Introduction
Breast cancer is a malignant tumor that has a
variety of clinicopathological features due to its heterogeneous
nature (1), which is mainly
classified on the basis of the specific immunohistochemical
indicators as luminal breast cancer, human epidermal growth factor
receptor 2 (HER-2)-overexpressed breast cancer and triple-negative
breast cancer (TNBC) (2). TNBC
patients are diagnosed with negative indicators of estrogen
receptor (ER), progesterone receptor (PR) and HER-2, which account
for ~10–24% of all cases with breast cancer (3). Compared with other subtypes, TNBC is
associated with the worst curative outcome and the highest
mortality owing to its biological characteristics of strong
invasion, high risk of early recurrence and rapid rate of distant
metastasis (4,5). Therefore, improving the efficacy of
the treatment of TNBC is currently one of the greatest challenges
in the research field of breast carcinoma.
Adriamycin (ADM) belongs to anthracyclines and has a
very broad antitumor spectrum and is commonly used in the
chemotherapy regimen for breast cancer (6). ADM acts as a suppressor of the
growth of tumor cells mainly by blocking the synthesis of nucleic
acids, for instance (7). However,
a high rate of resistance to ADM in clinical chemotherapy for TNBC
has been reported, but there is a lack of effective therapeutic
measures (8). Based on this
discovery, it is of great significance to explore the mechanism
underlying the resistance to ADM in TNBC cells so as to improve the
chemotherapeutic efficacy.
Circular RNA (circRNA) is a new type of RNA
different from linear RNA and is more stable in expression due to
its closed-loop molecular structure, which makes it less
susceptible to Ribonuclease R (RNase R) (9). It has been shown in previous studies
that circRNAs are widely present in the human body and have
biological functions, such as the participation as competing
endogenous RNAs (ceRNAs), the regulation of variable shearing and
transcription, and the translation of protein, in addition to the
control of normal physiological activities and the development of
tumors (10,11). At present, increasing evidence
suggests that circRNAs are closely engaged in the proliferation,
invasion, apoptosis and drug resistance of multiple tumor cells,
including breast cancer cells, and are expected to be the
therapeutic targets or prognostic markers for cancers (12–14). In a study regarding the aberrant
expression profile of circRNAs in drug-resistant breast cancer
cells, circRNA_0044556 was revealed to be upregulated (6), but the detailed mechanism remains to
be further elucidated.
In the present study, following the quantification
of the expression of circRNA_0044556 in TNBC, its role in
regulating the sensitivity of TNBC cells to ADM was investigated
and its related molecular mechanism was revealed by cell functional
experiments, aiming to provide a rationale for the selection of
circRNAs as molecular targets in improving the sensitivity of TNBC
to chemotherapy.
Materials and methods
Ethical statement and sample
collection
TNBC tissues and the corresponding adjacent normal
tissues were collected from 40 patients (aged ~26–58 years) with
TNBC who underwent tumor resection at Tangshan People's Hospital
(Tangshan, China) between April 2019 and July 2020, upon obtaining
written informed consents from the donors. ADM-resistant tissue
samples (n=15) and ADM-sensitive tissue samples (n=25) were
acquired from patients who met the Response Evaluation Criteria in
Solid Tumors (15). The present
study was approved (approval no. TNBC20190304) by the Ethics
Committee of Tangshan People's Hospital (Tangshan, China).
Cell culture
Normal mammary epithelial cell line MCF-10A
(CRL-10317), and TNBC cell lines MDA-MB-231 (CRM-HTB-26),
MDA-MB-453 (HTB-131), MDA-MB-157 (HTB-24) and BT549 (HTB-122) were
obtained from American Type Culture Collection (ATCC).
ADM-resistant cell line MDA-MB-231/ADM (IMD-003) was purchased from
Xiamen Immocell Biotechnology Co., Ltd. DMEM (cat. no. SNM-002C;
Sunncell) supplemented with 10% fetal bovine serum (FBS; cat. no.
SNS-002; Sunncell) was used for the culture of all cells at 37°C
with 5% CO2 as previously described (6). Subsequently, reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) was
carried out to quantify the expression of circRNA_0044556 in these
cells.
Cell transfection and grouping
To determine the role circRNA_0044556 plays in TNBC,
MDA-MB-231 and MDA-MB-231/ADM cells were subjected to the
transfection. CircRNA_0044556-overexpression plasmid was
synthesized and obtained from BersinBio, the empty plasmid as the
control, and then these plasmids were transfected at 37°C and for
48 h into the MDA-MB-231 cells which were used to establish the
groups of circRNA_0044556 and negative control (NC), respectively,
while the cells without any transfection or treatment served as the
control. Likewise, small interfering (si)RNA against
circRNA_0044556 (50 pmol, si-circRNA_0044556,
5′-AGCCACAAAGAGTCTACATGTCT-3′) and its negative control (50 pmol,
si-NC, 5′-UUCUCCGAACGUGUCACGU-3′), purchased from Shanghai
GenePharma Co., Ltd., were independently transfected into
MDA-MB-231/ADM cells, and the Control group was established as
well. In the further investigation on the molecular interplay, 100
nM microRNA (miR)-145 mimic (M, miR10000437-1-5,
5′-GUCCAGUUUUCCCAGGAAUCCCU-3′; Guangzhou RiboBio Co., Ltd.) or 100
nM mimic control (MC, 5′-UUCUUCGAACGUGUCACGUTT-3′; Guangzhou
RiboBio Co., Ltd.) was transfected into the parental MDA-MB-231
cells, and 100 nM miR-145 inhibitor (I, miR20000437-1-5,
5′-AGGGAUUCCUGGGAAAACUGGAC-3′; Guangzhou RiboBio Co., Ltd.) or 100
nM inhibitor control (IC, 5′-CAGUACUUUUGUGUAGUACAA-3′) was
transfected into MDA-MB-231/ADM cells at 37°C for 48 h. All
transfections on the cells were carried out using
Lipofectamine® 2000 (cat. no. 11668019; Invitrogen;
Thermo Fisher Scientific, Inc.), and the transfected cells were
assigned to the groups as follows: circRNA_0044556 group,
si-circRNA_0044556 group, NC group, si-NC group, circRNA_0044556+M
group, si-circRNA_0044556+I group, circRNA_0044556+MC group,
si-circRNA_0044556+IC group, si-NC+I group, NC+M group, NC+MC group
and si-NC+IC group. At 48 h after transfection, cells were
collected for subsequent experiments.
Total RNA isolation and RT-qPCR
Total RNA was separated from tissue samples and
cells by TRIzol reagent (cat. no. 15596026; Invitrogen; Thermo
Fisher Scientific, Inc.). Then, the extracted RNA was reversely
transcribed into complementary DNA (cDNA) using a reverse
transcription kit (cat. no. 18090010; Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocols.
Subsequently, the expression levels of circRNA_0044556, miR-145 and
NRAS proto-oncogene, GTPase (NRAS) were evaluated by RT-qPCR using
qPCR SYBR Green Master Mix (cat. no. Q121-02; Vazyme Biotech Co.,
Ltd.) on an ABI 7500 system (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The thermocycling conditions were set as
follows: Predenaturation at 95°C for 5 min, 40 cycles of 95°C for
10 sec and 60°C for 35 sec. Glyceraldehyde-3-phosphate
dehydrogenase (GAPDH) and U6 were used for the normalization of the
expression levels of circRNA_0044556, NRAS, and miR-145. The
expression levels of circRNA_0044556/NRAS (relative to GAPDH) and
miR-145 (relative to U6) were calculated by the 2−ΔΔCq
method (16). Primer sequences
used in this experiment were as follows: circRNA_0044556 forward,
5′-TGACGAGACCAAGAACTGCC-3′ and reverse, 5′-GCACCATCATTTCCACGAGC-3′;
miR-145 forward, 5′-CAGTCTTGTCCAGTTTTCCCAG-3′ and reverse,
5′-TATGCTTGTTCTCGTCTCTGTGTC-3′; NRAS forward,
5′-ATGACTGAGTACAAACTGGTGGT-3′ and reverse,
5′-CATGTATTGGTCTCTCATGGCAC-3′; U6 forward,
5′-ATTGGAACGATACAGAGAAGATT-3′ and reverse,
5′-GGAACGCTTCACGAATTTG-3′; and GAPDH forward,
5′-GAAGGTGAAGGTCGGAGTC-3′ and reverse,
5′-GAAGATGGTGATGGGATTTC-3′.
Cell viability evaluation
To explore the role of circRNA_0044556 in the
viability of TNBC cells to ADM, Cell Counting Kit-8 (CCK-8) assay
was applied. Firstly, parental and ADM-resistant MDA-MB-231 cells
(2×104) were seeded into a 96-well plate and treated
with different concentrations of ADM (0, 0.1, 0.2, 0.4, 0.8, 1.6, 3
and 6 µg/ml) at 37°C for 24 h, followed by the addition of 10 µl
CCK-8 solution (cat. no. CK04; Dojindo Molecular Technologies,
Inc.) to treat cells in each well at 37°C with 5% CO2
for 2 h. A microplate reader (Infinite M200; Tecan Group, Ltd.) was
used to measure the optical density (OD) value at a wavelength of
490 nm, and then the half maximal inhibitory concentration
(IC50) value was calculated.
Cell apoptosis analysis
Annexin V/FITC apoptosis detection kit (cat. no.
AD10; Dojindo Molecular Technologies, Inc.) was used for assessing
the apoptotic capacity of TNBC cells undergoing the indicated
treatments (parental and ADM-resistant MDA-MB-231 cells transfected
with circRNA_0044556, si-circRNA_0044556, miR-145 mimic, inhibitor
and their negative control). According to the description in the
manual, cell suspension (1×106) was prepared using 1X
Annexin V Binding Solution, and was then incubated with 5 µl
Annexin V/FITC solution and 5 µl PI solution at room temperature
for 15 min in the dark. Next, the stained samples were analyzed
using an Accuri C6 flow cytometer with CFlow software (v. 1.32; BD
Biosciences) to determine the apoptosis.
Cell migration detection
Transwell inserts (8 µm; product number 351184;
Corning, Inc.) were used to conduct the Transwell assay. Following
transfection, parental and ADM-resistant MDA-MB-231 cells were
resuspended at a density of 4×104 cells/well with 5 µl
serum-free medium in the upper chamber of the insert, whereas 500
µl culture medium containing 10% FBS was added in the lower chamber
at the same time. After 48 h of incubation at 37°C, the residual
cells on the upper chamber were removed with a cotton swab, and the
membrane was subjected to fixation with 4% paraformaldehyde (cat.
no. E672002; Sangon Biotech Co., Ltd.) at 4°C for 30 min and
staining was performed using 0.1% crystal violet for 15 min at room
temperature (cat. no. G1064; Beijing Solarbio Science and
Technology Co., Ltd.). Finally, a light microscope (×250,
magnification; CX23; Olympus Corporation) was used to observe the
migration of cells on the lower side of the membrane.
Bioinformatics analysis and
dual-luciferase reporter assay
The targeting relationship among circRNA_0044556,
miR-145, and NRAS in TNBC cells was predicted by circInteractome
(https://circinteractome.irp.nia.nih.gov/) and StarBase
v2.0 (http://starbase.sysu.edu.cn/index.php),
respectively.
For verifying the targeting relationship through
dual luciferase reporter assay, vectors (cat. no. E1330; Promega
Corporation) were used to construct wild-type (wt) reporter plasmid
of circRNA_0044556 (circRNA_0044556-wt;
5′-GGUGCCAAGGGUCUGACUGGAAG-3′), mutant (mut) plasmid of
circRNA_0044556 (circRNA_0044556-mut;
5′-GGUGCCAAGGGUCUGCCUCGUAG-3′), NRAS-wt reporter plasmid
(5′-CAAACCCUUUACCAUGACUGGAA-3′) and NRAS-mut reporter plasmid
(5′-CAAACCCUUUACCCUGUCUCGUA-3′). Next, MDA-MB-231 cells were
co-transfected with miR-145 mimic (5′-GUCCAGUUUUCCCAGGAAUCCCU-3′)
or its miR-NC (5′-UUCUUCGAACGUGUCACGUTT-3′) and the reporter
plasmids (circRNA_0044556-wt, circRNA_0044556-mut, NRAS-wt or
NRAS-mut) using Lipofectamine® 2000 reagent (Invitrogen;
Thermo Fisher Scientific, Inc.). After 48 h, the relative
luciferase activity was determined by a Dual Luciferase Reporter
Gene Assay Kit (cat. no. 11402ES60; Shanghai Yeasen Biotechnology
Co., Ltd.). The luciferase activity was normalized to the
Renilla luciferase activity.
RNA binding protein
immunoprecipitation (RIP)
RIP kit (cat. no. KT102-01), obtained from Guangzhou
Saicheng Biotechnology Co., Ltd. was used to determine the
connectivity between circRNA_0044556 and miR-145. Briefly, the
transfected cells (4×107 cells) were collected with
phosphate-buffered saline (cat. no. C0221A; Beyotime Institute of
Biotechnology) and lysed with cell lysis buffer. G/A beads (100 µl)
were prepared and divided into the groups argonaute2 (Ago2) and
immunoglobulin G (IgG) (4). Then,
the antibodies against Ago2 (product code ab32381, 1:1,000) and IgG
(product code ab133470, 1:1,000; both from Abcam) were added into
the lysates of cells in the indicated groups at 4°C. After 6 h of
incubation, the antibody-tagged beads were incubated with the
lysates at 4°C overnight. Subsequently, the beads were washed with
RIP buffer for five times by centrifugation at 1,500 × g for 2 min
at 4°C. Proteinase K was added and incubated for 45 min at 65°C to
remove the protein prior to RNA isolation, and RNA was isolated
using TRIzol® reagent (Thermo Fisher Scientific, Inc.)
as aforementioned. RNA was purified and precipitation according to
the manufacturer's protocols. The immunoprecipitated RNA was
subjected to RT-qPCR to determine the enrichment of circRNA_0044556
in MDA-MB-231 cells with miR-145 overexpression.
Statistical analysis
All data were analyzed using GraphPad Prism 8.0
software (GraphPad Software, Inc.), and measurement data were
expressed as the mean ± standard deviation. The differences between
two independent samples or two paired samples were analyzed by
independent samples t-test or paired samples t-test.
One-way analysis of variance with Tukey's post hoc test was applied
for single-factor differences between multiple groups, while
two-way analysis of variance for the two-factor differences between
multiple groups. The correlation among circRNA_0044556, miR-145 and
NRAS in TNBC was analyzed with Pearson's correlation test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Quantification of circRNA_0044556
expression in TNBC tissues and cells with or without ADM
treatment
RT-qPCR was first performed to quantify the
expression of circRNA_0044556 in tissues (TNBC tissues with or
without ADM resistance and the adjacent tissue) and cells (mammary
epithelial cell line MCF-10A, TNBC cell lines and MDA-MB-231/ADM
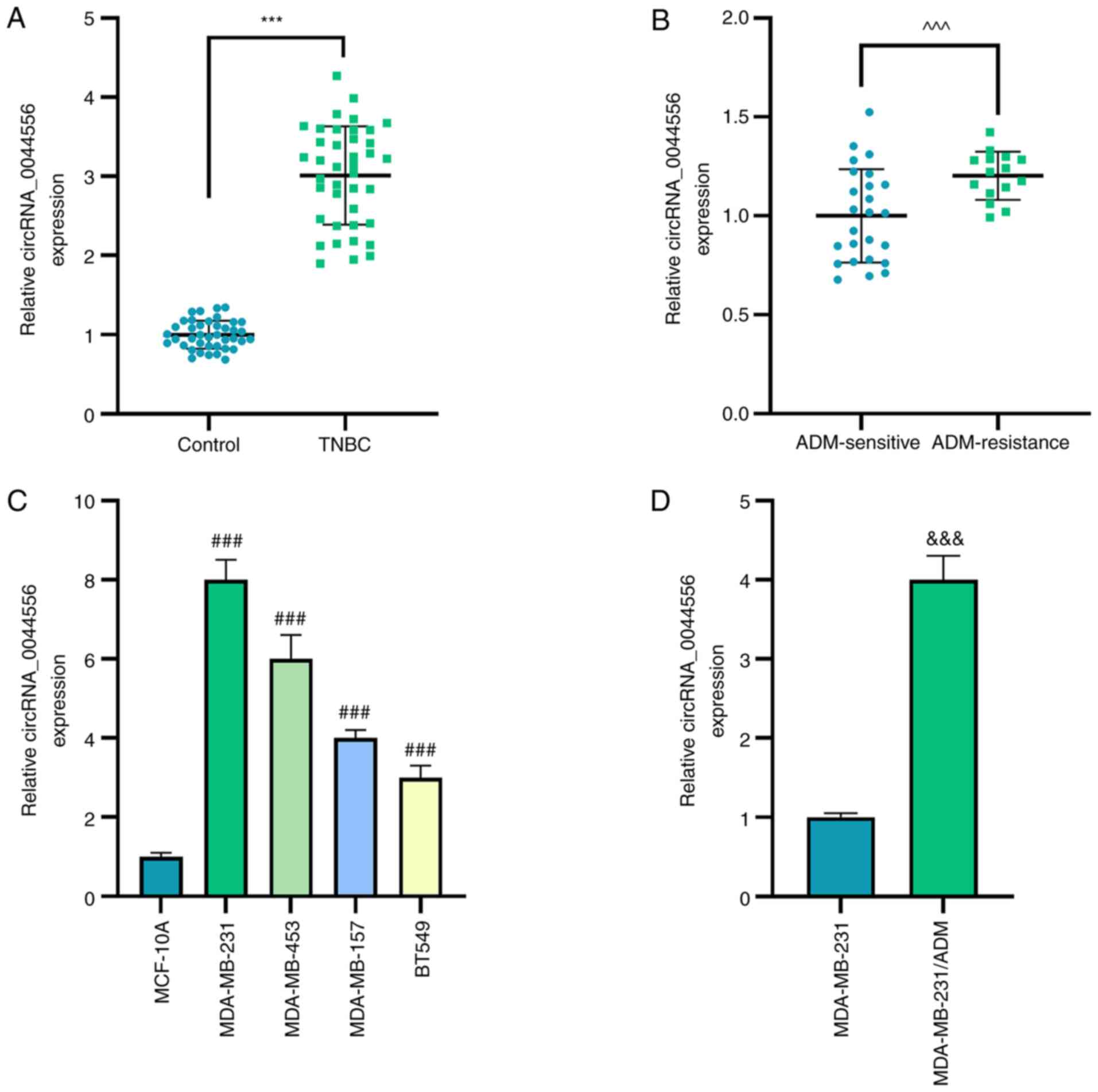
cells). As revealed in Fig. 1A-D,
circRNA_0044556 was highly expressed in TNBC tissues, ADM-resistant
TNBC tissues and TNBC cells (P<0.001). As the most significantly
high expression of circRNA_0044556 was detected in MDA-MB-231 cells
among the four other TNBC cell lines, MDA-MB-231 cells were
selected for subsequent experiments.
CircRNA_0044556 regulates the
viability of ADM-treated MDA-MB-231 and MDA-MB-231/ADM cells
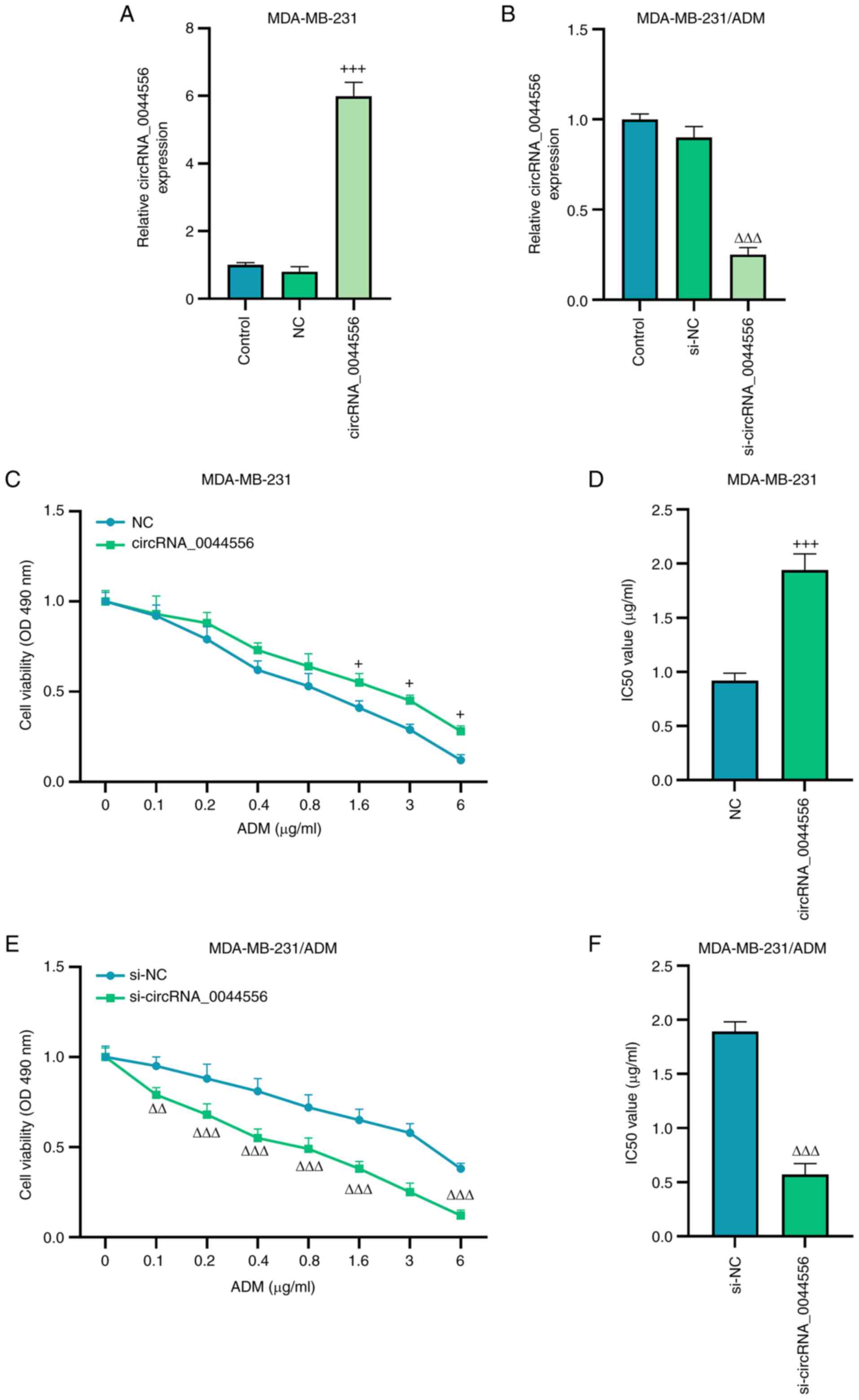
Subsequently, MDA-MB-231 cells were transfected with
circRNA_0044556 overexpression plasmid, and MDA-MB-231/ADM cells
were transfected with si-circRNA_0044556. RT-qPCR was used to
estimate the transfection efficiency and it was determined that the
expression of circRNA_0044556 was significantly promoted by the
overexpression plasmid of circRNA_0044556, yet it was suppressed by
si-circRNA_0044556 (Fig. 2A and
B; P<0.001). It was indicated in the results of the CCK-8
assay that after the treatment of ADM in gradient concentrations,
overexpressed circRNA_0044556 enhanced the viability of parental
MDA-MB-231 cells and increased the IC50 value (Fig. 2C and D; P<0.05), whereas the
silencing of circRNA_0044556 significantly reduced the viability of
MDA-MB-231/ADM cells and decreased the IC50 value
(Fig. 2E and F; P<0.01).
CircRNA_0044556 affects the apoptotic
and migratory capacities of TNBC cells with or without ADM
treatment
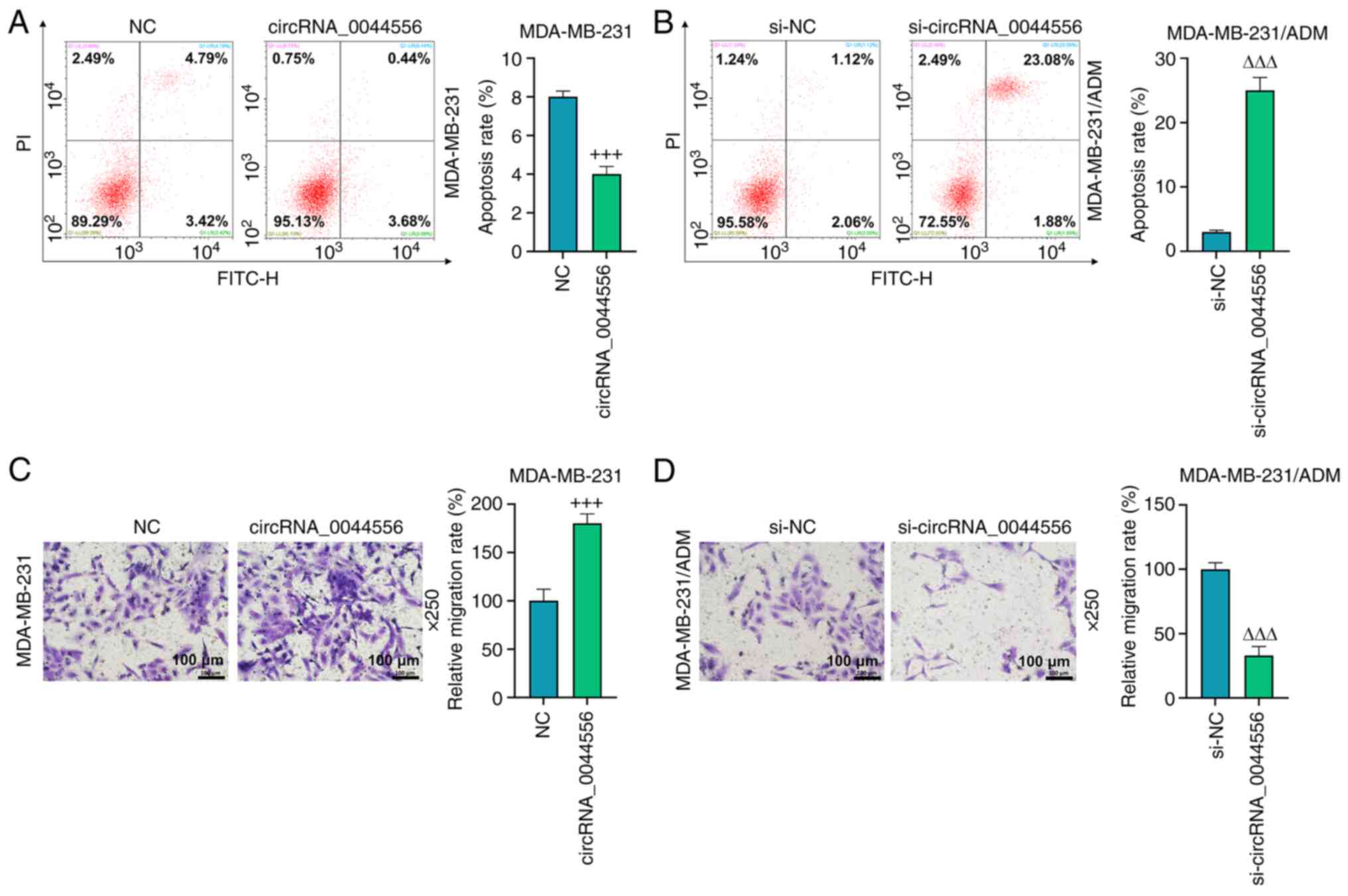
It was demonstrated in the apoptotic assay (Fig. 3A and B) that the overexpression of
circRNA_0044556 decreased the percentage of apoptotic parental TNBC
cells (P<0.001), whereas the silencing of circRNA_0044556
promoted apoptosis of the ADM-resistant cells (P<0.001). As
demonstrated in Fig. 3C and D, a
distinctly increased number of migratory TNBC cells were observed
after the overexpression of circRNA_0044556 compared with those in
the NC group (P<0.001), whereas the migratory capacity of
MDA-MB-231/ADM cells was significantly suppressed after the
inhibition of circRNA_0044556 as compared with the si-NC group
(P<0.001).
CircRNA_0044556 sponges miR-145, which
is expressed at a low level in TNBC tissues and TNBC cells with or
without ADM treatment
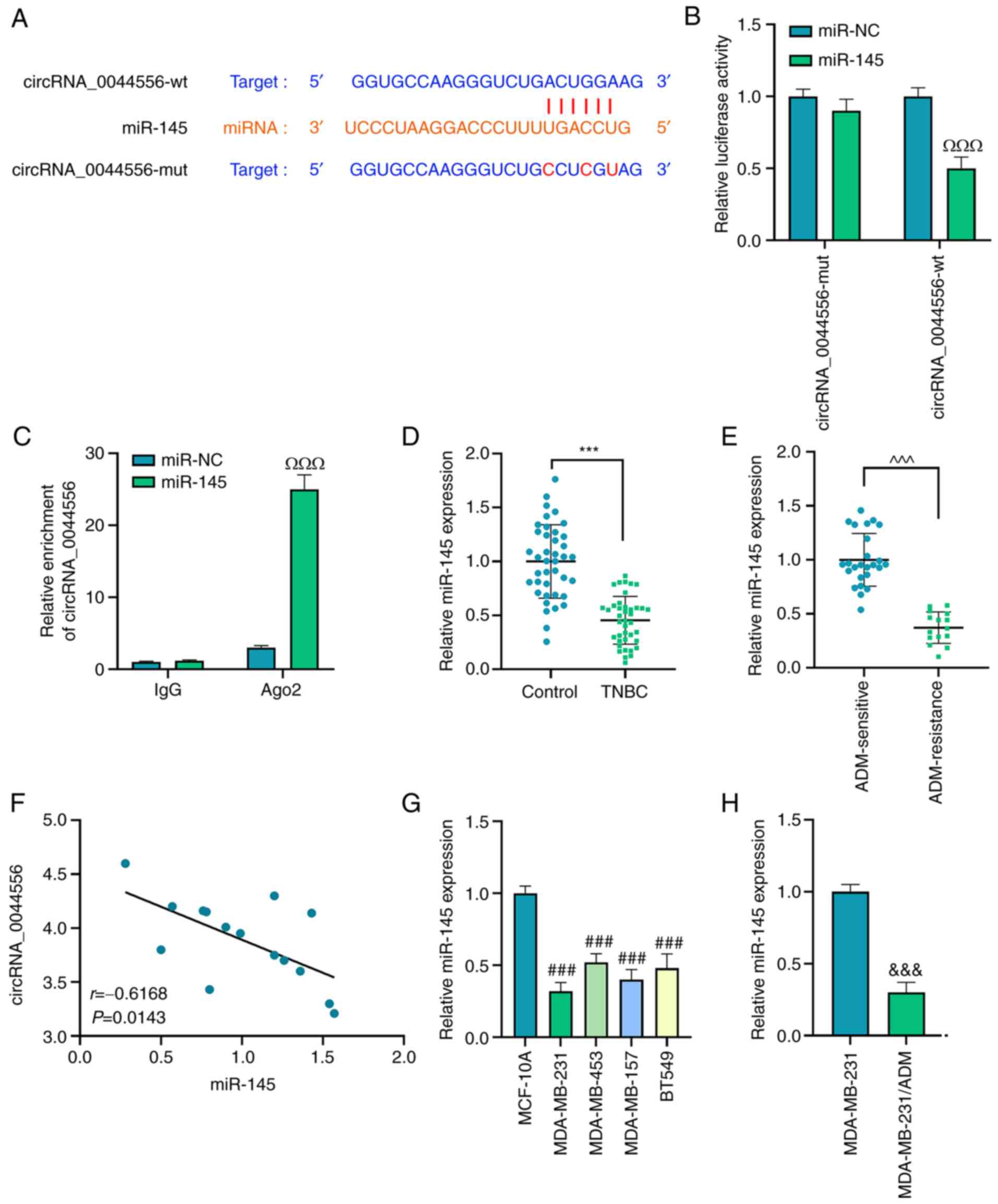
The binding site between the 3′-untranslated region
(UTR) of circRNA_0044556 and the seed region of miR-145 (Fig. 4A) was predicted by bioinformatics
analysis. Subsequently, the dual-luciferase reporter assay in
Fig. 4B indicated that miR-145
mimic significantly suppressed the luciferase activity of
circRNA_0044556-wt (P<0.001). The binding between
circRNA_0044556 and miR-145 in TNBC cells was further determined
via RIP assay, and it was revealed that circRNA_0044556 was
enriched in Ago2-tagged beads compared with the control group of
IgG (Fig. 4C; P<0.001).
Notably, detection with RT-qPCR revealed that the expression of
miR-145 was downregulated in TNBC tissues, and ADM-resistant TNBC
tissues in particular (Fig. 4D and
E; P<0.001). Additionally, according to Pearson's
correlation analysis (Fig. 4F),
miR-145 was negatively correlated with circRNA_0044556 in TNBC
patients with ADM resistance (r=−0.6168, P=0.0143).
Correspondingly, a lower expression of miR-145 in TNBC cell lines
and MDA-MB-231/ADM cells was also identified (Fig. 4G and H; P<0.001).
Overexpressed circRNA_0044556
attenuates apoptosis and enhances migration of TNBC cells with or
without ADM treatment by targeting miR-145
In order to understand the interplay between
circRNA_0044556 and miR-145 in TNBC, miR-145 mimic and
circRNA_0044556-overexpressing plasmid or miR-145 inhibitor and
si-circRNA_0044556 were co-transfected in parental and
ADM-resistant MDA-MB-231 cells, and the transfection efficiency was
evaluated by RT-qPCR. As revealed in Fig. 5A, the overexpression of
circRNA_0044556 downregulated miR-145 expression and miR-145 mimic
induced upregulation of miR-145 compared with the NC+MC group, the
trends of which were both overturned in the circRNA_0044556+M group
(P<0.001). Conversely, si-circRNA_0044556 upregulated miR-145
expression and miR-145 inhibitor led to decreased miR-145
expression compared with the si-NC+IC group, and these trends were
both reversed in the si-circRNA_0044556+I group (Fig. 5B; P<0.01). Subsequently, the
results of rescue experiments indicated that the high expression of
miR-145 induced the apoptosis, decreased the migration of parental
MDA-MB-231 cells, and abrogated the effects of overexpressed
circRNA_0044556 on the apoptosis and migration of parental cells
(Fig. 6A and C; P<0.05). In
ADM-resistant MDA-MB-231 cells, the knockdown of miR-145 dampened
apoptosis, facilitated migration, and reversed the effects of
circRNA_0044556 silencing (Fig. 6B
and D; P<0.05).
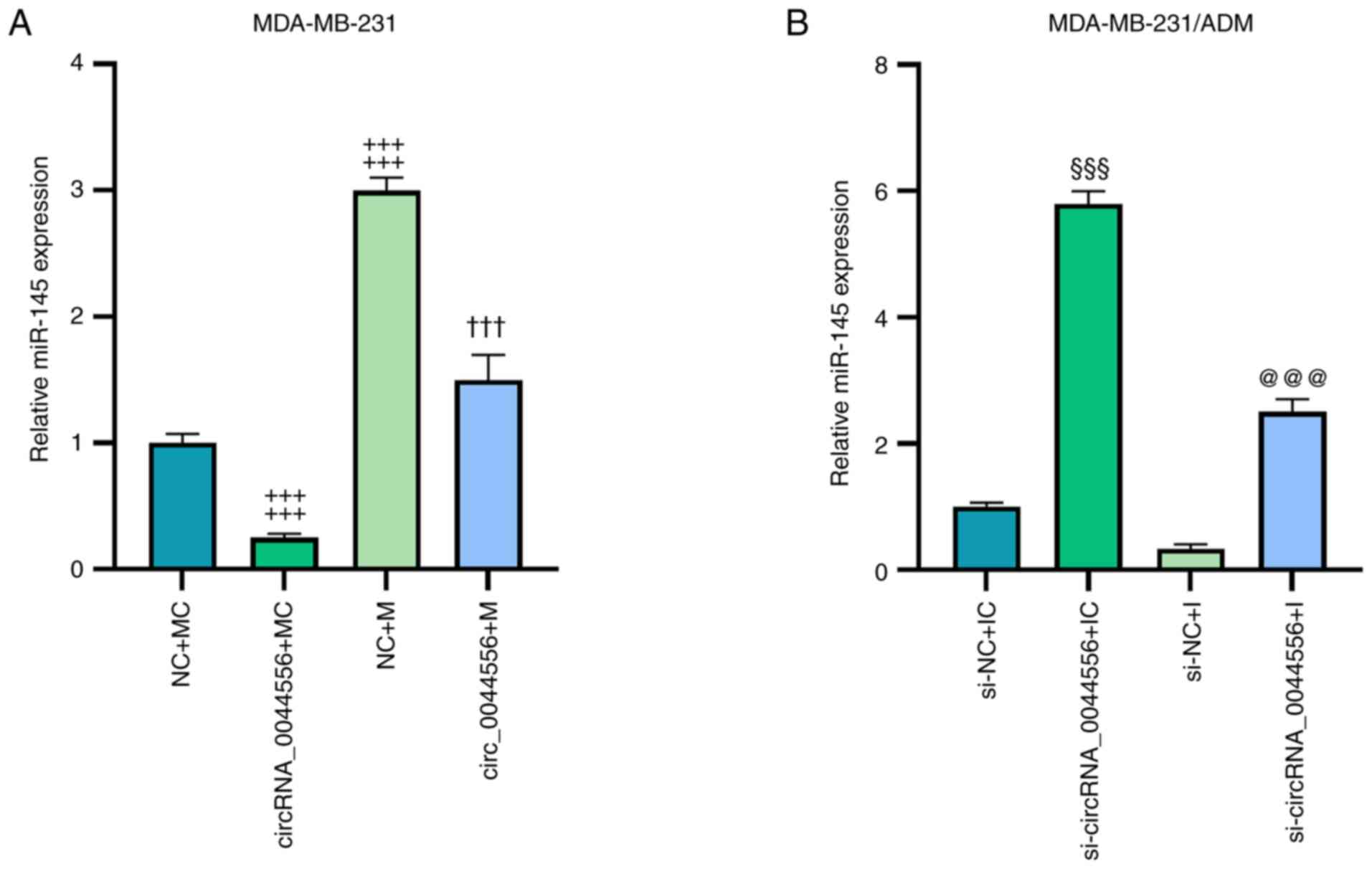 | Figure 5.CircRNA_0044556 regulates the
expression of miR-145 in TNBC cells with or without ADM treatment.
(A and B) MDA-MB-231 cells were transfected with circRNA_0044556
overexpression plasmids, miR-145 mimic or both and MDA-MB-231/ADM
cells were transfected with si-circRNA_0044556, miR-145 inhibitor
or both, and the expression of miR-145 was calculated by reverse
transcription-quantitative PCR. U6 was used as the internal
control. ‡‡‡P<0.001 vs. NC+MC;
†††P<0.001 vs. NC+M; §§§P<0.001 vs.
si-NC+IC; and @@@P<0.001 vs. si-NC+I. NC+MC:
MDA-MB-231 cells were transfected with empty plasmids (negative
control for circRNA_0044556) and mimic control (MC for miR-145);
circRNA_0044556+MC: MDA-MB-231 cells were transfected with
circRNA_0044556 overexpression plasmids and mimic control; NC+M:
MDA-MB-231 cells were transfected with empty plasmids (negative
control for circRNA_0044556) and miR-145 mimic; circRNA_0044556+M:
MDA-MB-231 cells were transfected with circRNA_0044556
overexpression plasmids and miR-145 mimic; si-NC+IC: MDA-MB-231/ADM
cells were transfected with si-NC (negative control for
si-circRNA_0044556) and inhibitor control (IC for miR-145);
si-circRNA_0044556+IC: MDA-MB-231/ADM cells were transfected with
si-circRNA_0044556 and inhibitor control; si-NC+I: MDA-MB-231/ADM
cells were transfected with si-NC and miR-145 inhibitor;
si-circRNA_0044556+I: MDA-MB-231/ADM cells were transfected with
si-circRNA_0044556 and miR-145 inhibitor. circRNA, circular RNA;
miR, microRNA; TNBC, triple-negative breast cancer; ADM,
adriamycin; si-, small interfering; NC, negative control; I,
inhibitor; M, mimic; IC, inhibitor control; MC, mimic control. |
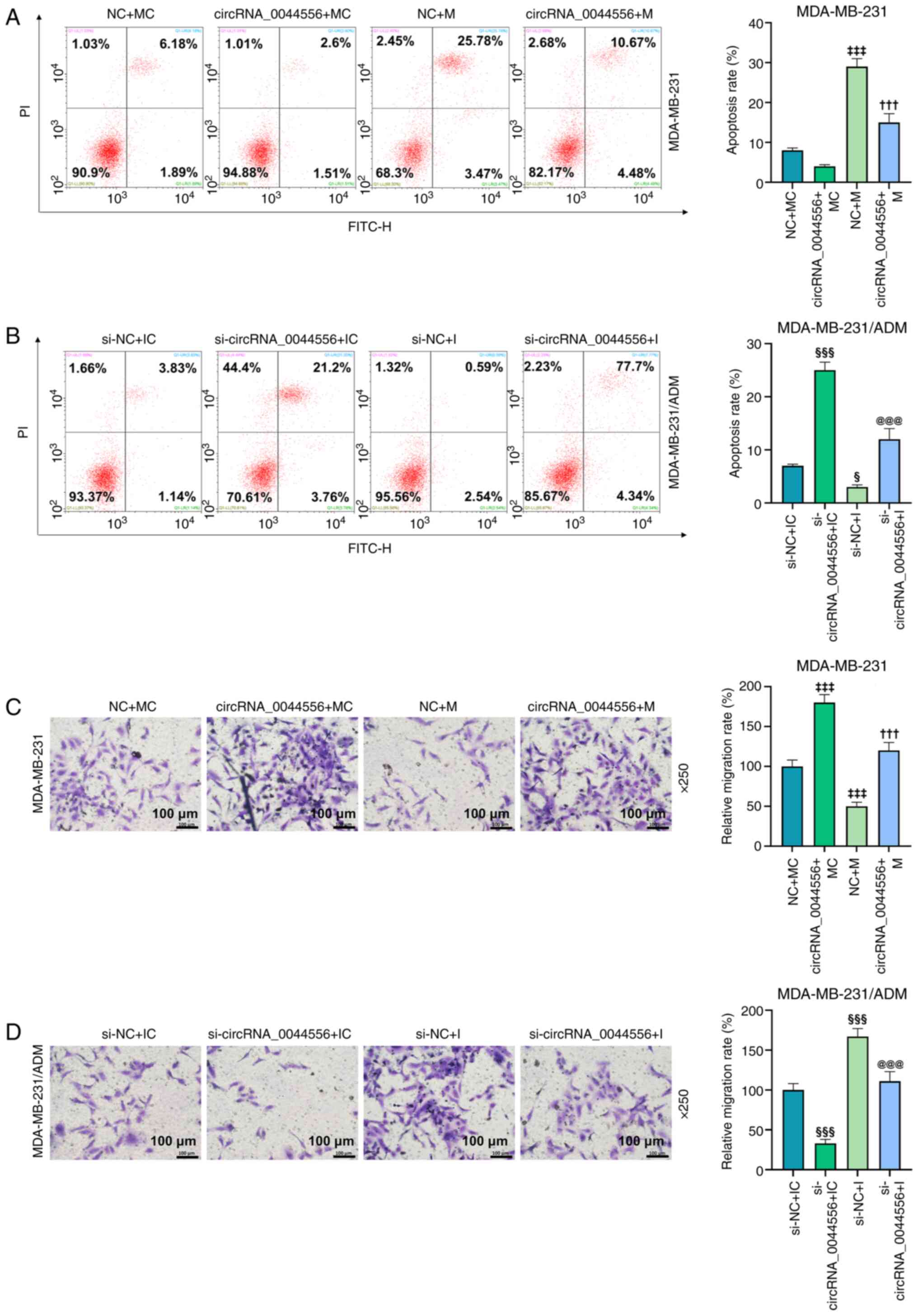 | Figure 6.Regulatory role of the
circRNA_0044556/miR-145 axis in the development of TNBC cells with
or without ADM treatment. (A and B) Flow cytometry was employed to
analyze the apoptosis after the overexpression or depletion of
circRNA_0044556, miR-145 alone or in combination in MDA-MB-231 and
MDA-MB-231/ADM cells. (C and D) The migratory capacities of TNBC
cells after various transfections were evaluated by Transwell
assay. ‡‡‡P<0.001 vs. NC+MC; †††P<0.001
vs. NC+M; §P<0.05 and §§§P<0.001 vs.
si-NC+IC; and @@@P<0.001 vs. si-NC+I. NC+MC:
MDA-MB-231 cells were transfected with empty plasmids (negative
control for circRNA_0044556) and mimic control (MC for miR-145);
circRNA_0044556+MC: MDA-MB-231 cells were transfected with
circRNA_0044556 overexpression plasmids and mimic control; NC+M:
MDA-MB-231 cells were transfected with empty plasmids (negative
control for circRNA_0044556) and miR-145 mimic; circRNA_0044556+M:
MDA-MB-231 cells were transfected with circRNA_0044556
overexpression plasmids and miR-145 mimic; si-NC+IC: MDA-MB-231/ADM
cells were transfected with si-NC (negative control for
si-circRNA_0044556) and inhibitor control (IC for miR-145);
si-circRNA_0044556+IC: MDA-MB-231/ADM cells were transfected with
si-circRNA_0044556 and inhibitor control; si-NC+I: MDA-MB-231/ADM
cells were transfected with si-NC and miR-145 inhibitor;
si-circRNA_0044556+I: MDA-MB-231/ADM cells were transfected with
si-circRNA_0044556 and miR-145 inhibitor. circRNA, circular RNA;
miR, microRNA; TNBC, triple-negative breast cancer; ADM,
adriamycin; si-, small interfering; NC, negative control; I,
inhibitor; M, mimic; IC, inhibitor control; MC, mimic control. |
MiR-145 targets NRAS, which is
overexpressed in TNBC tissues and cells with or without ADM
treatment
Following analysis by StarBase, NRAS was predicted
to contain the binding site for miR-145 (Fig. 7A), the existence of which was then
evidenced in the dual-luciferase reporter assay, where a decrease
of luciferase activity in TNBC cells co-transfected with miR-145
mimic and NRAS-wt was identified (Fig. 7B; P<0.001). In addition, NRAS
was revealed to be overexpressed in TNBC tissues and cells with or
without ADM treatment, according to the results of RT-qPCR
(Fig. 7C-F; P<0.001).
Furthermore, NRAS was negatively correlated with miR-145 (Fig. 7G; r=−0.6552, P=0.008), and
positively correlated with circRNA_0044556 in TNBC cells (Fig. 7H; r=0.6391, P=0.0103).
circRNA_0044556 overexpression promoted the expression of NRAS in
MDA-MB-231 cells, and silencing of circRNA_0044556 inhibited the
expression of NRAS in ADM-resistant MDA-MB-231 cells (Fig. 7I and J; P<0.001).
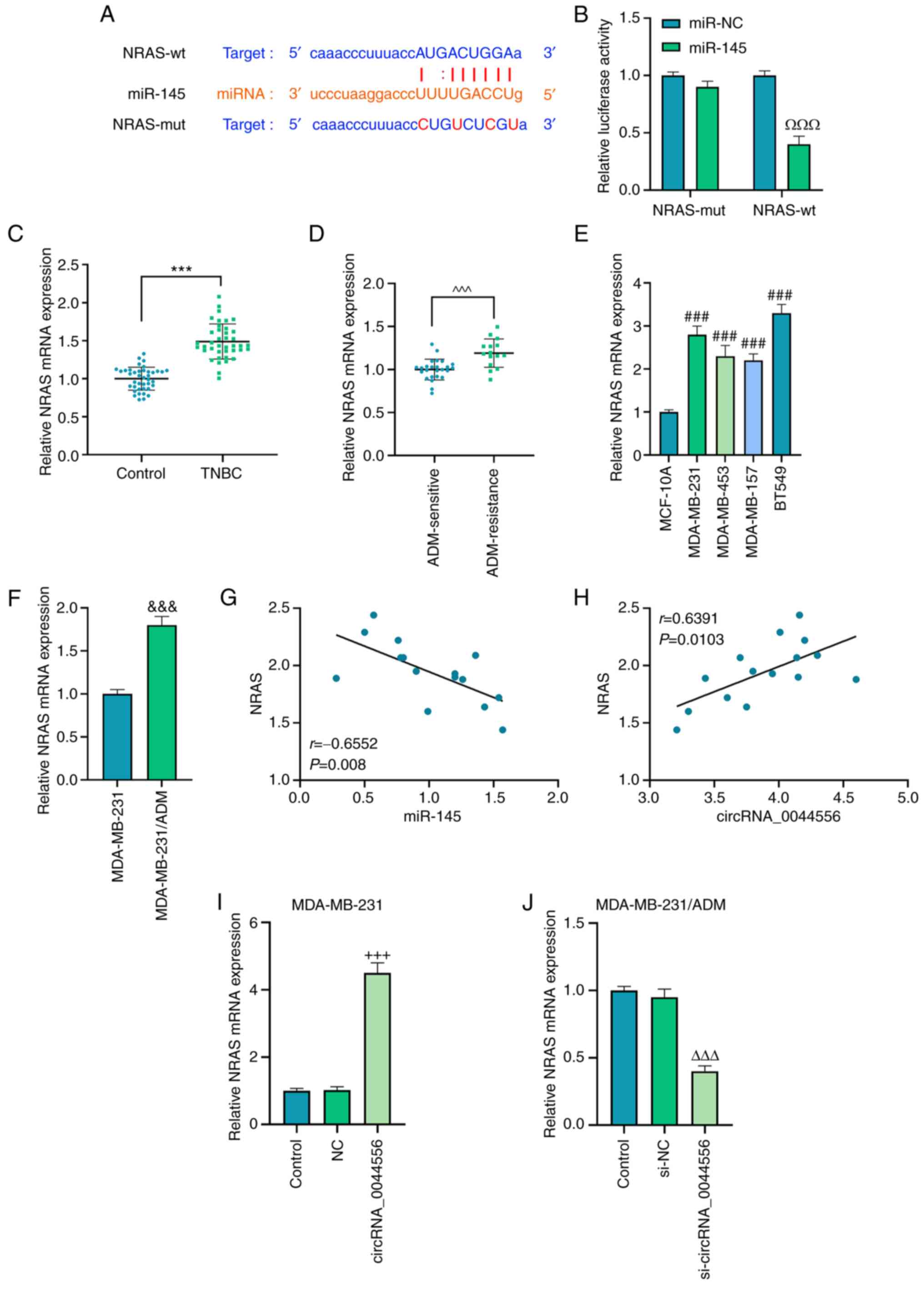 | Figure 7.NRAS is targeted by miR-145 and the
effect of circRNA_0044556/miR-145 axis on TNBC cells with or
without ADM treatment is mediated by NRAS. (A) The potential
binding site of NRAS for miR-145 was predicted by StarBase website.
(B) A dual luciferase reporter assay was carried out for the
validation that NRAS was targeted by miR-145. (C-F) The expression
of NRAS was determined in TNBC tissues, ADM-sensitive TNBC tissues,
ADM-resistant TNBC tissues, normal mammary epithelial cells and
cells with or without ADM-resistance by reverse
transcription-quantitative PCR. GAPDH was used as the internal
control. (G and H) The correlation between miR-145 and NRAS, or
between circRNA_0044556 and NRAS in TNBC was analyzed with
Pearson's correlation analysis. (I and J) The expression of NRAS
was detected after the overexpression or silencing of
circRNA_0044556 in MDA-MB-231 and MDA-MB-231/ADM cells by reverse
transcription-quantitative PCR. ΩΩΩP<0.001 vs.
miR-NC; ***P<0.001 vs. Control; ^^^P<0.001 vs.
ADM-sensitive; ###P<0.001 vs. MCF-10A;
&&&P<0.001 vs. MDA-MB-231;
+++P<0.001 vs. NC; and rrrP<0.001 vs. si-NC. NRAS,
NRAS proto-oncogene, GTPase; miR, microRNA; circRNA, circular RNA;
TNBC, triple-negative breast cancer; ADM, adriamycin. |
Discussion
Chemotherapy is an important part of the
comprehensive treatment of breast cancer (17). However, the resistance that
develops in TNBC patients receiving ADM greatly limits clinical
efficacy and is one of the culprits that ultimately leads to poor
prognosis (18). The discovery of
new chemotherapeutic drugs is a difficult and lengthy process, and
as such, unravelling the mechanism of resistance to ADM in TNBC
cells will facilitate the in-depth use of this original classical
chemotherapeutic agent.
Recent studies have demonstrated that the
development of drug resistance in cancer cells is closely linked to
the aberrant expression of one or more genes and the activation of
related signaling pathways (19,20). The aberrancy of circRNAs is
related to the sensitivity of breast cancer cells to
chemotherapeutic agents. Sang et al identified that the knockdown
of circRNA_0025202 in breast cancer bolsters tamoxifen resistance
and contributes to tumor progression by sponging miR-182-5p
(14). In addition, Liang et
al revealed that circRNA_KDM4C is a potential tumor suppressor
in breast cancer and markedly attenuates the resistance to ADM
(21). In addition, the
overexpression of circRNA_UBE2D2 in TNBC was revealed to facilitate
cell proliferation and metastasis, whilst decreasing the
sensitivity to doxorubicin (22).
Collectively, it is suggested that circRNAs are implicated in
promoting or suppressing chemosensitivity in breast cancer.
CircRNA_0044556 is a newly identified circRNA that is highly
expressed in colorectal cancer and positively associated to tumor
stage and lymphatic metastasis (23). Intriguingly, circRNA_0044556 was
aberrantly expressed in ADM-resistant TNBC. Nevertheless, its
participation in the development of resistance to ADM in TNBC and
its effects on the fate of TNBC cells have not been identified. In
the present study, it was revealed that circRNA_0044556 was highly
expressed in TNBC cells and in particular in ADM-resistant cells.
In accordance with a recent study, the main role of chemotherapy
drugs is to induce apoptosis in tumor cells and inhibit their
growth and metastasis (24). In
addition, suppressing circRNA_0044556 significantly enhanced the
sensitivity of TNBC cells to ADM, resulting in decreased cell
viability, increased apoptosis and attenuated migration. Based on
these findings, it was concluded that circRNA_0044556, which was
considerably expressed at a high level in TNBC cells, could be a
pivotal factor implicated in a mechanism via which TNBC cells
develop the resistance to ADM, suggesting that circRNA_0044556 was
indeed involved in the sensitivity of TNBC cells to ADM, which may
provide new therapeutic directions to overcome this obstacle to
tumor chemotherapy.
The ceRNA mechanism, as one of the biological
functions of circRNAs, is creating a wave of interest in cancer
research, where the circRNAs sponge miRNAs and competitively
regulate the expression of downstream target genes, thus exerting
their biological functions (25).
Through the screening of a database and the validation of target
miRNAs, it was revealed that circRNA_0044556 negatively regulated
miR-145 in TNBC cells with or without ADM-resistance. Subsequent
cellular functional experiments indicated that circRNA_0044556
interacted with miR-145 to affect the sensitivity of TNBC cells to
ADM. The study conducted by Gao et al revealed that miR-145
mimic could enhance the sensitivity of breast cancer to the
chemotherapy of ADM by targeting multidrug resistance-associated
protein 1 (MRP1) (26). Ding
et al demonstrated that downregulation of miR-145 promoted
the proliferative and migratory capacities of breast cancer cells
(27). Collectively, in our
present study, the underlying mechanism of the effect of
circRNA_0044556 on the ADM-resistance in TNBC was achieved by
regulating miR-145 expression.
NRAS is a member of the RAS gene family and
possesses the ability to bind GTP/GDP and GTPase, which controls
cell growth under normal physiological conditions (28). Previous studies have revealed that
NRAS is aggressively expressed in multiple cancers and its mutation
could promote the progression of tumors (29–31). In our subsequent study with regard
to the regulation of miR-145 on the expression of mRNA, it was
identified that NRAS was targeted by miR-145 and expressed at a
high level in ADM-resistant TNBC tissues and cells. Based on the
study of drug resistance in breast cancer by Song et al, miRNA-22
could sensitize tumor cells to paclitaxel by targeting NRAS
(32). Additionally,
circ_0000073/miR-145-5p/NRAS axis has been revealed to regulate the
methotrexate resistance of osteosarcoma (33). Based on our correlation analysis,
it was revealed that NRAS was negatively correlated with miR-145,
yet it was positively correlated with circRNA_0044556, providing
evidence that the circRNA_0044556/miR-145/NRAS axis may be
implicated in the development of ADM resistance in TNBC cells.
However, the present study had certain limitations; rescue
experiments regarding the roles of miR-145 and NRAS in
ADM-resistant TNBC cells were not performed.
Overall, the present study firstly demonstrated, to
the best of our knowledge, that circRNA_0044556 decreased the
sensitivity of TNBC cells to ADM by sponging miR-145, which may be
achieved by NRAS, indicating that circRNA_0044556 could be applied
as a biomarker for diagnostic purposes or as an adjunct for the
efficacy of chemotherapy using ADM in TNBC.
Acknowledgements
Not applicable.
Funding
The present study was supported by Hebei Province 2021 Medical
Science Research Project Plan (grant no. 20211662).
Availability of data and materials
The datasets used and/or analyzed generated during
the current study are available from the corresponding author on
reasonable request.
Authors' contributions
JC and PS made substantial contributions to the
conception and design of the study. JZ, YL, JM, YZ and HL performed
data acquisition, data analysis and interpretation. JC and PS
drafted the article or critically revised it for important
intellectual content. JC and PS confirm the authenticity of all the
raw data. All authors read and approved the final version of the
manuscript. All authors agreed to be accountable for all aspects of
the work in ensuring that questions related to the accuracy or
integrity of the work are appropriately investigated and
resolved.
Ethics approval and consent to
participate
All procedures performed in studies involving human
participants were in accordance with the ethical standards of the
institutional and/or national research committee and with the 1964
Helsinki declaration and its later amendments or comparable ethical
standards. The present study was approved (approval no.
TNBC20190304) by the Ethics Committee of Tangshan People's Hospital
(Tangshan, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zuo T, Zeng H, Li H, Liu S, Yang L, Xia C,
Zheng R, Ma F, Liu L, Wang N, et al: The influence of stage at
diagnosis and molecular subtype on breast cancer patient survival:
A hospital-based multi-center study. Chin J Cancer. 36:842017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Coates AS, Winer EP, Goldhirsch A, Gelber
RD, Gnant M, Piccart-Gebhart M, Thürlimann B, Senn HJ, Panel
Members, André F, et al: Tailoring therapies-improving the
management of early breast cancer: St gallen international expert
consensus on the primary therapy of early breast cancer 2015. Ann
Oncol. 26:1533–1546. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kumar P and Aggarwal R: An overview of
triple-negative breast cancer. Arch Gynecol Obstet. 293:247–269.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Parikh RR, Yang Q, Higgins SA and Haffty
BG: Outcomes in young women with breast cancer of triple-negative
phenotype: The prognostic significance of CK19 expression. Int J
Radiat Oncol Biol Phys. 70:35–42. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lehmann BD, Pietenpol JA and Tan AR:
Triple-negative breast cancer: Molecular subtypes and new targets
for therapy. Am Soc Clin Oncol Educ Book. e31–e39. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gao D, Zhang X, Liu B, Meng D, Fang K, Guo
Z and Li L: Screening circular RNA related to chemotherapeutic
resistance in breast cancer. Epigenomics. 9:1175–1188. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Song J, Yin J, Bai Z, Zhang J, Meng H, Cai
J, Deng W, Ma X and Zhang Z: The profile of serum microRNAs
predicts prognosis for resected gastric cancer patients receiving
platinum-based chemotherapy. Dig Dis Sci. 62:1223–1234. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang M, Li Y, Ruan Y, Lu Y, Lin D, Xie Y,
Dong B, Dang Q and Quan C: CLDN6 enhances chemoresistance to ADM
via AF-6/ERKs pathway in TNBC cell line MDAMB231. Mol Cell Biochem.
443:169–180. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li Z, Chen Z, Hu G and Jiang Y: Roles of
circular RNA in breast cancer: Present and future. Am J Transl Res.
11:3945–3954. 2019.PubMed/NCBI
|
|
10
|
Jahani S, Nazeri E, Majidzadeh AK, Jahani
M and Esmaeili R: Circular RNA; a new biomarker for breast cancer:
A systematic review. J Cell Physiol. 235:5501–5510. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Guo JU, Agarwal V, Guo H and Bartel DP:
Expanded identification and characterization of mammalian circular
RNAs. Genome Biol. 15:4092014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Meng S, Zhou H, Feng Z, Xu Z, Tang Y, Li P
and Wu M: CircRNA: Functions and properties of a novel potential
biomarker for cancer. Mol Cancer. 16:942017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shen Z, Zhou L, Zhang C and Xu J:
Reduction of circular RNA Foxo3 promotes prostate cancer
progression and chemoresistance to docetaxel. Cancer Lett.
468:88–101. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sang Y, Chen B, Song X, Li Y, Liang Y, Han
D, Zhang N, Zhang H, Liu Y, Chen T, et al: circRNA_0025202
regulates tamoxifen sensitivity and tumor progression via
regulating the miR-182-5p/FOXO3a axis in breast cancer. Mol Ther.
27:1638–1652. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Akram M and Siddiqui SA: Breast cancer
management: Past, present and evolving. Indian J Cancer.
49:277–282. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yin Y, Wang X, Li T, Ren Q, Li L, Sun X,
Zhang B, Wang X, Han H, He Y, et al: MicroRNA-221 promotes breast
cancer resistance to adriamycin via modulation of PTEN/Akt/mTOR
signaling. Cancer Med. 9:1544–1552. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vasan N, Baselga J and Hyman DM: A view on
drug resistance in cancer. Nature. 575:299–309. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nikolaou M, Pavlopoulou A, Georgakilas AG
and Kyrodimos E: The challenge of drug resistance in cancer
treatment: A current overview. Clin Exp Metastasis. 35:309–318.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liang Y, Song X, Li Y, Su P, Han D, Ma T,
Guo R, Chen B, Zhao W, Sang Y, et al: circKDM4C suppresses tumor
progression and attenuates doxorubicin resistance by regulating
miR-548p/PBLD axis in breast cancer. Oncogene. 38:6850–6866. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dou D, Ren X, Han M, Xu X, Ge X, Gu Y,
Wang X and Zhao S: CircUBE2D2 (hsa_circ_0005728) promotes cell
proliferation, metastasis and chemoresistance in triple-negative
breast cancer by regulating miR-512-3p/CDCA3 axis. Cancer Cell Int.
20:4542020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jing L, Wu J, Tang X, Ma M, Long F, Tian B
and Lin C: Identification of circular RNA hsa_circ_0044556 and its
effect on the progression of colorectal cancer. Cancer Cell Int.
20:4272020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yamaguchi R, Lartigue L and Perkins G:
Targeting Mcl-1 and other Bcl-2 family member proteins in cancer
therapy. Pharmacol Therap. 195:13–20. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tang YY, Zhao P, Zou TN, Duan JJ, Zhi R,
Yang SY, Yang DC and Wang XL: Circular RNA hsa_circ_0001982
promotes breast cancer cell carcinogenesis through decreasing
miR-143. DNA Cell Biol. 36:901–908. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gao M, Miao L, Liu M, Li C, Yu C, Yan H,
Yin Y, Wang Y, Qi X and Ren J: miR-145 sensitizes breast cancer to
doxorubicin by targeting multidrug resistance-associated protein-1.
Oncotarget. 7:59714–59726. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ding Y, Zhang C, Zhang J, Zhang N, Li T,
Fang J, Zhang Y, Zuo F, Tao Z, Tang S, et al: miR-145 inhibits
proliferation and migration of breast cancer cells by directly or
indirectly regulating TGF-β1 expression. Int J Oncol. 50:1701–1710.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen S, Li F, Xu D, Hou K, Fang W and Li
Y: The function of RAS mutation in cancer and advances in its drug
research. Curr Pharm Des. 25:1105–1114. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Druillennec S, Pouponnot C and Eychène A:
NRAS-driven melanoma: A RAF can hide another. Mol Cell Oncol.
4:e13447582017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Banys-Paluchowski M, Milde-Langosch K,
Fehm T, Witzel I, Oliveira-Ferrer L, Schmalfeldt B and Müller V:
Clinical relevance of H-RAS, K-RAS, and N-RAS mRNA expression in
primary breast cancer patients. Breast Cancer Res Treat.
179:403–414. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Prior IA, Lewis PD and Mattos C: A
comprehensive survey of Ras mutations in cancer. Cancer Res.
72:2457–2467. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Song YK, Wang Y, Wen YY, Zhao P and Bian
ZJ: MicroRNA-22 suppresses breast cancer cell growth and increases
paclitaxel sensitivity by targeting NRAS. Technol Cancer Res Treat.
17:15330338188099972018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li X, Liu Y, Zhang X, Shen J, Xu R, Liu Y
and Yu X: Circular RNA hsa_circ_0000073 contributes to osteosarcoma
cell proliferation, migration, invasion and methotrexate resistance
by sponging miR-145-5p and miR-151-3p and upregulating NRAS. Aging
(Albany NY). 12:14157–14173. 2020. View Article : Google Scholar : PubMed/NCBI
|