Introduction
Pregnancy produces important changes in the tissues
and homeostasis of women and the most important are those related
to the cardiovascular system, such as an increase in blood volume
and alterations in systemic vascular resistance (1–3).
Numerous studies have shown how the venous system is affected
during pregnancy, particularly with the development of chronic
venous disease (CVD), which is clinically diagnosed by the presence
of varicose veins (2–4). As CVD progresses, there is a
simultaneous decrease in blood flow velocity (5), an increase in leg vein diameter
(6) and valve closure time
(7). These hemodynamic alterations
during pregnancy can lead to venous hypertension and affect venous
return, with the appearance of varicose veins being the most
important clinical manifestation (8). Age, family history, occupation and
diet are other important risk factors, that have been associated
with the appearance of this condition (9).
Preeclampsia is one of the most important vascular
pathologies associated with pregnancy. It is a vascular alteration
characterized by systemic hypertension, that can seriously
compromise the health of the mother and the fetus, and in which
important changes in placental tissue also occurs (10). Gestational venous hypertension is a
lower-risk condition for both the mother and child, and our
previous studies revealed the association between CVD during
pregnancy, increased cell damage in the placental villi of those
women and increased cellular hypoxia (3,11). In
this regard, it is important to determine how the extracellular
matrix (ECM) behaves in these placental villi to reveal the
consequences of CVD during pregnancy. Proper functioning of the ECM
is key to the development of the placenta from the very earliest
stages (12). The process of
elastogenesis is fundamental for correct embryonic development
(13). The EGF-like
domain-containing protein 7 (EGFL7) plays a key role in the ECM
(14). Lelièvre et al
(15) demonstrated that EGFL7
regulated the catalytic activity of different components of elastic
fiber assembly, which affected ECM homeostasis. Alterations in the
composition of the ECM are present in a wide variety of pathologies
associated with pregnancy, such as preeclampsia and gestational
trophoblastic diseases (16).
Similarly, the placenta of patients with CVD during pregnancy was
found to have significant alterations in the composition of the
ECM, such as in the collagen fibers or in the calcifications of the
placental villi (17–19).
Therefore, the aim of the present study was to
analyze the gene and protein expression level of EGFL7 and the
components of the elastic fibers of the ECM [tropoelastin (TE),
fibulin 4 (FBLN-4), fibrillin 1 (FBN-1), lysyl oxidase (LOX) and
lysyl oxidase-like 1 (LOXL-1)] in the placental villi of women with
CVD during pregnancy.
Materials and methods
Study design
An observational, analytical and prospective study
was performed and included 114 women in the third trimester of
pregnancy (32 weeks). From these, 62 women were clinically
diagnosed with CVD according to CEAP classification (20), with a median age of 33 years
[interquartile range (IQR), 22–40 years] and a median gestational
age of 40.5 weeks (IQR, 39–41.5 weeks). Simultaneously, 52 controls
without a history of CVD [healthy controls (HC)] were also
included, with a median age of 34 years (IQR, 27–41 years) and a
median gestational age of 41 weeks (IQR, 39–42 weeks). The present
study was conducted according to the basic ethical principles of
autonomy, beneficence, non-maleficence and distributive justice.
The development of the research followed the regulations of Good
Clinical Practice, as well as the principles set forth in the last
Declaration of Helsinki (2013) and the Oviedo Convention (1997).
Patients were informed prior to enrolment, and each participant
provided their corresponding written consent. The current study was
approved by the Clinical Research Ethics Committee of the Central
University Hospital of Defence-University of Alcalá (37/17). During
the third trimester consultation, the clinical history was
recorded, a general physical examination was performed and lower
limb ultrasounds were conducted using an Eco-Doppler (Portable
M-Turbo Eco-Doppler; SonoSite, Inc.) at 7.5 MHz.
The inclusion criteria were defined as women over 18
years of age, with clinical evidence of lower limb venous
insufficiency (VI) in the third trimester, according to
Clinical-Etiology-Anatomy-Pathophysiology classification (≥1)
(20). The exclusion criteria
included women previously diagnosed with diabetes mellitus,
gestational diabetes mellitus or other endocrine diseases; high
blood pressure; autoimmune diseases; active infectious diseases;
venous malformations; heart, kidney and lung insufficiency;
preeclampsia and/or HELLP [an acronym for hemolysis (H), elevated
liver enzymes (EL) and a low platelet count (LP)] syndrome; known
causes of intrauterine growth restrictions; body mass index ≥25;
toxicological habits [tobacco (≥1 cigarette a day), alcohol (≥1
unit a day) or drugs (e.g., cannabis, heroin, cocaine,
amphetamines)]; existence of pathological injuries, such as
placental infarction, avascular villi, delayed villi maturation or
chronic villitis; as well as the appearance of any exclusion
criteria in the following months (until delivery); and previous
evidence of CVD. There were no significant differences between the
groups regarding the number of previous pregnancies: 33 (53.2%) for
women with CVD and 19 (36.5%) for women in the HC group (Table SI). There were also no significant
differences in the clinical characteristics between the CVD and HC
groups (gestational age, c-section delivery, previous pregnancies,
previous abortions, regular menstrual cycles and type of
profession-sedentary, Table
SI).
Placental samples
Placental biopsies were collected once they were
expelled after delivery. In all cases, 5 placental fragments were
obtained in all cases using a scalpel to ensure that the samples
included various cotyledons. These placental pieces were added to
two different sterile tubes: One containing Minimum Essential
Medium (MEM; Thermo Fisher Scientific, Inc.) with 1%
antibiotic/antimycotic (Streptomycin, Amphotericin B and
Penicillin) (Thermo Fisher Scientific, Inc.) and another with
RNAlater® (Ambion; Thermo Fisher Scientific, Inc.)
solution. The samples were processed in a class II laminar flow
hood (Telstar AV 30/70 Müller 220 V 50 MHz; Telstar; Azbil
Corporation) in a sterile environment. Preserved samples were
stored in 1 ml RNAlater® at −80°C until they were
processed for gene expression analysis. Conserved MEM placentas
were used for histological and immunodetection studies.
The samples stored in MEM were washed and rehydrated
five times in MEM without antibiotics to remove the blood cells,
then they were cut into fragments (2 cm) and fixed in F13 (60%
ethanol, 20% methanol, 7% polyethylene glycol and 13% distilled
water) following established protocols (20). The samples were then
paraffin-embedded in blocks using moulds. After the paraffin had
solidified, a HM 350 S rotation microtome (Thermo Fisher
Scientific, Inc.) was used to obtain 5-µm thick sections, which
were stretched in a hot water bath, then mounted on glass slides,
previously treated with 10% polylysine, allowing for improved
adhesion of the sections.
Gene expression studies using reverse
transcription-quantitative PCR (RT-qPCR)
RNA was extracted according to the guanidinium
thiocyanate-phenol-chloroform method (21,22)
and was used to analyze the mRNA expression levels of the genes of
interest.
RNA samples at a concentration of 50 ng/µl were used
to synthesize complementary DNA (cDNA) by reverse transcription; 4
µl of each sample is mixed with 4 µl of oligo-dT (15) 0.25 µg/µl solution (Thermo Fisher
Scientific, Inc.), and incubated at 65°C for 10 min in a dry bath
(AccuBlock, Labnet International Inc.), in order to denature the
RNA. After this, the samples were placed on ice and 10 µl per
sample of a reverse transcription mix containing the following
products was added for each sample: 2.8 µl First Strand Buffer 5X
(250 mM Tris-HCl and pH 8.3; 375 mM KCl; 15 mM MgCl2)
(Thermo Fisher Scientific, Inc.); 2 µl of 10 mM
deoxyribonucleotides triphosphate; 2 µl of 0.1 M dithiothreitol;
1.7 µl of DNase- and RNase-free water; 0.5 µl of RNase inhibitor
(RNase Out); 1 µl of reverse transcriptase enzyme (all from Thermo
Fisher Scientific, Inc.).
The RT process was carried out using a G-Storm GS1
thermal cycler (G-Storm Ltd.). The samples were incubated at 37°C
for 1 h and 15 min, to allow cDNA synthesis. The temperature was
then increased to 70°C and maintained for 15 min, thus causing the
denaturation of the reverse transcriptase enzyme, and the
temperature gradually decreased to 4°C.
To verify the absence of genomic DNA contamination
in the total RNA samples, a negative reverse transcription was
performed in parallel in which the M-MLV RT enzyme is replaced by
water free of DNases and RNases. The cDNA produced in RT was
diluted 1:20 using water free of DNases and RNases and stored at
−20°C until use.
Specific primers for all the genes studied (Table SII) were designed de novo
using the Primer-BLAST and AutoDimer online applications (23,24).
The constitutively expressed TATA-box binding protein (TBP) gene
was used to as a control to normalize the results (25). The gene expression units are
expressed as relative quantities of mRNA. RT-qPCR was performed on
a StepOnePlus™ System (Applied Biosystems; Thermo Fisher
Scientific, Inc.) and the relative standard curve method was used.
The total reaction volume was 20 µl and included: 5 µl sample
[mixed at 1:20 with 10 µl iQ™ SYBR® Green Supermix
(Bio-Rad Laboratories, Inc.)], 1 µl each forward and reverse
primers, and 3 µl DNase and RNase-free water, and added to a
MicroAmp® 96-well plate (Applied Biosystems; Thermo
Fisher Scientific, Inc.). The following thermocycling conditions
were used: Initial denaturation at 95°C for 10 min, denaturation at
95°C for 15 sec, annealing at variable temperatures depending on
the melting temperature of each primer pair for 30 sec, and
elongation at 72°C for 1 min, for 40–45 cycles. Followed by a
dissociation curve at 95°C for 15 sec, 60°C for 1 min, 95°C for 15
sec, and 60°C for 15 sec. Fluorescence detection was performed at
the end of each repeat cycle (amplification) and at each step of
the dissociation curve. The data obtained from each gene was added
in a standard curve made by serial dilutions of a mixture of the
samples, that were included in each plate according to the
constitutive expression of TBP (in accordance with the
manufacturer's protocols). All tests were performed in duplicate in
all samples of placenta tissue.
Immunohistochemistry studies for
protein expression analysis
Immunohistochemical studies were performed on
paraffin-embedded placental tissue samples. The antibody retrieval
step was described in the protocol specifications (Table SIII). The antigen/antibody
reactions were detected using the avidin-biotin complex method,
with avidin-peroxidase, as previously described (26). After incubation with the primary
antibody (1 h and 30 min; Table
SIII), the samples were incubated with 3% BSA Blocker (cat. no.
37525; Thermo Fisher Scientific, Inc.) and PBS overnight at 4°C.
Then, the cells were incubated with biotin-conjugated secondary
antibody, diluted in PBS, for 90 min at room temperature (RT;
Table SIII). The avidin-peroxidase
conjugate ExtrAvidin®-Peroxidase (Sigma-Aldrich; Merck
KGaA) was used for 60 min at RT (1:200 dilution with PBS), then the
protein expression level was determined using a chromogenic
diaminobenzidine (DAB) substrate kit (cat. no. SK-4100; Maravai
LifeSciences), which was prepared immediately before exposure (5 ml
distilled water, two drops buffer, four drops DAB and two drops
hydrogen peroxide). The signal was developed with the peroxidase
chromogenic substrate for 15 min at RT; this technique allows for
the detection of a brown stain. For the detection of each protein,
sections of the same tissue were assigned as negative controls,
substituting incubation with the primary antibody for a blocking
solution (PBS). In all the tissues, the contrast was performed with
Carazzi hematoxylin for 15 min at RT.
For each patient within the defined groups, 5
sections and 10 fields of view were randomly examined. The patients
were described as positive when the marked mean area in the
analyzed sample was ≥5% of the total, following the immunoreactive
score (IRS) from Remmele and Schicketanz (27) and Cristóbal et al (28). Immunostaining in the tissue was
assessed by two independent histologists, blinded to the outcome.
In each sample, immunohistochemical staining was scored using the
following scale: 0–1, minimum staining (≤25%); 2, moderate staining
(25–65%); and 3–4, strong staining (≥65-100%). Preparations were
viewed using a Zeiss Axiophot optical microscope (Zeiss GmbH).
Orcein stain
Once the sections were dried, they were
deparaffinized for 30 min in xylol at RT (PanReac AppliChem;
Illinois Tool Works, Inc.) and subsequently rehydrated using a
descending alcohol series until they were completely hydrated in
distilled water. After rehydration, the sections of the samples
were: i) Stained with alcoholic orcein for 30 min at RT, ii) washed
with distilled water for 30 min, iii) immersed in 96% alcohol for 5
min, iv) immersed in 100% alcohol for 15 min, v) discolored on the
bottom with acid alcohol for 2–10 min, vi) washed with water for 10
min, vii) stained with Carazzi hematoxylin for 20 min at RT, viii)
washed in running water for 10 min, viiii) dehydrated in 96%
alcohol for 5 min, x) dehydrated in 100% alcohol for 5 min, xi)
submerged in xylol for 10 min, and xii) mounted using Cytoseal™,
which allows for the visualization of the elastic fibers with a
brown color using an optical microscope (Zeiss GmbH).
Statistical analysis
For the statistical analysis, the GraphPad
Prism® v6.0 (GraphPad, Inc.) program was used. The
Mann-Whitney U test was used to compare the 2 groups, and the data
was expressed as the median and the IQR. For the categorical
variables, Pearson's χ2 or Fisher's exact test was used.
P<0.05 was used to indicate a statistically significant
difference.
Results
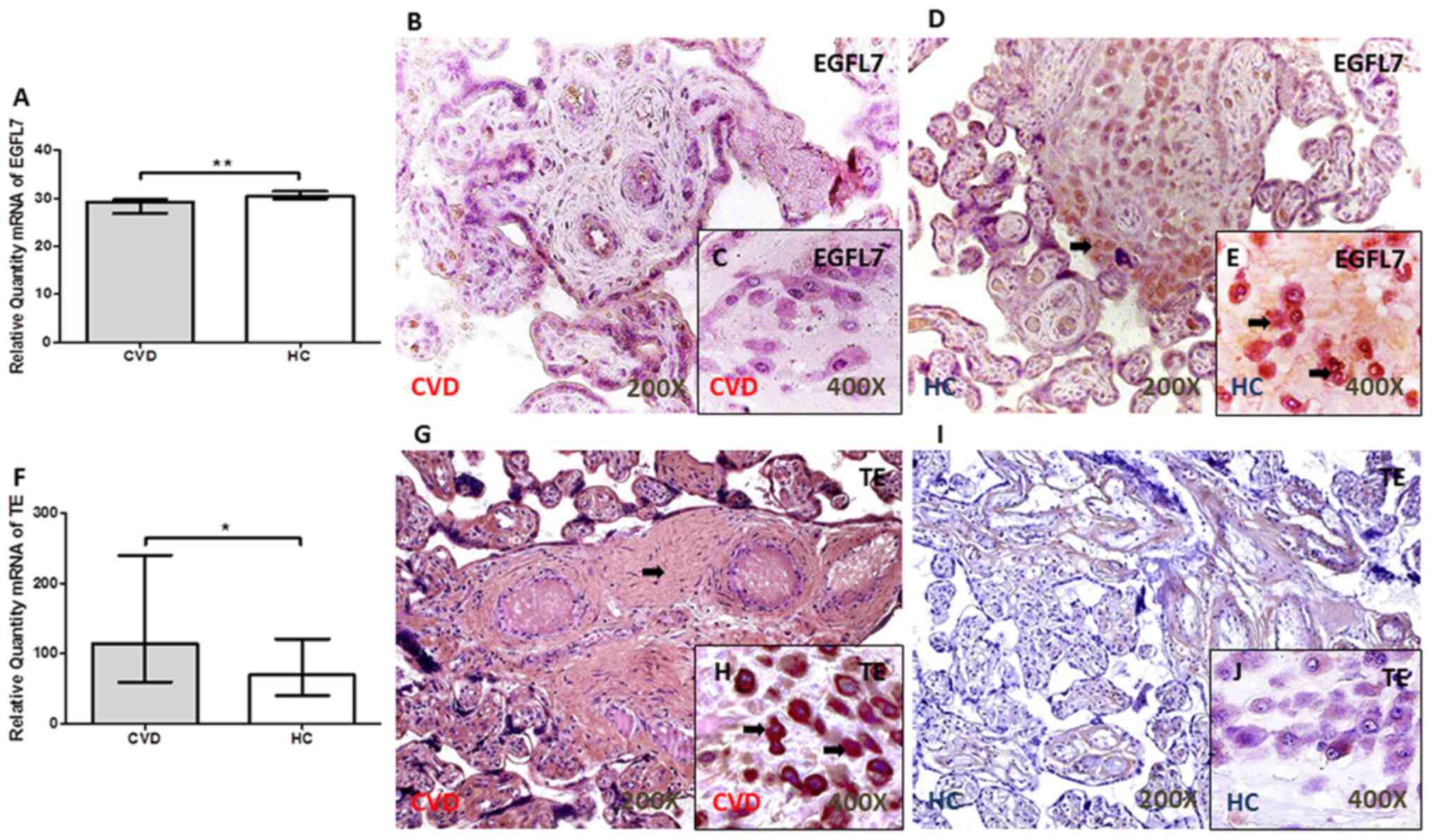
EGFL7 is expressed at low levels in
the placental villi of women with CVD during pregnancy
There was a significant decrease in the EGFL7 gene
expression level in the placental villi of women with CVD during
pregnancy (P=0.0012; Fig. 1A). The
results from the protein expression levels showed that the IRS
score was significantly lower in the placental villi from the CVD
group [CVD, 0.00 (IQR, 0.00–1.25); HC, 1.00 (IQR, 0.00–2.00)
P=0.0077; Fig. 1B and D]. Notably,
there was a decrease in the percentage of decidual cells with EGFL7
protein expression [CVD, 15.50% (IQR, 7.00–41.00%); HC, 31.00%
(IQR, 12.00–82.00%); P=0.0059; Fig. 1C
and E]. The arrows show the positive expression in the
tissue.
TE expression level is significantly
increased in the placenta of women with CVD during pregnancy
An increase in TE gene expression was observed in
the placental villi of women with CVD during pregnancy compared
with that in women from the HC group (P=0.0355; Fig. 1F). The analysis of TE protein
expression level using immunohistochemistry showed a significant
increase in the IRS in patients with CVD, with intense staining
throughout the ECM [CVD, 2.50 (IQR, 0.50–3.00); HC, 1.00 (IQR,
0.00–2.500); P=0.0003; Fig. 1G and
I]. The percentage of decidual cells with TE protein expression
level was significantly higher in the placenta of women with CVD
during pregnancy [CVD, 52.00 (IQR, 16.00–96.00%); HC, 22.00 (IQR,
10.00–54.00%); P=0.0005; Fig. 1H and
J]. The arrows show the positive expression in the tissue.
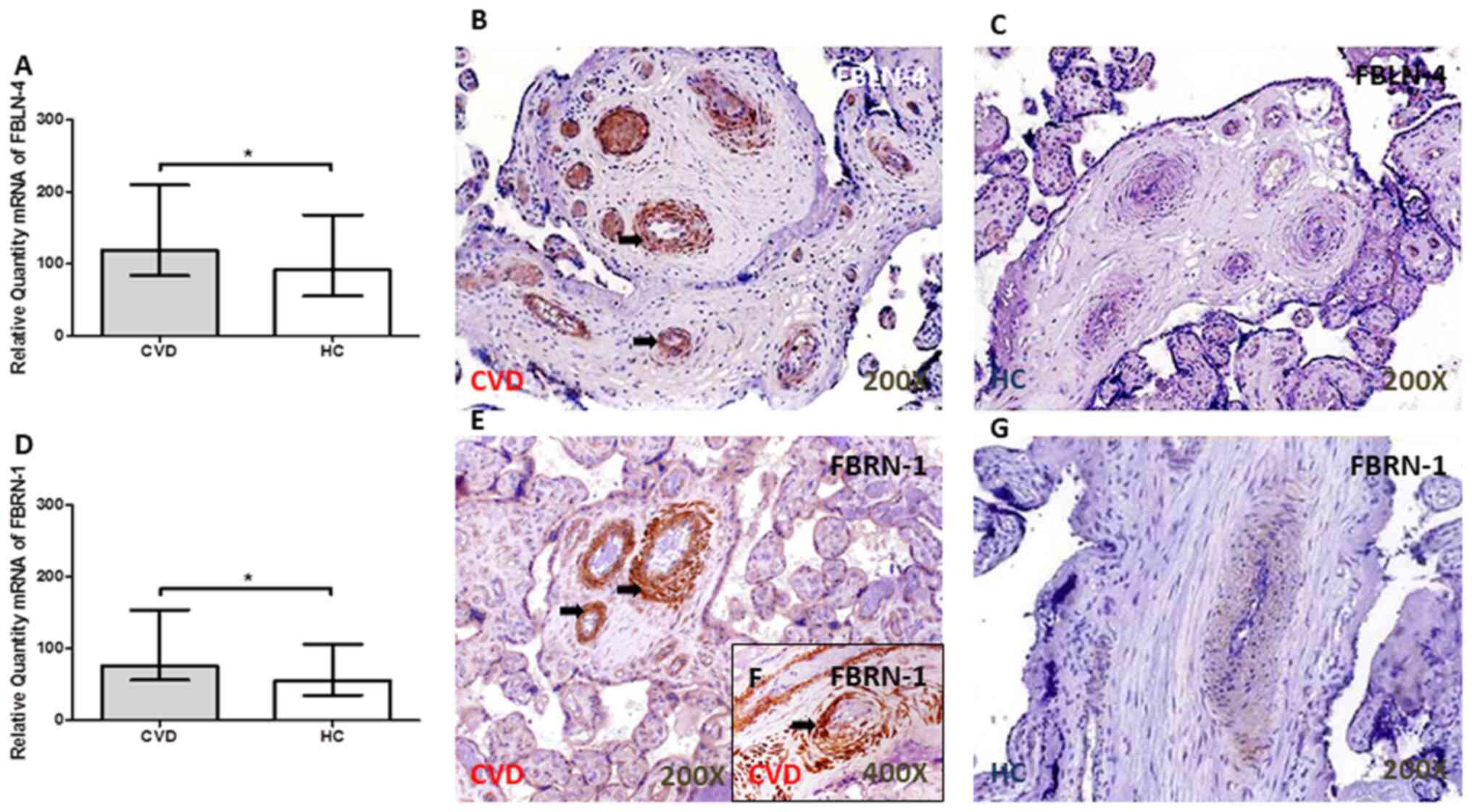
FBLN-4 and FBN-1 expression level is
increased in the placental villi of women with CVD during
pregnancy
The FBLN-4 gene was significantly increased in the
placental villi of women with CVD compared with that in women in
the HC group (P=0.0456; Fig. 2A).
An increase in FBN-1 gene expression was also observed in the CVD
group [CVD, 76.26 (IQR, 35.39–259.70); HC, 55.39 (IQR,
8.81–156.02); P=0.0185; Fig. 2D].
The arrows show the positive expression in the tissue.
The protein expression level of FBLN-4 did not
differ significantly in the placental villi between the 2 groups
[CVD, 0.87 (0.00–2.00); HC, 0.50 (IQR, 0.00–2.00); P=0.40], using
the IRS score; however, FBLN-4 protein expression was observed
around the large vessels in the placenta of women with CVD during
pregnancy (Fig. 2B and C). By
contrast, there was a significant increase in the IRS for FBN-1 in
the placental villi of women with CVD during pregnancy [CVD, 1.25
(IQR, 0.50–3.00); HC, 1.00 (IQR, 0.00–3.00); P=0.0188; Fig. 2E and G]. The protein expression
level of FBN-1 was particularly found around the large vessels in
the placenta of patients with CVD (indicated by the arrow; Fig. 2E and F). No protein expression of
FBLN-4 or FBN-1 was observed in decidual cells. The arrows show the
positive expression in the tissue.
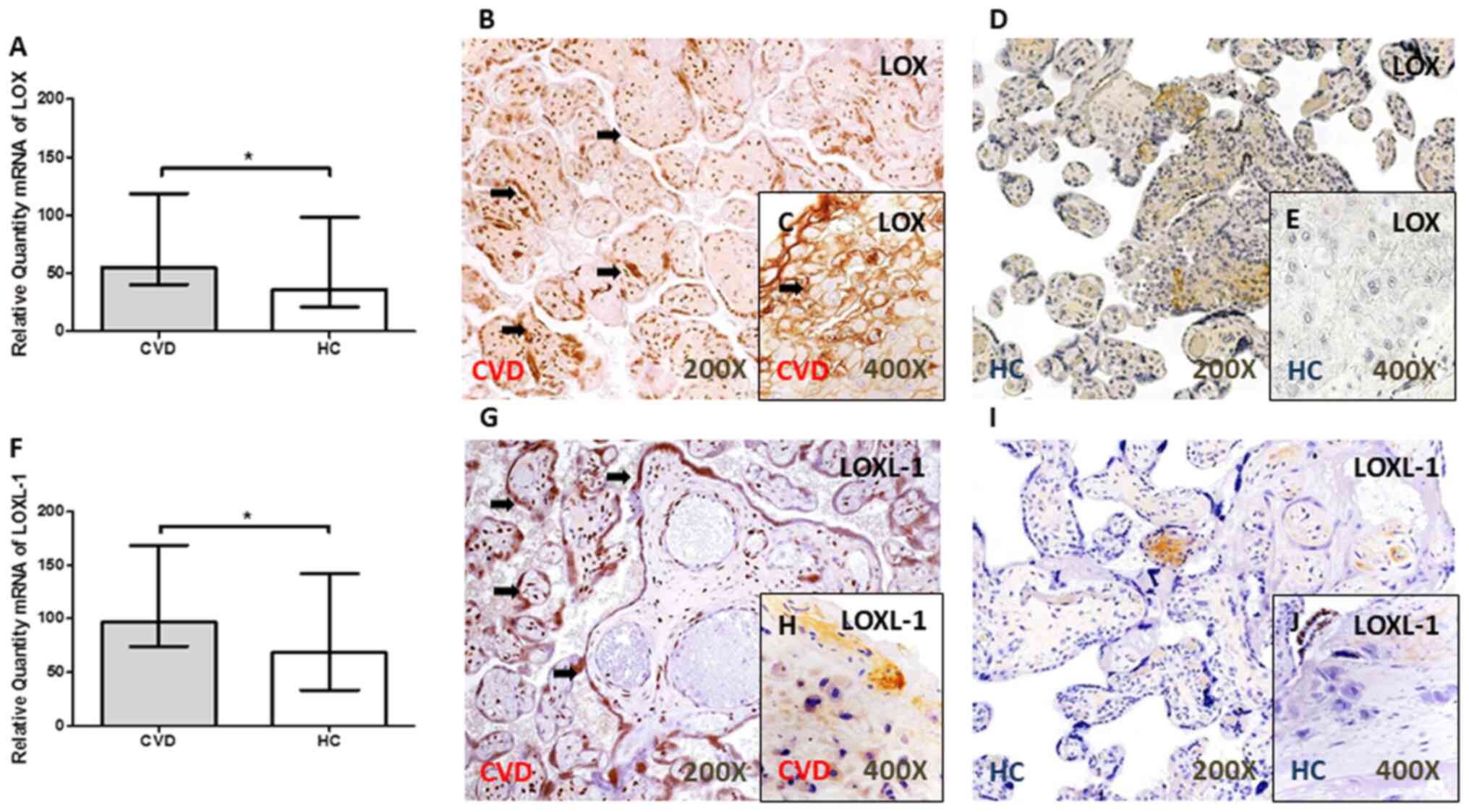
LOX and LOXL-1 expression level is
increased in the placental villi of women with CVD during
pregnancy
A significant increase in LOX gene expression level
was observed in the placental villi of women with CVD during
pregnancy [P=0.0344; Fig. 3A].
Similarly, the gene expression level of LOXL-1 was significantly
higher in the placental villi from women with CVD [CVD, 96.63 (IQR,
41.99–321.38); HC, 68.26 (IQR, 27.30–247.54); P=0.0390; Fig. 3F).
The results from protein expression showed an
increase in the IRS in the placental villi of women with CVD during
pregnancy for LOX [CVD, 2.00 (IQR, 0.50–3.00); HC, 1.00 (IQR,
0.00–2.50); P=0.0036] (Fig. 3B and
D) and LOXL-1 [CVD, 2.50 (IQR, 2.00–3.00); HC, 1.00 (IQR,
0.00–2.50); P<0.0001] (Fig. 3.G
and I). In addition, an increase in the protein expression level of
LOX [CVD, 50.50% (IQR, 21.00–85.00%); HC, 21.00 (IQR, 9.00–41.00%);
P<0.0001] (Fig. 3C and E) and
LOXL-1 [CVD, 18.00% (IQR, 7.00–45.00%); HC, 9.00% (6.00–21.00%),
P=0.0021] (Fig. 3H and J) was
observed in the placental decidual cells (Fig. 3.C and H) of women with CVD during
pregnancy. The arrows show the positive expression in the
tissue.
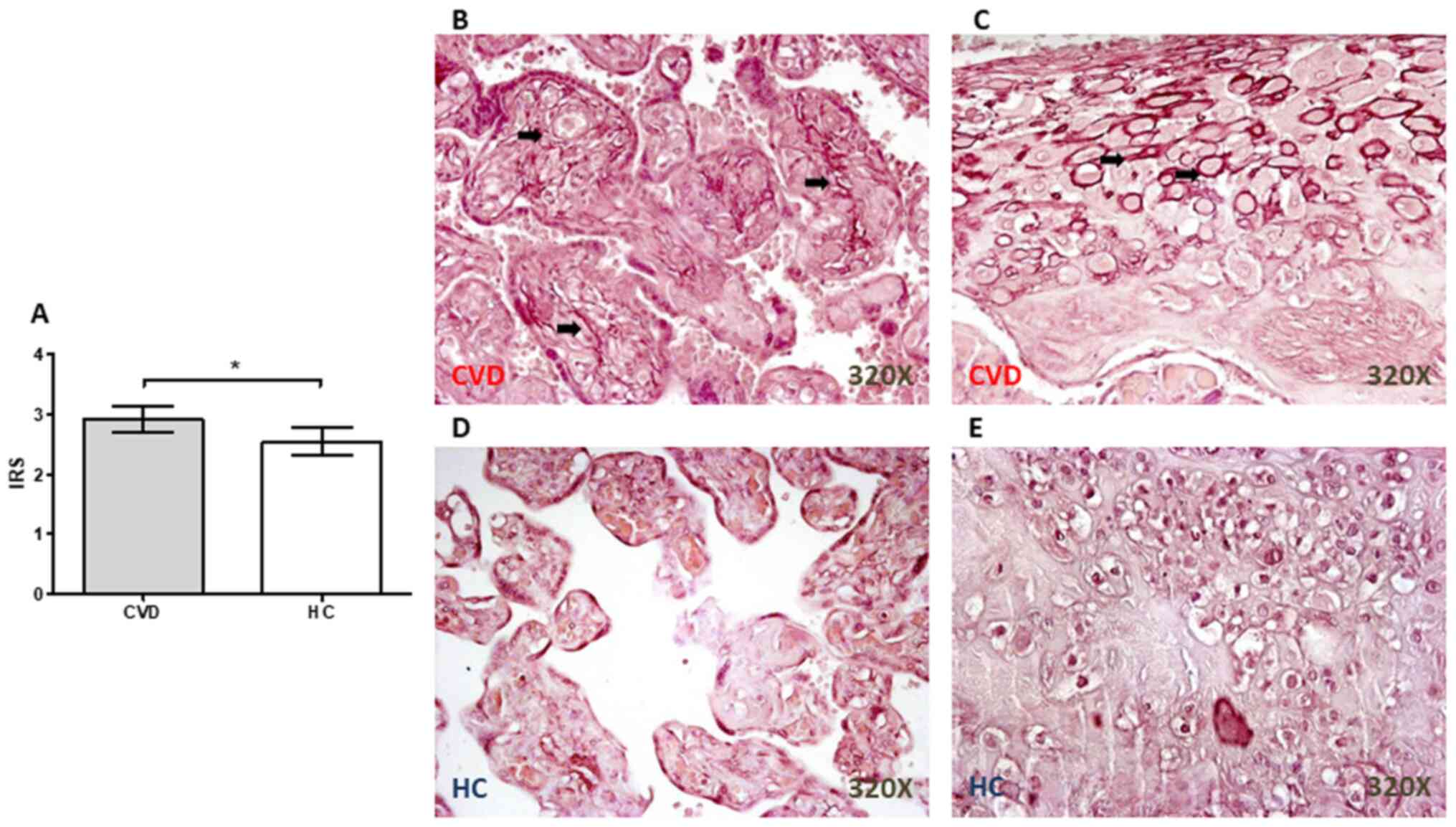
Significant increases in the number of
elastic fibers in the placental villi from women with CVD during
pregnancy
Orcein staining revealed the presence of a
significant increase in the elastic fibers in the placenta from
women with CVD during pregnancy (P=0.0110; Fig. 4A), particularly in the placental
villi (Fig. 4B) and around the
decidual cells (Fig. 4C). In the HC
group, the staining was not as intense in either the placental
villi (Fig. 4D) or in the decidual
cells (Fig. 4E).
Discussion
CVD is a frequent complication of pregnancy, that
can alter placental homeostasis and have important repercussions on
the health of the mother and the fetus (3,19). The
ECM is a dynamic network of macromolecules, that is constantly
being remodeled (29,30). The ECM plays a key role in the
correct development, implantation and separation of the placenta,
in addition to responding to hormonal changes, that occur during
pregnancy, particularly from progesterone (31,32).
In patients with CVD, the results from previous studies showed that
there was a redistribution of these hormonal receptors in the
venous tissue, affecting vascular homeostasis and the ECM (33,34).
The expression levels of the different ECM components of elastic
fibers were elevated in the placenta of women with CVD during
pregnancy, particularly the collagen fibers and metalloproteins in
the context of different hypertensive disorders, such as
preeclampsia (35).
The mechanical properties of tissues are fundamental
to their proper functioning, and the components of the ECM are
responsible for these mechanical properties (30). Notably, the rigidity/elasticity of
the cellular environment is key for the maintenance of tissue
homeostasis and changes in elasticity can affect the progression of
the disease in general (36).
Ortega et al (17)
demonstrated that there was a change in the mRNA and protein
expression profile of collagen fibers in the placental villi of
women with CVD during pregnancy, particularly with type III
collagen and in the collagen I/collagen III ratio, and there was a
significant increase in MMP-9 gene and protein expression levels in
the placental villi and decidual cells. However, the condition of
the elastic fibers in the placenta of women with CVD has not been
fully elucidated yet. These fibers are essential for providing
tissues with elastic properties, as well as for regulating the
bioavailability of components, such as TGF-β, and alterations in
this system arise in a wide variety of inherited or acquired
pathologies, such as Marfan or cancer (37,38).
It is important to investigate the elasticity of the placenta and
to develop techniques to evaluate its state under multiple
conditions (39). The results from
the present study showed that there was variation in the expression
level of TE, as well as in the different components of ECM, such as
FBLN-4, FBN-1 and the lysyl oxidase family (LOX and LOXL-1).
Lysyl oxidases are a set of fundamental enzymes
found in a wide variety of tissues, including the placenta, where
their importance in the regulation of the composition of the ECM
has been previously described (40). In prelabor rupture of fetal
membranes, increased levels of these enzymes have been associated
with changes in the cell cycle and in promoting oxidative stress in
some placental complications (41).
Recently, an association was found between CVD during pregnancy and
an increase in oxidative stress markers in these placentas
(11). The increased expression of
these enzymes in patients with CVD suggests that lysyl oxidases
could play an important role in CVD. These enzymes play roles in
the crosslinking of collagen fibers (36) and in the regulation and homeostasis
of elastic fibers (42). Higher
expression in the regulation of both components have been reported
in the vascular tissues of some placental complications, such as
fetal growth restriction (43). LOX
and LOXL-1 were found to interact directly with TE, promoting the
formation of mature elastic fibers (44). TE is a fundamental component of
elastic fibers and is a precursor to elastin, which participates in
cell attachment; any alteration in its expression could; therefore,
be associated with the requirement to meet the demands of a
hypertensive event (45,46). In this regard, one of the
limitations of the present study was that LOX activity was not
measured, as an alternative method of measuring the function of
LOX.
FBN-1 expression level was increased in the
placental villi of women with CVD during pregnancy, in the present
study. FBN-1 is a widely distributed protein in the stroma of
placental villi and is detected mainly as a thin layer, that
encapsulates decidual cells, and occurs in the form of fibrils,
that are in contact with these cells (47,48).
The expression level of FBN-1 has been associated with conferring a
certain stiffness to more elastic tissues (49). Costa et al (50) showed that this protein is part of an
important myofibroelastic system in the functionality of the
placental terminal villi and was upregulated in patients with
preeclampsia or systemic lupus erythematosus. Recently, Abbas et
al (51) demonstrated that this
molecule was also expressed in extravillous trophoblasts,
conferring greater tissue rigidity. The present study showed the
increase of FBN-1 expression in the placental tissue of women with
CVD during pregnancy. In addition, it has been shown that FBN-1
binds calcium molecules in the ECM of different tissues, such as
vascular structures (52). The
increase in mRNA expression level and IRS of FBN-1 was confirmed in
the present study. A previous study demonstrated an increase in the
calcifications of the placental villi in pregnant women with CVD
(18). Thus, the importance of
FBN-1 in responding to the changes in the placenta of women with
CVD during pregnancy should be considered.
FBLN-4 can significantly regulate the homeostasis of
elastic fibers (53). Notably,
FBLN-4 is fundamental to the process of sequential elastogenesis
(54). The results from the present
study showed that there was an increase in this component, showing
that it is involved in the elastogenesis process in the placentas
of women with CVD. The effect of deletions mutations in this
component has been described in some pathologies, such as aneurysms
(55); however, the effect of
overexpression in different pathologies is still under
investigation, such as placental complications. In addition, FBLN-4
has important functions beyond the regulation of elastic fibers;
therefore, an increase in its gene and protein expression could be
associated with different altered processes in placental tissue;
however, this hypothesis requires confirmation (53).
The results from the present study demonstrated an
association between CVD during pregnancy and the process of
elastogenesis in the placental villi. Proteins that may play a role
in elastogenesis were investigated, such as EGFL7, and the results
showed a decrease in the gene and protein expression level of EGFL7
in the placental villi of women with CVD. EGFL7 is an important
component in endothelial cells during their development (56). Lacko et al (57) showed that EGFL7 activity has
repercussions on the endothelial homeostasis of the maternal and
fetal vasculature, indicating the importance of this protein in
placental development and that its mRNA expression was reduced in
preeclampsia. Lelièvre et al (15) showed that EGFL7 interacted with the
catalytic subunit of lysyl oxidases in the vascular wall,
preventing the crosslinking of TE molecules and inhibiting the
formation of elastin polymers; thus, regulating the elastogenesis
process. The results from the present study indicated that it was
possible to observe a gene and protein expression between the
downregulation of EGFL7 by immunohistochemistry and RT-qPCR and a
change in the expression of elastic fiber promoters in the
placental tissue of women with CVD.
EGFL7 also plays a key role in trophoblasts,
particularly during the invasion and migration processes of these
cells (58). In an EGFL7 knockout
mice model, alterations in the morphogenesis of the chorionic villi
were observed, as were changes in vascular patterns, indicating the
possible involvement of this knockout in intrauterine growth
restriction (59). The results from
the present study further identified the changes that occur in
these processes in the placental villi. Shrestha et al
(60) showed that an increase in
body weight during pregnancy epigenetically regulated EGFL7
expression. A limitation to the present study was that it only
included in vitro results. Future studies should be aimed at
verifying the behavior of different cell types of placental villi
and confirming the viability of the ECM following EGFL7 inhibition,
as well as phenotyping decidual cells to understand their role in
vascular diseases.
Previous studies have observed how the placental
villi of pregnant women with CVD had a significant increase in
hypoxia-inducible factors, proteins related to apoptosis and
oxidative stress (3,11). In addition, a significant increase
in the number of placental villi was also observed in relation to
CVD (3,11,19).
Notably, it was found that there was a decrease in the fetal venous
pH at delivery (11), suggesting
that venous hypertension, as a result of CVD, triggers an adaptive
process, which enables the placental villi to combat the developing
hypoxia in the intervillous chamber. To the best of our knowledge,
the results from the present study showed an association between
the expression level of proteins related to elastogenesis and
gestational CVD for the first time.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The study (FIS-PI18/00912) was supported by the
Instituto de Salud Carlos III (grant no. Estatal de I + D+I
2013-2016) and co-financed by the European Development Regional
Fund ‘A way to achieve Europe’ and B2017/BMD-3804 MITIC-CM
(Comunidad de Madrid).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
NGH, JB and MAM contributed to the design of the
study, acquired funding and supervised the study. MAO, AA, SC and
FS was involved in the administration of the study. MAO, MAS, SC,
AA, OFM, JADLL, MAAM, CB and FS performed the experiments. NGH, JB,
MAM and MAO validated and curated the data in the study. SC and JB
confirm the authenticity of all the raw data All authors have read
and approved the final version of the manuscript.
Ethics approval and consent to
participate
This study was approved by the Clinical Research
Ethics Committee of the Central University Hospital of Defense-UAH
(37/17). The patients/participants provided their written informed
consent to participate in this study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ouzounian JG and Elkayam U: Physiologic
changes during normal pregnancy and delivery. Cardiol Clin.
30:317–329. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Troiano NH: Physiologic and hemodynamic
changes during pregnancy. AACN Adv Crit Care. 29:273–283. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
García-Honduvilla N, Ortega MA, Asúnsolo
Á, Álvarez-Rocha MJ, Romero B, De León-Luis J, Álvarez-Mon M and
Buján J: Placentas from women with pregnancy-associated venous
insufficiency show villi damage with evidence of hypoxic cellular
stress. Hum Pathol. 77:45–53. 2018. View Article : Google Scholar
|
|
4
|
Saliba Júnior OA, Rollo HA, Saliba O and
Sobreira ML: Graduated compression stockings effects on chronic
venous disease signs and symptoms during pregnancy. Phlebology.
35:46–55. 2020. View Article : Google Scholar
|
|
5
|
Ropacka-Lesiak M, Jarosław K and
Bręborowicz G: Pregnancy-dependent blood flow velocity changes in
lower extremities veins in venous insufficiency. Ginekol Pol.
86:659–665. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Iupatov EI, Ignat'ev IM and Fomina EE:
Ultrasonographic examination of major veins of lower limbs and
pelvic veins in pregnant women. Angiol Sosud Khir. 24:70–75.
2018.(In Russian). PubMed/NCBI
|
|
7
|
Asbeutah AM, Al-Azemi M, Al-Sarhan S,
Almajran A and Asfar SK: Changes in the diameter and valve closure
time of leg veins in primigravida women during pregnancy. J Vasc
Surg Venous Lymphat Disord. 3:147–153. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fanfera FJ and Palmer LH: Pregnancy and
varicose veins. Arch Surg. 96:33–35. 1968. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Davies AH: The seriousness of chronic
venous disease: A review of Real-World evidence. Adv Ther. 36
(Suppl 1):S5–S12. 2019. View Article : Google Scholar
|
|
10
|
El-Sayed AAF: Preeclampsia: A review of
the pathogenesis and possible management strategies based on its
pathophysiological derangements. Taiwan J Obstet Gynecol.
56:593–598. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ortega MA, Romero B, Asúnsolo Á,
Martínez-Vivero C, Sainz F, Bravo C, De León-Luis J, Álvarez-Mon M,
Buján J and García-Honduvilla N: Pregnancy-associated venous
insufficiency course with placental and systemic oxidative stress.
J Cell Mol Med. 24:4157–4170. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Smith SD, Choudhury RH, Matos P, Horn JA,
Lye SJ, Dunk CE, Aplin JD, Jones RL and Harris LK: Changes in
vascular extracellular matrix composition during decidual spiral
arteriole remodeling in early human pregnancy. Histol Histopathol.
31:557–571. 2016.PubMed/NCBI
|
|
13
|
Gauster M, Berghold VM, Moser G, Orendi K,
Siwetz M and Huppertz B: Fibulin-5 expression in the human
placenta. Histochem Cell Biol. 135:203–213. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schmidt M, De Mazière A, Smyczek T, Gray
A, Parker L, Filvaroff E, French D, van Dijk S, Klumperman J and Ye
W: The role of Egfl7 in vascular morphogenesis. Novartis Found
Symp. 283:18–28. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lelièvre E, Hinek A, Lupu F, Buquet C,
Soncin F and Mattot V: VE-statin/egfl7 regulates vascular
elastogenesis by interacting with lysyl oxidases. EMBO J.
27:1658–1670. 2008. View Article : Google Scholar
|
|
16
|
Rahat B, Sharma R, Bagga R, Hamid A and
Kaur J: Imbalance between matrix metalloproteinases and their
tissue inhibitors in preeclampsia and gestational trophoblastic
diseases. Reproduction. 152:11–22. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ortega MA, Asúnsolo Á, Álvarez-Rocha MJ,
Romero B, De León-Luis J, Álvarez-Mon M, Buján J and
García-Honduvilla N: Remodelling of collagen fibres in the
placentas of women with venous insufficiency during pregnancy.
Histol Histopathol. 33:567–576. 2018.PubMed/NCBI
|
|
18
|
Ortega MA, Saez MÁ, Asúnsolo Á, Romero B,
Bravo C, Coca S, Sainz F, Álvarez-Mon M, Buján J and
García-Honduvilla N: Upregulation of VEGF and PEDF in placentas of
women with lower extremity venous insufficiency during pregnancy
and its implication in villous calcification. Biomed Res Int.
2019:53209022019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ortega MA, Saez MA, Fraile-Martínez O,
Asúnsolo Á, Pekarek L, Bravo C, Coca S, Sainz F, Mon MÁ, Buján J
and García-Honduvilla N: Increased angiogenesis and
lymphangiogenesis in the placental villi of women with chronic
venous disease during pregnancy. Int J Mol Sci. 21:24872020.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lurie F, Passman M, Meisner M, Dalsing M,
Masuda E, Welch H, Bush RL, Blebea J, Carpentier PH, De Maeseneer
M, et al: The 2020 update of the CEAP classification system and
reporting standards. J Vasc Surg Venous Lymphat Disord. 8:342–352.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ortega MA, Asúnsolo Á, Leal J, Romero B,
Alvarez-Rocha MJ, Sainz F, Álvarez-Mon M, Buján J and
García-Honduvilla N: Implication of the PI3K/Akt/mTOR Pathway in
the process of incompetent valves in patients with chronic venous
insufficiency and the relationship with aging. Oxid Med Cell
Longev. 2018:14951702018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bustin SA, Benes V, Garson JA, Hellemans
J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL,
et al: The MIQE Guidelines: Minimum information for publication of
quantitative real-time PCR experiments. Clin Chem. 55:611–622.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ye J, Coulouris G, Zaretskaya I,
Cutcutache I, Rozen S and Madden TL: Primer-BLAST: A tool to design
target-specific primers for polymerase chain reaction. BMC
Bioinformatics. 13:1342012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vallone PM and Butler JM: AutoDimer: A
screening tool for primer-dimer and hairpin structures.
Biotechniques. 37:226–231. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ortega MA, Romero B, Asúnsolo Á, Sola M,
Álavrez-Rocha MJ, Sainz F, Álavrez-Mon M, Buján J and
García-Honduvilla N: Patients with incompetent valves in chronic
venous insufficiency show increased systematic lipid peroxidation
and cellular oxidative stress markers. Oxid Med Cell Longev.
2019:51645762019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Remmele W and Schicketanz KH:
Immunohistochemical determination of estrogen and progesterone
receptor content in human breast cancer: Computer-assisted image
analysis (QIC score) vs. subjective grading (IRS). Pathol Res
Pract. 189:862–866. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cristóbal L, Ortega MA, Asúnsolo Á, Romero
B, Álvarez-Mon M, Buján J, Maldonado AA and García-Honduvilla N:
Human skin model for mimic dermal studies in pathology with a
clinical implication in pressure ulcers. Histol Histopathol.
33:959–970. 2018.
|
|
29
|
Macura B and Śliwa L: Epigenetics in the
placenta, the current knowledge and future clinical perspectives.
Przegl Lek. 72:673–676. 2015.PubMed/NCBI
|
|
30
|
Chen Y, Peng W, Raffetto JD and Khalil RA:
Matrix metalloproteinases in remodeling of lower extremity veins
and chronic venous disease. Prog Mol Biol Transl Sci. 147:267–299.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chabbert-Buffet N, Meduri G, Bouchard P
and Spitz IM: Selective progesterone receptor modulators and
progesterone antagonists: Mechanisms of action and clinical
applications. Hum Reprod Update. 11:293–307. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Franczyk M, Lopucki M, Stachowicz N,
Morawska D and Kankofer M: Extracellular matrix proteins in healthy
and retained placentas, comparing hemochorial and
synepitheliochorial placentas. Placenta. 50:19–24. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
García-Honduvilla N, Asúnsolo Á, Ortega
MA, Sainz F, Leal J, Lopez-Hervas P, Pascual G and Buján J:
Increase and redistribution of sex hormone receptors in
premenopausal women are associated with varicose vein remodelling.
Oxid Med Cell Longev. 2018:39740262018. View Article : Google Scholar
|
|
34
|
Ortega MA, Asúnsolo Á, Romero B,
Álvarez-Rocha MJ, Sainz F, Leal J, Álvarez-Mon M, Buján J and
García-Honduvilla N: Unravelling the role of MAPKs (ERK1/2) in
venous reflux in patients with chronic venous disorder. Cells
Tissues Organs. 206:272–282. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ren Z, Cui N, Zhu M and Khalil RA:
Placental growth factor reverses decreased vascular and
uteroplacental MMP-2 and MMP-9 and increased MMP-1 and MMP-7 and
collagen types I and IV in hypertensive pregnancy. Am J Physiol
Heart Circ Physiol. 315:H33–H47. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Handorf AM, Zhou Y, Halanski MA and Li WJ:
Tissue stiffness dictates development, homeostasis, and disease
progression. Organogenesis. 11:1–15. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Baldwin AK, Simpson A, Steer R, Cain SA
and Kielty CM: Elastic fibres in health and disease. Expert Rev Mol
Med. 15:e82013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ortega MA, Romero B, Asúnsolo Á, Sainz F,
Martinez-Vivero C, Álvarez-Mon M, Buján J and García-Honduvilla N:
Behavior of smooth muscle cells under hypoxic conditions: Possible
implications on the varicose vein endothelium. Biomed Res Int.
2018:71561502018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Simon EG, Callé S, Perrotin F and
Remenieras JP: Measurement of shear wave speed dispersion in the
placenta by transient elastography: A preliminary ex vivo study.
PLoS One. 13:e01943092018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hein S, Yamamoto SY, Okazaki K,
Jourdan-LeSaux C, Csiszar K and Bryant-Greenwood GD: Lysyl
oxidases: Expression in the fetal membranes and placenta. Placenta.
22:49–57. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Polettini J, Silva MG, Kacerovsky M, Syed
TA, Saade GR and Menon R: Screening of lysyl oxidase (LOX) and
lysyl oxidase like (LOXL) enzyme expression and activity in preterm
prelabor rupture of fetal membranes. J Perinat Med. 44:99–109.
2016.PubMed/NCBI
|
|
42
|
Liu X, Zhao Y, Gao J, Pawlyk B, Starcher
B, Spencer JA, Yanagisawa H, Zuo J and Li T: Elastic fiber
homeostasis requires lysyl oxidase-like 1 protein. Nat Genet.
36:178–182. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
43
|
Saw SN, Tay JJH, Poh YW, Yang L, Tan WC,
Tan LK, Clark A, Biswas A, Mattar CNZ and Yap CH: Altered placental
chorionic arterial biomechanical properties during intrauterine
growth restriction. Sci Rep. 8:165262018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Thomassin L, Werneck CC, Broekelmann TJ,
Gleyzal C, Hornstra IK, Mecham RP and Sommer P: The Pro-regions of
lysyl oxidase and lysyl oxidase-like 1 are required for deposition
onto elastic fibers. J Biol Chem. 280:42848–42855. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hieber AD, Corcino D, Motosue J, Sandberg
LB, Roos PJ, Yu SY, Csiszar K, Kagan HM, Boyd CD and
Bryant-Greenwood GD: Detection of elastin in the human fetal
membranes: Proposed molecular basis for elasticity. Placenta.
18:301–312. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Baldock C, Oberhauser AF, Ma L, Lammie D,
Siegler V, Mithieux SM, Tu Y, Chow JY, Suleman F, Malfois M, et al:
Shape of tropoelastin, the highly extensible protein that controls
human tissue elasticity. Proc Natl Acad Sci USA. 108:4322–4327.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Jacobson SL, Kimberly D, Thornburg K and
Maslen C: Localization of fibrillin-1 in the human term placenta. J
Soc Gynecol Investig. 2:686–690. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Fleming S and Bell SC: Localization of
fibrillin-1 in human endometrium and decidua during the menstrual
cycle and pregnancy. Hum Reprod. 12:2051–2056. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Sherratt MJ, Baldock C, Louise Haston JL,
Holmes DF, Jones CJ, Shuttleworth CA, Wess TJ and Kielty CM:
Fibrillin microfibrils are stiff reinforcing fibres in compliant
tissues. J Mol Biol. 332:183–193. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Costa AM, Maximiano EB, Avvad-Portari E,
Jésus NR, Levy RA and Porto LC: Contractile cells and fibrillin-1
distribution is disturbed in terminal villi of placentae from
patients with preeclampsia and systemic lupus erythematosus.
Placenta. 27:234–243. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Abbas Y, Carnicer-Lombarte A, Gardner L,
Thomas J, Brosens JJ, Moffett A, Sharkey AM, Franze K, Burton GJ
and Oyen ML: Tissue stiffness at the human maternal-fetal
interface. Hum Reprod. 34:1999–2008. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Handford PA: Fibrillin-1, a calcium
binding protein of extracellular matrix. Biochim Biophys Acta.
1498:84–90. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Papke CL and Yanagisawa H: Fibulin-4 and
fibulin-5 in elastogenesis and beyond: Insights from mouse and
human studies. Matrix Biol. 37:142–149. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Kumra H, Nelea V, Hakami H, Pagliuzza A,
Djokic J, Xu J, Yanagisawa H and Reinhardt DP: Fibulin-4 exerts a
dual role in LTBP-4L-mediated matrix assembly and function. Proc
Natl Acad Sci USA. 116:20428–20437. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Burger J, van Vliet N, van Heijningen P,
Kumra H, Kremers GJ, Alves M, van Cappellen G, Yanagisawa H,
Reinhardt DP, Kanaar R, et al: Fibulin-4 deficiency differentially
affects cytoskeleton structure and dynamics as well as TGFβ
signaling. Cell Signal. 58:65–78. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
d'Audigier C, Susen S, Blandinieres A,
Mattot V, Saubamea B, Rossi E, Nevo N, Lecourt S, Guerin CL, Dizier
B, et al: Egfl7 represses the vasculogenic potential of human
endothelial progenitor cells. Stem Cell Rev Rep. 14:82–91. 2018.
View Article : Google Scholar
|
|
57
|
Lacko LA, Massimiani M, Sones JL, Hurtado
R, Salvi S, Ferrazzani S, Davisson RL, Campagnolo L and Stuhlmann
H: Novel expression of EGFL7 in placental trophoblast and
endothelial cells and its implication in preeclampsia. Mech Dev.
133:163–176. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Massimiani M, Vecchione L, Piccirilli D,
Spitalieri P, Amati F, Salvi S, Ferrazzani S, Stuhlmann H and
Campagnolo L: Epidermal growth factor-like domain 7 promotes
migration and invasion of human trophoblast cells through
activation of MAPK, PI3K and NOTCH signaling pathways. Mol Hum
Reprod. 21:435–451. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Lacko LA, Hurtado R, Hinds S, Poulos MG,
Butler JM and Stuhlmann H: Altered feto-placental vascularization,
feto-placental malperfusion and fetal growth restriction in mice
with Egfl7 loss of function. Development. 144:2469–2479.
2017.PubMed/NCBI
|
|
60
|
Shrestha D, Ouidir M, Workalemahu T, Zeng
X and Tekola-Ayele F: Placental DNA methylation changes associated
with maternal prepregnancy BMI and gestational weight gain. Int J
Obes (Lond). 44:1406–1416. 2020. View Article : Google Scholar : PubMed/NCBI
|