Introduction
Intervertebral disc degeneration (IDD) is considered
to be the root cause of the occurrence and development of
intervertebral disc (IVD) herniation, and its occurrence is
affected by a series of factors, including genetic susceptibility,
cell senescence, mechanical load, matrix degradation, inflammation
and apoptosis (1). With the
increasing aging population in the world, IDD has become the
leading cause of spinal-related disability worldwide (2). However, the disease cannot be
alleviated through drug therapy or surgical treatment in the clinic
at present (3). Therefore, there
is an urgent need for an effective treatment to alleviate the
progression of IDD.
IVD is an avascular organ composed of peripheral
ring and central nucleus pulposus, of which human nucleus pulposus
cells (HNPCs) are responsible for regulating the synthesis and
decomposition of extracellular matrix (ECM) components (4). In the pathogenesis of IDD, the
reduction in the number of NPCs and the loss of ECM are important
features (5). Previous studies
have shown that the process of IDD is closely associated with
inflammatory reactions (6,7).
The nucleus pulposus secretes pro-inflammatory molecules, such as
tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6 and IL-17,
of which TNF-α is the most prominent, promoting the degradation of
ECM, and leading to cell phenotypic changes and a series of
degenerative events (8). In
addition, endoplasmic reticulum (ER) stress is also one of the
potential factors for the induction of IDD by inducing NPC
apoptosis and ECM degradation (9). Thus, finding effective drugs to
inhibit ECM degradation, inflammatory response and ER
stress-induced apoptosis in HNPCs may be a feasible strategy for
the prevention and treatment of IDD.
Hyperoside is an active flavonoid glycoside present
in numerous medicinal plants such as Epimedium, Hypericum
perforatum and Hypericum (10). Previous studies have shown that
hyperoside has a wide range of pharmacological effects, including
anti-inflammation (11),
anti-oxidation (12) and
anti-apoptosis (13). For
example, hyperoside plays an anti-inflammatory role in
sepsis-related cardiac insufficiency (14), acute lung injury (15) and acute liver injury (16). In addition, hyperoside was able to
attenuate the IL-1β-induced ECM destruction of chondrocytes
(10). However, the role of
hyperoside in IDD has not been investigated thus far.
Sirtuin-1 (SIRT1) is an NAD+-dependent
deacetylase that functions in a variety of inflammatory and immune
responses (17). A previous study
suggested that upregulating SIRT1 inhibited the IL-1β-stimulated
apoptosis and inflammation of NPCs by activating the PI3K/Akt
signaling pathway, and regulated ECM remodeling (18). In addition, SIRT1 inhibited the
IL-1β-mediated inflammatory response in HNPCs by regulating the
Toll-like receptor (TLR)2/SIRT1/NF-κB pathway (19). Notably, hyperoside was able to
reduce lipopolysaccharide (LPS)-induced inflammation, oxidative
stress and apoptosis by upregulating SIRT1 (20). A pervious study showed that
hyperoside could attenuate H2O2-induced L02
cell damage by activating the nuclear factor E2-related factor 2
(Nrf2)-antioxidant responsive element (ARE) signaling pathway
(21). Hou et al (22) reported that hyperoside showed a
protective effect on myocardial ischemia-reperfusion injury by
inhibiting ER stress and activating the Nrf2 signaling pathway, and
activating the Kelch-like ECH-associated protein 1 (Keap1)/Nrf2/ARE
signaling pathway was conducive to reducing oxidative stress-IDD
degeneration (23). Thus, it was
hypothesized that hyperoside may play a protective role in IDD by
regulating the SIRT1/NF-κB and Nrf2/ARE signaling pathways.
As aforementioned, the purpose of the present study
was to investigate the effects of hyperoside on TNF-α-induced
apoptosis of NPCs, ECM degradation and inflammatory response, as
well as ER stress, and to assess the underlying mechanism. The
present results may provide a new basis for understanding the
molecular mechanism of the occurrence and development of IDD, and
may suggest the possibility of hyperoside becoming a candidate drug
for the treatment of IDD.
Materials and methods
Cell culture
HNPCs were obtained from AcceGen Biotechnology (cat.
no. ABI-TC102D). The cells were cultured in DMEM with F12 nutrient
mixture (Gibco; Thermo Fisher Scientific, Inc.) supplemented with
10% fetal bovine serum (HyClone; Cytiva), 100 U/ml penicillin and
100 µg/ml streptomycin (Invitrogen; Thermo Fisher Scientific, Inc.)
at 37°C in a humidified atmosphere with 5% CO2. Cells in
logarithmic growth phase were used for subsequent experiments.
Cell Counting Kit-8 (CCK-8) assay
A CCK-8 assay was performed to assess cell
viability. Briefly, cells were inoculated in 96-well plates at a
density of 8×103 cells/well, and then treated with
different concentrations (10, 20 and 50 µM) of hyperoside (Beijing
Solarbio Science & Technology Co., Ltd.; cat. no: 482-36-0;
Purity ≥98%) (20), 50 ng/ml
TNF-α, 1 µM EX527 (24) or 5 µM
ML385 (25) for 24 h. Next, 10 µl
CCK-8 reagent was added into each well, and the cells were cultured
for additional 4 h at 37°C with 5% CO2. The absorbance
in each well was then measured at a wavelength of 450 nm by using a
microplate reader (BioTek Instruments, Inc.).
Flow cytometric analysis
To quantitatively assess the induced apoptotic cell
death rate, an annexin V-FITC apoptosis detection assay was
performed according to the manufacturer's protocol (Beyotime
Institute of Biotechnology). Briefly, cells were inoculated on a
si-well plate and cultured with different treatments for 24 h at
37°C and 5% CO2. Subsequently, the cells were collected,
washed twice with PBS and resuspended in 500 µl with 1X binding
buffer at a concentration of 1×106 cells/ml prior to the
addition of 5 µl annexin V-FITC. The cells were then gently
vortexed and incubated for 20 min at room temperature in the dark.
Next, 10 µl of propidium iodide (PI) was added to and incubated for
additional 5 min at room temperature in the dark. The stained cells
were analyzed using a flow cytometer (BD FACSCalibur; BD
Biosciences), and labeled as viable (annexin V and PI negative),
early apoptotic (annexin V positive and PI negative) or late
apoptotic (annexin V and PI positive). The data were analyzed with
FlowJo software (version 10.2; FlowJo LLC).
ELISA
ELISA kits (Beyotime Institute of Biotechnology)
were applied to detect the levels of the inflammatory cytokines
IL-6 (cat. no. P1326) and IL-1β (cat. no. PI305).
Western blotting
Cells from each group were collected, and total
protein was extracted using RIPA lysis buffer (Beijing Solarbio
Science & Technology Co., Ltd.). Protein concentration was
determined using the BCA Protein Detection kit (Beyotime Institute
of Biotechnology) according to the manufacturer's protocol. Total
protein (30 µg per lane) was separated by 12% SDS-PAGE and
transferred into a PVDF membrane. The membranes were blocked in 5%
non-fat milk at room temperature for 4 h. Upon washing 3 times with
1X TBS-0.1% Tween 20 for 5 min each, the following primary
antibodies (all purchased from Abcam) were added to the membrane
and incubated overnight at 4°C: Anti-Bcl-2 (1:1,000; cat. no.
Ab32124), anti-Bax (1:1,000; cat. no. Ab32503),
anti-glucose-regulated protein (GRP)78 (1:1,000; cat. no. Ab21685),
anti-phosphorylated (p)-protein kinase RNA-like ER kinase (PERK)
(1:5,000; cat. no. Ab192591), anti-activating transcription factor
6 (ATF6) (1:1,000; cat. no. Ab122897), anti-the C/EBP homologous
protein (CHOP) (1:1,000; cat. no. Ab11419), anti-caspase 12
(1:1,000; cat. no. Ab62484), anti-PERK (1:1,000; cat. no.
Ab229912), anti-inducible nitric oxide synthase (iNOS) (1:10,000;
cat. no. Ab178945), anti-cyclooxygenase (COX)-2 (1:5,000; cat. no.
Ab62331), anti-aggrecan (1:1,000; cat. no. Ab3778), anti-collagen
II (1:1,000; cat. no. Ab34712), anti-MMP3 (1:1,000; cat. no.
Ab52915), anti-MMP13 (1:10,000; cat. no. Ab219620), anti-a
disintegrin and metalloproteinase with thrombospondin motifs
(ADAMTS)5 (1:5,000; cat. no. Ab41037), anti-SIRT1 (1:1,000; cat.
no. Ab110304), anti-p-NF-κB (1:1,000; cat. no. Ab239882),
anti-NF-κB (1:1,000; cat. no. Ab220803), anti-Nrf2 (1:1,000; cat.
no. Ab137550), anti-heme oxygenase-1 (HO-1) (1:10,000; cat. no.
Ab52947), anti-NAD(P)H quinone dehydrogenase 1 (NQO1) (1:5,000;
cat. no. Ab80588) and anti-GAPDH (1:1,000; cat. no. Ab8245).
Subsequently, the membranes were incubated with a horseradish
peroxidase-conjugated secondary antibody (cat. no. Ab150077;
1:5,000) at room temperature for 4 h. Protein bands were visualized
using an ECL solution and imaged with a gel imager (C150; Azure
Biosystems, Inc.). The gray value of the protein bands was analyzed
with ImageJ (version 1.51; National Institutes of Health), and
GAPDH as used as the loading control for normalization.
Statistical analysis
All data were analyzed using GraphPad Prism 7
software (GraphPad Software, Inc.). Data are presented as the mean
± SD from ≥3 independent experimental repeats. Statistical
differences between 2 groups were compared using an unpaired
Student's t-test, statistical differences among ≥2 groups were
compared using one-way ANOVA followed by Tukey's post hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Hyperoside enhances TNF-α-induced
HNPCs viability
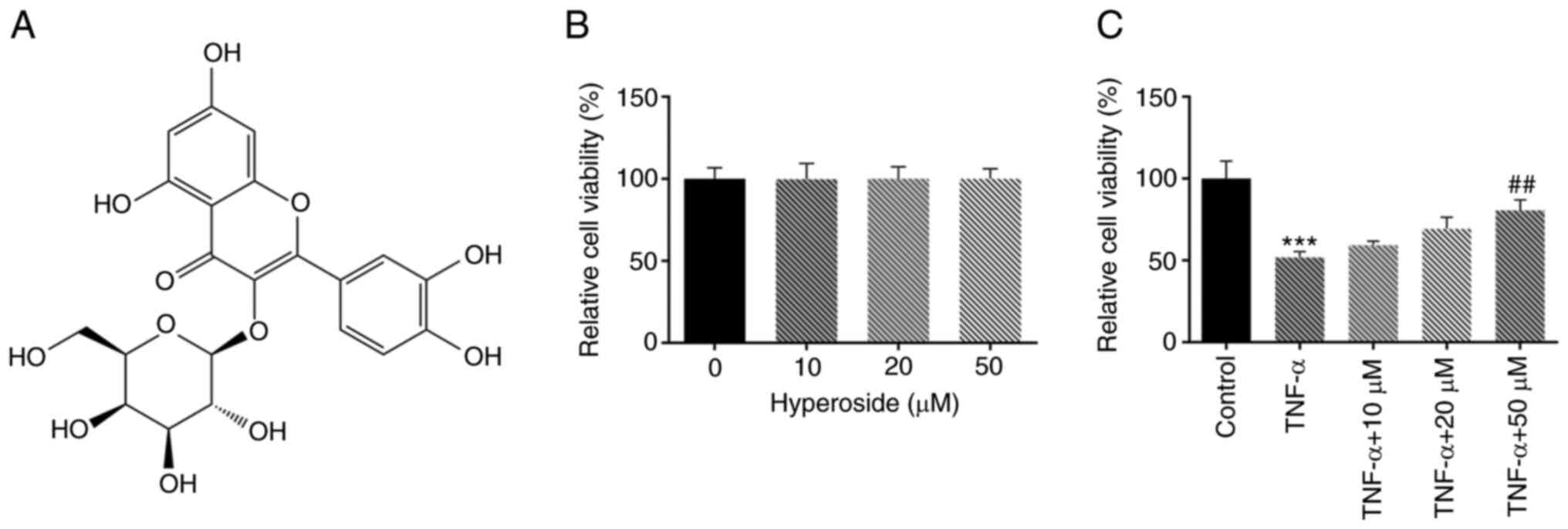
The viability of HNPCs was detected by CCK-8 assay.
The results showed that hyperoside (Fig. 1A) produced no obvious damage to
the viability of HNPCs at concentrations ≤50 µM, indicating that
hyperoside had a good biocompatibility (Fig. 1B). Subsequently, the effect of
hyperoside on TNF-α-induced HNPCs viability was examined. As
revealed in Fig. 1C, cell
viability was downregulated by 50% after TNF-α induction. Compared
with the TNF-α group, hyperoside intervention led to a
concentration-dependent increase in viability of HNPCs.
Hyperoside inhibits TNF-α-induced ER
stress-mediated apoptosis in HNPCs
The effect of hyperoside on TNF-α-induced apoptosis
was detected by flow cytometry. As revealed in Fig. 2A, TNF-α induced significant
apoptosis in HNPCs, and the percentage of late apoptosis reached
8.78%, while treatment with hyperoside reversed cell apoptosis in a
concentration-dependent manner. To further verify the protective
effect of hyperoside on ER stress-induced apoptosis, the expression
levels of apoptosis-related proteins and ER stress proteins were
examined by western blotting (Fig.
2B). The results demonstrated that, compared with those in the
TNF-α-induced group, the expression levels of the ER stress
proteins GRP78, p-PERK and ATF6 were significantly decreased in the
hyperoside-treated groups, while the expression levels of apoptotic
proteins, including CHOP, Bax and caspase 12, were also markedly
decreased in the hyperoside-treated groups, whereas the expression
levels of Bcl-2 were significantly increased.
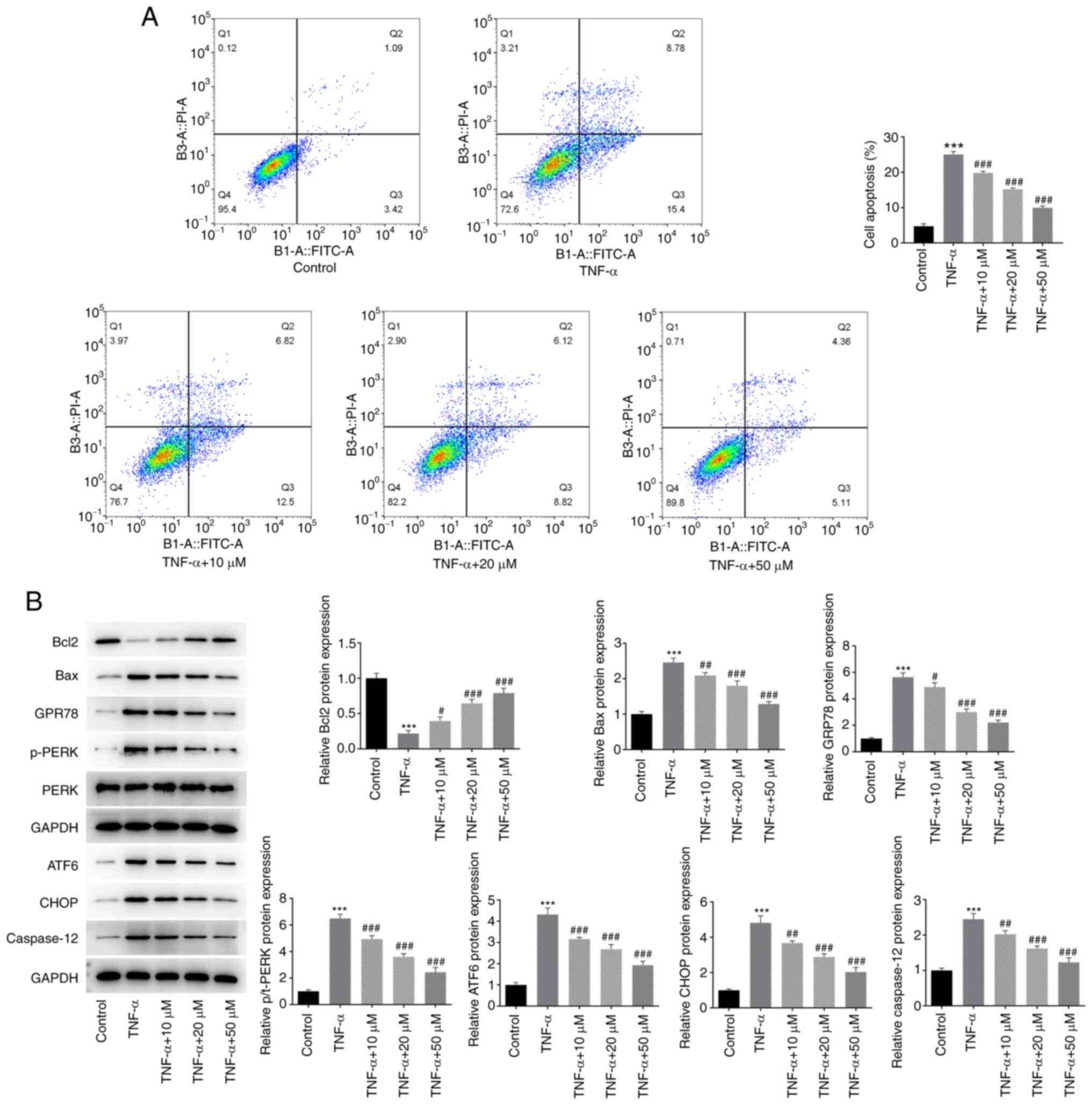 | Figure 2.Hyperoside inhibits TNF-α-induced
endoplasmic reticulum stress-mediated apoptosis in HNPCs. (A) Flow
cytometry was used to detect the effect of hyperoside on
TNF-α-induced apoptosis of HNPCs. (B) The expression levels of
Bcl-2, Bax, GRP78, p-PERK, ATF6, CHOP and caspase 12 proteins were
detected by western blot analysis. ***P<0.001 vs. control;
#P<0.05, ##P<0.01 and
###P<0.001 vs. TNF-α. HNPCs, human nucleus pulposus
cells; GRP, glucose-regulated protein; p-PERK, ATF, activating
transcription factor 6; p-, phosphorylated; PERK, protein kinase
RNA-like ER kinase; CHOP, C/EBP homologous protein. |
Hyperoside reduces TNF-α-induced
inflammation in HNPCs
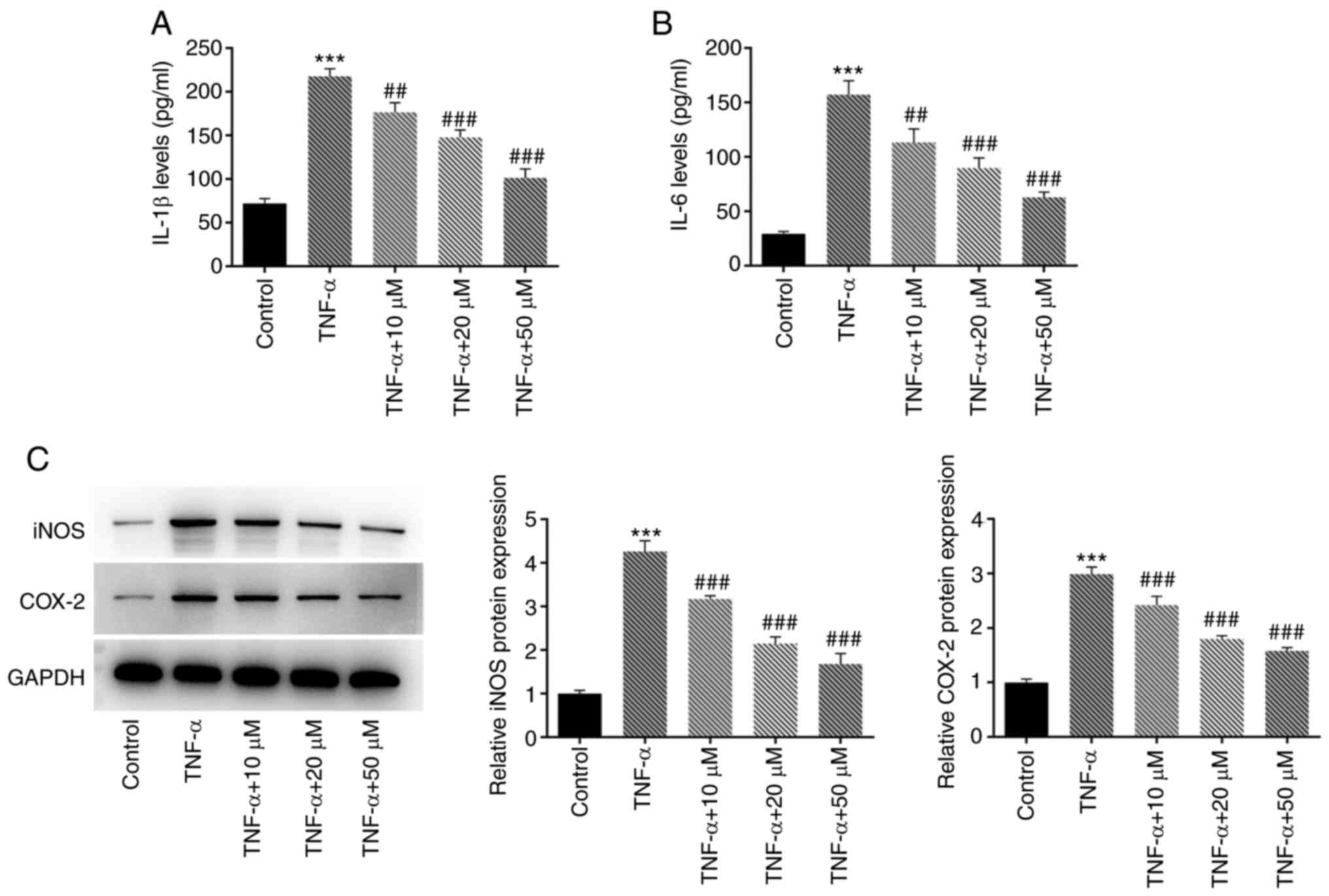
ELISA was used to evaluate the effect of hyperoside
on TNF-α-induced inflammatory factors in HNPCs (Fig. 3A and B). The results demonstrated
that, compared with that in the TNF-α group, hyperoside inhibited
the expression of IL-1β and IL-6 in a concentration-dependent
manner. Subsequently, the expression level of inflammation-related
proteins was detected (Fig. 3C).
The western blot results indicated that hyperoside could also
reduce the protein expression of iNOS and COX-2 in a
concentration-dependent manner compared with that in the TNF-α
group.
Hyperoside inhibits TNF-α-induced
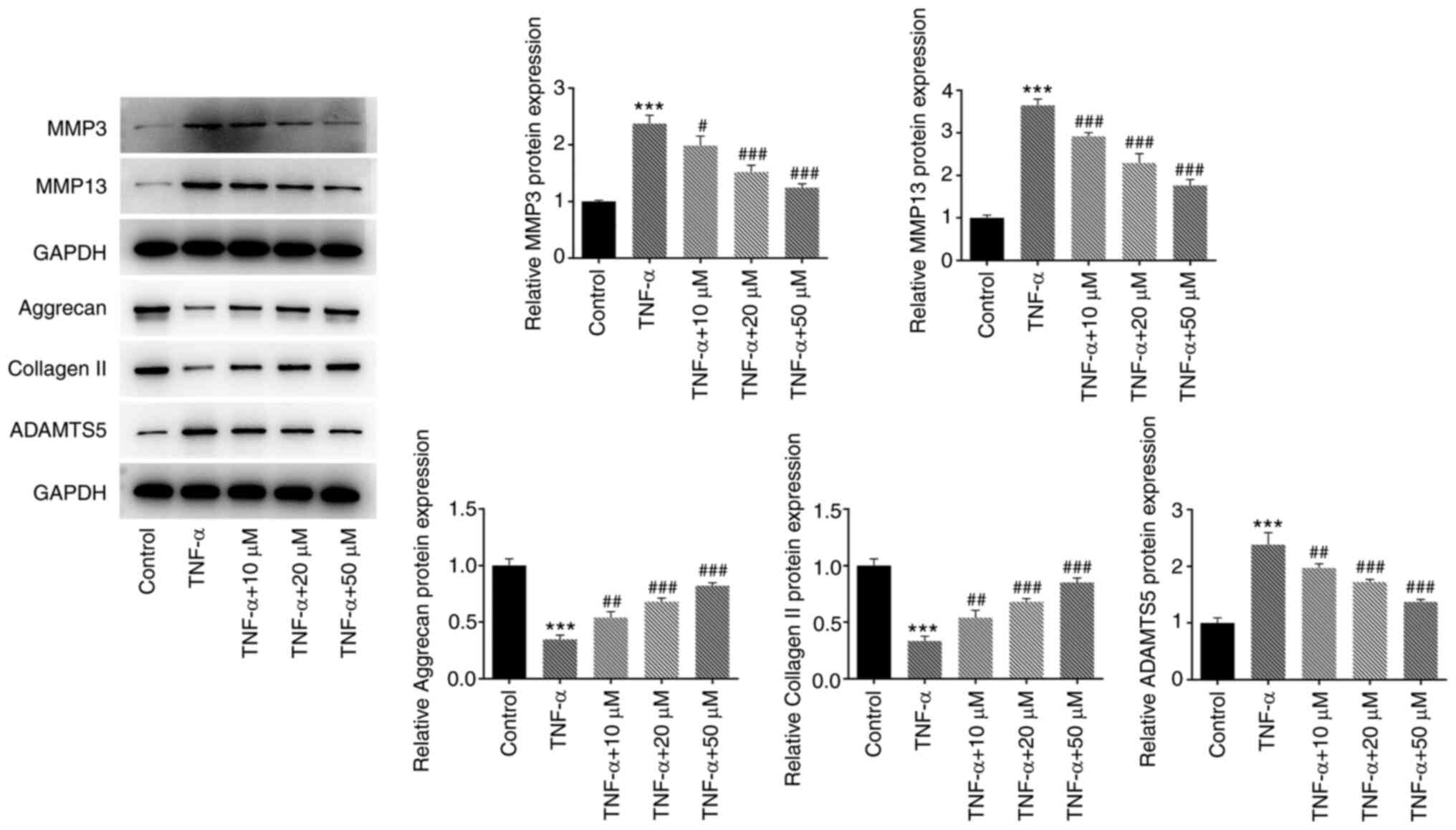
degradation of ECM in HNPCs
The expression of ECM degradation-related proteins
was detected by western blotting (Fig. 4). Compared with that of the TNF-α
group, treatment with hyperoside attenuated the TNF-α-induced
degradation of ECM in a concentration-dependent manner, and
upregulated the expression of aggrecan and collagen II. Hyperoside
also reduced the TNF-α-induced expression levels of MMP3, MMP13 and
ADAMTS5, thus exerting protective effects against TNF-α-induced ECM
degradation.
Hyperoside regulates the SIRT1/NF-κB
and Nrf2/ARE signaling pathways
To further study the mechanism of hyperoside,
western blotting was used to detect the expression of SIRT1/NF-κB
and Nrf2/ARE signaling pathways-related proteins (Fig. 5). The results revealed that TNF-α
induced the downregulation of SIRT1, Nrf2, HO-1 and NQO1 proteins,
and the upregulation of p-NF-κB p65 protein, while hyperoside
treatment concentration dependently reversed these effects. The
aforementioned results indicated that hyperoside may play its role
by regulating the SIRT1/NF-κB and Nrf2/ARE signaling pathways.
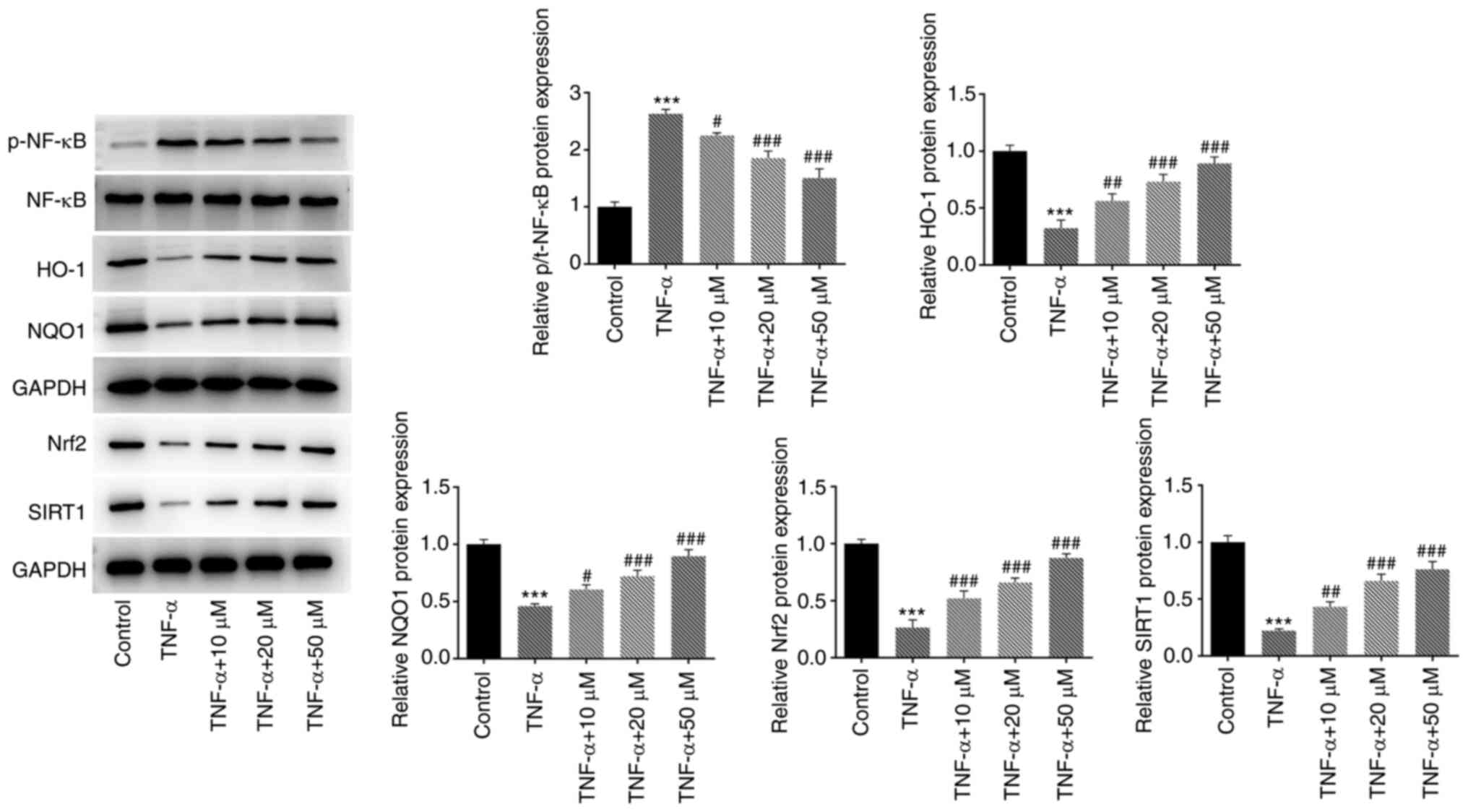 | Figure 5.Hyperoside regulates the SIRT1/NF-κB
and Nrf2/ARE signaling pathways. Western blot analysis was used to
detect the expression of SIRT1/NF-κB and Nrf2/ARE pathway-related
proteins (SIRT1, p-NF-κB, NF-κB, Nrf2, HO-1 and NQO1).
***P<0.001 vs. control; #P<0.05,
##P<0.01 and ###P<0.001 vs. TNF-α.
SIRT, sirtuin; Nrf, nuclear factor E2-related factor 2; ARE,
antioxidant responsive element; p-, phosphorylated; HO, heme
oxygenase; NQO1, NAD(P)H quinone dehydrogenase. |
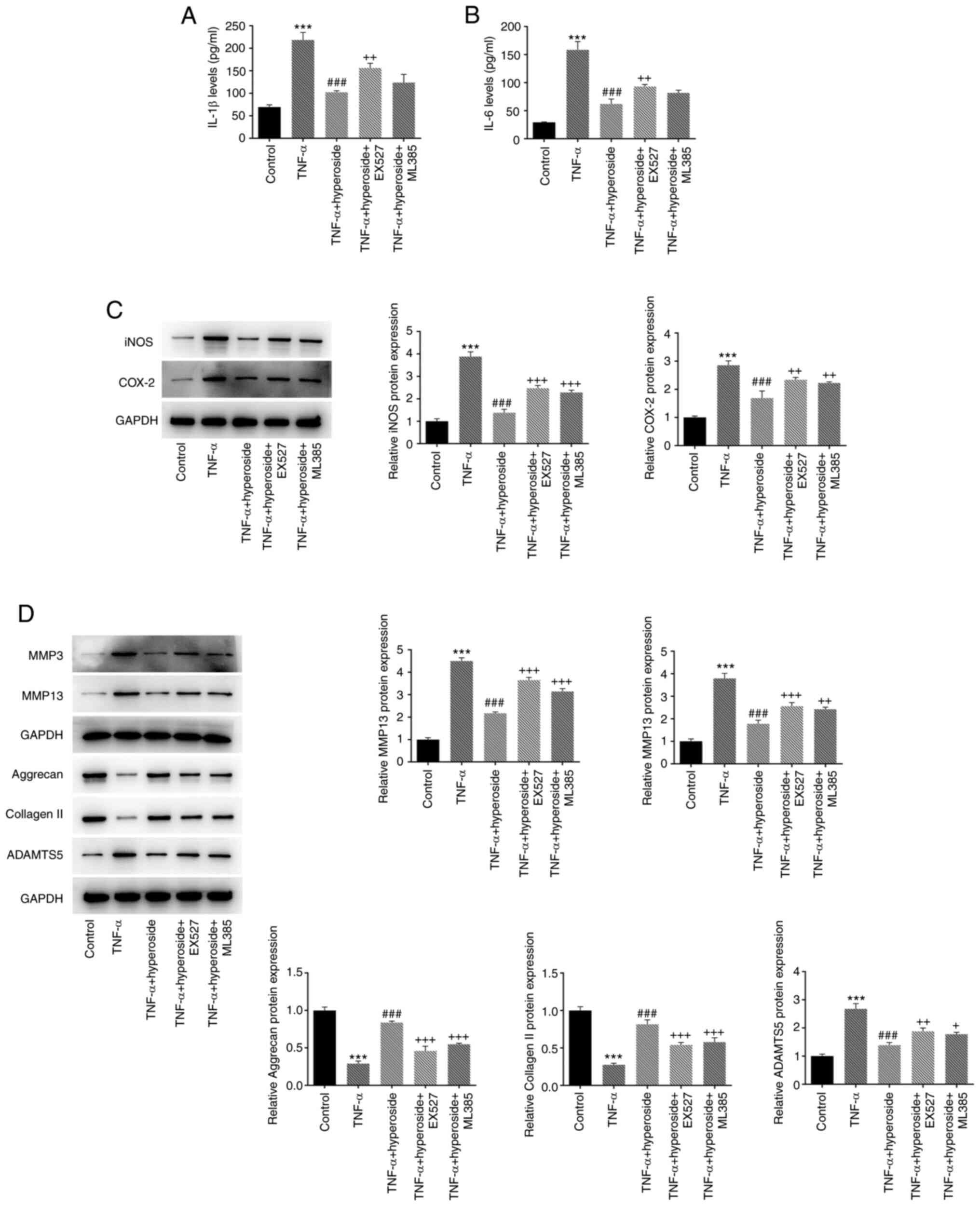
EX527 (a SIRT1 inhibitor) and ML385 (a
Nrf2 inhibitor) reverse the protective effect of hyperoside on
TNF-α-induced HNPCs
The SIRT1 inhibitor EX527 and the Nrf2 inhibitor
EX527 were employed to verify the mechanism of the protective
effect of hyperoside on TNF-α-induced HNPCs. CCK-8 assay (Fig. 6A) and flow cytometry (Fig. 6B) were performed to detect cell
viability and apoptosis, respectively. The results demonstrated
that both EX527 and ML385 could partially reverse the protective
effect of hyperoside on TNF-α-induced viability of HNPCs and
promote apoptosis. The expression levels of apoptosis-related
proteins (CHOP, Bax, caspase 12 and Bcl-2) and ER stress proteins
(GRP78, p-PERK and ATF6) further verified that EX527 and ML385
reversed the inhibitory effect of hyperoside on TNF-α-induced
apoptosis mediated by ER stress to a certain extent (Fig. 6C). Next, the effects of EX527 and
ML385 on the expression of intracellular inflammatory factors were
observed, and treatment with the inhibitor exacerbated the
expression of intracellular inflammatory factors (IL-1β and IL-6)
(Fig. 7A and B) and
inflammation-related proteins (iNOS and COX-2) (Fig. 7C) compared with the findings in
the TNF-α + hyperoside group. Finally, the expression of ECM
degradation-related proteins (aggrecan, collagen II, MMP3, MMP13
and ADAMTS5) in HNPCs was detected, and similar results were
obtained (Fig. 7D), suggesting
that hyperoside may improve TNF-α-induced inflammation, ECM
degradation and ER stress-mediated apoptosis through the
SIRT1/NF-κB and Nrf2/ARE signaling pathways.
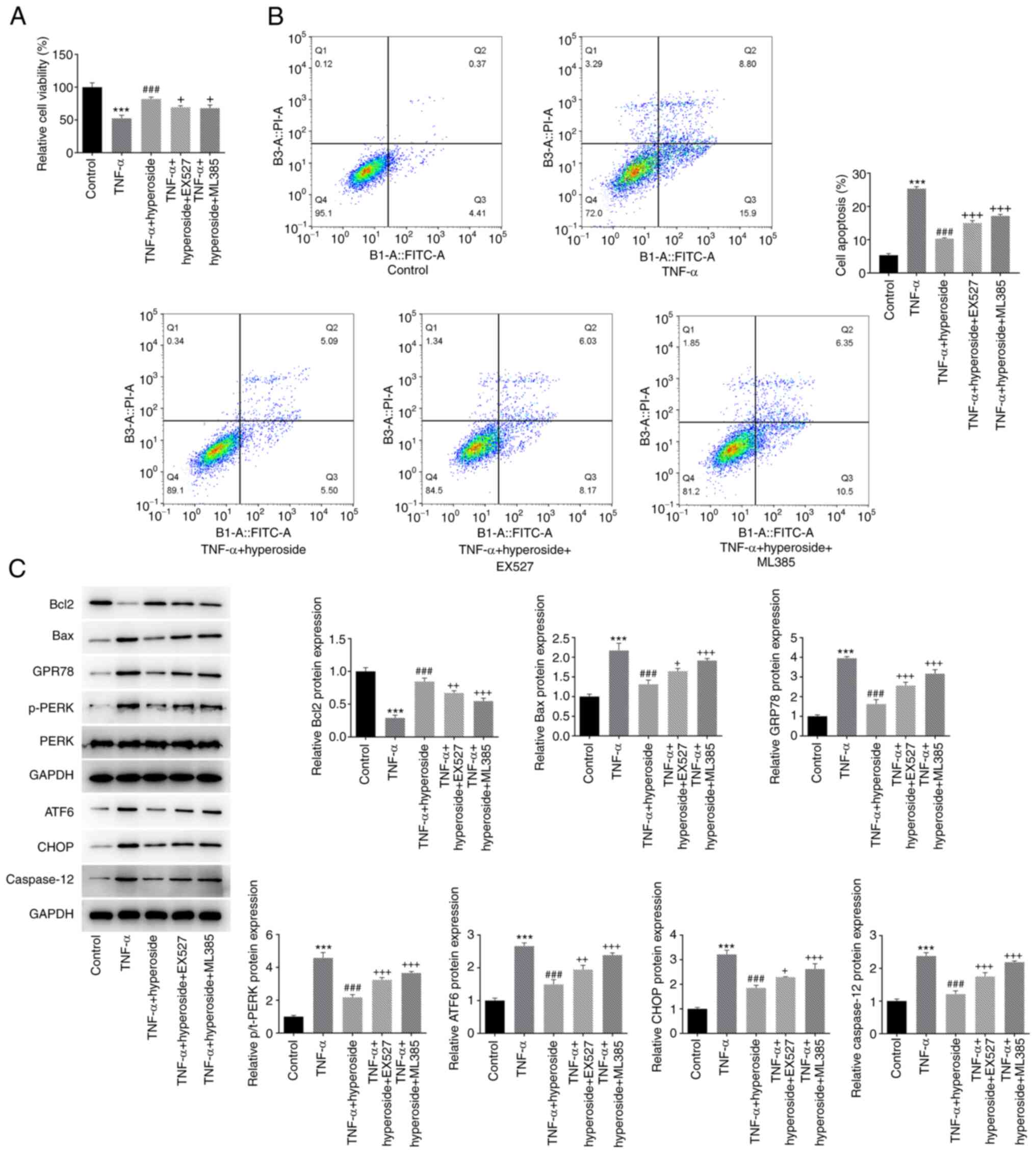 | Figure 6.EX527 and ML385 reverse the
inhibitory effect of hyperoside on TNF-α-induced endoplasmic
reticulum stress-mediated apoptosis of human nucleus pulposus
cells. (A) Cell viability and (B) apoptosis were detected by Cell
Counting Kit-8 and flow cytometry, respectively. (C) The expression
levels of Bcl-2, Bax, GRP78, p-PERK, ATF6, CHOP and caspase 12
proteins were detected by western blotting. ***P<0.001 vs.
control; ###P<0.001 vs. TNF-α; +P<0.05,
++P<0.01 and +++P<0.001 vs. TNF-α +
hyperoside. GRP, glucose-regulated protein; p-, phosphorylated;
PERK, protein kinase RNA-like ER kinase; ATF, activating
transcription factor 6; CHOP, C/EBP homologous protein. |
Discussion
IDD is one of the main causes of low back pain,
which seriously affects the life and health of patients (26). The development of IDD is
characterized by cellular and biochemical changes in the
microenvironment of the IVD, resulting in progressive functional
and structural impairment (27).
The main pathological characteristics of IDD include production of
pro-inflammatory mediators, loss of ECM, cell senescence and cell
death (1,28,29). These changes further lead to the
disruption of normal disc function. Inflammation is considered to
be the main factor leading to IDD (7). Shamji et al (30) showed that the expression levels of
macrophage products such as IL-4, IL-6, IL-12 and interferon γ in
herniated IVD tissue were significantly increased. Second, the
degradation of ECM, resulting in the loss of type II collagen and
nucleus pulposus (NP) proteoglycans, is also one of the
characteristics of IDD (31).
There is abundant evidence that TNF-α could stimulate the
expression of a variety of MMPs and ADAMTS5, leading to the
degradation of aggregates and collagen (8,32).
In addition, the ER is responsible for lipid biosynthesis, calcium
storage and protein folding (33). Previous studies have shown that
persistent ER stress could induce programmed cell death,
particularly apoptosis (34,35). A recent study has also reported
that cholesterol induces IDD by activating ER stress in NP cells
(36). In summary, controlling
the inflammatory response, ER stress-induced apoptosis and the
degradation of ECM is considered to be a potential and feasible
strategy for the treatment of patients with IDD.
Hyperoside, as one of the main components of
Traditional Chinese Medicine (Huangkui capsule, which a patented
drug), has multiple biological effects, including
anti-inflammatory, antiviral, antioxidant and anticancer effects
(37,38). Previous studies have confirmed
that hyperoside has an anti-apoptotic effect in hamster lung
fibroblast (V79-4) (39) and PC12
(40) cells. In addition,
hyperoside protects myocardium from ischemia-reperfusion injury by
inhibiting ER stress and activating the Nrf2 signaling pathway
(41). Hyperoside can upregulate
pituitary adenylate-cyclase-activating polypeptide to inhibit the
activation of NOD-, LRR- and pyrin domain-containing protein 3
inflammasomes, thus effectively inhibiting
N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced
neuroinflammation (42).
Therefore, the present study focused on the effects of hyperoside
on TNF-α-induced HNPCs inflammation, ECM degradation and ER stress.
Consistent with the protective effect of hyperoside previously
reported (41), in the present
study, hyperoside could concentration-dependently inhibit the
TNF-α-induced, ER stress-mediated apoptosis of HNPCs, and reduce
TNF-α-induced inflammation and ECM degradation.
Regarding the protective role of hyperoside in an
in vitro model of IDD, hyperoside was previously reported to
reduce LPS-induced inflammation, oxidative stress and apoptosis by
upregulating SIRT1, which activated Wnt/β-catenin (20). SIRT1 is the most important and
most widely studied member of the sirtuin family, and plays a role
in inflammatory, oxidative stress and immune responses (43,44). SIRT1 inhibits IL-1β-mediated NPCs
inflammation by regulating the Toll-like receptor 2/SIRT1/NF-κB
signaling pathway (19). In
addition, Jiang et al (45) reported that hyperoside is
considered an Nrf2 inducer, reducing the damage of
N-acetyl-para-amino-phenol to liver by reducing the production of
reactive oxygen species. Activation of the Keap1/Nrf2/ARE signaling
pathway helps to reduce oxidative stress-induced disc degeneration
(23). Shao et al
(46) found that quercetin
inhibited the expression of senescence-associated secreted
phenotype factor via the Nrf2/NF-κB axis, and improved the progress
of IDD. In addition, tea polyphenols could reduce oxidative
stress-induced disc degeneration by regulating the Keap1/Nrf2/ARE
signaling pathway (47).
Similarly, the present study showed that hyperoside treatment
relieved TNF-α-induced downregulation of SIRT1, Nrf2, HO-1 and NQO1
protein expressions and upregulation of p-NF-κB p65 protein
expression in a concentration-dependent manner, and the SIRT1
inhibitor EX527 as well as the Nrf2 inhibitor ML385 reversed the
protective effect of hyperoside on TNF-α-induced HNPCs. These
results suggested that hyperoside may play a role in IDD by
regulating the SIRT1/NF-κB and Nrf2/ARE signaling pathways.
Although the present study was the first to confirm
the inhibitory effect of hyperoside on TNF-α-induced inflammatory
response, ECM degradation and ER stress in HNPC cells, effects of
hyperoside on other aspects related to IDD, such as cell senescence
(48) and oxidative stress
(7), were not observed.
Furthermore, the present research results are only supported by
in vitro experiments, and further verification in
vivo will be the focus of our next study. Of note, the
protective mechanism of hyperoside may not only be associated with
the regulation of the SIRT1/NF-κB and Nrf2/ARE signaling pathways,
but other pathways and the optimal concentration of hyperoside need
to be further investigated.
In summary, to the best of our knowledge, the
present study is the first one to report that hyperoside improves
TNF-α-induced inflammation, ECM degradation and ER stress-mediated
apoptosis, indicating that it may play a protective role in IDD,
which is associated with the regulation of the SIRT1/NF-κB and
Nrf2/ARE signaling pathways.
Acknowledgements
Not applicable.
Funding
The present study was supported by Wuhan Municipal Health
Commission (grant no. WZ15B09).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TX and RP designed the study. JY, LM and PL
performed the experiments. TX and LM revised the manuscript. JY, LM
and PL collected and analyzed the data. RJ, TX and JY confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Risbud MV and Shapiro IM: Role of
cytokines in intervertebral disc degeneration: Pain and disc
content. Nat Rev Rheumatol. 10:44–56. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kos N, Gradisnik L and Velnar T: A brief
review of the degenerative intervertebral disc disease. Med Arch.
73:421–424. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dowdell J, Erwin M, Choma T, Vaccaro A,
Iatridis J and Cho SK: Intervertebral disk degeneration and repair.
Neurosurgery. 80 (3 Suppl):S46–S54. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li Z, Chen X, Xu D, Li S, Chan MTV and Wu
WKK: Circular RNAs in nucleus pulposus cell function and
intervertebral disc degeneration. Cell Prolif. 52:e127042019.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liao Z, Luo R, Li G, Song Y, Zhan S, Zhao
K, Hua W, Zhang Y, Wu X and Yang C: Exosomes from mesenchymal stem
cells modulate ER stress to protect against nucleus pulposus cell
death and ameliorate intervertebral disc degeneration in vivo.
Theranostics. 9:4084–4100. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Navone SE, Marfia G, Giannoni A, Beretta
M, Guarnaccia L, Gualtierotti R, Nicoli D, Rampini P and Campanella
R: Inflammatory mediators and signalling pathways controlling
intervertebral disc degeneration. Histol Histopathol. 32:523–542.
2017.PubMed/NCBI
|
|
7
|
Zhang GZ, Deng YJ, Xie QQ, Ren EH, Ma ZJ,
He XG, Gao YC and Kang XW: Sirtuins and intervertebral disc
degeneration: Roles in inflammation oxidative stress and
mitochondrial function. Clin Chim Acta. 508:33–42. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang Y, Che M, Xin J, Zheng Z, Li J and
Zhang S: The role of IL-1β and TNF-α in intervertebral disc
degeneration. Biomed Pharmacother. 131:1106602020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Constable MD and Knoblich G: Sticking
together? Re-binding previous other-associated stimuli interferes
with self-verification but not partner-verification. Acta Psychol
(Amst). 210:1031672020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rosi IM, Bombardieri F, Steri D,
Sternativo M and Rancati S: ‘Those plates that save me’:
Experiences of Italian patients with implantable cardioverter
defibrillator. Clin Nurs Res. 30:616–624. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ku SK, Zhou W, Lee W, Han MS, Na M and Bae
JS: Anti-inflammatory effects of hyperoside in human endothelial
cells and in mice. Inflammation. 38:784–99. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nirumand MC, Hajialyani M, Rahimi R,
Farzaei MH, Zingue S, Nabavi SM and Bishayee A: Dietary plants for
the prevention and management of kidney stones: Preclinical and
clinical evidence and molecular mechanisms. Int J Mol Sci.
19:7652018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Charachit N, Sukhamwang A,
Dejkriengkraikul P and Yodkeeree S: Hyperoside and quercitrin in
houttuynia cordata extract attenuate UVB-induced human keratinocyte
cell damage and oxidative stress via modulation of MAPKs and Akt
signaling pathway. Antioxidants (Basel). 11:2212022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang J, Liu Y and Liu L: Hyperoside
prevents sepsis–associated cardiac dysfunction through regulating
cardiomyocyte viability and inflammation via inhibiting miR-21.
Biomed Pharmacother. 138:1115242021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hu X, Li H, Fu L, Liu F, Wang H, Li M,
Jiang C and Yin B: The protective effect of hyperin on LPS-induced
acute lung injury in mice. Microb Pathog. 127:116–120. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang C, Yang Y, Li WX, Wu XQ, Li XF, Ma
TT, Zhang L, Meng XM and Li J: Hyperin attenuates inflammation by
activating PPAR-γ in mice with acute liver injury (ALI) and
LPS-induced RAW264.7 cells. Int Immunopharmacol. 29:440–447. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Han F, Li Z, Han S, Jia Y, Bai L, Li X and
Hu D: SIRT1 suppresses burn injury-induced inflammatory response
through activating autophagy in RAW264.7 macrophages. J Investig
Med. 69:761–767. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Qi W, Ren D, Wang P, Song Z, Wu H, Yao S,
Geng L, Su Y and Bai X: Upregulation of Sirt1 by tyrosol suppresses
apoptosis and inflammation and modulates ECM remodeling in
interleukin-1β-stimulated human nucleus pulposus cells through
activation of PI3K/Akt pathway. Int Immunopharmacol. 88:1069042020.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shen J, Fang J, Hao J, Zhong X, Wang D,
Ren H and Hu Z: SIRT1 inhibits the catabolic effect of IL-1β
through TLR2/SIRT1/NF-κB pathway in human degenerative nucleus
pulposus cells. Pain Physician. 19:E215–E226. 2016.PubMed/NCBI
|
|
20
|
Huang J, Zhou L, Chen J, Chen T, Lei B,
Zheng N, Wan X, Xu J and Wang T: Hyperoside attenuate inflammation
in HT22 cells via upregulating SIRT1 to activities
Wnt/β-catenin and sonic hedgehog pathways. Neural Plast.
10:87064002021.PubMed/NCBI
|
|
21
|
Xing HY, Cai YQ, Wang XF, Wang LL, Li P,
Wang GY and Chen JH: The cytoprotective effect of hyperoside
against oxidative stress is mediated by the Nrf2-ARE signaling
pathway through GSK-3β inactivation. PLoS One. 10:e01451832015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hou JY, Liu Y, Liu L and Li XM: Protective
effect of hyperoside on cardiac ischemia reperfusion injury through
inhibition of ER stress and activation of Nrf2 signaling. Asian Pac
J Trop Med. 9:76–80. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Edwards PD, Frenette-Ling C, Palme R and
Boonstra R: A mechanism for population self-regulation: Social
density suppresses GnRH expression and reduces reproductivity in
voles. J Anim Ecol. 90:784–795. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Du L Qian X, Li Y, Li XZ, He LL, Xu L, Liu
YQ, Li CC, Ma P, Shu FL, et al: Sirt1 inhibits renal tubular cell
epithelial-mesenchymal transition through YY1 deacetylation in
diabetic nephropathy. Acta Pharmacol Sin. 42:242–251. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang Z, Han N, Zhao K, Li Y, Chi Y and
Wang B: Protective effects of pyrroloquinoline quinine against
oxidative stress-induced cellular senescence and inflammation in
human renal tubular epithelial cells via Keap1/Nrf2 signaling
pathway. Int Immunopharmacol. 72:445–453. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Roh EJ, Darai A, Kyung JW, Choi H, Kwon
SY, Bhujel B, Kim KT and Han I: Genetic therapy for intervertebral
disc degeneration. Int J Mol Sci. 22:15792021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xue J, Hu B, Xing W, Li F, Huang Z, Zheng
W, Wang B, Zhu Y and Yang X: Low expression of miR-142-3p promotes
intervertebral disk degeneration. J Orthop Surg Res. 16:552021.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mascarenhas RO, Souza MB and Oliveira VC:
Treatment of fibromyalgia in the 21st century-reply. JAMA Intern
Med. 181:1011–1012. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhao Y, Qiu C, Wang W, Peng J, Cheng X,
Shangguan Y, Xu M, Li J, Qu R, Chen X, et al: Cortistatin protects
against intervertebral disc degeneration through targeting
mitochondrial ROS-dependent NLRP3 inflammasome activation.
Theranostics. 10:7015–7033. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shamji MF, Setton LA, Jarvis W, So S, Chen
J, Jing L, Bullock R, Isaacs RE, Brown C and Richardson WJ:
Proinflammatory cytokine expression profile in degenerated and
herniated human intervertebral disc tissues. Arthritis Rheum.
62:1974–1982. 2010.PubMed/NCBI
|
|
31
|
Sive JI, Baird P, Jeziorsk M, Watkins A,
Hoyland JA and Freemont AJ: Expression of chondrocyte markers by
cells of normal and degenerate intervertebral discs. Mol Pathol.
55:91–97. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Seguin CA, Pilliar RM, Roughley PJ and
Kandel RA: Tumor necrosis factor-alpha modulates matrix production
and catabolism in nucleus pulposus tissue. Spine (Phila Pa 1976).
30:1940–1948. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang G, Yang ZQ and Zhang K: ER stress
response in cancer: Molecular mechanism and therapeutic potential.
Am J Transl Res. 2:65–74. 2010.PubMed/NCBI
|
|
34
|
Fernandez A, Ordóñez R, Reiter RJ,
González-Gallego J and Mauriz JL: Melatonin and ER stress: Relation
to autophagy and apoptosis. J Pineal Res. 59:292–307. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yang L, Guan G, Lei L, Lv Q, Liu S, Zhan
X, Jiang Z and Gu X: Palmitic acid induces human osteoblast-like
Saos-2 cell apoptosis via ER stress and autophagy. Cell Stress
Chaperones. 23:1283–1294. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yan J, Li S, Zhang Y, Deng Z, Wu J, Huang
Z, Qin T, Xiao Y, Zhou J, Xu K and Ye W: Cholesterol induces
pyroptosis and matrix degradation via mSREBP1-driven ER stress in
intervertebral disc degeneration. Front Cell Dev Biol.
9:8031322021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sun K, Luo J, Jing X, Xiang W, Guo J, Yao
X, Liang S, Guo F and Xu T: Hyperoside ameliorates the progression
of osteoarthritis: An in vitro and in vivo study. Phytomedicine.
80:1533872021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li C, He Y, Yang Y, Gou Y, Li S, Wang R,
Zeng S and Zhao X: Antioxidant and inflammatory effects of Nelumbo
Nucifera Gaertn. Leaves. Oxid Med Cell Longev.
28:83759612021.PubMed/NCBI
|
|
39
|
Piao MJ, Kang KA, Zhang R, Ko DO, Wang ZH,
You HJ, Kim HS, Kim JS, Kang SS and Hyun JW: Hyperoside prevents
oxidative damage induced by hydrogen peroxide in lung fibroblast
cells via an antioxidant effect. Biochim Biophys Acta.
1780:1448–1457. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu Z, Tao X, Zhang C, Lu Y and Wei D:
Protective effects of hyperoside (quercetin-3-o-galactoside) to
PC12 cells against cytotoxicity induced by hydrogen peroxide and
tert-butyl hydroperoxide. Biomed Pharmacother. 59:481–490. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chiu MC and Hsieh MC: Latent human error
analysis and efficient improvement strategies by fuzzy TOPSIS in
aviation maintenance tasks. Appl Ergon. 54:136–147. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wan K, Lu C, Wang T, Qiao C, Lu L, Wu D,
Lu M, Chen R, Fan L and Tang J: Hyperoside suppresses NLRP3
inflammasome in parkinson's disease via pituitary adenylate
cyclase-activating polypeptide. Neurochem Int. 152:1052542022.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lou T, Huang Q, Su H, Zhao D and Li X:
Targeting sirtuin 1 signaling pathway by ginsenosides. J
Ethnopharmacol. 268:1136572021. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Xu C, Song Y, Wang Z, Jiang J, Piao Y, Li
L, Jin S, Li L, Zhu L and Yan G: Pterostilbene suppresses oxidative
stress and allergic airway inflammation through AMPK/Sirt1 and
Nrf2/HO-1 pathways. Immun Inflamm Dis. 9:1406–1417. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Jiang Z, Wang J, Liu C, Wang X and Pan J:
Hyperoside alleviated N-acetyl-para-amino-phenol-induced acute
hepatic injury via Nrf2 activation. Int J Clin Exp Pathol.
12:64–76. 2019.PubMed/NCBI
|
|
46
|
Shao Z, Wang B, Shi Y, Xie C, Huang C,
Chen B, Zhang H, Zeng G, Liang H, Wu Y, et al: Senolytic agent
quercetin ameliorates intervertebral disc degeneration via the
Nrf2/NF-κB axis. Osteoarthritis Cartilage. 29:413–422. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Song D, Ge J, Wang Y, Yan Q, Wu C, Yu H,
Yang M, Yang H and Zou J: Tea polyphenol attenuates oxidative
stress-induced degeneration of intervertebral discs by regulating
the Keap1/Nrf2/ARE pathway. Oxid Med Cell Longev.
7:66841472021.PubMed/NCBI
|
|
48
|
Chen D, Xia D, Pan Z, Xu D, Zhou Y, Wu Y,
Cai N, Tang Q, Wang C, Yan M, et al: Metformin protects against
apoptosis and senescence in nucleus pulposus cells and ameliorates
disc degeneration in vivo. Cell Death Dis. 7:e24412016. View Article : Google Scholar : PubMed/NCBI
|