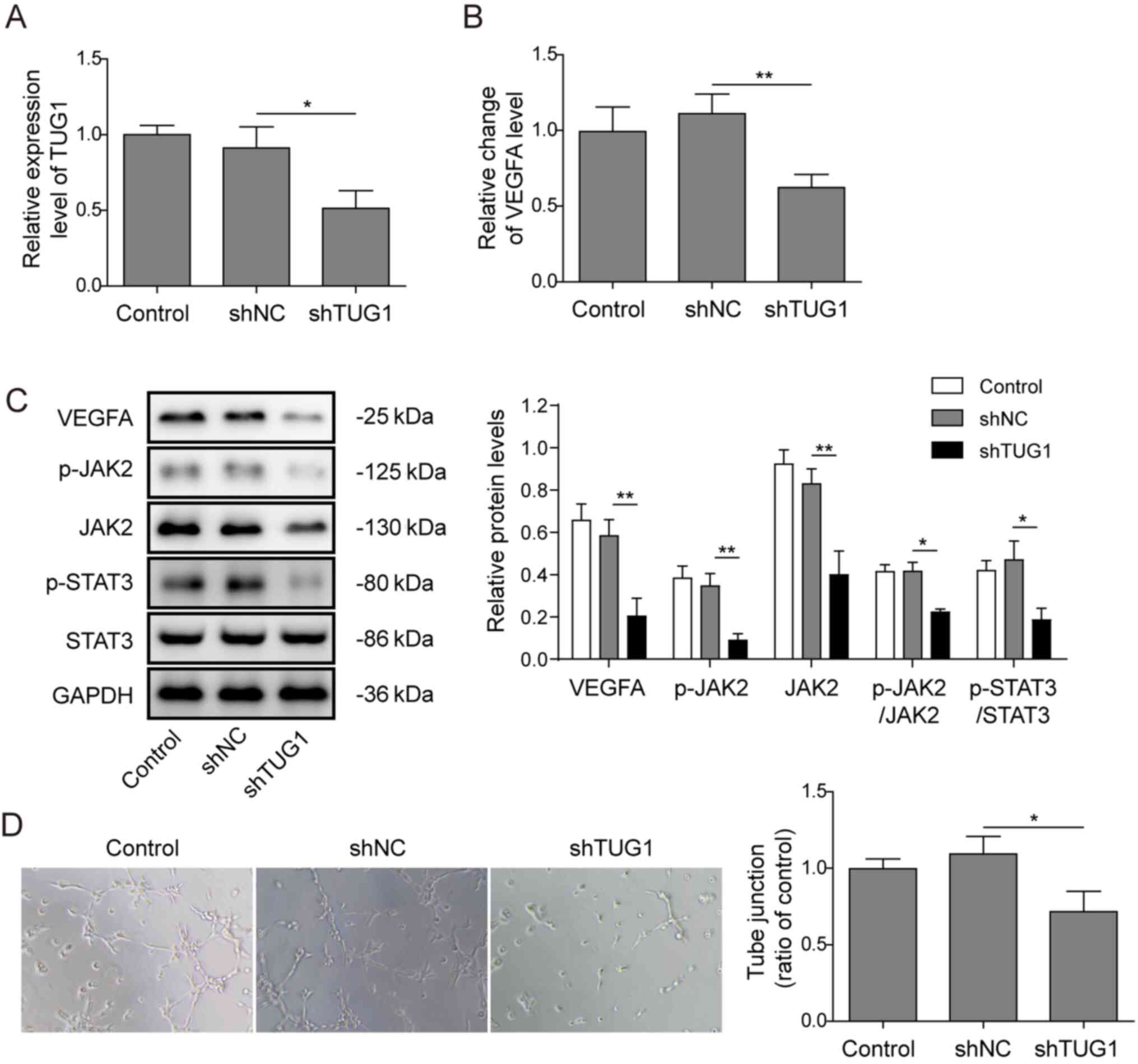
To investigate the role of TUG1 in tumour
angiogenesis, HuH-6 cells were selected for further study as the
expression of TUG1 was higher in HuH-6 cells. TUG1 expression was
knocked down via transfection with shTUG1 plasmid (shRNA for TUG1,
shRNA1, shRNA2, shRNA3) or a negative control shRNA (shNC), and
shRNA2 had the best silencing effect (Fig. S1). Thus, shRNA2 was used for the
subsequent experiments to knockdown TUG1 in HuH-6 cells (Fig. 2A), and then the CM were collected.
The mRNA expression level of VEGFA, the key signalling molecule
involved in pathological angiogenesis, was significantly decreased
after transfection of HuH-6 cells with shTUG1 (Fig. 2B), and similar results were obtained
for its protein expression (Fig.
2C). As presented in Fig. 2C,
it was observed that, knockdown of TUG1 resulted in significantly
decreased protein expression levels of VEGFA, p-JAK2, JAK2 and
p-STAT3. Moreover, a Matrigel-based tube formation assay using
HUVECs was also conducted. Reduced tube formation was observed in
HUVECs cultured in the presence of CM derived from HuH-6 cells
transfected with shTUG1 (Fig. 2D).
These data indicated that TUG1 was potentially involved in
angiogenesis in hepatoblastoma.
TUG1 acts as a sponge of
miR-204-5p
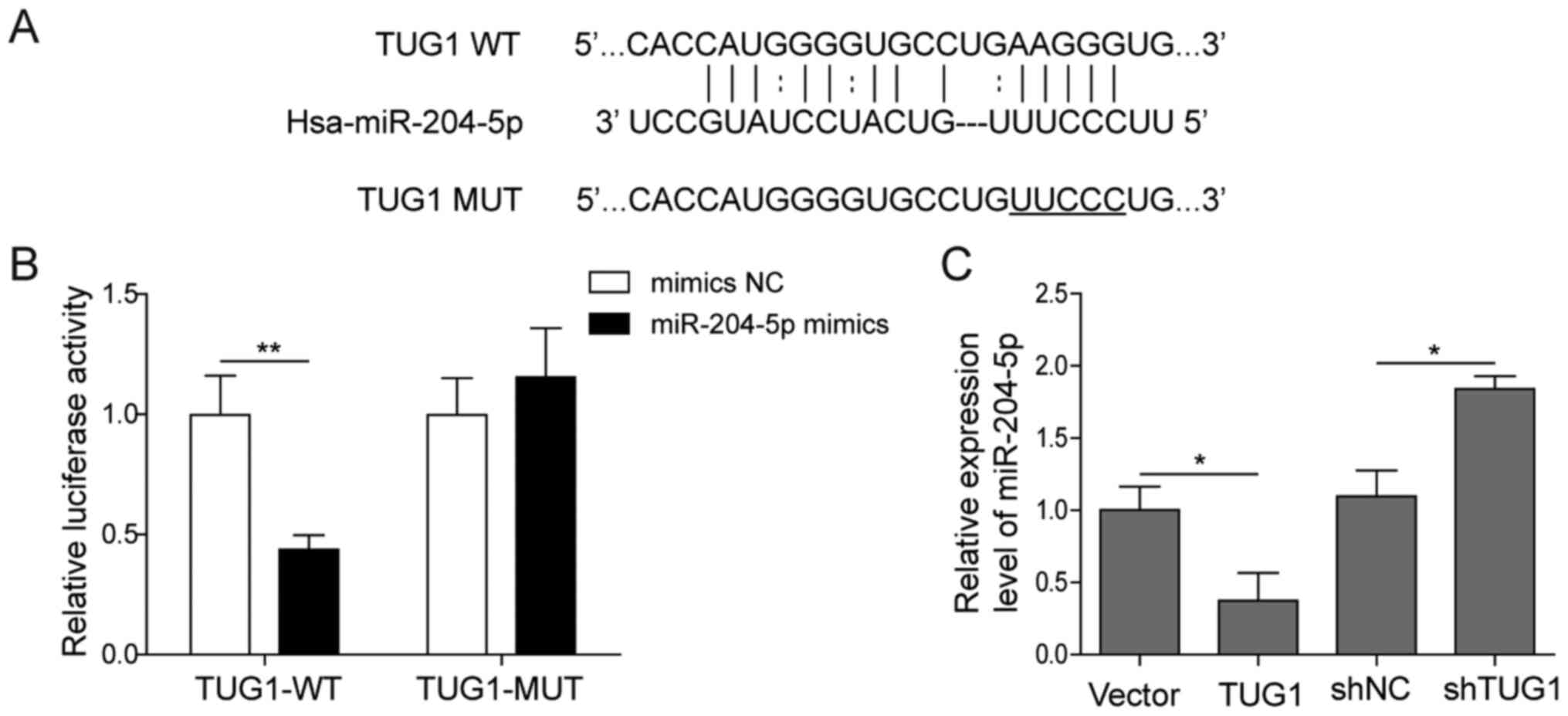
Since cytoplasmic lncRNA was found to act as a
natural miRNA sponge to interfere with miRNA-targeted genes at the
post-transcriptional level (22),
the present study further investigated the relationship between
TUG1 and miR-204-5p. The sequence of TUG1 containing the putative
binding site of miR-204-5p (Fig.
3A) (11) was cloned into the
3′-UTR of the luciferase reporter [Fluc-TUG1-wild-type (WT)] and
transfected into HuH-6 cells along with miR-204-5p mimics or the NC
mimics. As presented in Fig. 3B,
luciferase activity was decreased in HuH-6 cells co-transfected
with Fluc-TUG1-WT and miR-204-5p. However, when the putative
binding site of miR-204-5p on the TUG1 sequence was mutated
(Fluc-TUG1-MUT), there was no change in promoter activity compared
with the control, which was designed to prevent non-specific
binding. The effect of TUG1 on miR-204-5p was also demonstrated,
indicating the binding of TUG1 with miR-204-5p (Fig. S2). Overexpression of TUG1
significantly decreased the expression of miR-204-5p, whereas its
knockdown increased the expression of miR-204-5p (Fig. 3C). Collectively, these data
suggested that miR-204-5p interacted with TUG1 in hepatoblastoma
cells.
miR-204-5p directly targets JAK2 to
regulate the JAK2/STAT3 pathway
A putative binding site between miR-204-5p and JAK2
was predicted using StarBase (Fig.
4A). To determine whether miR-204-5p directly targeted JAK2 and
regulated the downstream signalling pathway in hepatoblastoma, a
luciferase reporter assay was conducted in HuH-6 cells. As shown in
Fig. 4B, it was found that
co-transfection of miR-204-5p mimics inhibited the luciferase
activity of the WT JAK2 3′-UTR, which contained the theoretical
miR-204-5p binding site, but failed to suppress that of the MUT
JAK2 3′-UTR. Transfection of miR-204-5p mimics increased the
expression of miR-204-5p, while transfection of miR-204-5p
inhibitor decreased the expression of miR-204-5p (Fig. 4C). In addition, overexpression of
miR-204-5p resulted in a reduction in JAK2 mRNA expression
(Fig. 4D), as well as in the
protein expression levels of JAK2, p-JAK2 and p-STAT3 (Fig. 4E). Moreover, addition of the
miR-204-5p inhibitor significantly increased JAK2 mRNA expression
(Fig. 4D) and the protein
expression levels of JAK2, p-JAK2 and p-STAT3 (Fig. 4E). These results demonstrated that
endogenous JAK2 was targeted by miR-204-5p, which further affected
the downstream STAT3 pathway in hepatoblastoma cells.
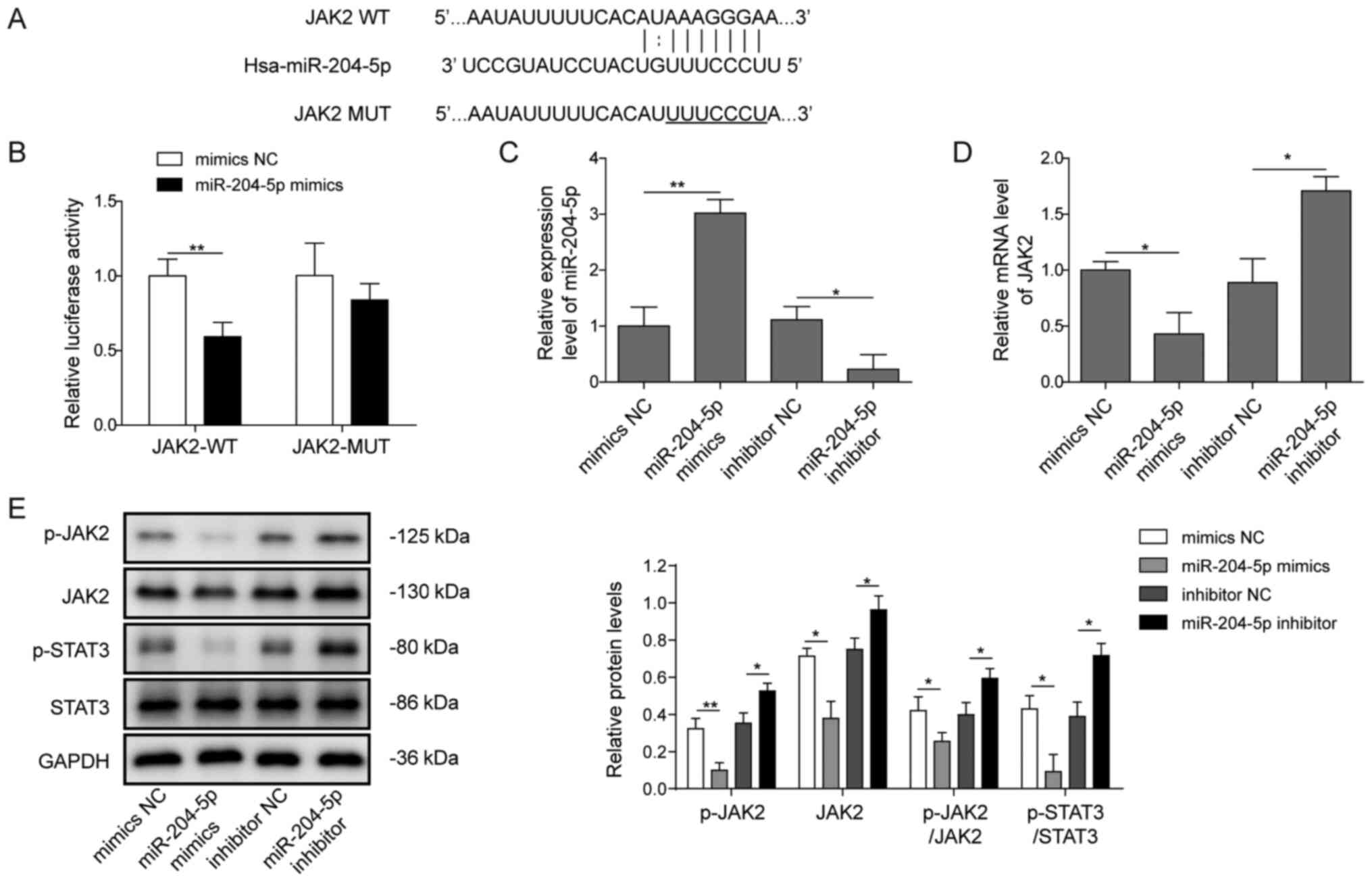 | Figure 4.miR-204-5p targets JAK2 to regulate
the JAK2/STAT3 pathway. (A) The putative binding site of miR-204-5p
on JAK2 was predicted using StarBase. (B) Relative luciferase
activity of JAK2-WT and JAK2-MUT co-transfected with mimics NC or
miR-204-5p mimics was reported using a dual luciferase reporter
assay. (C) miR-204-5p was detected via RT-qPCR after transfection
of HuH-6 cells with miR-204-5p mimics or inhibitor. (D) Relative
mRNA expression level of JAK2 after transfection with miR-204-5p
mimics or inhibitor, as detected via RT-qPCR. (E) Representative
western blotting images and semi-quantification of p-JAK2, JAK2,
p-STAT3 and STAT3 protein expression levels after transfection with
miR-204-5p mimics or inhibitor. *P<0.05, **P<0.01. p,
phosphorylated; NC, negative control; miR, microRNA; WT, wild-type;
MUT, mutant; JAK, Janus kinase 2; RT-qPCR, reverse
transcription-quantitative PCR. |
TUG1 promotes angiogenesis via the
miR-204-5p/JAK2/STAT3 network in hepatoblastoma
Next, it was evaluated whether TUG1 could regulate
the JAK2/STAT3 cascade via miR-204-5p. The mRNA expression of JAK2
was decreased following transfection with siJAK2 (Fig. S3). TUG1 knockdown increased the
expression of miR-204-5p (Fig. 5A)
and decreased the mRNA expression of JAK2 (Fig. 5B). The trend was reversed after
co-transfection with shTUG1 and miR-204-5p. However, the mRNA
expression of JAK2 was decreased following transfection with
siJAK2. The expression level of the secretory proangiogenic factor
VEGFA in the culture media of HuH-6 cells was decreased after
transfection with shTUG1, while the expression level of VEGFA was
increased after transfection with the miR-204-5p inhibitor.
Moreover, VEGFA expression was restored after co-transfection of
shTUG1 and the miR-204-5p inhibitor or miR-204-5p inhibitor and
siJAK2 (Fig. 5C).
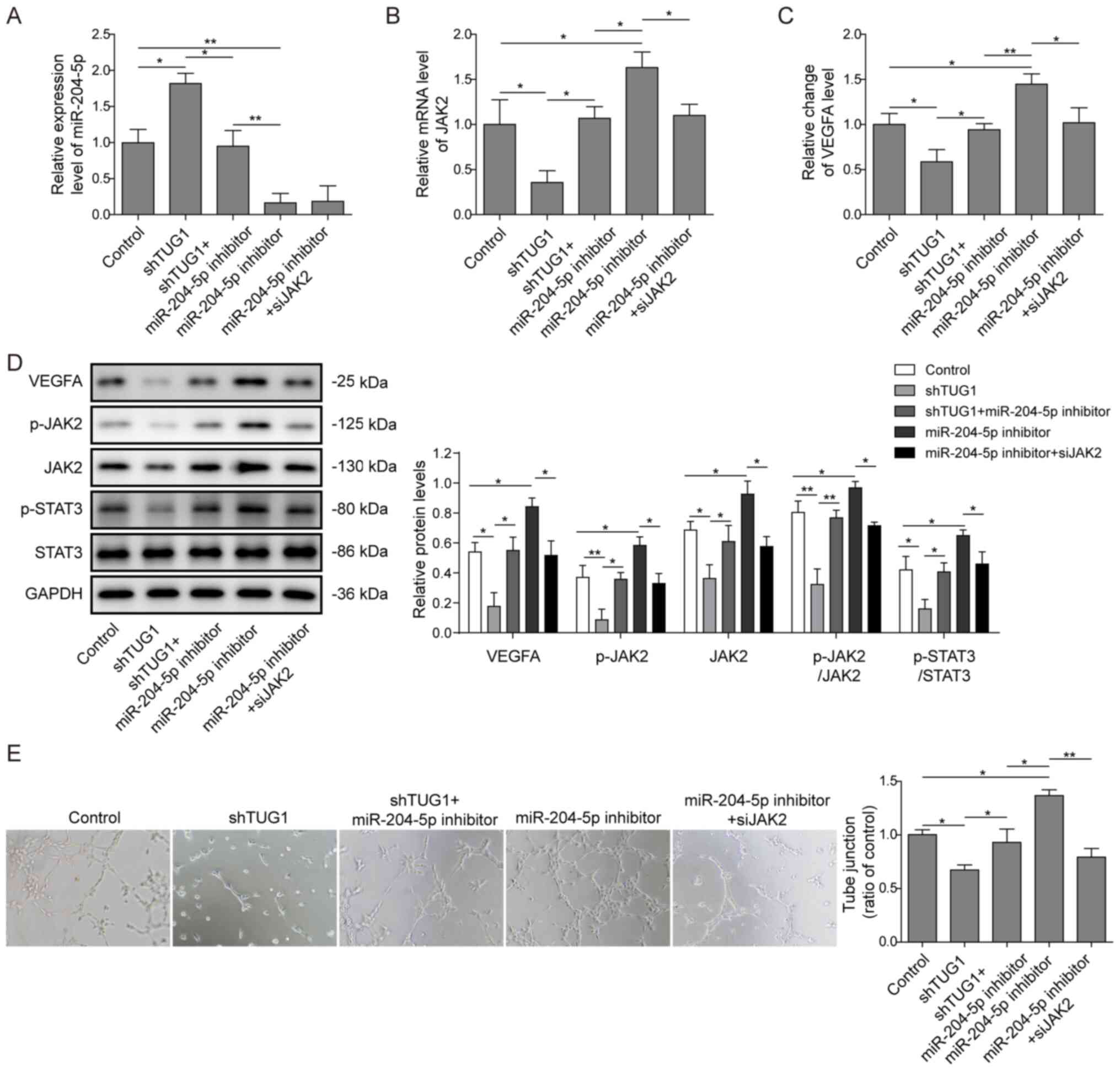 | Figure 5.TUG1 modulates angiogenesis via
miR-204-5p/JAK2/STAT3. HuH-6 cells were transfected with shTUG1 or
miR-204-5p inhibitor or co-transfected with shTUG1 and miR-204-5p
or siJAK2 and miR-204-5p. Relative expression levels of (A)
miR-204-5p and (B) JAK2 mRNA were detected via reverse
transcription-quantitative PCR. (C) The level of the secretory
proangiogenic factor VEGFA in the culture media of HuH-6 cells was
detected using ELISA. (D) Protein expression levels of VEGFA,
p-JAK2, JAK2, p-STAT3 and STAT3 were measured using western blot
analysis. (E) Human umbilical vein endothelial cells were cultured
in 96-well plates precoated with Matrigel and conditioned media
derived from treated HuH-6 cells. Representative graphs and tube
junctions in these groups were obtained via light microscopy at
×100 magnification. *P<0.05, **P<0.01. p, phosphorylated;
miR, microRNA; JAK2, Janus kinase 2; sh, short hairpin RNA; TUG1,
taurine upregulated 1; si, small interfering RNA. |
The protein expression levels of downstream
effectors of the JAK2/STAT3 pathway were also detected. Knockdown
of TUG1 significantly downregulated the protein expression levels
of JAK2, p-JAK2, p-STAT3 and VEGFA (Fig. 5D). The same trend was observed in
HepG2 cells (Fig. S4). However,
the decrease was reversed by the miR-204-5p inhibitor, which
suggested that TUG1 controlled the JAK2/STAT3 pathway via
miR-204-5p.
To determine the role of TUG1 and miR-204-5p in the
angiogenesis of hepatoblastoma, a tube formation assay was
performed using HUVECs. It was found that tube junctions in HUVECs
cultured in CM of shTUG1 HuH-6 cells were fewer compared with those
in the control group, whereas the loss of CM angiogenic activity
was countered by the addition of the miR-204-5p inhibitor to
shTUG1-transfected HuH-6 cells (Fig.
5E). When siJAK2 was transfected into HuH-6 cells, the
upregulation of VEGFA and the tube formation activity of the CM
induced by the miR-204-5p inhibitor was partially suppressed, which
confirmed that miR-204-5p regulated angiogenesis via JAK2/STAT3
signalling (Fig. 5D and E).
Overall, TUG1 acted as a sponge of miR-204-5p, which activated the
JAK2/STAT3 pathway and promoted the expression of VEGFA, leading to
increased angiogenesis activity.
Discussion
Hepatoblastoma is a type of paediatric cancer
arising from hepatic progenitors or hepatoblasts (23). In recent decades, there have been
considerable improvements in the treatment of hepatoblastoma, such
as the advances in cisplatin-based chemotherapy, and the overall
5-year survival has reached 70–80% (24). However, ~20% of children diagnosed
with hepatoblastoma have pulmonary metastasis, which is associated
with a very poor prognosis (25–50% survival rate) (25). Thus, it is of great clinical
necessity to improve the early diagnosis and inhibit the
progression of hepatoblastoma.
The dysregulation of lncRNAs and their correlation
with the prognosis of patients with hepatoblastoma have been widely
reported. Genome-wide analysis of lncRNA expression has also been
conducted to evaluate lncRNAs that can serve as potential clinical
targets or biomarkers for hepatoblastoma (26). The present study demonstrated that
lncRNA TUG1 was highly expressed in hepatoblastoma tumour tissues
and cell lines, which promoted the unusual hypervascularity.
Recent evidence has revealed the intricate interplay
among diverse RNA transcripts, including mRNAs, lncRNAs and miRNAs.
These molecules communicate and work together to co-regulate the
expression of targeted genes, and have gained substantial attention
(22). In the present study, the
interaction between TUG1 and miR-204-5p could be explained by the
ceRNA hypothesis. In normal hepatic cells, the expression levels of
TUG1 and miR-204-5p maintained a balance. However, in
hepatoblastoma cells, TUG1 was significantly upregulated, which
attenuated the inhibitory effect of miR-204-5p on VEGFA and
resulted in hypervascularity effects.
miRNAs are a class of small non-coding
single-stranded RNA molecules containing 19–25 nucleotides that
function as important post-transcriptional gene expression
regulators via base pairing with specific targeted mRNAs (3). Importantly, via gene manipulation,
miRNAs serve key roles in cell proliferation, invasion,
differentiation, angiogenesis and metastasis, amongst other
processes (27). Moreover, miRNAs
were found to participate in the activation of angiogenesis by
targeting the angiogenic factors VEGFA and MET (28). The present study identified that
miR-204-5p participated in the process of angiogenesis in
hepatoblastoma by promoting the production of VEGFA, which is
consistent with previous research (29). Furthermore, Tan et al
(30) reported that
TUG1/miR-145/ZEB2 was dysregulated in human bladder cancer and
promoted EMT, as well as radio resistance, which indicated the
tumour promoter role of TUG1 and was in accordance with the current
findings. However, whether TUG1 can regulate hepatoblastoma through
binding with other miRNA? It is needed to further exploration.
Aberration of the JAK2/STAT3 signalling pathway is
involved in several oncogenic processes in solid tumours (31). Mutations in JAK1 and JAK2, which
result in constitutive activation of STAT3, are frequently reported
in various haematopoietic malignancies (32). The present study demonstrated that
JAK2/STAT3 was involved in the angiogenesis of hepatoblastoma by
regulating the expression of VEGFA, which is in accordance with the
previously reported role of JAK2/STAT3 in cancer cells. For
example, Xue et al (33)
revealed that inhibition of JAK/STAT3 attenuated angiogenesis in an
endothelial cell/adipose-derived stromal cell co-culture 3D model,
and this process was mediated by decreasing VEGFA and cyclin D1
expression, which is consistent with the current observations. JAK
inhibitors are already under clinical evaluation for the treatment
of these diseases (33), and these
may be expected to be used in the inhibition of angiogenesis of
hepatoblastoma in further studies.
It is the limitations of the present study that TUG1
promote the angiogenesis of hepatoblastoma maybe through binding
other miRNA and pathways, and miR-204-5p maybe have other target
genes. However, we have only explored the
TUG1/miR-204-5p/JAK2/STAT3 pathway, and other pathways was required
further study.
In conclusion, the present study determined that
TUG1 promoted the angiogenesis of hepatoblastoma via the
miR-204-5p/JAK2/STAT3 axis. Further studies should investigate the
possibility of developing effective drugs targeting this pathway in
hepatoblastoma. Future studies will examine the molecular mechanism
of TUG1 in hepatoblastoma in animal models. Whether TUG1 can
regulate hepatoblastoma through other pathways requires further
study.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by Health Commission
Fund of Hunan Province. (grant no. 20200150).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
MXY was the guarantor of integrity of the entire
study, conceptualised and designed the study, defined the
intellectual content and performed the literature search, as well
as conducted the data/statistical analysis. MXY, CYJ, HQG, XYS, WXX
and QY performed the clinical studies. MXY, CYJ, HQG, XYS and WXX
performed the experimental studies and data acquisition. MXY and
CYJ prepared and edited the manuscript. QY reviewed the manuscript.
MXY and QY confirm the authenticity of the raw data. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Hunan Children's
Hospital Ethics Committee. All legal guardians of patients were
informed of the study and signed the written consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
lncRNAs
|
long non-coding RNAs
|
|
HUVECs
|
human umbilical vein endothelial
cells
|
|
CM
|
conditioned media
|
|
3′-UTR
|
3′-untranslated region
|
|
p-
|
phosphorylated
|
|
miRNA/miR
|
microRNA
|
|
ceRNA
|
competitive endogenous RNA
|
References
|
1
|
Yang T, Whitlock RS and Vasudevan SA:
Surgical management of hepatoblastoma and recent advances. Cancers
(Basel). 11:19442019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sharma D, Subbarao G and Saxena R:
Hepatoblastoma. Semin Diagn Pathol. 34:192–200. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cristóbal I, Sanz-Álvarez M, Luque M,
Caramés C, Rojo F and García-Foncillas J: The role of microRNAs in
hepatoblastoma tumors. Cancers (Basel). 11:4092019. View Article : Google Scholar
|
|
4
|
Venkatramani R, Wang L, Malvar J, Dias D,
Sposto R, Malogolowkin MH and Mascarenhas L: Tumor necrosis
predicts survival following neo-adjuvant chemotherapy for
hepatoblastoma. Pediatr Blood Cancer. 59:493–498. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bell D, Ranganathan S, Tao J and Monga SP:
Novel advances in understanding of molecular pathogenesis of
hepatoblastoma: A Wnt/β-catenin perspective. Gene Expr. 17:141–154.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Huarte M: The emerging role of lncRNAs in
cancer. Nat Med. 21:1253–1261. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Luo Z and Cao P: Long noncoding RNA PVT1
promotes hepatoblastoma cell proliferation through activating
STAT3. Cancer Manag Res. 20:8517–8527. 2019. View Article : Google Scholar
|
|
8
|
Chen LJ, Yuan MX, Ji CY, Zhang YB, Peng
YM, Zhang T, Gao HQ, Sheng XY, Liu ZY, Xie WX and Yin Q: Long
non-coding RNA CRNDE regulates angiogenesis in hepatoblastoma by
targeting the MiR-203/VEGFA axis. Pathobiology. 87:161–170. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xie F, Zhang L, Yao Q, Shan L, Liu J, Dong
N and Liang J: TUG1 promoted tumor progression by sponging
miR-335-5p and regulating CXCR4-Mediated infiltration of pro-tumor
immunocytes in CTNNB1-mutated hepatoblastoma. Onco Targets Ther.
14:3105–3115. 2020. View Article : Google Scholar
|
|
10
|
Li Q, Zhang J, Su DM, Guan LN, Mu WH, Yu
M, Ma X and Yang RJ: lncRNA TUG1 modulates proliferation,
apoptosis, invasion, and angiogenesis via targeting miR-29b in
trophoblast cells. Hum Genomics. 13:502019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lei H, Gao Y and Xu X: lncRNA TUG1
influences papillary thyroid cancer cell proliferation, migration
and EMT formation through targeting miR-145. Acta Biochim Biophys
Sin (Shanghai). 49:588–597. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhou H, Gao Z and Wan F:
Taurine-upregulated gene 1 contributes to cancers through sponging
microRNA. Acta Biochim Biophys Sin (Shanghai). 51:123–130. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
He C, Liu Z, Jin L, Zhang F, Peng X, Xiao
Y, Wang X, Lyu Q and Cai X: lncRNA TUG1-Mediated Mir-142-3p
downregulation contributes to metastasis and the
epithelial-to-mesenchymal transition of hepatocellular carcinoma by
targeting ZEB1. Cell Physiol Biochem. 48:1928–1941. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yu C, Li L, Xie F, Guo S, Liu F, Dong N
and Wang Y: lncRNA TUG1 sponges miR-204-5p to promote osteoblast
differentiation through upregulating Runx2 in aortic valve
calcification. Cardiovasc Res. 114:168–179. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hong BS, Ryu HS, Kim N, Kim J, Lee E, Moon
H, Kim KH, Jin MS, Kwon NH, Kim S, et al: Tumor suppressor
miRNA-204-5p regulates growth, metastasis, and immune
microenvironment remodeling in breast cancer. Cancer Res.
79:1520–1534. 2019.PubMed/NCBI
|
|
16
|
Tang J, Li Z, Zhu Q, Wen W, Wang J, Xu J,
Wu W, Zhu Y, Xu H and Chen L: miR-204-5p regulates cell
proliferation, invasion, and apoptosis by targeting IL-11 in
esophageal squamous cell carcinoma. J Cell Physiol. 235:3043–3055.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu X, Gao X, Zhang W, Zhu T, Bi W and
Zhang Y: MicroRNA-204 deregulation in lung adenocarcinoma controls
the biological behaviors of endothelial cells potentially by
modulating Janus kinase 2-signal transducer and activator of
transcription 3 pathway. IUBMB Life. 70:81–91. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang X, Crowe PJ, Goldstein D and Yang JL:
STAT3 inhibition, a novel approach to enhancing targeted therapy in
human cancers (review). Int J Oncol. 41:1181–1191. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dong R, Liu GB, Liu BH, Chen G, Li K,
Zheng S and Dong KR: Targeting long non-coding RNA-TUG1 inhibits
tumor growth and angiogenesis in hepatoblastoma. Cell Death Dis.
7:e22782016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen X, Mangala LS, Mooberry L, Bayraktar
E, Dasari SK, Ma S, Ivan C, Court KA, Rodriguez-Aguayo C, Bayraktar
R, et al: Identifying and targeting angiogenesis-related microRNAs
in ovarian cancer. Oncogene. 38:6095–6108. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Thomson DW and Dinger ME: Endogenous
microRNA sponges: Evidence and controversy. Nat Rev Genet.
17:272–283. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ranganathan S, Lopez-Terrada D and Alaggio
R: Hepatoblastoma and pediatric hepatocellular carcinoma: An
update. Pediatr Dev Pathol. 23:79–95. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Angelico R, Grimaldi C, Gazia C, Saffioti
MC, Manzia TM, Castellano A and Spada M: How do synchronous lung
metastases influence the surgical management of children with
hepatoblastoma? An update and systematic review of the literature.
Cancers (Basel). 11:16932019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Meyers RL, Katzenstein HM, Krailo M,
McGahren ED III and Malogolowkin MH: Surgical resection of
pulmonary metastatic lesions in children with hepatoblastoma. J
Pediatr Surg. 42:2050–2056. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dong R, Jia D, Xue P, Cui X, Li K, Zheng
S, He X and Dong K: Genome-wide analysis of long noncoding RNA
(lncRNA) expression in hepatoblastoma tissues. PLoS One.
9:e855992014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kong YW, Ferland-McCollough D, Jackson TJ
and Bushell M: MicroRNAs in cancer management. Lancet Oncol.
13:e249–e258. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hua Z, Lv Q, Ye W, Wong CK, Cai G, Gu D,
Ji Y, Zhao C, Wang J, Yang BB and Zhang Y: miRNA-directed
regulation of VEGF and other angiogenic factors under hypoxia. PLoS
One. 1:e1162006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wu Q, Zhao Y and Wang P: miR-204 inhibits
angiogenesis and promotes sensitivity to cetuximab in head and neck
squamous cell carcinoma cells by blocking JAK2-STAT3 signaling.
Biomed Pharmacother. 99:278–285. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tan J, Qiu K, Li M and Liang Y:
Double-negative feedback loop between long non-coding RNA TUG1 and
miR-145 promotes epithelial to mesenchymal transition and
radioresistance in human bladder cancer cells. FEBS Lett.
589B:B3175–B3181. 2015. View Article : Google Scholar
|
|
31
|
Yoshikawa T, Miyamoto M, Aoyama T, Soyama
H, Goto T, Hirata J, Suzuki A, Nagaoka I, Tsuda H, Furuya K and
Takano M: JAK2/STAT3 pathway as a therapeutic target in ovarian
cancers. Oncol Lett. 15:5772–5780. 2018.PubMed/NCBI
|
|
32
|
Marotta LL, Almendro V, Marusyk A,
Shipitsin M, Schemme J, Walker SR, Bloushtain-Qimron N, Kim JJ,
Choudhury SA, Maruyama R, et al: The JAK2/STAT3 signaling pathway
is required for growth of CD44+CD24− stem
cell-like breast cancer cells in human tumors. J Clin Invest.
121:2723–2735. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xue C, Xie J, Zhao D, Lin S, Zhou T, Shi
S, Shao X, Lin Y, Zhu B and Cai X: The JAK/STAT3 signalling pathway
regulated angiogenesis in an endothelial cell/adipose-derived
stromal cell co-culture, 3D gel model. Cell Prolif. 50:e123072017.
View Article : Google Scholar : PubMed/NCBI
|