Introduction
Osteoporosis is a systemic skeletal disease
characterized by a decrease in bone density (1). Bone homeostasis is maintained by
osteoclasts, which absorb bone, and osteoblasts, which form bone
(2). This homeostasis can be
unbalanced by various causes, such as menopause, aging and
steroidal side effects (3). Among
these, hormonal changes due to menopause are the cause of abnormal
osteoclast activity. Various drugs, such as bisphosphonates,
parathyroid hormone, denosumab and selective estrogen receptor
modulator, have been used to treat osteoporosis (4), but these drugs are not suitable for
long-term treatment due to serious side effects, including
mandibular necrosis and cardiovascular disease (5). Therefore, research and development of
a natural-based treatment for osteoporosis with fewer and less
severe side effects is required.
Solanum nigrum Line (SL) has been used as a
medicinal plant in East Asian countries, such as Korea, Japan and
China. SL is the above-ground part of Solanum nigrum Linné
(Solanaceae) and has traditionally been used to treat conditions
associated with inflammatory disease, such as boils, cancer and
chronic bronchitis (6). A previous
study reported that SL methanol extract inhibits osteoclast
differentiation (7). To the best of
our knowledge, however, the effects of ethanol extract, mechanism
of osteoclast inhibition and effects of SL in osteoporosis have not
been identified. In addition, inflammatory conditions and aging are
known to increase the risk of developing osteoporosis (8,9).
Various natural herbs showing anti-inflammatory effects have been
shown to be effective in treating osteoporosis (10,11).
In previous studies, SL has been shown to have anti-inflammatory
effects (12,13). Therefore, SL may serve as a
potential treatment for postmenopausal osteoporosis by suppressing
abnormal osteoclast activity.
Osteoclasts are multinucleated giant cells derived
from hematopoietic progenitors (14). Receptor activator of NF-κB ligand
(RANKL) is a member of the tumor necrosis factor (TNF) superfamily
and is a cytokine that serves an important role in osteoclast
differentiation and mature osteoclast activity (15). When RANKL binds to RANK, TNF
receptor-associated factor 6 expression is upregulated, leading to
activation of downstream signaling molecules, such as MAPK and
NF-κB. Thereafter, c-Fos and nuclear factor of activated T-cells,
cytoplasmic 1 (NFATc1), which are key transcription factors
involved in osteoclast differentiation, are sequentially expressed
(2). Finally, these factors induce
expression of factors associated with osteoclast differentiation
and bone resorption, such as tartrate-resistant acid phosphatase
(TRAP), cathepsin K (CTsK), matrix metallopeptidase-9 (MMP-9) and
carbonic anhydrase 2 (CA2) (16).
In the present study, the anti-osteoporotic effects
of SL were investigated using an ovariectomy (OVX)-induced
osteoporosis model, which is the most commonly used model of
postmenopausal osteoporosis (11,17–19).
In addition, to confirm the effects of SL on osteoclast
differentiation, its ability to inhibit osteoclast differentiation
and the underlying mechanism were assessed using a RANKL-induced
osteoclast model.
Materials and methods
Reagents
SL was purchased from Omniherb (Dongwoodang Pharmacy
Co., Ltd.); 17β-estradiol (E2), TRAP staining kit,
chlorogenic and caffeic acid, quercetin and protease and
phosphatase inhibitor cocktail were purchased from Sigma-Aldrich
(Merck KGaA). Minimum Essential Medium Eagle, α-Modification
(α-MEM), FBS, penicillin/streptomycin (P/S) and Dulbecco's PBS
(DPBS) were obtained from Gibco (Thermo Fisher Scientific, Inc.).
DMEM was purchased from Welgene, Inc. RANKL was obtained from
PeproTech, Inc. Anti-NFATc1 (cat. no. 556602) was purchased from BD
Pharmingen (BD Biosciences; used for western blotting) and
anti-c-Fos (cat. no. sc-447), anti-CTsK (cat. no. sc-48353),
anti-NFATc1 (cat. no. sc-7294; used for immunohistochemistry),
anti-β-actin (cat. no. sc-8432) and ECL solution were obtained from
Santa Cruz Biotechnology, Inc. The SuperScript® IV
reverse transcriptase (RT) kit and SYBR Green were purchased from
Invitrogen (Thermo Fisher Scientific, Inc.) and Taq polymerase was
purchased from MGmed. PCR primers were obtained from GenoTech Corp.
All reagents used in cell experiments were analytical grade.
Preparation of SL
SL was verified by Professor Yungmin Bu at the
Herbology Laboratory, College of Korean Medicine, Kyunghee
University (Seoul, South Korea). The plant specimens were stored in
the plant storage cabinet of the Anatomy Laboratory, College of
Korean Medicine, Kyunghee University. SL was extracted by immersion
in 80% ethanol (Et-OH) for 2 weeks at 4°C. Bottles containing SL
and Et-OH was shaken at the same time every day. Et-OH in the
solution was removed using a concentrator, and the extracts were
lyophilized at −20°C for 48 h to obtain a powder (yield, 13.88%).
The extracts were stored in a cryogenic refrigerator at −80°C until
required and diluted in DMSO (100 mg/ml) for use in
experiments.
Animals and OVX-induced osteoporosis
in Sprague Dawley (SD) rats
The in vivo experiments were approved by the
Kyunghee University Institutional Animal Care and Use Committee
[approval no. KHUASP(SE)-17-052]. A total of 40 12-week-old female
SD rats (weight, 230–250 g) were purchased from Koatech. The
animals were housed at 22±2°C with 55±10% humidity and a 12-h
light/dark cycle. Rats were provided ad libitum access to
food and water and allowed to acclimatize for 1 week. Body weight
was measured weekly. Humane end points were as follows: i) weight
loss ≥20% compared with other rats of the same age; ii) difficultly
ingesting food or water due to uncomfortable walking; iii)
difficulty maintaining a normal posture due to low energy; iv)
unconsciousness or lack of reaction to external stimuli; and v)
severe infection, laceration and bleeding at the surgical site.
In order to induce osteoporosis, 32 SD rats were
deeply anesthetized using O2 diluted with 5% isoflurane.
After removing the hair from the surgical site, the epidermis and
muscles were incised and both ovaries were removed. Additionally,
eight SD rats underwent the same procedure but the ovaries were not
removed (sham operation). During surgery, the concentration of
isoflurane was maintained at 2–3%. No rats died during surgery. In
order to prevent infection following surgery, gentamycin (4 mg/kg)
was given intraperitoneally for 3 days. After 1 week stabilization,
administration of distilled water (DW), SL and E2
(positive control) was initiated. Experimental groups were as
follows: i) Sham, mock surgery then daily oral administration of
DW; ii) OVX, OVX surgery then daily oral administration of DW; iii)
E2, OVX surgery then daily oral administration of 100
µg/kg E2; iv) SL-low, OVX surgery then daily oral
administration of 50 mg/kg SL; and v) SL-high, OVX surgery then
daily oral administration of 100 mg/kg SL. The dosage of SL was
calculated based on the following criteria: In Korean medicine, an
adult human dose of 60 kg is a daily dose of SL of 8 g. Given that
the SL used in the present experiment was lyophilized powder, and
the yield was 13.88%, 18.5 mg/kg was equivalent to the recommended
adult dose. In addition, rats generally metabolize drugs faster
than humans (20). SL-low group
were administered ~2 times the calculated amount and the SL-high
group was administered ~5 times the calculated amount. After the
8-week dosing period, SD rats were sacrificed via cervical
dislocation after collecting 10 ml blood via cardiac puncture under
deep anesthesia with O2 diluted with 5% isoflurane. In
order to determine the success of OVX surgery, the ovaries were
collected and weighed and the right femur was extracted for
micro-computed tomography (CT) analysis and histological
examination.
Micro-CT analysis
In order to confirm the anti-osteoporotic effects of
SL, changes in bone microstructure of the femoral head of the right
femur were scanned using a high-resolution cone beam micro-CT
system (SkyScan1176; Bruker Corporation) with an aluminum filter of
0.5 mm, source set at 50 kV/200 µA and 8.9 µm isotropic resolution.
Starting with the growth plate of the femoral head, 200 slides were
taken and visualized using Data Viewer software (Skyscan version
1.6.10.1; Bruker Corporation). Bone microstructure indexes, such as
bone mineral density (BMD), bone volume fraction (BV/TV),
trabecular number (Tb.N) and trabecular separation (Tb.Sp) were
measured using Skyscan.
Serum analysis
Blood extracted at the time of sacrifice was stored
at room temperature for 30 min. Then, the serum was separated by
centrifugation at 14,310 × g for 20 min at 4°C. In order to measure
TRAP activity in the serum, 50 µl serum and 50 µl TRAP solution
(4.93 mg para-nitrophenyl phosphate + 850 µl 0.5 M acetate solution
+ 150 µl tartrate solution) were reacted at 37°C for 1 h. The
reaction was terminated with 50 µl 0.5 M NaOH and TRAP activity was
measured at an absorbance of 405 nm using an ELISA reader
(Versamax; Molecular Devices, LLC).
Histological examination
The femur was fixed for 2 days using 10% (v/v)
neutral buffered formalin at room temperature. The tissue was
demineralized for 8 weeks using a solution of EDTA-2Na at room
temperature. The demineralized tissue was dehydrated using Et-OH
(at gradient concentration of 70, 80, 90 and 100% for 5 min per
concentration), cleared with xylene and embedded in paraffin. The
embedded tissue was sectioned to a thickness of 5 µm using a rotary
microtome. Histomorphological changes were assessed by 7%
hematoxylin and 1% eosin (H&E) staining at room temperature.
Moreover, to measure the number of osteoclasts in the femur, a TRAP
staining kit was used according to the manufacturer's protocol. For
immunohistochemical (IHC) staining, tissue antigens were retrieved
with proteinase K (Thermo Fisher Scientific, Inc.) at 37°C for 1 h.
Endogenous peroxidase was blocked using 3%
H2O2 diluted in methanol at room temperature
for 30 min. Normal serum (Gibco; Thermo Fisher Scientific, Inc.)
was added at room temperature for 1 h to block the binding of
non-specific proteins. Antibodies were diluted in tris-buffered
saline containing 0.5% bovine serum albumin and reacted at 4°C
overnight with anti-NFATc1 (1:100; cat. no. sc-7294) and anti-CTsK
(1:100; cat. no. sc-48353). Subsequently, the secondary antibody
(1:100; rabbit; cat. no. BA-1000) was added at room temperature for
1 h, and then VECTASTAIN Elite ABC kit (cat. no. PK-6100; both
Vector Laboratories, Inc.; Maravai LifeSciences) was used according
to the manufacturer's instructions. The tissue was reacted with
3,3′-diaminobenzidine (Vector Laboratories, Inc.; Maravai
LifeSciences) at room temperature for 5 min and counterstained with
7% hematoxylin for 30 sec at room temperature. Dyed tissues were
observed using a light microscope (magnification, ×100) and imaged
using a DP73 camera (Olympus Corporation). The trabecular area and
positive cells for each indicator were assessed using ImageJ
software (version 1.51j8; National Institutes of Health).
Cell culture and viability assay
RAW 264.7 cells were purchased from the Korean Cell
Line Bank (cat no. 40071; lot no. 41484). RAW 264.7 cells were
cultured in DMEM containing 10% FBS and 1% P/S and sub-cultured
every 2 days. MC3T3-E1 Subclone 4 cells were purchased from the
American Type Culture Collection (cat no. CRL-2593). MC3T3-E1 cells
were cultured in α-MEM without ascorbic acid containing 10% FBS and
1% P/S, and sub-cultured every 3 days. Both cell lines were
maintained in a humidified incubator at 37°C, 95% humidity and 5%
CO2. In order to measure the cytotoxicity of SL, RAW
264.7 cells were seeded in 96-well plates at a density of
5×103 cells/well and stabilized for 1 day. In addition,
in order to investigate the toxicity of SL to osteoclasts, RAW
264.7 cells were stimulated for 5 days using 100 ng/ml RANKL and 5,
10, 20 or 40 µg/ml SL in a humidified incubator at 37°C. To
determine the effect of SL on cell viability, cells were treated
with SL for 24 h and 20 µl Cell Counting Kit-8 (Dojindo Molecular
Technologies, Inc.) solution was subsequently added for 2 h. The
absorbance in each well was measured at a wavelength of 450 nm
using a microplate reader. SL was considered to be toxic when cell
survival rate was ≤90% compared with untreated cells. In order to
measure the effect of SL on cell necrosis, RAW 264.7 cells were
seeded in 96-well plates at a density of 5×103
cells/well and stabilized for 1 day. Thereafter, cells were treated
with 5, 10, 20 or 40 µg/ml SL for 24 h in a humidified incubator at
37°C, and cell necrosis rate was measured using a lactate
dehydrogenase (LDH) assay kit (Dojindo Molecular Technologies,
Inc.) (21), according to the
manufacturer's protocol. LDH is released from cells upon cell
death, thus LDH levels in the culture medium were used as an
indicator of cell death (21).
TRAP staining and pit formation
assays
TRAP is an osteoclast-specific marker and is used to
determine whether osteoclasts are differentiated (22). In order to induce osteoclast
differentiation, RAW 264.7 cells were cultured in α-MEM containing
10% FBS and 1% P/S (5×103 cells/well) in a humidified
incubator at 37°C with 5% CO2 and stabilized for 1 day.
Subsequently, the cells were exposed to 100 ng/ml RANKL and 5, 10,
20 or 40 µg/ml SL for 5 days. The medium was replaced with fresh
medium every 2 days. After osteoclast differentiation was
completed, cells were fixed with 10% formalin solution at room
temperature for 10 min and stained using the TRAP staining kit as
aforementioned. In order to test the effects of SL on osteoclast
ability to absorb bone, RAW 264.7 cells were cultured in
hydroxyapatite-coated plates (Corning, Inc.) with 5, 10, 20 or 40
µg/ml SL in a humidified incubator at 37°C for 5 days. The medium
was replaced with fresh medium every 2 days. Thereafter, the cells
were lysed with 4% NaClO, washed three times with DPBS and dried
completely. Plates were imaged using a light microscope at ×100
magnification in five random fields of view per well. The absorbed
area is expressed as a percentage of the total area.
Actin ring formation assay
In order to measure filamentous (F-)actin ring
formation, RAW 264.7 cells were cultured in α-MEM containing 10%
FBS and 1% P/S (5×103 cells/well) and stabilized for 1
day. Subsequently, the cells were exposed to 100 ng/ml RANKL and
medium containing 5, 10, 20 or 40 µg/ml SL for 5 days. The
differentiated cells were fixed with 4% paraformaldehyde at room
temperature for 20 min and permeabilized with PBS containing 0.1%
Triton X-100 at room temperature for 5 min. Subsequently, the cells
were stained with 200 µl 100 nM Acti-Stain™ Fluorescent Phalloidins
for 30 min at room temperature (cat. no. PHDG1; Cytoskeleton, Inc.)
and the nuclei were counterstained with 200 µl 100 nM DAPI in PBS
for 30 sec at room temperature (Sigma-Aldrich; Merck KGaA). The
F-actin formation was imaged using an immunofluorescence microscope
at ×100 magnification (Celena; Logos Biosystems).
Western blotting
In order to determine protein expression following
SL treatment, RAW 264.7 cells were seeded in a 60-mm dish at a
density of 5×105 cells/well and stabilized for 1 day.
The cells were treated with 100 ng/ml RANKL and 5, 10, 20 or 40
µg/ml SL in a humidified incubator at 37°C for 1 day. In order to
extract total and nuclear protein, the cells were washed three
times using DPBS, and lysed using RIPA buffer and NE-PER™ nuclear
and cytoplasmic extraction reagents (Thermo Fisher Scientific,
Inc.). The lysates were maintained on ice for 30 min and
centrifuged at 58,440 × g for 20 min at 4°C. Protein concentration
was quantified using a bicinchoninic acid assay kit (Sigma-Aldrich;
Merck KGaA). Equal quantities (30 µg) of protein were loaded on a
10% SDS-gel, and resolved using SDS-PAGE at 100 V for 1.5 h. The
resolved proteins were transferred to nitrocellulose membranes
(Whatman plc; Cytiva) at 100 V for 1 h. Non-specific proteins in
the membrane were blocked using TBST (0.5% Tween-20) and 5% skimmed
milk for 1 h at room temperature. The membrane was reacted with
primary antibodies against NFATc1 (1:1,000; cat. no. 556602), c-Fos
(1:200; cat. no. sc-447) and β-actin (1:500; cat. no. sc-8432) at
4°C overnight. Subsequently, the membranes were incubated with a
horseradish peroxidase-conjugated secondary antibody for 1 h at
room temperature (1:10,000; mouse; cat. no. 115-035-062; Jackson
ImmunoResearch Laboratories, Inc.), and the expression of each
indicator was visualized using ECL solution. The expression of each
indicator was measured using ImageJ version 1.51j8 (National
Institutes of Health) and normalized to β-actin.
RT-semi-quantitative PCR
In order to measure mRNA expression following SL
treatment, RAW 264.7 cells were seeded in 6-well-plates at a
density 2×104 cells/well and stabilized for 1 day. The
cells were exposed to 100 ng/ml RANKL and 5, 10, 20 or 40 µg/ml SL
in a humidified incubator at 37°C for 4 days. Thereafter, the cells
were washed 3 times using DPBS and were lysed using
TRIzol® reagent (Takara Bio, Inc.). The extracted mRNA
was quantified using NanoDrop (Thermo Fisher Scientific, Inc.), and
2 µg RNA was reverse transcribed into cDNA using the
SuperScript® IV RT kit according to the manufacturer's
protocol. cDNA was amplified using Taq polymerase and target
primers in a C1000 Touch™ Thermal Cycler (Bio-Rad Laboratories,
Inc.). The following thermocycling conditions were used for qPCR:
22–40 cycles of 30 sec at 94°C (denaturation); 30 sec at 53–58°C
(annealing); and 30 sec at 72°C (extension). The target primer
sequence and annealing temperature are listed in Table I. The PCR reactants were
electrophoresed on SYBR-Green-dyed 2% agarose gels diluted in 1%
Tris acetate-EDTA buffer. The expression of each mRNA was measured
using ImageJ software (version 1.51j8; National Institutes of
Health) with β-actin as the loading control.
 | Table I.Primers used for reverse
transcription-quantitative PCR. |
Table I.
Primers used for reverse
transcription-quantitative PCR.
| Gene | Sequence,
5′-3′ | Accession
number | Tm, °C | Base pair |
|---|
| NFATc1 | Forward: TGC TCC
TCC TCC TGC TGC TC | NM_198429.2 | 58 | 480 |
|
(Nfatc1) | Reverse: CGT CTT
CCA CCT CCA CGT CG |
|
|
|
| c-Fos | Forward: ATG GGC
TCT CCT GTC AAC AC | NM_010234.3 | 55 | 480 |
| (Fos) | Reverse: GGC TGC
CAA AAT AAA CTC CA |
|
|
|
| TRAP | Forward: ACT TCC
CCA GCC CTT ACT ACC G | NM_007388.3 | 58 | 381 |
| (Acp5) | Reverse: TCA GCA
CAT AGC CCA CAC CG |
|
|
|
| RANK | Forward: AAA CCT
TGG ACC AAC TGC AC | NM_009399.3 | 53 | 377 |
|
(Tnfrsf11a) | Reverse: ACC ATC
TTC TCC TCC CHA GT |
|
|
|
| CTsK | Forward: AGG CGG
CTA TAT GAC CAC TG | NM_007802.4 | 58 | 403 |
| (Ctsk) | Reverse: CCG AGC
CAA GAG AGC ATA TC |
|
|
|
| CA2 | Forward: CTC TCA
GGA CAA TGC AGT GCT GA | NM_001357334.1 | 58 | 411 |
| (Ca2) | Reverse: ATC CAG
GTC ACA CAT TCC AGC A |
|
|
|
| MMP-9 | Forward: CGA CTT
TTG TGG TCT TCC CC | NM_013599.4 | 58 | 258 |
| (Mmp9) | Reverse: TGA AGG
TTT GGA ATC GAC CC |
|
|
|
| ATP6v0d2 | Forward: ATG GGG
CCT TGC AAA AGA AAT CTG | NM_175406.3 | 58 | 504 |
|
(Atp6v0d2) | Reverse: CGA CAG
CGT CAA ACA AAG GCT TGT A |
|
|
|
| OSCAR | Forward: CTG CTG
GTA ACG GAT CAG CTC CCC AGA | NM_001290377.1 | 53 | 310 |
| (Oscar) | Reverse: CCA AGG
AGC CAG AAC CTT CGA AAC T |
|
|
|
| Actin | Forward: TTC TAC
AAT GAG CTG CGT GT | NM_007393 | 58 | 456 |
| (Actb) | Reverse: CTC ATA
GCT CTT CTC CAG GG |
|
|
|
Alizarin red S staining
In order to induce osteoblast differentiation,
MC3T3-E1 cells were cultured in α-MEM without ascorbic acid
containing 10% FBS and 1% P/S (1.5×104 cells/24-well
plate) in a humidified incubator at 37°C and stabilized for 1 day.
Subsequently, the cells were exposed to 25 µg/ml ascorbic acid, 10
mM β-glycerophosphate and medium containing SL (5, 10, 20 µg/ml) in
a humidified incubator at 37°C for 14 days. The medium was replaced
with fresh culture medium every 3 days. After osteoblast
differentiation was completed, calcified nodules in the plate were
fixed with 80% Et-OH at 4°C for 1 h and stained using 0.1% alizarin
red S solution (Duksan Pharmaceutical Co., Ltd.) at room
temperature for 5 min. The nodules were imaged using a camera and
light microscope (magnification, ×100). The stained dye was
extracted using 10 mM sodium phosphate (pH 7.0) diluted in 10%
cetylpyridinium chloride and measured at absorbance at 405 nm.
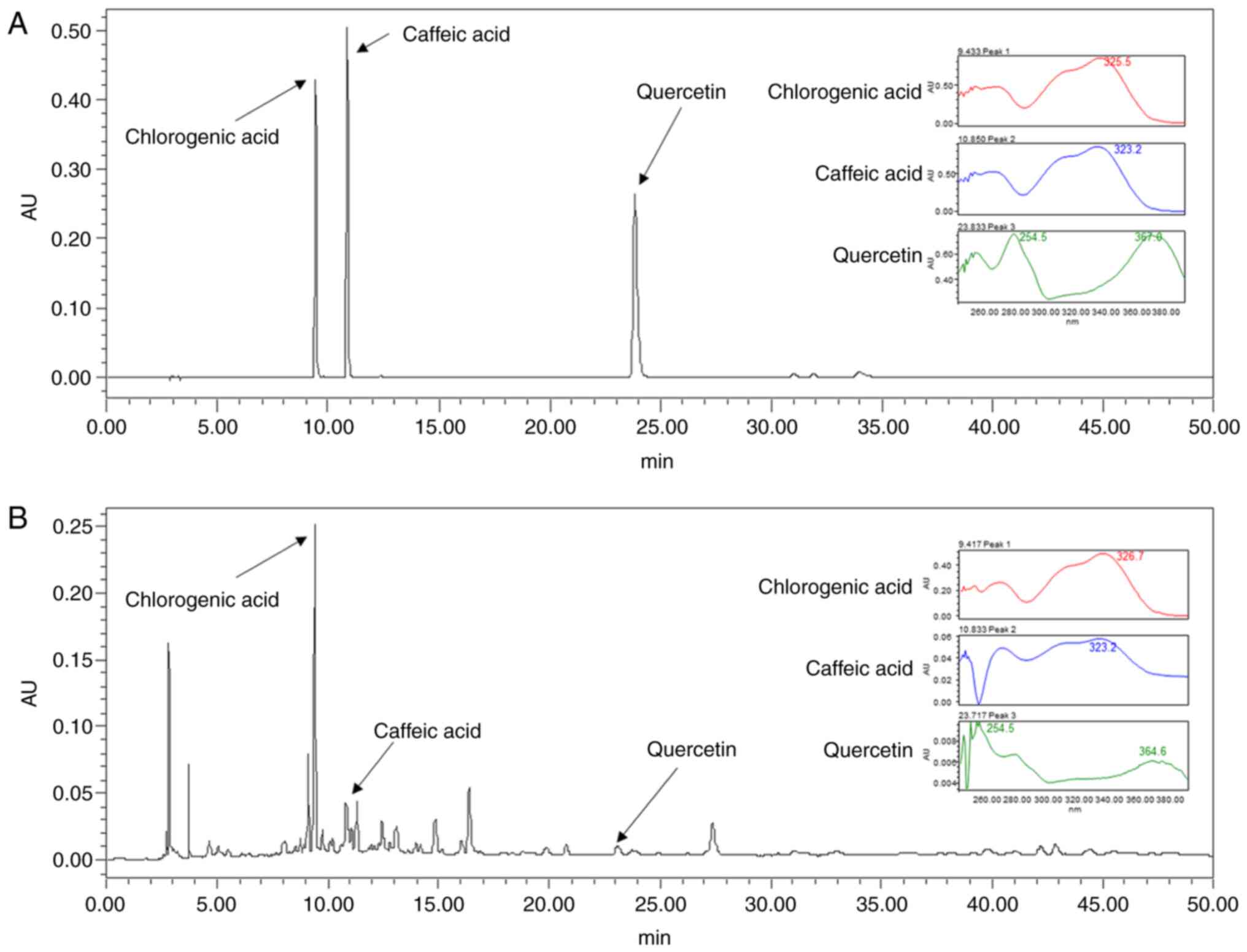
High-performance liquid chromatography
(HPLC) analysis
Chlorogenic acid, caffeic acid and quercetin are
active ingredients of SL (23,24).
In order to analyze the SL extracts, HPLC was performed. Absorbance
was measured using a UV detector (2996 Waters 2695). Xbridge C18
(250.0×4.6 mm, 5 µm) was used as the column and proceeded at 30°C
for 50 min at a flow rate of 1 ml/min. Samples were injected in a
volume of 10 µl. As the mobile phase, (A) acetonitrile and (B)
H2O diluted with 1% acetic acid were used (ratio, 9:1).
Ingredients were detected at an absorbance of 280 nm.
Statistical analysis
Data are presented as the mean ± SEM (n≥3) and were
analyzed using GraphPad prism software (version 5.01; GraphPad
Software Inc.). Comparisons between groups were performed using
one-way ANOVA with post hoc Tukey's test. P<0.05 was considered
to indicate a statistically significant difference.
Results
SL decreases loss of bone density and
changes in bone microstructure caused by OVX
In order to investigate the effects of SL on
menopausal osteoporosis, SL was administered following removal of
both ovaries. Changes in bone density of the femoral head were
measured using micro-CT (Fig. 1A).
The density of trabecular bone in the femoral head was lower in the
OVX group than in the sham group. The positive control group
E2 exhibited a reduced decrease in bone density; the
SL-low and -high groups also exhibited a suppressed decrease in
bone density. As a result of analyzing the microstructure of the
femur images using CT analyzer software, BMD and BV/TV were
decreased due to OVX, and E2, SL-low and SL-high groups
suppressed this reduction. The difference between the E2
and SL-high group was significant (Fig.
1B and C). In addition, Tb.N and Tb.Sp of the femur were
decreased and increased by OVX, respectively. For Tb.N,
E2, SL-low and SL-high groups were significantly
different from the sham group; for Tb.Sp, E2 and SL-high
were significantly different. These results indicated that the
effect of SL on bone density recovery was comparable with that of
the positive control group (E2 treatment). Analyzing the
TRAP activity in serum revealed that OVX did not significantly
affect the expression of TRAP, but the SL-low and SL-high groups
showed a notable ability to inhibit TRAP activity (Fig. 1F). The OVX group exhibited increased
body weight and decreased uterine weight compared with the sham
group. In addition, administration of E2 significantly
suppressed this change and the SL-low and SL-high groups exhibited
no effect on changes in weekly body and uterine weight (Fig. 1G and H).
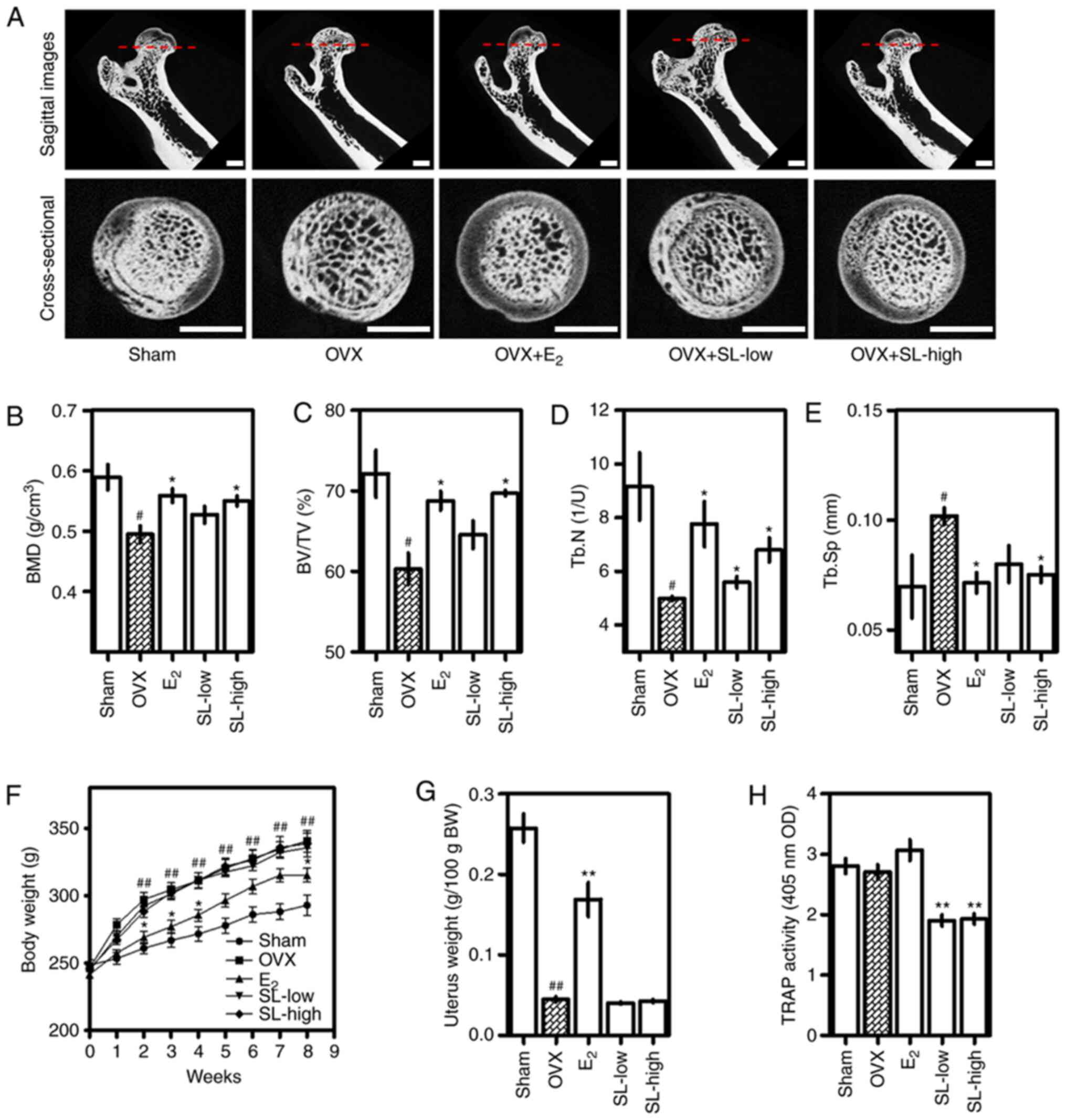 | Figure 1.Effect of SL on bone density in an
OVX-induced osteoporosis model. Osteoporosis was induced in Sprague
Dawley rats (12-weeks-old) via OVX. Rats were then treated with SL
or E2 for 8 weeks. (A) Changes in the bone
microstructure in the femoral tissue induced by OVX were imaged
using micro-CT (scale bar, 2 mm). (B) BMD, (C) BV/TV, (D) Tb.N and
(E) Tb.Sp was analyzed using micro-CT. (F) Body weight was measured
weekly. (G) Uterine weight was measured after sacrifice. (H) TRAP
activity in serum was measured using ELISA. The results are
expressed as the mean ± SEM (n=8). #P<0.05 and
##P<0.01 vs. sham; *P<0.05 and **P<0.01 vs.
OVX. OVX, ovariectomy; SL, Solanum nigrum Line;
E2, 17β-estradiol; CT, computed tomography; BMD, bone
mineral density; BV/TV, bone volume/total volume; Tb.N, trabecular
number; Tb.Sp, trabecular separation; TRAP, tartrate-resistant acid
phosphatase; OD, optical density; BW, body weight. |
SL inhibits trabecular bone loss,
osteoclast formation and expression of NFATc1 and CTsK in femoral
tissue
H&E, TRAP and IHC staining were performed to
observe histological and histochemical changes in the femoral head.
The trabecular area of the femoral head was decreased following
OVX; this was suppressed by administration of E2 and
SL-high (Fig. 2A and E). Measuring
the area revealed a significant difference between the
E2 and SL-high and OVX group. Consistent with the
H&E staining results, the number of osteoclasts in the femoral
head was increased following OVX and decreased following treatment
with E2, SL-low and SL-high (Fig. 2B and F). IHC staining was performed
to measure the protein expression of NFATc1 and CTsK in the femoral
head. Expression of NFATc1 in the femoral head was increased in the
OVX group compared with the sham group (Fig. 2C and G). Treatment with
E2 inhibited this expression. In particular, the SL-high
group notably inhibited expression of NFATc1 compared with the OVX
group. Consistent with the NFATc1 staining results, the expression
of CTsK in the femoral head was induced following OVX and decreased
by E2 and SL (Fig. 2D and
H).
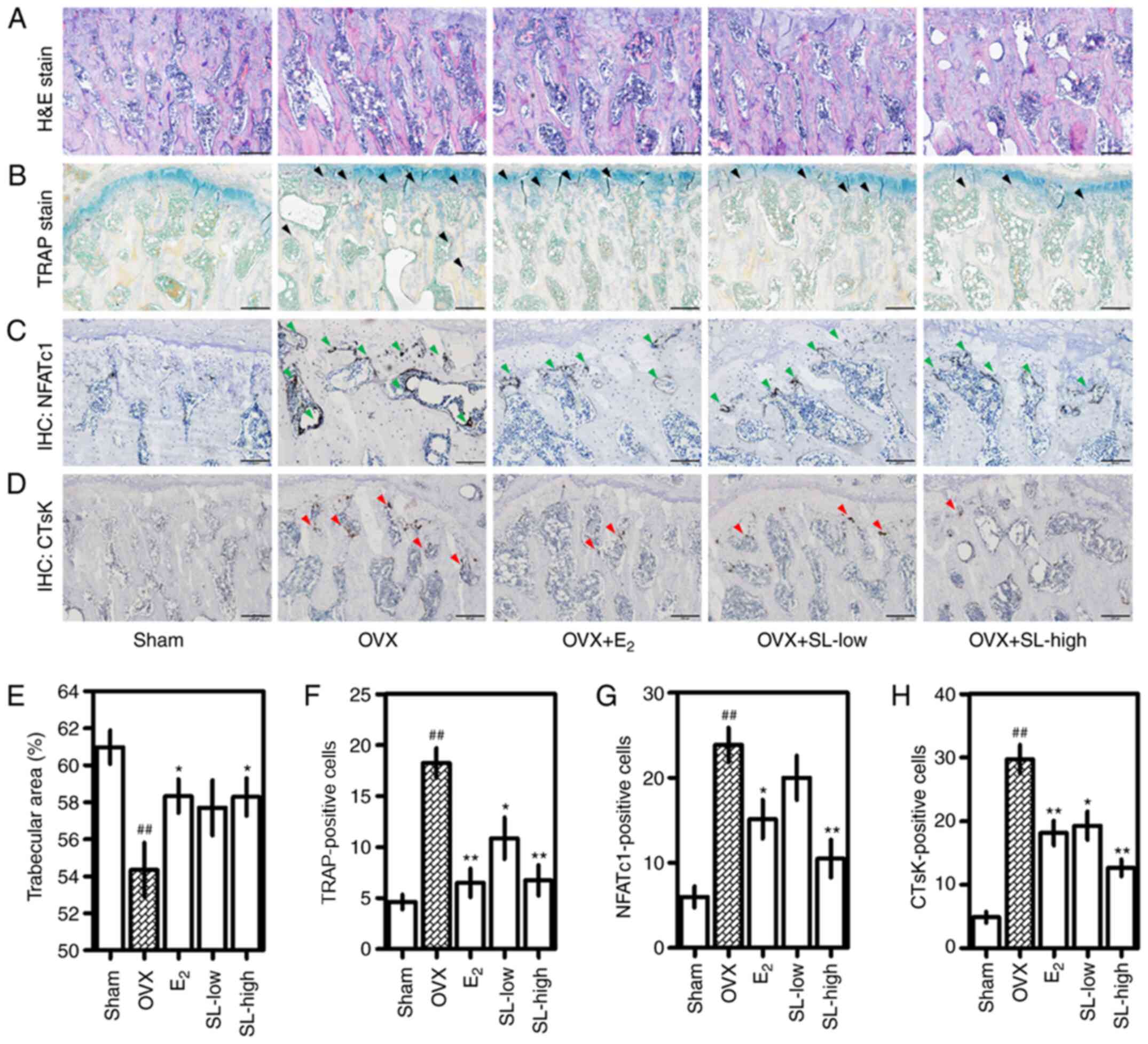 | Figure 2.Effect of SL on histological changes
in femoral tissue. (A) Decrease in the density of trabecular bone
in the femoral tissue induced by OVX was analyzed by H&E
staining. (B) Number of osteoclasts in the femoral head was
detected using a TRAP staining kit. TRAP-positive cells are marked
with black arrows. (C) NFATc1 (green) and (D) CTsK (red) protein
expression in the tissue was detected via IHC. All images were
captured using a phase-contrast microscope. Magnification, ×100;
scale bar, 200 µm. (E) Trabecular area was measured using ImageJ.
Number of (F) TRAP-, (G) NFATc1- and (H) CTsK-positive cells were
counted. The results are expressed as the mean ± SEM (n=8).
##P<0.01 vs. sham; *P<0.05, **P<0.01 vs. OVX.
OVX, ovariectomy; SL, Solanum nigrum Line; E2,
17β-estradiol; H&E, hematoxylin and eosin; TRAP,
tartrate-resistant acid phosphatase; IHC, immunohistochemistry;
NFATc1, nuclear factor-activated T cells c1; CTsK, cathepsin K. |
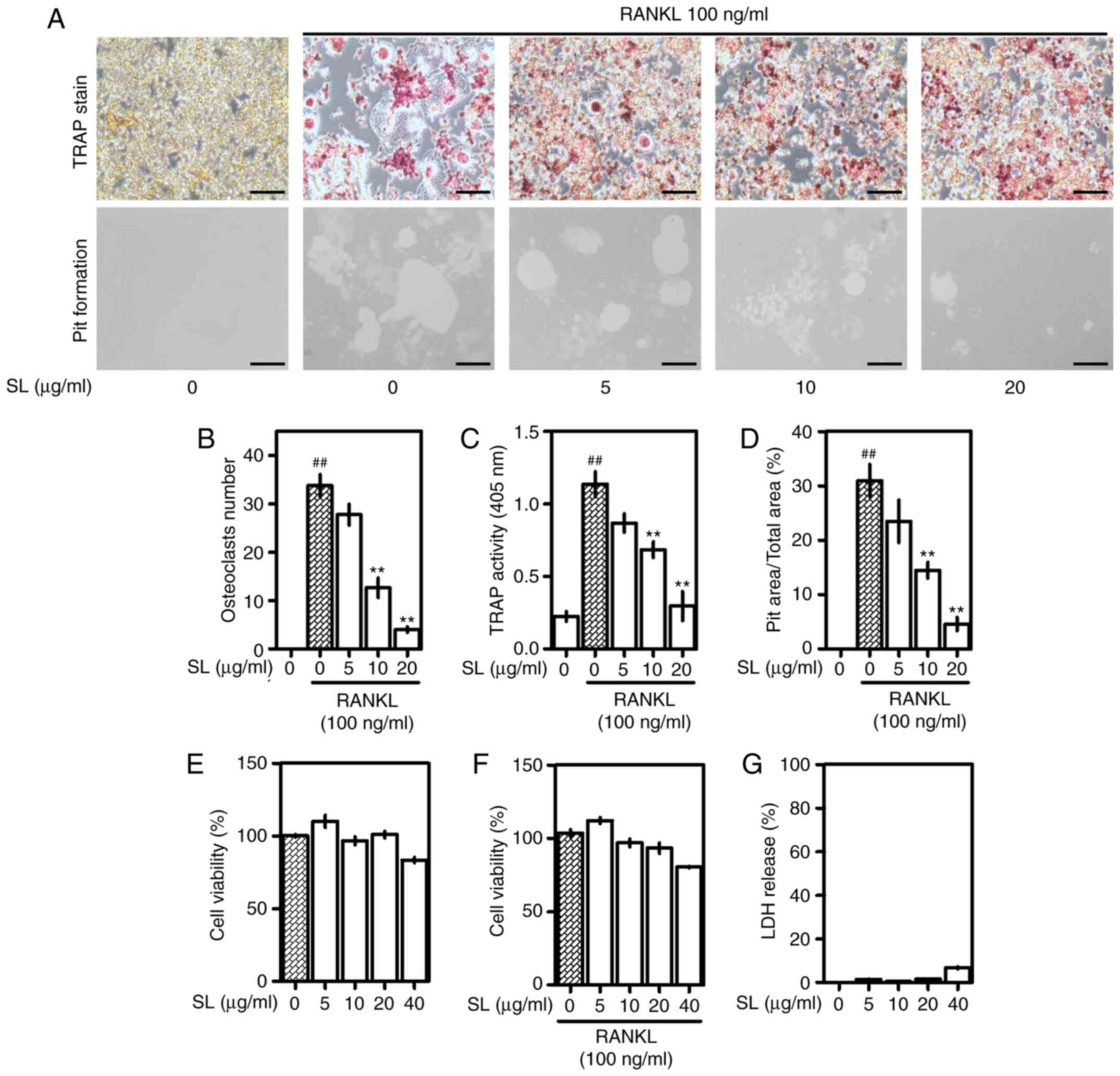
SL inhibits osteoclast differentiation
and bone absorption capacity
After confirming the positive effects of SL in the
osteoporotic in vivo model, the effects of SL in the
osteoclast model were confirmed using a RANKL-induced in
vitro model. RAW 264.7 cells were cultured for 5 days in a
medium containing RANKL (Fig. 3A).
Differentiated osteoclasts were stained using the TRAP staining kit
and multinucleated red giant cells were observed. SL decreased the
area and number of TRAP-positive cells in a dose-dependent manner.
In the pit formation assay, the absorbed area of the plate was used
to measure the activity of osteoclasts. The absorbed area induced
by RANKL treatment was decreased in cells treated with SL.
Consistent with this, measuring the number of osteoclasts and TRAP
activity in the medium demonstrated that SL inhibited osteoclast
differentiation and activity (Fig. 3B
and C). In addition, measuring the pit area formed by
osteoclasts showed that SL also suppressed the absorbed area in a
concentration-dependent manner (Fig.
3D). At 40 µg/ml, SL decreased viability of RAW 264.7 cells and
osteoclasts (Fig. 3E and F).
Therefore, 40 µg/ml was considered toxic and subsequent cell
experiments were performed using 0–20 µg/ml SL. In addition, LDH
was slightly increased following 40 µg/ml SL treatment but was not
detected at 0–20 µg/ml SL, indicating that 0–20 µg/ml SL did not
induce necrosis (Fig. 3G).
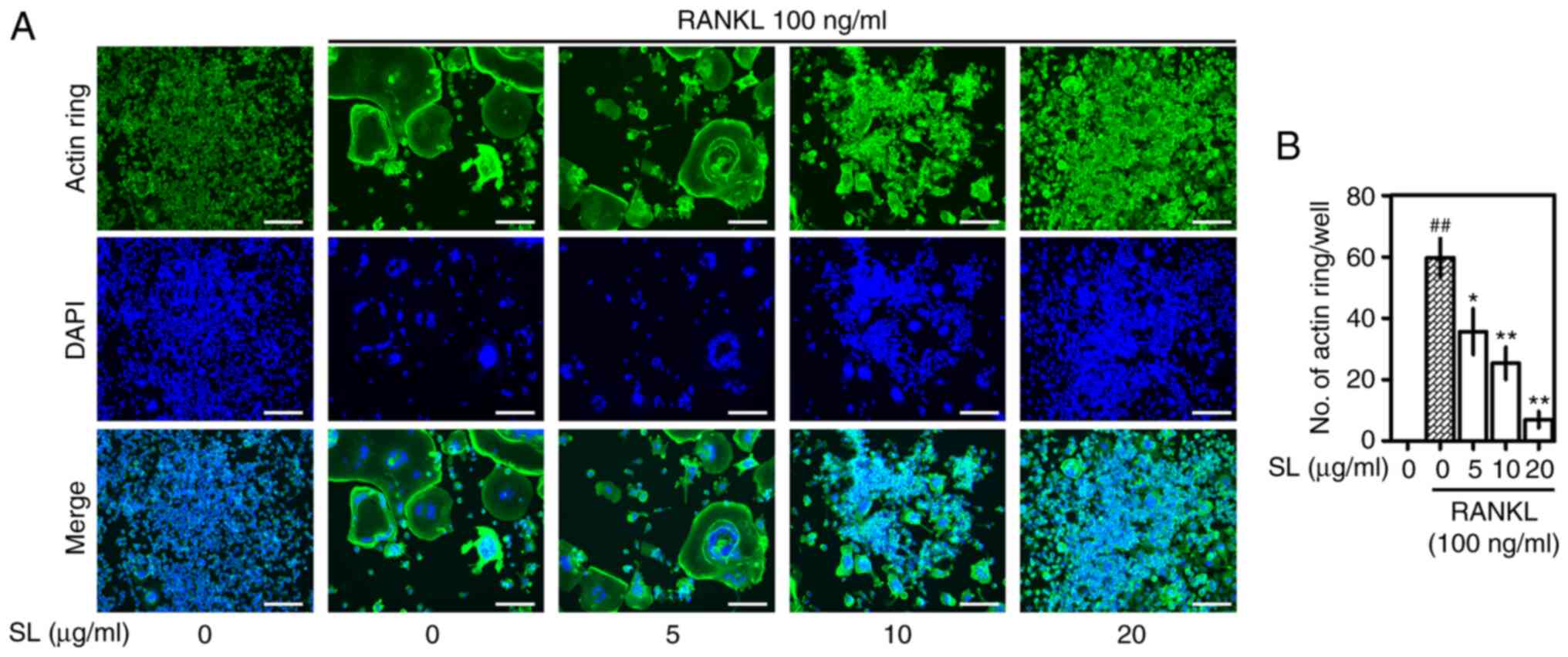
SL suppresses formation of actin
rings
The formation of an actin ring is an important
target to measure bone absorption capacity via pit formation assay
(25). The actin ring formation of
osteoclasts treated with RANKL was observed via immunofluorescence
analysis (Fig. 4). F-actin was
observed in RANKL-treated RAW 264.7 cells, and the number of nuclei
stained through DAPI decreased as cells fused during
differentiation. SL treatment significantly decreased formation of
F-actin in a concentration-dependent manner, which was consistent
with the results of TRAP staining and pit formation assay.
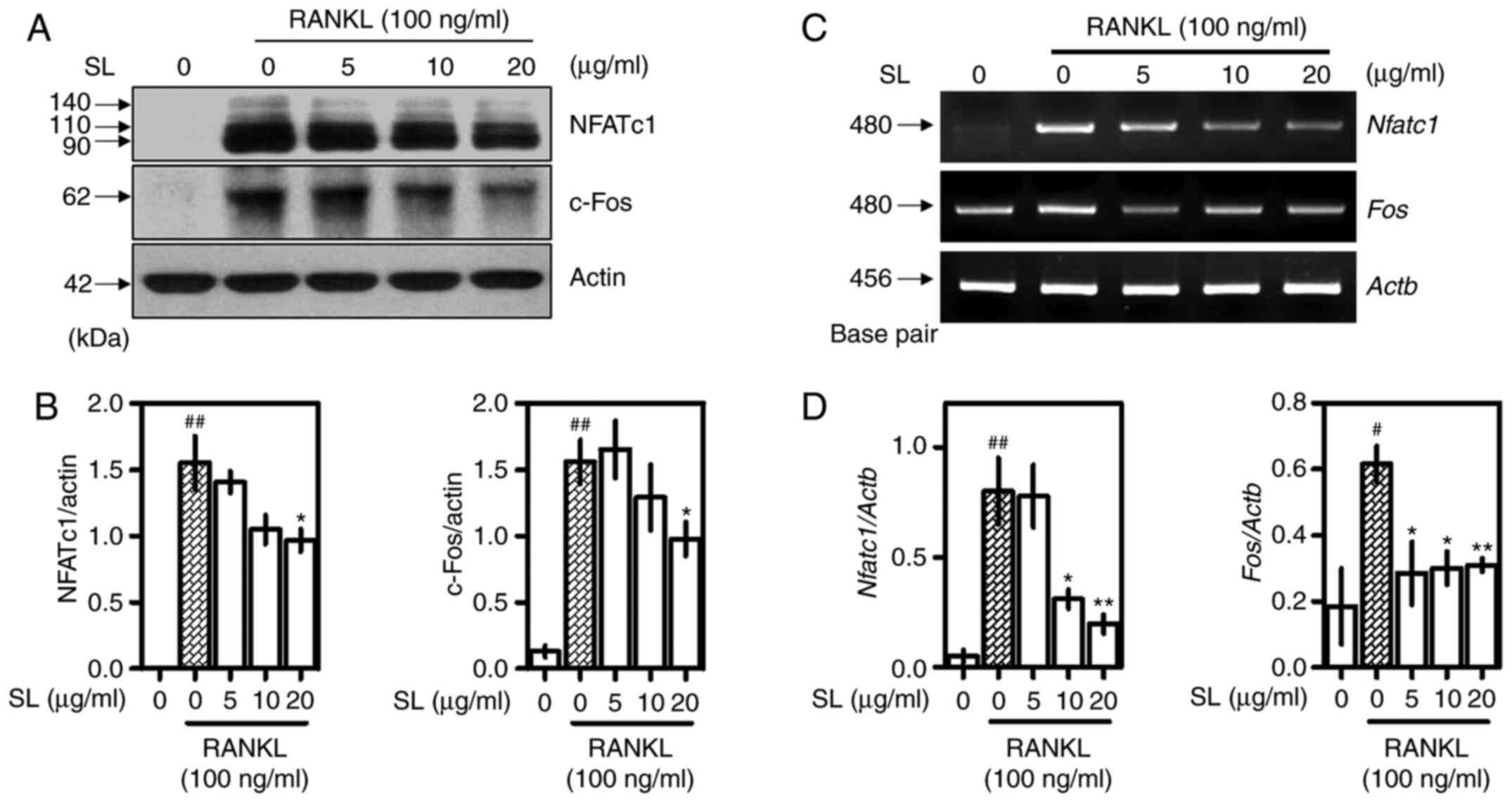
SL inhibits expression of NFATc1 and
c-Fos
Western blotting and RT-qPCR were used confirm
protein and gene expression of NFATc1 and c-Fos, which are
important transcription factors for osteoclast differentiation
(2). Following incubation for 24 h
with RANKL and SL, proteins were extracted to confirm the effect of
SL on expression of NFATc1 and c-Fos (Fig. 5A). These indicators were
significantly inhibited when treated with 20 µg/ml SL (Fig. 5B). mRNA expression was observed 4
days after RANKL and SL treatment (Fig.
5C). RANKL treatment upregulated expression of both indicators.
SL showed inhibitory effects on NFATc1 expression levels at
concentrations of 10 and 20 µg/ml. In addition, Fos expression was
inhibited at all concentrations of SL (Fig. 5D). Given the differences in sampling
times between western blotting and RT-qPCR experiments, it was
hypothesized that the inhibitor effect of SL on osteoclasts was
exerted in the late, rather than the early, stage of
differentiation.
SL suppresses expression of
osteoclast-associated genes
The effects of SL on osteoclast-associated genes was
demonstrated using RT-qPCR. Osteoclast-associated genes, such as
Acp5 (TRAP), Tnfrsf11a (RANK), Ctsk, Mmp9,
osteoclast-associated immunoglobulin-like receptor (OSCAR,
Oscar), ATPase H+ transporting V0 subunit d2
(ATP6v0d2, Atp6v0d2) and Ca2 were increased by RANKL
treatment; SL treatment decreased the expression of these genes
(Fig. 6A). Normalized to Actb,
Acp5, Ctsk and Atp6v0d2 were significantly suppressed
following treatment with 20 µg/ml SL compared with the RANKL-alone
group. In addition, Tnfrsf11a, Ca2, Mmp9 and Oscar
were significantly decreased when treated with 10 and 20 µg/ml SL
(Fig. 6B).
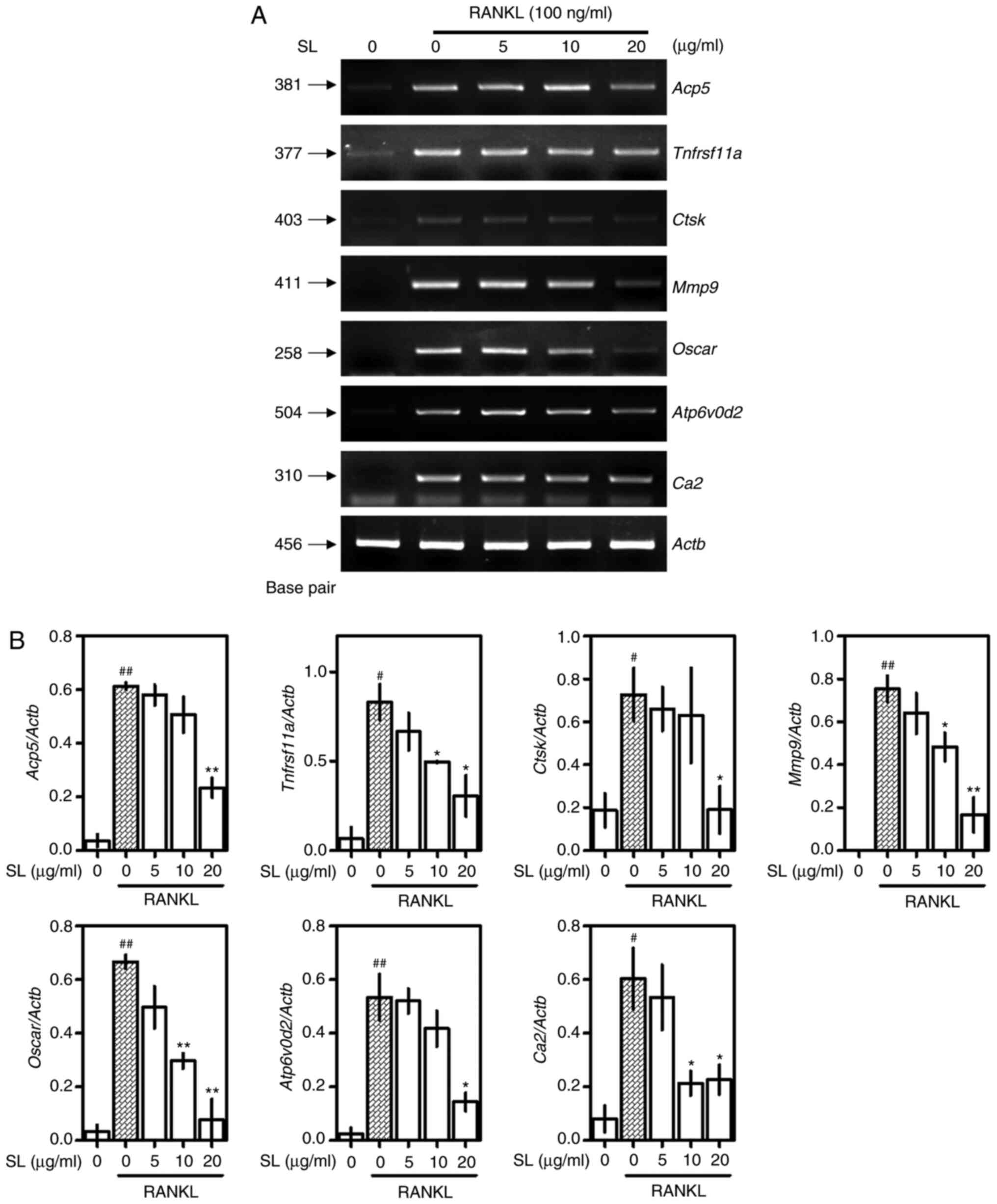 | Figure 6.Effect of SL on expression of
osteoclast-associated genes. (A) Inhibitory effects of SL on
expression of osteoclast-associated mRNA was verified by reverse
transcription-quantitative PCR and (B) normalized to Actb.
The results are expressed as the mean ± SEM (n=3).
#P<0.05, ##P<0.01 vs. untreated;
*P<0.05 and **P<0.01 vs. RANKL-alone. SL, Solanum
nigrum Line; RANKL, receptor activator of NF-κB ligand;
NFATc1, nuclear factor-activated T cells c1;
Tnfrsf11a, receptor activator of NF-κB; Acp5,
tartrate-resistant acid phosphatase; Ctsfk, cathepsin K;
Mmp9, matrix metallopeptidase 9; Oscar,
osteoclast-associated immunoglobulin-like receptor;
Atp6v0ds, ATPase H+ transporting V0 subunit d2;
Ca2, carbonic anhydrase II; Actb, β-actin. |
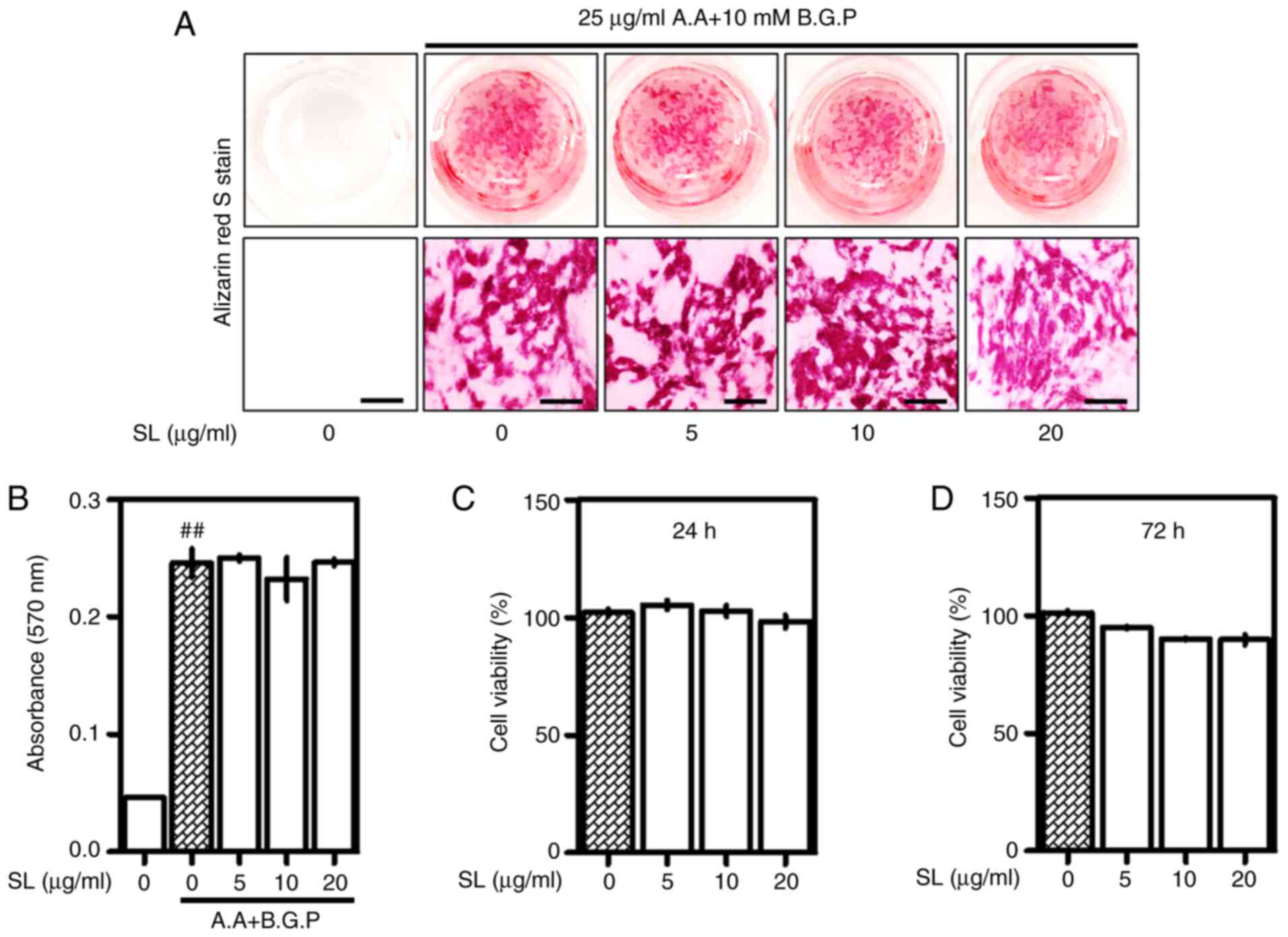
SL does not significantly affect
osteoblast differentiation
After demonstrating the inhibitory effects of SL in
an osteoclastogenesis in vitro model, the effects of SL in
an osteoblast model were confirmed using a MC3T3-E1 cell model. SL
did not significantly affect osteoblast differentiation and
formation of calcified nodules. These results indicate that SL
specifically acted on osteoclasts rather than osteoblasts (Fig. 7A and B). The concentration of SL did
not affect the viability of MC3T3-E1 cells (Fig. 7C and D).
Quantitative analysis of SL
Chlorogenic acid, caffeic acid and quercetin are
well-known active ingredients of SL (23,24).
The chromatography peak of ingredient standard is shown in Fig. 8A. In the SL Et-OH extract, peaks
were observed from 0 to 50 min and each peak was detected at the
same retention time (chlorogenic acid, 9.420; caffeic acid, 10.836;
quercetin, 23.722 min) as the ingredient standard (Fig. 8B).
Discussion
In vivo, SL inhibited expression of NFATc1
and CTsK in the femoral head and significantly decreased bone
density and osteoclast differentiation. In vitro, SL
inhibited the expression of NFATc1/c-Fos during early and late
osteoclast differentiation and suppressed expression of genes
associated with differentiation and bone resorption. As a result,
SL inhibited osteoclast differentiation, bone resorption activity
and formation of actin rings.
Osteoporosis is characterized by a decrease in bone
density and fragility. However, previous studies have shown that
measuring bone volume and BMD is insufficient to determine
improvement in osteoporosis, and that structural changes of
trabecular bone must also be assessed (26,27).
In the present study, the administration of SL in the OVX
osteoporosis model not only increased BMD and bone volume, but also
improved the trabecular bone microstructure. These results
indicated that SL suppressed a decrease in bone density, which is a
phenotype of osteoporosis, and improved bone quality via improved
bone microstructure. The OVX-induced osteoporosis model shows the
phenotype of postmenopausal osteoporosis and is used to research
osteoporosis treatments (28,29).
Following OVX, an increase in body weight and a decrease in uterine
weight due to hormonal changes are considered to indicate
successful surgery (17). In the
present study, the E2 group exhibited suppressed changes
in body and uterine weight, whereas the SL group exhibited no
effect. These results indicated that OVX surgery was successful and
SL did not exert a hormone-associated effect similar to that of
E2 (30).
RAW 264.7 cells are monocyte/macrophages extracted
from male BALB/c mice and are used as a cell model for various
pathological diseases, such as inflammation, antioxidant and
osteoclast differentiation (31–34).
When RAW 264.7 cells are treated with RANKL, which is a member of
the TNF superfamily and a type of cytokine, the monocytes induce
differentiation, fusion, function and maturation of osteoclasts
(35). Osteoclasts express various
phenotypic markers, the most representative of which is TRAP, as
its concentration in serum is used as a biochemical indicator of
osteoclast activity and bone resorption (36). In the present study, SL decreased
serum levels and activity of TRAP and the number of TRAP-positive
cells in the femur of OVX-induced osteoporotic rats and inhibited
the differentiation of RANKL-induced TRAP-positive cells. These
results suggested that the anti-osteoporotic effect of SL is
mediated by suppression of osteoclast differentiation.
Osteoclast differentiation involves essential
transcription factors such as NFATc1 (37). The importance of NFATc1 in
osteoclast differentiation has been demonstrated in transgenic mice
and cell models. Aliprantis et al (38) found that cells of NFATc1-deficient
mice do not differentiate into osteoclasts, leading to
osteopetrosis. The embryonic stem cells from which NFATc1 was
removed from did not differentiate into osteoclasts, even following
stimulation with RANKL, whereas embryonic stem cells overexpressing
NFATc1 differentiate into osteoclasts without the need for
stimulation with RANKL (39). In
the present study, SL significantly inhibited the expression of
NFATc1 in femoral tissue and RANKL-induced RAW 264.7 cells. These
results indicated that the inhibitory effect of SL on osteoclast
differentiation is mediated by NFATc1. In addition, NFATc1
regulates expression of various osteoclast differentiation and bone
resorption factors, such as CTsK, MMP-9, OSCAR and ATP6v0d2
(40). CTsK and MMP-9 are expressed
in mature osteoclasts attached to the bone surface and degrade the
bone. According to Saftig et al (41), osteoclasts extracted from
CTsK-knockout mice impair bone resorption. In the present study, SL
suppressed expression of CTsK in femoral tissue and cell models of
osteoclast differentiation. These results indicated that SL served
an important role not only in inhibiting osteoclast
differentiation, but also in suppressing its ability to absorb
bone. OSCAR is expressed in preosteoclasts and mature osteoclasts
and is an etiological factor in osteoporosis and rheumatoid
arthritis (42). ATP6v0d2 serves an
important role in cell-cell fusion and actin ring formation
(10,43). The actin ring is associated with the
‘sealing zone’ that is formed when osteoclasts attach to bone,
which is a structural factor essential for osteoclast bone
absorption (44). In the present
study, SL inhibited the formation of actin rings and expression of
ATP6v0d2, suggesting that SL controlled cell fusion during the
early stages of osteoclast differentiation, as well as formation of
the skeletal structure of the mature osteoclast.
c-Fos is a representative osteoclast transcription
factor that controls expression of NFATc1 (40). c-Fos recruits the NFATc1 promoter in
the early stages of osteoclast differentiation (45,46).
In addition, c-Fos-deficient cells cause disorders in NFATc1
expression and osteoclast differentiation via induction of RANKL,
which is improved by overexpression of NFATc1 (46). c-Fos also controls expression of
CA2, which serves an important role in osteoclast bone absorption
function (47). CA2 acidifies the
bone surface prior to osteoclast-mediated bone absorption, creating
an environment in which various enzymes can function (48,49).
In the present study, SL inhibited both c-Fos and CA2 expression.
These results showed that the inhibitory effect of SL on NFATc1
expression was mediated by regulation of c-Fos. In conclusion, it
was confirmed that SL inhibited osteoclast differentiation and
function by suppressing the expression of NFATc1/c-Fos, and thus,
significantly suppressing OVX-induced decreases in bone density.
These results highlight the possibility of SL as a therapeutic
agent for management of osteoporosis.
The present study had certain limitations. The
activity of osteoclasts was investigated via stimulation of RANKL.
Various factors, such as inflammation, steroids and aging, cause
osteoporosis (50–52). However, the effect of SL on these
factors was not investigated in the present study. Research on
treatment of osteoporosis research has focused on patients with
postmenopausal osteoporosis (53).
However, with an increase in the elderly population, male and
senile osteoporosis is becoming increasingly important (54). Additionally, treatment with steroids
results in potential social implications for patients due to
adverse side effects (50).
Therefore, studying the effect of SL on other osteoporosis-causing
factors such as inflammation, steroids and aging may highlight more
generalizable targets and mechanisms. Previous studies have shown
that the fruit of SL contains solanine, which is known to be toxic
(55–57). However, a previous study
demonstrated that it displays hepatoprotective effects (58). Additional studies on the
administration method and toxic concentration of SL are required.
Expression of NFATc1 is controlled by MAPK, NF-κB and c-Fos
(2). However, only c-Fos expression
was investigated in the present study. In order to confirm the
anti-osteoporotic and inhibitory effects of SL on osteoclast
differentiation, the effects of SL on phosphorylation of MAPK, ERK,
JNK and p38 and expression of NF-κB induced by RANKL should be
studied. The present study demonstrated the effect of SL on
osteoclast differentiation but did not investigate the active
ingredients in SL. In previous studies, SL has been shown to
contain several active ingredients (24,55),
some of which have an effect on osteoclasts and osteoporosis. For
example, diosgenin and ferulic acid inhibit differentiation of
osteoclasts via NF-κB (59,60). According to Wu et al
(61), protocatechuic acid inhibits
osteoclast differentiation via apoptosis of osteoclasts. In
addition, Rutin inhibits osteoclast differentiation via its
antioxidant effect (62). However,
most of the components of SL have not been studied yet. Therefore,
analysis of the structure and anti-osteoclastogenic effect of each
component will be helpful in understanding the anti-osteoporosis
mechanism of SL. It is necessary to verify the inhibitory effect of
active ingredients in SL on osteoclasts in future.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Research Foundation of Korea grant funded by the Korean government
(grant nos. 2020R1A2C1007836 and 2020R1A6A3A01098984).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YS and HSJ conceptualized the study. JHK and HS
performed all experiments. JHK, MK and HSJ contributed to the
statistical analysis. YS, SK and KS interpreted the results. JHK
and HS drafted the manuscript. JHK and HS confirm the authenticity
of all the raw data All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All animal experiments were approved by Kyunghee
University Animal Committee [approval no. KHUASP(SE)-17-052].
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sözen T, Özışık L and Başaran NC: An
overview and management of osteoporosis. Eur J Rheumatol. 4:46–56.
2017. View Article : Google Scholar
|
|
2
|
Boyle WJ, Simonet WS and Lacey DL:
Osteoclast differentiation and activation. Nature. 423:337–342.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Teitelbaum SL: Bone resorption by
osteoclasts. Science. 289:1504–1508. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tu KN, Lie JD, Wan CKV, Cameron M, Austel
AG, Nguyen JK, Van K and Hyun D: Osteoporosis: A Review of
Treatment Options. P&T. 43:92–104. 2018.
|
|
5
|
Skjødt MK, Frost M and Abrahamsen B: Side
effects of drugs for osteoporosis and metastatic bone disease. Br J
Clin Pharmacol. 85:1063–1071. 2019. View Article : Google Scholar
|
|
6
|
Herbology Editorial Committee of Korean
Medicine, . Herbology; Younglimsa, Seoul: 2004
|
|
7
|
Youn YN, Lim E, Lee N, Kim YS, Koo MS and
Choi SY: Screening of Korean medicinal plants for possible
osteoclastogenesis effects in vitro. Genes Nutr. 2:375–380. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ginaldi L, Di Benedetto MC and De Martinis
M: Osteoporosis, inflammation and ageing. Immun Ageing. 2:142005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Souza PP and Lerner UH: The role of
cytokines in inflammatory bone loss. Immunol Invest. 42:555–622.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim K, Lee SH, Ha Kim J, Choi Y and Kim N:
NFATc1 induces osteoclast fusion via up-regulation of Atp6v0d2 and
the dendritic cell-specific transmembrane protein (DC-STAMP). Mol
Endocrinol. 22:176–185. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim M, Kim HS, Kim JH, Kim EY, Lee B, Lee
SY, Jun JY, Kim MB, Sohn Y and Jung HS: Chaenomelis fructus
inhibits osteoclast differentiation by suppressing NFATc1
expression and prevents ovariectomy-induced osteoporosis. BMC
Complement Med Ther. 20:352020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zakaria ZA, Gopalan HK, Zainal H, Mohd
Pojan NH, Morsid NA, Aris A and Sulaiman MR: Antinociceptive,
anti-inflammatory and antipyretic effects of Solanum nigrum
chloroform extract in animal models. Yakugaku Zasshi.
126:1171–1178. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zakaria ZA, Sulaiman MR, Morsid NA, Aris
A, Zainal H, Pojan NH and Kumar GH: Antinociceptive,
anti-inflammatory and antipyretic effects of Solanum nigrum
aqueous extract in animal models. Methods Find Exp Clin Pharmacol.
31:81–88. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Miyamoto T and Suda T: Differentiation and
function of osteoclasts. Keio J Med. 52:1–7. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Clohisy JC, Frazier E, Hirayama T and
Abu-Amer Y: RANKL is an essential cytokine mediator of
polymethylmethacrylate particle-induced osteoclastogenesis. J
Orthop Res. 21:202–212. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Takayanagi H: The role of NFAT in
osteoclast formation. Ann NY Acad Sci. 1116:227–237. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim JH, Kim EY, Lee B, Min JH, Song DU,
Lim JM, Eom JW, Yeom M, Jung HS and Sohn Y: The effects of Lycii
Radicis Cortex on RANKL-induced osteoclast differentiation and
activation in RAW 264.7 cells. Int J Mol Med. 37:649–658. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee KY, Kim JH, Kim EY, Yeom M, Jung HS
and Sohn Y: Water extract of Cnidii Rhizoma suppresses
RANKL-induced osteoclastogenesis in RAW 264.7 cell by inhibiting
NFATc1/c-Fos signaling and prevents ovariectomized bone loss in
SD-rat. BMC Complement Altern Med. 19:2072019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yeom M, Kim EY, Kim JH, Jung HS and Sohn
Y: High doses of Bupleurum falcatum partially prevents
estrogen deficiency-induced bone loss with anti-osteoclastogenic
activity due to enhanced iNOS/NO signaling. Front Pharmacol.
9:13142018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tschöp MH, Speakman JR, Arch JR, Auwerx J,
Brüning JC, Chan L, Eckel RH, Farese RV Jr, Galgani JE, Hambly C,
et al: A guide to analysis of mouse energy metabolism. Nat Methods.
9:57–63. 2011. View Article : Google Scholar
|
|
21
|
Chan FK, Moriwaki K and De Rosa MJ:
Detection of necrosis by release of lactate dehydrogenase activity.
Methods Mol Biol. 979:65–70. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hayman AR: Tartrate-resistant acid
phosphatase (TRAP) and the osteoclast/immune cell dichotomy.
Autoimmunity. 41:218–223. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Campisi A, Acquaviva R, Raciti G, Duro A,
Rizzo M and Santagati NA: Antioxidant activities of Solanum
Nigrum L. leaf extracts determined in in vitro cellular models.
Foods. 8:82019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huang HC, Syu KY and Lin JK: Chemical
composition of Solanum nigrum linn extract and induction of
autophagy by leaf water extract and its major flavonoids in AU565
breast cancer cells. J Agric Food Chem. 58:8699–8708. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Matsubara T, Myoui A, Ikeda F, Hata K,
Yoshikawa H, Nishimura R and Yoneda T: Critical role of cortactin
in actin ring formation and osteoclastic bone resorption. J Bone
Miner Metab. 24:368–372. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Burstein AH, Reilly DT and Martens M:
Aging of bone tissue: Mechanical properties. J Bone Joint Surg Am.
58:82–86. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Osterhoff G, Morgan EF, Shefelbine SJ,
Karim L, McNamara LM and Augat P: Bone mechanical properties and
changes with osteoporosis. Injury. 47 (Suppl 2):S11–S20. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kalu DN: The ovariectomized rat model of
postmenopausal bone loss. Bone Miner. 15:175–191. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim EY, Kim JH, Kim M, Park JH, Sohn Y and
Jung HS: Abeliophyllum distichum Nakai alleviates
postmenopausal osteoporosis in ovariectomized rats and prevents
RANKL-induced osteoclastogenesis in vitro. J Ethnopharmacol.
257:1128282020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yousefzadeh N, Kashfi K, Jeddi S and
Ghasemi A: Ovariectomized rat model of osteoporosis: A practical
guide. EXCLI J. 19:89–107. 2020.PubMed/NCBI
|
|
31
|
Taciak B, Białasek M, Braniewska A, Sas Z,
Sawicka P, Kiraga Ł, Rygiel T and Król M: Evaluation of phenotypic
and functional stability of RAW 264.7 cell line through serial
passages. PLoS One. 13:e01989432018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Funk JL, Feingold KR, Moser AH and
Grunfeld C: Lipopolysaccharide stimulation of RAW 264.7 macrophages
induces lipid accumulation and foam cell formation.
Atherosclerosis. 98:67–82. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kong L, Smith W and Hao D: Overview of
RAW264.7 for osteoclastogensis study: Phenotype and stimuli. J Cell
Mol Med. 23:3077–3087. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yeom M, Kim JH, Min JH, Hwang MK, Jung HS
and Sohn Y: Xanthii fructus inhibits inflammatory responses in
LPS-stimulated RAW 264.7 macrophages through suppressing NF-κB and
JNK/p38 MAPK. J Ethnopharmacol. 176:394–401. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Collin-Osdoby P and Osdoby P:
RANKL-mediated osteoclast formation from murine RAW 264.7 cells.
Methods Mol Biol. 816:187–202. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ballanti P, Minisola S, Pacitti MT,
Scarnecchia L, Rosso R, Mazzuoli GF and Bonucci E:
Tartrate-resistant acid phosphate activity as osteoclastic marker:
Sensitivity of cytochemical assessment and serum assay in
comparison with standardized osteoclast histomorphometry.
Osteoporos Int. 7:39–43. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kim JH and Kim N: Regulation of NFATc1 in
osteoclast differentiation. J Bone Metab. 21:233–241. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Aliprantis AO, Ueki Y, Sulyanto R, Park A,
Sigrist KS, Sharma SM, Ostrowski MC, Olsen BR and Glimcher LH:
NFATc1 in mice represses osteoprotegerin during osteoclastogenesis
and dissociates systemic osteopenia from inflammation in cherubism.
J Clin Invest. 118:3775–3789. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Takayanagi H, Kim S, Koga T, Nishina H,
Isshiki M, Yoshida H, Saiura A, Isobe M, Yokochi T, Inoue J, et al:
Induction and activation of the transcription factor NFATc1 (NFAT2)
integrate RANKL signaling in terminal differentiation of
osteoclasts. Dev Cell. 3:889–901. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhao Q, Wang X, Liu Y, He A and Jia R:
NFATc1: Functions in osteoclasts. Int J Biochem Cell Biol.
42:576–579. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Saftig P, Hunziker E, Wehmeyer O, Jones S,
Boyde A, Rommerskirch W, Moritz JD, Schu P and von Figura K:
Impaired osteoclastic bone resorption leads to osteopetrosis in
cathepsin-K-deficient mice. Proc Natl Acad Sci USA. 95:13453–13458.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kim K, Kim JH, Lee J, Jin HM, Lee SH,
Fisher DE, Kook H, Kim KK, Choi Y and Kim N: Nuclear factor of
activated T cells c1 induces osteoclast-associated receptor gene
expression during tumor necrosis factor-related activation-induced
cytokine-mediated osteoclastogenesis. J Biol Chem. 280:35209–35216.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wu H, Xu G and Li YP: Atp6v0d2 is an
essential component of the osteoclast-specific proton pump that
mediates extracellular acidification in bone resorption. J Bone
Miner Res. 24:871–885. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Han G, Zuo J and Holliday LS: Specialized
roles for actin in osteoclasts: Unanswered questions and
therapeutic opportunities. Biomolecules. 9:92019. View Article : Google Scholar
|
|
45
|
Anderson DM, Maraskovsky E, Billingsley
WL, Dougall WC, Tometsko ME, Roux ER, Teepe MC, DuBose RF, Cosman D
and Galibert L: A homologue of the TNF receptor and its ligand
enhance T-cell growth and dendritic-cell function. Nature.
390:175–179. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
46
|
Matsuo K, Galson DL, Zhao C, Peng L,
Laplace C, Wang KZ, Bachler MA, Amano H, Aburatani H, Ishikawa H,
et al: Nuclear factor of activated T-cells (NFAT) rescues
osteoclastogenesis in precursors lacking c-Fos. J Biol Chem.
279:26475–26480. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
David JP, Rincon M, Neff L, Horne WC and
Baron R: Carbonic anhydrase II is an AP-1 target gene in
osteoclasts. J Cell Physiol. 188:89–97. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kim M, Kim M, Kim JH, Hong S, Kim DH, Kim
S, Kim EY, Jung HS and Sohn Y: Crataegus pinnatifida bunge
inhibits RANKL-induced osteoclast differentiation in RAW 264.7
cells and prevents bone loss in an ovariectomized rat model. Evid
Based Complement Alternat Med. 2021:55215622021. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lehenkari P, Hentunen TA, Laitala-Leinonen
T, Tuukkanen J and Väänänen HK: Carbonic anhydrase II plays a major
role in osteoclast differentiation and bone resorption by effecting
the steady state intracellular pH and Ca2+. Exp Cell
Res. 242:128–137. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Manolagas SC: Steroids and osteoporosis:
The quest for mechanisms. J Clin Invest. 123:1919–1921. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Rochira V, Balestrieri A, Madeo B, Zirilli
L, Granata AR and Carani C: Osteoporosis and male age-related
hypogonadism: Role of sex steroids on bone (patho)physiology. Eur J
Endocrinol. 154:175–185. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Tella SH and Gallagher JC: Prevention and
treatment of postmenopausal osteoporosis. J Steroid Biochem Mol
Biol. 142:155–170. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Thulkar J and Singh S: Overview of
research studies on osteoporosis in menopausal women since the last
decade. J Midlife Health. 6:104–107. 2015.PubMed/NCBI
|
|
54
|
Christensen K, Doblhammer G, Rau R and
Vaupel JW: Ageing populations: The challenges ahead. Lancet.
374:1196–1208. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Gu XY, Shen XF, Wang L, Wu ZW, Li F, Chen
B, Zhang GL and Wang MK: Bioactive steroidal alkaloids from the
fruits of Solanum nigrum. Phytochemistry. 147:125–131. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Shen KH, Liao AC, Hung JH, Lee WJ, Hu KC,
Lin PT, Liao RF and Chen PS: α-Solanine inhibits invasion of human
prostate cancer cell by suppressing epithelial-mesenchymal
transition and MMPs expression. Molecules. 19:11896–11914. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Zhao L, Wang L, Di SN, Xu Q, Ren QC, Chen
SZ, Huang N, Jia D and Shen XF: Steroidal alkaloid solanine A from
Solanum nigrum Linn. exhibits anti-inflammatory activity in
lipopolysaccharide/interferon γ-activated murine macrophages and
animal models of inflammation. Biomed Pharmacother. 105:606–615.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Lin HM, Tseng HC, Wang CJ, Lin JJ, Lo CW
and Chou FP: Hepatoprotective effects of Solanum nigrum Linn
extract against CCl(4)-induced oxidative damage in rats. Chem Biol
Interact. 171:283–293. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Doss HM, Samarpita S, Ganesan R and Rasool
M: Ferulic acid, a dietary polyphenol suppresses osteoclast
differentiation and bone erosion via the inhibition of RANKL
dependent NF-κB signalling pathway. Life Sci. 207:284–295. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Shishodia S and Aggarwal BB: Diosgenin
inhibits osteoclastogenesis, invasion, and proliferation through
the downregulation of Akt, I kappa B kinase activation and NF-kappa
B-regulated gene expression. Oncogene. 25:1463–1473. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Wu YX, Wu TY, Xu BB, Xu XY, Chen HG, Li XY
and Wang G: Protocatechuic acid inhibits osteoclast differentiation
and stimulates apoptosis in mature osteoclasts. Biomed
Pharmacother. 82:399–405. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Kyung TW, Lee JE, Shin HH and Choi HS:
Rutin inhibits osteoclast formation by decreasing reactive oxygen
species and TNF-alpha by inhibiting activation of NF-kappaB. Exp
Mol Med. 40:52–58. 2008. View Article : Google Scholar : PubMed/NCBI
|