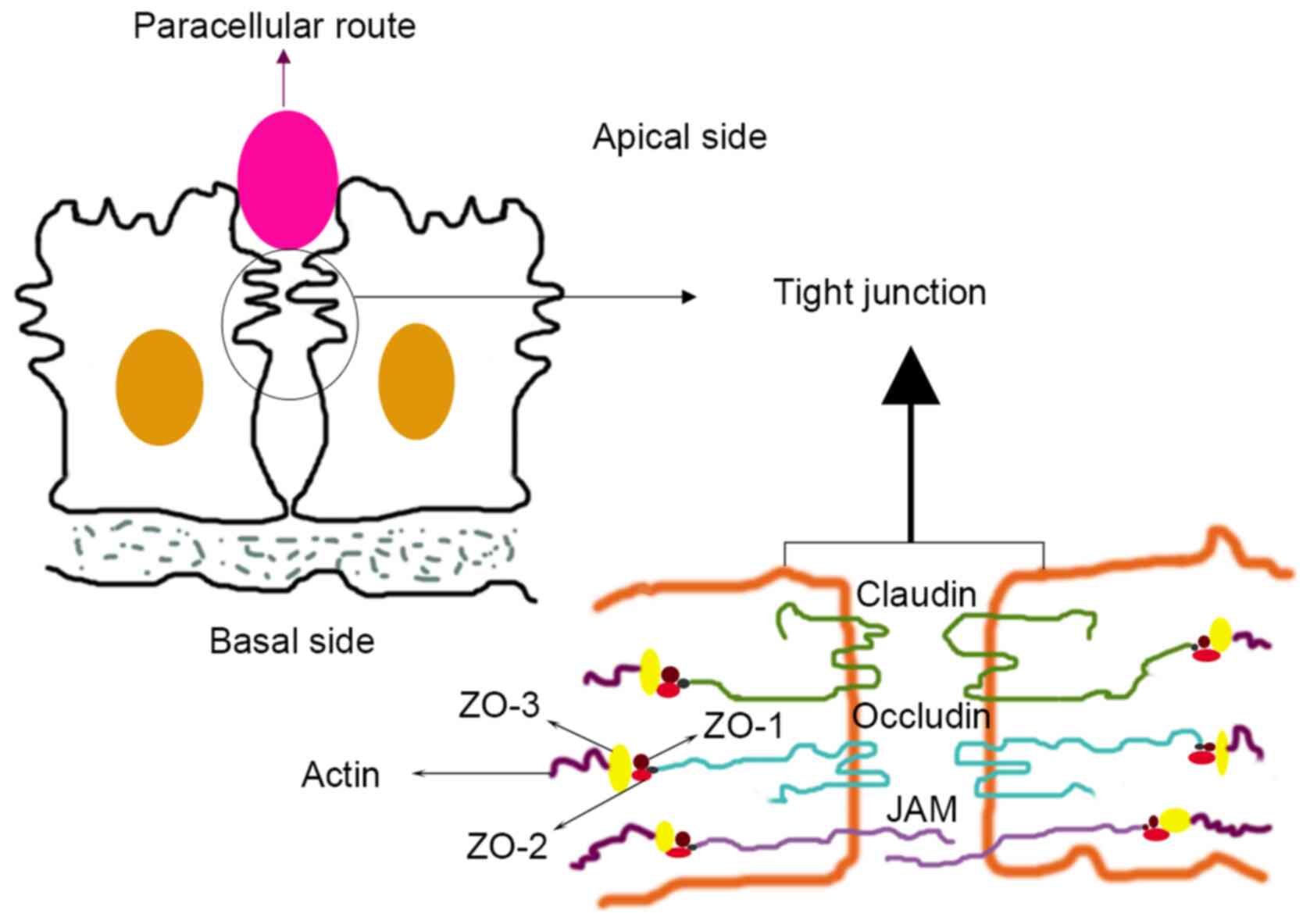
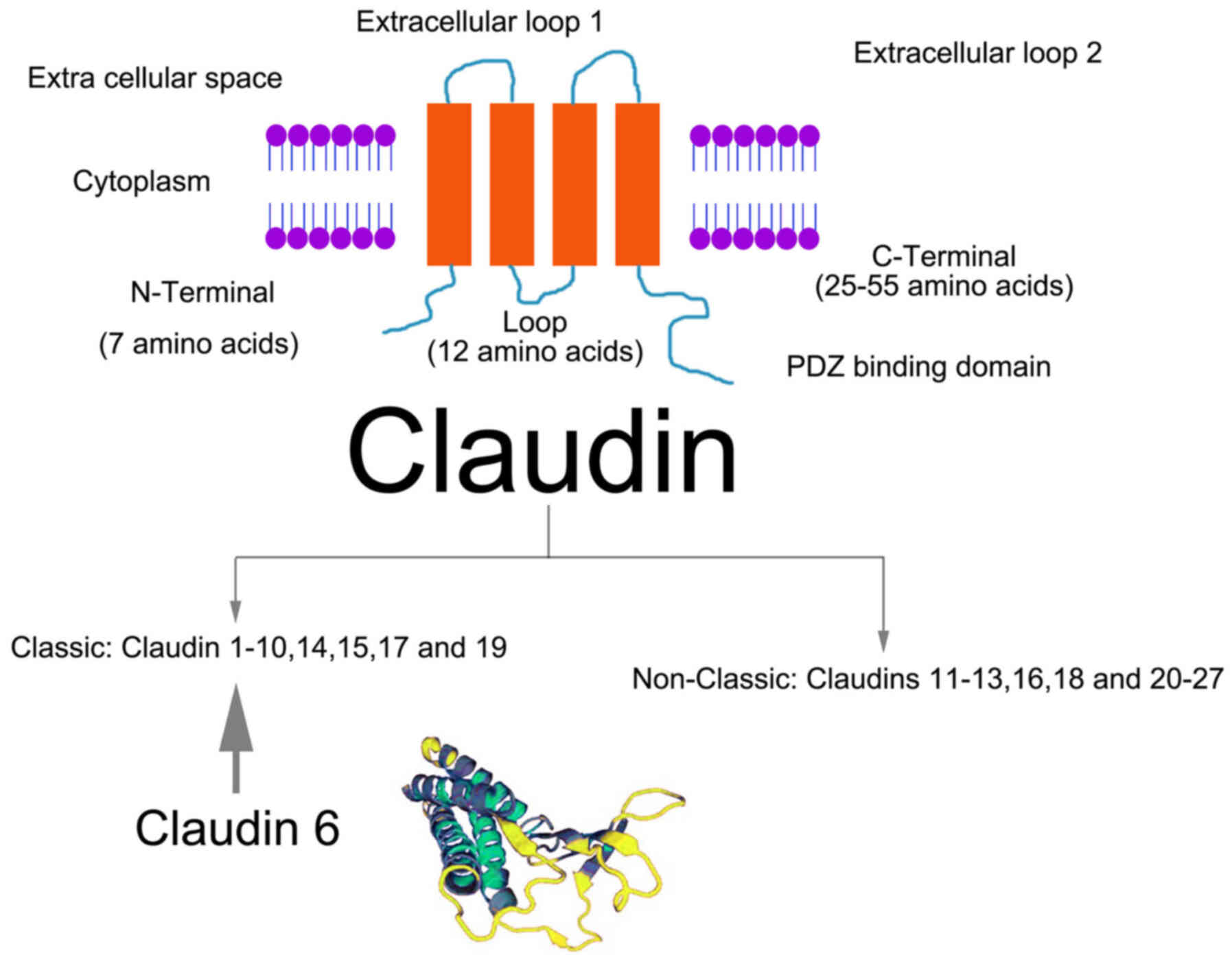
Introduction
The monolayer epithelium is the largest tissue
lining in a number of human organs, and its integrity is maintained
by the cell junction complex. Tight junctions (TJs) exist in the
junctional complexes of epithelial and endothelial cells, and serve
crucial roles in cell polarity, adhesion and permeability (1). TJs also maintain the integrity of the
tissue structure and participate in transmembrane movement and
signal transduction inside and outside of cells (2). A decrease in TJ integrity and an
increase in cell bypass permeability are characteristics of tumours
and inflamed tissues (3). TJs also
have adhesive properties and can prevent the shedding of epithelial
cells. The initial stage of tumour metastasis is a disconnect
between tumour and endothelial cells. Therefore, TJs are the first
obstacle that must be overcome in tumour metastasis (4). The decrease in TJ integrity leads to
the perfusion and extravasation of cancer cells through the
endothelial barrier (5). TJs are
composed of three basic membrane proteins: Occludin, claudin (CLDN)
and junction adhesion molecules. The CLDN protein family serves a
key role in the functions of TJs (6) (Fig.
1), including helping regulate defence and barrier functions,
and differentiation and polarity in epithelial and endothelial
cells (7). Tumour progression is
characterized by the migration, invasion and metastasis of cancer
cells. CLDNs are believed to serve a significant role in these
processes as their loss contributes to the loss of cell junctions
in a tissue-dependent manner. CLDN expression can affect cancer
progression in several ways: First, changes in CLDN expression
cause TJ disorder and leakage, which is conducive to tumour
metastasis and invasion; second, a decrease in cell polarity
increases the supply of nutrition and growth factors to the tumour,
and increases tumour cell expansion; and third, a decrease in
intercellular adhesion may increase the risk of metastasis and
promote tumour invasion (8). The
CLDN family forms homologous and heterologous interactions between
adjacent cells and is a key regulator of tumorigenesis and
metastasis. Classic CLDNs (CLDNs 1–10, 14, 15, 17, and 19) also
exhibit a much stronger sequence homology than the non-classic
CLDNs (CLDNs 11–13, 16, 18, and 20–27) (9) (Fig.
2). Recent findings have shown that abnormal expression of
CLDNs is closely related to the occurrence, progression and
prognosis of tumours (10).
Specifically, the CLDN family member CLDN6 is expressed in numerous
tumours but rarely found in adult normal tissues (11). Although some studies have reported
results that contradict this, the high expression of CLDN6 in
certain tumours makes CLDN6 a potential therapeutic target in these
tumour types. Therefore, the present study reviewed the literature
on CLDN6, including information on its structure, expression in
different tumours, regulatory mechanisms and therapeutic
prospects.
Structural characteristics of CLDN6
CLDN6 is one of 27 members of the CLDN family; it is
located on chromosome 16p13.3 and has a molecular weight of 23 kDa
(12). CLDN6 has four transmembrane
domains and a PDZ-binding region at the carboxyl end of the
cytoplasm. This CLDN can bind with signal proteins and cytoskeletal
proteins, and participate in the cellular response to external and
intracellular signal transmission (13). CLDN6 is the only CLDN to potentially
show specificity, as it can activate cell adhesion signals and
regulate the activity of nuclear receptors (14). CLDN6 is expressed in a variety of
embryonic epithelia, induces epithelial cell junction formation and
polarity, and participates in the differentiation of stem cells
into epithelial cells. Overall, CLDN6 is an important component of
the CLDN family and serves a substantial role in maintaining the
function of TJs (15).
Expression and regulation mechanisms of
CLDN6 in tumours
Previous findings have shown that changes in CLDN
expression cause TJ disorders and leakage (16,17).
This decrease in cell polarity increases the supply of nutrition
and growth factors for tumour cells. In addition, the decrease in
intercellular adhesion may increase the metastatic potential
(18). Previous findings have shown
that as a member of the CLDN family, CLDN6 is strongly expressed in
the foetal stomach, lung and kidney, but not in healthy adult
tissues (19,20). However, other studies have not
reached the same conclusion (21,22),
and the specific expression and regulatory mechanisms for CLDN6
remain unclear or controversial. Regardless, it is accepted that
CLDN6 is expressed in a variety of tumours and serves an important
role in the occurrence and development of tumours. The known
expression and regulatory mechanisms of CLDN6 in various tumour
types are shown in Table I.
 | Table I.Expression of claudin 6 in human
tumours. |
Table I.
Expression of claudin 6 in human
tumours.
|
| (Refs.) |
|---|
|
|
|
|---|
| Cancer type | Cell | Tissue |
|---|
| Breast invasive
carcinoma | (17,25–35,65,66) | (21–24) |
| Gastric cancer | (37,39,41,43–45) | (36,38–40,42) |
| Hepatocellular
carcinoma | (49) |
|
| Cervical
carcinoma | (50) | (51) |
| Non-small cell lung
cancer | (52) | (53) |
| Ovarian cancer |
| (54) |
| Endometrial
carcinoma | (55) |
|
| Atypical
teratoid/rhabdoid tumours |
| (56–58) |
| Oesophageal
squamous cell carcinoma |
| (59) |
Breast cancer
CLDN6 cannot be detected in human breast cancer cell
lines and tissues or is expressed at lower levels than in healthy
breast tissue (21,22). In addition, inhibiting CLDN6
expression leads to an increase in the resistance of tumour cells
to various types of apoptosis, enhancement of anchoring independent
growth characteristics and promotion of breast cancer progression.
Conversely, overexpression of CLDN6 restores the integrity of TJs,
inhibits the epithelial mesenchymal transition (EMT) of breast
cancer cells, inhibits the proliferation of breast cancer cells,
decreases the migration and invasion of tumour cells, and induces
the apoptosis of tumour cells (23). Knocking out CLDN6 can eliminate the
inhibition of EMT-related genes and promote cell migration and
invasion. In addition, the expression of CLDN6 is independently
associated with BRCA1-related breast cancer (24). Intratumoral overexpression compared
with the healthy surrounding tissue was significantly more frequent
in BRCA1 mutation carriers for CLDN6. However, compared with all
sporadic breast cancer cases, the incidence of breast cancer with
low CLDN expression (CLDN-low; E-cadherin and CLDN3, 4, and 7)
breast cancer was significantly higher among BRCA1 mutation
carriers (23). Finally, CLDN6 can
act as a tumour suppressor, and upregulating the expression of
CLDN6 may be helpful in preventing breast cancer (17).
There are numerous studies on the role of CLDN6 in
breast cancer (25), which involves
different pathways (Fig. 3).
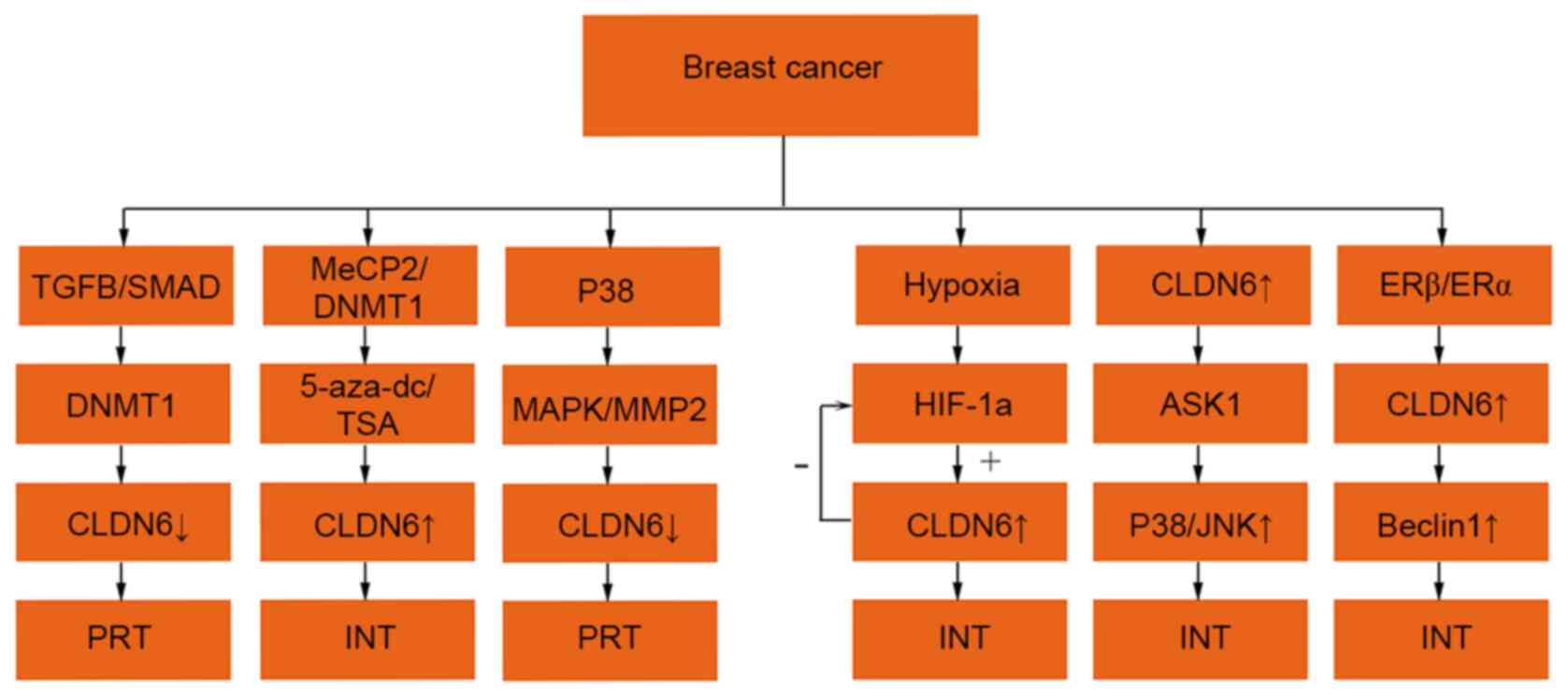 | Figure 3.Regulatory mechanisms of CLDN6
expression in breast cancer. The expression ↑ is increased, ↓
decreased, + is promoting effect and -is inhibiting effect. CLDN6,
claudin 6; TGF-β, transforming growth factor-β; MeCP2, methyl
cytosine binding protein-2; DNMT1,DNA methyltransferase 1;
5-aza-dc, 5-aza-2-deoxycytidine; AKT, protein kinase B; MAPK,
mitogen-activated protein kinase; ASK1, apoptosis signal-regulated
kinase 1; MMP2, matrix metalloproteinase 2; HIF-1α, hypoxia
inducible factor-1α; SMAD, small mothers against decapentaplegic;
mall mothers against decapentaplegic; JNK, c-Jun N-terminal kinase;
PRT, promote tumour progression; INT, inhibition of tumour
progression. |
TGF-β/Smad pathway
It has been suggested that silencing CLDN6 may be
related to DNA methylation mediated by DNA methyltransferase 1
(DNMT1), which is regulated by the TGF-β/Smad pathway, and thereby
has an effect on the invasion and metastasis of breast cancer cells
(26).
Methyl CpG binding protein 2
(MeCP2)/DNMT1/5-aza-2-deoxycytidine (5-aza-dC)/trichostatin A (TSA)
pathway
Li et al (27) hypothesized that DNA methylation of
CLDN6 promotes the migration and invasion of breast cancer cells
through the recruitment of MeCP2 and deacetylation. In one study, a
methyltransferase inhibitor (5-aza-dC) and a histone deacetylase
inhibitor, TSA, acted synergistically and induced the expression of
CLDN6 in breast cancer cells (28).
In addition, 5-aza-dC treatment can induce the demethylation of
CLDN6, inhibit the binding of MeCP2 and the CLDN6 promoter, ease
the chromatin structure of the CLDN6 gene and upregulate the
expression of CLDN6 in breast cancer cells, thereby inhibiting the
migration and invasion of tumour cells. CLDN6 silencing restores
the migration and invasiveness of tumour cells (28).
MAPK/MMP2 pathway
As reported by Ren et al (29), CLDN6 gene silencing may promote the
proliferation and migration of human breast epithelial cells
through the MAPK pathway and increase the activity of MMP2.
Moreover, the inhibition of CLDN6 expression may enhance the
resistance of tumour cells to apoptosis, leading to tumour
progression.
Hypoxia-inducible factor 1A (HIF-1α)
pathway
Due to the rapid growth of tumours and abnormalities
in the tumour vascular system, hypoxia is prevalent in breast
cancer. Therefore, HIF-1α is closely related to breast cancer
metastasis (22). Researchers
discovered a negative feedback loop between CLDN6 and HIF-1α, in
which HIF-1α increased and upregulated CLDN6 expression during
hypoxia. After overexpression of CLDN6, negative feedback inhibited
the expression of HIF-1α to decrease the metastasis of breast
cancer (29). The downregulation of
CLDN6 expression was positively correlated with lymph node
metastasis in breast cancer (30).
Apoptosis signal regulated kinase 1
(ASK1)-p38/c-Jun N-terminal kinase (JNK) pathway
In a previous study, it was found that CLDN6 gene
expression was related to the expression level of ASK1 (31), which induces apoptosis of breast
cancer cells by regulating the ASK1-p38/JNK signalling pathway.
ASK1 is a mitogen-activated protein kinase, which is involved in
the activation of the JNK and p38 pathways. The expression of CLDN6
in breast cancer cells decreases the phosphorylation of ASK1,
induces the activation of downstream target proteins JNK and p38
kinase, and leads to tumour cell death (32).
Οestrogen receptor β (Erβ)/Erα-Beclin1
pathway
Oestrogen has been found to regulate the expression
of CLDN6 at the transcriptional level through Erβ (33). Erβ can induce autophagy in tumour
cells through CLDN6 and inhibit the migration and invasion of
breast cancer cells. In addition, Beclin1 is a key regulator of
autophagy, and the expression of Erβ and CLDN6 in breast cancer has
been positively correlated with Beclin1 expression. The prognosis
of patients with breast cancer with high expression of Erβ, CLDN6
and Beclin1 was improved compared with that of patients with low
expression of these proteins (33).
Knocking out the Beclin1 gene can also reverse the autophagy
induced by CLDN6 and the inhibition of CLDN6 on breast cancer
metastasis (34). Wu et al
(35) also suggested that the
expression of CLDN6 in breast cancer cells is regulated by the ERα
pathway, and that upregulation of CLDN6 expression may be helpful
in preventing breast cancer.
Gastric cancer
One report indicated that the expression of CLDN6
differed between gastric cancer and adjacent tissues (36). Additional studies showed that CLDN6
expression differed in gastric cancer compared with healthy tissue
(37–41), and according to Zavala-Zendejas
et al (37), the expression
of CLDN6 in gastric cancer tissues was higher compared with that in
adjacent tissues. CLDN6 is a gene promoter in gastric cancer, and
its upregulation enhances the tumorigenicity of gastric cancer
cells (37). Several studies
reported that the upregulation of CLDN6 expression was positively
correlated with a decrease in overall survival rate in gastric
cancer, while CLDN6 silencing inhibited the proliferation and
invasion of tumour cells (39,42).
However, according to Gao et al (39), the expression level of CLDN6 in
gastric cancer tissues was lower compared with that in adjacent
tissues. Results of the study showed that the expression level of
CLDN6 was associated with age, lymph node metastasis, pathological
stage and distant metastasis. The expression of CLDN6 in patients
<50 years of age was higher compared with that in patients
>50 years of age. Furthermore, the survival rate of patients
with high expression of CLDN6 was significantly higher compared
with that of patients with low CLDN6 expression (40). Decreasing the expression of CLDN6
may destroy the integrity of epithelial cells, leading to
paracellular leakage, an increased nutrient supply and promotion of
metastasis (41) (Fig. 4).
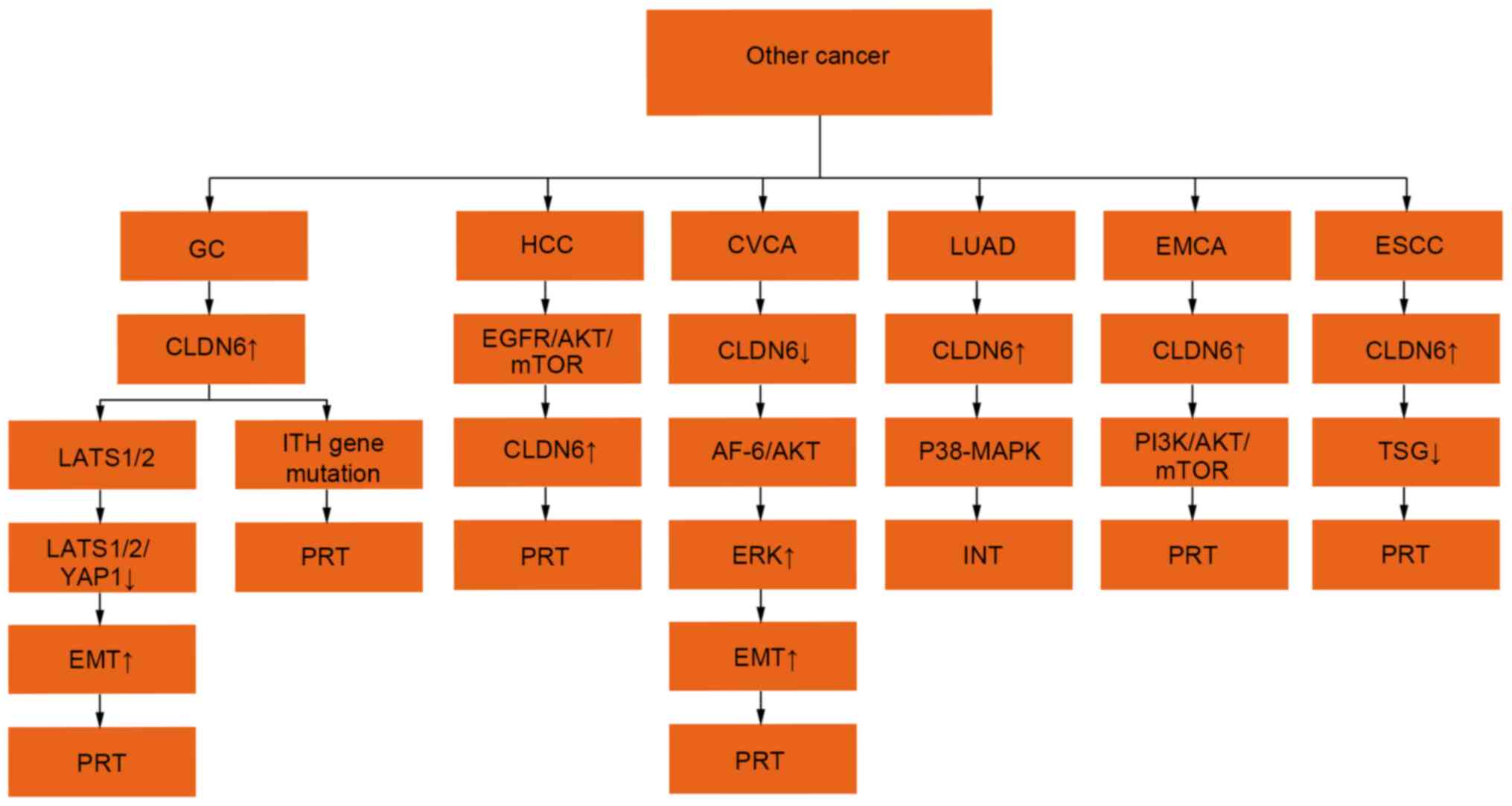 | Figure 4.Regulatory mechanisms of CLDN6
expression in other tumours. The expression ↑ is increased, ↓
decreased, + is promoting effect and -is inhibiting effect. CLDN6,
claudin 6; LATS1/2, large tumor suppressor 1 and 2; EMT, epithelial
mesenchymal transition; YAP1, Yes-associated protein1; PI3K,
phosphatidylinositol 3-kinase; MAPK, mitogen-activated protein
kinase; ERK, extracellular regulated protein kinases; AKT, protein
kinase B; EGFR, epidermal growth factor receptor; mTOR, mammalian
target of rapamycin; ITH, intratumoral heterogeneity; AF-6, Protein
AF-6; TSG, tumour suppressor gene; PRT, promote tumour progression,
INT, inhibition of tumour progression. |
An additional regulatory role for CLDN6 involves the
large tumour suppressor kinase (LATS)1/2/yes-associated protein 1
(YAP1) pathway and intratumoral heterogeneity (ITH) gene mutation.
CLDN6 may promote the proliferation and invasion of gastric cancer
by affecting the Yap1 and Yap1-snail axes (43). The interaction between CLDN6 and
LATS1/2 in the Hippo signalling pathway decreases the
phosphorylation of LATS1/2 and Yap1, affects Yap1 entry into the
nucleus, changes target gene modification, affects the EMT process
and enhances the invasion ability of gastric cancer cells (43). CLDN1 is a pre-MMP2 activator, while
CLDN6 promotes the migration and invasion of gastric cancer cells
by promoting the expression of CLDN6 and inducing the activation of
MMP2 (44). Previous findings have
shown that CLDN6 may change TJ function in gastric cancer through
gene mutations and in this way may promote the occurrence of
cancer. The CLDN6 gene contains not only a frameshift mutation, but
also an ITH mutation (44).
Previous findings have shown that lipopolysaccharides from
Helicobacter pylori can increase the expression of CLDN-4,
−6, −7 and −9 in gastric cancer cells by inducing Toll-like
receptor TLR2 expression (45).
Overall, the expression of CLDN6 in gastric cancer is associated
with a poor prognosis and a short survival period (45).
Hepatocellular carcinoma (HCC)
HCC is one of the most common malignant tumours of
the digestive system, with high incidence and mortality rates. In
particular, China has a high incidence of hepatitis B and liver
cancer (46). Therefore, it is
important to identify potential therapeutic targets for HCC. CLDN6
is highly expressed in HCC and is also expressed in the liver
during hepatitis C virus (HCV) replication. CLDN6 is the receptor
for HCV and is therefore necessary for the entry of HCV into cells
(47,48). In addition, CLDN6 can promote EMT
and enhance the proliferation and invasion of hepatoma cells.
Moreover, the upregulation of CLDN6 expression may act as an
oncogene in HCC through the EGFR/Akt/mTOR signalling pathway, and
may promote the proliferation, metastasis and invasion of HepG2
cells. By contrast, CLDN6 silencing inhibits the proliferation and
metastasis of hepatoma cells (49).
Cervical carcinoma
CLDN6 expression was downregulated in cervical
cancer cells (50,51), which is inconsistent with the
conclusion of Reinhard et al (11). Zhang et al (50) hypothesised that the occurrence of
cervical cancer may be related to the decreased expression of CLDN6
and that increased expression of CLDN6 may inhibit the
proliferation of tumour cells (51). CLDN6 may also promote the apoptosis
of cervical cancer cells by inhibiting the activity of the AKT
signalling pathway. Similarly, it may reverse EMT and inhibit the
invasion of cervical cancer cells by binding to AF-6, thereby
inhibiting the ERK signalling pathway (51). In this way, CLDN6 may act as a
tumour suppressor gene in cervical cancer, and loss of CLDN6 may
enhance the carcinogenicity of cervical cancer cells (50).
Non-small cell lung cancer
(NSCLC)
CLDN6 protein levels showed significant positivity
in NSCLC cells, but were negative in normal lung cells and
bronchial epithelial cells (52).
In this study, the expression of CLDN6 was related to lymph node
metastasis and Tumour-Node-Metastasis stage. However, in the study
by Wang et al (53), the
expression of CLDN6 in adjacent tissues was higher compared with
that in NSCLC tissues. The survival rate of patients with low CLDN6
expression was significantly lower compared with that of patients
with high CLDN6 expression. In addition, low CLDN6 expression may
be associated with poor prognosis in patients with NSCLC (53).
Ovarian cancer
CLDN6 was highly expressed in ovarian cancer tissues
and showed low expression in normal ovarian epithelium. The high
expression of CLDN6 in ovarian cancer cells suggests promotion of
ovarian cancer cell proliferation. In addition, high CLDN6
expression enhanced cell migration and invasion abilities, and
inhibition of the apoptosis of ovarian cancer cells. CLDN6 may
serve an active role in the invasion and metastasis of ovarian
cancer (54).
Endometrial cancer
The expression of CLDN6 was upregulated in
endometrial carcinoma. CLDN6 gene knockout may inhibit the
proliferation and migration of endometrial carcinoma HEC-1-B cells
through the PI3K/Akt/mTOR signalling pathway. The expression of
CLDN6 may be related to the overall survival rate of patients with
endometrial carcinoma (55).
Atypical teratoid/rhabdoid tumours
(AT/RTs)
AT/RTs are highly aggressive brain tumours in
children (56). CLDN6 was
moderately or highly expressed in AT/RTs (57), but the expression level of CLDN6 was
not associated with survival rate or clinical behaviour (58).
Oesophageal squamous cell
carcinoma
The methylation frequency of CLDN6 in oesophageal
carcinoma was higher compared with that in non-invasive tissues.
Methylation of CLDN6 can result in the silencing of tumour
suppressor genes and the development of oesophageal cancer
(59). According to Tsunoda et
al (59), CLDN6 may silence
tumour suppressor genes through high-frequency methylation, thus
promoting the occurrence and development of oesophageal cancer.
CLDN6 and drug resistance in cancer
Previous findings have shown that cancer stem cells
(CSCs) are responsible for cancer treatment failure and drug
resistance, and that CSCs serve an important role in tumour
recurrence, metastasis and drug resistance. Therefore,
understanding the factors that regulate CSCs has become very
important (60,61). In mouse stem cells, CLDN6 may
trigger epithelial morphogenesis, while the expression of CLDN6
constitutes an early marker of embryonic stem cells (62,63).
Therefore, it is possible that CLDN6 may affect the biological
functions of CSCs.
Chemotherapy is one of the primary treatments for
breast cancer, and chemotherapy resistance is the main obstacle in
breast cancer treatment (64). In a
previous study (65), the
expression of CLDN6 was significantly higher in the MCF-7/MDR cell
line than in the MCF-7 cell line. The high expression of CLDN6 in
MCF-7/MDR cells increased the resistance to Adriamycin (ADM),
5-fluorouracil and cisplatin, while the silencing of CLDN6 enhanced
the killing effect of those drugs on the MCF-7/MDR cell line. It is
also possible that CLDN6 is involved in the regulation of drug
resistance in human breast cancer cells. The mechanism may be
through CLDN6 regulating glutathione S-transferase π1 (GSTP1) to
promote the chemotherapeutic resistance of MCF-7 cells through p53,
as GSTP1 expression and enzyme activity is regulated by p53. In
cells that overexpress MCF-7, the expression of GSTP1 was
upregulated (65). Furthermore,
CLDN6 interacts with p53 to inhibit the transfer of p53 from the
cell nucleus to the cytoplasm, leading to drug resistance. CLDN6
can also enhance the drug resistance of breast cancer cells to ADM
by activating the AF-6/ERK signalling pathway and upregulating the
characteristics of breast cancer cells (66). These findings may provide a new
target and strategy for cancer treatment.
Prospect of CLDN6 in the treatment of
tumours
In the study by Reinhard et al (11), CLDN6 was specifically expressed in a
variety of tumours but not in healthy tissues. Although a number of
research conclusions contradict this (39,50,51),
CLDN6 still has the potential to act as a tumour gene target in
cancer types, such as liver, ovarian and endometrial cancer, and to
provide new strategies for tumour treatment. The treatment
modalities for this include antibody drug-binding targets,
radionuclide therapeutic targets and new antibody therapeutic
targets, among others.
A new patent for a monoclonal antibody against CLDN6
has been shown to be effective against testicular germ cell tumours
(TGCTs). The use of CLDN6 antibody can block tumour growth and
prolong the life of patients with TGCT. The presence of
tumour-associated antigens can induce strong and persistent
antigen-specific T-cell responses, break the immune system
tolerance to endogenous self-antigens and produce effective
antitumour effects (67).
Antibody drug conjugates (ADCs) are monoclonal
antibodies that bind to cytotoxic drugs (68). The antibodies used by ADCs have high
specificity for tumour cell surface proteins and can specifically
kill tumour cells. ADCs can transport drugs to tumour sites through
targeted drug delivery and decrease the toxicity of cytotoxic drugs
to normal tissues (69,70). As CLDN6 is a specific antigen found
in some tumours, it may be a potential target for ADC drugs.
Targeted radionuclide therapy (TRT) uses a molecular
carrier with a high affinity for tumour cell surface antigens to
transport nuclides to tumour cells and sites of metastasis for
treatment. Drugs containing radionuclides are targeted to
accumulate in tumour tissues, which directly affects cancer cells
and avoids damaging normal cells (71). TRT combines the specificity of
molecular targeting and the cytotoxicity of ionising radiation to
reduce toxicity to healthy tissues and provides a more effective
tumour treatment method (72). This
may be an important direction for tumour treatment in the future.
As a specific antigen in some tumours, CLDN6 may be an effective
tumour-targeting vector.
Recent findings have shown that chimeric antigen
receptor (CAR)-T cells can be used to treat tumours expressing
chimeric antigen receptors after the gene transformation of B-cell
malignant tumours (11). However,
due to the limitations of tumour-specific targets, CAR-T cell
therapy is ineffective in patients with solid tumours (11). CLDN6 is a surface antigen of
carcinoembryonic cells and has an ideal expression profile for
CAR-T cells. Although some research conclusions are inconsistent on
this topic, study results show that CLDN6 LPX can cause CLDN6-CAR-T
proliferation and survival effectively in vivo, allowing
CAR-T cells to treat solid tumours (73). This provides a new strategy for the
treatment of solid tumours using CAR-T cells.
In addition, it has been found that CLDN6-mediated
measles virus (MV) can induce tumour-associated antigen-specific
humoral immunity. Mice inoculated with the CLDN6 vaccine showed
targeted complement-dependent cytotoxicity with cytolytic activity.
MV combined with a tumour lytic effect and the CLDN6 tumour vaccine
was able to break the tolerance to endogenous tumour antigens and
produce a highly effective and specific antitumour immune response
(74). These studies suggest that
CLDN6 may provide a new strategy for tumour vaccine treatments of
solid tumours.
Discussion
Reinhard et al (11) believed that CLDN6 is a strictly
carcinoembryonic antigen that is expressed exclusively in tumour
tissues. However, the present study reviewed the expression of
CLDN6 in various tumours and found that the research conclusions
for several tumour types were not completely consistent or were
contradictory. Possible reasons for inconsistent conclusions
include heterogeneity in tumours, use of monoclonal antibodies,
different experimental methods and the interpretation of the
results. In addition, the role of CLDN6 differs by tumour type, as
it promotes some tumours, inhibits others and shows inconsistent
(promotional and inhibitory) effects in some tumours.
However, in liver, ovarian, endometrial and
oesophageal cancer types, the expression of CLDN6 is consistently
expressed in tumour tissues, compared with no or less expression in
the surrounding normal tissues. Therefore, CLDN6 has potential as a
carcinoembryonic antigen and a therapeutic target for these
tumours.
The reason for CLDN6 expression being upregulated in
some tumours and downregulated in others remains unclear. Possible
reasons include detection errors or CLDN6 having different roles in
different tumours. This area requires further research. Moreover,
currently, there are no studies of whether a relationship exists
between these tumours; this needs to be further explored. The
expression of other CLDNs and their synergistic effects with CLDN6
have been reported. In breast cancer, overexpression of CLDN3, −4
and −7 was primarily dependent on oestrogen receptor-status,
whereas overexpression of CLDN6 and high membranous expression of
CLDN1 were independent of other characteristics (24). Another study showed that CLDN5,
CLDN9, CLDN12 and CLDN13 were not expressed in breast carcinoma
tissues or non-neoplastic tissues (22). In addition, CLDN1, CLDN3, CLDN8 and
CLDN10 were expressed in breast carcinoma and non-neoplastic
tissues, but there was no significant difference between the
expression of all these CLDN proteins. The expression of CLDN2,
CLDN6 and CLDN14 was downregulated, while the expression of CLDN11
was upregulated in breast carcinoma compared with those in
non-neoplastic tissues. In addition, a distinct prognostic
significance in the expression of CLDN3 and mostly of CLDN4 between
triple-negative and luminal breast carcinomas was identified
(75). In gastric cancer, the
expression of CLDN2 and CLDN6 was downregulated in gastric cancer
tissue, while the expression of CLDN11 was upregulated (36). CLDN6 induces MMP2 activation through
CLDN1 membrane expression, which in turn promotes cell migration
and invasiveness (44). CLDN6
expression was high in both intestinal- and diffuse-type gastric
adenocarcinomas. CLDN7 was expressed mainly in the diffuse-type,
whereas CLDN9 was primarily found in the apical membrane of the
gland cells in the intestinal-type (40). In addition, CLDN7, −3 and −4 have
been detected in gastric cancer (76–78).
CLDN6 has more than one regulatory mechanism in a
variety of tumours, such as breast and gastric cancer. These
mechanisms may work synergistically. However, the primary
mechanism, as well as the effect(s) of different regulatory
mechanisms, remain to be determined and require further study.
Although there are still a number of controversies
regarding the expression and regulation mechanisms of CLDN6, CLDN6
is a potential antigen and has the potential to be used as a target
for a variety of tumours and treatments.
Conclusion
CLDN6 is expressed in a variety of tumours, and its
expression levels differ by tumour type. In some tumours, research
consistently shows that CLDN6 is expressed in tumour tissues, but
is not expressed or expressed at low levels in surrounding tissues.
These include liver, ovarian, endometrial and oesophageal cancer,
and AT/RTs, among others. In these tumours, CLDN6 has potential as
a carcinoembryonic antigen and a therapeutic target. However, in
other tumours, the expression levels of CLDN6 between tumour and
surrounding tissues differ from one another or are even the
opposite. These include breast, gastric, cervical and lung cancer.
In the future, CLDN6 may be used as a target for a variety of
therapeutic types such as antibody-drug radionuclide-targeted drugs
and ADCs.
Acknowledgements
Not applicable.
Funding
This study was supported by the Mianyang Science and
Technology Bureau (grant no. 15-S01-3).
Availability of data and materials
Not applicable.
Authors' contributions
XD contributed to the conception and design of this
study. HD, XY and JF searched for relevant literature. HD wrote the
manuscript. XD provided advice and was responsible for revising the
manuscript. All authors read and approved the final version of the
manuscript. Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CLDN6
|
claudin 6
|
|
TJs
|
tight junctions
|
References
|
1
|
Li J, Ananthapanyasut W and Yu AS:
Claudins in renal physiology and disease. Pediatr Nephrol.
26:2133–2142. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Singh AB, Uppada SB and Dhawan P: Claudin
proteins, outside-in signaling, and carcinogenesis. Pflugers Arch.
469:69–75. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hu CA, Hou Y, Yi D, Qiu Y, Wu G, Kong X
and Yin Y: Autophagy and tight junction proteins in the intestine
and intestinal diseases. Anim Nutr. 1:123–127. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
English DP and Santin AD: Claudins
overexpression in ovarian cancer: Potential targets for clostridium
perfringens enterotoxin (CPE) based diagnosis and therapy. Int J
Mol Sci. 14:10412–10437. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tabariès S and Siegel PM: The role of
claudins in cancer metastasis. Oncogene. 36:1176–1190. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ushiku T, Shinozaki-Ushiku A, Maeda D,
Morita S and Fukayama M: Distinct expression pattern of claudin-6,
a primitive phenotypic tight junction molecule, in germ cell
tumours and visceral carcinomas. Histopathology. 61:1043–1056.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gowrikumar S, Singh AB and Dhawan P: Role
of claudin proteins in regulating cancer stem cells and
chemoresistance-potential implication in disease prognosis and
therapy. Int J Mol Sci. 21:532019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kwon MJ: Emerging roles of claudins in
human cancer. Int J Mol Sci. 14:18148–18180. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Singh AB and Dhawan P: Claudins and
cancer: Fall of the soldiers entrusted to protect the gate and keep
the barrier intact. Semin Cell Dev Biol. 42:58–65. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen S, Liu X and Luo W: Advances in the
application of claudins to tumor therapy. Sheng Wu Gong Cheng Xue
Bao. 35:931–941. 2019.(In Chinese). PubMed/NCBI
|
|
11
|
Reinhard K, Rengstl B, Oehm P, Michel K,
Billmeier A, Hayduk N, Klein O, Kuna K, Ouchan Y, Wöll S, et al: An
RNA vaccine drives expansion and efficacy of claudin-CAR-T cells
against solid tumors. Science. 367:446–453. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Singh AB, Sharma A and Dhawan P: Claudin
family of proteins and cancer: An overview. J Oncol.
2010:5419572010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lin D, Guo Y, Li Y, Ruan Y, Zhang M, Jin
X, Yang M, Lu Y, Song P, Zhao S, et al: Bioinformatic analysis
reveals potential properties of human claudin-6 regulation and
functions. Oncol Rep. 38:875–885. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Anderson WJ, Zhou Q, Alcalde V, Kaneko OF,
Blank LJ, Sherwood RI, Guseh JS, Rajagopal J and Melton DA: Genetic
targeting of the endoderm with claudin-6CreER. Dev Dyn.
237:504–512. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sugimoto K, Ichikawa-Tomikawa N, Kashiwagi
K, Endo C, Tanaka S, Sawada N, Watabe T, Higashi T and Chiba H:
Cell adhesion signals regulate the nuclear receptor activity. Proc
Natl Acad Sci USA. 116:24600–24609. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hoevel T, Macek R, Swisshelm K and Kubbies
M: Reexpression of the TJ protein CLDN1 induces apoptosis in breast
tumor spheroids. Int J Cancer. 108:374–383. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yafang L, Qiong W, Yue R, Xiaoming X, Lina
Y, Mingzi Z, Ting Z, Yulin L and Chengshi Q: Role of estrogen
receptor-α in the regulation of claudin-6 expression in breast
cancer cells. J Breast Cancer. 14:20–27. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mullin JM: Potential interplay between
luminal growth factors and increased tight junction permeability in
epithelial carcinogenesis. J Exp Zool. 279:484–489. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Stadler CR, Bähr-Mahmud H, Plum LM,
Schmoldt K, Kölsch AC, Türeci Ö and Sahin U: Characterization of
the first-in-class T-cell-engaging bispecific single-chain antibody
for targeted immunotherapy of solid tumors expressing the oncofetal
protein claudin 6. Oncoimmunology. 5:e10915552015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ben-David U, Nudel N and Benvenisty N:
Immunologic and chemical targeting of the tight-junction protein
claudin-6 eliminates tumorigenic human pluripotent stem cells. Nat
Commun. 4:19922013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu Q, Liu Y, Ren Y, Xu X, Yu L, Li Y and
Quan C: Tight junction protein, claudin-6, downregulates the
malignant phenotype of breast carcinoma. Eur J Cancer Prev.
19:186–194. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jia H, Chai X, Li S, Wu D and Fan Z:
Identification of claudin-2, −6, −11 and −14 as prognostic markers
in human breast carcinoma. Int J Clin Exp Pathol. 12:2195–2204.
2019.PubMed/NCBI
|
|
23
|
Wu Q, Liu YF, Ren Y, Xu XM, Yu LN, Li YL
and Quan CS: Effects of stable up-regulation of tight junction
protein claudin-6 upon biological phenotypes of breast cancer cell
MCF-7. Zhonghua Yi Xue Za Zhi. 90:407–412. 2010.(In Chinese).
PubMed/NCBI
|
|
24
|
Heerma van Voss MR, van Diest PJ, Smolders
YH, Bart J, van der Wall E and van der Groep P: Distinct claudin
expression characterizes BRCA1-related breast cancer.
Histopathology. 65:814–827. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xu X, Jin H, Liu Y, Liu L, Wu Q, Guo Y, Yu
L, Liu Z, Zhang T, Zhang X, et al: The expression patterns and
correlations of claudin-6, methy-CpG binding protein 2, DNA
methyltransferase 1, histone deacetylase 1, acetyl-histone H3 and
acetyl-histone H4 and their clinicopathological significance in
breast invasive ductal carcinomas. Diagn Pathol. 7:332012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lu Y, Wang L, Li H, Li Y, Ruan Y, Lin D,
Yang M, Jin X, Guo Y, Zhang X and Quan C: SMAD2 inactivation
inhibits CLDN6 methylation to suppress migration and invasion of
breast cancer cells. Int J Mol Sci. 18:18632017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li Q, Zhu F and Chen P: miR-7 and miR-218
epigenetically control tumor suppressor genes RASSF1A and claudin-6
by targeting HoxB3 in breast cancer. Biochem Biophys Res Commun.
424:28–33. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu Y, Jin X, Li Y, Ruan Y, Lu Y, Yang M,
Lin D, Song P, Guo Y, Zhao S, et al: DNA methylation of claudin-6
promotes breast cancer cell migration and invasion by recruiting
MeCP2 and deacetylating H3Ac and H4Ac. J Exp Clin Cancer Res.
35:1202016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ren Y, Wu Q, Liu Y, Xu X and Quan C: Gene
silencing of claudin-6 enhances cell proliferation and migration
accompanied with increased MMP-2 activity via p38 MAPK signaling
pathway in human breast epithelium cell line HBL 100. Mol Med Rep.
8:1505–1510. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jia Y, Guo Y, Jin Q, Qu H, Qi D, Song P,
Zhang X, Wang X, Xu W, Dong Y, et al: A SUMOylation-dependent
HIF-1α/CLDN6 negative feedback mitigates hypoxia-induced breast
cancer metastasis. J Exp Clin Cancer Res. 39:422020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Guo Y, Xu X, Liu Z, Zhang T, Zhang X, Wang
L, Wang M, Liu Y, Lu Y, Liu Y and Quan C: Apoptosis
signal-regulating kinase 1 is associated with the effect of
claudin-6 in breast cancer. Diagn Pathol. 7:1112012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Guo Y, Lin D, Zhang M, Zhang X, Li Y, Yang
R, Lu Y, Jin X, Yang M, Wang M, et al: CLDN6-induced apoptosis via
regulating ASK1-p38/JNK signaling in breast cancer MCF-7 cells. Int
J Oncol. 48:2435–2444. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Song P, Li Y, Dong Y, Liang Y, Qu H, Qi D,
Lu Y, Jin X, Guo Y, Jia Y, et al: Estrogen receptor β inhibits
breast cancer cells migration and invasion through CLDN6-mediated
autophagy. J Exp Clin Cancer Res. 38:3542019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Figueiredo NB, Cestari SH, Conde SJ,
Luvizotto RA, De Sibio MT, Perone D, Katayama ML, Carraro DM,
Brentani HP, Brentani MM and Nogueira CR: Estrogen-responsive genes
overlap with triiodothyronine-responsive genes in a breast
carcinoma cell line. ScientificWorldJournal. 2014:9694042014.
View Article : Google Scholar : PubMed/NCBIPubMed/NCBIPubMed/NCBI
|
|
35
|
Wu Q, Liu X, Liu YF, Lu Y, Wang LP, Zhang
XW, Li YL and Quan CS: Inhibition of p38 activity reverses
claudin-6 induced cell apoptosis, invasion, and migration. Chin Med
J (Engl). 126:3539–3544. 2013.PubMed/NCBI
|
|
36
|
Lin Z, Zhang X, Liu Z, Liu Q, Wang L, Lu
Y, Liu Y, Wang M, Yang M, Jin X and Quan C: The distinct expression
patterns of claudin-2, −6, and −11 between human gastric neoplasms
and adjacent non-neoplastic tissues. Diagn Pathol. 8:1332013.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zavala-Zendejas VE, Torres-Martinez AC,
Salas-Morales B, Fortoul TI, Montaño LF and Rendon-Huerta EP:
Claudin-6, 7, or 9 overexpression in the human gastric
adenocarcinoma cell line AGS increases its invasiveness, migration,
and proliferation rate. Cancer Invest. 29:1–11. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kohmoto T, Masuda K, Shoda K, Takahashi R,
Ujiro S, Tange S, Ichikawa D, Otsuji E and Imoto I: Claudin-6 is a
single prognostic marker and functions as a tumor-promoting gene in
a subgroup of intestinal type gastric cancer. Gastric Cancer.
23:403–417. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gao F, Li M, Xiang R, Zhou X, Zhu L and
Zhai Y: Expression of CLDN6 in tissues of gastric cancer patients:
Association with clinical pathology and prognosis. Oncol Lett.
17:4621–4625. 2019.PubMed/NCBI
|
|
40
|
Rendón-Huerta E, Teresa F, Teresa GM,
Xochitl GS, Georgina AF, Veronica ZZ and Montaño LF: Distribution
and expression pattern of claudins 6, 7, and 9 in diffuse- and
intestinal-type gastric adenocarcinomas. J Gastrointest Cancer.
41:52–59. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lu YZ, Li Y, Zhang T and Han ST: Claudin-6
is down-regulated in gastric cancer and its potential pathway.
Cancer Biomark. 28:329–340. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Resnick MB, Gavilanez M, Newton E, Konkin
T, Bhattacharya B, Britt DE, Sabo E and Moss SF: Claudin expression
in gastric adenocarcinomas: A tissue microarray study with
prognostic correlation. Hum Pathol. 36:886–892. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yu S, Zhang Y, Li Q, Zhang Z, Zhao G and
Xu J: CLDN6 promotes tumor progression through the YAP1-snail1 axis
in gastric cancer. Cell Death Dis. 10:9492019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Torres-Martínez AC, Gallardo-Vera JF,
Lara-Holguin AN, Montaño LF and Rendón-Huerta EP: Claudin-6
enhances cell invasiveness through claudin-1 in AGS human
adenocarcinoma gastric cancer cells. Exp Cell Res. 350:226–235.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chavarría-Velázquez CO, Torres-Martínez
AC, Montaño LF and Rendón-Huerta EP: TLR2 activation induced by
H. pylori LPS promotes the differential expression of
claudin-4, −6, −7 and −9 via either STAT3 and ERK1/2 in AGS cells.
Immunobiology. 223:38–48. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhu ZX, Huang JW, Liao MH and Zeng Y:
Treatment strategy for hepatocellular carcinoma in China:
Radiofrequency ablation versus liver resection. Jpn J Clin Oncol.
46:1075–1080. 2016.PubMed/NCBI
|
|
47
|
Zheng A, Yuan F, Li Y, Zhu F, Hou P, Li J,
Song X, Ding M and Deng H: Claudin-6 and claudin-9 function as
additional coreceptors for hepatitis C virus. J Virol.
81:12465–12471. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Osanai M, Takasawa A, Murata M and Sawada
N: Claudins in cancer: Bench to bedside. Pflugers Arch. 469:55–67.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Huang L, Zhao C, Sun K, Yang D, Yan L, Luo
D, He J, Hu X, Wang R, Shen X, et al: Downregulation of CLDN6
inhibits cell proliferation, migration, and invasion via regulating
EGFR/AKT/mTOR signalling pathway in hepatocellular carcinoma. Cell
Biochem Funct. 38:541–548. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhang X, Ruan Y, Li Y, Lin D and Quan C:
Tight junction protein claudin-6 inhibits growth and induces the
apoptosis of cervical carcinoma cells in vitro and in vivo. Med
Oncol. 32:1482015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhang X, Ruan Y, Li Y, Lin D, Liu Z and
Quan C: Expression of apoptosis signal-regulating kinase 1 is
associated with tight junction protein claudin-6 in cervical
carcinoma. Int J Clin Exp Pathol. 8:5535–5541. 2015.PubMed/NCBI
|
|
52
|
Micke P, Mattsson JS, Edlund K, Lohr M,
Jirström K, Berglund A, Botling J, Rahnenfuehrer J, Marincevic M,
Pontén F, et al: Aberrantly activated claudin 6 and 18.2 as
potential therapy targets in non-small-cell lung cancer. Int J
Cancer. 135:2206–2214. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wang Q, Zhang Y, Zhang T, Han ZG and Shan
L: Low claudin-6 expression correlates with poor prognosis in
patients with non-small cell lung cancer. Onco Targets Ther.
8:1971–1977. 2015.PubMed/NCBI
|
|
54
|
Wang L, Jin X, Lin D, Liu Z, Zhang X, Lu
Y, Liu Y, Wang M, Yang M, Li J and Quan C: Clinicopathologic
significance of claudin-6, occludin, and matrix
metalloproteinases-2 expression in ovarian carcinoma. Diagn Pathol.
8:1902013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Cao X and He GZ: Knockdown of CLDN6
inhibits cell proliferation and migration via PI3K/AKT/mTOR
signaling pathway in endometrial carcinoma cell line HEC-1-B. Onco
Targets Ther. 11:6351–6360. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Birks DK, Kleinschmidt-DeMasters BK,
Donson AM, Barton VN, McNatt SA, Foreman NK and Handler MH: Claudin
6 is a positive marker for atypical teratoid/rhabdoid tumors. Brain
Pathol. 20:140–150. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Sullivan LM, Yankovich T, Le P, Martinez
D, Santi M, Biegel JA, Pawel BR and Judkins AR: Claudin-6 is a
nonspecific marker for malignant rhabdoid and other pediatric
tumors. Am J Surg Pathol. 36:73–80. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Antonelli M, Hasselblatt M, Haberler C, Di
Giannatale A, Garrè ML, Donofrio V, Lauriola L, Ridola V, Arcella
A, Frühwald M and Giangaspero F: Claudin-6 is of limited
sensitivity and specificity for the diagnosis of atypical
teratoid/rhabdoid tumors. Brain Pathol. 21:558–563. 2011.PubMed/NCBI
|
|
59
|
Tsunoda S, Smith E, De Young NJ, Wang X,
Tian ZQ, Liu JF, Jamieson GG and Drew PA: Methylation of CLDN6,
FBN2, RBP1, RBP4, TFPI2, and TMEFF2 in esophageal squamous cell
carcinoma. Oncol Rep. 21:1067–1073. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Phi LTH, Sari IN, Yang YG, Lee SH, Jun N,
Kim KS, Lee YK and Kwon HY: Cancer stem cells (CSCs) in drug
resistance and their therapeutic implications in cancer treatment.
Stem Cells Int. 2018:54169232018. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Abdullah LN and Chow EK: Mechanisms of
chemoresistance in cancer stem cells. Clin Transl Med. 2:32013.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Wang L, Xue Y, Shen Y, Li W, Cheng Y, Yan
X, Shi W, Wang J, Gong Z, Yang G, et al: Claudin 6: A novel surface
marker for characterizing mouse pluripotent stem cells. Cell Res.
22:1082–1085. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Turksen K and Troy TC: Claudin-6: A novel
tight junction molecule is developmentally regulated in mouse
embryonic epithelium. Dev Dyn. 222:292–300. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Gonzalez-Angulo AM, Morales-Vasquez F and
Hortobagyi GN: Overview of resistance to systemic therapy in
patients with breast cancer. Adv Exp Med Biol. 608:1–22. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Yang M, Li Y, Shen X, Ruan Y, Lu Y, Jin X,
Song P, Guo Y, Zhang X, Qu H, et al: CLDN6 promotes chemoresistance
through GSTP1 in human breast cancer. J Exp Clin Cancer Res.
36:1572017. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Yang M, Li Y, Ruan Y, Lu Y, Lin D, Xie Y,
Dong B, Dang Q and Quan C: CLDN6 enhances chemoresistance to ADM
via AF-6/ERKs pathway in TNBC cell line MDAMB231. Mol Cell Biochem.
443:169–180. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Chieffi P, De Martino M and Esposito F:
New anti-cancer strategies in testicular germ cell tumors. Recent
Pat Anticancer Drug Discov. 14:53–59. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Thomas A, Teicher BA and Hassan R:
Antibody-drug conjugates for cancer therapy. Lancet Oncol.
17:e254–e262. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Birrer MJ, Moore KN, Betella I and Bates
RC: Antibody-drug conjugate-based therapeutics: State of the
science. J Natl Cancer Inst. 111:538–549. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Tsuchikama K and An Z: Antibody-drug
conjugates: Recent advances in conjugation and linker chemistries.
Protein Cell. 9:33–46. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Gudkov SV, Shilyagina NY, Vodeneev VA and
Zvyagin AV: Targeted radionuclide therapy of human tumors. Int J
Mol Sci. 17:332015. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Gill MR, Falzone N, Du Y and Vallis KA:
Targeted radionuclide therapy in combined-modality regimens. Lancet
Oncol. 18:e414–e423. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Schneider IC, Hartmann J, Braun G, Stitz
J, Klamp T, Bihi M, Sahin U and Buchholz CJ: Displaying
tetra-membrane spanning claudins on enveloped virus-like particles
for cancer immunotherapy. Biotechnol J. 13:e17003452018. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Hutzler S, Erbar S, Jabulowsky RA, Hanauer
JRH, Schnotz JH, Beissert T, Bodmer BS, Eberle R, Boller K, Klamp
T, et al: Antigen-specific oncolytic MV-based tumor vaccines
through presentation of selected tumor-associated antigens on
infected cells or virus-like particles. Sci Rep. 7:168922017.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Kolokytha P, Yiannou P, Keramopoulos D,
Kolokythas A, Nonni A, Patsouris E and Pavlakis K: Claudin-3 and
claudin-4: Distinct prognostic significance in triple-negative and
luminal breast cancer. Appl Immunohistochem Mol Morphol.
22:125–131. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Wu Z, Shi J, Song Y, Zhao J, Sun J, Chen
X, Gao P and Wang Z: Claudin-7 (CLDN7) is overexpressed in gastric
cancer and promotes gastric cancer cell proliferation, invasion and
maintains mesenchymal state. Neoplasma. 65:349–359. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Danilova NV, Anikina KA, Oleynikova NA,
Vychuzhanin DV and Malkov PG: Claudin-3 expression in gastric
cancer. Arkh Patol. 82:5–11. 2020.(In Russian). View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Liu JX, Wei ZY, Chen JS, Lu HC, Hao L and
Li WJ: Prognostic and clinical significance of claudin-4 in gastric
cancer: A meta-analysis. World J Surg Oncol. 13:2072015. View Article : Google Scholar : PubMed/NCBI
|