Introduction
Lung cancer is one of the most common malignant
tumors and the main cause of cancer-associated mortality, with
>1.7 million deaths reported worldwide in 2018 (1). Lung cancer can be broadly divided into
two major categories based on its histological characteristics:
Small cell lung cancer (SCLC) and non-small cell lung cancer
(NSCLC) (2,3). Compared with SCLC, NSCLC is the main
type of lung cancer and accounts for >84% of cases of lung
cancer (4). The clinical efficacy
of current therapeutic strategies of lung cancer is greatly
dependent on the stage of disease, where poor outcomes are closely
associated with diagnosis at advanced stages and propensity for
metastasis (5). By contrast, good
outcomes are associated with diagnosis at early stages (5). However, most patients with NSCLC are
typically diagnosed at advanced or metastatic stages of the disease
(6). Although major improvements
have been made in the treatment of NSCLC with the development of
targeted therapies and immunotherapy, the 5-year survival rate of
patients with lung cancer has remained unchanged over the past
number of decades (7). NSCLC is
also the archetype of a genomics-driven malignancy, such as
YES1 (a member of SRC family kinases) mutation, which showed
a promising therapeutic target in lung cancer (8). However, YES1 status can only be
used as a predictive biomarker for treatment in a selected subset
of patients, which means that only a small proportion of patients
with lung cancer can benefit from targeted therapy (8). Therefore, it is imperative to identify
new druggable targets to enhance the efficacy of the currently
available therapies and to reduce the mortality rate of lung
cancer.
Tumor endothelial marker 8 (TEM8) is an
integrin-like cell surface transmembrane protein that was first
identified as a marker in the tumor endothelium in colorectal
cancer by St Croix et al (9)
in 2000. TEM8 has been found to be upregulated in the stroma of
numerous solid tumors, including osteosarcoma (10), colorectal cancer (9), lung cancer (11), melanoma (12) and triple-negative breast cancer
(13). This makes it an attractive
target for studying any potentially novel anticancer therapeutics,
due to its overexpression on the cell surface of solid tumors.
Previous studies in human tumor xenografts have reported that
either blocking or knocking down TEM8 expression can inhibit tumor
growth (14,15). In addition, previous studies have
confirmed that TEM8 knockdown could inhibit the migration and
invasion of Xuanwei Lung Cancer (XWLC)-05 cells in vitro
(11). Since the XWLC-05 cell line
has been proved to exhibit histological characteristics that are
similar to those observed in NSCLC (16), the present study mainly focused on
the effects of silencing TEM8 expression on NSCLC tumor growth
in vivo.
Angiogenesis has been previously shown to be an
important process in promoting tumor growth and metastasis
(17), such that a number of
anti-angiogenic agents (e.g., bevacizumab plus irinotecan,
fluorouracil and leucovorin) are currently being applied clinically
(18). TEM8 has been shown to be
required for angiogenesis in melanoma, breast and colon cancer
(15). However, TEM8 knockdown in
mice was found not to affect the angiogenic process during normal
development or wound healing (19).
Taken together, these previously reported effects suggest that TEM8
is required for tumor angiogenesis, but not physiological
angiogenesis. However, most of the known vascular endothelial
markers are not only expressed in tumor tissues, but also in normal
tissues, meaning that targeting these markers can lead to side
effects (19,20). Therefore, treatments that target
TEM8 may inhibit tumor growth with less severe side effects.
Nevertheless, little is known concerning the function of TEM8 in
lung cancer in vivo.
In the present study, the effects of silencing TEM8
expression on tumor growth in a murine xenograft model of lung
cancer was investigated. Specifically, the relationship between
TEM8 expression and tumor angiogenesis was examined. Furthermore,
the underlying signaling pathways that are activated following TEM8
knockdown was also measured. These findings may provide a novel
theoretical basis for early lung cancer diagnosis and improving the
outcome of targeted therapies.
Materials and methods
Cell lines
The XWLC-05 cell line was provided by Dr Wang Li,
Radiotherapy Center of The Third Affiliated Hospital of Kunming
Medical University (Kunming, China). The cells were cultured in
RPMI-1640 medium (HyClone; Cytiva) supplemented with 10% FBS
(HyClone; Cytiva) at 37°C in a humidified incubator under 5%
CO2 atmosphere in T25 flasks. When the cell density
reached >90%, cells were passaged at reasonable ratios for
further study.
Experimental animals
A total of 18 male BALB/c-nu/nu mice (4–6 weeks old;
weighing 16–18 g, mean 16.9±0.5 g) were purchased from Beijing
Vital River Laboratory Animal Technology Co., Ltd. All mice were
randomly divided into three groups with six mice each. The mice
were housed under a 12-h light/dark cycle at a constant temperature
(22–24°C) and humidity (40–70%) in a specific pathogen-free
environment with free access to food and water. The mice were
acclimated to the housing conditions for 1 week before inoculation.
All animal experiments were approved by the Committee on the Ethics
of Animal Experiments of The Third Affiliated Hospital of Kunming
Medical University (approval no. 16KY-LA00135).
Creation of the TEM8-short hairpin RNA
(shRNA/sh) stable transfection cell line
The pGLVH1/GFP/Puro (PGLV3) vectors encoding
TEM8-shRNA and negative control (NC) shRNA constructs were
purchased from Shanghai GenePharma Co., Ltd. The lentivirus was
synthesized by Shanghai GenePharma Co., Ltd. XWLC-05 cells were
plated into a 96-well plate at a density of 5×104
cells/well and cultured in RPMI-1640 medium with 10% FBS at 37°C
overnight. The XWLC-05 cells were then infected with lentiviral or
control virus (NC) in RPMI-1640 medium with 10% FBS and polybrene
at a final concentration of 5 µg/ml according to the manufacturer's
instructions. After 24 h, the cells were washed three times with
PBS and cultured in RPMI-1640 medium supplemented with 10% FBS at
37°C for 72 h. Efficiency of TEM8 silencing was determined by
reverse transcription-quantitative PCR (RT-qPCR) and western
blotting under different conditions, namely untreated control
XWLC-05 cells, shNC-transfected cells and shTEM8-transfected
cells.
After 72 h transfection, the transfected or
untransfected XWLC-05 cells were plated into a six-well plate at
densities of 5×105 cells/well and fresh medium
containing 2 µg/ml puromycin (Sigma-Aldrich; Merck KGaA) was added
to select for stably transfected XWLC-05 cells. Subsequently, 2
µg/ml puromycin was used for maintenance of the stably transfected
cell line. The stably transfected XWLC-05 cells were harvested and
subsequently amplified.
In vivo tumorigenicity assays
All BALB/c-nu/nu mice were randomly divided into the
following three groups (n=6/group): i) Control group; ii) shNC
group; and iii) shTEM8 group. XWLC-05 cells in the indicated groups
were harvested and resuspended in PBS to a final concentration of
1×107 cells/ml. The mice were then given subcutaneous
injections in the right dorsal flank with the prepared cells
(2×106 cells/mouse), after which they were monitored
every day. After the subcutaneous tumors become visible, the tumor
volumes were measured with a vernier caliper once every 2 days and
the tumor size was calculated using the following formula: (Length
× width × height)/2. At 28 days post-injection, the maximum tumor
size reached ~1,200 mm3 in the control group all mice
were sacrificed by cervical dislocation, and tumors were excised
and weighed.
RT-qPCR
Total RNA was extracted from tumor tissues or cells
using TRIzol® reagent (Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. The quality and
concentration of extracted RNA was measured using a UV/VIS
spectrophotometer. First-strand cDNAs were synthesized using the
High-Capacity cDNA Reverse Transcription kit (Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. qPCR
was performed using an ABI 7500 Real-Time PCR detection system
(Applied Biosciences; Thermo Fisher Scientific, Inc.) with
SuperReal PreMix Plus (SYBR-Green) (Tiangen Biotech Co., Ltd.). The
following qPCR thermocycling conditions were used: Pre-denaturation
at 95°C for 10 min; followed by 40 cycles of 95°C for 15 sec and
60°C for 60 sec. The primers used for the RT-qPCR are shown in
Table I, where GAPDH was used as
the internal reference. The relative mRNA level was calculated
using the 2−ΔΔCq method (21). Each experiment was repeated ≥3
times.
 | Table I.Primers used for reverse
transcription-quantitative PCR. |
Table I.
Primers used for reverse
transcription-quantitative PCR.
| Gene | Primer sequences
(5′→3′) | Production size
(bp) |
|---|
| TEM8 | F:
GCTGCACCACTGGAATGAAATCT | 153 |
|
| R:
AGGCCTTGACGGATTTGTTCTCT |
|
| ERK | F:
TTACTGCGCTTCAGACATGAGA | 111 |
|
| R:
ATCTGTTTCCATGAGGTCCTGT |
|
| Bcl-2 | F:
TGTGTGTGGAGAGCGTCAAC | 142 |
|
| R:
GGGCCGTACAGTTCCACAAA |
|
| GAPDH | F:
TCTCTGCTCCTCCTGTTCGA | 122 |
|
| R:
GCGCCCAATACGACCAAATC |
|
Immunohistochemistry and
immunofluorescence staining
Tumor tissues from each mouse were harvested at the
end of the study, fixed with 4% paraformaldehyde for 12 h at room
temperature and embedded in paraffin. The paraffin sections at a
thicknesses of 4 µm were prepared, dewaxed with xylene and
rehydrated in ethanol, and antigen retrieval was performed in 0.01
mol/l citrate buffer (pH 6.0) in a microwave oven for 20 min at
98–100°C. The sections were then treated with 3% hydrogen peroxide
methanol for 10 min at room temperature to block the endogenous
peroxidase activity.
For immunohistochemistry, the sections were blocked
with 1% BSA (Beijing Solarbio Science & Technology Co., Ltd.)
in PBS containing 0.05% Tween-20 (PBST) for 40 min at room
temperature. The sections were incubated with the anti-TEM8 primary
antibody (cat. no. ab21270; 1:200; Abcam) overnight at 4°C. After
washing three times with PBS, the sections were incubated with
HRP-conjugated goat anti-rabbit (cat. no. ab6721; 1:500; Abcam)
secondary antibodies at 37°C for 2 h. The sections were then
stained with diaminobenzidine (Beijing Solarbio Science &
Technology Co., Ltd.) at 37°C for 30 min after washing with PBS.
The sections were counterstained with hematoxylin for 1 min at room
temperature after the DAB reaction was stopped. The sections were
then dehydrated in a graded ethanol series, transparentized with
xylene, and mounted in neutral gum. The immunostained sections were
examined using a fluorescence microscope at ×200 magnification
(Olympus Corporation).
For immunofluorescence, the sections were blocked
with 1% BSA (Beijing Solarbio Science & Technology Co., Ltd.)
in PBST for 40 min at room temperature. The sections were then
incubated with the primary antibody against CD34 (cat. no. ab81289;
1:200; Abcam) overnight at 4°C. After three washes with PBS,
samples were incubated with the Alexa Fluor®
594-conjugated secondary antibody (cat. no. ab150084; 1:200; Abcam)
for 2 h at room temperature. After washing three times with PBS,
cell nuclei were stained with 4′,6-diamidino-2-phenylindole (cat.
no. 36308ES11; Shanghai Yeasen Biotech Co., Ltd.) solution, and
stored in the dark for 5 min at room temperature. The sections were
imaged by fluorescence microscopy at ×200 magnification (Olympus
Corporation).
To determine the microvessel density (MVD) values,
CD34-stained MVD was enumerated using the Weidner method (22). The positive microvessel quantity
stained with CD34 in five different visual fields was counted under
a light microscope (magnification, ×200) to calculate the average
as the value of MVD.
Hematoxylin and eosin (H&E)
staining
For H&E staining, the sections were dewaxed in
xylene and rehydrated in a graded alcohol series. Then, the
sections were rinsed with tap water for 1 min, immersed in
hematoxylin (Beyotime Institute of Biotechnology) solution for 4
min at room temperature and rinsed with tap water for 30 sec.
Sections were differentiated using a 1% acid ethanol solution for 2
sec and rinsed with tap water for 5 min. Then, the sections were
stained with eosin (Beyotime Institute of Biotechnology) solution
for 10 sec at room temperature and rinsed with tap water, followed
by dehydration with graded alcohol and clearing in xylene. Finally,
the sections were dried and mounted with neutral gum seal. Sections
were examined under a fluorescence microscope at ×200 magnification
(Olympus Corporation).
Western blotting
The tumor tissues or cells were lysed with ice-cold
RIPA buffer (Beyotime Institute of Biotechnology) mixed with
protease and phosphatase inhibitors (Beyotime Institute of
Biotechnology). Following 30 min of lysis on ice, the tissues or
cells were centrifuged at 12,000 × g for 12 min at 4°C and the
supernatant was collected. Then, the protein concentration was
quantified using a bicinchoninic acid protein assay kit (Beyotime
Institute of Biotechnology). Protein samples (~50 µg) were
separated via 10% SDS-PAGE, and then transferred onto
polyvinylidene difluoride membranes (EMD Millipore). The membranes
were blocked in 5% skimmed milk powder for 2 h at room temperature,
and subsequently incubated overnight at 4°C with the following
primary antibodies: Anti-TEM8 (cat. no. ab21270; 1:1,000; Abcam),
anti-extracellular signal-regulated kinase (ERK)1/2 (cat. no. 4695;
1:1,000; Cell Signaling Technology, Inc.), anti-Bcl-2 (cat. no.
4223; 1:1,000; Cell Signaling Technology, Inc.),
anti-phosphorylated (p)-ERK1/2 (cat. no. 4370; 1:2,000; Cell
Signaling Technology, Inc.) and anti-GAPDH (cat. no. 5174; 1:1,000;
Cell Signaling Technology, Inc.). After washing three times with
TBS containing 0.05% Tween-20 (TBST), the membranes were incubated
with HRP-conjugated goat anti-rabbit secondary antibodies (cat. no.
7074; 1:5,000; Cell Signaling Technology, Inc.) for 2 h at room
temperature. The immunoreactive bands were detected using the
SuperSignal™ West Pico chemiluminescent substrate (Thermo Fisher
Scientific, Inc.). The blots were semi-quantified using the
Quantity One software version 4.62 (Bio-Rad Laboratories, Inc.) to
analyze the gray value of each antibody band. The relative
integrated optical density for TEM8, ERK1/2, p-ERK1/2 and Bcl-2
were normalized to that of GAPDH in the same sample.
Statistical analysis
Statistical differences were determined using a
one-way ANOVA followed by a Tukey's post hoc test. All data were
analyzed using SPSS software version 17.0 (SPSS, Inc.) and are
expressed as the mean ± SD. All experiments were repeated ≥3 times.
P<0.05 was considered to indicate a statistically significant
difference.
Results
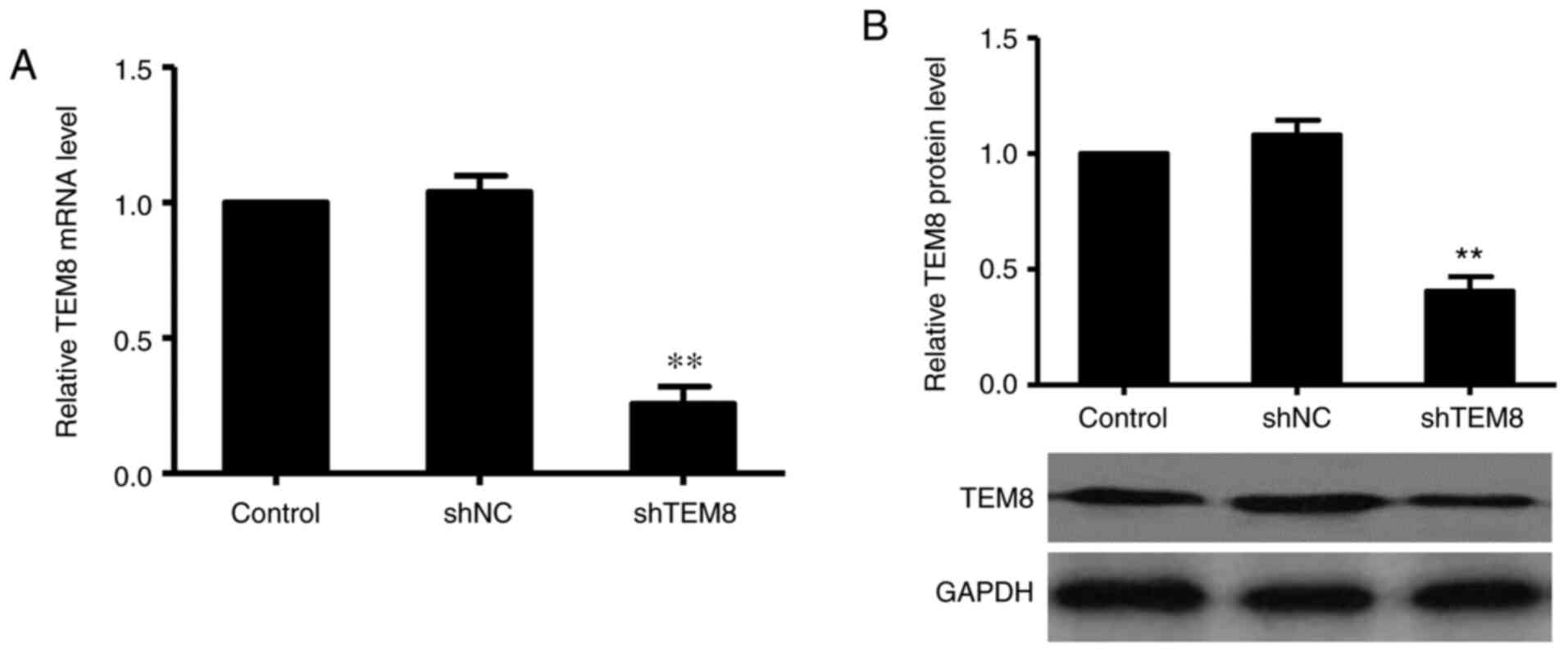
Successful knockdown of TEM8
expression via transfection with shTEM8 in XWLC-05 cells
To evaluate the efficiency of gene silencing using
shTEM8, XWLC-05 cells were transfected with either shTEM8 or shNC.
After 72 h of transfection, TEM8 expression was measured using
RT-qPCR and western blotting. The RT-qPCR results showed that TEM8
mRNA expression was significantly decreased in the shTEM8 group
compared with that in the control group (Fig. 1A). Subsequently, western blotting
revealed that TEM8 protein expression was also significantly
suppressed by transfection with shTEM8 compared with that in the
control group (Fig. 1B). These
results suggested that shTEM8 transfection could successfully knock
down TEM8 expression in XWLC-05 cells.
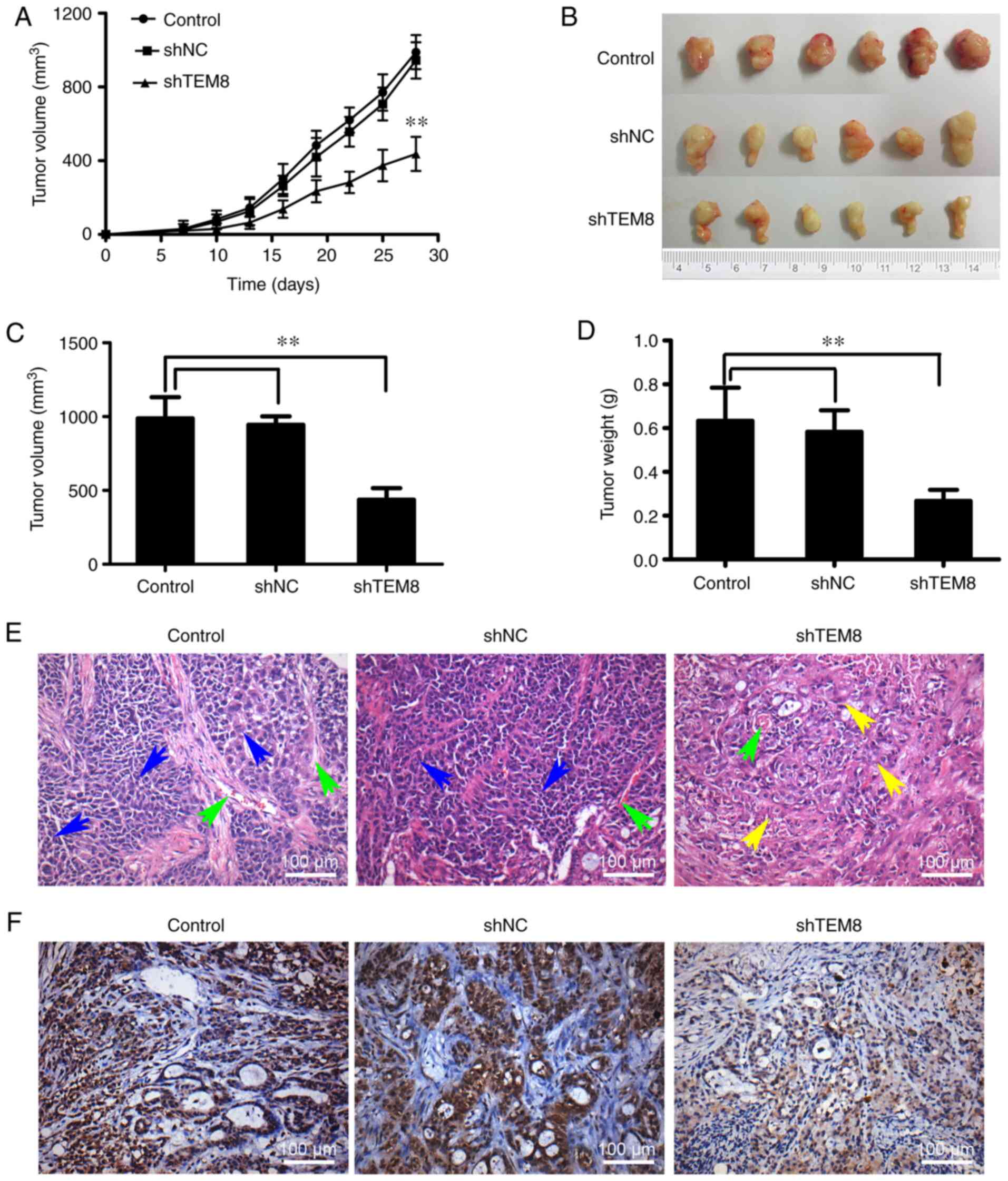
TEM8 knockdown inhibits tumor growth
in a xenograft mouse model
A previous study reported that TEM8 expression was
significantly increased in human lung cancer tissues compared with
that in the normal tissue (11). To
investigate whether TEM8 knockdown has any influence on lung cancer
cell tumorgenicity in vivo, shTEM8-transfected,
shNC-transfected or untreated XWLC-05 cells were subcutaneously
injected into nude mice. As shown in Fig. 2A, tumor growth was significantly
decreased in the shTEM8 group compared with that in the shNC and
control groups. Both final tumor size (Fig. 2B and C) and weight (Fig. 2D) were significantly decreased in
the shTEM8 group compared with those in the shNC and control
groups. Histological analysis was also performed to macroscopically
analyze tumor growth after TEM8 expression was knocked down. The
results showed that the number of tumor cells, vascular tissues and
necrotic foci in the tumor tissue of shNC and control groups were
markedly decreased compared with those in the shTEM8 group
(Fig. 2E). Knock down of TEM8 was
verified by immunohistochemistry. Tumors formed by XWLC-05 cells
transfected with shTEM8 exhibited notably lower TEM8 expression
compared with those in the shNC and control groups (Fig. 2F). These results suggested that TEM8
knockdown could significantly inhibit lung cancer tumor growth
in vivo.
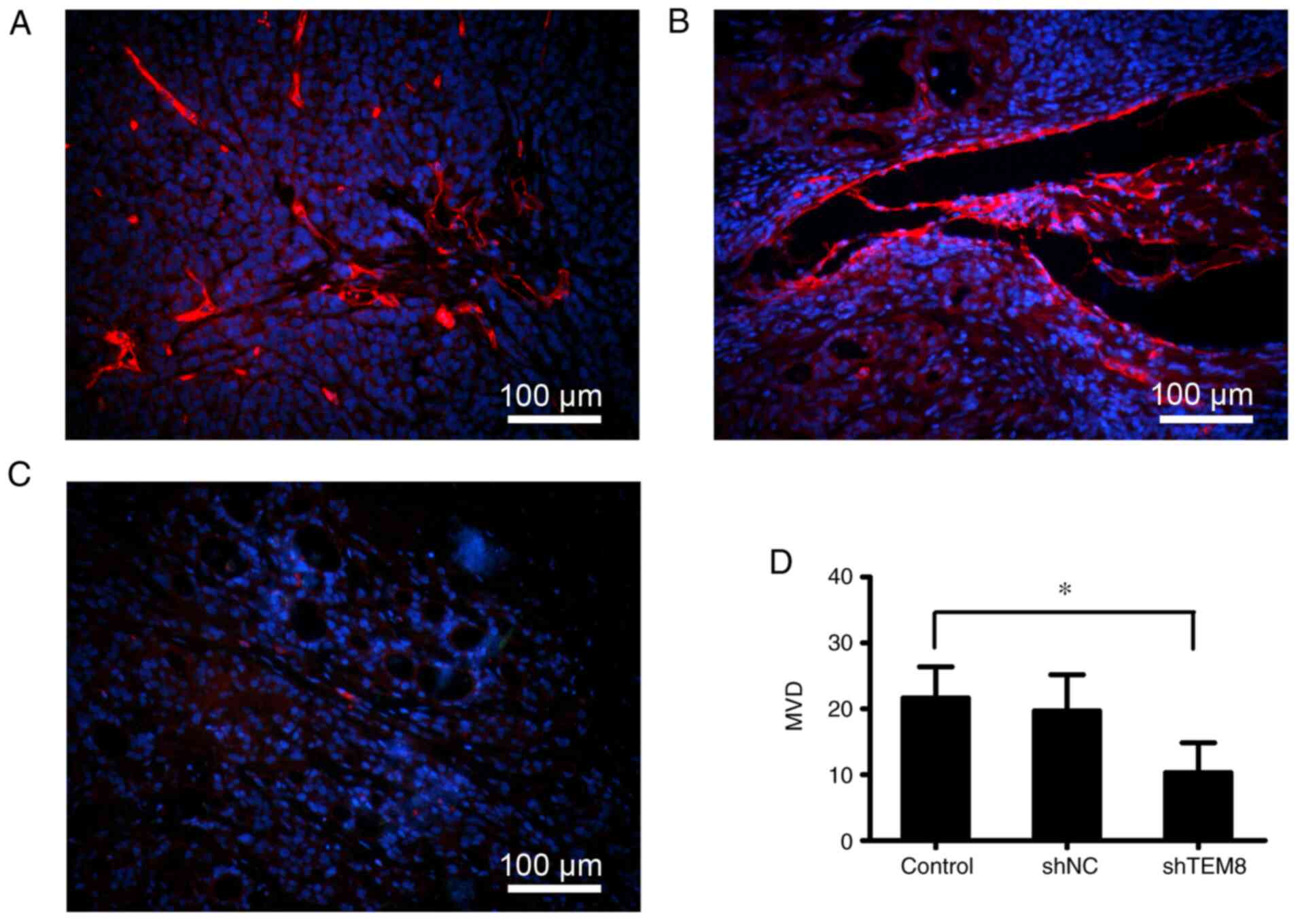
TEM8 knockdown attenuates tumor
angiogenesis in mouse tumor tissues
Previous studies have reported that tumor growth is
associated with angiogenesis (23,24).
To determine if TEM8 knockdown affects angiogenesis in vivo,
the expression of the endothelial surface marker CD34 was detected
by immunofluorescence staining using an anti-CD34 antibody.
Compared with that in the control (Fig.
3A) and shNC groups (Fig. 3B),
the expression of CD34 was notably decreased in the shTEM8 group
(Fig. 3C). The MVD of tumor-bearing
tissues was significantly decreased in the shTEM8 group compared
with that in the shNC and control groups (Fig. 3D). Therefore, TEM8 knockdown could
potently suppress angiogenesis in this xenograft tumor model.
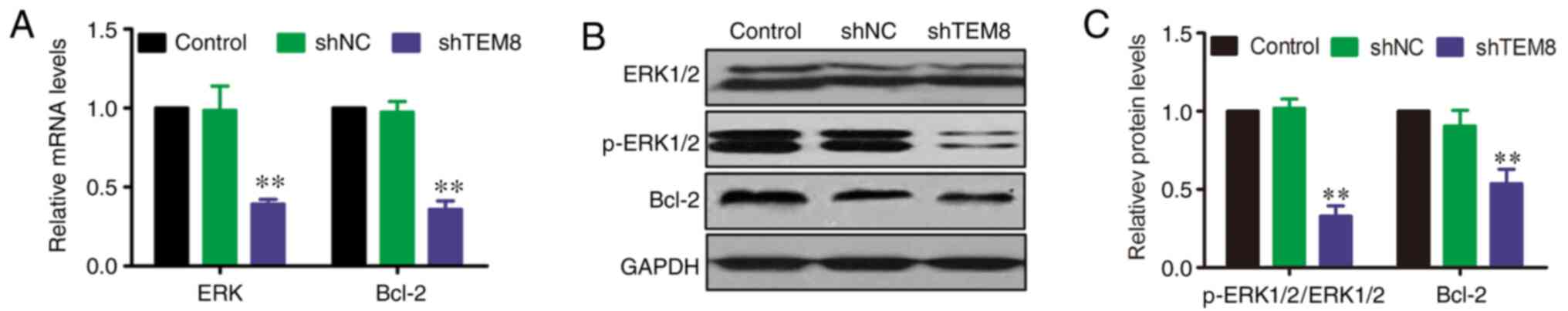
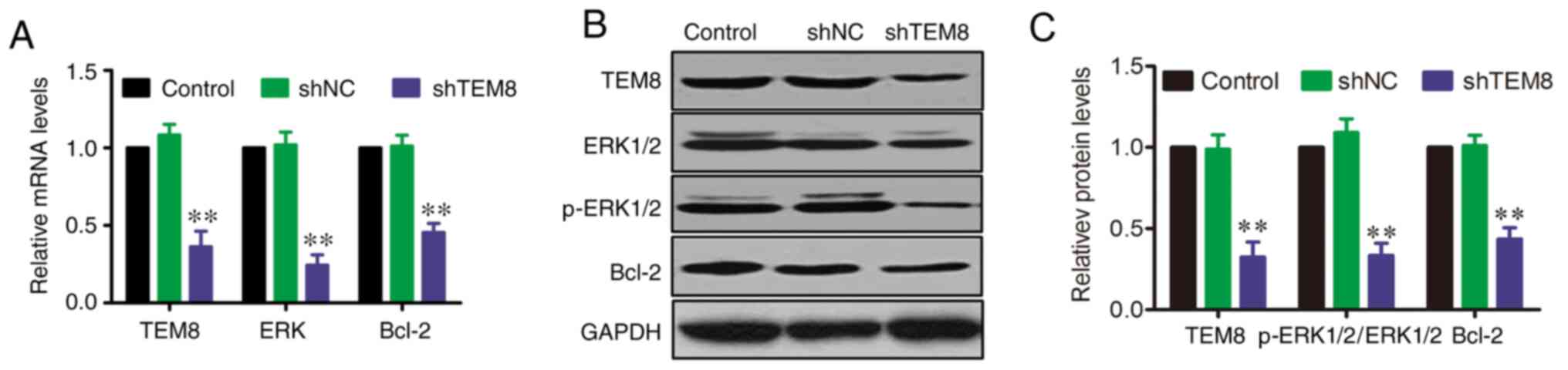
TEM8 knockdown reduces ERK1/2
activation and Bcl-2 expression in XWLC-05 cells
A previous study reported that the ERK signaling
pathway serves an important role in the pathophysiology of lung
cancer (25). However, the effect
of TEM8 on the ERK signaling pathway in XWLC-05 cells remains
unknown. Therefore, the mRNA and protein expression levels of
ERK1/2 and Bcl-2 were evaluated by RT-qPCR and western blotting at
the cellular level after TEM8 gene silencing. RT-qPCR results
showed that the mRNA levels of ERK and Bcl-2 were significantly
decreased in shTEM8-transfected XWLC-05 cells compared with those
in shNC or control XWLC-05 cells (Fig.
4A). In addition, western blot analysis showed that the protein
expression levels of p-ERK1/2 and Bcl-2 were significantly
decreased in shTEM8-transfected cells compared with those in shNC
or control XWLC-05 cells, although there were no variations in
total ERK1/2 expression (Fig. 4B and
C).
TEM8 knockdown reduces the
phosphorylation of ERK1/2 and Bcl-2 expression in xenograft tumor
tissues
To evaluate further if TEM8 regulates the ERK
signaling pathway, the mRNA and protein levels of ERK1/2 and Bcl-2
in isolated xenograft tumor tissues were measured by RT-qPCR and
western blotting. RT-qPCR results showed that the mRNA expression
levels of ERK and Bcl-2 were significantly decreased in the shTEM8
group compared with that in the shNC and control groups (Fig. 5A). Moreover, western blotting
analysis results revealed that the protein expression levels of
p-ERK1/2 and Bcl-2 were significantly decreased in the shTEM8 group
compared with those in the shNC and control groups (Fig. 5B and C).
Discussion
The present study sought to investigate the effects
of TEM8 in a murine xenograft model of lung cancer and its
underlying mechanisms. It was found that: i) TEM8 knockdown
significantly inhibited tumor growth in a murine xenograft model of
lung cancer; ii) TEM8 knockdown prominently attenuated tumor
angiogenesis in tumor tissues of the lung cancer xenografts; and
iii) TEM8 regulation of tumor growth and angiogenesis was in part
mediated by activation of the ERK/Bcl-2 signaling pathway.
Previous studies have reported that TEM8 serves an
important role in lung cancer carcinogenesis (9,11,15),
which is also important for tumor progression and growth (26). In the present study, XWLC-05 cells
were used to induce stable knock down of TEM8 expression in
vitro. Lung cancer in Xuanwei Country (Yunnan province, China)
has been previously demonstrated to be a histological subtype of
NSCLC (27). The XWCL-05 cell line
established by Yan et al (16) as a human lung adenocarcinoma cell
line possesses the characteristics of lung adenocarcinoma. In the
present study, TEM8 knockdown was found to significantly inhibit
tumor growth in a murine xenograft model of lung cancer induced by
injection with the XWLC-05 cell line, which is consistent with
previously reported preclinical cancer models, including colon
cancer, melanoma tumors and infantile hemangioma (28–30). A
number of studies have demonstrated that TEM8 is highly expressed
in tumor tissues, including colon cancer, osteosarcoma and breast
cancer, where it participates in tumor angiogenesis (10,31,32).
Importantly, a previous study also found that TEM8 is highly
expressed in lung cancer tumor tissues compared with adjacent
normal lung tissues (11).
Therefore, it was hypothesized that the significantly inhibited
tumor growth in mice injected with TEM8-knockdown lung cancer cells
compared with that in control mice was associated with tumor
angiogenesis. CD34 is a capillary endothelial cell marker, which
has been used as an angiogenesis marker in previous studies
(33–35) and to measure the intensity of the
microvasculature (36). CD34
staining was therefore used as an angiogenesis marker in the
present study to evaluate if TEM8 knockdown could inhibit tumor
angiogenesis in the lung cancer tumor tissues. TEM8 knockdown was
found to significantly inhibit tumor angiogenesis in tumor tissues,
which was in consistent with previous findings (14,15).
Although previous studies have confirmed that TEM8
knockdown can significantly inhibit tumor growth in numerous cancer
types (10,37), the underlying mechanism remains
poorly understood. TEM8 antibodies have been reported to reduce
tumor growth in mice through an antibody-dependent cellular
cytotoxicity mechanism (15). The
ERK signaling pathway was previously shown to participate in the
progression and metastasis of lung cancer (38), where ERK1/2 phosphorylation can
promote epithelial-to-mesenchymal transition and treatment
resistance in lung cancer (39).
ERK1/2 activity has been demonstrated to serve an important role in
tumorigenesis by regulating a variety of biological processes,
including apoptosis, proliferation and migration (40,41).
The Bcl-2 family of proteins are central regulators of apoptosis,
which are traditionally divided into two types, pro-apoptotic or
anti-apoptotic, where Bcl-2 is the prototypic anti-apoptotic
protein (42). Several studies have
previously knocked down Bcl-2 expression to evaluate anticancer
efficacy in different cancer types (43,44).
ERK signaling has also been shown to positively regulate Bcl-2
expression in human pancreatic cancer. (45). Blocking the ERK signaling pathway
has been demonstrated to exert different outcomes on Bcl-2
expression (42,45). Chang et al (46) previously reported that inhibition of
ERK did not affect the expression of Bcl-2 in lung cancer. Although
ERK may be involved in the dysregulation of Bcl-2 expression,
little is known concerning the role of the ERK signaling pathway in
the regulation of tumor growth in lung cancer after TEM8 knockdown.
To investigate the expression statuses of ERK and Bcl-2 underlying
the regulation of lung cancer tumor growth and angiogenesis by
TEM8, RT-qPCR and western blotting were performed. TEM8 knockdown
was shown to significantly inhibit ERK phosphorylation and Bcl-2
expression at both the transcriptional and protein levels. These
results suggested that TEM8 knockdown in tumor tissues
significantly inhibited the ERK/Bcl-2 signaling pathway.
The present study had some limitations. For example,
different lung cancer cell lines were not used to verify the
results, and ERK gene interference was not performed to verify the
role of TEM8 in regulating NSCLCs via the ERK/Bcl-2 signaling
pathway. The occurrence and development of NSCLC is a complex
biological process involving multiple signaling pathways; however,
the present study only focused on the ERK signaling pathway.
Therefore, further studies are required.
In conclusion, the present study suggested that TEM8
serves an important role in human lung cancer, as it was found that
silencing of TEM8 expression could inhibit xenograft tumor growth
of lung cancer by suppressing the ERK/Bcl-2 signaling pathways.
Therefore, targeting TEM8-mediated molecular mechanisms may prove
to be an effective therapeutic strategy for patients with
NSCLC.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Basic
Research Program of Yunnan Province-Joint Project of Kunming
Medical University (grant no. 2018FE001-255) and Health Science and
Technology Project of Yunnan Province (grant nos. 2017NS174 and
2018NS0049).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LZ participated in the study design and revised the
manuscript. QG and JD performed the experiments and were
responsible for writing the manuscript. LZ and CZ analyzed the
data. CF and XW participated in statistical analysis and organized
the figures. QG and LZ confirm the authenticity of all the raw
data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The experimental protocols used in the present study
were approved by the Committee on the Ethics of Animal Experiments
of Third Affiliated Hospital of Kunming Medical University
(approval no. 16KY-LA00135).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lancet T: Lung cancer: Some progress, but
still a lot more to do. Lancet. 394:18802019. View Article : Google Scholar
|
|
2
|
Herbst RS, Heymach JV and Lippman SM: Lung
cancer. N Engl J Med. 359:1367–1380. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bordoloi D, Banik K, Padmavathi G,
Vikkurthi R, Harsha C, Roy NK, Singh AK, Monisha J, Wang H, Kumar
AP and Kunnumakkara AB: TIPE2 induced the proliferation, survival,
and migration of lung cancer cells through modulation of
Akt/mTOR/NF-κB signaling cascade. Biomolecules. 9:8362019.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cohen JD, Li L, Wang Y, Thoburn C, Afsari
B, Danilova L, Douville C, Javed AA, Wong F, Mattox A, et al:
Detection and localization of surgically resectable cancers with a
multi-analyte blood test. Science. 359:926–930. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Didkowska J, Wojciechowska U, Mańczuk M
and Łobaszewski J: Lung cancer epidemiology: Contemporary and
future challenges worldwide. Ann Transl Med. 4:1502016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Martin P and Leighl NB: Review of the use
of pretest probability for molecular testing in non-small cell lung
cancer and overview of new mutations that may affect clinical
practice. Ther Adv Med Oncol. 9:405–414. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Garmendia I, Pajares MJ, Hermida-Prado F,
Ajona D, Bértolo C, Sainz C, Lavín A, Remírez AB, Valencia K,
Moreno H, et al: YES1 drives lung cancer growth and progression and
predicts sensitivity to dasatinib. Am J Respir Crit Care Med.
200:888–899. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
St Croix B, Rago C, Velculescu V, Traverso
G, Romans KE, Montgomery E, Lal A, Riggins GJ, Lengauer C,
Vogelstein B and Kinzler KW: Genes expressed in human tumor
endothelium. Science. 289:1197–1202. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cao C, Wang Z, Huang L, Bai L, Wang Y,
Liang Y, Dou C and Wang L: Down-regulation of tumor endothelial
marker 8 suppresses cell proliferation mediated by ERK1/2 activity.
Sci Rep. 6:234192016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gong Q, Liu C, Wang C, Zhuang L, Zhang L
and Wang X: Effect of silencing TEM8 gene on proliferation,
apoptosis, migration and invasion of XWLC-05 lung cancer cells. Mol
Med Rep. 17:911–917. 2018.PubMed/NCBI
|
|
12
|
Koo HM, VanBrocklin M, McWilliams MJ,
Leppla SH, Duesbery NS and Vande Woude GF: Apoptosis and
melanogenesis in human melanoma cells induced by anthrax lethal
factor inactivation of mitogen-activated protein kinase kinase.
Proc Natl Acad Sci USA. 99:3052–3057. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Byrd TT, Fousek K, Pignata A, Szot C,
Samaha H, Seaman S, Dobrolecki L, Salsman VS, Oo HZ, Bielamowicz K,
et al: TEM8/ANTXR1-Specific CAR T cells as a targeted therapy for
triple-negative breast cancer. Cancer Res. 78:489–500. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fernando S and Fletcher BS: Targeting
tumor endothelial marker 8 in the tumor vasculature of colorectal
carcinomas in mice. Cancer Res. 69:5126–5132. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chaudhary A, Hilton MB, Seaman S, Haines
DC, Stevenson S, Lemotte PK, Tschantz WR, Zhang XM, Saha S, Fleming
T and St Croix B: TEM8/ANTXR1 blockade inhibits pathological
angiogenesis and potentiates tumoricidal responses against multiple
cancer types. Cancer Cell. 21:212–226. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yan FC, Wang QQ, Ruan YH, Ma LJ, Jia JT
and Jin KW: Establishment and biological characteristics of lung
cancer cell line XWLC-05. Ai Zheng. 26:21–25. 2007.(In Chinese).
PubMed/NCBI
|
|
17
|
Carmeliet P and Jain RK: Angiogenesis in
cancer and other diseases. Nature. 407:249–257. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hurwitz H, Fehrenbacher L, Novotny W,
Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S,
Holmgren E, et al: Bevacizumab plus irinotecan, fluorouracil, and
leucovorin for metastatic colorectal cancer. N Engl J Med.
350:2335–2342. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Verheul HM and Pinedo HM: Possible
molecular mechanisms involved in the toxicity of angiogenesis
inhibition. Nat Rev Cancer. 7:475–485. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen HX and Cleck JN: Adverse effects of
anticancer agents that target the VEGF pathway. Nat Rev Clin Oncol.
6:465–477. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Weidner N: Current pathologic methods for
measuring intratumoral microvessel density within breast carcinoma
and other solid tumors. Breast Cancer Res Treat. 36:169–180. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hernandez JL, Padilla L, Dakhel S, Coll T,
Hervas R, Adan J, Masa M, Mitjans F, Martinez JM, Coma S, et al:
Therapeutic targeting of tumor growth and angiogenesis with a novel
Anti-S100A4 monoclonal antibody. PLoS One. 8:e724802013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Craig M, Ying C and Loberg RD:
Co-inoculation of prostate cancer cells with U937 enhances tumor
growth and angiogenesis in vivo. J Cell Biochem. 103:1–8. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lu Z, Ding L, Hong H, Hoggard J, Lu Q and
Chen YH: Claudin-7 inhibits human lung cancer cell migration and
invasion through ERK/MAPK signaling pathway. Exp Cell Res.
317:1935–1946. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kerbel RS: Tumor angiogenesis. N Engl J
Med. 358:2039–2049. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li R, Liu Y, Wang T, Tang J, Xie L, Yao Z,
Li K, Liao Y, Zhou L, Geng Z, et al: The characteristics of lung
cancer in Xuanwei County: A review of differentially expressed
genes and noncoding RNAs on cell proliferation and migration.
Biomed Pharmacother. 119:1093122019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jinnin M, Medici D, Park L, Limaye N, Liu
Y, Boscolo E, Bischoff J, Vikkula M, Boye E and Olsen BR:
Suppressed NFAT-dependent VEGFR1 expression and constitutive VEGFR2
signaling in infantile hemangioma. Nat Med. 14:1236–1246. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Carson-Walter EB, Watkins DN, Nanda A,
Vogelstein B, Kinzler KW and St Croix B: Cell surface tumor
endothelial markers are conserved in mice and humans. Cancer Res.
61:6649–6655. 2001.PubMed/NCBI
|
|
30
|
Nanda A, Carson-Walter EB, Seaman S,
Barber TD, Stampfl J, Singh S, Vogelstein B, Kinzler KW and St
Croix B: TEM8 interacts with the cleaved C5 domain of collagen
alpha 3(VI). Cancer Res. 64:817–820. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rmali KA, Watkins G, Harrison G, Parr C,
Puntis MC and Jiang WG: Tumour endothelial marker 8 (TEM-8) in
human colon cancer and its association with tumour progression. Eur
J Surg Oncol. 30:948–953. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen D, Bhat-Nakshatri P, Goswami C, Badve
S and Nakshatri H: ANTXR1, a stem cell-enriched functional
biomarker, connects collagen signaling to cancer stem-like cells
and metastasis in breast cancer. Cancer Res. 73:5821–5833. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Popravka ES, Dyatlova AS, Lin'kova NS,
Krylova YS, Polyakova VO and Kvetnoi IM: Role of LIF Cytokine and
CD34 angiogenesis marker in non-developing pregnancy. Bull Exp Biol
Med. 163:772–776. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Vieira SC, Silva BB, Pinto GA, Vassallo J,
Moraes NG, Santana JO, Santos LG, Carvasan GA and Zeferino LC: CD34
as a marker for evaluating angiogenesis in cervical cancer. Pathol
Res Pract. 201:313–318. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tanaka F, Otake Y, Yanagihara K, Kawano Y,
Miyahara R, Li M, Ishikawa S and Wada H: Correlation between
apoptotic index and angiogenesis in non-small cell lung cancer:
Comparison between CD105 and CD34 as a marker of angiogenesis. Lung
Cancer. 39:289–296. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ceyran AB, Şenol S, Güzelmeriç F, Tunçer
E, Tongut A, Özbek B, Şavluk Ö, Aydın A and Ceyran H: Effects of
hypoxia and its relationship with apoptosis, stem cells, and
angiogenesis on the thymus of children with congenital heart
defects: A morphological and immunohistochemical study. Int J Clin
Exp Pathol. 8:8038–8047. 2015.PubMed/NCBI
|
|
37
|
Opoku-Darko M, Yuen C, Gratton K, Sampson
E and Bathe OF: Tumor endothelial marker 8 overexpression in breast
cancer cells enhances tumor growth and metastasis. Cancer Invest.
29:676–682. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Heigener DF, Gandara DR and Reck M:
Targeting of MEK in lung cancer therapeutics. Lancet Respir Med.
3:319–327. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kim E, Youn H, Kwon T, Son B, Kang J, Yang
HJ, Seong KM, Kim W and Youn B: PAK1 tyrosine phosphorylation is
required to induce epithelial-mesenchymal transition and
radioresistance in lung cancer cells. Cancer Res. 74:5520–5531.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chang CH, Li JR, Shu KH, Fu YC and Wu MJ:
Hydronephrotic urine in the obstructed kidney promotes urothelial
carcinoma cell proliferation, migration, invasion through the
activation of mTORC2-AKT and ERK signaling pathways. PLoS One.
8:e743002013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Koyama T, Ogawara K, Kasamatsu A, Okamoto
A, Kasama H, Minakawa Y, Shimada K, Yokoe H, Shiiba M, Tanzawa H
and Uzawa K: ANGPTL3 is a novel biomarker as it activates ERK/MAPK
pathway in oral cancer. Cancer Med. 4:759–769. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Galante JM, Mortenson MM, Bowles TL,
Virudachalam S and Bold RJ: ERK/BCL-2 pathway in the resistance of
pancreatic cancer to anoikis. J Surg Res. 152:18–25. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lariche N, Lahouel M, Benguedouar L and
Zellagui A: Ferulenol, a sesquiterpene coumarin, induce apoptosis
via mitochondrial dysregulation in lung cancer induced by
Benzo[a]pyrene: Involvement of Bcl2 protein. Anticancer Agents Med
Chem. 17:1357–1362. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Beider K, Begin M, Abraham M, Wald H,
Weiss ID, Wald O, Pikarsky E, Zeira E, Eizenberg O, Galun E, et al:
CXCR4 antagonist 4F-benzoyl-TN14003 inhibits leukemia and multiple
myeloma tumor growth. Exp Hematol. 39:282–292. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Boucher MJ, Morisset J, Vachon PH, Reed
JC, Lainé J and Rivard N: MEK/ERK signaling pathway regulates the
expression of Bcl-2, Bcl-X(L), and Mcl-1 and promotes survival of
human pancreatic cancer cells. J Cell Biochem. 79:355–369. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chang GC, Hsu SL, Tsai JR, Wu WJ, Chen CY
and Sheu GT: Extracellular signal-regulated kinase activation and
Bcl-2 downregulation mediate apoptosis after gemcitabine treatment
partly via a p53-independent pathway. Eur J Pharmacol. 502:169–183.
2004. View Article : Google Scholar : PubMed/NCBI
|