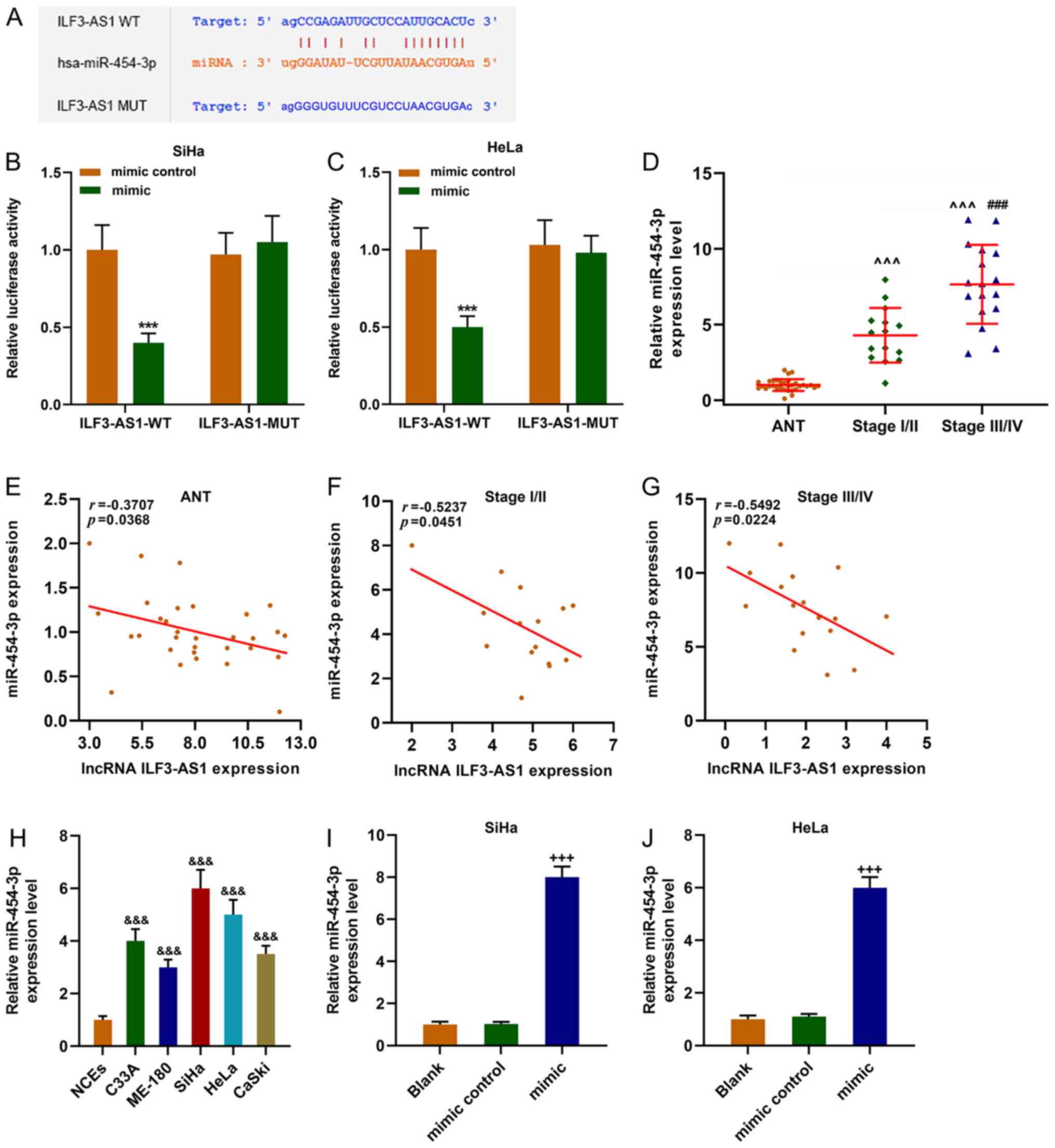
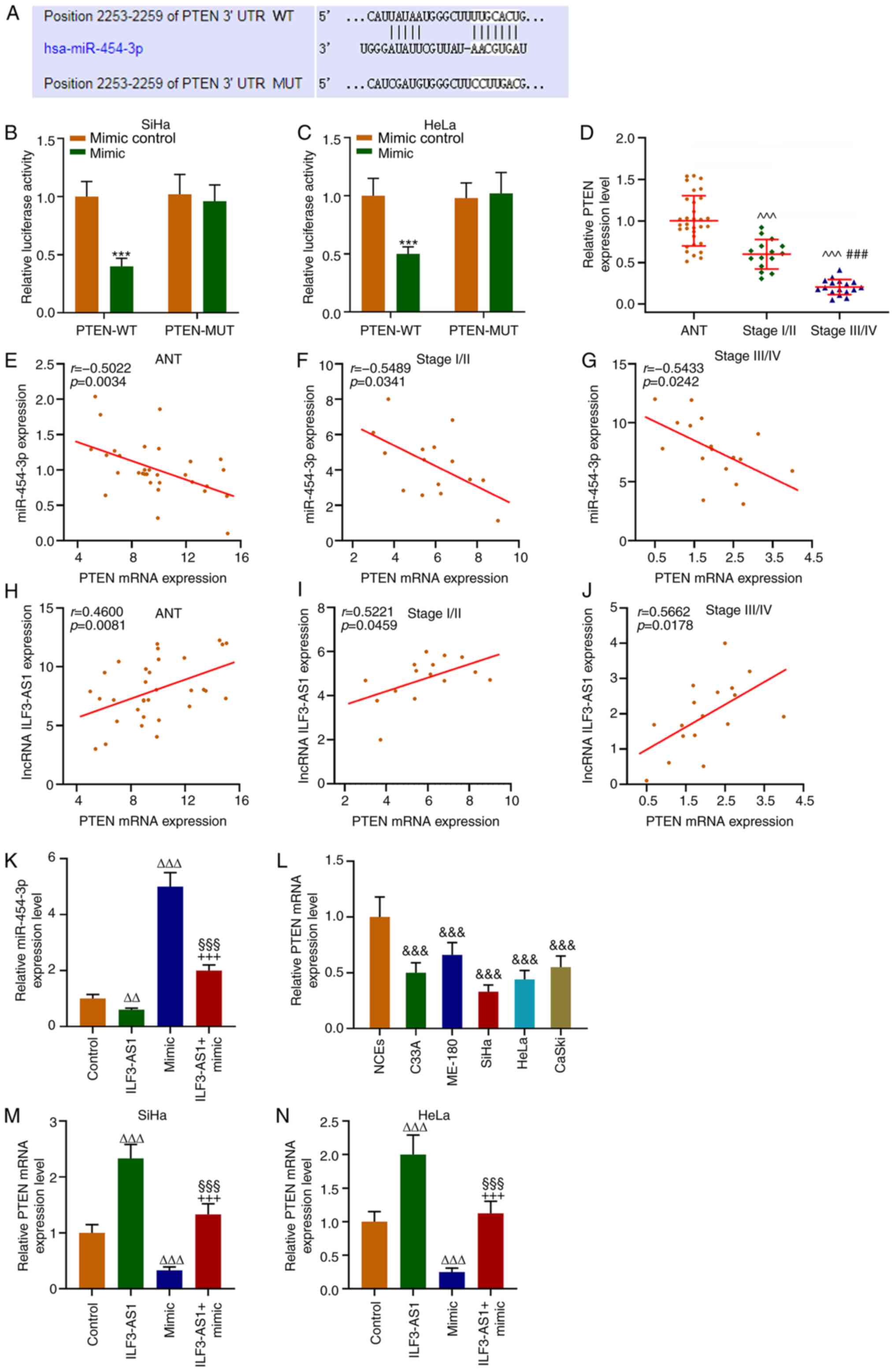
|
1
|
Burki TK: Novel mutations in cervical
cancer. Lancet Oncol. 18:e1372017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bychkovsky BL, Ferreyra ME,
Strasser-Weippl K, Herold CI, de Lima Lopes G Jr, Dizon DS,
Schmeler KM, Del Carmen M, Randall TC, Nogueira-Rodrigues A, et al:
Cervical cancer control in Latin America: A call to action. Cancer.
122:502–514. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Arbyn M, Weiderpass E, Bruni L, de Sanjosé
S, Saraiya M, Ferlay J and Bray F: Estimates of incidence and
mortality of cervical cancer in 2018: a worldwide analysis. Lancet
Glob Health. 8:e191–e203. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fang J, Zhang H and Jin S: Epigenetics and
cervical cancer: From pathogenesis to therapy. Tumour Biol.
35:5083–5093. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kanyina EW, Kamau L and Muturi M: Cervical
precancerous changes and selected cervical microbial infections,
Kiambu County, Kenya, 2014: A cross sectional study. BMC Infect
Dis. 17:6472017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tavakoli F, Khatami SS, Momeni F,
Azadbakht J and Ghasemi F: Cervical cancer diagnosis: Insights into
biochemical biomarkers and Imaging techniques. Comb Chem High
Throughput Screen. Aug 31–2020.(Epub ahead of print).
|
|
7
|
zur Hausen H: Papillomaviruses and cancer:
From basic studies to clinical application. Nat Rev Cancer.
2:342–350. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
zur Hausen H: Papillomaviruses in the
causation of human cancers-a brief historical account. Virology.
384:260–265. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gillison ML: Human papillomavirus-related
diseases: Oropharynx cancers and potential implications for
adolescent HPV vaccination. J Adolesc Health. 43 (Suppl 4):S52–S60.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mattick JS and Makunin IV: Non-coding RNA.
Hum Mol Genet. 15:R17–R29. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hombach S and Kretz M: Non-coding RNAs:
Classification, biology and functioning. Adv Exp Med Biol.
937:3–17. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cabianca DS, Casa V and Gabellini D: A
novel molecular mechanism in human genetic disease: A DNA
repeat-derived lncRNA. RNA Biol. 9:1211–1217. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jarroux J, Morillon A and Pinskaya M:
History, discovery, and classification of lncRNAs. Adv Exp Med
Biol. 1008:1–46. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim J, Piao HL, Kim BJ, Yao F, Han Z, Wang
Y, Xiao Z, Siverly AN, Lawhon SE, Ton BN, et al: Long noncoding RNA
MALAT1 suppresses breast cancer metastasis. Nat Genet.
50:1705–1715. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang Y, He L, Du Y, Zhu P, Huang G, Luo J,
Yan X, Ye B, Li C, Xia P, et al: The long noncoding RNA lncTCF7
promotes self-renewal of human liver cancer stem cells through
activation of Wnt signaling. Cell Stem Cell. 16:413–425. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hu X, Feng Y, Zhang D, Zhao SD, Hu Z,
Greshock J, Zhang Y, Yang L, Zhong X, Wang LP, et al: A functional
genomic approach identifies FAL1 as an oncogenic long noncoding RNA
that associates with BMI1 and represses p21 expression in cancer.
Cancer Cell. 26:344–357. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu WJ, Shen Y, Sui J, Li CY, Yang S, Xu
SY, Zhang M, Yin LH, Pu YP and Liang GY: Integrated analysis of
long non-coding RNA competing interactions revealed potential
biomarkers in cervical cancer: Based on a public database. Mol Med
Rep. 17:7845–7858. 2018.PubMed/NCBI
|
|
18
|
Luan X and Wang Y: LncRNA XLOC_006390
facilitates cervical cancer tumorigenesis and metastasis as a ceRNA
against miR-331-3p and miR-338-3p. J Gynecol Oncol. 29:e952018.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu Y, Yang Y, Li L, Liu Y, Geng P, Li G
and Song H: LncRNA SNHG1 enhances cell proliferation, migration,
and invasion in cervical cancer. Biochem Cell Biol. 96:38–43. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen X, Liu S, Zhao X, Ma X, Gao G, Yu L,
Yan D, Dong H and Sun W: Long noncoding RNA ILF3-AS1 promotes cell
proliferation, migration, and invasion via negatively regulating
miR-200b/a/429 in melanoma. Biosci Rep. 37:BSR201710312017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gao G, Li W, Liu S, Han D, Yao X, Jin J,
Han D, Sun W and Chen X: The positive feedback loop between ILF3
and lncRNA ILF3-AS1 promotes melanoma proliferation, migration, and
invasion. Cancer Manag Res. 10:6791–6802. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hu XH, Dai J, Shang HL, Zhao ZX and Hao
YD: SP1-mediated upregulation of lncRNA ILF3-AS1 functions a ceRNA
for miR-212 to contribute to osteosarcoma progression via
modulation of SOX5. Biochem Biophys Res Commun. 511:510–517. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhou M, Hu L, Zhang Z, Wu N, Sun J and Su
J: Recurrence-associated long non-coding RNA signature for
determining the risk of recurrence in patients with colon cancer.
Mol Ther Nucleic Acids. 12:518–529. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ye G, Guo L, Xing Y, Sun W and Yuan M:
Identification of prognostic biomarkers of prostate cancer with
long non-coding RNA-mediated competitive endogenous RNA network.
Exp Ther Med. 17:3035–3040. 2019.PubMed/NCBI
|
|
25
|
Wu W, Sui J, Liu T, Yang S, Xu S, Zhang M,
Huang S, Yin L, Pu Y and Liang G: Integrated analysis of two-lncRNA
signature as a potential prognostic biomarker in cervical cancer: A
study based on public database. PeerJ. 7:e67612019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mao X, Qin X, Li L, Zhou J, Zhou M, Li X,
Xu Y, Yuan L, Liu QN and Xing H: A 15-long non-coding RNA signature
to improve prognosis prediction of cervical squamous cell
carcinoma. Gynecol Oncol. 149:181–187. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tsikouras P, Zervoudis S, Manav B, Tomara
E, Iatrakis G, Romanidis C, Bothou A and Galazios G: Cervical
cancer: Screening, diagnosis and staging. J BUON. 21:320–325.
2016.PubMed/NCBI
|
|
28
|
Li JH, Liu S, Zhou H, Qu LH and Yang JH:
starBase v2.0: Decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA
interaction networks from large-scale CLIP-Seq data. Nucleic Acids
Res. 42((Database Issue)): D92–D97. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Agarwal V, Bell GW, Nam JW and Bartel DP:
Predicting effective microRNA target sites in mammalian mRNAs.
Elife. 4:e050052015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Qu BL, Yu W, Huang YR, Cai BN, Du LH and
Liu F: 6-OH-BDE-47 promotes human lung cancer cells epithelial
mesenchymal transition via the AKT/Snail signal pathway. Environ
Toxicol Pharmacol. 39:271–279. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wong SHM, Fang CM, Chuah LH, Leong CO and
Ngai SC: E-cadherin: Its dysregulation in carcinogenesis and
clinical implications. Crit Rev Oncol Hematol. 121:11–22. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
van Roy F: Beyond E-cadherin: Roles of
other cadherin superfamily members in cancer. Nat Rev Cancer.
14:121–134. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Angst BD, Marcozzi C and Magee AI: The
cadherin superfamily: Diversity in form and function. J Cell Sci.
114:629–641. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Osorio LA, Farfán NM, Castellón EA and
Contreras HR: SNAIL transcription factor increases the motility and
invasive capacity of prostate cancer cells. Mol Med Rep.
13:778–786. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lamouille S, Xu J and Derynck R: Molecular
mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell
Biol. 15:178–196. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Diepenbruck M and Christofori G:
Epithelial-mesenchymal transition (EMT) and metastasis: Yes, no,
maybe? Curr Opin Cell Biol. 43:7–13. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cheng N, Wu J, Yin M, Xu J, Wang Y, Chen
X, Nie Z and Yin J: LncRNA CASC11 promotes cancer cell
proliferation in hepatocellular carcinoma by inhibiting
miRNA-188-5p. Biosci Rep. 39:BSR201902512019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Slaby O, Laga R and Sedlacek O:
Therapeutic targeting of non-coding RNAs in cancer. Biochem J.
474:4219–4251. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ren L, Chen H, Song J, Chen X, Lin C,
Zhang X, Hou N, Pan J, Zhou Z, Wang L, et al: MiR-454-3p-mediated
Wnt/β-catenin signaling antagonists suppression promotes breast
cancer metastasis. Theranostics. 9:449–465. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Song Y, Guo Q, Gao S and Hua K: miR-454-3p
promotes proliferation and induces apoptosis in human cervical
cancer cells by targeting TRIM3. Biochem Biophys Res Commun.
516:872–879. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang S, Zhang G, Zheng W, Xue Q, Wei D,
Zheng Y and Yuan J: MiR-454-3p and miR-374b-5p suppress migration
and invasion of bladder cancer cells through targetting ZEB2.
Biosci Rep. 38:BSR201814362018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Shao N, Xue L, Wang R, Luo K, Zhi F and
Lan Q: miR-454-3p is an exosomal biomarker and functions as a tumor
suppressor in glioma. Mol Cancer Ther. 18:459–469. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Li W, Feng Y, Ma Z and Lu L: Expression of
miR-454-3p and its effect on proliferation, invasion and metastasis
of colon cancer. Nan Fang Yi Ke Da Xue Xue Bao. 38:1421–1426.
2018.(In Chinese). PubMed/NCBI
|
|
45
|
Malaney P, Uversky VN and Davé V: PTEN
proteoforms in biology and disease. Cell Mol Life Sci.
74:2783–2794. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chen CY, Chen J, He L and Stiles BL: PTEN:
Tumor suppressor and metabolic regulator. Front Endocrinol
(Lausanne). 9:3382018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ortega-Molina A and Serrano M: PTEN in
cancer, metabolism, and aging. Trends Endocrinol Metab. 24:184–189.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Nitulescu GM, Van De Venter M, Nitulescu
G, Ungurianu A, Juzenas P, Peng Q, Olaru OT, Grădinaru D, Tsatsakis
A, Tsoukalas D, et al: The Akt pathway in oncology therapy and
beyond (Review). Int J Oncol. 53:2319–2331. 2018.PubMed/NCBI
|
|
49
|
Milella M, Falcone I, Conciatori F, Cesta
Incani U, Del Curatolo A, Inzerilli N, Nuzzo CM, Vaccaro V, Vari S,
Cognetti F and Ciuffreda L: PTEN: Multiple functions in human
malignant tumors. Front Oncol. 5:242015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ye Z, Li Q, Guo Q, Xiong Y, Guo D, Yang H
and Shu Y: Ketamine induces hippocampal apoptosis through a
mechanism associated with the caspase-1 dependent pyroptosis.
Neuropharmacology. 128:63–75. 2018. View Article : Google Scholar : PubMed/NCBI
|