Introduction
Immature granulocytes (IGs) include metamyelocytes,
myelocytes and promyelocytes, and are the precursors of
neutrophils. The percentage of IGs can be measured using automated
hematology analyzers, and elevated IG count values found in
peripheral blood indicate an enhanced bone marrow activity
(1).
IGs have been evaluated in numerous clinical
conditions. In a previous study, the IG count was reported as an
independent prognostic biomarker in patients with severe acute
pancreatitis (1), while in
another study, the IG count was found to be independently
associated with the development of acute respiratory distress
syndrome in patients with acute pancreatitis (2). Furthermore, according to certain
studies, the IG count is a useful marker for discriminating between
infected and non-infected patients early during systemic
inflammatory response syndrome (3); a low IG count can exclude sepsis
with high specificity, and the IG count is more positively
associated with infections and positive blood cultures than the
white blood cell count (4,5).
Moreover, a post-operative increase in the IG count has been shown
to be associated with post-operative organ failure and may be used
to identify patients who are at risk of developing infectious
complications following open-heart surgery under cardiopulmonary
bypass (6).
Neutrophils are leukocytes derived from bone marrow,
and are considered to play a major role in the host defense during
bacterial and fungal infections. Neutrophils are also involved in
the antiviral immune response, and are the first and predominant
cell population that reaches affected tissues after viral infection
(7). The beneficial and harmful
effects of neutrophils in viral infections, as well as the
antiviral mechanisms of neutrophils have been previously reported
(7,8). The role of neutrophils has been
mostly studied in the context of influenza A virus infection, a
human respiratory virus that causes severe disease with high rates
of mortality, particularly among the elderly (9). The importance of neutrophil function
has also been studied in infections from other viruses, such as
herpes simplex virus types 1 and 2, cytomegalovirus, vesicular
stomatitis virus (9), West Nile
virus (10) and respiratory
syncytial virus (11).
Coronavirus disease 2019 (COVID-19) is an infectious
disease caused by severe acute respiratory coronavirus 2
(SARS-CoV-2) (12), which was
first identified in December, 2019 in Wuhan, China, and has since
spread globally, resulting in an ongoing pandemic (13). Several researchers have reported
the role of neutrophils in COVID-19 infection, demonstrating that
the percentage of neutrophils along with other markers may be
predictors of the severity of COVID-19 infection (14,15) and that an increased neutrophil
count and neutrophil-to-lymphocyte ratio are predominant in
critical cases or non-survivors (16).
However, to date, there has been limited research
concerning the role of neutrophil precursors in viral infections,
including SARS-CoV-2 infection, at least to the best of our
knowledge. The present study thus aimed to evaluate the role of the
IG count in patients with COVID-19 infection.
Materials and methods
Study design
The design of the present study was prospective.
Data were collected at the ‘Laiko General Hospital’ between
October, 2020 and June, 2021. The present study was approved by the
Institutional Board of Laiko General Hospital (protocol no. 716)
and was in line with the declaration of Helsinki in 1995 (as
revised in Edinburgh 2000). Written informed was obtained from all
patients.
Study participants and data
collection
In the present study, adult patients who visited or
were hospitalized at the COVID-19 Unit of Laiko General Hospital
due to SARS-CoV-2 infection, were enrolled. The patients were
predominantly infected with the alpha variant and were all
unvaccinated. All patients were treated according to the National
Institutes of Health (NIH) protocols (17). Patients suffering from a disease
or receiving medication that affects bone marrow neutrophil
production were excluded from the study. More specifically, the
exclusion criteria were the following: Hematological disorders,
active cancer with or without chemotherapy or radiation treatment,
autoimmune disorders and the administration of immunosuppressive
agents. SARS-CoV-2 infection was confirmed by the positive
detection of SARS-CoV-2 nucleic acid in examined nasopharyngeal
samples with the use of reverse transcription-polymerase chain
reaction (RT-PCR). Whole blood from the participants upon admission
was collected and used for the measurement of the IG count using an
automated Sysmex ΧΕ 2100 hematology analyzer (TOA Medical
Electronics). The patients were classified into the following
severity of illness categories: Mild/moderate, severe and critical
based on the clinical spectrum of SARS-CoV-2 infection (17).
The following data were collected from all patients:
i) Demographics: Age and sex; ii) the presence of comorbidities;
iii) outcomes (recovery, intubation, mortality); iv) the duration
of hospitalization; v) the development of disease-related
complications (pulmonary embolism, myocardial infarction,
hemorrhage, mesenteric ischemia, pneumothorax, hematological
complications). The IG count was associated with disease severity,
outcomes, the duration of hospitalization and the development of
complications.
Statistical analysis
Categorical variables are summarized as the number
(percentage) and continuous variables as the mean ± standard
deviation. The normal distribution of variables was assessed using
the Kolmogorov-Smirnov test. Normally distributed variables were
compared using an independent samples Student's t-test on factors
with two groups and one-way analysis of variance (ANOVA) with
Bonferroni post hoc pairwise comparisons on factors with three
groups. Multivariate logistic regression analysis was performed to
identify independent variables. Odds ratios (ORs) with 95%
confidence intervals (CIs) are presented. Values of P<0.05 were
considered to indicate statistically significant differences.
Statistical analysis was performed using IBM SPSS-Statistics
version 26.0 (IBM Corp.).
Results
A total of 1,005 patients, 581 (57.8%) males and 424
(42.2%) females with COVID-19 were enrolled in the study. The mean
age of the patients was 62.07±16.83 years. A total of 798 (79.4%)
patients had comorbidities. In addition, 134 (13.1%) patients had
mild/moderate disease, 627 (62.4%) patients had severe disease and
243 (24.2%) patients had critical disease. Furthermore, 712 (70.8%)
patients had a duration of hospitalization >10 days, 27 (2.7%)
patients developed complications, 92 (9.2%) patients were intubated
and 142 (14.1%) patients did not survive (Table I).
 | Table I.Characteristics of the study
population. |
Table I.
Characteristics of the study
population.
| Characteristic | No. of patients (%),
n=1,005 |
|---|
| Sex |
|
| Male | 581 (57.8) |
|
Female | 424 (42.2) |
| Age (years ± SD) | 62.07±16.83 |
| Disease severity |
|
|
Mild/moderate | 134 (13.1%) |
|
Severe | 627 (62.4%) |
|
Critical | 243 (24.2%) |
| Outcomes |
|
|
Hospitalization >10
days | 712 (70.8%) |
|
Complications | 27 (2.7%) |
|
Recovery | 863 (85.9%) |
|
Intubation | 92 (9.2%) |
|
Mortality | 142 (14.1%) |
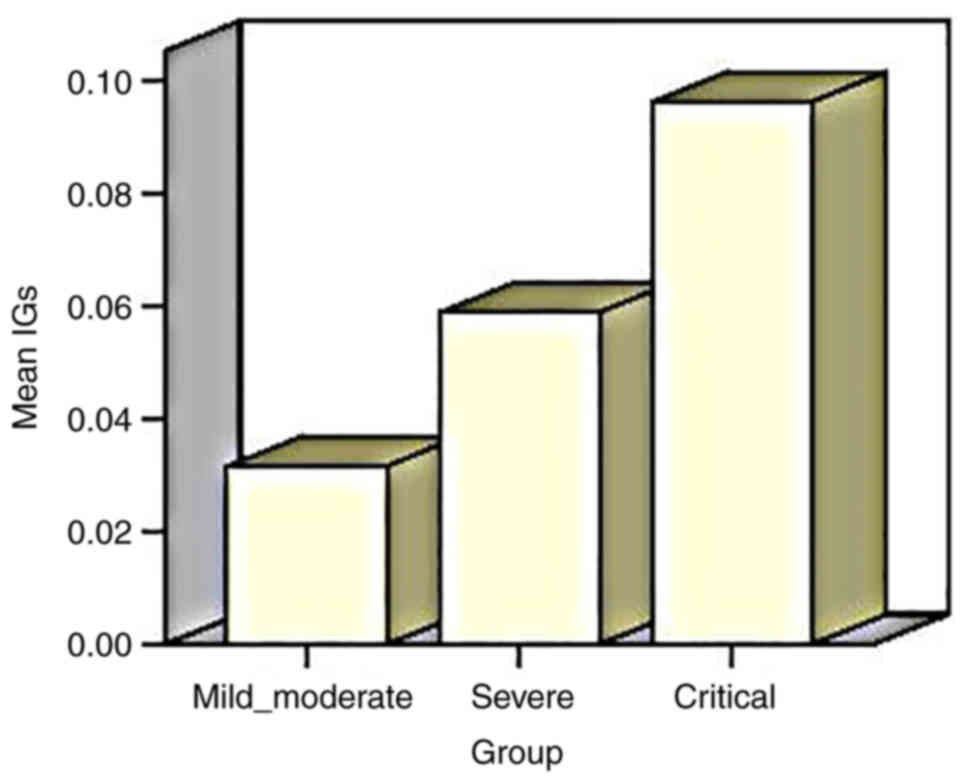
The mean IG count was 0.03±0.02×109/l in
patients with mild/moderate disease, 0.05±0.10×109/l in
patients with severe disease and 0.09±0.15×109/l in
patients with critical disease. There was a statistically
significant difference in the mean values of the IG counts between
the three groups of disease severity, with the greatest mean value
of IG observed in patients with critical disease (P=0.0001)
(Fig. 1 and Table II).
 | Table II.Mean IG counts in the study
groups. |
Table II.
Mean IG counts in the study
groups.
| Study group | IG count (mean±SD
×109/l) | P-value |
|---|
| Mild/moderate | 0.03±0.02 | 0.0001 |
| Severe | 0.05±0.10 |
|
| Critical | 0.09±0.15 |
|
| Hospitalization
<10 days | 0.05±0.09 | 0.029 |
| Hospitalization
>10 days | 0.07+0.12 |
|
| Complications | 0.07±0.06 | 0.66 |
| No
complications | 0.06±0.11 |
|
| Intubation | 0.11±0.15 | 0.002 |
| No intubation | 0.06±0.10 |
|
| Mortality | 0.11±0.15 | 0.001 |
| Recovery | 0.05±0.10 |
|
The mean IG count was 0.05±0.09×109/l in
patients with a duration of hospitalization <10 days and
0.07+0.12×109/l in patients with a duration of
hospitalization >10 days. There was a statistically significant
difference in the mean values of IG counts between the patients
with a duration of hospitalization >10 and <10 days (P=0.029;
Table II).
The mean IG count was 0.07±0.06×109/l in
patients who developed complications and 0.06±0.11×109/l
in patients who did not develop complications. Although the mean
value of IG count was higher in patients who developed
complication, no statistically significant difference was observed
(P=0.66; Table II).
The mean IG count was 0.11±0.15×109/l in
patients who were intubated and 0.06±0.10×109/l in
patients that were not. There was a statistically significant
difference in the mean IG count between patients who were intubated
and patients who were not intubated (P=0.002; Table II).
The mean IG count was 0.11±0.15×109/l in
patients who did not survive and 0.05±0.10×109/l in
patients who recovered. There was a statistically significant
difference in the mean IG count between patients who did not
survive and patients who recovered (P=0.001; Table II).
Following multivariate logistic regression analysis,
including as confounders age, male sex and the presence of
comorbidities, an independent association was found between the IG
count and intubation (OR, 13.98; 95% CI, 3.7-52.83; P= 0.003)
(Table III). An independent
association was also detected between the IG count and mortality
(OR, 42.17; 95% CI, 10.23-173.85; P= 0.001) (Table IV).
 | Table III.Multivariate logistic regression
analysis of factors independently associated with intubation. |
Table III.
Multivariate logistic regression
analysis of factors independently associated with intubation.
| Parameter | Odds ratio | 95% Confidence
interval | P-value |
|---|
| Age | 1.854 | 1.160-2.964 | 0.010 |
| Male sex | 1.021 | 1.006-1.036 | 0.006 |
| IGs | 13.986 | 3.703-52.823 | 0.003 |
| Comorbidities | 3.795 | 1.458-9.879 | 0.006 |
 | Table IV.Multivariate logistic regression
analysis of factors independently associated with mortality. |
Table IV.
Multivariate logistic regression
analysis of factors independently associated with mortality.
| Parameter | Odds ratio | 95% Confidence
interval | P-value |
|---|
| Age | 1.084 | 1.066-1.102 | 0.001 |
| Male sex | 2.237 | 1.463-3.421 | 0.001 |
| IGs | 42.173 | 10.230-173.856 | 0.001 |
| Comorbidities | 7.409 | 1.732-31.695 | 0.007 |
Discussion
Severe stressors, including sepsis, trauma and viral
infections, can trigger emergency granulopoiesis, a hematological
response mechanism that rapidly enhances de novo neutrophil
production to cope with increased demands (18). This process leads to the presence
of both immature and mature neutrophils in the peripheral
circulation, which can have immunosuppressive or pro-inflammatory
effects (19). Despite a lack of
understanding of the role of mature and immature neutrophils in the
immune response, as well as their distinct characteristics,
clinical interest in these cells is increasing due to their
increasingly apparent association with disease severity and
treatment response in a variety of pathologies, including sepsis
and severe influenza (20). It
has been reported that the process of emergency granulopoiesis and
the availability of numerous freshly generated granulocytes may
increase their destructive capacity (21).
According to the results of the present study, the
IG count was associated with disease severity, with greater IG
count values being detected in severe and critical cases. In
addition, greater IG count values were found to be associated with
a longer duration of hospitalization. Furthermore, the IG count was
an independent prognostic biomarker of intubation and mortality in
patients with COVID-19.
Several studies have demonstrated the efficacy of
standard blood tests performed upon patient admission to the
hospital for the diagnosis and prediction of the severity of
COVID-19 infection (15,22–24). The IG% is a metric that is poorly
understood and underutilized by clinicians. In a routine blood
count, this parameter may be measured inexpensively and swiftly
(22). The early release of
immature neutrophils from bone marrow into peripheral blood has
been linked to inflammation, particularly in numerous infectious
diseases (24). As a result, the
IG% may be a reliable biomarker, which may be extremely useful in
the context of the prediction of the severity of COVID-19 infection
and patient outcomes. Moreover, the half-life of IGs is 3 h and a
short half-life marker easily reflects the inflammation status
compared to other parameters with a longer half-life (25).
The IG count has been reported as an indicator of
the severity of COVID-19 infection in a some studies.
Kuri-Cervantes et al (26)
demonstrated that patients with severe COVID-19 infection were
distinguished from those with mild or moderate infection by the
extensive induction and activation of various immune lineages,
including the modulation of innate lymphocytes and granulocytes,
manifested as changes in the frequency and phenotype of circulating
IGs. Carissimo et al (24)
performed the comprehensive flow cytometric analysis of whole blood
samples from patients with SARS-CoV-2, which revealed a significant
increase in the number of IGs. This increase was found to be
prominently associated with disease severity (24). Schulte-Schrepping et al
(27), in their study, mentioned
that severe COVID-19 infection was marked by the presence of
neutrophil precursors, as evidence of emergency myelopoiesis,
indicating that COVID-19 induces marked alterations in the
neutrophil compartment.
According to the study by Combadière et al
(28), increased proportions of
circulating IGs expressing either CD123 or lectin-like oxidized
low-density lipoprotein receptor-1 in critical COVID-19 cases were
associated with disease severity and thromboembolic complications.
In addition, in their study on 368 patients with SARS-CoV-2
infection, Myari et al (22) discovered that the IG count may be
a useful indicator of critical disease.
The delta neutrophil index (DNI) represents a marker
obtained by calculating the fraction of circulating IGs. Birben
et al (29), in their
study on 388 patients with COVID-19 requiring intensive care,
concluded that the DNI could be used as an effective prognostic
biomarker for mortality in these patients. Moreover, according to
the study by Karagol et al (30), there was a statistically
significant association between the DNI levels and the severity of
multisystem inflammatory syndrome in children with COVID-19.
Notably, Daix et al (31) reported that the IG count could aid
in the identification of pulmonary bacterial infections in patients
in the intensive care unit hospitalized for acute respiratory
distress syndrome caused by COVID-19. To the best of our knowledge,
the present study is the first to mention a statistically
significant association between the IG count and the duration of
hospitalization, and between the IG count and intubation and
mortality in patients hospitalized in general hospital wards due to
COVID-19.
However, there are some limitations to the present
study. Although the strong points of the study are the large number
of participants and reliable follow-up, this was a single-center
study. In addition, patients vulnerable to SARS-CoV-2 infection,
such as those suffering from hematological disorders, active cancer
and autoimmune disorders were excluded. Thus, further comprehensive
multicenter, prospective studies are required for more detailed
results.
In conclusion, the present study demonstrates that
the IG count is associated with the severity of COVID-19 infection,
with greater IG count values observed in severe and critical cases.
In addition, greater IG count values were found to be associated
with a longer duration of hospitalization. Furthermore, the IG
count was found to be an independent prognostic indicator of
intubation and mortality in patients with COVID-19.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
AG, DAS and PP conceptualized the study. VEG, SM,
MT, SS, PMV, CVP, AA and NVS were involved in the design of the
study and prepared the draft of the manuscript. VEG and NVS
provided critical revisions. PS, GC, NT and EX obtained the data,
and prepared the tables and figures. VEG and NVS confirm the
authenticity of all the data. All authors contributed to manuscript
revision and have read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Ethical approval for this study was obtained from
the Research Ethics Committee of Laiko General Hospital (protocol
no. 716). The study was in line with the declaration of Helsinki in
1995 (as revised in Edinburgh 2000). Written informed consent was
obtained from all patients. A copy of the written consent is
available for review by the Editor-in-Chief of this journal on
request.
Patient consent for publication
Not applicable.
Competing interests
DAS is the Editor-in-Chief for the journal, but had
no personal involvement in the reviewing process, or any influence
in terms of adjudicating on the final decision, for this article.
The other authors declare that they have no competing
interests.
References
|
1
|
Lipiński M and Rydzewska G: Immature
granulocytes predict severe acute pancreatitis independently of
systemic inflammatory response syndrome. Prz Gastroenterol.
12:140–144. 2017.
|
|
2
|
Huang Y, Xiao J, Cai T, Yang L, Shi F,
Wang Y, Li Y, Shi T, Li C, Peng Y, et al: Immature granulocytes: A
novel biomarker of acute respiratory distress syndrome in patients
with acute pancreatitis. J Crit Care. 50:303–308. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nierhaus A, Klatte S, Linssen J, Eismann
NM, Wichmann D, Hedke J, Braune SA and Kluge S: Revisiting the
white blood cell count: Immature granulocytes count as a diagnostic
marker to discriminate between SIRS and sepsis-a prospective,
observational study. BMC Immunol. 14:82013. View Article : Google Scholar
|
|
4
|
Ayres LS, Sgnaolin V and Munhoz TP:
Immature granulocytes index as early marker of sepsis. Int J Lab
Hematol. 41:392–396. 2019. View Article : Google Scholar
|
|
5
|
Ansari-Lari MA, Kickler TS and Borowitz
MJ: Immature granulocyte measurement using the Sysmex XE-2100.
Relationship to infection and sepsis. Am J Clin Pathol.
120:795–799. 2003. View Article : Google Scholar
|
|
6
|
Daix T, Guérin E, Tavernier E, Marsaud JP,
Hacan A, Gauthier F, Piccardo A, Vignon P, Feuillard J and François
B: Immature granulocytes: A risk factor of infection after cardiac
surgery. Cytometry B Clin Cytom. 94:887–894. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Galani IE and Andreakos E: Neutrophils in
viral infections: Current concepts and caveats. J Leukoc Biol.
98:557–564. 2015. View Article : Google Scholar
|
|
8
|
Naumenko V, Turk M, Jenne CN and Kim SJ:
Neutrophils in viral infection. Cell Tissue Res. 371:505–516. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Daher KA, Selsted ME and Lehrer RI: Direct
inactivation of viruses by human granulocyte defensins. J Virol.
60:1068–1074. 1986. View Article : Google Scholar
|
|
10
|
Bai F, Kong KF, Dai J, Qian F, Zhang L,
Brown CR, Fikrig E and Montgomery RR: A paradoxical role for
neutrophils in the pathogenesis of West Nile virus. J Infect Dis.
202:1804–1812. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Faden H, Hong JJ and Ogra PL: Interaction
of polymorphonuclear leukocytes and viruses in humans: Adherence of
polymorphonuclear leukocytes to respiratory syncytial
virus-infected cells. J Virol. 52:16–23. 1984. View Article : Google Scholar
|
|
12
|
Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J,
Wang B, Xiang H, Cheng Z, Xiong Y, et al: Clinical characteristics
of 138 hospitalized patients with 2019 novel coronavirus-infected
pneumonia in Wuhan, China. JAMA. 323:1061–1069. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hui DS, I Azhar E, Madani TA, Ntoumi F,
Kock R, Dar O, Ippolito G, Mchugh TD, Memish ZA, Drosten C, et al:
The continuing 2019-nCoV epidemic threat of novel coronaviruses to
global health-the latest 2019 novel coronavirus outbreak in Wuhan,
China. Int J Infect Dis. 91:264–266. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu Y, Yang Y, Zhang C, Huang F, Wang F,
Yuan J, Wang Z, Li J, Li J, Feng C, et al: Clinical and biochemical
indexes from 2019-nCoV infected patients linked to viral loads and
lung injury. Sci China Life Sci. 63:364–374. 2020. View Article : Google Scholar
|
|
15
|
Georgakopoulou VE, Lembessis P, Skarlis C,
Gkoufa A, Sipsas NV and Mavragani CP: Hematological abnormalities
in COVID-19 disease association with type I interferon pathway
activation and disease outcomes. Front Med (Lausanne).
9:8504722022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen R, Sang L, Jiang M, Yang Z, Jia N, Fu
W, Xie J, Guan W, Liang W, Ni Z, et al: Longitudinal hematologic
and immunologic variations associated with the progression of
COVID-19 patients in China. J Allergy Clin Immunol. 146:89–100.
2020. View Article : Google Scholar
|
|
17
|
National Institutes of Health, . COVID-19
treatment guidelines panel: Coronavirus disease 2019 (COVID-19)
treatment guidelines. https://www.covid19treatmentguidelines.nih.gov/October
20–2021
|
|
18
|
Scapini P, Marini O, Tecchio C and
Cassatella MA: Human neutrophils in the saga of cellular
heterogeneity: Insights and open questions. Immunol Rev. 273:48–60.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Manz MG and Boettcher S: Emergency
granulopoiesis. Nat Rev Immunol. 14:302–314. 2014. View Article : Google Scholar
|
|
20
|
De Santo C, Salio M, Masri SH, Lee LY,
Dong T, Speak AO, Porubsky S, Booth S, Veerapen N, Besra GS, et al:
Invariant NKT cells reduce the immunosuppressive activity of
influenza A virus-induced myeloid-derived suppressor cells in mice
and humans. J Clin Invest. 118:4036–4048. 2008. View Article : Google Scholar
|
|
21
|
Reusch N, De Domenico E, Bonaguro L,
Schulte-Schrepping J, Baßler K, Schultze JL and Aschenbrenner AC:
Neutrophils in COVID-19. Front Immunol. 12:6524702021. View Article : Google Scholar
|
|
22
|
Myari A, Papapetrou E and Tsaousi C:
Diagnostic value of white blood cell parameters for COVID-19: Is
there a role for HFLC and IG? Int J Lab Hematol. 44:104–111. 2022.
View Article : Google Scholar
|
|
23
|
Georgakopoulou VE, Garmpis N, Damaskos C,
Valsami S, Dimitroulis D, Diamantis E, Farmaki P, Papageorgiou CV,
Makrodimitri S, Gravvanis N, et al: The impact of peripheral
eosinophil counts and eosinophil to lymphocyte ratio (ELR) in the
clinical course of COVID-19 patients: A retrospective study. In
Vivo. 35:641–648. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Carissimo G, Xu W, Kwok I, Abdad MY, Chan
YH, Fong SW, Puan KJ, Lee CY, Yeo NK, Amrun SN, et al: Whole blood
immunophenotyping uncovers immature neutrophil-to-VD2 T-cell ratio
as an early marker for severe COVID-19. Nat Commun. 11:52432020.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nahm CH, Choi JW and Lee J: Delta
neutrophil index in automated immature granulocyte counts for
assessing disease severity of patients with sepsis. Ann Clin Lab
Sci. 38:241–246. 2008.
|
|
26
|
Kuri-Cervantes L, Pampena MB, Meng W,
Rosenfeld AM, Ittner CAG, Weisman AR, Agyekum RS, Mathew D, Baxter
AE, Vella LA, et al: Comprehensive mapping of immune perturbations
associated with severe COVID-19. Sci Immunol. 5:eabd71142020.
View Article : Google Scholar
|
|
27
|
Schulte-Schrepping J, Reusch N, Paclik D,
Baßler K, Schlickeiser S, Zhang B, Krämer B, Krammer T, Brumhard S,
Bonaguro L, et al: Severe COVID-19 is marked by a dysregulated
myeloid cell compartment. Cell. 182:1419–1440.e23. 2020. View Article : Google Scholar
|
|
28
|
Combadière B, Adam L, Guillou N, Quentric
P, Rosenbaum P, Dorgham K, Bonduelle O, Parizot C, Sauce D, Mayaux
J, et al: LOX-1-expressing immature neutrophils identify
critically-Ill COVID-19 patients at risk of thrombotic
complications. Front Immunol. 12:7526122021. View Article : Google Scholar
|
|
29
|
Birben B, Birben OD, Akın T, Akkurt G,
Surel AA, Yakısık E and Erdem D: Efficacy of the delta neutrophil
index in predicting 30-day mortality in COVID-19 patients requiring
intensive care. Int J Clin Pract. 75:e139702021. View Article : Google Scholar
|
|
30
|
Karagol C, Tehci AK, Gungor A, Ekici Tekin
Z, Çelikel E, Aydın F, Kurt T, Sezer M, Tekgöz N, Coşkun S, et al:
Delta neutrophil index and C-reactive protein: A potential
diagnostic marker of multisystem inflammatory syndrome in children
(MIS-C) with COVID-19. Eur J Pediatr. 181:775–781. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Daix T, Jeannet R, Hernandez Padilla AC,
Vignon P, Feuillard J and François B: Immature granulocytes can
help the diagnosis of pulmonary bacterial infections in patients
with severe COVID-19 pneumonia. J Intensive Care. 9:582021.
View Article : Google Scholar : PubMed/NCBI
|