Introduction
Silent information regulator 1 (Sirt1) is an
NAD+-dependent deacetylase that can deacetylate
histones, non-histone proteins and various transcription factors
(1,2). Sirt1 is a member of the sirtuin
family that has close evolutionary homology with silent mating-type
information regulation 2 of yeast and serves a vital role in
mammals. Moreover, Sirt1 is usually related to complex
physiological processes, including oxidative stress, metabolism,
apoptosis and aging (1–4). Sirt1 is highly expressed in the
nuclei of embryonic mouse heart cells and its expression gradually
decreases with further development of the organ. It is also
expressed in both the cytoplasm and nuclei of mature cardiomyocytes
(5,6).
Sirt1 is involved in the regulation of aging, gene
transcription, energy balance and oxidative stress in the
cardiovascular system, possesses anti-inflammatory, antioxidant and
anti-aging properties, and also helps resist the development of
heart disease and the formation of atherosclerotic plaques
(7). Furthermore, Sirt1 has been
reported to be involved with ischemic/hypoxic conditions (8). Sirt1 protein expression levels are
lower in the hearts of elderly patients with heart failure compared
with those of young subjects (7).
Sirt1 expression and left ventricular contractile function are also
significantly reduced in the hearts of elderly mice, whereas
overexpression of Sirt1 leads to an improvement in cardiac function
and a reduced mortality (7,9).
Moreover, it has been demonstrated that the increased cardiac
apoptosis rates and infarction size induced by ischemia/reperfusion
in animal models are related to reduced Sirt1 activity (10,11). Studies have demonstrated that
overexpression of Sirt1 in the mouse heart can promote upregulation
of the antioxidant genes manganese superoxide dismutase and
thioredoxin-1, thereby resisting oxidative stress induced by
oxidants or ischemia/reperfusion and leading to the protection of
cells. However, the high levels of Sirt1 expression can create
oxidative damage via induction of mitochondrial dysfunction
(12,13). In our previous study, it was
observed that endothelin-1 (ET-1) could stimulate the expression of
nicotinamide adenine dinucleotide phosphate oxidase 4 (NOX4), as
well as hydrogen peroxide production, involved in the regulation of
atrial natriuretic factor (ANF) secretion in isolated beating rat
atria under normoxia or hypoxia (14). ANF is the first member of a family
of cardiac natriuretic peptides that has been demonstrated to be
involved in regulating body fluid volume and blood pressure
homeostasis. It also possesses important anti-inflammatory and
antioxidant properties that help protect cells (15).
The effect of NOX4 on Sirt1 expression and its role
in regulating ANF secretion has not been fully elucidated,
especially under hypoxic conditions. The aim of the present study
was to therefore investigate these aspects during hypoxia in
isolated perfused beating rat atria.
Materials and methods
Reagents
The NOX4 inhibitor GLX351322, Sirt1-specific
inhibitor selisistat (EX527), nuclear factor erythroid-2-related
factor 2 (Nrf2)-specific inhibitor ML385 and ET-1 were purchased
from MedChemExpress. The dosages of these inhibitors were
determined based on the IC50 concentration (GLX351322, 5
µM; EX527, 123 nM; and ML385, 1.9 µM) and the results of the
pre-tests.
Isolated perfused beating left atrium
preparation
A total of 90 male Sprague-Dawley rats were obtained
from Laboratory Animal Center of Yanbian University (Yanji, China;
weight, 260–300 g; age, 18 weeks). Rats were housed in 45–65%
humidity, at a constant temperature 24±2°C, under 12 h light/dark
cycles and were given a free access to food and water. The rats
were divided into control, hypoxia (Hy), inhibitors (GLX351322,
EX527, ML385) + Hy, ET-1, EX527 + ET-1 and inhibitor only groups
(36 rats were in the control group and the other groups had 6
rats/group). The animal procedures used in the present study were
approved by the Animal Care and Use Committee of Yanbian University
[approval no. SCXK (Ji) 2011–006] and were in accordance with the
Guide for the Care and Use of Laboratory Animals published by the
National Institutes of Health (16). Rats were anaesthetized by
intraperitoneal injection of sodium pentobarbital (>90 mg/kg)
leading to euthanasia and isolated perfused beating left atria were
prepared using a previously described method (17). Briefly, the heart was rapidly
removed and placed in physiological saline at 36°C for washing and
the left atrium was dissected. An atrial cannula (length, 8 cm;
outer diameter, 1.5 mm) containing two small catheters was inserted
into the left atrium and the cannula was secured by ligatures. The
outer tip of the atrial cannula was open to allow outflow from the
atrium. The cannulated atrium was transferred to an organ chamber
containing HEPES buffer. The atrium was maintained at 36°C and
perfused with HEPES buffer solution (1.0 ml/min). The composition
of the buffer was the following: 118 mM NaCl, 4.7 mM KCl, 2.5 mM
CaCl2, 1.2 mM MgCl2, 25 mM NaHCO3,
10 mM glucose, 10 mM HEPES (pH 7.4 with NaOH) and 0.1% bovine serum
albumin (Beijing Yuanheng Shengma Institute of Biotechnology). The
atria from each perfusion was stimulated at 1.5 Hz (0.3 msec; 30–40
V) with a luminal electrode.
Experimental design
The atria were perfused for 60 min to stabilize
atrial dynamics and ANF secretion, after which formal experiments
were conducted. The perfusates were collected at 2 min intervals at
4°C to measure ANF levels. The normoxic perfused atrium was
supplied with sufficient 100% of O2 and normal HEPES
buffer. The hypoxic atrial model was prepared with 100% of
N2 instead of O2 and normal HEPES buffer was
replaced with N2-saturated HEPES buffer. Two cycles of
control (an experimental cycle=12 min) were followed by infusion of
N2-saturated HEPES buffer for four cycles to determine
changes in atrial dynamics and ANF levels in the perfusates. Atrial
tissue was immediately frozen and stored at −80°C for western
blotting.
To identify the effect of NOX4 on Sirt1 and Nrf2
expression levels, as well as its role in the regulation of ANF
secretion during hypoxia, another series of experiments were
performed. After the control period, one cycle of treatment was
followed by four cycles of treatment agent plus hypoxia infusion.
The treatment agents used were: GLX351322, EX527, ML385 and ET-1 (3
nM).
Furthermore, to clarify the effective dosages of
inhibitors used in the present study, 18 male Sprague-Dawley rats
were selected for preliminary tests. After two control cycles,
whereby the atrium was perfused with normal HEPES buffer without
further treatment, one cycle of treatment was followed by four
cycles of inhibitors plus hypoxia infusion.
ANF and atrial pulse pressure
determination
As described previously (14), immunoreactive ANF levels in
perfusates were quantified using an Iodine (125I) ANF
Radioimmunoassay Kit (Beijing North Institute of Biotechnology Co.,
Ltd.), used according to the manufacturer's protocol. The levels of
secreted immunoreactive ANF were expressed as ng/min/g wet atrial
tissue and the following formula was used: Changes in ANF secretion
(fold)=(value of ANF-mean value of basal ANF)/mean value of basal
ANF.
Intra-atrial pressure was recorded using a
physiograph (RM6240EC; Chengdu Capital Instrument Factory) via a
pressure transducer (YPJ01; Chengdu Capital Instrument Factory) and
pulse pressure was assessed using the difference between systolic
and diastolic pressures: Changes in atrial pulse pressure
(fold)=(value of pulse pressure-mean value of basal pulse
pressure)/mean value of basal pulse pressure.
Western blotting
As described in our previous study (14), the radioimmunoprecipitation assay
(RIPA) buffer containing PMSF (cat. no. R0020; Beijing Solarbio
Science & Technology Co., Ltd.) was used to extract total
protein from the left atrium. Protein concentrations were
determined using the Enhanced BCA Protein Assay Kit (cat. no.
P0010; Beyotime Institute of Biotechnology). After quantification,
protein samples (40 µM protein/lane) were separated via SDS-PAGE on
8 or 10% gels, which was subsequently transferred to a PVDF
membrane. The PVDF membranes were blocked in 5% nonfat dry milk at
room temperature for 2 h, were washed three times (15 min/time)
using PBS containing 0.1% Tween-20 and were incubated with primary
antibodies overnight at 4°C. Following the primary incubation, the
membranes were incubated with secondary antibody for 2 h at room
temperature after being washed three times (15 min/time). Target
bands were visualized using the ECL Western Blot Substrate (cat.
no. 180–501; Tanon Science and Technology Co., Ltd.) using a
bioanalytical imaging system. Results were semi-quantified using
ImageJ version l.48 software (National Institutes of Health).
β-actin was used as the internal reference gene.
The primary antibodies used in these experiments
were as follows: Sirt1 (1:1,000; cat. no. DF6033; Affinity
Biosciences), Nrf2 (1:1,000; cat. no. bs-1074R; BIOSS), total-Akt
(1:10,000; cat. no. ab179463; Abcam), phosphorylated (p)-Akt
(1:1,000; cat. no. AF0016; Affinity Biosciences), sequestosome 1
(p62; 1:1,000; cat. no. bs55207R; BIOSS), Kelch-like ECH-associated
protein 1 (Keap1; 1:5,000; cat. no. 10503-2-AP; Wuhan Sanying
Biotechnology), T cell factor (TCF)3 (1,000; cat. no. DF4573;
Affinity Biosciences) and TCF4 (1:1,000; cat. no. DF7622; Affinity
Biosciences), lymphoid enhancer factor 1 (LEF1; 1:1,000; cat. no.
DF7570; Affinity Biosciences), activating transcription factor
(ATF)3 (1:1,000; cat. no. DF6660; Affinity Biosciences) and ATF4
(1:1,000; cat. no. DF6008; Affinity Biosciences) and β-actin
(1:1,000; cat. no. BM3873; Boster Biological Technology). The
secondary antibody used was HRP-conjugated goat anti-rabbit IgG
(Heavy chain + Light chain), which was obtained from Nachuan
Biotech Co. (1:3,000; cat. no. AP132P).
Immunofluorescence staining
The fresh atrial tissue was fixed in 4%
paraformaldehyde at room temperature for 24 h. The deparaffinized
and rehydrated (using a descending ethanol gradient and distilled
water; 5 min/step) atrial tissue sections (5.0-µm sections) were
immersed in 0.1 mol/l sodium citrate solution for antigen retrieval
and placed in a microwave oven to heating by medium-high fire
(80–90°C) for 8 min until the solution had been boiled and the
samples were left in the oven for 10 min after the fire had been
switched off. After repeating this step three times the solution
was cooled naturally. The sections were immersed in PBS to washing
three times (5 min/time) and were then incubated with 0.1% Triton
X-100 at room temperature for 15 min (cat. no. ZLI-9308; OriGene
Technologies, Inc.). The penetrated sections were washed in PBS
three times (5 min/time) using a decoloring shaker and then blocked
by using 10% goat-derived antibody blocking solution (cat. no.
G2010; Wuhan Servicebio Technology Co., Ltd.) at room temperature
for 30 min. After removal of the blocking solution, three
antibodies against Sirt1 (1:200; cat. no. DF6033; Affinity
Biosciences), Nrf2 (1:200; cat. no. bs-1074R; BIOSS) and LEF1
(1:200; cat. no. DF7570; Affinity Biosciences) were added to the
sections and incubated overnight at 4°C. Subsequently, fluorescent
dye-labelled secondary antibodies [Cy3 conjugated with goat
anti-rabbit IgG (1:200; cat. no. GB21303; Wuhan Servicebio
Technology Co., Ltd.) and Alexa Fluor® 488-conjugated
with goat anti-rabbit IgG (1:200; cat. no. GB25303; Wuhan
Servicebio Technology Co., Ltd.)] were added to the sections, which
were incubated at room temperature for 30 min in the dark.
Subsequently, samples were incubated for a further 8 min with 2
µg/ml DAPI (cat. no. G1012; Wuhan Servicebio Technology Co., Ltd.)
at room temperature for cell nuclear staining. Finally,
Fluoromount-G (cat. no. G1401; Wuhan Servicebio Technology Co.,
Ltd.) was added and covered with a cover slide, according to the
manufacturer's instructions. The sections were mounted in an
antifade mounting medium. An inverted fluorescent biological
microscope was used to image the samples (BDS 400; ChongQing Optec
Instrument Co., Ltd.).
Statistical analysis
Prism software (version 7; GraphPad Software, Inc.)
was used to analyze the data. All data are presented as the mean ±
SE and experiments were repeated six times. Significant differences
were statistically compared using one-way ANOVA, followed by
Bonferroni's multiple comparisons test for more than two groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Effects of NOX4 inhibition on Sirt1
and Nrf2 expression levels during hypoxia
To clarify the effective dosages of inhibitors used
in the present study, two dosages of inhibitors were used in the
preliminary tests. The results demonstrated that the optimal doses
of GLX351322, EX3527 and ML385 were 35, 0.25 and 10 µM,
respectively, rather than their IC50 concentrations
(GLX351322, 5 µM; EX527, 123 nM; and ML385, 1.9 µM), which
significantly inhibited the hypoxia-induced increase of atrial ANF
secretion (Fig. S1). Therefore,
the effective dosages of these inhibitors were used in subsequent
experiments. Furthermore, to clarify the effect of NOX4 on Sirt1
and Nrf2 expression and its role in the regulation of ANF secretion
under hypoxic conditions, experiments were performed with a NOX4
inhibitor in isolated beating rat hypoxic atria. The results
demonstrated that compared with the control group hypoxia
significantly increased ANF secretion and inhibited atrial pulse
pressure (Fig. 1A and B)
concomitantly with the significant upregulation of Sirt1 and Nrf2
protein expression levels (Fig.
1C-F). The hypoxia-induced protein expression of Sirt1 was
significantly inhibited by treatment with the NOX4 inhibitor,
GLX351322, compared with the hypoxia group, whereas Nrf2 protein
expression induced by hypoxia was significantly abolished by EX527
treatment, an inhibitor of Sirt1, compared with the hypoxia group.
These results indicated that NOX4 may stimulate Sirt1 activation
and its downstream factor Nrf2 in beating rat atria under hypoxic
conditions.
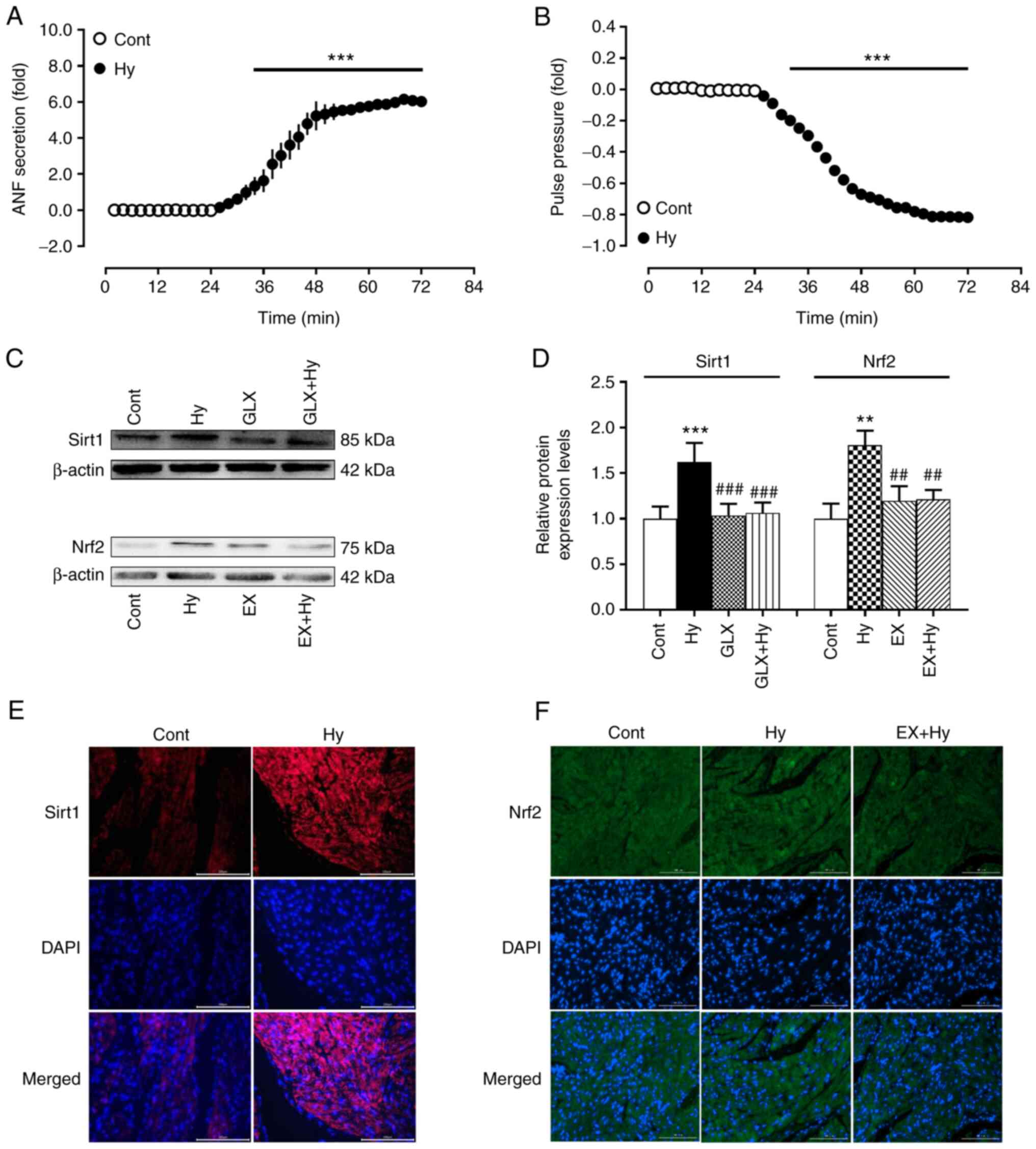 | Figure 1.Effects of hypoxia on ANF secretion,
pulse pressure and the protein expression levels of Sirt1 and Nrf2
in beating rat atria. (A) ANF secretion; (B) pulse pressure; (C-F)
protein expression levels of Sirt1 and Nrf2. Protein expression
levels have been analyzed using western blotting, quantification of
protein levels and immunofluorescence staining (scale bar, 100 µm).
Data are presented as the mean ± SE. A and B, n=6; B and C, n=5.
**P<0.01, ***P<0.001 vs. control; ##P<0.01,
###P<0.001 vs. Hy. ANF, atrial natriuretic factor;
sirt1, silent information regulator 1; Nrf2, nuclear factor
erythroid-2-related factor 2; cont, control; hy, hypoxia; GLX,
GLX351322; EX, EX527. |
Effect of Sirt1 inhibition on p-Akt,
p62 and Keap1 expression under hypoxia
To explore the mechanism by which Sirt1 regulates
Nrf2 protein expression levels, the effect of Sirt1 inhibition on
the protein expression levels of p-Akt, p62 and Keap1 induced by
ET-1 and hypoxia were examined. The results demonstrated that the
protein expression levels of p-Akt and p62 were significantly
upregulated by exogenous ET-1 treatment, whereas Keap1 protein
expression levels were significantly downregulated in the ET-1
group compared with the control (Fig.
2). The ET-1-mediated effects on p-Akt, p62 and Keap1 were
significantly eliminated with EX527 treatment compared with the
control. Moreover, the hypoxia-induced significant upregulation of
p-Akt and p62 protein expression levels, as well as the significant
downregulation of Keap1 protein expression levels compared with the
control, were also significantly reversed by EX527 treatment in the
EX + Hy group compared with the hypoxia group. These results
suggested that Sirt1 may regulate Nrf2 protein expression levels
via the downregulation of Keap1 and the activation of Akt and
p62.
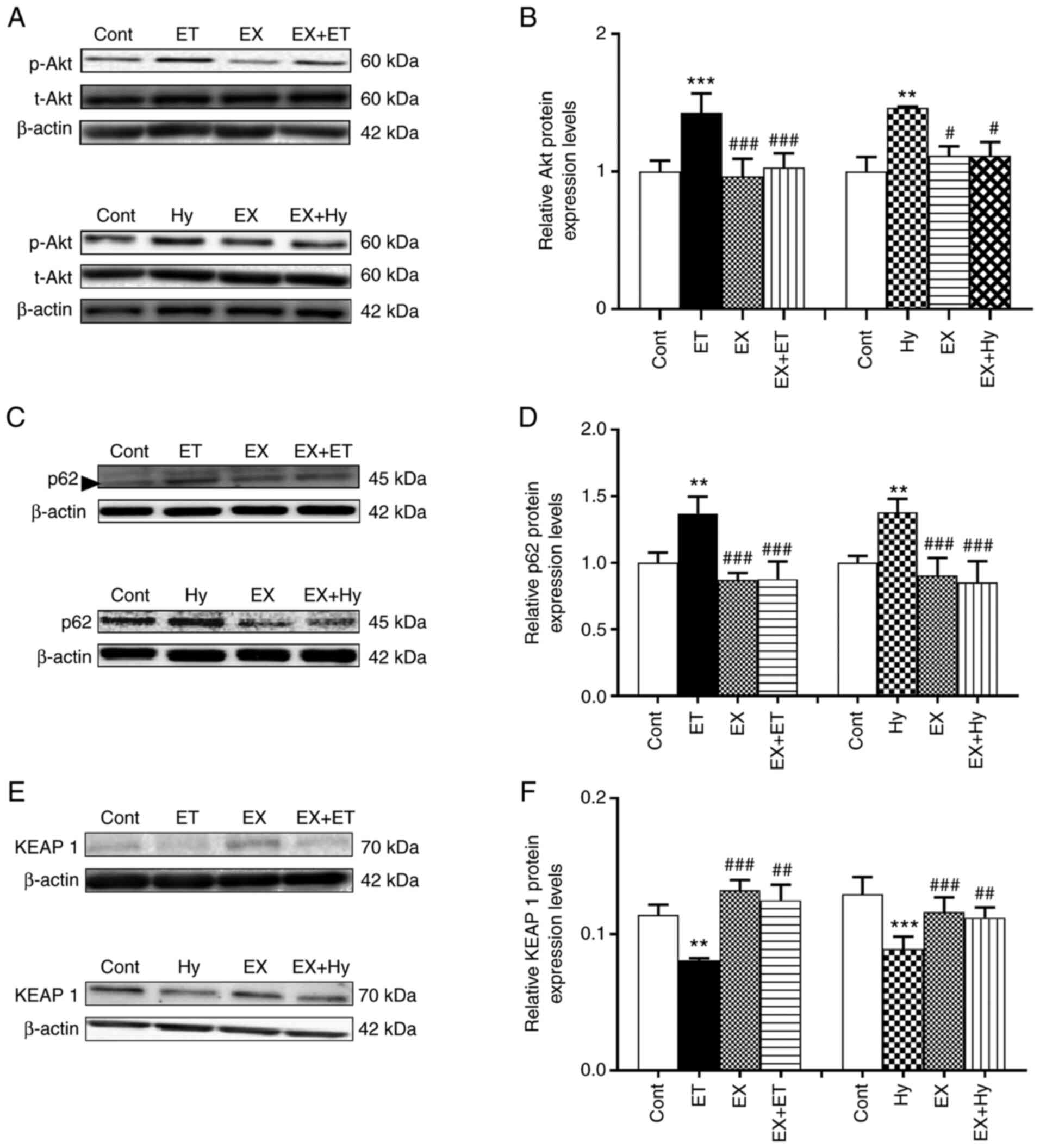 | Figure 2.Effects of Sirt1 inhibitor on the
levels of p-Akt, p62 and Keap1 induced by ET-1 (under normoxia) or
hypoxia in beating rat atria. (A, C and E) Representative western
blots (in C, the band next to the p62 band is a miscellaneous band;
size, ~47 kDa). (B, D and F) Semi-quantification of protein
expression levels. Data are presented as the mean ± SE. n=5.
**P<0.01, ***P<0.001 vs. control; #P<0.05 m
##P<0.01, ###P<0.001 vs. hypoxia. p62,
sequestosome 1; Keap1, Kelch-like ECH-associated protein 1; cont,
control; ET/ET-1, endothelin-1; hy, hypoxia; EX, EX527; t, total;
p, phosphorylated. |
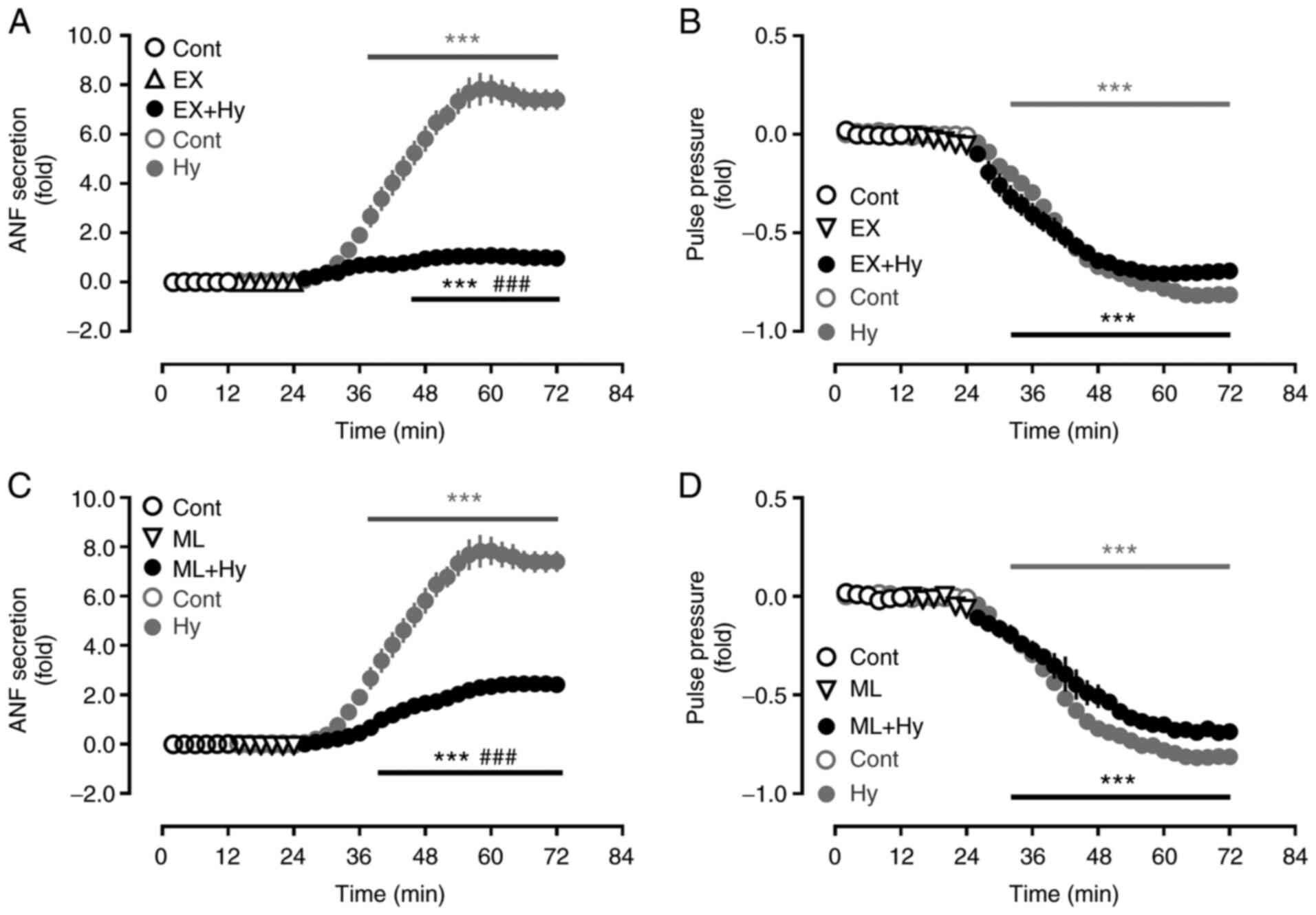
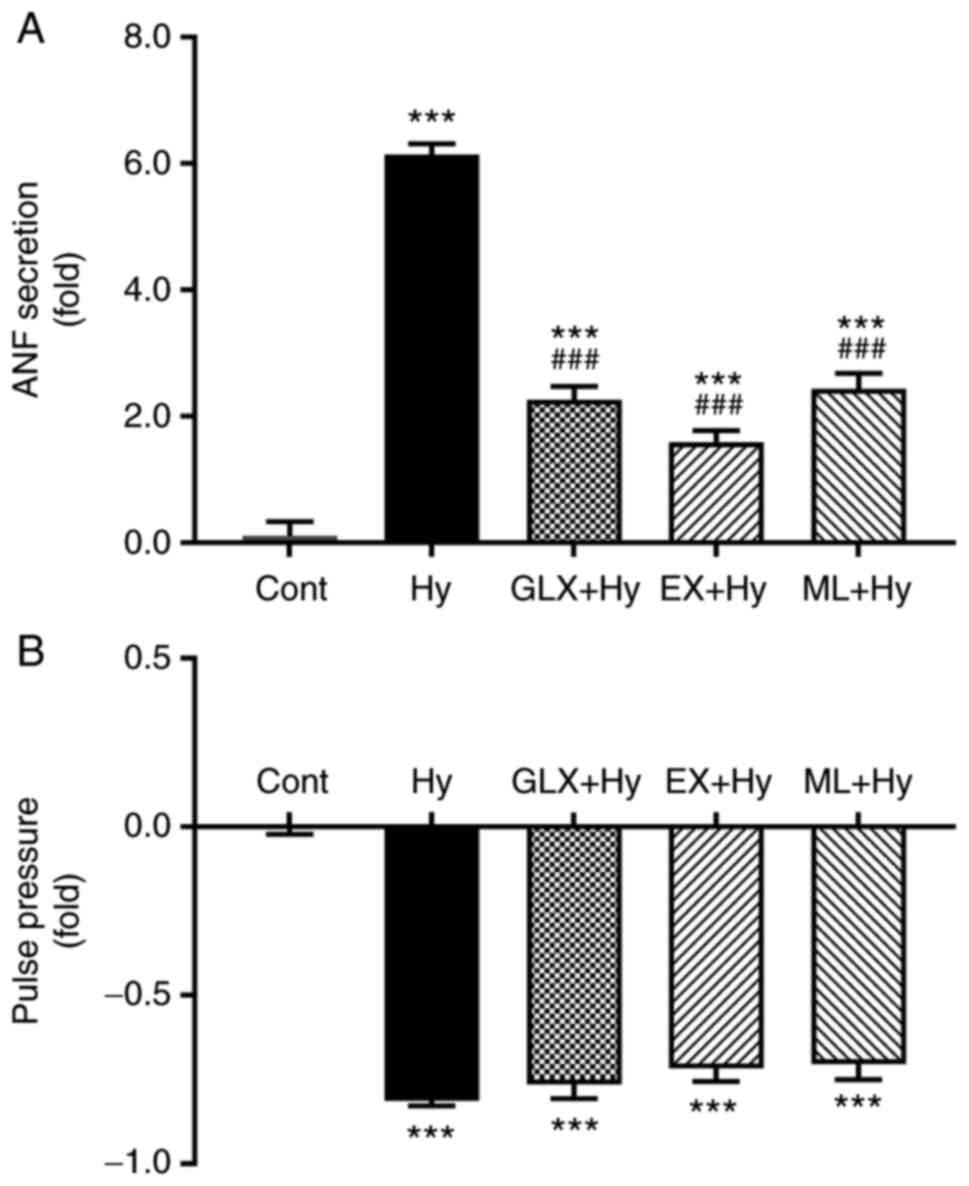
Effect of Sirt1 and Nrf2 inhibition on
ANF secretion during hypoxia
To determine the effect of Sirt1 and Nrf2 on ANF
secretion in beating hypoxic atria, inhibitors of Sirt1 and Nrf2
were used. Hypoxia significantly stimulated the atrial secretion of
ANF (Fig. 3A and C) and also
significantly suppressed pulse pressure, compared with the control
group (Fig. 3B and D). The
hypoxia-induced increase in ANF secretion was significantly
attenuated by the Sirt1 and Nrf2 inhibitors, EX527 and ML385,
respectively, compared with the hypoxia group. The atrial pulse
pressure induced by hypoxia was not significantly affected by EX527
and ML385. These data suggested that Sirt1 and Nrf2, controlled by
NOX4, may be involved in the regulation of ANF secretion during
hypoxia.
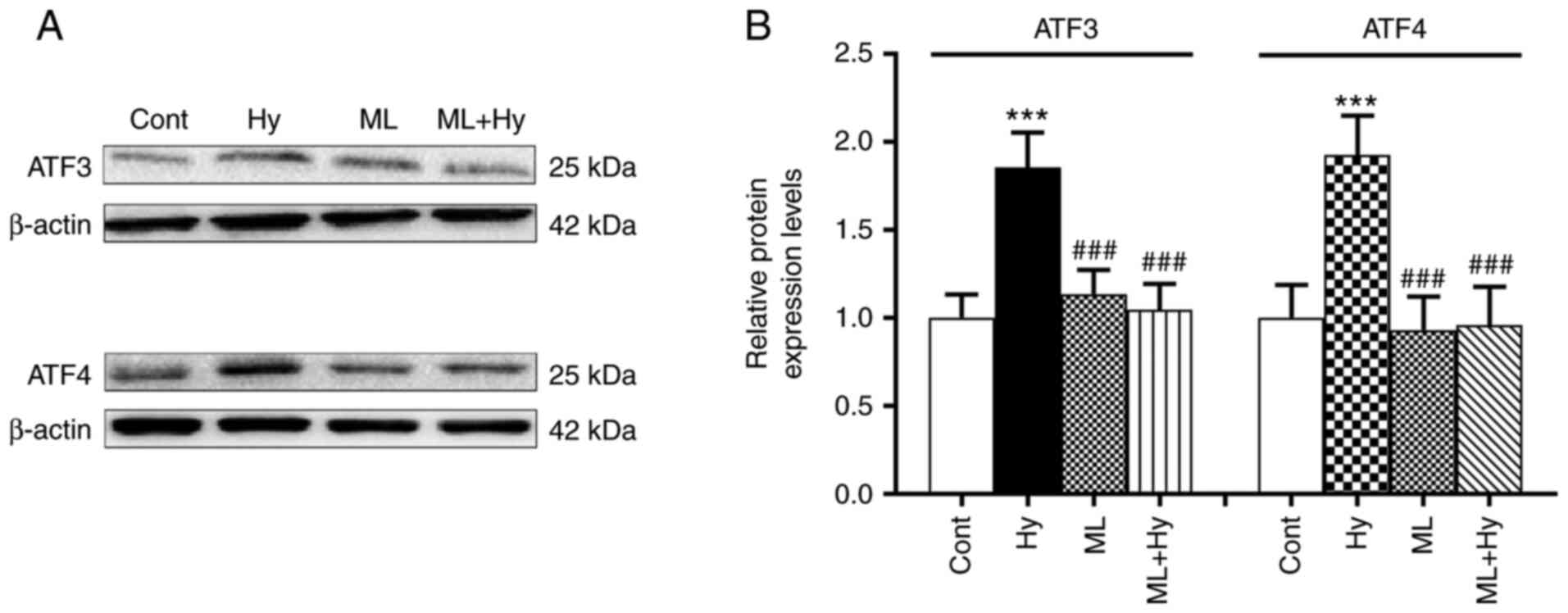
Effect of hypoxia on the regulation of
ATF3 and ATF4 protein expression levels
Due to the role of Nrf2 in the regulation of ATF
activity (18), the present study
further investigated the effects of hypoxia on ATF3 and ATF4
expression levels. The results demonstrated that hypoxia
significantly upregulated both ATF3 and ATF4 protein expression
levels, compared with the control (Fig. 4). In the presence of the Nrf2
inhibitor, ML385, the hypoxia-induced upregulation of ATF3 and ATF4
protein expression was not observed. The basal levels of ATF3 and
ATF4 protein expression levels were not affected by ML385 alone.
These data indicated that the activities of ATF3 and ATF4 may be
controlled by Nrf2 during hypoxia.
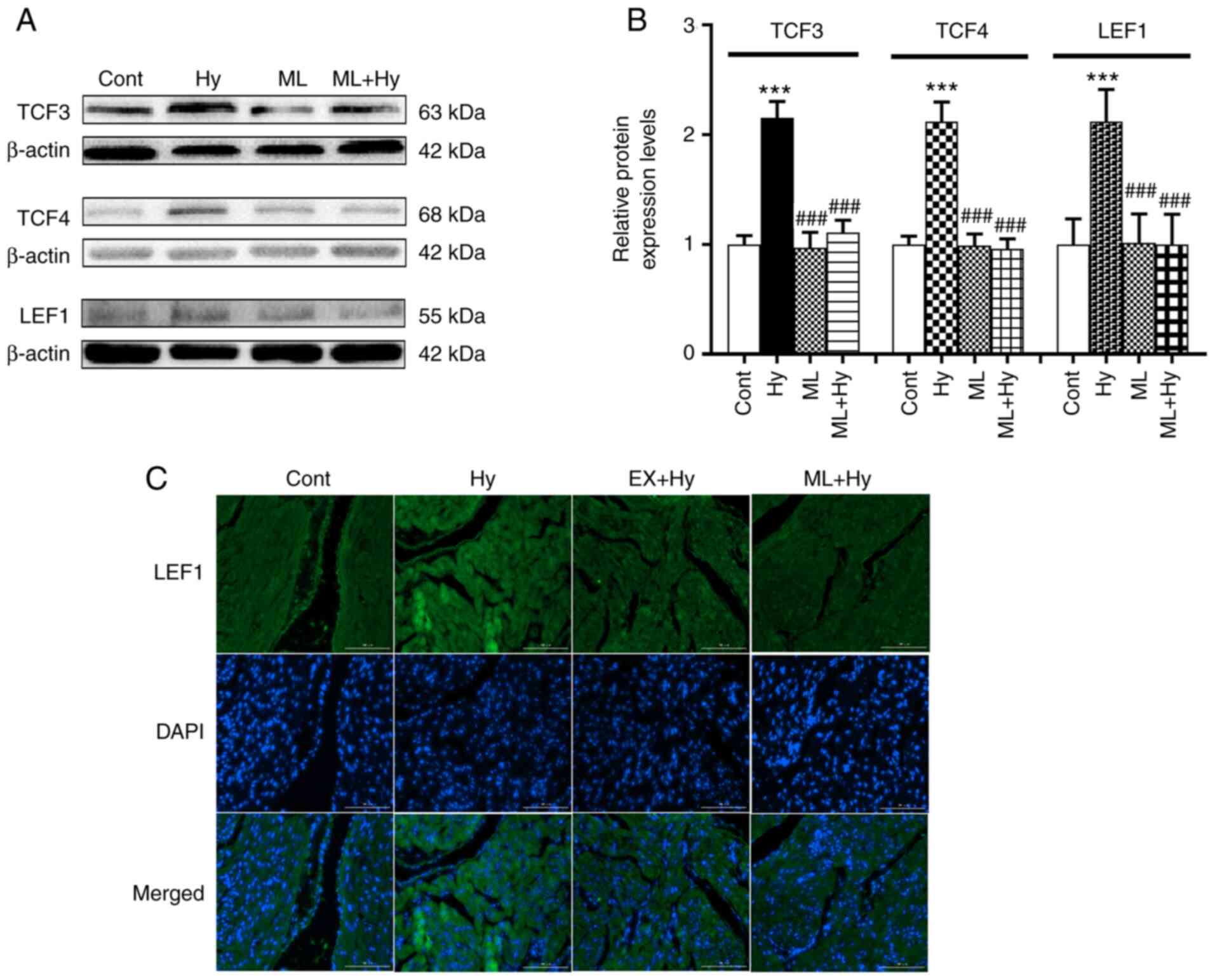
Effect of hypoxia on TCF3, TCF4 and
LEF1 protein expression levels
A previous study reported the influence of ATF on
TCF/LEF1, as well as the role of LEF1 in regulating ANF promoter
activity (19). Therefore, the
effects of hypoxia on TCF3, TCF4 and LEF1 protein expression levels
were examined. The results demonstrated that hypoxia significantly
elevated TCF3, TCF4 and LEF1 protein expression levels compared
with the control (Fig. 5). ML385
significantly reduced the effect of hypoxia on these three
proteins, compared with the hypoxia group. The results also
demonstrated that ML385 also significantly attenuated the
hypoxia-induced increase in ANF secretion, compared with the
hypoxia group (Fig. 6A). The
hypoxia-induced inhibition of atrial pulse pressure was not
significantly changed by ML385 treatment (Fig. 6B). These results indicated that
Nrf2 may be involved in regulating ANF secretion via the activation
of TCF/LEF1 signaling.
Discussion
In the present study, hypoxic conditions resulted in
the significant upregulation of Sirt1 and Nrf2 protein expression
levels, along with significantly increased ANF secretion, in
isolated beating rat atria. The hypoxia-induced expression of Sirt1
was blocked by an inhibitor of NOX4 and Nrf2 expression was
abolished by the Sirt1 inhibitor. Hypoxia also significantly
elevated the protein expression levels of p-AKT and p62, whereas
Keap1 protein expression levels were significantly downregulated.
Moreover, these effects were blocked by treatment with a Sirt1
inhibitor, which prevented hypoxia-induced Nrf2 protein expression.
Furthermore, the Nrf2 inhibitor abolished the hypoxia-induced
upregulation of ATF3, ATF4 and TCF3, as well as TCF4/LEF1 protein
expression levels, accompanied by the significant attenuation of
hypoxia-induced ANF secretion. These results suggested that NOX4
possibly regulated Sirt1 and its downstream protein Nrf2, which
stimulated TCF3 and TCF4/LEF1 signaling by activating ATF3 and
ATF4, thereby participating in the regulation of ANF secretion
during hypoxia (Fig. 7).
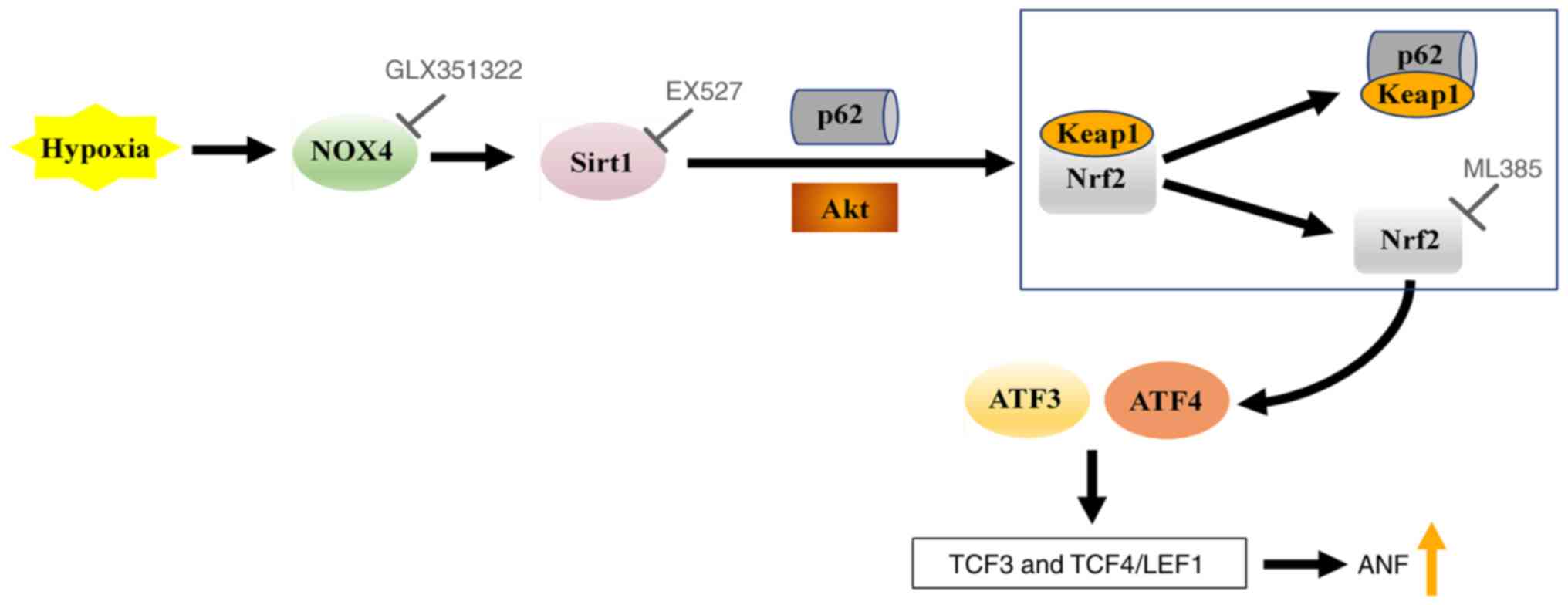 | Figure 7.Schematic of the NOX4/Sirt1/Nrf2
signaling pathway, which regulates ANF secretion during hypoxia.
NOX4, NADPH oxidase 4; Sirt1, silent information regulator 1; Nrf2,
nuclear factor erythroid-2-related factor 2; p62, sequestosome 1;
Keap1, Kelch-like ECH-associated protein 1; ATF, activating
transcription factor; TCF, T-cell factor; LEF1, lymphoid enhancer
factor 1; ANF, atrial natriuretic factor. |
Sirt1 has been reported to be localized to both the
cytoplasm and nucleus under basal conditions and is shuttled to the
nucleus in response to certain stressors in the heart (5). Acute ischemic preconditioning,
pressure overload, nutrient starvation and exercise, can also
upregulate Sirt1 expression in the heart (12,13). Furthermore, Sirt1 prevents
apoptosis of cardiomyocytes and protects the heart from
ischemia/reperfusion-induced damage (20,21). Sirt1 acts as an upstream signaling
molecule for the transcription factor Nrf2, which serves a role in
resisting ischemia/reperfusion injury via activation of Nrf2 and
its downstream signaling pathway (22,23). The results of the present study
demonstrated that hypoxia can significantly increase ANF secretion
and significantly inhibit atrial pulse pressure, as well as
significantly upregulate Sirt1 and Nrf2 protein expression levels.
This upregulation was significantly blocked by treatment with a
NOX4 inhibitor. Adding a Sirt1 antagonist also abolished Nrf2
protein expression levels during hypoxic conditions without
significant changes to atrial pulse pressure. Moreover, the
significantly upregulated levels of p-Akt and p62, as well as the
significantly downregulated levels of Keap1, induced by exogenous
ET-1 under normoxic or hypoxic conditions were also significantly
abolished using the Sirt1 antagonist. These results indicated that
Sirt1 possibly downregulated Keap1 by activating Akt and p62,
thereby upregulating Nrf2 protein expression. The data in the
present study are consistent with the aforementioned cited studies
and support the notion that p62 aggregation leads to the
recruitment of Keap1 for the degradation and release of Nrf2 into
the nucleus (24).
ATF3 and ATF4, members of the transcription factor
ATF/cyclic AMP-reactive element binding protein family, are
expressed in the cardiovascular system in response to a variety of
stimuli, including pathological stressors, such as pressure
overload, stretch, ischemia/reperfusion, ischemic preconditioning,
hypoxia, ET-1 and H2O2 (25,26). Previously, it has been confirmed
that Nrf2 can resist oxidative stress and protects the heart via
activation of ATF (18).
Moreover, members of the ATF family participate in the regulation
of the transcriptional processes via activation of the
Wnt/β-catenin signaling pathway downstream effectors TCF and LEF
(27). The roles of TCF and LEF
in the regulation of ANF promoter activity and transcription were
also demonstrated in rat cultured cardiomyocytes (19). In the present study, the results
demonstrated that hypoxia not only significantly upregulated the
protein expression levels of ATF3 and ATF4, but also significantly
increased the protein expression levels of TCF3, TCF4 and LEF1,
which may lead to the promotion of ANF secretion. The
hypoxia-induced increases in ATF3, ATF4, TCF3, TCF4 and LEF1 were
significantly abolished by adding a specific antagonist of Nrf2,
accompanied by a significant attenuation of hypoxia-augmented
secretion of ANF. These results demonstrated that Nrf2, controlled
by Sirt1, may stimulate TCF3 and TCF4/LEF1 via activation of ATF3
and ATF4, leading to promoted ANF secretion in beating rat atria
during hypoxia. The results of the present study support the
previously aforementioned studies. In our previous study, we
demonstrated that endogenous ET-1 participates in the regulation of
ANF secretion by regulating the NOX4/proto-oncogene
tyrosine-protein kinase Src signaling pathway in rat beating atria
during hypoxia (14). Therefore,
it can be hypothesized that the regulation of hypoxia-induced ANF
secretion by NOX4/Sirt1/Nrf2 in the present study is another
important regulatory mechanism by which endogenous ET-1 regulates
hypoxia-induced ANF secretion via NOX4.
In summary, it was demonstrated that hypoxia
significantly upregulated the expression levels of Sirt1 and its
downstream molecule Nrf2 via NOX4, which may have activated TCF3
and TCF4/LEF1 via ATF3 and ATF4 signaling. This cascade ultimately
resulted in increased ANF secretion. Therefore, the present study
indicated that the Sirt1/Nrf2/ATF axis is potentially involved in a
cardioprotective mechanism against oxidative stress damage during
hypoxia. A limitation of the present study is the lack of
inhibitors for ATF and TCF/LEF1, it is therefore necessary to
further verify the results of the study by knockout or knockdown of
these factors in future work. Intervening with the Sirt1/Nrf2/ATF
signaling pathway may be useful for the prevention of hypoxic heart
disease.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Natural Science
Foundation of China (grant nos. 81960099 and 81660089).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding authors on reasonable
request.
Authors' contributions
ZYL and YL performed the atrial perfusion
experiments and ANP measurements. YYW and XL performed the western
blotting analysis. ZNH and LH performed the statistical analysis of
the experimental data. XC and YSL designed the experiments and
wrote the manuscript. XC and YSL confirm the authenticity of all
the raw data. All authors read and agreed to the final
manuscript.
Ethics approval and consent to
participate
The animal procedures used in the present study were
approved by the Animal Care and Use Committee of Yanbian University
[approval no. SCXK (Ji) 2011–006] and were in accordance with the
Guide for the Care and Use of Laboratory Animals published by the
National Institutes of Health (16).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kumari S, Chaurasia SN, Nayak MK, Mallick
RL and Dash D: Sirtuin inhibition induces apoptosis-like changes in
platelets and thrombocytopenia. J Biol Chem. 290:12290–12299. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Boutant M and Canto C: SIRT1 metabolic
actions: Integrating recent advances from mouse models. Mol Metab.
3:5–18. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tsai KL, Cheng YY, Leu HB, Lee YY, Chen
TJ, Liu DH and Kao CL: Investigating the role of Sirt1-modulated
oxidative stress in relation to benign paroxysmal positional
vertigo and Parkinson's disease. Neurobiol Aging. 36:2607–2616.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Poulose N and Raju R: Sirtuin regulation
in aging and injury. Biochim Biophys Acta. 1852:2442–2455. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tanno M, Sakamoto J, Miura T, Shimamoto K
and Horio Y: Nucleocytoplasmic shuttling of the NAD+-dependent
histone deacetylase SIRT1. J Biol Chem. 282:6823–6832. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Moynihan KA, Grimm AA, Plueger MM,
Bernal-Mizrachi E, Ford E, Cras-Méneur C, Permutt MA and Imai SI:
Increased dosage of mammalian Sir2 in pancreatic beta cells
enhances glucose-stimulated insulin secretion in mice. Cell Metab.
2:105–117. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
D'Onofrio N, Servillo L and Balestrieri
ML: SIRT1 and SIRT6 signaling pathways in cardiovascular disease
protection. Antioxid Redox Signal. 28:711–732. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Meng X, Tan J, Li M, Song S, Miao Y and
Zhang Q: Sirt1: Role under the condition of ischemia/hypoxia. Cell
Mol Neurobiol. 37:17–28. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gu XS, Wang ZB, Ye Z, Lei JP, Li L, Su DF
and Zheng X: Resveratrol, an activator of SIRT1, upregulates AMPK
and improves cardiac function in heart failure. Genet Mol Res.
13:323–335. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cattelan A, Ceolotto G, Bova S, Albiero M,
Kuppusamy M, De Martin S, Semplicini A, Fadini GP, de Kreutzenberg
SV and Avogaro A: NAD(+)-dependent SIRT1 deactivation has a key
role on ischemia-reperfusion-induced apoptosis. Vascul Pharmacol.
70:35–44. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shalwala M, Zhu SG, Das A, Salloum FN, Xi
L and Kukreja RC: Sirtuin 1 (SIRT1) activation mediates sildenafil
induced delayed cardioprotection against ischemia-reperfusion
injury in mice. PLoS One. 9:e869772014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Alcendor RR, Gao S, Zhai P, Zablocki D,
Holle E, Yu X, Tian B, Wagner T, Vatner SF and Sadoshima J: Sirt1
regulates aging and resistance to oxidative stress in the heart.
Circ Res. 100:1512–1521. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hsu CP, Zhai P, Yamamoto T, Maejima Y,
Matsushima S, Hariharan N, Shao D, Takagi H, Oka S and Sadoshima J:
Silent information regulator 1 protects the heart from
ischemia/reperfusion. Circulation. 122:2170–2182. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu CZ, Li X, Hong L, Han ZN, Liu Y, Wei CX
and Cui X: NOX4/Src regulates ANP secretion through activating
ERK1/2 and Akt/GATA4 signaling in beating rat hypoxic atria. Korean
J Physiol Pharmacol. 25:159–166. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim HY, Cho KW, Xu DY, Kang DG and Lee HS:
Endogenous ACh tonically stimulates ANP secretion in rat atria. Am
J Physiol Heart Circ Physiol. 305:H1050–H1056. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals, . Guide for the Care and Use of Laboratory Animals. 8th
edition. National Academies Press; Washington, DC: 2011
|
|
17
|
Li X, Han ZN, Liu Y, Hong L, Cui BR and
Cui X: Endogenous ET-1 promotes ANP secretion through activation of
COX2-L-PGDS-PPARγ signaling in hypoxic beating rat atria. Peptides.
122:1701502019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen QM and Maltagliati AJ: Nrf2 at the
heart of oxidative stress and cardiac protection. Physiol Genomics.
50:77–97. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang CG, Jia ZQ, Li BH, Zhang H, Liu YN,
Chen P, Ma KT and Zhou CY: beta-Catenin/TCF/LEF1 can directly
regulate phenylephrine-induced cell hypertrophy and Anf
transcription in cardiomyocytes. Biochem Biophys Res Commun.
390:258–262. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu Y, Zhou A, Zhao S, Huber WE and Li Q:
Quadruple atrioventricular nodal pathways: involved in orthodromic
atrioventricular reentrant tachycardia. Tex Heart Inst J.
37:706–709. 2010.PubMed/NCBI
|
|
21
|
Huang L, He H, Liu Z, Liu D, Yin D and He
M: Protective effects of isorhamnetin on cardiomyocytes against
anoxia/reoxygenation-induced injury is mediated by SIRT1. J
Cardiovasc Pharmacol. 67:526–537. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yu L, Li S, Tang X, Li Z, Zhang J, Xue X,
Han J, Liu Y, Zhang Y, Zhang Y, et al: Diallyl trisulfide
ameliorates myocardial ischemia-reperfusion injury by reducing
oxidative stress and endoplasmic reticulum stress-mediated
apoptosis in type 1 diabetic rats: Role of SIRT1 activation.
Apoptosis. 22:942–954. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen X, Yan L, Guo Z, Chen Z, Chen Y, Li
M, Huang C, Zhang X and Chen L: Adipose-derived mesenchymal stem
cells promote the survival of fat grafts via crosstalk between the
Nrf2 and TLR4 pathways. Cell Death Dis. 7:e23692016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Deng S, Essandoh K, Wang X, Li Y, Huang W,
Chen J, Peng J, Jiang DS, Mu X, Wang C, et al: Tsg101 positively
regulates P62-Keap1-Nrf2 pathway to protect hearts against
oxidative damage. Redox Biol. 32:1014532020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhou H, Li N, Yuan Y, Jin YG, Guo H, Deng
W and Tang QZ: Activating transcription factor 3 in cardiovascular
diseases: A potential therapeutic target. Basic Res Cardiol.
113:372018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ameri K and Harris AL: Activating
transcription factor 4. Int J Biochem Cell Biol. 40:14–21. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Grumolato L, Liu G, Haremaki T, Mungamuri
SK, Mong P, Akiri G, Lopez-Bergami P, Arita A, Anouar Y, Mlodzik M,
et al: β-Catenin-independent activation of TCF1/LEF1 in human
hematopoietic tumor cells through interaction with ATF2
transcription factors. PLoS Genet. 9:e10036032013. View Article : Google Scholar : PubMed/NCBI
|