Introduction
Diabetic nephropathy (DN) is a common microvascular
complication of diabetes mellitus and is a major cause of chronic
kidney failure and end-stage renal disease (ESRD) (1,2). The
increasing incidence of DN worldwide affects the quality of life of
patients and has a heavy burden on society and the economy.
According to a US Renal Data System report, 20–40% of diabetic
patients in the United States display different degrees of renal
injury, and DN is the first secondary factor leading to ESRD
(3). The main clinical features of
DN are proteinuria, progressive renal impairment and hypertension
(4,5), and its prognosis and outcome are
associated with a variety of mechanisms, such as hemodynamic
changes, oxidative stress and inflammation. Therefore, research
should be conducted to further understand the pathogenesis of DN,
in order to develop novel therapeutic agents.
Recent studies have reported that autophagy was
closely associated with the occurrence and development of DN
(6,7). The process of autophagy is an
effective method to remove damaged proteins and senescent
organelles in cells to maintain the stability of the intracellular
environment (8,9). The PI3K/Akt/mTOR signaling pathway is
the most typical signaling pathway regulating autophagy, and mTOR
is a key target of autophagy regulation and it negatively regulates
autophagy (10,11). mTOR is a downstream target of
P13K/Akt, and its activity is dependent on regulation of the
PI3K/Akt signaling pathway. Under normal physiological
circumstances, a tyrosine kinase receptor activates PI3K, which
then converts its substrate phosphatidylinositol diphosphate into
inositol triphosphate (PIP3). Then, PIP3 and
phosphoinositide-dependent kinase-1 synergistically activate Akt,
which then transmits the signals for mTOR activation (12). Activation of mTOR and its downstream
factors promotes protein synthesis and cell proliferation,
accelerates cell metabolism and inhibits cell autophagy (13,14).
Beclin-1 and LC3 are important autophagy markers.
Beclin-1, as one of the related genes regulating autophagy
formation, is an important component of the autophagy core complex,
and it serves a key role in maintaining autophagy activity
(15). The LC3 gene also belongs to
the autophagy system, and it is involved in the encoding of
proteins associated with autophagy. The encoded proteins are mainly
expressed on the surface of the pre-autophagosome and autophagosome
membranes, and serve a major role in the construction of
autophagosome (16). Moreover, the
proportion of LC3II/LC3I can reflect the degree of autophagy
(17,18). Therefore, the LC3II/LC3I ratio and
Beclin-1 expression are usually used as references to evaluate the
level of autophagy (19,20).
Dendrobium mixture (DMix) is a preparation
used at The Second Affiliated Hospital of Fujian Traditional
Chinese Medical University (batch. no. Min Q/YZ-2012-315; patent.
no. ZL201110408411.0), which was developed by Professor Hong Shi
for the long-term clinical treatment of diabetes and its
complications (21,22). DMix is composed of Dendrobium,
Astragalus, Schisandra, Radix puerariae, Salvia miltiorrhiza,
Rehmanniae and Rhizoma anemarrhenae. In clinical
applications, it can lower glucose and lipid levels, and alleviate
insulin resistance (22); however,
the molecular mechanisms underlying its action remain unknown.
The PI3K/Akt signaling pathway is a signal
transduction pathway that promotes cell survival and proliferation
in response to extracellular signals (23). It has been reported that the
PI3K/Akt/mTOR signaling pathway serves an important role in the
pathogenesis of diabetes mellitus; therefore, the regulation of
this signaling pathway may be a potential therapeutic target
(24). In the present study, a DN
rat model was established using a high-sugar and high-fat feed
diet, combined with a low-dose streptozocin (STZ) peritoneal
injection. Based on the regulatory effect of the PI3K/Akt/mTOR
signaling pathway on autophagy, the mechanism of DMix in renal
protection in rats with DN was further examined to provide an
experimental basis for clinical treatment of DN.
Materials and methods
Drugs
The DMix decoction consisting of the seven herbs
listed in Table I (15 g
Dendrobium, 20 g Astragalus, 8 g Schisandra,
15 g Radix puerariae, 20 g Salvia miltiorrhiza, 18 g
Rehmanniae and 12 g Rhizoma anemarrhenae) was
purchased from the Guoyitang Clinic of Fujian University of
Traditional Chinese Medicine. The aforementioned herbs were added
to distilled water and soaked for 30 min, boiled for 1 h and
filtered. The filtered liquid was concentrated to 1.6 g/ml by
boiling and stored at this concentration for use. Gliquidone
tablets (batch. no. 1140573) were purchased from Beijing Wanhui
Shuanghe Pharmaceutical Co., Ltd.
 | Table I.Composition of Dendrobium
mixture. |
Table I.
Composition of Dendrobium
mixture.
| Ingredient, plant
name | Plant organ | Weight, g |
|---|
|
Dendrobium | Stem | 15 |
|
Astragalus | Radix | 20 |
|
Schisandra | Fruit | 8 |
| Radix
puerariae | Radix | 15 |
| Salvia
miltiorrhiza | Radix and
Rhizoma | 20 |
|
Rehmanniae | Root tuber | 18 |
| Rhizoma
anemarrhenae | Rhizoma | 12 |
Animals
A total of 50 specific-pathogen free healthy male
Sprague-Dawley rats (age, 6 weeks; weight, 180–220 g) were provided
by Shanghai Slyke Experimental Animals Co., Ltd. [license. no. SCXK
(HU) 2017-0005]. Rats were kept in a specific-pathogen free
environment (temperature, 22–25°C; humidity, 50–60%; 12-h
light/dark cycles) at the Experimental Animal Center of Fujian
University of Traditional Chinese Medicine, with free access to a
standard diet and water. All animal experiments were conducted in
accordance with internationally recognized animal welfare
guidelines (25) and were approved
by the Medical Ethics Committee of Fujian University of Traditional
Chinese Medicine (approval. no. 2019-031). Rats were rapidly
euthanized via cervical dislocation under deep anesthesia using 20%
urethane (1,000 mg/kg; intraperitoneal).
Experimental design
All rats were fed an adaptive routine diet for 1
week. In total, 10 rats were randomly selected as the normal
control group and were fed a routine diet. The remaining 40 rats
were given a high-sugar and high-fat diet (main ingredients: 60.7%
basic feed, 10% lard, 15% sucrose, 10% egg yolk powder, 4%
cholesterol and 0.3% cholate). After 6 weeks of feeding, the rats
were fasted for 12 h and given an intraperitoneal injection of 25
mg/kg STZ (Sigma-Aldrich; Merck KGaA) dissolved in 0.1 mol/l
citrate buffer (pH 4.2). The control group was given an
intraperitoneal injection of equal volume of citrate buffer. After
72 h, a second intraperitoneal injection of the same dose was given
using the same method. Then, 3 days later, tail vein blood sampling
was performed for detecting random blood glucose levels of ≥16.7
mmol/l, and the unqualified rats (blood glucose level, <16.7
mmol/l) were excluded from the experiment. Rat urine was collected
from a metabolic cage for 24 h, and the urinary albumin excretion
rate (UAER) was measured. Blood glucose level at ≥16.7 mmol/l and
an UAER of ≥30 mg/24 h were used as the establishment criteria for
DN (26). In total, 30 rats with DN
successfully met the DN model criteria and were randomly allocated
into three groups: DN group (n=10), DMix group (n=10) and
gliquidone group (n=10). Gliquidone (cat. no. 1140573; Beijing
Wanhui Double Crane Pharmaceutical Co., Ltd.) is an anti-DN drug
and was used as a positive control. At 9:00 a.m every day, all
animals were treated via intragastric administration at the
clinical equivalent dose for adults with body weight of 60 kg. The
rats in the DMix group were administered 8 g/(kg·d) DMix, and those
in the gliquidone group were given 3 mg/(kg·d) gliquidone via
gastric gavage. The control rats and rats with DN were administered
the same amount of normal saline daily via gastric gavage for 8
weeks.
Measurement of fasting blood glucose
(FBG), body weight, kidney weight, kidney index, blood lipid level,
insulin and renal function
During the treatment period, the rats were weighed,
and FBG was measured every 2 weeks. The rats were fasted (with free
access to water) for 12 h, and the tails of the rats were pierced
with disposable sterile blood collection needles. After collecting
0.1 ml tail blood, the FBG of the rats was measured using a Roche
glucose meter (Roche Diagnostics). At the end of 0, 4 and 8 weeks,
rat urine was collected from a metabolic cage for 24 h; then, the
urine volume was measured using a volumetric canister and the urine
protein concentration was measured using a urine protein
quantitative kit (cat. no. C035-2, Nanjing Jiancheng Bioengineering
Institute), according to the manufacturer's protocol. Values of the
total urine volume and protein concentration were used to calculate
the UAER.
After 8 weeks of treatment, all rats were fasted for
12 h, with water available, and then anesthetized using
intraperitoneal 20% urethane (1,000 mg/kg). Blood was collected
from the abdominal aorta and the serum was separated via
centrifugation at 4°C for 15 min at 1,200 × g. Serum total
cholesterol (TC; cat. no. A111-2), triglyceride (TG; cat. no.
A110-2), low-density lipoprotein cholesterol (LDL-C, cat. no.
A113-1), high-density lipoprotein cholesterol (HDL-C; cat. no.
A112-1), hemoglobin A1c (HbA1c; cat. no. A056-2), blood urea
nitrogen (BUN; cat. no. C013-2) and serum creatinine (Scr; cat. no.
C011-2) levels were measured using biochemical analysis kits
according to the manufacturer's protocols. All biochemical analysis
kits were purchased from the Nanjing Jiancheng Bioengineering
Institute. Serum insulin levels were measured with an ELISA kit
(cat. no. F15960; Shanghai Xitang Biotechnology Co., Ltd.)
according to the manufacturer's protocol. After the rats were
sacrificed, both kidneys were removed, washed with normal saline
and weighed. Kidney index was calculated as follows: Kidney
index=weight (mg)/body weight (g).
Renal histology
Kidney tissue was fixed in 4% paraformaldehyde
solution at room temperature for 24 h, embedded in paraffin and cut
into 4-µm-thick sections. H&E, Periodic Acid-Schiff (PAS) and
Masson's staining were used to evaluate the pathological changes in
the kidney tissue. All staining steps were performed at room
temperature. The stained kidney sections were examined under a
light microscope (Nikon Corporation) at a magnification of
×400.
H&E staining
The dried kidney tissue sections were dewaxed using
xylene, graded alcohol (100, 95, 90, 80 and 70%) and distilled
water. The sections were stained with hematoxylin for 10 min,
differentiated with 1% hydrochloric acid alcohol for 5 sec and then
immersed in eosin for 3 min. Dehydration and transparent sealing
were then performed for examination under a light microscope.
PAS staining
The dried kidney tissue sections were dewaxed using
xylene, graded alcohol and distilled water. Sections were immersed
in iodic acid oxidation solution for 5 min, followed by immersion
in Schiff reagent for 15 min. After counterstaining with
hematoxylin for 1 min, 1% hydrochloric acid alcohol differentiation
for 3 sec, dehydration and transparent sealing, examination was
performed under a light microscope.
Masson staining
The dried kidney tissue sections were dewaxed using
xylene, gradient alcohol and distilled water. The sections were
fixed for 1 h in the Bouin's fixative solution (15:5:1 of picric
acid saturated liquid, formaldehyde and glacial acetic acid),
immersed in Masson composite dyeing solution for 10 min and
differentiated in 1% phosphomolybdate for 10 min. The collagen
fiber showed a reddish color and was immersed in 2% aniline blue
solution for 5 min. Dehydration and transparent sealing were then
performed for examination under a light microscope.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from rat kidney tissue using
the RNAiso Plus reagent (cat. no. 9108; Takara Bio, Inc.), and the
concentration was determined. Then, cDNA was synthesized using a
PrimeScript RT Reagent kit (cat. no. RR047A; Takara Bio, Inc.) at
37°C for 15 min and 85°C for 5 sec. PCR was performed using a PCR
kit (cat. no. RR420A; Takara Bio, Inc.) under the following
reaction conditions: Initial denaturation at 95°C for 30 sec;
followed by 40 cycles of denaturation at 95°C for 5 sec, annealing
at 55°C for 30 sec and extension at 72°C for 30 sec. SDS2.4
software (Thermo Fisher Scientific, Inc.) was used to analyze the
Cq values of the samples detected during the PCR process, using
GAPDH as the internal reference and the ΔΔCq method for relative
quantitative analysis, with 2−∆∆Cq as a quantity for the
relative expression of the target RNA. PCR primers (Table II) were designed and provided by
Fuzhou Shangya Biotechnology Co., Ltd.
 | Table II.Primers used for reverse
transcription-quantitative PCR. |
Table II.
Primers used for reverse
transcription-quantitative PCR.
| Gene | Primer
sequence | Product length,
bp |
|---|
| LC3 | Forward:
5′-GCGAGTTGGTCAAGATCATCC-3′ | 138 |
|
| Reverse:
5′-CGTCTTCATCCTTCTCCTGTTC-3′ |
|
| Beclin-1 | Forward:
5′-AATCTAAGGAGTTGCCGTTGT-3′ | 191 |
|
| Reverse:
5′-GCCTCCAGTGTCTTCAATCTT-3′ |
|
| GAPDH | Forward:
5′-ACGGCAAGTTCAACGGCACAG-3′ | 149 |
|
| Reverse:
5′-GAAGACGCCAGTAGACTCCACGAC-3′ |
|
Immunohistochemistry
The kidney tissue was fixed in 4% paraformaldehyde
solution at room temperature for 24 h, embedded in paraffin and cut
into 4-µm-thick sections. The paraffin-embedded kidney tissue was
cut into 4-µm-thick slices, adhered to microscope slides, baked at
58°C for 2 h, dewaxed using xylene twice, hydrated with gradient
alcohol (100, 95, 90, 80 and 70%), placed into boiled sodium
citrate solution for antigen repair and naturally cooled to room
temperature (18–30°C). The sections were rinsed with PBS three
times, co-incubated with an endogenous peroxidase blocker at room
temperature for 10 min, rinsed with PBS three times and
co-incubated with non-immunized animal serum (10%; Fuzhou Maixin
Biotech Co., Ltd.) at room temperature for 10 min. After removing
the serum, the following primary antibodies were added in a
dropwise manner: Rabbit anti-LC3 polyclonal antibody (1:200; cat.
no. ab48394; Abcam) and rabbit anti-Beclin-1 polyclonal antibody
(1:500; cat. no. ab62557; Abcam). Slides were then incubated
overnight at 4°C and then rinsed with PBS three times.
Biotin-labeled sheep anti-rabbit IgG (ready to use; cat. no.
KIT-9710; Fuzhou Maixin Biotech Co., Ltd.) was added and slides
were incubated at room temperature for 10 min, after which they
were rinsed with PBS thrice, incubated with streptavidin-peroxidase
(Fuzhou Maixin Biotech Co., Ltd.) at room temperature for 10 min
and rinsed with PBS three times. Then, the sections were treated
with DAB (Wuhan Boster Biological Technology Co., Ltd.) for color
development at room temperature for 3 min, rinsed with distilled
water, dyed with hematoxylin at room temperature for 1 min and
rinsed using tap water for blueness. Gradient alcohol was used for
dehydration, sections were dried and made transparent using xylene,
following which neutral gum was used for sealing. Brown staining
indicated positive expression as observed under an optical
microscope (magnification, ×400). Image-pro Plus 6.0 software
(Media Cybernetics, Inc.) was used for analysis, and the relative
protein expression was represented in terms of mean density.
Western blotting
The kidney tissues stored in liquid nitrogen were
lysed in RIPA buffer (cat. no. P0013B; Beyotime Institute of
Biotechnology) and fully ground to create tissue homogenate. After
centrifugation (4°C; 1,200 × g; 15 min), the supernatant was
absorbed to obtain the total protein of kidney tissue, and the
protein concentration was determined using a BCA assay. Then, 30 µg
each sample was used for 10% SDS-PAGE, after which the samples were
transferred to a PVDF membrane, and the membrane was blocked with
5% skim milk at room temperature for 1 h. Next, the membrane was
incubated with primary antibodies overnight at 4°C. After rinsing
with TBS-0.1% Tween 20 (TBST), the membrane was incubated with a
secondary antibody at room temperature for 1 h. After rinsing with
TBST, the membrane was stained using ECL (cat. no. P0018S; Beyotime
Institute of Biotechnology) reagent and viewed with a gel imaging
system (Bio-Rad Laboratories, Inc.). The antibodies and dilutions
used were as follows: β-actin (1:1,000; cat. no. ab8226; Abcam),
PI3K (1:1,000; cat. no. ab191606; Abcam), phosphorylated (p)-PI3K
(1:500; cat. no. ab182651; Abcam), Akt (1:500; cat. no. ab8805;
Abcam), p-Akt (1:500; cat. no. ab222489; Abcam), mTOR (1:10,000;
cat. no. ab134903; Abcam), p-mTOR (1:1,000; cat. no. ab137133;
Abcam), LC3 (1:2,000; cat. no. ab48394; Abcam), Beclin-1 (1:1,000;
cat. no. ab62557; Abcam), goat anti-mouse IgG secondary antibody
(1:2,000; cat. no. A0216; Biyuntian Biotechnology Research
Institute) and goat anti-rabbit IgG secondary antibody (1:1,000;
cat. no. A0208; Biyuntian Biotechnology Research Institute). Image
Lab software (version 5.2.1; Bio-Rad Laboratories, Inc.) was used
to analyze and semi-quantify the images.
Statistical analysis
All experiments were repeated three times. SPSS 22.0
statistical software (IBM Corp.) was used to analyze the data,
which are presented as the mean ± SD if they followed a normal
distribution. If not, the data are expressed as median and
interquartile range. If the data conformed to the normal
distribution pattern, differences among multiple groups were
analyzed using one-way and mixed two-way ANOVA. Kruskal-Wallis test
was used for analyzing data that did not follow a normal
distribution. The Bonferroni method was used for pairwise
comparison between groups when the variances were homogeneous, and
Dunnett's T3 comparison was used when the variances were
heterogeneous. P<0.05 was considered to indicate a statistically
significant difference.
Results
General conditions of rats during the
treatment period
The rats in the control group displayed a quick
reaction, good mental state and glossy hair. The rats in the DN
group showed slow reaction and weight loss, and increased diet,
water intake and urine output, compared with the control group. In
comparison with the DN group, these characteristics were improved
to varying degrees in the DMix and gliquidone groups.
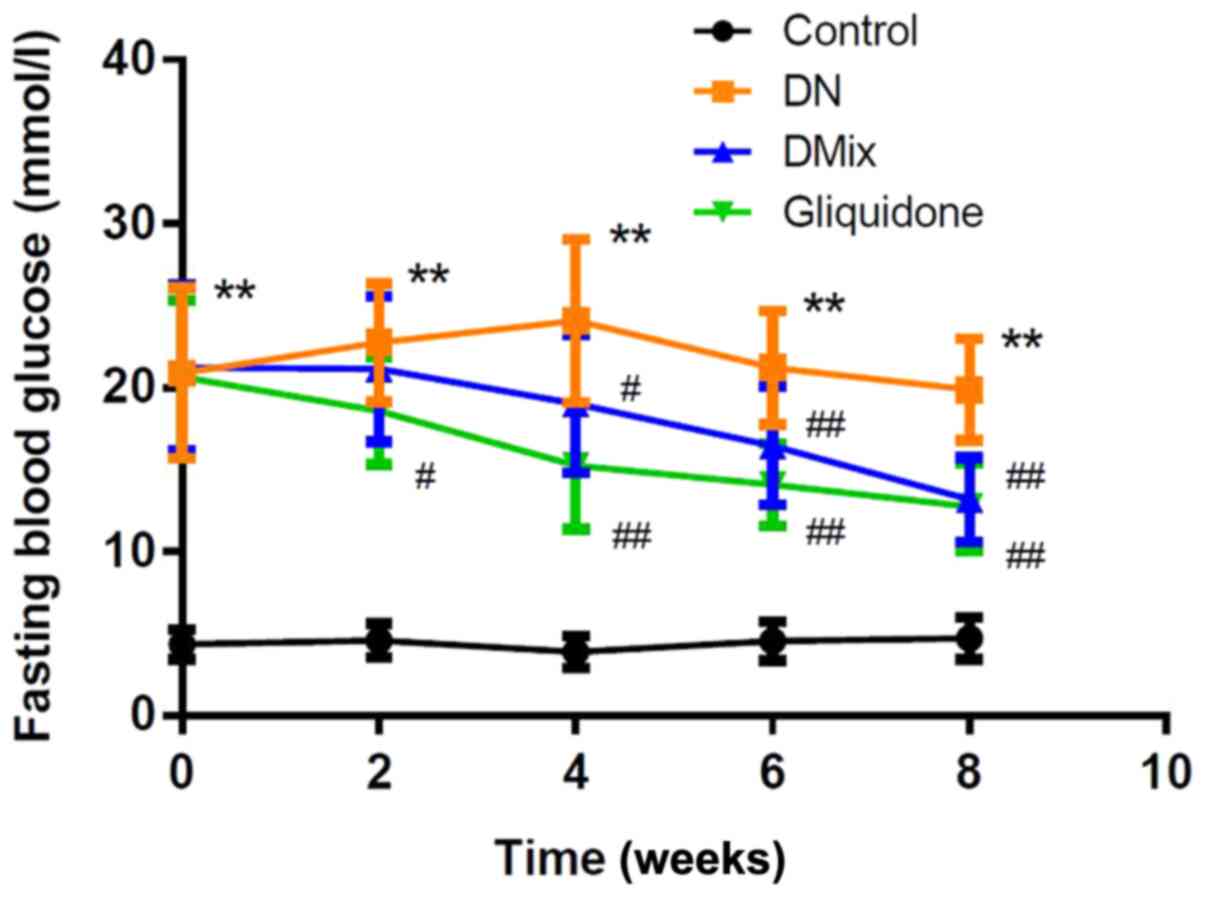
DMix lowers FBG levels in rats with
DN
As shown in Fig. 1,
the FBG levels of rats in the DN group were significantly increased
compared with those in the control group (P<0.01). After
treatment with DMix, FBG levels were gradually decreased with
treatment time, and they were significantly different from those in
the DN group from the 4th week onwards (week 4, P=0.034; week 6,
P=0.009; and week 8, P<0.01); the difference was not
statistically significant compared with the gliquidone group
(P=0.767).
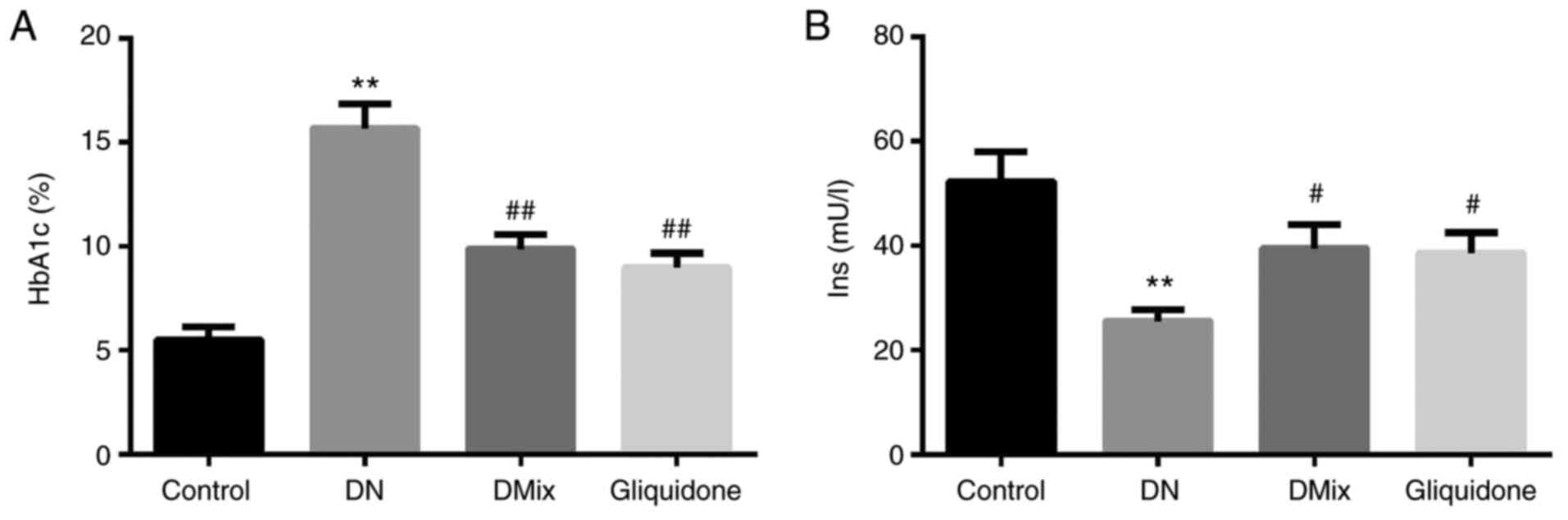
Effect of DMix on serum HbA1c and
insulin levels of rats with DN
It was found that compared with the control group,
HbA1c levels of rats in the DN group were increased significantly
(P=0.000; Fig. 2A), and insulin
levels were decreased significantly (P<0.01; Fig. 2B). Compared with the DN group, HbA1c
and insulin levels in the DMix group were significantly decreased
and increased, respectively (HbA1c, P<0.01; insulin,
P=0.033).
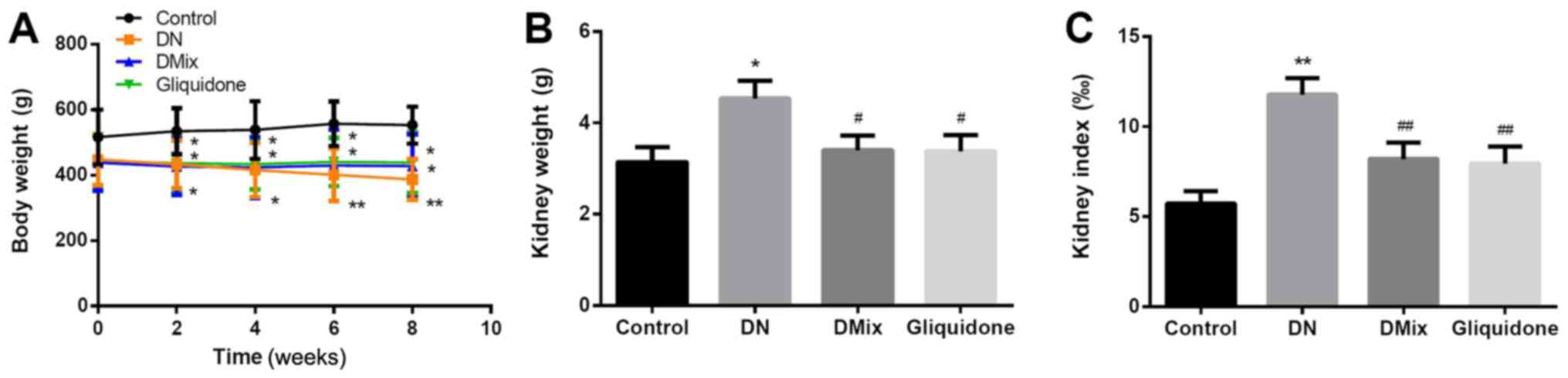
Comparison of body weight, kidney
weight and kidney index in each group
Compared with the control group, the DN group
continued to lose weight over time (week 2, P=0.043; week 4,
P=0.022; week 6, P=0.004; and week 8, P=0.002). However, after
treatment with DMix, further weight loss of the rats was inhibited
(P=0.006; Fig. 3A). The kidney
weight and index of the DN group were significantly higher compared
with those of the normal group (kidney weight, P=0.011; kidney
index, P<0.01; Fig. 3B and C),
suggesting that the renal tissue was damaged. After 8 weeks of
treatment with DMix, the kidney weight and index of the rats were
significantly decreased (kidney weight, P=0.034; kidney index,
P=0.008; Fig. 3B and C), which
showed alleviated renal tissue injury.
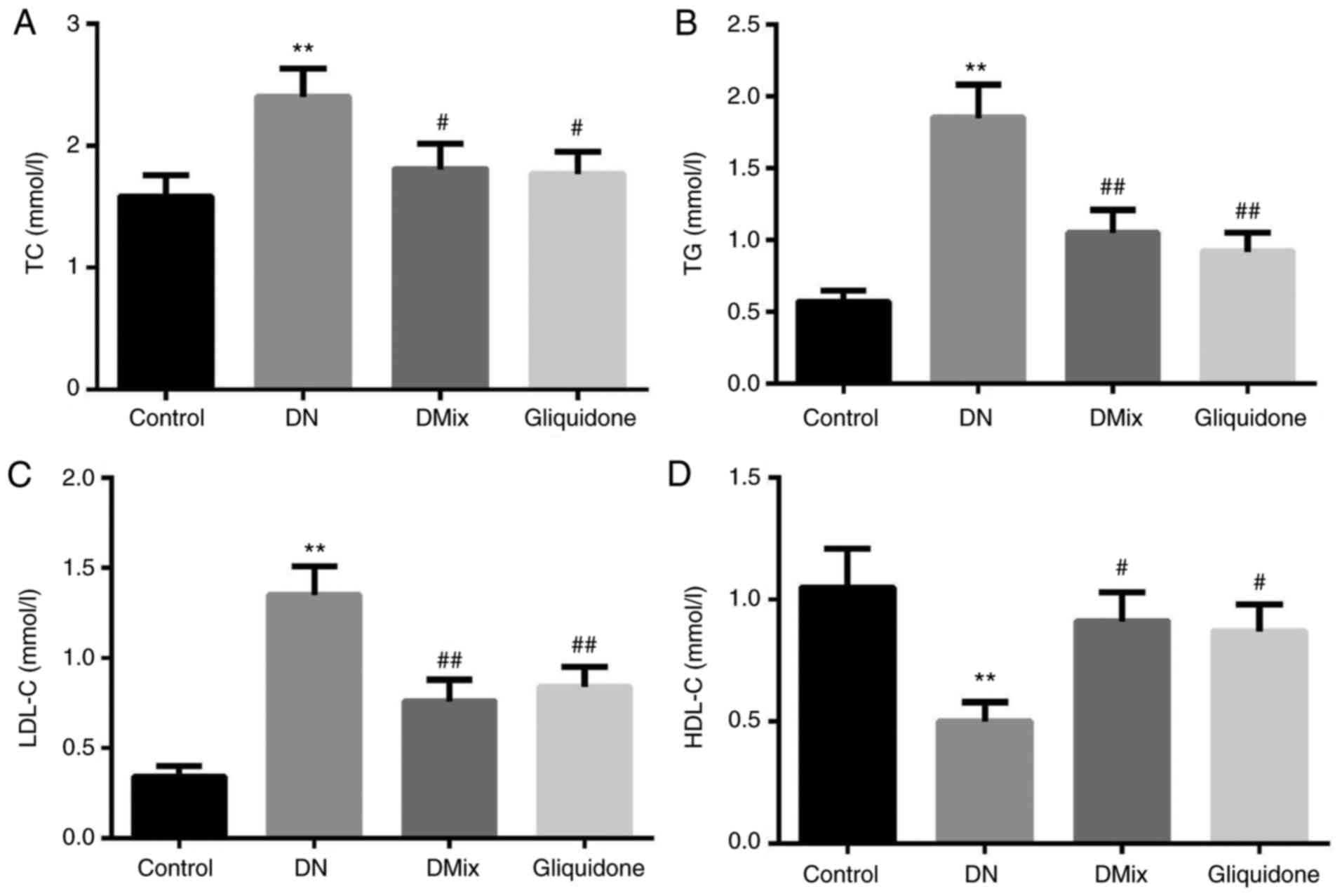
Effects of DMix on serum TC, TG, LDL-C
and HDL-C levels in rats with DN
As shown in Fig. 4,
the serum TC, TG and LDL-C levels of rats in the DN group were
significantly higher compared with those in the control group (TC,
P=0.009; TG, P<0.01; LDL-C, P<0.01), while HDL-C levels were
significantly lower (P=0.005). Moreover, compared with the DN
group, TC, TG and LDL-C levels were significantly lower in the DMix
group (TC, P=0.049; TG, P=0.048; LDL-C, P=0.003), while HDL-C
levels were significantly higher (P=0.029). There was no
statistically significant difference between the DMix and
gliquidone groups (TC, P=0.894; TG, P=0.579; LDL-C, P=0.664; HDL-C,
P=0.848).
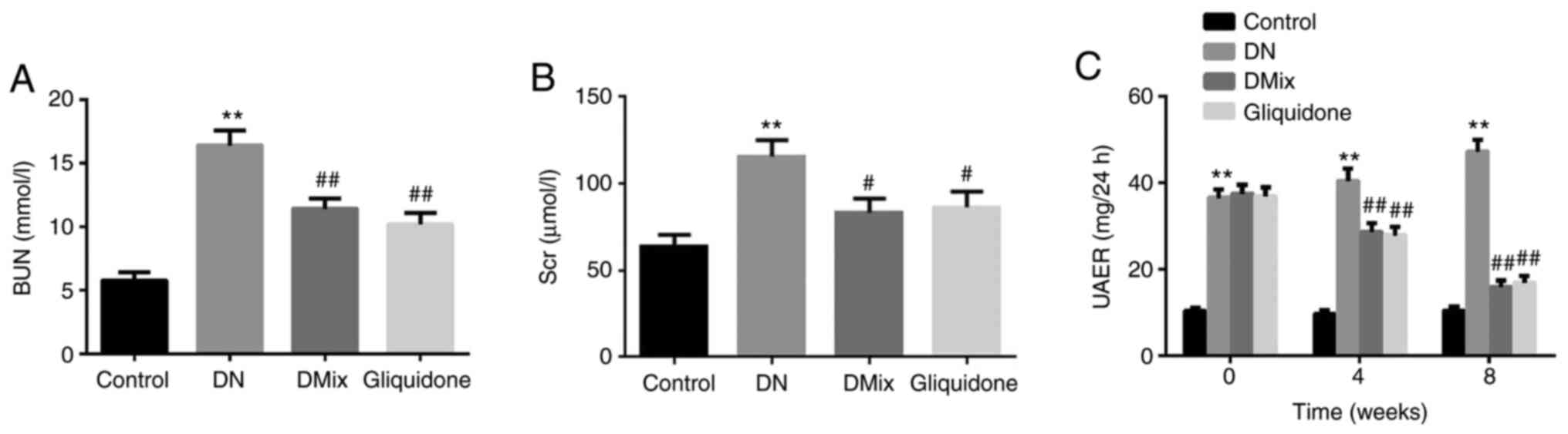
DMix improves renal function in rats
with DN
Indices of renal function, including BUN, Scr and
UAER, were detected in each group, and compared with those of the
control group. All indices in the DN group were found to be
significantly increased (BUN, P<0.01; Scr, P<0.01; UAER,
P<0.01; Fig. 5A-C). Renal
insufficiency was observed in rats with DN. Compared with the DN
group, BUN and Scr levels in the DMix group were significantly
reduced (BUN, P=0.001; Scr, P=0.013; Fig. 5A and B), but the difference was not
statistically significant compared with that of the gliquidone
group (BUN, P=0.347; Scr, P=0.792). UAER was measured every 4
weeks. It was identified that the UAER in the DN group was
gradually increased (week 4, P=0.293; week 8, P=0.011), and it was
significantly decreased after treatment with DMix (week 4, P=0.001;
week 8, P<0.01; Fig. 5C). These
data indicated that DMix protected renal function in rats with
DN.
Effect of DMix on pathological renal
morphology of rats with DN
H&E, PAS and Masson staining revealed that the
kidney structure of the control rats was clear and complete, the
size and shape of the glomeruli were normal, the number of matrix
and mesangial cells was not increased and the renal tubular lumen
was not obvious. Moreover, the epithelial cells were intact, there
was no glycogen deposition, no signs of fibrous tissue hyperplasia
were observed and the basement membrane was not thickened (Fig. 6). In the DN group, the glomerular
volume was higher, the number of mesangial cells and extracellular
matrix deposition were increased, the mesangial area was wider and
there were signs of vacuolar degeneration of the renal tubular
epithelial cells. In addition, the number of renal interstitial
cells was increased, the amount of red-stained glycogen deposits
was increased and there was obvious collagen fiber accumulation
(Fig. 6). Compared with the DN
group, both the DMix and gliquidone groups had significantly
improved observable morphology, as the proliferation of glomerular
mesangial cells was significantly reduced, the deposition of
extracellular matrix was decreased and the basement membrane was
thinner than that of the DN group. Furthermore, the structure of
renal tubules was essentially restored to normal, a small amount of
glycogen was observed and the deposition of collagen fibers was
decreased (Fig. 6).
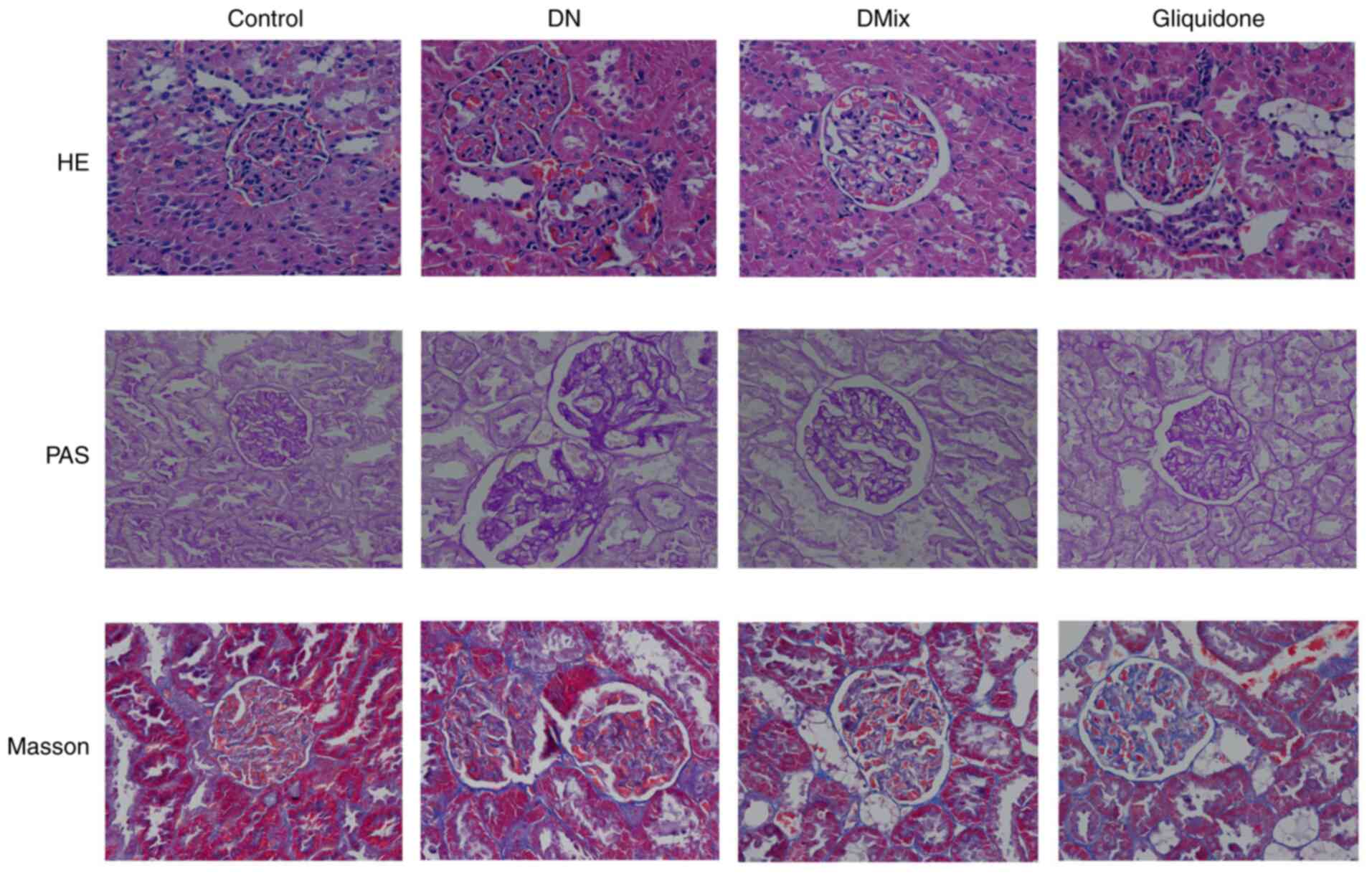 | Figure 6.Photomicrographs of H&E, PAS and
Masson staining of rat kidneys from each group, as observed under a
light microscope (magnification, ×400). The kidney specimen of the
DN group showed markedly severe destruction in glomerular and
tubulointerstitial lesions, such as glomerular hypertrophy,
increased mesangial cell number and extracellular matrix
deposition, interstitial cell infiltration and accumulation of
glycogen and collagen fibres. After treatment with DMix, the
overall morphology of glomerular and tubulointerstitial lesions
improved significantly. DMix, Dendrobium mixture; DN,
diabetic nephropathy; PAS, Periodic Acid-Schiff. |
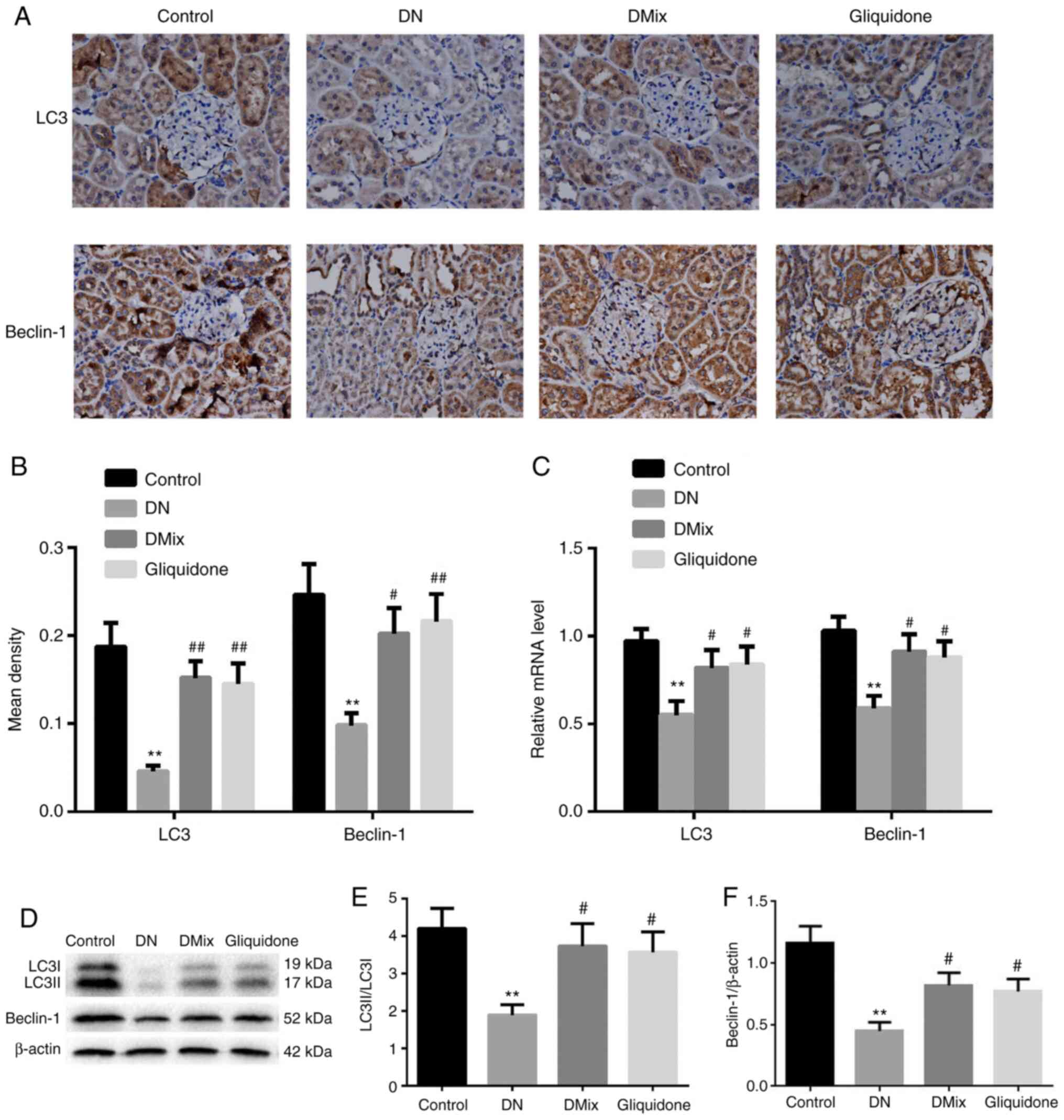
DMix increases the protein and mRNA
expression levels of LC3 and Beclin-1 in the kidneys of rats with
DN
Immunohistochemical staining and western blot
analysis demonstrated that the protein expression levels of LC3 and
Beclin-1, and the ratio of LC3II to LC3I in rat kidney tissues of
the DN group were significantly lower compared with those in the
control group (immunohistochemical: LC3, P<0.01; Beclin-1,
P=0.002; western blotting: LC3II/LC3I, P=0.004; Beclin-1,
P<0.01; Fig. 7A, B, D-F).
However, the protein expression levels of LC3 and Beclin-1 and the
ratio of LC3II to LC3I were significantly increased in the DMix and
gliquidone groups (immunohistochemical, DMix: LC3, P=0.001;
Beclin-1, P=0.019; immunohistochemical, gliquidone: LC3, P=0.002;
Beclin-1, P=0.008; western blotting, DMix: LC3II/LC3I, P=0.018;
Beclin-1, P=0.023; western blotting, gliquidone: LC3II/LC3I,
P=0.029; Beclin-1, P=0.049; Fig. 7A, B,
D-F). Additionally, the mRNA expression levels of LC3 and
Beclin-1 in rat kidney tissues of the DN group were significantly
lower (LC3, P=0.003; Beclin-1, P=0.002; Fig. 7C). DMix upregulated the mRNA
expression levels of LC3 and Beclin-1 in rats with DN (LC3,
P=0.048; Beclin-1, P=0.017; Fig.
7C). These results indicated that DMix may promote the
expression levels of autophagy-related proteins in the kidney
tissues of rats with DN to protect renal function.
DMix inhibits the PI3K/Akt/mTOR signal
transduction pathway in the renal tissues of rats with DN
It is well known that the PI3K/Akt/mTOR signaling
pathway is one of the main pathways regulating autophagy (27). In order to understand the molecular
mechanism underlying the effect of DMix on renal autophagy in DN,
the effect of DMix on the PI3K/Akt/mTOR signaling pathway was
examined (Fig. 8). The results
demonstrated that the protein expression levels of p-PI3K, p-Akt
and p-mTOR in the DN group were significantly higher compared with
those in the control group (p-PI3K, P<0.01; p-Akt, P<0.01;
p-mTOR, P<0.01), indicating that the PI3K/Akt/mTOR signaling
pathway was more active in the kidney tissues of rats with DN.
After 8 weeks of DMix treatment, the protein expression levels of
p-PI3K, p-Akt and p-mTOR were significantly decreased (p-PI3K,
P=0.036; p-Akt, P=0.014; p-mTOR, P=0.022), but there were no
significant changes in the expression levels of PI3K, Akt and mTOR
proteins. Furthermore, there was no statistically significant
difference between the DMix and gliquidone groups (p-PI3K, P=0.716;
p-Akt, P=0.531; p-mTOR, P=0.522). This indicated that DMix
inhibited the PI3K/Akt/mTOR signaling pathway in the kidney tissues
of rats with DN.
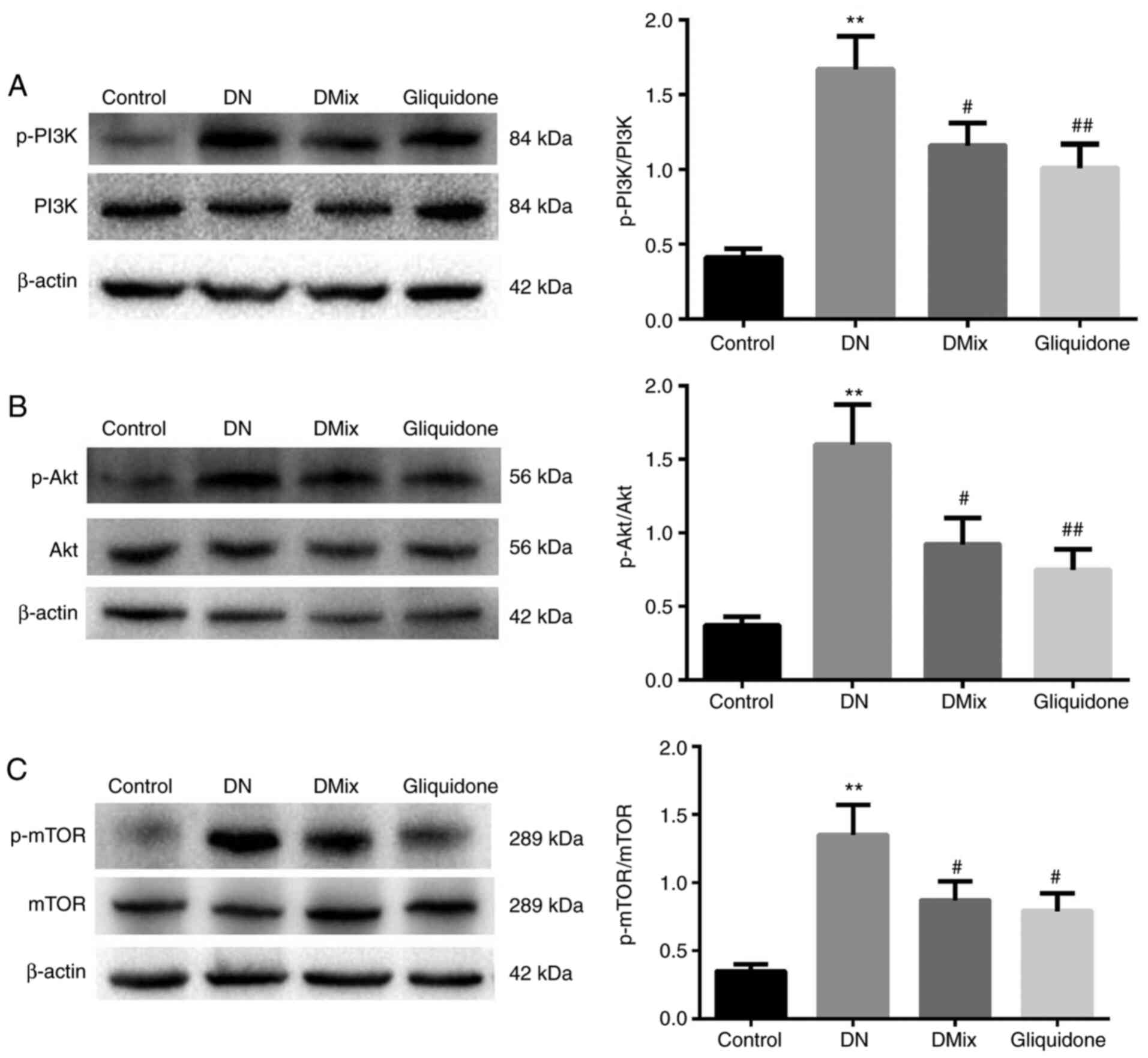 | Figure 8.DMix inhibits the PI3K/Akt/mTOR
signal transduction pathway in the renal tissues of rats with DN.
The protein expression levels of (A) PI3K, p-PI3K, (B) Akt, p-Akt,
(C) mTOR and p-mTOR were detected using western blotting. β-actin,
PI3K, Akt and mTOR were used as the internal control. The relative
ratios of (A) p-PI3K/PI3K, (B) p-Akt/Akt and (C) p-mTOR/mTOR were
used to analyze the gray value. **P<0.01 vs. control;
#P<0.05, ##P<0.01 vs. DN. DMix,
Dendrobium mixture; DN, diabetic nephropathy; p-,
phosphorylated. |
Discussion
In the present study, it was identified that DMix
inhibited the PI3K/Akt/mTOR signaling pathway, which promoted renal
autophagy and improved renal function. Moreover, DMix inhibited the
phosphorylation of PI3K, Akt and mTOR, increased the expression
levels of LC3 and Beclin-1, and alleviated renal damage based on
the morphological analysis of the kidneys, thereby delaying the
progression of DN. The basic pathological changes in DN are
glomerular mesangial cell proliferation, increase in the
extracellular matrix contents, glomerular basement membrane
thickening and glomerular sclerosis (28–30).
Its pathogenesis is complex, and there are a lack of effective
treatments for this condition.
Recently, Traditional Chinese Medicine (TCM) has
achieved good efficacy in the treatment of DN. Numerous TCMs have
been used in clinical practice and have shown promising results in
the treatment of diabetes and its complications (31,32).
Unlike a single compound that has only one active chemical
component, TCM is usually a complex combination of chemicals that
work synergistically. Moreover, any active ingredient of TCM alone
cannot reflect the final therapeutic effect of the whole medicine
(33,34). In preliminary experimental studies
and clinical practice, DMix has been proved to have a good
therapeutic effect against diabetes and its complications (21,22).
The present study used a high-sugar and high-fat
feed combined classic model of STZ-induced DN to successfully
reproduce the typical pathological changes of DN in rats. The
biochemical indexes of these rats were abnormal to varying degrees,
and these were significantly improved after DMix treatment, which
was consistent with the findings of our previous studies (21,22).
Furthermore, the current study demonstrated the therapeutic effects
of DMix in protecting renal function and preventing renal fibrosis.
Gliquidone is a sulfonylurea-based hypoglycemic agent that can bind
to the specific receptors on the pancreatic β cell membrane to
promote insulin secretion; it has a certain protective effect on
the kidney (35). Gliquidone is a
commonly used drug in the clinical treatment of DN and was used as
a positive control in the present study. The current results
demonstrated that the clinical equivalent dose of DMix had effects
similar to those of gliquidone.
Autophagy is considered an important potential
mechanism underlying the development of DN; thus, activation of
autophagy may be a potential therapeutic target for the treatment
of DN (36–38). Our previous studies confirmed that
Beclin-1 and LC3 levels in the kidneys of rats with DN were
downregulated, suggesting that autophagy was a self-repairing
mechanism of the body to fight against the damage caused by stress
(39,40). The results of the present study also
indicated that the autophagy activity in the renal tissue of DN was
reduced, and that DMix enhanced autophagy and improved renal
function.
Previous studies have reported that in DN, the renal
PI3K/Akt/mTOR signaling pathway was abnormally activated and
autophagy was inhibited, leading to kidney injury, glomerular
hypertrophy and accelerated renal fibrosis (41–43).
p-PI3K, p-Akt and p-mTOR are key participants in the PI3K/Akt/mTOR
signaling pathway that activate this signaling pathway (44,45).
In the current study, the intervention of DMix significantly
inhibited phosphorylation of PI3K, Akt and mTOR. These results
suggest that DMix may restore renal autophagy activity and serve a
protective role in DN kidney tissue by inhibiting the PI3K/Akt/mTOR
signaling pathway.
Although the results support the current hypothesis,
this study has some limitations. For example, the duration of drug
therapy was relatively short; the present findings are similar to
studies of multiple other drug mixtures (46,47);
and other mechanisms that have not been investigated here cannot be
excluded. Therefore, further studies in larger cohorts over a
longer duration, as well as clinical trials, are required to verify
the present findings.
In conclusion, the present study demonstrated that
DMix has a protective effect on the kidneys of rats with DN, which
may be due to the negative regulation of the PI3K/Akt/mTOR
signaling pathway, promotion of renal autophagy and inhibition of
renal fibrosis, thereby delaying the progression of DN. Therefore,
DMix could be used as a potentially beneficial TCM for the
prevention and treatment of DN.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Fujian
University of Traditional Chinese Medicine's research platform
management project (grant. no. X2019001-platform), the Natural
Science Foundation of Fujian Province (grant. no. 2018J01873) and
the National Natural Science Foundation of China (grant. no.
81973827).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YC, YFZ, XHL, JPZ, FL and HS participated in the
study design. YC, YFZ and XHL performed the experiments. YC, YFZ,
XHL, JPZ, FL and HS performed the data analysis. YC and HS confirm
the authenticity of all the raw data. YC drafted the manuscript.
All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was carried out in strict
accordance with the requirements in the Guide for the Care and Use
of Laboratory Animals of the National Institutes of Health. The
experiments were carried out strictly in line with the requirements
of the International Association for the Study of Pain. The present
study was approved by the Medical Ethics Committee of Fujian
University of Traditional Chinese Medicine (Fujian, China;
approval. no. 2019-031).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
DMix
|
Dendrobium mixture
|
|
DN
|
diabetic nephropathy
|
|
STZ
|
streptozocin
|
|
FBG
|
fasting blood glucose
|
|
UAER
|
urinary albumin excretion rate
|
|
BUN
|
blood urea nitrogen
|
|
Scr
|
serum creatinine
|
|
HbA1c
|
hemoglobin A1c
|
References
|
1
|
Li X, Wang L and Ma H: Betaine alleviates
high glucose-induced mesangial cell proliferation by inhibiting
cell proliferation and extracellular matrix deposition via the
AKT/ERK1/2/p38 MAPK pathway. Mol Med Rep. 20:1754–1760.
2019.PubMed/NCBI
|
|
2
|
Hsieh AR, Huang YC, Yang YF, Lin HJ, Lin
JM, Chang YW, Wu CM, Liao WL and Tsai FJ: Lack of association of
genetic variants for diabetic retinopathy in Taiwanese patients
with diabetic nephropathy. BMJ Open Diabetes Res Care.
8:e0007272020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Perkovic V, Jardine MJ, Neal B, Bompoint
S, Heerspink HJL, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull
S, et al: Canagliflozin and renal outcomes in type 2 diabetes and
nephropathy. N Engl J Med. 380:2295–2306. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang Q, Yang M, Xiao Y, Han Y, Yang S and
Sun L: Towards better drug repositioning: Targeted
immunoinflammatory therapy for diabetic nephropathy. Curr Med Chem.
8:1003–1024. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Oraby MA, El-Yamany MF, Safar MM, Assaf N
and Ghoneim HA: Amelioration of early markers of diabetic
nephropathy by linagliptin in fructose-streptozotocin-induced type
2 diabetic rats. Nephron. 141:273–286. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li X, Zhu Q, Zheng R, Yan J, Wei M, Fan Y,
Deng Y and Zhong Y: Puerarin attenuates diabetic nephropathy by
promoting autophagy in podocytes. Front Physiol. 11:732020.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang C, Chen XC, Li ZH, Wu HL, Jing KP,
Huang XR, Ye L, Wei B, Lan HY and Liu HF: SMAD3 promotes autophagy
dysregulation by triggering lysosome depletion in tubular
epithelial cells in diabetic nephropathy. Autophagy. 10:1–20. 2020.
View Article : Google Scholar
|
|
8
|
Kim JH, Kim KM, Jeong JU, Shin JH, Shin JM
and Bang KT: Nrf2-Heme oxygenase-1 modulates autophagy and inhibits
apoptosis triggered by elevated glucose levels in renal tubule
cells. Kidney Res Clin Pract. 38:318–325. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cao T, Xu R, Xu Y, Liu Y, Qi D and Wan Q:
The protective effect of cordycepin on diabetic nephropathy through
autophagy induction in vivo and in vitro. Int Urol Nephrol.
51:1883–1892. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dai H, Liu Q and Liu B: Research progress
on mechanism of podocyte depletion in diabetic nephropathy. J
Diabetes Res. 2017:26152862017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen Y, Liu Q, Shan Z, Mi W, Zhao Y, Li M,
Wang B, Zheng X and Feng W: Catalpol ameliorates podocyte injury by
stabilizing cytoskeleton and enhancing autophagy in diabetic
nephropathy. Front Pharmacol. 10:14772019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang MZ, Jang H and Nussinov R: The
structural basis for ras activation of PI3Kα lipid kinase. Phys
Chem Chem Phys. 21:12021–12028. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang X, Wang D, Liu B, Jin X, Wang X, Pan
J, Tu W and Shao Y: IMP3 accelerates the progression of prostate
cancer through inhibiting PTEN expression in a SMURF1-dependent
way. J Exp Clin Cancer Res. 39:1902020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Clement E, Inuzuka H, Nihira NT, Wei W and
Toker A: Skp2-dependent reactivation of AKT drives resistance to
PI3K inhibitors. Sci Signal. 11:eaao38102018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen XB, Sun Y, Wang BR and Wang HH:
Prognostic significance of autophagy-related genes beclin1 and LC3
in ovarian cancer: A meta-analysis. J Int Med Res.
48:3000605209682992020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yu H, Shao J, Huang R, Guan Y, Li G, Chen
S, Zhou F, Yao Q and Shen J: Targeting PTEN to regulate autophagy
and promote the repair of injured neurons. Brain Res Bull.
165:161–168. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jung G, Roh J, Lee H, Gil M, Yoon DH, Suh
C, Jang S, Park CJ, Huh J and Park CS: Autophagic markers beclin1
and LC3 are associated with prognosis of multiple myeloma. Acta
Haematol. 134:17–24. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ebrahim N, Ahmed IA, Hussien NI, Dessouky
AA, Farid AS, Elshazly AM, Mostafa O, Gazzar WBE, Sorour SM, Seleem
Y, et al: Mesenchymal stem cell-derived exosomes ameliorated
diabetic nephropathy by autophagy induction through the mTOR
signaling pathway. Cells. 7:2262018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fu L, Wu W, Sun X and Zhang P:
Glucocorticoids enhanced osteoclast autophagy through the
PI3K/Akt/mTOR signaling pathway. Calcif Tissue Int. 107:60–71.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vurusaner B, Gargiulo S, Testa G, Gamba P,
Leonarduzzi G, Poli G and Basaga H: The role of autophagy in
survival response induced by 27-hydroxycholesterol in human
promonocytic cells. Redox Biol. 17:400–410. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu Q, Liu Y, Cong YB, Zheng YY, Zhang JP
and Shi H: Gene expression and microarray investigation of
dendrobium mixture as progressive therapy for the treatment of type
2 diabetes mellitus. Trop J Pharmaceutical Res. 12:195–201.
2013.
|
|
22
|
Lin X, Shi H, Cui Y, Wang X, Zhang J, Yu W
and Wei M: Dendrobium mixture regulates hepatic gluconeogenesis in
diabetic rats via the phosphoinositide-3-kinase/protein kinase B
signaling pathway. Exp Ther Med. 16:204–212. 2018.PubMed/NCBI
|
|
23
|
Nitulescu GM, Margina D, Juzenas P, Peng
Q, Olaru OT, Saloustros E, Fenga C, Spandidos DA, Libra M and
Tsatsakis AM: Akt inhibitors in cancer treatment: The long journey
from drug discovery to clinical use. Int J Oncol. 48:869–885. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nitulescu GM, Van De Venter M, Nitulescu
G, Ungurianu A, Juzenas P, Peng Q, Olaru OT, Grădinaru D, Tsatsakis
A, Tsoukalas D, et al: The Akt pathway in oncology therapy and
beyond. Int J Oncol. 53:2319–2331. 2018.PubMed/NCBI
|
|
25
|
Brown MJ, Symonowicz C, Medina LV,
Bratcher NA, Buckmaster CA, Klein H and Anderson LC: Culture of
care: Organizational responsibilities. Management of Animal Care
and Use Programs in Research, Education, and Testing. 2nd edition.
Boca Raton (FL): CRC Press/Taylor & Francis; 2018, PubMed/NCBI
|
|
26
|
Tesch GH and Allen TJ: Rodent models of
streptozotocin-induced diabetic nephropathy. Nephrology (Carlton).
12:261–266. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu K, Yang Y, Zhou F, Xiao Y and Shi L:
Inhibition of PI3K/AKT/mTOR signaling pathway promotes autophagy
and relieves hyperalgesia in diabetic rats. Neuroreport.
31:644–649. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zeng LF, Xiao Y and Sun L: A glimpse of
the mechanisms related to renal fibrosis in diabetic nephropathy.
Adv Exp Med Biol. 1165:49–79. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Qi C, Mao X, Zhang Z and Wu H:
Classification and differential diagnosis of diabetic nephropathy.
J Diabetes Res. 2017:86371382017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dong Z, Sun Y, Wei G, Li S and Zhao Z:
Ergosterol ameliorates diabetic nephropathy by attenuating
mesangial cell proliferation and extracellular matrix deposition
via the TGF-β1/smad2 signaling pathway. Nutrients. 11:4832019.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Park CH, Hiratani K, Natazuka T and
Yokozawa T: Therapeutic effect of Chinese prescription Kangen-Karyu
in patients with diabetic nephropathy. Drug Discov Ther. 14:84–88.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang J, Ma Q, Li Y, Li P, Wang M, Wang T,
Wang C, Wang T and Zhao B: Research progress on Traditional Chinese
Medicine syndromes of diabetes mellitus. Biomed Pharmacother.
121:1095652020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cheng T, Ye J, Li H, Dong H, Xie N, Mi N,
Zhang Z, Zou J, Jin H and Zhang W: Hybrid multidimensional data
acquisition and data processing strategy for comprehensive
characterization of known, unknown and isomeric compounds from the
compound dan zhi tablet by UPLC-TWIMS-QTOFMS. Rsc Advances.
9:8714–8727. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shen H, Qu Z, Harata-Lee Y, Aung TN, Cui
J, Wang W, Kortschak RD and Adelson DL: Understanding the
mechanistic contribution of herbal extracts in compound kushen
injection with transcriptome analysis. Front Oncol. 9:6322019.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tian HY, Yang JB, Xie ZC and Liu JL:
Gliquidone alleviates diabetic nephropathy by inhibiting
notch/snail signaling pathway. Cell Physiol Biochem. 51:2085–2097.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kim H, Dusabimana T, Kim SR, Je J, Jeong
K, Kang MC, Cho KM, Kim HJ and Park SW: Supplementation of
abelmoschus manihot ameliorates diabetic nephropathy and hepatic
steatosis by activating autophagy in mice. Nutrients. 10:17032018.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu WJ, Huang WF, Ye L, Chen RH, Yang C,
Wu HL, Pan QJ and Liu HF: The activity and role of autophagy in the
pathogenesis of diabetic nephropathy. Eur Rev Med Pharmacol Sci.
22:3182–3189. 2018.PubMed/NCBI
|
|
38
|
Tu Q, Li Y, Jin J, Jiang X, Ren Y and He
Q: Curcumin alleviates diabetic nephropathy via inhibiting podocyte
mesenchymal transdifferentiation and inducing autophagy in rats and
MPC5 cells. Pharm Biol. 57:778–786. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jiang Y, Zhao Y, Zhu X, Liu Y, Wu B, Guo
Y, Liu B and Zhang X: Effects of autophagy on macrophage adhesion
and migration in diabetic nephropathy. Ren Fail. 41:682–690. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wu W, Zhang M, Liu Q, Xue L, Li Y and Ou
S: Piwil 2 gene transfection changes the autophagy status in a rat
model of diabetic nephropathy. Int J Clin Exp Pathol.
8:10734–10742. 2015.PubMed/NCBI
|
|
41
|
Ribback S, Cigliano A, Kroeger N, Pilo MG,
Terracciano L, Burchardt M, Bannasch P, Calvisi DF and Dombrowski
F: PI3K/AKT/mTOR pathway plays a major pathogenetic role in
glycogen accumulation and tumor development in renal distal tubules
of rats and men. Oncotarget. 6:13036–13048. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Huang C, Lin MZ, Cheng D, Braet F, Pollock
CA and Chen XM: KCa3.1 mediates dysfunction of tubular autophagy in
diabetic kidneys via PI3k/Akt/mTOR signaling pathways. Sci Rep.
6:238842016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yang F, Qu Q, Zhao C, Liu X, Yang P, Li Z,
Han L and Shi X: Paecilomyces cicadae-fermented Radix astragali
activates podocyte autophagy by attenuating PI3K/AKT/mTOR pathways
to protect against diabetic nephropathy in mice. Biomed
Pharmacother. 129:1104792020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhang Q, Wang X, Cao S, Sun Y, He X, Jiang
B, Yu Y, Duan J, Qiu F and Kang N: Berberine represses human
gastric cancer cell growth in vitro and in vivo by inducing
cytostatic autophagy via inhibition of MAPK/mTOR/p70S6K and akt
signaling pathways. Biomed Pharmacother. 128:1102452020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Li D, Lu Z, Xu Z, Ji J, Zheng Z, Lin S and
Yan T: Spironolactone promotes autophagy via inhibiting
PI3K/AKT/mTOR signalling pathway and reduce adhesive capacity
damage in podocytes under mechanical stress. Biosci Rep.
36:e003552016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yang P, Tian YM, Deng WX, Cai X, Liu WH,
Li L and Huang HY: Sijunzi decoction may decrease apoptosis via
stabilization of the extracellular matrix following cerebral
ischaemia-reperfusion in rats. Exp Ther Med. 18:2805–2812.
2019.PubMed/NCBI
|
|
47
|
Lee D, Lee SH, Lee M, Lee SH, Shin YJ, Lee
JY, Kim H, Kim YS and Song J: Effects of Siwu decoction on
chondrocyte proliferation of growth plate in adolescent rats. J
Ethnopharmacol. 236:108–113. 2019. View Article : Google Scholar : PubMed/NCBI
|