Introduction
Sinusoidal obstruction syndrome (SOS), also called
central venous occlusion, is caused by severe liver damage
following liver transplantation, hematopoietic stem cell
transplantation and some anticancer drug treatments (1–8).
SOS develops mainly in zone 3 of the hepatic acinus and exhibits
portal hypertension symptoms, such as splenomegaly and
thrombocytopenia (9,10); notably, zone 3 is more susceptible
to drugs due to the low amount of glutathione, which is responsible
for drug detoxification (8,11).
The presence of platelet aggregation in the space of
Disse around zone 3 has been reported in the livers of transplant
recipients who developed SOS, and in patients with colorectal liver
metastasis who received preoperative chemotherapy with
oxaliplatin-based regimens (1–3).
This phenomenon is known as extravasated platelet aggregation (EPA)
(1–3).
In a monocrotaline (MCT)-induced SOS rat model, EPA
has been detected in the space of Disse, and the effect of
anti-platelet drugs (PDEIII inhibitors) on the prevention and
reduction of SOS has been reported (12,13). In addition, the severity of SOS
can be reduced by the protective effect of recombinant
thrombomodulin on liver endothelial cells (LSECs) in a SOS mouse
model (14,15). Based on these findings, LSEC
detachment and EPA may serve important roles in the mechanism of
SOS; however, it is difficult to predict the onset of SOS,
determine its severity and assess therapeutic approaches. Some
biomarkers of SOS have been reported, such as urine metabonomic
profiles; however, none are specific (16). Therefore, it is necessary to
identify biomarkers to determine the status of SOS.
We previously reported that platelets invading the
space of Disse not only adhere to hepatocytes but are also taken up
into the hepatocytes of patients who develop SOS after liver
transplantation (2). Several
reports on the clearance of platelets have described mechanisms
involving Fc receptor-mediated splenic macrophages, integrins,
apoptosis and Ashwell-Morell receptors (AMR) (17). Aging platelets and platelets in
patients with sepsis are phagocytized by hepatocytes through AMR
and thrombopoietin (TPO), which is produced by the JAK2-STAT3
signaling pathway (18).
TPO is a hematopoietic factor that promotes the
proliferation and differentiation of megakaryocytes. TPO was
successfully cloned by multiple groups in 1994 (19). TPO is produced mainly by
hepatocytes, where the amino acid sequence in the N-terminal region
binds to cell surface-expressed receptors (c-Mpl) on platelets and
megakaryocytes (19). Upon
binding to the megakaryocyte receptor, TPO induces activation of
multiple signaling pathways, including JAK, STAT and MAP kinases,
suppresses megakaryocyte apoptosis, and increases megakaryocyte
number, size and nuclear ploidy; as a result, platelet production
is enhanced (19). Two TPO
receptor agonists, romiplostim and eltrombopag, are currently
approved for the treatment of idiopathic thrombocytopenic purpura,
and their use is increasing worldwide. In previous years, it has
also been suggested that TPO receptors may be an effective
treatment for aplastic anemia (19,20).
The aim of the present study was to examine the
significance of TPO levels as a biomarker of SOS in a drug-induced
SOS mouse model.
Materials and methods
Reagents
A 20 mg/ml solution of MCT (Wako Pure Chemical
Industries, Ltd.) was prepared by dissolving 1,000 mg MCT in 1.0 N
HCl, and the pH was adjusted to 7.4 with 0.5 N NaOH. The solution
was diluted in phosphate-buffered saline (PBS; pH 7.4), to increase
the total volume to 50 ml (21).
Animal model of SOS
A total of 54 female Crl:CD1 mice (Charles River
Laboratories, Inc.) weighing 20-30 g at 6-8 weeks of age were used
in this study. The Animal Research Committee of Kanazawa University
(Kanazawa, Japan) approved all experiments (approval no. 183934).
Mice were maintained under the following conditions: Temperature,
23±3°C; humidity, 55±10%; 12-h light/dark cycle, 8:45 lights on,
20:45 lights off; access to food and water, ad libitum. The
mice were randomly divided into two groups: SOS model and control
(n=27/group). After fasting for 12 h, MCT (270 mg/kg) was
administered via intraperitoneal injection in the SOS model group,
and the control group received the same volume of PBS (14,15,22). The mice were sacrificed 12, 24 and
48 h after MCT or PBS treatment (n=16 mice sacrificed at each time
point, n=8/group), and blood samples (~1 ml) and liver tissues were
collected. The blood samples were centrifuged at 1,500 × g for 15
min at 4°C to collect serum. Liver tissue lysate was collected as
follows: Liver tissue was homogenized with RIPA buffer (50 mM
Tris-HCl, pH 8.0; 150 mM sodium chloride; 0.5 w/v% sodium
deoxycholate; 0.1 w/v% sodium dodecyl sulfate; 1.0 w/v% NP-40
substitute) containing a protease inhibitor; while cooling on ice,
sonication at 19.5-20.5 kHz was performed approximately three times
(10–15 sec each). After storage on ice, the sample was centrifuged
at 15,000 × g for 15 min at 4°C and the supernatant was collected.
All animals were monitored for health and behavior once a day.
Animals were euthanized by decapitation, and the cause of death was
respiratory and cardiac arrest. In addition, 15 mice were used for
surgical stabilization and optimization of SOS modeling.
Biochemical analysis
Platelet counts were measured using an automated
blood cell counter (Celltac α MEK-6458; Nihon Kohden). Serum and
liver tissue concentrations of TPO were measured using ELISA (cat.
no. MTP00; R&D Systems, Inc.). ELISA was performed according to
the manufacturer's protocol.
Liver histology
The liver tissues were fixed in 10% neutral buffered
formalin at room temperature overnight and embedded in paraffin.
Slides were prepared (4 µm) and were stained with hematoxylin and
eosin. Hematoxylin staining was performed for 4 min and eosin
staining was performed for 2 min at room temperature. All sections
were examined using a BX51 light microscope (Olympus
Corporation).
Immunohistochemistry
The liver tissues were fixed in 4% paraformaldehyde
in PBS for 3 days at room temperature and embedded in O.C.T.
compound solution (Sakura Finetek Japan Co., Ltd.) and 30% sucrose
in 0.1 M phosphate buffer (pH 7.4) containing 0.05%
NaN3. All tissue samples were sectioned at 6 µm using a
cryostat (Thermo Fisher Scientific, Inc.). Slides were
immunostained with a primary antibody against TPO (1:200; cat. no.
ab216884; Abcam). Briefly, deparaffinized sections were autoclaved
with 10% citric acid buffer (pH 8.0) at 120°C for 15 min. After
treatment with Peroxidase Blocking Solution containing 10%
Na3PO4, 5% H2O2, 3%
NaH2PO4 and H2O (Agilent
Technologies, Inc.) for 5 min at room temperature, sections were
incubated with a primary antibody at 4°C overnight. After the
sections were washed in PBS, the sections were incubated with
EnVision+ Single Reagent (HRP. Rabbit) (cat. no. K4003, not
diluted; Agilent Technologies, Inc.) for 45 min at room
temperature. Immunoreactivity was visualized using EnVision reagent
(Agilent Technologies, Inc.), and the slides were developed with
diaminobenzidine and counterstained with hematoxylin for 1 min at
room temperature. All sections were examined using a BX51 light
microscope (Olympus Corporation).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was isolated from liver tissue using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's instructions. Total RNA was
reverse transcribed into cDNA using the Affinity Script qPCR cDNA
Synthesis Kit (Agilent Technologies, Inc.) according to the
manufacturer's protocol. Amplification and real-time fluorescence
detection were performed using the Mx3005P Real-Time QPCR system
(Stratagene; Agilent Technologies, Inc.) with the following
protocol: Activation step (95°C, 10 min), followed by 40 cycles of
denaturation (95°C, 30 sec), annealing (55°C, 60 sec), and
extension (72°C, 60 sec). The relative abundance of transcripts was
normalized according to the expression of β-actin using the
2−ΔΔCq method (23).
The primer sequences were as follows: TPO forward,
5′-CACAGCTGTCCCAAGCAGTA-3′ and reverse, 5′-CATTCACAGGTCCGTGTGTC-3′;
and β-actin forward, 5′-TCCATCGAAGTGTGACGT-3′ and reverse,
5′-GAGCAATGATCTTGATCTTCAT-3′.
Statistical analysis
All results are expressed as the mean ± standard
deviation. The groups were compared using one-way ANOVA followed by
Dunnett's test or two-way ANOVA followed by Tukey's test. All
analyses were performed using SPSS II 23.0 software (IBM Corp.).
P<0.05 was considered to indicate a statistically significant
difference.
Results
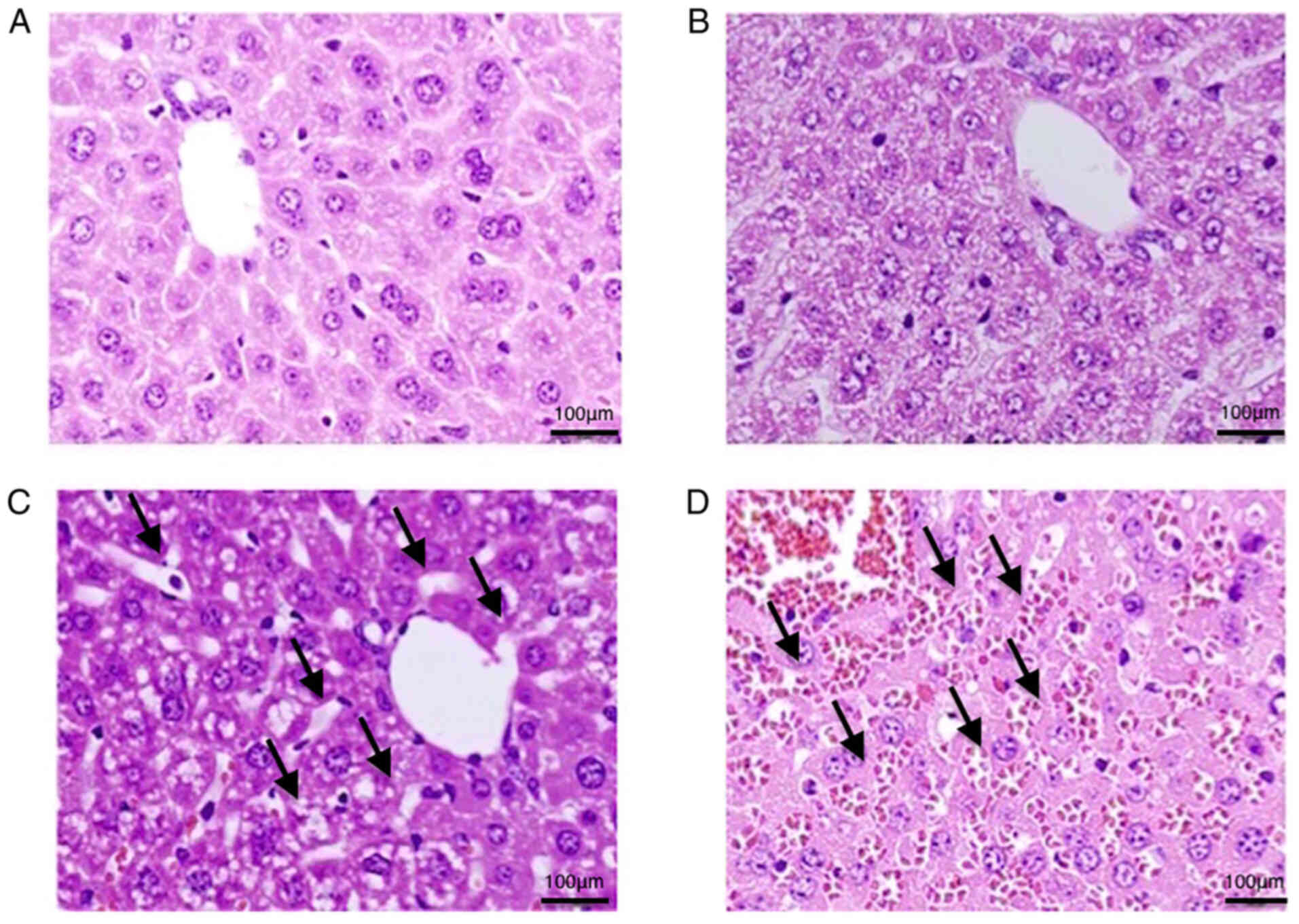
Pathological findings in the SOS
model
Sinusoid dilation 24 h after MCT treatment was
observed in zone 3. Red blood cells could be observed in the
dilated sinusoid at 48 h, indicating congestion. These findings are
pointed to by black arrows. These changes were not observed at 0
and 12 h (Fig. 1).
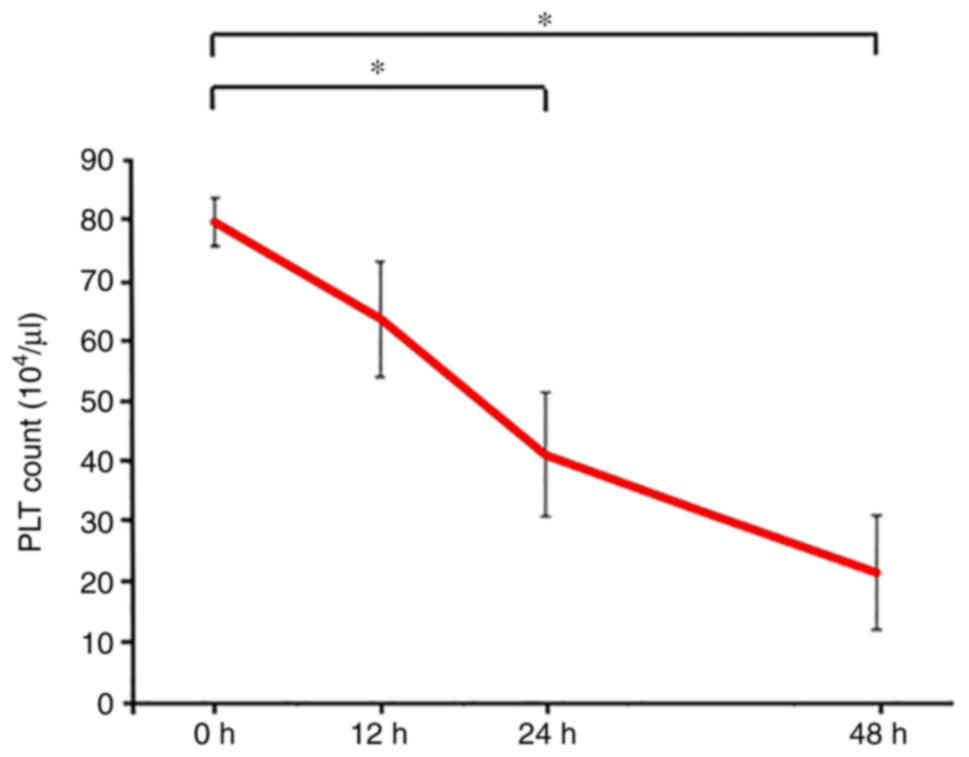
Platelet counts are decreased in the
SOS model
The platelet counts in peripheral blood samples were
significantly decreased after 24 h in the SOS model (0 h,
79.8±4.0×104; 12 h, 63.8±9.7×104; 24 h,
41.2±10.3×104 and 48 h, 21.6×104; P=0.337,
P=0.0231 and P<0.001, respectively) (Fig. 2). Pathological changes and
thrombocytopenia was confirmed in the SOS model, which is
consistent with the results of previous reports (14,15,22).
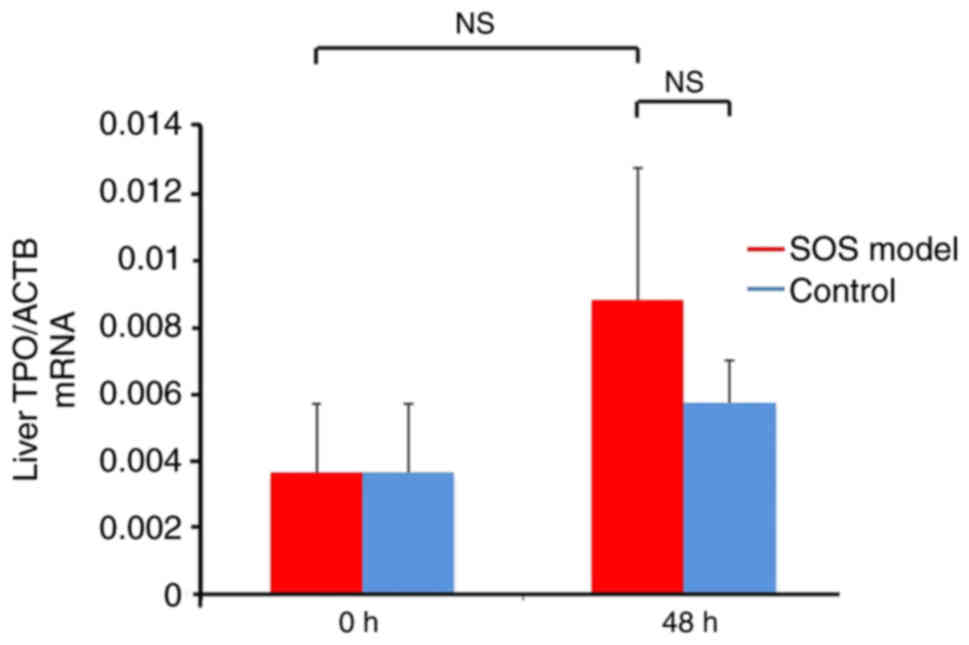
TPO mRNA expression are not affected
in the SOS model
TPO mRNA expression levels in liver tissues were not
significantly different between the SOS model and control groups.
Non-significant increases in TPO levels were detected in both
groups at 48 h (vs. control group at 48 h, P=0.0731; vs. 0h SOS
model group, 0.521; Fig. 3).
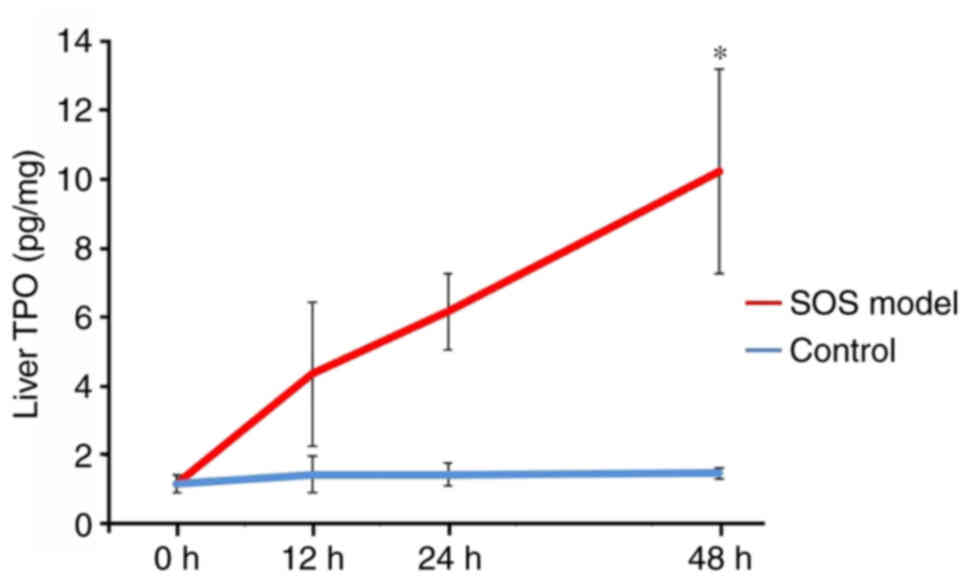
TPO protein levels are increased in
the SOS model
The TPO protein levels in liver tissue increased
over time, especially at 48 h, in the SOS model group. By contrast,
TPO protein levels did not increase in the control group (12 h:
4.37±2.09 pg/mg vs. 1.46±0.52 pg/mg, P=0.891; 24 h: 6.18±1.11 pg/mg
vs. 1.15±0.33 pg/mg, P=0.480; 48 h: 9.61±2.71 pg/mg vs. 1.49±0.34
pg/mg, P=0.037; Fig. 4).
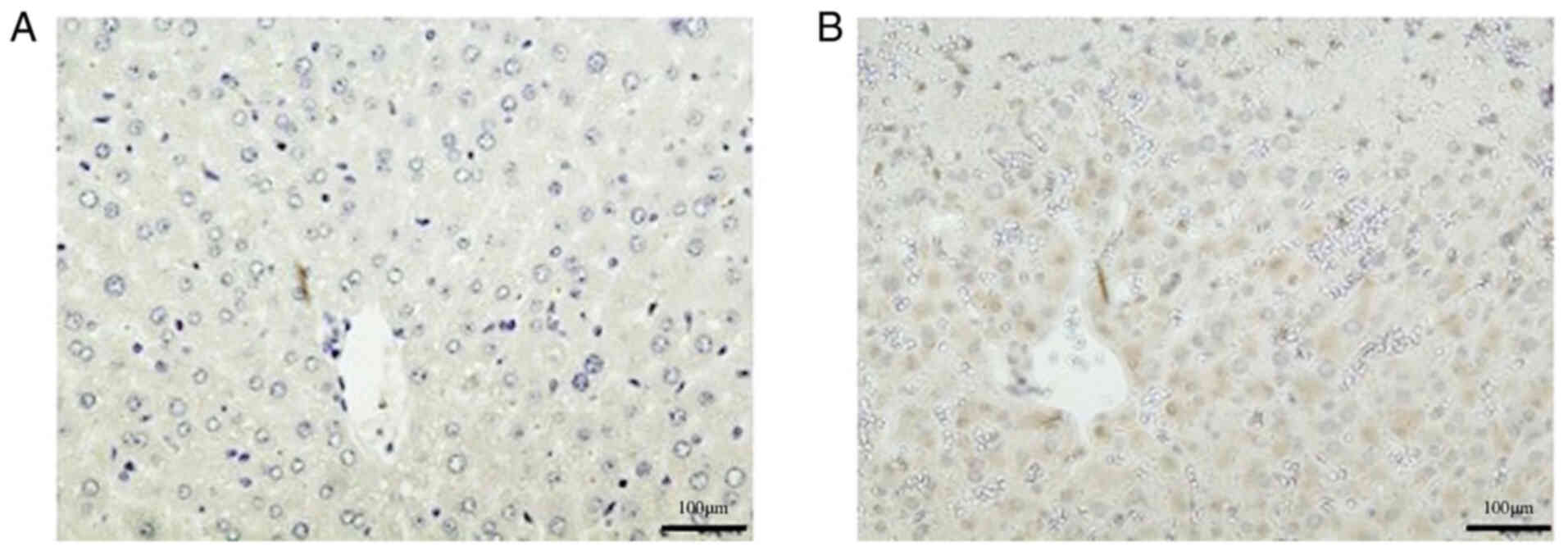
To verify these findings, immunohistochemistry was
also performed. The TPO protein expression levels were verified by
immunohistochemistry in the SOS model group 48 h after treatment
(Fig. 5). A representative image
of a slide created from multiple mice is shown. Similar images were
seen in all SOS model mice
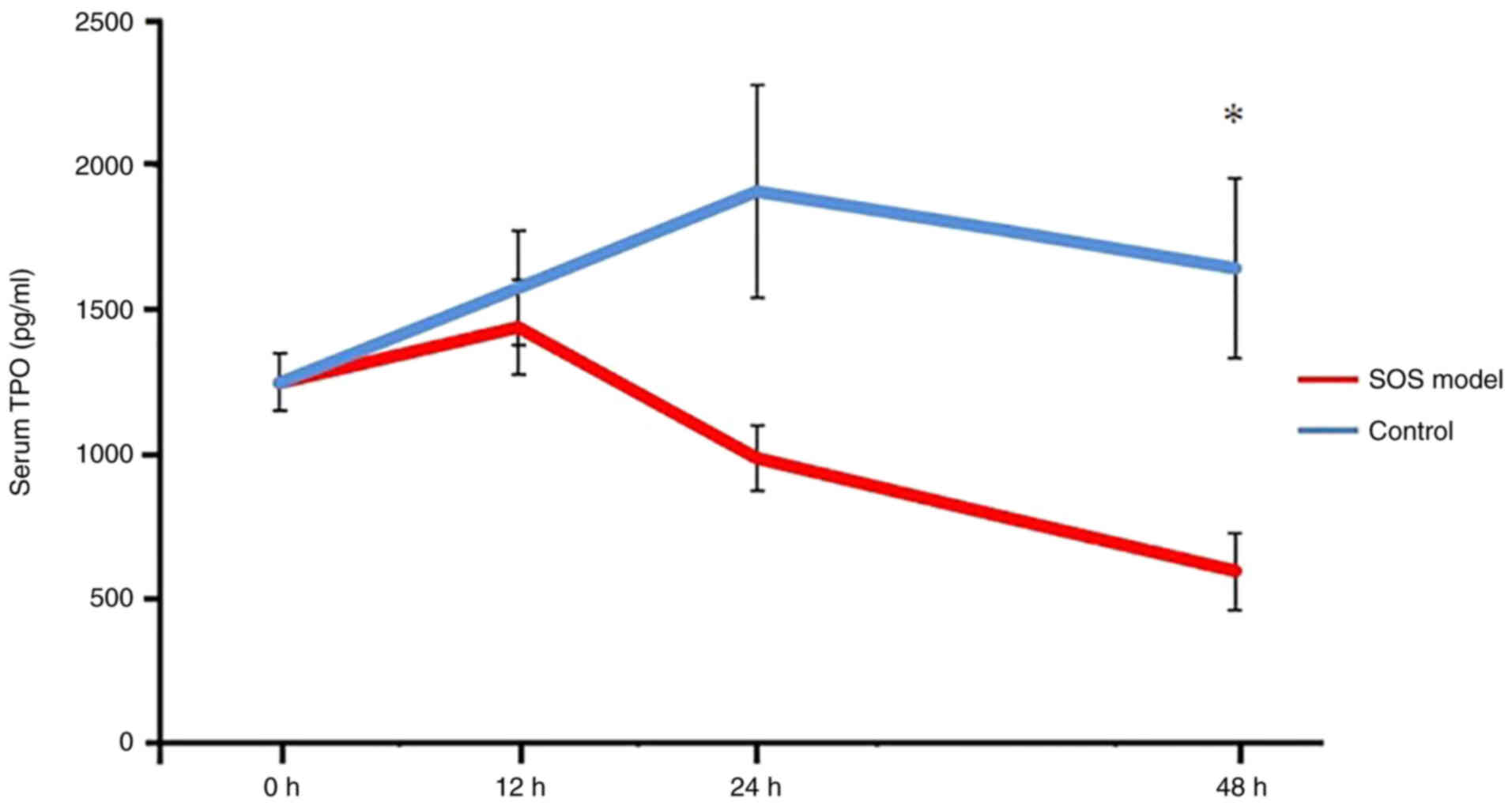
TPO serum levels are decreased in the
SOS model
In contrast to TPO protein levels in liver tissues,
the serum levels of TPO were significantly decreased in the SOS
model group at 48 h compared with in the control group. By
contrast, in the control group, TPO serum levels in peripheral
blood showed no change over time. (12 h: 1,439.3±163.5 pg/ml vs.
1,575.2±196.0 pg/ml, P=0.999; 24 h: 987.4±112.2 pg/ml vs.
1,909.7±369.3 pg/ml, P=0.091; 48 h: 596.0±133.8 pg/ml vs.
1,644±313.5 pg/ml, P=0.022; Fig.
6).
Discussion
SOS is a fatal complication following liver
transplantation, hematopoietic stem cell transplantation and
treatment with anticancer drugs. Morbidity ranges from 5 to
>50%, and the mortality rate of severe SOS is as high as 80%
(24).
In the present study, dilation of the sinusoid was
observed in the liver in a SOS mouse model after 24 h and
congestion of the sinusoid was observed after 48 h. In our previous
studies, destruction and fibrosis of LSECs were detected 48 h after
SOS induction (12–15,22).
Decreased platelet counts in the peripheral blood
are generally thought to result from hypersplenism due to portal
hypertension in patients with SOS; however, thrombocytopenia has
been reported to occur before splenomegaly (3). The cause of early thrombocytopenia
in patients with SOS could be explained by the consumption of
platelets due to EPA in the liver.
Biomarkers that predict the onset of SOS and the
effectiveness of therapies following SOS onset have not yet been
established. To the best of our knowledge, the present study was
the first to focus on TPO, which is involved in platelet
homeostasis. Aging platelets and senescent platelets in a septic
environment are desialylated, internalized by hepatocyte AMR and
induce TPO expression via the JAK2-STAT3 signaling pathway
(18). Platelets that invade the
space of Disse not only adhere to hepatocytes but are taken up by
hepatocytes in the liver of patients with SOS following liver
transplantation (2). The present
study hypothesized that when EPA-induced platelets are taken up by
hepatocytes, the serum levels of TPO may increase. In the present
study, peripheral blood platelet counts were significantly
decreased 24 h after induction of SOS in a mouse model, as
previously reported (15).
Unexpectedly, serum TPO levels were significantly reduced in the
SOS mouse model, despite increased TPO levels in the liver. This
discrepancy may be due to the prevention of TPO produced in the
liver from being transferred to the blood under SOS conditions,
resulting in TPO protein accumulation in liver tissue. Although
slightly increased, the mRNA expression levels of TPO were not
significant altered in the SOS model. Thus, TPO changes in the
liver may be largely due to the influence of physiological
expression. That is, although the increase in TPO production by
platelet uptake in the SOS model was not significant, the blood
concentration decreased as a result of inhibition of the secretion
of physiologically produced TPO.
Unlike general capillary endothelial cells, LSECs do
not have a basement membrane. Instead, the space of Disse is
present between sinusoidal endothelial cells and hepatocytes. In
addition, the combined sinusoidal endothelial cells have a number
of fenestrae with a diameter of ~100 nm in the cell gap, through
which various substances are exchanged between blood flowing
through the sinusoid and hepatocytes (25–27). Narita et al (21) reported that overexpression of CD34
within liver parenchyma was associated with an abnormal indocyanine
green retention rate. This association was due to SOS-induced liver
damage caused by chemotherapy for colorectal liver metastasis. The
SOS was caused by impaired substance exchange due to capillary
vascularization of the sinusoids. This situation, known as
sinusoidal capillarization, can result in the deposition of a dense
extracellular matrix in the space of Disse, the formation of a
basement membrane and the disappearance of small pores in the
endothelial cells (25–27). Sinusoidal capillarization in SOS
model rats was previously reported by immunostaining with CD34,
which was suppressed by PDE III inhibitors (12,13). Plasminogen activator inhibitor-1
and transforming growth factor-β released by EPA-activated
platelets have been shown to promote hepatic fibrosis but may also
contribute to sinusoidal capillarization (2,3,12,13). In the present study, TPO secretion
may be impaired by capillary angiogenesis, but at an earlier stage,
EPA aggregation may cause substance exchange failure. In the
future, further investigations, such as confirmation of platelet
localization in hepatocytes by immunostaining and TPO accumulation
by electron microscopy, are required. In addition, although the
model in the present study was a model of acute-phase SOS, further
investigation is needed for the chronic phase with fibrosis.
In conclusion, the present study analyzed the
involvement of TPO in platelet homeostasis and revealed that serum
TPO levels were significantly reduced in a mouse model of SOS
compared with those in a control group at 48 h; therefore, TPO may
be used as a biomarker for future clinical studies.
Acknowledgements
Not applicable.
Funding
This work was supported by JSPS KAKENHI (grant no.
JP20K09029).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HY, HidT, YY and TO designed this study. SM taught
the experimental methods of RT-qPCR and ELISA. HY performed the
experiments. SM, MO, YO, SN, IM, HirT and TM contributed to the
data analysis. All authors have read and approved the final
manuscript. HY and HT confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
All institutional and national guidelines for the
care and use of laboratory animals were followed. Animals were
treated in accordance with the Fundamental Guidelines for the
Proper Conduct of Animal Experiments and Related Activities in
Academic Research Institutions, under the jurisdiction of the
Ministry of Education, Culture, Sports, Science, and Technology of
Japan. All animal experiments were approved by the Committee on
Animal Experimentation of Kanazawa University (approval no.
183934).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Takamura H, Nakanuma S, Hayashi H, Tajima
H, Kakinoki K, Kitahara M, Sakai S, Makino I, Nakagawara H,
Miyashita T, et al: Severe veno-occlusive disease/sinusoidal
obstruction syndrome after deceased-donor and living-donor liver
transplantation. Transplant Proc. 46:3523–3535. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nakanuma S, Miyashita T, Hayashi H, Tajima
H, Takamura H, Tsukada T, Okamoto K, Sakai S, Makino I, Kinoshita
J, et al: Extravasated platelet aggregation in liver zone 3 may
correlate with the progression of sinusoidal obstruction syndrome
following living donor liver transplantation: A case report. Exp
Ther Med. 9:1119–1124. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tajima H, Ohta T, Miyashita T, Nakanuma S,
Matoba M, Miyata T, Sakai S, Okamoto K, Makino I, Kinoshita J, et
al: Oxaliplatin-based chemotherapy induces extravasated platelet
aggregation in the liver. Mol Clin Oncol. 3:555–558. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dignan FL, Wynn RF, Hadzic N, Karani J,
Quaglia A, Pagliuca A, Veys P and Potter MN; Haemato-oncology Task
Force of British Committee for Standards in Haematology, ; British
Society for Blood and Marrow Transplantation, : BCSH/BSBMT
guideline: Diagnosis and management of veno-occlusive disease
(sinusoidal obstruction syndrome) following haematopoietic stem
cell transplantation. Br J Haematol. 163:444–457. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tamandl D, Klinger M, Eipeldauer S,
Herberger B, Kaczirek K, Gruenberger B and Gruenberger T: Sinusoid
obstruction syndrome impairs long-term outcome of colorectal liver
metas- tases treated with resection after neoadjuvant chemotherapy.
Ann Surg Oncol. 18:421–430. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Duret-Aupy N, Lagarce L, Blouet A, Kettani
S, Conte C, Bourneau-Martin D, Drablier G, Umlil A and Briet M:
Liver sinusoidal obstruction syndrome associated with trastuzumab
emtansine treatment for breast cancer. Therapie. 74:675–677. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bornhäuser M, Illmer T, Oelschlaegel U,
Schetelig J, Ordemann R, Schaich M, Hänel M, Schuler U, Thiede C,
Kiani A, et al: Gemtuzumab ozogamicin as part of reduced-intensity
conditioning for allogeneic hematopoietic cell transplantation in
patients with relapsed acute myeloid leukemia. Clin Cancer Res.
14:5585–5593. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Efrati E, Zuckerman T, Ben-Ami E and
Krivoy N: MTHFR C677T/A1298C genotype: A possible risk factor for
liver sinusoidal obstruction syndrome. Bone Marrow Transplant.
49:726–727. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rubbia-Brandt L, Audard V, Sartoretti P,
Roth AD, Brezault C, Le Charpentier M, Dousset B, Morel P, Soubrane
O, Chaussade S, et al: Severe hepatic sinusoidal obstruction
associated with oxaliplatin-based chemotherapy in patients with
metastatic colorectal cancer. Ann Oncol. 15:460–466. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Morine Y, Shimada M and Utsunomiya T:
Evaluation and management of hepatic injury induced by
oxaliplatin-based chemotherapy in patients with hepatic resection
for colorectal liver metastasis. Hepatol Res. 44:59–69. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
DeLeve LD and Wang X: Role of oxidative
stress and glutathione in busulfan toxicity in cultured murine
hepatocytes. Pharmacology. 60:143–154. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hirata M, Tajima H, Miyashita T, Miyata T,
Nakanuma S, Makino I, Hayashi H, Oyama K, Takamura H, Ninomiya I,
et al: Extravasated platelet aggregation in the livers of rats with
drug induced hepatic sinusoidal obstruction syndrome. Mol Med Rep.
15:3147–3152. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Miyata T, Tajima H, Hirata M, Nakanuma SI,
Makino I, Hayashi H, Oyama K, Miyashita T, Takamura H, Ninomiya I,
et al: Phosphodiesterase III inhibitor attenuates rat sinusoidal
obstruction syndrome through inhibition of platelet aggregation in
Disse's space. J Gastroenterol Hepatol. 33:950–957. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Takada S, Miyashita T, Yamamoto Y, Kanou
S, Munesue S, Ohbatake Y, Nakanuma S, Okamoto K, Sakai S, Kinoshita
J, et al: Soluble thrombomodulin attenuates endothelial cell damage
in hepatic sinusoidal obstruction syndrome. In Vivo. 32:1409–1417.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kanou S, Miyashita T, Yamamoto Y, Takada
S, Nakura M, Okazaki M, Ohbatake Y, Nakanuma S, Makino I, Tajima H,
et al: Prophylactic effect of recombinant human soluble
thrombomodulin for hepatic sinusoidal obstruction syndrome model
mice. In Vivo. 34:1037–1045. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Conotte R and Colet JM: A metabonomic
evaluation of the monocrotaline-induced sinusoidal obstruction
syndrome (SOS) in rats. Toxicol Appl Pharmacol. 276:147–156. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Grozovsky R, Giannini S, Falet H and
Hoffmeister KM: Regulating billions of blood platelets: Glycans and
beyond. Blood. 126:1877–1884. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Grozovsky R, Begonja AJ, Liu K, Visner G,
Hartwig JH, Falet H and Hoffmeister KM: The Ashwell-Morell receptor
regulates hepatic thrombopoietin production via JAK2-STAT3
signaling. Nat Med. 21:47–54. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hitchcock IS and Kaushansky K:
Thrombopoietin from beginning to end. Br J Haematol. 165:259–268.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kuter DJ: New thrombopoietic growth
factors. Blood. 109:4607–4616. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Narita M, Hatano E, Ikai I,
Miyagawa-Hayashino A, Yanagida A, Nagata H, Asechi H, Taura K and
Uemoto S: A phosphodiesterase III inhibitor protects rat liver from
sinusoidal obstruction syndrome through heme oxygenase-1 induction.
Ann Surg. 249:806–813. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nakura M, Miyashita T, Yamamoto Y, Takada
S, Kanou S, Tajima H, Takamura H and Ohta T: Inhibitory effects of
beraprost sodium in murine hepatic sinusoidal obstruction syndrome.
Anticancer Res. 40:5171–5180. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yakushijin K, Atsuta Y, Doki N, Yokota A,
Kanamori H, Miyamoto T, Ohwada C, Miyamura K, Nawa Y, Kurokawa M,
et al: Sinusoidal obstruction syndrome after allogeneic
hematopoietic stem cell transplantation: Incidence, risk factors
and outcomes. Bone Marrow Transplant. 51:403–409. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Narita M, Oussoultzoglou E, Chenard MP,
Fuchshuber P, Rather M, Rosso E, Addeo P, Jaeck D and Bachellier P:
Liver injury due to chemotherapy-induced sinusoidal obstruction
syndrome is associated with sinusoidal capillarization. Ann Surg
Oncol. 19:2230–2237. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hickey PL, McLean AJ, Angus PW, Choo EF
and Morgan DJ: Increased sensitivity of propranolol clearance to
reduced oxygen delivery in the isolated perfused cirrhotic rat
liver. Gastroenterology. 111:1039–1048. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schaffner F and Poper H: Capillarization
of hepatic sinusoids in man. Gastroenterology. 44:239–242. 1963.
View Article : Google Scholar : PubMed/NCBI
|