Introduction
In 1827, the German professors Friedrich Tiedemann
and Leopold Gmelin became the pioneers who isolated the currently
known taurine originating from Ox bile and they named it
Gallen-Asparagin (1). Later, it was
named taurus, after the Latin Bos taurus which means Ox. However,
the currently used name (taurine) first appeared in the literature
in 1838 by von H. Demarcay (2).
Since 1975, the implication of taurine in homeostasis has intrigued
the scientific community. Initially, it was reported that taurine
deficiency leads to retinal degeneration in cats (3). Diminished taurine levels appeared to
be implicated in the development of other pathological conditions,
such as cardiomyopathy and developmental defects in many species,
therefore suggesting a stringent requirement of taurine for
homeostasis (4).
The role of taurine in homeostasis
Taurine (2-aminoethanesulfonic acid;
NH2CH2CH2SO3H) is a
non-essential amino acid, not participating in protein synthesis
because it is devoid of a carboxyl group. It is not metabolized and
not involved in gluconeogenesis, thereby not constituting a direct
energy source (5). Taurine is an
abundant amino acid in many mammalian tissues, including the
retina, skeletal muscle, liver, platelets, and leukocytes, exerting
many physiological activities, especially in electrically excitable
tissues (heart and brain) (6).
Since taurine is a sulfonic amino acid with strong acidity and it
comprises a zwitterionic amino acid (pK1=1.5, pK2=8.8), it is
plausible that it exhibits strong water-soluble and poor lipophilic
properties over the physiological pH range. Even though taurine has
low membrane permeability, it passes through the cells via the
sodium/chloride dependent taurine transporter (TauT), which is
committed to the transport of taurine from the extracellular to the
intracellular compartments (7). For
this reason, it passes through the lipophilic cellular membrane,
and not diffused through, thereby forming a steep concentration
gradient. For example, plasma taurine levels range from 40 to 100
µΜ, reaching very high concentrations in tissues such as the heart
(30 mM) (8), suggesting that plasma
taurine levers are 100-fold lower than in other tissue (7).
The biosynthesis of taurine occurs primarily in the
liver, in the kidney, to a smaller extent, in the brain (4) and its content relies on
cysteine/methionine metabolism (9).
Endogenously, there are two distinct routes of taurine
biosynthesis. Firstly, cysteine dioxygenase (CDO) participates in
the oxidation of cysteine to cysteine sulfinic acid, where the
cysteine sulfinic acid decarboxylase (CSAD) enzyme exerts its
action, converting cysteine sulfinic acid to hypotaurine, with the
final step being the oxidation of hypotaurine to taurine (10,11).
The importance of the CSAD enzyme is confirmed by the death of
third generation (G3) CSAD KO (knock-out) mouse models within 24 h
from their birth. Alternatively, cysteine can also be conjugated to
coenzyme A (CoA) and in this way, cysteamine is released during CoA
turnover. In this procedure, the 2-aminoethomethiol dioxygenase
(ADO) enzyme is actively involved in converting cysteamine to
hypotaurine. Notably, the biosynthetic capacity of taurine is high
in prenatal life and starts to decline in adulthood, reaching its
lowest concentrations in the elderly and in certain pathological
conditions (trauma, sepsis). Therefore, taurine biosynthesis does
not produce the amount of taurine required for homeostasis and for
this reason, exogenous dietary supply of taurine becomes necessary
(12). The amount of daily taurine
intake is estimated to range from 40 to 400 mg (13) and taurine content depends on the
dietary intake of animal/sea origin (12). Characteristically, it has been shown
that taurine distribution in vegans is half compared to individuals
that follow an omnivore diet (14).
Accordingly, the urinary fractional excretion of taurine ranges
from 0.5 to 80.0% based on the diet (5,15).
The physiochemical properties of taurine potentially
make it an ideal modulator of various basic processes, including
osmoregulation, modulation of protein phosphorylation, calcium ion
regulation, anti-oxidant response, membrane stabilization, bile
acid conjugation, lipid metabolism, glucose regulation (16,17).
The prevailing view regarding the contribution of taurine to bile
acid excretion is supported by the notion that taurine conjugates
to cholesterol, promoting cholesterol excretion through bile salt
formation and fat digestion (18).
Furthermore, taurine plays a significant role in reducing lipid
peroxidation (LPO) products, thereby protecting cells from tissue
damage (19). Notably, taurine is
considered to be fundamental in maintaining homeostasis, mainly due
to functioning as an intracellular osmolyte in the brain and renal
medulla (5,20). It has been reported that taurine is
recruited to hypertonic solutions and is released towards lower
osmolarity solutions (21). In this
way, the osmoregulatory properties of taurine can influence cell
volume in tissues. For example, activation of the Fas receptor in
Jurkat T lymphocytes is accompanied by high taurine efflux, thereby
contributing to cell shrinkage (22). Apart from the potential of taurine
to regulate cell volume (23), its
osmolytic activity can also contribute to protein folding in the
endoplasmic reticulum (ER) (24).
For example, taurine stimulates proper protein folding and membrane
trafficking of the mutant cystic fibrosis transmembrane conductance
regulator (CFTR) protein (delta508 CFTR) in the ER (25). Besides, various reports have also
suggested the suppressive impact of taurine on infection, oxidative
stress, inflammation, as well as its anti-microbial, its
anti-diabetic and its antitumor activity (26).
The anti-apoptotic nature of taurine
underlying its effectiveness
Several examples support that taurine predominantly
inhibits apoptosis. Following intestinal ischemia-reperfusion (IR)
injury, treatment with taurine appears to abolish mucosal damage
and to protect intestinal epithelial cells from apoptosis in IR
rats (27). Accordingly, the size
of infarction in ischemic-induced brain injury seems to be
significantly reduced through the downregulation of pro-apoptotic
proteins following treatment with taurine (28). In the case of acute myocardial
infarction, exogenous taurine administration seems to confer
protection in glucose deprived rat cardiomyocytes from
mitochondrial and endoplasmic reticulum stress, by interfering with
mitochondria-dependent apoptosis and unfolded protein response
(UPR)-related apoptosis (29). In
another example, the treatment of UV-treated cells with taurine
protect them from oxidative stress, owing to the downregulation of
p53-Chk1 pathway (30). This
anti-apoptotic property of taurine has also been demonstrated in
injuries mediated by toxic insults or drugs, where taurine
apparently reverses doxorubicin (DOX)-mediated liver damage
(31). In the same context,
taurine's anti-apoptotic ability may ameliorate oxidized
low-density lipoprotein (oxLDL)-induced cytotoxicity, as shown by
experiments in oxLDL exposed human renal proximal tubular
epithelial (HK-2) cells (32). The
cytoprotective effect of taurine has also been confirmed in
osteocytes, by preventing oxidative stress-induced cell death
(33). In the same context, taurine
exerts a protective effect on hydrogen peroxide
(H2O2)-mediated damaged osteoblasts, through
stimulation of extracellular-signal-regulated kinases (ERK) and
Wnt/β-catenin pathway (34).
Since taurine is fundamental to certain aspects of
normal mammalian development, the positive relationship between
taurine loss and various pathological insults is somewhat
anticipated. In this frame, pathologies such as mitochondrial
myopathy, encephalopathy, lactic acidosis, stroke-like episodes
(MELAS) and myoclonic epilepsy and ragged-red fiber syndrome
(MERRF) have been attributed to taurine deficiency (35,36),
because taurine functions as a substrate for mitochondrial tRNA,
thereby protecting mitochondria against excessive superoxide anion
(O2−) generation and ensuring normal
adenosine triphosphate (ATP) biosynthesis. Recently, taurine
administration seems to alleviate negative effects of mitochondrial
oxidative stress found in induced pluripotent stem cells (iPSCs) of
a patient with MELAS, by normalizing glutathione (GSH) stores and
epithelial-mesenchymal transition (EMT) (37).
If one considers that certain pathological lesions
are related to low taurine levels (38), it is reasonable to suggest that
taurine possibly exhibits a therapeutic effect against
cardiovascular diseases, hypercholesterolemia, epileptic seizures,
muscular degeneration, Alzheimer's disease, hepatic disorders,
alcoholism, and cystic fibrosis.
The role of taurine against oxidative stress
and its underlying molecular mechanisms
Taurine's anti-oxidant activity has been
acknowledged for over a decade. On one hand, the high concentration
of taurine and its transporter in lymphocytes (39–41),
provided significant evidence that taurine was actively involved in
the defense against oxidative stress (42). On the other hand, low taurine serum
levels have been closely associated with many oxidative
stress-mediated pathologies, including hepatic disorders, epilepsy,
cardiomyopathy, cystic fibrosis, alcoholism, Alzheimer's disease,
growth retardation and retinal degeneration (43,44).
When taurine levels decrease, energy metabolism suffers elevations
in the NADH/NAD+ ratio, a process followed by the
inhibition of key dehydrogenases (45). The citric acid cycle appears
vulnerable to increases in the NADH/NAD+ ratio, as three
NADH sensitive enzymes (α-ketoglutarate dehydrogenase, isocitrate
dehydrogenase, and citrate synthase) are prone to inhibition by
elevations in the NADH/NAD+ ratio (45). Experiments using taurine transporter
inhibitor (β-alanine) have highlighted the inhibition of
oxidative metabolism through impaired synthesis of mitochondrial
proteins and reduced activity of respiratory chain complexes I and
III, which may in turn lead to compromised mitochondrial integrity
and decreased ATP production (46,47).
We should take into consideration that a decline in electron
transport chain (ETC) is mainly mediated through changes in
oxidative phosphorylation (OXPHOS), contributing to the development
of multiple diseases, despite the NADH/NAD+ contribution
being largely unknown in this process (48). As a consequence, citric acid flux
appears to be significantly reduced, leading to inefficient
oxidation of endogenous fatty acids and exogenous acetate in the
taurine-depleted muscle and favoring the conversion of glucose to
lactate for NADH production (45).
For example, inhibition of pyruvate dehydrogenase dephosphorylation
seems to strengthen its activity, with a concomitant reduction in
the expression of fatty acid metabolism-related genes, such as
muscle-type carnitine palmitoyl transferase-1 (mCPT-1) (45). The latter makes sense if one
considers that fatty acid oxidation genes are under the control of
both peroxisome proliferator-activated receptor alpha (PPARα) and
5′ AMP-activated protein kinase (AMPK) and that the expression of
the AMPKβ2 subunit is reduced to a minimum in the skeletal
muscle of taurine transporter knock-out (TauTKO) mice [33]. In
support of this, AMPK modulates the protein expression of acetyl
CoA carboxylase (ACC), which facilitates the transport of
long-chain fatty acids into the mitochondria for β-oxidation
(49), thus supporting impaired
fatty acid oxidation in taurine-deficient conditions.
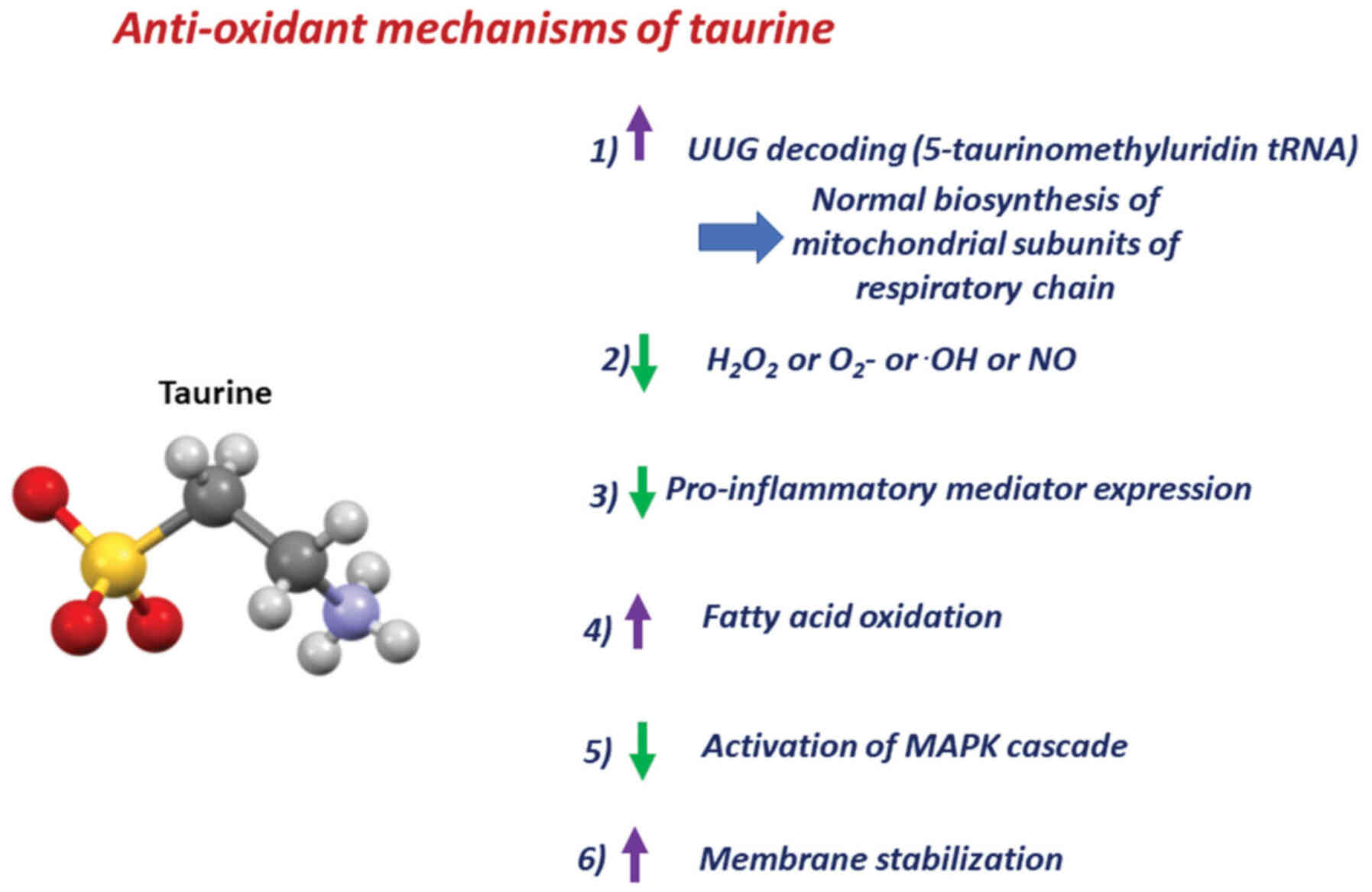
A plethora of underlying molecular mechanisms
reportedly explain taurine's beneficial action in oxidative
stress-associated pathologies (Fig.
1). First of all, taurine is involved in the anti-oxidant
defense, as it is conjugated to the tRNA (46). In normal mitochondria, UUG codons of
mitochondrial mRNAs are decoded due to the conjugation of taurine
with a uridine in the AAU anticodons of tRNA (46). When 5-taurinomethyluridine-tRNA is
formed, the UUG codons of mRNA can interact with the AAU anticodons
of tRNA (46). This way, following
treatment with taurine, decoding of proteins containing UUG codons
is enhanced, thereby protecting cells from mitochondrial oxidative
stress (50) and
mitochondria-dependent apoptosis (51,52).
In contrast, when the taurine substrate is lost, the conjugated
form of taurine [5 taurinomethyluridine-tRNA] is diminished,
causing a significant decline in specific mitochondria-encoded
proteins that contain multiple UUG codons, such as NADH-ubiquinone
oxidoreductase chain 6 (36).
Notably, there is a decrease in respiratory chain complex I subunit
biosynthesis, thereby inhibiting the appropriate assembly of
subunits required for normal ATP generation (36,46).
In addition to playing a fundamental role within
mitochondria, taurine's anti-oxidant activities can take place
outside the mitochondria (53). The
cytoprotective action of taurine is also evidenced through its
potential to detoxify hydrogen peroxide
(H2O2) (54),
hydroxyl radicals (.OH) (55) and nitric oxide (NO) (12), without acting as the classical
scavenger of reactive oxygen species (ROS) formation. For example,
when cardiomyocytes were incubated with the taurine transporter
antagonist (guanidinoethane sulfonate-GES) or taurine transporter
inhibitor (β-alanine), mitochondrial oxidative stress increased and
was accompanied by an accumulation of superoxide anions
(O2−), glutathione oxidation (GSSG), and
inactivation of anti-oxidant enzymes such as aconitase (46,50).
However, treatment with taurine appeared to reverse the oxidative
stress by increasing the electron transport chain activity, thereby
protecting mitochondria against excessive O2−
generation (46). In line with
previous findings, the notion that taurine counteracts the side
effects of oxidative stress either in mitochondria or in the
endoplasmic reticulum following exposure of glucose deprived rat
cardiomyocytes to taurine has been confirmed by another research
group (29). Specifically, taurine
seemed to exert its preventive action via maintaining an intact
membrane potential (Δψm), via regulating anti-oxidant
enzymes, via suppression of intracellular calcium levels, and
through downregulation of UPR-related proteins (29).
Taurine can also act as a cytoprotective molecule
against oxidative stress conditions, by decreasing LPO (19) or calcium overload (56–58).
For example, in experiments with rats receiving tamoxifen,
taurine's anti-oxidant properties have been manifested through
attenuation of mitochondrial LPO, thereby protecting the animals
from damage (59). Similarly,
taurine has been shown to confer protection against oxidative
stress through normalization of glutathione (GSH) stores in rats
following nicotine administration (60,61).
In another case, it has been reported that taurine confers a
benefit to NMDA-administered Sprague-Dawley rats bearing retinal
injury (62). Indeed, taurine
ameliorates retinal oxidative stress, through augmenting
glutathione levels and action of anti-oxidant enzymes (62). Alternatively, taurine acts as an
indirect anti-oxidant, as it has the capacity to stabilize the
biological membrane and to suppress alterations in membrane
permeability caused by oxidative stress factors (56,63).
In the same context, the indirect anti-oxidant capacity of taurine
is manifested through the formation of taurine derivatives
(N-bromotaurine or N-chlorotaurine), which have been produced from
the reaction of taurine with either hypobromous acid (HOBr) or
hypochlorous acid (HOCl) (26).
Taurine derivatives appear to play an important role in protecting
phagocytic cells from their self-imposed oxidative injury (26,64).
In addition, taurine modulates the renin-angiotensin
system as a defense mechanism against oxidative stress. Several
lines of evidence support taurine's indirect anti-oxidant role by
its capacity to modulate angiotensin II (Ang II) signaling, through
its effect on the nicotinamide adenine dinucleotide phosphate
(NADPH) oxidase. Notably, NADPH oxidase is activated following
induction of protein kinase C (PKC) by Ang II signaling and
contributes to O2− generation (51). In another case, increased generation
of ROS and p38 mitogen-activated protein kinase (MAPK)
phosphorylation, as well as increased angiotensin secretion have
been observed in renal proximal tubular cells following
administration of high glucose (25 mM), all of which are
neutralized following taurine administration (65).
Even though taurine has not been tightly associated
with inhibiting ROS accumulation, it is believed to exert its
action through modulation of anti-oxidant enzymes (66,67).
Several reports have supported the notion that taurine enhances the
activity of anti-oxidant enzymes (superoxide dismutase-SOD,
catalase-CAT, glutathione peroxidase-GPx, glutathione reductase-GR)
(68–70), preserving redox levels and GSH
stores (71).
In this review, we outline the oxidative stress
conditions which taurine exerts its action in, thereby normalizing
the disturbance of redox status. The administration of taurine to
patients with oxidative stress-related pathologies (hepatotoxicity;
renal disorders; epilepsy; cardiomyopathy; cystic fibrosis;
Alzheimer's disease; growth retardation and retinal degeneration)
appears to have a beneficial effect, due to its anti-oxidant
activity (46). In recent years,
taurine has been identified as an attractive protective agent for a
broad spectrum of oxidative stress-induced pathologies caused by
chemical agents (72,73), toxins (74,75),
certain diseases (76,77) and several potential therapeutic
options (74,78,79).
The beneficial effect of taurine against
neuro-associated disorders
Taurine seems to be abundant in the central nervous
system (80), constituting the
major component of electrically excitable tissues (including the
brain) and determining the regeneration of the central nervous
system (16). Indeed, taurine has
been acknowledged as an enhancer of GABAAR and
GABABR receptors (81–84),
knowing that gamma-aminobutyric acid (GABA) is the main inhibitory
neurotransmitter of the mammalian central nervous system (CNS)
(85). When neuronal C6 cells are
subjected to morphine-induced oxidative insult and apoptosis,
taurine has a positive counteracting effect on oxidative stress, as
opposed to anti-oxidant activity conferred by the activation of
GABA receptors (86). In this way,
taurine has been considered a significant neuromodulator, through
its potential to inhibit glutamate transmission (87).
In brain, taurine has been shown to exert both
metabolic and anti-oxidant activity. Firstly, taurine probably
serves as a potential anti-oxidant agent in cerebral cell-defense,
as shown by the direct interaction of taurine with either
H2O2 or O2− or OH
(55). Secondly, taurine's
neuroprotective activity has been explained by its positive effect
on carbohydrate metabolism, its inhibitory impact on LPO, its
potential to sustain the action of enzymes involved in electron
transport chain such as succinate dehydrogenase (SDH) to normal
levels, its capacity to reduce phenomena of either post-ischemic
defect or hypercoagulation (88).
Thirdly, the function of taurine also contributes to increased
hyperpolarizing chloride-mediated conductance and increased
membrane stability (89). Fourthly,
taurine exerts a strong neuroprotective function through the
inhibition of extracellular calcium influx and the outflow of
calcium from intracellular pools and through the regulation of
neurotransmitters (57,90).
Taurine's neuro-modulating activity is important not
only in normal situations, but also in neuronal damage. In
neurodegenerative diseases, cell death is caused by
glutamate-mediated hyperexcitability and impaired mitochondrial
membrane potential (91). Following
activation of glutamate receptors, mitochondrial respiratory chain
defects along with calcium overload and ROS accumulation seem to be
the major routes that are induced, allowing the stimulation of
apoptosis (91,92). Among the known signal transduction
pathways related to apoptosis is the activation of UPR pathways
during ER stress, which leads to the activation of caspase-12, CHOP
(C/EBP homologous protein) and c-Jun NH-terminal kinase (JNK)
proteins (93,94). In this context, taurine can function
as a repair system against glutamate toxicity by inhibiting ER
stress-mediated apoptosis and calcium overload (89,95).
Interestingly, taurine seems to attenuate peroxisomal oxidative
malfunction, by reducing malondialdehyde (MDA) levels, expression
levels of peroxisomal membrane proteins and by upregulating
anti-oxidant enzymes in rats injected intracerebroventricularly
with Aβ 1–42 (96). Similarly,
taurine's protective mode against glutamate-induced oxidative
neurotoxicity appears to happen through an increase in heme
oxygenase-1 (HO-1) expression in HT22 mouse hippocampal neuronal
cells (97). In particular, taurine
has been shown to upregulate the transactivation rate of nuclear
factor-E2-related factor 2 (Nrf2), which is the key transcription
factor for HO-1 expression (97).
Use of p38 inhibitors has confirmed that taurine-mediated HO-1
expression is caused by the induction of the p38 signaling cascade
(97). In addition to affecting the
HO-1 expression, taurine has also been documented to enhance
anti-oxidant responses in many other cases of neurotoxicity.
Interestingly, taurine administration appears to increase the
action of anti-oxidant enzymes (SOD, CAT, and GPx), in an attempt
to overcome morphine-induced oxidative stress (86) and hexabromocyclododecane
(HBCD)-induced cytotoxicity (98).
In perfluorooctane sulfonate (PFOS)-induced neurotoxicity, taurine
has been shown to exert potent anti-oxidant properties through its
ROS-scavenging capacity in PC12 cells, originating from the
embryonic neural crest (98).
In keeping with the anti-oxidant role of taurine, it
has been reported that taurine can ameliorate neuro-associated
changes arising in the cerebrum, following exposure to toxins such
as arsenic (99–101). For example, taurine can preserve
the viability of arsenite-exposed primary cortical neurons, through
upregulation of PI3K/Akt pathway (102). Taurine emerges as a key protective
factor against the activation of autophagy in the cerebrum of
arsenic oxidoarsenous trioxide
(As2O3)-intoxicated mice (103), given that the self-degradation
process of autophagy is the main cause of arsenic-induced
neurotoxicity (104,105). Specifically, the expression levels
of microtubule-associated protein light chain 3B (LC3II) and its
bound p62 protein are reduced in cerebral neuronal cells of
As2O3-intoxicated mice following taurine
administration, thereby highlighting taurine's neuroprotective role
(103). If one considers that the
Nrf2 transcription factor is transactivated following oxidative
stress so as to stimulate autophagic flux (106,107), it is possible that taurine can
attenuate the expression levels of Nrf2 transcription factor in the
cerebrum of As2O3-exposed mice (103). Accordingly, it has been reported
that taurine causes a marked decline of autophagy marker such as
LC3II, following methamphetamine (METH) and taurine administration
in PC12 cells, originating from the embryonic neural crest
(108).
The beneficial effect of taurine on stroke-related
injury has also been examined in humans (109). The advantageous effect of taurine
on stroke events has been attributed to its anti-oxidant and
anti-inflammatory properties. Specifically, taurine was shown to
enhance anti-oxidant enzyme activity by reducing
O2− production, thereby improving
mitochondrial function in traumatic/ischemic brain injury (TBI)
(110). Such evidence is in
agreement with the involvement of taurine in mitochondrial protein
synthesis and increased ETC activity (111). In another case, Lotocki et
al (112) demonstrated that
expression levels of pro-inflammatory cytokines (TNF-α, IL-6, IL-1α
and IL-1β) were substantially reduced in both spinal cord injury
(SCI) and traumatic/ischemic brain injury (TBI), following taurine
treatment (113), proving
taurine's anti-inflammatory properties in injured brain cells
(113). Accordingly, the
anti-oxidant and the anti-inflammatory properties of taurine
account for its regenerative and protective capacity in TBI cells
(114). Furthermore, taurine
levels have been increased as a defense mechanism against
excitotoxicity and hypoxia (115).
Specifically, taurine has been shown to ameliorate damage in rat
cortical brain slices subjected to a hypoxia-reoxygenation insult
by suppressing brain cell swelling (116); this is probably due to taurine's
inhibitory action on the substrate of NADH oxidase (45,117),
via which it mediates the downregulation of Nox2/Nox4 (NADH oxidase
isoforms) (118).
The anti-oxidant efficacy of taurine against
cardiac- associated oxidative stress
Initial efforts highlighted that a taurine-deficient
diet causes dilated cardiomyopathy in the cat and fox (119,120). Later on, it was proposed that
taurine ablation mediated by either taurine transporter inhibitor
(β-alanine) or taurine transporter antagonist [guanidinoethane
sulfonate (GES)] leads to cardiac injuries in mice or rats
(121). Following these
observations, it was proposed that low plasma taurine is associated
with myocardial failure (119).
The hallmarks of taurine deficiency-induced cardiomyopathy are as
follows: Remodeling of ventricular cardiomyocytes, ultrastructural
damages of myofilament and mitochondria, and overexpression of
markers of heart failure, such as atrial natriuretic peptide (ANP),
brain natriuretic peptide (BNP) and beta-myosin heavy chain (MYH7)
(122,123). In a clinical setting, it has been
recorded that patients with chronic congestive heart failure have
twice the amount of taurine as compared to matched controls,
highlighting an inconsistency between the results obtained from
patients and the results derived from inhibitor-mediated taurine
loss (124).
Over 50 percent of the total free amino acid pool in
the heart is reportedly occupied by taurine (125), which appears to have a positive
inotropic action on cardiac tissue (126) and to lower high blood pressure
(127,128). Taurine protects cardiomyocytes
from damage mediated by either excessive or inadequate calcium ion
levels due to its regulatory effect on the activity of the
voltage-dependent calcium and sodium channels (129). At the same time, taurine acts on
many other ion channels and transporters, even though its mechanism
of action is not quite specific (14). Importantly, it has also been
suggested that taurine stabilizes membrane potential through its
interference with membrane-bound Na+K+ATPase
(130).
The inhibitory effect of taurine on
atherogenesis
Considering the multiple functions of taurine, its
administration appears to cause a regression of atherogenesis
through several possible mechanisms. First of all, in the majority
of studies, taurine administration caused a marked decline in
cholesterol levels in atherogenic animals (131–133). During the regression period of
atherogenesis, hepatic cholesterol levels were rapidly decreased in
taurine treated animals, due to taurine's ability to accelerate the
degradation of cholesterol, as indicated by the increase in
cholesterol 7α-hydroxylase (CYP7A1) activity (132–135). At the same time, taurine
administration inhibited hepatic biosynthesis of cholesterol esters
and triglycerides through the decreased activity of
3-hydroxy-3-methylglutaryl CoA reductase (HMG-CoA reductase)
(136). Since the latter plays a
determinant role in the assembly of lipoproteins in the endoplasmic
reticulum, it was considered that taurine abrogated the assembly
and secretion of lipoproteins containing the apolipoprotein B100,
the primary structural constituent of both LDL and VLDL (132,137). In another study, taurine prevented
the passage of glucose-induced and oxidized LDL (oxLDL)-induced
endothelial cells in vascular tissue, thereby alleviating the risk
of atherosclerosis (138). In
support of the above, taurine appeared to attenuate atherogenesis
by importing reduced levels of oxidized LDL, through downregulation
of lectin-like oxidized low-density lipoprotein LDL receptor-1
(LOX-1) (139). Indeed, taurine's
cholesterol-lowering effect has been analyzed both on experimental
animal models with atherosclerosis and on animal models with
inducible hypercholesterolemia (131,140,141). In the case of hypercholesterolemia
induced by the high cholesterol/sodium and cholate diet, serum
cholesterol and lipid levels are reduced following taurine
supplementation, given that cholate is known to increase
cholesterol absorption (142). In
the same model, taurine appears to mediate its action via an
increase of CYP7A1 mRNA levels (142). The latter is considered to be the
rate-controlling enzyme for the catabolic conversion of hepatic
cholesterol to bile acids for subsequent elimination in the feces.
On the contrary, taurine does not appear to exert an effect in
cases of endogenous hypercholesterolemia that are caused by
phenobarbital (PB) or by polychlorinated biphenyl (PCB) (143,144).
The ameliorative action of taurine
against endothelial dysfunction
In addition, it has been suggested that taurine
protects endothelial cells from the homocysteine-induced ER stress
and apoptosis, and from toxicity through the inhibition of
hyperhomocysteinemia (145). Since
both hyperglycemia and hyperhomocysteinemia can be characteristics
of oxidative stress, resulting from the accumulation of asymmetric
dimethylarginine (ADMA), which is a major endogenous nitric oxide
synthase inhibitor (146). In
another case, taurine supplementation has been shown to confer
benefit against oxLDL mediated endothelial dysfunction, as shown by
in vitro and in vivo experiments (146). In the in vitro setting,
administration of taurine (1 or 5 microg/ml) has been shown to
alleviate signs of oxidative stress in human umbilical vein
endothelial cells (HUVECs) treated with oxLDL, as demonstrated by
reduced levels of lactate dehydrogenase (LDH), tumor necrosis
factor-a (TNF-α), MDA and NO (146). In the in vivo setting,
taurine supplementation in endothelial cells treated with oxLDL (60
or 180 mg/kg) has been demonstrated to contribute to
endothelium-dependent vasorelaxation, through the inhibition of NO
(146). Accordingly, Elvevoll
et al have suggested that taurine acts synergistically with
n-3 fatty acid supplementation, enhancing the beneficial effects of
fatty acids on total cholesterol, LDL cholesterol and triglycerides
in human subjects (147). In
another study, taurine has been proved to play an important role in
ameliorating endothelial dysfunction via protein kinase B (Akt) and
extracellular-signal-regulated kinase (ERK) signal transduction
cascades, without inducing vascular inflammation and permeability
in vitro and in vivo (148). Recently, Katakawa et al
explained why humans on a diet that included taurine and magnesium
supplementation are characterized by a minimal risk of developing
atherogenesis due to a decrease in oxidative stress and improved
endothelial function (149).
An increasing amount of evidence highlights the
cytoprotective properties of taurine, which ameliorate the cardiac
damage induced by various toxins (74,75),
by attenuating mitochondrial dysfunction (150,151). For example, it has been suggested
that taurine can overcome cardiac dysfunction mediated by either
methionine or doxorubicin (DOX), by reducing ROS generation or
intracellular calcium overload, respectively (150,151). In addition, it has been reported
that taurine exerts a protective effect against arsenic-induced
cardiac oxidative stress, playing a pivotal role in modulating ROS
production, calcium overload and apoptotic cell death (151), taking into consideration that
taurine ameliorates arsenic-induced neuro-associated changes in the
cerebrum through the downregulation of autophagy (103). In this case, it has been
highlighted that downregulation of p38 MAPK and JNK signaling
pathways appear to be the main mechanisms underlying the protective
action of taurine, ultimately attenuating nuclear factor-κB (NF-κB)
transcription and blocking the apoptotic signaling pathway
(151). In the same context, it
has been mentioned that an important problem of cirrhosis is the
heart injury, which can be assimilated with the use of bile duct
ligated (BDL) animals. When taurine is administered to BDL animals
for 42 consecutive days, it can rescue heart injury, by restoring
mitochondrial function in the myocardium of animals (152). Taurine can ameliorate heart
failure, by increasing energy expenditure (NAD+/NADH)
and by interfering with acetylation of p53 transcription factor
(153).
The beneficial effect of taurine has also been
confirmed in experimental genetically modified animal models of
oxidative stress. For example, in methionine sulfoxide reductase A
gene knockout mice (MsrA−/−) exposed to
H2O2 insult that are characterized by
mitochondrial dysfunction in the myocardium, taurine has been shown
to protect MsrA−/− cardiac myocytes, exposed to
H2O2 insult. Specifically, cardiac
disturbance was determined in 8-months old MsrA−/− mice
through a marked decline of ejection fraction (EF) and fraction
shortening (FS), as compared to control mice. Prolonged
administration of taurine (5 months) to MsrA−/− mice
exposed to H2O2 insult was shown to improve
mitochondrial dysfunction, thereby suggesting a protective role for
taurine in MsrA−/− cardiac myocytes from protein
oxidation. Nonetheless, taurine treatment on its own did not appear
to have a significant impact on the oxidative status of
MsrA−/− hearts (154).
Similarly, taurine favorably contributes to normal cardiac
function, as shown by the reduction of LDH and uric acid, which
constitute serum markers of cardiac disorders (155) and by the inhibition of enzymes
degrading extracellular matrix such as matrix metalloproteinase-2
(MMP-2) (156). As a result,
taurine exerts its beneficial action against heart failure, through
modulation of calcium homeostasis, regression of atherogenesis, its
interaction with endothelial cells. Indeed, taurine exerts its
multiple actions by modulating signaling pathways to restore
cardiac tissue taurine levels, to ameliorate potential cardiac
abnormalities.
The regulatory importance of taurine in
ischemia and reperfusion
Numerous studies have reported that the harmful
consequences of ischemic oxidative stress include the following: i)
Reduced myocardial anti-oxidants, ii) disturbed mitochondrial
membrane potential (Δψm), and iii) superoxide anion
(O2−) accumulation (47,157).
Taurine molecule is one of the several factors that have been
proposed to alleviate symptoms of ischemia-reperfusion (110). In particular, several
investigators have suggested that taurine could be incorporated to
cardioplegic solutions (158) or
uploaded to the donor's hearts before transplantation (159,160). Taurine's therapeutic effectiveness
against ischemia-reperfusion injury and cardiovascular diseases,
including congestive heart failure, has been well-demonstrated. For
example, oral taurine supplementation (3 mg/kg) has been shown to
improve systolic left ventricular function within the space of 6
weeks, compared to treatment with coenzyme Q10 in 17 patients with
congestive heart failure. Consistent with clinical trials, animal
studies have also produced similar results. For example, taurine
perfusion has been shown to improve oxidative injury (i.e. LPO),
ventricular function, and infarct size during reperfusion in
isolated rat hearts (161).
Excessive accumulation of calcium (Ca2+)
is cytotoxic to the heart (162).
Calcium overload is not only capable of the induction of proteases
and lipases, but it also facilitates mitochondrial permeability
transition, eventually causing the release of pro-apoptotic factors
from the mitochondria to the cytoplasm (52,163).
It has been shown that the taurine transporter uptakes lower
taurine concentrations during an ischemia-reperfusion insult,
causing the respective loss of intracellular sodium
(Na+) (164). It should
be taken into consideration that a vicious cycle is observed
between sodium and calcium, and more sodium is available for
calcium entry via the Na+/Ca2+ exchanger,
exacerbating calcium overload (165). Firstly, taurine exerts great
anti-oxidant activity by preventing calcium overload during
reperfusion (166–168) and by protecting the myocardium
from LPO products (169). In
particular, taurine has also been shown to regulate the activity of
the sarcoplasmic reticulum (SR) Ca2+ATPase, given that
ATPase retains cytosolic calcium to normal levels, promoting
cellular calcium homeostasis (170). Even though the effect of taurine
on protein phosphorylation remains to be stablished, enough
experimental evidence has been obtained to suggest that taurine
loss induces the reduction of SR Ca2+ATPase activity and
the phosphorylation state of phospholamban, which is accompanied by
delayed myocardial relaxation and ROS accumulation (46,76,165).
This positive effect on ischemic oxidative stress relies on
reducing osmotic stress and calcium overload (165,171). As a result, both the systolic and
diastolic heart function are disturbed, as SR Ca2+ pump
activity is reduced to a minimum.
Secondly, taurine contributes to the inhibition of
K+-ATP channels and slows down the decrease of
intracellular ATP in ischemic hearts (172). The suppressive effect of taurine
against the opening of K+-ATP channels is
cytoprotective, not only in terms of ATP content but also in the
regulation of calcium homeostasis. In this regard, taurine exerts
protective action against ischemia-reperfusion injury through
regulation of potassium efflux, which in turn modulates the influx
of calcium ions, thereby leading to the sustenance of the ATP
content (172,173). Notably, it seems that
administration of taurine can reverse ischemic oxidative stress
through its ability to reduce ROS formation, by the mitochondrial
electron transport chain and through its anti-inflammatory activity
with the formation of N-chlorotaurine (174).
Thirdly, taurine can serve either as anti-apoptotic
or as an apoptotic agent in various circumstances. On one hand,
taurine appears to be effective in inhibiting myocardial ischemia
due to the induction of apoptosis through interference with the
apoptosome, which is comprised of the apoptotic protease activating
factor-1 (Apaf-1)/caspase-9 and the Akt/caspase-9 pathway (175). On the other hand, inhibition of
apoptosis has also been identified as the underlying mechanism by
which cardiomyocytes continue their proliferation, neutralizing the
NADPH oxidase, and calpain activation (176). In the same context, taurine has
been found to act as an anti-apoptotic agent in cardiomyocytes
subjected to experimental ischemia-reperfusion (108). Of particular interest has been the
ability of taurine to maintain energy levels and to preserve
myocardium homeostasis against DNA damage signals before or during
ischemia (110).
In addition, ischemia-reperfusion has emerged as a
potential challenge in patients undergoing ovarian transplantation.
Taurine seems to exert its beneficial effect, circumventing the
ischemia-reperfusion, by increasing angiogenesis and by hindering
apoptosis as well as oxidative stress (177).
Overall, one of the most important actions of
taurine against ischemia is its regulatory impact on intracellular
calcium levels and its anti-oxidant activity. Primarily, taurine
counteracts ischemic oxidative damage by attenuating intracellular
calcium levels (166–168). Intriguingly, taurine loss
contributes to the activation of K+-ATP channels and
slows down the decrease of intracellular ATP levels in ischemic
hearts (172). Also, taurine's
anti-oxidant activity is mediated by the attenuation of xanthine
oxidase and the ROS-forming inflammatory reaction, thus limiting
ROS synthesis via complex I of ETC (174).
The anti-oxidant efficacy of taurine against
muscle- associated disorders
Skeletal muscle is one of the tissues that
accumulates the higher amount of the body's taurine, through the
action of taurine transporter (TauT). Primarily, the significance
of taurine in skeletal muscle emerged through its deficiency in
TauT−/− mice. In particular, those taurine deficient
mice presented with a 90% decrease in taurine content, showing
abnormalities of muscle structure. The impaired mitochondrial
function and disturbed fatty acid oxidation accounted for the
phenotype of skeletal muscle in TauT−/− mice (178). As expected, the high concentration
of taurine is important for normal muscle performance and
excitation-contraction coupling. The main action of taurine in
skeletal muscle appears to be the regulation of mitochondrial
protein synthesis, which is achieved by increasing electron
transport chain activity and by preventing excessive superoxide
anion (O2−) formation, thereby establishing
normal adenosine triphosphate (ATP) biosynthesis (46). Apart from the regulatory effect of
taurine on mitochondrial function and respiratory chain, it has
also been reported that taurine facilitates calcium homeostasis and
displays strong anti-oxidant properties (179). Specifically, taurine seems to be
significantly involved in the regulation of calcium release from
the sarcoplasmic reticulum (SR) and to improve the sensitivity of
contractile elements to intracellular calcium levels (179). This way, contractile properties
and force production of skeletal muscle are regulated in a
taurine-dependent manner (179).
In support of this, several studies have evidenced the contribution
of taurine to calcium homeostasis. For example, administration of
20 mM taurine in isolated skinned myofibers accounted for the
excess amount of calcium into SR, thus enabling for the greater
depolarization-mediated contraction of myofibers (179). The effects of long-term taurine
administration were similar to its short-term supplementation
(180). When both type I and II
human myofibers were exposed to 10–20 mM taurine in the long-term,
there was a remarkable increase in calcium uptake, thereby leading
to augmented sensitivity of contractile apparatus of myofibers to
calcium (181). Accordingly, high
levels of taurine have been shown to promote calcium homeostasis by
inducing active re-absorption by the SR (124) and by controlling ion channels in
the skeletal muscle cells (182).
It is important to note that taurine is
concentrated in the skeletal muscle against gradient, not only due
to its effect on calcium, but also on chloride channels. The
contribution of taurine to the regulation of chloride channels has
been highlighted in taurine-deficient conditions, caused by taurine
transporter antagonist or inhibitor, in which taurine elimination
accounted for reduced chloride conductance (gCl), accompanied by
the increased sarcolemmal excitability (183). These results acquire additional
significance, if one considers that resting gCl plays a pivotal
role in total membrane conductance of sarcolemma and maintains the
sarcolemmal electrical stability by shunting the
depolarization-driven potassium accumulation (184).
The positive effect of taurine on
muscle during intense and prolonged exercise
Several reports have highlighted a favorable effect
of taurine on animal skeletal muscle during steer exercise. Intense
and prolonged exercise is believed to reduce rat skeletal muscle
taurine levels, by exerting a negative effect on skeletal muscle
performance (185,186). Taurine-treated rats display a
normal taurine content during exercise and enhanced endurance until
the running time is increased to exhaustion, as compared to control
rats (187). Apart from the
anti-oxidant role of taurine, taurine administration has been shown
to alter hepatic glucose tolerance, to exert a stimulatory effect
on hepatic gluconeogenesis and to enhance endurance time during
exercise (188).
When taurine is supplemented in the long-term, its
effects on skeletal muscle are strengthened during exercise, as
evidenced by subsequent taurine-mediated changes in the metabolism
and inflammation. For example, long-term administration of taurine
(5% taurine in drinking water for 12 months) appeared to exert a
positive effect on glucose tolerance and to inhibit triglyceride
(TG) deposition, reinforcing the glucose uptake in skeletal muscle
post-exercise (189). This was
suggested to have occurred through the activation of 5′
AMP-activated protein kinase (AMPK) and the reduction of total AMPK
protein content in the gastrocnemius (GAST) muscle of ob/ob mice
(189), thereby favoring an
increase in carbohydrate oxidation, rather than an increase in
fatty acid oxidation. In another study, taurine supplementation
returned taurine levels to normal rates and prevented the increase
of plasma creatinine and TG levels under prolonged exercise-induced
muscle damage (190). Taurine was
proved to minimize oxidative stress by reducing the cytosolic
content of LDH and creatine kinase (CK) (191). Also, the long-term administration
of taurine has been evidenced to be beneficial against in
vivo eccentric exercise damage, as shown in rats (192). The mechanism underlying the
protective mode of taurine was based on the inhibition of either
protein carbonylated (PC) content or oxidized thiols, without
modulating the activities of anti-oxidant enzymes (192).
Besides, the well-established anti-inflammatory
properties of taurine were identified in the muscles of rats under
an intensive exercise protocol, where it seemed to contribute to
muscle repair, through its capacity to alleviate the signs of
inflammation (193). Specifically,
TNF-α and IL-6 appeared to be preferentially reduced in the stromal
cells of skeletal muscle, accompanied by a strong reduction of
accumulated CD68 signal intensities within the muscle fibers
(194). In the same context,
taurine has been proved to confer benefit against nitrosative
inflammation and muscle DNA damage during strenuous exercise,
through interference with inducible nitric oxide synthase (iNOS)
(195). Notably, the adverse
effects of exercise in the form of injury have mainly been observed
in type I muscle fibers which are composed of a higher number of
mitochondria in comparison to type II muscle fibers (196); therefore, type I muscle fibers
appear to generate higher levels of free radicals during exercise,
as well as at rest, due to greater polyunsaturated fatty acid
(PUFAs) gene expression, which renders them more susceptible to
lipid peroxidation (LPO) and other types of oxidative damage
(197,198).
In addition to the above, a treatment scheme
including a combination of caffeine and taurine over 2 weeks seemed
to increase the run time of mice on a treadmill, thereby resulting
in increased endurance time of mice during exercise due to
diminished muscle lactate levels (199). Indeed, the combination scheme
(taurine and caffeine) displayed ergogenic properties, whilst also
enhancing muscle power output (199). Accordingly, another combination
scheme including taurine and fish oil has been shown to enhance
fatty acid oxidation by upregulating the activity of acyl-CoA
oxidase (ACO), which is regarded as the rate-limiting enzyme of
peroxisomal fatty acid β-oxidation (200). The same combination scheme has
also been shown to improve glycolysis, via increasing GLUT4
distribution, thereby facilitating diffusion from circulating
glucose (200). However, taurine
supplemented in combination with branched-chain amino acids (BCAA)
(9.6 grammars of BCAA and 6 grammars of taurine/day) did not seem
to have a direct impact on the skeletal muscle of men following
intense exercise, but it seemed to reduce the serum levels of
muscle damage markers, such as LDH, lactate aldolase and
8-hydroxydeoxyguanosine (8-OHdG) (201).
The effect of taurine on human
skeletal muscle
Even though taurine supplementation has been proved
to be very successful in alleviating signs of muscle injury in
various animal models, its effect on human skeletal muscle has not
been completely elucidated. Relatively high doses of taurine
supplementation (1.66 grammars) have been shown to increase plasma
taurine levels by 15-fold (from 50 mmol/l to over 750 mmol/l)
within 2 h, and high plasma taurine levels of 350 mmol/l can be
sustained for 4 h (202). At this
point, subsequent acute dietary supplementation of taurine (1.66
grammars) can accelerate the concentration of taurine in the plasma
to 1,000 mmol/l within the space of one hour, and then it follows a
rapid falling trend, reaching the 150 mmol/l within 4 h (202). Besides, acute dietary
supplementation of taurine increases plasma taurine levels in a way
similar to that caused by creatinine supplementation (190,203).
When taurine was given in an acute manner (i.e., in
short time intervals), researchers examined its impact on endurance
time in the skeletal muscle of various trained subjects. Acute
dietary supplementation of taurine (1.66 grammars), one hour before
a 90 min submaximal cycle did not appear to affect time trial
performance in human subjects, and prolonged supplementation of
taurine (5 grammars/day for 7 days) did not appear to have any
effect on human skeletal muscle (202). Experiments in well-trained
cyclists showed the high dietary intake of taurine (204). In this context, Rutherford et
al supported that taurine exerts its action through the
activation of adenyl cyclase, by increasing cyclic adenosine
monophosphate (cAMP) and therefore by increasing lipolysis and
subsequently fat oxidation (204).
In particular, fat oxidation was increased by a rate of 16%, when
taurine was given in conjunction with a 90 min submaximal exercise
period (204). Similar results
were obtained with acute dietary supplementation of 1 g taurine 2 h
before a 3 km treadmill exercise in yet another study (205). No alterations were observed in
parameters such as heart rate, perceived exertion, oxygen
consumption, or blood lactate concentrations, whereas skeletal
muscle performance was slightly improved (205).
At molecular setting, taurine has been shown to
overcome oxidative stress induced by intense exercise in young men,
through alterations in calcium homeostasis. This mechanism of
action is based on increasing intracellular calcium levels released
from the SR and therefore makes contractile filaments more
vulnerable to intracellular calcium levels (179). The positive effect of taurine
supplementation on calcium homeostasis following intense exercise
also leads to increased muscle strength, reduced muscle soreness,
as well as decreased serum LDH activity, serum creatine kinase
activity, PC content and LPO (206). Nonetheless, taurine does not seem
to affect the expression levels of pro-inflammatory markers [TNF-α,
IL-1b, IL-10] in the human skeletal muscle (206). On the contrary, clinical studies
recently highlighted that taurine exerts an incremental effect on
intracellular calcium levels excreted by the SR into either type of
fiber, without altering maximum calcium rates (181).
Interestingly, taurine supplementation seems very
promising in improving vascular endothelial function following a
high-intensity exercise. Specifically, 29 healthy men were divided
into two groups: Taurine-treated and taurine-untreated;
taurine-treated men received 6 grammars of taurine for 2 weeks
prior to exercise and for 3 days following exercise; taurine
supplementation improved endothelial function, as demonstrated by
increasing relative and absolute values of brachial artery
flow-mediated dilation (FMD), whilst also preventing a resistance
exercise-induced arterial stiffness (207).
Taurine has been shown to minimize oxidative stress
and muscle damage in humans; taurine administered to 11 sedentary
young men at 2 grammars per 3 times per 7 days leads to a marked
decline of white blood cell DNA migration 24 h after an exhaustive
maximal VO2 test (208). Even though taurine supplementation
appeared to be effective in reducing LPO before exercise, it did
not seem to significantly affect serum thiobarbituric-acid reactive
substance (TBARS) levels after exercise (208).
Besides, taurine administration seems to ameliorate
the side effects of exercise in older women, namely the cognitive
dysfunction and neurodegeneration, by reducing the myeloperoxidase
(MPO) activity (209).
Equally important seems to be the notion that
taurine seems to be involved in the regeneration of muscle fibers
in patients with muscle atrophy (210). Specifically, taurine improves the
dexmedetomidine-induced muscle atrophy in C2C12 myocytes by keeping
the expression of atrophy-associated genes to normal levels
(211,212). However, taurine does not seem to
exert any positive effect on skeletal muscle atrophy under
pathological conditions (185,211). On a similar basis, taurine has
been reported to affect the progression of duchenne muscular
dystrophy (DMD) (213). In DMD
patients, expression of the dystrophin gene is impaired, resulting
in the replacement of skeletal muscle with nonfunctional fibrotic
tissue and in high urinary excretion levels of taurine (213). Despite the reported therapeutic
effectiveness of glucocorticoids against DMD, their use is limited
due to related adverse effects. In vivo experiments have
demonstrated that taurine enhances the effects of classical
glucocorticoid [α-methyl-prednisolone (PDN)] in the
fast-twitch muscle on MDX mice, by attenuating the overactivity of
voltage-independent cation channels and by preserving calcium
homeostasis in extensor digitorum longus (EDL) myofibrils and
alleviating signs of DMD (214).
The anti-oxidant efficacy of taurine against
hepatic- associated stress
Oxidative stress in hepatocytes is closely
associated with hepatic damage (215). Taurine is essential for liver
homeostasis and taurine deficiency in hepatocytes causes severe
hepatic damage, followed by compensatory hepatocyte proliferation
(216). The phenotype of
TauT−/−mice is characterized by various manifestations
of hepatic dysfunction, hepatitis, and liver fibrosis, all of which
are mediated by impaired mitochondrial function and inefficient
control of the respiratory chain (216). Interestingly, taurine deficiency
appears to have a profound effect on Kupffer and sinusoidal
endothelial cells but not in parenchymal cells (216).
Taurine has emerged as an attractive therapeutic
agent against liver injury as it is actively involved in the
reduction of hepatic oxidative burst, which is accompanied by a
remarkable increase in anti-oxidant enzymes and by attenuation of
inflammatory injury (217). In
cases where ethanol is the cause of hepatic oxidative stress,
hepatic MDA levels are decreased, whereas the activity of hepatic
superoxide dismutase (SOD) is significantly increased, following
taurine treatment (217).
Similarly, taurine has been considered as a palliative agent to
combat renal and hepatic oxidative stress of alcohol-supplemented
mice, through its advantageous effect on GSH, MDA levels and
anti-oxidant enzymes (218).
Taurine has also been shown to limit the possibility of hepatic
steatosis, by reducing TNF-α expression levels and by increasing
fatty acid oxidation through activation of carnitine
palmitoyltransferase 1a (CPT1a) (217). This is of utmost importance if one
considers that peroxisome proliferator-activated receptor alpha
(PPARα) and 5′AMP-activated protein kinase (AMPK) are essential for
the regulation of genes involved in the oxidation of long-chain
fatty acids (glutathione peroxidase 3 and carnitine palmitoyl
transferase 2) and that their levels decline in cases of impaired
fatty acid oxidation, such as in the skeletal muscle of
TauT−/− mice (178). In
a rat model of chronic alcohol consumption, taurine has been shown
to impair lipogenesis, lymphocyte infiltration as well as iNOS,
C-reactive protein (CRP), and inflammatory cytokine expression
through TLR4/MyD88 signal transduction, proving its
anti-inflammatory properties (219). Accordingly, taurine ameliorates
hepatic steatosis, by reducing ROS, lipid accumulation and
preserving mitochondrial membrane potential (220). In an iron-overload murine model of
oxidative stress, which mimics features of chronic liver diseases
(such as alcoholic liver disease and chronic viral hepatitis),
taurine has been shown to reduce the accumulation of ROS, thereby
exhibiting strong anti-oxidant activity (221). In another experimental setting,
administration of 2% taurine (2/100 g diet) to rats fed a 50%
calcium-deficient diet, has been shown to inhibit LPO in the liver,
as compared to control rats (222). This makes sense if one considers
that calcium is a crucial component of cholesterol and lipid
metabolism and that taurine has been shown to counteract the
effects of oxidative stress by regulating both calcium and lipid
homeostasis (222). In other case,
rats supplemented with a 5% taurine diet demonstrated a reduction
in their visceral fat weight, because of reduced cholesterol
levels, downregulation of fatty acid synthase (FAS) and
upregulation of carnitine palmitoyltransferase 1α (CPT1a) (30). In another case, it has been
substantiated that taurine provides protection against hepatorenal
injury caused by fipronil (FPN), a commonly used pesticide
(223). In FPN-induced hepatorenal
side effects, the anti-oxidant properties of taurine emerge through
reduction of GSH, LPO, NO formation and upregulation of
anti-oxidant enzymes (223).
Apart from its significant anti-oxidant and
anti-inflammatory role, taurine exerts a great effect on
cholesterol levels. In particular, taurine has been documented to
be a direct liver X receptor α (LXR-α) ligand, which
modulates the expression of genes implicated in reverse cholesterol
transport in macrophages, this way compromising cholesterol to
lipid accumulation without increasing hepatic fatty acid synthesis
(224). Paradoxically, increased
nuclear translocation of sterol regulatory element-binding
protein-1c (SREBP-1c) results in lipogenesis in the liver, through
induction of LXR-α (225). In this
direction, low-dose taurine administration (0.1 g/kg body
weight/day for 5 weeks) has also been shown to restore structural
changes in the liver of an MSG-induced obesity model (226). Consistent with the above, it has
been reported that taurine negatively affects the serum cholesterol
levels of rats on a high cholesterol diet (134). From a clinical point of view,
taurine has been suggested as a potential effective therapeutic
agent for hyperlipidemia and calcium deficiency-related metabolic
disorders. Indeed, taurine has been shown to lower triglyceride and
cholesterol levels in healthy young human adults in a dose of 6
grammars of taurine per day for 21 days, following a high fat and
cholesterol diet (227). On a
similar note, it has been reported that administration of 3
grammars of taurine per day for 42 days reduces plasma triglyceride
and total cholesterol to normal levels in overweight human subjects
(228).
The beneficial effect of taurine on
hepatic side-effects
In addition, the beneficial effect of taurine
against toxin-mediated hepatic damage has been substantiated by
several examples. According to Miyazaki et al, taurine can
reverse carbon tetrachloride (CCL4)-induced hepatic
damage; histologically, fibrotic infiltrations of both pericentral
and periportal regions are restored following treatment with
taurine, as shown by immunohistochemical stainings (229). In parallel, it has been shown that
a combination of taurine and hesperidin can afford benefit to
CCL4-administered rats from acute kidney and testicular
damage (230). The antithrombotic
effect of taurine has been proved very significant in patients with
acute kidney damage, who have increased risk for atherothrombotic
and cardioembolic danger (231,232). Recently, the European Dialysis
Working Group has presented convincing evidence about the benefits
of antiplatelet and anticoagulant drugs, recommending their use in
patients with chronic kidney disease requiring dialysis (231). In another case, acetaminophen
(N-acetyl-p-aminophenol, APAP) was shown to induce hepatic failure,
causing significant alterations in hepatic metabolism (233); it was observed that, following the
entry of APAP in hepatocytes, the greatest proportion of APAP (90%)
is converted to innocuous molecules, and only a small fraction of
APAP (5–10%) yields the toxic electrophile
N-acetyl-para-benzoquinoimine (NAPQI), due to the activity of
cytochrome P450 isoforms, mainly CYP 2E1, CYP 1A2, CYP 3A2
(234). Specifically, all the
APAP-mediated changes are mediated by the binding of NAPQI to
mitochondrial proteins of the respiratory chain, thus attenuating
ATP formation and culminating in excessive
O2− formation (235). Several mechanisms have been
suggested to account for the liver damage caused by APAP, including
disturbed hepatic GSH reserves, inhibition of cytochrome P450
enzyme activity, elevation of intracellular calcium levels,
increased LPO and ROS formation (236). In addition to depleting
intrahepatic GSH, APAP seems to decrease the activity of
anti-oxidant enzymes (SOD, CAT, GPx), as well as of enzymes
involved in glutathione redox cycling (glutathione reductase, GR),
utilization (glutathione S-transferase, GST) and synthesis
(gamma-glutamylcysteine synthetase) (237). In this context, taurine has been
shown to improve the effects of APAP on both oxidative stress and
glutathione redox metabolism (238). Taurine administered at a dose of
2.4 mmol/kg for 30 min following administration of 800 mg/kg APAP
appeared to successfully alleviate both DNA damage signals and
oxidative indices (238). This was
achieved by i) reducing MDA formation, ii) regulating glutathione
cycling-utilization-synthesis cycle, iii) increasing the already
reduced glutathione to glutathione disulfide (GSH/GSSG) ratio and
iv) increasing the activity of all anti-oxidant enzymes (238). Notably, hepatic damage mediated by
paracetamol intake can be monitored through plasma and urine
taurine levels (239). In addition
to the above, taurine has been reported to alleviate hepatic damage
caused by hexavalent chromium and tamoxifen in mice (240,241), as well as lead, cadmium and copper
in rats (242–244). In the case of thioacetamide
(TAA)-induced liver damage, taurine supplementation has been proved
to be advantageous due to its ROS-scavenging capacity and its
potential to normalize mitochondrial energy expenditure via
suppressing mitochondrial swelling (245). Taurine administration at doses of
400 mg/kg, intraperitoneally (every 12 h beginning at 24 h prior to
the first TAA injection) has been reported to reduce serum
transaminase activity and hepatic LPO, possibly due to its
scavenging properties (245). In
addition, taurine supplementation has been shown to regulate
glutathione metabolizing enzymes without affecting the activity of
hepatic anti-oxidant enzymes in TAA-treated rats (246).
On a similar note, epidemiological studies have
demonstrated that chronic oxidearsenous trioxide
(As2O3) hepatic insult can lead to liver
injury, which may present as fibrosis or cirrhosis or even cancer
(247). Four years ago, Li et
al found that exposure to As2O3 causes
extensive hepatic injury, as hepatocytes convert oxidearsenous
trioxide to dimethyl arsenic acid and monomethyl arsenic acid
(248). Liver injury is manifested
via increased expression levels of alkaline phosphatase (ALP) and
alanine aminotransferase (ALT) in serum, which represent reliable
markers of liver damage (248). In
this context, taurine appears to attenuate
As2O3 mediated toxicity (249), possibly by reducing the expression
levels of phosphorylated JNK kinases, thereby ruling out the
possibility that hepatic cells follow the apoptotic route after
taurine treatment in As2O3-administered rats
(248). In the meantime, the
As2O3-induced toxicity has been
differentially documented in terms of autophagy. While older
studies have suggested that low As2O3
supplementation suppresses autophagy (250), more recent data have demonstrated
the induction of autophagic cell death in INS-1 cells or HepG2
cells following NaAsO2 or As2O3
treatment (251). In this frame,
Qiu et al highlighted the contribution of arsenic exposure
to nonalcoholic steatohepatitis (NASH): Arsenic trioxide
(As2O3) treatment was shown to activate NASH
in mice via the induction of autophagy and NLRP3 inflammasome
activation, ultimately leading to dysregulation of lipid-related
genes and to lipid accumulation. Interestingly, taurine
supplementation appeared to reverse
As2O3-mediated liver inflammation by
inhibiting the autophagic-CTSB-NLRP3 inflammasomal pathway rather
than decreasing lipid accumulation (252). Furthermore, taurine can suppress
autophagy and oxidative burst by inducing the expression of the
nuclear hormone receptor such as peroxisome proliferator-activated
receptor gamma (PPAR-γ) in both HepG2 cells and liver tissues of
animal models (251).
Interestingly, taurine's activity can be amplified
when used in combination with other anti-oxidant drugs. For
example, when taurine is combined with dimercaptosuccinate, it
appears to significantly enhance the activity of anti-oxidant
enzymes in the liver (253).
Elsewhere, taurine has been shown to alleviate liver injury caused
by carbon tetrachloride (CCL4) and to reverse the
characteristic symptoms of liver fibrosis such as portal
inflammation, severe centrilobular necrosis, and excessive
deposition of collagen (254).
Taurine counteracts CCL4-mediated damage by maintaining
energy homeostasis, preventing ROS accumulation, and enhancing the
interaction of Thioredoxin 1 (Txn1) with numerous proteins involved
in glycolysis (254). In diethyl
nitrosamine (DEN)-mediated liver injury, a combination treatment
scheme consisting of curcumin and taurine displayed strong
anti-inflammatory properties, by reducing the expression levels of
IL-2 and IFN-γ, thereby preventing mitochondrial disturbance
in malignant cells (255).
Besides, the viability of cultured human hepatoma cells was
significantly decreased when taurine was administered in
combination with curcumin, as opposed to those two compounds
administered separately (256).
Furthermore, a combination treatment scheme consisting of tea
polyphenols (TPs) and taurine has been shown to decrease oxidative
damage markers (alanine transaminase and aspartate transaminase)
and lipid destruction (257). In
the case of hepatic damage induced by chronic ethanol
administration (258) or
methotrexate (anti-leukemic drug) (259), taurine's therapeutic action
appears to rely on decreasing the MDA content and restoring GSH
levels. Accordingly, the carnosine and taurine combined treatment
scheme appeared to protect the livers of animal models from D
galactose (GAL) mediated liver damage through a significant
reduction of MDA and of PC content and through a slight
upregulation of anti-oxidant enzymes, including SOD and GPx
(260). It has therefore been
proposed that taurine rescues injured hepatocytes from various
toxins due to its cytoprotective role as an intracellular calcium
flux regulator, an anti-oxidant, and an osmoregulator.
Last but not least, metabolomic data analysis has
highlighted a diagnostic importance for taurine in the detection of
hyperlactatemia following alcohol consumption (261). Increased quantities of
N-acetyltaurine (NAT), which uses taurine as the main substrate for
its synthesis, have been observed in the urine samples of
Cyp2e1-null mice following ethanol consumption (261). Accordingly, taurine levels are
significantly reduced in certain liver disorders and
xenobiotic-mediated liver damage and are therefore used for the
clinical detection of hepatic injury in plasma and urine samples
(262,263).
The anti-oxidant properties of taurine in
various toxic-mediated insults
The cytoprotective role of taurine has been
substantiated in various toxin-mediated oxidative stress
conditions. For example, taurine has been demonstrated to inhibit
oxidative stress-induced lung damage (264), whereas more recently taurine
supplementation has been shown to increase the activity of
anti-oxidant enzymes in the lungs (265). In other cases, taurine has shown
to exert anti-oxidant activity against bleomycin-induced lung
injury (266) or against
amiodarone (267) or paraquat
(268,269) or sumatriptan mediated lipid
peroxidation (LPO), and mitochondrial injury (270). Other studies have indicated that
oral supplementation of taurine probably serves as a protective
agent against damage mediated by nitrogen dioxide (NO2)
(271). Interestingly, the
treatment of MRC-5 fibroblasts with 20 mM of taurine has appeared
to inhibit the lipopolysaccharide (LPS)-induced ROS formation and
the activation of the MAPK signaling pathway (272). Similarly, taurine seems to confer
protection against testicular toxicity. For example, taurine has
been found to counteract the effects of sodium arsenite
(NaAsO2)-mediated oxidative insults to the testes
(273). It seems that taurine
serves as an anti-oxidant and as an anti-apoptotic agent by
inhibiting the activation of two members of the MAPK family, ERK1/2
and p38 MAPK (273). Probably, in
this way, taurine protects the testes from damage caused by sodium
arsenite exposure, through inhibition of lipid peroxidation. In
another study, taurine has been shown to alleviate the arsenic-
associated adverse effects in the testes by functioning as a
capacitating agent or as a sperm motility factor (274). In the same context, taurine
appears to be remarkably useful against endosulfan-mediated adverse
effects. In an experimental rat model using endosulfan to cause
testicular toxicity, endosulfan has appeared to induce oxidative
stress and apoptosis, as demonstrated by increased
H2O2 levels, lipid accumulation and reduced
activity of anti-oxidant enzymes (SOD, CAT, GPx), as well as
altered reduced GSH content and mitochondrial cytochrome c release
(275). In addition to providing
complementary insights into the mode of action of
endosulfan-induced toxicity, taurine has been shown to reverse all
of the endosulfan-induced effects, by acting as a protective agent
against testicular toxicity in rats (275). In another case, taurine emerges as
an effective cytoprotective agent against cisplatin-mediated
testicular apoptosis, due to its anti-oxidant nature (276). Indeed, taurine ameliorates
testicular damage in rats, through upregulation of anti-oxidant
enzymes (276). Finally, the
protective properties of taurine have been confirmed in murine
testicular Leydig TM3 cells, preventing oxidative stress through
activation of autophagy (277).
Last but not least, the combination of curcumin and taurine exerts
beneficial effect on Bisphenol A (BPA)-mediated testicular
dysfunction in rats, though its inhibitory action on MDA levels and
its possibility to upregulate anti-oxidant enzymes (278).
Conclusions
Taurine is a cytoprotective molecule, which is
implicated in a wide spectrum of processes such as energy
production, neuromodulation, calcium homeostasis and
osmoregulation, all of which support its anti-oxidant nature. To
our knowledge, this is the first study that discusses the
anti-oxidant nature of taurine and its underlying molecular
mechansims of action in various pathological circumstances related
to oxidative stress. Indeed, taurine emerges as a promising
therapeutic approach against CNS-related disorders, as well as
defects in the cardiovascular system, and defects in the skeletal
muscle and metabolism. Leveraging next-generation sequencing, the
multilayered characterization of taurine therapeutic targets will
give comprehensive insights into the potential clinical use of
taurine.
However, an important limitation of this review
article is the paucity of clinical studies to examine taurine's
therapeutic efficacy. The pharmacodynamic and pharmacokinetic
properties of taurine are requested to elucidate taurine's
effectiveness in a clinical setting, allowing the correct design of
clinical trials. In this way, we will acquire information about
taurine's duration and dose administration in patients bearing
various oxidative stress-related diseases. Indeed, clinical trials
will confirm the benefits of taurine's action in patients
undergoing oxidative stress in a safe manner.
Acknowledgements
Not applicable.
Funding
The present study was supported by The State
Scholarships Foundation (I.K.Y; grant no. 2018-050-0502-13155).
This work was also supported by the European Infrastructure For
Translational Medicine-Greece project (grant no. MIS 5028091) which
is implemented under the Action ‘Reinforcement of the Research and
Innovation Infrastructure’, funded by the Operational Program
‘Competitiveness, Entrepreneurship and Innovation’ (grant no. NSRF
2014-2020) and co-financed by Greece and the European Union
(European Regional Development Fund).
Availability of data and materials
Not applicable.
Authors' contributions
SB, MA, DAS, PI, VZ, AP, MIP, IC and AMK were
involved in the conception and design of the study. SB performed
the literature search, designed the figure, interpreted the data
and wrote the manuscript. SB, MA, PI and VZ contributed to editing
the manuscript. SB, MA, DAS, PI, VZ, AP, MIP, IC and AMK
contributed to the acquisition of data. SB and PI contributed to
the analysis and interpretation of data. All authors have read and
approved the final manuscript. Data sharing is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
DAS is the Editor-in-Chief for the journal, but had
no personal involvement in the reviewing process, or any influence
in terms of adjudicating on the final decision for this article.
The other authors declare that they have no competing
interests.
References
|
1
|
Tiedemann F and Gmelin L: Einige neue
bestandtheile der galle des ochsen. Ann Phys. 85:326–337. 1827.
View Article : Google Scholar
|
|
2
|
Kim SJ, Lee HW and Gupta RC: Taurine, Bone
growth and bone development. Curr Nutr Food Sci. 4:135–144. 2008.
View Article : Google Scholar
|
|
3
|
Hayes KC, Carey RE and Schmidt SY: Retinal
degeneration associated with taurine deficiency in the cat.
Science. 188:949–951. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Aerts L and Van Assche FA: Taurine and
taurine-deficiency in the perinatal period. J Perinat Med.
30:281–286. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chesney RW: Taurine: Its biological role
and clinical implications. Adv Pediatr. 32:1–42. 1985.PubMed/NCBI
|
|
6
|
Lee JY, Jung DW, Park HA, Kim SJ, Chung
JH, Moon CK and Kim YC: Effect of taurine on biliary excretion and
metabolism of acetaminophen in male hamsters. Biol Pharm Bull.
27:1792–1796. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu QR, López-Corcuera B, Nelson H,
Mandiyan S and Nelson N: Cloning and expression of a cDNA encoding
the transporter of taurine and beta-alanine in mouse brain. Proc
Natl Acad Sci USA. 89:12145–12149. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chesney RW, Lippincott S, Gusowski N,
Padilla M and Zelikovic I: Studies on renal adaptation to altered
dietary amino acid intake: Tissue taurine responses in nursing and
adult rats. J Nutr. 116:1965–1976. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Räihä NC, Heinonen K, Rassin DK and Gaull
GE: Milk protein quantity and quality in low-birthweight infants:
I. Metabolic responses and effects on growth. Pediatrics.
57:659–684. 1976.
|
|
10
|
Park E, Park SY, Wang C, Xu J, LaFauci G
and Schuller-Levis G: Cloning of murine cysteine sulfinic acid
decarboxylase and its mRNA expression in murine tissues. Biochim
Biophys Acta. 1574:403–406. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bella DL, Kwon YH, Hirschberger LL and
Stipanuk MH: Post-transcriptional regulation of cysteine
dioxygenase in rat liver. Adv Exp Med Biol. 483:71–85. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Redmond HP, Stapleton PP, Neary P and
Bouchier-Hayes D: Immunonutrition: The role of taurine. Nutrition.
14:599–604. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wójcik OP, Koenig KL, Zeleniuch-Jacquotte
A, Costa M and Chen Y: The potential protective effects of taurine
on coronary heart disease. Atherosclerosis. 208:19–25. 2010.
View Article : Google Scholar
|
|
14
|
Hansen SH: The role of taurine in diabetes
and the development of diabetic complications. Diabetes Metab Res
Rev. 17:330–346. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chesney RW, Han X and Patters AB: Taurine
and the renal system. J Biomed Sci. 17 (Suppl 1):S42010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huxtable RJ: Physiological actions of
taurine. Physiol Rev. 72:101–163. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sarsour EH, Kumar MG, Chaudhuri L, Kalen
AL and Goswami PC: Redox control of the cell cycle in health and
disease. Antioxid Redox Signal. 11:2985–3011. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chiang JY: Bile acid metabolism and
signaling. Compr Physiol. 3:1191–212. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Goodman CA, Horvath D, Stathis C, Mori T,
Croft K, Murphy RM and Hayes A: Taurine supplementation increases
skeletal muscle force production and protects muscle function
during and after high-frequency in vitro stimulation. J Appl
Physiol (1985). 107:144–154. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sturman JA and Chesney RW: Taurine in
pediatric nutrition. Pediatr Clin North Am. 42:879–897. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lien YH, Shapiro JI and Chan L: Effects of
hypernatremia on organic brain osmoles. J Clin Invest.
85:1427–1435. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lang F, Madlung J, Uhlemann AC, Risler T
and Gulbins E: Cellular taurine release triggered by stimulation of
the Fas(CD95) receptor in Jurkat lymphocytes. Pflugers Arch.
436:377–383. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Schaffer S, Takahashi K and Azuma J: Role
of osmoregulation in the actions of taurine. Amino Acids.
19:527–546. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yancey PH: Organic osmolytes as
compatible, metabolic and counteracting cytoprotectants in high
osmolarity and other stresses. J Exp Biol. 208:2819–2830. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Howard M, Fischer H, Roux J, Santos BC,
Gullans SR, Yancey PH and Welch WJ: Mammalian osmolytes and
S-nitrosoglutathione promote delta F508 cystic fibrosis
transmembrane conductance regulator (CFTR) protein maturation and
function. J Biol Chem. 278:35159–35167. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Marcinkiewicz J and Kontny E: Taurine and
inflammatory diseases. Amino Acids. 46:7–20. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sukhotnik I, Aranovich I, Ben Shahar Y,
Bitterman N, Pollak Y, Berkowitz D, Chepurov D, Coran AG and
Bitterman A: Effect of taurine on intestinal recovery following
intestinal ischemia-reperfusion injury in a rat. Pediatr Surg Int.
32:161–168. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gharibani P, Modi J, Menzie J,
Alexandrescu A, Ma Z, Tao R, Prentice H and Wu JY: Comparison
between single and combined post-treatment with
S-Methyl-N,N-diethylthiolcarbamate sulfoxide and taurine following
transient focal cerebral ischemia in rat brain. Neuroscience.
300:460–473. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang Y, Zhang Y, Liu X, Zuo J, Wang K, Liu
W and Ge J: Exogenous taurine attenuates mitochondrial oxidative
stress and endoplasmic reticulum stress in rat cardiomyocytes. Acta
Biochim Biophys Sin (Shanghai). 45:359–367. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang Z, Ohata Y, Watanabe Y, Yuan Y,
Yoshii Y, Kondo Y, Nishizono S and Chiba T: Taurine improves lipid
metabolism and increases resistance to oxidative stress. J Nutr Sci
Vitaminol (Tokyo). 66:347–356. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nagai K, Fukuno S, Oda A and Konishi H:
Protective effects of taurine on doxorubicin-induced acute
hepatotoxicity through suppression of oxidative stress and
apoptotic responses. Anticancer Drugs. 27:17–23. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chang CY, Shen CY, Kang CK, Sher YP, Sheu
WH, Chang CC and Lee TH: Taurine protects HK-2 cells from oxidized
LDL-induced cytotoxicity via the ROS-mediated mitochondrial and
p53-related apoptotic pathways. Toxicol Appl Pharmacol.
279:351–363. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Prideaux M, Kitase Y, Kimble M, O'Connell
TM and Bonewald LF: Taurine, an osteocyte metabolite, protects
against oxidative stress-induced cell death and decreases
inhibitors of the Wnt/β-catenin signaling pathway. Bone.
137:1153742020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lou J, Han D, Yu H, Yu G, Jin M and Kim
SJ: Cytoprotective effect of taurine against hydrogen
peroxide-induced oxidative stress in UMR-106 cells through the
Wnt/β-catenin signaling pathway. Biomol Ther (Seoul). 26:584–590.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Suzuki T, Suzuki T, Wada T, Saigo K and
Watanabe K: Taurine as a constituent of mitochondrial tRNAs: New
insights into the functions of taurine and human mitochondrial
diseases. EMBO J. 21:6581–6589. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Schaffer SW, Jong CJ, Ito T and Azuma J:
Role of taurine in the pathologies of MELAS and MERRF. Amino Acids.
46:47–56. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Homma K, Toda E, Osada H, Nagai N, Era T,
Tsubota K, Okano H and Ozawa Y: Taurine rescues
mitochondria-related metabolic impairments in the patient-derived
induced pluripotent stem cells and epithelial-mesenchymal
transition in the retinal pigment epithelium. Redox Biol.
41:1019212021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sturman JA: Taurine in development.
Physiol Rev. 73:119–147. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lima L, Obregón F, Urbina M, Carreira I,
Baccichet E and Peña S: Taurine concentration in human blood
peripheral lymphocytes. In: Taurine 5. Advances in Experimental
Medicine and Biology. Lombardini JB, Schaffer SW and Azuma J: 526.
Springer; Boston, MA: pp. 297–304. 2013
|
|
40
|
Iruloh CG, D'Souza SW, Speake PF, Crocker
I, Fergusson W, Baker PN, Sibley CP and Glazier JD: Taurine
transporter in fetal T lymphocytes and platelets: Differential
expression and functional activity. Am J Physiol Cell Physiol.
292:C332–C341. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Oja SS and Saransaari P: Taurine 6.
Advances in Experimental Medicine and Biology. 583. Springer; New
York, NY: pp. p5562006
|
|
42
|
Lubec B, Ya-hua Z, Pertti S, Pentti T,
Kitzmüller E and Lubec G: Distribution and disappearance of the
radiolabeled carbon derived from L-arginine and taurine in the
mouse. Life Sci. 60:2373–2381. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Menzie J, Pan C, Prentice H and Wu JY:
Taurine and central nervous system disorders. Amino Acids.
46:31–46. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Schaffer SW, Jong CJ, Ramila KC and Azuma
J: Physiological roles of taurine in heart and muscle. J Biomed
Sci. 17 (Suppl 1):S22010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Schaffer SW, Shimada-Takaura K, Jong CJ,
Ito T and Takahashi K: Impaired energy metabolism of the
taurine-deficient heart. Amino Acids. 48:549–558. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Jong CJ, Azuma J and Schaffer S: Mechanism
underlying the antioxidant activity of taurine: Prevention of
mitochondrial oxidant production. Amino Acids. 42:2223–2232. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Jong C, Ito T, Mozaffari M, Azuma J and
Schaffer S: Effect of beta-alanine treatment on mitochondrial
taurine level and 5-taurinomethyluridine content. J Biomed Sci. 17
(Suppl 1):S252010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Vafai SB and Mootha VK: Mitochondrial
disorders as windows into an ancient organelle. Nature.
491:374–833. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Jansen M, Ten Klooster JP, Offerhaus GJ
and Clevers H: LKB1 and AMPK family signaling: The intimate link
between cell polarity and energy metabolism. Physiol. 89:777–798.
2009.PubMed/NCBI
|
|
50
|
Jong CJ, Azuma J and Schaffer SW: Role of
mitochondrial permeability transition in taurine deficiency-induced
apoptosis. Exp Clin Cardiol. 16:125–128. 2011.PubMed/NCBI
|
|
51
|
Ricci C, Pastukh V, Leonard J, Turrens J,
Wilson G, Schaffer D and Schaffer SW: Mitochondrial DNA damage
triggers mitochondrial-superoxide generation and apoptosis. Am J
Physiol Cell Physiol. 294:C413–C422. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Shetewy A, Shimada-Takaura K, Warner D,
Jong CJ, Mehdi AB, Alexeyev M, Takahashi K and Schaffer SW:
Mitochondrial defects associated with β-alanine toxicity: Relevance
to hyper-beta-alaninemia. Mol Cell Biochem. 416:11–22. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Schuller-Levis G and Park E: Is taurine a
biomarker? Adv Clin Chem. 41:1–21. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Cozzi R, Ricordy R, Bartolini F, Ramadori
L, Perticone P and De Salvia R: Taurine and ellagic acid: Two
differently-acting natural antioxidants. Environ Mol Mutagen.
26:248–254. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Aruoma OI, Halliwell B, Hoey BM and Butler
J: The antioxidant action of taurine, hypotaurine and their
metabolic precursors. Biochem J. 256:251–255. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Timbrell JA, Seabra V and Waterfield CJ:
The in vivo and in vitro protective properties of taurine. Gen
Pharmacol. 26:453–462. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Chen WQ, Jin H, Nguyen M, Carr J, Lee YJ,
Hsu CC, Faiman MD, Schloss JV and Wu JY: Role of taurine in
regulation of intracellular calcium level and neuroprotective
function in cultured neurons. J Neurosci Res. 66:612–619. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Yamauchi-Takihara K, Azuma J, Kishimoto S,
Onishi S and Sperelakis N: Taurine prevention of calcium
paradox-related damage in cardiac muscle. Its regulatory action on
intracellular cation contents. Biochem Pharmacol. 37:2651–2658.
1988. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Parvez S, Tabassum H, Banerjee BD and
Raisuddin S: Taurine prevents tamoxifen-induced mitochondrial
oxidative damage in mice. Basic Clin Pharmacol Toxicol.
102:382–387. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Sener G, Ozer Sehirli A, Ipçi Y, Cetinel
S, Cikler E, Gedik N and Alican I: Taurine treatment protects
against chronic nicotine-induced oxidative changes. Fundam Clin
Pharmacol. 155–164. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Şener G, Şehirli Ö, İpçi Y, Çetinel Ş,
Çikler E, Gedik N and Alican I: Protective effects of taurine
against nicotine-induced oxidative damage of rat urinary bladder
and kidney. Pharmacology. 74:37–44. 2005. View Article : Google Scholar
|
|
62
|
Jafri AJA, Agarwal R, Iezhitsa I, Agarwal
P and Ismail NM: Taurine protects against NMDA-induced retinal
damage by reducing retinal oxidative stress. Amino Acids.
51:641–646. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Gordon RE and Heller RF and Heller RF:
Taurine protection of lungs in hamster models of oxidant injury: A
morphologic time study of paraquat and bleomycin treatment.
Taurine. Advances in Experimental Medicine and Biology. Lombardini
JB, Schaffer SW and Azuma J: 315. Springer; Boston, MA: pp.
319–328. 1992, View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Kim C and Cha YN: Taurine chloramine
produced from taurine under inflammation provides anti-inflammatory
and cytoprotective effects. Amino Acids. 46:89–100. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Hsieh TJ, Zhang SL, Filep JG, Tang SS,
Ingelfinger JR and Chan JS: High glucose stimulates angiotensinogen
gene expression via reactive oxygen species generation in rat
kidney proximal tubular cells. Endocrinology. 143:2975–2985. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Kettle AJ, van Dalen CJ and Winterbourn
CC: Peroxynitrite and myeloperoxidase leave the same footprint in
protein nitration. Redox Rep. 3:257–258. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Schaffer S, Solodushko V, Pastukh V, Ricci
C and Azuma J: Possible cause of taurine-deficient cardiomyopathy:
Potentiation of angiotensin II action. J Cardiovasc Pharmacol.
41:751–759. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Vohra BP and Hui X: Taurine protects
against carbon tetrachloride toxicity in the cultured neurons and
in vivo. Arch Physiol Biochem. 109:90–94. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Nonaka H, Tsujino T, Watari Y, Emoto N and
Yokoyama M: Taurine prevents the decrease in expression and
secretion of extracellular superoxide dismutase induced by
homocysteine: Amelioration of homocysteine-induced endoplasmic
reticulum stress by taurine. Circulation. 104:1165–1170. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Anand P, Rajakumar D, Jeraud M, Felix AJ
and Balasubram T: Effects of taurine on glutathione peroxidase,
glutathione reductase and reduced glutathione levels in rats. Pak J
Biol Sci. 14:219–225. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Oudit GY, Trivieri MG, Khaper N, Husain T,
Wilson GJ, Liu P, Sole MJ and Backx PH: Taurine supplementation
reduces oxidative stress and improves cardiovascular function in an
iron-overload murine model. Circulation. 109:1877–1885. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Das J and Sil PC: Taurine ameliorates
alloxan-induced diabetic renal injury, oxidative stress-related
signaling pathways and apoptosis in rats. Amino Acids.
43:1509–1523. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Sayed RH, Salem HA and El-Sayeh BM:
Potential protective effect of taurine against
dibromoacetonitrile-induced neurotoxicity in rats. Environ Toxicol
Pharmacol. 34:849–857. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Das J, Ghosh J, Manna P and Sil PC:
Taurine protects rat testes against doxorubicin-induced oxidative
stress as well as p53, Fas and caspase 12-mediated apoptosis. Amino
Acids. 42:1839–1855. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Bhavsar TM, Cantor JO, Patel SN and
Lau-Cam CA: Attenuating effect of taurine on
lipopolysaccharide-induced acute lung injury in hamsters. Pharmacol
Res. 60:418–428. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Ito T, Schaffer SW and Azuma J: The
potential usefulness of taurine on diabetes mellitus and its
complications. Amino Acids. 42:1529–1539. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Rikimaru M, Ohsawa Y, Wolf AM, Nishimaki
K, Ichimiya H, Kamimura N, Nishimatsu S, Ohta S and Sunada Y:
Taurine ameliorates impaired the mitochondrial function and
prevents stroke-like episodes in patients with MELAS. Intern Med.
51:3351–3357. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Hagar HH, El Etter E and Arafa M: Taurine
attenuates hypertension and renal dysfunction induced by
cyclosporine a in rats. Clin Exp Pharmacol Physiol. 33:189–196.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Shao X, Hu Z, Hu C, Bu Q, Yan G, Deng P,
Lv L, Wu D, Deng Y, Zhao J, et al: Taurine protects
methamphetamine-induced developmental angiogenesis defect through
antioxidant mechanism. Toxicol Appl Pharmacol. 260:260–270. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Brosnan JT and Brosnan ME: The
sulfur-containing amino acids: An overview. J Nutr. 136 (Suppl
6):1636S–1640S. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Wenting L, Ping L, Haitao J, Meng Q and
Xiaofei R: Therapeutic effect of taurine against aluminum-induced
impairment on learning, memory and brain neurotransmitters in rats.
Neurol. 35:1579–1584. 2014.PubMed/NCBI
|
|
82
|
Albrecht J and Schousboe A: Taurine
interaction with neurotransmitter receptors in the CNS: An update.
Neurochem Res. 30:1615–1621. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Wu H, Jin Y, Wei J, Jin H, Sha D and Wu
JY: Mode of action of taurine as a neuroprotector. Brain Res.
1038:123–131. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Chen C, Xia S, He J, Lu G, Xie Z and Han
H: Roles of taurine in cognitive function of physiology,
pathologies and toxication. Life Sci. 231:1165842019. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Ochoa-de la Paz L, Zenteno E,
Gulias-Cañizo R and Quiroz-Mercado H: Taurine and GABA
neurotransmitter receptors, a relationship with therapeutic
potential? Expert Rev Neurother. 19:289–291. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Zhou J, Li Y, Yan G, Bu Q, Lv L, Yang Y,
Zhao J, Shao X, Deng Y, Zhu R, et al: Protective role of taurine
against morphine-induced neurotoxicity in C6 cells via inhibition
of oxidative stress. Neurotox Res. 20:334–342. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Bulley S and Shen W: Reciprocal regulation
between taurine and glutamate response via
Ca2+-dependent pathways in retinal third-order neurons.
J Biomed Sci. 17 (Suppl 1):S52010. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Makarova LM, Pogorelyĭ VE, Voronkov AV and
Novikova NA: Modern notions about the role of taurine in the
central nervous system. Eksp Klin Farmakol. 77:38–44. 2014.(In
Russian). PubMed/NCBI
|
|
89
|
Wu JY and Prentice H: Role of taurine in
the central nervous system. J Biomed Sci. 17 (Suppl 1):S12010.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Zhang C, Liu F, Liu X and Chen D:
Protective effect of N-acetylcysteine against BDE-209-induced
neurotoxicity in primary cultured neonatal rat hippocampal neurons
in vitro. Int J Dev Neurosci. 28:521–528. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Abramov AY and Duchen MR: Mechanisms
underlying the loss of mitochondrial membrane potential in
glutamate excitotoxicity. Biochim Biophys Acta. 1777:953–964. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Prentice H, Modi JP and Wu JY: Mechanisms
of neuronal protection against excitotoxicity, endoplasmic
reticulum stress, and mitochondrial dysfunction in stroke and
neurodegenerative diseases. Oxid Med Cell Longev. 2015:9645182015.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
McCullough KD, Martindale JL, Klotz LO, Aw
TY and Holbrook NJ: Gadd153 sensitizes cells to endoplasmic
reticulum stress by down-regulating Bcl2 and perturbing the
cellular redox state. Mol Cell Biol. 21:1249–1259. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Urano F, Wang X, Bertolotti A, Zhang Y,
Chung P, Harding HP and Ron D: Coupling of stress in the ER to
activation of JNK protein kinases by transmembrane protein kinase
IRE1. Science. 287:664–666. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Zhang R, Jiang M, Zhang J, Qiu Y, Li D, Li
S, Liu J, Liu C, Fang Z and Cao F: Regulation of the
cerebrovascular smooth muscle cell phenotype by mitochondrial
oxidative injury and endoplasmic reticulum stress in simulated
microgravity rats via the PERK-eIF2α-ATF4-CHOP pathway. Biochim
Biophys Acta Mol Basis Dis. 1866:1657992020. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Aslan Karakelle N, Dinçer S and Yar Sağlam
AS: The effect of intracerebroventricular amyloid beta 1–42
application on cognitive functions in aged rats supplemented with
taurine and the change of peroxisomal proteins in this process.
Brain Res Bull. 172:89–97. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Lee DS and Cheong SH: Taurine have
neuroprotective activity against oxidative damage-induced HT22 Cell
death through heme oxygenase-1 pathway. Taurine 10. Advances in
Experimental Medicine and Biology. Lee DH, Schaffer SW, Park E and
Kim HW: 975. Springer; Dordrecht: pp. 159–171. 2017, View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Zhang X, Wang X, Zhang J, Pan X, Jiang J
and Li Y: Effects of taurine on alterations of neurobehavior and
neurodevelopment key proteins expression in infant rats by exposure
to hexabromocyclododecane. Taurine 10. Advances in Experimental
Medicine and Biology. Lee DH, Schaffer SW, Park E and Kim HW: 975.
Springer; Dordrecht: pp. 119–30. 2017, View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Chen R, Liu S, Piao F, Wang Z, Qi Y, Li S,
Zhang D and Shen J: 2,5-hexanedione induced apoptosis in
mesenchymal stem cells from rat bone marrow via
mitochondria-dependent caspase-3 pathway. Ind Health. 53:222–235.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Liu S, Piao F, Sun X, Bai L, Peng Y, Zhong
Y, Ma N and Sun W: Arsenic-induced inhibition of hippocampal
neurogenesis and its reversibility. Neurotoxicology. 33:1033–1039.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Yorifuji T, Kato T, Ohta H, Bellinger DC,
Matsuoka K and Grandjean P: Neurological and neuropsychological
functions in adults with a history of developmental arsenic
poisoning from contaminated milk powder. Neurotoxicol Teratol.
53:75–80. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Li K, Wang D, Zhou X, Shao J, Li Y, Liu X,
Zhang C, Zuo E, Shi X, Piao F and Li S: Taurine protects against
arsenic-induced apoptosis Via PI3K/Akt pathway in primary cortical
neurons. In: Taurine 11. Advances in Experimental Medicine and
Biology. Hu J, Piao F, Schaffer SW, El Idrissi A and Wu JY: 1155.
Springer; Singapore: pp. 747–754. 2019, PubMed/NCBI
|
|
103
|
Piao F, Zhang Y, Yang L, Zhang C, Shao J,
Liu X, Li Y and Li S: Taurine attenuates As2O3-induced autophagy in
cerebrum of mouse through Nrf2 pathway. Adv Exp Med Biol.
975:863–870. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Cholanians AB, Phan AV, Ditzel EJ,
Camenisch TD, Lau SS and Monks TJ: From the cover: Arsenic induces
accumulation of α-synuclein: Implications for synucleinopathies and
neurodegeneration. Toxicol Sci. 153:271–281. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Escudero-Lourdes C: Toxicity mechanisms of
arsenic that are shared with neurodegenerative diseases and
cognitive impairment: Role of oxidative stress and inflammatory
responses. Neurotoxicology. 53:223–235. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Lee J, Giordano S and Zhang J: Autophagy,
mitochondria and oxidative stress: Cross-talk and redox signalling.
Biochem J. 441:523–540. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Zhu XX, Yao XF, Jiang LP, Geng CY, Zhong
LF, Yang G, Zheng BL and Sun XC: Sodium arsenite induces
ROS-dependent autophagic cell death in pancreatic β-cells. Food
Chem Toxicol. 70:144–150. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Li Y, Hu Z, Chen B, Bu Q, Lu W, Deng Y,
Zhu R, Shao X, Hou J, Zhao J, et al: Taurine attenuates
methamphetamine-induced autophagy and apoptosis in PC12 cells
through mTOR signaling pathway. Toxicol Lett. 215:1–7. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Menzie J, Prentice H and Wu JY:
Neuroprotective mechanisms of taurine against ischemic stroke.
Brain Sci. 3:877–907. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Schaffer SW, Jong CJ, Ito T and Azuma J:
Effect of taurine on ischemia-reperfusion injury. Amino Acids.
46:21–30. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Yamori Y, Liu L, Mori M, Sagara M,
Murakami S, Nara Y and Mizushima S: Taurine as the nutritional
factor for the longevity of the japanese revealed by a world-wide
epidemiological survey. In: Taurine 7. Advances in Experimental
Medicine and Biology. Azuma J, Schaffer SW and Ito T: 643.
Springer; New York, NY: pp. 13–25. 2009, PubMed/NCBI
|
|
112
|
Lotocki G, de Rivero Vaccari JP, Perez ER,
Sanchez-Molano J, Furones-Alonso O, Bramlett HM and Dietrich WD:
Alterations in blood-brain barrier permeability to large and small
molecules and leukocyte accumulation after traumatic brain injury:
Effects of post-traumatic hypothermia. J Neurotrauma. 26:1123–1134.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Sun Q, Hu H, Wang W, Jin H, Feng G and Jia
N: Taurine attenuates amyloid β 1–42-induced mitochondrial
dysfunction by activating of SIRT1 in SK-N-SH cells. Biochem
Biophys Res Commun. 447:485–489. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Niu X, Zheng S, Liu H and Li S: Protective
effects of taurine against inflammation, apoptosis, and oxidative
stress in brain injury. Mol Med Rep. 18:4516–4522. 2018.PubMed/NCBI
|
|
115
|
Saransaari P and Oja SS: Enhanced taurine
release in cultured cerebellar granule cells in cell-damaging
conditions. Amino Acids. 17:323–334. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Ricci L, Valoti M, Sgaragli G and Frosini
M: Protection by taurine of rat brain cortical slices against
oxygen glucose deprivation- and reoxygenation-induced damage. Eur J
Pharmacol. 621:26–32. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Abramov AY, Scorziello A and Duchen MR:
Three distinct mechanisms generate oxygen free radicals in neurons
and contribute to cell death during anoxia and reoxygenation. J
Neurosci. 27:1129–1138. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Han Z, Gao LY, Lin YH, Chang L, Wu HY, Luo
CX and Zhu DY: Neuroprotection of taurine against reactive oxygen
species is associated with inhibiting NADPH oxidases. Eur J
Pharmacol. 777:129–135. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Pion PD, Kittleson MD, Rogers QR and
Morris JG: Myocardial failure in cats associated with low plasma
taurine: A reversible cardiomyopathy. Science. 237:764–768. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Moise NS, Pacioretty LM, Kallfelz FA,
Stipanuk MH, King JM and Gilmour RF Jr: Dietary taurine deficiency
and dilated cardiomyopathy in the fox. Am Heart J. 121:541–547.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Lake N: Loss of cardiac myofibrils:
Mechanism of contractile deficits induced by taurine deficiency. Am
J Physiol. 264:H1323–H1326. 1993.PubMed/NCBI
|
|
122
|
Ito T, Kimura Y, Uozumi Y, Takai M,
Muraoka S, Matsuda T, Ueki K, Yoshiyama M, Ikawa M, Okabe M, et al:
Taurine depletion caused by knocking out the taurine transporter
gene leads to cardiomyopathy with cardiac atrophy. J Mol Cell
Cardiol. 44:927–937. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Ito T, Oishi S, Takai M, Kimura Y, Uozumi
Y, Fujio Y, Schaffer SW and Azuma J: Cardiac and skeletal muscle
abnormality in taurine transporter-knockout mice. J Biomed Sci. 17
(Suppl 1):S202010. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Huxtable R and Bressler R: Taurine
concentrations in congestive heart failure. Science. 184:1187–1188.
1974. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Jacobsen JG and Smith LH: Biochemistry and
physiology of taurine and taurine derivatives. Physiol Rev.
48:424–511. 1968. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Huxtable RJ and Sebring LA: Cardiovascular
actions of taurine. Prog Clin Biol Res. 125:5–37. 1983.PubMed/NCBI
|
|
127
|
Suwanich A, Wyss JM and Roysommuti S:
Taurine supplementation in spontaneously hypertensive rats:
Advantages and limitations for human applications. World J Cardiol.
5:404–409. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Bousquet P, Feldman J, Bloch R and
Schwartz J: Central cardiovascular effects of taurine: Comparison
with homotaurine and muscimol. J Pharmacol Exp Ther. 219:213–218.
1981.PubMed/NCBI
|
|
129
|
Satoh H: Cardioprotective actions of
taurine against intracellular and extracellular calcium-induced
effects. Taurine in Health and Disease. Advances in Experimental
Medicine and Biology. Huxtable RJ and Michalk D: 359. Springer;
Boston, MA: pp. 181–196. 1994, View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Qi B, Yamagami T, Naruse Y, Sokejima S and
Kagamimori S: Effects of taurine on depletion of erythrocyte
membrane Na-K ATPase activity due to ozone exposure or cholesterol
enrichment. J Nutr Sci Vitaminol (Tokyo). 41:627–634. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Petty MA, Kintz J and DiFrancesco GF: The
effects of taurine on atherosclerosis development in
cholesterol-fed rabbits. Eur J Pharmacol. 180:119–127. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Murakami S, Sakurai T, Tomoike H, Sakono
M, Nasu T and Fukuda N: Prevention of hypercholesterolemia and
atherosclerosis in the hyperlipidemia- and atherosclerosis-prone
Japanese (LAP) quail by taurine supplementation. Amino Acids.
38:271–278. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Murakami S, Nara Y and Yamori Y: Taurine
accelerates the regression of hypercholesterolemia in stroke-prone
spontaneously hypertensive rats. Life Sci. 58:1643–1651. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Yokogoshi H, Mochizuki H, Nanami K, Hida
Y, Miyachi F and Oda H: Dietary taurine enhances cholesterol
degradation and reduces serum and liver cholesterol concentrations
in rats fed a high-cholesterol diet. J Nutr. 129:1705–1712. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Lam NV, Chen W, Suruga K, Nishimura N,
Goda T and Yokogoshi H: Enhancing effect of taurine on CYP7A1 mRNA
expression in Hep G2 cells. Amino Acids. 30:43–48. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Bellentani S, Pecorari M, Cordoma P,
Marchegiano P, Manenti F, Bosisio E, De Fabiani E and Galli G:
Taurine increases bile acid pool size and reduces bile saturation
index in the hamster. J Lipid Res. 28:1021–1027. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Yanagita T, Han SY, Hu Y, Nagao K,
Kitajima H and Murakami S: Taurine reduces the secretion of
apolipoprotein B100 and lipids in HepG2 cells. Lipids Health Dis.
7:382008. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Ulrich-Merzenich G, Zeitler H, Vetter H
and Bhonde RR: Protective effects of taurine on endothelial cells
impaired by high glucose and oxidized low density lipoproteins. Eur
J Nutr. 46:431–438. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Gokce G, Ozsarlak-Sozer G, Oran I, Oktay
G, Ozkal S and Kerry Z: Taurine suppresses oxidative
stress-potentiated expression of lectin-like oxidized low-density
lipoprotein receptor and restenosis in balloon-injured rabbit iliac
artery. Clin Exp Pharmacol Physiol. 38:811–818. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Matsushima Y, Sekine T, Kondo Y, Sakurai
T, Kameo K, Tachibana M and Murakami S: Effects of taurine on serum
cholesterol levels and development of atherosclerosis in
spontaneously hyperlipidaemic mice. Clin Exp Pharmacol Physiol.
30:295–299. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Murakami S, Kondo-Ohta Y and Tomisawa K:
Improvement in cholesterol metabolism in mice given chronic
treatment of taurine and fed a high-fat diet. Life Sci. 64:83–91.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Chen W, Guo JX and Chang P: The effect of
taurine on cholesterol metabolism. Mol Nutr Food Res. 56:681–690.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Mochizuki H, Takido J and Yokogoshi H:
Effect of dietary taurine on endogenous hypercholesterolemia in
rats fed on phenobarbital-containing diets. Biosci Biotechnol
Biochem. 63:1298–1300. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Mochizuki H, Oda H and Yokogoshi H:
Dietary taurine potentiates polychlorinated biphenyl-induced
hypercholesterolemia in rats*. J Nutr Biochem. 12:109–115. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Zulli A, Lau E, Wijaya BP, Jin X, Sutarga
K, Schwartz GD, Learmont J, Wookey PJ, Zinellu A, Carru C and Hare
DL: High dietary taurine reduces apoptosis and atherosclerosis in
the left main coronary artery: Association with reduced
CCAAT/enhancer binding protein homologous protein and total plasma
homocysteine but not lipidemia. Hypertension. 53:1017–1022. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Tan B, Jiang DJ, Huang H, Jia SJ, Jiang
JL, Hu CP and Li YJ: Taurine protects against low-density
lipoprotein-induced endothelial dysfunction by the DDAH/ADMA
pathway. Vascul Pharmacol. 46:338–345. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Elvevoll EO, Eilertsen KE, Brox J, Dragnes
BT, Falkenberg P, Olsen JO, Kirkhus B, Lamglait A and Østerud B:
Seafood diets: Hypolipidemic and antiatherogenic effects of taurine
and n-3 fatty acids. Atherosclerosis. 200:396–402. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
di Wu Q, Wang JH, Fennessy F, Redmond HP
and Bouchier-Hayes D: Taurine prevents high-glucose-induced human
vascular endothelial cell apoptosis. Am J Physiol. 277:C1229–C1238.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Katakawa M, Fukuda N, Tsunemi A, Mori M,
Maruyama T, Matsumoto T, Abe M and Yamori Y: Taurine and magnesium
supplementation enhances the function of endothelial progenitor
cells through antioxidation in healthy men and spontaneously
hypertensive rats. Hypertens Res. 39:848–856. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Chang L, Xu J, Yu F, Zhao J, Tang X and
Tang C: Taurine protected myocardial mitochondria injury induced by
hyperhomocysteinemia in rats. Amino Acids. 27:37–48. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Ghosh J, Das J, Manna P and Sil PC:
Taurine prevents arsenic-induced cardiac oxidative stress and
apoptotic damage: Role of NF-kappa B, p38 and JNK MAPK pathway.
Toxicol Appl Pharmacol. 240:73–87. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Mousavi K, Niknahad H, Ghalamfarsa A,
Mohammadi H, Azarpira N, Ommati MM and Heidari R: Taurine mitigates
cirrhosis-associated heart injury through mitochondrial-dependent
and antioxidative mechanisms. Clin Exp Hepatol. 6:207–219. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Liu J, Ai Y, Niu X, Shang F, Li Z, Liu H,
Li W, Ma W, Chen R, Wei T, et al: Taurine protects against cardiac
dysfunction induced by pressure overload through SIRT1-p53
activation. Chem Biol Interact. 317:1089722020. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Chen G, Nan C, Tian J, Jean-Charles P, Li
Y, Weissbach H and Huang XP: Protective effects of taurine against
oxidative stress in the heart of MsrA knockout mice. J Cell
Biochem. 113:3559–3566. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Rashid K, Das J and Sil PC: Taurine
ameliorate alloxan induced oxidative stress and intrinsic apoptotic
pathway in the hepatic tissue of diabetic rats. Food Chem Toxicol.
51:317–329. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Sevin G, Ozsarlak-Sozer G, Keles D, Gokce
G, Reel B, Ozgur HH, Oktay G and Kerry Z: Taurine inhibits
increased MMP-2 expression in a model of oxidative stress induced
by glutathione depletion in rabbit heart. Eur J Pharmacol.
706:98–106. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Hansen SH, Andersen ML, Cornett C,
Gradinaru R and Grunnet N: A role for taurine in mitochondrial
function. J Biomed Sci. 17 (Suppl 1):S232010. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Oriyanhan W, Yamazaki K, Miwa S, Takaba K,
Ikeda T and Komeda M: Taurine prevents myocardial
ischemia/reperfusion-induced oxidative stress and apoptosis in
prolonged hypothermic rat heart preservation. Heart Vessels.
20:278–285. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Sahin MA, Yucel O, Guler A, Doganci S,
Jahollari A, Cingoz F, Arslan S, Gamsizkan M, Yaman H and
Demirkilic U: Is there any cardioprotective role of Taurine during
cold ischemic period following global myocardial ischemia? J
Cardiothorac Surg. 6:312011. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Venturini A, Ascione R, Lin H, Polesel E,
Angelini GD and Suleiman MS: The importance of myocardial amino
acids during ischemia and reperfusion in dilated left ventricle of
patients with degenerative mitral valve disease. Mol Cell Biochem.
330:63–70. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Xu YJ, Arneja AS, Tappia PS and Dhalla NS:
The potential health benefits of taurine in cardiovascular disease.
Exp Clin Cardiol. 13:57–65. 2008.PubMed/NCBI
|
|
162
|
Jacobus WE, Tiozzo R, Lugli G, Lehninger
AL and Carafoli E: Aspects of energy-linked calcium accumulation by
rat heart mitochondria. J Biol Chem. 250:7863–7870. 1975.
View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Rasola A and Bernardi P: Mitochondrial
permeability transition in Ca(2+)-dependent apoptosis and necrosis.
Cell Calcium. 50:222–233. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Zhang Y, Yang L, Yang YJ, Liu XY, Jia JG,
Qian JY, Wang KQ, Zuo J and Ge J: Low-dose taurine upregulates
taurine transporter expression in acute myocardial ischemia. Int J
Mol Med. 31:817–824. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Schaffer S, Solodushko V and Azuma J:
Taurine-deficient cardiomyopathy: Role of phospholipids, calcium
and osmotic stress. Taurine 4. Advances in Experimental Medicine
and Biology. Della Corte L, Huxtable RJ, Sgaragli G and Tipton KF:
483. Springer; Boston, MA: pp. 57–69. 2002, View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Bagchi D, Wetscher GJ, Bagchi M, Hinder
PR, Perdikis G, Stohs SJ, Hinder RA and Das DK: Interrelationship
between cellular calcium homeostasis and free radical generation in
myocardial reperfusion injury. Chem Biol Interact. 104:65–85. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Grace PA: Ischaemia-reperfusion injury. Br
J Surg. 81:637–647. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Lemasters JJ, Nieminen AL, Qian T, Trost
LC and Herman B: The mitochondrial permeability transition in
toxic, hypoxic and reperfusion injury. Mol Cell Biochem.
174:159–165. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Kaplan B, Aricioglu A, Erbas D, Erbas S
and Turkozkan N: The effects of taurine on perfused heart muscle
malondialdehyde levels. Gen Pharmacol. 24:1411–1413. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Ramila KC, Jong CJ, Pastukh V, Ito T,
Azuma J and Schaffer SW: Role of protein phosphorylation in
excitation-contraction coupling in taurine deficient hearts. Am J
Physiol-Heart Circ Physiol. 308:H232–H239. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Modi P and Suleiman MS: Myocardial
taurine, development and vulnerability to ischemia. Amino Acids.
26:65–70. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Tricarico D, Barbieri M and Camerino DC:
Taurine blocks ATP-sensitive potassium channels of rat skeletal
muscle fibres interfering with the sulphonylurea receptor. Br J
Pharmacol. 130:827–834. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Tricarico D, Barbieri M and Conte Camerino
D: Voltage-dependent antagonist/agonist actions of taurine on
Ca(2+)-activated potassium channels of rat skeletal muscle fibers.
J Pharmacol Exp Ther. 298:1167–1171. 2001.PubMed/NCBI
|
|
174
|
Shimada K, Jong CJ, Takahashi K and
Schaffer SW: Role of ROS Production and Turnover in the Antioxidant
Activity of Taurine. Taurine. 9:Marcinkiewicz J and Schaffer SW:
581–96. 2015.
|
|
175
|
Takatani T, Takahashi K, Uozumi Y, Shikata
E, Yamamoto Y, Ito T, Matsuda T, Schaffer SW, Fujio Y and Azuma J:
Taurine inhibits apoptosis by preventing formation of the
Apaf-1/caspase-9 apoptosome. Am J Physiol Cell Physiol.
287:C949–C953. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Li Y, Arnold JM, Pampillo M, Babwah AV and
Peng T: Taurine prevents cardiomyocyte death by inhibiting NADPH
oxidase-mediated calpain activation. Free Radic Biol Med. 46:51–61.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Ahmadi S and Mehranjani MS: Taurine
improves follicular survival and function of mice ovarian grafts
through increasing CD31 and GDF9 expression and reducing oxidative
stress and apoptosis. Eur J Pharmacol. 903:1741342021. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Ito T, Yoshikawa N, Schaffer SW and Azuma
J: Tissue taurine depletion alters metabolic response to exercise
and reduces running capacity in mice. J Amino Acids.
2014:9646802014. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Bakker AJ and Berg HM: Effect of taurine
on sarcoplasmic reticulum function and force in skinned fast-twitch
skeletal muscle fibres of the rat. J Physiol. 538:185–194. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Thirupathi A, Pinho RA, Baker JS, István B
and Gu Y: Taurine reverses oxidative damages and restores the
muscle function in overuse of exercised muscle. Front Physiol.
11:5824492020. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Dutka TL, Lamboley CR, Murphy RM and Lamb
GD: Acute effects of taurine on sarcoplasmic reticulum
Ca2+ accumulation and contractility in human type I and
II skeletal muscle fibers. J Appl Physiol. 117:797–805. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
182
|
De Luca A, Pierno S and Camerino DC:
Taurine: The appeal of a safe amino acid for skeletal muscle
disorders. J Transl Med. 13:2432015. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
De Luca A, Pierno S and Camerino DC:
Effect of taurine depletion on excitation-contraction coupling and
Cl-conductance of rat skeletal muscle. Eur J Pharmacol.
296:215–222. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Jentsch TJ: CLC chloride channels and
transporters: From genes to protein structure, pathology and
physiology. Crit Rev Biochem Mol Biol. 43:3–36. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Hamilton EJ, Berg HM, Easton CJ and Bakker
AJ: The effect of taurine depletion on the contractile properties
and fatigue in fast-twitch skeletal muscle of the mouse. Amino
Acids. 31:273–278. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Tallis J, Higgins MF, Cox VM, Duncan MJ
and James RS: Does a physiological concentration of taurine
increase acute muscle power output, time to fatigue, and recovery
in isolated mouse soleus (slow) muscle with or without the presence
of caffeine? Can J Physiol Pharmacol. 92:42–49. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Yatabe Y, Miyakawa S, Miyazaki T,
Matsuzaki Y and Ochiai N: Effects of taurine administration in rat
skeletal muscles on exercise. J Orthop Sci. 8:415–419. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Ohmori H, Matsumura M, Komine S, Kobayashi
H, Kobayashi Y, Shiromoto J and Miyakawa S: The production of a rat
model that inhibits phosphoenolpyruvate carboxykinase (PEPCK), a
rate-limiting enzyme of hepatic gluconeogenesis. Taurine 11.
Advances in Experimental Medicine and Biology. Hu J, Piao F,
Schaffer SW, El Idrissi A and Wu JY: 1155. Springer; Singapore: pp.
113–118. 2019, View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Zachariah Tom R, Garcia-Roves PM, Sjögren
RJ, Jiang LQ, Holmström MH, Deshmukh AS, Vieira E, Chibalin AV,
Björnholm M and Zierath JR: Effects of AMPK activation on insulin
sensitivity and metabolism in leptin-deficient ob/ob mice.
Diabetes. 63:1560–1571. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Beloshapka AN, de Godoy MR, Carter RA,
Fascetti AJ, Yu Z, McIntosh BJ, Swanson KS and Buff PR:
Longitudinal changes in blood metabolites, amino acid profile, and
oxidative stress markers in American foxhounds fed a
nutrient-fortified diet. J Anim Sci. 96:930–940. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Dawson R Jr, Biasetti M, Messina S and
Dominy J: The cytoprotective role of taurine in exercise-induced
muscle injury. Amino Acids. 22:309–324. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Silva LA, Silveira PC, Ronsani MM, Souza
PS, Scheffer D, Vieira LC, Benetti M, De Souza CT and Pinho RA:
Taurine supplementation decreases oxidative stress in skeletal
muscle after eccentric exercise. Cell Biochem Funct. 29:43–49.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Marcinkiewicz J: Taurine bromamine: A new
therapeutic option in inflammatory skin diseases. Pol Arch Med
Wewn. 119:673–676. 2009.PubMed/NCBI
|
|
194
|
Kato T, Okita S, Wang S, Tsunekawa M and
Ma N: The effects of Taurine administration against inflammation in
heavily exercised skeletal muscle of rats. Taurine 9. Advances in
Experimental Medicine and Biology. Marcinkiewicz J and Schaffer SW:
803. Springer; New York, NY: pp. 773–784. 2015, View Article : Google Scholar : PubMed/NCBI
|
|
195
|
Sugiura H, Okita S, Kato T, Naka T,
Kawanishi S, Ohnishi S, Oshida Y and Ma N: Protection by taurine
against INOS-dependent DNA damage in heavily exercised skeletal
muscle by inhibition of the NF-κB signaling pathway. Taurine 8.
Advances in Experimental Medicine and Biology. El Idrissi A and
L'Amoreaux WJ: 775. Springer; New York, NY: pp. 237–246. 2013,
View Article : Google Scholar : PubMed/NCBI
|
|
196
|
Moyes CD: Controlling muscle mitochondrial
content. J Exp Biol. 206:4385–4391. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
197
|
Nikolaidis MG and Mougios V: Effects of
exercise on the fatty-acid composition of blood and tissue lipids.
Sports Med. 34:1051–1057. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Nikolaidis MG, Petridou A and Mougios V:
Comparison of the phospholipid and triacylglycerol fatty acid
profile of rat serum, skeletal muscle and heart. Physiol Res.
55:259–265. 2006.PubMed/NCBI
|
|
199
|
Imagawa TF, Hirano I, Utsuki K, Horie M,
Naka A, Matsumoto K and Imagawa S: Caffeine and taurine enhance
endurance performance. Int J Sports Med. 30:485–488. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
200
|
Mikami N, Hosokawa M and Miyashita K:
Dietary combination of fish oil and taurine decreases fat
accumulation and ameliorates blood glucose levels in type 2
diabetic/obese KK-A(y) mice. J Food Sci. 77:H114–H120. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
201
|
Ra SG, Miyazaki T, Ishikura K, Nagayama H,
Komine S, Nakata Y, Maeda S, Matsuzaki Y and Ohmori H: Combined
effect of branched-chain amino acids and taurine supplementation on
delayed onset muscle soreness and muscle damage in high-intensity
eccentric exercise. J Int Soc Sports Nutr. 10:512013. View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Galloway SD, Talanian JL, Shoveller AK,
Heigenhauser GJ and Spriet LL: Seven days of oral taurine
supplementation does not increase muscle taurine content or alter
substrate metabolism during prolonged exercise in humans. J Appl
Physiol (1985). 105:643–651. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
203
|
D'Antona G, Nabavi SM, Micheletti P, Di
Lorenzo A, Aquilani R, Nisoli E, Rondanelli M and Daglia M:
Creatine, L-carnitine, and ω3 polyunsaturated fatty acid
supplementation from healthy to diseased skeletal muscle. Biomed
Res Int. 2014:6138902014.
|
|
204
|
Rutherford JA, Spriet LL and Stellingwerff
T: The effect of acute taurine ingestion on endurance performance
and metabolism in well-trained cyclists. Int J Sport Nutr Exerc
Metab. 20:322–329. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
205
|
Balshaw TG, Bampouras TM, Barry TJ and
Sparks SA: The effect of acute taurine ingestion on 3-km running
performance in trained middle-distance runners. Amino Acids.
44:555–561. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
206
|
da Silva LA, Tromm CB, Bom KF, Mariano I,
Pozzi B, da Rosa GL, Tuon T, da Luz G, Vuolo F, Petronilho F, et
al: Effects of taurine supplementation following eccentric exercise
in young adults. Appl Physiol Nutr Metab. 39:101–104. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
207
|
Ra SG, Choi Y, Akazawa N, Kawanaka K,
Ohmori H and Maeda S: Effects of taurine supplementation on
vascular endothelial function at rest and after resistance
exercise. Taurine 11. Advances in Experimental Medicine and
Biology. Hu J, Piao F, Schaffer SW, El Idrissi A and Wu JY: 1155.
Springer; Singapore: pp. 407–414. 2019, View Article : Google Scholar : PubMed/NCBI
|
|
208
|
Zhang M, Izumi I, Kagamimori S, Sokejima
S, Yamagami T, Liu Z and Qi B: Role of taurine supplementation to
prevent exercise-induced oxidative stress in healthy young men.
Amino Acids. 26:203–207. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
209
|
Chupel MU, Minuzzi LG, Furtado GE, Santos
ML, Ferreira JP, Filaire E and Teixeira AM: Taurine supplementation
reduces myeloperoxidase and matrix-metalloproteinase-9 levels and
improves the effects of exercise in cognition and physical fitness
in older women. Amino Acids. 53:333–435. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
210
|
De Paepe B, Martin JJ, Herbelet S,
Jimenez-Mallebrera C, Iglesias E, Jou C, Weis J and De Bleecker JL:
Activation of osmolyte pathways in inflammatory myopathy and
Duchenne muscular dystrophy points to osmoregulation as a
contributing pathogenic mechanism. Lab Invest. 96:872–884. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
211
|
Pierno S, Liantonio A, Camerino GM, De
Bellis M, Cannone M, Gramegna G, Scaramuzzi A, Simonetti S, Nicchia
GP, Basco D, et al: Potential benefits of taurine in the prevention
of skeletal muscle impairment induced by disuse in the
hindlimb-unloaded rat. Amino Acids. 43:431–445. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
212
|
Uozumi Y, Ito T, Hoshino Y, Mohri T, Maeda
M, Takahashi K, Fujio Y and Azuma J: Myogenic differentiation
induces taurine transporter in association with taurine-mediated
cytoprotection in skeletal muscles. Biochem J. 394:699–706. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
213
|
Terrill JR, Grounds MD and Arthur PG:
Taurine deficiency, synthesis and transport in the mdx mouse model
for Duchenne muscular dystrophy. Int J Biochem Cell Biol.
66:141–148. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
214
|
Cozzoli A, Nico B, Sblendorio VT,
Capogrosso RF, Dinardo MM, Longo V, Gagliardi S, Montagnani M and
De Luca A: Enalapril treatment discloses an early role of
angiotensin II in inflammation- and oxidative stress-related muscle
damage in dystrophic mdx mice. Pharmacol Res. 64:482–492. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
215
|
Jaeschke H: Reactive oxygen and mechanisms
of inflammatory liver injury. J Gastroenterol Hepatol. 15:718–724.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
216
|
Warskulat U, Borsch E, Reinehr R,
Heller-Stilb B, Mönnighoff I, Buchczyk D, Donner M, Flögel U,
Kappert G, Soboll S, et al: Chronic liver disease is triggered by
taurine transporter knockout in the mouse. FASEB J. 20:574–576.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
217
|
Chen X, Sebastian BM, Tang H, McMullen MM,
Axhemi A, Jacobsen DW and Nagy LE: Taurine supplementation prevents
ethanol-induced decrease in serum adiponectin and reduces hepatic
steatosis in rats. Hepatology. 49:1554–1562. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
218
|
Goc Z, Kapusta E, Formicki G, Martiniaková
M and Omelka R: Effect of taurine on ethanol-induced oxidative
stress in mouse liver and kidney. Chin J Physiol. 62:148–156. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
219
|
Lin CJ, Chiu CC, Chen YC, Chen ML, Hsu TC
and Tzang BS: Taurine attenuates hepatic inflammation in chronic
alcohol-fed rats through inhibition of TLR4/MyD88 signaling. J Med
Food. 18:1291–1298. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
220
|
Murakami S, Ono A, Kawasaki A, Takenaga T
and Ito T: Taurine attenuates the development of hepatic steatosis
through the inhibition of oxidative stress in a model of
nonalcoholic fatty liver disease in vivo and in vitro. Amino Acids.
50:1279–1288. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
221
|
Zhang Z, Liu D, Yi B, Liao Z, Tang L, Yin
D and He M: Taurine supplementation reduces oxidative stress and
protects the liver in an iron-overload murine model. Mol Med Rep.
10:2255–2262. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
222
|
Choi MJ and Jung YJ: Effects of taurine
and vitamin d on antioxidant enzyme activity and lipids profiles in
rats fed diet deficient calcium. Taurine 10. Advances in
Experimental Medicine and Biology. Lee DH, Schaffer SW, Park E and
Kim HW: 975. Springer; Dordrecht: pp. 1081–1092. 2017, View Article : Google Scholar : PubMed/NCBI
|
|
223
|
Abdel-Daim MM, Dessouki AA, Abdel-Rahman
HG, Eltaysh R and Alkahtani S: Hepatorenal protective effects of
taurine and N-acetylcysteine against fipronil-induced injuries: The
antioxidant status and apoptotic markers expression in rats. Sci
Total Environ. 650:2063–2073. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
224
|
Hoang MH, Jia Y, Jun H, Lee JH, Hwang KY,
Choi DW, Um SJ, Lee BY, You SG and Lee SJ: Taurine is a liver X
receptor-α ligand and activates transcription of key genes in the
reverse cholesterol transport without inducing hepatic lipogenesis.
Mol Nutr Food Res. 56:900–911. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
225
|
Geyeregger R, Zeyda M and Stulnig TM:
Liver X receptors in cardiovascular and metabolic disease. Cell Mol
Life. 63:524–539. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
226
|
Cao PJ, Jin YJ, Li ME, Zhou R and Yang MZ:
PGC-1α may associated with the anti-obesity effect of taurine on
rats induced by arcuate nucleus lesion. Nutr Neurosci. 19:86–93.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
227
|
Mizushima S, Nara Y, Sawamura M and Yamori
Y: Effects of oral taurine supplementation on lipids and
sympathetic nerve tone. Taurine 2. Advances in Experimental
Medicine and Biology. Huxtable RJ, Azuma J, Kuriyama K, Nakagawa M
and Baba A: 403. Springer; Boston, MA: pp. 615–622. 1996,
View Article : Google Scholar : PubMed/NCBI
|
|
228
|
Zhang M, Bi LF, Fang JH, Su XL, Da GL,
Kuwamori T and Kagamimori S: Beneficial effects of taurine on serum
lipids in overweight or obese non-diabetic subjects. Amino Acids.
26:267–271. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
229
|
Miyazaki T, Karube M, Matsuzaki Y, Ikegami
T, Doy M, Tanaka N and Bouscarel B: Taurine inhibits oxidative
damage and prevents fibrosis in carbon tetrachloride-induced
hepatic fibrosis. J Hepatol. 43:117–125. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
230
|
Abd-Elhakim YM, Ghoneim MH, Ebraheim LLM
and Imam TS: Taurine and hesperidin rescues carbon
tetrachloride-triggered testicular and kidney damage in rats via
modulating oxidative stress and inflammation. Life Sci.
254:1177822020. View Article : Google Scholar : PubMed/NCBI
|
|
231
|
Burlacu A, Genovesi S, Ortiz A, Combe C,
Basile C, Schneditz D, van der Sande F, Popa GT, Morosanu C and
Covic A: Pros and cons of antithrombotic therapy in end-stage
kidney disease: A 2019 update. Nephrol Dial Transplant. 34:923–933.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
232
|
Ijiri Y, Ikarugi H, Tamura Y, Ura M,
Morishita M, Hamada A, Mori M, Mori H, Yamori Y, Ishii H and
Yamamoto J: Antithrombotic effect of taurine in healthy Japanese
people may be related to an increased endogenous thrombolytic
activity. Thromb Res. 131:158–161. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
233
|
Lee WM: Acetaminophen toxicity: Changing
perceptions on a social/medical issue. Hepatology. 46:966–970.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
234
|
Bessems JG and Vermeulen NP: Paracetamol
(acetaminophen)-induced toxicity: Molecular and biochemical
mechanisms, analogues and protective approaches. Crit Rev Toxicol.
31:55–138. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
235
|
Jaeschke H, Knight TR and Bajt ML: The
role of oxidant stress and reactive nitrogen species in
acetaminophen hepatotoxicity. Toxicol Lett. 144:279–288. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
236
|
Hinson JA, Roberts DW and James LP:
Mechanisms of acetaminophen-induced liver necrosis. Adverse Drug
Reactions. Handbook of Experimental Pharmacology. Uetrecht J: 196.
Springer; Berlin: pp. 369–405. 2010, View Article : Google Scholar : PubMed/NCBI
|
|
237
|
Acharya M and Lau-Cam CA: Comparison of
the protective actions of N-acetylcysteine, hypotaurine and taurine
against acetaminophen-induced hepatotoxicity in the rat. J Biomed
Sci. 17 (Suppl 1):S352010. View Article : Google Scholar : PubMed/NCBI
|
|
238
|
Acharya M and Lau-Cam CA: Comparative
evaluation of the effects of taurine and thiotaurine on alterations
of the cellular redox status and activities of antioxidant and
glutathione-related enzymes by acetaminophen in the rat. Taurine 8.
Advances in Experimental Medicine and Biology. El Idrissi A and
L'Amoreaux WJ: 776. Springer; New York, NY: pp. 199–215. 2013,
View Article : Google Scholar : PubMed/NCBI
|
|
239
|
Ghandforoush-Sattari M and Mashayekhi S:
Evaluation of taurine as a biomarker of liver damage in paracetamol
poisoning. Eur J Pharmacol. 581:171–176. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
240
|
Boşgelmez İİ, Söylemezoğlu T and Güvendik
G: The protective and antidotal effects of taurine on hexavalent
chromium-induced oxidative stress in mice liver tissue. Biol Trace
Elem Res. 125:46–58. 2008. View Article : Google Scholar
|
|
241
|
Tabassum H, Rehman H, Banerjee BD,
Raisuddin S and Parvez S: Attenuation of tamoxifen-induced
hepatotoxicity by taurine in mice. Clin Chim Acta. 370:129–136.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
242
|
Patrick L: Lead toxicity part II: The role
of free radical damage and the use of antioxidants in the pathology
and treatment of lead toxicity. Altern Med Rev. 11:114–127.
2006.PubMed/NCBI
|
|
243
|
Hwang DF, Wang LC and Cheng HM: Effect of
taurine on toxicity of copper in rats. Food Chem Toxicol.
36:239–244. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
244
|
Hwang D and Wang LC: Effect of taurine on
toxicity of cadmium in rats. Toxicology. 167:173–180. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
245
|
Jamshidzadeh A, Heidari R, Abasvali M,
Zarei M, Ommati MM, Abdoli N, Khodaei F, Yeganeh Y, Jafari F, Zarei
A, et al: Taurine treatment preserves brain and liver mitochondrial
function in a rat model of fulminant hepatic failure and
hyperammonemia. Biomed Pharmacother. 86:514–520. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
246
|
Doğru-Abbasoğlu S, Kanbağli O, Balkan J,
Cevikbaş U, Aykaç-Toker G and Uysal M: The protective effect of
taurine against thioacetamide hepatotoxicity of rats. Hum Exp
Toxicol. 20:23–27. 2001. View Article : Google Scholar
|
|
247
|
Bardach AE, Ciapponi A, Soto N, Chaparro
MR, Calderon M, Briatore A, Cadoppi N, Tassara R and Litter MI:
Epidemiology of chronic disease related to arsenic in Argentina: A
systematic review. Sci Total Environ. 538:802–816. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
248
|
Li S, Yang L, Dong G and Wang X: Taurine
protects mouse liver against arsenic-induced apoptosis through JNK
pathway. Taurine 10. Advances in Experimental Medicine and Biology.
Lee DH, Schaffer SW, Park E and Kim HW: 975. Springer; Dordrecht:
pp. 855–862. 2017, View Article : Google Scholar : PubMed/NCBI
|
|
249
|
Taranukhin AG, Taranukhina EY, Saransaari
P, Pelto-Huikko M, Podkletnova IM and Oja SS: Taurine protects
cerebellar neurons of the external granular layer against
ethanol-induced apoptosis in 7-day-old mice. Amino Acids.
43:1705–1711. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
250
|
Lau A, Zheng Y, Tao S, Wang H, Whitman SA,
White E and Zhang DD: Arsenic inhibits autophagic flux, activating
the Nrf2-Keap1 pathway in a p62-dependent manner. Mol Cell Biol.
33:2436–2446. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
251
|
Bai J, Yao X, Jiang L, Zhang Q, Guan H,
Liu S, Wu W, Qiu T, Gao N, Yang L, et al: Taurine protects against
As2O3-induced autophagy in livers of rat offsprings through PPARγ
pathway. Sci Rep. 6:277332016. View Article : Google Scholar : PubMed/NCBI
|
|
252
|
Qiu T, Pei P, Yao X, Jiang L, Wei S, Wang
Z, Bai J, Yang G, Gao N, Yang L, et al: Taurine attenuates
arsenic-induced pyroptosis and nonalcoholic steatohepatitis by
inhibiting the autophagic-inflammasomal pathway. Cell Death Dis.
9:9462018. View Article : Google Scholar : PubMed/NCBI
|
|
253
|
Flora SJS, Chouhan S, Kannan GM, Mittal M
and Swarnkar H: Combined administration of taurine and monoisoamyl
DMSA protects arsenic induced oxidative injury in rats. Oxid Med
Cell Longev. 1:39–45. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
254
|
Cao W, Zhou Y, Li Y, Zhang X, He M, Zang
N, Zhou Y and Liao M: iTRAQ-based proteomic analysis of combination
therapy with taurine, epigallocatechin gallate, and genistein on
carbon tetrachloride-induced liver fibrosis in rats. Toxicol Lett.
232:233–245. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
255
|
El-Houseini ME, El-Agoza IA, Sakr MM and
El-Malky GM: Novel protective role of curcumin and taurine
combination against experimental hepatocarcinogenesis. Exp Ther
Med. 13:29–36. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
256
|
Abd-Rabou AA, Zoheir KMA and Ahmed HH:
Potential impact of curcumin and taurine on human hepatoma cells
using Huh-7 cell line. Clin Biochem. 45:1519–1521. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
257
|
Pan MH, Yang G, Li S, Li MY, Tsai ML, Wu
JC, Badmaev V, Ho CT and Lai CS: Combination of citrus
polymethoxyflavones, green tea polyphenols, and Lychee extracts
suppresses obesity and hepatic steatosis in high-fat diet induced
obese mice. Mol Nutr Food Res. 61:2017. View Article : Google Scholar
|
|
258
|
Balkan J, Kanbagli Ö, Hatipoglu A, Küçük
M, Çevikbas U, Aykaç-Toker G and Uysal M: Improving effect of
dietary taurine supplementation on the oxidative stress and lipid
levels in the plasma, liver and aorta of rabbits fed on a
high-cholesterol diet. Biosci Biotechnol Biochem. 66:1755–1758.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
259
|
Cetiner M, Sener G, Sehirli AO,
Ekşioğlu-Demiralp E, Ercan F, Sirvanci S, Gedik N, Akpulat S,
Tecimer T and Yeğen BC: Taurine protects against
methotrexate-induced toxicity and inhibits leukocyte death. Toxicol
Appl Pharmacol. 209:39–50. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
260
|
Kalaz EB, Çoban J, Aydın AF, Doğan-Ekici
I, Doğru- Abbasoğlu S, Öztezcan S and Uysal M: Carnosine and
taurine treatments decreased oxidative stress and tissue damage
induced by D-galactose in rat liver. J Physiol Biochem. 70:15–25.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
261
|
Shi X, Yao D and Chen C: Identification of
N-acetyltaurine as a novel metabolite of ethanol through
metabolomics-guided biochemical analysis. J Biol Chem.
287:6336–6349. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
262
|
Heidari R, Babaei H and Eghbal MA:
Amodiaquine-induced toxicity in isolated rat hepatocytes and the
cytoprotective effects of taurine and/or N-acetyl cysteine. Res
Pharm Sci. 9:97–105. 2014.PubMed/NCBI
|
|
263
|
Timbrell JA and Waterfield CJ: Changes in
taurine as an indicator of hepatic dysfunction and biochemical
perturbations. Taurine 2. Advances in Experimental Medicine and
Biology. Huxtable RJ, Azuma J, Kuriyama K, Nakagawa M and Baba A:
403. Springer; Boston, MA: pp. 125–134. 1996, View Article : Google Scholar : PubMed/NCBI
|
|
264
|
Gordon RE, Park E, Laskin D and
Schuller-Levis GB: Taurine protects rat bronchioles from acute
ozone exposure: A freeze fracture and electron microscopic study.
Exp Lung Res. 24:659–674. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
265
|
Venkatachalam S, Kuppusamy P, Kuppusamy B
and Dhanapal S: The potency of essential nutrient taurine on
boosting the antioxidant status and chemopreventive effect against
benzo (a)pyrene induced experimental lung cancer. Biomed Prev Nutr.
4:251–255. 2014. View Article : Google Scholar
|
|
266
|
Gurujeyalakshmi G, Wang Y and Giri SN:
Suppression of bleomycin-induced nitric oxide production in mice by
taurine and niacin. Nitric Oxide. 4:399–411. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
267
|
Wang Q, Hollinger MA and Giri SN:
Attenuation of amiodarone-induced lung fibrosis and
phospholipidosis in hamsters by taurine and/or niacin treatment. J
Pharmacol Exp Ther. 262:127–132. 1992.PubMed/NCBI
|
|
268
|
Izumi K, Nagata R, Motoya T, Yamashita J,
Hirokane T, Nagata T, Satoh Y, Sawada Y, Ishibashi M, Yoshida H, et
al: Preventive effect of taurine against acute paraquat
intoxication in beagles. Jpn J Pharmacol. 50:229–233. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
269
|
Li S, Wang J, Wei BK, Dong G and Wang X:
Protective effect of taurine on paraquat-induced lung epithelial
cell injury. Taurine 11. Advances in Experimental Medicine and
Biology. Hu J, Piao F, Schaffer SW, El Idrissi A and Wu JY: 1155.
Springer; Singapore: pp. 739–746. 2019, View Article : Google Scholar : PubMed/NCBI
|
|
270
|
Khalili Fard J, Hamzeiy H, Sattari M and
Eghbal MA: Protective roles of N-acetyl cysteine and/or taurine
against sumatriptan-induced hepatotoxicity. Adv Pharm Bull.
6:627–637. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
271
|
Gordon RE, Shaked AA and Solano DF:
Taurine protects hamster bronchioles from acute
NO2-induced alterations. A histologic, ultrastructural,
and freeze-fracture study. Am J Pathol. 125:585–600.
1986.PubMed/NCBI
|
|
272
|
Jeon SH, Lee MY, Rahman MM, Kim SJ, Kim
GB, Park SY, Hong CU, Kim SZ, Kim JS and Kang HS: The antioxidant,
taurine reduced lipopolysaccharide (LPS)-induced generation of ROS,
and activation of MAPKs and Bax in cultured pneumocytes. Pulm
Pharmacol Ther. 22:562–566. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
273
|
Das J, Ghosh J, Manna P, Sinha M and Sil
PC: Taurine protects rat testes against NaAsO(2)-induced oxidative
stress and apoptosis via mitochondrial dependent and independent
pathways. Toxicol Lett. 187:201–210. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
274
|
Meizel S: Molecules that initiate or help
stimulate the acrosome reaction by their interaction with the
mammalian sperm surface. Am J Anat. 174:285–302. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
275
|
Aly HA and Khafagy RM: Taurine reverses
endosulfan-induced oxidative stress and apoptosis in adult rat
testis. Food Chem Toxicol. 64:1–9. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
276
|
Azab SS, kamel I, Ismail NN, El Din Hosni
H and El Fatah MA: The defensive role of taurine against
gonadotoxicity and testicular apoptosis effects induced by
cisplatin in rats. J Infect Chemother. 26:51–57. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
277
|
Yahyavy S, Valizadeh A, Saki G and
Khorsandi L: Taurine induces autophagy and inhibits oxidative
stress in mice Leydig cells. JBRA Assist Reprod. 24:250–256.
2020.PubMed/NCBI
|
|
278
|
Kalender S, Apaydin FG and Kalender Y:
Testicular toxicity of orally administrated bisphenol A in rats and
protective role of taurine and curcumin. Pak J Pharm Sci.
32:1043–1047. 2019.PubMed/NCBI
|