Parkinson's disease (PD) is the second most common
age-related neurodegenerative disorder after Alzheimer's disease
(AD). With the increase in the proportion of the aging population,
the incidence of PD is increasing (1). The prevalence of PD is 0.3% in the
general population, and as high as 1% in the elderly over the age
of 60 years, and 3% in individuals >80 years in industrialized
countries (2). A major
characteristic of PD is the accumulation of the misfolded
α-synuclein (α-syn) protein in cerebral nerve cells, eventually
leading to the loss of dopaminergic (DA) neurons in the substantia
nigra pars compacta (SNpc) or the death of nerve tissue (3). This results in large areas of dead
brain tissue, and the promotion of the formation of eosinophilic
inclusion bodies, such as Lewis bodies (LBs) and Lewy neuritis
(LN), in the cytoplasm (4). With
the death of neurons in the brain, the clinical manifestations of
PD comprise a static tremor, muscle rigidity, bradykinesia,
abnormal posture gait and a series of non-motor symptoms, such as
olfactory disorders, anxiety and depression, cognitive decline,
sleep disorders, autonomic dysfunction, fatigue and pain (5,6).
Several studies have demonstrated that mitochondrial dysfunction
and oxidative stress (OS) serve a key role in the pathogenesis of
PD, as they cause a loss of DA neurons (7–10).
Several genes and signaling pathways are involved in the initiation
and development of PD. For example, the familial autosomal
recessive genes PTEN induced kinase 1 (PINK1)/parkin and the
autosomal dominant mutations of Leucine-rich repeat kinase 2
(LRRK2) regulate mitochondrial dysfunction and PD pathogenesis
(11,12). Furthermore, it has been reported
that a mutated LRRK2 gene can induce PD pathogenesis, which is
dependent on the PINK1/parkin pathway via independent mechanisms
(13). In addition, several genes,
such as GTP cyclohydrolase 1 (GCH1) (14), coiled-coil-helix-coiled-coil-helix
domain containing 2 (CHCHD2/PARK22) (15) and VPS35 retromer complex component
(VPS35/PARK17) (16) are involved
in the induction of mitochondrial dysfunction of patients with PD.
Environmental factors are also a pathogenic factor of PD. For
example, the occupational and environmental exposure to pesticides
and cytokine pathways (17), the
influence of genetic polymorphisms on pesticides (18) and the dysregulation of the microRNA
(miRNA/miR) network caused by pesticide exposure (19), all serve an important role in the
pathophysiology of PD via neurodegeneration of SNpc DA neurons,
mitochondrial dysfunction or oxidative damage (20). Some of the related genes, such as
miRNAs, may serve as novel non-invasive early biomarkers for the
prediction and prognosis of PD (21). It has also been demonstrated that
exposure to polystyrene microplastics can induce intestinal injury
and neurodegeneration through increased production of reactive
oxygen species (ROS) in Caenorhabditis elegans (C.
elegans) (22). Recent reports
have reported that coronavirus 2019 (COVID-19) colonizes in the gut
and the central nervous system (CNS), where it triggers
neuroinflammation and neurodegenerative processes, suggesting that
patients infected with COVID-19 may be susceptible to certain
neurodegenerative disorders, such as PD (23,24).
However, the molecular mechanism underlying the development and
progression of PD requires further investigation.
Gastrointestinal dysfunction, an important non-motor
symptom of PD, not only has a high incidence, but also appears
several years prior to the characteristic motor symptoms (25). Currently, the influence of
intestinal microbiota on PD has been studied extensively by
scientists, and has been termed the microbiota-gut-brain axis
(MGBA) (26). Numerous studies have
demonstrated that abnormal intestinal microbiotas are not only
closely associated with gastrointestinal dysfunction in patients
with PD but may also be an important mechanism underlying the
pathological process of PD (27,28).
Recently, it has been reported that dysfunction of gastrointestinal
microbiomes (GM) occurs earlier than/or at the same time as PD, and
the pathology of PD is closely associated with the changes of the
GM (29). Nielsen et al
(30) demonstrated that individuals
infected with Helicobacter pylori are more likely to induce
the development of PD. Devos et al (31) reported that most patients with PD
have colitis, which can enhance the peripheral and brain
inflammatory response, and promote the pathogenesis of PD.
α-syn consists of 140 amino acids and the gene
encoding it, synuclein α (SNCA), is comprised of five exons and is
located at chromosome 4q21.3-q22 (32). It is widely expressed in the CNS,
mainly in the presynaptic terminals, and is involved in the
regulation of neurotransmission and synaptic homeostasis (33). The α-syn family of proteins contains
three members, synapsin-I, synapsin-II and synapsin-III (34). According to the C-terminal splicing
structure, α-syn can be divided into α and β subtypes (34). Currently, five synapsin proteins
(synapsin-Iα, synapsin-Iβ, synapsin-IIα, synapsin-IIβ and
synapsin-IIIα) have been detected (35). A pathological characteristic of PD
is the accumulation of the misfolded α-syn protein involved in slow
and progressive degeneration of DA neurons in the SNpc (36). α-syn exhibits characteristics of
prion-like protein during PD pathogenesis; the misfolded α-syn is
an ‘infectious’ protein spreading pathology into the CNS via the
vagus nerve (VN) by forming a template that seeds misfolding for
nearby α-syn proteins, turning the endogenous physiological protein
into a pathogenic protein (37).
Previous studies have demonstrated that neurons can absorb α-syn
in vitro and in vivo, and α-syn can also transmit
between two neurons to neighboring neurons via endocytosis
(38,39). However, further studies are required
to determine the specific mechanisms. In addition, studies have
reported that an abnormal GM can enhance the levels of inflammation
via the induction of immune responses, leading to the misfolding of
α-syn in patients with PD (40,41).
The dysfunction of the enteric nervous system (ENS), and the
accumulation of anti-α-syn immune response proteins were detected
in the ENS ganglia in mice with α-syn mutations (either A53T or
A30P from insertions of an entire human SNCA gene) when they were 3
months old (42). Braak et
al (43) demonstrated that PD
is initiated by unknown pathogens that traverse the gastric
epithelial lining and lead to the formation of misfolded α-syn in
nerve cells of the submucosal plexus. The pathological formation of
α-syn is retrogradely propagated along the axonal and transneuronal
axis along with the VN to reach the CNS (37). Braak et al (43) also reported that the accumulation of
misfolded α-syn in the peripheral nervous system (PNS) occurred
earlier than that in the CNS of patients with PD. Several studies
have speculated that the initiation of PD originates from the PNS,
and is retrogradely transported towards the CNS via the PNS
(44,45). These results suggest that the
abnormal GM can affect the development of PD by inducing α-syn
misfolding, abnormal aggregation and transmission from VN to
CNS.
Several studies have reported that GM serves an
important role in the development of PD by regulating misfolded and
abnormal aggregation of α-syn and the MGBA (46,47).
The present review focuses on the association between GM, α-syn and
MGBA in patients with PD, with an emphasis on the mechanisms of the
GM and its role in the pathogenesis of PD (Fig. 1). In addition, adaptable novel
potential treatment strategies are discussed. The present review
offers an insight into the role of α-syn and MGBA in the
pathological progression of PD, and highlights the potential of
α-syn and MGBA as relevant drug targets, as well as discussing
potential therapeutic candidates.
Numerous studies have confirmed that the GM
regulates the autonomic nervous system and CNS via the immune
system, neuroendocrine system, direct neural conduction and the
interaction of the MGBA (48–50).
Changes in GM are associated with the pathological process of PD
(51).
Changes to the GM have been widely reported amongst
patients with PD. Scheperjans et al (48) reported that ~77.6% of patients with
PD exhibited a reduction of Prevotellaceae (family), and a
notable increase in several pathogenic Gram-negative bacteria, such
as Enterobacteriaceae (family), Verrucomicrobiaceae
(family) and Escherichia coli (species) from their feces.
Gerhardt and Mohajeri (52)
demonstrated that there was a significant decrease of
Prevotellaceae (family), Prevotella (genus) and
Prevotella Copri (species) in the intestines of patients
with PD. Lubomski et al (53) reported a notable alteration in the
microbial population of Firmicutes in the GM of patients
with PD compared with healthy individuals. The quantity of
Lactobacillaceae (family) and Lactobacillus (genus)
increased, whereas the abundance of Lachnospiraceae
(family), such as Ruminococcus (genus), Blautia
(genus), Dorea (genus), Roseburia (genus) and
Faecalibacterium (genus) decreased. According to these
results, the researchers hypothesized that the types of
pro-inflammatory bacteria in the intestines of patients with PD
significantly increased, whereas the types of probiotic bacteria
decreased. Keshavarzian et al (54) further confirmed this hypothesis,
demonstrating that the proportion of pro-inflammatory bacteria in
the intestines of patients with PD increased by studying the
mucosal-associated bacteria and fecal microbiota. The results
demonstrated that the diversity of fecal microbiota communities was
not significantly altered between PD and healthy control (HC)
individuals, but the α-diversity at the phylum level and the
richness of the genus level were significantly different.
Specifically, at the phylum level, the abundance of
Bacteroidetes, Proteobacteria and Verrucomicrobia in
the fecal microbiota of the PD group significantly increased,
whereas the presence of Firmicutes in the fecal microbiota
of HC was higher in the HC group. Similar results were obtained by
assessing the fecal samples of the PD group compared with the HC
group at the genus level, in which the abundance of
pro-inflammatory bacteria, such as the Akkermansia,
Oscillospira and Bacteroides were increased, and the
abundance of butyrate-producing bacteria that produce
anti-inflammatory short chain fatty acids (SCFAs), such as
Blautia, Coprococcus and Roseburia were significantly
reduced in the fecal samples of the PD group. The abundance of
Coprobacillaceae (family), Dorea (genus) and
anti-inflammatory Faecalibacterium (genus) were rich in the
mucosal-associated bacterial populations of the HC group, whereas
the Oxalobac-teraceae (family) and Ralstonia (genus)
were richer in the PD group (55).
Li et al (56) reported that
the composition of GM was slightly altered between the healthy and
Han Chinese patients with PD using next generation sequencing to
analyze the feces. Consistent with other studies, the abundance of
Bacteroides (genus) and Prevotellaceae (family)
significantly increased in the healthy Han Chinese individuals,
whereas the abundance of Ruminococcaceae (family),
Verrucomicrobiaceae (family), Porphyromonaceae
(family), Hydrogenoanaerobacterium (family) and
Lachnospiraceae NK4A (family) increased significantly in the
Han Chinese patients with PD. In addition, these studies failed to
demonstrate an increase in the abundance of Bifidobacterium
(family) and Enterobacteriaceae (family) in the feces of Han
Chinese patients with PD, an inconsistent result compared with the
study of Caucasian patients with PD (57–59).
In summary, numerous changes to the GM are involved in the
pathological process of PD (Table
I).
Due to changes in the gut microbiota composition
that occur during the course of PD, several studies have
investigated fecal microbiota transplant (FMT), a novel therapeutic
method, which involves the transfer of gut microbiota from a
healthy individual to another via oral administration of fecal
material in rodents, or via medication that alters the GM or
gastrointestinal endoscopy in humans, as a method to improve the
symptoms of constipation in patients with PD (60,61).
Tan et al (62) reported
that the FMT procedure is an effective treatment in 65.6% of
patients with PD, and that several patients exhibited an increase
in spontaneous bowel movements by 1–2 times per week in the process
of FMT treatment. In a PD mouse model, FMT is necessary for the
neuroprotective effects of osteocalcin (63). In addition, FMT alleviates
dyskinesia and neurodegeneration of striatal DA, reduces
neuroinflammation and activates microglia and astrocytes in the
brain of PD mice. Furthermore, FMT can also increase the levels of
5-hydroxytryptophan, decrease fecal SCFAs and improve gut microbial
dysbiosis in the intestinal tracts of PD mice (64).
The barrier functions of the gut epithelium serve an
important role during host-microbiome interactions, and the
disruption of this barrier can lead to intestinal inflammation,
production of reactive oxygen/nitrogen species in the gut, and a
shift in microbial composition towards pro-inflammatory bacteria
(65). Several studies have
demonstrated that one of the potential mechanisms by which α-syn
enters into the mucosal neuronal tissue is the generation of OS and
the disruption of intestinal barrier integrity via aberrant changes
to the GM in patients with PD (66,67).
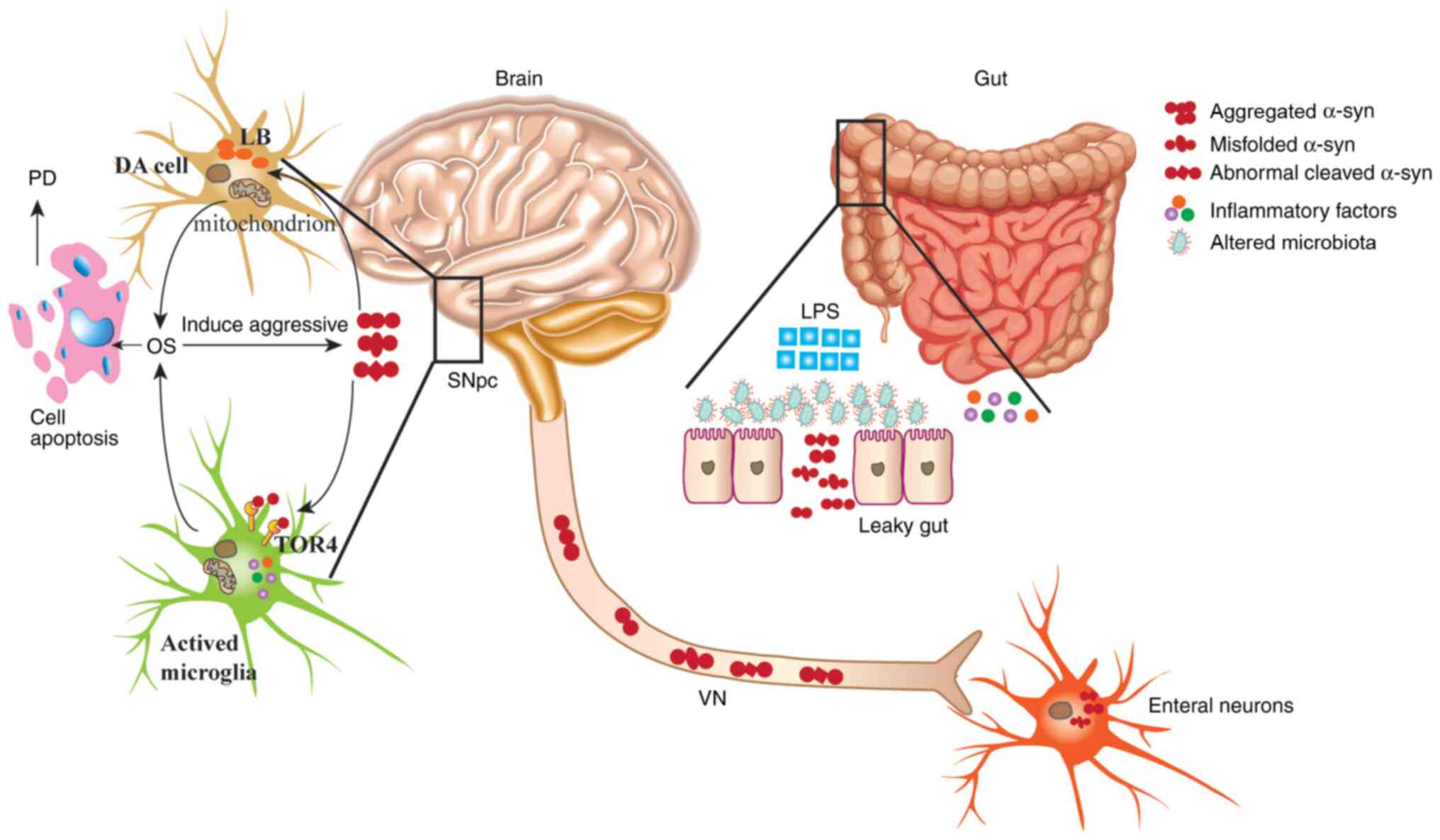
Given the effect of aberrant GM on the gastrointestinal barrier,
the resultant translocation of bacteria or their products, such as
lipopolysaccharide (LPS) can increase OS and intestinal
inflammation, which in turn increases the mucosal intestinal
permeability, also known as leaky gut, and increases the ability of
α-syn to communicate with the ENS (53). Immunohistochemical staining of
postmortem analyses of intestines in patients with PD exhibited an
inevitable association between impaired intestinal barrier
integrity, the increase in the intestinal bacterial flora, the high
levels of expression of inflammatory genes and the abnormal
accumulation of α-syn in the ENS (68–70).
Other studies have reported that the volatile SCFA, particularly
butyrate, serves a vital role in maintaining intestinal barrier
integrity, and a lack of SCFA can increase intestinal permeability,
which has been confirmed in studies of patients with inflammatory
bowel disease (71,72). Further studies on the GM have
reported that Prevotellaceae is involved in the formation of
intestinal mucins and the production of SCFA through fiber
fermentation in the sigmoid (73,74).
Thus, the decrease of Prevotellaceae in the intestines of
patients with PD can lead to a decrease in intestinal mucus and an
increase in intestinal permeability, and this serves as a
prerequisite for entry of α-syn into the ENS via the intestinal
barrier, and to maintain excessive α-syn expression or even promote
its misfolding (75). The treatment
of a colitis mouse model with butyrate can reduce the expression of
TNF-α in the intestines and reduce cell shedding (76).
An aberrant GM or their products are involved in the
misfolding, abnormal aggregation and presence of truncated
fragments of α-syn in the ENS of patients with PD (66,69).
In particular, the LPS generated by inflammatory bacteria can
increase the nitration and oligomerization of α-syn by upregulating
the expression of inducible nitric oxide synthase (iNOS),
suggesting that LPS not only increases the inflammatory response to
induce gut leakiness and the communication of α-syn, but can also
accelerate the neurodegenerative process via the influence of α-syn
(55). The
monocyte/macrophage-related signaling pathway is involved in the
aforementioned biological processes (77). The LPS generated from the
gram-negative bacteria can downregulate the expression of tight
junction proteins in the intestinal epithelial cells, such as
occludin, and upregulate the expression of TNF-α, which activates
the macrophages and promotes the expression of α-syn in a mouse
model (78,79). Bhattacharyya et al (80) demonstrated that LPS binds to the
α-helical intermediates of α-syn to form a lipid-protein complex
that acts as a scaffold for growth of α-syn fibers, and rapid
nucleation based on CD spectra analysis. LPS also accelerates
abnormal aggregation of α-syn by increasing the production and
reducing the half-life and lag time of α-syn (81). Wang et al (82) reported that inflammatory activators,
such as LPS, aluminum potassium sulfate crystals, nigericin and
vitamin K3 (menadione) can activate the inflammasome, including
caspase-1, by inducing the cleavage of procaspase-1 to the active
caspase-1, which is directly involved in cleaving and inducing the
aggregation of α-syn. Furthermore, the 10 or 30-residues of α-syn
N-terminal truncations alter the conformation of fibril, thus
contributing to a reduction in its stability and induce their
compatibility with normal α-syn. The 20-residues of α-syn
C-terminal truncations result in it exhibiting unique prion-like
properties (83). The prion
conformation of α-syn interferes with the lysosomal and proteasomal
degradation processes, and ultimately promotes aberrant
accumulation (84).
The primary pathological characteristic of PD is the
abnormal aggregation of α-syn in the SNpc (85). Due to its prion-like protein
activation mechanism, α-syn can misfold to cause self-abnormal
aggregation, and can also transmit signals amongst nervous cells,
and spread throughout the nervous system (86). Several studies have indicated that
the ENS may be the channel by which α-syn is transferred from the
PNS to the CNS (41,87).
Misfolded α-syn can transfer from affected to
unaffected cells and serve as a template for pathophysiological
aggregation of α-syn in neuronal cells (88–91).
It has been speculated that α-syn may be transmitted to the dorsal
nucleus of the VN via VN fibers at a speed of 5–10 mm/day, and it
accumulated in the neurons to trigger cell apoptosis after α-syn
was injected into the intestinal wall of rats (92). Kim et al (93) injected 25 µg misfolded α-syn,
preformed fibrils (PFF), into the pyloric stomach and upper
duodenum (UD) of mice, and detected the distribution of PFF in the
brain tissues after 1, 3, 7 and 10 months. The results demonstrated
that PFF aggregated in the stomach and spread into the VN 1 month
after injection. In the following 3–10 months, the distribution of
PFF was detected consecutively in the medulla oblongata, locus
coeruleus in the pons, SNc in the ventral mesencephalon brain
(VMB), prefrontal cortex and olfactory bulb. The number of DA
neurons in the SNpc of mice significantly decreased, and this was
accompanied by a decrease in forelimb strength, grip strength, the
hindlimb strength, and muscle and motor coordination ability of
mice based on a rotarod test 7 months after injection. However, PFF
was not detected in the aforementioned areas of the brain in the
mice whose VN fibers near UD and neck were cut by truncal vagotomy
7 months after injection with the same dosage of PFF. The death of
neuronal cells and the production of LBs were not detected. Braak
et al (94) demonstrated
that several misfolded α-syn proteins had accumulated in a portion
of the ENS that regulated intestinal function and connected the gut
to the brain of patients with PD. They speculated that the ENS may
be the α-syn accumulation starting point, and α-syn was transported
from the VN to the VMB, where the SNpc killed DA cells selectively,
resulting in PD.
The abnormal α-syn in DA cells transported from MGBA
often involves mitochondrial dysfunction and the generation of OS,
which serve important roles in the development of PD (95,96).
It has been reported that overexpression of α-syn in GT1-7 cells
increases mitochondrial volume and abnormal vacuolated cristae
(97). Hu et al (98) demonstrated that in the
α-syn-overexpressing SH-SY5Y cells, α-syn can interact with
ATP-dependent Clp protease (ClpP) to inhibit the activity of ClpP,
leading to mitochondrial oxidative damage and neurotoxicity. Chong
et al (99) performed
experiments using an N27 immortalized rat mesencephalic
dopaminergic neuronal model of PD cell lines that stably expressed
wild-type human α-syn, and hypothesized that the expression levels
of caspase-3 and 9 increased as the OS increased, and the
expression of Akt gradually decreased, resulting in cells
undergoing mitochondrial dependent apoptosis. This suggests that
α-syn promotes apoptosis by increasing OS. Dryanovski et al
(100) and Tapias et al
(101) reported that the increase
in levels of α-syn in DA cells promotes the generation of OS.
Musgrove et al (102)
detected large quantities of nitrated α-syn in the brain tissues of
patients with PD after death using an oxidized modified antibody.
The study of SH-SY5Y human neuroblastoma cells stably expressing
the C-terminal half of Venus YFP-tagged α-syn or the N-terminal
half of Venus YFP-tagged α-syn demonstrated that the cells treated
with 25 µm paraquat exhibited a stronger fluorescent signal due to
the increase in OS compared with the control groups. However, after
co-culturing with 25 µM paraquat and oxidized modified inhibitor,
the intensity of the fluorescent signal in the experimental group
decreased compared with the control group. Therefore, it was
hypothesized that the increased OS was the result of increased
spreading of α-syn from the dorsal motor nucleus of the VN to the
rostral brain regions, resulting in enhanced cell-to-cell α-syn
transmission in patients with PD. Martin et al (103) and Stichel et al (104) reported that LBs were formed in
transgenic mice that expressed the human mutational α-syn.
Furthermore, the activity of neuronal mitochondrial complexes I and
IV significantly reduced. Conversely, inhibiting the activity of
mitochondrial complex I promoted the accumulation of α-syn in mice
brains, suggesting that increased OS resulted in increased levels
of oxidatively modified forms of α-syn, resulting in increased
α-syn aggregation and thus, cell-to-cell transmission (105). Thus, an increase in OS and the
aggregation of α-syn forms a positive feedback loop throughout the
progression of PD (95).
In the brain, microglia act as innate immune cells
and serve a vital role in the pathogenesis of PD (106). During the early stages of PD,
microglias are activated and are positive for human leukocyte
antigen-DR (HLA-DR) (107).
Microglias maintain continuous activation through the development
of the disease (108). The
activated microglia release several pro-inflammatory factors that
target the blood vessel endothelial cells of the blood-brain
barrier (BBB) and promote the expression of adhesion molecules on
their surface (109). The adhesion
molecules induce T cells and monocytes to enter the brain through
the BBB, which further release more pro-inflammatory factors,
resulting in neuroinflammation and neuronal apoptosis (110). α-syn is involved in the activity
of microglial cells in patients with PD (111). Studies on brain tissue samples of
patients with PD revealed high expression of α-syn in neurons, and
the microglia were HLA-DR positive (112,113). The microglial cells treated with
different (from low to high) concentrations of α-syn can increase
the pro-inflammatory effect of microglial cells, and the mRNA
expression levels of TNF-α, interleukin (IL)-1β, cyclooxygenase-2
and iNOS levels also increase, which causes apoptosis of nerve
cells (114). In the brain,
toll-like receptor 4 (TLR4) is primarily expressed by microglial
cells, and a small amount of TLR4 is expressed by astrocytes,
oligodendrocytes and neurons (115,116). TLR4 can induce the activity of
microglial cells, and subsequently induce the secretion of
inflammatory chemokines and cytokines (117,118). Fellner et al (119) demonstrated that α-syn can active
microglial cells by targeting TLR4, and inducing the production of
nuclear factor-κB (NF-κB) and the secretion of cytokines by
treating TLR4-deficient and wild-type mice with different forms of
α-syn (full length soluble, fibrillized and C-terminally
truncated). Further research demonstrated that the C-terminally
truncated α-syn was the most effective inducer of TLR4-dependent
microglial activity (120). Choi
et al (121) indicated that
α-syn also reduces cell autophagy by decreasing lysosomal and
proteasomal degradation, which further results in increased
expression of TLR4 and further strengthened TLR4-dependent
microglial activity. Thus, α-syn is considered an inducer of
microglial activation in patients with PD. Notably, several studies
have reported that NF-κB, matrix metalloproteinase and protease
activated receptor 1 are also involved in microglial activity via
monomers, polymers and nitration of α-syn (122,123), which further induces the
production of excessive ROS in microglia, and thus, the death of DA
neurons (124).
Activated microglia cells inhibit the activity of
nuclear factor E2-related factor 2 (Nrf2), an antioxidant
transcription factor associated with the anti-inflammatory capacity
of microglia, via oxidative modification (125). The expression levels of TNF-α,
IL-6, IL-1β and iNOS are significantly upregulated in
Nrf2-deficient microglia (126).
Shavali et al (127)
reported that activated microglia further promote nitrification of
α-syn through nitric oxide, thereby inducing the continuous spread
of α-syn to the adjacent neurons and resulting in neuronal cell
death. It has been demonstrated that TNF-α produced by
α-syn-activated microglia can result in impaired function of
mitochondrial complex I and the generation of OS; in turn, the OS
promotes further aggregation of α-syn in neuronal cells (128). Subsequently, the aggregated α-syn
increases OS further, forming a positive feedback loop between
neuroinflammation, OS and the aggregation and spread of α-syn in
patients with PD (129).
The current treatments available for patients with
PD not only have no effect on its relentless progression but may
also induce several side effects. There are no effective
therapeutic strategies that target the MGBA to slow or halt the
neurodegenerative process, or reduce motor and non-motor symptoms.
Nutrition based probiotics or prebiotics-based interventions
inhibit neuroinflammation and ameliorate the diffusion of α-syn in
the MGBA, which offer a novel therapeutic strategy for the
treatment of PD and overcome the disadvantages of current therapies
(130). An overview of the studies
summarizing the novel treatments based on MGBA and α-syn for the
management of PD is presented in Fig.
2.
The death of cerebral DA neurons in patients with PD
leads to a decrease in secretion of dopamine and other
neurotransmitters, resulting in the motor symptoms. Currently,
pharmacological therapeutic strategies primarily compensate for the
loss of dopamine and other neurotransmitters to alleviate motor
symptoms temporarily. The drugs often include dopamine receptor
activators and/or the dopamine precursor L-3,
4-dihydrophenylalanine (levodopa). However, levodopa not only
relieves the neurodegenerative processes, but also causes several
effects, including nausea, emesis, abnormal involuntary movement of
head, face, tongue, upper limbs, depression and dysuria.
Conversely, levodopa administered orally provides a favorable
gastrointestinal environment required for the drug absorption
(131). However, most patients
with PD also exhibit gastrointestinal disorders, resulting in poor
drug absorption (132). Several
drug trials on healthy volunteers had demonstrated that levodopa
can delay the time of gastric emptying, and long-term use of
levodopa can exacerbate the gastrointestinal dysfunction in
patients with PD (133). Further
studies have reported that long-term use of levodopa may lead to
fluctuation of symptoms and resistance to levodopa (134,135). Tyrosine decarboxylase (TDC), which
is involved in the transformation of levodopa to dopamine and
prevents the uptake of levodopa in the small intestine of patients
with PD, is specially coded in the genome of Lactobacillus
and Enterococcus in the intestine (136). The content of TDC is negatively
correlated with the dosage of levodopa in the feces of patients,
which may be used as a biomarker to evaluate the therapeutic effect
of levodopa (137). The
combination of a TDC inhibitor and levodopa can reduce the dosage
and the dependence on levodopa for more effective treatment of PD
(138).
Prebiotics, such as Galacto-Oligosaccharides and
Fructo-Oligosaccharide, are non-digestible oligosaccharides,
primarily synthesized from lactose or fructose (150). Prebiotics selectively activate
certain probiotics in the gut, such as Bifidobacteria
(151), promote its metabolism and
produce SCFAs to maintain the integrity of the intestinal epithelia
and regulate the mucosal immune response (152). Savignac et al (153) demonstrated that prebiotics can
increase the expression levels of brain derived neurotrophic
factor, which serves a protective role in neurons in the dentate
gyrus of the hippocampus of rats. Taken together, prebiotics can
selectively reduce intestinal permeability and inflammation via
activation of the metabolism of probiotics. Thus, it is
hypothesized that the combination of FMT, prebiotics and
traditional drugs may both improve intestinal dysfunction and
protect neurons, as well as effectively reducing the dosage of
levodopa or other drugs to reduce drug dependence (154).
Given that α-syn serves a key role in the
development of PD, an increasing number of studies have
demonstrated that the inhibition of α-syn expression and abnormal
aggregation is an important method for the treatment of PD
(155). Preclinical gene therapy
using RNA interference (RNAi) targeting α-syn mRNA via
gene-silencing is being assessed, and the results have indicated
that it can effectively inhibit the expression of α-syn in
fibroblasts (156). In addition,
miRNAs can downregulate the translation of target mRNAs as
post-transcriptional regulators, through binding to the
3′-untranslated region (UTR) complementary sequences (157), which can be used as another means
of gene therapy for the treatment of PD. Junn et al
(158) reported that miR-7 can
bind to the 3′-UTR of α-syn mRNA complementary sequences to inhibit
the expression of α-syn, effectively relieving its inhibitory
effect on the proteasome, and thus promoting the degradation of
α-syn. Masliah et al (159)
demonstrated that PD transgenic mice immunized with full length
human α-syn protein exhibit decreased accumulation of the misfolded
α-syn in neuronal cell bodies and synapses, resulting in reduced PD
symptoms, and the mice that produced high affinity antibodies
exhibited a greater capacity to reduce α-syn expression compared
with the mice that produced lower affinity and/or lower titers of
anti-α-syn antibodies. Ghochikyan et al (160) also indicated that a PD mouse model
immunized with full length human α-syn protein can produce high
affinity antibodies, which can reduce the accumulation of the
aggregated forms of α-syn in neuronal cells to relieve
neurodegeneration. Other studies indicated that monoclonal
antibodies that target α-syn can inhibit the expression and
aggregation of α-syn in a PD mouse model, as well as in patients
(161,162). Upregulation of α-syn in the
extracellular matrix can be recognized by specific antibodies and
cleared by the macrophages through phagocytosis (163,164). In addition, α-syn specific
antibodies can reduce the activation of CD4+ T cells by
clearing cytokines, such as IL-2 and TNF-β, which are released by
the activated CD4+ T cell-mediated neurodegeneration
(165). A study demonstrated that
monoclonal antibodies against α-syn can decrease LB/LN formation to
reduce the loss of primary cultured neuronal cells by preventing
the uptake of α-syn-PFF (166).
Due to the α-syn form, the oligomers and fibrils, as well as the
post-translational modifications of α-syn in patients with PD, such
as acetylation, phosphorylation and truncation, several studies
have demonstrated that D10, a single chain antibody of α-syn, had
the highest affinity with α-syn; the single chain antibody D5 can
effectively target the oligomer of α-syn; the single chain antibody
syn-O1, -O2 and -O4 can effectively reduce neuroinflammation and
the loss of neurons by specifically targeting the oligomer of α-syn
in the CA3 area of the hippocampus; the single chain antibody
syn-10h targets α-syn trimers, and antibodies syn-F1 and F2 prevent
neuronal loss and facilitated amelioration in behavioral defects by
specifically recognizing and clearing α-syn fibers (167,168). In addition, a specific antibody,
LS4-2G12, which recognizes phospho-α-syn (ser129), is highly
sensitive to the aggregation in the tissues of patients with PD,
and protects neurons from damage mediated by the immune system
(169,170). TLR4 inhibitors can be used as a
potential drug for the treatment of PD (171). The inhibitor of TLR4 can
effectively reduce intestinal inflammation and permeability in
patients with PD, which can be used as a competitive inhibitor of
mutant α-syn in the brain of patients with PD to inhibit the
activation of microglia (172).
Taken together, the use of small molecules or antibodies to reduce
the toxicity of α-syn may serve as an effective method to inhibit
the pathogenesis and development of PD (173).
The failure to eliminate α-syn is also an important
cause of PD, and autophagy serves a vital role in the elimination
of abnormally aggregated α-syn (174). mTOR complexes are widely involved
in regulating apoptosis and autophagy (175). MSDC-0160, a mitochondrial pyruvate
carrier inhibitor, enhances autophagy by inhibiting the activation
of mTOR in cells (176). It has
been reported that MSDC-0160 can effectively protect DA neurons in
an MPTP-induced mouse model, and decreases α-syn-induced neural
toxicity by increasing autophagy in a C. elegans model of PD
(177). In nerve cells, the
expression of Beclin 1 can reduce apoptosis and enhance autophagy
(178). Spencer et al
(179) overexpressed Beclin 1 in a
PD mouse model using lentivirus to activate autophagy and reduce
the accumulation of α-syn; however, the study only assessed this
method in a mouse model. The levels of activated C-Abl, a tyrosine
kinase, in the brain of patients with PD increased due to α-syn
aggregation (180). Nilotinib, an
antitumor drug, can promote intracellular autophagy by inhibiting
the PI3k/Akt/mTOR signaling pathway to inhibit C-Abl (181). A recent non-placebo-controlled
study reported that the lower dose of Nilotinib is effective and
safe for the treatment of PD by effectively inhibiting α-syn
aggregation (182). Taken
together, these findings suggest that enhancing autophagy and
inducing the degradation of α-syn are potential therapeutic
strategies for the treatment of PD.
The intestinal microbiota contributes to the
pathogenesis of PD, which has changed the previous view that the
etiology of PD is concentrated on the brain. Misfolded and
abnormally aggregated α-syn in the intestines of patients with PD
is transported from the intestines to the brain via the MGBA. The
spreading and abnormal aggregation of α-syn in the brain results in
the formation of LBs in DA neurons, the activation of microglia,
the production of inflammatory factors, an increase in OS, and
ultimately apoptosis. MGBA not only serves an important role in the
stability of the digestive system, but also serves a key role in
the pathogenesis of PD. MGBA abnormalities may be one of the causes
of PD. Currently, several novel clinical therapeutic strategies
that target the MGBA to slow or halt the neurodegenerative process
or reduce the motor and non-motor symptoms, such as food-based
therapies, vagotomy, inhibiting the expression and abnormal
aggregation of α-syn in patients with PD using RNAi or other gene
modification measures, as well as FMT have been suggested. In
addition, several genes serve prominent roles between MGBA and the
progression of PD, such as the aforementioned PINK1/parkin pathway,
LRRK2, TLRs and the PI3k/Akt/mTOR pathway. These genes/pathways may
serve as potential therapeutic targets for PD clinical therapy via
modulation of the MGBA in the near future. Thus, an improved
understanding of the molecular mechanisms that are involved between
the gut microbiota, MGBA and the brain in patients with PD may
assist in the development of novel therapeutic strategies for the
treatment of PD.
Although several changes in the taxa of the GM have
been found in patients with PD, several questions regarding the
association between the MGBA and PD remain unanswered. For example,
similar changes to the taxa of the GM have been found in other
diseases, such as decreased levels of prevotella in type I
diabetes and an increased abundance of Lactobacillus in type
II diabetes (183,184). Thus, identifying specific
PD-associated bacterial taxa as a method to diagnose PD may not be
viable. In the future, studies should concentrate on confirming the
changes to the bacterial taxa in the intestinal tract that may
serve as biomarkers for diagnosis of PD. Furthermore, although
several studies have attempted to understand the association
between the changes in the GM and the pathogenesis of PD, specific
molecular mechanisms regarding the associations between the
constituents of the intestinal bacterial taxa and PD remain to be
determined. Notably, whether the changes in the bacterial taxa are
the cause or consequence of PD should be addressed.
Despite recognizing the pathogeny of PD and the
role of abnormal aggregation of α-syn, Horsager et al
(185) reported that PD can be
separated into two hypotypes based on the initial position of the
pathological α-syn aggregation. The initial site of the build-up of
α-syn aggregates has not been determined, thus, further studies are
required to determine the starting position of α-syn polymers and
the associated mechanisms. In addition, in the gastrointestinal
tract, why α-syn tends to oligomerization or polymer and its
functional roles remain unclear. Barbut et al (186) reported that monomeric α-syn did
not exhibit the ability of antimicrobial infection, but aggregated
α-syn exhibited a property of antimicrobial peptides (AMPs), which
is concentration-dependent aggregation in the gastrointestinal
tract of patients with PD. Briefly, the ‘intrinsically disordered’
α-syn assumes no specific structure in the aqueous solvent.
However, α-syn exhibits a net of cationic charge by the N-terminal
(~60 amino acid residue) in the presence of lipid membranes, and
interacts with the phospholipids of lipid membranes that contain an
abundance of negatively charged headgroups, such as the
phosphatidyl serine. Subsequently, the α-syn is pulled toward the
lipid membranes electrostatically, which increases the
concentration of α-syn on the lipid membrane. The bound α-syn
molecules are closer to one another and begin to aggregate,
exhibiting the characteristic of concentration-dependent
aggregation, which has been described for numerous AMPs (187,188). Despite the understanding of the
process of α-syn aggregation, the downstream targeted molecules of
aggregated α-syn in the lipid membrane remain unclear. Goya et
al (144) demonstrated that a
probiotic strain, B. subtilis PXN21, can effectively clear
aggregated α-syn. However, whether probiotic can remove α-syn that
has bound to the lipid membrane remains unknown. Abbott et
al (189) reported that the
unbalance of lipid metabolism is an important pathogenesis of PD.
Whether the unbalance of lipid metabolism can increase an affinity
between α-syn and lipid membrane requires further investigation. If
such means are exposed, it can unravel a new wave of therapies that
target PD from the foundational pathophysiology, rather than just
suppressing the symptoms.
Not applicable.
The present review was funded by the Innovative
Team of Functional Neurosurgery of Kunming Medical University
(grant no. CXTD201703).
Not applicable.
QL, TW, JY and GS conceived and designed the
present review. QL, TW, JW, XH, YG, YW, JY and GS performed the
literature review and acquired the relevant data. QL, TW, JY and GS
drafted the initial manuscript. Data authentication is not
applicable. All authors have read and approved the final
manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Wu S, Lei L, Song Y, Liu M, Lu S, Lou D,
Shi Y, Wang Z and He D: Mutation of hop-1 and pink-1 attenuates
vulnerability of neurotoxicity in C. elegans: The role of
mitochondria-associated membrane proteins in Parkinsonism. Exp
Neurol. 309:67–78. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Balestrino R and Schapira AHV: Parkinson
disease. Eur J Neurol. 27:27–42. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mahoney-Sanchez L, Bouchaoui H, Ayton S,
Devos D, Duce JA and Devedjian JC: Ferroptosis and its potential
role in the physiopathology of Parkinson's disease. Prog Neurobiol.
196:1018902021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Samii A, Nutt JG and Ransom BR:
Parkinson's disease. Lancet. 363:1783–1793. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Khoo TK, Yarnall AJ, Duncan GW, Coleman S,
O'Brien JT, Brooks DJ, Barker RA and Burn DJ: The spectrum of
nonmotor symptoms in early Parkinson disease. Neurology.
80:276–281. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lopiano L, Modugno N, Marano P, Sensi M,
Meco G, Cannas A, Gusmaroli G, Tamma F, Mancini F, Quatrale R, et
al: Motor outcomes in patients with advanced Parkinson's disease
treated with levodopa/carbidopa intestinal gel in Italy: An interim
analysis from the GREENFIELD observational study. Neurol Sci.
37:1785–1792. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kalinderi K, Bostantjopoulou S and Fidani
L: The genetic background of Parkinson's disease: Current progress
and future prospects. Acta Neurol Scand. 134:314–326. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Malpartida AB, Williamson M, Narendra DP,
Wade-Martins R and Ryan BJ: Mitochondrial dysfunction and mitophagy
in Parkinson's disease: From mechanism to therapy. Trends Biochem
Sci. 46:329–343. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tysnes OB and Storstein A: Epidemiology of
Parkinson's disease. J Neural Transm (Vienna). 124:901–905. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ascherio A and Schwarzschild MA: The
epidemiology of Parkinson's disease: Risk factors and prevention.
Lancet Neurol. 15:1257–1272. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ahier A, Dai CY, Kirmes I, Cummins N, Hung
GCC, Götz J and Zuryn S: PINK1 and parkin shape the organism-wide
distribution of a deleterious mitochondrial genome. Cell Rep.
35:1092032021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tolosa E, Vila M, Klein C and Rascol O:
LRRK2 in Parkinson disease: Challenges of clinical trials. Nat Rev
Neurol. 16:97–107. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wauters F, Cornelissen T, Imberechts D,
Martin S, Koentjoro B, Sue C, Vangheluwe P and Vandenberghe W:
LRRK2 mutations impair depolarization-induced mitophagy through
inhibition of mitochondrial accumulation of RAB10. Autophagy.
16:203–222. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Terbeek J, Martin S, Imberechts D, Kinnart
I, Vangheluwe P, Nicholl D and Vandenberghe W: Increased superoxide
in GCH1 mutant fibroblasts points to a dopamine-independent
toxicity mechanism. Parkinsonism Relat Disord. 82:10–12. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Imai Y, Meng H, Shiba-Fukushima K and
Hattori N: Twin CHCH proteins, CHCHD2, and CHCHD10: Key molecules
of Parkinson's disease, amyotrophic lateral sclerosis, and
frontotemporal dementia. Int J Mol Sci. 20:9082019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sassone J, Reale C, Dati G, Regoni M,
Pellecchia MT and Garavaglia B: The role of VPS35 in the
pathobiology of Parkinson's disease. Cell Mol Neurobiol.
41:199–227. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gangemi S, Gofita E, Costa C, Teodoro M,
Briguglio G, Nikitovic D, Tzanakakis G, Tsatsakis AM, Wilks MF,
Spandidos DA and Fenga C: Occupational and environmental exposure
to pesticides and cytokine pathways in chronic diseases (Review).
Int J Mol Med. 38:1012–1020. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Teodoro M, Briguglio G, Fenga C and Costa
C: Genetic polymorphisms as determinants of pesticide toxicity:
Recent advances. Toxicol Rep. 6:564–570. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Costa C, Teodoro M, Rugolo CA, Alibrando
C, Giambo F, Briguglio G and Fenga C: MicroRNAs alteration as early
biomarkers for cancer and neurodegenerative diseases: New
challenges in pesticides exposure. Toxicol Rep. 7:759–767. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Srivastav S, Fatima M and Mondal AC:
Important medicinal herbs in Parkinson's disease pharmacotherapy.
Biomed Pharmacother. 92:856–863. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Titze-de-Almeida SS, Soto-Sanchez C,
Fernandez E, Koprich JB, Brotchie JM and Titze-de-Almeida R: The
promise and challenges of developing miRNA-Based therapeutics for
Parkinson's disease. Cells. 9:8412020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yu Y, Chen H, Hua X, Dang Y, Han Y, Yu Z,
Chen X, Ding P and Li H: Polystyrene microplastics (PS-MPs)
toxicity induced oxidative stress and intestinal injury in nematode
caenorhabditis elegans. Sci Total Environ. 726:1386792020.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Schirinzi T, Landi D and Liguori C:
COVID-19: Dealing with a potential risk factor for chronic
neurological disorders. J Neurol. 268:1171–1178. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ribeiro DE, Oliveira-Giacomelli A, Glaser
T, Arnaud-Sampaio VF, Andrejew R, Dieckmann L, Baranova J, Lameu C,
Ratajczak MZ and Ulrich H: Hyperactivation of P2X7 receptors as a
culprit of COVID-19 neuropathology. Mol Psychiatry. 26:1044–1059.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fasano A, Visanji NP, Liu LW, Lang AE and
Pfeiffer RF: Gastrointestinal dysfunction in Parkinson's disease.
Lancet Neurol. 14:625–639. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mayer EA, Tillisch K and Gupta A:
Gut/brain axis and the microbiota. J Clin Invest. 125:926–938.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ghaisas S, Maher J and Kanthasamy A: Gut
microbiome in health and disease: Linking the microbiome-gut-brain
axis and environmental factors in the pathogenesis of systemic and
neurodegenerative diseases. Pharmacol Ther. 158:52–62. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Felice VD, Quigley EM, Sullivan AM,
O'Keeffe GW and O'Mahony SM: Microbiota-gut-brain signalling in
Parkinson's disease: Implications for non-motor symptoms.
Parkinsonism Relat Disord. 27:1–8. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pfeiffer R: Beyond here be dragons: SIBO
in Parkinson's disease. Mov Disord. 28:1764–1765. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nielsen HH, Qiu J, Friis S, Wermuth L and
Ritz B: Treatment for helicobacter pylori infection and risk of
Parkinson's disease in denmark. Eur J Neurol. 19:864–869. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Devos D, Lebouvier T, Lardeux B, Biraud M,
Rouaud T, Pouclet H, Coron E, Bruley des Varannes S, Naveilhan P,
Nguyen JM, et al: Colonic inflammation in Parkinson's disease.
Neurobiol Dis. 50:42–48. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mehra S, Sahay S and Maji SK: α-synuclein
misfolding and aggregation: Implications in Parkinson's disease
pathogenesis. Biochim Biophys Acta Proteins Proteom. 1867:890–908.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li X, Li YH, Han JY, Yu S and Chen B: cDNA
cloning, prokaryotic expression and purification of rat
alpha-synuclein. Neurosci Bull. 22:29–33. 2006.PubMed/NCBI
|
|
34
|
Pogorelov VM, Kao HT, Augustine GJ and
Wetsel WC: Postsynaptic mechanisms render Syn I/II/III mice highly
responsive to psychostimulants. Int J Neuropsychopharmacol.
22:453–465. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Thiel G: Synapsin I, synapsin II, and
synaptophysin: Marker proteins of synaptic vesicles. Brain Pathol.
3:87–95. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang L, Zhang C, Zhu Y, Cai Q, Chan P,
Uéda K, Yu S and Yang H: Semi-quantitative analysis of
alpha-synuclein in subcellular pools of rat brain neurons: An
immunogold electron microscopic study using a C-terminal specific
monoclonal antibody. Brain Res. 1244:40–52. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Braak H, Rub U, Gai WP and Del Tredici K;
Idiopathic Parkinson's disease, : Possible routes by which
vulnerable neuronal types may be subject to neuroinvasion by an
unknown pathogen. J Neural Transm (Vienna). 110:517–536. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Luk KC, Kehm VM, Zhang B, O'Brien P,
Trojanowski JQ and Lee VM: Intracerebral inoculation of
pathological alpha-synuclein initiates a rapidly progressive
neurodegenerative alpha-synucleinopathy in mice. J Exp Med.
209:975–986. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zheng H, Shi C, Luo H, Fan L, Yang Z, Hu
X, Zhang Z, Zhang S, Hu Z, Fan Y, et al: alpha-synuclein in
Parkinson's disease: Does a prion-like mechanism of propagation
from periphery to the brain play a role? Neuroscientist.
27:367–387. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Perez-Pardo P, Kliest T, Dodiya HB,
Broersen LM, Garssen J, Keshavarzian A and Kraneveld AD: The
gut-brain axis in Parkinson's disease: Possibilities for food-based
therapies. Eur J Pharmacol. 817:86–95. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Dogra N, Mani RJ and Katare DP: The
gut-brain axis: Two ways signaling in Parkinson's disease. Cell Mol
Neurobiol. 2:10072021.
|
|
42
|
Kuo YM, Li Z, Jiao Y, Gaborit N, Pani AK,
Orrison BM, Bruneau BG, Giasson BI, Smeyne RJ, Gershon MD and
Nussbaum RL: Extensive enteric nervous system abnormalities in mice
transgenic for artificial chromosomes containing Parkinson
disease-associated alpha-synuclein gene mutations precede central
nervous system changes. Hum Mol Genet. 19:1633–1650. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Braak H, Del Tredici K, Rüb U, de Vos RA,
Steur EN and Braak E: Staging of brain pathology related to
sporadic Parkinson's disease. Neurobiol Aging. 24:197–211. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Borghammer P and Van Den Berge N:
Brain-first versus gut-first Parkinson's disease: A hypothesis. J
Parkinsons Dis. 9:S281–S295. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Comi C, Magistrelli L, Oggioni GD,
Carecchio M, Fleetwood T, Cantello R, Mancini F and Antonini A:
Peripheral nervous system involvement in Parkinson's disease:
Evidence and controversies. Parkinsonism Relat Disord.
20:1329–1334. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Nair AT, Ramachandran V, Joghee NM, Antony
S and Ramalingam G: Gut microbiota dysfunction as reliable
non-invasive early diagnostic biomarkers in the pathophysiology of
Parkinson's disease: A critical review. J Neurogastroenterol Motil.
24:30–42. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Rani L and Mondal AC: Unravelling the role
of gut microbiota in Parkinson's disease progression: Pathogenic
and therapeutic implications. Neurosci Res. 168:100–112. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Scheperjans F, Aho V, Pereira PA, Koskinen
K, Paulin L, Pekkonen E, Haapaniemi E, Kaakkola S, Eerola-Rautio J,
Pohja M, et al: Gut microbiota are related to Parkinson's disease
and clinical phenotype. Mov Disord. 30:350–358. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Mulak A and Boaz B: Brain-gut-microbiota
axis in Parkinson's disease. World J Gastroenterol. 21:10609–10620.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Cirstea MS, Yu AC, Golz E, Sundvick K,
Kliger D, Radisavljevic N, Foulger LH, Mackenzie M, Huan T, Finlay
BB and Appel-Cresswell S: Microbiota composition and metabolism are
associated with gut function in Parkinson's disease. Mov Disord.
35:1208–1217. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lee HS, Lobbestael E, Vermeire S, Sabino J
and Cleynen I: Inflammatory bowel disease and Parkinson's disease:
Common pathophysiological links. Gut. 70:408–417. 2021.PubMed/NCBI
|
|
52
|
Gerhardt S and Mohajeri MH: Changes of
colonic bacterial composition in Parkinson's disease and other
neurodegenerative diseases. Nutrients. 10:7082018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Lubomski M, Tan AH, Lim SY, Holmes AJ,
Davis RL and Sue CM: Parkinson's disease and the gastrointestinal
microbiome. J Neurol. 267:2507–2523. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Keshavarzian A, Green SJ, Engen PA, Voigt
RM, Naqib A, Forsyth CB, Mutlu E and Shannon KM: Colonic bacterial
composition in Parkinson's disease. Mov Disord. 30:1351–1360. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Baizabal-Carvallo JF and Alonso-Juarez M:
The link between gut dysbiosis and neuroinflammation in Parkinson's
disease. Neuroscience. 432:160–173. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Li F, Wang P, Chen Z, Sui X, Xie X and
Zhang J: Alteration of the fecal microbiota in North-Eastern Han
Chinese population with sporadic Parkinson's disease. Neurosci
Lett. 707:1342972019. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Hill-Burns EM, Debelius JW, Morton JT,
Wissemann WT, Lewis MR, Wallen ZD, Peddada SD, Factor SA, Molho E,
Zabetian CP, et al: Parkinson's disease and Parkinson's disease
medications have distinct signatures of the gut microbiome. Mov
Disord. 32:739–749. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Elfil M, Kamel S, Kandil M, Koo BB and
Schaefer SM: Implications of the gut microbiome in Parkinson's
disease. Movement disorders: Mov Disord. 35:921–933. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Sorrentino ZA, Xia Y, Gorion KM, Hass E
and Giasson BI: Carboxy-terminal truncations of mouse α-synuclein
alter aggregation and prion-like seeding. FEBS Lett. 594:1271–1283.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Cryan JF, O'Riordan KJ, Cowan CSM, Sandhu
KV, Bastiaanssen TFS, Boehme M, Codagnone MG, Cussotto S, Fulling
C, Golubeva AV, et al: The microbiota-gut-brain axis. Physiol Rev.
99:1877–2013. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Koszewicz M, Jaroch J, Brzecka A, Ejma M,
Budrewicz S, Mikhaleva LM, Muresanu C, Schield P, Somasundaram SG,
Kirkland CE, et al: Dysbiosis is one of the risk factor for stroke
and cognitive impairment and potential target for treatment.
Pharmacol Res. 164:1052772021. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Tan AH, Lim SY, Chong KK, Manap AM, Hor
JW, Lim JL, Low SC, Chong CW, Mahadeva S and Lang AE: Probiotics
for constipation in Parkinson disease: A randomized
placebo-controlled study. Neurology. 96:e772–e782. 2021.PubMed/NCBI
|
|
63
|
Hou YF, Shan C, Zhuang SY, Zhuang QQ,
Ghosh A, Zhu KC, Kong XK, Wang SM, Gong YL, Yang YY, et al: Gut
microbiota-derived propionate mediates the neuroprotective effect
of osteocalcin in a mouse model of Parkinson's disease. Microbiome.
9:342021. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Sun MF, Zhu YL, Zhou ZL, Jia XB, Xu YD,
Yang Q, Cui C and Shen YQ: Neuroprotective effects of fecal
microbiota transplantation on MPTP-induced Parkinson's disease
mice: Gut microbiota, glial reaction and TLR4/TNF-α signaling
pathway. Brain Behav Immun. 70:48–60. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Kayisoglu O, Weiss F, Niklas C, Pierotti
I, Pompaiah M, Wallaschek N, Germer CT, Wiegering A and Bartfeld S:
Location-specific cell identity rather than exposure to GI
microbiota defines many innate immune signalling cascades in the
gut epithelium. Gut. 70:687–697. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Kelly LP, Carvey PM, Keshavarzian A,
Shannon KM, Shaikh M, Bakay RA and Kordower JH: Progression of
intestinal permeability changes and alpha-synuclein expression in a
mouse model of Parkinson's disease. Mov Disord. 29:999–1009. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Uemura N, Yagi H, Uemura MT, Hatanaka Y,
Yamakado H and Takahashi R: Inoculation of α-synuclein preformed
fibrils into the mouse gastrointestinal tract induces lewy
body-like aggregates in the brainstem via the vagus nerve. Mol
Neurodegener. 13:212018. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Forsyth CB, Shannon KM, Kordower JH, Voigt
RM, Shaikh M, Jaglin JA, Estes JD, Dodiya HB and Keshavarzian A:
Increased intestinal permeability correlates with sigmoid mucosa
alpha-synuclein staining and endotoxin exposure markers in early
Parkinson's disease. PLoS One. 6:e280322011. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
George S and Brundin P: Immunotherapy in
Parkinson's disease: Micromanaging alpha-synuclein aggregation. J
Parkinsons Dis. 5:413–424. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Wang Y, Liu Y, Sidhu A, Ma Z, McClain C
and Feng W: Lactobacillus rhamnosus GG culture supernatant
ameliorates acute alcohol-induced intestinal permeability and liver
injury. Am J Physiol Gastrointest Liver Physiol. 303:G32–G41. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Bischoff SC, Barbara G, Buurman W,
Ockhuizen T, Schulzke JD, Serino M, Tilg H, Watson A and Wells JM:
Intestinal permeability-a new target for disease prevention and
therapy. BMC Gastroenterol. 14:1892014. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Ploger S, Stumpff F, Penner GB, Schulzke
JD, Gäbel G, Martens H, Shen Z, Günzel D and Aschenbach JR:
Microbial butyrate and its role for barrier function in the
gastrointestinal tract. Ann N Y Acad Sci. 1258:52–59. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Unger MM, Spiegel J, Dillmann KU,
Grundmann D, Philippeit H, Burmann J, Faßbender K, Schwiertz A and
Schäfer KH: Short chain fatty acids and gut microbiota differ
between patients with Parkinson's disease and age-matched controls.
Parkinsonism Relat Disord. 32:66–72. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Zhou X, Zhang B, Zhao X, Lin Y, Wang J,
Wang X, Hu N and Wang S: Chlorogenic acid supplementation
ameliorates hyperuricemia, relieves renal inflammation, and
modulates intestinal homeostasis. Food Funct. 12:5637–5649. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Chen ZJ, Liang CY, Yang LQ, Ren SM, Xia
YM, Cui L, Li XF and Gao BL: Association of Parkinson's disease
with microbes and microbiological therapy. Front Cell Infect
Microbiol. 11:6193542021. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Watson AJ and Hughes KR: TNF-α-induced
intestinal epithelial cell shedding: Implications for intestinal
barrier function. Ann N Y Acad Sci. 1258:1–8. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Resnikoff H, Metzger JM, Lopez M,
Bondarenko V, Mejia A, Simmons HA and Emborg ME: Colonic
inflammation affects myenteric alpha-synuclein in nonhuman
primates. J Inflamm Res. 12:113–126. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Choi JG, Kim N, Ju IG, Eo H, Lim SM, Jang
SE, Kim DH and Oh MS: Oral administration of proteus mirabilis
damages dopaminergic neurons and motor functions in mice. Sci Rep.
8:12752018. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Brown GC: The endotoxin hypothesis of
neurodegeneration. J Neuroinflammation. 16:1802019. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Bhattacharyya D, Mohite GM, Krishnamoorthy
J, Gayen N, Mehra S, Navalkar A, Kotler SA, Ratha BN, Ghosh A,
Kumar R, et al: Lipopolysaccharide from gut microbiota modulates
α-synuclein aggregation and alters its biological function. ACS
Chem Neurosci. 10:2229–2236. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Huang C, Zhu L, Li H, Shi FG, Wang GQ, Wei
YZ, Liu J and Zhang F: Adulthood exposure to lipopolysaccharide
exacerbates the neurotoxic and inflammatory effects of rotenone in
the substantia nigra. Front Mol Neurosci. 10:1312017. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Wang W, Nguyen LT, Burlak C, Chegini F,
Guo F, Chataway T, Ju S, Fisher OS, Miller DW, Datta D, et al:
Caspase-1 causes truncation and aggregation of the Parkinson's
disease-associated protein alpha-synuclein. Proc Natl Acad Sci USA.
113:9587–9592. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Terada M, Suzuki G, Nonaka T, Kametani F,
Tamaoka A and Hasegawa M: The effect of truncation on prion-like
properties of α-synuclein. J Biol Chem. 293:13910–13920. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Woerman AL, Kazmi SA, Patel S, Freyman Y,
Oehler A, Aoyagi A, Mordes DA, Halliday GM, Middleton LT, Gentleman
SM, et al: MSA prions exhibit remarkable stability and resistance
to inactivation. Acta Neuropathol. 135:49–63. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Davie CA: A review of Parkinson's disease.
Br Med Bull. 86:109–127. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Prusiner SB: Cell biology. A unifying role
for prions in neurodegenerative diseases. Science. 336:1511–1513.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Bhattacharyya D and Bhunia A: Gut-brain
axis in Parkinson's disease etiology: The role of
lipopolysaccharide. Chem Phys Lipids. 235:1050292021. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Lee SJ, Desplats P, Sigurdson C, Tsigelny
I and Masliah E: Cell-to-cell transmission of non-prion protein
aggregates. Nat Rev Neurol. 6:702–706. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Luk KC, Kehm V, Carroll J, Zhang B,
O'Brien P, Trojanowski JQ and Lee VM: Pathological α-synuclein
transmission initiates Parkinson-like neurodegeneration in
nontransgenic mice. Science. 338:949–953. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Pan-Montojo F, Schwarz M, Winkler C,
Arnhold M, O'Sullivan GA, Pal A, Said J, Marsico G, Verbavatz JM,
Rodrigo-Angulo M, et al: Environmental toxins trigger PD-like
progression via increased alpha-synuclein release from enteric
neurons in mice. Sci Rep. 2:8982012. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Mezias C, Rey N, Brundin P and Raj A:
Neural connectivity predicts spreading of alpha-synuclein pathology
in fibril-injected mouse models: Involvement of retrograde and
anterograde axonal propagation. Neurobiol Dis. 134:1046232020.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Holmqvist S, Chutna O, Bousset L,
Aldrin-Kirk P, Li W, Björklund T, Wang ZY, Roybon L, Melki R and Li
JY: Direct evidence of Parkinson pathology spread from the
gastrointestinal tract to the brain in rats. Acta Neuropathol.
128:805–820. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Kim S, Kwon SH, Kam TI, Panicker N,
Karuppagounder SS, Lee S, Lee JH, Kim WR, Kook M, Foss CA, et al:
Transneuronal propagation of pathologic alpha-synuclein from the
gut to the brain models Parkinson's disease. Neuron. 103:627–641.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Braak H, Sastre M, Bohl JR, de Vos RA and
Del Tredici K: Parkinson's disease: Lesions in dorsal horn layer I,
involvement of parasympathetic and sympathetic pre- and
postganglionic neurons. Acta Neuropathol. 113:421–429. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Dodiya HB, Forsyth CB, Voigt RM, Engen PA,
Patel J, Shaikh M, Green SJ, Naqib A, Roy A, Kordower JH, et al:
Chronic stress-induced gut dysfunction exacerbates Parkinson's
disease phenotype and pathology in a rotenone-induced mouse model
of Parkinson's disease. Neurobiol Dis. 135:1043522020. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Santos SF, de Oliveira HL, Yamada ES,
Neves BC and Pereira A Jr: The gut and Parkinson's disease-a
bidirectional pathway. Front Neurol. 10:5742019. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Hsu LJ, Sagara Y, Arroyo A, Rockenstein E,
Sisk A, Mallory M, Wong J, Takenouchi T, Hashimoto M and Masliah E:
alpha-synuclein promotes mitochondrial deficit and oxidative
stress. Am J Pathol. 157:401–410. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Hu D, Sun X, Liao X, Zhang X, Zarabi S,
Schimmer A, Hong Y, Ford C, Luo Y and Qi X: Alpha-synuclein
suppresses mitochondrial protease ClpP to trigger mitochondrial
oxidative damage and neurotoxicity. Acta Neuropathol. 137:939–960.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Chong W, Jimenez J, Mc IM, Saito MA and
Kwakye GF: α-Synuclein enhances cadmium uptake and neurotoxicity
via oxidative stress and caspase activated cell death mechanisms in
a dopaminergic cell model of Parkinson's disease. Neurotox Res.
32:231–246. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Dryanovski DI, Guzman JN, Xie Z, Galteri
DJ, Volpicelli-Daley LA, Lee VM, Miller RJ, Schumacker PT and
Surmeier DJ: Calcium entry and α-synuclein inclusions elevate
dendritic mitochondrial oxidant stress in dopaminergic neurons. J
Neurosci. 33:10154–10164. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Tapias V, Hu X, Luk KC, Sanders LH, Lee VM
and Greenamyre JT: Synthetic alpha-synuclein fibrils cause
mitochondrial impairment and selective dopamine neurodegeneration
in part via iNOS-mediated nitric oxide production. Cell Mol Life
Sci. 74:2851–2874. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Musgrove RE, Helwig M, Bae EJ, Aboutalebi
H, Lee SJ, Ulusoy A and Di Monte DA: Oxidative stress in vagal
neurons promotes parkinsonian pathology and intercellular
alpha-synuclein transfer. J Clin Invest. 129:3738–3753. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Martin LJ, Pan Y, Price AC, Sterling W,
Copeland NG, Jenkins NA, Price DL and Lee MK: Parkinson's disease
alpha-synuclein transgenic mice develop neuronal mitochondrial
degeneration and cell death. J Neurosci. 26:41–50. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Stichel CC, Zhu XR, Bader V, Linnartz B,
Schmidt S and Lübbert H: Mono- and double-mutant mouse models of
Parkinson's disease display severe mitochondrial damage. Hum Mol
Genet. 16:2377–2393. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Ding H, Xiong Y, Sun J, Chen C, Gao J and
Xu H: Asiatic acid prevents oxidative stress and apoptosis by
inhibiting the translocation of α-synuclein into mitochondria.
Front Neurosci. 12:4312018. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Ho MS: Microglia in Parkinson's disease.
Adv Exp Med Biol. 1175:335–353. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Harms AS, Delic V, Thome AD, Bryant N, Liu
Z, Chandra S, Jurkuvenaite A and West AB: α-synuclein fibrils
recruit peripheral immune cells in the rat brain prior to
neurodegeneration. Neuropathol Commun. 5:852017. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Politis M, Su P and Piccini P: Imaging of
microglia in patients with neurodegenerative disorders. Front
Pharmacol. 3:962012. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Joers V, Tansey MG, Mulas G and Carta AR:
Microglial phenotypes in Parkinson's disease and animal models of
the disease. Prog Neurobiol. 155:57–75. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Whitton PS: Inflammation as a causative
factor in the aetiology of Parkinson's disease. Br J Pharmacol.
150:963–976. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Zhang YN, Fan JK, Gu L, Yang HM, Zhan SQ
and Zhang H: Metabotropic glutamate receptor 5 inhibits
α-synuclein-induced microglia inflammation to protect from
neurotoxicity in Parkinson's disease. J Neuroinflammation.
18:232021. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Croisier E, Moran LB, Dexter DT, Pearce RK
and Graeber MB: Microglial inflammation in the parkinsonian
substantia nigra: Relationship to alpha-synuclein deposition. J
Neuroinflammation. 2:142005. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Williams GP, Marmion DJ, Schonhoff AM,
Jurkuvenaite A, Won WJ, Standaert DG, Kordower JH and Harms AS: T
cell infiltration in both human multiple system atrophy and a novel
mouse model of the disease. Acta Neuropathol. 139:855–874. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Roodveldt C, Labrador-Garrido A,
Gonzalez-Rey E, Fernandez-Montesinos R, Caro M, Lachaud CC, Waudby
CA, Delgado M, Dobson CM and Pozo D: Glial innate immunity
generated by non-aggregated alpha-synuclein in mouse: Differences
between wild-type and Parkinson's disease-linked mutants. PLoS One.
5:e134812010. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Vaure C and Liu Y: A comparative review of
toll-like receptor 4 expression and functionality in different
animal species. Front Immunol. 5:3162014. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Uhlén M, Fagerberg L, Hallström BM,
Lindskog C, Oksvold P, Mardinoglu A, Sivertsson A, Kampf C,
Sjöstedt E, Asplund A, et al: Proteomics. Tissue-based map of the
human proteome. Science. 347:12604192015. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Wardill HR, Van Sebille YZ, Mander KA,
Gibson RJ, Logan RM, Bowen JM and Sonis ST: Toll-like receptor 4
signaling: A common biological mechanism of regimen-related
toxicities: An emerging hypothesis for neuropathy and
gastrointestinal toxicity. Cancer Treat Rev. 41:122–128. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Rahimifard M, Maqbool F, Moeini-Nodeh S,
Niaz K, Abdollahi M, Braidy N, Nabavi SM and Nabavi SF: Targeting
the TLR4 signaling pathway by polyphenols: A novel therapeutic
strategy for neuroinflammation. Ageing Res Rev. 36:11–19. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Fellner L, Irschick R, Schanda K, Reindl
M, Klimaschewski L, Poewe W, Wenning GK and Stefanova N: Toll-like
receptor 4 is required for α-synuclein dependent activation of
microglia and astroglia. Glia. 61:349–360. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Mariucci G, Pagiotti R, Galli F, Romani L
and Conte C: The potential role of toll-like receptor 4 in
mediating dopaminergic cell loss and alpha-synuclein expression in
the acute MPTP mouse model of Parkinson's disease. J Mol Neurosci.
64:611–618. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Choi I, Zhang Y, Seegobin SP, Pruvost M,
Wang Q, Purtell K, Zhang B and Yue Z: Microglia clear
neuron-released α-synuclein via selective autophagy and prevent
neurodegeneration. Nat Commun. 11:13862020. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Wilms H, Rosenstiel P, Romero-Ramos M,
Arlt A, Schäfer H, Seegert D, Kahle PJ, Odoy S, Claasen JH,
Holzknecht C, et al: Suppression of MAP kinases inhibits microglial
activation and attenuates neuronal cell death induced by
alpha-synuclein protofibrils. Int J Immunopathol Pharmacol.
22:897–909. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Lee EJ, Woo MS, Moon PG, Baek MC, Choi IY,
Kim WK, Junn E and Kim HS: Alpha-synuclein activates microglia by
inducing the expressions of matrix metalloproteinases and the
subsequent activation of protease-activated receptor-1. J Immunol.
185:615–623. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Zhang W, Dallas S, Zhang D, Guo JP, Pang
H, Wilson B, Miller DS, Chen B, Zhang W, McGeer PL, et al:
Microglial PHOX and Mac-1 are essential to the enhanced
dopaminergic neurodegeneration elicited by A30P and A53T mutant
alpha-synuclein. Glia. 55:1178–1188. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Lastres-Becker I, Ulusoy A, Innamorato NG,
Sahin G, Rábano A, Kirik D and Cuadrado A: α-Synuclein expression
and Nrf2 deficiency cooperate to aggravate protein aggregation,
neuronal death and inflammation in early-stage Parkinson's disease.
Hum Mol Genet. 21:3173–3192. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Sanchez-Guajardo V, Tentillier N and
Romero-Ramos M: The relation between α-synuclein and microglia in
Parkinson's disease: Recent developments. Neuroscience. 302:47–58.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Shavali S, Combs CK and Ebadi M: Reactive
macrophages increase oxidative stress and alpha-synuclein nitration
during death of dopaminergic neuronal cells in co-culture:
Relevance to Parkinson's disease. Neurochem Res. 31:85–94. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Stefanova N, Fellner L, Reindl M, Masliah
E, Poewe W and Wenning GK: Toll-like receptor 4 promotes
alpha-synuclein clearance and survival of nigral dopaminergic
neurons. Am J Pathol. 179:954–963. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Campos SS, Alza NP and Salvador GA: Lipid
metabolism alterations in the neuronal response to A53T α-synuclein
and Fe-induced injury. Arch Biochem Biophys. 655:43–54. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Liao JF, Cheng YF, You ST, Kuo WC, Huang
CW, Chiou JJ, Hsu CC, Hsieh-Li HM, Wang S and Tsai YC:
Lactobacillus plantarum PS128 alleviates neurodegenerative
progression in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced
mouse models of Parkinson's disease. Brain Behav Immun. 90:26–46.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Martin WRW, Miles M, Zhong Q, Hartlein J,
Racette BA, Norris SA, Ushe M, Maiti B, Criswell S, Davis AA, et
al: Is levodopa response a valid indicator of parkinson's disease?
Mov Disord. 36:948–954. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Tomlinson CL, Stowe R, Patel S, Rick C,
Gray R and Clarke CE: Systematic review of levodopa dose
equivalency reporting in Parkinson's disease. Mov Disord.
25:2649–2653. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Epprecht L, Schreglmann SR, Goetze O,
Woitalla D, Baumann CR and Waldvogel D: Unchanged gastric emptying
and visceral perception in early Parkinson's disease after a high
caloric test meal. J Neurol. 262:1946–1953. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Schrag A and Quinn N: Dyskinesias and
motor fluctuations in Parkinson's disease. A community-based study.
Brain. 123((Pt 11)): 2297–2305. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Nonnekes J, Timmer MH, de Vries NM, Rascol
O, Helmich RC and Bloem BR: Unmasking levodopa resistance in
Parkinson's disease. Mov Disord. 31:1602–1609. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Zhu H, Xu G, Zhang K, Kong X, Han R, Zhou
J and Ni Y: Crystal structure of tyrosine decarboxylase and
identification of key residues involved in conformational swing and
substrate binding. Sci Rep. 6:277792016. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Rekdal VM, Bess EN, Bisanz JE, Turnbaugh
PJ and Balskus EP: Discovery and inhibition of an interspecies gut
bacterial pathway for levodopa metabolism. Science.
364:eaau63232019. View Article : Google Scholar
|
|
138
|
van Kessel SP, Frye AK, El-Gendy AO,
Castejon M, Keshavarzian A, van Dijk G and El Aidy S: Gut bacterial
tyrosine decarboxylases restrict levels of levodopa in the
treatment of Parkinson's disease. Nat Commun. 10:3102019.
View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Sampson TR, Debelius JW, Thron T, Janssen
S, Shastri GG, Ilhan ZE, Challis C, Schretter CE, Rocha S,
Gradinaru V, et al: Gut microbiota regulate motor deficits and
neuroinflammation in a model of Parkinson's disease. Cell.
167:1469–1480. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Varankovich NV, Nickerson MT and Korber
DR: Probiotic-based strategies for therapeutic and prophylactic use
against multiple gastrointestinal diseases. Front Microbiol.
6:6852015. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Liu Y, Yin F, Huang L, Teng H, Shen T and
Qin H: Long-term and continuous administration of Bacillus subtilis
during remission effectively maintains the remission of
inflammatory bowel disease by protecting intestinal integrity,
regulating epithelial proliferation, and reshaping microbial
structure and function. Food Funct. 12:2201–2210. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Ait-Belgnaoui A, Durand H, Cartier C,
Chaumaz G, Eutamene H, Ferrier L, Houdeau E, Fioramonti J, Bueno L
and Theodorou V: Prevention of gut leakiness by a probiotic
treatment leads to attenuated HPA response to an acute
psychological stress in rats. Psychoneuroendocrinology.
37:1885–1895. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Evrensel A and Ceylan ME: Fecal microbiota
transplantation and its usage in neuropsychiatric disorders. Clin
Psychopharm Neu. 14:231–237. 2016.PubMed/NCBI
|
|
144
|
Goya ME, Xue F, Sampedro-Torres-Quevedo C,
Arnaouteli S, Riquelme-Dominguez L, Romanowski A, Brydon J, Ball
KL, Stanley-Wall NR and Doitsidou M: Probiotic bacillus subtilis
protects against α-synuclein aggregation in C. elegans. Cell Rep.
30:367–380. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Metta V, Leta V, Mrudula KR, Prashanth LK,
Goyal V, Borgohain R, Chung-Faye G and Chaudhuri KR:
Gastrointestinal dysfunction in Parkinson's disease: Molecular
pathology and implications of gut microbiome, probiotics, and fecal
microbiota transplantation. J Neurol. 21:10072021.
|
|
146
|
Khoruts A: Faecal microbiota
transplantation in 2013: Developing human gut microbiota as a class
of therapeutics. Nat Rev Gastroenterol Hepatol. 11:79–80. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Liang S, Wang T, Hu X, Luo J, Li W, Wu X,
Duan Y and Jin F: Administration of Lactobacillus helveticus NS8
improves behavioral, cognitive, and biochemical aberrations caused
by chronic restraint stress. Neuroscience. 310:561–577. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Vendrik KEW, Ooijevaar RE, de Jong PRC,
Laman JD, van Oosten BW, van Hilten JJ, Ducarmon QR, Keller JJ,
Kuijper EJ and Contarino MF: Fecal microbiota transplantation in
neurological disorders. Front Cell Infect Microbiol. 10:982020.
View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Fang X: Microbial treatment: The potential
application for Parkinson's disease. Neurol Sci. 40:51–58. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Gibson GR, Hutkins R, Sanders ME, Prescott
SL, Reimer RA, Salminen SJ, Scott K, Stanton C, Swanson KS, Cani
PD, et al: Expert consensus document: The international scientific
association for probiotics and prebiotics (ISAPP) consensus
statement on the definition and scope of prebiotics. Nat Rev
Gastroenterol Hepatol. 14:491–502. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Gibson GR and Roberfroid MB: Dietary
modulation of the human colonic microbiota: Introducing the concept
of prebiotics. J Nutr. 125:1401–1412. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Kovacs Z, Benjamins E, Grau K, Ur Rehman
A, Ebrahimi M and Czermak P: Recent developments in manufacturing
oligosaccharides with prebiotic functions. Adv Biochem Eng
Biotechnol. 143:257–295. 2014.PubMed/NCBI
|
|
153
|
Savignac HM, Corona G, Mills H, Chen L,
Spencer JP, Tzortzis G and Burnet PW: Prebiotic feeding elevates
central brain derived neurotrophic factor, N-methyl-D-aspartate
receptor subunits and D-serine. Neurochem Int. 63:756–764. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Armstrong MJ and Okun MS: Diagnosis and
treatment of Parkinson disease: A review. JAMA. 323:548–560. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Emamzadeh FN and Surguchov A: Parkinson's
disease: Biomarkers, treatment, and risk factors. Front Neurosci.
12:6122018. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Takahashi M, Suzuki M, Fukuoka M, Fujikake
N, Watanabe S, Murata M, Wada K, Nagai Y and Hohjoh H:
Normalization of overexpressed α-synuclein causing Parkinson's
disease by a moderate gene silencing with RNA interference. Mol
Ther Nucleic Acids. 4:e2412015. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Ding L, Gu H, Xiong X, Ao H, Cao J, Lin W,
Yu M, Lin J and Cui Q: MicroRNAs involved in carcinogenesis,
prognosis, therapeutic resistance and applications in human
triple-negative breast cancer. Cells. 8:14922019. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Junn E, Lee KW, Jeong BS, Chan TW, Im JY
and Mouradian MM: Repression of alpha-synuclein expression and
toxicity by microRNA-7. Proc Natl Acad Sci USA. 106:13052–13057.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Masliah E, Rockenstein E, Adame A, Alford
M, Crews L, Hashimoto M, Seubert P, Lee M, Goldstein J, Chilcote T,
et al: Effects of alpha-synuclein immunization in a mouse model of
Parkinson's disease. Neuron. 46:857–868. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Ghochikyan A, Petrushina I, Davtyan H,
Hovakimyan A, Saing T, Davtyan A, Cribbs DH and Agadjanyan MG:
Immunogenicity of epitope vaccines targeting different B cell
antigenic determinants of human alpha-synuclein: Feasibility study.
Neurosci Lett. 560:86–91. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Vaikath NN, Hmila I, Gupta V, Erskine D,
Ingelsson M and El-Agnaf OMA: Antibodies against alpha-synuclein:
Tools and therapies. J Neurochem. 150:612–625. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Rabenstein M, Agbo DB, Wolf E, Dams J,
Nicolai M, Roeder A, Bacher M, Dodel RC and Noelker C: Effect of
naturally occurring α-synuclein-antibodies on toxic
alpha-synuclein-fragments. Neurosci Lett. 704:181–188. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Wang Z, Gao G, Duan C and Yang H: Progress
of immunotherapy of anti-alpha-synuclein in Parkinson's disease.
Biomed Pharmacother. 115:1088432019. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Lashuel HA, Overk CR, Oueslati A and
Masliah E: The many faces of alpha-synuclein: From structure and
toxicity to therapeutic target. Nat Rev Neurosci. 14:38–48. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Sulzer D, Alcalay RN, Garretti F, Cote L,
Kanter E, Agin-Liebes J, Liong C, McMurtrey C, Hildebrand WH, Mao
X, et al: T cells from patients with Parkinson's disease recognize
α-synuclein peptides. Nature. 546:656–661. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Tran HT, Chung CH, Iba M, Zhang B,
Trojanowski JQ, Luk KC and Lee VM: Alpha-synuclein immunotherapy
blocks uptake and templated propagation of misfolded
alpha-synuclein and neurodegeneration. Cell Rep. 7:2054–2065. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Zhou C, Emadi S, Sierks MR and Messer A: A
human single-chain Fv intrabody blocks aberrant cellular effects of
overexpressed alpha-synuclein. Mol Ther. 10:1023–1031. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
168
|
El-Agnaf O, Overk C, Rockenstein E, Mante
M, Florio J, Adame A, Vaikath N, Majbour N, Lee SJ, Kim C, et al:
Differential effects of immunotherapy with antibodies targeting
alpha-synuclein oligomers and fibrils in a transgenic model of
synucleinopathy. Neurobiol Dis. 104:85–96. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Rutherford NJ, Brooks M and Giasson BI:
Novel antibodies to phosphorylated α-synuclein serine 129 and NFL
serine 473 demonstrate the close molecular homology of these
epitopes. Acta Neuropathol Commun. 4:802016. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Wang N, Garcia J, Freeman R and Gibbons
CH: Phosphorylated alpha-synuclein within cutaneous autonomic
nerves of patients with Parkinson's disease: The implications of
sample thickness on results. J Histochem Cytochem. 68:669–678.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Ciesielska A, Matyjek M and Kwiatkowska K:
TLR4 and CD14 trafficking and its influence on LPS-induced
pro-inflammatory signaling. Cell Mol Life Sci. 78:1233–1261. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Perez-Pardo P, Dodiya HB, Engen PA,
Forsyth CB, Huschens AM, Shaikh M, Voigt RM, Naqib A, Green SJ,
Kordower JH, et al: Role of TLR4 in the gut-brain axis in
Parkinson's disease: A translational study from men to mice. Gut.
68:829–843. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Brundin L, Bryleva EY and Rajamani KT:
Role of inflammation in suicide: From mechanisms to treatment.
Neuropsychopharmacology. 42:271–283. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Decressac M, Mattsson B, Weikop P,
Lundblad M, Jakobsson J and Bjorklund A: TFEB-mediated autophagy
rescues midbrain dopamine neurons from α-synuclein toxicity. Proc
Natl Acad Sci USA. 110:E1817–E1826. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Maiese K, Chong ZZ, Shang YC and Wang S:
mTOR: On target for novel therapeutic strategies in the nervous
system. Trends Mol Med. 19:51–60. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Sardi SP, Cedarbaum JM and Brundin P:
Targeted therapies for Parkinson's Disease: From genetics to the
clinic. Mov Disord. 33:684–696. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Ghosh A, Tyson T, George S, Hildebrandt
EN, Steiner JA, Madaj Z, Schulz E, Machiela E, McDonald WG, Escobar
Galvis ML, et al: Mitochondrial pyruvate carrier regulates
autophagy, inflammation, and neurodegeneration in experimental
models of Parkinson's disease. Sci Transl Med. 8:368ra1742016.
View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Erlich S, Shohami E and Pinkas-Kramarski
R: Neurodegeneration induces upregulation of beclin 1. Autophagy.
2:49–51. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Spencer B, Potkar R, Trejo M, Rockenstein
E, Patrick C, Gindi R, Adame A, Wyss-Coray T and Masliah E: Beclin
1 gene transfer activates autophagy and ameliorates the
neurodegenerative pathology in alpha-synuclein models of
Parkinson's and lewy body diseases. J Neurosci. 29:13578–13588.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Savitt D and Jankovic J: Targeting
α-synuclein in Parkinson's disease: Progress towards the
development of disease-modifying therapeutics. Drugs. 79:797–810.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Hussain T, Zhao D, Shah SZA, Sabir N, Wang
J, Liao Y, Song Y, Dong H, Mangi MH, Ni J, et al: Nilotinib: A
tyrosine kinase inhibitor mediates resistance to intracellular
mycobacterium via regulating autophagy. Cells. 8:5062019.
View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Pagan F, Hebron M, Valadez EH,
Torres-Yaghi Y, Huang X, Mills RR, Wilmarth BM, Howard H, Dunn C,
Carlson A, et al: Nilotinib effects in Parkinson's disease and
dementia with lewy bodies. J Parkinsons Dis. 6:503–517. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
183
|
de Groot P, Nikolic T, Pellegrini S, Sordi
V, Imangaliyev S, Rampanelli E, Hanssen N, Attaye I, Bakker G,
Duinkerken G, et al: Faecal microbiota transplantation halts
progression of human new-onset type 1 diabetes in a randomised
controlled trial. Gut. 70:92–105. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Gurung M, Li Z, You H, Rodrigues R, Jump
DB, Morgun A and Shulzhenko N: Role of gut microbiota in type 2
diabetes pathophysiology. EBioMedicine. 51:1025902020. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Horsager J, Andersen KB, Knudsen K,
Skjærbæk C, Fedorova TD, Okkels N, Schaeffer E, Bonkat SK, Geday J,
Otto M, et al: Brain-first versus body-first Parkinson's disease: A
multimodal imaging case-control study. Brain. 143:3077–3088. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Barbut D, Stolzenberg E and Zasloff M:
Gastrointestinal immunity and alpha-synuclein. J Parkinsons Dis.
9:S313–S322. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Perni M, Galvagnion C, Maltsev A, Meisl G,
Müller MB, Challa PK, Kirkegaard JB, Flagmeier P, Cohen SI,
Cascella R, et al: A natural product inhibits the initiation of
α-synuclein aggregation and suppresses its toxicity. Proc Natl Acad
Sci USA. 114:E1009–E1017. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Das T and Eliezer D: Membrane interactions
of intrinsically disordered proteins: The example of
alpha-synuclein. Biochim Biophys Acta Proteins Proteom.
1867:879–889. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Abbott SK, Li H, Muñoz SS, Knoch B,
Batterham M, Murphy KE, Halliday GM and Garner B: Altered ceramide
acyl chain length and ceramide synthase gene expression in
Parkinson's disease. Mov Disord. 29:518–526. 2014. View Article : Google Scholar : PubMed/NCBI
|