Introduction
Vascular dementia (VD) is one of the leading causes
of neurological disorder following Alzheimer's disease and accounts
for 15% of patients with neurological disorders worldwide (1). Patients with VD suffer from memory
loss and cognitive impairment, which progressively worsen over the
time (2). Numerous vascular risk
factors have been identified that are associated with the
development of VD and its progression (3). It has been suggested that VD may
affect more individuals in the future as the population ages, and
the average survival of patients following stroke and
cardiovascular disorders increases (4). The symptoms of VD include cognitive
impairment and a reduction in memory. Currently, several compounds
including, memantine, galantamine, donepezil and rivastigmine, are
undergoing clinical trials for treatment of VD but the obtained
results are not satisfactory. Thus, the development of effective
treatment for VD is urgently needed to prevent neurological damage
in more individuals.
Autophagy is the cellular process leading to
self-degradation that regulates stability in the body environment
by eliminating damaged cellular components, such as mitochondria
(5,6). Increased autophagy causes cell death
via self-digestion, and degrades cellular organelles and proteins
(7). The ischemia-induced
activation of autophagy leads to neuronal damage in brain tissues
(8,9). This suggests that autophagy may have a
vital role in inducing neuronal damage to the central nervous
system in patients with ischemia. Mammalian target of rapamycin
(mTOR) serves a leading role in the regulation of cellular growth,
survival, protein translation and autophagy (10). A variety of autophagic cellular
processes are controlled by phosphorylated (p)-mTOR/mTOR signaling
pathways (11).
Microtubule-associated protein light chain 3 (LC3) II is an
autophagy-related factor, which has been widely studied as an
autophagic protein (12). LC3II
serves an important role in the development of autophagosomes and
their maturation, and is therefore used for monitoring the
autophagic activity in cells (12).
Naturally obtained compounds from diverse sources
have demonstrated potential pharmacological activities in multiple
diseases. Asiaticoside is a saponin compound with a monomeric
structure that is obtained from the medicinal plant Centella
asiatica. Pharmacological screening has identified various
properties of asiaticoside, including hepatoprotective (13), antioxidant (14), neuroprotective (15) and anti-inflammatory (16) activities. In addition, numerous
saponins have been shown to possess a diverse range of
pharmacological activities; notably, some saponins are in the
clinical trials stage and a few have been approved as therapeutic
drugs by the Food and Drug Administration (14,16).
Additionally, saponins exhibit their activity through multiple
pathways in different types of diseases/disorders. The present
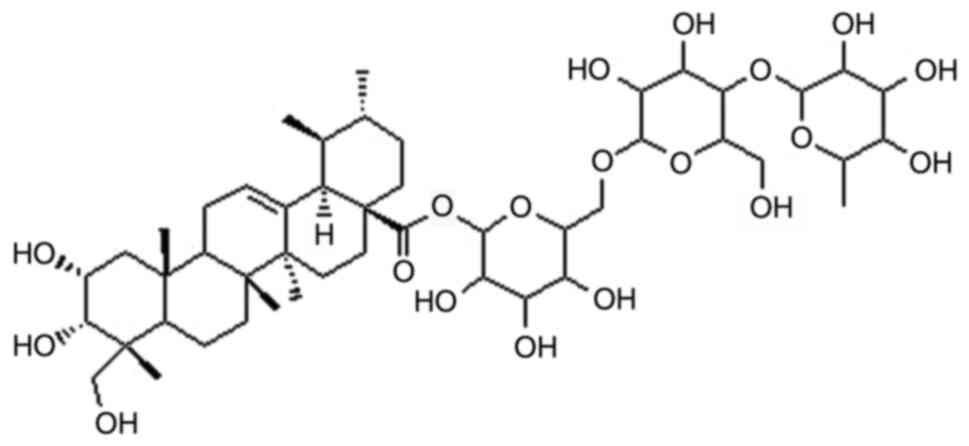
study evaluated the potential role or asiaticoside (Fig. 1) in the treatment of a rat model of
VD and its inhibitory effects on autophagy in hippocampal
tissues.
Materials and methods
Animals and grouping
A total of 50 male Sprague-Dawley rats (age, 8
weeks; weight, 240–270 g) were obtained from the Animal Center,
Shenyang Medical University. The rats were individually maintained
in sterile cages in an animal center under ~55% humidity and at
23±2°C, and were exposed to 12-h light/dark cycles. All rats were
allowed free access to water and a laboratory rodent diet. The
experimental procedures involving rats were conducted in accordance
with the guidelines of the Care and Use of Laboratory Animals
Committee China Medical University (15). The present study was approved by the
Animal Ethics Committee, Dongying District People's Hospital
(Dongying, China; approval. no. DSH/2017/067). The rats were
separated into five groups (n=10/group): Sham group, VD group, VD +
asiaticoside group, rapamycin group and rapamycin + asiaticoside
group. The rats in all groups, with the exception of the sham
group, were subjected to bilateral occlusion of carotid arteries
(17). Chloral hydrate (10%; 300
mg/kg) anesthesia was intraperitoneally administered to the rats,
after which they were fixed supine on hot pads and a ventral
midline incision was made to the neck. No signs of peritonitis were
observed in the rats following anesthesia with 10% chloral hydrate.
Muscles on either side of the trachea were incised carefully to
expose the carotid arteries. Subsequently, double ligation was
performed for permanent occlusion of the arteries. The same
procedure without vessel ligation was repeated in the sham group.
The rapamycin and rapamycin + asiaticoside groups were injected
with rapamycin (50 µl) 1 day before surgery directly into the
ventricle using a catheter. Asiaticoside dissolved in physiological
saline at 5 mg/kg body weight was given to treatment groups via the
intragastric route as a single dose after surgery.
Behavioral assessment using T-maze
tests
The spatial memory of rats was assessed using the
T-maze test 28 days after surgery, using previously reported
methodology (18). Each trial in
the T-maze test involves two runs: A sample run and a choice run.
The sample run consisted of forcing the rats to enter one of the
two arms of the maze in order to obtain sugar placed in left arm,
while the second arm of the maze was closed using a sliding door.
During the choice run, the door was opened and rats were left to
choose one of the arms freely. The time duration set between the
two runs was 10 sec, and the rats entering the previously unvisited
arm were rewarded. Subsequently, the time duration between the two
runs was increased to 90 and 180 sec. Each session involved five
trials every day and the time gap between two trials was set at 10
min. The number of corrections was taken as the number of times
rats entered the arm that was previously unvisited.
Morris water maze (MWM) test
The MWM test was used to assess cognitive ability of
the animals 28 days after surgery (17). Briefly, the rats were given four
training trials every day for 5 consecutive days. The training
trials consisted of placing the rats alternately in four different
quadrants of a pool of water and allowing them to locate a platform
during 120 sec. The rats were then given 20 sec to rest on the
platform; time taken to find the platform was counted as escape
latency. The rats unable to locate the platform during the assigned
duration were guided towards it and allowed to rest for 20 sec. The
platform was removed from the pool on the 6th day of the probe test
and rats were permitted to swim freely to locate the removed
platform. The swimming activity of the rats was monitored and
recorded video-graphically. The platform crossings were recorded by
calculating the platform location crossed by each rat.
Extraction of brain tissues
The rats were sacrificed using pentobarbital
overdose. Subsequently, normal saline and paraformaldehyde (4%) in
sodium phosphate buffer were perfused transcardially. The rat
brains were dissected and then subjected to fixing in 4%
paraformaldehyde at 4°C. After 3 days of fixing, the brain samples
were embedded in paraffin and sliced into 2-µm sections.
Transmission electron microscopy
(TEM)
The brain tissues were fixed with 2.5%
glutaraldehyde in PBS at 4°C for 2 h, and then with 1% osmium
tetroxide (Ph 7.4) for 2.5 h at 4°C. Subsequently, the tissues were
stained with uranyl acetate (1% aqueous) solution overnight at 4°C
prior to embedding in Durcupan (Sigma-Aldrich; Merck KGaA). An
ultracut microtome (Leica Microsystems, Inc.) was used to cut
hippocampal tissues into ~60-nm sections, which were placed on
formvar-coated copper grids. The sample sections were subjected to
staining for 5 min with uranyl acetate (2.5%) and lead citrate (3%)
followed by examination under a 7650 transmission electron
microscope (Hitachi High-Technologies Corporation).
Western blotting
The rats were sacrificed using pentobarbital
overdose. Subsequently, normal saline and 4% paraformaldehyde in
sodium phosphate buffer was perfused transcardially. The
hippocampal tissues were excised and then homogenized with RIPA
lysis buffer (Beyotime Institute of Biotechnology) mixed with PMSF.
The lysates were centrifuged at 12,000 × g for 15 min at 4°C,
followed by protein content determination using the BCA assay kit
(Beyotime Institute of Biotechnology). The protein samples (30 µg)
were separated by SDS-PAGE on 12% gels and were then transferred to
PVDF membranes, which were blocked using 3% BSA (Sigma-Aldrich;
Merck KGaA) in Tris-buffered saline for 40 min at room temperature.
The membranes were probed with primary antibodies at 4°C overnight,
washed with PBS and then incubated for 2 h with horseradish
peroxidase-conjugated secondary antibodies (cat. no. 7074; 1:2,000;
Cell Signaling Technology, Inc.) at room temperature. Detection and
visualization of protein bands were performed using an enhanced
chemiluminescence system (EMD Millipore) and the blots were
semi-quantified by Quantity-One software version 4.6.3 (Bio-Rad
Laboratories, Inc.). The primary antibodies used were: Anti-mTOR
(cat. no. 2972; dilution 1:400; Cell Signaling Technology, Inc.),
anti-LC3B (cat. no. 2775; dilution 1:1,000; Cell Signaling
Technology, Inc.), anti-phosphorylated (p)-mTOR (cat. no. 2971;
dilution 1:250; Cell Signaling Technology, Inc.), anti-Beclin 1
(cat. no. sc-48341; dilution 1:1,000; Santa Cruz Biotechnology,
Inc.) and anti-β-actin (cat. no. 4967; dilution 1:1,000, Cell
Signaling Technology, Inc.).
Nissl staining
The rats were sacrificed after anaesthetization with
overdose of sodium pentobarbital. Transcardial perfusion was
performed with normal saline (0.9%) and then with 4%
paraformaldehyde in 0.1 M sodium phosphate buffer (pH 7.3).
Dissection of the brain was followed by fixing with 4%
paraformaldehyde at 4°C for 3-days and then paraffin embedding.
Brains were sliced into 5-µm sections followed by staining at 60°C
for 45 min with 1% Toluidine Blue. Neuronal survival was determined
by the examination of Nissl-positive cells in the rat hippocampus.
Light microscope (model, BX53; Olympus Corporation) was used for
calculation of normal neurons in the CA1 subfield at ×400
magnification. The average number of neurons was calculated in
three sections and five representative fields were randomly chosen
for each section.
Histological analysis using H&E
staining
The rats were sacrificed after anaesthetization with
200 mg/kg sodium pentobarbital intraperitoneally. Hippocampal
tissues were extracted, subjected to PBS washing and then fixed in
10% neutral buffered formalin at room temperature for 45 min. The
tissues were decalcified using EDTA (10%) followed by embedding in
paraffin and were then sliced into 3-µm sections. The sections were
subjected to H&E staining for 40 min at room temperature and
were then examined under a light microscope (Olympus IX81; Olympus
Corporation) at ×200 magnification.
Statistical analysis
The data are presented as the mean ± standard
deviation of three experiments. Data analysis was performed using
SPSS 16.0 statistical software (SPSS, Inc.). The statistical
differences between groups were determined using one-way ANOVA
followed by Tukey's post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Asiaticoside improves cognitive
function in a rat model of VD
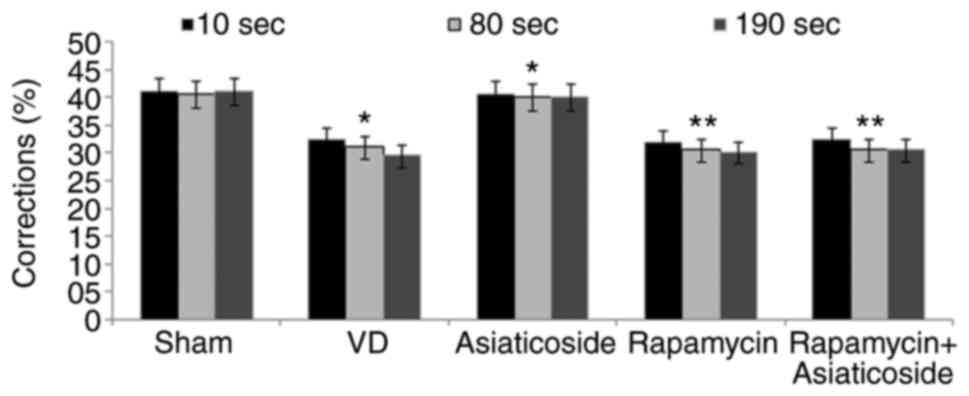
Changes in behaviors were significantly impaired in
the VD rat model group compared with in the sham group (Fig. 2). Asiaticoside treatment of rats
with VD significantly improved the changes in behaviors. In
addition, rapamycin administration also mediated an impairment in
spontaneously altered behaviors in the rats. Asiaticoside failed to
significantly alleviate spontaneously altered behavior impairment
in VD rats treated with rapamycin. These findings indicated that
asiaticoside effectively improved VD-mediated changes in behavior
compared with the VD group.
Asiaticoside shortens escape latency
in a rat model of VD
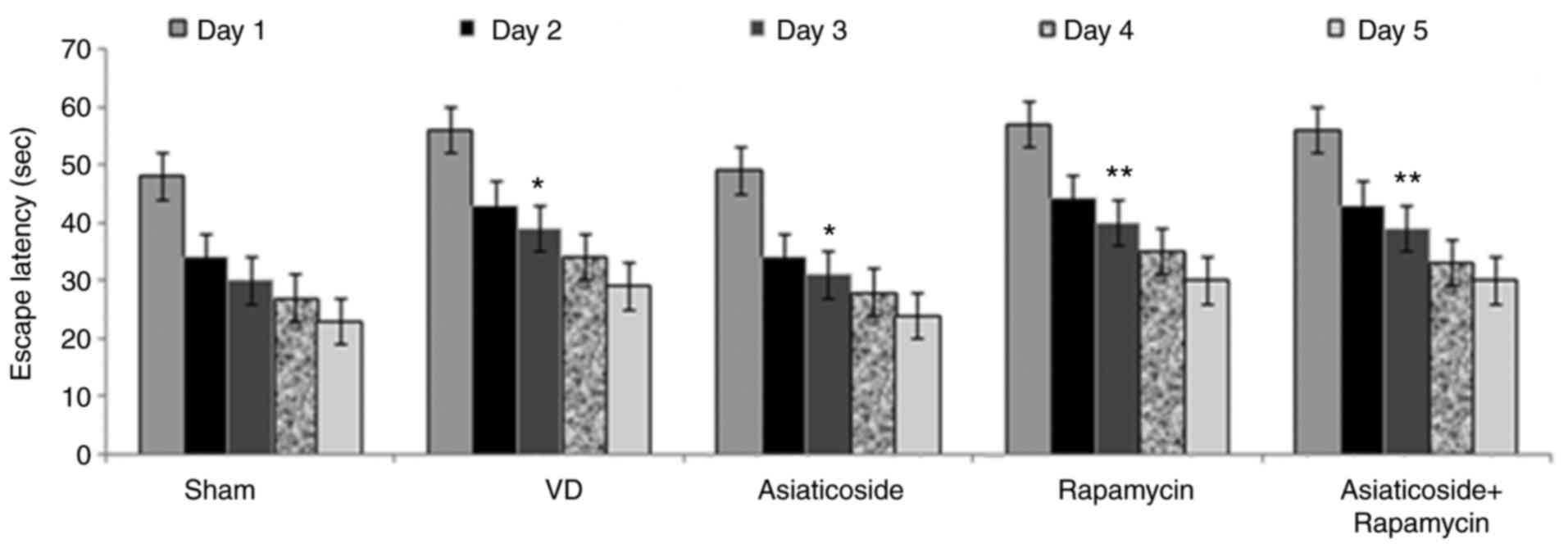
Escape latency exhibited a significant increase in
the VD model group compared with in the sham group (P<0.05;
Fig. 3). Treatment of VD rats with
asiaticoside caused a significant reduction in escape latency
(P<0.05). Rapamycin administration also increased escape
latency. The rapamycin-mediated increase in escape latency in rats
could not be significantly reduced by asiaticoside treatment.
Asiaticoside increases swimming time
in a rat model of VD
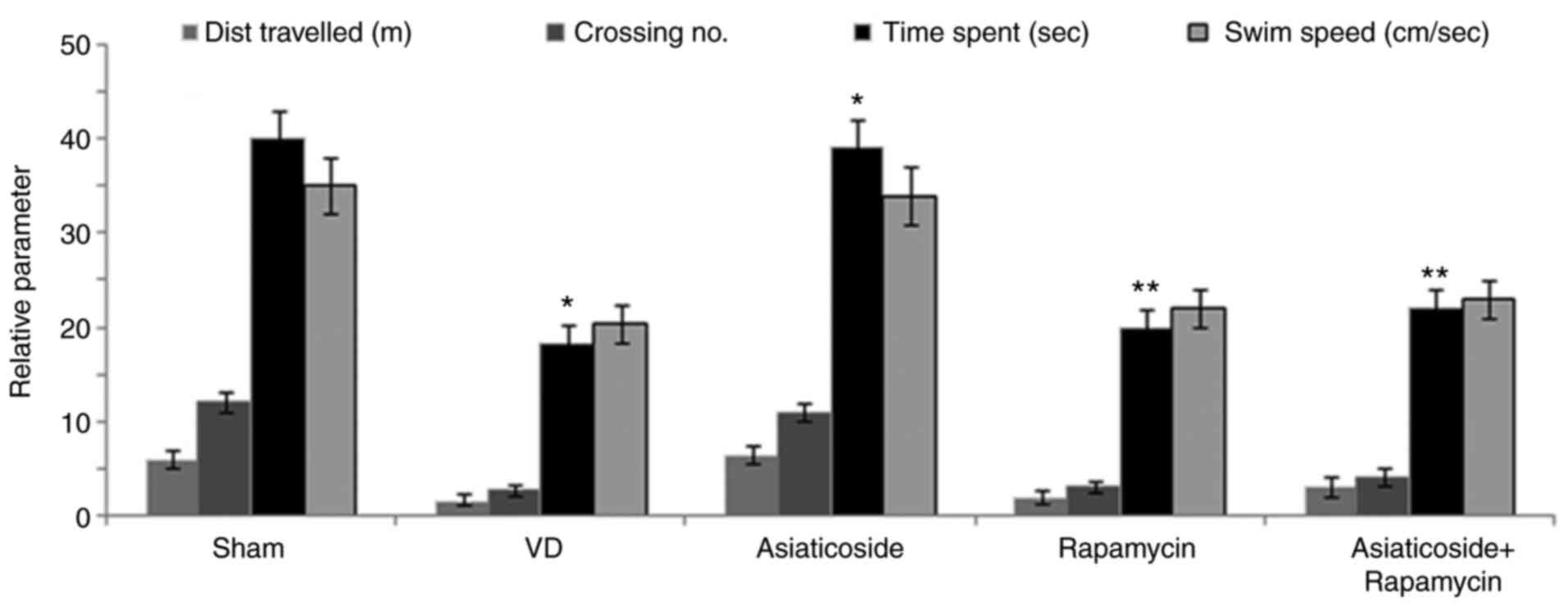
The distance travelled, swim speed, swim time and
number of platform crossings were significantly reduced for rats in
the VD group compared with in the sham group (P<0.05; Fig. 4). However, asiaticoside
significantly alleviated VD-mediated decreases in distance
travelled, swim time and number of platform crossings (P<0.05).
Administration of rapamycin also significantly reduced distance
travelled, swim time and number of platform crossings (P<0.05).
However, asiaticoside treatment could not significantly alleviate
the rapamycin-mediated reduction in distance travelled, swim time
and number of platform crossings.
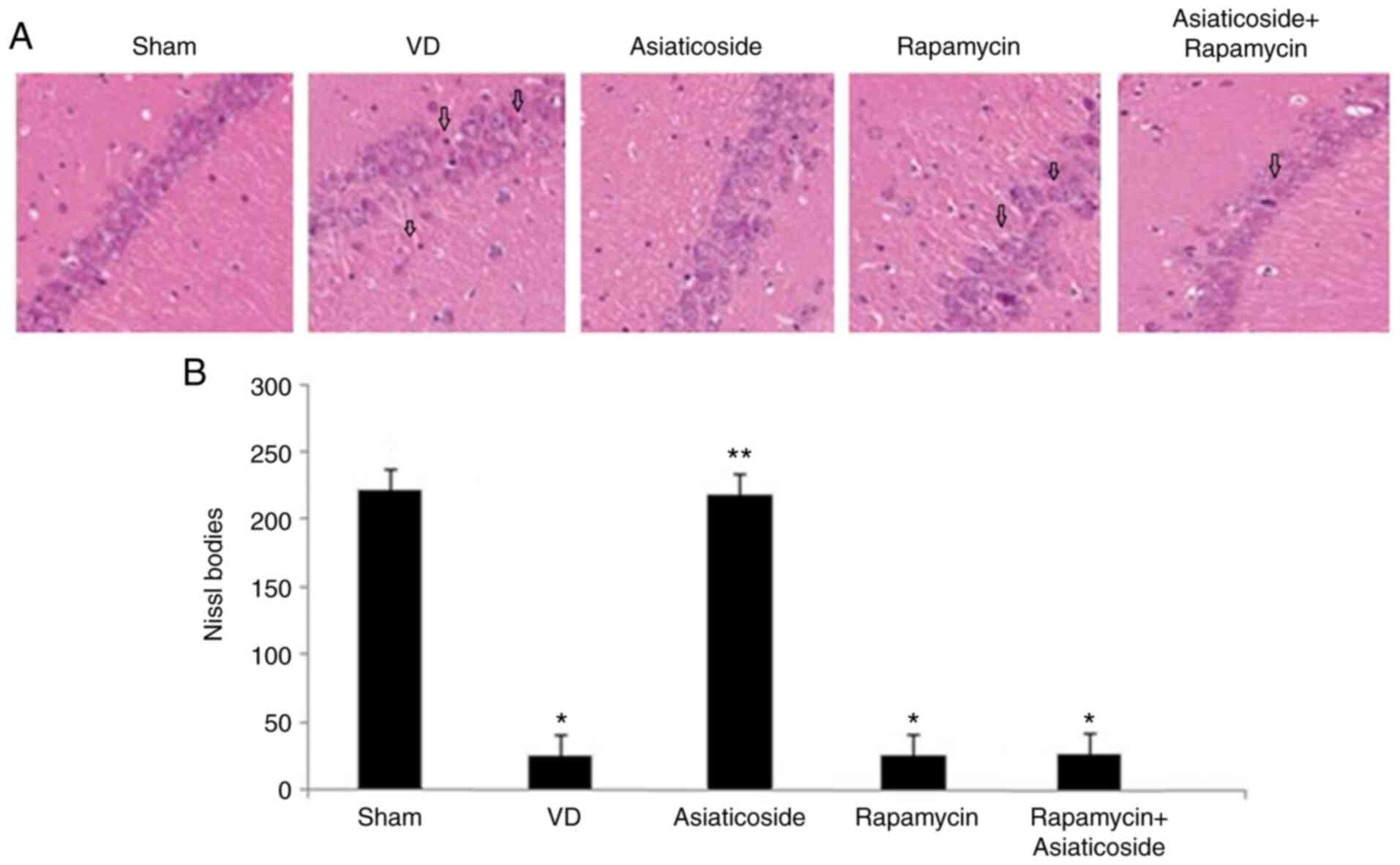
Asiaticoside promotes neuronal
survival in rats with VD
Abnormalities in the hippocampal tissues of rats
were detected using H&E staining (Fig. 5A) and analysis of Nissl bodies
(Fig. 5B). The abnormalities were
markedly evident in the hippocampus of VD rats compared with in the
sham group. Neuronal apoptosis and degeneration were clearly
detected in the hippocampus of VD rats. Conversely, VD-mediated
hippocampal tissue damage was significantly alleviated by
asiaticoside treatment of the rats (P<0.05). In addition,
abnormalities were also induced in the hippocampal tissues of rats
treated with rapamycin. However, no significant improvement in
rapamycin-induced hippocampal tissue damage was observed following
treatment with asiaticoside.
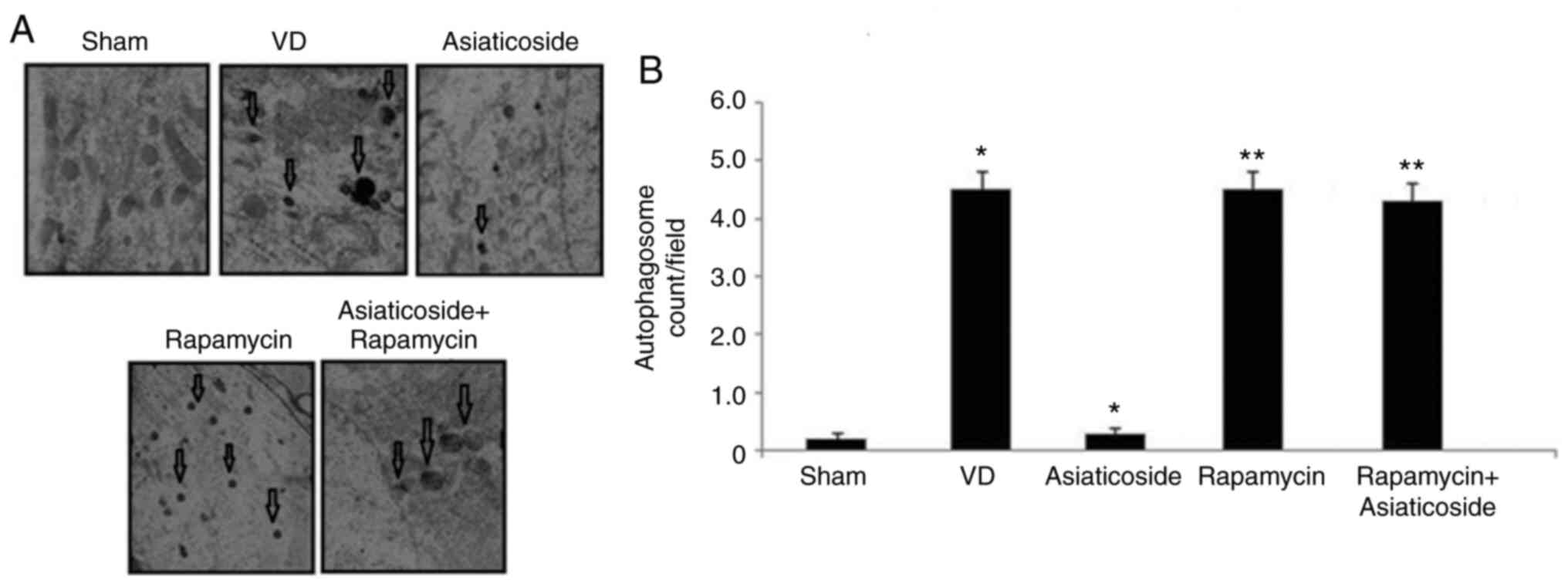
Asiaticoside inhibits autophagosome
formation in the hippocampus of rats with VD
Autophagosome formation was clearly detected near
the nuclei in the hippocampus of rats in the VD group, but was
absent in the sham group (Fig. 6A and
B). The primary autophagosome count was also increased in the
hippocampus of rats with VD. Treatment of rats in the VD group with
asiaticoside alleviated formation of autophagosomes and markedly
suppressed the number of primary lysosomes. In
rapamycin-administered rats, the numbers of autophagosomes were
markedly increased. No significant reduction in autophagosome
formation was observed in rapamycin-administered rats treated with
asiaticoside.
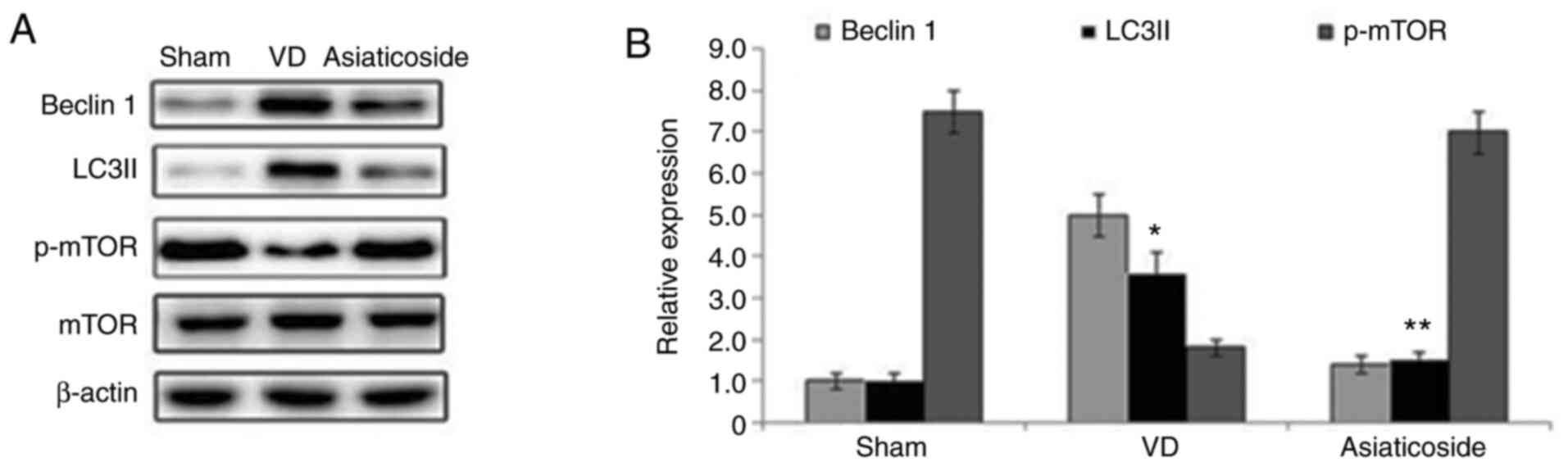
Asiaticoside upregulates p-mTOR
expression and suppresses autophagy-related proteins in a rat model
of VD
The expression levels of Beclin 1 and LC3II were
significantly elevated in the hippocampal tissues of rats in the VD
group compared with in the sham group (Fig. 7A and B). Treatment with asiaticoside
alleviated VD-mediated increases in Beclin 1 and LC3II expression
in the rat hippocampal tissues. The phosphorylation of mTOR in the
hippocampal tissues of rats in the VD group was markedly suppressed
compared with in the sham group. Conversely, asiaticoside treatment
prevented suppression of mTOR phosphorylation in the hippocampal
tissues of rats with VD.
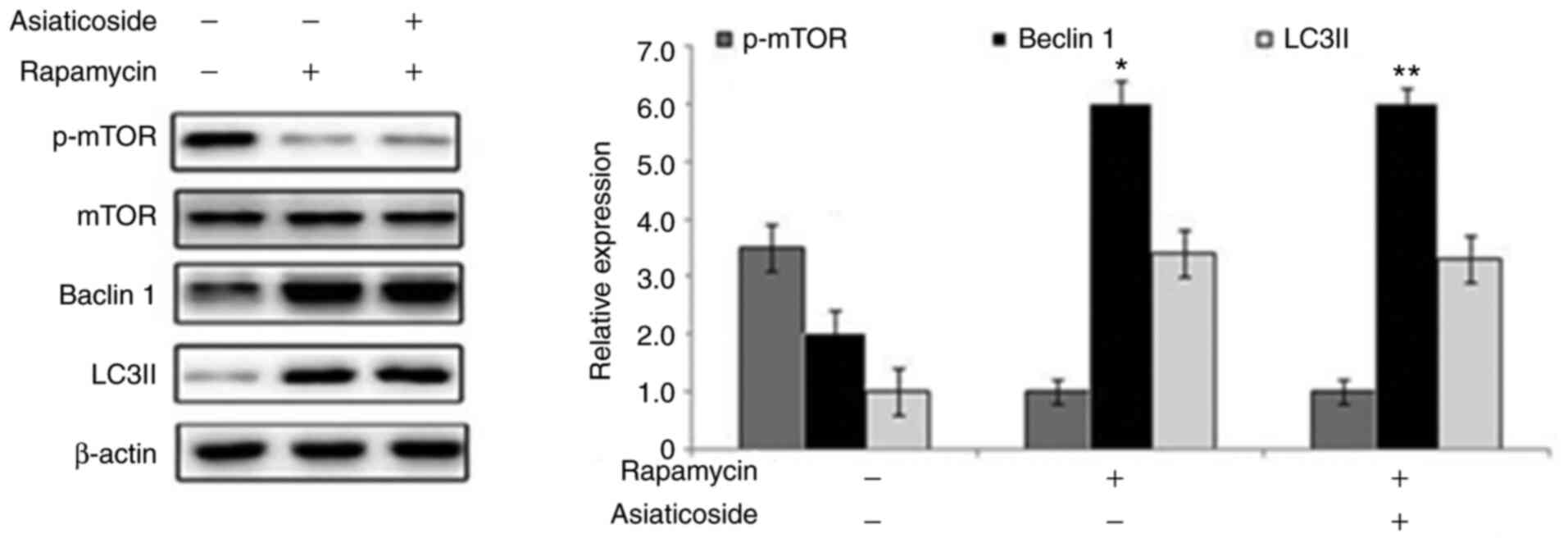
Asiaticoside inhibits autophagy in
rats with VD via the mTOR pathway
Rapamycin administration markedly suppressed
activation of mTOR in rat hippocampal tissues compared with in the
sham group (Fig. 8). Conversely,
the protein expression levels of Beclin 1 and LC3II were markedly
upregulated by rapamycin in the hippocampus of rats. The
rapamycin-mediated suppression of p-mTOR, and elevation of Beclin 1
and LC3II expression in the rat hippocampus could not be alleviated
by asiaticoside treatment.
Discussion
VD is a commonly diagnosed type of dementia, which
is generally caused by cerebrovascular diseases and is associated
with several other factors, including smoking, high blood pressure
and diabetes (19). The
pathological mechanism of VD is not clearly known; therefore,
studies are urgently required to develop effective treatments.
Autophagy is a cellular adaptive process, which is induced by
various stress factors in multi-cellular organisms and is
associated with dynamic catabolism (20). Excessive autophagy leads to cell
death and contributes significantly to ischemia-induced neuronal
damage (21,22). Previous studies reported that
neurological impairments caused by cerebral ischemia were modulated
by targeting autophagy induction (23,24).
Autophagy induction is regulated by mTOR kinase, and it has been
reported that autophagy may be suppressed by mTOR activation and
enhanced by inhibition of mTOR (25). Autophagy is specifically induced in
cells by administration of rapamycin (mTOR inhibitor), which
directly inhibits mTOR (26). In
addition, hippocampal neuronal damage caused by hypoxia-mediated
injury has been reported to be protected by downregulation of the
mTOR pathway (27). The present
study established a rat model of VD using the reported protocol,
and evaluated the effects of asiaticoside treatment on cognitive
memory improvement using MWM and T-maze tests (17). The data revealed that asiaticoside
effectively prevented VD-mediated cognitive memory impairment in
rats. However, asiaticoside was ineffective against cognitive
impairment induced by VD in rats administered with rapamycin
(autophagy agonist). In rats with VD, asiaticoside treatment
prevented neuronal damage, as evidenced by a marked increase in
Nissl-positive cell proportion compared with in the VD group.
Conversely, the neuronal damage in VD rats administered with
rapamycin could not be prevented by treatment with asiaticoside.
These findings suggested that asiaticoside prevented VD-induced
neuronal damage and cognitive impairment in rats, but could not
alleviate the effect of rapamycin. Thus, asiaticoside inhibits
induction of autophagy in rats to prevent VD-induced damage to the
neurons.
Autophagy is associated with the transport of
denatured intracellular proteins, senescent proteins and damaged
organelles to lysosomes, where degradation and digestion takes
place. Autophagy is a cellular defense mechanism against adverse
environmental conditions encountered during various pathological
processes. Previous studies have demonstrated that, in
cardiomyocytes, ischemia and hypoxia induced activation of
autophagy via promotion of mTOR and LC3II expression (28,29).
Cardiomyocytes are protected by an appropriate level of autophagy;
however, increased autophagy during ischemia and hypoxia may result
in injury to myocardial cells (30). Exposure of myocardial cells to
extreme levels of ischemia has been shown to lead to increased
autophagy and may promote apoptosis during reperfusion (31). In addition, it has been suggested
that ischemia or hypoxia may induce autophagy, which could
subsequently lead to neuronal death (32–35).
The present study revealed that VD induced formation of
autophagosomes and enhanced the number of lysosomes in cells. TEM
examination exhibited an absence of autophagosomes and
significantly reduced count of lysosomes in rats with VD treated
with asiaticoside. The inhibition of VD-mediated autophagy
activation by asiaticoside was also confirmed by western blotting.
In VD rats, the protein expression levels of LC3II and Beclin-1
were markedly higher compared with in the sham group. By contrast,
treatment of rats in the VD group with asiaticoside alleviated
VD-mediated upregulation of LC3II and Beclin-1 expression. These
data indicated that asiaticoside may prevent VD-mediated neuronal
damage via inhibition of autophagy. The effect of asiaticoside on
mTOR, which is a downstream executer of autophagy, was also
evaluated in rats with VD. The present study demonstrated that
asiaticoside treatment markedly promoted phosphorylation of mTOR in
the hippocampal tissues of VD rats; however, in rats with VD
administered with rapamycin, asiaticoside could not inhibit the
induction of autophagy.
In conclusion, asiaticoside may effectively prevent
cerebral ischemia-mediated cognitive impairment and neuronal damage
in rats. Moreover, autophagy was inhibited and the mTOR pathway was
activated in rats with VD treated with asiaticoside. Therefore,
asiaticoside may be studied further as therapeutic agent for the
treatment of dementia.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MG, JX and SW conducted the experimental work,
performed the literature survey and analyzed the data. BD designed
the study and wrote the manuscript. All authors read and approved
the final manuscript. MG and BD confirm the authenticity of all the
raw data.
Ethics approval and consent to
participate
The present study was approved by the Animal Ethics
Committee, Dongying District People's Hospital (Dongying, China;
approval. no. DSH/2017/067).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
O'Brien JT and Thomas A: Vascular
dementia. Lancet. 386:1698–1706. 2015. View Article : Google Scholar
|
|
2
|
Smith EE: Clinical presentations and
epidemiology of vascular dementia. Clin Sci (Lond). 131:1059–1068.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jayant S and Sharma B: Selective modulator
of cannabinoid receptor type 2 reduces memory impairment and
infarct size during cerebral hypoperfusion and vascular dementia.
Curr Neurovasc Res. 13:289–302. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Levine DA and Langa KM: Vascular cognitive
impairment: Disease mechanisms and therapeutic implications.
Neurotherapeutics. 8:361–373. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Voigt O and Pöggeler S: Self-eating to
grow and kill: Autophagy in filamentous ascomycetes. Appl Microbiol
Biotechnol. 97:9277–9290. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bharadwaj PR, Verdile G, Barr RK, Gupta V,
Steele JW, Lachenmayer ML, Yue Z, Ehrlich ME, Petsko G, Ju S, et
al: Latrepirdine (dimebon) enhances autophagy and reduces
intra-cellular GFP-Aβ42 levels in yeast. J Alzheimers Dis.
32:949–967. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ouyang L, Shi Z, Zhao S, Wang FT, Zhou TT,
Liu B and Bao JK: Programmed cell death pathways in cancer: A
review of apoptosis, autophagy and programmed necrosis. Cell
Prolif. 45:487–498. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lu J, Qian HY, Liu LJ, Zhou BC, Xiao Y,
Mao JN, An GY, Rui MZ, Wang T and Zhu CL: Mild hypothermia
alleviates excessive autophagy and mitophagy in a rat model of
asphyxial cardiac arrest. Neurol Sci. 35:1691–1699. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fujita S, Sakurai M, Baba H, Abe K and
Tominaga R: Autophagy-mediated stress response in motor neurons
after hypothermic spinal cord ischemia in rabbits. J Vasc Surg.
62:1312–1319. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hwang JY, Gertner M, Pontarelli F,
Court-Vazquez B, Bennett MV, Ofengeim D and Zukin RS: Global
ischemia induces lysosomal-mediated degradation of mTOR and
activation of autophagyin hippocampal neurons destined to die. Cell
Death Differ. 24:317–329. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chang H, Li X, Cai Q, Li C, Tian L, Chen
J, Xing X, Gan Y, Ouyang W and Yang Z: The PI3K/Akt/mTOR pathway is
involved in CVB3-induced autophagy of HeLa cells. Int J Mol Med.
40:182–192. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schaaf MB, Keulers TG, Vooijs MA and
Rouschop KM: LC3/GABARAP family proteins: Autophagy-(un)related
functions. FASEB J. 30:3961–3978. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dong MS, Jung SH, Kim HJ, Kim JR, Zhao LX,
Lee ES, Lee EJ, Yi JB, Lee N, Cho YB, et al: Structure-related
cytotoxicity and anti-hepatofibric effect of asiatic acid
derivatives in rat hepatic stellate cell-line, HSC-T6. Arch Pharm
Res. 27:512–517. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guo JS, Cheng CL and Koo MW: Inhibitory
effects of Centella asiatica water extract and asiaticoside
on inducible nitric oxide synthase during gastric ulcer healing in
rats. Planta Med. 70:1150–1154. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen S, Yin ZJ, Jiang C, Ma ZQ, Fu Q, Qu R
and Ma SP: Asiaticoside attenuates memory impairment induced by
transient cerebral ischemia-reperfusion in mice through
anti-inflammatory mechanism. Pharmacol Biochem Behav. 122:7–15.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yun KJ, Kim JY, Kim JB, Lee KW, Jeong SY,
Park HJ, Jung HJ, Cho YW, Yun K and Lee KT: Inhibition of
LPS-induced NO and PGE2 production by asiatic acid via NF-kappa B
inactivation in RAW 264.7 macrophages: Possible involvement of the
IKK and MAPK pathways. Int Immunopharmacol. 8:431–441. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xing M, Sun Q, Wang Y, Cheng Y and Zhang
N: Hydroxysafflor yellow A increases BDNF and NMDARs in the
hippocampus in A vascular dementia rat model. Brain Res.
1642:419–425. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Deacon RM and Rawlins JN: T-maze
alternation in the rodent. Nat Protoc. 1:7–12. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pulsinelli WA, Brierley JB and Plum F:
Temporal profile of neuronal damage in a model of transient
forebrain ischemia. Ann Neurol. 11:491–498. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Levine B, Mizushima N and Virgin HW:
Autophagy inimmunity and inflammation. Nature. 469:323–335. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhao Y, Huang G, Chen S, Gou Y, Dong Z and
Zhang X: Homocysteine aggravates cortical neural cell injury
through neuronal autophagy overactivation following rat cerebral
ischemia-reperfusion. Int J Mol Sci. 17:11962016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zou W, Song Y, Li Y, Du Y, Zhang X and Fu
J: The role of autophagy in the correlation between neuron damage
and cognitive impairmentin ratchronic cerebral hypoperfusion. Mol
Neurobiol. 55:776–791. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li L, Chen J, Sun S, Zhao J, Dong X and
Wang J: Effects of estradiol on autophagy and Nrf-2/ARE signals
after cerebral ischemia. Cell Physiol Biochem. 41:2027–2036. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim YC and Guan KL: mTOR: A pharmacologic
target for autophagy regulation. J Clin Invest. 125:25–32. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang D, Lin Q, Su S, Liu K, Wu Y and Hai
J: URB597 improves cognitive impairment induced by chronic cerebral
hypoperfusion by inhibiting mTOR-dependent autophagy. Neuroscience.
344:293–304. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Klionsky DJ, Abdalla FC, Abeliovich H,
Abraham RT, Acevedo-Arozena A, Adeli K, Agholme L, Agnello M,
Agostinis P, Aguirre-Ghiso JA, et al: Guidelines for the use and
interpretation of assays for monitoring autophagy in higher
eukaryotes. Autophagy. 8:445–544. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang J, Meng F, Cottrell JE, Sacktor TC
and Kass IS: Metabotropic actions of the volatile anaesthetic
sevoflurane increase protein kinase M synthesis and induce
immediate preconditioning protection of rat hippocampal slices. J
Physiol. 590:4093–4107. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dai S, Xu Q, Liu S, Yu B, Liu J and Tang
J: Role of autophagy and its signaling pathways in
ischemia/reperfusion injury. Am J Transl Res. 9:4470–4480.
2017.PubMed/NCBI
|
|
29
|
Zhao P, Zhang BL, Liu K, Qin B and Li ZH:
Overexpression of miR-638 attenuated the effects of
hypoxia/reoxygenation treatment on cell viability, cell apoptosis
and autophagy by targeting ATG5 in the human cardiomyocytes. Eur
Rev Med Pharmacol Sci. 22:8462–8471. 2018.PubMed/NCBI
|
|
30
|
Ravikumar B, Sarkar S, Davies JE, Futter
M, Garcia-Arencibia M, Green-Thompson ZW, Jimenez-Sanchez M,
Korolchuk VI, Lichtenberg M, Luo S, et al: Regulation of mammalian
autophagy in physiology and pathophysiology. Physiol Rev.
90:1383–1435. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang SS, Liu YB, Yu JB, Fan Y, Tang SY,
Duan WT, Wang Z, Gan RT and Yu B: Rapamycin protects heart from
ischemia/reperfusion injury independent of autophagy by activating
PI3 kinase-Akt pathway and mitochondria K(ATP) channel. Pharmazie.
65:760–765. 2010.PubMed/NCBI
|
|
32
|
Kiriyama Y and Nochi H: The function of
autophagy in neurode- generative diseases. Int J Mol Sci.
16:26797–26812. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Che H, Yan Y, Kang XH, Guo F, Yan ML, Liu
HL, Hou X, Liu T, Zong DK, Sun LL, et al: MicroRNA-27a promotes
inefficient lysosomal clearance in the hippocampi of rats following
chronic brain hypoperfusion. Mol Neurobiol. 54:2595–2610. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jia Y, Jin W, Xiao Y, Dong Y, Wang T, Fan
M, Xu J, Meng N, Li L and Lv P: Lipoxin A4 methyl ester alleviates
vascular cognition impairment by regulating the expression of
proteins related to autophagy and ER stress in the rat hippocampus.
Cell Mol Biol Lett. 20:475–487. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yang X, He C, Liu P, Song X, Thomas T,
Tshimanga S, Wang F, Niu J, Sun T and Li PA: Inhibition of mTOR
pathway by rapamycin reduces brain damage in rats subjected to
transient forebrain ischemia. Int J Biol Sci. 11:1424–1435. 2015.
View Article : Google Scholar : PubMed/NCBI
|