Introduction
Cardiac arrest (CA), also known as cardiopulmonary
arrest or circulatory arrest, involves a sudden cessation of normal
blood circulation due to the failure of the heart to pump blood
adequately (1). CA induces
whole-body ischemia, which causes damage to multiple organs,
including the brain, heart, kidneys and liver. The majority of
research studies involving CA over the past half-century have
focused on improving the rate of successful return of spontaneous
circulation (ROSC), with significant progress (2–4). Although
immediate resuscitation may improve ROSC, the survival rate with a
poor prognosis is a concern (5–7). Post-cardiac arrest syndrome
(PCAS) refers to the pathophysiological consequences of ROSC
following successful cardiopulmonary resuscitation (CPR) following
CA (8). PCAS is the main cause of
decreased survival following ROSC (9). The early-period PCAS survival rate
in patients is only 30% (5).
There is no doubt that the heart and brain are important organs in
PCAS. Meanwhile, studies have rarely investigated renal failure
following CA (1,10,11). Transient impaired renal function
is common in patients surviving CA (12). The incidence and impact of kidney
dysfunction following CA are not well described (13). In addition, our previous study
suggested that the low early survival rate following ROSC in
experimental studies (14) may be
strongly related to renal failure such as acute kidney injury. One
of the most common causes of acute kidney injury is CA (15). Acute kidney injury is a common
PCAS developing in ~30% of in-hospital patients with CA (16).
Reactive oxygen species (ROS) are composed of a
series of oxygen intermediates, including the free radical
superoxide anion (O2•−), the nonradical
hydrogen peroxide (H2O2), the highly reactive
hydroxyl free radical (OH•), peroxynitrite (ONOO−) and
singlet oxygen (1O2) (17). ROS have been revealed to play an
essential role in several experimental renal conditions, such as
acute ischemic renal failure, renal graft rejection, acute
glomerulonephritis and toxic renal diseases (18). There is significant evidence
supporting the synthesis of ROS immediately following acute
ischemic stroke (19) and acute
myocardial infarction (20). ROS
are known to be important in ischemic diseases, such as stroke and
myocardial infarction. For example, Hackenhaar et al
(21) reported that ROS are
generated in the blood of patients with PCAS; however, studies have
rarely investigated ROS formation in the kidney following CA during
the early post-PCAS period (22,23).
For this reason, it was hypothesized that ROS are
important in kidney injury following CA and contribute to the low
survival rate in the early stages of PCAS. To examine this
hypothesis, asphyxial CA was induced in rats and the survival rate
during the early stages of PCAS was observed. Additionally,
immediate and delayed hypothermia were performed to increase the
low survival rate associated with PCAS following ROSC. Furthermore,
the renal dysfunction was analyzed histopathologically and the
changes induced by ROS, such as copper-zinc superoxide dismutase
(SOD-1), manganese superoxide dismutase (SOD-2), catalase (CAT) and
glutathione peroxidase (GPX) were assessed via immunohistochemical
analysis following ROSC.
Materials and methods
Experimental animals and groups
A total of 62 male Sprague-Dawley (SD) rats
(weight,270–300 g; age, 10 weeks) were obtained from the
Experimental Animal Center of Jeonbuk National University (Iksan,
Republic of Korea). They were housed at a temperature of 23±2°C and
humidity of 60±10% under a 12-h light/dark cycle. They were
supplied with free access to food and water. All experimental
protocols were approved based on ethical procedures and scientific
care by the Institutional Animal Care and Use Committee of Jeonbuk
National University (approval no. JBNU2020–084).
Experimental animals were stratified in three
categories [a sham operation group, CA under normothermia, and CA
and hypothermia treatment (HT)] as follows: i) group I, a sham
group (n=5) was maintained under normothermia conditions without
CA; ii) group II, a normothermia group without hypothermia (33°C)
treatment following CA (n=17); and iii) group III (n=40), a group
that underwent CA under normothermia and were treated with HT after
CA for 2 h (n=17), 4 h (n=13) and 6 h (n=10) following ROSC, where
all rats were reheated to normothermia.
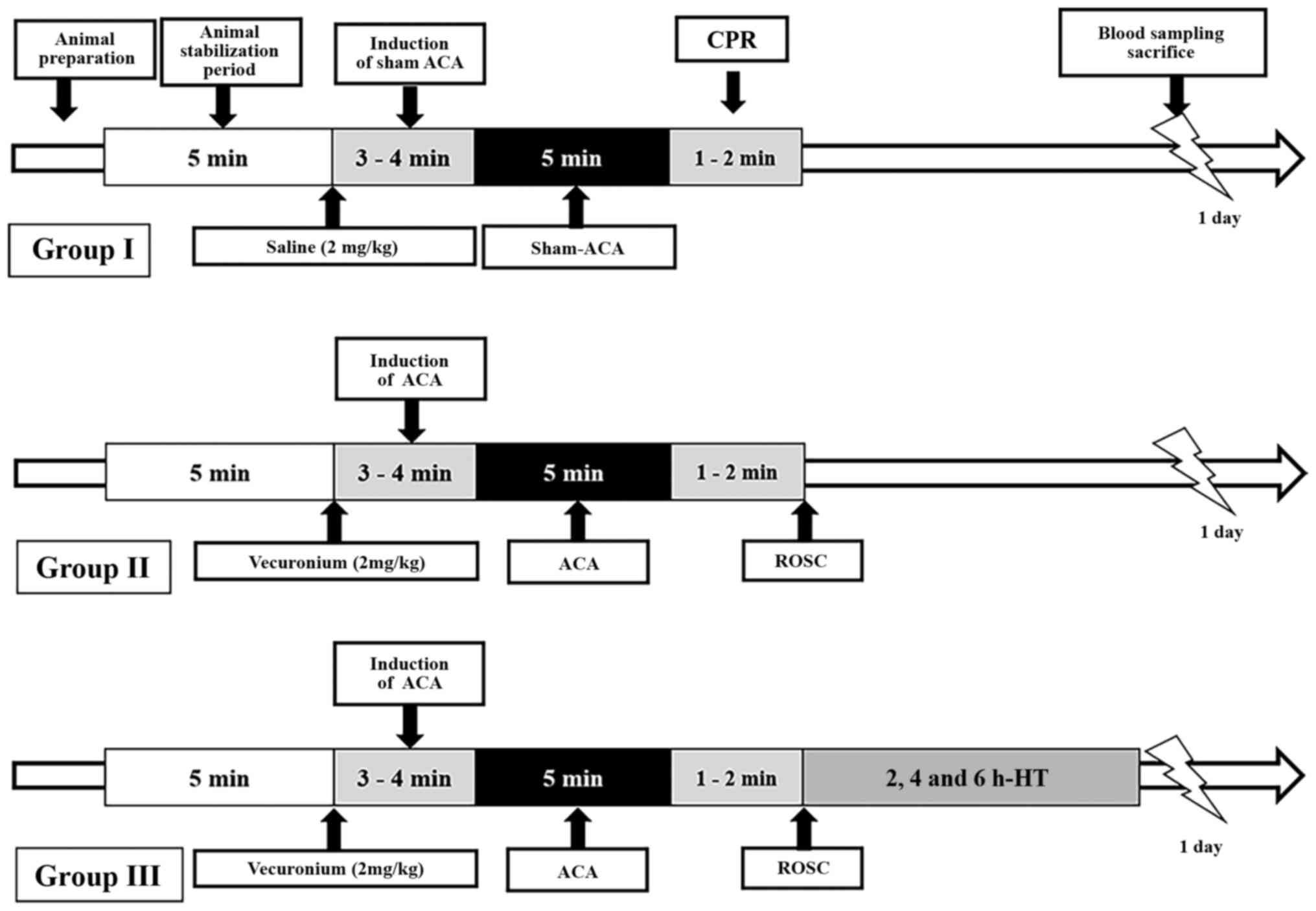
CA induction and CPR
CA and CPR were performed as previously described
(24,25) with minor modifications (Fig. 1). Briefly, the rats were
anesthetized with2–3% isoflurane and mechanically ventilated to
maintain respiration using a rodent ventilator (Harvard Apparatus).
To monitor peripheral oxygen saturation (SpO2), a pulse
oximetry oxygen saturation probe (Nonin Medical, Inc.) was attached
to the left foot. Body temperature was maintained at 37±0.5°C
during and following the CA surgery. To monitor electrocardiogram
(ECG) changes, electrocardiographic probes (Cytiva) were placed on
the limbs to provide three-lead data, and which were monitored
continuously. The left femoral artery and right femoral vein were
separately cannulated to monitor the mean arterial pressure (MAP)
(MLT 1050/D; ADInstruments, Ltd.) and intravenous injection.
Following a 5-min stabilization period, vecuronium
bromide (2 mg/kg; Gensia Sicor Pharmaceuticals, Inc.) was
intravenously administered, anesthesia was stopped and mechanical
ventilation was withdrawn. A MAP below 25 mm-Hg and subsequent
pulseless electric activity were used to define CA (25,26). CA was confirmed at3–4 min
following vecuronium bromide injection. At 5 min following CA, CPR
was initiated by intravenously administering a bolus injection of
epinephrine (0.005 mg/kg; Sigma-Aldrich; Merck KGaA) and sodium
bicarbonate (1 mEq/kg; Sigma-Aldrich; Merck KGaA) followed by
mechanical ventilation with 100% oxygen and manual chest
compressions at a rate of 300/min until MAP reached 60 mm-Hg and
electrocardiographic activity was observed. Once the animal was
hemodynamically stable and spontaneously breathing (usually at 1 h
following ROSC), the catheters were removed and the animal was
extubated.
Temperature management among the
groups
The body temperature of the normothermia group was
maintained at 37±0.5°C during and following the CA surgery and
maintained until the rats were sacrificed according to the time
schedule. In the hypothermia group, CA was established at normal
temperature; then, the body temperature was maintained with ice
packs and fans at 33±0.5°C immediately following CPR for 2, 4 and 6
h, and they were rewarmed rapidly with the heating pad until the
desired temperature (37±0.5°C) was achieved. The rats were then
returned to their cages until they were sacrificed one day
following CPR/ROSC. The body temperature was monitored using a
rectal temperature sensor (27).
Serum biochemical analysis
An intraperitoneal injection of 30 mg/kg
pentobarbital sodium (JW Pharm Co., Ltd.) was used to anesthetize
all animals. Blood was collected from the abdominal veins of each
animal in each group. Serum was collected by blood centrifugation
(2,774 × g, 15 min, 4°C) and was preserved at −80°C until analysis.
The levels of blood urea nitrogen (BUN) and creatinine in the serum
were determined according to methods outlined by the International
Federation of Clinical Chemistry (28) using an automated chemical analyzer
Hitachi 2070 (Hitachi, Ltd.). All assays were conducted in
triplicate using fresh serum.
Tissue processing
The rats were deeply anesthetized by an
intraperitoneal injection of 200 mg/kg pentobarbital sodium (JW
Pharm Co., Ltd.), and they were perfused transcardially with 0.1 M
of phosphate-buffered saline (PBS; pH 7.4), followed by 4%
paraformaldehyde in 0.1 M of phosphate buffer (PB; pH 7.4). Kidneys
were isolated from each animal and fixed with 4% paraformaldehyde
in 0.1 M PB (pH 7.4) at room temperature during the 1 week, then
sliced sagittally, embedded in paraffin and sectioned (6 µm).
Hematoxylin and eosin (H&E),
Periodic Acid-Schiff (PAS) and Masson's trichrome staining
H&E staining was performed to examine
pathological changes in the kidneys according to a previously
described procedure (29). PAS
staining was performed to examine changes in glomeruli according to
previously described procedures (30–32). Masson's trichrome
staining method was used for defining tubular injury, considering
tubular dilatation, tubular atrophy, tubular cast formation,
vacuolization, degeneration, interstitial fibrosis and sloughing of
tubular epithelial cells, or thickening of the tubular basement
membrane according to previously described procedures (33,34).
A total of 2 experienced pathologists evaluated
histopathological changes in a double-blinded manner. Images of 10
stained sections/rat were captured at ×400 magnifications using a
Leica DM 2500 light microscope (Leica Microsystems GmbH). A total
of 10 fields were analyzed in each section. Histopathological
analysis of renal lesions was performed according to previously
described procedures (35,36).
Briefly, lesions were categorized as no significant microscopic
lesions (NSML), minimal, mild, moderate, or marked lesions,
respectively, graded using the following scale with the blind test:
normal, 0 points; <25% damage, 1 point;26–50% damage, 2
points;51–75% damage, 3 points; and76–100% damage, 4 points.
Glomerular lesions were defined by loss of cellular elements,
collapse of capillary lumen, amorphous hyaline material with or
without adhesions to the Bowman's capsule (30–32) and scored by the
following numeric scales: no damage, 0 points; very mild, 1 point;
mild, 2 points; moderate, 3 points; and severe, 4 points. Tubular
injury was scored by the following scoring system: no tubular
injury, 0 points;1–9% of tubules injured, 1 point;10–25% of tubules
injured, 2 points;26–50% of tubules injured, 3 points; 51–75% of
tubules injured, 4 points; and at least 76% of tubules injured, 5
points (33,34).
Malondialdehyde (MDA)
MDA concentration in the renal cortex was evaluated
according to a previously described protocol (23,37,38). In short, the homogenization and
centrifugation of the renal tissues were performed at 8,832 × g for
10 min at 4°C, and the supernatant was collected and stored at
−80°C for MDA analysis. MDA content was determined according to the
instructions of TBARS assay kit (cat. no. 10009055; Cayman Chemical
Company).
Immunohistochemistry (IHC) for
antioxidant enzymes
IHC was performed with SOD-1, SOD-2, CAT and GPX to
study changes in antioxidant immunoreactivities in the kidney. IHC
was carried out according to our previously described method
(22). In brief, the sections (6
µm) were incubated with primary goat anti-SOD1 (1:500; cat. no.
SAB2500976; Sigma-Aldrich; Merck KGaA), goat anti-SOD2 (1:1,000;
cat. no. SAB2501676; Sigma-Aldrich; Merck KGaA), rabbit anti-CAT
(1:1,000; cat. no. ab16731; Abcam) and rabbit anti-GPX (1:1,000;
cat. no. ab22604; Abcam) overnight at 4°C, followed by the
biotinylated-conjugated anti-rabbit (1:250; cat. no. BA-1000-1.5;
Vector Laboratories, Inc.) and the biotinylated-conjugated
anti-goat (1:250; cat. no. BA-5000-1.5; Vector Laboratories, Inc.)
secondary antibodies for 2 h at 24°C and developed using Vectastain
ABC (Vector Laboratories, Inc.). Then, they were visualized with
3,3′-diaminobenzidine solution (in 0.1 M Tris-HCl buffer).
Leica DM 2500 microscope was used to image the
sections at a magnification of ×400. A total of 10 sections/rat
were selected and 10 areas were captured. ImageJ threshold analysis
software version 1.52a (National Institutes of Health) was used to
measure the percent (%) of relative optical density (ROD).
Statistical analysis
All experiments were repeated in triplicate. Graph
Pad Prism version 5.0 (GraphPad Software, Inc.) was used to analyze
the data, which were expressed as the means ± standard error of the
mean (SEM) values. Survival was analyzed using Kaplan-Meier
statistics and the log-rank test. MAP and peripheral oxygen were
compared using one- and two-way repeated-measures of analysis of
variance to assess the effect of time. To determine the
significance of differences, post hoc analyses were conducted using
Tukey's test for all pairwise multiple comparisons. P<0.05 was
considered to indicate a statistically significant difference.
Results
Physiological changes, the survival
rate and serum biochemical variables
There was no statistically significant difference
among the groups regarding baseline characteristics, including body
weight, MAP and SpO2 (Table I and Fig. 2). The induction of CA occurred 3–4
min following the intravenous injection of vecuronium bromide (2
mg/kg). CA was confirmed with an isoelectric ECG, SpO2
and MAP, and these changed as expected according to the
experimental protocol (Fig.
2A-C). As revealed in Fig.
2D, the body temperature was different among all groups
following ROSC.
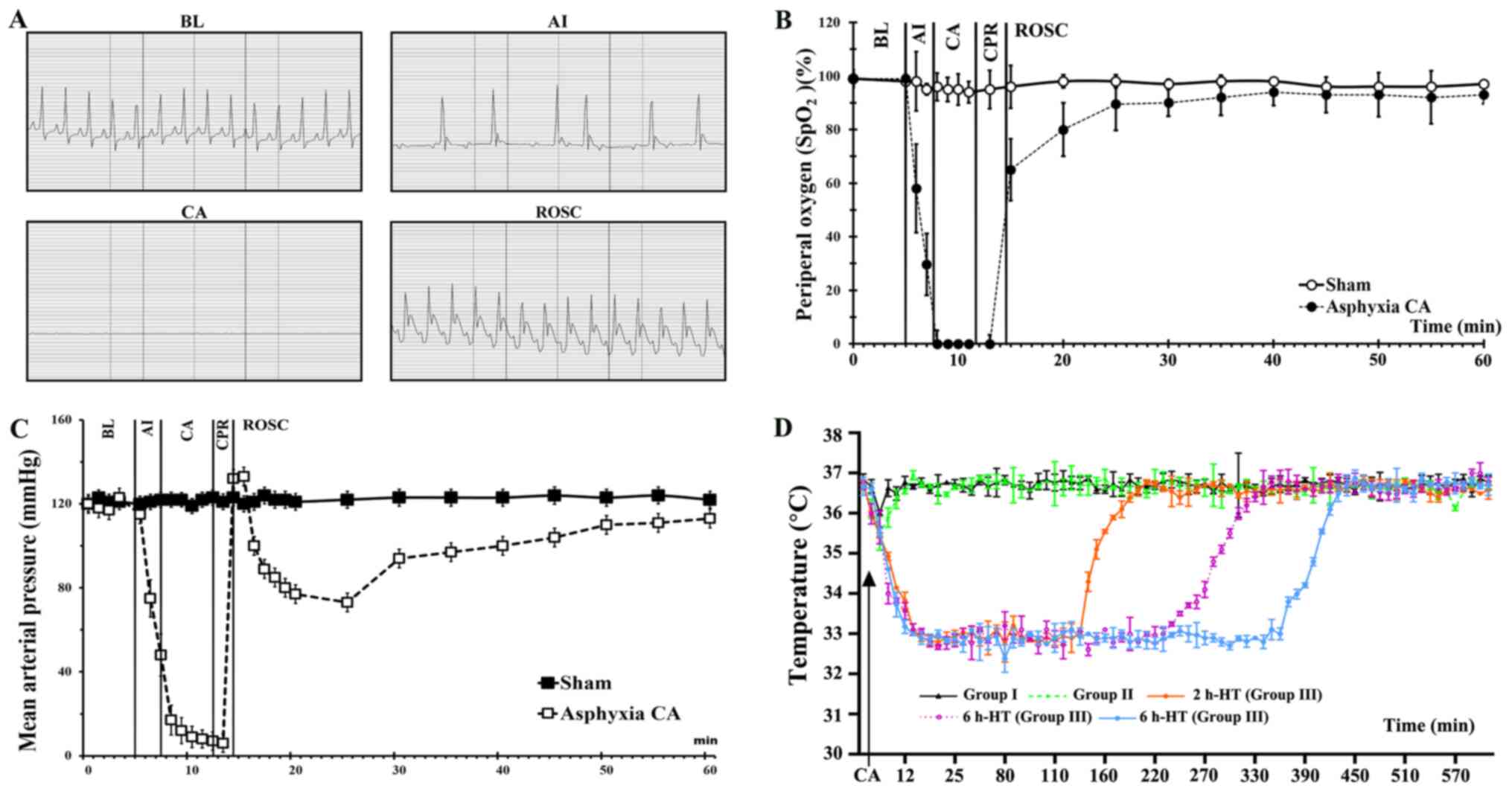 | Figure 2.Physiological variables in Groups I,
II and III. (A) Electrocardiogram from a representative animal at
BL, AI, CA and ROSC. Pulseless electrical activity is shown during
CA, although it is often visible during CA. (B) SpO2
levels were revealed during CA, CPR and ROSC. (C) Mean arterial
pressure is shown during CA, CPR and ROSC. (D) Temperature
management following ROSC. Data are expressed as the means ± SEM.
HT, hypothermia treatment; CA, cardiac arrest; ROSC, return of
spontaneous circulation; CPR, cardiopulmonary resuscitation; BL,
baseline; AI, asphyxia induction. |
 | Table I.Physiological condition, asphyxia
time and CPR time in Groups I, II and III before CA. |
Table I.
Physiological condition, asphyxia
time and CPR time in Groups I, II and III before CA.
|
|
|
| Group III |
|---|
|
|
|
|
|
|---|
| Parameters | Group I | Group II | 2 h-HT | 4 h-HT | 6 h-HT |
|---|
| Body weight, g | 355.44±17.33 | 354.13±17.04 | 284.14±9.89 | 278.14±19.41 | 283.71±12.08 |
| Heart rate,
beats/min | 335.14±8.51 | 336.34±6.51 | 339.78±16.13 | 333.50±9.65 | 338.67±9.55 |
| Room temperature,
°C | 36.60±0.53 | 23.90±0.87 | 25.07±0.53 | 25.06±0.68 | 24.83±0.72 |
| Asphyxia time to
CA, sec | - | 162.12±16.36 | 145.44±28.79 | 148.89±19.65 | 150.56±24.55 |
| CPR time, sec | - | 71.12±12.49 | 72.03±7.97 | 69.78±10.57 | 73.89±9.93 |
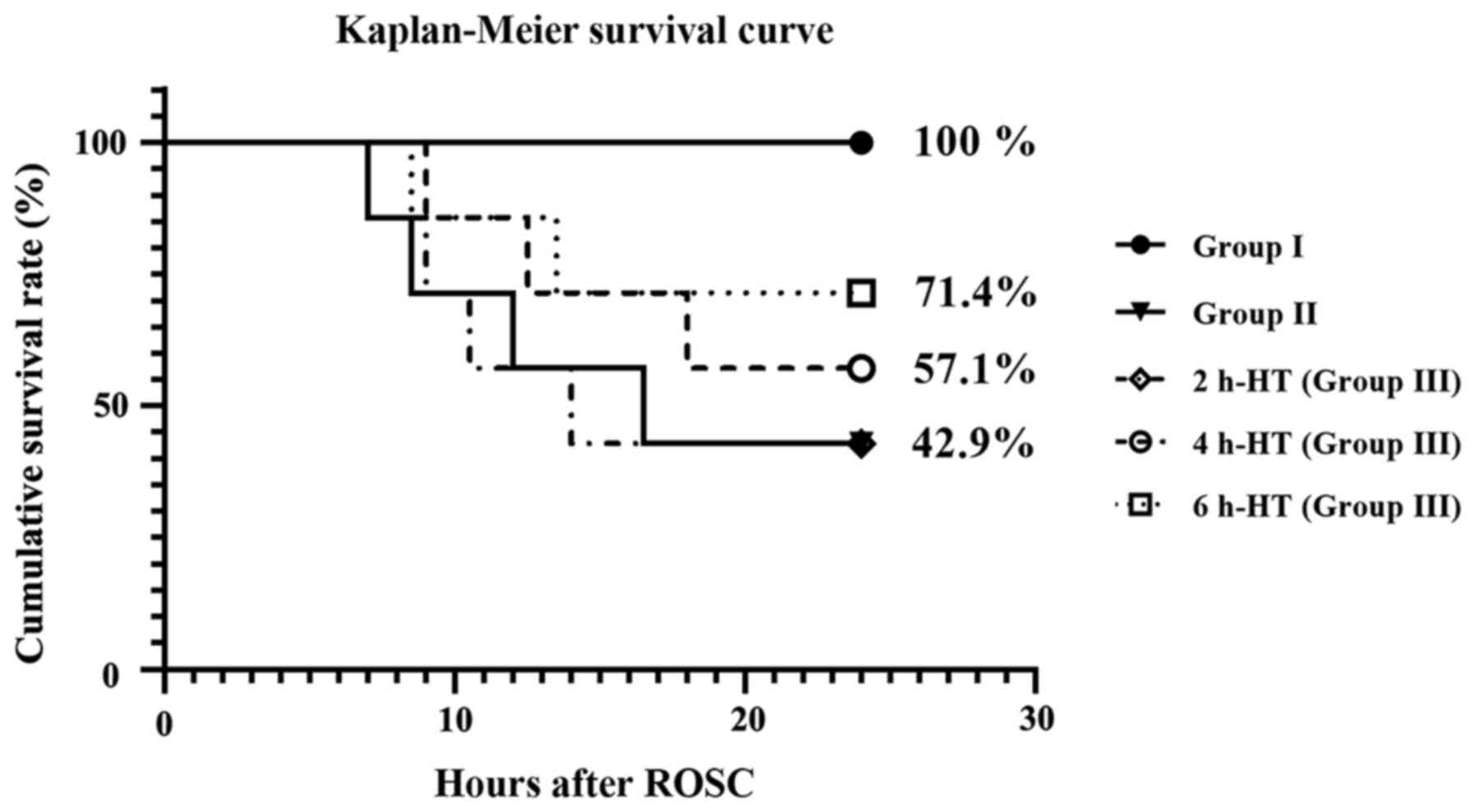
As revealed in Fig.
3, the survival rate of each group was evaluated at one day
post-CA. The rate of Group II was 42.9%. In group III, the rate
following 2 h-HT was 42.9%, the rate following 4 h-HT was 57.1% and
the rate following 6 h-HT was 71.4%. In this experiment, there was
no difference between the rats in Group II and the rats with 2 h-HT
in Group III.
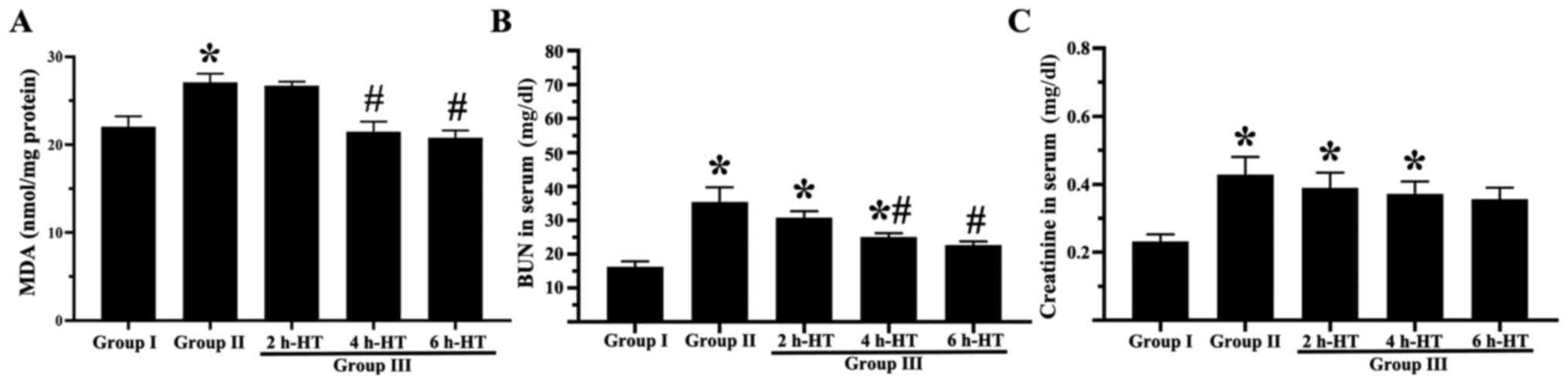
As demonstrated in Fig. 4B, the serum BUN level in Group I
was 13.8 mg/dl one day post-CA. In group II, the BUN level was
significantly increased to 35.3 mg/dl. In Group III, the BUN level
was 30.7 mg/dl following 2 h-HT, 25.0 mg/dl following 4 h-HT and
22.7 mg/dl following 6 h-HT. In addition, as revealed in Fig. 4C, the serum creatinine level in
Group I was 0.23 mg/dl. In group II, the creatinine level was
significantly increased to 0.43 mg/dl. In group III, the creatinine
levels were lower than that observed in Group II as follows: 0.39
mg/dl following 2 h-HT, 0.37 mg/dl following 4 h-HT and 0.36 mg/dl
following 6 h-HT.
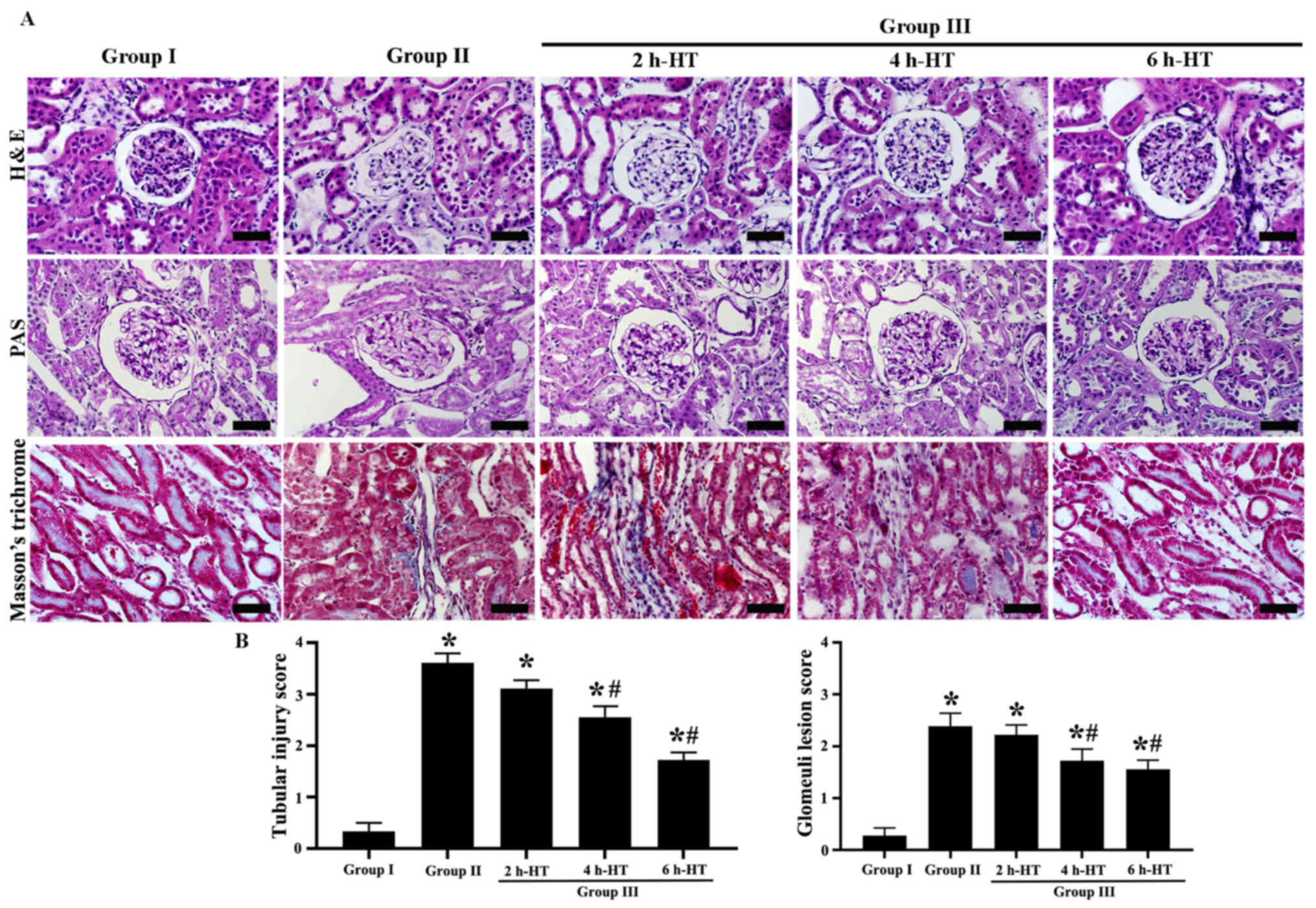
Histopathological findings
In Group I (sham), intact histological structures
were revealed by H&E, PAS and Masson's trichrome staining
(Fig. 5A). The interstitial
fibrosis was not detected in all groups, meanwhile in Group II,
CA-induced renal histopathology was examined at one day following
ROSC using H&E, PAS and Masson's trichrome staining. Severe
CA-induced kidney injury was significantly increased in the
proximal tubules and glomeruli; particularly, the brush borders of
the renal tubular epithelial cells were seriously eroded (Fig. 5A and B). In addition, in this
group, glomerular capillaries were dilated with inflammatory cells,
and interstitial edema and acute renal tubular necrosis were
serious as compared with those observed in Group I (Fig. 5A).
In Group III, kidney injury was attenuated at one
day following ROSC (Fig. 5A and
B). In particular, CA-induced injury in the proximal tubules
was significantly decreased following 6 h-HT as compared with that
observed in Group II. In addition, local expansion of the proximal
tubules was decreased as compared with that revealed in Group II
(Fig. 5A). For the glomeruli, 6
h-HT significantly attenuated glomerular injury as compared with
that revealed in Group II (Fig. 5A
and B).
MDA level
As revealed in Fig.
4A, the level of MDA in the renal cortex was significantly
increased at one day following CA in Group II compared with Group
I. However, in Group III, the level of MDA was significantly
decreased following 4 h- and 6 h-HT. It was also decreased in the 2
h-HT, but there was no statistically significant difference
compared with Group II.
Findings of antioxidant enzyme
immunoreactivities
In Group I, normal SOD-1, SOD-2, GPX and CAT
immunoreactivities were evaluated, revealing that they were
principally located in the tubules (Fig. 6A). In Group II, SOD-1, SOD-2, GPX
and CAT immunoreactivities were significantly reduced at one day
following ROSC as compared with those revealed in Group I (Fig. 6A and B).
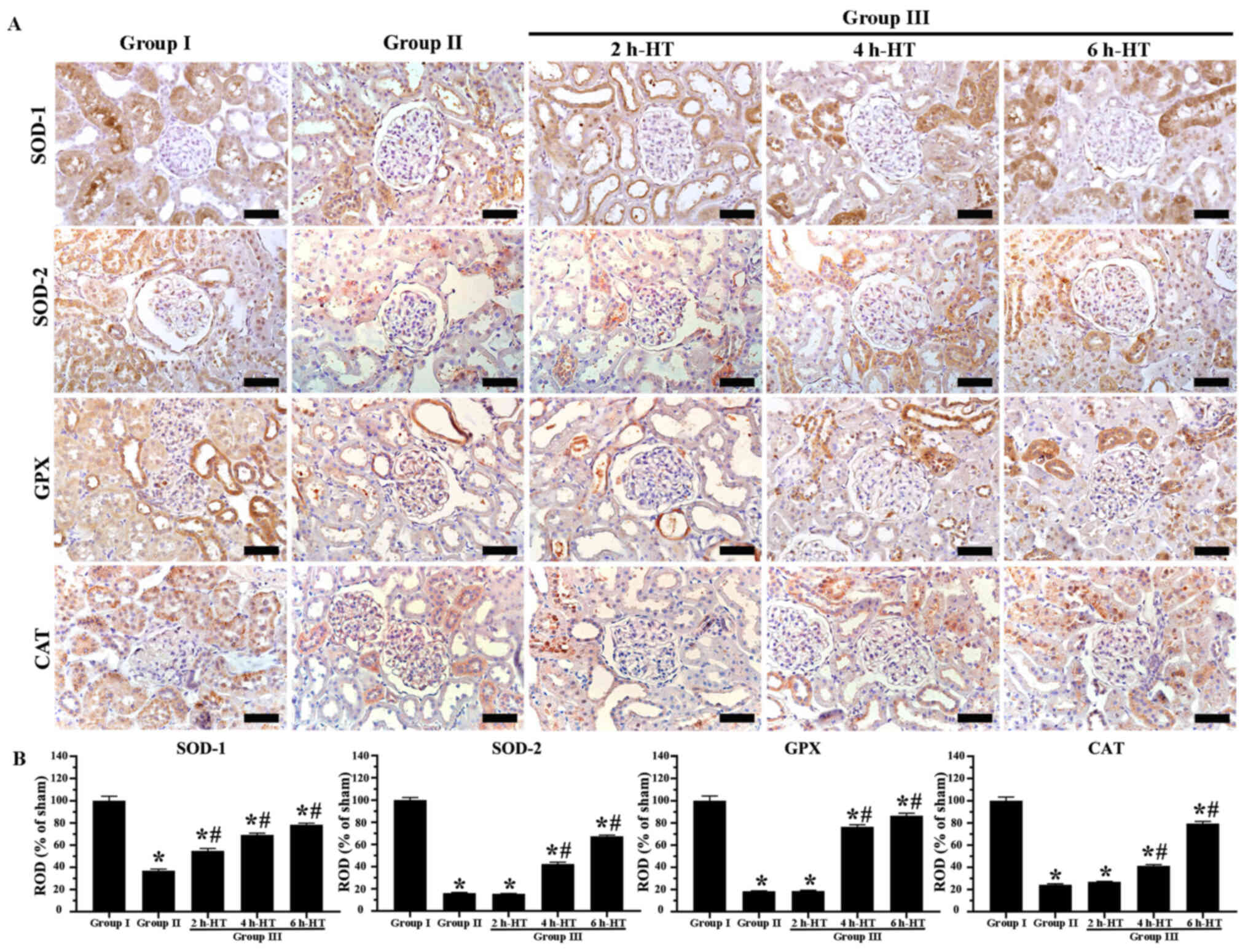 | Figure 6.Immunohistochemistry analysis of
antioxidant enzyme expression in renal cortex tissue. (A)
Immunoreactivities of antioxidant enzymes (SOD-1, SOD-2, GPX and
CAT) in the kidneys of Groups I, II and III were altered. All
immunoreactivities in Group III with 6 h-HT were markedly increased
when compared with Group II. Original magnification, ×400. Scale
bar, 50 µm. (B) ROD of SOD-1, SOD-2, GPX and CAT
immunoreactivities. The ROD in Group III with 6 h-HT was
significantly increased when compared with Group II. *P<0.05 vs.
Group I; #P<0.05 vs. Group II. SOD, superoxide
dismutase; GPX, glutathione peroxidase; CAT, catalase; ROD,
relative optical density; HT, hypothermia treatment. |
In Group III, SOD-1, SOD-2, GPX and CAT
immunoreactivities following 2 h-HT were not significantly
different from those observed in Group II (Fig. 6A and B). In the case of 4 h-HT,
the four immunoreactivities were significantly higher than those
revealed in Group II (Fig. 6A and
B), demonstrating that, in particular, GPX immunoreactivity was
significantly higher compared with the other immunoreactivities. In
the case of 6 h-HT, all immunoreactivities were higher than those
identified in the rats which received 4 h-HT, revealing that the
ROD of each SOD-1, SOD-2, GPX and CAT immunoreactivity was 78.4,
67.4, 86.5 and 79.5%, respectively, as compared with Group I
(Fig. 6A and B).
Discussion
In animal studies, the heart and brain are the most
affected organs following ischemia/reperfusion (I/R) injury after
CA (39,40). Nevertheless, certain studies have
reported that acute kidney injury has an impact on neurological
recovery (41,42). Therefore, it is important to
investigate acute kidney injury following CA and CPR. In the
present study, adult male SD rats were used for asphyxial CA by
injecting vecuronium bromide. CA was confirmed 3–4 min following
induction of asphyxia and CPR was performed 5 min after CA. MAP,
ECG and SpO2 were altered as expected during CA and
following ROSC. In our present study, the survival rate in Group
III was 42.9% one day following ROSC in rats exposed to 2 h-HT,
57.1% in rats treated with 4 h-HT, and 71.4% in rats subjected to 6
h-HT. Che et al (26)
reported that the survival rate was 40% two days following ROSC in
a rat model of asphyxial CA. In addition, Wang et al
(43) reported that in rats, the
combination of hypothermia and levosimendan (a calcium sensitizer
and potassium-channel opener) following ROSC significantly
increased survival. Based on these findings, HT in rats with CA may
increase the survival rate a few days following ROSC. However, in
humans, HT after CA hardly increased the survival rate following
ROSC (44).
Renal dysfunction was reported in 12–28% of patients
with CA following successful resuscitation (13). In addition, acute kidney injury
developed in 43% of patients resuscitated after CA, and more than
75% of these episodes occurred within three days following CA
(45). In animal models, acute
kidney injury induced by I/R (i.e., ROSC following CA) was
significantly attenuated by HT (43,46,47). For example, Tissier et al
(47) reported significant
attenuation of kidney lesions by HT in a rabbit model of CA, based
on histopathology and electron microscopy, compared with the
control group. In our present study, the histopathological
glomerular and tubular lesion scores of kidneys in Group II were
apparently enhanced one day following ROSC. In this group, serum
BUN and creatinine levels were significantly increased following
ROSC when compared with the sham group (Group I). These results
were similar to those of previous studies involving canine, rabbit
and piglet CA models (47–49). Thus, kidney injury was severely
increased in the early phase following CA in experimental animals.
In our present study, renal glomerular and tubular lesions and
histopathological scores in Group III were significantly reduced
following 4 h- and 6 h-HT one day following ROSC compared with
Group II. Ribeiro et al (46) and Souza et al (50) reported that HT was effective in
animal models of renal I/R injury. Islam et al (23) and Jawad et al (22) determined that HT reduced the
severity of renal injury and increased the survival rate in an
asphyxial CA model. The findings suggested that HT has a
significant renal-protective effect, which was associated with an
increased survival rate.
Endogenous antioxidant enzymes mainly include SODs,
CAT and GPX. These enzymes provide a first line of defense against
O2•− and OH•. SOD-1 and SOD-2 provide a
defense against oxidative stress by catalyzing the dismutation of
O2•− into O2 and
H2O2 (51).
Oxidative stress is a crucial factor in organ injury and
hemodynamic dysfunction during PCAS and the generation of ROS
during I/R injury. The activity of antioxidant enzymes is altered
by I/R injury following CA (21).
Our study revealed that SOD-1, SOD-2, GPX and CAT levels were
decreased following ROSC in Group II compared with Group I. The
levels of these antioxidant enzymes are reduced following I/R,
which causes cell damage and death due to the consumption of
endogenous antioxidants as a result of ROS release (52).
Xia et al (53) reported increased antioxidant
activity in kidney tissues of mice exposed to HT in renal I/R
injury. Hackenhaar et al (21) observed a significant increase in
the activity of SOD-1, SOD-2, GPX and CAT after 6, 12, 36 and 72-h
HT in humans following ROSC. In previous studies using a rat model
of asphyxial CA, Islam et al (23) reported that HT following CA
reduced oxidative stress in the kidney and Jawad et al
(22) reported that HT following
CA protected the kidney against injury induced by CA, demonstrating
that Nrf2/HO-1 was increased in the kidney. In our present study,
the immunoreactivities of SOD-1, SOD-2, GPX and CAT were
significantly increased following 4 h-HT and 6 h-HT in Group III
when compared with Group II, suggesting that HT activated
antioxidant enzymes and reduced the oxidative stress.
Based on the survival, histopathology, biochemical
and immunohistochemical results of this study, it was determined
that renal dysfunction is common and associated with mortality in
the early stages of PCAS following ROSC, in our rat model of
asphyxial CA. However, 4 h- or 6 h-HT following ROSC significantly
reduced renal injury, suggesting that HT induces activation of
antioxidant enzymes, such as SOD-1, SOD-2, GPX and CAT, resulting
in reduced oxidative stress in the kidneys. As a result, it was
hypothesized that HT reduces renal injury by an antioxidant
mechanism and increases the early survival rate. However, western
blot analysis is required to elucidate the mechanism of renal
injury and HT in CA following ROSC. This is a potential limitation
of the present study and underscores the need for further
studies.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Basic Science Research
Program through the National Research Foundation of Korea funded by
the Ministry of Education (grant nos. NRF-2019R1C1C1002564,
NRF-2019R1F1A1062696 and NRF-2021R1F1A1059992).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SEK, HYS, MHW and HJT were responsible for the
experimental design, data acquisition, data analysis and manuscript
writing. EYL, YJY, RHK, JHC and TKL performed the experiments and
data analysis. DCA, BYP, JCY, SKH and ISK performed the data
analysis and made critical comments on the entire process of the
study. All authors have read and approved the final manuscript. HYS
and HJT confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
All experimental protocols were approved based on
ethical procedures and scientific care by the Institutional Animal
Care and Use Committee of Jeonbuk National University (approval no.
JBNU 2020-084; Jeonju, South Korea).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Girotra S, Chan PS and Bradley SM:
Post-resuscitation care following out-of-hospital and in-hospital
cardiac arrest. Heart. 101:1943–1949. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Roh YI, Jung WJ, Hwang SO, Kim S, Kim HS,
Kim JH, Kim TY, Kang HS, Lee JS and Cha KC: Shorter defibrillation
interval promotes successful defibrillation and resuscitation
outcomes. Resuscitation. 143:100–105. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xanthos T, Iacovidou N, Pantazopoulos I,
Vlachos I, Bassiakou E, Stroumpoulis K, Kouskouni E, Karabinis A
and Papadimitriou L: Ischaemia-modified albumin predicts the
outcome of cardiopulmonary resuscitation: An experimental study.
Resuscitation. 81:591–595. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yeh ST, Cawley RJ, Aune SE and Angelos MG:
Oxygen requirement during cardiopulmonary resuscitation (CPR) to
effect return of spontaneous circulation. Resuscitation.
80:951–955. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
López-Herce J, del Castillo J, Matamoros
M, Canadas S, Rodriguez-Calvo A, Cecchetti C, Rodríguez-Núnez A and
Carrillo Á; Iberoamerican Pediatric Cardiac Arrest Study Network
RIBEPCI, : Post return of spontaneous circulation factors
associated with mortality in pediatric in-hospital cardiac arrest:
A prospective multicenter multinational observational study. Crit
Care. 18:6072014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mongardon N, Dumas F, Ricome S, Grimaldi
D, Hissem T, Pène F and Cariou A: Postcardiac arrest syndrome: From
immediate resuscitation to long-term outcome. Ann Intensive Care.
1:452011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Neumar RW, Nolan JP, Adrie C, Aibiki M,
Berg RA, Böttiger BW, Callaway C, Clark RS, Geocadin RG, Jauch EC,
et al: Post-cardiac arrest syndrome: Epidemiology, pathophysiology,
treatment, and prognostication. A consensus statement from the
International Liaison Committee on Resuscitation (American Heart
Association, Australian and New Zealand Council on Resuscitation,
European Resuscitation Council, Heart and Stroke Foundation of
Canada, InterAmerican Heart Foundation, Resuscitation Council of
Asia, and the Resuscitation Council of Southern Africa); the
American Heart Association Emergency Cardiovascular Care Committee;
the Council on Cardiovascular Surgery and Anesthesia; the Council
on Cardiopulmonary, Perioperative, and Critical Care; the Council
on Clinical Cardiology; and the Stroke Council. Circulation.
118:2452–2483. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nolan JP, Neumar RW, Adrie C, Aibiki M,
Berg RA, Böttiger BW, Callaway C, Clark RS, Geocadin RG, et al:
Post-cardiac arrest syndrome: epidemiology, pathophysiology,
treatment, and prognostication. A Scientific Statement from the
International Liaison Committee on Resuscitation; the American
Heart Association Emergency Cardiovascular Care Committee; the
Council on Cardiovascular Surgery and Anesthesia; the Council on
Cardiopulmonary, Perioperative, and Critical Care; the Council on
Clinical Cardiology; the Council on Stroke. Resuscitation.
79:350–79. 2008.doi: 10.1016/j.resuscitation.2008.09.017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jentzer JC, Chonde MD and Dezfulian C:
Myocardial Dysfunction and shock after cardiac arrest. BioMed Res
Int. 2015:3147962015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Madl C and Holzer M: Brain function after
resuscitation from cardiac arrest. Curr Opin Crit Care. 10:213–217.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Roberts BW, Kilgannon JH, Chansky ME,
Mittal N, Wooden J, Parrillo JE and Trzeciak S: Multiple organ
dysfunction after return of spontaneous circulation in postcardiac
arrest syndrome. Crit Care Med. 41:1492–1501. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zeiner A, Sunder-Plassmann G, Sterz F,
Holzer M, Losert H, Laggner AN and Müllner M: The effect of mild
therapeutic hypothermia on renal function after cardiopulmonary
resuscitation in men. Resuscitation. 60:253–261. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yanta J, Guyette FX, Doshi AA, Callaway CW
and Rittenberger JC; Post Cardiac Arrest Service, : Renal
dysfunction is common following resuscitation from out-of-hospital
cardiac arrest. Resuscitation. 84:1371–1374. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee JH, Lee TK, Kim IH, Lee JC, Won MH,
Park JH, Ahn JH, Shin MC, Ohk TG, Moon JB, et al: Changes in
histopathology and tumor necrosis factor-α levels in the hearts of
rats following asphyxial cardiac arrest. Clin Exp Emerg Med.
4:160–167. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Uchino S, Kellum JA, Bellomo R, Doig GS,
Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, et al:
Beginning Ending Supportive Therapy for the Kidney (BEST Kidney)
Investigators Acute renal failure in critically ill patients: A
multinational, multicenter study. JAMA. 294:813–818. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mattana J and Singhal PC: Prevalence and
determinants of acute renal failure following cardiopulmonary
resuscitation. Arch Intern Med. 153:235–239. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sachse A and Wolf G: Angiotensin
II-induced reactive oxygen species and the kidney. J Am Soc
Nephrol. 18:2439–2446. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Baud L and Ardaillou R: Reactive oxygen
species: Production and role in the kidney. Am J Physiol.
251:F765–F776. 1986.PubMed/NCBI
|
|
19
|
Rodrigo R, Fernández-Gajardo R, Gutiérrez
R, Matamala JM, Carrasco R, Miranda-Merchak A and Feuerhake W:
Oxidative stress and pathophysiology of ischemic stroke: Novel
therapeutic opportunities. CNS Neurol Disord Drug Targets.
12:698–714. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shahzad S, Hasan A, Faizy AF, Mateen S,
Fatima N and Moin S: Elevated DNA damage, oxidative stress, and
impaired response defense system inflicted in patients with
myocardial infarction. Clin Appl Thromb Hemost. 24:780–789. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hackenhaar FS, Medeiros TM, Heemann FM,
Behling CS, Putti JS, Mahl CD, Verona C, da Silva ACA, Guerra MC,
Gonçalves CAS, et al: Therapeutic hypothermia reduces oxidative
damage and alters antioxidant defenses after cardiac arrest. Oxid
Med Cell Longev. 2017:87043522017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jawad A, Yoo YJ, Cho JH, Yoon JC, Tian W,
Islam MS, Lee EY, Shin HY, Kim SE, Kim K, et al: Therapeutic
hypothermia effect on asphyxial cardiac arrest-induced renal
ischemia/reperfusion injury via change of Nrf2/HO-1 levels. Exp
Ther Med. 22:10312021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Islam A, Kim SE, Yoon JC, Jawad A, Tian W,
Yoo YJ, Kim IS, Ahn D, Park BY, Hwang Y, et al: Protective effects
of therapeutic hypothermia on renal injury in an asphyxial cardiac
arrest rat model. J Therm Biol. 94:1027612020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Drabek T, Janata A, Wilson CD, Stezoski J,
Janesko-Feldman K, Tisherman SA, Foley LM, Verrier JD and Kochanek
PM: Minocycline attenuates brain tissue levels of TNF-α produced by
neurons after prolonged hypothermic cardiac arrest in rats.
Resuscitation. 85:284–291. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Han F, Boller M, Guo W, Merchant RM, Lampe
JW, Smith TM and Becker LB: A rodent model of emergency
cardiopulmonary bypass resuscitation with different temperatures
after asphyxial cardiac arrest. Resuscitation. 81:93–99. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Che D, Li L, Kopil CM, Liu Z, Guo W and
Neumar RW: Impact of therapeutic hypothermia onset and duration on
survival, neurologic function, and neurodegeneration after cardiac
arrest. Crit Care Med. 39:1423–1430. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Park Y, Ahn JH, Cho JH, Tae HJ, Lee TK,
Kim B, Lee JC, Park JH, Shin MC, Ohk TG, et al: Effects of
hypothermia on inflammatory cytokine expression in rat liver
following asphyxial cardiac arrest. Exp Ther Med. 21:6262021.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Varming K, Forsum U, Bruunshuus I and
Olesen H: International Federation of Clinical Chemistry and
Laboratory Medicine: Scientific Division. EJIFCC. 15:10–13.
2004.PubMed/NCBI
|
|
29
|
Nam SM, Kim JW, Yoo DY, Choi JH, Kim W,
Jung HY, Won MH, Hwang IK, Seong JK and Yoon YS: Effects of
treadmill exercise on neural stem cells, cell proliferation, and
neuroblast differentiation in the subgranular zone of the dentate
gyrus in cyclooxygenase-2 knockout mice. Neurochem Res.
38:2559–2569. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Duarte CG, Zhang J and Ellis S: Effects of
radiocontrast and endothelin administration on systolic blood
pressure and renal damage in male spontaneously hypertensive and
Wistar Kyoto rats with phentolamine-induced adrenergic blockade.
Invest Radiol. 33:104–112. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Raij L, Azar S and Keane W: Mesangial
immune injury, hypertension, and progressive glomerular damage in
Dahl rats. Kidney Int. 26:137–143. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Slaughter TN, Paige A, Spires D, Kojima N,
Kyle PB, Garrett MR, Roman RJ and Williams JM: Characterization of
the development of renal injury in Type-1 diabetic Dahl
salt-sensitive rats. Am J Physiol Regul Integr Comp Physiol.
305:R727–R734. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Canales BK, Reyes L, Reinhard MK, Khan SR,
Goncalves CG and Meguid MM: Renal glomerular and tubular injury
after gastric bypass in obese rats. Nutrition. 28:76–80. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kang DH, Kim YG, Andoh TF, Gordon KL, Suga
S, Mazzali M, Jefferson JA, Hughes J, Bennett W, Schreiner GF, et
al: Post-cyclosporine-mediated hypertension and nephropathy:
Amelioration by vascular endothelial growth factor. Am J Physiol
Renal Physiol. 280:F727–F736. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Açıkgöz Ş, Edebali N, Barut F, Can M,
Tekin İÖ, Büyükuysal Ç and Açıkgöz B: Ischemia modified albumin
increase indicating cardiac damage after experimental subarachnoid
hemorrhage. BMC Neurosci. 15:332014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Klopfleisch R: Multiparametric and
semiquantitative scoring systems for the evaluation of mouse model
histopathology - a systematic review. BMC Vet Res. 9:1232013.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li J, Jiang H, Wu P, Li S, Han B, Yang Q,
Wang X, Han B, Deng N, Qu B, et al: Toxicological effects of
deltamethrin on quail cerebrum: Weakened antioxidant defense and
enhanced apoptosis. Environ Pollut. 286:1173192021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yang D, Yang Q, Fu N, Li S, Han B, Liu Y,
Tang Y, Guo X, Lv Z and Zhang Z: Hexavalent chromium induced heart
dysfunction via Sesn2-mediated impairment of mitochondrial function
and energy supply. Chemosphere. 264:1285472021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Nguyen Thi PA, Chen MH, Li N, Zhuo XJ and
Xie L: PD98059 protects brain against cells death resulting from
ROS/ERK activation in a cardiac arrest rat model. Oxid Med Cell
Longev. 2016:37237622016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yin XL, Shen H, Zhang W and Yang Y:
Inhibition of endoplasm reticulum stress by anisodamine protects
against myocardial injury after cardiac arrest and resuscitation in
rats. Am J Chin Med. 39:853–866. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chou AH, Lee CM, Chen CY, Liou JT, Liu FC,
Chen YL and Day YJ: Hippocampal transcriptional dysregulation after
renal ischemia and reperfusion. Brain Res. 1582:197–210. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Schuck PF, Alves L, Pettenuzzo LF,
Felisberto F, Rodrigues LB, Freitas BW, Petronilho F, Dal-Pizzol F,
Streck EL and Ferreira GC: Acute renal failure potentiates
methylmalonate-induced oxidative stress in brain and kidney of
rats. Free Radic Res. 47:233–240. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang CH, Chang WT, Tsai MS, Huang CH and
Chen WJ: Synergistic effects of moderate therapeutic hypothermia
and levosimendan on cardiac function and survival after
asphyxia-induced cardiac arrest in rats. J Am Heart Assoc.
9:e0161392020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Safar PJ and Kochanek PM: Therapeutic
hypothermia after cardiac arrest. N Engl J Med. 346:612–613. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Tujjar O, Mineo G, Dell'Anna A,
Poyatos-Robles B, Donadello K, Scolletta S, Vincent JL and Taccone
FS: Acute kidney injury after cardiac arrest. Crit Care.
19:1692015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ribeiro GB, Santos EBD, Bona SR, Schaefer
PG, Garcez TA, Rabolini EB, Smaniotto GP, Marroni NP and Corso CO:
The effects of local ischemic preconditioning and topical
hypothermia in renal ischemia/reperfusion injury in rats. Acta Cir
Bras. 32:816–826. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Tissier R, Giraud S, Quellard N, Fernandez
B, Lidouren F, Darbera L, Kohlhauer M, Pons S, Chenoune M, Bruneval
P, et al: Kidney protection by hypothermic total liquid ventilation
after cardiac arrest in rabbits. Anesthesiology. 120:861–869. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Bleske BE, Song J, Chow MS, Kluger J and
White CM: Hematologic and chemical changes observed during and
after cardiac arrest in a canine model - a pilot study.
Pharmacotherapy. 21:1187–1191. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Hang CC, Li CS, Wu CJ and Yang J: Acute
kidney injury after cardiac arrest of ventricular fibrillation and
asphyxiation swine model. Am J Emerg Med. 32:208–215. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Souza PC, Santos EBD, Motta GL, Bona SR,
Schaefer PG, Campagnol D, Bortolini T and Corso CO: Combined
effects of melatonin and topical hypothermia on renal
ischemia-reperfusion injury in rats. Acta Cir Bras. 33:197–206.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Tae HJ, Park JH, Cho JH, Kim IH, Ahn JH,
Lee JC, Kim JD, Park J, Choi SY and Won MH: Oenanthe javanica
extract increases immunoreactivities of antioxidant enzymes in the
rat kidney. Chin Med J (Engl). 127:3758–3763. 2014.PubMed/NCBI
|
|
52
|
Sehitoglu MH, Karaboga I, Kiraz A and
Kiraz HA: The hepatoprotective effect of Aloe vera on
ischemia-reperfusion injury in rats. North Clin Istanb. 6:203–209.
2018.PubMed/NCBI
|
|
53
|
Xia Z, Wang W, Xiao Q, Ye Q, Zhang X and
Wang Y: Mild hypothermia protects renal function in
ischemia-reperfusion kidney: an experimental study in mice.
Transplant Proc. 50:3816–3821. 2018. View Article : Google Scholar : PubMed/NCBI
|