Introduction
Exercise intervention, as well as mechanical stress
stimulation, has become one of the most effective methods to
prevent and treat osteoporosis because exercise has no toxic side
effects and possesses osteogenic benefits (1,2).
Stress exists in all intracellular environments and can regulate a
number of cell and biological functions, such as proliferation and
differentiation (3). Mechanical
stress stimulation is one of the necessary conditions for
maintaining the stability of the skeletal system. A lack of
mechanical stimulation will cause bone metabolism disorders, bone
microstructure degradation, bone loss and, ultimately, osteoporosis
(4,5). Osteoporosis is a common age-related
disease, which seriously affects the health and quality of life of
the elderly (6). At present, it is
considered that possible mechanisms underlying the beneficial
effects exercise therapy in prevention and treatment of
osteoporosis mainly include the mechanical stimulation effects of
exercise, the changes in the levels of hormones and cytokines
induced by exercise and the regulation of the signal transduction
pathways of bone metabolism (5–7). The
exact mechanism of exercise prevention and treatment of
osteoporosis, however, has yet to be elucidated. Clarifying the
effect of exercise or mechanical stress on bone cells and its
mechanism may provide a theoretical basis for further research on
the prevention and treatment of osteoporosis by exercise.
MicroRNAs (miRNAs/miRs) are a type of non-coding RNA
with a length of ~22 nucleotides. Studies have confirmed that
miRNAs are widely involved in the regulation of various
physiological processes in bone metabolism (8,9).
miRNAs can target osteogenic factors, bone resorption factors or
other key molecules that regulate bone metabolism, and then
regulate the proliferation and differentiation of bone marrow
mesenchymal stem cells (BMSCs), osteoblasts and osteoclasts
(10–12). Previous studies have shown that
mechanical stress can also cause the differential expression of
miRNAs in BMSCs and osteoblasts, which indicates that miRNAs may be
one of the important transducers of exercise or mechanical stress
to promote bone formation (13–15).
Another type of non-coding RNA with >200 nucleotides, known as
long non-coding RNAs (lncRNAs), have also been reported to be able
to mediate the regulation of the differentiation of BMSCs (16).
The present study intended to simulate mechanical
stress stimulation through treadmill exercise experiments, thus
exploring the key miRNAs or lncRNAs mediating osteoblastic
differentiation in femur and tibia by using miRNA and lncRNA
sequencing and aimed to provide a basis for further research on the
molecular mechanisms underlying the regulation of osteoporosis by
exercise.
Materials and methods
Rat exercise model
A total of 12 male 8-week-old Sprague Dawley rats
(weight, 280–320 g) provided by Beijing Vital River Laboratory
Animal Technology Co., Ltd. were randomly divided into an exercise
group and a control group (n=6). The animals were fed ad
libitum with clean food and water under a 12-h light/dark
cycle. The constant temperature of the animal room was 20–25°C and
the relative humidity was 20–25%. The exercise group was treated
with 8 weeks of moderate treadmill exercise. In the first 3 days,
the initial speed, slope and time were set as 10 m/min at a 0°
slope for 20 min, which increased to 20 m/min at a 5° slope for 60
min after 2 days of rest. The exercise was carried out once a day,
5 days a week for 8 weeks. Apart from treadmill exercise, the rats
in the exercise group were free to roam their cages, whereas those
in the control group moved freely in their cages all day. In the
end of the experiments, rat were euthanized using 100%
CO2 anesthesia using an air displacement rate of 20% of
the chamber volume/min. It was determined that the rats had
succumbed when they had stopped breathing, the heart had stopped
completely and the pupils were dilated. All rats were successfully
sacrificed by CO2. All rat experiments (including
euthanasia) were performed between October 2019 and March 2020).
Finally, the femur and tibia were collected and isolated for
further investigation. All animal experiments were conducted
according to relevant national and international guidelines and
approved by the Animal Care and Use Committee of Nanfang Hospital,
Southern Medical University. The animal protocol no. for these
experiments was NFYY-2019-58.
Reverse transcription-quantitative
(RT-q) PCR
Total RNA was isolated from the femur and tibia
using TRIzol® (Thermo Fisher Scientific, Inc.),
according to the manufacturer's instructions. Total RNA (1 µg) was
reverse transcribed using a reverse transcription kit (cat. no.
DBI-2220; DBI; http://www.yisonbio.com/yisen001-Products-14675239/),
according to the manufacturer's protocol using the following
temperature conditions: 42°C for 30 min followed by 80°C for 5 min.
qPCR was performed using a PCR kit (cat. no. AOPR-1200;
GeneCopoeia, Inc.). The 2−∆∆Cq method was used for
quantification (17). The initial
denaturation was at 95°C for 10 sec, followed by 40 cycles of 55°C
for 20 sec and 72°C for 35 sec. β-actin and U6 were
used as internal reference for mRNAs, miRNAs and lncRNAs. The
primers (including reference gene) used in RT-qPCR are shown in
Tables I and II.
 | Table I.Primers (mRNAs) used in reverse
transcription-quantitative PCR. |
Table I.
Primers (mRNAs) used in reverse
transcription-quantitative PCR.
| Gene | Gene ID | Primer sequence
(5′-3′) | Length (bp) |
|---|
| Runx2 | NM_001145920.2 | Forward:
TGGCTTGGGTTTCAGGTTAG | 104 |
|
|
| Reverse:
GGTTTCTTAGGGTCTTGGAGTG |
|
| PPARγ | NM_001127330.2 | Forward:
GAACCTGCATCTCCACCTTATT | 125 |
|
|
| Reverse:
TGGAAGCCTGATGCTTTATCC |
|
| TGFβ1 | NM_011577.2 | Forward:
GGTGGTATACTGAGACACCTTG | 103 |
|
|
| Reverse:
CCCAAGGAAAGGTAGGTGATAG |
|
| TGFβR1 | NM_009370.3 | Forward:
CCTTGAGTCACTGGGTGTTATG | 117 |
|
|
| Reverse:
CCACTTAGCTGTCACCCTAATC |
|
| Smad2 | NM_001252481.1 | Forward:
GCTGAGTGCCTAAGTGATAGTG | 103 |
|
|
| Reverse:
TACAGCCTGGTGGGATCTTA |
|
| β-actin
(reference) | NM_007393.5 | Forward:
GAGGTATCCTGACCCTGAAGTA | 104 |
|
|
| Reverse:
CACACGCAGCTCATTGTAGA |
|
 | Table II.Primers (miRNAs and lncRNAs) used in
reverse transcription-quantitative PCR. |
Table II.
Primers (miRNAs and lncRNAs) used in
reverse transcription-quantitative PCR.
| Gene (miRNAs) | Primer sequence
(5′-3′) |
|---|
|
miR-9942-3p | Forward:
CGGGCGAGGGCCGGGC |
|
| Reverse:
CCTGTTGTCTCCAGCCACAAAAGAGCACAATATTTCAGGAGACAACAGGCCCGCCC |
|
miR-1260 | Forward:
CGCCGATCCCACCGCT |
|
| Reverse:
CCTGTTGTCTCCAGCCACAAAAGAGCACAATATTTCAGGAGACAACAGGTGGTGGC |
|
miR-30d-3p | Forward:
CGCCGCTTTCAGTCAGATGT |
|
| Reverse:
CCTGTTGTCTCCAGCCACAAAAGAGCACAATATTTCAGGAGACAACAGGGCAGCAA |
|
miR-7704 | Forward:
CGCCGCCGGGGTCGGCG |
|
| Reverse:
CCTGTTGTCTCCAGCCACAAAAGAGCACAATATTTCAGGAGACAACAGGACATCGC |
|
miR-5100-p5 | Forward:
CGCCGATCCCAGCGGT |
|
| Reverse:
CCTGTTGTCTCCAGCCACAAAAGAGCACAATATTTCAGGAGACAACAGGTGGAGGC |
| U6 | Forward:
CTCGCTTCGGCAGCACA |
|
| Reverse:
AACGCTTCACGAATTTGCGT |
|
| Gene
(lncRNAs) | Primer sequence
(5′-3′) |
|
|
MSTRG.7497 | Forward:
GTCGTTGGTGGCAGCAG |
|
| Reverse:
GGCCGAGCTTAGAACGC |
|
MSTRG.2625 | Forward:
GCCAGAGGTGACCTGTGAAG |
|
| Reverse:
TGAACACGAAGGTTTGAGCC |
|
MSTRG.1557 | Forward:
TTTCAGTTCCGCCAATCCAAC |
|
| Reverse:
TCTTTCCCATCAGGGTCAGCA |
|
MSTRG.691 | Forward:
CTGCTGCTCCTCTACTGTTCTG |
|
| Reverse:
ACCTTCGTTTGTCTGACTTGC |
|
MSTRG.13994 | Forward:
GATTCCCACTGTCCCTACCTA |
|
| Reverse:
CCTCCCACTTATTCTACACCTC |
| β-actin
(reference) | Forward:
GCAAGGATACTGAGAGCAAGAG |
|
| Reverse:
GGATGGAATTGTGAGGGAGATG |
Western blotting
The femur and tibia were lysed using ice-cold RIPA
lysis buffer (Fdbio Science) supplemented with protease inhibitor
(Fdbio Science) and total proteins were subsequently extracted and
quantified using a BCA Protein Assay kit (Fdbio Science). Equal
amounts of proteins (60 µg) were loaded in each lane, separated via
10% SDS-PAGE (Biosharp Life Sciences) and then transferred to a
PVDF membrane (EMD Millipore). After blocking with 5% FBS (Gibco;
Thermo Fisher Scientific, Inc.) for 2 h at 25°C, the membranes was
successively incubated with primary antibodies overnight at 4°C and
secondary antibodies for 2 h at room temperature. Each membrane was
visualized using enhance chemiluminescence reagent (cat. no.
11520709001; Roche Applied Science). Densitometric analysis was
performed using ImageJ (version 1.8.0; National Institutes of
Health). The antibodies used for western blotting are given in
Table III.
 | Table III.Antibodies used in western
blotting. |
Table III.
Antibodies used in western
blotting.
| Antibody | Supplier | Cat. no. | Isotype | Dilution |
|---|
| Anti-Runx2 | BIOSS | bs-1134R | Rabbit | 1:500 |
| Anti-PPARγ | BIOSS | bsm-33436M | Rabbit | 1:1,000 |
| Anti-Smad2 | BIOSS | bs-0718R | Mouse | 1:1,000 |
| Anti-TGFβ1 | BIOSS | bs-0103R | Rabbit | 1:500 |
| Anti-TGFβR1 | BIOSS | bs-0638R | Rabbit | 1:1,000 |
| HRP-Goat Anti-Mouse
IgG | Jackson
ImmunoResearch Laboratories, Inc. | 115-035-003 |
| 1:10,000 |
| HRP-Goat
Anti-Rabbit IgG | Jackson
ImmunoResearch Laboratories, Inc. | 111-035-003 |
| 1:10,000 |
Immunofluorescence
Sections of femur and tibia for were fixed for 20
min with 4% paraformaldehyde at 25°C and the sections permeabilized
for another 30 min at 37°C with 0.3% Triton X-100. After washing
with 0.1 M PBS, they were incubated with peroxisome
proliferator-activated receptor γ (PPARγ) primary antibody (1:400;
cat. no. MAB3872; Sigma-Aldrich; Merck KGaA) at 4°C overnight.
Next, Alexa Fluor 488-conjugated AffiniPure Goat Anti-Rabbit IgG
(1:500; cat. no. BF05002, Beijing Biodragon Immunotechnologies Co.,
Ltd.) was used at room temperature for 90 min as a secondary
antibody. The samples were incubated with DAPI at 25°C for 20 sec
to stain the nucleus. Fluorescent staining and images were captured
in five randomly selected fields using a fluorescence microscope
(magnification, ×200). The image were processed using NIS-Elements
software (V4.2.2; Nikon Corporation) and ImageJ 1.8.0 software
(National Institutes of Health; contrast enhancement).
miRNA sequencing
Total RNA was extracted from the femurs and tibias
using TRIzol reagent (Thermo Fisher Scientific, Inc.), according to
the manufacturer's instructions. The experimental procedure were
conducted according to the standard process provided by Illumina,
Inc., including the preparation of libraries and sequencing. Small
RNA sequencing libraries were prepared using truseq small RNA
sample prep kits (Illumina, Inc.). After the preparation of each
library, the libraries were sequenced with an Illumina hiseq
2000/2500 (Illumina, Inc.) and the read length was 1×50 bp.
Downstream bioinformatics analysis was performed by ACGT101-miR (LC
Sciences).
lncRNA sequencing
Total RNA was extracted from the femurs and tibias
using TRIzol reagent (Thermo Fisher Scientific, Inc.), according to
the manufacturer's instructions. rRNA deletion was used to
construct strand-specific libraries for lncRNA sequencing. An
Illumina Novaseq™ 6000 (Illumina, Inc.) was used after each library
passed quality inspection. The read length was 2×150 bp (PE150),
paired end read length in single channel. After reads were
assembled using the transcriptional assembly software stringtie
(18), known mRNAs and transcripts
<200 bp were removed and the remaining transcripts were further
subjected to lncRNA prediction. The prediction software used was
coding potential calculator (https://github.com/biocoder/cpc) and coding noncoding
index (https://github.com/www-bioinfo-org/CNCI#install-cnci).
If remaining transcripts had the potential to encode proteins, they
were classified as novel mRNAs and then filtered from the lncRNA
dataset. The expression levels of lncRNAs by were measured by
fragments per kilobase of exon model per million mapped reads
(FPKM) and the expression abundance of known genes in different
samples counted by FPKM values.
Analysis of data of miRNA and lncRNA
sequencing
Kyoto Encyclopedia of Genes and Genomes (KEGG) was
used to analysis the target genes of differentially expressed
miRNAs (DEMs), and Gene Ontology (GO) enrichment analysis was
carried out for these. The edgerR (Bioconductor software package,
https://bioconductor.org/packages/release/bioc/html/edgeR.html;
http://www.R-project.org/) was used for
statistical analysis of miRNA sequencing and lncRNA sequencing
data: i) Genes with biological duplication and |log2fold
change|≥1 and P-value ≤0.05 were accepted and then counted; and ii)
genes without biological duplication and |log2fold
change|≥1 and P-value <1 were accepted and then counted.
Statistical analyses
RT-qPCR and western blotting experiments were
performed in triplicate and the data were shown as mean ± SD.
Unpaired Student's t-test was used for statistical analysis.
Statistical significance (P-value) was calculated by SPSS 17.0
(SPSS, Inc.). P<0.05 was considered to indicate a statistically
significant difference.
Results
Effects of exercise on biomarkers of
osteogenesis or adipogenesis and the TGFβ1/Smad2 pathway in femurs
and tibias
An exercise rat model was established through
treadmill exercise based on previous research (19,20),
and the optimization of pre-experiments and corresponding
biological indices were measured. The rats were randomly divided
into an exercise group and a control group. Rats in the exercise
group were treated with 8 weeks of moderate treadmill exercise.
Subsequently, the expression levels of runt-related transcription
factor 2 (Runx2), a biomarker of osteogenesis (21); PPARγ, a biomarker of
adipogenesis (22); and TGFβ1,
TGFβR1 and Smad2 were detected by qPCR (Fig. 1A-E) and western blotting (Fig. 1F). The results demonstrated that
compared with the control group, the femurs and tibias collected
from the exercise group demonstrated significantly higher
osteogenic activity, represented by an upregulation of Runx2
and lower adipogenic activity, represented by the downregulation of
PPARγ. In addition, the levels of TGFβ1, TGFβR1 and
Smad2 were all upregulated in the exercise group, which was
consistent with our previous study regarding MSCs loading stress
in vitro (23). These
results also demonstrated that exercise could promote the
osteogenic differentiation while inhibiting their adipogenic
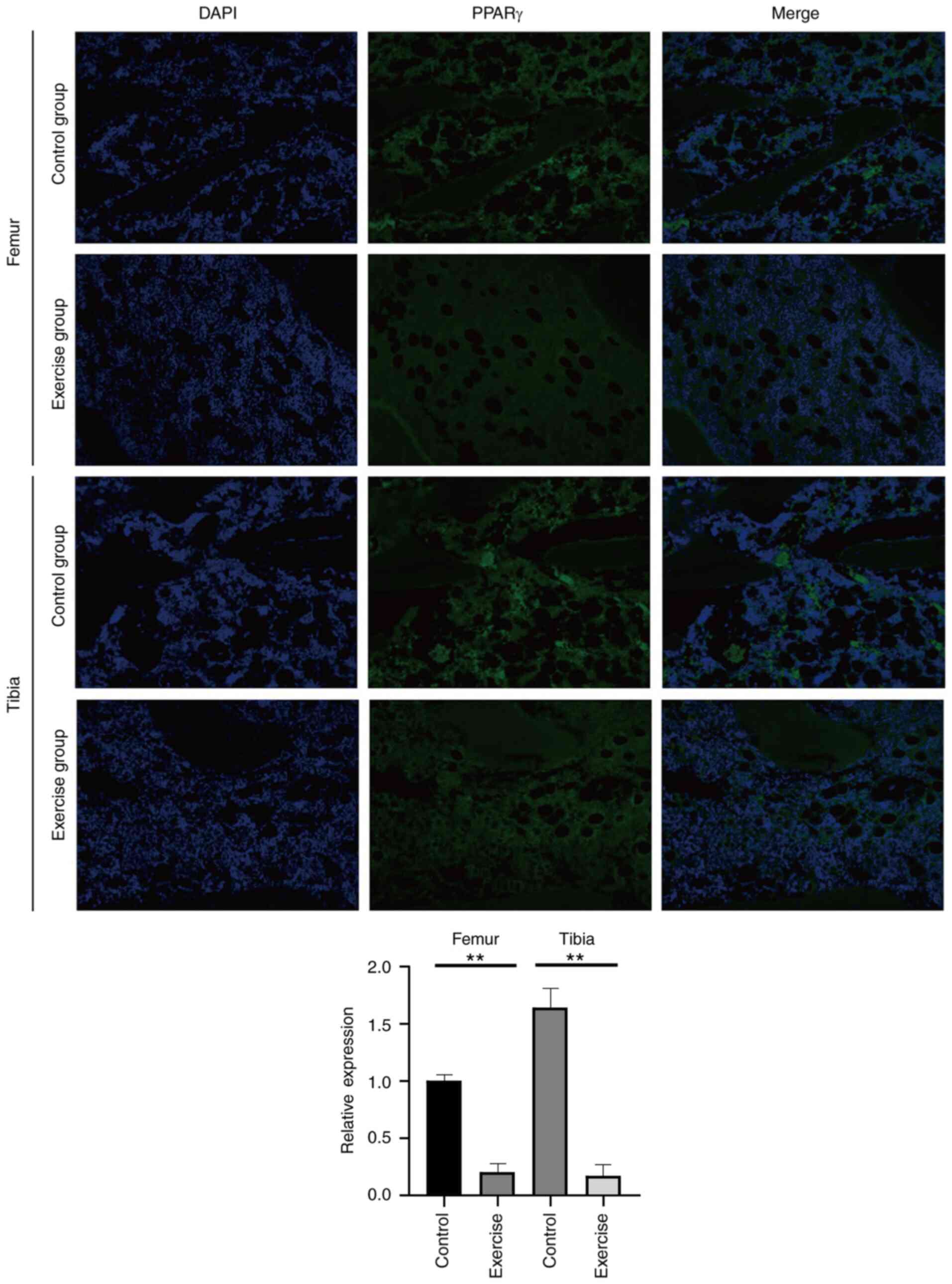
differentiation in femur and tibia. In addition, immunofluorescence
detection of PPARγ revealed its downregulated expression (Fig. 2). Finally, miRNA and lncRNA
sequencing were performed from femurs and tibias.
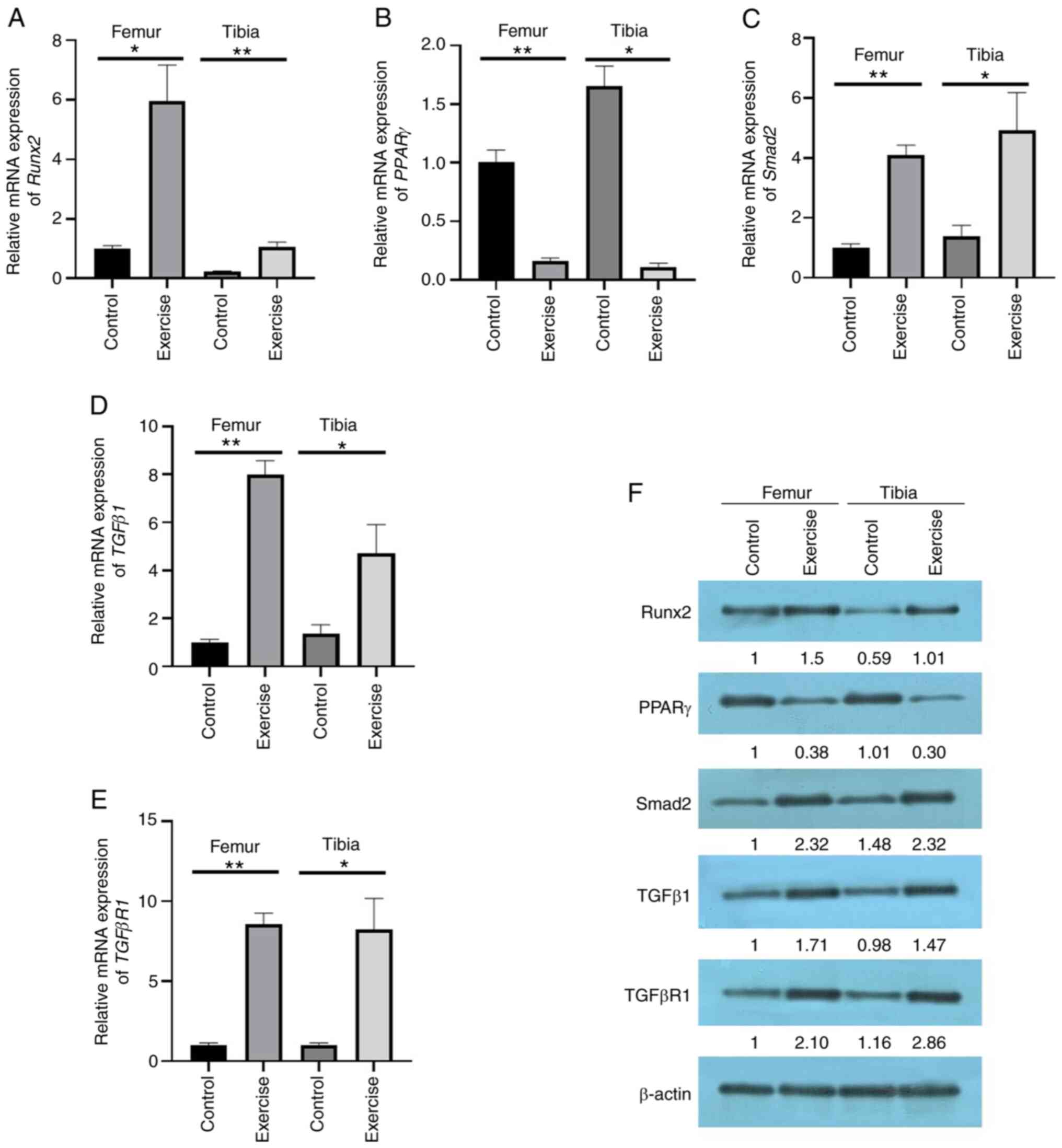 | Figure 1.Measurement of biological indices in
a rat exercise model. The mRNA expression of (A) Runx2, (B)
PPARγ, (C) Smad2, (D) TGFβ1 and (E)
TGFβR1 in the femur and tibia of rats in both
exercise and control groups was detected by reverse
transcription-quantitative PCR. (F) The protein expression of
Runx2, PPARγ, Smad2, TGFβ1 and TGFβR1 in the femur and tibia of
rats in both an exercise and control group was detected by western
blotting. Data are presented as the mean ± SD (n≥3), *P<0.05,
**P<0.01 (t-test). Runx2, runt-related transcription
factor 2; PPARγ, peroxisome proliferator-activated receptor
γ; TGFβR1, TGFβ receptor 1. |
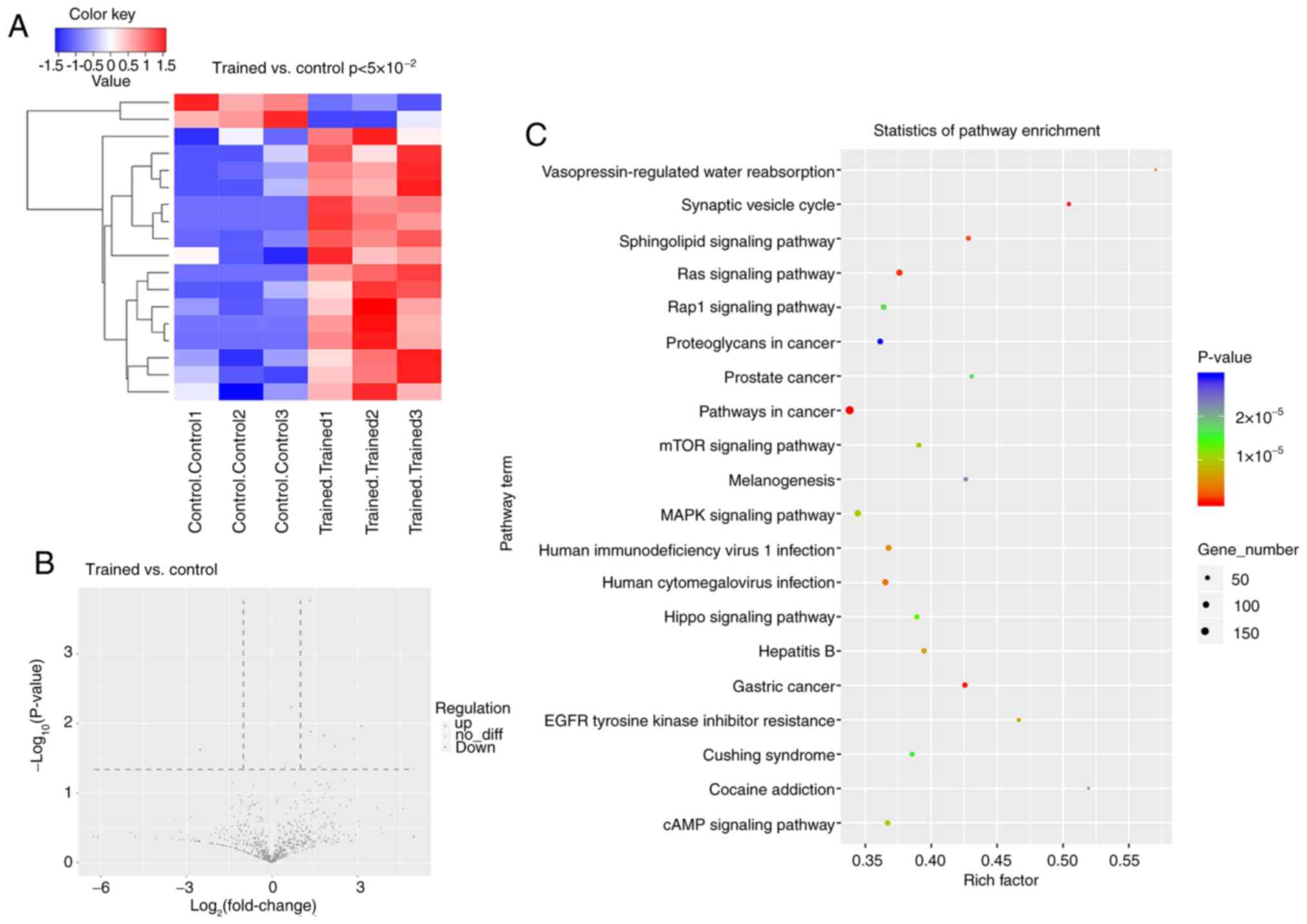
Exploration of key miRNAs in femur and
tibia with mechanical stress by miRNA sequencing
As shown in Fig. 3A,
based on the screening criteria of having a log2fold
change >1 and P<0.05, 16 miRNAs were upregulated and two
miRNAs were downregulated in the exercise group. There were five
upregulated miRNAs and one downregulated miRNA identified when
P<0.01 (Fig. 3B). Enrichment
analysis was performed based on the target genes of the identified
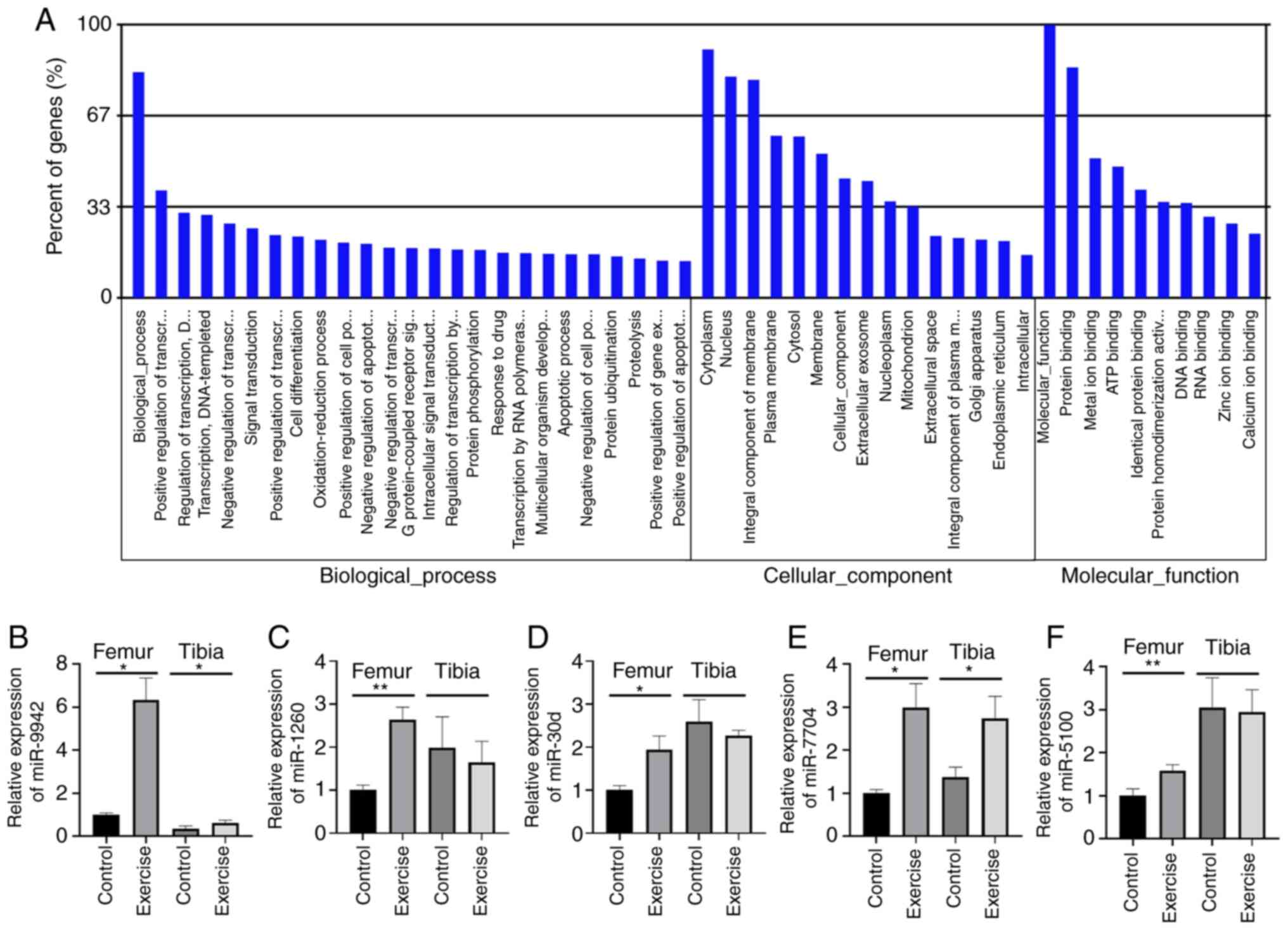
DEMs. As shown in Fig. 3C, KEGG
analysis demonstrated that target genes of DEMs were most
significantly enriched in ‘pathways in cancer’. GO enrichment
analysis, by contrast, demonstrated that the target genes of DEMs
were most enriched in the ‘positive regulation of transfer’ in
biological processes, in ‘nucleus’ and ‘integral component of
membrane’ in the cellular component and in ‘protein binding’ in
terms of molecular functions (Fig.
4A). Finally, five upregulated miRNAs were selected, including
miR-9942, miR-30d, miR-7704, miR-5100 and miR-1260, all with
P<0.01, for further verification in the femur and tibia by qPCR.
The results indicated an increased expression of miR-9942 and
miR-7704 in both the femur and tibia, as well as the upregulation
of the other three specifically in the femur (Fig. 4B-F).
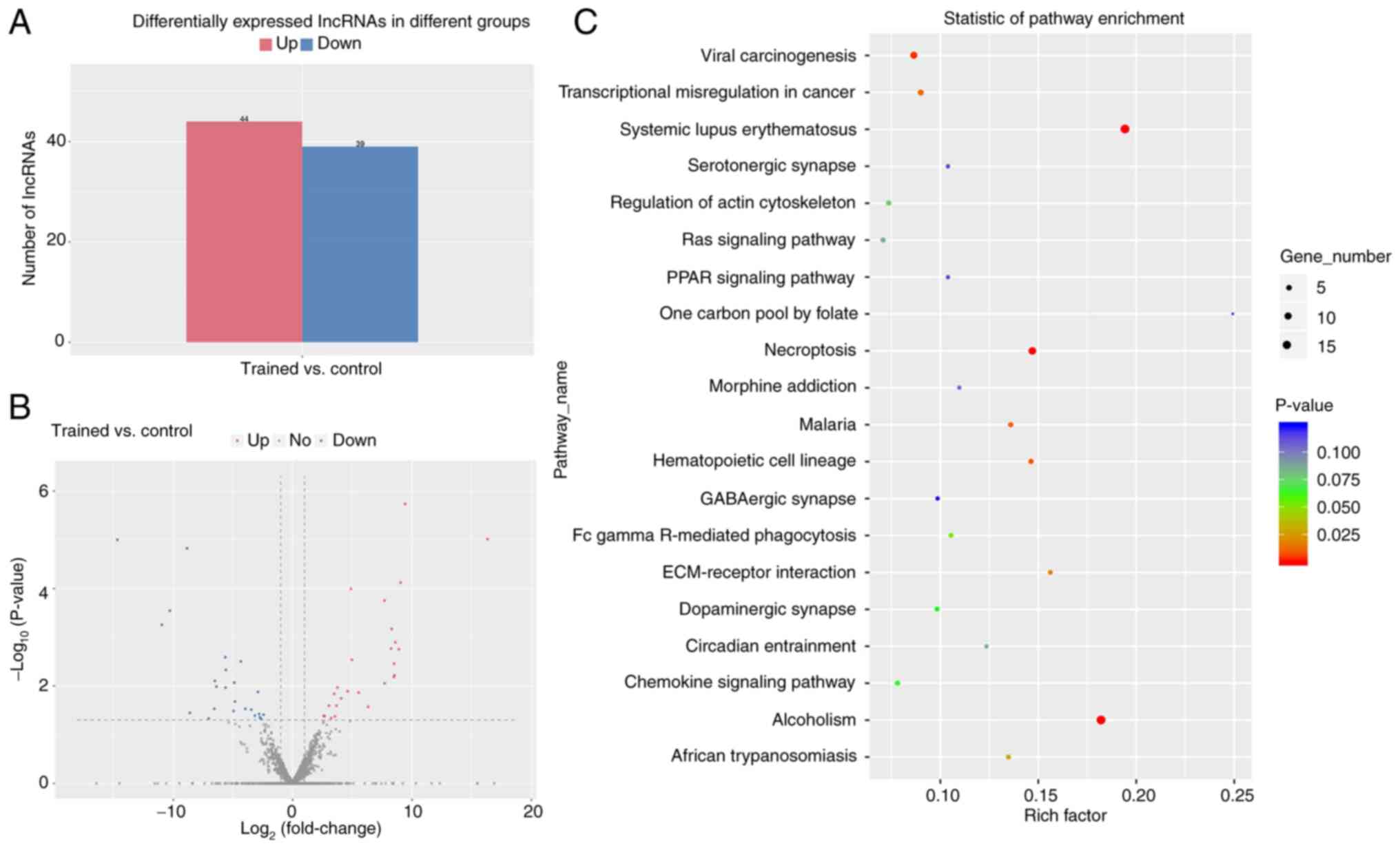
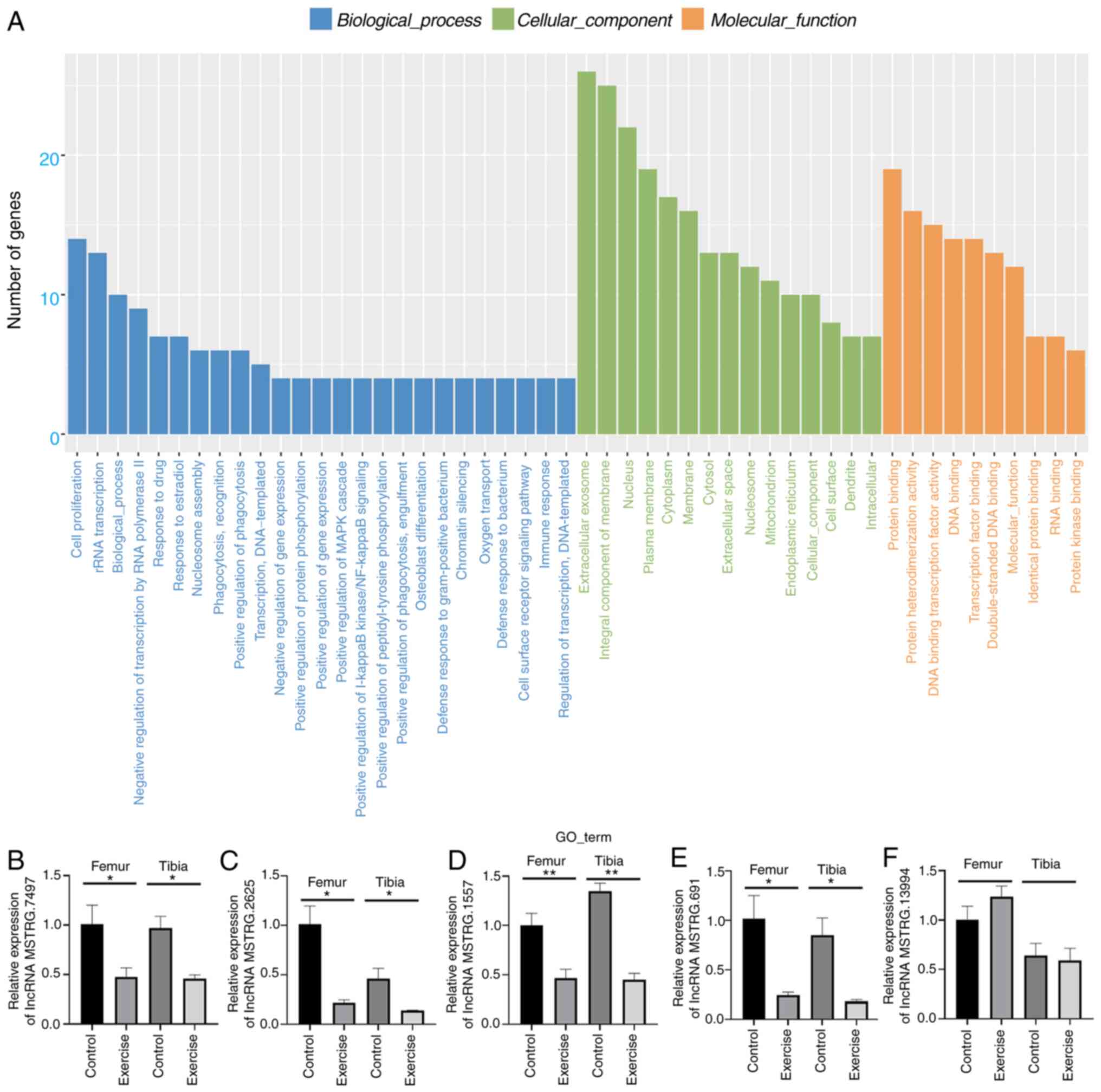
Exploration of key lncRNAs in femur
and tibia with mechanical stress by lncRNA sequencing
As shown in Fig. 5A and
B, based on the screening criteria of having a log2
fold change >1 and P<0.05, 44 lncRNAs were found to be
upregulated and 39 lncRNAs to be downregulated in the exercise
group. In addition, enrichment analysis was performed based on the
identified differentially expressed lncRNAs (DELs). As is shown in
Fig. 5C, the most significantly
enriched signaling pathways obtained from KEGG analysis was
‘systemic lupus erythematosus’. By contrast, the GO enrichment
analysis demonstrated that the target genes of DELs were most
enriched in ‘cell proliferation’ in terms of biological processes,
in ‘extracellular exosome’ in terms of cellular component and in
‘protein binding’ in terms of molecular functions (Fig. 6A). Finally, the five most
downregulated lncRNAs, including lncRNA MSTRG.2625, lncRNA
MSTRG.1557, lncRNA MSTRG.691, lncRNAMSTRG.7497 and lncRNA
MSTRG.13994, were selected for further verification in the femur
and tibia by qPCR. The qPCR results were the same as those observed
by sequencing, except for lncRNA MSTRG.13994, which further
validated the previous enrichment analysis (Fig. 6B-F).
Discussion
It has been reported that osteoporosis exhibit
increased bone marrow fat, while bone mass is reduced (24). To study the mechanism by which
exercise improves bone metabolism in more depth, mechanical stress
was used to interfere with BMSCs, osteoblasts, osteocytes and
osteoclasts, simulating the mechanical stimulation of exercise on
the bones. BMSCs are primitive multipotent cells that can
differentiate into osteoblasts, adipocytes, chondrocytes and other
cells under specific induction conditions (25). BMSCs serve an important role in bone
growth and metabolism and age-related osteoporosis and their
ability to proliferate and transform into osteoblasts decreases as
the body ages, which may be one of the mechanisms driving
age-related osteoporosis (26).
Some studies have revealed that appropriate mechanical stress
stimulation can promote the osteogenic differentiation of BMSCs,
inhibit their adipogenic differentiation, promote the proliferation
and differentiation of osteoblasts, reduce the activity and number
of osteoclasts, promote bone formation and inhibit bone resorption
(27–29). In our previous in vitro
studies, it has been demonstrated that mechanical stretch could
regulate MSCs osteogenic/adipogenic differentiation through the
TGFβ/Smad signaling pathway and high intensity stress could damage
the biological activity of cells due to the imbalance of oxygen
free radical system (23,30–32).
In order to further explore the molecular mechanisms underlying the
regulation of osteoporosis by exercise, animal experiment on
mechanical stress are performed in vivo, using a treadmill
exercise system. The present study demonstrated the
exercise-induced promotion of osteogenic differentiation and
inhibition of adipogenic differentiation in femur and tibia and
identified 16 upregulated and two downregulated miRNAs in the
exercise group, as well as 44 upregulated lncRNAs and 39
downregulated lncRNAs in the exercise group through miRNA and
lncRNA sequencing analyses. The results provide a valuable resource
for further exploring the molecular mechanisms underlying the
regulation of osteoporosis by exercise.
It has been noted that miRNAs mainly target
osteogenic factors, bone resorption factors, or other key factors
involved in the regulation of bone metabolism to exert their
regulatory effects on the proliferation or differentiation of
osteoblasts and osteoclasts (14).
A type of miRNA can enhance the proliferation and differentiation
of osteoblasts and inhibit the proliferation of osteoclasts,
thereby promoting bone formation. For example, miR-19a-3p can
alleviate the progression of osteoporosis by downregulating the
expression of histone deacetylases (HDACs). HDAC4 can inhibit the
function of Runx2 and miR-19a-3p exerts its osteogenic effects by
targeting the expression of HDAC4 (33). Yang et al (34) found that miR-21 could promote the
migration and osteogenic differentiation of BMSCs by increasing the
activation of the AKT pathway and hypoxia inducible factor1α. Huang
et al (35) demonstrated
that the expression level of miR-488 was significantly lower during
the process of psoralen-induced osteogenic differentiation in
BMSCs. In addition, experimental verification of bioinformatics
analysis and luciferase report analysis identified Runx2 as a
potential target of miR-488, which provides a possible target for
the treatment of osteoporosis (36). More specifically, mechanical stress
stimulation can upregulate the expression of osteogenic factors,
such as ALP, osterix, osteocalcin and Runx2; inhibit the expression
of bone resorption markers, such as receptor activator of NF-κB
ligand and tartrate-resistant acid phosphatase; promote the
differentiation of mesenchymal stem cells into osteoblasts; and
enhance the proliferation and differentiation of osteoblasts via
specific miRNA (15). Notably,
miRNAs are one of the important molecules for mechanical stress to
exert its osteogenesis effects (36). Research by Guo et al
(37) demonstrated that mechanical
traction can increase ALP activity, upregulate the expression of
osteogenic markers and induce the differential expression of
miR-218, miR-191, miR-3070a and miR-33. A previous study
demonstrated that mechanical stress upregulates the protein
expression of Runx2, which promotes the proliferation and
differentiation of osteoblasts and also inhibits the expression of
miR-103a (38). Additionally,
luciferase reporter gene experiments have confirmed that Runx2 is a
target of miR-103a, which indicates that mechanical stress may
increase the expression of Runx2 by inhibiting the expression of
miR-103a and thus promote bone formation (38). By contrast, it has been shown that
the mechanism of bone metabolism disorder and osteoporosis caused
by a long-term lack of mechanical stress stimulation also may be
related to miRNA (39). Sun et
al (40) studied the
proliferation of MC3T3-E1 osteoblasts in a microgravity environment
and found that the proliferation of osteoblasts is inhibited when
the expression of miR-103 is upregulated, while inhibiting the
expression of miR-103 could promote the proliferation of
osteoblasts.
Among the five miRNAs identified and verified in the
present study as being upregulated in exercise-treated rats, the
biological functions of miR-9942 and miR-5100 have not been
investigated previously to the best of the authors' knowledge. It
has been noted that miR-30d-3p is significantly upregulated during
the differentiation of pancreatic stem cells into β cells, may
serve an important role in the epigenetic mechanism of β cell
differentiation and could be useful in the diagnosis and treatment
of diabetes (41). It has been
reported that the expression of miR-7704 increases in
hepatocellular carcinoma and liver cirrhosis and exerts a negative
regulatory effect on lncRNA HAGLR (42). In addition, Siglec1 overexpression
increases the expression of miR-1260, thus degrading IκBα through
its untranslated region to serve a pro-inflammatory role and
promote the progression of chronic obstructive pulmonary disease
(43).
Accumulating evidence has shown that lncRNAs also
serve an important role in the regulation of osteogenic
differentiation and adipogenic differentiation of BMSCs,
occasionally acting as competing endogenous RNAs to regulate
miRNAs. For example, lncRNA KCNQ1OT1 and lncRNA MSC-AS1 were found
to be able to upregulate BMP2 thus promoting the osteogenic
differentiation of BMSCs by sequestering miR-214 and miR-140-5p,
respectively (44,45). Previous studies reveal that
lncRNAMEG3 is involved in the regulation of differentiation and
metabolism of mesenchymal stem cell by interacting and regulating
miRNAs. For example, lncRNA MEG3 can regulate the adipogenic and
osteogenic differentiation of human adipose stem cells through
miR-140-5p, while the osteogenic differentiation of human
periodontal ligament stem cells in periodontitis was affected
through the miR-27a-3p/IGF1 axis (46,47).
Notably, lncRNAs also can act as a mediator in the regulation of
BMSC differentiation. For example, lncRNA H19 acts as a competing
endogenous RNA for miR-138 to upregulate the expression level of
downstream focal adhesion kinase, thereby acting as a positive
regulator of the BMSC osteogenic differentiation induced by
mechanical stress stimulation (48).
The lack of BMD MRI measurements of bone content and
strength are the limitations of the present study.
In summary, considering the key roles served by
miRNAs and lncRNAs in the differentiation, the present study
established an animal model through treadmill exercise experiments
and performed miRNA and lncRNA sequencing to identify
differentially expressed miRNAs and lncRNAs in femur and tibia that
may be key mediators of differentiation. The results of the present
study are a valuable resource for further exploring the molecular
mechanisms driving the development of osteoporosis and allow the
search for therapeutic targets.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural
Sciences Foundation of China (grant no. 81101366) and the Natural
Science Foundation of Guangdong Province (grant nos. 2018A030313640
and 2019A1515012176).
Availability of data and materials
The datasets generated and/or analyzed during the
current study are available in the NCBI's Gene Expression Omnibus
repository (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE168713).
Authors' contributions
RL designed this program. YQ, GZ, SY and YQ operated
the cell and animal experiments. YQ, GZ, CZ, ZY and SZ conducted
the data acquisition and analysis. GZ and CZ uploaded all the data
onto an online repository. RL, YQ, GZ and CZ examined the revised
the paper. YQ, GZ, CZ and RL produced the manuscript, which was
checked by RL and YQ. RL and YQ are responsible for confirming the
authenticity of the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
All animal experiments were conducted according to
relevant national and international guidelines and approved by the
Animal Care and Use Committee of Nanfang Hospital, Southern Medical
University. The animal protocol no. for these experiments was
NFYY-2019-58.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Christianson MS and Shen W: Osteoporosis
prevention and management: Nonpharmacologic and lifestyle options.
Clin Obstet Gynecol. 56:703–710. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Todd JA and Robinson RJ: Osteoporosis and
exercise. Postgrad Med J. 79:3202003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Matamoro-Vidal A and Levayer R: Multiple
influences of mechanical forces on cell competition. Curr Biol.
29:R762–R774. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hemmatian H, Bakker AD, Klein-Nulend J and
van Lenthe GH: Aging, osteocytes, and mechanotransduction. Curr
Osteoporos Rep. 15:401–411. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sandino C, McErlain DD, Schipilow J and
Boyd SK: Mechanical stimuli of trabecular bone in osteoporosis: A
numerical simulation by finite element analysis of
microarchitecture. J Mech Behav Biomed. 66:19–27. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yuan Y, Chen X, Zhang L, Wu J, Guo J, Zou
D, Chen B, Sun Z, Shen C and Zou J: The roles of exercise in bone
remodeling and in prevention and treatment of osteoporosis. Prog
Biophys Mol Biol. 122:122–130. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tong X, Chen X, Zhang S, Huang M, Shen X,
Xu J and Zou J: The effect of exercise on the prevention of
osteoporosis and bone angiogenesis. Biomed Res Int.
2019:81718972019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mohr AM and Mott JL: Overview of microRNA
biology. Semin Liver Dis. 35:3–11. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rupaimoole R and Slack FJ: MicroRNA
therapeutics: Towards a new era for the management of cancer and
other diseases. Nat Rev Drug Discov. 16:203–222. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chapurlat RD and Confavreux CB: Novel
biological markers of bone: From bone metabolism to bone
physiology. Rheumatology. 55:1714–1725. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Taipaleenmäki H: Regulation of bone
metabolism by microRNAs. Curr Osteoporos Rep. 16:1–12. 2018.
View Article : Google Scholar
|
|
12
|
Tang P, Xiong Q, Ge W and Zhang L: The
role of microRNAs in osteoclasts and osteoporosis. Rna Biol.
11:1355–1363. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yuan Y, Guo J, Zhang L, Tong X, Zhang S,
Zhou X, Zhang M, Chen X, Lei L, Li H, et al: MiR-214 attenuates the
osteogenic effects of mechanical loading on osteoblasts. Int J
Sports Med. 40:931–940. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang J, Liu S, Li J, Zhao S and Yi Z:
Roles for miRNAs in osteogenic differentiation of bone marrow
mesenchymal stem cells. Stem Cell Res Ther. 10:1972019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yuan Y, Zhang L, Tong X, Zhang M, Zhao Y,
Guo J, Lei L, Chen X, Tickner J, Xu J and Zou J: Mechanical stress
regulates bone metabolism through MicroRNAs. J Cell Physiol.
232:1239–1245. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang X, Zhao D, Zhu Y, Dong Y and Liu Y:
Long non-coding RNA GAS5 promotes osteogenic differentiation of
bone marrow mesenchymal stem cells by regulating the
miR-135a-5p/FOXO1 pathway. Mol Cell Endocrinol. 496:1105342019.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Barra GB, Santa Rita TH, Almeida ALSC,
Jácomo RH and Nery LFA: Serum has higher proportion of janus kinase
2 V617F mutation compared to paired EDTA-whole blood sample: A
model for somatic mutation quantification using qPCR and the
2−∆∆Cq method. Diagnostics (Basel). 10:1532020.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pertea M, Kim D, Pertea GM, Leek JT and
Salzberg SL: Transcript-level expression analysis of RNA-seq
experiments with HISAT, StringTie and Ballgown. Nat Protoc.
11:1650–1667. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tamaki H, Akamine T, Goshi N, Kurata H and
Sakou T: Effects of exercise training and etidronate treatment on
bone mineral density and trabecular bone in ovariectomized rats.
Bone. 23:147–153. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kannus P, Sievänen H, Järvinen TL,
Järvinen M, Kvist M, Oja P, Vuori I and Jozsa L: Effects of free
mobilization and low- to high-intensity treadmill running on the
immobilization-induced bone loss in rats. J Bone Miner Res.
9:1613–1619. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bruderer M, Richards R, Alini M and
Stoddart M: Role and regulation of RUNX2 in osteogenesis. Eur Cells
Mater. 28:269–286. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ali AT, Hochfeld WE, Myburgh R and Pepper
MS: Adipocyte and adipogenesis. Eur J Cell Biol. 92:229–236. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li R, Liang L, Dou Y, Huang Z, Mo H, Wang
Y and Yu B: Mechanical stretch inhibits mesenchymal stem cell
adipogenic differentiation through TGFβ1/Smad2 signaling. J
Biomech. 48:3665–3671. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang C, Meng H, Wang X, Zhao C, Peng J and
Wang Y: Differentiation of bone marrow mesenchymal stem cells in
osteoblasts and adipocytes and its role in treatment of
osteoporosis. Med Sci Monit. 22:226–233. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Naji A, Eitoku M, Favier B, Deschaseaux F,
Rouas-Freiss N and Suganuma N: Biological functions of mesenchymal
stem cells and clinical implications. Cell Mol Life Sci.
76:3323–3348. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Infante A and Rodríguez CI: Osteogenesis
and aging: Lessons from mesenchymal stem cells. Stem Cell Res Ther.
9:2442018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Menuki K, Mori T, Sakai A, Sakuma M,
Okimoto N, Shimizu Y, Kunugita N and Nakamura T: Climbing exercise
enhances osteoblast differentiation and inhibits adipogenic
differentiation with high expression of PTH/PTHrP receptor in bone
marrow cells. Bone. 43:613–620. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lee J, Park H and Kim KS: Intrinsic and
extrinsic mechanical properties related to the differentiation of
mesenchymal stem cells. Biochem Biophys Res Commun. 473:752–757.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fu X, Halim A, Tian B, Luo Q and Song G:
MT1-MMP downregulation via the PI3K/Akt signaling pathway is
required for the mechanical stretching-inhibited invasion of
bone-marrow-derived mesenchymal stem cells. J Cell Physiol.
234:14133–14144. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li R, Liang L, Dou Y, Huang Z, Mo H, Wang
Y and Yu B: Mechanical strain regulates osteogenic and adipogenic
differentiation of bone marrow mesenchymal stem cells. Biomed Res
Int. 2015:8732512015.PubMed/NCBI
|
|
31
|
Li R, Chen B, Wang G, Yu B, Ren G and Ni
G: Effects of mechanical strain on oxygen free radical system in
bone marrow mesenchymal stem cells from children. Injury.
42:753–757. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhu G, Qian Y, Wu W and Li R: Negative
effects of high mechanical tensile strain stimulation on
chondrocyte injury in vitro. Biochem Biophys Res Commun. 510:48–52.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen R, Qiu H, Tong Y, Liao F, Hu X, Qiu Y
and Liao Y: MiRNA-19a-3p alleviates the progression of osteoporosis
by targeting HDAC4 to promote the osteogenic differentiation of
hMSCs. Biochem Biophys Res Commun. 516:666–672. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yang C, Liu X, Zhao K, Zhu Y, Hu B, Zhou
Y, Wang M, Wu Y, Zhang C, Xu J, et al: miRNA-21 promotes
osteogenesis via the PTEN/PI3K/Akt/HIF-1α pathway and enhances bone
regeneration in critical size defects. Stem Cell Res Ther.
10:652019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Huang Y, Hou Q, Su H, Chen D, Luo Y and
Jiang T: miR-488 negatively regulates osteogenic differentiation of
bone marrow mesenchymal stem cells induced by psoralen by targeting
Runx2. Mol Med Rep. 20:3746–3754. 2019.PubMed/NCBI
|
|
36
|
Wei F, Yang S and Wang S: MicroRNAs: A
critical regulator under mechanical force. Histol Histopathol.
33:335–342. 2018.PubMed/NCBI
|
|
37
|
Guo Y, Wang Y, Liu Y, Liu Y, Zeng Q, Zhao
Y and Zhang X and Zhang X: MicroRNA-218, microRNA-191*,
microRNA-3070a and microRNA-33 are responsive to mechanical strain
exerted on osteoblastic cells. Mol Med Rep. 12:3033–3038. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zuo B, Zhu J, Li J, Wang C, Zhao X, Cai G,
Li Z, Peng J, Wang P, Shen C, et al: microRNA-103a functions as a
mechanosensitive microRNA to inhibit bone formation through
targeting Runx2. J Bone Miner Res. 30:330–345. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Blaber EA, Dvorochkin N, Torres ML, Yousuf
R, Burns BP, Globus RK and Almeida EA: Mechanical unloading of bone
in microgravity reduces mesenchymal and hematopoietic stem
cell-mediated tissue regeneration. Stem Cell Res. 13:181–201. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sun Z, Cao X, Hu Z, Zhang L, Wang H, Zhou
H, Li D, Zhang S and Xie M: MiR-103 inhibits osteoblast
proliferation mainly through suppressing Cav1.2 expression in
simulated microgravity. Bone. 76:121–128. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Coskun E, Ercin M and Gezginci-Oktayoglu
S: The role of epigenetic regulation and pluripotency-related
MicroRNAs in differentiation of pancreatic stem cells to beta
cells. J Cell Biochem. 119:455–467. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Mahlab-Aviv S, Boulos A, Peretz AR,
Eliyahu T, Carmel L, Sperling R and Linial M: Small RNA sequences
derived from pre-microRNAs in the supraspliceosome. Nucleic Acids
Res. 46:11014–11029. 2018.PubMed/NCBI
|
|
43
|
Li S, Jiang L, Yang Y, Cao J, Zhang Q,
Zhang J, Wang R, Deng X and Li Y: Siglec1 enhances inflammation
through miR-1260-dependent degradation of IκBα in COPD. Exp Mol
Pathol. 113:1043982020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang CG, Liao Z, Xiao H, Liu H, Hu YH,
Liao QD and Zhong D: LncRNA KCNQ1OT1 promoted BMP2 expression to
regulate osteogenic differentiation by sponging miRNA-214. Exp Mol
Pathol. 107:77–84. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang N, Hu X, He S, Ding W, Wang F, Zhao
Y and Huang Z: LncRNA MSC-AS1 promotes osteogenic differentiation
and alleviates osteoporosis through sponging microRNA-140-5p to
upregulate BMP2. Biochem Biophys Res Commun. 519:790–796. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Li Z, Jin C, Chen S, Zheng Y, Huang Y, Jia
L, Ge W and Zhou Y: Long non-coding RNA MEG3 inhibits adipogenesis
and promotes osteogenesis of human adipose-derived mesenchymal stem
cells via miR-140-5p. Mol Cell Biochem. 433:51–60. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Liu Y, Liu C, Zhang A, Yin S, Wang T, Wang
Y, Wang M, Liu Y, Ying Q, Sun J, et al: Down-regulation of long
non-coding RNA MEG3 suppresses osteogenic differentiation of
periodontal ligament stem cells (PDLSCs) through miR-27a-3p/IGF1
axis in periodontitis. Aging (Albany NY). 11:5334–5350. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wu J, Zhao J, Sun L, Pan Y, Wang H and
Zhang W: Long non-coding RNA H19 mediates mechanical
tension-induced osteogenesis of bone marrow mesenchymal stem cells
via FAK by sponging miR-138. Bone. 108:62–70. 2018. View Article : Google Scholar : PubMed/NCBI
|