Osteoporosis is a systemic bone disease
characterised by low bone mass and degeneration of bone tissue
micro-architecture, leading to increased bone fragility and
susceptibility to fractures (1).
Strategies to prevent and treat osteoporosis include preventive
(such as increased vitamin D and protein intake, exercise, smoking
cessation, avoidance of excessive alcohol intake and prevention of
falls) and therapeutic measures (2). Clinically used drugs include
bisphosphonates (such as alendronate, risedronate and ibandronate),
antibodies against nuclear factor-κB ligand receptor activator
(RANKL; for example, denosumab), selective oestrogen receptor
modulators (SERMs; for example, raloxifene), parathyroid hormone
(PTH), PTH-related peptides (such as teriparatide and
abaloparatide) and calcitonin (such as salmon calcitonin). However,
clinical use of most anti-bone resorption drugs is limited by side
effects of long-term inhibition of bone resorption, such as upper
gastrointestinal bleeding, acute-phase reaction, hypocalcaemia,
secondary hyperparathyroidism and most drugs on the market are
expensive (3,4). With the ageing world population, the
incidence of osteoporosis is increasing annually, with an increase
of 70.1% in 2019 compared with 1990, endangering quality of life of
middle-aged and older adults and imposing a burden on society and
families (5).
Flavonoids are compounds formed when two benzene
rings (A and B rings) are joined by a pyran heterocyclic ring (C
ring) consisting of a central three-carbon chain (6). The compounds are found in the roots,
stems, leaves, fruit and seeds of plants such as strawberries,
onions and cucumbers (7).
Flavonoids are diverse in structure and most are similar to
oestrogen and exert anti-inflammatory, antibacterial, anti-cancer,
antioxidant, osteogenic, osteoclast-inhibitory and oestrogen-like
effects (8).
Multiple studies have confirmed that flavonoids have
a promoting effect on osteogenesis and the underlying mechanism has
received attention and become a hotspot in developing novel
osteoporosis drugs (9,10). The present review focused on the
role of flavonoids in osteoporosis.
According to the World Health Organization (WHO),
osteoporosis is defined as a bone disease characterized by a
decrease in bone strength that puts a person at increased risk of
fracture. Bone strength primarily reflects a combination of bone
density and bone mass. The majority of the patient population with
this disease is postmenopausal women (11). Postmenopausal osteoporosis is
defined as bone density that is ≤2.5 standard deviations the mean
for women aged 25-50 years. (T-score ≤2.5) (12,13). Therefore, clinical diagnosis and
assessment of osteoporosis are based on measuring bone mineral
density (BMD) (14). The results
of an epidemiological survey on osteoporosis conducted by the
National Health Council of China in 2019 demonstrated that the
prevalence of osteoporosis in China is 3.2 for those aged 40-49,
19.2 for those aged 50-64 years and 32.0% for those aged ≥65 years
(15). In the United States, ~10
million people aged >50 years have osteoporosis (16). In the United Kingdom, 50% of women
and 20% of men aged >50 years suffer from osteoporotic fractures
(17,18). Fragility fractures caused by
osteoporosis lead to increased morbidity and mortality, resulting
in a social burden (19). Thus,
research on the prevention and treatment of osteoporosis has become
an urgent priority (20).
Flavonoids are compounds that are widely found in
nature, and so far, >9,000 flavonoids have been reported
(21,22) Flavonoids comprise subclasses,
including flavonoids (such as lignans, rutin, bryophyllin and
baicalin), flavanones (such as naringenin and hesperidin),
isoflavones (such as soy flavonoids and genistein),
pro-anthocyanidins, flavanols (such as catechins and epicatechin)
and flavonols (such as kaempferol, yohimbine and quercetin), which
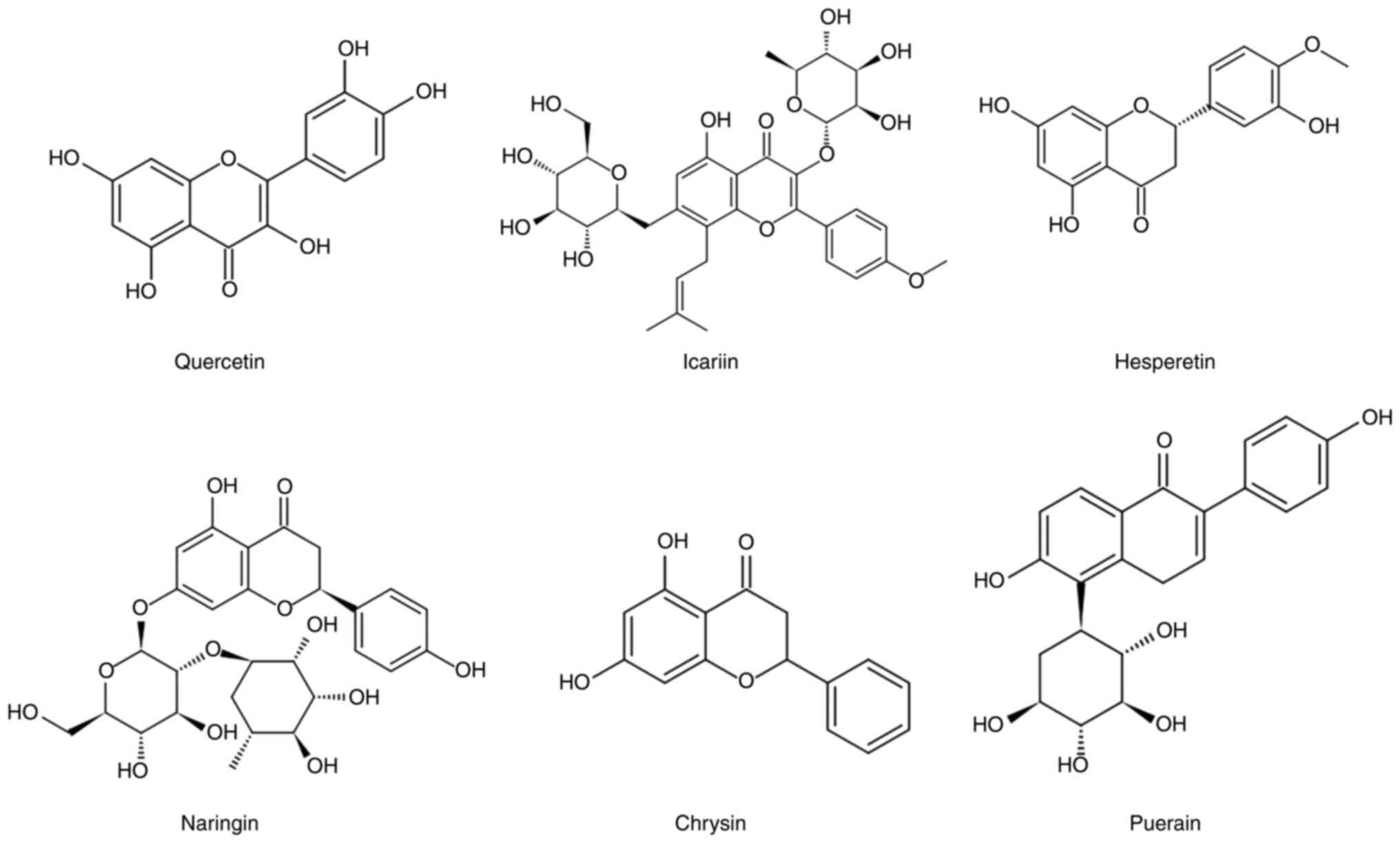
all have a basic flavan structure (2-phenylchroman) (23). The present review focuses on six
well-studied flavonoids (Fig.
1).
With the development of modern separation techniques
and ethnopharmacology, increasing evidence has shown that ICA is
one of the primary biologically active monomers extracted from
Epimedium (44,45). Epimedium, also known as
Ninebark, was recorded 400 years ago in the traditional Chinese
medical text Shennong Ben Cao Jing and is used in various herbal
formulations (46). The herb is
considered a complementary and alternative medicine and has been
demonstrated to possess therapeutic effects in fractures, joint
disease and gonadal dysfunction (36). There are >40 species of the
genus worldwide, primarily found in southwest and central China,
with a total of 27 species and four varieties, accounting for ~70%
of the total global number (47).
More than 260 components have been extracted from Epimedium,
including 141 flavonoids and 31 lignans. Among them, flavonoid
glycosides have been identified as key pharmacologically active
ingredients (48,49). ICA is the primary active
ingredient of Epimedium and was selected as a chemical
marker for quality control of Epimedium in the Chinese
Pharmacopoeia (44). It is a
light-yellow powder with a molecular formula of
C33H40O15 and a molecular weight
of 676.67 g/mol. ICA has a variety of pharmacological activities,
including anti-inflammatory, anti-oxidant, anti-cancer,
anti-osteoporosis, anti-hepatotoxic, anti-depressant and
neuroprotective effects and protects against cardiac ischaemia and
atherosclerosis (50,51).
Hesperitin (3′,5,-trihydroxy-4-methyl-7-xanthone) is
a key component of citrus plants in the Rutaceae family. It
has the molecular formula C16H14O6
and is a member of the flavonoid subclass flavones, primarily found
in citrus fruits (52). Like most
flavonoids, hesperetin naturally occurs in the form of glycosides,
known as hesperidin, first isolated from citrus peel by the French
chemist Le Breton (53). Citrus
bioflavonoids (including hesperidin) appear safe and do not cause
side effects even during pregnancy. Dietary hesperidin is
deglycosylated to hesperetin by intestinal bacteria before
absorption (54,55). Its structure consists of ketone
carbonyl, ether, methoxy and phenolic hydroxyl groups, allowing for
a wide range of pharmacological effects, such as antioxidant,
anti-allergic, and anti-inflammatory effects (56–59).
Naringin is a flavonoid and key secondary
metabolite. Bioactive naringin compounds are found in plant-based
foods, such as vegetables, fruit, tea and wine (60). Naringin-derived drugs are used in
traditional medicine because of their non-addictive and non-toxic
properties (61). Studies have
demonstrated that naringin has antioxidant, anti-microbial,
anti-mutagenic, anti-cancer, bone-protective, anti-inflammatory and
cholesterol-lowering effects (62–65).
Chrysin, also known as poplar flavonoid, is
5,7-dihydroxyflavone. It is found in propolis, honey, passion
fruit, mushrooms and other plant sources. Due to its multiple
pharmacological (anticancer, antitumor, antidiabetic, antioxidant
stress, antiinflammatory, anti-obesity, antiallergic,
hepatoprotective, reproductive organ-protective, neuroprotective
and cardioprotective) effects and low toxicity. Therefore, chrysin
has potential medicinal value (66–76).
Bone is in a constant state of remodeling, which is
key for maintaining structure and function. Imbalances can lead to
disease, such as osteoporosis. Numerous types of cell and cytokine,
hormones and signaling pathway are involved in bone remodeling.
Osteoblasts and osteoclasts are responsible for new bone formation
and resorption, respectively (89–91).
BMPs are members of the transforming growth factor β
superfamily and were first identified in 1960 (92). However, they were not isolated and
purified until the late 1980s (93,94). To date, ~20 BMPs have been
identified. BMP signaling is associated with bone formation
(95,96). BMP is the initial inducer of
osteoblastogenesis during bone development. It binds to type II BMP
receptors to phosphorylate them; activated type II BMP receptors
phosphorylate type I BMP receptors and bind to form complexes
(97). The activated complex
further activates the downstream BMP signaling protein receptor
(R-Smad). Phosphorylated R-Smad binds to Smad4 and migrates to the
nucleus, where it serves as a transcriptional enhancer and
interacts with the transcription factors Runx2 and Osterix to
affect the transcription of osteogenic-associated genes (98). Differentiation of bone precursor
cells and initiation of osteoblast-specific factors (such as
alkaline phosphatase) promote bone formation (99–101).
Quercetin has been shown to possess positive
pharmacological effects on bone metabolism, such as preventing bone
loss (43,102). Zhou et al (103) showed that 10 and 50 µM quercetin
stimulates gene expression of the osteoblast markers, bone
morphogenetic protein 2 (BMP-2), Runx2, osteopontin (OPN),
osteocalcin (OCN), collagen type 1 (COL-1) and osterix in mouse
adipose stem cells (mASCs) in vitro but does not affect
proliferation. The osteogenic effect of quercetin at certain
concentrations has not yet been determined (104). By contrast to Zhou, another
study showed that quercetin enhances proliferation of bone marrow
mesenchymal stem cells (BMSCs) on days one, four and seven after
dosing in a dose-dependent manner with the greatest effect at a
concentration of 2 µM. In the aforementioned study, quercetin
enhanced alkaline phosphatase (ALP) activity and especially the
middle and late markers (bone sialoprotein (BSP), BMP-2, OPN and
OCN) in a dose-dependent manner, with the greatest stimulation
occurring at 2 µM. Furthermore, quercetin not only promoted
osteogenic differentiation of BMSCs, but also promoted secretion of
angiogenic factors in a dose-dependent manner, with the most
significant effect at a concentration of 2 µM (103). Liu et al (105) found that naringin addition had a
bidirectional effect on the cell proliferation and ALP activity of
human amniotic fluid-derived stem cells (hAFSCs). At a
concentration of 200 µg/ml, naringin inhibited the growth and
moderately increased the ALP activity of hAFSCs; while at lower
concentrations (1–100 µg/ml), naringin significantly enhanced the
proliferative capacity and ALP activity of hAFSCs in a
dose-dependent manner. In addition, naringin promotes osteogenic
differentiation of hAFSCs via BMP signalling pathways; this
finding, however, has only been assessed in vitro. Menon
et al (106) showed that
chrysin released from a chitosa/carboxymethyl
cellulose/nanohydroxyapatite stent stimulates proliferation of
mouse mesenchymal stem cells (mMSCs) and promotes osteoblast
differentiation; this may be due to upregulation of Runx2 and
downregulation of Runx2 co-repressors.
The Wnt/β-catenin signalling pathway activates
transcription of Wnt gene in the nucleus via β-catenin (107). When the extraneous osteoblast
Wnt factor binds to the membrane receptor frizzled, a series of
membrane and cytoplasmic protein interactions lead to dimer
formation. This results in β-catenin accumulation in the cytoplasm
and subsequent entry into the nucleus (108). T cell/lymph enhancement factors
combine to form a complex, which activates transcription of
downstream target genes and promotes differentiation and
proliferation of osteoblasts (109).
The MAPK signaling pathway is key for regulation of
osteoblast proliferation and differentiation. MAPKs are a group of
serine/threonine kinases that serve key signaling transducer roles
in translating extracellular stimuli into cellular responses
(111). Activation of the MAPK
cascade occurs via sequential phosphorylation of three protein
kinases. Upon stimulus, MAPKK kinases (MAPKKKs) is activated,
phosphorylating MAPK kinases (MAPKKs), which then phosphorylates
MAPK (112). Certain studies
have suggested that there are three primary pathways involved in
MAPK signaling: Extracellular signal-regulated protein kinase
(ERK), c-Jun N-terminal kinase (JNK) and p38 pathway; different
pathways receive different stimuli and serve different roles
(113,114).
Osteoporosis is a global public health issue and is
considered the second most common health problem after coronary
heart disease by the WHO. Current approaches toward osteoporosis
prevention and treatment focus on drug therapy including
bisphosphonates, calcitonin and SERMs. However, because these drugs
have side effects, such as increased risk of cardiovascular events,
breast cancer and venous thromboembolism, researchers have turned
to traditional Chinese medicine (142,143).
Traditional Chinese medicine has developed over
thousands of years based on a different perspective from Western
medical knowledge. The natural abundance flavonoids, low price,
high osteogenesis rate and low immune rejection during clinical
treatment have made the application of flavonoids in bone tissue
engineering research more widespread. However, current
investigations have certain limitations. First, most research on
flavonoids remains at the cellular level and research at the animal
level needs to be more extensive. Additionally, the pharmacological
effect of flavonoids is a complex process that involves multiple
systems. Although certain mechanisms have been elucidated, further
research is needed. In addition to the aforementioned signalling
pathways, there are other overlapping signalling pathways that
interact with each other. Current research has limitations, such as
focusing on only independent pathways and molecular targets and
there are few clinical studies (50).
More in-depth research on flavonoids is needed to
develop effective and inexpensive novel drugs for clinical
applications.
Not applicable.
The present study was supported by open project of Key
Laboratory of Shanxi Province (grant no. KF2020-02).
Not applicable.
LC wrote the manuscript. FT, JW and YZ edited the
manuscript. CW revised the manuscript. Data authentication is not
applicable. All authors have read and approved the final
manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Christiansen C: Consensus development
conference: Diagnosis, prophylaxis, and treatment of osteoporosis.
Osteoporosis Int. 295:914–915. 1987.
|
|
2
|
Management of osteoporosis in
postmenopausal women, . 2010 position statement of The North
American Menopause Society. Menopause. 17:25–56. 2010. View Article : Google Scholar
|
|
3
|
Langdahl BL and Harslof T: Medical
treatment of osteoporotic vertebral fractures. Ther Adv
Musculoskel. 3:17–29. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Leong KH: Medical treatment of
osteoporosis-increasing options. Ann Acad Med Singap. 31:43–47.
2002.PubMed/NCBI
|
|
5
|
Nahas NE, Samy AM and Omer MO: Global,
regional, and national burden of bone fractures in 204 countries
and territories, 1990-2019: A systematic analysis from the global
burden of disease study 2019. Lancet Healthy Longev. 2:e580–e592.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Harborne JB and Baxter H: The Handbook of
Natural Flavonoids. 2:pp18001999.
|
|
7
|
Kimira M, Arai Y, Shimoi K and Watanabe S:
Japanese intake of flavonoids and isoflavonoids from foods. J
Epidemiol. 8:168–175. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cushnie T and Lamb AJ: Antimicrobial
activity of flavonoids. Int J Antimicrob Ag. 26:343–356. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim SY, Lee JY, Park YD, Kang KL, Lee JC
and Heo JS: Hesperetin alleviates the inhibitory effects of high
glucose on the osteoblastic differentiation of periodontal ligament
stem cells. PLoS One. 8:e675042013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang P, Dai KR and Yan SG: Effects of
naringin on the proliferation and osteogenic differentiation of
human bone mesenchymal stem cell. Eur J Pharmacol. 607:1–5. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Black DM and Rosen CJ: Clinical practice.
Postmenopausal osteoporosis. N Engl J Med. 374:254–262. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Assessment of fracture risk and its
application to screening for postmenopausal osteoporosis. Report of
a WHO Study Group. World Health Organ Tech Rep Ser. 843:1–129.
1994.PubMed/NCBI
|
|
13
|
Kanis JA, Melton LJ III, Christiansen C,
Johnston CC and Khaltaev N: The diagnosis of osteoporosis. J Bone
Miner Res. 9:1137–1141. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Johnell O, Kanis JA, Oden A, Johnell O,
Kanis JA, Oden A, Johansson H, De Laet C, Delmas P, Eisman JA, et
al: Predictive value of BMD for hip and other fractures. J Bone
Miner Res. 20:1185–1194. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jianning Y: Osteoporosis prevalence and
community-based diagnosis and management of osteoporosis-related
chronic pain in China. Chin Gen Pract. 23:2223–2228. 2020.
|
|
16
|
Office of the Surgeon General (US), . Bone
Health and Osteoporosis: A report of the surgeon general. Rockville
(MD): Office of the Surgeon General (US); 2004
|
|
17
|
Clynes MA, Harvey NC, Curtis EM, Fuggle
NR, Dennison EM and Cooper C: The epidemiology of osteoporosis. Br
Med Bull. 133:105–117. 2020.PubMed/NCBI
|
|
18
|
van Staa T, Dennison EM, Leufkens H and
Cooper C: Epidemiology of fractures in England and Wales. Bone.
29:517–522. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ensrud KE and Crandall CJ: Osteoporosis.
Ann Intern Med. 167:ITC17–ITC32. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dewan N, Macdermid JC, Grewal R and
Beattie K: Risk factors predicting subsequent falls and
osteoporotic fractures at 4 years after distal radius fracture-a
prospective cohort study. Arch Osteoporos. 13:322018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Balasuriya B and Rupasinghe H: Plant
flavonoids as angiotensin converting enzyme inhibitors in
regulation of hypertension. Funct Foods Health Dis. 5:172–188.
2010.
|
|
22
|
Wang TY, Li Q and Bi KS: Bioactive
flavonoids in medicinal plants: Structure, activity and biological
fate. Asian J Pharm Sci. 13:12–23. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Alvarez-Arellano L, Salazar-García M and
Corona JC: Neuroprotective effects of quercetin in pediatric
neurological diseases. Molecules. 25:55972020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Manca ML, Castangia I, Caddeo C, Pando D,
Escribano E, Valenti D, Lampis S, Zaru M, Fadda AM and Manconi M:
Improvement of quercetin protective effect against oxidative stress
skin damages by incorporation in nanovesicles. Colloids Surf B
Biointerfaces. 123:566–574. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Andres S, Pevny S, Ziegenhagen R, Bakhiya
N, Schäfer B, Hirsch-Ernst KI and Lampen A: Safety aspects of the
use of quercetin as a dietary supplement. Mol Nutr Food Res.
622018.doi: 10.1002/mnfr.201700447. PubMed/NCBI
|
|
26
|
David A, Arulmoli R and Parasuraman S:
Overviews of biological importance of quercetin: A bioactive
flavonoid. Pharmacogn Rev. 10:84–89. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mehrbod P, Abdalla MA, Fotouhi F,
Heidarzadeh M, Aro AO, Eloff JN, McGaw LJ and Fasina FO:
Immunomodulatory properties of quercetin-3-O-α-L-rhamnopyranoside
from Rapanea melanophloeos against influenza a virus. BMC
Complement Altern Med. 18:1842018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Flores IR, Vásquez-Murrieta MS,
Franco-Hernández MO, Márquez-Herrera CE, Ponce-Mendoza A and Del
Socorro López-Cortéz M: Bioactive compounds in tomato (Solanum
lycopersicum) variety saladette and their relationship with soil
mineral content. Food Chem. 344:1286082021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Torres N, Martínez-Lüscher J, Porte E, Yu
R and Kurtural SK: Impacts of leaf removal and shoot thinning on
cumulative daily light intensity and thermal time and their
cascading effects of grapevine (Vitis vinifera L.) berry and
wine chemistry in warm climates. Food Chem. 343:1284472020.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Maria P, Vivian O, Tatiana P and Sandra P:
Physicochemical stability, antioxidant activity, and acceptance of
beet and orange mixed juice during refrigerated storage. Beverages.
3:362017. View Article : Google Scholar
|
|
31
|
Santiago B, Calvo AA, Gullón B, Feijoo G,
Moreira MT and González-García S: Production of flavonol quercetin
and fructooligosaccharides from onion (Allium cepa L.)
waste: An environmental life cycle approach. Chem Eng J.
392:1237722020. View Article : Google Scholar
|
|
32
|
Ribes-Moya AM, Adalid AM, Raigón MD,
Hellín P, Fita A and Rodríguez-Burruezo A: Variation in flavonoids
in a collection of peppers (Capsicum sp.) under organic and
conventional cultivation: Effect of the genotype, ripening stage,
and growing system. J Sci Food Agr. 100:2208–2223. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sun J, Janisiewicz WJ, Takeda F, Evans B,
Wayne JM, Mengliang Z, Liangli Y and Chen P: Effect of nighttime
UV-C irradiation of strawberry plants on phenolic content of fruit:
Targeted and non-targeted metabolomic analysis. J Berry Res.
10:365–380. 2020. View Article : Google Scholar
|
|
34
|
Zhou W, Liang X, Dai P, Chen Y, Zhang Y,
Zhang M, Lu L, Jin C and Lin X: Alteration of phenolic composition
in lettuce (Lactuca sativa L.) by reducing nitrogen supply
enhances its anti-proliferative effects on colorectal cancer cells.
Int J Mol Sci. 20:42052019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Formica JV and Regelson W: Review of the
biology of Quercetin and related bioflavonoids. Food Chem Toxicol.
33:1061–1080. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nugroho A, Hesty H, Choi JS and Park HJ:
Identification and quantification of flavonoids in Carica papaya
leaf and peroxynitrite-scavenging activity. Asian Pac J Trop
Biomed. 7:208–213. 2017. View Article : Google Scholar
|
|
37
|
Bolling BW, Mckay DL and Blumberg JB: The
phytochemical composition and antioxidant actions of tree nuts.
Asia Pac J Clin Nutr. 19:117–123. 2010.PubMed/NCBI
|
|
38
|
Stavric B: Quercetin in our diet: From
potent mutagen to probable anticarcinogen. Clin Biochem.
27:245–248. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Batiha ES, Beshbishy AM, Ikram M, Mulla
ZS, El-Hack MEA, Taha AE, Algammal AM and Elewa YHA: The
pharmacological activity, biochemical properties, and
pharmacokinetics of the major natural polyphenolic flavonoid:
Quercetin. Foods. 9:3742020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Beecher GR, Warden BA and Merken H:
Analysis of tea polyphenols. Proc Soc Exp Biol Med. 220:267–270.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hirpara KV, Aggarwal P, Mukherjee AJ,
Joshi N and Burman AC: Quercetin and its derivatives: Synthesis,
pharmacological uses with special emphasis on anti-tumor properties
and prodrug with enhanced bio-availability. Anticancer Agents Med
Chem. 9:138–161. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Boots AW, Haenen GR and Bast A: Health
effects of quercetin: From mechanism to nutraceutical. Eur J
Pharmacol. 585:325–337. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yamaguchi M and Weitzmann MN: Quercetin, a
potent suppressor of NF-κB and Smad activation in osteoblasts. Int
J Mol Med. 28:521–525. 2011.PubMed/NCBI
|
|
44
|
Chen M, Wu J, Luo Q, Mo S, Lyu Y, Wei Y
and Dong J: The Anticancer properties of Herba Epimedii and its
main bioactive Componentsicariin and Icariside II. Nutrients.
8:5632016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Huang W, Zeng S, Xiao G, Wei G, Liao S,
Chen J, Sun W, Lv H and Wang Y: Elucidating the biosynthetic and
regulatory mechanisms of flavonoid-derived bioactive components in
Epimedium sagittatum. Front Plant Sci. 6:6892015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Huang KC: The pharmacology of Chinese
herbs. Pharmacol Chin Herbs. 1993.
|
|
47
|
Makarova MN, Pozharitskaya ON, Shikov AN,
Tesakova SV, Makarov VG and Tikhonov VP: Effect of lipid-based
suspension of Epimedium koreanum Nakai extract on sexual behavior
in rats. J Ethnopharmacol. 114:412–416. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ma H, He X, Yang Y, Li M, Hao D and Jia Z:
The genus Epimedium: An ethnopharmacological and phytochemical
review. J Ethnopharmacol. 134:519–541. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Xie X, Pei F, Wang H, Tan Z, Yang Z and
Kang P: Icariin: A promising osteoinductive compound for repairing
bone defect and osteonecrosis. J Biomater Appl. 30:290–299. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wang Z, Wang D, Yang D, Zhen W, Zhang J
and Peng S: The effect of icariin on bone metabolism and its
potential clinical application. Osteoporos Int. 29:535–544. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
He C, Wang Z and Shi J: Pharmacological
effects of icariin. Adv Pharmacol. 87:179–203. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Gary AB and McIntosh C: Radioimmunoassay
for the quantitative determination of hesperidin and analysis of
its distribution in Citrus sinensis. Phytochemistry. 27:249–254.
1988. View Article : Google Scholar
|
|
53
|
Rady H: Pharmacographia: A History of the
principal Drugs of Vegetable Origin met with in Great Britain and
British India. Nature. 11:42–44. 1874. View Article : Google Scholar
|
|
54
|
Garg A, Garg S, Zaneveld LJ and Singla AK:
Chemistry and pharmacology of the Citrus bioflavonoid hesperidin.
Phytother Res. 15:655–669. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Parhiz H, Roohbakhsh A, Soltani F, Rezaee
R and Iranshahi M: Antioxidant and anti-inflammatory properties of
the citrus flavonoids hesperidin and hesperetin: An updated review
of their molecular mechanisms and experimental models. Phytother
Res. 29:323–331. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Li R, Cai L, Xie XF, Peng L, Wu TN and Li
J: 7,3′-dimethoxy hesperetin inhibits inflammation by inducing
synovial apoptosis in rats with adjuvant-induced arthritis.
Immunopharmacol Immunotoxicol. 35:139–146. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Yang Z, Liu Z, Wang J and Zhu H:
Antioxidative effects of hesperetin against lead acetate-induced
oxidative stress in rats. Indian J Pharmacol. 45:395–398. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Itoh K, Masuda M, Naruto S, Murata K and
Matsuda H: Antiallergic activity of unripe Citrus hassaku fruits
extract and its flavanone glycosides on chemical substance-induced
dermatitis in mice. J Nat Med. 63:443–450. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Trzeciakiewicz A, Habauzit V, Mercier S,
Lebecque P, Davicco MJ, Coxam V, Demigne C and Horcajada MN:
Hesperetin stimulates differentiation of primary rat osteoblasts
involving the BMP signalling pathway. J Nutr Biochem. 21:424–431.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Ashraful AM, Nusrat S, Mahbubur RM, Uddin
SJ, Reza HM and Sarker SD: Effect of citrus flavonoids, naringin
and naringenin, on metabolic syndrome and their mechanisms of
action. Adv Nutr. 5:404–417. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
E. P. O. Additives and Products or
Substances used in Animal Feed, . Scientific Opinion on the safety
and efficacy of naringin when used as a sensory additive for all
animal species. EfSA J. 9:24162011.
|
|
62
|
Joshi R, Kulkarni YA and Wairkar S:
Pharmacokinetic, pharmacodynamic and formulations aspects of
Naringenin: An update. Life Sci. 215:43–56. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Wang Y, Yin L, Li Y, Liu P and Cui Q:
Preventive effects of puerarin on alcohol-induced osteonecrosis.
Clin Orthop Relat Res. 466:1059–1067. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Akintunde JK, Akintola TE, Hammed MO, Amoo
CO, Adegoke AM and Ajisafe LO: Naringin protects against
Bisphenol-A induced oculopathy as implication of cataract in
hypertensive rat model. Biomed Pharmacother. 126:1100432020.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Zhang YF, Meng NN, Li HZ, Wen YJ, Liu JT,
Zhang CL, Yuan XH and Jin XD: Effect of naringin on oxidative
stress and endoplasmic reticulum stress in diabetic cardiomyopathy.
Zhongguo Zhong Yao Za Zhi. 43:596–602. 2018.(In Chinese).
PubMed/NCBI
|
|
66
|
Choi JH and Yun JW: Chrysin induces brown
fat-like phenotype and enhances lipid metabolism in 3T3-L1
adipocytes. Nutrition. 32:1002–1010. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Mohammadian F, Abhari A, Dariushnejad H,
Nikanfar A, Pilehvar-Soltanahmadi Y and Zarghami N: Effects of
Chrysin-PLGA-PEG nanoparticles on proliferation and gene expression
of miRNAs in gastric cancer cell line. Iran J Cancer Prev.
9:e41902016. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Kang MK, Park SH, Kim YH, Lee EJ, Antika
LD, Kim DY, Choi YJ and Kang YH: Chrysin ameliorates podocyte
injury and slit diaphragm protein loss via inhibition of the
PERK-eIF2α-ATF-CHOP pathway in diabetic mice. Acta Pharmacol Sin.
38:1129–1140. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Shoieb SM, Esmat A, Khalifa AE and
Abdel-Naim AB: Chrysin attenuates testosterone-induced benign
prostate hyperplasia in rats. Food Chem Toxicol. 111:650–659. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Zeinali M, Rezaee SA and Hosseinzadeh H:
An overview on immunoregulatory and anti-inflammatory properties of
chrysin and flavonoids substances. Biomed Pharmacother.
92:998–1009. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Vedagiri A and Thangarajan S: Mitigating
effect of chrysin loaded solid lipid nanoparticles against Amyloid
β25-35 induced oxidative stress in rat hippocampal region: An
efficient formulation approach for Alzheimer's disease.
Neuropeptides. 58:111–125. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Khan R, Khan AQ, Qamar W, Lateef A, Ali F,
Rehman MU, Tahir M, Sharma S and Sultana S: Chrysin abrogates
cisplatin-induced oxidative stress, p53 expression, goblet cell
disintegration and apoptotic responses in the jejunum of Wistar
rats. Br J Nutr. 108:1574–1585. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Khan MS, Devaraj H and Devaraj N: Chrysin
abrogates early hepatocarcinogenesis and induces apoptosis in
N-nitrosodiethylamine-induced preneoplastic nodules in rats.
Toxicol Appl Pharmacol. 251:85–94. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Shen Y, Tian P, Li D, Wu Y, Wan C, Yang T,
Chen L, Wang T and Wen F: Chrysin suppresses cigarette
smoke-induced airway inflammation in mice. Int J Clin Exp Med.
8:2001–2008. 2015.PubMed/NCBI
|
|
75
|
Rehman MU, Ali N, Rashid S, Jain T, Nafees
S, Tahir M, Khan AQ, Lateef A, Khan R, Hamiza OO, et al:
Alleviation of hepatic injury by chrysin in cisplatin administered
rats: Probable role of oxidative and inflammatory markers.
Pharmacol Rep. 66:1050–1059. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Ravishankar D, Salamah M, Attina A, Pothi
R, Vallance TM, Javed M, Williams HF, Alzahrani EMS, Kabova E,
Vaiyapuri R, et al: Ruthenium-conjugated chrysin analogues modulate
platelet activity, thrombus formation and haemostasis with enhanced
efficacy. Sci Rep. 7:57382017. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Shibata S, Murakami T, Nishikawa Y and
Harada M: The constituents of pueraria root. Chem Pharm Bull.
7:134–136. 1959. View Article : Google Scholar
|
|
78
|
Keung WM and Vallee BL: Kudzu root: An
ancient Chinese source of modern antidipsotropic agents.
Phytochemistry. 47:499–506. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Brenner R, Perez GJ, Bonev AD, Eckman DM,
Kosek JC, Wiler SW, Patterson AJ, Nelson MT and Aldrich RW:
Vasoregulation by the beta1 subunit of the calcium-activated
potassium channel. Nature. 407:870–876. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Hsu FL, Liu IM, Kuo DH, Chen WC, Su HC and
Cheng JT: Antihyperglycemic effect of puerarin in
streptozotocin-induced diabetic rats. J Nat Prod. 66:788–792. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Hao LN, Zhang YQ, Shen YH, Wang ZY and
Wang YH: Inducible nitric oxide synthase and Fas/FasL with C3
expression of mouse retinal pigment epithelial cells in response to
stimulation by peroxynitrite and antagonism of puerarin. Chin Med
J. 124:2522–2529. 2011.PubMed/NCBI
|
|
82
|
Shao HM, Tang YH, Jiang PJ, Zhu HQ, Zhang
YC, Ji JM, Ji O and Shen Q: Inhibitory effect of flavonoids of
puerarin on proliferation of different human acute myeloid leukemia
cell lines in vitro. Zhongguo Shi Yan Xue Ye Xue Za Zhi.
18:296–299. 2010.(In Chinese). PubMed/NCBI
|
|
83
|
Cheng Y, Zhu G, Guan Y, Liu Y, Hu Y and Li
Q: Protective effects of puerarin against
1-methyl-4-phenylpyridinium-induced mitochondrial apoptotic death
in differentiated SH-SY5Y cells. Zhongguo Zhong Yao Za Zhi.
36:1222–1226. 2011.(In Chinese). PubMed/NCBI
|
|
84
|
Zou Y, Hong B, Fan L, Zhou L, Liu Y, Wu Q,
Zhang X and Dong M: Protective effect of puerarin against
beta-amyloid-induced oxidative stress in neuronal cultures from rat
hippocampus: Involvement of the GSK-3β/Nrf2 signaling pathway. Free
Radical Res. 47:55–63. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Song JL, Baek HJ, Chang HL and Kim HP:
Antiinflammatory activity of isoflavonoids from Pueraria
radix and biochanin A derivatives. Arch Pharm Res. 17:31–35. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Overstreet DH, Kralic JE, Morrow AL, Ma
ZZ, Zhong ZM and Lee D: NPI-031G (puerarin) reduces anxiogenic
effects of alcohol withdrawal or benzodiazepine inverse or 5-HT2C
agonists. Pharmacol Biochem Behav. 75:619–625. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Yong PH and Jeong HG: Mechanism of
phytoestrogen puerarin-mediated cytoprotection following oxidative
injury: Estrogen receptor-dependent up-regulation of PI3K/Akt and
HO-1. Toxicol Appl Pharmacol. 233:371–381. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Cho HJ, Jun HJ, Ji HL, Jia Y, Hoang MH,
Shim JH, Park KH and Lee SJ: Acute effect of high-dose isoflavones
from Pueraria lobata (Willd.) Ohwi on lipid and bone metabolism in
ovariectomized mice. Phytother Res. 26:1864–1871. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Chen X, Wang Z, Duan N, Zhu G, Schwarz EM
and Xie C: Osteoblast-osteoclast interactions. Connect Tissue Res.
59:99–107. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Compston JE, McClung MR and Leslie WD:
Osteoporosis. Lancet. 393:364–376. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Lerner UH, Kindstedt E and Lundberg P: The
critical interplay between bone resorbing and bone forming cells. J
Clin Periodontol. 46 (Suppl 21):S33–S51. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Urist MR: Bone: Formation by
autoinduction. Science. 150:893–899. 1965. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Luyten FP, Cunningham NS, Ma S,
Muthukumaran N, Hammonds RG, Nevins WB, Woods WI and Reddi AH:
Purification and partial amino acid sequence of osteogenin, a
protein initiating bone differentiation. J Biol Chem.
264:13377–13380. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Wozney JM, Rosen V, Celeste AJ, Mitsock
LM, Whitters MJ, Kriz RW, Hewick RM and Wang EA: Novel regulators
of bone formation: Molecular clones and activities. Science.
242:1528–1534. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Xu C and Di C: The BMP signaling and in
vivo bone formation. Gene. 357:1–8. 2005. View Article : Google Scholar
|
|
96
|
Hinck AP, Mueller TD and Springer TA:
Structural biology and evolution of the TGF-β family. Cold Spring
Harb Perspect Biol. 8:a221032016. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
von Bubnoff A and Cho KW: Intracellular
BMP signaling regulation in vertebrates: Pathway or network? Dev
Biol. 239:1–14. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Nohe A, Keating E, Knaus P and Petersen
NO: Signal transduction of bone morphogenetic protein receptors.
Cell Signal. 16:291–299. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Javed A, Afzal F, Bae JS, Gutierrez S,
Zaidi K, Pratap J, van Wijnen AJ, Stein JL, Stein GS and Lian JB:
Specific residues of RUNX2 are obligatory for formation of
BMP2-induced RUNX2-SMAD complex to promote osteoblast
differentiation. Cells Tissues Organs. 189:133–137. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Phimphilai M, Zhao Z, Boules H, Roca H and
Franceschi RT: BMP signaling is required for RUNX2-dependent
induction of the osteoblast phenotype. J Bone Mineral Res.
21:637–646. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Tan X, Weng T, Zhang J, Wang J, Li W, Wan
H, Lan Y, Cheng X, Hou N, Liu H, et al: Smad4 is required for
maintaining normal murine postnatal bone homeostasis. J Cell Sci.
120:2162–2170. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Liang W and Luo Z, Ge S, Li M, Du J, Yang
M, Yan M, Ye Z and Luo Z: Oral administration of quercetin inhibits
bone loss in rat model of diabetic osteopenia. Eur J Pharmacol.
670:317–324. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Zhou C and Lin Y: Osteogenic
differentiation of adipose-derived stem cells promoted by
quercetin. Cell Prolif. 47:124–132. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Sharan K, Mishra JS, Swarnkar G, Siddiqui
JA, Khan K, Kumari R, Rawat P, Maurya R, Sanyal S and Chattopadhyay
N: A novel quercetin analogue from a medicinal plant promotes peak
bone mass achievement and bone healing after injury and exerts an
anabolic effect on osteoporotic bone: The role of aryl hydrocarbon
receptor as a mediator of osteogenic action. J Bone Miner Res.
26:2096–2111. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Liu M, Li Y and Yang ST: Effects of
naringin on the proliferation and osteogenic differentiation of
human amniotic fluid-derived stem cells. J Tissue Eng Regen Med.
11:276–284. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Menon AH, Soundarya SP, Sanjay V, Chandran
SV, Balagangadharan K and Selvamurugan N: Sustained release of
chrysin from chitosan-based scaffolds promotes mesenchymal stem
cell proliferation and osteoblast differentiation. Carbohyd Polym.
195:356–367. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Willert K and Nusse R: Beta-catenin: A key
mediator of Wnt signaling. Curr Opin Genet Dev. 8:95–102. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Clevers H and Nusse R: Wnt/β-catenin
signaling and disease. Cell. 149:1192–1205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Kestler HA and Kühl M: From individual Wnt
pathways towards a Wnt signalling network. Philos Trans R Soc Lond
B Biol Sci. 363:1333–1347. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Lin FX, Du SX, Liu DZ, Hu QX, Yu GY, Wu
CC, Zheng GZ, Xie D, Li XD and Chang B: Naringin promotes
osteogenic differentiation of bone marrow stromal cells by
up-regulating Foxc2 expression via the IHH signaling pathway. Am J
Transl Res. 8:5098–5107. 2016.PubMed/NCBI
|
|
111
|
Johnson GL and Lapadat R:
Mitogen-activated protein kinase pathways mediated by ERK, JNK, and
p38 protein kinases. Science. 298:1911–1912. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Mercedes SS, Diniz FF, Gomes GN and Diana
B: The Mitogen-activated protein kinase (MAPK) pathway: Role in
immune evasion by trypanosomatids. Front Microbiol.
7:1832016.PubMed/NCBI
|
|
113
|
Arthur JS and Ley SC: Mitogen-activated
protein kinases in innate immunity. Nat Rev Immunol. 13:679–692.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Ronkina N and Gaestel M: MAPK-activated
protein kinases: Servant or partner? Annu Rev Biochem. Feb
18–2022.(Epub ahead of print). doi:
10.1146/annurev-biochem-081720-114505. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Wu Y, Xia L, Zhou Y, Xu Y and Jiang X:
Icariin induces osteogenic differentiation of bone mesenchymal stem
cells in a MAPK-dependent manner. Cell Prolif. 48:375–384. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Liu L, Zheng J, Yang Y, Ni L, Chen H and
Yu D: Hesperetin alleviated glucocorticoid-induced inhibition of
osteogenic differentiation of BMSCs through regulating the ERK
signaling pathway. Med Mol Morphol. 54:1–7. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Xue D, Chen E, Zhang W, Gao X, Wang S,
Zheng Q, Pan Z, Li H and Liu L: The role of hesperetin on
osteogenesis of human mesenchymal stem cells and its function in
bone regeneration. Oncotarget. 8:21031–21043. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Yang X, Yang Y, Zhou S, Gong X, Dai Q,
Zhang P and Jiang L: Puerarin Stimulates osteogenic differentiation
and bone formation through the ERK1/2 and p38-MAPK signaling
pathways. Curr Mol Med. 17:488–496. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Franke TF, Hornik CP, Segev L, Shostak GA
and Sugimoto C: PI3K/Akt and apoptosis: Size matters. Oncogene.
22:8983–8998. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Lien EC, Dibble CC and Toker A: PI3K
signaling in cancer: Beyond AKT. Curr Opin Cell Biol. 45:62–71.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Kashii Y, Uchida M, Kirito K, Tanaka M,
Nishijima K, Toshima M, Ando T, Koizumi K, Endoh T, Sawada K, et
al: A member of Forkhead family transcription factor, FKHRL1, is
one of the downstream molecules of phosphatidylinositol
3-kinase-Akt activation pathway in erythropoietin signal
transduction. Blood. 96:941–949. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Gu YX, Du J, Si MS, Mo JJ, Qiao SC and Lai
HC: The roles of PI3K/Akt signaling pathway in regulating MC3T3-E1
preosteoblast proliferation and differentiation on SLA and SLActive
titanium surfaces. J Biomed Mater Res A. 101:748–754. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Xi JC, Zang HY, Guo LX, Xue HB, Liu XD,
Bai YB and Ma YZ: The PI3K/AKT cell signaling pathway is involved
in regulation of osteoporosis. J Recept Signal Transduct Res.
35:640–645. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Zhai YK, Guo XY, Ge BF, Zhen P, Ma XN,
Zhou J, Ma HP, Xian CJ and Chen KM: Icariin stimulates the
osteogenic differentiation of rat bone marrow stromal cells via
activating the PI3K-AKT-eNOS-NO-cGMP-PKG. Bone. 66:189–198. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Lv H, Che T, Tang X, Liu L and Cheng J:
Puerarin enhances proliferation and osteoblastic differentiation of
human bone marrow stromal cells via a nitric oxide/cyclic guanosine
monophosphate signaling pathway. Mol Med Rep. 12:2283–2290. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Zhang Y, Yan M, Yu QF, Yang PF, Zhang HD,
Sun YH, Zhang ZF and Gao YF: Puerarin prevents LPS-induced
osteoclast formation and bone loss via inhibition of Akt
activation. Biol Pharm Bull. 39:2028–2035. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Simonet WS, Lacey DL, Dunstan CR, Kelley
MC and Boyle WJ: Osteoprotegerin: A novel secreted protein involved
in the regulation of bone density. Cell. 89:309–319. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Lacey DL, Timms E, Tan HL, Kelley MJ,
Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S,
et al: Osteoprotegerin ligand is a cytokine that regulates
osteoclast differentiation and activation. Cell. 93:165–176. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Xu J, Tan JW, Huang L, Gao XH, Laird R,
Liu D, Wysocki S and Zheng MH: Cloning, sequencing, and functional
characterization of the rat homologue of receptor activator of
NF-kappaB ligand. Bone. 15:2178–2186. 2000.PubMed/NCBI
|
|
130
|
Burgess TL, Qian Y, Kaufman S, Ring BD,
Van G, Capparelli C, Kelley M, Hsu H, Boyle WJ, Dunstan CR, et al:
The ligand for osteoprotegerin (OPGL) directly activates mature
osteoclasts. J Cell Biol. 145:527–538. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Mizuno A, Amizuka N, Irie K, Murakami A,
Fujise N, Kanno T, Sato Y, Nakagawa N, Yasuda H, Mochizuki S, et
al: Severe osteoporosis in mice lacking osteoclastogenesis
inhibitory factor/osteoprotegerin. Biochem Biophys Res Commun.
247:610–615. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Yasuda H, Shima N, Nakagawa N, Mochizuki
SI, Yano K, Fujise N, Sato Y, Goto M, Yamaguchi K, Kuriyama M, et
al: Identity of osteoclastogenesis inhibitory factor (OCIF) and
osteoprotegerin (OPG): A mechanism by which OPG/OCIF inhibits
osteoclastogenesis in vitro. Endocrinology. 139:1329–1337. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Kostenuik P and Shalhoub V:
Osteoprotegerin: A physiological and pharmacological inhibitor of
bone resorption. Curr Pharm Design. 7:613–635. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Hofbauer LC, Kühne CA and Viereck V: The
OPG/RANKL/RANK system in metabolic bone diseases. J Musculoskelet
Neuronal Interact. 4:268–275. 2004.PubMed/NCBI
|
|
135
|
Yuan SY, Sheng T, Qi L, Zhang YL, Liu XM,
Ma T, Zheng H, Yan Y, Ishimi Y and Wang XX: Puerarin prevents bone
loss in ovariectomized mice and inhibits osteoclast formation in
vitro. Chin J Nat Med. 14:265–269. 2016.PubMed/NCBI
|
|
136
|
Shan Z, Cheng N, Huang R, Zhao B and Zhou
Y: Puerarin promotes the proliferation and differentiation of
MC3T3-E1 cells via microRNA106b by targeting receptor activator of
nuclear factor-κB ligand. Exp Ther Med. 15:55–60. 2018.PubMed/NCBI
|
|
137
|
Turner RT, Maran A, Lotinun S, Hefferan T
and Sibonga JD: Animal models for osteoporosis. Rev Endocr Metab
Disord. 2:117–127. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Huo JF, Zhang ML, Wang XX and Zou DH:
Chrysin induces osteogenic differentiation of human dental pulp
stem cells. Exp Cell Res. 400:1124662021. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Liu H, Li W, Ge X, Jia S and Li B:
Coadministration of puerarin (low dose) and zinc attenuates bone
loss and suppresses bone marrow adiposity in ovariectomized rats.
Life Sci. 166:20–26. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Huang J, Bao Y, Xiang W, Jing XZ, Guo JC,
Yao XD, Wang R and Guo FJ: Icariin regulates the bidirectional
differentiation of bone marrow mesenchymal stem cells through
canonical Wnt signaling pathway. Evid Based Complement Alternat
Med. 2017:80853252017. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Wang D, Ma W, Wang F, Dong J, Wang D, Sun
B and Wang B: Stimulation of Wnt/β-catenin signaling to improve
bone development by naringin via interacting with AMPK and Akt.
Cell Physiol Biochem. 36:1563–1576. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Rossouw JE, Anderson GL, Prentice RL,
LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA,
Howard BV, Johnson KC, et al: Risks and benefits of estrogen plus
progestin in healthy postmenopausal women: Principal results From
the Women's Health Initiative randomized controlled trial. JAMA.
288:321–333. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Ettinger B, Black DM, Mitlak BH,
Knickerbocker RK, Nickelsen T, Genant HK, Christiansen C, Delmas
PD, Zanchetta JR, Stakkestad J, et al: Reduction of vertebral
fracture risk in postmenopausal women with osteoporosis treated
with raloxifene: Results from a 3-year randomized clinical trial.
Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators.
JAMA. 282:637–645. 1999. View Article : Google Scholar : PubMed/NCBI
|