Introduction
Transient global cerebral ischemia refers to a
pathological deprivation of oxygen and glucose in the brain within
a short time period, which can be caused by cardiac arrest,
cardiopulmonary bypass surgery and other situations (1). During the process of cerebral
ischemia, the hippocampal neurons are vulnerable to injury and
delayed neuronal death could be triggered when the cerebral blood
supply is recovered (2,3). Numerous factors, such as calcium
overload, oxidative stress and activation of apoptotic pathways,
have been proposed to lead to ischemia/reperfusion-induced neuronal
death (1–3). Furthermore, accumulating evidence has
shown that autophagy may serve a crucial role in regulation of
ischemia/reperfusion-induced neuronal death (4–7).
Autophagy degrades long-lived proteins or damaged
organelles through the lysosomal pathway, which is different from
the ubiquitin-proteasome system, which clears intracellular
short-lived proteins (3). As a
determinant of cell destiny, autophagy serves dual roles in the
regulation of cell function; autophagy protects cells against the
damage induced by interior or exterior stresses, whereas
over-activated autophagy can lead to excessive auto-digestion of
cellular constituents and the initiation of autophagic cell death
(programmed cell death) (4). It has
been reported that induction of autophagy may prevent epilepsy- or
neurodegenerative disease-induced brain damage (4); however, accumulating evidence has
demonstrated that autophagy may also contribute to
hypoxic-ischemia- or head trauma-induced brain injury (5,6).
Previous reports have suggested that suppression of autophagy could
inhibit transient ischemia-induced brain injury, whereas other
studies have revealed that induction of autophagy may alleviate
transient ischemia-induced brain damage (7,8).
Therefore, the role of autophagy in regulation of transient
ischemia-induced brain damage remains controversial.
Lycopene is a lipid-soluble carotenoid compound with
potent anti-oxidative capacity (9).
Previous reports have demonstrated that transient ischemia-induced
organ damage, such as heart, kidney, testis and liver damage, could
be prevented by lycopene treatment (10–13).
Notably, lycopene has been reported to act as an effective
neuroprotectant, given that it not only prevents brain damage
induced by transient focal ischemia or global ischemia (14–16),
but also alleviates neuronal injury induced by various neurotoxic
compounds, including colchicine, methylmercury, rotenone, amyloid
β, trimethyltin and 6-hydroxydopamine (17–22).
In addition, it has been shown that lycopene may protect against
cell damage through numerous pathways, such as inhibiting oxidative
stress, suppressing inflammation and regulating iron metabolism
(14,17,18,23).
However, the role of lycopene in autophagy regulation remains
elusive. Chen et al (24)
reported that lycopene protected H9C2 cardiomyocytes against
hypoxia and reoxygenation through activation of autophagy. By
contrast, Zeng et al (25)
demonstrated that lycopene prevented hyperglycemia-induced damage
in endothelial progenitor cells via inhibition of autophagy.
Insufficient cerebral blood supply-induced oxygen-glucose
deprivation (OGD) is a known important pathological basis that can
induce neuronal death, and a common feature of focal ischemia and
global ischemia (26). The human
neuroblastoma SH-SY5Y cells used in the present study have similar
characteristics to neurons, including morphology, neurochemistry
and electrophysiology (26). The
present study used an SH-SY5Y cell model of OGD to simulate
cerebral ischemia, and investigated the role of autophagy in the
protective effects of lycopene against neuronal damage and its
underlying mechanism.
Materials and methods
Reagents and antibodies
Primary antibodies against AMPK (cat. no. ab32047),
phosphorylated (p)-AMPK (cat. no. ab92701), LC3B (cat. no.
ab192890), autophagy protein 5 (ATG5; cat. no. ab108327), p62 (cat.
no. ab109012), mTOR (cat. no. ab2732), p-mTOR (cat. no. ab109268),
xCT (cat. no. ab175186) and β-actin (cat. no. ab8226) were
purchased from Abcam. 3-Methyladenine (3MA), bafilomycin A1 and
lycopene were all purchased from Sigma-Aldrich; Merck KGaA.
Lycopene was dissolved in tetrahydrofuran (THF) before each
experiment. Other reagents were all purchased from Sigma-Aldrich;
Merck KGaA, unless otherwise specified.
Cell culture and OGD
The human neuroblastoma SH-SY5Y cell line was
obtained from The Cell Bank of Type Culture Collection of The
Chinese Academy of Sciences and was verified using short tandem
repeat analysis. Cells were cultured at 37°C in an atmosphere
containing 5% CO2 in DMEM (Sigma-Aldrich; Merck KGaA)
containing 10% fetal bovine serum (Gibco; Thermo Fisher Scientific,
Inc.), 2 mmol/l glutamine (Gibco; Thermo Fisher Scientific, Inc.),
100 U/ml penicillin and 100 µg/ml streptomycin. The medium was
replaced twice a week. OGD was induced according to a previously
published protocol (3). The cells
underwent OGD for 3, 6, 12 and 24 h, or were pretreated for 1 h at
37°C with target chemicals 3MA (5 mmol/l), bafilomycin A1 (1.5
µmol/l) and GSH (10 mmol/l; Sigma-Aldrich; Merck KGaA) and lycopene
(0.5, 2.0 and 8.0 µmol/l), and then underwent OGD at 37°C for 24 h.
Moreover, the cells were treated with scrambled small interfering
RNA (siRNA), ATG5 siRNA or AMPK siRNA prior to undergoing OGD at
37°C for 24 h.
Lactate dehydrogenase (LDH) release
cell death assay
Elevated LDH was used to detect cell death caused by
cellular membrane damage using a detection kit (Beyotime Institute
of Biotechnology). Control cells were treated with the lycopene
solvent THF (0.1%), and the other groups of cells were treated with
lycopene at 0.5, 2.0 and 8 µmol/l prior to OGD at 37°C for 24 h.
LDH release was detected according to the manufacture's protocol.
The absorbance value of each sample was read at 490 nm, as
specified by the manufacturer's protocol. The death ratio was
calculated using the following formula: Cell death ratio (%) = (A
sample-A control/A max-A control) ×100. A sample refers to the
sample absorbance value; A control refers to the absorbance value
of the control group; A max refers to the absorbance value of the
positive group (which consisted of cells treated with Triton
X-100).
Measurement of total intracellular
GSH
The DTNB-GSSH reductase recycling assay kit
(Beyotime Institute of Biotechnology) was used to detect total
intracellular GSH according to the manufacturer's instructions.
Briefly, the collected cells (1×107) were treated with
protein-removing buffer S, homogenized on ice with a homogenizer
and centrifuged at 10,000 × g for 10 min at 4°C, in order to obtain
the supernatant used to assess intracellular total GSH. GSH content
was detected at an absorbance value of 412 nm and was expressed as
a ratio to the absorbance value of the control cells. GSH was
purchased from Sigma-Aldrich; Merck KGaA.
Measurement of total intracellular
cysteine
A cysteine assay kit (Nanjing Jiancheng
Bioengineering Institute) was used to detect intracellular
cysteine. The experiment was performed according to the
manufacturer's instructions. Briefly, the collected cells
(1×107) were treated with reagent A, homogenized on ice
and centrifuged at 8,000 × g for 4 min at 4°C, in order to obtain
the supernatant used to assess cysteine levels. After the protein
concentration of the supernatant was measured using the Pierce BCA
protein assay kit (Pierce; Thermo Fisher Scientific, Inc.), a 20-µl
sample was incubated with 100 µl reagent B and 100 µl reagent C for
15 min at room temperature and read at an absorbance of 600 nm
using a microplate reader. Finally, the results were expressed as a
ratio to the absorbance value of the control cells.
Measurement of intracellular reactive
oxygen species (ROS)
Intracellular ROS levels were detected using the ROS
probe DCFH-DA (Beyotime Institute of Biotechnology). Firstly,
SH-SY5Y cells (1×106) were seeded onto 96-well plates
and after 24 h, OGD was performed at 3, 6, 12 and 24 h. Secondly,
the cells were washed in PBS twice and then stained with 20 µmol/l
DCFH-DA for 30 min in the dark at 37°C. Thirdly, these cells were
dissolved with 1% Triton X-100, followed by fluorescence detection
using a spectrometer (HTS 7000; Perkin Elmer, Inc.). The excitation
wavelength was 485 nm and the emission wavelength was 530 nm.
Furthermore, SH-SY5Y cells (3×106) seeded onto a 3-cm
dish were observed under a fluorescence microscope (Olympus IX71;
Olympus Corporation). This group of cells were also treated with
OGD and stained with 20 µmol/l DCFH-DA as aforementioned.
Measurement of intracellular
malondialdehyde (MDA)
An MDA assay kit (Nanjing Jiancheng Bioengineering
Institute) was used to detect intracellular MDA levels. The
experiment was performed according to the manufacturer's
instructions. Briefly, the collected cells (1×107) were
added to RIPA lysis buffer (cat. no. P0013C; Beyotime Institute of
Biotechnology), homogenized on ice and centrifuged at 1,600 × g for
10 min at 4°C to obtain the supernatant. Once the protein
concentration of the supernatant was measured using the Pierce BCA
protein assay kit, a 100-µl sample was incubated with 100 µl test
solution for 15 min at 100°C. Once the samples were cooled to room
temperature, they were centrifuged at 1,000 × g for 10 min at 37°C
to obtain the supernatant, the absorbance of which was measured at
530 nm using a microplate reader. MDA content was expressed as a
ratio to the absorbance value of the control cells.
Transfection of siRNA and GFP-LC3
lentiviral vectors
SH-SY5Y cells (2×105) were seeded onto
10-cm dishes. The siRNAs were transfected into the cells using
Lipofectamine® 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions
(Opti-MEM:Lipofecta-mine 3000:siRNA, 100:1:5; concentration of
siRNA transfected, 20 ng/µl). Cells were then treated with OGD
after overnight siRNA transfection. The siRNAs used in the present
study were purchased from Shanghai GenePharma Co., Ltd. and are
listed as follows: ATG5 siRNA, 5-3):GACGUUGGUAACUGACAAATT; AMPK
siRNA, 5-3):GCGUGUACGAAGGAAGAAUTT; scrambled siRNA,
5-3):UUCUCCGAACGUGUCACGUTT-3.
For GFP-LC3 lentiviral vector transfection, SH-SY5Y
cells (1×106) were seeded onto 6-well plates and
cultured for 18 h, followed by infection with GFP-LC3 lentiviral
vectors (Shanghai GeneChem Co., Ltd.) for 12 h (MOI, 2.5). After
being incubated in normal culture medium for 48 h and undergoing
OGD, the cells were observed by confocal microscopy (FV10i; Olympus
Corporation).
Gel electrophoresis and western
blotting
SH-SY5Y cells were lysed with ice-cold cellular
membrane lysis buffer (Beyotime Institute of Biotechnology) and
homogenized with a glass Pyrex microhomogenizer (20 strokes). The
protein content of the supernatant was obtained after the
homogenates were centrifuged at 1,000 × g for 10 min at 4°C;
protein concentration was detected using the Bio-Rad Protein Assay
kit (Bio-Rad Laboratories, Inc.).
Equal amounts of protein (20 µg) were separated by
SDS-PAGE on 7% gels and transferred to PVDF membranes. After
blocking with 3% bovine serum albumin (Gibco; Thermo Fisher
Scientific, Inc.) for 1 h at room temperature, PVDF membranes were
immunoblotted with primary antibodies against AMPK (1:1,000),
p-AMPK (1:1,000), LC3 (1:1,000), ATG5 (1:1,500), p62 (1:1,000), xCT
(1:1,000), mTOR (1:1,000) and p-mTOR (1:1,000) overnight at 4°C.
β-actin (1:1,000) was used as a loading control. The membranes were
then washed three times in PBS containing 0.1% Tween-20 and
incubated with horseradish peroxidase-conjugated secondary
antibodies: Goat anti-rabbit IgG (cat. no. ab6721) and horse
anti-mouse IgG (cat. no. ab6789) (1:2,000 dilution; Abcam) at room
temperature for 2 h. Finally, blots were washed three times. The
immunoreactive proteins were visualized using an ECL kit (cat. no.
P0018F; Beyotime Institute of Biotechnology) and analyzed using
ImageJ software (version 1.8.0; National Institutes of Health).
Light microscopy
The SH-SY5Y cells (1×107/well) were
seeded on a 6-well plate and underwent OGD at 37°C for 24 h in the
presence or absence of lycopene (8 µmol/l). Subsequently,
morphological changes of the cells were observed under a light
microscope (Olympus BX51; Olympus Corporation). Representative
images were obtained by a researcher who was blinded to the cell
groups.
Statistical analysis
SPSS statistical software (version 19.0; IBM Corp.)
was utilized for data analysis. All data shown in the present study
represent at least four independent experiments and are presented
as the mean ± SEM. Data were statistically analyzed using one-way
or two-way ANOVA, followed by LSD post hoc test if there were less
than four groups assessed or Tukey's post hoc method if there were
more than three groups assessed. P<0.05 was considered to
indicate a statistically significant difference.
Results
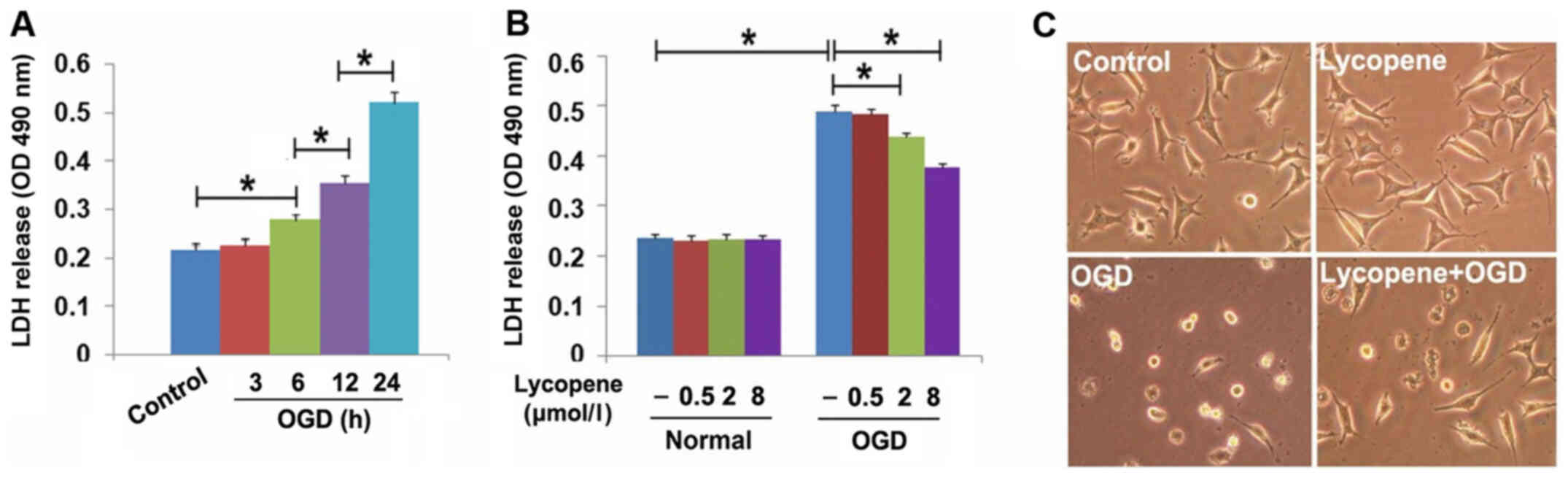
Lycopene inhibits OGD-induced SH-SY5Y
cell death
Since the LDH release assay is a common method used
to evaluate cell death, the present study used this method to
detect the effects of lycopene on OGD-induced SH-SY5Y cell death.
As shown in Fig. 1A, OGD
significantly induced SH-SY5Y cell death at 6 h, which was markedly
increased when OGD treatment time was extended to 12 and 24 h, thus
indicating that OGD induced SH-SY5Y cell death in a time-dependent
manner. Subsequently, the cells were treated with 0.5, 2.0 and 8.0
µmol/l lycopene for 1 h, and then underwent OGD for 24 h. LDH
release assay revealed that OGD-induced SH-SY5Y cell death was
prevented by 2 µmol/l lycopene, and this prevention was more
apparent when the dosage of lycopene was increased to 8 µmol/l
(Fig. 1B). Moreover, light
microscopy revealed that most of the cells that underwent OGD
became shrunken and exhibited a round shape compared with the cells
in the control group, which exhibited a polygonal shape. By
contrast, pretreatment with 8 µmol/l lycopene inhibited OGD-induced
morphological changes in SH-SY5Y cells (Fig. 1C). These results indicated that
lycopene may protect against the lethal effects of OGD on SH-SY5Y
cells.
Lycopene inhibits OGD-induced
autophagic death of SH-SY5Y cells
Induction of lethal autophagy has been reported to
be a pathway accounting for OGD-triggered death in SH-SY5Y cells
(8). To elucidate the mechanism
underlying the protective effect of lycopene on OGD-induced SH-SY5Y
cell death, the present study examined the effect of lycopene on
OGD-induced autophagy.
Firstly, OGD-induced changes in the expression
levels of autophagy marker proteins were detected through western
blotting. OGD upregulated the protein expression levels of ATG5 and
LC3II (autophagy marker proteins), and downregulated the expression
levels of p62 (substrate of autophagy) in a time-dependent manner
(Fig. 2A). To determine whether OGD
induced formation of autophagosomes, the GFP-LC3 lentiviral vector
was transfected into SH-SY5Y cells, which then did or did not
undergo OGD. As revealed by laser scanning confocal microscopy,
numerous green puncta were induced to form in the cytoplasm by OGD
when compared with the control cells (Fig. 2B). Subsequently, the cells were
treated with 3MA and bafilomycin A1 prior to OGD treatment. As an
inhibitor of activated PI3K vps34, 3MA effectively prevents
autophagy initiation (4).
Bafilomycin A1 inactivates autophagic flux by preventing the fusion
of autophagosomes with lysosomes (4). The results of the LDH release assay
revealed that pretreatment with either 3MA (5 mmol/l) or
bafilomycin A1 (1.5 µmol/l) for 1 h significantly prevented
OGD-induced SH-SY5Y cell death (Fig.
2C). Moreover, laser scanning confocal microscopy demonstrated
that OGD-induced formation of green puncta was decreased by
pretreatment with 3MA, but increased in the presence of bafilomycin
A1 (Fig. 2B). These findings
indicated that OGD may induce activation of autophagic flux, which
could contribute to OGD-induced SH-SY5Y cell death.
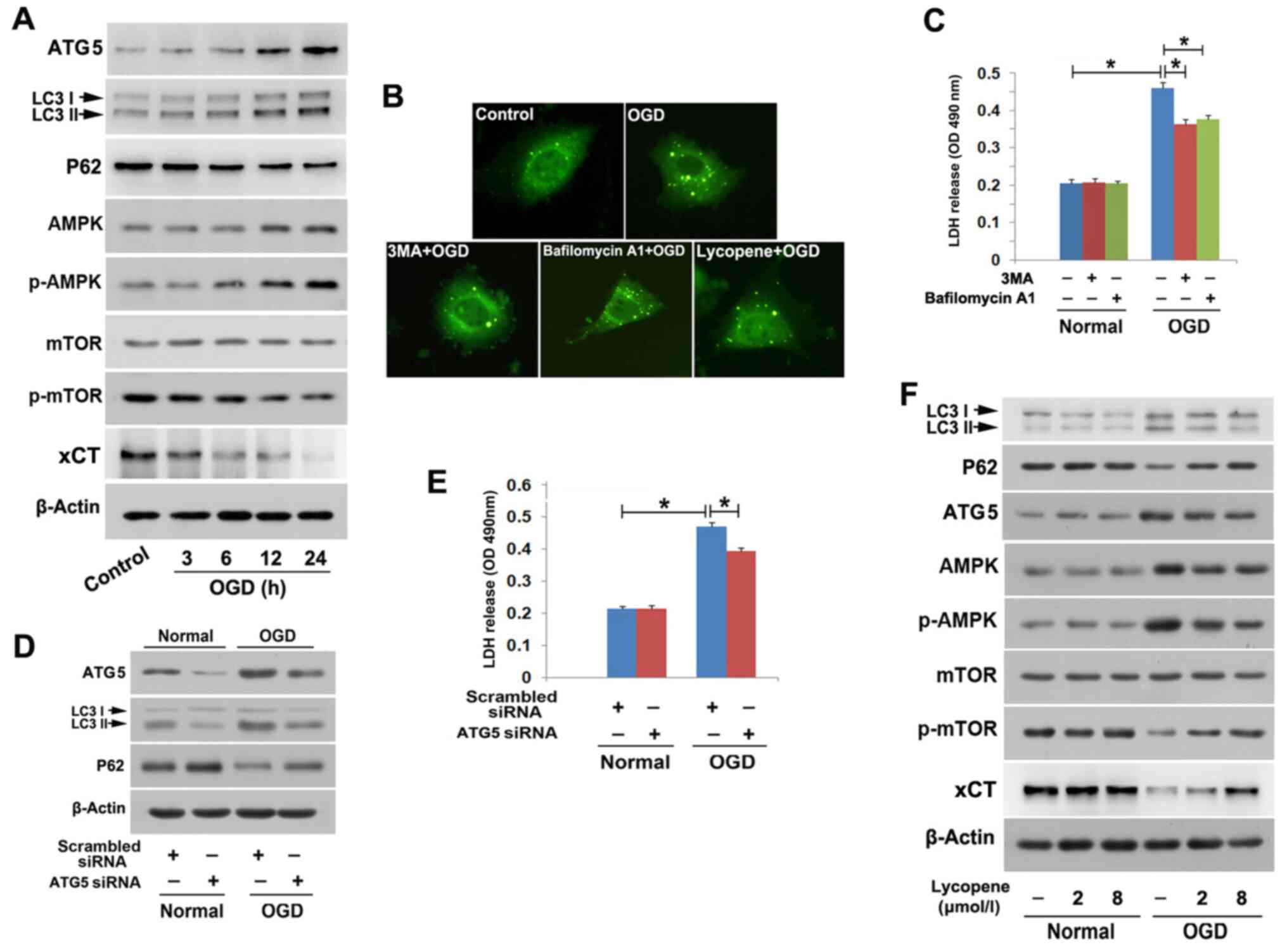 | Figure 2.Lycopene inhibits OGD-induced
autophagy. (A) Western blotting proved that OGD increased the
expression levels of ATG5 and LC3II, and decreased the expression
levels of p62 in a time-dependent manner. Moreover, OGD induced
upregulation of AMPK, p-AMPK and p-mTOR, but downregulated xCT. (B)
Laser scanning microscopy (magnification, ×40) showed that
OGD-induced formation of green puncta in cells transfected with
lentivirus-GFP-LC3 was inhibited by pretreatment with 3MA or
lycopene, but reinforced by bafilomycin A1. (C) LDH release assay
demonstrated that both 3MA and bafilomycin A1 inhibited OGD-induced
SH-SY5Y cell death. (D) Western blotting revealed that knockdown of
ATG5 with siRNA attenuated OGD-induced upregulation of LC3II and
downregulation of p62. (E) LDH release assay revealed that
knockdown of ATG5 with siRNA prevented OGD-induced SH-SY5Y cell
death. (F) Lycopene pretreatment obviously reversed OGD-induced
changes in LC3II, p62, ATG5, AMPK, p-AMPK, p-mTOR and xCT
expression. LDH release data were presented as the mean ± SEM (n=5
per group). *P<0.01. 3MA, 3-methyladenine; ATG5, autophagy
protein 5; LDH, lactate dehydrogenase; OGD, oxygen-glucose
deprivation; p, phosphorylated; siRNA, small interfering RNA. |
To further verify the role of autophagy in
OGD-induced SH-SY5Y cell death, the present study introduced siRNA
to knockdown ATG5 and examined OGD-induced SH-SY5Y cell death. The
results demonstrated that knockdown of ATG5 not only reversed
OGD-induced upregulation of LC3II and downregulation of p62, but
also prevented OGD-induced SH-SY5Y cell death (Fig. 2D and E). Therefore, these results
indicated that OGD may induce lethal autophagy in SH-SY5Y
cells.
Notably, western blotting revealed that OGD-induced
upregulation of ATG5 and LC3II, and downregulation of p62, were all
inhibited following treatment with 2 µmol/l lycopene (Fig. 2F); the effects of lycopene were more
apparent when the dosage was increased to 8 µmol/l. Meanwhile,
laser scanning microscopy demonstrated that 8 µmol/l lycopene
effectively inhibited OGD-induced formation of green puncta in the
cytoplasm of SH-SY5Y cells (Fig.
2B), which was different from the effect of bafilomycin A1, but
similar to the effect of 3MA. Collectively, these data suggested
that lycopene may prevent OGD-induced lethal autophagy in SH-SY5Y
cells through inhibition of autophagy initiation, not through
blocking OGD-induced activation of autophagy flux.
Lycopene inhibits OGD-induced
activation of the AMPK/mTOR pathway
Since AMPK, which senses dysfunctional energy
metabolism, serves a key role in promoting autophagy occurrence
(27), the present study assessed
whether AMPK was involved in OGD-induced autophagic death in
SH-SY5Y cells. As shown in Fig. 2A,
OGD upregulated the protein expression levels of AMPK and p-AMPK
(active form of AMPK) in SH-SY5Y cells, which became more apparent
with the extension of OGD duration. Concomitantly, OGD decreased
the expression levels of p-mTOR in a time-dependent manner, despite
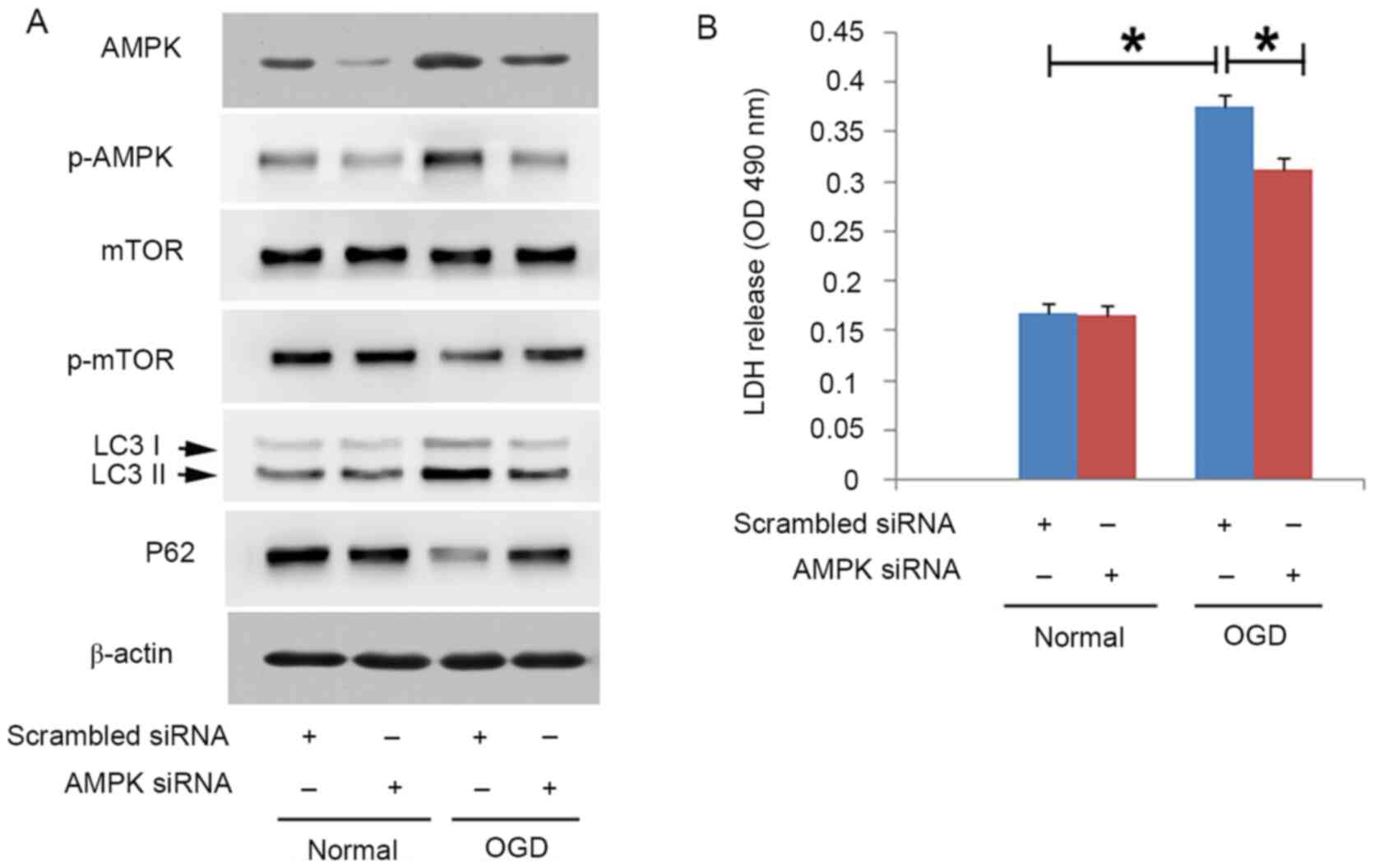
no obvious changes in mTOR at each indicated time point (Fig. 2A). In addition, knockdown of AMPK
with siRNA not only prevented OGD-induced upregulation of p-AMPK,
but also prevented the downregulation of p-mTOR (Fig. 3A). Since de-phosphorylation of mTOR
is considered a downstream biochemical event of activated AMPK
(3), it was hypothesized that OGD
activated the AMPK/mTOR pathway in SH-SY5Y cells. Additionally,
knockdown of AMPK using siRNA not only inhibited OGD-induced
upregulation of LC3II and downregulation of p62 (Fig. 3A), but also significantly prevented
OGD-induced SH-SY5Y cell death (Fig.
3B). Therefore, these results indicated that exposure to OGD
may lead to autophagic death through activation of the AMPK/mTOR
pathway in SH-SY5Y cells.
Notably, pretreatment with 2 or 8 µmol/l lycopene
markedly decreased OGD-induced upregulation of p-AMPK and
downregulation of p-mTOR (Fig. 2A).
Moreover, the inhibitory effect of 8 µmol/l lycopene on OGD-induced
activation of the AMPK/mTOR pathway was more obvious than 2 µmol/l
lycopene. These findings demonstrated that lycopene inhibited
OGD-induced activation of the AMPK/mTOR pathway and consequently
prevented autophagic death.
Lycopene prevents OGD-induced
oxidative stress
Given that oxidative stress is one of the important
factors that activates the AMPK/mTOR pathway in SH-SY5Y cells
(28), the present study assessed
whether lycopene could inhibit OGD-induce oxidative stress in
SH-SY5Y cells.
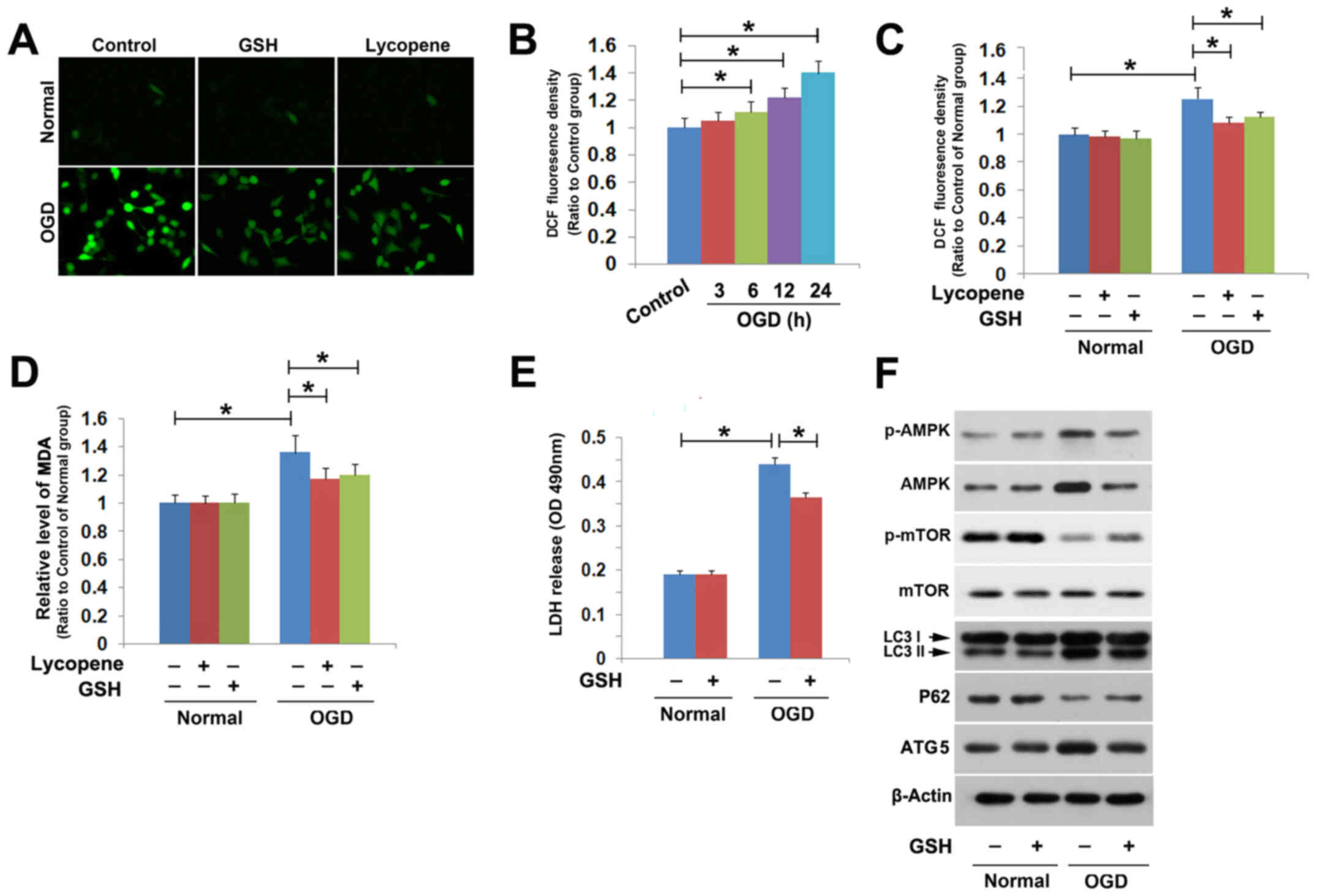
The ROS probe DCFH-DA was used to detect
intracellular ROS. Fluorescence microcopy revealed that green
fluorescence (ROS) was much brighter in the cells stressed with OGD
for 24 h compared with that in the control group (Fig. 4A). Statistical analysis of the
fluorescence density revealed that OGD significantly increased
intracellular ROS levels at 6 h, and higher levels were detected at
12 and 24 h (Fig. 4B). However,
pretreatment with the antioxidant GSH (10 mmol/l) for 1 h
significantly mitigated OGD-induced increases in ROS levels
(Fig. 4A and C). In addition, the
levels of OGD-induced MDA, which is a product of lipid oxidation,
were reduced by GSH (Fig. 4D). GSH
pretreatment also inhibited OGD-induced SH-SY5Y cell death
(Fig. 4E). Therefore, these results
indicated that OGD induced SH-SY5Y cell death through the oxidative
stress pathway.
Western blotting revealed that mitigation of
intracellular ROS with GSH effectively prevented OGD-induced
phosphorylation of AMPK and dephosphorylation of mTOR (Fig. 4F). Furthermore, it was revealed that
OGD-induced upregulation of ATG5 and LC3II, and downregulation of
p62, was also reversed in the presence of GSH (Fig. 4F). These findings indicated that
oxidative stress may be required for OGD-induced activation of the
AMPK/mTOR pathway and subsequent lethal autophagy in SH-SY5Y
cells.
Notably, pretreatment with 8 µmol/l lycopene for 1 h
effectively attenuated OGD-induced increases in ROS and MDA
(Fig. 4A, C and D), indicating that
lycopene may inhibit the oxidative stress caused by OGD.
Collectively, these results suggested that lycopene inhibited
OGD-induced activation of the AMPK/mTOR pathway and lethal
autophagy through mitigation of oxidative stress.
Lycopene inhibits OGD-induced
depletion of intracellular antioxidant GSH
Given that GSH is an interior antioxidant within
cells, in order to uncover the mechanism underlying the inhibitory
effects of lycopene on OGD-induced oxidative stress in SH-SY5Y
cells, OGD-induced GSH levels were compared between the cells
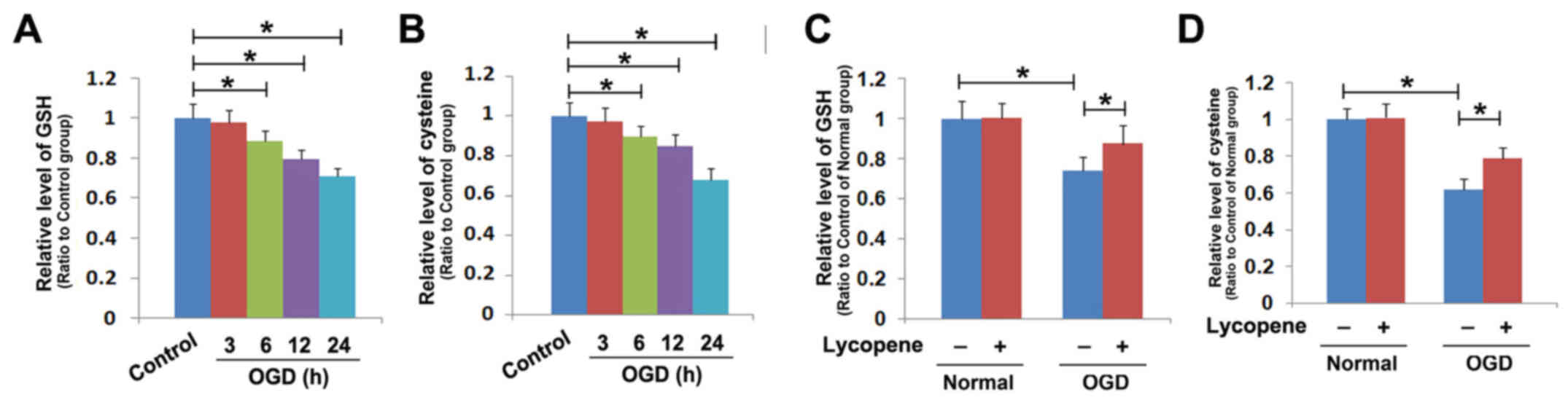
pretreated with or without lycopene. As shown in Fig. 5A, OGD decreased the intracellular
levels of GSH in SH-SY5Y cells at 6 h, which was exacerbated when
the exposure time to OGD was extended to 12 and 24 h. Cysteine that
is transformed from cystine is a material used for GSH synthesis
(29), the present study thus
tested whether OGD could deplete intracellular cysteine. The
results revealed that exposure to OGD significantly reduced
cysteine in SH-SY5Y cells at 6 h, which was aggravated at 12 and 24
h (Fig. 5B). These findings
indicated that OGD inhibited the levels of intracellular GSH and
cysteine in a time-dependent manner. However, the OGD-induced
decrease in GSH levels was prevented by treatment with 8 µmol/l
lycopene for 1 h (Fig. 5C).
Moreover, pretreatment with lycopene markedly prevented the
OGD-induced reduction in cysteine (Fig.
5D). These findings indicated that the inhibitory effect of
lycopene on OGD-induced depletion of GSH may be associated with
maintaining intracellular levels of cysteine.
Cystine, which could be used to generate cysteine,
is transported into cells through the cystine/glutamate antiporter
(29). The present study further
examined the protein expression levels of xCT (also known as
SLC7A11), which is a specific light-chain subunit of the
cystine/glutamate antiporter, in the cells that did or did not
undergo OGD by western blotting. It was revealed that OGD
downregulated the protein expression levels of xCT in a
time-dependent manner (Fig. 2A).
However, the downregulation of xCT caused by OGD was markedly
prevented in the cells pretreated with 2 µmol/l lycopene for 1 h;
the downregulation was further inhibited when the dosage of
lycopene was increased to 8 µmol/l (Fig. 2F). There results demonstrated that
lycopene inhibited OGD-induced downregulation of xCT, which may
account for the inhibitory effect of lycopene on OGD-induced
depletion of cysteine and GSH.
Discussion
The present study provided evidence that OGD induced
autophagic death in SH-SY5Y cells, which was accompanied by a
time-dependent upregulation of the autophagy marker proteins ATG5
and LC3II, and downregulation of the autophagy substrate p62.
Conversely, OGD-induced cell death was inhibited when ATG5 was
knocked down with siRNA, or in the presence of autophagy inhibitors
3MA or bafilomycin A1. Notably, it was demonstrated that lycopene
not only prevented OGD-induced SH-SY5Y cell death, but also
effectively inhibited OGD-induced changes in the protein expression
levels of ATG5, LC3 and p62 in a dosage-dependent manner.
Mechanistically, lycopene inhibited OGD-induced activation of the
AMPK/mTOR pathway via mitigation of oxidative stress by preventing
the depletion of the intracellular antioxidant GSH. Further studies
revealed that lycopene inhibited the OGD-induced decrease in
cysteine and attenuated the downregulation of xCT. Collectively,
these data demonstrated that lycopene prevented OGD-induced lethal
autophagy via maintaining intracellular GSH levels in SH-SY5Y cells
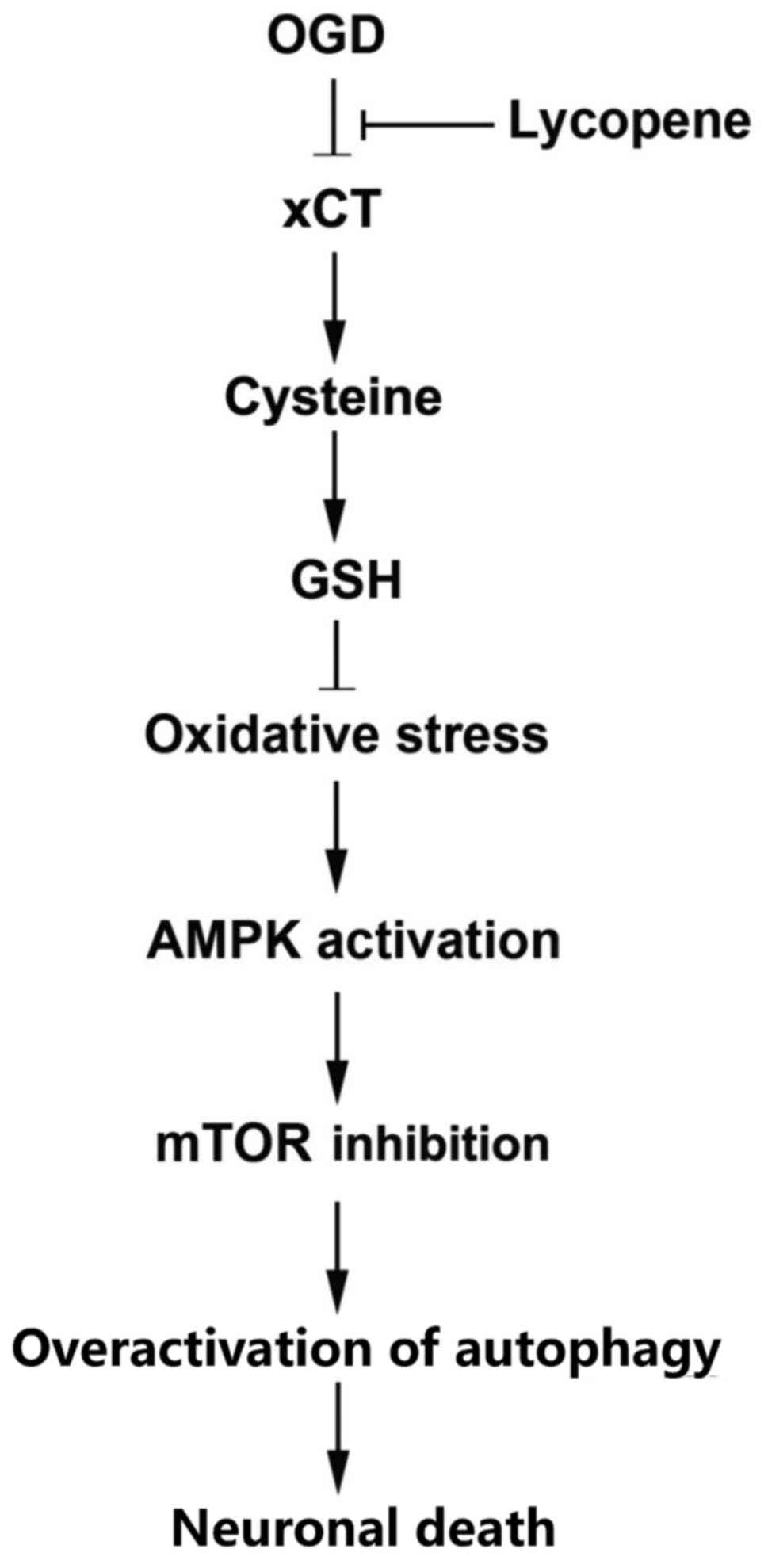
(Fig. 6).
Autophagy is known to be an evolutionarily conserved
and highly regulated homeostatic process by which cells acquire
nutrients through degradation of cytoplasmic macromolecules and
organelles using the lysosomal system (30). Overactivated autophagy leads to cell
death, which is named autophagic death or type II programmed death.
Similar to apoptosis, autophagy is one of the crucial factors
leading to SH-SY5Y cell death under the OGD condition, which is
commonly used as an ischemic model in vitro (31,32).
The present study revealed that OGD-induced upregulation of LC3II
was attenuated by 3MA, which could inhibit autophagy initiation,
but was enhanced by bafilomycin A1, which could block combination
of autophasomes with lysosomes. Consistently, OGD-induced formation
of green puncta in the cells transfected with LC3 was decreased by
3MA, whereas it was strengthened by bafilomycin A1. Thus, these
results indicated that OGD activated autophagy in SH-SY5Y cells. It
was previously reported that several compounds, such as picroside
II (31) and cornin (32), could protect SH-SY5Y cells against
OGD-induced death by weakening overactivated autophagy. Therefore,
targeting autophagy is thought to be a strategy to prevent
OGD-induced damage. Although accumulating evidence has revealed
that lycopene may inhibit neuronal apoptosis induced by amyloid-β,
subarachnoid hemorrhage and cerebral ischemia, the role of lycopene
in autophagy remains unclear (16,33,34).
In contrast to a previous report, which revealed that lycopene
protected H9C2 cardiomyocytes against hypoxia/reoxygenation-induced
apoptosis through increased protective autophagy (24), the present study demonstrated that
pretreatment with lycopene not only inhibited OGD-induced
upregulation of ATG5 and LC3II, and downregulation of p62, but also
attenuated OGD-induced SH-SY5Y cell death. Thus, these data
indicated that lycopene inhibited OGD-triggered neuronal death via
preventing the activation of lethal autophagy.
The AMPK/mTOR signaling pathway is well established
as an important regulator in the pathological process of neuronal
damage caused by head trauma and cerebral ischemia (35,36).
Moreover, it has been reported to be involved in autophagic death
of various cell types, such as cardiomyocytes, vascular endothelial
cells and renal tubular cells (37–39).
Thus, it was hypothesized that autophagic neuronal death could be
rescued through inhibition of the AMPK/mTOR signaling pathway. Li
et al (37) demonstrated
that thioredoxin-2 attenuated OGD-induced autophagic death in H9c2
cardiomyocytes via suppression of AMPK/mTOR signaling. Sun et
al (40) reported that propofol
protected neurons against OGD-induced lethal autophagy via
inhibition of the AMPK/mTOR pathway. Similarly, the present study
demonstrated that lycopene effectively reversed OGD-induced
phosphorylation of AMPK and de-phosphorylation of mTOR, indicating
that the preventive effects of lycopene against OGD-induced lethal
autophagy in SH-SY5Y cells were associated with inhibition of the
AMPK/mTOR pathway. In addition to the AMPK/mTOR pathway, activated
JNK and HIF1-α have been reported to contribute to OGD-induced
lethal autophagy (31,41). Notably, previous studies have shown
that lycopene could suppress the activation of JNK, as well as
inhibit the activity of HIF1-α (31,42).
Therefore, lycopene may inhibit OGD-induced lethal autophagy via
multiple pathways.
Oxidative stress is a crucial factor leading to
AMPK/mTOR activation, and it is characterized by intracellular
accumulation of ROS resulting from a disrupted equilibrium between
ROS generation and clearance (28).
It was reported previously that ROS not only upregulated the
protein expression levels of AMPK, but also promoted
phosphorylation of AMPK. Similarly, the present study revealed that
OGD not only induced upregulation of both AMPK and p-AMPK, but also
promoted intracellular levels of ROS. Lycopene has been shown to
act as a potent antioxidant; previous studies have demonstrated
that lycopene prevented neuronal death induced by
ischemia/reperfusion, colchicine, methylmercury, rotenone and
amyloid β through inhibition of oxidative stress (16–20).
Moreover, it was previously reported that lycopene significantly
inhibited hydrogen peroxide (H2O2)-induced
autophagic SH-SY5Y cell death (43). The present study revealed that
lycopene markedly suppressed OGD-induced intracellular accumulation
of ROS and MDA, indicating that lycopene inhibited OGD-induced
AMPK/mTOR activation via inhibition of oxidative stress. Previous
studies have reported that lycopene may serve anti-oxidative roles
through numerous mechanisms, such as inhibition of mitochondrial
superoxide generation, activation of the Nrf2/HO-1 signaling
pathway, inhibition of the nitric oxide pathway, and maintaining
the protein levels and activity of catalase that could degrade
H2O2 (13,15,17,43).
Moreover, it has also been shown that lycopene could inhibit
H2O2-induced apoptosis of SH-SY5Y cells via inhibition of oxidative
stress-activated caspase-3 and nuclear translocation of AIF
(43). By contrast, the present
study demonstrated that lycopene inhibited OGD-induced SH-SY5Y cell
death via inhibition of excessive activation of autophagy.
Apoptosis is morphologically characterized by cell membrane
blebbing, chromatin condensation and formation of apoptotic bodies
(43); however, autophagy is
characterized by the cytoplasmic formation of numerous vacuoles
containing cytosolic components or organelles (35,36).
At molecular levels, autophagy activation is associated with ATG5
and ATG12, whereas apoptosis is related to cascade activation of
caspase-9 and caspase-3 (35,36,43).
Thus, apoptosis and autophagic death are two different types of
programmed cell death, despite the fact that they can both be
induced under the condition of oxidative stress. Therefore, the
novelty of the present study is that lycopene exerted a protective
effect on SH-SY5Y cells via inhibition of autophagy.
GSH is an intracellular antioxidant, which can be
used by glutathione peroxidase 4 to reduce intracellular ROS and
lipid oxidation products (29). The
present study revealed that OGD-induced depletion of GSH was
reversed in the presence of lycopene. Furthermore, cystine can be
converted into cysteine, which can then be used as material for GSH
synthesis, and as a specific light-chain subunit of the
cystine/glutamate antiporter, xCT is required for transporting
extracellular cystine into cells (29). The present results demonstrated that
lycopene inhibited OGD-induced decreases in cysteine and
downregulation of xCT (SLC7A11). Thus, it was suggested that the
inhibitory role of lycopene in OGD-induced oxidative stress was
associated with maintaining GSH levels by preventing OGD-induced
dysfunction of the cystine/glutamate antiporter.
Endoplasmic reticulum (ER) stress could be activated
by intracellular ROS and has been suggested as a key regulator of
autophagy (44). Furthermore, in
previous studies, ER stress not only promoted
H2O2-induced autophagic death, but also
contributed to OGD-induced intracellular accumulation of ROS in
SH-SY5Y cells (26,28). Although the present study did not
investigate whether lycopene could inhibit OGD-induced ER stress,
it has previously been reported that lycopene protected
cardiomyocytes against the damage induced by hypoxia/reoxygenation
via inhibition of ER stress (45,46).
Collectively, these findings indicated the inhibitory role of
lycopene in OGD-induced oxidative stress and autophagic death may
be associated with inhibition of ER stress.
In conclusion, the present study demonstrated that
lycopene protected against OGD-induced autophagic death via
inhibition of oxidative stress-dependent activation of the
AMPK/mTOR signaling pathway in SH-SY5Y cells. Moreover, it was
revealed that the inhibitory effect of lycopene on OGD-induced GSH
depletion was associated with the preventive effects of lycopene
against OGD-induced depletion of intracellular cysteine and
downregulation of xCT. Therefore, lycopene may be considered a
potential treatment that could prevent OGD-induced autophagic
neuronal death.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Wu Jieping
Medical Foundation of China (grant no. 320.6750.16030).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TL, YZ and YQ conducted the experiments, and
acquired, analyzed and interpreted the data. TL drafted the
manuscript, figures, and revised the manuscript. TL and YZ confirm
the authenticity of all the raw data. YZ and YQ drafted the
manuscript and critically revised it for important intellectual
content. HL made substantial contributions to the conception and
design of the study, conceived and supervised the project, and
approved the final version of the manuscript. All authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
OGD
|
oxygen-glucose deprivation
|
|
LDH
|
lactate dehydrogenase
|
|
MDA
|
malondialdehyde
|
|
ER
|
endoplasmic reticulum
|
References
|
1
|
Arumugam TV, Baik SH, Balaganapathy P,
Sobey CG, Mattson MP and Jo DG: Notch signaling and neuronal death
in stroke. Prog Neurobiol. 165-167:103–116. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kirino T: Delayed neuronal death in the
gerbil hippocampus following ischemia. Brain Res. 239:57–69. 1982.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li Y, Luo Y, Luo T, Lu B, Wang C, Zhang Y,
Piao M, Feng C and Ge P: Trehalose inhibits protein aggregation
caused by transient ischemic insults through preservation of
proteasome activity, not via induction of autophagy. Mol Neurobiol.
54:6857–6869. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ouyang L, Shi Z, Zhao S, Wang FT, Zhou TT,
Liu B and Bao JK: Programmed cell death pathways in cancer: A
review of apoptosis, autophagy and programmed necrosis. Cell
Prolif. 45:487–498. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xu Y, Tian Y, Tian Y, Li X and Zhao P:
Autophagy activation involved in hypoxic-ischemic brain injury
induces cognitive and memory impairment in neonatal rats. J
Neurochem. 139:795–805. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Luo CL, Li BX, Li QQ, Chen XP, Sun YX, Bao
HJ, Dai DK, Shen YW, Xu HF, Ni H, et al: Autophagy is involved in
traumatic brain injury-induced cell death and contributes to
functional outcome deficits in mice. Neuroscience. 184:54–63. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zheng Y, Hou J, Liu J, Yao M, Li L, Zhang
B, Zhu H and Wang Z: Inhibition of autophagy contributes to
melatonin-mediated neuroprotection against transient focal cerebral
ischemia in rats. J Pharmacol Sci. 124:354–364. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Luo T, Liu G, Ma H, Lu B, Xu H, Wang Y, Wu
J, Ge P and Liang J: Inhibition of autophagy via activation of
PI3K/Akt pathway contributes to the protection of ginsenoside Rb1
against neuronal death caused by ischemic insults. Int J Mol Sci.
15:15426–15442. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen D, Huang C and Chen Z: A review for
the pharmacological effect of lycopene in central nervous system
disorders. Biomed Pharmacother. 111:791–801. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tong C, Peng C, Wang L, Zhang L, Yang X,
Xu P, Li J, Delplancke T, Zhang H and Qi H: Intravenous
administration of lycopene, a tomato extract, protects against
myocardial ischemia-reperfusion injury. Nutrients. 8:1382016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kaya C, Karabulut R, Turkyilmaz Z, Sonmez
K, Kulduk G, Gülbahar Ö, Köse F and Basaklar AC: Lycopene has
reduced renal damage histopathologically and biochemically in
experimental renal ischemia-reperfusion injury. Ren Fail.
37:1390–1395. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hekimoglu A, Kurcer Z, Aral F, Baba F,
Sahna E and Atessahin A: Lycopene, an antioxidant carotenoid,
attenuates testicular injury caused by ischemia/reperfusion in
rats. Tohoku J Exp Med. 218:141–147. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bayramoglu G, Bayramoglu A, Altuner Y,
Uyanoglu M and Colak S: The effects of lycopene on hepatic
ischemia/reperfusion injury in rats. Cytotechnology. 67:487–491.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hsiao G, Fong TH, Tzu NH, Lin KH, Chou DS
and Sheu JR: A potent antioxidant, lycopene, affords
neuroprotection against microglia activation and focal cerebral
ischemia in rats. In Vivo. 18:351–356. 2004.PubMed/NCBI
|
|
15
|
Lei X, Lei L, Zhang Z and Cheng Y:
Neuroprotective effects of lycopene pretreatment on transient
global cerebral ischemia reperfusion in rats: The role of the
Nrf2/HO 1 signaling pathway. Mol Med Rep. 13:412–418. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fujita K, Yoshimoto N, Kato T, Imada H,
Matsumoto G, Inakuma T, Nagata Y and Miyachi E: Lycopene inhibits
ischemia/reperfusion-induced neuronal apoptosis in gerbil
hippocampal tissue. Neurochem Res. 38:461–469. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Prakash A and Kumar A: Lycopene protects
against memory impairment and mito-oxidative damage induced by
colchicine in rats: An evidence of nitric oxide signaling. Eur J
Pharmacol. 721:373–381. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kaur H, Chauhan S and Sandhir R:
Protective effect of lycopene on oxidative stress and cognitive
decline in rotenone induced model of Parkinson's disease. Neurochem
Res. 36:1435–1443. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Qu M, Nan X, Gao Z, Guo B, Liu B and Chen
Z: Protective effects of lycopene against methylmercury-induced
neurotoxicity in cultured rat cerebellar granule neurons. Brain
Res. 1540:92–102. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Qu M, Li L, Chen C, Li M, Pei L, Chu F,
Yang J, Yu Z, Wang D and Zhou Z: Protective effects of lycopene
against amyloid β-induced neurotoxicity in cultured rat cortical
neurons. Neurosci Lett. 505:286–290. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Qu M, Zhou Z, Chen C, Li M, Pei L, Chu F,
Yang J, Wang Y, Li L, Liu C, et al: Lycopene protects against
trimethyltin-induced neurotoxicity in primary cultured rat
hippocampal neurons by inhibiting the mitochondrial apoptotic
pathway. Neurochem Int. 59:1095–1103. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
di Matteo V, Pierucci M, Di Giovanni G,
Dragani LK, Murzilli S, Poggi A and Esposito E: Intake of
tomato-enriched diet protects from 6-hydroxydopamine-induced
degeneration of rat nigral dopaminergic neurons. J Neural Transm
Suppl. 73:333–341. 2009.PubMed/NCBI
|
|
23
|
Zhao Y, Xin Z, Li N, Chang S, Chen Y, Geng
L, Chang H, Shi H and Chang YZ: Nano-liposomes of lycopene reduces
ischemic brain damage in rodents by regulating iron metabolism.
Free Radic Biol Med. 124:1–11. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen F, Sun ZW, Ye LF, Fu GS, Mou Y and Hu
SJ: Lycopene protects against apoptosis in hypoxia/reoxygenation
induced H9C2 myocardioblast cells through increased autophagy. Mol
Med Rep. 11:1358–1365. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zeng YC, Peng LS, Zou L, Huang SF, Xie Y,
Mu GP, Zeng XH, Zhou XL and Zeng YC: Protective effect and
mechanism of lycopene on endothelial progenitor cells (EPCs) from
type 2 diabetes mellitus rats. Biomed Pharmacother. 92:86–94. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang HF, Wang ZQ, Ding Y, Piao MH, Feng
CS, Chi GF, Luo YN and Ge PF: Endoplasmic reticulum stress
regulates oxygen-glucose deprivation-induced parthanatos in human
SH-SY5Y cells via improvement of intracellular ROS. CNS Neurosci
Ther. 24:29–38. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cui L, Li C, Gao G, Zhuo Y, Yang L, Cui N
and Zhang S: FTY720 inhibits the activation of pancreatic stellate
cells by promoting apoptosis and suppressing autophagy via the
AMPK/mTOR pathway. Life Sci. 217:243–250. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gao Z, Wang H, Zhang B, Wu X, Zhang Y, Ge
P, Chi G and Liang J: Trehalose inhibits
H2O2-induced autophagic death in dopaminergic
SH-SY5Y cells via mitigation of ROS-dependent endoplasmic reticulum
stress and AMPK activation. Int J Med Sci. 15:1014–1024. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shin CS, Mishra P, Watrous JD, Carelli V,
D'Aurelio M, Jain M and Chan DC: The glutamate/cystine xCT
antiporter antagonizes glutamine metabolism and reduces nutrient
flexibility. Nat Commun. 8:150742017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mariño G, Madeo F and Kroemer G: Autophagy
for tissue homeostasis and neuroprotection. Curr Opin Cell Biol.
23:198–206. 2011. View Article : Google Scholar
|
|
31
|
Wang T, Zhu L, Liu H, Yu G and Guo Y:
Picroside II protects SH-SY5Y cells from autophagy and apoptosis
following oxygen glucose deprivation/reoxygen injury by inhibiting
JNK signal pathway. Anat Rec (Hoboken). 302:2245–2254. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ding C, Zhang J, Li B, Ding Z, Cheng W,
Gao F, Zhang Y, Xu Y and Zhang S: Cornin protects SH SY5Y cells
against oxygen and glucose deprivation induced autophagy through
the PI3K/Akt/mTOR pathway. Mol Med Rep. 17:87–92. 2018.PubMed/NCBI
|
|
33
|
Hwang S, Lim JW and Kim H: Inhibitory
effect of lycopene on amyloid-β-induced apoptosis in neuronal
cells. Nutrients. 9:92017.
|
|
34
|
Wu A, Liu R, Dai W, Jie Y, Yu G, Fan X and
Huang Q: Lycopene attenuates early brain injury and inflammation
following subarachnoid hemorrhage in rats. Int J Clin Exp Med.
8:14316–14322. 2015.PubMed/NCBI
|
|
35
|
Lin CJ, Chen TH, Yang LY and Shih CM:
Resveratrol protects astrocytes against traumatic brain injury
through inhibiting apoptotic and autophagic cell death. Cell Death
Dis. 5:e11472014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang M, Li YJ, Ding Y, Zhang HN, Sun T,
Zhang K, Yang L, Guo YY, Liu SB, Zhao MG, et al: Silibinin prevents
autophagic cell death upon oxidative stress in cortical neurons and
cerebral ischemia-reperfusion injury. Mol Neurobiol. 53:932–943.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li YY, Xiang Y, Zhang S, Wang Y, Yang J,
Liu W and Xue FT: Thioredoxin-2 protects against oxygen-glucose
deprivation/reperfusion injury by inhibiting autophagy and
apoptosis in H9c2 cardiomyocytes. Am J Transl Res. 9:1471–1482.
2017.PubMed/NCBI
|
|
38
|
Zhang L, Wei J, Ren L, Zhang J, Wang J,
Jing L, Yang M, Yu Y, Sun Z and Zhou X: Endosulfan induces
autophagy and endothelial dysfunction via the AMPK/mTOR signaling
pathway triggered by oxidative stress. Environ Pollut. 220:843–852.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang L, Mao N, Tan RZ, Wang HL, Wen J, Liu
YH, Furhad M and Fan JM: Ginsenoside Rg1 reduces
aldosterone-induced autophagy via the AMPK/mTOR pathway in NRK-52E
cells. Int J Mol Med. 36:518–526. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sun B, Ou H, Ren F, Huan Y, Zhong T, Gao M
and Cai H: Propofol inhibited autophagy through
Ca2+/CaMKKβ/AMPK/mTOR pathway in OGD/R-induced neuron
injury. Mol Med. 24:582018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Niu G, Zhu D, Zhang X, Wang J, Zhao Y and
Wang X: Role of hypoxia-inducible factors 1α (HIF1α) in SH-SY5Y
cell autophagy induced by oxygen-glucose deprivation. Med Sci
Monit. 24:2758–2766. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Upadhyay J, Kesharwani RK and Misra K:
Comparative study of antioxidants as cancer preventives through
inhibition of HIF-1 alpha activity. Bioinformation. 4:233–236.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Feng C, Luo T, Zhang S, Liu K, Zhang Y,
Luo Y and Ge P: Lycopene protects human SH SY5Y neuroblastoma cells
against hydrogen peroxide induced death via inhibition of oxidative
stress and mitochondria associated apoptotic pathways. Mol Med Rep.
13:4205–4214. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Xiang C, Wang Y, Zhang H and Han F: The
role of endoplasmic reticulum stress in neurodegenerative disease.
Apoptosis. 22:1–26. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Xu J, Hu H, Chen B, Yue R, Zhou Z, Liu Y,
Zhang S, Xu L, Wang H and Yu Z: Lycopene protects against
hypoxia/reoxygenation injury by alleviating er stress induced
apoptosis in neonatal mouse cardiomyocytes. PLoS One.
10:e01364432015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Gao Y, Jia P, Shu W and Jia D: The
protective effect of lycopene on hypoxia/reoxygenation-induced
endoplasmic reticulum stress in H9C2 cardiomyocytes. Eur J
Pharmacol. 774:71–79. 2016. View Article : Google Scholar : PubMed/NCBI
|