Introduction
The ends of chromosomes, termed telomeres, consist
of 5–15 kb double-stranded (ds) telomeric repeats 5′-TTAGGG-3′,
which result in a single-stranded (ss) 3′ G-overhang. G-overhangs
can form a G-quadruplex structure, which is crucial for telomeric
stability (1). Telomeres are
important for ensuring chromosomal integrity and their importance
to cellular function is indicated by the evolutionary conservation
of their repetitive sequences. However, due to the inability of DNA
polymerase to replicate the end of the chromosome during lagging
strand synthesis, there is a gradual loss of telomeric repeats in
each cell division, with telomeres eventually becoming critically
short, leading to cell senescence. If cells fail to undergo
senescence, progressive telomere shortening can lead to the
formation of chromosome fusions, genomic instability and eventually
loss of cell viability and tumor development. This end replication
issue is overcome with the enzyme telomerase, which is expressed in
specific cell types (2).
Telomerase is a reverse transcriptase, responsible
for the replication of telomeres. Telomerase binds to the 3′
G-overhang, which functions as its substrate, to begin elongating
the telomeric sequence. When the first repeat is added, the 3′-end
is repositioned, and telomerase is able to add more repeats.
Telomerase consists of two subunits human (h)telomerase RNA (TR)
and human (h)telomerase reverse transcriptase (TERT). The TR
component contains the RNA template for telomeric amplification and
specialized structures for the enzymatic stability. The TERT
subunit, which is the catalytic one, consists of four functional
domains, the telomerase N-terminal domain, the TR-binding domain,
the reverse transcriptase domain and the C-terminal extension.
Telomeres are protected by the Shelterin complex
which consists of six proteins TTAGGG repeat binding factor (TRF)1,
TRF2, TPP1, RAP1, TIN2 and POT1, and binds to telomeres, playing a
critical role in telomere stability and telomerase activity. TRF1
and TRF2 form homodimers and have a low affinity for DNA, while
POT1 binds to ss telomeric DNA. TPP1 does not interact with DNA;
however, it contributes to telomerase recruitment to the tip of the
telomeres through interaction with hTERT and by base pairing
between the template region in the hTR and the G-overhang. In the
absence of certain Shelterin components, such as TRF1, TRF2 and
POT1, telomeres become unprotected and often become critically
short. This phenomenon triggers a DNA damage response [mainly
through the ataxia-telangiectasia mutated (ATM)/ataxia
telangiectasia and Rad3-related (ATR) kinases], in order to arrest
the cell cycle and repair/protect telomeres preventing telomere
fusions and breaks, a great source of genomic instability (3).
Telomerase has been a target for therapeutic
interventions for several decades, as its regulation is associated
with numerous diseases. More precisely, telomerase overexpression
is related to cancer development, whereas telomerase inhibition is
combined with age-related diseases (4–8).
As a consequence, telomerase is a potential target in various
diseases, and the investigation of telomerase modulators and
regulators is of critical importance. In particular, since
telomerase is not detected in the majority of normal tissues,
telomerase inhibitors enable a more specific treatment of cancer
compared with conventional chemotherapy drugs, which are not
selective, acting also on healthy cells (9). On the other hand, telomerase
activators can be potent drugs against age-related diseases
associated with telomere shortening. Several studies have
demonstrated that telomere shortening in humans is associated with
an increased mortality rate due to heart disease, stroke, or
infection, while individuals with chronic stress or infections have
accelerated telomere shortening compared to healthy individuals of
the same age (10).
The present systematic review summarizes all the
known synthetic or non-synthetic telomerase-specific regulators,
including genetic regulators, intracellular pathways controlling
telomerase activity and natural components, which affect telomerase
activity associated with cancer, chronic diseases and aging.
Data and methods
Aim and scope of the systematic
review
The main aim of the present systematic review was to
determine telomerase inhibitors and activators which impact aging
and cancer that are referred to in in vitro and in
vivo studies. Additionally, the present systematic review also
provides a description of the actions of these
inhibitors/activators and the type of substance (synthetic or
not).
Search strategy
The review protocol followed PRISMA 2020 (Preferred
Reporting Items for Systematic Reviews and Meta-Analyses)
guidelines (11). Studies were
identified through the PubMed and Scopus databases (between 2002 to
2021). The search strategy included the use of the following terms:
‘telomerase inhibitors in aging’, ‘telomerase inhibitors in
cancer’, ‘telomerase activators in aging’, ‘telomerase activators
in cancer’ ‘telomerase activators in cancer and in aging’ and
‘telomerase inhibitors in aging and in cancer’. Articles were
included if they met the following criteria: They referred to
telomerase modulators in aging and in cancer and were in
vitro and/or in vivo studies, while studies that did not
provide sufficient data or studies not written in the English
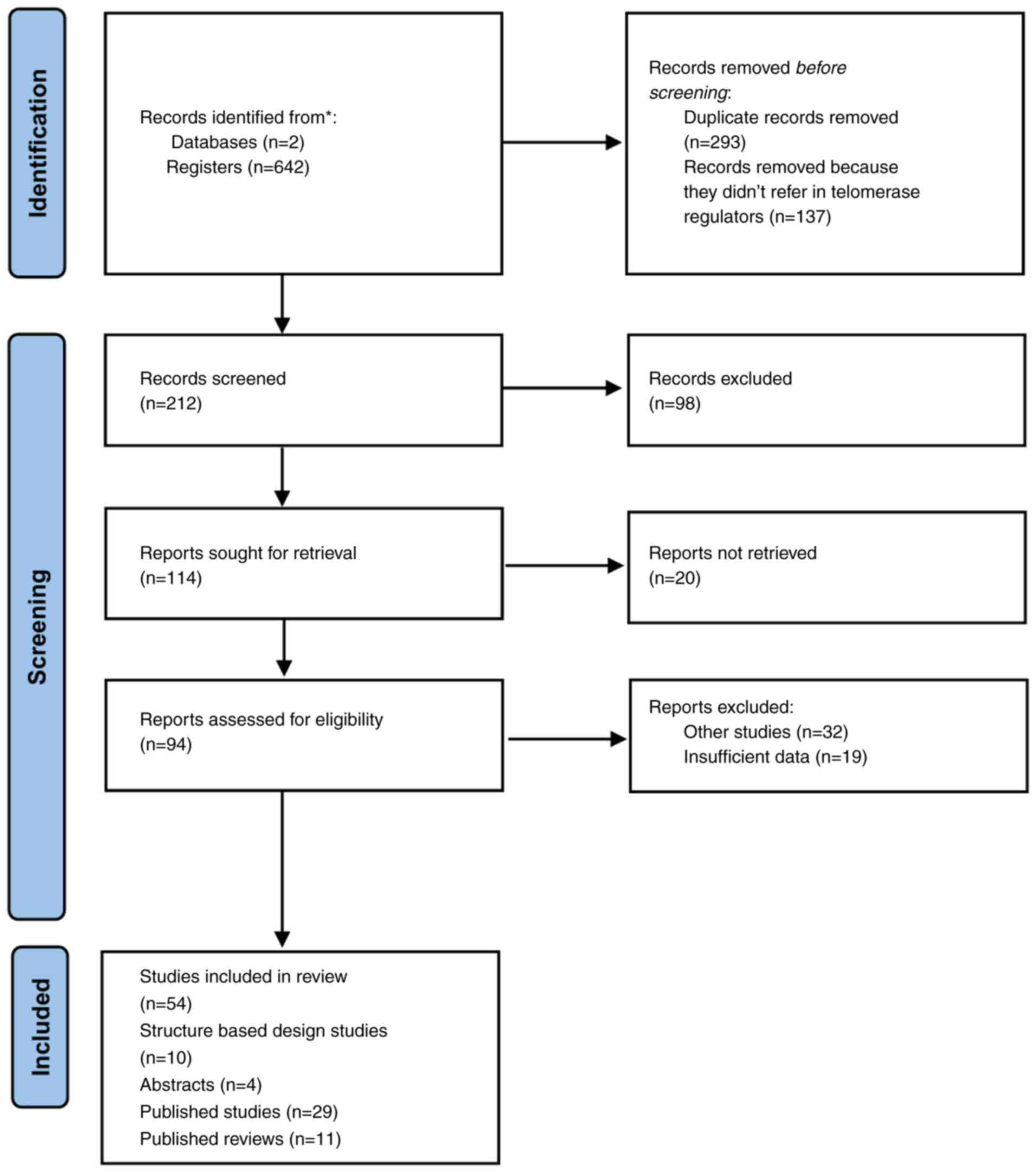
language were excluded. A total of 293 duplicate records were
removed prior to screening and 137 records were removed due to no
reference to telomerase regulators. In total, 212 records were
screened, 114 of which were sought for retrieval, while 94 reports
were assessed for eligibility. Finally, 54 studies were included in
the present systematic review, 10 of which are structure-based
design studies, 4 were abstracts, 29 were published studies and 11
were published reviews (Fig.
1).
Article analysis
According to the existing literature, there were 29
molecules, as reliable telomerase regulators that referred to aging
and cancer. Taking into consideration their origin (synthetic or
non-synthetic) and their mechanisms of action, these molecules were
segmented into synthetic direct inhibitors, synthetic indirect
inhibitors, synthetic activators, non-synthetic genetic inducers,
non-synthetic natural inhibitors, non-synthetic natural activators.
Telomerase inhibitors and telomerase activators are summarized in
Tables I and II, respectively.
 | Table I.Telomerase inhibitors. |
Table I.
Telomerase inhibitors.
| Authors/(Refs),
year | Molecule | Mechanism of
action |
|---|
| Wang and Yang
(13), 2021; Seimiya et al
(12), 2002 | MST-312 and
MST-199a | The precise
mechanism of telomerase inhibition by MST remains unclear |
| Altamura et
al (14), 2021; Pandya et
al (15), 2021; |
BIBR1532a | Inhibits the
formation of long reaction products, leading to an overall
reduction in the number of added TTAGGG repeats |
| Pascolo et
al (16), 2002 |
|
|
| Wang et al
(17), 2016 |
N-substituted-dihydropyrazole
derivativesa | These compounds
have an active site that binds to the hTERT subunit of telomerase
and inhibit telomerase activity |
| Nasiri et al
(19), 2013; Thelen et al
(18), 2004 |
Silibinina | Decreases
telomerase catalytic subunit mRNA; combined with curcuminoids,
downregulates hTERT gene expression in breast cancer cells |
| Taka et al
(41), 2014 | Curcuminoid
derivativesa | Play critical role
in the affinity between telomerase and telomeric DNA |
| Chen et al
(20), 2018 | Ethenesulfonyl
fluoride derivativesa | These compounds
exhibited potent inhibitory activities against telomerase though
interaction with hTERT |
| Man et al
(21), 2016 | Imidazole-4-one
derivativesa | Alkyl group and the
aliphatic substitutes with double bonds, are important moieties for
telomerase inhibition, while longer carbon chains demonstrated
inferior inhibition |
| Hu et al
(22), 2020 | DIZ-3a | Is bound in
G-quadruplex, inhibiting the ALT process as well as disrupting the
T-loop structure |
| Thompson et
al (23), 2018; Tremblay and
Mascarenhas (25), 2021; Burchett
et al (24), 2017 |
GRN163La | Binds directly to
the telomerase RNA component and inhibit telomerase activity |
| Ghareghomi et
al (26), 2021 | siRNAsa | A novel strategy
for silencing hTERT expression, following the RNAi method |
| Roe et al
(27), 2015 | Acridine
compoundsa | Bind to HSP90
chaperone, and form bifunctional acridine-HSP90 inhibitor ligands,
acting as telomerase inhibitors |
| Meng et al
(28), 2018 |
Coumarinsa | Acts as telomerase
inhibitor targeting c-myc promoter elements |
| Wei et al
(38), 2016 |
Oxoisoaporphineb | Binds to the
G-quadruplex and stabilizes the telomeric structure preventing
telomerase from replicating telomeres |
| Herz et al
(39), 2014 | MTBITC,
erucinb | The mechanism of
telomerase inhibition remains unclear |
| Adler et al
(40), 2011 | I3Cb | Inhibits telomerase
activity and hTERT mRNA expression in prostatic cell lines |
| Adler et al
(40), 2011 | DESb | DES combined with
I3C and increases the inhibitory effect on telomerase activity,
gene expression, and cell viability |
 | Table II.Telomerase activators. |
Table II.
Telomerase activators.
| Authors/(Refs),
year | Molecule | Activity |
|---|
| Yu et al
(42), 2018; Chu and Hickson
(44), 2009 | Cycloastragenol or
TA-65b | Upregulates the
telomerase expression through the ERK pathway; induces the
expression of JAK2; is a signal transducer and activator of STAT5b
and modulates the telomerase expression through the JAK/STAT
pathway |
| Kim et al
(30), 2018 | PROX1b | Novel
transcriptional activator of TERT with strong binding affinity for
the mutant hTERT promoter |
| Li et al
(31), 2017 | CDC5Lb | Novel hTERT
promoter-binding protein and its knockdown inhibited tumor growth
by down-regulating hTERT expression; it is also a transcriptional
activator of hTERT |
| Chen et al
(32), 2015 | SPT5b | Acts as a novel
tumor-specific hTERT promoter-binding protein and activator in
colon cancer cells |
| Qin et al
(33), 2015 | RFPL3 and
CBPb | RFPL3 binds to
hTERT promoter; CBP is a co-activator promoting interaction between
RFPL3 and hTERT promoter; RFPL3 act together with CBP to upregulate
hTERT through the CBP-induced acetylation of RFPL3 protein and
their co-anchoring at hTERT promoter region |
| Le Saux et
al (29), 2013 | GRN510a | Induces the
upregulation of TERT |
| Sanokawa-Akakura
et al (36), 2016 | Hydrogen
sulfideb | Maintains the
expression of hTERT in a NAMPT-and SIRT1-dependent manner, delaying
the onset of replicative senescence |
| Stefanou et
al (34), 2010 | Leptinb | Modulates hTERT
transcription by the binding of STAT3 and Myc/Max/Mad network
proteins on the hTERT promoter |
| Yu et al
(35), 2018 | ZEB1b | It binds to the
hTERT promoter and upregulates hTERT transcription by activating
the YAP co-activator |
| Tsoukalas et
al (48), 2019 | Centella
asiatica extract formulation (08AGTLF)b | Enhances telomerase
activity, the mechanism remains unclear |
| Akiyama et
al (37), 2011 | EPOb | Regulates
telomerase via 2 separate mechanisms: i) On the transcriptional
level, by regulating hTERT gene transcription through Janus
tyrosine kinase 2/STAT5/c-Myc; and ii) on the post transcriptional
level, by controlling hTERT protein phosphorylation by
phosphatidylinositol 3-kinase/AKT |
| Uchiumi et
al (45), 2011 |
Resveratrolb | Increases Werner's
syndrome gene promoter activity, and that expression of its gene
and protein is accompanied by up-regulation of telomerase in HeLa
S3 cells, without affecting cell viability |
Results
According to the PRISMA Statement Criteria 2020
(11), a systematic review was
performed, by reclaiming data from 54 studies as follows: A total
of 10 structure-based design studies, four abstracts, 29 published
studies and 11 published reviews. Telomerase regulators are
summarized into the following categories.
Synthetic direct telomerase
inhibitors
Several synthetic telomerase inhibitors have been
designed to downregulate its activity by directly binding to the
enzyme and they are used in various diseases, including cancer.
Specifically, 14 studies in the present systematic review referred
to synthetic direct telomerase inhibitors. MST-312
[N,N'-bis(2,3-dihydroxybenzoyl)-1,2-phenylenediamine,
dihydroxybenzoyl-1,3-phenylenediamine] and MST-199
(N-[2-(3,4-dihydroxyphenyl)-4-oxo-4H-chromen-3-yl]-3,4
dihydroxybenzamide) are synthesized on basis of epigallocatechin
gallate, a major tea catechin that has been found to decrease
telomerase activity. More specifically, the MST treatment of the
U937 cell line has been shown to result in cell growth inhibition
following a long-term period, which led to telomere shortening, as
indicated by Southern blot analysis. In addition, treatment with
MST-312 resulted in a high number of senescent cells, as measured
by senescence-associated β-galactosidase staining (12). Furthermore, a recently published
study indicated that MST-312 was a potent inhibitor of
hepatocellular carcinoma (HCC) cells that overexpressed stathmin 1,
an oncoprotein that promotes cancer cell migration and invasion
(13). However, the precise
mechanisms of telomerase inhibition by MST remain unclear (12).
BIBR1532 is a non-nucleosidic compound that is used
as a selective telomerase inhibitor. It directly targets telomerase
core components, inhibiting telomerase reconstitution from hTR and
recombinant hTERT, similarly with the native enzyme derived from
tumor cells. According to the analyzed inhibition profile, BIBR1532
is a mixed-type non-competitive inhibitor, since telomerase has
different, yet functionally interdependent, binding sites for
deoxyribonucleotides and BIBR1532. It inhibits the formation of
long reaction products, leading to an overall reduction in the
number of added TTAGGG repeats, as indicated by TRAP, telomerase
repeated amplification protocol (TRAP) assays and RNA affinity
chromatography. This suggests that BIBR1532 does not block the
basic catalytic steps involved in template copying, but
specifically impairs the elongation of the DNA substrate. A recent
in vitro study demonstrated that BIBR1532 functions
synergistically with anticancer treatment, such as chemotherapy or
radiotherapy, which amplifies its anticancer effect (14). Nevertheless, it is important to
state that the action of BIBR1532 is found to cause a severe toxic
effect in various cell types. There is also evidence to indicate
that in low, non-toxic doses, BIBR1532 is not effective against
telomerase activity (15).
However, a detailed understanding of the molecular basis of
BIBR1532 inhibition will require the crystal structure analysis of
the telomerase-inhibitor complex (16).
N-substituted-dihydropyrazole derivatives appear to
inhibit telomerase activity and exert a potent antitumor effect
against four types of cancer, namely gastric, breast and prostate
cancer, and human hepatoma, while they exhibit good selectivity on
tumor cells over somatic cells. Docking analysis has revealed that
these compounds have an active site that binds to the hTERT subunit
of telomerase. More specifically, dihydropyazole derivatives form a
hydrogen bond with the lysine (Lys)710 residue of hTERT (17).
Silibinin, a polyphenolic flavonoid, is an
anticancer drug that appears to downregulate telomerase activity
and prostate-specific antigen (PSA) levels, together with the
co-activator of the androgen receptor prostate epithelium specific
Ets transcription factor. Silibinin has been shown to downregulate
PSA mRNA expression and PSA secretion in conditioned medium, as
shown by reverse transcription-quantitative PCR (RT-qPCR).
Moreover, following silibinin treatment, telomerase catalytic
subunit mRNA levels decreased significantly, while telomerase
activity was downregulated (18).
In addition, silibinin combined with curcuminoids, has been proven
to downregulate hTERT gene expression in breast cancer cells
(19). These results suggest the
possible therapeutic use of silibinin as an anti-proliferative
agent in intervention treatments for prostate cancer (18).
A structure-based design study of ethenesulfonyl
fluoride derivatives, including certain 2-(hetero)
arylethenesulfonyl fluoride and 1,3-dienylsulfonyl fluoride
derivatives, indicates another group of chemical compounds that may
be used as synthetic telomerase inhibitors and by extension in
anti-cancer therapy. Some of these compounds exhibit potent
inhibitory activities against telomerase though interaction with
hTERT, as indicated by the modified TRAP assay (20).
Imidazole-4-one derivatives are selected telomerase
inhibitors that have been shown to inhibit telomerase activity by
TRAP analysis. Moreover, an extensive structure-activity
association study demonstrated that the alkyl group and the
aliphatic substitutes with double bonds, are important moieties for
telomerase inhibition, while longer carbon chains demonstrated
inferior inhibition (21). More
specifically, dimeric imidazole is bound in the G-quadruplex,
inhibiting the alternative lengthening of telomeres process, as
well as a disruption in the T-loop structure (22).
Another potent direct synthetic inhibitor of
telomerase is imetelstat (GRN163L). Imetelstat is a 13-base
oligonucleotide (5′-TAGGGTTAGACAA-3′), that binds directly to the
telomerase RNA component. It is used as a treatment in numerous
diseases, such as myelofibrosis, thrombocytopenia and various types
of cancer. The treatment of a variety of cell lines with imetelstat
has revealed that it is able to control cell cycle progression
through the inhibition of telomerase activity. More specifically,
telomerase inhibition by imetelstat attenuates the removal of DNA
damage signals from telomeres in telomerase-positive cancer cells
and affects the G2/M progression of the cell cycle, a mechanism
that is common in response to telomere dysfunction (23). Another study analyzed the effects
of imetelstat on two pancreatic cancer cell lines, AsPC1 and
L3.6pl. In both cell lines, chronic exposure to imetelstat
initially led to telomere shortening followed by the stabilization
of critically short telomeres. In addition to the direct effects of
imetelstat on telomerase activity, its function when combined with
other drugs includes the further activation of intracellular
pathways (24). Currently,
imetelstat is being tested in a phase 3 clinical trial for
myelofibrosis (25). The
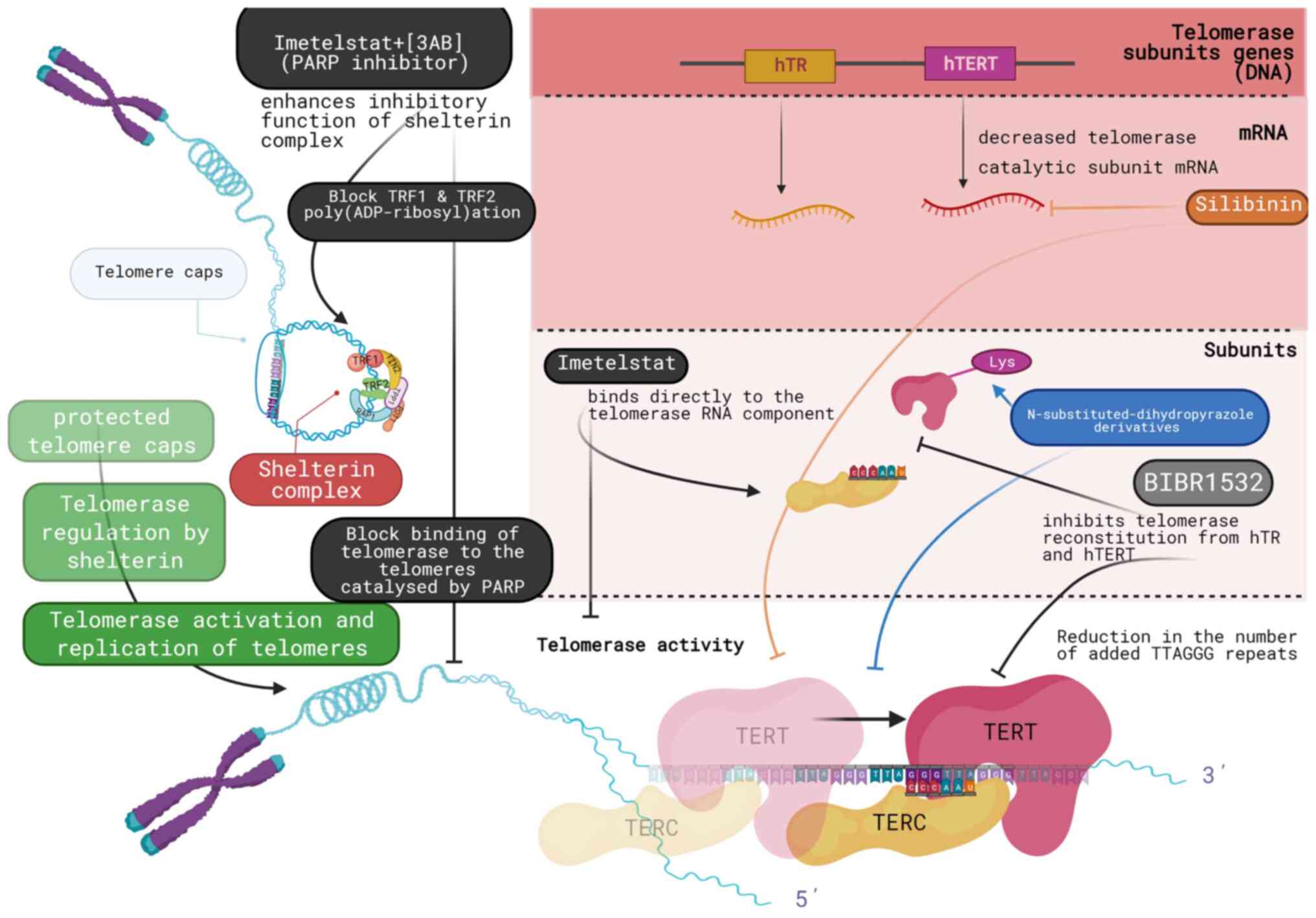
Shelterin complex, as aforementioned, is a telomere-associated
complex that limits the access of telomerase to telomeres. The
inhibitory function of this complex is enhanced by drugs that block
the poly-ADP-ribosylation of TRF1 and TRF2, components of the
Shelterin complex that are crucial for the binding of telomerase to
the telomeres. This process is catalyzed by poly(ADP-ribose)
polymerase (PARP) family enzymes, a group of enzymes involved in
DNA repair and more specifically, in the ss break repair mechanism.
The combined treatment of the GRN163L-resistant L3.6pl cells with
imetelstat and 3-aminobenzamide (3AB), a general PARP inhibitor,
has been shown to lead to additional telomere shortening and to
limit the lifespan of the resistant cells (24) (Fig.
2).
Finally, small interfering RNAs (siRNAs) are a novel
strategy for silencing the hTERT expression, following the RNA
interference method. More specifically, Ghareghomi et al
indicated that siRNAs in combination with magnetic nanoparticles
may be an effective treatment for ovarian cancer (26).
Synthetic indirect telomerase
inhibitors
There are also additional synthetic compounds that
act indirectly on telomerase activity by activating intracellular
pathways. Acridine compounds, such as geldanamycin, GA alkyn and
bis-amido chloroacridine do not bind directly to telomerase;
however, they bind to heat shock protein (HSP)90 chaperone and form
bifunctional acridine-HSP90 inhibitor ligands, further functioning
as telomerase inhibitors. Acridine compounds can be used as potent
anticancer drugs, since HSP90 is required for telomerase activity
and as a consequence, the binding of acridine to HSP90 leads to the
inhibition of telomerase activity and the downregulation of
telomerase expression (27).
Other synthetic telomerase inhibitors are eight
platinum complexes with substituted 3-(2′-benzimidazolyl)
coumarins, which have been found to exhibit high cytotoxic activity
in vitro against the cisplatin-resistant Sloan-Kettering
ovarian cancer cisplatin-resistant cells (SK-OV-3/DDP) cancer
cells, but low cytotoxicity one normal HL-7702 cells. Further
analyses revealed that three of the eight complexes induced the
apoptosis of SK-OV-3/DDP cancer cells via mitochondria dysfunction
signaling pathways, while one of them acted as telomerase inhibitor
targeting c-myc promoter elements. More specifically, it was
demonstrated using western blot analysis and RT-qPCR that platinum
complexes act on transcription and the expression of c-myc and
hTERT, by inhibiting the c-myc promoter, leading to the induction
of apoptosis via mitochondrial dysfunction. Therefore, platinum
complexes are used as potent drugs in various cancer treatments
(28).
Synthetic telomerase activators
The development and synthesis of telomerase
activators has gained a increasing attention due to the emergence
of age-related diseases or diseases associated with telomere
dysfunction and shortening, including AIDS, aplastic anemia and
pulmonary fibrosis. GRN510 is a novel small molecule activator,
that was tested as a potential drug to limit fibrosis induced by
bleomycin in mTERT heterozygous mice. Treatment with GRN510 was
shown to lead to a 2–4 fold increase in telomerase activity both
ex vivo and in vivo. GRN510 suppressed the
development of fibrosis and the accumulation of senescent cells in
the lungs via a mechanism dependent on telomerase activation,
indicating that small molecule activators of telomerase can be used
in therapies against idiopathic pulmonary fibrosis (29).
Non-synthetic-natural direct
telomerase activators
Various genetic products function as regulators of
telomerase on a transcriptional or posttranscriptional level and
can serve as potential therapeutic targets in cancer and various
diseases. In particular, somatic mutations in the hTERT promoter
are related to telomerase activation and frequently occur at two
hotspots in various types of cancer. Prospero homeobox protein 1
(PROX1) was identified as a novel transcriptional activator of TERT
with strong binding affinity for the mutant hTERT promoter. TERT
promoter mutations can enhance the promoter activity in HCC cells
expressing PROX1, while PROX1 has been found to be necessary for
the upregulation of TERT in Hep3B and HepG2 cells. These
observations suggest that PROX1 may serve as potential target in
therapy against HCC (30).
Another protein functioning as an activator of
telomerase by binding to hTERT promoter is the cell division cycle
5-like (CDC5L). CDC5L is a protein encoded by CDC5L gene, and is
highly expressed in certain types of cancer cells, particularly in
colorectal cancer. CDC5L was identified as a novel hTERT
promoter-binding protein and its knockdown has been found to
inhibit tumor growth by downregulating hTERT expression. CDC5L has
also been shown to be a transcriptional activator of hTERT in a
luciferase reporter assay. These findings indicate that CDC5L may
serve as a potential therapeutic target for human colorectal cancer
(31).
Furthermore, suppressor of Ty homolog-5 (SPT5) is
also a protein encoded by the suppressor of Ty 5 homolog
(SUPT5H) gene and acts as a novel tumor-specific hTERT
promoter-binding protein and activator in colon cancer cells. It
has a tumor-specific binding activity to hTERT promoter in
vivo and in vitro and it exhibits high expression levels
in colorectal cancer cell lines and primary human colorectal cancer
tissues. The inhibition of SUPT5H expression has been found
to significantly suppress telomerase activity and telomere
shortening, while it induces senescence in colon cancer cells,
suppressing cancel cell growth and migration. According to these
findings, since SPT5 contributes to the upregulation of hTERT,
leading to tumor growth, its gene SUPT5H may serve as a
potent therapeutic target and a novel tumor biomarker for colon
cancer (32).
In the category of telomerase inducers through hTERT
promoter regulation belong the ret finger protein like 3 (RFPL3)
and CREB binding protein (CBP) proteins, leptin and zinc finger
E-box binding homeobox 1 (ZEB1). RFPL3 and CBP co-localize in the
nucleus and appear to be overexpressed in human lung cancer cells.
They function synergistically to induce hTERT expression and
activity and tumor cell growth. More precisely, RFPL3 binds to the
hTERT promoter, whereas CBP is a co-activator, promoting the
interaction between RFPL3 and hTERT promoter. It has also been
found that RFPL3 act together with CBP to upregulate hTERT through
the CBP-induced acetylation of RFPL3 protein and their co-anchoring
at hTERT promoter region, suggesting that RFPL3/CBP/hTERT pathway
may be used as a novel target for lung cancer treatment (33).
Leptin is encoded by human obese gene and was found
to be associated with hTERT expression. It has been found to
modulate hTERT transcription by the binding of signal transducer
and activator of transcription (STAT)3 and Myc/Max/Mad network
proteins on the hTERT promoter and may be a potent regulator of the
malignancy of HCC (34).
Finally ZEB1 is a transcriptional regulator in
various types of cancer, as it binds to the hTERT promoter and
upregulates hTERT transcription by activating the YAP co-activator
(35).
Further endogenous proteins exert an effect on
telomerase activity not by directly binding to the hTERT promoter,
but via the activation of intracellular pathways that can also
serve as potential therapeutic targets. In a previous study,
hydrogen sulfide (H2S) was shown to exert anti-aging
effects and to protect against replicative senescence. In
particular, the expression of H2S and its producing
enzyme appeared to be diminished in adult human dermal fibroblasts
undergoing replicative senescence. This reduced production
coincided with the appearance of hallmarks of replicative
senescence, such as an enhanced expression of p16, p21 and
ribonucleotide-diphosphate reductase subunit M2 B. On the other
hand, a decrease was observed in the levels of hTERT, sirtuin 1
(SIRT1), nicotinamide phosphoribosyltransferase (NAMPT) and the
nicotinamide adenine dinucleotide (NAD)/NADH ratio, which was
rescued by the addition of exogenous H2S. H2S maintained expression
of hTERT in a NAMPT- and SIRT1-dependent manner, delaying the onset
of replicative senescence (36).
Erythropoietin (EPO) also exerts an effect on
telomerase activity. It has been found that in human
erythroleukemic JAS-REN-A cells, EPO regulates telomerase via two
separate mechanisms: i) On the transcriptional level, by regulating
hTERT gene transcription through Janus tyrosine kinase
2/STAT5/c-Myc; and ii) on the post-transcriptional level, by
controlling hTERT protein phosphorylation by phosphatidylinositol
3-kinase/AKT (37).
Non-synthetic-natural telomerase
inhibitors
Several natural compounds that function as
telomerase inhibitors are used as potent drugs in cancer therapy.
Oxoisoaporphine, an alkaloid isolated from Menispermum
dauricum, is known for its antitumor effects. Oxoisoaporphine
metal complexes bind strongly to the G-quadruplex and stabilize the
telomeric structure, preventing telomerase from replicating
telomeres. The binding properties of these two oxoisaporphine
compounds to telomerase were previously examined using molecular
docking and TRAP-silver staining assay (38).
Another small molecule that is used as an anticancer
drug, particularly in HCC, is 4-methylthiobutyl isothiocyanate
(MTBITC, erucin), which is a metabolite of sulforaphane, a small
molecule found in broccoli. Sulforaphane is known for its cancer
therapeutic potential in vitro and in vivo, and is
rapidly metabolized into MTBITC, which was previously identified as
potent selective inducer of apoptosis in HCC cells. MTBITC was
shown to be effective against telomerase and its function was
independent from TP53, which is also a suppressor of telomerase
(39).
Finally, another natural inhibitor of telomerase
used in therapy against prostate cancer is Indole-3-carbinol (I3C),
a phytochemical in cruciferous vegetables. A previous study
demonstrated that I3C significantly inhibited telomerase activity
and hTERT mRNA expression in prostate cancer cell lines. I3C
becomes more potent when combined with diethylstilbestrol (DES),
significantly enhancing the inhibitory effect on telomerase
activity, gene expression and cell viability. These results
suggested that I3C combined with DES may aid in the treatment of
prostate cancer (40).
Non-synthetic-natural indirect
telomerase activators
Several natural compounds acting as positive
telomerase regulators are also used in age-related diseases
associated with telomere dysfunction. Curcuminoids are natural
polyphenol compounds derived from turmeric that appear to enhance
telomerase activity. Although they are not considered to bind
directly to the telomerase enzyme, they play a critical role in the
affinity between telomerase and the telomeric DNA. A number of
curcuminoid derivatives have been found to enhance telomerase
activity in an in vitro TRAP assay and the minimal
requirement for this enhanced telomerase activity is a curcuminoid
core with at least one n-pentylpyridine side chain (41).
Another natural telomerase activator is
cycloastragenol (CAG, GRN665 or TA65), a triterpenoid saponin
compound and a hydrolytic product of the main active ingredient in
Astragalus membranaceus. CAG is involved in two cell
signaling pathways. One pathway includes the phosphorylation of
extracellular signal-regulated kinase (ERK), and upregulates the
telomerase expression through the ERK pathway, while the other
induces the expression of Janus kinase 2 (JAK2) and STAT5b, and
modulates telomerase expression through the JAK/STAT pathway
(42). CAG has a variety of
properties, such as in anti-hypoxic and anti-ischemic, anti-viral
and anti-fibrotic properties, while it also ameliorates metabolism
and wound healing. According to existing studies and clinical
trials, CAG is safe and effective in telomere maintenance and
lengthening, and has a broad range of applications (42). However, according to Akbarizare
et al (43), CAG affected
human dermal fibroblasts (HDFs) in a different manner. More
specifically in HDFs, CAG interferes with TERT mRNA splicing, and
inhibits telomerase activity. As a consequence, further studies are
required to fully understand its efficacy and ensure the proper
clinical use of CAG to treat diseases (42,43).
Finally, resveratrol is a natural polyphenol used in
the treatment of Werner's syndrome (WS). Patients with WS undergo
premature aging accompanied by chromosomal instability by mutations
in the WRN gene, which is involved in DNA repair and
telomere maintenance. It has been found that resveratrol increases
WRN promoter activity, and that the expression of its gene
and protein is accompanied by the upregulation of telomerase in
HeLa S3 cells, without affecting cell viability. Therefore, its
ability to maintain telomere length without affecting cell
proliferation renders it an important tool in the treatment of WS
(44,45).
In another study by Tsoukalas et al (46), an in vitro model was used
and it was revealed that Centella asiatica extract
formulation (08AGTLF) induced telomerase activation in peripheral
blood mononuclear cells from healthy donors. Previous research has
demonstrated that rats treated with Centella asiatica
exhibited an enhanced cognitive ability and a higher expression of
mitochondrial and antioxidant genes in the brain and liver, which
may contribute to cognitive improvement (46). Furthermore it has been
demonstrated that TERT expression in the brains of middle-aged rats
was restored following the administration of a Centella
asiatica containing supplement (Reverse™) for
3-month period (46). Behavioral
effects have also been reported in a complementary behavioral
study, where the administration of the same supplement
(Reverse™) improved locomotor activity and significantly
decreased stress in a dose-dependent manner in aged rats (47). However, future studies on humans
are warranted to examine its effect on telomere length, aging and
human health (48).
Discussion
Telomerase is the riboenzyme that contributes to the
preservation of chromosomal integrity and stability by elongating
the telomeric regions and eliminating the effects of DNA damage.
Telomeric activity contributes to a prolonged cell life and this
mechanism effectively serves the needs of malignant cells. Indeed,
telomerase is highly expressed in tumorous cells of all cancer
types, whereas it is normally absent in the vast majority of
somatic cells (with an exception of the embryonic and stem cells
that undergo fast differentiation and repetitive divisions)
(49). However, the inefficient
telomerase enzymatic performance has been linked to multiple
age-related diseases (10).
In a time that the clinical community needs novel
approaches for cancer treatment, telomerase appears to be a potent
therapeutic target and biomarker. The pre-existing in-depth
knowledge of the genetic and enzymatic background of telomerase is
an important advantage for its use, as it will be easier to predict
the possible pathways that need to be followed for the more
effective controlling of its actions. It has now been a decade
since scientists have been trying to take advantage of the
regulation of telomerase for the treatment of cancer and other
diseases (50).
There are three approaches to controlling
telomerase. One is by blocking the expression of the enzyme.
Telomerase consists of several different subunits, formed by the
translation of genes found in different chromosomal regions. A gene
therapy that includes regulators that target the desired loci can
successfully lead to the suppression of the enzyme. The second
effective method for telomerase inhibition depends upon the
principle of targeting the catalytic subunits of enzyme. As long as
the mechanisms of action and the intracellular pathways of this
enzyme are understood, there is ample information provided on which
of these steps could be blocked in order to prevent the catalytic
activity of telomerase. The third alternative of telomerase
inhibition is immunotherapy. Clinical cancer treatments have
already been applied that can trigger an immune response in
patients with h-TERT epitope injections (51). Over 25 known telomerase peptides
induce an immune specific response process through human leukocyte
antigens, which are finally destroyed. GV1001 and GRNVAC1 are two
of the already released vaccines that improved the response of
cancer patients and contributed to a prolonged survival rate
(21).
It has been revealed that telomerase is active in
85–95% of cancers. TERT expression and telomerase activity in
tumors are affected by TERT promoter mutations and the highest TERT
promoter mutation rates occur in glioblastoma, melanoma, bladder
urothelial carcinoma and lower-grade glioma (52). An intermediate mutation frequency
range is observed in HCC and thyroid carcinoma. However, the lowest
level of mutation frequency is detected in kidney, lung, prostate
and gastrointestinal cancers, and in leukemia (52,53). In another study, it was found that
the highest telomerase activity occurred ovarian, adrenal cortical,
esophageal and lung cancer (54).
In addition, it has been found that hTERT expression is increased
in the early stages of colorectal cancer (55).
As aforementioned, telomerase is poorly expressed in
somatic cells. This selective expression of the enzyme in
malignancies could allow for novel cancer treatments to be more
targeted into the desired tumors, while at the same time
eliminating the side-effects. Synthetic and natural inhibitors are
the molecules that play a significant role in telomerase
inhibition, either by directly blocking the enzymatic subunits
(such as MST-312, BIBR1532 etc.) or by blocking the formation of
the enzymatic entity through the inhibition of the translation
process (13,14). Synthetic drugs that bind to the
template region of telomerase, such as imetelstat, have already
begun to be tested in both in vivo and in vitro
experiments; of note, impressive results have been obtained in
several cancer types (melanoma, glioblastoma and pancreatic
carcinoma, etc.) (23,52). Furthermore, systematic therapy by
the intranasal administration of GRN163 and GRN163L was proven to
be effective in brain tissue cases. The drugs overpass the brain
blood barrier targeting only the cancer cells, with a low toxicity,
so that the normal brain cells remain intact (56).
Possibly, there may be other alternatives when
trying to affect the enzymatic activity of telomerase. For example,
reactive oxygen species (ROS) scavengers have been widely used for
years for attenuating the aging process. In HCC cells, the
estimated rates of ROS are higher compared to normal cells. At the
same time, ROS are involved in the Akt pathway, a regulatory
telomerase system. Thus, probably NAC particles (antioxidants that
directly affect ROS levels in the intracellular redox state) may
aid telomerase inhibition through cell apoptosis (53).
As regards synthetic and natural factors, even
everyday habits may have consequences on telomerase regulation.
Food ingredients that enter the body can significantly affect the
controlling process of telomerase. For example, catechin and
sulfoquinorosyldiacylglycerol are molecules that block the activity
of the enzyme, while retinoic acid and tocotrienol interfere with
the pathways of telomerase regulation by negatively affecting the
expression of the hTERT subunit. There are numerous xenobiotics and
bioactive compounds, such as endocrine disrupting chemicals, that
have been found to affect telomerase activity and have been linked
to multiple age-related diseases (57).
The specificity of a drug is probably one of the
most important qualifications that need to be considered when
trying to develop alternative therapies for cancer and other
diseases. From a clinical aspect, there are indications that
telomerase is an effective target that results in the improvement
of a number of human cancer types, while exerting minimal
side-effects (58,59). Apart from its advantageous
effects, there are some details that should not be neglected. It is
always important to examine the in vivo telomerase
enzymology in different cancer types. Clinicians should consider
each patient as a separate incident and decide for the most
appropriate treatment concerning the telomeric
inhibition/activation in order to reach the highest potential for
any disease.
Furthermore, there is always the possibility of
telomeric resistance to specific types of synthetic and natural
modulators (60). For example,
due to additional mutation patterns on the genetic loci of our
interest. This could be a parameter that enhances the stability of
the enzyme. Finally, telomerase treatment should be an
additional-if not a secondary-therapy for cancer and age-related
diseases (61) until it will
become efficiently reliable to replace traditionally applied
therapies (as chemotherapy, radiation, and immunotherapy).
The present systematic review provides data
regarding telomerase activators and inhibitors with effects on
aging and cancer. Telomerase is not a universal target for cancer
treatment and for aging prevention; however, targeting telomere
maintenance mechanisms is crucial for future research in aging and
cancer. Nevertheless, there are some limitations to the present
study which should be mentioned. The majority of the research has
been conducted using animal models; thus, there are no clinical
data available regarding the use of these activators and inhibitors
in human subjects. In addition, the present systematic review did
not assess the risk of bias; however, the authors aim to perform
such a risk analysis in the future. There is a need to search for
unpublished data and to provide data other than those published in
the Web of Science and PubMed databases. Data from clinical trial
registries and regulatory agency websites were not included herein;
thus, there was a lack of graphical and statistical analyses for
bias assessment, such as funnel plot and Egger's test, as well the
use the Cochrane collaboration risk of bias tool.
Future intervention studies on humans are warranted
to examine the effects of telomerase modulators on aging and cancer
since the treatment with telomerase activating procedures and an
extended cell lifespan may accumulate rare genetic and epigenetic
aberrations that may contribute to malignant transformation. To
this end, it a potential therapeutic approach has been designed in
which telomerase is induced temporarily and selectively in aged
cells without promoting cancer growth by using a recombinant
adeno-associated virus vector (62). In addition, the identification of
novel telomerase modulators is an important aspect for increasing
the tools that are currently available for telomerase approaches to
cancer therapeutics and to aging prevention. For the past two
decades, telomerase-based therapeutic cancer vaccines have been
under clinical investigation since telomerase has a universal
presence in cancer, and it is essential for tumor growth.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
All authors (PF, ER, KK, EK, EA, EV, CM, DAS and AT)
contributed to the conception and design of the study. PF and KK
searched the literature for inclusion in the study that this was
then examined and reviewed by EK, EA, EV and EV. PF, KK, EK, EA and
EV drafted and wrote the manuscript. AT, DAS and CM provided advice
on the experimental design, interpreted the results and critically
revised the manuscript. ER and EK designed the figures. KK and EV
designed the tables. PF and KK confirm the authenticity of all the
raw data. All authors have read and approved the final version of
the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
DAS is the Editor-in-Chief for the journal, but had
no personal involvement in the reviewing process, or any influence
in terms of adjudicating on the final decision, for this article.
The other authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ds
|
double-stranded
|
|
ss
|
single-stranded
|
|
(h)TR
|
(human) telomerase RNA
|
|
(h)TERT
|
(human) telomerase reverse
transcriptase
|
|
TRF
|
TTAGGG repeat binding factor
|
|
ATM kinase
|
ataxia-telangiectasia mutated
kinase
|
|
ATR kinase
|
ataxia telangiectasia and Rad3-related
kinase
|
|
TRAP
|
telomerase repeated amplification
protocol
|
|
Lys
|
lysine
|
|
PSA
|
prostate-specific antigen
|
|
PARP
|
poly(ADP-ribose) polymerase
|
|
HSP90
|
heat shock protein 90
|
|
PROX
|
prospero homeobox protein
|
|
HCC
|
hepatocellular carcinoma
|
|
CDC5L
|
cell division cycle 5-like
|
|
SK-OV-3/DDP
|
Sloan-Kettering ovarian cancer
cisplatin-resistant cells
|
|
SPT5
|
suppressor of Ty homolog-5
|
|
SUPT5H
|
suppressor of Ty 5 homolog
|
|
RFPL3
|
ret finger protein like 3
|
|
CBP
|
CREB binding protein
|
|
STAT
|
signal transducer and activator of
transcription
|
|
SIRT1
|
sirtuin 1
|
|
NAMPT
|
nicotinamide
phosphoribosyltransferase
|
|
EPO
|
erythropoietin
|
|
MTBITC
|
4-methylthio-3-butenyl
isothiocyanate
|
|
I3C
|
indole-3-carbinol
|
|
DES
|
diethylstilbestrol
|
|
CAG
|
cycloastragenol
|
|
ERK
|
extracellular signal-regulated
kinase
|
|
JAK2
|
Janus kinase 2
|
|
EPO
|
erythropoietin
|
References
|
1
|
Lu W, Zhang Y, Liu D, Songyang Z and Wan
M: Telomere-strucutre, function, and regulation. Exp Cell Res.
319:133–141. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Giardini MA, Segatto M, Da Silva MS, Nunes
VS and Cano MIN: Telomere and telomerase biology. Prog Mol Biol
Transl Sci. 125:1–40. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sandin S and Rhodes D: Telomerase
structure. Curr Opin Struct Biol. 25:104–110. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fragkiadaki P, Nikitovic D, Kalliantasi K,
Sarandi E, Thanasoula M, Stivaktakis PD, Nepka C, Spandidos DA,
Tosounidis T and Tsatsakis A: Telomere length and telomerase
activity in osteoporosis and osteoarthritis. Exp Ther Med.
19:1626–1632. 2020.PubMed/NCBI
|
|
5
|
Fragkiadaki P, Tsoukalas D, Fragkiadoulaki
I, Psycharakis C, Nikitovic D, Spandidos DA and Tsatsakis AM:
Telomerase activity in pregnancy complications (Review). Mol Med
Rep. 14:16–21. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Razgonova MP, Zakharenko AM, Golokhvast
KS, Thanasoula M, Sarandi E, Nikolouzakis K, Fragkiadaki P,
Tsoukalas D, Spandidos DA and Tsatsakis A: Telomerase and telomeres
in aging theory and chronographic aging theory (Review). Mol Med
Rep. 22:1679–1694. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vakonaki E, Tsiminikaki K, Plaitis S,
Fragkiadaki P, Tsoukalas D, Katsikantami I, Vaki G, Tzatzarakis MN,
Spandidos DA and Tsatsakis AM: Common mental disorders and
association with telomere length. Biomed Rep. 8:111–116.
2018.PubMed/NCBI
|
|
8
|
Vasilopoulos E, Fragkiadaki P, Kalliora C,
Fragou D, Docea AO, Vakonaki E, Tsoukalas D, Calina D, Buga AM,
Georgiadis G, et al: The association of female and male infertility
with telomere length (Review). Int J Mol Med. 44:375–389.
2019.PubMed/NCBI
|
|
9
|
Murofushi Y, Nagano S, Kamizono J,
Takahashi T, Fujiwara H, Komiya S, Matsuishi T and Kosai K: Cell
cycle-specific changes in hTERT promoter activity in normal and
cancerous cells in adenoviral gene therapy: A promising implication
of telomerase-dependent targeted cancer gene therapy. Int J Oncol.
29:681–688. 2006.PubMed/NCBI
|
|
10
|
Yeh JK, Lin MH and Wang CY: Telomeres as
therapeutic targets in heart disease. JACC Basic to Transl Sci.
4:855–865. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Page MJ, McKenzie JE, Bossuyt PM, Boutron
I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan
SE, et al: The PRISMA 2020 statement: An updated guideline for
reporting systematic reviews. BMJ. 372:n712021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Seimiya H, Oh-hara T, Suzuki T, Naasani I,
Shimazaki T, Tsuchiya K and Tsuruo T: Telomere shortening and
growth inhibition of human cancer cells by novel synthetic
telomerase inhibitors MST-312, MST-295, and MST-1991. Mol Cancer
Ther. 1:657–665. 2002.PubMed/NCBI
|
|
13
|
Wang SJ and Yang PM: A bioinformatics
analysis identifies the telomerase inhibitor MST-312 for treating
High-STMN1-expressing hepatocellular carcinoma. J Pers Med.
11:3322021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Altamura G, Degli Uberti B, Galiero G, De
Luca G, Power K, Licenziato L, Maiolino P and Borzacchiello G: The
small molecule BIBR1532 exerts potential anti-cancer activities in
preclinical models of feline oral squamous cell carcinoma through
inhibition of telomerase activity and down-regulation of TERT.
Front Vet Sci. 7:6207762021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pandya VA, Crerar H, Mitchell JS and
Patani R: A non-toxic concentration of telomerase inhibitor
BIBR1532 fails to reduce TERT expression in a feeder-free induced
pluripotent stem cell model of human motor neurogenesis. Int J Mol
Sci. 22:32562021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pascolo E, Wenz C, Lingner J, Hauel N,
Priepke H, Kauffmann I, Garin-Chesa P, Rettig WJ, Damm K and
Schnapp A: Mechanism of human telomerase inhibition by BIBR1532, a
synthetic, non-nucleosidic drug candidate. J Biol Chem.
277:15566–15572. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang Y, Cheng FX, Yuan XL, Tang WJ, Shi
JB, Liao CZ and Liu XH: Dihydropyrazole derivatives as telomerase
inhibitors: Structure-based design, synthesis, SAR and anticancer
evaluation in vitro and in vivo. Eur J Med Chem. 112:231–251. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Thelen P, Wuttke W, Jarry H, Grzmil M and
Ringert RH: Inhibition of telomerase activity and secretion of
prostate specific antigen by silibinin in prostate cancer cells. J
Urol. 171:1934–1938. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nasiri M, Zarghami N, Koshki KN,
Mollazadeh M, Moghaddam MP, Yamchi MR, Esfahlan RJ, Barkhordari A
and Alibakhshi A: Curcumin and silibinin inhibit telomerase
expression in T47D human breast cancer cells. Asian Pac J Cancer
Prev. 14:3449–3453. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen X, Zha GF, Wang JQ and Liu XH:
Ethenesulfonyl fluoride derivatives as telomerase inhibitors:
structure-based design, SAR, and anticancer evaluation in vitro. J
Enzyme Inhib Med Chem. 33:1266–1270. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Man RJ, Chen LW and Zhu HL: Telomerase
inhibitors: A patent review (2010–2015). Expert Opin Ther Pat.
26:679–688. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hu MH, Lin XT, Liu B and Tan JH: Dimeric
aryl-substituted imidazoles may inhibit ALT cancer by targeting the
multimeric G-quadruplex in telomere. Eur J Med Chem.
186:1118912020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Thompson CAH, Gu A, Yang SY, Mathew V,
Fleisig HB and Wong JMY: Transient telomerase inhibition with
imetelstat impacts DNA damage signals and cell-cycle kinetics. Mol
Cancer Res. 16:1215–1225. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Burchett KM, Etekpo A, Batra SK, Yan Y and
Ouellette MM: Inhibitors of telomerase and poly(ADP-ribose)
polymerases synergize to limit the lifespan of pancreatic cancer
cells. Oncotarget. 8:83754–83767. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tremblay D and Mascarenhas J: Next
generation therapeutics for the treatment of myelofibrosis. Cells.
10:10342021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ghareghomi S, Ahmadian S, Zarghami N and
Hemmati S: hTERT-molecular targeted therapy of ovarian cancer cells
via folate-functionalized PLGA nanoparticles co-loaded with
MNPs/siRNA/wortmannin. Life Sci. 277:1196212021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Roe S, Gunaratnam M, Spiteri C, Sharma P,
Alharthy RD, Neidle S and Moses JE: Synthesis and biological
evaluation of hybrid acridine-HSP90 ligand conjugates as telomerase
inhibitors. Org Biomol Chem. 13:8500–8504. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Meng T, Qin QP, Wang ZR, Peng LT, Zou HH,
Gan ZY, Tan MX, Wang K and Liang FP: Synthesis and biological
evaluation of substituted 3-(2′-benzimidazolyl)coumarin
platinum(II) complexes as new telomerase inhibitors. J Inorg
Biochem. 189:143–150. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Le Saux CJ, Davy P, Brampton C, Ahuja SS,
Fauce S, Shivshankar P, Nguyen H, Ramaseshan M, Tressler R, Pirot
Z, et al: A novel telomerase activator suppresses lung damage in a
murine model of idiopathic pulmonary fibrosis. PLoS One.
8:e584232013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim YJ, Yoo JE, Jeon Y, Chong JU, Choi GH,
Song DG, Jung SH, Oh BK and Park YN: Suppression of PROX1-mediated
TERT expression in hepatitis B viral hepatocellular carcinoma. Int
J Cancer. 143:3155–3168. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li J, Zhang N, Zhang R, Sun L, Yu W, Guo
W, Gao Y, Li M, Liu W, Liang P, et al: CDC5L promotes hTERT
expression and colorectal tumor growth. Cell Physiol Biochem.
41:2475–2488. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen R, Zhu J, Dong Y, He C and Hu X:
Suppressor of Ty homolog-5, a novel tumor-specific human telomerase
reverse transcriptase promoter-binding protein and activator in
colon cancer cells. Oncotarget. 6:32841–32855. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Qin Y, Chen W, Xiao Y, Yu W, Cai X, Dai M,
Xu T, Huang W, Guo W, Deng W and Wu T: RFPL3 and CBP
synergistically upregulate hTERT activity and promote lung cancer
growth. Oncotarget. 6:27130–27145. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Stefanou N, Papanikolaou V, Furukawa Y,
Nakamura Y and Tsezou A: Leptin as a critical regulator of
hepatocellular carcinoma development through modulation of human
telomerase reverse transcriptase. BMC Cancer. 10:4422010.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yu P, Shen X, Yang W, Zhang Y, Liu C and
Huang T: ZEB1 stimulates breast cancer growth by up-regulating
hTERT expression. Biochem Biophys Res Commun. 495:2505–2511. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sanokawa-Akakura R, Akakura S and
Tabibzadeh S: Replicative senescence in human fibroblasts is
delayed by hydrogen sulfide in a NAMPT/SIRT1 dependent manner. PLoS
One. 11:e01647102016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Akiyama M, Kawano T, Mikami-Terao Y,
Agawa-Ohta M, Yamada O, Ida H and Yamada H: Erythropoietin
activates telomerase through transcriptional and
posttranscriptional regulation in human erythroleukemic JAS-REN-A
cells. Leuk Res. 35:416–418. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wei ZZ, Qin QP, Chen JN and Chen ZF:
Oxoisoaporphine as potent telomerase inhibitor. Molecules.
21:15342016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Herz C, Hertrampf A, Zimmermann S, Stetter
N, Wagner M, Kleinhans C, Erlacher M, Schüler J, Platz S, Rohn S,
et al: The isothiocyanate erucin abrogates telomerase in
hepatocellular carcinoma cells in vitro and in an orthotopic
xenograft tumour model of HCC. J Cell Mol Med. 18:2393–4203. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Adler S, Rashid G and Klein A:
Indole-3-carbinol inhibits telomerase activity and gene expression
in prostate cancer cell lines. Anticancer Res. 31:3733–3737.
2011.PubMed/NCBI
|
|
41
|
Taka T, Changtam C, Thaichana P,
Kaewtunjai N, Suksamrarn A, Lee TR and Tuntiwechapikul W:
Curcuminoid derivatives enhance telomerase activity in an in vitro
TRAP assay. Bioorg Med Chem Lett. 24:5242–5246. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yu Y, Zhou L, Yang Y and Liu Y:
Cycloastragenol: An exciting novel candidate for age-associated
diseases. Exp Ther Med. 16:2175–2182. 2018.PubMed/NCBI
|
|
43
|
Akbarizare M, Ofoghi H and Hadizadeh M:
Dual effect of sapogenins extracted from spirulina platensis on
telomerase activity in two different cell lines. Mol Biol Res
Commun. 10:1–4. 2021.PubMed/NCBI
|
|
44
|
Chu WK and Hickson ID: RecQ helicases:
Multifunctional genome caretakers. Nat Rev Cancer. 9:644–654. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Uchiumi F, Watanabe T, Hasegawa S, Hoshi
T, Higami Y and Tanuma S: The effect of resveratrol on the Werner
syndrome RecQ helicase gene and telomerase activity. Curr Aging
Sci. 4:1–7. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Tsoukalas D, Buga AM, Docea AO, Sarandi E,
Mitrut R, Renieri E, Spandidos DA, Rogoveanu I, Cercelaru L,
Niculescu M, et al: Reversal of brain aging by targeting
telomerase: A nutraceutical approach. Int J Mol Med. 48:1992021.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Tsoukalas D, Zlatian O, Mitroi M, Renieri
E, Tsatsakis A, Izotov BN, Burada F, Sosoi S, Burada E, Buga AM, et
al: A novel nutraceutical formulation can improve motor activity
and decrease the stress level in a murine model of middle-age
animals. J Clin Med. 10:6242021. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Tsoukalas D, Fragkiadaki P, Docea AO,
Alegakis AK, Sarandi E, Thanasoula M, Spandidos DA, Tsatsakis A,
Razgonova MP and Calina D: Discovery of potent telomerase
activators: Unfolding new therapeutic and anti-aging perspectives.
Mol Med Rep. 20:3701–3708. 2019.PubMed/NCBI
|
|
49
|
Looi LM, Ng MH and Cheah PL: Telomerase
activation in neoplastic cell immortalization and tumour
progression. Malays J Pathol. 29:33–35. 2007.PubMed/NCBI
|
|
50
|
Shay JW and Keith N: Targeting telomerase
for cancer therapeutics. Br J Cancer. 98:677–683. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Andrews LG and Tollefsbol TO: Methods of
telomerase inhibition. Methods Mol Biol. 405:1–7. 2008. View Article : Google Scholar
|
|
52
|
Trybek T, Kowalik A, Góźdź S and Kowalska
A: Telomeres and telomerase in oncogenesis. Oncol Lett.
20:1015–1027. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Bu X, Jia F, Wang W, Guo X, Wu M and Wei
L: Coupled down-regulation of mTOR and telomerase activity during
fluorouracil-induced apoptosis of hepatocarcinoma cells. BMC
Cancer. 7:2082007. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Barthel FP, Wei W, Tang M,
Martinez-Ledesma E, Hu X, Amin SB, Akdemir KC, Seth S, Song X, Wang
Q, et al: Systematic analysis of telomere length and somatic
alterations in 31 cancer types. Nat Genet. 49:349–357. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Nikolouzakis TK, Vakonaki E, Stivaktakis
PD, Alegakis A, Berdiaki A, Razos N, Souglakos J, Tsatsakis A and
Tsiaoussis J: Novel prognostic biomarkers in metastatic and locally
advanced colorectal cancer: Micronuclei frequency and telomerase
activity in peripheral blood lymphocytes. Front Oncol.
11:6836052021. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Hashizume R, Ozawa T, Gryaznov SM, Bollen
AW, Lamborn KR, Frey WH II and Deen DF: New therapeutic approach
for brain tumors: Intranasal delivery of telomerase inhibitor
GRN163. Neuro Oncol. 10:112–120. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Renieri E, Vakonaki E, Karzi V,
Fragkiadaki P and Tsatsakis AM: Telomere length: associations with
nutrinets and xenobiotics. In: Toxicological Risk Assessment and
Multi-System Health Impacts from Exposure. Academic Press.
pp295–306. 2021.
|
|
58
|
Shay JW: Telomerase therapeutics:
Telomeres recognized as a DNA damage signal: Commentary re: K.
Kraemer et al, antisense-mediated hTERT inhibition
specifically reduces the growth of human bladder cancer cells.
Clin. Cancer Res. 9:3794–3800. 2003.Clin Cancer Res 9: 3521–3525,
2003. PubMed/NCBI
|
|
59
|
Martinez P and Blasco MA: Telomere-driven
diseases and telomere-targeting therapies. J Cell Biol.
216:875–887. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Sugarman ET, Zhang G and Shay JW: In
perspective: An update on telomere targeting in cancer. Mol
Carcinog. 58:1581–1588. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Smith-Sonneborn J: Telomerase biology
associations offer keys to cancer and aging therapeutics. Curr
Aging Sci. 13:11–21. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Bernardes de Jesus B, Vera E, Schneeberger
K, Tejera AM, Ayuso E, Bosch F and Blasco MA: Telomerase gene
therapy in adult and old mice delays aging and increases longevity
without increasing cancer. EMBO Mol Med. 4:691–704. 2012.
View Article : Google Scholar : PubMed/NCBI
|