Introduction
Inflammation is the body's defense response against
various damage factors, including bacteria, viruses and tissue
damage, and regulated inflammatory responses play a vital role in
coping with pathogens and preventing tissue damage (1). However, abnormal inflammatory
responses can facilitate the progression of a wide range of types
of disease, including rheumatoid arthritis, chronic hepatitis,
Alzheimer's disease, inflammatory bowel disease and cancer
(2,3). Thus, effective control of inflammatory
responses is pivotal for the prevention and treatment of several
diseases, including cancer (3).
Inflammatory diseases are complex and difficult to cure, thus
inflammatory models are used to screen for anti-inflammatory drugs.
For example, the lipopolysaccharide (LPS)-induced inflammatory
response model is widely used in inflammation research (4–6).
NF-κB is an important, multi-directional, functional
regulator of the anti-inflammatory response (7). The main method for preventing chronic
inflammation-mediated disorders is to regulate the secretion of
proinflammatory cytokines (8). The
production or secretion of proinflammatory cytokines results in the
activation of NF-κB, which in turn stimulates several transcription
factors that control the gene expression of proinflammatory
cytokines, including ILs, inducible nitric oxide synthase (iNOS)
and cyclooxygenease-2 (COX-2) (9).
The activation of NF-κB also plays an indispensable role in the
development of several types of disease, including rheumatoid
arthritis, inflammatory bowel disease and autoimmunity, as well as
diseases comprising a significant inflammatory component, such as
cancer and atherosclerosis (10).
TNF-associated factor 6 (TRAF6) is a key regulator
of NF-κB and plays an important regulatory role in inflammation
(11). NOD-like receptor family
CARD domain containing 3 (NLRC3) has been found to exert inhibitory
effects on proinflammatory signaling transduction, the
ubiquitination of TRAF6 and nuclear translocation of NF-κB p65
(12). In addition, NLRC3 was
discovered to inhibit a major inflammatory pathway controlled by
NF-κB, which directly interacts with TRAF6 and forms a new protein
complex called the ‘TRAFasome’ (13).
Nuclear factor-erythroid 2-related factor 2 (Nrf2)
is a pivotal and significant transcription factor, which controls
several antioxidant enzymes including heme oxygenase-1 (HO-1) and
NAD(P)H quinone dehydrogenase 1 (NQO1) (14). HO-1 plays a key role in the
antioxidant processes and suppressing the immune response (15). Nrf2, coupled with its target genes
to act as an inflammation regulatory system, has been reported to
downregulate the expression levels of several proinflammatory
cytokines, which could antagonize NF-κB activation (16,17).
Loranthus tanakae Franch. & Sav, which
grows on the trees of Quercus L. and Betula, has been
found to have various biological properties, including
anti-microbial, antitumor and antioxidant effects (18). It has been reported that its
methanol extracts possess various antitumor activities (19). Natural products represent novel
compounds that have been shown to prevent different types of
disease, such as cancer, infectious diseases and cardiovascular
diseases (20). Epigallocatechin,
curcumin and other natural phenolic compounds have been established
to possess anti-inflammatory and antioxidant activities (21–23).
α-rhamnrtin-3-α-rhamnoside (ARR; Fig.
1A), a phenolic flavonoid compound, is the main active
ingredient of Loranthus tanakae Franch. & Sav (24). However, to the best of our
knowledge, the pharmacological activities and anti-inflammatory
molecular mechanisms of ARR remain unknown. Thus, the present study
aimed to investigate the anti-inflammatory effect of ARR in
RAW264.7 cells to determine whether it occurred via the NF-κB and
Nrf2 signaling pathway. In addition, the study sought to elucidate
its underlying molecular mechanism of action to provide a
preliminary basis for the development of ARR into an
anti-inflammatory drug.
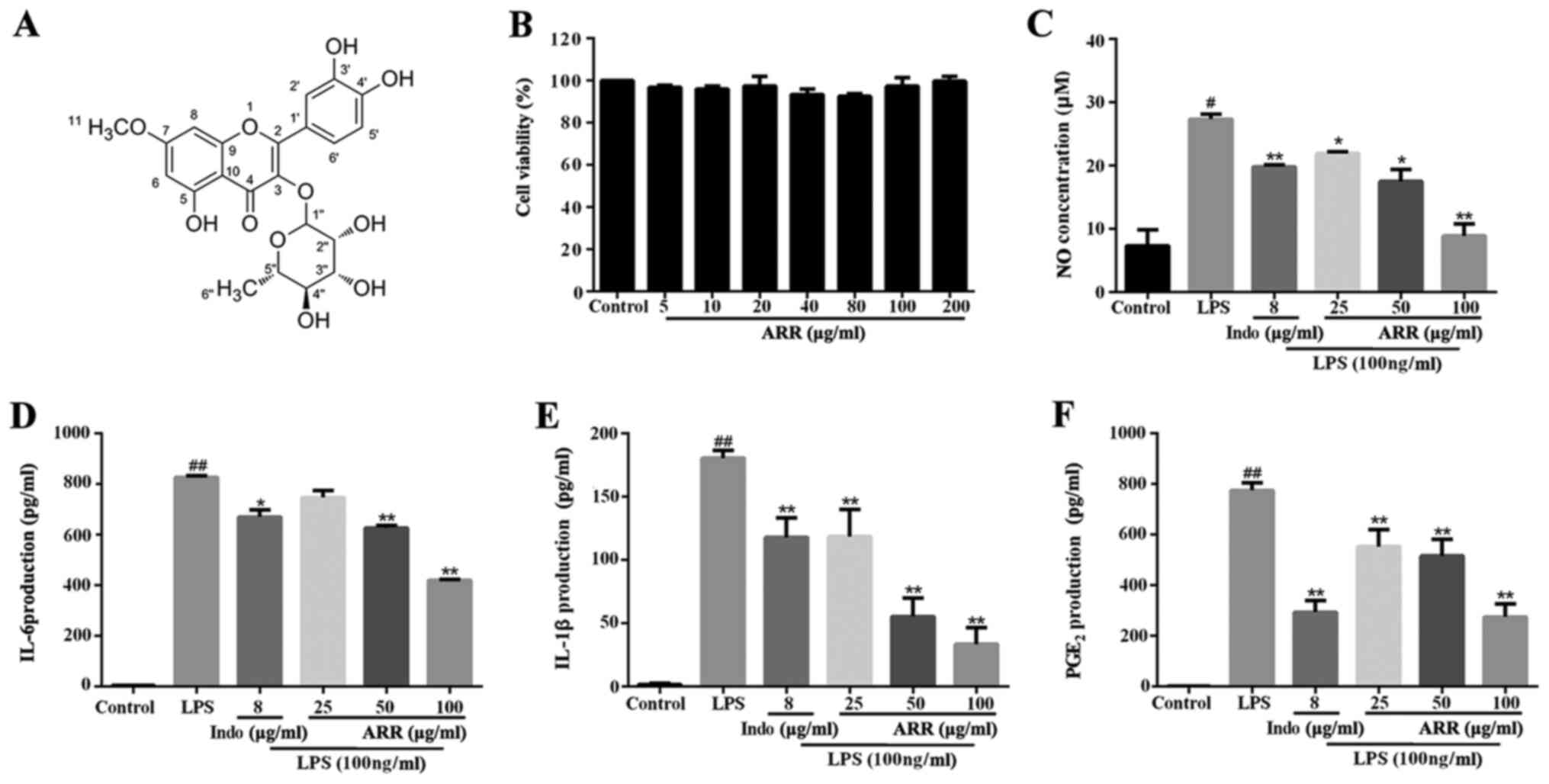 | Figure 1.ARR inhibits the inflammatory
response. (A) ARR is a flavonoid compound extracted from the
Loranthus tanakae Franch. & Sav. (B) RAW264.7 cells were
treated with different concentrations of ARR (0, 5, 10, 20, 40, 80,
100 or 200 µg/ml) for 24 h. A Cell Counting Kit-8 assay was
performed to assess cell viability. RAW264.7 cells were pretreated
with different concentrations of ARR (0, 25, 50 or 100 µg/ml) or
Indo (positive control, 8 µg/ml) at 37°C for 2 h, and incubated
with or without LPS (100 ng/ml) at 37°C for 24 h. (C) Levels of NO
in the culture media were determined using a NO colorimetric assay
kit. The effect of ARR on (D) IL-6, (E) IL-1β and (F)
PGE2 cytokine production was detected using ELISA kits.
Data are presented as the mean ± SD. #P<0.05,
##P<0.01 vs. untreated control group; *P<0.05,
**P<0.01 vs. LPS group. ARR, α-rhamnrtin-3-α-rhamnoside; Indo,
indomethacin; LPS, lipopolysaccharide; NO, nitric oxide;
PGE2, prostaglandin E2. |
Materials and methods
Reagents and chemicals
ARR (purity >95%) was isolated from Loranthus
tanakae Franch. & Sav. in our laboratory, as previously
described (21). The structure of
ARR was elucidated by nuclear magnetic resonance. LPS and
indomethacin (Indo) were purchased from Sigma-Aldrich; Merck KGaA.
DMEM, FBS and penicillin-streptomycin were purchased from Gibco;
Thermo Fisher Scientific, Inc. Primary rabbit monoclonal
antibodies, including NF-κB p65 (cat. no. ab16502),
phosphorylated-(p)-NF-κB-p65 (cat. no. ab76302), Nrf2 (cat. no.
ab92946), NQO1 (cat. no. ab80588), HO-1 (cat. no. ab13243), TRAF6
(cat. no. ab137452), β-actin (cat. no. ab8227) and Histone H3 (cat.
no. ab1791) were purchased from Abcam and the NLRC3 antibody (cat.
no. DF13411) was obtained from Affinity Biosciences. The nitric
oxide (NO) colorimetric kit (cat. no. E-BC-K035-M) and the cytokine
mouse ELISA kits specific for prostaglandin E2 (PGE2; cat. no.
E-EL-0034c), IL-6 (cat. no. E-EL-M0044c) and IL-1β (cat. no.
E-EL-M0037c) were purchased from Elabscience.
Cell lines and culture
Leukemia cells from mouse mononuclear macrophages
(RAW264.7; cat. no. CL-0190) were obtained from Procell Life
Science & Technology Co. Ltd., and cultured in DMEM
supplemented with 10% heat-inactivated FBS and 1%
penicillin-streptomycin, at 37°C with 5% CO2.
Cell viability assay
A Cell Counting Kit-8 (CCK-8) assay (which uses
WST-8 for the colorimetric reaction) was performed to assess cell
viability. Briefly, RAW264.7 macrophages (4×104
cells/ml) were seeded into 96-well plates (100 µl/well) and treated
with different concentrations of ARR (5, 10, 20, 40, 80, 100 or 200
µg/ml) for 24 h at 37°C. Following the incubation, 10 µl CCK-8
reagent (Sigma-Aldrich; Merck KGaA) was added to each well and
incubated for an additional 2 h at 37°C. In the presence of the
electronic coupling reagent, 1-Methoxy PMS, WST-8 was transformed
to orange-yellow water-soluble formazan. The optical density was
determined using a microplate reader at a wavelength of 450 nm.
NO assay
For the NO assay, 1×106/ml RAW264.7
macrophages were seeded into 6-well plates. Following incubation
for 24 h with different concentrations of ARR (0, 25, 50 and 100
µg/ml) or the positive control drug, Indo (8 µg/ml; commonly used
to treat inflammation) for 2 h, LPS (100 ng/ml) was added, and
cells were incubated for an additional 24 h. According to the
manufacturer's instructions, reagents were added into each well and
cells were incubated at 37°C for 30 min in the dark. The absorbance
was measured using a microplate reader at a wavelength of 550 nm.
The dosage of ARR used was determined according to the preliminary
experiments (data not shown), and the dosage of LPS was selected
based on our previous study (25).
ELISA
The levels of the cytokines, IL-6, IL-1β and
PGE2, in the macrophage supernatants (obtained by
centrifugation at 1,000 × g at room temperature for 5 min) from
each group were determined using IL-6, IL-1β and PGE2
ELISA kits, according to the manufacturer's instructions. The
absorbance was measured using a microplate reader at a wavelength
of 450 nm.
Reverse transcription-quantitative
(RT-q)PCR
Total RNA was extracted from RAW264.7 cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.), and the quality and concentration of the isolated RNA were
determined using a Nanodrop 1000 spectrophotometer (Thermo Fisher
Scientific, Inc.). First-strand cDNA was synthesized using an
RT-qPCR synthesis kit (cat. no. AT311; Beijing TransGen Biotech
Co., Ltd.), according to the manufacturer's protocol. Relative
expression levels of IL-6, IL-1β, COX-2, iNOS and GAPDH were
determined using a PerfectStart™ Green qPCR SuperMix (Beijing
TransGen Biotech Co., Ltd.). The following thermocycling conditions
were used for the qPCR: Initial denaturation at 94°C for 30 sec;
followed by 45 cycles at 94°C for 5 sec and 60°C for 30 sec. The
mRNA expression levels of IL-6, IL-1β, COX-2 and iNOS were
calculated using the 2−ΔΔCq method (26) and normalized to GAPDH. The primer
sequences used for the qPCR are listed in Table I.
 | Table I.Primer sequences used for reverse
transcription-quantitative PCR. |
Table I.
Primer sequences used for reverse
transcription-quantitative PCR.
| Gene | Primer sequence
(5′→3′) |
|---|
| IL-6 | F:
GAGTCCTTCAGAGAGATACAG |
|
| R:
CTGTGACTCCAGCTTATCTG |
| IL-1β | F:
AAATACCTGTGGCCTTGGGC |
|
| R:
CTTGGGATCCACACTCTCCAG |
| COX-2 | F:
GAAGATTCCCTCCGGTGTTT |
|
| R:
CCCTTCTCACTGGCTTATGTAG |
| iNOS | F:
GCTTGGGTCTTGTTCACTCC |
|
| R:
GGCCTTGTGGTGAAGAGTGT |
| GAPDH | F:
CCTTCCGTGTTCCTACCCCC |
|
| R:
AGCCCAAGATGCCCTTCAGT |
Western blotting
Total protein was extracted from RAW264.7 cells
using a Whole Cell Lysis assay (Nanjing KeyGen Biotech Co., Ltd.).
Total nuclear and cytoplasmic proteins were extracted using the
Nuclear and Cytoplasmic Protein Extraction kit (Nanjing KeyGen
Biotech Co., Ltd.). Protein concentration was measured using a BCA
protein assay kit (Nanjing KeyGen Biotech Co., Ltd.) and 20 µg
protein/lane was separated via 10% SDS-PAGE. The separated proteins
were subsequently transferred onto PVDF membranes and blocked with
5% BSA (Thermo Fisher Scientific, Inc.) at room temperature for 1
h. The membranes were then incubated overnight at 4°C with primary
antibodies against NF-κB p65 (1:1,000), p-NF-κB p65 (1:1,000), Nrf2
(1:1,000), HO-1 (1:1,000), NQO1 (1:1,000), NLRC3 (1:1,000), TRAF6
(1:1,000), β-actin (1:2,000) or Histone H3 (1:2,000). Following the
primary antibody incubation, the membranes were incubated with an
HRP-conjugated anti-rabbit secondary antibody (Abcam; cat. no.
ab6721; 1:5,000) at room temperature for 1 h. The membranes were
washed multiple times with TBS-Tween-20 buffer and the protein
bands were visualized using enhanced chemiluminescence reagent
(cat. no. G2020; Wuhan Servicebio Technology Co., Ltd.) and a
chemiluminescence imaging system (Bio-Rad Laboratories, Inc.).
Densiometric analysis was performed using Image Lab software
(version 6.0; Bio-Rad Laboratories, Inc.).
Immunofluorescence staining
RAW264.7 cells (1×105/ml) were seeded
onto glass coverslips, plated into the bottom of 6-well plates and
fixed with 4% paraformaldehyde at room temperature for 15 min.
Cells were subsequently permeabilized with 0.1% Triton X-100 and
blocked with 10% goat serum (Elabscience) at room temperature for
30 min. Cells were then incubated with a rabbit anti-NF-κB p65
antibody (1:1,000) at 37°C for 1 h and Cy3-conjugated goat
anti-rabbit IgG (H+L) secondary antibody (Elabscience; cat. no.
E-IR-R321; 1:5,000) at 37°C for 1 h. Nuclei were stained with DAPI
(Pierce; Thermo Fisher Scientific, Inc.) at 37°C for 5 min, and a
drop of anti-fluorescence quenching mounting solution was added
prior to visualization using a fluorescence microscope
(magnification, ×400). Analysis was performed using ImageJ software
(version 1.80; National Institutes of Health).
Immunohistochemistry staining
Immunohistochemistry analysis was performed as
previously described (22). The
primary antibodies used were as follows: Anti-NLRC3 (1:500),
anti-TRAF6 (1:500) and anti-NF-κB p65 (1:500), and an
HRP-conjugated anti-rabbit antibody was used as the secondary
antibody (Abcam; cat. no. ab6721; 1:500). Following the antibody
incubations, all samples were stained with DAB at room temperature
for 2 min. Samples were observed using a fluorescence microscope
(magnification, ×400) and quantified using ImageJ software.
Statistical analysis
All experiments were repeated at least three times.
Statistical analyses were performed using SPSS software (version
26.0; IBM Corp.) and GraphPad Prism software (version 6.0; GraphPad
Software, Inc.). Data are presented as the mean ± SD. Significant
differences between groups were determined using a one-way ANOVA
followed by a Tukey's or Dunnett's T3 post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Effect of ARR on viability in
LPS-stimulated RAW 264.7 cells
To investigate the effect of ARR on cell viability,
RAW264.7 cells were incubated with 0–200 µg/ml ARR for 24 h. As
presented in Fig. 1B, 5–200 µg/ml
ARR exerted no significant effect on the viability of RAW264.7
cells. Thus, doses of 25–100 µg/ml ARR were used for subsequent
experimentation. The doses of ARR were used according to our prior
trial (data not shown).
Effect of ARR on inflammatory
mediators in LPS-stimulated RAW264.7 cells
As the LPS-induced inflammatory response model is
widely used in inflammation research of anti-inflammatory drugs
(4–6), the present study established an
LPS-induced inflammatory response model in RAW264.7 cells to
evaluate the anti-inflammatory effect of ARR. Following incubation
with LPS, NO expression was significantly increased compared with
the control group; however, the addition of ARR at all doses
significantly suppressed the LPS-induced secretion of NO (Fig. 1C). Among them, the high dose of ARR
(100 µg/ml) exhibited the strongest inhibitory effect. These
results suggested that ARR markedly suppresses NO production in
RAW264.7 cells.
IL-6, IL-1β and PGE2 are critical
inflammatory cytokines involved in mediating inflammatory responses
(27); thus, in the present study,
the expression levels of IL-6, IL-1β and PGE2 were
detected in RAW264.7 cell supernatants using ELISA kits. As
presented in Fig. 1D-F, LPS
significantly upregulated the levels of IL-6, IL-1β and
PGE2 compared with the control group, while ARR
treatment significantly decreased the expression levels of the
three inflammatory cytokines compared with the LPS group. Taken
together, these results suggested that ARR may exert
anti-inflammatory effects by inhibiting the release of IL-6, IL-1β
and PGE2. In addition, the anti-inflammatory effect of
ARR seems to occur in a dose-dependent manner, and works best at a
concentration of 100 µg/ml.
Effect of ARR on the gene
transcription of proinflammatory factors in LPS-stimulated RAW264.7
cells
To determine whether the regulation of inflammatory
factors by ARR occurred at the mRNA level, the expression levels of
various inflammatory factors were detected via RT-qPCR analysis. As
presented in Fig. 2A-D, LPS
significantly upregulated the mRNA expression levels of iNOS, IL-6,
IL-1β and COX-2 compared with the control group. Compared with the
LPS group, the mRNA levels of inflammatory factors, iNOS, IL-6,
IL-1β and COX-2 (except 25 µg/ml ARR treatment), were significantly
downregulated following the addition of ARR, whereby the effects of
ARR were most notable at 100 µg/ml. These findings are consistent
with the ELISA results, suggesting that ARR may exert an
anti-inflammatory effect by inhibiting the expression of several
inflammatory factors at both the mRNA and protein levels.
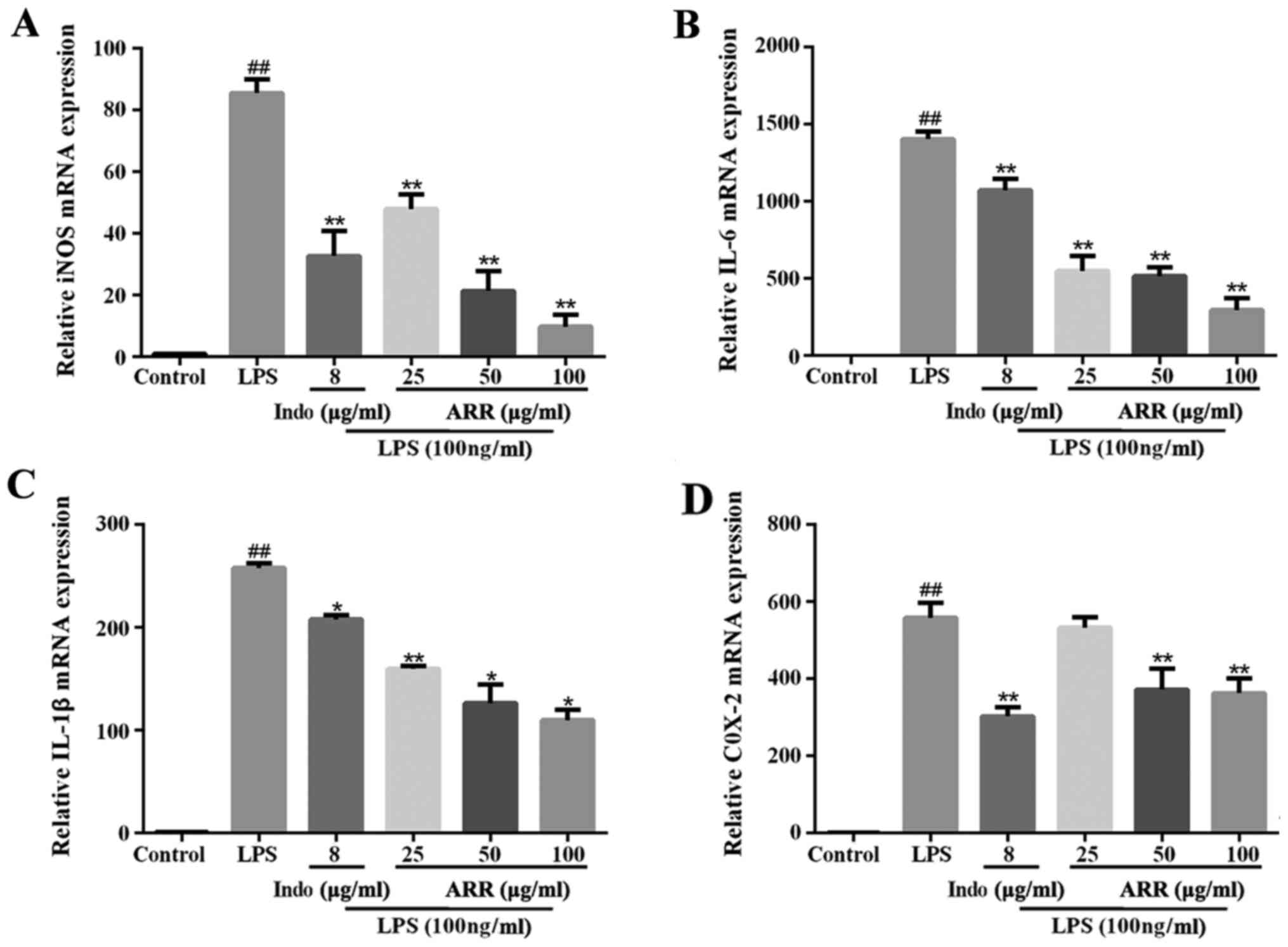 | Figure 2.ARR decreases the levels of
proinflammatory factors. RAW264.7 cells were pretreated with
different concentrations of ARR (0, 25, 50 or 100 µg/ml) or Indo
(positive control, 8 µg/ml) for 2 h, and incubated with or without
LPS (100 ng/ml) for 24 h. Reverse transcription-quantitative PCR
analysis was performed to detect the mRNA expression levels of (A)
iNOS, (B) IL-6, (C) IL-1β and (D) COX-2. Data are presented as the
mean ± SD. (n=3). ##P<0.01 vs. untreated control
group; *P<0.05, **P<0.01 vs. LPS group. ARR,
α-rhamnrtin-3-α-rhamnoside; Indo, indomethacin; LPS,
lipopolysaccharide; iNOS, inducible nitric oxide synthase; COX-2,
cyclooxygenase-2. |
Effect of ARR on the Nrf2 signaling
pathway in LPS-stimulated RAW264.7 cells
The effect of ARR on the Nrf2 signaling pathway in
LPS-stimulated RAW264.7 cells was also investigated. The western
blotting results demonstrated that, compared with the control
group, LPS significantly upregulated the expression levels of NQO1,
while the expression levels of Nrf2 and HO-1 were not significantly
altered. Treatment with ARR notably induced the expression levels
of Nrf2 protein and its target molecule, HO-1, compared with the
LPS group (Fig. 3D-F). Taken
together, these results suggest that ARR can also exert
anti-inflammatory effects via the Nrf2 signaling pathway.
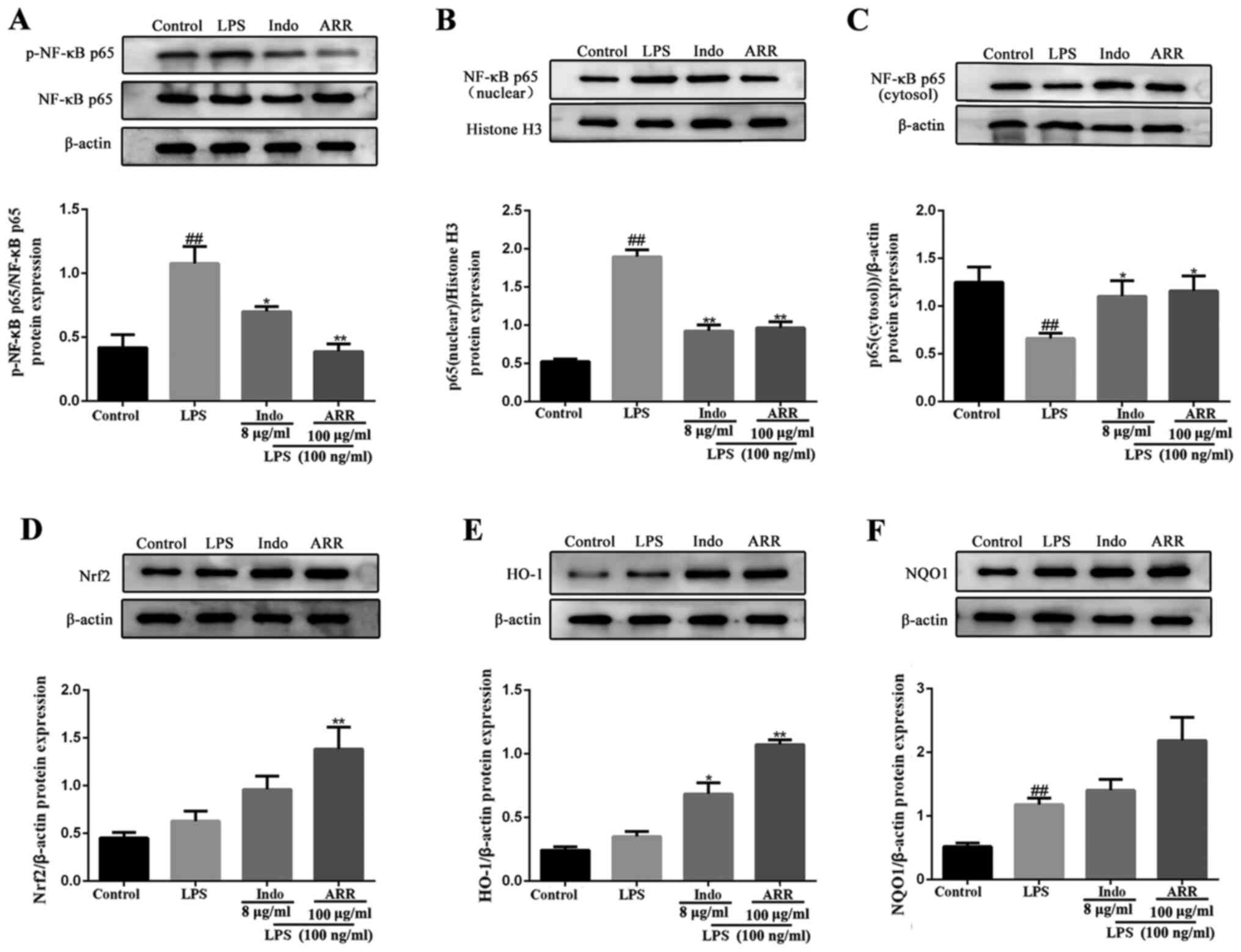 | Figure 3.ARR inhibits the NF-κB signaling
pathway and activates the Nrf2 signaling pathway. RAW264.7 cells
were treated with ARR (100 µg/ml) or Indo (positive control, 8
µg/ml) at 37°C for 24 h, with or without LPS (100 ng/ml). The
protein expression levels of (A) total NF-κB p65 and p-NF-κB p65,
(B) NF-κB p65 in the nucleus, (C) NF-κB p65 in the cytosol, (D)
Nrf2, (E) HO-1 and (F) NQO1 were detected using western blotting
and semi-quantified using Image Lab software. LPS represents
proteins from the 100 ng/ml LPS-treated group. Data are presented
as the mean ± SD. ##P<0.01 vs. control group;
*P<0.05, **P<0.01 vs. LPS group. ARR,
α-rhamnrtin-3-α-rhamnoside; Nrf2, nuclear factor-erythroid
2-related factor 2; Indo, indomethacin; LPS, lipopolysaccharide;
HO-1, heme oxygenase-1; NQO1, NAD(P)H quinone dehydrogenase 1; p-,
phosphorylated. |
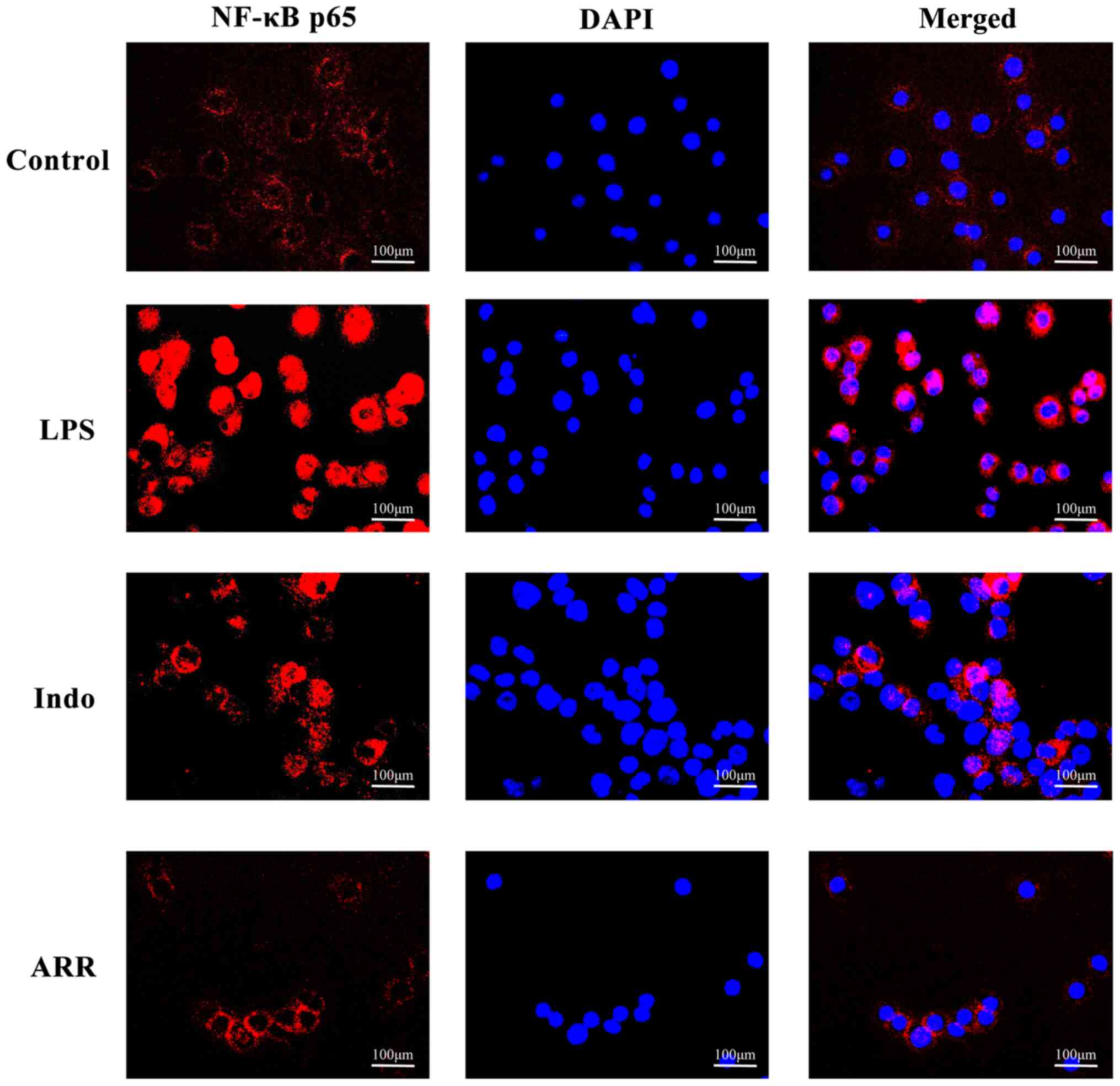
Effect of ARR on NF-κB p65
translocation in LPS-stimulated RAW264.7 cells
NF-κB is a well-known transcription factor that is
involved in the inflammatory response (28). Western blotting analysis was
performed to detect the phosphorylation of p65 and translocation of
NF-κB p65 to the nucleus. LPS significantly increased the
phosphorylation of NF-κB p65 compared with the control group, while
the addition of ARR significantly suppressed the phosphorylation of
NF-κB p65 in RAW264.7 cells (Fig.
3A). Stimulation with LPS also induced the translocation of
NF-κB p65 to the nucleus (Fig.
3B-C). However, addition of ARR significantly suppressed
LPS-induced NF-κB p65 nuclear translocation of RAW264.7
macrophages. Similar results for NF-κB p65 nuclear translocation
were observed via immunofluorescence microscopy. As expected, LPS
markedly increased NF-κB p65 nuclear translocation compared with
the control group (Fig. 4).
Notably, ARR markedly suppressed the nuclear translocation of NF-κB
p65 compared with the LPS group. Taken together, these results
suggested that ARR may exert anti-inflammatory effects by
inhibiting NF-κB p65 translocation.
Effect of ARR on NLRC3, TRAF6 and
NF-κB p65 protein expression in LPS-stimulated RAW264.7 cells
To further investigate the effect of ARR on the
NF-κB signaling pathway, immunohistochemistry staining and western
blotting were performed to determine the expression levels of
NLRC3, TRAF6 and NF-κB p65 in RAW264.7 cells. Treatment with LPS
did not significantly affect NLRC3 expression, while the expression
levels of TRAF6 and NF-κB p65 were upregulated compared with the
control group (Fig. 5A-D). However,
NLRC3 expression was markedly upregulated following the addition of
ARR, while the expression levels of TRAF6 and NF-κB p65 were
downregulated compared with the LPS group. Taken together, these
results suggested that ARR may significantly upregulate NLRC3 and
downregulate TRAF6 and NF-κB p65 expression levels in the
inflammatory response.
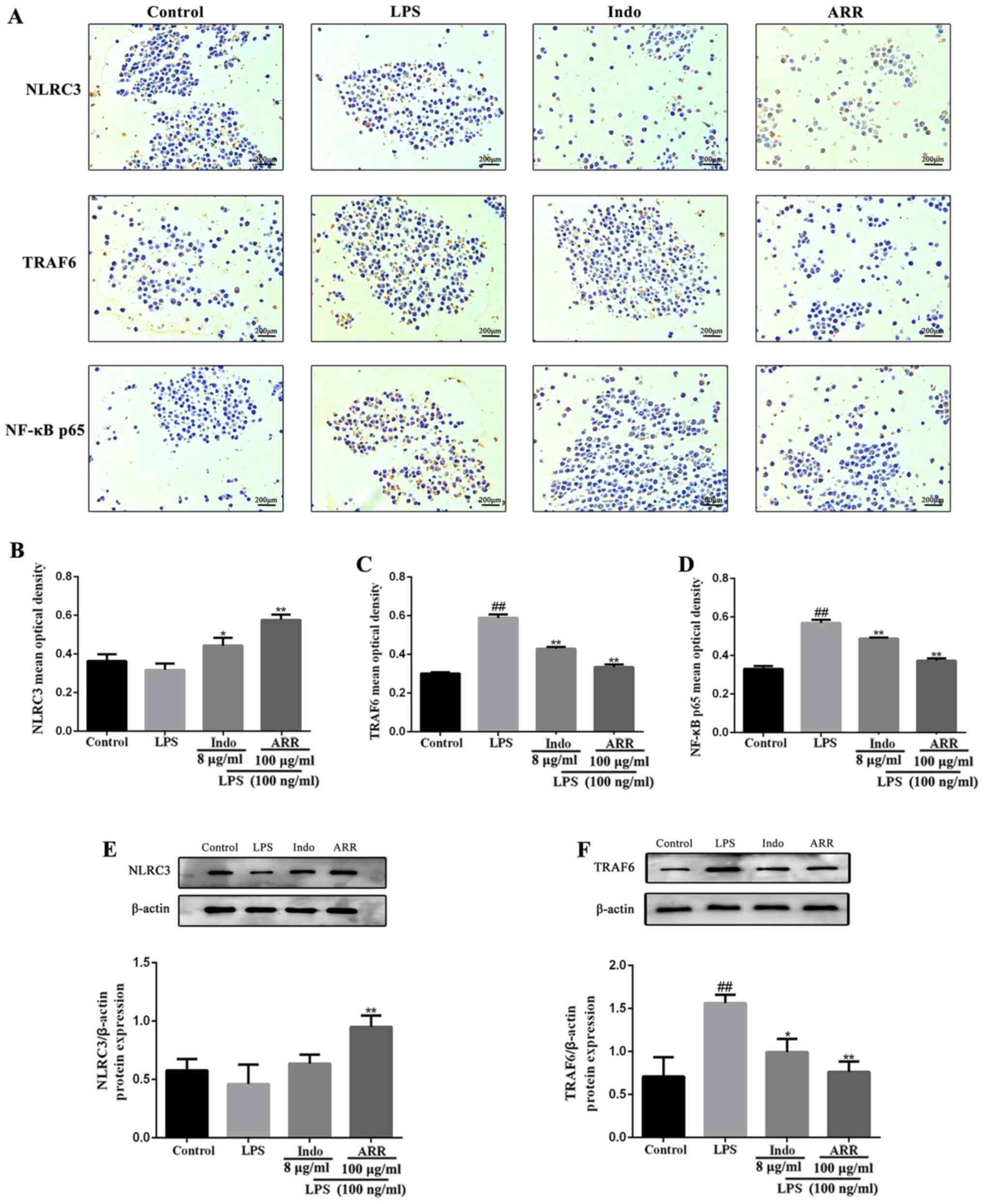 | Figure 5.ARR downregulates the protein
expression of TRAF6 and NF-κB p65 by increasing the content of
NLRC3 protein molecules. (A) Expression levels of NLRC3, TRAF6 and
NF-κB p65 in LPS-stimulated RAW264.7 cells were detected using
immunohistochemistry staining (magnification, ×400; scale bar,
200-µm). The mean optical density values of (B) NLRC3, (C) TRAF6
and (D) NF-κB p65 were quantified using ImageJ software. The
protein expression levels of (E) NLRC3 and (F) TRAF6 were detected
using western blotting and semi-quantified using Image Lab
software. LPS represents protein from the 100 ng/ml LPS-treated
group; Indo represents protein from the 8 µg/ml Indo and 100 ng/ml
LPS-treated group; ARR represents protein from the 100 µg/ml ARR
and 100 ng/ml LPS-treated group. Data are presented as the mean ±
SD (n=3). ##P<0.01 vs. control group; *P<0.05,
**P<0.01 vs. LPS group. ARR, α-rhamnrtin-3-α-rhamnoside; TRAF6,
tumor necrosis factor-associated factor 6; NLRC3, NOD-like receptor
family CARD domain containing 3; LPS, lipopolysaccharide; Indo,
indomethacin. |
Discussion
Inflammation is a natural host defense reaction
process, which is divided into acute and chronic inflammation
according to the duration (29).
The main symptoms of acute inflammation include redness, swelling
and pain (30,31). Chronic inflammation is caused by the
persistence of inflammatory factors and damage to the tissues,
which is manifested by the degeneration, exudation and
proliferation of local tissues (32,33).
ARR is a flavonoid compound extracted from the Loranthus
tanakae Franch. & Sav (19). Flavonoids have been reported to
exert anti-inflammatory (34),
anticancer (35) and
cardioprotective effects (36).
However, the effect of ARR on inflammation and its underlying
molecular mechanism remain unclear.
When macrophages are activated, they produce various
inflammatory cytokines that cause inflammation (9). LPS is a macrophage stimulus, which can
cause macrophages to secrete proinflammatory factors, including NO,
PGE2, IL-6 and IL-1β (37). The present study established an
LPS-induced inflammatory response in vitro model to evaluate
the anti-inflammatory effect of ARR on RAW264.7 cells. The use of
an LPS-induced macrophage line is a well-established
anti-inflammatory in vitro model, which is widely deemed as
a standard and reliable model to determine the potential of novel
anti-inflammatory drug candidates, and therefore predominantly
adopted by researchers of this field (38–41).
Exposure to high levels of NO can cause an innate immune response
and result in tissue disruption or cell injury (42). The cytokines, IL-6 and IL-1β, cause
tissue damage and play an essential role in mediating various types
of inflammatory disease (43). The
results of the present study demonstrated that ARR notably
suppressed the secretion of the proinflammatory factors, IL-6 and
IL-1β.
Inflammatory responses are accompanied by the
systematic activation of several signaling pathways (44). NF-κB is crucial to inflammatory
responses as it releases proinflammatory cytokines and p65
translocation plays a key role in the activation of NF-κB (45), which was also discovered to be the
main signaling pathway for LPS to induce inflammation in
macrophages (46,47). Suppression of NF-κB activation has
been found to represent a promising anti-inflammatory strategy
(48). NF-κB downregulates the
expression levels of iNOS, COX-2 and other inflammatory-related
genes by activating transcription (49). NO is a free gaseous signaling
molecule synthesized by iNOS, and excess production of NO mediated
by iNOS induces an inflammatory response (50). COX-2 is known to generate
proinflammatory prostaglandins, such as PGE2, which
induce inflammation (51). A
variety of natural compounds, including flavonoids, quercetin,
genistein and kaempferol have been considered as natural COX-2
inhibitors (52,53). Kim et al (54) demonstrated that
formononetin-7-O-phosphate inhibited COX-2 expression by inhibiting
NF-κB nuclear translocation.
The present study also investigated whether ARR
exerts anti-inflammatory effects via the NF-κB signaling pathway.
As expected, the results demonstrated that ARR not only
downregulated iNOS and COX-2 mRNA expression levels, but also
suppressed NO and PGE2 content, in a dose-dependent
manner. In addition, ARR markedly blocked NF-κB p65 translocation.
To the best of our knowledge, the present study was the first to
demonstrate that ARR can inhibit the inflammatory response via the
NF-κB signaling pathway in LPS-induced RAW264.7 cells.
NLRC3 serves as a checkpoint to prevent dysregulated
inflammation. Following stimulation of RAW264.7 cells with LPS,
NLRC3 was observed to serve as a de-ubiquitinating enzyme to remove
the ubiquitination of TRAF6 and inhibited the nuclear translocation
of the NF-κB p65 subunit to reduce the release of IL-1β (55). The results of the present study
demonstrated that ARR upregulated NLRC3 expression to inhibit the
activation of the NF-κB pathway, which is consistent with previous
findings (55,56).
The Nrf2 signaling pathway is another important
regulator of inflammation. The activation of Nrf2 and its target
molecules, such as HO-1 and NQO1, is considered an intracellular
protective mechanism against oxidative stress and inflammatory
responses (57). It has been
reported that the activation of Nrf2 could disrupt the crosstalk
between NF-κB and its target molecules, thereby controlling the
inflammatory response (58). In
addition, HO-1 and NQO1 were discovered to inhibit the
transcription of inflammatory adhesive molecules mediated by NF-κB
(41). Moreover, previous studies
have revealed that the regulation of NF-κB may be associated with
the Nrf2 signaling pathway, and it was reported that Nrf2 knockdown
promoted the transcriptional activity of NF-κB (59–61).
The results of the present study demonstrated that ARR upregulated
Nrf2 expression and inhibited the nuclear translocation of NF-κB.
Therefore, it was suggested that ARR-induced Nrf2 activation may
prevent the increase of inflammatory cytokines mediated by NF-κB.
However, the underlying molecular mechanism by which ARR affects
the crosstalk between Nrf2 and NF-κB requires further
investigation.
The aim of the present study was to explore the
effects of ARR on LPS-induced RAW264.7 macrophages and to
investigate the potential underlying mechanism. The western
blotting, immunofluorescence and immunohistochemistry experimental
results indicated that ARR inhibited the LPS-induced activation of
TRAF6 and NF-κB p65 signaling molecules. Furthermore, ARR could
upregulate NLRC3, HO-1, NQO1 and Nrf2 expression. The experiments
performed and parameters evaluated in the present work suggested
that ARR may exert anti-inflammatory effects, at least in part, by
downregulating NF-κB and activating Nrf2-mediated inflammatory
responses. In future studies, more in-depth investigations on the
anti-inflammatory effect of ARR, and the specific relationship
between NLRC3, NLRC3 and NF-κB should be performed.
In conclusion, the results of the present study
demonstrated that ARR exerted anti-inflammatory effects in
LPS-stimulated RAW264.7 cells, at least partially through the
modulation of NF-κB- and Nrf2-mediated inflammatory responses.
These results suggested that ARR may be an attractive candidate for
the treatment of inflammation-related diseases. However, as this
study was only performed using one macrophage cell line, future
studies should be conducted on a wider variety of cells to verify
the current study findings. Currently, numerous studies have
evaluated the biological activities and mechanisms of tested
compounds by comparing the treatment group (tested compound plus
challenge) with the model group (only challenge), seldom employing
a group treated with the sole test compound without challenge
(62–66). Following this experimental setup,
this type of grouping was employed for the LPS-stimulated RAW264.7
cell model in the present work. Hence, in future studies, more
in-depth investigations on the anti-inflammatory effects of ARR,
including the involvement of an ARR group without LPS challenge and
in vivo animal models, should be conducted to gain further
insight into the mechanism of action. These future studies should
broaden the current understanding of the anti-inflammatory
mechanism and highlight the potential of ARR as anti-inflammatory
candidate drug.
Acknowledgements
Not applicable.
Funding
The present study was funded by the Central
Government Guides Local Scientific and Technological Development
Fund Projects (grant no. YDZX20201400001443), Shanxi International
Science and Technology Cooperation Project (grant no.
201803D421065), the National Natural Science Foundation of China
(grant nos. 30672621 and 81173473) and the Taiyuan City Science and
Technology Project Special Talents Star Project (grant no.
120247-08).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JTZ and GY conceived and designed the experiments.
KDR, JC and JH performed the experiments and analyzed the data. JTZ
and GY confirmed the authenticity of all the raw data. JTZ and KDR
drafted the initial manuscript and prepared the figures. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yang SH, Le B, Androutsopoulos VP,
Tsukamoto C, Shin TS, Tsatsakis AM and Chung G: Anti-inflammatory
effects of soyasapogenol I-αa via downregulation of the MAPK
signaling pathway in LPS-induced RAW 264.7 macrophages. Food Chem
Toxicol. 113:211–217. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Venkatesan T, Park EJ, Choi YW, Lee J and
Kim YK: Anti-inflammatory activity of Ternstroemia gymnanthera stem
bark extracts in bacterial lipopolysaccharide-stimulated RAW264.7
murine macrophage cells. Pharm Biol. 55:837–846. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Venkatesan T, Choi YW, Lee J and Kim YK:
Falcarindiol inhibits LPS-induced inflammation via attenuating MAPK
and JAK-STAT signaling pathways in murine macrophage RAW 264.7
cells. Mol Cell Biochem. 445:169–178. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vašíček O, Lojek A and Číž M: Serotonin
and its metabolites reduce oxidative stress in murine RAW264.7
macrophages and prevent inflammation. J Physiol Biochem. 76:49–60.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Park SM, Lee TH, Zhao R, Kim YS, Jung JY,
Park CA, Jegal KH, Ku SK, Kim JK, Lee CW, et al: Amelioration of
inflammatory responses by Socheongryong-Tang, a traditional herbal
medicine, in RAW 264.7 cells and rats. Int J Mol Med. 41:2771–2783.
2018.PubMed/NCBI
|
|
6
|
Novilla A, Djamhuri DS, Nurhayati B,
Rihibiha DD, Afifah E and Widowati W: Anti-inflammatory properties
of oolong tea (Camellia sinensis) ethanol extract and
epigallocatechin gallate in LPS-induced RAW 264.7 cells. Asian
Pacific J Tropical Biomed. 7:1005–1009. 2017. View Article : Google Scholar
|
|
7
|
Liang N, Sang Y, Liu W, Yu W and Wang X:
Anti-inflammatory effects of gingerol on
lipopolysaccharide-stimulated RAW 264.7 cells by inhibiting NF-κB
signaling pathway. Inflammation. 41:835–845. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shim SY, Sung SH and Lee M:
Anti-inflammatory activity of mulberrofuran K isolated from the
bark of Morus bombycis. Int Immunopharmacol. 58:117–124. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Alam MB, Ju MK, Kwon YG and Lee SH:
Protopine attenuates inflammation stimulated by carrageenan and LPS
via the MAPK/NF-κB pathway. Food Chem Toxicol. 131:1105832019.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Olajide OA, Akande IS, Filho C,
Lepiarz-Raba I and de Sousa DP: Methyl 3,4,5-trimethoxycinnamate
suppresses inflammation in RAW264.7 macrophages and blocks
macrophage-adipocyte interaction. Inflammopharmacology.
28:1315–1326. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gültekin Y, Eren E and Özören N:
Overexpressed NLRC3 acts as an anti-inflammatory cytosolic protein.
J Innate Immun. 7:25–36. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schneider M, Zimmermann AG, Roberts RA,
Zhang L, Swanson KV, Wen H, Davis BK, Allen IC, Holl EK, Ye Z, et
al: The innate immune sensor NLRC3 attenuates toll-like receptor
signaling via modification of the signaling adaptor TRAF6 and
transcription factor NF-κB. Nat Immunol. 13:823–831. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang L, Mo J, Swanson KV, Wen H,
Petrucelli A, Gregory SM, Zhang Z, Schneider M, Jiang Y, Fitzgerald
KA, et al: NLRC3, a member of the NLR family of proteins, is a
negative regulator of innate immune signaling induced by the DNA
sensor STING. Immunity. 40:329–341. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu A, Yang Z, Huang Y, Yuan H, Lin C, Wang
T, Zhao Z, Zhou Y and Zhu C: Natural phenylethanoid glycosides
isolated from Callicarpa kwangtungensis suppressed
lipopolysaccharide-mediated inflammatory response via activating
Keap1/Nrf2/HO-1 pathway in RAW 264.7 macrophages cell. J
Ethnopharmacol. 258:1128572020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Younis NS and Mohamed ME: Protective
effects of myrrh essential oil on isoproterenol-induced myocardial
infarction in rats through antioxidant, anti-inflammatory,
Nrf2/HO-1 and apoptotic pathways. J Ethnopharmacol. 270:1137932021.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kobayashi EH, Suzuki T, Funayama R,
Nagashima T, Hayashi M, Sekine H, Tanaka N, Moriguchi T, Motohashi
H, Nakayama K and Yamamoto M: Nrf2 suppresses macrophage
inflammatory response by blocking proinflammatory cytokine
transcription. Nat Commun. 7:116242016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim HN, Kim JD, Park SB, Son HJ, Park GH,
Eo HJ, Kim HS and Jeong JB: Anti-inflammatory activity of the
extracts from Rodgersia podophylla leaves through activation of
Nrf2/HO-1 pathway, and inhibition of NF-κB and MAPKs pathway in
mouse macrophage cells. Inflamm Res. 69:233–244. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Qiu HX and Lin YR: Loranthaceae Juss.
Flora of China. Editorial Committee of Flora of China, Chinese
Academy of Sciences (eds), . 24. Science Press; Beijing: pp.
p1011988
|
|
19
|
Kim YK, Kim YS, Choi SU and Ryu SY:
Isolation of flavonol rhamnosides from Loranthus tanakae and
cytotoxic effect of them on human tumor cell lines. Arch Pharm Res.
27:44–47. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen Y, Lin Y, Li Y and Li C: Total
flavonoids of Hedyotis diffusa willd inhibit inflammatory responses
in LPS-activated macrophages via suppression of the NF-kappaB and
MAPK signaling pathways. Exp Ther Med. 11:1116–1122. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Feng H, He Y, La L, Hou C, Song L, Yang Q,
Wu F, Liu W, Hou L, Li Y, et al: The flavonoid-enriched extract
from the root of Smilax China L. inhibits inflammatory responses
via the TLR-4-mediated signaling pathway. J Ethnopharmacol.
256:1127852020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cho BO, Che DN, Kim JS, Kim JH, Shin JY,
Kang HJ and Jang SI: In vitro anti-inflammatory and anti-oxidative
stress activities of kushenol C isolated from the roots of sophora
flavescens. Molecules. 25:17682020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chiu YH, Wu YW, Hung JI and Chen MC:
Epigallocatechin gallate/L-ascorbic acid-loaded poly-γ-glutamate
microneedles with antioxidant, anti-inflammatory, and
immunomodulatory effects for the treatment of atopic dermatitis.
Acta Biomater. 130:223–233. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ningning X, Yune B, Qiangqiang X, Shouyuan
Z and Guane Y: Preliminary experiments on the chemical constituents
of the parasitic mulberry. Chinese Medicines and Clinics.
12:762–763. 2012.(In Chinese).
|
|
25
|
Zhou J, Wang T, Dou Y, Huang Y, Qu C, Gao
J, Huang Z, Xie Y, Huang P, Lin Z and Su Z: Brusatol ameliorates
2,4,6-trinitrobenzenesulfonic acid-induced experimental colitis in
rats: Involvement of NF-κB pathway and NLRP3 inflammasome. Int
Immunopharmacol. 64:264–274. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu S, Man Y and Zhao L: Sinomenine
inhibits lipopolysaccharide-induced inflammatory injury by
regulation of miR-101/MKP-1/JNK pathway in keratinocyte cells.
Biomed Pharmacother. 101:422–429. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yuan S, Liu H, Yuan D, Xu J, Chen Y, Xu X,
Xu F and Liang H: PNPLA3 I148M mediates the regulatory effect of
NF-kB on inflammation in PA-treated HepG2 cells. J Cell Mol Med.
24:1541–1552. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Son ES, Park JW, Kim SH, Park HR, Han W,
Kwon OC, Nam JY, Jeong SH and Lee CS: Anti-inflammatory activity of
3,5,6,7,3′,4′-hexamethoxyflavone via repression of the NF-κB and
MAPK signaling pathways in LPS-stimulated RAW264.7 cells. Mol Med
Rep. 22:1985–1993. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li LQ, Song AX, Yin JY, Siu KC, Wong WT
and Wu JY: Anti-inflammation activity of exopolysaccharides
produced by a medicinal fungus Cordyceps sinensis Cs-HK1 in cell
and animal models. Int J Biol Macromol. 149:1042–1050. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lee M, Hong S, Park C, Han MH, Kim SO,
Hong SH, Kim GY and Choi YH: Anti-inflammatory effects of
Daehwangmokdantang, a traditional herbal formulation, in
lipopolysaccharide-stimulated RAW 264.7 macrophages. Exp Ther Med.
14:5809–5816. 2017.PubMed/NCBI
|
|
32
|
Leal NRF, Vigliano MV, Pinto FA, de Sousa
TV, Velozo LSM, Sabino KC, da Graça Justo M and Coelho MG:
Anti-inflammatory effect of diterpenes-enriched fractions from
Pterodon polygalaeflorus through inhibition of macrophage migration
and cytokine production. J Pharm Pharmacol. 70:808–820. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kim SJ, Ko WK, Jo MJ, Arai Y, Choi H,
Kumar H, Han IB and Sohn S: Anti-inflammatory effect of
Tauroursodeoxycholic acid in RAW 264.7 macrophages, Bone
marrow-derived macrophages, BV2 microglial cells, and spinal cord
injury. Sci Rep. 8:31762018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kim YJ: Rhamnetin attenuates melanogenesis
by suppressing oxidative stress and pro-inflammatory mediators.
Biol Pharm Bull. 36:1341–1347. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jnawali HN, Lee E, Jeong KW, Shin A, Heo
YS and Kim Y: Anti-inflammatory activity of rhamnetin and a model
of its binding to c-Jun NH2-terminal kinase 1 and p38 MAPK. J Nat
Prod. 77:258–263. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mendis S, Lindholm LH, Anderson SG, Alwan
A, Koju R, Onwubere BJ, Kayani AM, Abeysinghe N, Duneas A, Tabagari
S, et al: Total cardiovascular risk approach to improve efficiency
of cardiovascular prevention in resource constrain settings. J Clin
Epidemiol. 64:1451–1462. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kim KH, Kim EJ, Kwun MJ, Lee JY, Bach TT,
Eum SM, Choi JY, Cho S, Kim SJ, Jeong SI and Joo M: Suppression of
lung inflammation by the methanol extract of Spilanthes acmella
Murray is related to differential regulation of NF-κB and Nrf2. J
Ethnopharmacol. 217:89–97. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wu H, Wang Y, Zhang Y, Xu F, Chen J, Duan
L, Zhang T, Wang J and Zhang F: Breaking the vicious loop between
inflammation, oxidative stress and coagulation, a novel
anti-thrombus insight of nattokinase by inhibiting LPS-induced
inflammation and oxidative stress. Redox Biol. 32:1015002020.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ying Y, Sun CB, Zhang SQ, Chen BJ, Yu JZ,
Liu FY, Wen J, Hou J, Han SS, Yan JY, et al: Induction of autophagy
via the TLR4/NF-κB signaling pathway by astragaloside contributes
to the amelioration of inflammation in RAW264.7 cells. Biomed
Pharmacother. 137:1112712021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li M, Dong L, Du H, Bao Z and Lin S:
Potential mechanisms underlying the protective effects of
Tricholoma matsutake singer peptides against LPS-induced
inflammation in RAW264.7 macrophages. Food Chem. 353:1294522021.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Guo C, Bi J, Li X, Lyu J, Liu X, Wu X and
Liu J: Immunomodulation effects of polyphenols from thinned peach
treated by different drying methods on RAW264.7 cells through the
NF-κB and Nrf2 pathways. Food Chem. 340:1279312021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kim ME, Na JY and Lee JS:
Anti-inflammatory effects of trans-cinnamaldehyde on
lipopolysaccharide-stimulated macrophage activation via MAPKs
pathway regulation. Immunopharmacol Immunotoxicol. 40:219–224.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hwang SJ, Ahn EY, Park Y and Lee HJ: An
aqueous extract of Nomura's jellyfish ameliorates inflammatory
responses in lipopolysaccharide-stimulated RAW264.7 cells and a
zebrafish model of inflammation. Biomed Pharmacother. 100:583–589.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Khajuria V, Gupta S, Sharma N, Tiwari H,
Bhardwaj S, Dutt P, Satti N, Nargotra A, Bhagat A and Ahmed Z:
Kaempferol-3-o-β-d-glucuronate exhibit potential anti-inflammatory
effect in LPS stimulated RAW 264.7 cells and mice model. Int
Immunopharmacol. 57:62–71. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Su Y, Xiong S, Lan H, Xu L and Wei X:
Molecular mechanism underlying anti-inflammatory activities of
lirioresinol B dimethyl ether through suppression of NF-κB and MAPK
signaling in in vitro and in vivo models. Int Immunopharmacol.
73:321–332. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hunto ST, Kim HG, Baek KS, Jeong D, Kim E,
Kim JH and Cho JY: Loratadine, an antihistamine drug, exhibits
anti-inflammatory activity through suppression of the NF-κB
pathway. Biochem Pharmacol. 177:1139492020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ha DT, Long PT, Hien TT, Tuan DT, An NT,
Khoi NM, Oanh HV and Hung TM: Anti-inflammatory effect of
oligostilbenoids from Vitis heyneana in LPS-stimulated RAW 264.7
macrophages via suppressing the NF-κB activation. Chem Cent J.
12:142018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Tao MQ, Ji CL, Wu YJ, Dong JY, Li Y,
Olatunji OJ and Zuo J: 1,7-Dihydroxy-3,4-dimethoxyxanthone inhibits
lipopolysaccharide-induced inflammation in RAW264.7 macrophages by
suppressing TLR4/NF-κB signaling cascades. Inflammation.
43:1821–1831. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Eleazu C, Suleiman JB, Othman ZA, Zakaria
Z, Nna VU, Hussain NH and Mohamed M: Bee bread attenuates high fat
diet induced renal pathology in obese rats via modulation of
oxidative stress, downregulation of NF-κB mediated inflammation and
Bax signalling. Arch Physiol Biochem. 2:1–17. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Huang YF, Zhou JT, Qu C, Dou YX, Huang QH,
Lin ZX, Xian YF, Xie JH, Xie YL, Lai XP and Su ZR:
Anti-inflammatory effects of Brucea javanica oil emulsion by
suppressing NF-kappaB activation on dextran sulfate sodium-induced
ulcerative colitis in mice. J Ethnopharmacol. 198:389–398. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Huang C, Li W, Zhang Q, Chen L, Chen W,
Zhang H and Ni Y: Anti-inflammatory activities of Guang-Pheretima
extract in lipopolysaccharide-stimulated RAW 264.7 murine
macrophages. BMC Complement Altern Med. 18:462018. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Lee SG, Brownmiller CR, Lee SO and Kang
HW: Anti-inflammatory and antioxidant effects of anthocyanins of
trifolium pratense (Red Clover) in lipopolysaccharide-stimulated
RAW-267.4 macrophages. Nutrients. 12:10892020. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Karki S, Park HJ, Nugroho A, Kim EJ, Jung
HA and Choi JS: Quantification of major compounds fromIxeris
dentata, Ixeris dentata Var. albiflora, and Ixeris sonchifolia and
their comparative anti-inflammatoryactivity in
lipopolysaccharide-stimulated RAW 264.7 cells. J Med Food.
18:83–94. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Kim MS, Park JS, Chung YC, Jang S, Hyun CG
and Kim SY: Anti-inflammatory effects of formononetin
7-O-phosphate, a novel biorenovation product, on LPS-stimulated RAW
264.7 macrophage cells. Molecules. 24:39102019. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Li ZT, Liu H and Zhang WQ: NLRC3
alleviates hypoxia/reoxygenation induced inflammation in RAW264.7
cells by inhibiting K63-linked ubiquitination of TRAF6.
Hepatobiliary Pancreat Dis Int. 19:455–460. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Biliktu M, Senol SP, Temiz-Resitoglu M,
Guden DS, Horat MF, Sahan-Firat S, Sevim S and Tunctan B:
Pharmacological inhibition of soluble epoxide hydrolase attenuates
chronic experimental autoimmune encephalomyelitis by modulating
inflammatory and anti-inflammatory pathways in an
inflammasome-dependent and -independent manner.
Inflammopharmacology. 28:1509–1524. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Cho YC, Park J and Cho S:
Anti-inflammatory and anti-oxidative effects of
luteolin-7-O-glucuronide in LPS-stimulated murine macrophages
through TAK1 inhibition and Nrf2 activation. Int J Mol Sci.
21:20072020. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Kwon MY, Park J, Kim SM, Lee J, Cho H,
Park JH and Han IO: An alpha-lipoic acid-decursinol hybrid compound
attenuates lipopolysaccharide-mediated inflammation in BV2 and
RAW264.7 cells. BMB Rep. 52:508–513. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Li CL, Liu XH, Qiao Y, Ning LN, Li WJ, Sun
YS, Liu DS, Gao W and Ma CM: Allicin alleviates inflammation of
diabetic macroangiopathy via the Nrf2 and NF-κB pathway. Eur J
Pharmacol. 876:1730522020. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Wardyn JD, Ponsford AH and Sanderson CM:
The Keap1/Nrf2 pathway in health and disease dissecting molecular
cross-talk between Nrf2 and NF-κB response pathways. Biochem Soc
Trans. 43:621–626. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Ren J, Su D, Li L, Cai H, Zhang M, Zhai J,
Li M, Wu X and Hu K: Anti-inflammatory effects of Aureusidin in
LPS-stimulated RAW264.7 macrophages via suppressing NF-κB and
activating ROS- and MAPKs-dependent Nrf2/HO-1 signaling pathways.
Toxicol Appl Pharmacol. 387:1148462019. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Zheng Y, Tian C, Fan C, Xu N, Xiao J, Zhao
X, Lu Z, Cao H, Liu J and Yu L: Sheng-Mai Yin exerts
anti-inflammatory effects on RAW 264.7 cells and zebrafish. J
Ethnopharmacol. 267:1134972020. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Park YJ, Cheon SY, Lee DS, Cominguez DC,
Zhang Z, Lee S and An HJ: Anti-inflammatory and antioxidant effects
of carpesium cernuum L. Methanolic extract in LPS-stimulated RAW
264.7 macrophages. Mediators Inflammation. 2020:31642392020.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Baek SH, Park T, Kang MG and Park D:
Anti-inflammatory activity and ROS regulation effect of
sinapaldehyde in LPS-stimulated RAW 264.7 macrophages. Molecules.
25:40892020. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Kumar A, Sawhney G, Nagar RK, Chauhan N,
Gupta N, Kaul A, Ahmed Z, Sangwan PL, Kumar PS and Yadav G:
Evaluation of the immunomodulatory and anti-inflammatory activity
of Bakuchiol using RAW 264.7 macrophage cell lines and in animal
models stimulated by lipopolysaccharide (LPS). Int Immunopharmacol.
91:1072642021. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Sewwandi S, Dissanayake CY, Natraj P, Lee
YJ and Han CH: Anti-inflammatory effect of sulforaphane on
LPS-stimulated RAW 264.7 cells and ob/ob mice. J Vet Sci.
21:e912020. View Article : Google Scholar : PubMed/NCBI
|