Introduction
Uric acid (UA) is the final oxidation product of
purine metabolism (1). It has
been reported that high levels of UA in the serum are closely
associated with the development of gout and kidney stones (2). UA crystallization triggers robust
inflammation and immune activation, which serves an important role
in the development of numerous diseases, including hypertension,
atherosclerosis and diabetes (3,4).
Increasing epidemiological evidence suggests that hyperuricemia is
an independent risk factor for the development of cardiovascular
diseases, metabolic syndrome and chronic kidney diseases (5,6).
Furthermore, hyperuricemia contributes to vascular endothelial
dysfunction and the development of hypertension (7). High serum UA levels have been
observed in hypertensive adolescents, where UA reduction can be
used to treat hypertension (8).
Therefore, it is important to determine the role of UA in vascular
endothelial cells.
Fibroblast growth factor (FGF) 21 (FGF21) is a
hormone-like member of the FGF family that can regulate energy
homeostasis, systemic glucose and lipid metabolism (9,10).
FGF21 has been reported to alleviate HUVEC apoptosis by inhibiting
the Fas signaling pathway, which ameliorates atherosclerosis in
apoE-/-mice (11). Furthermore,
FGF21 has been found to alleviate oxidized low-density lipoprotein
(ox-LDL)-induced HUVEC pyroptosis through the tet methylcytosine
dioxygenase (TET2)/ubiquinol cytochrome c reductase core protein I
(UQCRC1)/reactive oxygen species (ROS) signaling pathway (12). Another previous study also
demonstrated FGF21 to protect HUVECs against high glucose-induced
oxidative stress and apoptosis by activating the PI3K/AKT/FOXO3a
signaling pathway (13). However,
the role of FGF21 in vascular endothelial cell injury induced by UA
remains unclear. It has previously been reported that FGF21
knockdown can reverse the inhibitory effects of the microRNA-149-5p
inhibitor on lipid accumulation induced by high UA levels (14). Therefore, the present study aimed
to investigate the role of FGF21 in vascular endothelial cell
injury induced by UA.
In the present study, HUVECs were induced using
different concentrations of UA to establish a vascular endothelial
cell injury model in vitro, which was then applied to
investigate the role of FGF21 in vascular endothelial cell injury
induced by UA.
Materials and methods
Cell culture and treatment
The human umbilical vein endothelial HUVEC-C cell
line (CRL-1730) was purchased from the American Type Culture
Collection and maintained in DMEM (Gibco; Thermo Fisher Scientific,
Inc.) supplemented with 10% fetal bovine serum (Gibco; Thermo
Fisher Scientific, Inc.) and 1% penicillin/streptomycin
(Invitrogen; Thermo Fisher Scientific, Inc.). Cells were maintained
at 37°C in a humidified atmosphere containing 5% CO2.
HUVECs were stimulated with 6, 9 and 12 mg/dl concentrations of UA
(cat. no. U2625; Sigma-Aldrich; Merck KGaA) for 24 h at room
temperature. Sirtuin 1 (sirt1) expression was inhibited by EX527
(10 µM; Beyotime Institute of Biotechnology) (15). Untreated cells were used as the
control group.
Cell transfection
For transfection, the FGF21 overexpression plasmid
(Ov-FGF21; 10 nM) and the control empty vector (Ov- NC; 10 nM) were
purchased from Shanghai GenePharma Co., Ltd. Subsequently, Ov-FGF21
or Ov-NC, were transfected into cells (2×106 cells/well)
for 48 h at 37°C using Lipofectamine® 2000 reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. HUVECs were used for subsequent
experimentation 48 h post-transfection and reverse transcription
(RT)-quantitative (q)PCR analysis was performed to confirm
transfection efficiency. In addition, cells were co-treated with
EX527. After transfection for 48 h, cells were then exposed to UA
for 24 h.
RT-qPCR
Total RNA was extracted from HUVECs using
TRIzol® (Thermo Fisher Scientific, Inc.) following the
manufacturer's instructions. RT was performed using the PrimeScript
RT reagent kit (Takara Bio, Inc.) according to the manufacturer's
instructions. For RT the following temperature protocol was used;
15 min at 42°C; followed by 5 min at 98°C. The reaction volume was
20 µl. qPCR was performed using the SYBR® Green
Quantitative RT-qPCR Kit (cat. no. QR0100; Sigma-Aldrich; Merck
KGaA). The following thermocycling conditions were used for the
qPCR: Initial denaturation at 95°C for 30 sec; followed by 40
cycles at 95°C for 5 sec and 60°C for 30 sec. The reaction volume
was 25 µl. The mRNA expression levels of FGF21, sirt1, tumor
necrosis factor α (TNF-α), interleukin-1β (IL-1β) and interleukin-6
(IL-6) were quantified using the 2−ΔΔCq method (16) and normalized to the internal
reference gene, GAPDH. Primer sequences were synthesized by
Shanghai GenePharma Co., Ltd. and are displayed in Table I.
 | Table I.Sequences of primers used for reverse
transcription-quantitative PCR. |
Table I.
Sequences of primers used for reverse
transcription-quantitative PCR.
| Gene | Sequence (5′→3′) |
|---|
| FGF21 | F:
CTGTGGGTTTCTGTGCTGG |
|
| R:
CCGGCTTCAAGGCTTTCAG |
| SIRT1 | F:
AAGTTGACTGTGAAGCTGTACG |
|
| R:
TGCTACTGGTCTTACTTTGAGGG |
| TNF-α | F:
TCTCGAACCCCGAGTGACAA |
|
| R:
TATCTCTCAGCTCCACACCCA |
| IL-1β | F:
GGCCCTAAACAGATGAAGTG |
|
| R:
GTAGTGGTGGTCGGAGATTC |
| IL-6 | F:
CCTTCTCCACAAGCGCCTTC |
|
| R:
GGCAAGTCTCCTCATTGAATC |
| GAPDH | F:
GGAGCGAGATCCCTCCAAAAT |
|
| R:
GGCTGTTGTCATACTTCTCATGG |
Western blotting
Total protein was extracted from HUVECs using the
RIPA lysis buffer (cat. no. P0013C; Beyotime Institute of
Biotechnology) and protein concentration was determined using a BCA
Protein Assay Kit (cat. no. P0012A; Beyotime Institute of
Biotechnology). Total protein (30 µg/lane) was separated via
SDS-PAGE on a 10% gel (Beyotime Institute of Biotechnology) and
transferred onto PVDF membranes (EMD Millipore). The membranes were
blocked with 5% skimmed milk for 2 h at room temperature, followed
by overnight incubation at 4°C with primary antibodies. Following
incubation with the primary antibody and washing with TBST (0.1%
Tween-20; 10 min at room temperature), the membranes were incubated
with a goat HRP-conjugated anti-rabbit secondary antibody (1:5,000;
cat. no. S0001; Affinity Biosciences) for 2 h at room temperature.
Protein bands were visualized using an ECL Kit (Beyotime Institute
of Biotechnology). Protein expression levels were semi-quantified
using Image-Pro Plus software version 6.0 (Media Cybernetics, Inc.;
Roper Technologies, Inc.). The primary antibodies used were as
follows: FGF21 (1:1,000; cat no. ab64857; Abcam), sirt1 (1:2,000;
cat. no. ab12193; Abcam), activating transcription factor 4 (ATF4;
1:500; cat. no. ab216839; Abcam), NLR family pyrin domain
containing 3 (NLRP3; 1:500; cat. no. ab214185; Abcam), pro-caspase
1 (1:1,000; cat. no. ab179515; Abcam), apoptosis-associated
speck-like protein containing a CARD (ASC; 1:1,000; cat. no.
ab70627; Abcam), phosphorylated (p)-AKT (1:500; cat. no. ab38449;
Abcam), AKT (1:500; cat. no. ab8805; Abcam), p-endothelial nitric
oxide synthase (p-eNOS; 1:500; cat. no. ab184154; Abcam), eNOS
(1:1,000; cat. no. ab5589; Abcam), GAPDH (1:2,500; cat. no. ab9485;
Abcam), C/EBP homologous protein (CHOP; 1:1,000; cat. no. DF6025;
Affinity Biosciences), p-eukaryotic initiation factor 2 (p-eIF2A;
1:500; cat. no. AF7188; Affinity Biosciences) and eIF2A (1:1,000;
cat. no. AF6087; Affinity Biosciences).
Assessment of ROS and nitric oxide
(NO) levels
Intracellular ROS generation was quantified as
previously described (17).
HUVECs were inoculated in six-well plates (5×105
cells/well) and incubated with 10 µM dichloro-dihydro-fluorescein
diacetate (DCFH-DA; Sigma-Aldrich; Merck KGaA) at 37°C for 30 min
in the dark. In the presence of ROS, DCFH-DA is oxidized and
produces fluorescence (18).
Cells were washed three times with pre-cooled PBS and ROS levels
were determined using a fluorescence microscope (magnification,
×200), with excitation and emission wavelengths of 485 and 520 nm,
respectively. ROS levels were also quantified using a Fluorometric
Intracellular ROS Kit (cat. no. MAK143; Sigma-Aldrich; Merck KGaA),
according to the manufacturer's protocols.
NO levels were quantified using a NO Assay Kit (cat.
no. A012-1-2; Nanjing Jiancheng Bioengineering Institute.).
According to the manufacturer's protocols, the mixed reagents were
added sequentially to the samples. After standing at room
temperature for 40 min, the samples were centrifuged at 1,000 × g
at 4°C for 10 min. The supernatant was removed and color developer
was added. After 10 min at room temperature, the absorbance was
detected at 550 nm using a microplate reader (Bio-Rad Laboratories,
Inc.).
Statistical analysis
Statistical analysis was performed using GraphPad
Prism 8.0 software (GraphPad Software, Inc.). Data are presented as
the mean ± standard deviation of ≥ three independent experiments.
One-way ANOVA was used to compare differences between three or more
groups followed by Tukey's post hoc test. P<0.05 was considered
to indicate a statistically significant difference.
Results
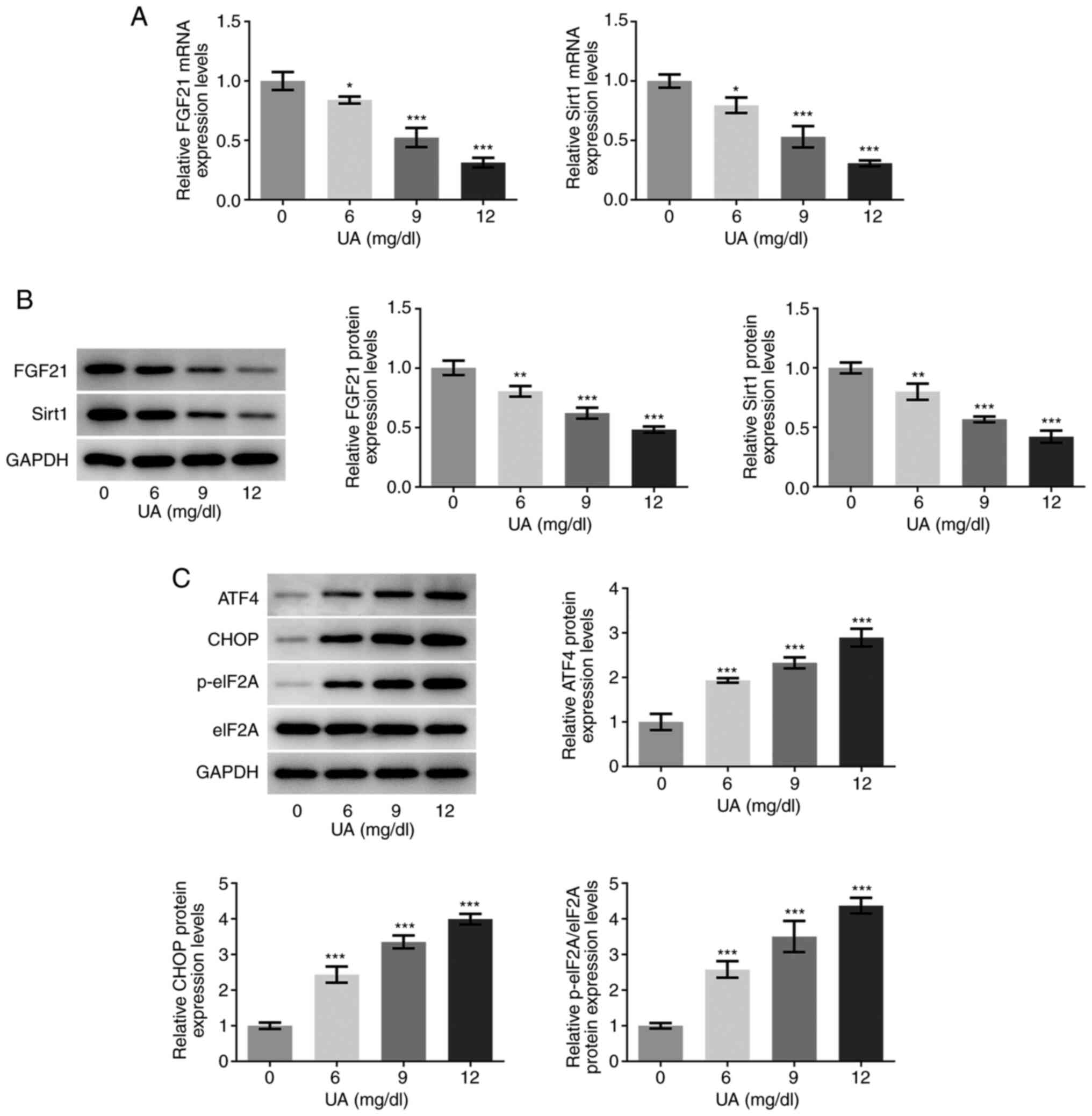
High UA inhibits FGF21 and Sirt1
expression levels and promotes endoplasmic reticulum (ER) stress in
HUVECs
The mRNA and protein expression levels of FGF21 and
sirt1 in UA-stimulated HUVECs were determined via RT-qPCR and
western blotting, respectively. As presented in Fig. 1A and B, the mRNA and protein
expression levels of FGF21 and sirt1 were significantly decreased
following UA stimulation in a dose-dependent manner compared with
those in the 0 mg/dl UA group (control group). Furthermore, the
protein expression levels of ER stress-associated proteins ATF4,
CHOP and the p-eIF2A/eIF2A ratio were also semi-quantified using
western blotting. As shown in Fig.
1C, the protein expression levels of ATF4, CHOP and eIF2A
phosphorylation were all significantly increased in UA-stimulated
HUVECs, in a dose-dependent manner compared with those in the 0
mg/dl UA group. Furthermore, when the concentration of UA was 12
mg/dl, the highest difference was observed compared with that in
the 0 mg/dl UA group. Therefore, 12 mg/dl UA was selected for
subsequent experimentation. These results suggest that the
expression levels of FGF21 and sirt1 may be closely associated with
the progression of UA-induced vascular endothelial cell injury.
Overexpression of FGF21 activates
sirt1 and suppresses ER stress in HUVECs
FGF21 was overexpressed via transfection of HUVEC
with the Ov-FGF21 plasmid. RT-qPCR analysis demonstrated that the
Ov-FGF21 plasmid was successfully transfected into HUVECs, since
the Ov-FGF21 plasmid transfection significantly increased FGF21
expression compared with that in HUVECs transfected with Ov-NC
(Fig. 2A). FGF21 was
significantly upregulated in the UA + Ov-FGF21 group compared with
that in the UA + Ov-NC group, indicating that the Ov-FGF21 plasmid
was also successfully transfected into UA-induced HUVECs (Fig. 2B and C). Subsequently, Sirt1 mRNA
and protein expression levels in the transfected cells were
measured (Fig. 2D and E).
Compared with those in the UA + Ov-NC group, overexpression of
FGF21 was demonstrated to significantly increase sirt1 mRNA and
protein expression levels. The protein expression levels of ATF4,
CHOP and eIF2A phosphorylation were significantly decreased in the
UA + Ov-FGF21 group compared with those in the UA + Ov-NC group
(Fig. 2F). Collectively, these
results suggest that FGF21 may activate sirt1 and inhibit ER
stress.
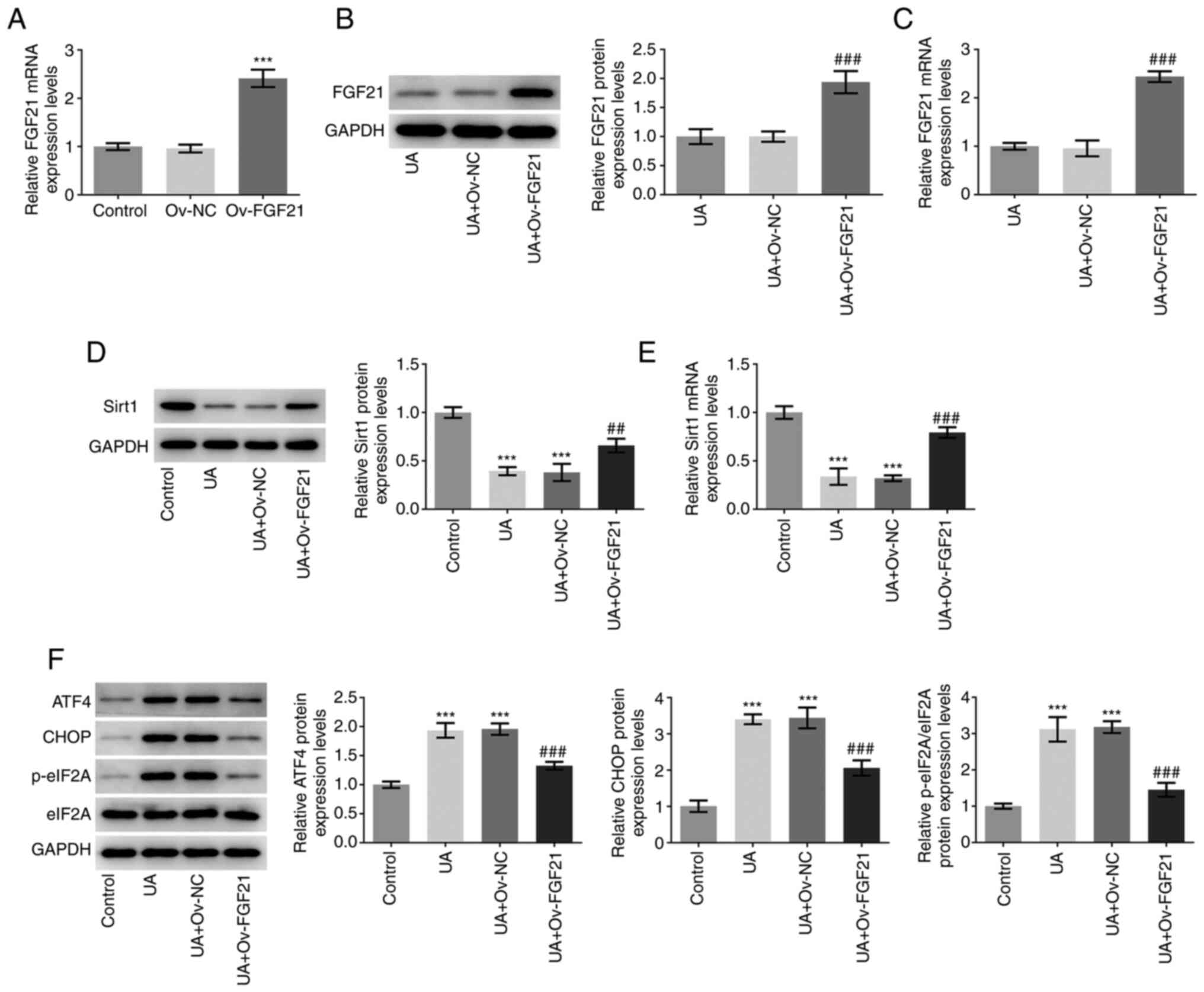 | Figure 2.Overexpression of FGF21 induces Sirt1
activation and suppresses endoplasmic reticulum stress in HUVECs.
(A) FGF21 mRNA expression levels were determined using RT-qPCR. (B)
Western blotting was performed to measure FGF21 protein expression
levels. (C) RT-qPCR was performed to detect FGF21 mRNA expression
levels. (D) Western blotting was performed to detect Sirt1 protein
expression levels. (E) RT-qPCR was performed to detect Sirt1 mRNA
expression levels. (F) Western blotting analysis was performed to
detect the protein levels of ATF4, CHOP, p-eIF2A and eIF2A.
***P<0.001 vs. control; ##P<0.01 and
###P<0.001 vs. UA + Ov-NC. UA, uric acid; FGF21,
fibroblast growth factor 21; Sirt1, sirtuin 1; RT-qPCR, reverse
transcription-quantitative PCR; ATF4, activating transcription
factor 4; eIF2A, eukaryotic initiation factor 2; p-,
phosphorylated; NC, negative control; Ov, overexpressed. |
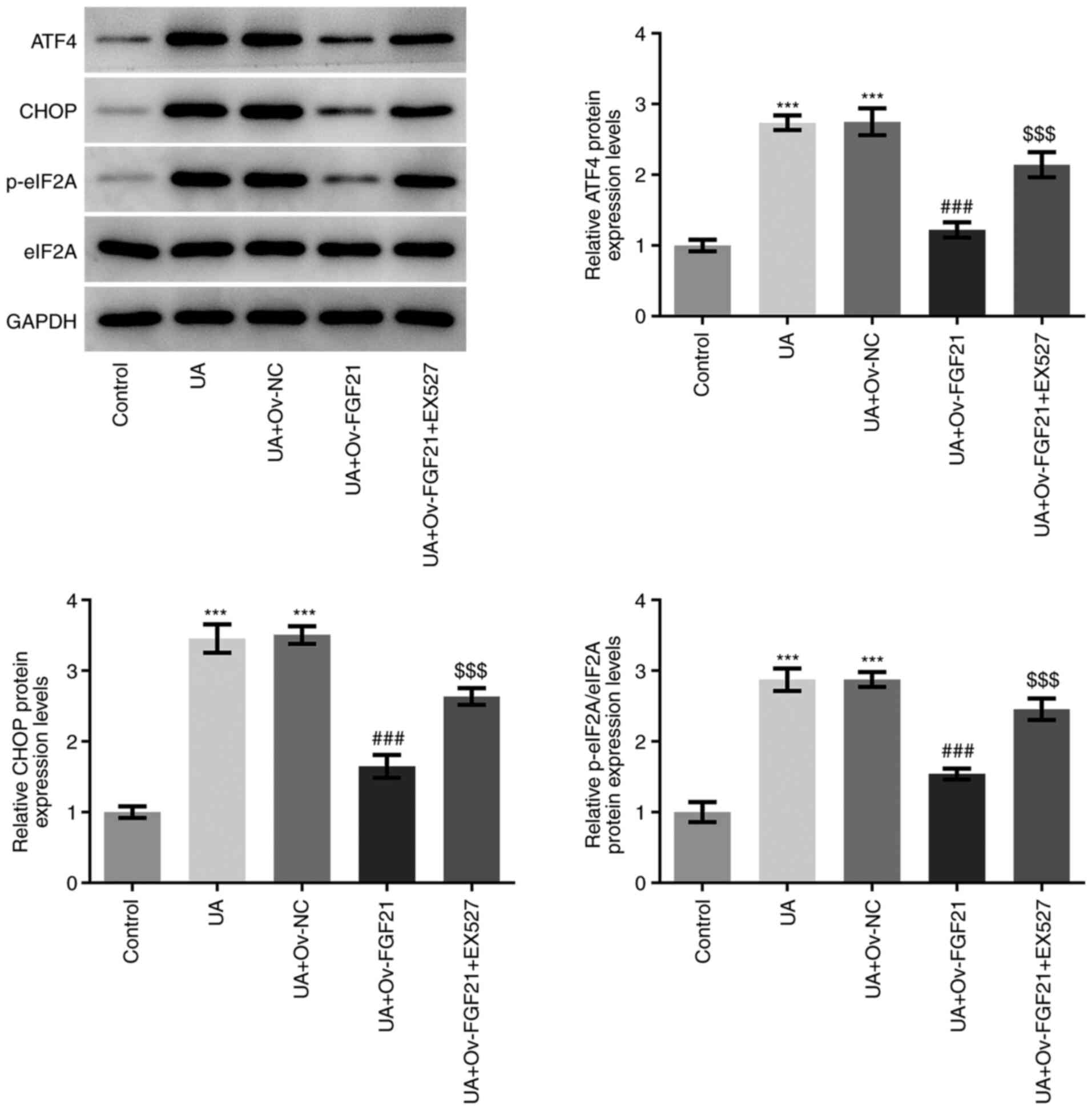
Overexpression of FGF21 attenuates ER
stress and the inflammatory response in high UA-induced HUVECs by
activating sirt1
EX527 was used as a sirt1 inhibitor for subsequent
experiments. As presented in Fig.
3, the protein expression levels of ATF4, CHOP and eIF2A
phosphorylation in HUVECs were significantly higher in the UA +
Ov-FGF21 + EX527 group compared with those in the UA + Ov-FGF21
group. These results suggest that EX527 may reverse the suppressive
effects of FGF21 overexpression on ER stress in HUVECs.
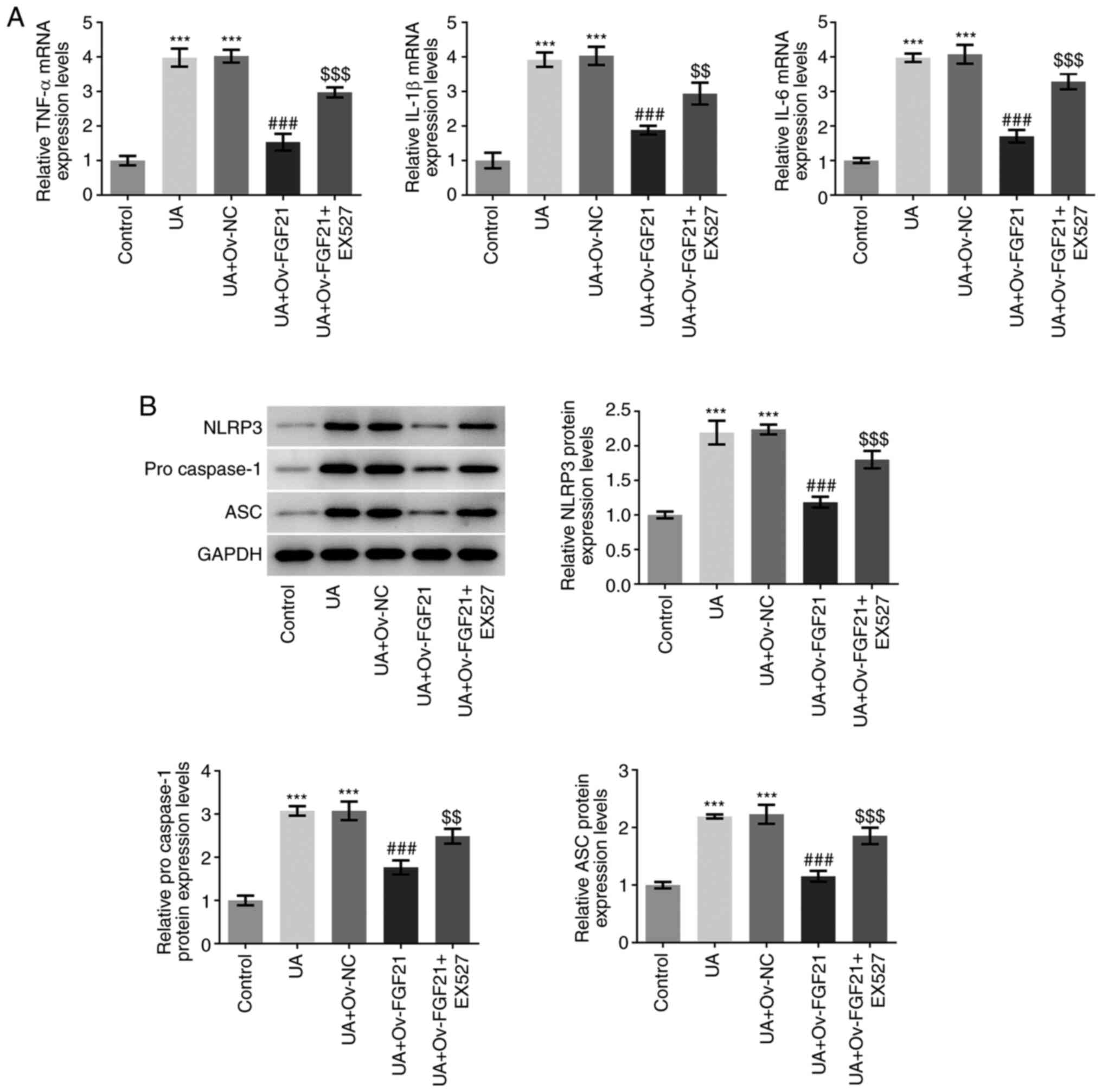
The mRNA expression levels of TNFα, IL-1β and IL-6
were next detected by RT-qPCR analysis. As presented in Fig. 4A, UA stimulation significantly
promoted the expression of inflammatory cytokines TNFα, IL-1β and
IL-6, compared with that in the control group. Following FGF21
overexpression, the mRNA expression levels of these inflammatory
cytokines were significantly suppressed compared with those in the
UA + Ov-NC group. However, after EX527 was added, this suppression
of inflammatory cytokine mRNA expression levels by FGF21 was
significantly reversed compared with that in the UA + Ov-FGF21
group. The protein expression levels of NLRP3, pro-caspase 1 and
ASC, which are inflammasome proteins, were measured using western
blotting (19). As presented in
Fig. 4B, the protein expression
levels of NLRP3, pro-caspase 1 and ASC were significantly increased
following UA stimulation compared with those in the control group.
By contrast, compared with those in the UA + Ov-NC group their
protein expression levels were significantly inhibited following
the overexpression of FGF21. Furthermore, EX527 significantly
reversed the inhibitory effects of FGF21 on the protein expression
levels of NLRP3, pro-caspase 1 and ASC compared with those in the
UA + Ov-FGF21 group. These results suggest that the overexpression
of FGF21 may attenuate ER stress and inflammatory injury in high
UA-induced HUVECs by activating Sirt1.
Overexpression of FGF21 attenuates
oxidative stress and vascular endothelial cell dysfunction in high
UA-induced HUVECs by activating Sirt1
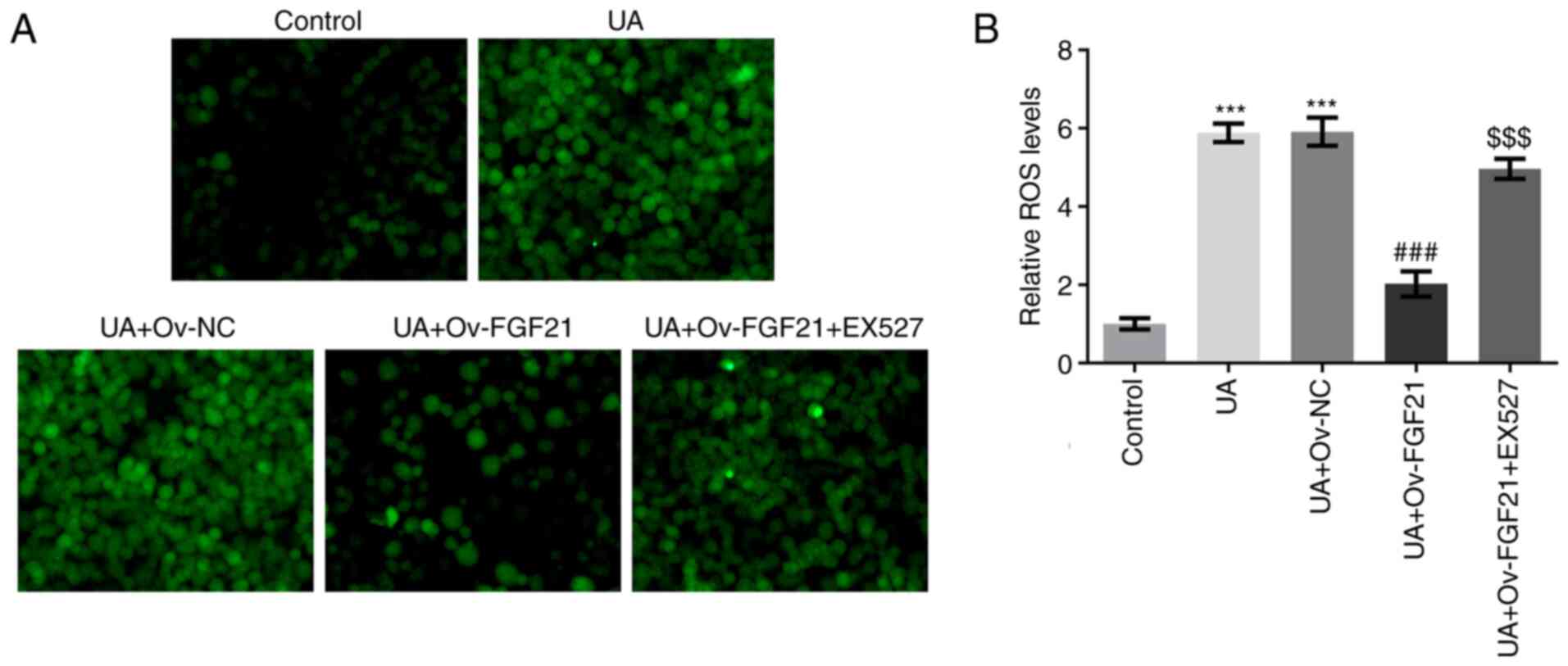
The levels of ROS and NO in HUVECs were measured.
Results from DCFH-DA staining and commercial assay kits
demonstrated that UA stimulation significantly promoted ROS
generation compared with that in the control group. Furthermore,
overexpression of FGF21 significantly inhibited ROS generation in
UA-stimulated HUVECs compared with that in the UA + Ov-NC group,
whereas the sirt1 inhibitor EX527 significantly reversed this
effect compared with that in the UA + Ov-FGF21 group (Fig. 5A and B). Collectively, these
results suggest that FGF21 can potentially inhibit ROS production
by activating Sirt1.
As shown in Fig.
6A, NO levels were significantly decreased following UA
stimulation compared with those in the control group. However, NO
levels were significantly increased following the overexpression of
FGF21 compared with those in the UA + Ov-NC group. Furthermore,
treatment with the Sirt1 inhibitor EX527 significantly attenuated
NO levels following the overexpression of FGF21 compared with those
in the UA + Ov-FGF21 group. In addition, UA stimulation
significantly decreased the ratios of p-AKT/AKT and p-eNOS/eNOS
compared with those in the control group. These effects were
significantly reversed following overexpression of FGF21 compared
with those in the UA + Ov-NC group, which were partially but
significantly reversed by the Sirt1 inhibitor EX527 compared with
those in the UA + Ov-FGF21 group (Fig. 6B and C). These results suggest
that EX527 may reverse the suppressive effects of FGF21
overexpression on vascular endothelial cell dysfunction. Therefore,
overexpression of FGF21 may attenuate oxidative stress and vascular
endothelial cell dysfunction in high UA-induced HUVECs by
activating Sirt1.
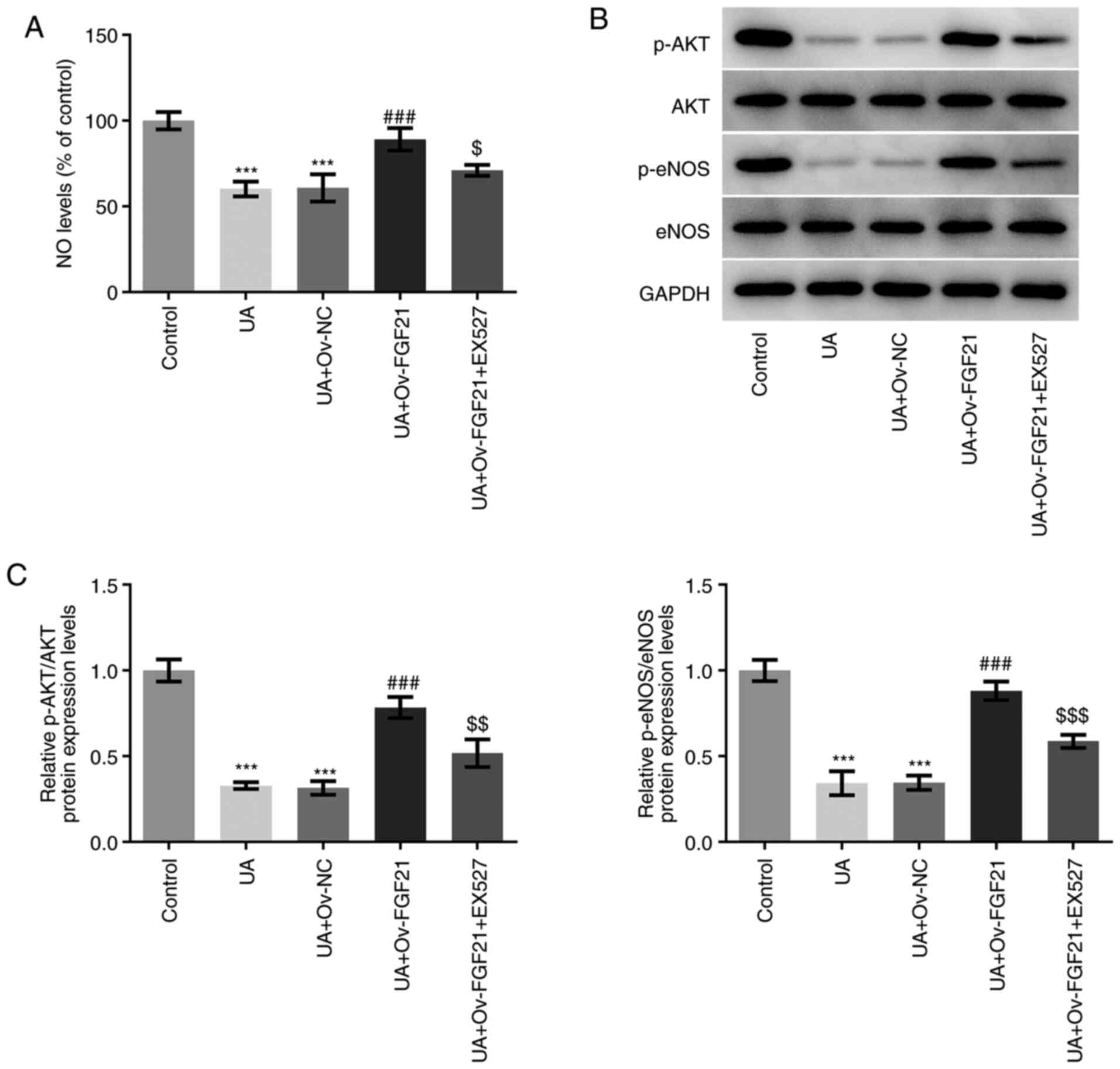 | Figure 6.Overexpression of FGF21 attenuates
vascular endothelial cell dysfunction in high UA-induced HUVECs by
activating Sirtuin 1. (A) NO levels were quantified using a NO
assay kit. (B) Western blotting was performed to detect the protein
levels of p-AKT, AKT, p-eNOS and eNOS. (C) Semi-quantification of
p-AKT/AKT and p-eNOS/eNOS ratios. ***P<0.001 vs. control;
###P<0.001 vs. UA + Ov-NC; $P<0.05,
$$P<0.01 and $$$P<0.001 vs. UA +
Ov-FGF21. FGF21, fibroblast growth factor 21; UA, uric acid; NO,
nitric oxide; eNOS, endothelial NO synthase; Ov, overexpressed; NC,
negative control; p-, phosphorylated. |
Discussion
Hyperuricemia occurs when UA levels are elevated in
the blood, increasing the risk of gout and nephrolithiasis
(20). Hyperuricemia is
considered a risk factor for a number of disorders, including
diabetes mellitus, cardiovascular diseases and metabolic syndrome
(21). Accumulating evidence
suggests that UA can cause endothelial injury and dysfunction,
though the mechanism remain unclear (22,23). Apoptosis induced by ox-LDL in
vascular endothelial cells is an important process in the
progression of atherosclerosis (11). FGF21 has been reported to be
closely associated with metabolic dysfunction, including the
development of vascular calcification and atherosclerotic disease
(24–26). In the present study, UA
stimulation was found to significantly decrease FGF21 mRNA and
protein expression, suggesting a potential association between
FGF21 and UA-induced vascular endothelial cell injury. Therefore,
the present study aimed to investigate the role of FGF21 in a
vascular endothelial cell injury model induced by UA.
Activation of ER stress, which frequently occurs
using diabetes and metabolic syndrome, can cause endothelial
dysfunction (27). The
eIF2A/ATF4/CHOP signaling pathway serves an important role in
regulating ER stress (28). The
results of the present study demonstrated that UA stimulation
significantly activated ER stress. Notably, overexpression of FGF21
suppressed the activation of ER stress in UA-stimulated HUVECs,
which suggested that FGF21 may exert protective effects on HUVECs
against ER stress. To investigate the detailed mechanism underlying
the role of FGF21 in UA-stimulated cells, a series of in
vitro experiments were performed.
A previous study reported that FGF21 can protect
against angiotensin II-induced cardiac hypertrophy and dysfunction
in a Sirt1-dependent manner (29). Furthermore, FGF21 has been found
to promote the formation of aerobic myofibers through the
Sirt1-mediated signaling pathway (30). Results of the present study
demonstrated that Sirt1 mRNA and protein expression were
significantly decreased following UA stimulation. However,
overexpression of FGF21 significantly increased Sirt1 expression.
Therefore, the Sirt1 inhibitor, EX527, was used to determine
whether Sirt1 was a downstream molecule of FGF21. The results of
the present study demonstrated that EX527 significantly reversed
the suppressive effects of FGF21 overexpression on ER stress in
UA-stimulated HUVECs. ER stress can activate the NLRP3 inflammasome
to induce inflammatory responses and oxidative stress (31). However, FGF21 can ameliorate
atherosclerosis by inhibiting NLRP3 inflammasome-related cell
pyroptosis and ER stress in vascular endothelial cells (32). In the present study,
overexpression of FGF21 significantly decreased the expression
levels of inflammatory cytokines and components of the NLRP3
inflammasome, which were partially reversed by the Sirt1 inhibitor.
Taken together, these results suggested that FGF21 may exert a
suppressive effect on ER stress and inflammation by activating
Sirt1.
Generation of ROS, reduction in eNOS activity and
therefore decreased NO release are considered to be a prominent
mechanism underlying endothelial injury (22). It has been previously reported
that NO production is reduced in a the high UA-induced
hyperuricemia model (22), which
is consistent with the results of the present study. The results
demonstrated that UA stimulation induced oxidative stress and
vascular endothelial cell dysfunction, as demonstrated by the
significantly increased ROS production, significant decline in NO
release and the significant decrease in eNOS activity in
UA-stimulated HUVECs in the present study. However, these effects
were significantly reversed following overexpression of FGF21.
EX527 significantly abrogated the suppressive effects of FGF21
overexpression on oxidative stress and vascular endothelial cell
dysfunction. These results suggest that FGF21 may attenuate
UA-induced ER stress, inflammation and vascular endothelial cell
dysfunction by activating sirt1.
In conclusion, results of the present study suggest
that FGF21 can attenuate ER stress, inflammatory injury and
endothelial cell dysfunction caused by high UA by Sirt1 activation.
However, the experimental sirt1 inhibitor, EX527, only partially
reversed the effects of FGF21, suggesting that FGF21 may also act
through other signaling pathways, which warrants further
exploratory studies.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Scientific Research Fund
of Sichuan Health and Health Committee (grant no. 19PJ114).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
RO, XZ, RZ and DD conceived and designed the study.
RO, XZ, RZ, JY and SL collected and analyzed the data. All authors
were involved in the writing of the manuscript and revised the
manuscript. RO and DD confirm the authenticity of all the raw data.
All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yang X, Gu J, Lv H, Li H, Cheng Y, Liu Y
and Jiang Y: Uric acid induced inflammatory responses in
endothelial cells via up-regulating(pro)renin receptor. Biomed
Pharmacother. 109:1163–1170. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ramirez-Sandoval JC and Madero M:
Treatment of hyperuricemia in chronic kidney disease. Contrib
Nephrol. 192:135–146. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ng G, Chau EM and Shi Y: Recent
developments in immune activation by uric acid crystals. Arch
Immunol Ther Exp (Warsz). 58:273–277. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stodle GS, Silva GB, Tangeras LH, Gierman
LM, Nervik I, Dahlberg UE, Sun C, Aune MH, Thomsen LCV, Bjørge L
and Iversen AC: Placental inflammation in pre-eclampsia by Nod-like
receptor protein (NLRP)3 inflammasome activation in trophoblasts.
Clin Exp Immunol. 193:84–94. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang S, Wang Y, Cheng J, Huangfu N, Zhao
R, Xu Z, Zhang F, Zheng W and Zhang D: Hyperuricemia and
cardiovascular disease. Curr Pharm Des. 25:700–709. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Johnson RJ, Bakris GL, Borghi C, Chonchol
MB, Feldman D, Lanaspa MA, Merriman TR, Moe OW, Mount DB, Sanchez
Lozada LG, et al: Hyperuricemia, acute and chronic kidney disease,
hypertension, and cardiovascular disease: Report of a scientific
workshop organized by the national kidney foundation. Am J Kidney
Dis. 71:851–865. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Choi YJ, Yoon Y, Lee KY, Hien TT, Kang KW,
Kim KC, Lee J, Lee MY, Lee SM, Kang DH and Lee BH: Uric acid
induces endothelial dysfunction by vascular insulin resistance
associated with the impairment of nitric oxide synthesis. FASEB J.
28:3197–3204. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Assadi F: Allopurinol enhances the blood
pressure lowering effect of enalapril in children with
hyperuricemic essential hypertension. J Nephrol. 27:51–56. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Alonge KM, Meares GP and Hillgartner FB:
Glucagon and insulin cooperatively stimulate fibroblast growth
factor 21 gene transcription by increasing the expression of
activating transcription factor 4. J Biol Chem. 292:5239–5252.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Brahma MK, Adam RC, Pollak NM, Jaeger D,
Zierler KA, Pöcher N, Schreiber R, Romauch M, Moustafa T, Eder S,
et al: Fibroblast growth factor 21 is induced upon cardiac stress
and alters cardiac lipid homeostasis. J Lipid Res. 55:2229–2241.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yan X, Gou Z, Li Y, Wang Y, Zhu J, Xu G
and Zhang Q: Fibroblast growth factor 21 inhibits atherosclerosis
in apoE-/- mice by ameliorating Fas-mediated apoptosis. Lipids
Health Dis. 17:2032018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen JJ, Tao J, Zhang XL, Xia LZ, Zeng JF,
Zhang H, Wei DH, Lv YC, Li GH and Wang Z: Inhibition of the
ox-LDL-induced pyroptosis by FGF21 of human umbilical vein
endothelial cells through the TET2-UQCRC1-ROS pathway. DNA Cell
Biol. 39:661–670. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Guo D, Xiao L, Hu H, Liu M, Yang L and Lin
X: FGF21 protects human umbilical vein endothelial cells against
high glucose-induced apoptosis via PI3K/Akt/Fox3a signaling
pathway. J Diabetes Complications. 32:729–736. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen S, Chen D, Yang H, Wang X, Wang J and
Xu C: Uric acid induced hepatocytes lipid accumulation through
regulation of miR-149-5p/FGF21 axis. BMC Gastroenterol. 20:392020.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu ZH, Zhang Y, Wang X, Fan XF, Zhang Y,
Li X, Gong YS and Han LP: SIRT1 activation attenuates cardiac
fibrosis by endothelial-to-mesenchymal transition. Biomed
Pharmacother. 118:1092272019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu X, Pan C, Chen R, Zhang S, Zhai Y and
Guo H: BML-111 attenuates high glucose-induced inflammation,
oxidative stress and reduces extracellular matrix accumulation via
targeting Nrf2 in rat glomerular mesangial cells. Int
Immunopharmacol. 79:1061082020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Aranda A, Sequedo L, Tolosa L, Quintas G,
Burello E, Castell JV and Gombau L: Dichloro-dihydro-fluorescein
diacetate (DCFH-DA) assay: A quantitative method for oxidative
stress assessment of nanoparticle-treated cells. Toxicol In Vitro.
27:954–963. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu HD, Li W, Chen ZR, Hu YC, Zhang DD,
Shen W, Zhou ML, Zhu L and Hang CH: Expression of the NLRP3
inflammasome in cerebral cortex after traumatic brain injury in a
rat model. Neurochem Res. 38:2072–2083. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li L, Zhang Y and Zeng C: Update on the
epidemiology, genetics, and therapeutic options of hyperuricemia.
Am J Transl Res. 12:3167–3181. 2020.PubMed/NCBI
|
|
21
|
George C and Minter DA: Hyperuricemia.
StatPearls (Internet). StatPearls Publishing; Treasure Island, FL:
2021
|
|
22
|
Hong Q, Wang L, Huang Z, Feng Z, Cui S, Fu
B, Cai G, Chen X and Wu D: High concentrations of uric acid and
angiotensin II act additively to produce endothelial injury.
Mediators Inflamm. 2020:83876542020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang B, Li S, Zhu J, Huang S, Zhang A, Jia
Z, Ding G and Zhang Y: miR-214 protects against uric acid-induced
endothelial cell apoptosis. Front Med (Lausanne). 7:4112020.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Durnwald C, Mele L, Landon MB, Varner MW,
Casey BM, Reddy UM, Wapner RJ, Rouse DJ, Tita ATN, Thorp JM Jr, et
al: Fibroblast growth factor 21 and metabolic dysfunction in women
with a prior glucose-intolerant pregnancy. Am J Perinatol.
38:1380–1385. 2020.PubMed/NCBI
|
|
25
|
Lee SY, Burns SF, Ng KKC, Stensel DJ,
Zhong L, Tan FHY, Chia KL, Fam KD, Yap MMC, Yeo KP, et al:
Fibroblast growth factor 21 mediates the associations between
exercise, aging, and glucose regulation. Med Sci Sports Exerc.
52:370–380. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wu L, Qian L, Zhang L, Zhang J, Zhou J, Li
Y, Hou X, Fang Q, Li H and Jia W: Fibroblast growth factor 21 is
related to atherosclerosis independent of nonalcoholic fatty liver
disease and predicts atherosclerotic cardiovascular events. J Am
Heart Assoc. 9:e0152262020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Osman A, El-Gamal H, Pasha M, Zeidan A,
Korashy HM, Abdelsalam SS, Hasan M, Benameur T and Agouni A:
Endoplasmic reticulum (ER) stress-generated extracellular vesicles
(microparticles) self-perpetuate ER stress and mediate endothelial
cell dysfunction independently of cell survival. Front Cardiovasc
Med. 7:5847912020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Guo Y, Guo R, Su Y, Fu J, Wang S, Kong Y,
Wu C, Wang J, Tan C, Mo C and Zhao B: The PERK/eIF2alpha/ATF4/CHOP
pathway plays a role in regulating monocrotaline-induced
endoplasmic reticulum stress in rat liver. Res Vet Sci.
130:237–239. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li S, Zhu Z, Xue M, Yi X, Liang J, Niu C,
Chen G, Shen Y, Zhang H, Zheng J, et al: Fibroblast growth factor
21 protects the heart from angiotensin II-induced cardiac
hypertrophy and dysfunction via SIRT1. Biochim Biophys Acta Mol
Basis Dis. 1865:1241–1252. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu X, Wang Y, Hou L, Xiong Y and Zhao S:
Fibroblast growth factor 21 (FGF21) promotes formation of aerobic
myofibers via the FGF21-SIRT1-AMPK-PGC1α pathway. J Cell Physiol.
232:1893–1906. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li W, Cao T, Luo C, Cai J, Zhou X, Xiao X
and Liu S: Crosstalk between ER stress, NLRP3 inflammasome, and
inflammation. Appl Microbiol Biotechnol. 104:6129–6140. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zeng Z, Zheng Q, Chen J, Tan X, Li Q, Ding
L, Zhang R and Lin X: FGF21 mitigates atherosclerosis via
inhibition of NLRP3 inflammasome-mediated vascular endothelial
cells pyroptosis. Exp Cell Res. 393:1121082020. View Article : Google Scholar : PubMed/NCBI
|