Introduction
Anorectal pathologies such as hemorrhoids and
fistula are common conditions affecting normal functions of anus
and rectum (1). Hemorrhoids are
symptomatic enlargement and distal displacement of vascular anal
cushions. It is estimated that 75% of the world population
experiences enlarged hemorrhoids at a certain point of time in
their lives (2). The incidence of
hemorrhoids is equally in males and females, with the highest
occurrence rate between 45 and 65 years of age (2,3). A
total of 40% of patients with hemorrhoids exhibit no symptoms
(3). However, pain, itching,
swelling, anal discomfort and rectal bleeding are observed in
symptomatic patients (4). Multiple
factors contribute to the development of hemorrhoids that include
prolonged straining, diarrhea, constipation, overweight, obesity,
pregnancy and old age (4–6). Previous studies indicated that severe
inflammatory reaction takes place in the vascular wall and the
surrounding connective tissue of the hemorrhoids and it gradually
leads to mucosal ulceration, ischemia and thrombosis (7). In this area, tissue damage induces an
inflammatory response, which manifests as infiltration of
T-lymphocytes, macrophages, neutrophils, monocytes, mast cells and
dendritic cells (8). These cells
secrete both anti- and proinflammatory cytokines. However, due to
excessive production of proinflammatory cytokines, the
proinflammatory response gradually overtakes the anti-inflammatory
response (8,9). IL-1β, IL-6, regulated upon activation,
normal T cell expressed and presumably secreted (RANTES), VEGF and
TNF-α are considered proinflammatory cytokines which have a
critical role in human health and disease (10,11).
RANTES is a C-C motif chemokine ligand 5, has a critical role in
inflammation by mobilizing different types of immune cells
(12,13). The proangiogenic factor VEGF is
highly present at these sites and involved in the advancement of
hemorrhoidal disease by regulating increased vascular density
(7).
An anal fistula is considered an epithelialized
tract or a connection between the anal canal and the perianal skin.
Classic anal fistulas are the outcome of a perineal infection and
abscess formation (14). Fistulas
are also associated with inflammatory bowel disease, Crohn's
disease, radiation, malignancy, chronic diarrhea or pre-existing
incontinence (15). Expression of
RANTES and VEGF is observed in Crohn's disease-related perianal
fistula and arteriovenous fistulas (16,17).
Fistulectomy is a common operative procedure for the treatment of
fistula (18). These procedures are
associated with postoperative complications, anal incontinence and
recurrence (19). Hemorrhoids and
fistulas are associated with inflammation. Different types of
cells, such as macrophages, neutrophils, and fibroblasts, are
present in the inflammatory environment of hemorrhoids and fistulas
(8,15,20–22).
Natural remedies for hemorrhoids and fistula, including laxatives,
analgesics and anti-inflammatory agents, have drawn much attention
recently due to their relatively higher effectiveness, low cost and
lower invasiveness (23,24). A study by our group, for the first
time, reported that Anoac-H, a polyherbal formulation, exhibits
safety and efficacy in treating bleeding hemorrhoids (25). The present study aimed to
investigate the mechanism of action of Anoac-H in the treatment of
bleeding hemorrhoids and fistula by studying its effect on the
expression of proinflammatory and proangiogenic factors.
Materials and methods
Cell culture
Fibroblasts and macrophages were used as a model for
inflammatory cells present in hemorrhoids and fistulas. The human
foreskin fibroblast cell line BJ and the mouse
monocyte/macrophage-like cell line RAW 264.7 were purchased from
the American Type Culture Collection. BJ and RAW 264.7 cells were
cultured in MEM(E) (HiMedia Laboratories LLC) and RPMI (Gibco;
Thermo Fisher Scientific, Inc.) media respectively, supplemented
with 10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.)
and 100 units of penicillin and 100 µg/ml streptomycin at 37°C in a
humidified atmosphere with 5% CO2. All treatments were
performed in complete medium.
Drug preparation
Anoac-H/PiloTab (Healing Hands & Herbs,
http://healinghandsandherbs.in/) is an
ayurvedic polyherbal formulation in the form of a tablet. Anoac-H
tablet consists of Lajjalu (Mimosa pudica), Dugdhika
(Euphorbia hirta), Nagkesar (Messua ferrea) and
Daruharidra (Berberis aristata) extracts, as described in a
previous study (25). For the
treatment of the cells, the tablet was minced and dissolved in
sterile water to prepare a stock of 100 mg/ml.
Cell viability assay
Cell viability was studied using the MTT assay, as
per the previously described protocol (26). In brief, BJ/RAW 264.7 cells
(1×104) were seeded into 96-well flat-bottom microplates
and treated with vehicle control (equal volume of water) or Anoac-H
at different concentrations (25–500 µg/ml) for 24 h. To each well,
MTT (0.5 mg/ml) solution was added, followed by incubation for 4 h
at 37°C. Isopropanol was then added to dissolve the formazan
crystals and the optical density of the formazan solution was
measured at 570 nm using an automated microplate reader (EPOCH2;
Agilent Technologies, Inc.). All experiments were performed in
biological triplicates.
Migration assay
Cell migration was studied by a wound closure assay
as per the standard procedure described previously (27,28).
In brief, BJ/RAW 264.7 (2×105) cells were seeded in
12-well plates and allowed to attain confluency. When cells
achieved confluency, a line-shaped scratch was made in the
monolayers with a sterile 200-µl pipette tip. Cells were either
treated with vehicle control (equal volume of water) or Anoac-H at
different concentrations (100 and 250 µg/ml). Images were captured
at 0 and 12/16 h using a Nikon phase-contrast microscope (Nikon
Corp.). The area of wound closure was analyzed by Image-Pro Plus
6.0 software (National Institutes of Health).
Western blot analysis
Western blot was performed to examine the expression
of proangiogenic and proinflammatory proteins in control or treated
cells according to a standard protocol (29). In brief, cells were harvested and
lysed using RIPA lysis buffer. Protein concentration of cell
lysates was determined using a Bradford assay. Equal amounts of
total protein (30 µg) were resolved by SDS-PAGE (10 or 12.5% gels).
The resolved proteins were then transferred onto a PVDF membrane
(Bio-Rad Laboratories, Inc.) and processed for analysis. The
membrane was incubated with antibodies to RANTES (Santa Cruz
Biotechnology, Inc., cat. no. sc-1410, 1:1,000 dilution), VEGF
(Santa Cruz Biotechnology, Inc., cat. no. sc-7269, 1:1,000
dilution), IL-1β (Elabscience Biotechnology Inc; cat. no.
E-AB-52153, 1:1,000 dilution) overnight at 4°C, followed by
respective horseradish peroxidase (HRP) antibodies (anti-goat HRP,
cat. no. sc-2020, anti-rabbit HRP, cat. no. sc-2005, anti-mouse
HRP, cat. no. sc-2004, Santa Cruz Biotechnology, Inc., 1:2,000
dilution) for 1 h at room temperature. β-actin (Santa Cruz
Biotechnology, Inc., cat. no. sc-1615, 1:2,000 dilution) was used
as a loading control. All the blots were visualized using Clarity
Western ECL (Bio-Rad Laboratories, Inc.) reagent.
Anorectal specimen analysis
The present study was approved by the Institutional
Ethics Committee of Healing Hands Clinic (Pune, India). Human
hemorrhoid and fistula specimens were collected with the help of a
histopathologist from Healing Hands Clinic (Pune, India) with
informed consent and the study was performed between February 2020
and December 2020. Paraffin-embedded tissue blocks were cut into
5-µm sections and deposited on poly-L-lysine coated slides.
Immunohistochemistry (IHC) was performed using the Super Sensitive
Polymer-HRP IHC Detection System (BioGenex) as per the
manufacturer's instructions. In brief, the sections were
deparaffinized in xylene and subjected to antigen retrieval in a
citrate buffer at 90°C for 15 min following rehydration. Sections
were covered with peroxide block (3% hydrogen peroxide in water)
and followed by power block (casein and proprietary additives in
PBS with 0.09% sodium azide) of Super Sensitive Polymer-HRP IHC
Detection system (BioGenex Laboratories) to block endogenous
peroxidase activity and non-specific binding sites, respectively.
Sections were incubated with primary antibodies, such as RANTES
(Santa Cruz Biotechnology, Inc., sc-365826, 1:100 dilution) and
VEGF (Santa Cruz Biotechnology, Inc., sc-7269, 1:100 dilution),
overnight at 4°C and subsequently with poly-HRP reagent (from Super
Sensitive Polymer-HRP IHC Detection system) for 1 h at room
temperature. Liquid DAB chromogen was added at room temperature for
10 min and images were captured using a Nikon Eclipse microscope
(Nikon Corp.).
Statistical analysis
All experiments were performed in biological
triplicates. GraphPad Prism 5 software (GraphPad Software, Inc.)
was used for the statistical analysis of the data. Unless indicated
otherwise, the results are expressed as the mean ± standard error
of the mean. Statistical significance between two groups was
determined by Student's t-test, while one-way ANOVA or the
Kruskal-Wallis test was used to determine statistical significance
in the case of multiple doses of drug treatments with the Dunn's
post-hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Effect of Anoac-H on cell
viability
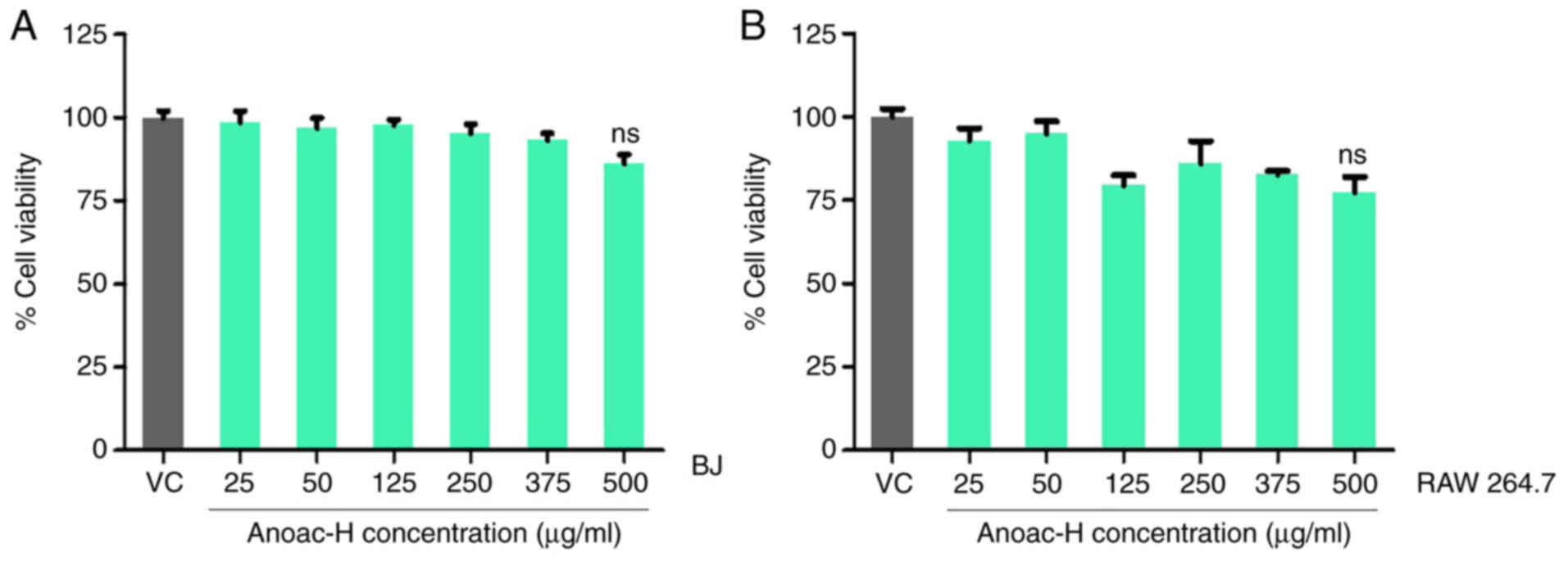
Fibroblasts were treated with the polyherbal
formulation Anoac-H to examine its effect on cell viability of the
cells. An MTT assay was performed after treatment with vehicle
control/Anoac-H at different concentrations (25–500 µg/ml) for 24
h, as per an earlier protocol (26). Kruskal-Wallis was performed to test
for statistically significant differences among the groups. The
results indicated that Anoac-H did not significantly affect the
viability of BJ human fibroblasts as compared to the vehicle
control (Fig. 1A). The same
experiment was performed on RAW 264.7 mouse macrophage-like cells
to observe the effect of Anoac-H on these cells and similarly, the
cell viability was not markedly affected (Fig. 1B). From these observations, it may
be inferred that Anoac-H does not affect the viability of
fibroblasts and macrophages.
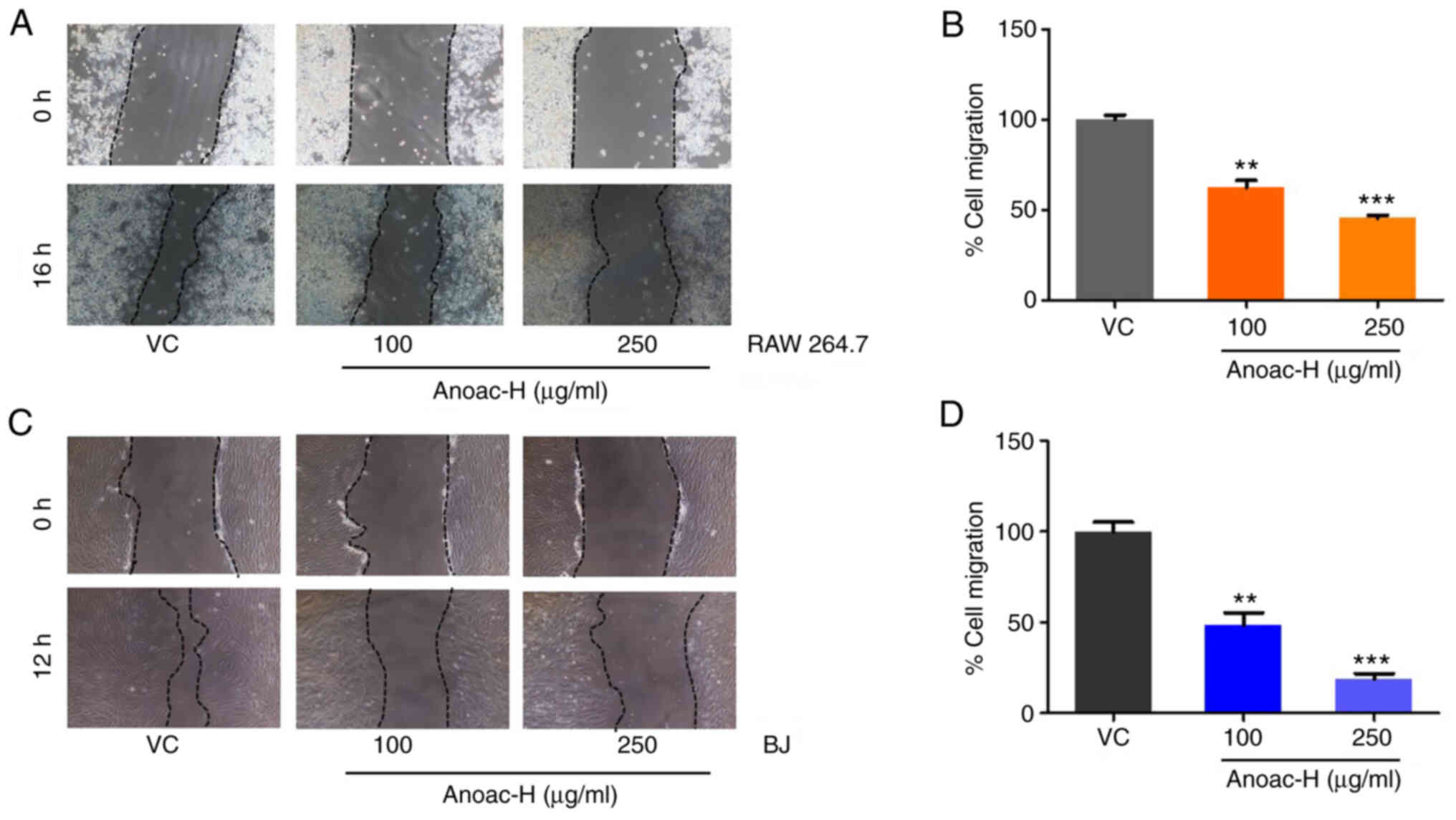
Anoac-H suppresses the cell migratory
potential
Several cell types infiltrate the site of
hemorrhoids and fistula to induce an inflammatory reaction
(8,15,20–22).
Several cell types, including macrophages and fibroblasts, migrate
to the site of inflammation and have a critical role in the
inflammatory reaction (20–22). Hence, in the present study, the
effect of Anoac-H on the migration of macrophages and fibroblasts
was assessed by performing a conventional wound migration assay.
Monolayers of fibroblasts and macrophages were wounded and treated
with either vehicle control or different concentrations of Anoac-H
(100 and 250 µg/ml). The effect of Anoac-H on the migration of
fibroblasts and macrophages was statistically examined using
Student's t-test. The results indicated that Anoac-H significantly
suppressed the migration of RAW 264.7 and BJ cells (Fig. 2A-D). Taken together, the present
data suggested that Anoac-H exhibits antimigratory effects.
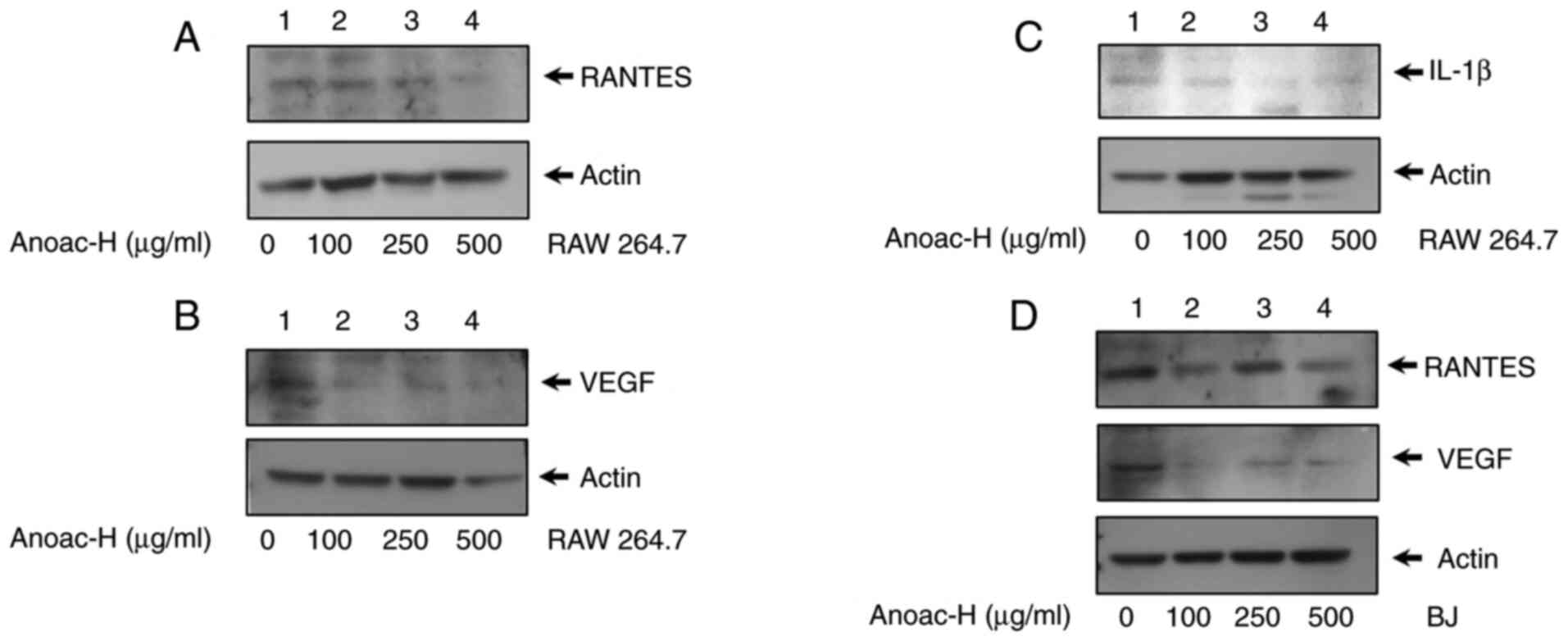
Anoac-H suppresses the expression of
proinflammatory and proangiogenic factors
Proinflammatory and angiogenic factors have a
critical role in shaping the inflammatory environment during
fistula and hemorrhoidal disease progression (10,16,17,20).
In the present study, the effect of Anoac-H on the expression of
the proinflammatory factors RANTES and IL-1β and the proangiogenic
factor VEGF were examined by western blot analysis. The results
revealed that the expression of RANTES and VEGF was significantly
downregulated upon Anoac-H treatment as compared to the vehicle
control in RAW 264.7 cells (Fig. 3A and
B). In addition, the expression of another inflammatory
cytokine, IL-1β, was also reduced by Anoac-H treatment in RAW 264.7
cells (Fig. 3C). A similar
experiment was performed using BJ cells. Though expression of these
cytokines was less in BJ cells, the results revealed that the
expression of RANTES and VEGF was considerably reduced in
Anoac-H-treated BJ cells as compared to the vehicle control
(Fig. 3D). Collectively, these
results demonstrated that Anoac-H significantly suppressed the
expression of proinflammatory and proangiogenic factors, RANTES,
IL-1β and VEGF, in both macrophages and fibroblasts.
Correlation of RANTES and VEGF
expression in patients with anorectal disease
Formalin-fixed, paraffin-embedded human hemorrhoids
(n=8) and fistula specimens (n=6) were subjected to histological
examination by staining with H&E. Out of the 14 patients with
anorectal disease, 6 patients were treated with Anoac-H, whereas 8
patients were untreated. The age distribution of patients was
between 29–65 years (Table SI). A
reduction in inflammation was observed in treated specimens as
compared to untreated anorectal tissues (Fig. 4A-D). These specimens were also
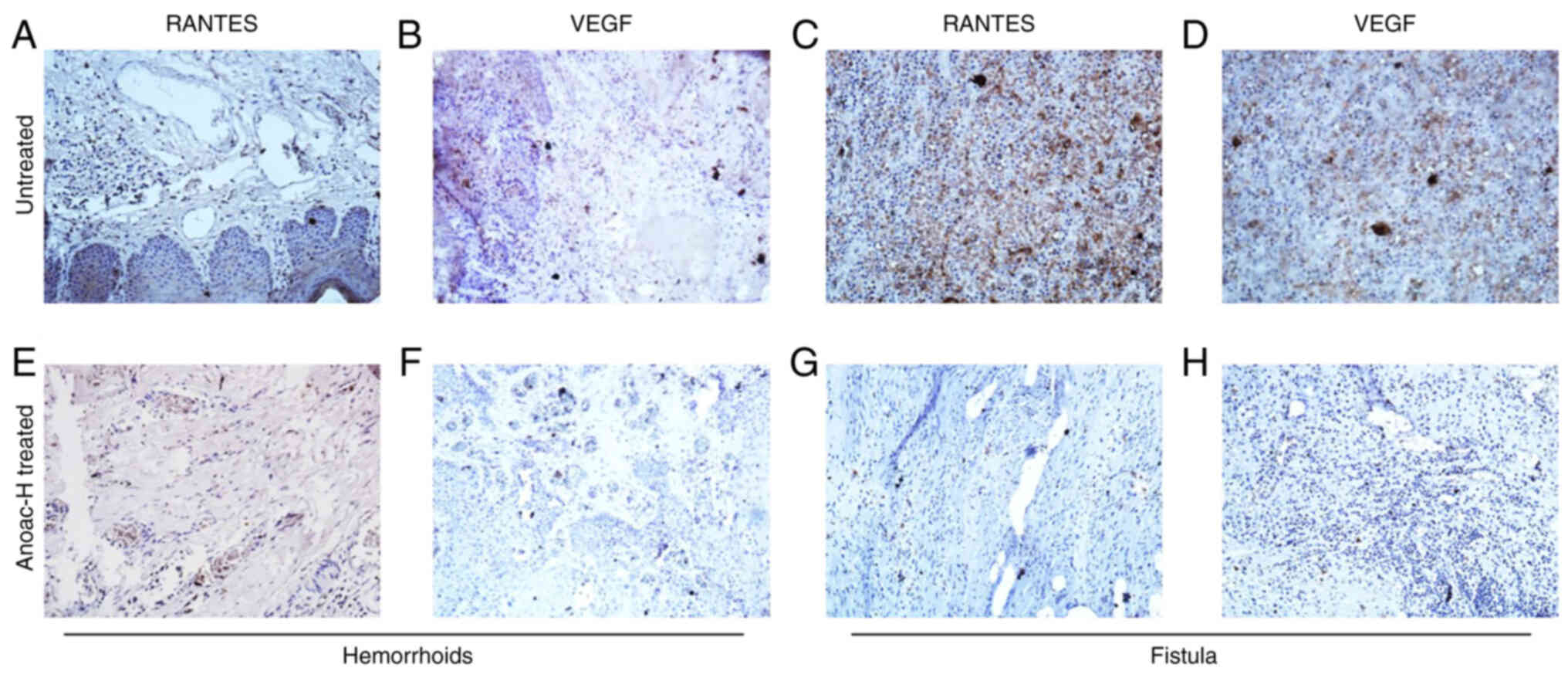
analyzed by IHC to determine the expression of RANTES and VEGF. The
IHC data confirmed the expression of RANTES and VEGF in hemorrhoids
(Fig. 5A and B). An elevated
expression of RANTES and VEGF was also observed in fistula
specimens (Fig. 5C and D).
Furthermore, it was observed that the expression of RANTES was
associated with the expression of VEGF in these tissues (Fig. 5A-D). In a previous study by our
group, it was determined that Anoac-H helps in the management of
hemorrhoids and relieving disease-associated symptoms (25). To determine the association of the
expression of RANTES and VEGF with the alleviation of the disease,
IHC was performed on Anoac-H-treated hemorrhoids and fistula
specimens. Of note, the results indicated that the expression of
RANTES and VEGF was drastically reduced in Anoac-H treated
hemorrhoid tissues as compared with untreated patients (Fig. 5E and F). In addition, the results
also suggested that the expression of RANTES and VEGF was
drastically reduced in Anoac-H treated fistula tissues as compared
with untreated patients (Fig. 5G and
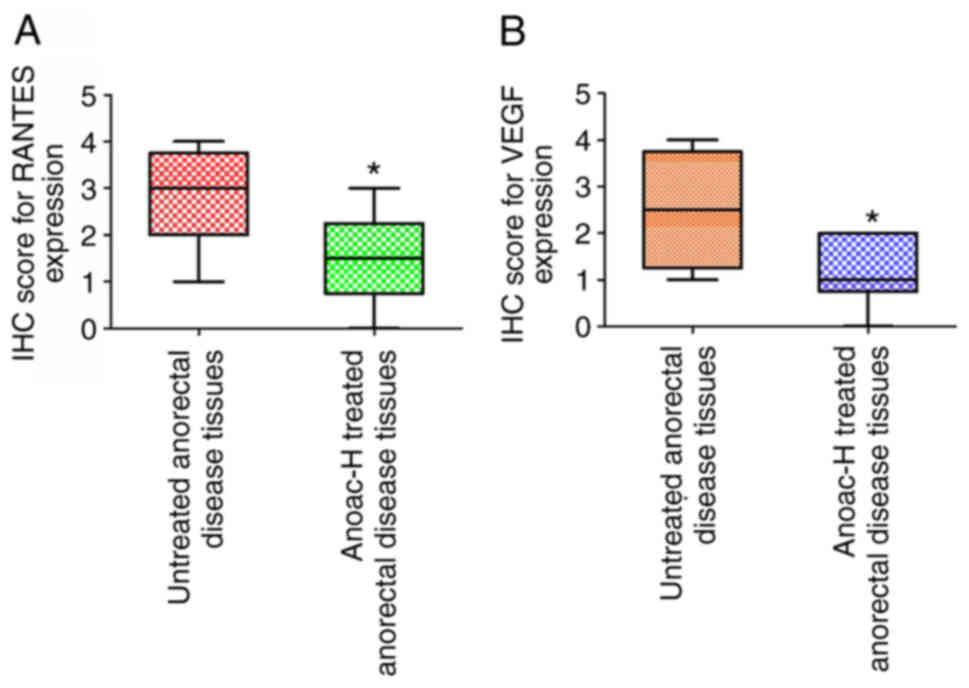
H). IHC scoring for staining intensity also indicated a
significant reduction of RANTES and VEGF expression in the Anoac-H
treatment group as compared to untreated anorectal disease tissues
(Fig. 6A and B). This is consistent
with the present in vitro findings and Anoac-H inhibiting
the expression of RANTES and VEGF may thus reduce inflammation and
affect the vasculature to alleviate the disease.
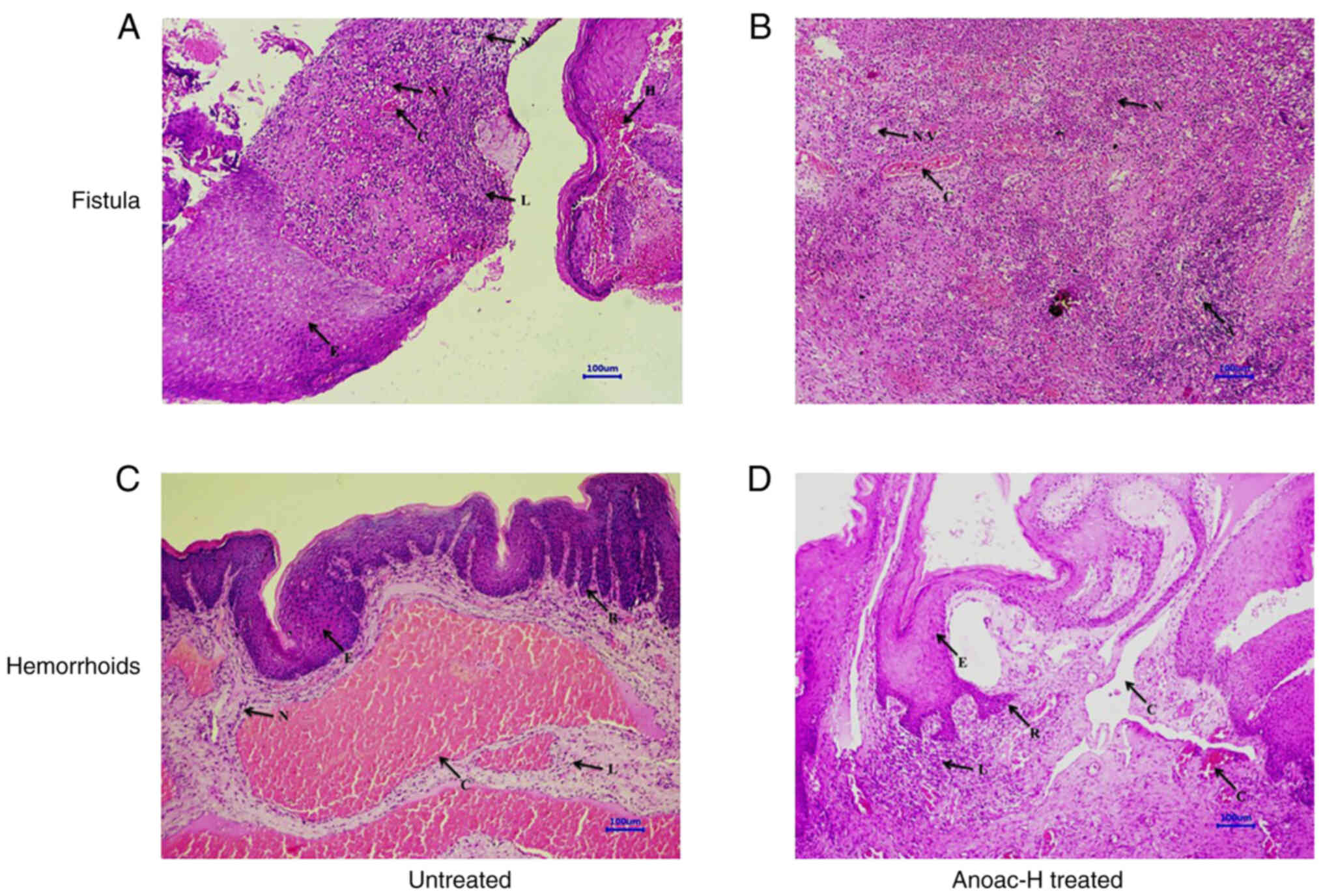 | Figure 4.Histological examination of the
effect of Anoac-H on tissues of anorectal disease. Untreated or
treated hemorrhoids and fistula specimens were stained with
H&E. (A) Untreated fistula, (B) treated fistula, (C) untreated
hemorrhoids, (D) treated hemorrhoids. The following features were
indicated: N, neutrophil infiltration; NV, neovascularization; L,
lymphocyte infiltration; E, epithelial hyperplasia; C, congestion;
A, microabscess formation; R, rete ridge formation; and H,
hemorrhages (images were captured using ×10 objective; scale bar,
100 µm). |
Discussion
Bleeding hemorrhoids and fistula are common
anorectal conditions in humans, but pathologically, they are
complex. Patients who present with signs and symptoms of
hemorrhoids and fistula require to be carefully evaluated to
exclude other masking morbidities (30,31).
Hemorrhoids and fistulas are responsible for unrelenting discomfort
and cause of distressing pain in patients (4,15,23,30).
These anorectal disorders are associated with severe inflammatory
reaction. Hemorrhoids are multifactorial diseases and increased
vascular density and enlargement of vascular component, as well as
disruption of stromal scaffolding are observed in hemorrhoids
(7,32). The treatment of hemorrhoids aims at
three different aspects. That includes alleviating the immediate
symptoms, preventing further exacerbation of the injury and
resolving the underlying cause (4,33).
Hemorrhoidectomy and fistulectomy are the surgical procedure for
removal and management of hemorrhoids and fistula, respectively
(18,34). However, these procedures are
invasive and associated with postoperative pain, bleeding and fecal
incontinence (7,19). Moggia et al (35) have indicated that treatment of
hemorrhoid-associated severe bleeding with embolization of superior
rectal arteries using coils is safe and effective. Furthermore,
relapse of disease is a major limitation of these procedures after
a certain postoperative period. Different treatment modalities have
been designed to minimize post-operative pain associated with the
spasm of the internal sphincter (36,37).
Patti et al (38) have
reported that the administration of botulinum toxin into the
internal anal sphincter after hemorrhoidectomy was successful in
decreasing maximum resting pressure, time of wound healing and pain
both in the resting state and during defecation without any side
effects and complications. Khan et al (39) have achieved efficacy in wound
healing and pain relief when they performed trials with a
combination of 0.2% glyceryl trinitrate and 2% lignocaine ointments
after Milligan Morgan hemorrhoidectomy. Another study has also
reported that treatment of post-hemorrhoidectomy wounds with
triclosan solution is safe and it reduces the wound healing time
compared to the control (40). Yet
another study revealed that administration of mesoglycan prevented
postoperative thrombosis and reduced pain after an open diathermy
excisional hemorrhoidectomy surgery (41). Furthermore, non-surgical treatments
such as topical and pharmacological approaches have not been fully
effective. Treatment with Proctosoll Allevia® was proven
effective against I–II degree symptomatic hemorrhoids with a good
profile of tolerability and safety (42). A clinical study by our group
suggested that treatment with Anoac-H polyherbal formulation
exhibited efficacy and safety in patients with bleeding hemorrhoids
(25). However, the mechanisms of
its action on bleeding hemorrhoids and fistula have remained to be
fully elucidated. Previous studies indicated that the treatment
modalities that were demonstrated to alleviate inflammation
achieved great clinical successes in the management of anorectal
diseases (7). In particular,
traditional medicine has proven effective for the clinical control
of hemorrhoids and fistula by reducing inflammation (24,43).
In the present study, it was demonstrated that Anoac-H, a
traditional medicine, exerts its action on bleeding hemorrhoids,
possibly by suppressing migration of immune and other cell types.
The in vitro assays as well as clinical specimen analyses
indicated that treatment with Anoac-H led to downregulation of
RANTES and VEGF in cell lines as well as tissues. Anoac-H may
reduce inflammation by downregulating RANTES and VEGF thereby
controls hemorrhoids and fistula. VEGF is also known to be involved
in angiogenesis as a proangiogenic factor. Anoac-H may reduce
vascular density by downregulating VEGF in cell lines and clinical
tissues, as vascular density drastically increases in these
diseases (7). Anoac-H formulation
consists of the extracts of Mimosa pudica, Euphorbia hirta,
Messua ferrea and Berberis aristate. All of these
traditional medicines were reported to exhibit anti-inflammatory
activities in in vitro/in vivo models (44–47).
The present results are consistent with previous studies that
support the anti-inflammatory activity of these medicinal plants.
An earlier clinical study by our group reported that 95% of the
patients treated with Anoac-H had recovered from bleeding
hemorrhoids. Furthermore, in the present study, it was demonstrated
that the action of Anoac-H on bleeding hemorrhoids was due to its
effect on migration, inflammation and downregulation of RANTES and
VEGF. Although conclusive results may be obtained with a smaller
number of patient samples, the number of anorectal samples is a
major limitation of the present study. In the future, further
experiments will be performed to validate the present results with
a larger number of samples and the molecular mechanisms of the
anti-inflammatory effects of Anoac-H will be comprehensively
investigated.
In conclusion, the present study identified that
Anoac-H does not affect the viability of fibroblasts and
macrophages; however, it significantly decreases the migration of
these cells. Furthermore, it suppressed the expression of RANTES
and VEGF. It may be speculated that Anoac-H exerts its effects on
hemorrhoids and fistula by alleviating inflammation and affecting
the vasculature at the lesion sites. The present results highlight
the implications of Anoac-H in the management of bleeding
hemorrhoids and fistula.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
Experiments were conceptually designed by AP, GCK,
GB and RB. Herbal material was prepared by AP and GB. Experiments
were performed and the manuscript was written by RB. AP, GCK and RB
were conducted the analysis. The study was supervised by GCK. The
manuscript was revised by AP, GCK and GB. AP and GCK confirmed the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Institutional Ethics
Committee of Healing Hands Clinic (Pune, India). Written informed
consent for participation in the study or use of their tissue was
obtained from all participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests. GB and RB worked for the company Healing Hands &
Herbs.
References
|
1
|
Foxx-Orenstein AE, Umar SB and Crowell MD:
Common anorectal disorders. Gastroenterol Hepatol (NY). 10:294–301.
2014.PubMed/NCBI
|
|
2
|
Guindic LC: Treatment of uncomplicated
hemorrhoids with a Hemor-Rite® cryotherapy device: A
randomized, prospective, comparative study. J Pain Res. 7:57–63.
2014.PubMed/NCBI
|
|
3
|
Lorenzo-Rivero S: Hemorrhoids: Diagnosis
and current management. Am Surg. 75:635–642. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sun Z and Migaly J: Review of hemorrhoid
disease: Presentation and management. Clin Colon Rectal Surg.
29:22–29. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Riss S, Weiser FA, Riss T, Schwameis K,
Mittlböck M and Stift A: Haemorrhoids and quality of life.
Colorectal Dis. 13:e48–e52. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Peery AF, Sandler RS, Galanko JA,
Bresalier RS, Figueiredo JC, Ahnen DJ, Barry EL and Baron JA: Risk
factors for hemorrhoids on screening colonoscopy. PLoS One.
10:e01391002015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lohsiriwat V: Hemorrhoids: From basic
pathophysiology to clinical management. World J Gastroenterol.
18:2009–2017. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shrivastava L, da Silva Borges G and
Shrivastava R: Clinical efficacy of a dual action, topical
anti-edematous and antiinflammatory device for the treatment of
external hemorrhoids. Clin Exp Pharmacol. 8:1–7. 2018. View Article : Google Scholar
|
|
9
|
Srivastava A, Yadav SK, Yachha SK, Thomas
MA, Saraswat VA and Gupta RK: Pro-inflammatory cytokines are raised
in extrahepatic portal venous obstruction, with minimal hepatic
encephalopathy. J Gastroenterol Hepatol. 26:979–986. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wojdasiewicz P, Poniatowski ŁA and
Szukiewicz D: The role of inflammatory and anti-inflammatory
cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm.
2014:5614592014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gulati K, Guhathakurta S, Joshi J, Rai N
and Ray AJ: Cytokines and their role in health and disease: A brief
overview. MOJ Immunol. 4:1–9. 2016.
|
|
12
|
Schall TJ: Biology of the RANTES/SIS
cytokine family. Cytokine. 3:165–183. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Appay V, Brown A, Cribbes S, Randle E and
Czaplewski LG: Aggregation of RANTES is responsible for its
inflammatory properties. Characterization of nonaggregating,
noninflammatory RANTES mutants. J Biol Chem. 274:27505–27512. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nottingham JM and Rentea RM: Anal
Fistulotomy (Seton Placement). StatPearls Publishing; Treasure
Island, FL: 2020
|
|
15
|
Gardner IH, Siddharthan RV and Tsikitis
VL: Benign anorectal disease: Hemorrhoids, fissures, and fistulas.
Ann Gastroenterol. 33:9–18. 2020.PubMed/NCBI
|
|
16
|
Haddow JB, Musbahi O, MacDonald TT and
Knowles CH: Comparison of cytokine and phosphoprotein profiles in
idiopathic and Crohn's disease-related perianal fistula. World J
Gastrointest Pathophysiol. 10:42–53. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang K, Deng P, Sun Y, Ye P, Zhang A, Wu
C, Yue Z, Chen Z and Xia J: MicroRNA-155 promotes neointimal
hyperplasia through smooth muscle-like cell-derived RANTES in
arteriovenous fistulas. J Vasc Surg. 67:933–944.e3. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sheikh P and Baakza A: Management of
fistula-in-ano-the current evidence. Indian J Surg. 76:482–486.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Emile SH: Recurrent anal fistulas: When,
why, and how to manage? World J Clin Cases. 8:1586–1591. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Azeemuddin M, Viswanatha GL, Rafiq M,
Thippeswamy AH, Baig MR, Kavya KJ, Patki PS and Shyam R: An
improved experimental model of hemorrhoids in rats: Evaluation of
antihemorrhoidal activity of an herbal formulation. ISRN Pharmacol.
2014:5309312014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Barnhoorn MC, Hakuno SK, Bruckner RS,
Rogler G, Hawinkels LJAC and Scharl M: Stromal cells in the
pathogenesis of inflammatory bowel disease. J Crohns Colitis.
14:995–1009. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
van Onkelen RS, Gosselink MP, van Meurs M,
Melief MJ, Schouten WR and Laman JD: Pro-inflammatory cytokines in
cryptoglandular anal fistulas. Tech Coloproctol. 20:619–625. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rakinic J and Poola VP: Hemorrhoids and
fistulas: New solutions to old problems. Curr Probl Surg.
51:98–137. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hashempur MH, Khademi F, Rahmanifard M and
Zarshenas MM: An evidence-based study on medicinal plants for
hemorrhoids in medieval Persia. J Evid Based Complementary Altern
Med. 22:969–981. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Porwal A, Gandhi P and Kulkarni D: A
prospective, open-label, single arm, single center study to
evaluate safety and efficacy of ANOAC-H tablet in the treatment of
bleeding hemorrhoids grades 1–3. Int J Ayurveda. 2018.
|
|
26
|
Kumar D, Haldar S, Gorain M, Kumar S,
Mulani FA, Yadav AS, Miele L, Thulasiram HV and Kundu GC:
Epoxyazadiradione suppresses breast tumor growth through
mitochondrial depolarization and caspase-dependent apoptosis by
targeting PI3K/Akt pathway. BMC Cancer. 18:522018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Leopold JA, Walker J, Scribner AW, Voetsch
B, Zhang YY, Loscalzo AJ, Stanton RC and Loscalzo J:
Glucose-6-phosphate dehydrogenase modulates vascular endothelial
growth factor-mediated angiogenesis. J Biol Chem. 278:32100–32106.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Butti R, Nimma R, Kundu G, Bulbule A,
Kumar TV, Gunasekaran VP, Tomar D, Kumar D, Mane A, Gill SS, et al:
Tumor-derived osteopontin drives the resident fibroblast to
myofibroblast differentiation through Twist1 to promote breast
cancer progression. Oncogene. 40:2002–2017. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kumar D, Kumar S, Gorain M, Tomar D, Patil
HS, Radharani NN, Kumar TV, Patil TV, Thulasiram HV and Kundu GC:
Notch1-MAPK signaling axis regulates CD133+ cancer stem
cell-mediated melanoma growth and angiogenesis. J Invest Dermatol.
136:2462–2474. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tse GN: Practical management of
hemorrhoids: Pitfalls and plain sailing. Can Fam Physician.
34:655–659. 1988.PubMed/NCBI
|
|
31
|
Scharl M and Rogler G: Pathophysiology of
fistula formation in Crohn's disease. World J Gastrointest
Pathophysiol. 5:205–212. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pata F, Sgró A, Ferrara F, Vigorita V,
Gallo G and Pellino G: Anatomy, physiology and pathophysiology of
haemorrhoids. Rev Recent Clin Trials. 16:75–80. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mott T, Latimer K and Edwards C:
Hemorrhoids: Diagnosis and treatment options. Am Fam Physician.
97:172–179. 2018.PubMed/NCBI
|
|
34
|
Lohsiriwat V: Treatment of hemorrhoids: A
coloproctologist's view. World J Gastroenterol. 21:9245–9252. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Moggia E, Talamo G, Gallo G, Bianco A,
Barattini M, Salsano G, Zefiro D, Stefanini T and Berti S: Do we
have another option to treat bleeding hemorrhoids? The Emborrhoid
technique: Experience in 16 patients. Rev Recent Clin Trials.
16:81–86. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lu M, Shi GY, Wang GQ, Wu Y, Liu Y and Wen
H: Milligan-Morgan hemorrhoidectomy with anal cushion suspension
and partial internal sphincter resection for circumferential mixed
hemorrhoids. World J Gastroenterol. 19:5011–5015. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Nienhuijs S and de Hingh I: Conventional
versus LigaSure hemorrhoidectomy for patients with symptomatic
Hemorrhoids. Cochrane Database Syst Rev. Jan 21–2009.(Epub ahead of
print). doi: 10.1002/14651858.CD006761.pub2. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Patti R, Almasio PL, Muggeo VM, Buscemi S,
Arcara M, Matranga S and Di Vita G: Improvement of wound healing
after hemorrhoidectomy: A double-blind, randomized study of
botulinum toxin injection. Dis Colon Rectum. 48:2173–2179. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Khan KI, Waqas A, Akmal M, Mahmood S and
Iqbal A: Efficacy of combination of 0.2% GTN and lignocaine
ointments in wound healing and pain relief after Milligan Morgan
hemorrhoidectomy-a comparison with lignocaine and 0.2% GTN
ointments separately. Int J Surg. 12:329–333. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Giannini I, Pecorella G, Pennisi D,
Santangelo G, Digennaro R, Latorre F, Giuliani G and Altomare DF:
Control of post-hemorrhoidectomy symptoms and wound healing by
Triclosan: A randomized, double-blind, controlled trial. Minerva
Chir. 69:75–82. 2014.PubMed/NCBI
|
|
41
|
Gallo G, Mistrangelo M, Passera R, Testa
V, Pozzo M, Perinotti R, Lanati I, Lazzari I, Tonello P, Ugliono E,
et al: Efficacy of mesoglycan in pain control after excisional
hemorrhoidectomy: A pilot comparative prospective multicenter
study. Gastroenterol Res Pract. 2018:64238952018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sturiale A, Gallo G, Brusciano L, Cacace
C, Cafaro D, Celedon Porzio F and Naldini G: Safety and efficacy of
proctosoll allevia in the management of haemorrhoidal disease in
adults: A prospective randomized clinical trial. Rev Recent Clin
Trials. 15:152–159. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Derakhshan AR: Natural treatments for
fissure in ano used by traditional Persian scholars, Razi (Rhazes)
and Ibn Sina (Avicenna). J Evid Based Complementary Altern Med.
22:324–333. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Mistry S, Patidar R, Vyas V, Jena J and
Dutt KR: Anti-inflammatory activity of Mimosa Pudica Linn.
(Mimosaceae) leaves: An ethnpharmacological study. Int J Pharm Sci
Res. 4:17892012.
|
|
45
|
Xia M, Liu L, Qiu R, Li M, Huang W, Ren G
and Zhang J: Anti-inflammatory and anxiolytic activities of
Euphorbia hirta extract in neonatal asthmatic rats. AMB
Express. 8:1792018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Murthuza S and Manjunatha BK: In vitro and
in vivo evaluation of anti-inflammatory potency of Mesua ferrea,
Saraca asoca, Viscum album and Anthocephalus cadamba in
murine macrophages raw 264.7 cell lines and wistar albino rats.
Beni-Seuf Univ J Appl Sci. 7:719–723. 2018.
|
|
47
|
Nimisha, Rizvi DA, Fatima Z, Neema and
Kaur CD: Antipsoriatic and anti-inflammatory studies of Berberis
aristata extract loaded nanovesicular gels. Pharmacogn Mag. 13
(Suppl 3):S587–S594. 2017. View Article : Google Scholar : PubMed/NCBI
|