Introduction
Sepsis (SP), a complex clinical condition, is
characterized by a systemic inflammatory response that results in
tissue or organ hypoperfusion and hypoxia, as well as organ
dysfunction (1). Despite
significant efforts in the development of therapeutic strategies,
SP remains life-threatening and is one of the most common causes of
mortality (15.9% in United States) in intensive care units (ICUs)
(1–3). Recent studies focusing on the
pathophysiology of SP have reported that systemic inflammation
leads to disorders of inflammation/anti-inflammation and
oxidant/antioxidant processes, the increased production of adhesion
molecules and chemokines, and the dysregulation of apoptotic cell
death (4,5). It is considered that neutrophils
serve an important role in the host response to infection and SP,
and are recruited in large numbers to the site of damage tissue
(6).
Statins, which are 3-hydroxy-3-methylglutaryl
coenzyme A reductase inhibitors, are a widely used medication for
controlling hypercholesterolemia via a lipid-lowering effect
(7). Recently, numerous studies
have reported that statins possess a variety of other biological
functions, including regulation of the inflammatory response,
exerting immunomodulatory effects and protecting against cell
apoptosis in animal models of SP (8,9).
Autophagy, a well-conserved homeostatic cellular system responsible
for removing damaged or dysfunctional cellular organelles, is
essential for the protection of cell survival against stressors,
such as invasive pathogenic organisms and reactive oxygen species
(ROS) (10). Previous studies
have shown that the regulatory functions of autophagy may
contribute to SP-related organ dysfunction (11,12). Moreover, several recent reports
have identified the relationship between autophagy activity and
simvastatin (13,14). Based on the aforementioned
evidence, the present study aimed to investigate the impact of
simvastatin on inflammation and ROS regulation, as well as the
association of these processes with simvastatin-induced autophagy
in neutrophils from patients with SP.
Materials and methods
Reagents and antibodies
Simvastatin (Sigma-Aldrich; Merck KGaA) was
dissolved in DMSO to make a 50 mM stock solution, which was
maintained at 4°C before use. The MTT and
2′,7′-Dichlorodihydrofluorescein diacetate (DCFH-DA) assays were
purchased from Sigma-Aldrich (Merck KGaA) and Nanjing KeyGen
Biotech Co., Ltd., respectively. Antibodies targeted against Beclin
1, LC3 and GAPDH were purchased from Abcam. RPMI-1640 and FBS were
purchased from Gibco (Thermo Fisher Scientific, Inc.). ELISA kits
were purchased from Hangzhou Multi Sciences (Lianke) Biotech Co.,
Ltd.
Human neutrophil isolation
Peripheral venous blood samples (10 ml) were
obtained from six healthy volunteers (HP) and six patients with
gram-negative SP, who were admitted to the ICU of the Department of
Anesthesiology of The First Affiliated Hospital of Soochow
University (Suzhou, China) between January 2019 and December 2019.
All study participants or their relatives (age, ≥18 years) provided
written informed consent in accordance with the Declaration of
Helsinki. The present study was approved by the Institutional
Review Board and Ethics committee of The First Affiliated Hospital
of Soochow University (approval no. 138/2018). The inclusion
criteria were as follows: i) Age, ≥18 years; and ii) diagnosed with
SP based on the third international consensus definitions for
sepsis and septic shock (Sepsis-3) (15). Furthermore, the exclusion criteria
were as follows: i) HIV infection; ii) organ transplantation; and
iii) pregnancy. The mean age of the participants was 55.0±10.9
years for the SP group and 57.8±10.5 years for the HP group. There
were three males and three females in both the SP and HP groups.
Venous blood samples were collected within 1 h after enrollment.
Human neutrophils were isolated by discontinuous density gradient
centrifugation as previously described (16). Briefly, the leukocyte-enriched
pellets were collected by centrifugation at 250 × g for 6 min at
room temperature. Subsequently, in a centrifuge tube, 3 ml 65%
Percoll solution (Pharmacia Biotech) was layered onto 3 ml 75%
Percoll solution, followed by the layering of 4 ml of peripheral
blood. The tube was then centrifuged at 330 × g for 25 min at room
temperature and the interface between the 75 and 65% layers was
carefully aspirated. After hypotonic lysis of erythrocytes by RBC
Lysis Buffer (Roche Diagnostics), neutrophils were isolated to
reach 98% purity as determined by Giemsa staining. After isolation,
1×106 cells were fixed in methanol for 5 min at room temperature
and stained with Giemsa solution for 1 min at room temperature and
were subsequently imaged using light microscopy. The viability of
neutrophils was >95% as indicated by Trypan blue staining.
Briefly, neutrophil suspensions were added to an equal volume of
0.4% trypan blue solution in a microtube and mixed for 2 min on
ice. Neutrophil viability was analyzed using a Neubauer chamber and
an optic microscope.
Cell culture and stimulation
Neutrophils were cultured in a humidified atmosphere
containing 5% CO2 at 37°C. Cells were adjusted to
~1×106 cells/ml in RPMI-l640 medium supplemented with
10% FBS and 2 mM L-glutamine, and then plated in 96-well culture
plates. Cells were treated with vehicle (DMSO) or simvastatin (5
µM) for 24 h. For inhibitory assays, cells were pre-incubated with
the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase
inhibitor diphenyleneiodonium (DPI; 20 µM) or the autophagy
inhibitor 3-methyladenine (3-MA; 5 nM) for 1 h at 37°C before each
experiment as previously described (17,18). 3-MA and DPI were both dissolved in
DMSO and diluted to the working concentrations. DMSO was used as
the negative control. Neutrophil media were centrifuged at 400 × g
for 5 min at 4°C, and the supernatants were used for quantification
of cytokines (TNF-α and IL-6).
Cell viability assay
Cell viability was detected using the MTT assay.
After treatment for 24 h, MTT solution was added to the medium and
incubated at 37°C for 4 h. Subsequently, the MTT solution was
replaced with DMSO (200 µl) for 30 min at 37°C. The optical density
was assessed using a microplate reader (Bio-Rad Laboratories,
Inc.). Neutrophil viability was analyzed at different doses of SIM
(0, 1, 5, 10 or 15 µM) for 24 h to assess the lethal concentration
of SIM. The mean absorbance values were recorded and normalized to
the values obtained for cells cultured with vehicle.
Measurement of intracellular ROS
levels
For intracellular ROS assessment, cells were seeded
into plates (1×105 cells/well). After treatment, cells
were incubated with DCFH-DA (50 µM; Nanjing KeyGen Biotech Co.,
Ltd.) at 37°C for 20 min. DCF fluorescence was determined and
analyzed using a flow cytometer (FACSVerse; BD Biosciences) and
FlowJo version 10 (Tree Star, Inc.).
Western blotting
Cells were lysed with lysis buffer (20 mM Tris-HCl
(pH 7.5), 150 mM NaCl, 1% NP-40, 0.25% Na-deoxycholate, 1 mM EDTA
and protease inhibitor cocktail). After centrifugation at 12,000 ×
g for 10 min at 4°C, the supernatants were collected. For western
blotting, 30 µg of protein/lane was separated by SDS-PAGE on a 15%
gel and then transferred to a PVDF membrane. After blocking with 5%
non-fat milk in TBST (10 mM Tris, 150 mM NaCl and 0.1% Tween-20)
for 1 h at 37°C, the membranes were probed with primary antibodies
targeted against rabbit anti-Beclin-1 (1:2,000; cat. no. ab210498;
Abcam), rabbit anti-LC3 (1:1,000; cat. no. ab192890; Abcam) or
rabbit anti-GAPDH (1:1,000; cat. no. ab181602; Abcam) with gentle
agitation overnight at 4°C. After washing with TBST, the membranes
were further incubated with HRP-conjugated goat anti-rabbit
secondary antibodies (1:5,000; cat. no. ab205718; Abcam) in
blocking buffer for 1 h at room temperature. Specific protein bands
were visualized using the ECL detection kit (Amersham; Cytiva). The
blots were scanned, and the intensity of the obtained blot bands
was semi-quantified and analyzed using Science Lab Image Gauge
software (version 4.0; FUJIFILM Wako Pure Chemical
Corporation).
Quantification of cytokines
The concentrations of TNF-α and IL-6 in the
supernatants were determined using Human TNF-α Quantikine ELISA Kit
(cat. no. DTA00D; R&D Systems, Inc.) and Human IL-6 Quantikine
ELISA Kit (cat. no. D6050; R&D Systems, Inc.) according to the
manufacturer's protocols. Absorbance was determined at a wavelength
of 450 nm using a VersaMax microplate reader (Molecular Devices,
LLC). The concentrations were calculated according to calibration
curves.
Statistical analysis
Statistical analyses were performed using SPSS
software (version 22.0; IBM Corp.). Data are presented as the mean
± SD from three replicates. Comparisons among multiple groups were
analyzed using one-way or two-way ANOVA followed by Bonferroni's
post hoc test. The unpaired Student's t-test was used for
comparisons between two groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
Simvastatin protects neutrophils in
SP
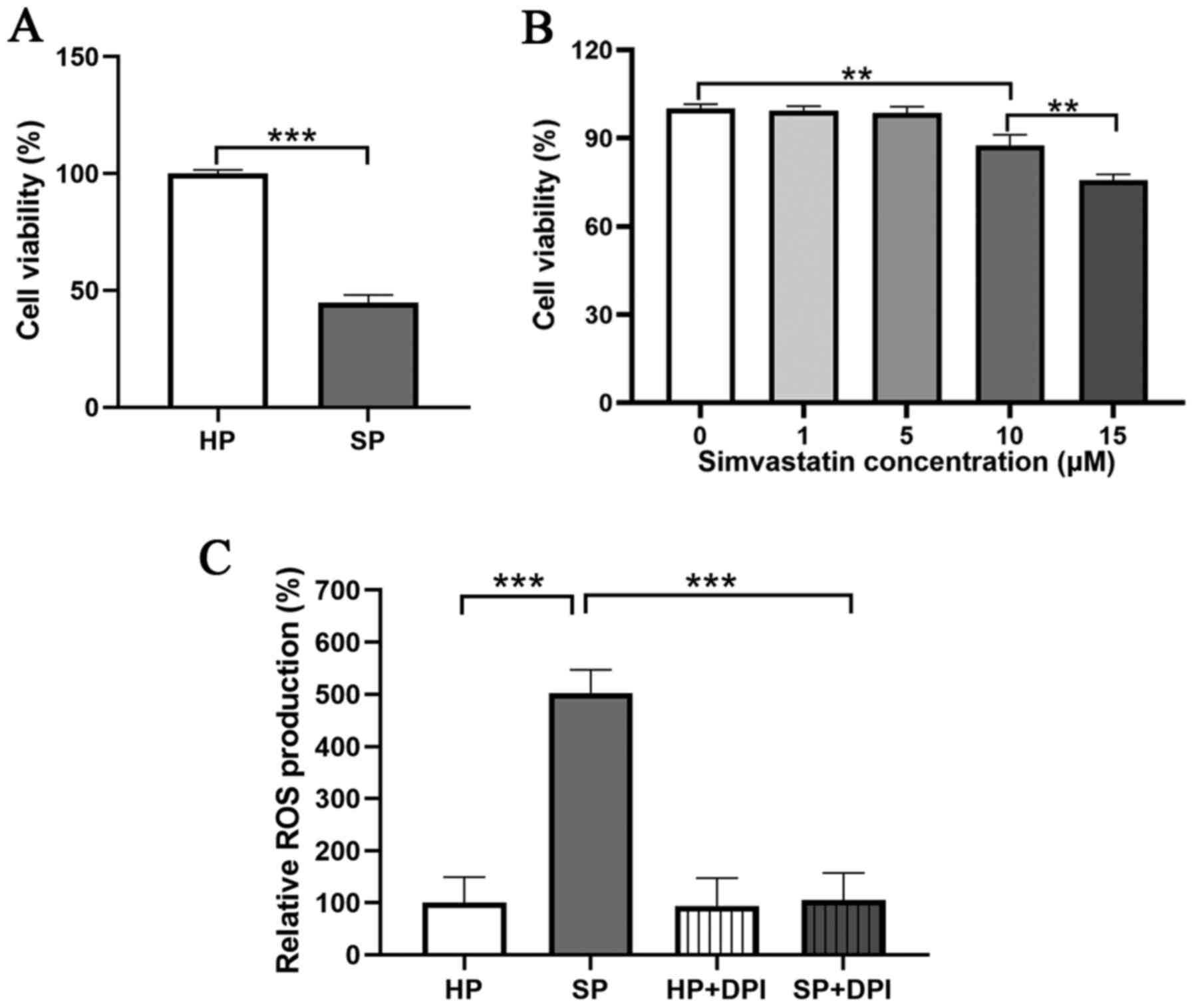
To investigate the viability of neutrophils in SP,
an MTT assay was performed. The results demonstrated that the
viability of neutrophils was significantly decreased in the SP
group compared with that in the HP group (Fig. 1A).
Next, whether simvastatin could protect neutrophils
from SP was investigated. To obtain a non-toxic dose of the
simvastatin, a viability test was performed, in which the
neutrophils from the HP group were treated with different doses of
simvastatin (0, 1, 5, 10 or 15 µM) for 24 h to assess the lethal
concentration. A significant decrease in neutrophil viability was
observed following treatment with 10 or 15 µM simvastatin compared
with that in untreated neutrophils (Fig. 1B). Moreover, there was no
significant difference in viability between the 0 and 5 µM
simvastatin groups, thus 5 µM was considered as the optimal
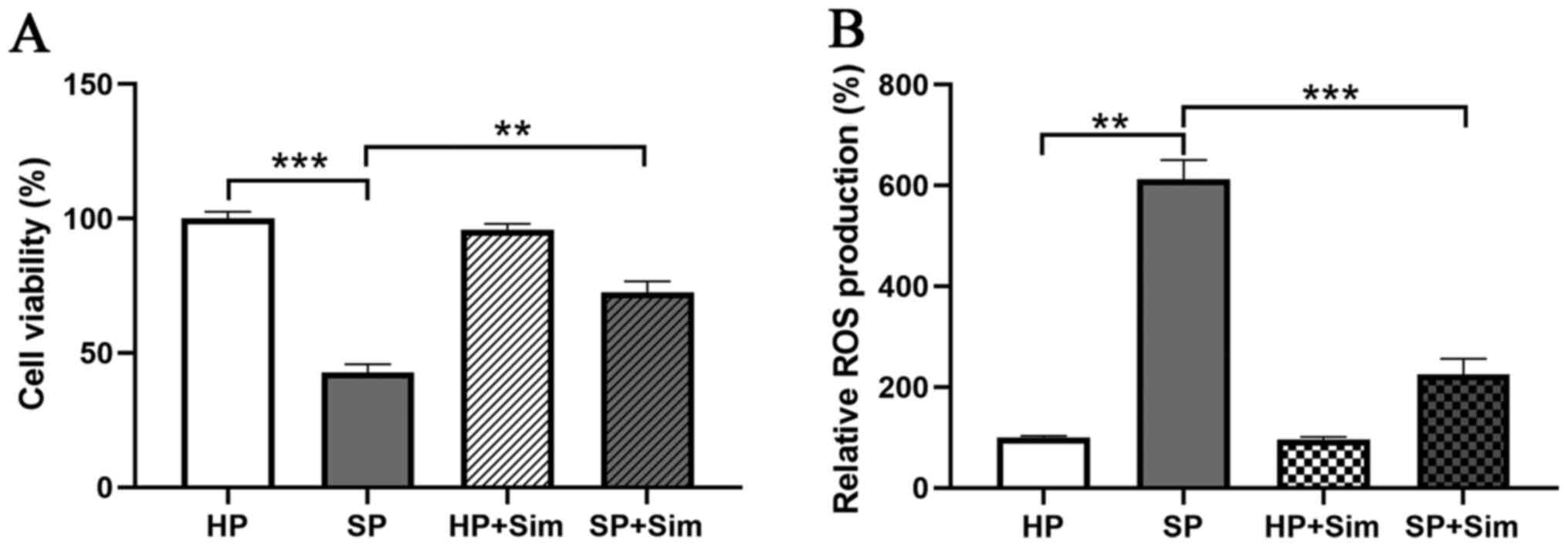
concentration in the present study. Subsequently, neutrophils from
the SP and HP groups were treated with simvastatin (5 µM), and the
results indicated that the viability of neutrophils was 98.33% in
the HP group, but 42.00% in the SP group (Fig. 2A). Moreover, simvastatin
significantly inhibited the decreased viability of neutrophils in
the SP group (42.00 vs. 71.33%, P<0.01).
Simvastatin decreases intracellular
ROS levels in neutrophils
Subsequently, ROS production in neutrophils was
assessed. The results demonstrated that the neutrophils from the SP
group displayed a significantly higher production of ROS compared
with neutrophils from the HP group. In addition, the elevated level
of ROS was abrogated with treatment by DPI, an inhibitor of NADPH
oxidase (Figs. 1C and S1). Following treatment with
simvastatin, neutrophils in the SP group displayed significantly
decreased levels of ROS (223.33±30.86%) compared with those from
the untreated SP group (606.67±38.24%, P<0.001; Fig. 2B). Furthermore, simvastatin did
not affect ROS production in neutrophils from the HP group.
Simvastatin reduces inflammation in
SP
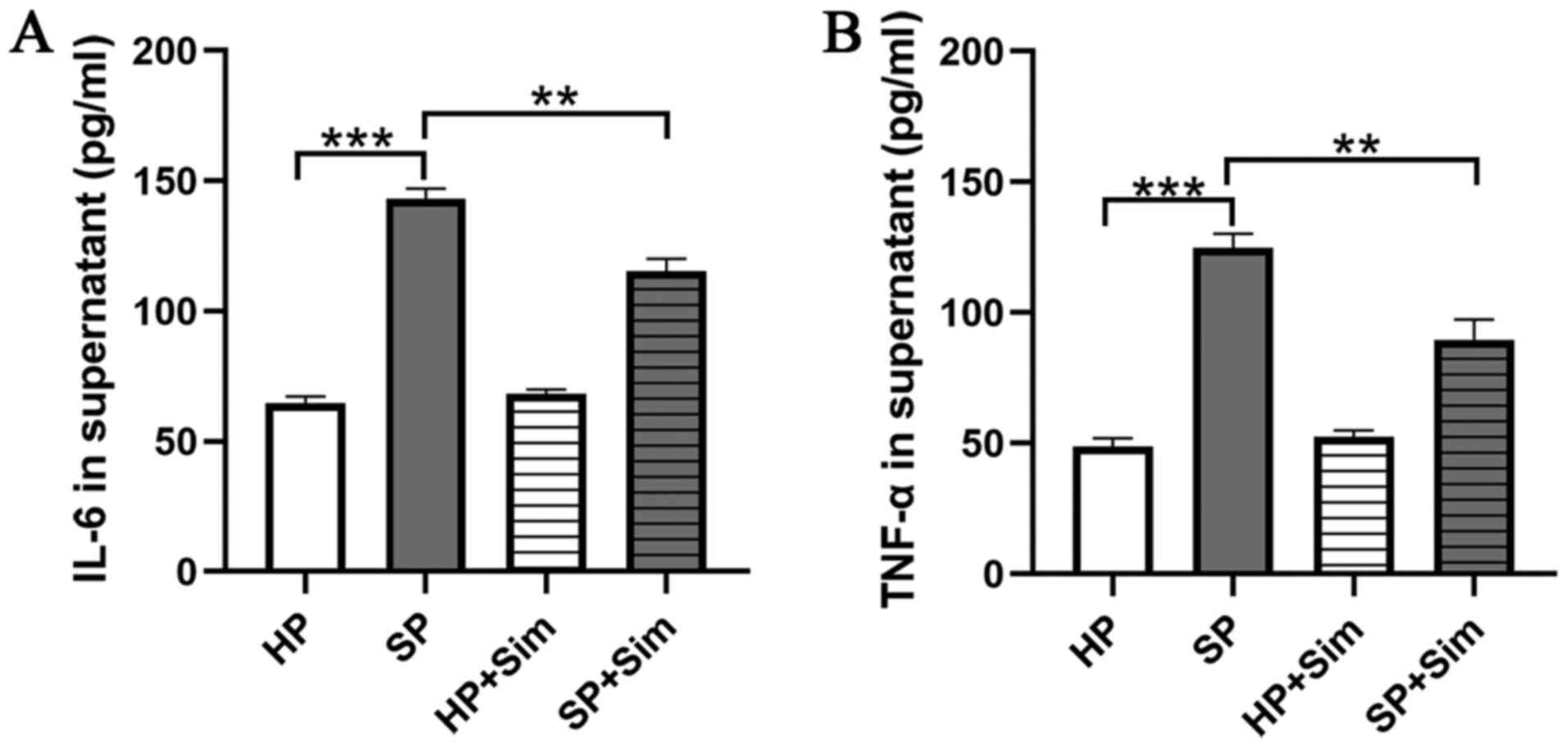
To investigate whether simvastatin had an effect on
the inflammatory response in neutrophils, the concentrations of
TNF-α and IL-6 in the supernatants of neutrophil-conditioned media
were assessed. The results demonstrated that the concentrations of
TNF-α and IL-6 were significantly higher in the SP
neutrophil-conditioned media compared with those in the HP
neutrophil-conditioned media (Fig. 3A
and B). However, TNF-α and IL-6 concentrations were
significantly reduced after treatment with simvastatin in septic
neutrophils (SP vs. SP + Sim TNF-α, 124.67±5.51 vs. 89.33±8.14
pg/ml, P=0.003; SP vs. SP + Sim, IL-6, 142.67±4.04 vs. 115.33±4.73
pg/ml, P=0.002).
Simvastatin inhibits inflammation and
oxidative stress via autophagy induction in SP
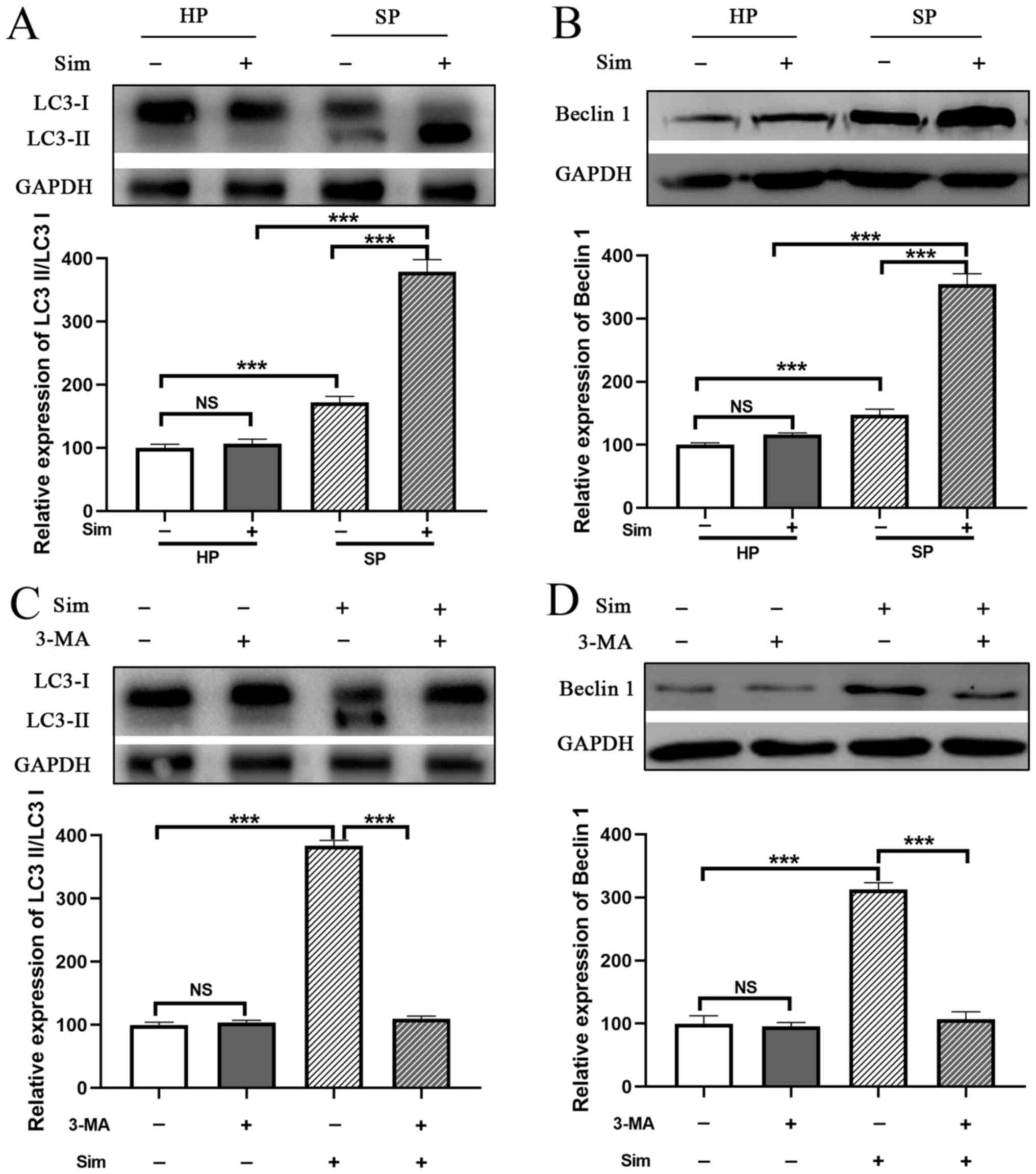
Subsequently, whether simvastatin affected autophagy
in septic neutrophils was investigated. The conversion of LC3I to
LC3II was significantly increased in neutrophils from the SP group
compared with that in the HP group (Fig. 4A). Moreover, SP neutrophils
exhibited a significant increase in Beclin 1 protein expression
(another autophagy-related molecule) compared with HP neutrophils
(P<0.001; Fig. 4B). Notably,
treatment with simvastatin promoted the conversion of LC3I to LC3II
and elevated the expression level of Beclin1 in SP neutrophils
compared with the untreated SP group, and these effects were
abrogated by treatment with 3-MA, a well-known autophagy inhibitor
(Fig. 4C and D). These results
indicated that simvastatin enhanced autophagy in neutrophils from
the SP group.
To investigate the importance of autophagy
activation in the protective effect of simvastatin, neutrophils
were treated with 3-MA. The results demonstrated that 3-MA
administration completely blocked the protective effect of
simvastatin, as evidenced by the decreased viability of neutrophils
compared with that in the SP group treated with simvastatin alone
(SP + Sim vs. SP + Sim + 3-MA, 72.33±6.03 vs. 41.67±3.51%, P=0.002;
Fig. 5A). Furthermore, the
inhibition of ROS production by simvastatin was reversed by
treatment with 3-MA in septic neutrophils (SP + Sim vs. SP + Sim +
3-MA, 217.67±18.23 vs. 566.67±31.34, P<0.001; Fig. 5B). Subsequently, the role of
autophagy in the inflammatory regulation of simvastatin was
examined. It was found that the suppression of autophagy using 3-MA
eliminated the inflammatory inhibitory effect of simvastatin in
septic neutrophils, as evidenced by the increased concentrations of
IL-6 (SP + Sim vs. SP + Sim + 3-MA, 109.00±6.00 vs. 143.67±5.69
pg/ml, P=0.002) and TNF-α (SP + Sim vs. SP + Sim + 3-MA, 81.67±3.06
vs. 114.00±6.24 pg/ml, P=0.001; Fig.
5C and D).
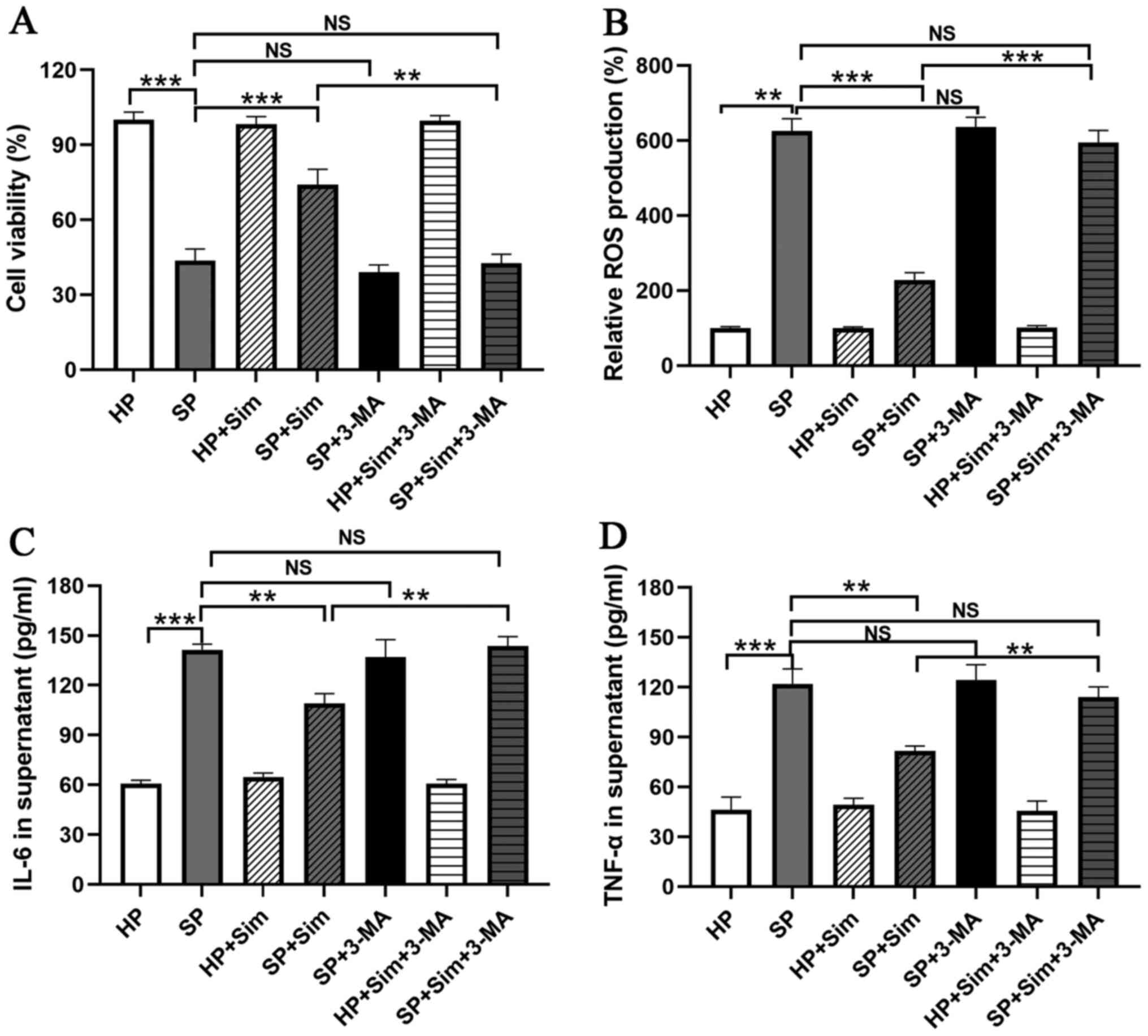 | Figure 5.Sim inhibits inflammation and
oxidative stress via autophagy induction. Treatment with 3-MA, an
autophagy inhibitor, blocked the protective effect of Sim on
neutrophils in the SP group, including (A) decreasing the cell
viability of neutrophils, (B) increasing ROS production and
increasing the concentrations of (C) IL-6 and (D) TNF-α in
neutrophil-conditioned media. **P<0.01 and ***P<0.001. Sim,
simvastatin; 3-MA, 3-methyladenine; SP, sepsis; ROS, reactive
oxygen species; HP, healthy volunteers; NS, not significant; |
Discussion
SP is a life-threatening organ dysfunction disease
caused by a dysregulated host response to infection, with a
mortality rate of 28.6% in patients with severe SP in the United
States (19). Neutrophils, the
most abundant inflammatory cells in the circulating bloodstream,
serve an important role in the SP, which phagocytose and eradicate
invasive substances via phagocytosis, degranulation, the release of
inflammatory cytokines and the generation of neutrophil
extracellular traps (8,9,20).
Numerous studies demonstrated a protective effect of simvastatin
against SP in an animal model of abdominal SP and the majority of
studies focusing on the association between statins and SP have
been conducted in experimental animals (21,22). In the present study, simvastatin
treatment led to the protection of neutrophils, decreased
proinflammatory cytokine concentrations and attenuated oxidative
stress in human neutrophils from patients with SP via autophagy
enhancement.
The excessive production of ROS causes significant
cellular dysfunctions in organs, which may also contribute to
multi-organ system failures (23). High levels of ROS promoted
apoptosis by opening mitochondrial permeability transition pores
during experimental SP (24),
whereas inhibition of ROS generation exhibited a protective effect
in mice with SP (25,26). In the present study, the levels of
ROS were elevated in human septic neutrophils. Moreover, treatment
with DPI, an NADPH oxidase inhibitor, reversed the high generation
of ROS in human septic neutrophils, indicating the sources of ROS
in human septic neutrophils primarily rely on NADPH oxidase. NADPH
oxidase is an important enzyme-rich cytoplasmic granule contained
in neutrophils that can convert molecular oxygen into free radicals
of oxygen, superoxide anions and H2O2
(27). In addition, treatment
with simvastatin significantly attenuated ROS levels in neutrophils
isolated from patients with SP, which was consistent with that of a
previous study that reported that simvastatin administration
reduced brain oxidative stress in experimental SP (28).
Furthermore, the role of simvastatin in
proinflammatory cytokine expression in SP neutrophils was
investigated. Cytokines have been shown to serve critical roles in
SP via the regulation of the immune response to infection (29,30). The present study observed that
simvastatin decreased the concentrations of IL-6 and TNF-α in human
septic neutrophils. IL-6 and TNF-α are two major proinflammatory
cytokines that stimulate systematic inflammation. These act as
endogenous pyrogens, upregulating the generation of other
proinflammatory cytokines, as well as promoting the generation of
acute phase proteins (31). A
previous study reported that IL-6 was the key cytokine in the
pathogenesis of severe SP and an increased level of IL-6 was
associated with a higher mortality rate in patients with SP
(32). Another proinflammatory
cytokine, TNF-α, has also been revealed to be significantly
increased in patients who developed and died of SP (33). In addition, use of a soluble TNF-α
surface receptor blocked cytokine functions, and subsequently
reduced the morbidity and mortality associated with septic shock in
animal models (34).
The extent of immune cell death is strongly
associated with the severity and mortality of SP; however, the
pathological role of immune cell death in SP is not completely
understood (35). The following
mechanisms are considered to be important, including
damage-associated molecular patterns, histone release, neutrophil
extracellular traps and autophagy (36,37). Autophagy is an essential
intracellular degradation process that is critical for cell
survival and homoeostasis maintenance, which has been shown to
serve a crucial role for autophagy in the regulation of SP
(38,39). In the present study, it was found
that autophagy in neutrophils from patients with SP served an
important role in maintaining the innate effector functions of
neutrophils. Neutrophils isolated from the SP group exhibited
elevated autophagy induction. Moreover, simvastatin intervention
enhanced autophagy activity in neutrophils isolated from patients
with SP via the elevated expression of Beclin 1 and the increased
conversion of LC3I to LC3II. Bruiners et al (13) demonstrated that simvastatin
treatment of human cells had transcriptional effects on mechanistic
target of rapamycin complex 1 and AMP-activated protein kinase
signaling pathways, thereby promoting autophagy. Moreover,
lysosome-associated membrane glycoprotein-1 and Unc-51 like
autophagy activating kinase 1 have been shown to be involved in
simvastatin-induced autophagy (40). Recently, the mechanism of action
underlying autophagy in SP has received increased attention.
Autophagy has been revealed as a cellular adaptive protective
mechanism in SP, where it could eliminate damaged proteins,
bacteria and pathogens in the cytoplasm (39). Herein, the present study also
demonstrated that inhibition of autophagy using 3-MA reversed the
effects of simvastatin on cell viability, the production of ROS and
the release of proinflammatory mediators (TNF-α and IL-6) in septic
neutrophils. These findings were consistent with previous studies
that reported that the autophagy regulation mechanism in SP was
involved in oxidative stress, immune regulation and inflammation
regulation in animal models (41,42).
In conclusion, the present study provided novel
evidence for the simvastatin-induced autophagic process of
neutrophils involved in human SP, which subsequently attenuated the
inflammatory response and oxidative stress. These results suggested
that autophagy served a crucial role in the protective effect of
simvastatin in human SP and that augmentation of neutrophil
autophagy may be a potential therapeutic target for patients with
SP. Moreover, the relationship between simvastatin and autophagy in
human septic neutrophils should be investigated further in future
studies.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the Suzhou
Science and Technology Bureau's Program of China (grant nos.
KJXW2016006 and SYS2018038).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available on reasonable request from the corresponding
author.
Authors' contributions
LX and XW performed the cell experiments. XW
performed the MTT and flow cytometry assays. YKZ and MH performed
the western blot assays. LX and MH performed the ELISAs. LX and JC
contributed to the study design and writing of the manuscript. LX
and JC confirm the authenticity of all the raw data. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Board and Ethics committee of The First Affiliated Hospital
of Soochow University (Suzhou, China; approval no. 138/2018).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rhee C, Dantes R, Epstein L, Murphy DJ,
Seymour CW, Iwashyna TJ, Kadri SS, Angus DC, Danner RL, Fiore AE,
et al: CDC prevention epicenter, program, Incidence and trends of
sepsis in US hospitals using clinical vs claims data, 2009-2014.
JAMA. 318:1241–1249. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rello J, Valenzuela-Sánchez F,
Ruiz-Rodriguez M and Moyano S: Sepsis: A review of advances in
management. Adv Ther. 34:2393–2411. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lelubre C and Vincent JL: Mechanisms and
treatment of organ failure in sepsis. Nat Rev Nephrol. 14:417–427.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Savi FF, de Oliveira A, de Medeiros GF,
Bozza FA, Michels M, Sharshar T, Dal-Pizzol F and Ritter C: What
animal models can tell us about long-term cognitive dysfunction
following sepsis: A systematic review. Neurosci Biobehav Rev.
124:386–404. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Steinhagen F, Schmidt SV, Schewe JC,
Peukert K, Klinman DM and Bode C: Immunotherapy in sepsis - brake
or accelerate? Pharmacol Ther. 208:1074762020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shen X, Cao K, Zhao Y and Du J: Targeting
neutrophils in Sepsis: From mechanism to translation. Front
Pharmacol. 12:6442702021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dobesh PP and Olsen KM: Statins role in
the prevention and treatment of sepsis. Pharmacol Res. 88:31–40.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Braga Filho JA, Abreu AG, Rios CE, Trovão
LO, Silva DL, Cysne DN, Nascimento JR, Fortes TS, Silva LA, Guerra
RN, et al: Prophylactic treatment with simvastatin modulates the
immune response and increases animal survival following lethal
sepsis infection. Front Immunol. 9:21372018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
de Jesus Oliveira FM,
Gonçalves-de-Albuquerque CF, de Moraes IM, Reis PA, Rocha VN, Bozza
PT, Silva AR and de Castro Faria Neto HC: Simvastatin posttreatment
controls inflammation and improves bacterial clearance in
experimental sepsis. Mediators Inflamm. Oct 14–2020.(Epub ahead of
print). doi: 10.1155/2020/1839762. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kang Y, Li Y, Wen H, Zhu J, Zheng J and
Feng Z: Prevention of renal ischemia and reperfusion injury by
penehyclidine hydrochloride through autophagy activation. Mol Med
Rep. 21:2182–2192. 2020.PubMed/NCBI
|
|
11
|
Wang Y, Zhu J, Liu Z, Shu S, Fu Y, Liu Y,
Cai J, Tang C, Liu Y, Yin X, et al: The PINK1/PARK2/optineurin
pathway of mitophagy is activated for protection in septic acute
kidney injury. Redox Biol. 38:1017672021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Quach C, Song Y, Guo H, Li S, Maazi H,
Fung M, Sands N, O'Connell D, Restrepo-Vassalli S, Chai B, et al: A
truncating mutation in the autophagy gene UVRAG drives inflammation
and tumorigenesis in mice. Nat Commun. 10:56812019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bruiners N, Dutta NK, Guerrini V, Salamon
H, Yamaguchi KD, Karakousis PC and Gennaro ML: The anti-tubercular
activity of simvastatin is mediated by cholesterol-driven autophagy
via the AMPK-mTORC1-TFEB axis. J Lipid Res. 61:1617–1628. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Carloni S and Balduini W: Simvastatin
preconditioning confers neuroprotection against hypoxia-ischemia
induced brain damage in neonatal rats via autophagy and silent
information regulator 1 (SIRT1) activation. Exp Neurol.
324:1131172020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Singer M, Deutschman CS, Seymour CW,
Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche
JD, Coopersmith CM, et al: The third international consensus
definitions for sepsis and septic shock (Sepsis-3). JAMA.
315:801–810. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Maianski NA, Mul FP, van Buul JD, Roos D
and Kuijpers TW: Granulocyte colony-stimulating factor inhibits the
mitochondria-dependent activation of caspase-3 in neutrophils.
Blood. 99:672–679. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sha LL, Wang H, Wang C, Peng HY, Chen M
and Zhao MH: Autophagy is induced by anti-neutrophil cytoplasmic
Abs and promotes neutrophil extracellular traps formation. Innate
Immun. 22:658–665. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xie T, Duan Z, Sun S, Chu C and Ding W:
β-Lactams modulate neutrophil extracellular traps formation
mediated by mTOR signaling pathway. Biochem Biophys Res Commun.
534:408–414. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Angus DC, Linde-Zwirble WT, Lidicker J,
Clermont G, Carcillo J and Pinsky MR: Epidemiology of severe sepsis
in the United States: Analysis of incidence, outcome, and
associated costs of care. Crit Care Med. 29:1303–1310. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Paudel S, Baral P, Ghimire L, Bergeron S,
Jin L, DeCorte JA, Le JT, Cai S and Jeyaseelan S: CXCL1 regulates
neutrophil homeostasis in pneumonia-derived sepsis caused by
Streptococcus pneumoniae serotype 3. Blood. 133:1335–1345. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang S, Rahman M, Zhang S, Qi Z and
Thorlacius H: Simvastatin antagonizes CD40L secretion, CXC
chemokine formation, and pulmonary infiltration of neutrophils in
abdominal sepsis. J Leukoc Biol. 89:735–742. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang S, Luo L, Wang Y, Rahman M, Lepsenyi
M, Syk I, Jeppsson B and Thorlacius H: Simvastatin protects against
T cell immune dysfunction in abdominal sepsis. Shock. 38:524–531.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hoetzenecker W, Echtenacher B, Guenova E,
Hoetzenecker K, Woelbing F, Brück J, Teske A, Valtcheva N, Fuchs K,
Kneilling M, et al: ROS-induced ATF3 causes susceptibility to
secondary infections during sepsis-associated immunosuppression.
Nat Med. 18:128–134. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Durand A, Duburcq T, Dekeyser T, Neviere
R, Howsam M, Favory R and Preau S: Involvement of mitochondrial
disorders in septic cardiomyopathy. Oxid Med Cell Longev.
2017:40763482017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang Y, Li L, Hang Q, Fang Y, Dong X, Cao
P, Yin Z and Luo L: γ-glutamylcysteine exhibits anti-inflammatory
effects by increasing cellular glutathione level. Redox Biol.
20:157–166. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhou L, Wang F, Sun R, Chen X, Zhang M, Xu
Q, Wang Y, Wang S, Xiong Y, Guan KL, et al: SIRT5 promotes IDH2
desuccinylation and G6PD deglutarylation to enhance cellular
antioxidant defense. EMBO Rep. 17:811–822. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Javeshghani D, Magder SA, Barreiro E,
Quinn MT and Hussain SN: Molecular characterization of a
superoxide-generating NAD(P)H oxidase in the ventilatory muscles.
Am J Respir Crit Care Med. 165:412–418. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Catalão CH, Santos-Júnior NN, da Costa LH,
Souza AO, Alberici LC and Rocha MJ: Brain oxidative stress during
experimental sepsis is attenuated by simvastatin administration.
Mol Neurobiol. 54:7008–7018. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Morrow KN, Coopersmith CM and Ford ML:
IL-17, IL-27, and IL-33: A novel axis linked to immunological
dysfunction during sepsis. Front Immunol. 10:19822019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Grondman I, Pirvu A, Riza A, Ioana M and
Netea MG: Biomarkers of inflammation and the etiology of sepsis.
Biochem Soc Trans. 48:1–14. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chaudhry H, Zhou J, Zhong Y, Ali MM,
McGuire F, Nagarkatti PS and Nagarkatti M: Role of cytokines as a
double-edged sword in sepsis. In Vivo. 27:669–684. 2013.PubMed/NCBI
|
|
32
|
Wu HP, Chen CK, Chung K, Tseng JC, Hua CC,
Liu YC, Chuang DY and Yang CH: Serial cytokine levels in patients
with severe sepsis. Inflamm Res. 58:385–393. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Finnerty CC, Herndon DN, Chinkes DL and
Jeschke MG: Serum cytokine differences in severely burned children
with and without sepsis. Shock. 27:4–9. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dinarello CA: The proinflammatory
cytokines interleukin-1 and tumor necrosis factor and treatment of
the septic shock syndrome. J Infect Dis. 163:1177–1184. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cheng Z, Abrams ST, Toh J, Wang SS, Wang
Z, Yu Q, Yu W, Toh CH and Wang G: The critical roles and mechanisms
of immune cell death in sepsis. Front Immunol. 11:19182020.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Meng W, Paunel-Görgülü A, Flohé S,
Hoffmann A, Witte I, MacKenzie C, Baldus SE, Windolf J and Lögters
TT: Depletion of neutrophil extracellular traps in vivo results in
hypersusceptibility to polymicrobial sepsis in mice. Crit Care.
16:R1372012. View
Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cheng Z, Abrams ST, Alhamdi Y, Toh J, Yu
W, Wang G and Toh CH: Circulating histones are major mediators of
multiple organ dysfunction syndrome in acute critical illnesses.
Crit Care Med. 47:e677–e684. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yang YM, Li YH, Ding LL, Fu Y and Li N:
Regulatory effect of lncRNA NKILA on autophagy induced by sepsis
kidney injury. Eur Rev Med Pharmacol Sci. 24:40572020.PubMed/NCBI
|
|
39
|
Oami T, Watanabe E, Hatano M, Teratake Y,
Fujimura L, Sakamoto A, Ito C, Toshimori K, Swanson PE and Oda S:
Blocking liver autophagy accelerates apoptosis and mitochondrial
injury in hepatocytes and reduces time to mortality in a murine
sepsis model. Shock. 50:427–434. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Piplani H, Marek-Iannucci S, Sin J, Hou J,
Takahashi T, Sharma A, de Freitas Germano J, Waldron RT,
Saadaeijahromi H, Song Y, et al: Simvastatin induces autophagic
flux to restore cerulein-impaired phagosome-lysosome fusion in
acute pancreatitis. Biochim Biophys Acta Mol Basis Dis.
165530:20191865.PubMed/NCBI
|
|
41
|
Baechler BL, Bloemberg D and Quadrilatero
J: Mitophagy regulates mitochondrial network signaling, oxidative
stress, and apoptosis during myoblast differentiation. Autophagy.
15:1606–1619. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Giegerich AK, Kuchler L, Sha LK, Knape T,
Heide H, Wittig I, Behrends C, Brüne B and von Knethen A:
Autophagy-dependent PELI3 degradation inhibits proinflammatory IL1B
expression. Autophagy. 10:1937–1952. 2014. View Article : Google Scholar : PubMed/NCBI
|