Introduction
Pulmonary fibrosis is a chronic, irreversible
interstitial lung disease, primarily affecting middle-aged and
elderly populations (1). The
incidence of pulmonary fibrosis is increasing globally as the
population ages; however, its pathogenesis is still poorly
understood and there is a lack of effective therapeutic agents
(2,3). Previous studies have reported the
presence of a large number of lung fibroblasts that maintain the
structural and functional integrity of lung tissue by synthesizing
extracellular matrix (ECM) and cytokines (4,5).
However, when lung tissue is damaged, fibroblasts become
dysfunctional and over-proliferate or convert to ECM, ultimately
leading to inflammatory and fibrotic processes in the lung
(6,7). Therefore, inhibition of excessive
lung fibroblast proliferation, inflammation and ECM deposition may
be key for improving the treatment of pulmonary fibrosis.
Transforming growth factor-β1 (TGF-β1) is regarded
as the most potent pro-fibrotic cytokine and regulates numerous
cellular processes, such as proliferation, growth inhibition,
migration and ECM remodeling via the SMAD-2/3 and β-catenin
signaling pathways (8–10). An increasing number of studies
have reported that TGF-β1 signaling is involved in numerous
fibrosis-associated diseases, such as renal (11) and in particular, pulmonary
fibrosis (12). Furthermore, Liu
et al (13) reported that
inhibition of TGF-β1 protects against liver fibrosis. The results
of a recent study further demonstrated that knockdown of TGF-β1
alleviates high mechanical stress-induced chondrocyte fibrosis
(14). Moreover, Leask and
Abraham (15) reported the
involvement of TGF-β1 signaling in the development of pulmonary
fibrosis. Therefore, inhibiting TGF-β1 activity may be an effective
strategy for inhibiting pulmonary fibrosis.
Platycodon grandiflorus (PG), a widely used
edible, traditional Chinese medicinal herb, is commonly used as a
pulmonary adjuvant to enhance the effect of other drugs in the
management of lung disease (16,17). It was previously reported that
bronchitis, asthma and tuberculosis, as well as inflammatory
disease, can be treated using PG extract (18). Platyconic acid A (PA) is the
active component of Platyconic saponin, which possess
similar effects to PG in the treatment of lung disease (19). A previous study reported that PA
inhibits TGF-β1-induced liver fibrosis by blocking SMAD signaling
pathway and activating the peroxisome proliferator-activated
receptor γ signaling pathway (20). These results suggest that PA may
serve as an important factor in the pathological process of
pulmonary fibrosis; however, the specific underlying mechanisms are
yet to be fully elucidated.
Protein phosphatase
Mg2+/Mn2+-dependent 1A (PPM1A) is a Ser/Thr
protein phosphatase that belongs to the protein phosphatase 2C
family, which regulates cell cycle progression, proliferation,
differentiation and apoptosis (21–23). A previous study reported that
PPM1A is an important inhibitory regulator in the TGF-β1 signaling
pathway (24). Moreover, numerous
studies have demonstrated that upregulation of PPM1A is associated
with anti-pulmonary fibrosis effects (25–27). These data suggested that PPM1A may
serve an inhibitory role in pulmonary fibrosis via inhibition of
TGF-β1.
The present study analyzed TGF-β1-induced pulmonary
fibrosis cell models; it was hypothesized that PA may be involved
in TGF-β1-induced pulmonary fibrosis via effects on the
SMAD/β-catenin signaling pathway and regulation of PPM1A.
Materials and methods
Cell culture and transfection
The BeNa Culture Collection (Beijing Beina Chunglian
Institute of Biotechnology) supplied the human lung fibroblast
MRC-5 cell line. Cells were incubated in 10% FBS (Thermo Fisher
Scientific, Inc.), 100 U/ml penicillin and 100 µg/ml streptomycin
in DMEM (Thermo Fisher Scientific, Inc.) at 37°C with 5%
CO2. To establish an in vitro cell model of
pulmonary fibrosis, MRC-5 cells were induced with 10 ng/ml TGF-β1
(R&D Systems, Inc.) for 48 h at 37°C and subsequently harvested
using 0.25% trypsin for further analysis, as previously described
(28). Small interfering (si)RNA
targeting PPM1A [si-PPM1A#1 (cat. no. siB05113142945-1-5;) and
si-PPM1A#2 (siB05113142946-1-5;)] and corresponding negative
control (NC; siN0000001-1-5;) were designed and manufactured by
Guangzhou RiboBio Co., Ltd. Transfection of the aforementioned
siRNAs (60 nM) into MRC-5 cells was performed using
Lipofectamine® 2000 (Thermo Fisher Scientific, Inc.) at
37°C for 24 h. At 48 h after transfection with si-PPM1A#1/2,
reverse transcription-quantitative PCR (RT-qPCR) and western
blotting were used to assess the mRNA and protein expression levels
of PPM1A.
Cell counting kit (CCK)-8
analysis
To determine whether PA was toxic to MRC-5 cells,
0.5, 1.0, 2.0, 5.0 and 10.0 µM PA (Shanghai Yuanye Bio-Technology
Co., Ltd.) was used to treat cells for 24 h at 37°C (19). Following TGF-β1 induction and
treatment with PA, MRC-5 cells were cultured in 96-well plates
(1×104) for 24 h at 37°C. Each well was supplemented
with 10 µl CCK-8 solution (Beijing AVIVA Systems Biology) for 4 h
at 37°C. A Synergy™ 2 multifunctional microplate reader
(BioTek Instruments, Inc.) was used to assess the optical density
at 450 nm.
Wound healing analysis
Following treatment of TGF-β1 (10 ng/ml; R&D
Systems, Inc.) and PA (0.5, 1.0, 2.0, 5.0 and 10.0 µm; Shanghai
Yuanye Bio-Technology Co., Ltd.), transfected or untransfected
MRC-5 cells were cultured at the bottom of a 60-mm culture dish.
When 100% confluence was reached, a wound was created in the
confluent monolayer using a sterile pipette tip (20 µl). Following
rinsing with PBS, MRC-5 cells were cultured again in serum-free
DMEM at 37°C for 24 h. The width of the scratch was assessed using
a light microscope (CKX53; Olympus Corporation) at 0 and 24 h
(magnification, ×100). The wound width was visualized using ImageJ
software version 1.8.0 (National Institutes of Health).
Immunofluorescent staining
Cells treated by TGF-β1 (10 ng/ml; R&D Systems,
Inc.) for 48 h at 37°C and PA (0.5, 1.0, 2.0, 5.0 and 10.0 µm;
Shanghai Yuanye Bio-Technology Co., Ltd.) for 24 h at 37°C were
grown to 100% confluence and fixed using 4% paraformaldehyde for 30
min at room temperature. Subsequently, 0.5% Triton X-100 (MP
Biomedicals, LLC) was added to permeabilize the cells at room
temperature. Cells were incubated with primary antibody against
α-SMA (1:300; cat. no. ab124964; Abcam) overnight at 4°C. Following
rinsing with PBS, cells were incubated with Alexa Fluor®
488-conjugated secondary antibody (1:500; cat. no. ab150077; Abcam)
at room temperature for 1 h. DAPI was used to counterstain the cell
nuclei at room temperature for 15 min and cells were visualized
under a fluorescence microscope (DM6000; Leica Microsystems GmbH).
Images were processed by ImageJ Software version 1.52s (National
Institutes of Health) to assess the fluorescence intensity.
Western blotting
MRC-5 cells were lysed using RIPA buffer (Beyotime
Institute of Biotechnology) containing protease and phosphatase
inhibitors to obtain total protein, which was quantified using a
BCA Protein Assay kit (Thermo Fisher Scientific, Inc.) to determine
protein concentration. Following electrophoresis with 8% SDS-PAGE
gels, protein samples (30 µg per lane) were transferred onto pure
nitrocellulose membranes (Pall Life Sciences). Then, the membranes
were washed three times with 0.1% Tween-20 TBS solution (TBST) and
blocked with 5% skimmed milk (Sigma-Aldrich; Merck KGaA) for 1 h at
room temperature before incubation with primary antibodies against
fibronectin 1 (Fn1; 1:1,000; cat. no. ab45688; Abcam), collagen 1
(1:1,000; cat. no. ab34710; Abcam), matrix metalloproteinase
(MMP)-1 (1:1,000; cat. no. ab134184; Abcam), α-smooth muscle actin
(SMA; 1:1,000; cat. no. ab124964; Abcam), phosphorylated (p)-NF-κB
(1:1,000; cat. no. sc-136548; Santa Cruz Biotechnology, Inc.),
NF-κB (1:1,000; cat. no. ab288751; Abcam), PPM1A (1:1,000; cat. no.
ab231893; Abcam), p-SMAD-2 (1:1,000; cat. no. ab280888; Abcam),
p-SMAD-3 (1:2,000; cat. no. ab52903; Abcam), β-catenin (1:10,000;
cat. no. ab32572; Abcam) and SMAD-2/3 (1:1,000; cat. no. ab202445;
Abcam) overnight at 4°C. Following primary incubation, membranes
were incubated with HRP-conjugated goat anti-rabbit IgG (1:2,000;
cat. no. ab6721; Abcam) and goat anti-mouse IgG (1:2,000; cat. no.
ab6789; Abcam) for 1 h at room temperature. Protein bands were
visualized using BeyoECL Plus kit (Beyotime Institute of
Biotechnology) and imaged using the Odyssey CLX Infrared Imaging
System. The intensity of signals was semi-quantified using ImageJ
1.8.0 software (National Institutes of Health).
ELISA
Protein expression levels of TNF-α, IL-1β and IL-6
in MRC-5 cells were determined using human TNF-α (cat. no. DTA00D;
R&D Systems, Inc.), IL-1β (cat. no. DLB50; R&D Systems,
Inc.) and IL-6 ELISA kits (cat. no. D6050; R&D Systems, Inc.),
according to the manufacturer's protocol. Absorbance values were
read at a wavelength of 540 nm and levels of TNF-α, IL-1β and IL-6
were calculated using the standard curve.
RT-qPCR
RT-qPCR was performed to quantify the mRNA
expression levels of PPM1A in MRC-5 cells before and after
transfection. RNA was isolated from MRC-5 cells using
TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.) and
RNA was reverse-transcribed into complementary (c)DNA using a High
Capacity cDNA Reverse Transcription Kit (Applied Biosystems; Thermo
Fisher Scientific, Inc.) according to the manufacturer's
instructions. qPCR was performed using a Maxima SYBR Green/ROX qPCR
Master Mix kit (Fermentas; Thermo Fisher Scientific, Inc.) on a
StepOnePlus™ Real-Time PCR system (Applied Biosystems;
Thermo Fisher Scientific, Inc.). The thermocycling conditions were
as follows: Initial denaturation at 95°C for 3 min, followed by 40
cycles at 95°C for 10 sec, 60°C for 30 sec and 72°C for 30 sec. The
specific primer sequences used were as follows: PPM1A forward (F),
5′-AGGGGCAGGGTAATGGGTT-3′ and reverse (R),
5′-GATCACAGCCGTATGTGCATC-3′ and GAPDH F, 5′-GCACCGTCAAGGCTGAGAAC-3′
and R, 5′-GGATCTCGCTCCTGGAAGATG-3′. mRNA expression levels were
quantified using the 2−ΔΔCq method (29).
Molecular target analysis
SwissTargetPrediction (swisstargetprediction.ch/)
was used to identify PPM1A as a potential target for PA.
Statistical analysis
All data are presented as the mean ± standard
deviation of at least three independent experiments and analyzed
using SPSS 22.0 (IBM Corp.). For comparisons between two groups,
unpaired Student's t-test was used and one-way ANOVA followed by
Tukey's post hoc test was used to determine differences between ≥3
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
PA inhibits TGF-β1-induced
over-proliferation of MRC-5 cells
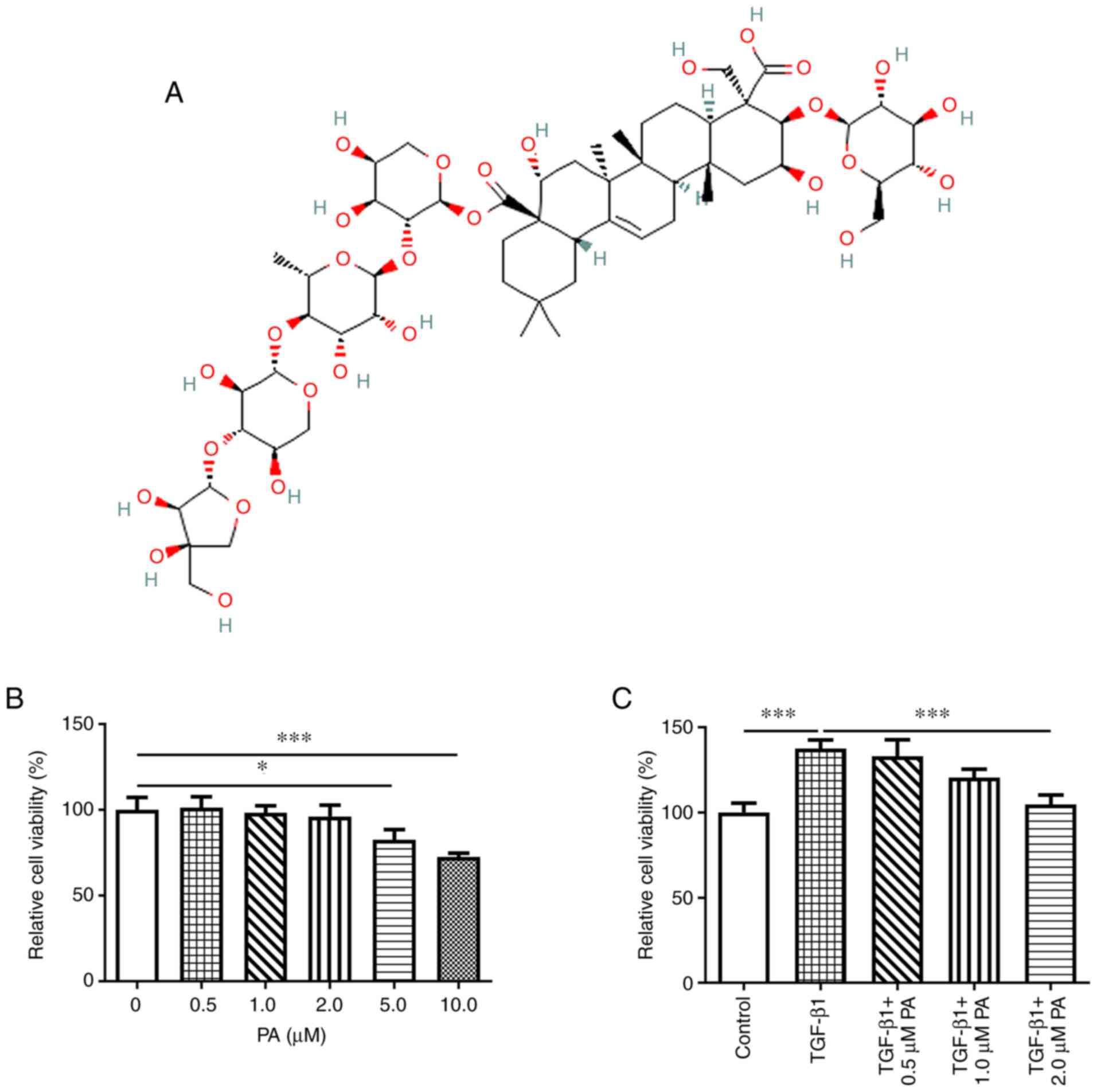
The chemical structure of PA is presented in
Fig. 1A. The effect of different
concentrations of PA on the viability of MRC-5 cells was assessed.
PA at concentrations of 0.5, 1.0 and 2.0 µM exhibited no
significant effect on cell viability compared with the control,
whereas a significant decrease was demonstrated in viability of
MRC-5 cells treated with PA concentrations of 5 and 10 µM (Fig. 1B). These results demonstrated that
5 and 10 µM PA were toxic to MRC-5 cells. Therefore, PA at
concentrations of 0.5, 1.0 and 2.0 µM was selected for use in
subsequent experiments. Following the induction of MRC-5 cells with
TGF-β1, the effect of PA on cell viability was evaluated. TGF-β1
significantly increased cell proliferation compared with the
control and PA markedly suppressed TGF-β1-induced proliferation in
MRC-5 cells (Fig. 1C).
Furthermore, the higher the concentration of PA, the lower the
levels of cell proliferation. And the proliferation of
TGF-β1-challenged MRC-5 cells was the most significantly suppressed
by 2 µM PA by contrast with the TGF-β1 group. These results
supported the hypothesis that PA inhibited TGF-β1-induced
over-proliferation of MRC-5 cells in a dose-dependent manner.
PA inhibits TGF-β1-induced migration
and ECM deposition in MRC-5 cells
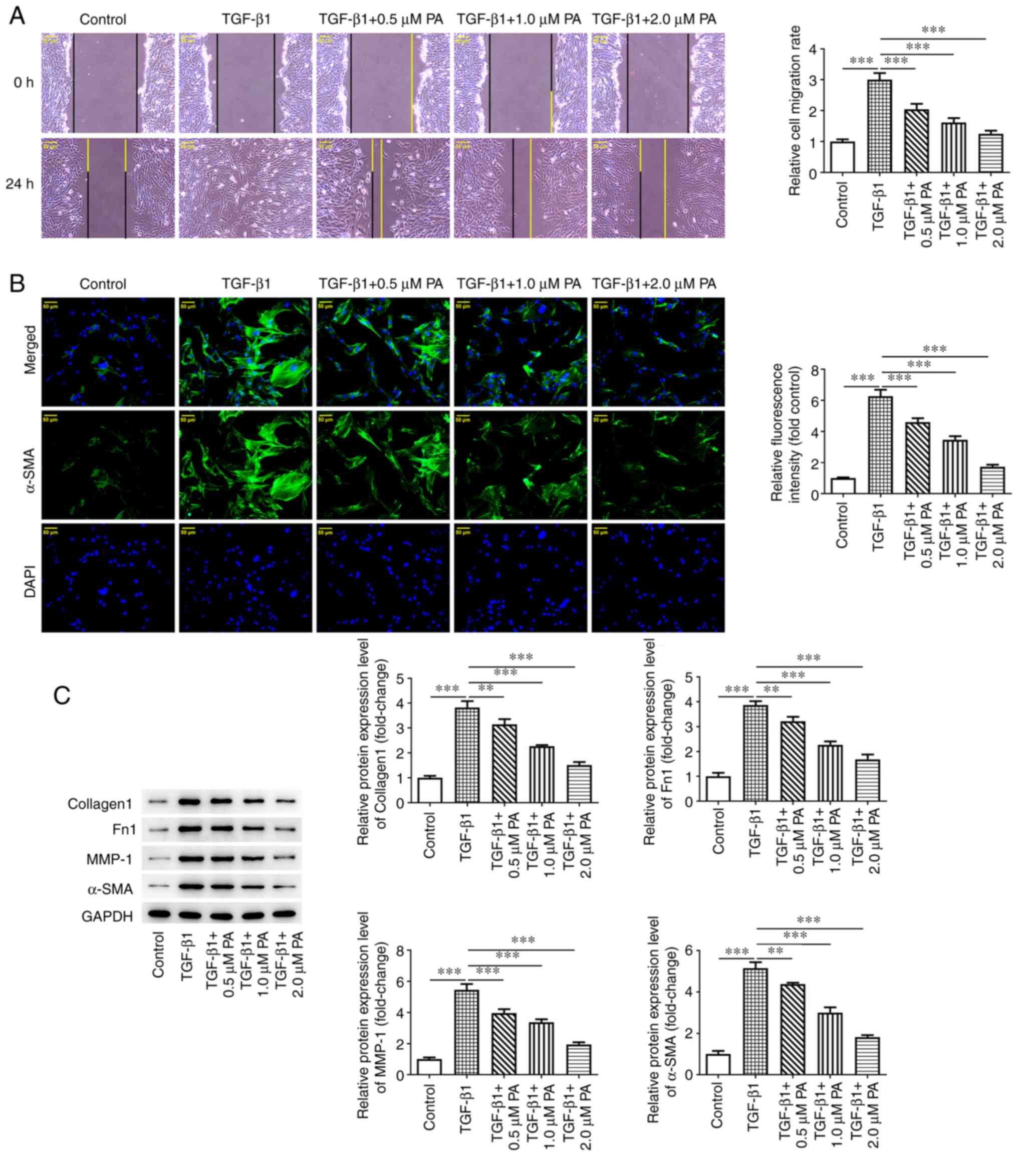
To elucidate the effect of PA on cell migration and
ECM deposition, wound healing assay was. Cell migration was
significantly higher following TGF-β1 induction compared with the
control (Fig. 2A). However, cell
migration was significantly decreased in the TGF-β1 + PA (0.5, 1.0
and 2.0 µM) groups compared with the TGF-β1 group. Furthermore,
protein expression levels of the myofibroblast marker α-SMA were
significantly elevated in the TGF-β1 group compared with the
control and these levels declined significantly in a dose-dependent
manner following treatment with different concentrations of PA
(0.5, 1.0 and 2.0 µM; Fig. 2B)
compared with the TGF-β1 group. Protein expression levels of
ECM-associated proteins, including Collagen I, Fn1, MMP-1 and α-SMA
were significantly elevated in TGF-β1-induced MRC-5 cells compared
with the control and these levels were significantly decreased in a
dose-dependent manner following treatment with PA at concentrations
of 0.5, 1.0 and 2.0 µM compared with the TGF-β1 group (Fig. 2C). Overall, these findings
demonstrated that PA exerted suppressive effects on TGF-β1-induced
migration and ECM deposition in MRC-5 cells.
PA inhibits the TGF-β1-induced
inflammatory response in MRC-5 cells
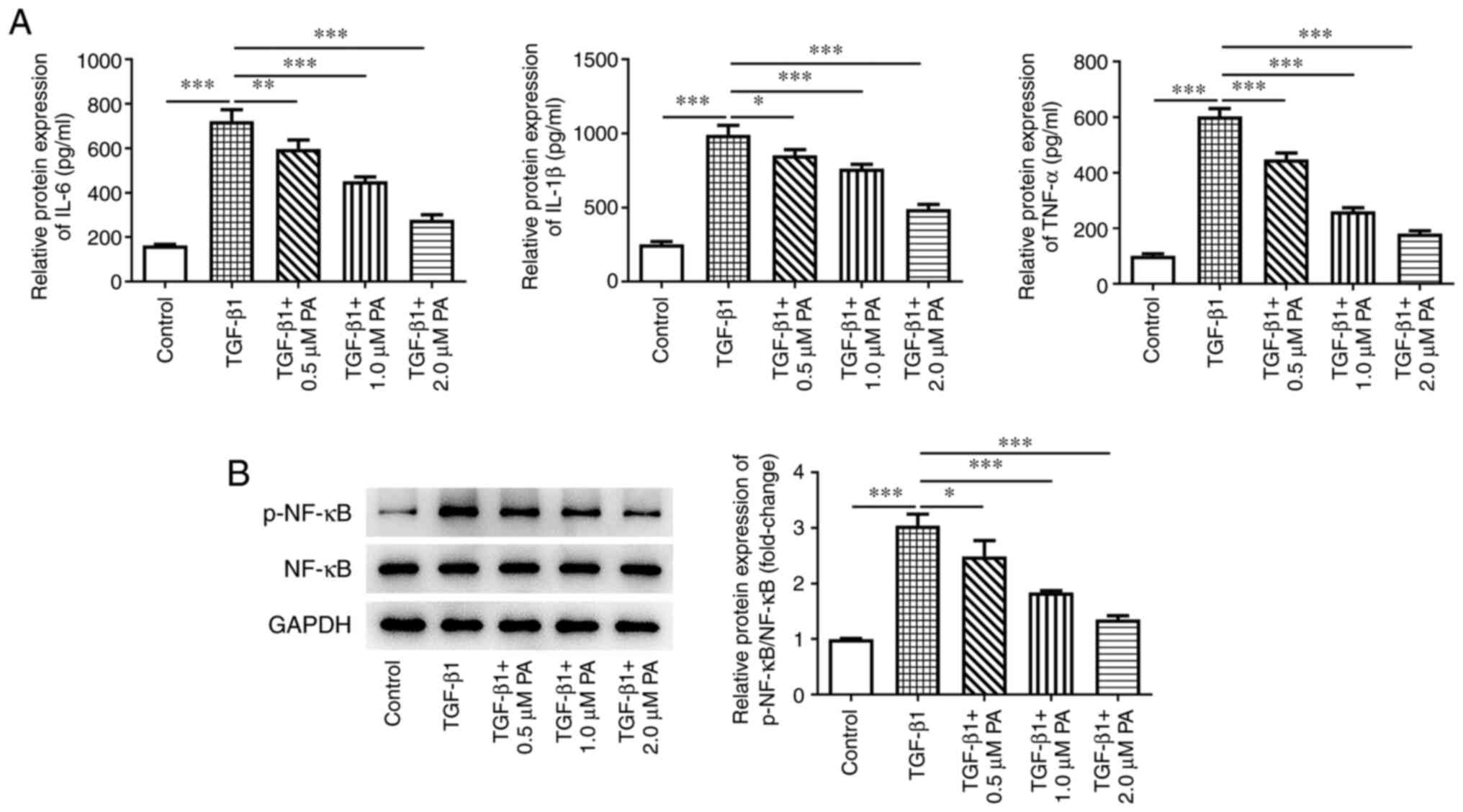
The present study assessed whether PA affected the
TGF-β1-induced inflammatory response in MRC-5 cells. ELISA
demonstrated a significant elevation in the protein expression
levels of the inflammatory cytokines IL-6, IL-1β and TNF-α in the
TGF-β1 group compared with the control (Fig. 3A). Moreover, significantly
decreased protein expression levels of IL-6, IL-1β and TNF-α in all
TGF-β1 + PA groups were observed compared with the TGF-β1 group.
The results of the present study demonstrated that TGF-β1 induced a
significant increase in protein expression levels of p-NF-κB in
MRC-5 cells compared with the control group (Fig. 3B). Furthermore, the protein
expression levels of p-NF-κB were significantly decreased in MRC-5
cells following all PA treatments compared with the control. As PA
exerted a greater inhibitory effect at a concentration of 2 µM,
this concentration was selected for subsequent experiments. These
results demonstrated that PA notably inhibited the TGF-β1-induced
inflammatory response in MRC-5 cells.
PA upregulates PPM1A expression in
TGF-β1-induced MRC-5 cells and inhibits TGF-β1-induced
proliferation and ECM deposition in MRC-5 cells via the
SMAD/β-catenin signaling pathway
PPM1A was predicted as a potential target for PA
using the SwissTargetPrediction website (data not shown). To
evaluate whether PA exerted effects on PPM1A in MRC-5 cells, mRNA
and protein expression levels of PPM1A were assessed in
TGF-β1-induced cells in the presence or absence of PA treatment.
PPM1A expression levels were significantly decreased in
TGF-β1-induced MRC-5 cells compared with those in the control group
and significantly elevated following PA treatment compared with the
TGF-β1 group (Fig. 4A and B).
These results indicated that PA may have upregulated PPM1A
expression. MRC-5 cells were transfected with si-PPM1A#1/2 for
verification. It was discovered that si-PPM1A#1/2 both demonstrated
significantly reduced mRNA and protein expression levels compared
with si-NC. MRC-5 cells transfected with si-PPM1A#1 exhibited
markedly lower PPM1A expression levels, compared with cells
transfected with si-PPM1A#2 (Fig. 4C
and D). Therefore, si-PPM1A#1 was selected for use in
subsequent experiments and referred to as si-PPM1A. The results of
the CCK-8 analysis demonstrated that the proliferation of
TGF-β1-induced MRC-5 cells treated with PA was increased
significantly in TGF-β 1 + PA + si-PPM1A group compared with the
TGF-β 1 + PA + si-NC group (Fig.
4E). Moreover, migration of TGF-β1-induced MRC-5 cells treated
with PA was significantly increased in TGF-β 1 + PA + si-PPM1A
group compared with the TGF-β 1 + PA + si-NC group (Fig. 4F). A significant increase in
expression of α-SMA was also demonstrated in TGF-β1-induced MRC-5
cells treated with PA following transfection with si-PPM1A compared
with NC (Fig. 4G). PPM1A
knockdown resulted in significantly increased protein expression
levels of ECM-associated proteins, including Collagen1, Fn1, MMP-1
and α-SMA in TGF-β1-induced MRC-5 cells treated with PA compared
with NC (Fig. 4H and I).
Collectively, these results suggested that PA suppressed
TGF-β1-induced proliferation and ECM deposition in MRC-5 cells via
the SMAD/β-catenin signaling pathway.
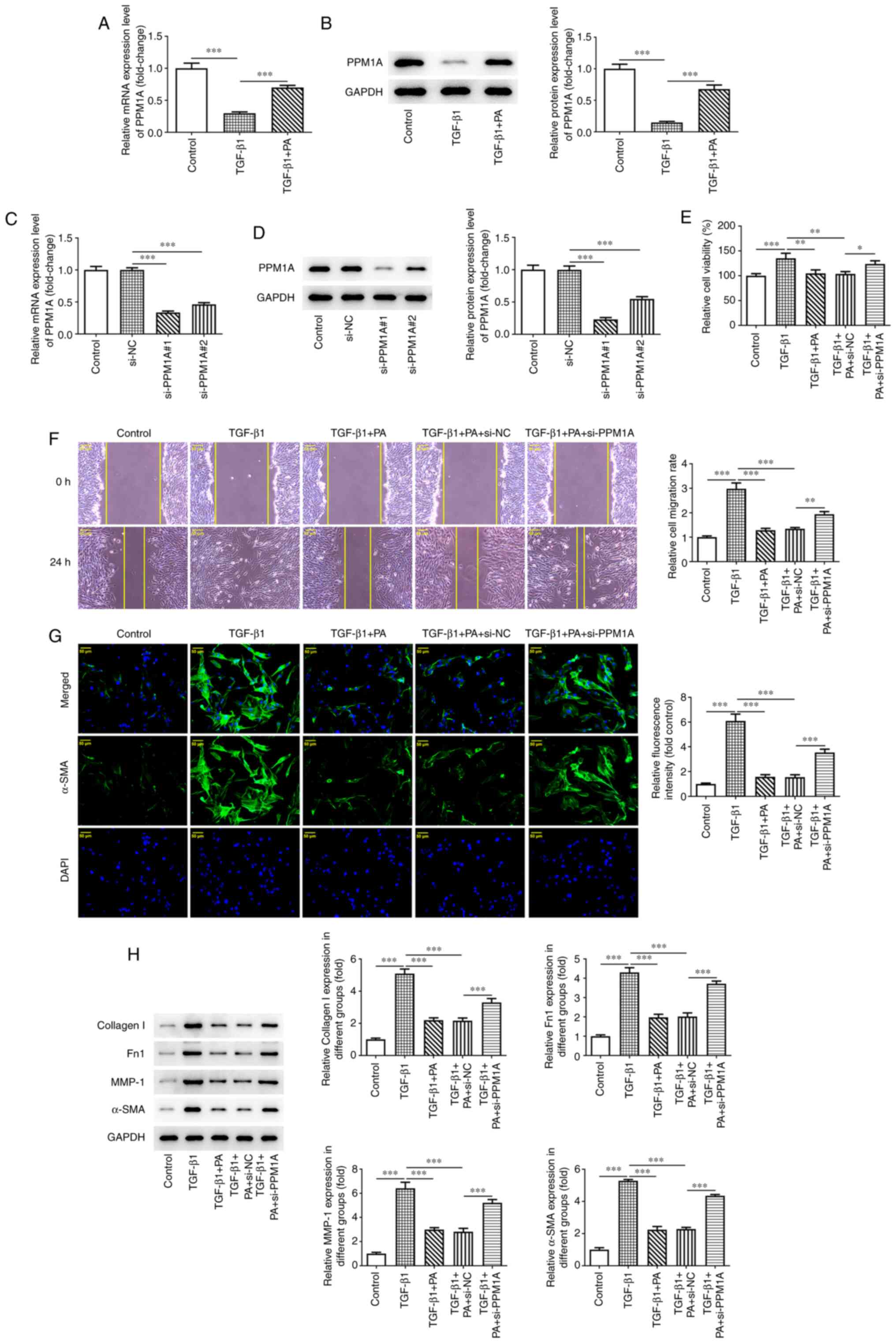 | Figure 4.PA upregulates PPM1A expression in
TGF-β1-induced MRC-5 cells and inhibits TGF-β1-induced
proliferation and ECM deposition in MRC-5 cells via the
SMAD/β-catenin signaling pathways. The mRNA and protein expression
levels of PPM1A in TGF-β1-induced MRC-5 cells in the absence and
presence of PA were assessed using (A) RT-qPCR and (B) western
blotting. PPM1A mRNA and protein expression levels following its
knockdown in MRC-5 cells were assessed using (C) RT-qPCR and (D)
western blotting. (E) Viability of TGF-β1-induced MRC-5 cells
treated with PA and transfected with si-PPM1A was assessed using
via Cell Counting Kit-8 assay. (F) Migration capacity in
TGF-β1-induced MRC-5 cells treated with PA following knockdown of
PPM1A was assessed using wound healing. (G) Protein expression of
α-SMA in TGF-β1-induced MRC-5 cells treated with PA following
knockdown of PPM1A was evaluated using immunofluorescent staining.
(H) Protein expression levels of ECM-associated proteins in
TGF-β1-induced MRC-5 cells treated with PA following knockdown of
PPM1A were semi-quantified using western blotting. Scale bar, 50
µm. *P<0.05, **P<0.01 and ***P<0.001. PA, platyconic acid
A; PPM1A, protein phosphatase
Mg2+/Mn2+-dependent 1A; RT-qPCR, reverse
transcription-quantitative PCR; Fn1, fibronectin 1; MMP-1, matrix
metalloprotease 1; TGF-β1, transforming growth factor-β1; ECM,
extracellular matrix; si small interfering; NC, negative
control. |
PA inhibits TGF-β1-induced
inflammation in MRC-5 cells via the SMAD/β-catenin signaling
pathway
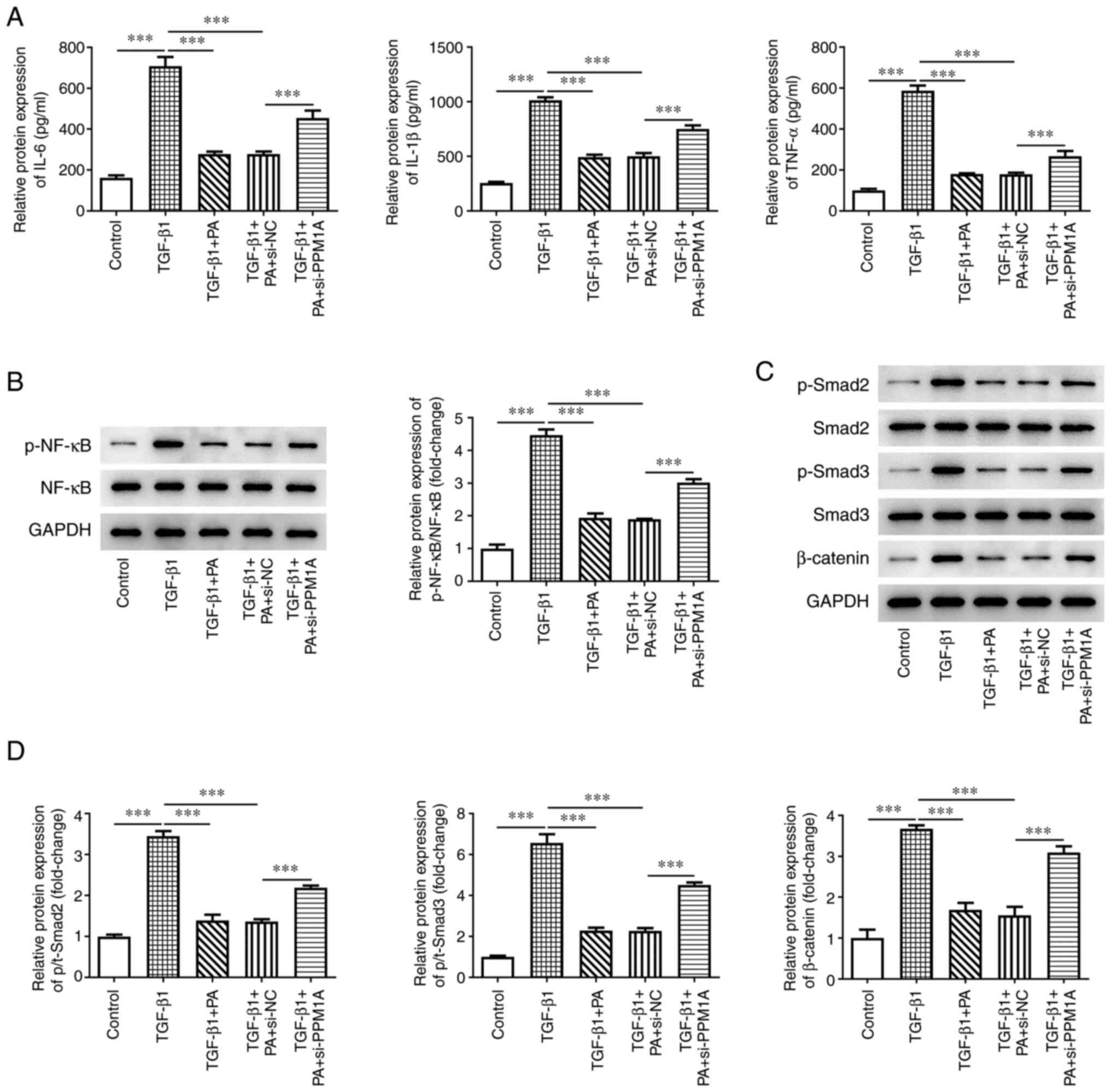
To assess the effect of PA binding to PPM1A on
TGF-β1-induced inflammation of MRC-5 cells, ELISA and western
blotting were performed. The results of the present study
demonstrated that protein expression levels of the inflammatory
cytokines IL-6, IL-1β and TNF-α were significantly elevated in
MRC-5 cells transfected with si-PPM1A compared with NC (Fig. 5A). Moreover, p-NF-κB protein
expression levels were also significantly increased in the TGF-β 1
+ PA + si-PPM1A group compared with the TGF-β 1 + PA + si-NC group
(Fig. 5B). Furthermore, the
expression levels of SMAD/β-catenin-associated proteins, including
p-SMAD-2, p-SMAD-3 and β-catenin, were significantly elevated in
TGF-β1-induced MRC-5 cells compared with the control; these levels
were significantly decreased following treatment with PA compared
with the TGF-β1 group. However, PPM1A knockdown significantly
increased the protein expression levels of p-SMAD-2, p-SMAD-3 and
β-catenin compared with NC (Fig. 5C
and D). These data demonstrated that the inhibitory effects of
PA on TGF-β1-induced inflammation of MRC-5 cells were achieved
following upregulation of PPM1A and the SMAD/β-catenin signaling
pathway.
Discussion
Pulmonary fibrosis is characterized by varying
degrees of inflammation and ECM deposition, often leading to lung
dysfunction and death (30). To
date, only a small number of drugs have been approved for the
treatment of pulmonary fibrosis-associated diseases, such as
idiopathic pulmonary fibrosis (IPF), which exhibits a poor
prognosis (31). The results of
previous studies have demonstrated the role of PG in treatment of
pulmonary disease and the anti-liver fibrotic effect of the active
component PA (32,33). However, the specific mechanisms
involved in the inhibitory effect of PA on pulmonary fibrosis are
yet to be fully elucidated. Therefore, the present study evaluated
the potential mechanisms underlying PA inhibition in lung
fibroblasts.
TGF-β1 is a key element in lung injury and pulmonary
fibrosis that activates fibroblast proliferation and
differentiation and increases accumulation of ECM in the lung,
leading to development of pulmonary fibrosis (34). Therefore, the present study used
TGF-β1 to induce MRC-5 cells to establish an in vitro
pulmonary fibrosis model. Results of the present study demonstrated
that PA exerted no effect on MRC-5 cell viability at concentrations
of 0.5, 1.0 and 2.0 µM. A previous study reported that PA inhibits
TGF-β1-induced proliferation of hepatic fibroblasts in a
dose-dependent manner (33). The
results of the present study demonstrated that TGF-β1 induction
significantly increased proliferation of MRC-5 cells; however, PA
treatment markedly inhibited the over-proliferation induced by
TGF-β1. Furthermore, TGF-β1 is a regulator of cell migration
(35). The results of the present
study demonstrated that cell migration was significantly increased
following TGF-β1 induction and treatment with PA significantly
inhibited the increased cell migration in a dose-dependent
manner.
A previous study reported that treatment with TGF-β1
leads to ECM production by increasing protein expression levels of
α-SMA and Collagen I (36). The
results of the present study also demonstrated a significant
increase in α-SMA protein expression levels in TGF-β1-induced MRC-5
cells. Moreover, previous studies reported that aqueous extracts
and saponins extracted from Platycodi radix inhibit
expression levels of α-SMA and Collagen1 in rat models induced by
carbon tetrachloride, dimethyl-nitrosamine and a high-fat diet
(37–39). The results of the present study
demonstrated that PA significantly inhibited TGF-β1-induced cell
migration and ECM deposition in a dose-dependent manner by
significantly suppressing the protein expression levels of α-SMA
and ECM-associated markers, Collagen1, Fn1 and MMP-1.
Notably, imbalanced inflammation contributes to
development of pulmonary fibrosis (40). Under normal conditions,
inflammation and repair is controlled in the lung; however, when
tissue injury occurs, TGF-β1 is released in large amounts and
chemotactic neutrophils secrete pro-inflammatory molecules
(41). The function of
chemotactic neutrophils shifts to resolving inflammation and repair
during the healing phase (41).
The results of the present study demonstrated that protein
expression levels of pro-inflammatory factors IL-6, IL-1β and TNF-α
were significantly increased in TGF-β1-induced MRC-5 cells.
Furthermore, the combination of PG and cisplatin has been reported
to significantly decrease inflammation in lung tissue (42). The results of the present study
demonstrated that PA significantly inhibited the production of
pro-inflammatory factors. The NF-κB pathway is commonly regarded as
a pro-inflammatory signaling pathway (43,44). Moreover, NF-κB is involved in the
development and progression of fibrosis via regulation of
transcription factors associated with fiber growth, such as
platelet-derived growth factor and TGF-β1 (45). A previous study reported that
activation of the NF-κB signaling pathway occurs in the lung tissue
of patients with IPF and mice with pulmonary fibrosis (46). The results of the present study
demonstrated that the protein expression levels of p-NF-κB were
significantly increased in TGF-β1-induced MRC-5 cells, which was
consistent with previous studies (45,46). Notably, PG or PG-derived
components increase AMP-activated protein kinase (AMPK) signaling
in numerous types of cell, such as macrophages and lung carcinoma
cells, hepatocellular carcinoma cells (47–49). Moreover, AMPK activation relieves
the inflammatory response via suppression of NF-κB activation and
downregulation of IL-1β (50).
The results of these aforementioned studies suggested that
components of PG may inhibit NF-κB. The results of the present
study demonstrated that PA significantly inhibited TGF-β1-induced
phosphorylation of NF-κB in MRC-5 cells, demonstrating that PA
successfully inhibited the TGF-β1-induced inflammatory response in
MRC-5 cells.
PPM1A is a member of the protein phosphatase 2C
family of Ser/Thr protein phosphatases (23). SwissTargetPrediction database
demonstrated that PPM1A may be a potential target for PA. A
previous study reported that PPM1A protein expression is decreased
in bleomycin-induced pulmonary fibrosis in rats (25). The present study demonstrated that
PPM1A mRNA and protein expression levels were significantly
decreased in TGF-β1-induced MRC-5 cells. However, the mRNA and
protein expression levels of PPM1A were significantly increased in
TGF-β1 + PA group in MRC-5 cells compared with TGF-β1 group,
demonstrating that PA may upregulate PPM1A expression. Previous
studies reported that upregulation of PPM1A is associated with
antifibrotic effects (23–25)
and it could be hypothesized that knockdown of PPM1A may reverse
these effects. Therefore, PPM1A knockdown was performed in the
present study and the results demonstrated that PPM1A knockdown led
to significantly increased cell viability and migration, ECM
deposition and protein expression levels of pro-inflammatory
factors compared with NC, which suggested that the inhibition of
pulmonary fibrosis by PA was mediated by PPM1A.
A previous study reported that the pro-fibrotic
process of TGF-β1 is mediated by SMAD-2/3 and the β-catenin
signaling pathway (10).
Activation of β-catenin-dependent genes including cyclin D1 and
c-Myc leads to fibroblast activation and fibrogenesis (10,51,52). The results of the present study
demonstrated that the protein expression levels of p-SMAD2, p-SMAD3
and β-catenin were significantly elevated following TGF-β1
induction compared with the control. Furthermore, previous studies
reported that PPM1A promotes its nuclear export via
dephosphorylation of SMAD2/3, leading to termination of the
TGF-β1/SMAD signaling cascade (24,53). However, the present study
demonstrated that PPM1A knockdown significantly increased
expression levels of SMAD/β-catenin signaling pathway-associated
proteins. These data suggested that inhibition of TGF-β1 via PPM1A
was achieved via inhibition of the SMAD/β-catenin signaling
pathways.
The present study had limitations. Only one cell
line was used and other cell lines need to be included to confirm
the results in future investigations. The function of PPM1A in
TGF-β1 induced pulmonary fibrosis model needs to be confirmed by
overexpressing PPM1A to determine whether this reverses the effect
induced by TGF-β1. There was no reliable method for cell-to-animal
dose conversion and acute toxicity testing should be used to
confirm the appropriate concentration of PA in vivo. Whether
PA treatment decreases both mRNA and protein levels of PPM1A and
whether knockdown of PPM1A without TGF-β1 treatment affects cell
migration needs to be assessed.
In conclusion, the results of the present study
demonstrated that PA inhibited TGF-β1-induced proliferation,
inflammation and ECM of lung fibroblasts via upregulation of PPM1A
via the SMAD/β-catenin signaling pathways. The present study
therefore provides a novel theoretical basis for the treatment of
pulmonary fibrosis.
Acknowledgements
Not applicable.
Funding
The present study was funded by the 2019 Nantong Municipal
Health Commission Scientific Research Project (grant no. QA2019017)
and the 2020 Nantong Science and Technology Bureau Science and
Technology Plan Project (grant no. JCZ20091).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HHo and CS designed and conceived the study. CS and
YT performed the experiments. CS, YT, CW and HHu analyzed the data.
CS drafted the manuscript. All authors have read and approved the
final manuscript. CS and YT confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sharif R: Overview of idiopathic pulmonary
fibrosis (IPF) and evidence-based guidelines. Am J Manag Care. 23
(11 Suppl):S176–S182. 2017.PubMed/NCBI
|
|
2
|
Wu Q, Zhang KJ, Jiang SM, Fu L, Shi Y, Tan
RB, Cui J and Zhou Y: p53: A key protein that regulates pulmonary
fibrosis. Oxid Med Cell Longev. 2020:66357942020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Oldham JM, Ma SF, Martinez FJ, Anstrom KJ,
Raghu G, Schwartz DA, Valenzi E, Witt L, Lee C, Vij R, et al:
TOLLIP, MUC5B, and the response to N-acetylcysteine among
individuals with idiopathic pulmonary fibrosis. Am J Respir Crit
Care Med. 192:1475–1482. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yadav SK, Shah SD and Penn RB: Give me a
fork: Can autophagy research solve the riddle of airway remodeling
in asthma? Am J Respir Cell Mol Biol. 60:494–496. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kaczmarek KA, Clifford RL and Knox AJ:
Epigenetic changes in airway smooth muscle as a driver of airway
inflammation and remodeling in asthma. Chest. 155:816–824. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rodrigues APD, Bortolozzo ASS,
Arantes-Costa FM, Saraiva-Romanholo BM, de Souza FCR, Brüggemann
TR, Santana FPR, de Brito MV, Bonturi CR, Nunes NNDS, et al: A
plant proteinase inhibitor from Enterolobium contortisiliquum
attenuates airway hyperresponsiveness, inflammation and remodeling
in a mouse model of asthma. Histol Histopathol. 34:537–552.
2019.PubMed/NCBI
|
|
7
|
McAlinden KD, Deshpande DA, Ghavami S,
Xenaki D, Sohal SS, Oliver BG, Haghi M and Sharma P: Autophagy
activation in asthma airways remodeling. Am J Respir Cell Mol Biol.
60:541–553. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bujak M, Ren G, Kweon HJ, Dobaczewski M,
Reddy A, Taffet G, Wang XF and Frangogiannis NG: Essential role of
Smad3 in infarct healing and in the pathogenesis of cardiac
remodeling. Circulation. 116:2127–2138. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dobaczewski M, Bujak M, Li N,
Gonzalez-Quesada C, Mendoza LH, Wang XF and Frangogiannis NG: Smad3
signaling critically regulates fibroblast phenotype and function in
healing myocardial infarction. Circ Res. 107:418–428. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guo Y, Gupte M, Umbarkar P, Singh AP, Sui
JY, Force T and Lal H: Entanglement of GSK-3β, β-catenin and TGF-β1
signaling network to regulate myocardial fibrosis. J Mol Cell
Cardiol. 110:109–120. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Loboda A, Sobczak M, Jozkowicz A and Dulak
J: TGF-β1/Smads and miR-21 in renal fibrosis and inflammation.
Mediators Inflamm. 2016:83192832016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Boutanquoi PM, Burgy O, Beltramo G,
Bellaye PS, Dondaine L, Marcion G, Pommerolle L, Vadel A, Spanjaard
M, Demidov O, et al: TRIM33 prevents pulmonary fibrosis by
impairing TGF-β1 signalling. Eur Respir J. 55:19013462020.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu N, Feng J, Lu X, Yao Z, Liu Q, Lv Y,
Han Y, Deng J and Zhou Y: Isorhamnetin inhibits liver fibrosis by
reducing autophagy and inhibiting extracellular matrix formation
via the TGF-β1/Smad3 and TGF-β1/p38 MAPK pathways. Mediators
Inflamm. 2019:61750912019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang YZ, Zhao L, Zhu Y, Tian SJ, Zhang W,
Liu S and Ge JF: Interrupting TGF-β1/CCN2/integrin-α5β1 signaling
alleviates high mechanical-stress caused chondrocyte fibrosis. Eur
Rev Med Pharmacol Sci. 25:1233–1241. 2021.PubMed/NCBI
|
|
15
|
Leask A and Abraham DJ: TGF-beta signaling
and the fibrotic response. FASEB J. 18:816–827. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Si-Cong L, Chaoqin R, Ge L, Xu-Ting L,
Jin-Liang L, Bin W, Min Z, Wei H, Liang C and Xue G: Platycodon
grandiflorum extract attenuates lipopolysaccharide-induced
acute lung injury via TLR4/NF-κBp65 pathway in rats. Pak J Pharm
Sci. 34:2213–2218. 2021.PubMed/NCBI
|
|
17
|
Ji MY, Bo A, Yang M, Xu JF, Jiang LL, Zhou
BC and Li MH: The pharmacological effects and health benefits of
Platycodon grandiflorus-a medicine food homology species.
Foods. 9:1422020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim JW, Park SJ, Lim JH, Yang JW, Shin JC,
Lee SW, Suh JW and Hwang SB: Triterpenoid saponins isolated from
Platycodon grandiflorum inhibit hepatitis C virus
replication. Evid Based Complement Alternat Med. 2013:5604172013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Choi JH, Jin SW, Kim HG, Choi CY, Lee HS,
Ryu SY, Chung YC, Hwang YJ, Um YJ, Jeong TC and Jeong HG: Saponins,
especially platyconic acid A, from Platycodon grandiflorum
reduce airway inflammation in ovalbumin-induced mice and
PMA-exposed A549 cells. J Agric Food Chem. 63:1468–1476. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yu Q, Cheng P, Wu J and Guo C: PPARγ/NF-κB
and TGF-β1/Smad pathway are involved in the anti-fibrotic effects
of levo-tetrahydropalmatine on liver fibrosis. J Cell Mol Med.
25:1645–1660. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Das AK, Helps NR, Cohen PT and Barford D:
Crystal structure of the protein serine/threonine phosphatase 2C at
2.0 A resolution. EMBO J. 15:6798–6809. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ofek P, Ben-Meir D, Kariv-Inbal Z, Oren M
and Lavi S: Cell cycle regulation and p53 activation by protein
phosphatase 2C alpha. J Biol Chem. 278:14299–14305. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shohat M, Ben-Meir D and Lavi S: Protein
phosphatase magnesium dependent 1A (PPM1A) plays a role in the
differentiation and survival processes of nerve cells. PLoS One.
7:e324382012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lin X, Duan X, Liang YY, Su Y, Wrighton
KH, Long J, Hu M, Davis CM, Wang J, Brunicardi FC, et al: PPM1A
functions as a Smad phosphatase to terminate TGFbeta signaling.
Cell. 125:915–928. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li L, Li Q, Wei L, Wang Z, Ma W, Liu F,
Shen Y, Zhang S, Zhang X, Li H and Qian Y: Chemokine (C-X-C motif)
ligand 14 contributes to lipopolysaccharide-induced fibrogenesis in
mouse L929 fibroblasts via modulating PPM1A. J Cell Biochem.
120:13372–13381. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li L, Zhang S, Wei L, Wang Z, Ma W, Liu F,
Shen Y, Zhang S, Zhang X, Hang Y and Qian Y: Anti-fibrotic effect
of melittin on TRIM47 expression in human embryonic lung fibroblast
through regulating TRIM47 pathway. Life Sci. 256:1178932020.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhou J, Lan Q, Li W, Yang L, You J, Zhang
YM and Ni W: Tripartite motif protein 52 (TRIM52) promoted fibrosis
in LX-2 cells through PPM1A-mediated Smad2/3 pathway. Cell Biol
Int. Jul 22–2019.(Epub ahead of print).
|
|
28
|
Chen T, Guo Y, Wang J, Ai L, Ma L, He W,
Li Z, Yu X, Li J, Fan X, et al: LncRNA CTD-2528L19.6 prevents the
progression of IPF by alleviating fibroblast activation. Cell Death
Dis. 12:6002021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wilson MS and Wynn TA: Pulmonary fibrosis:
Pathogenesis, etiology and regulation. Mucosal Immunol. 2:103–121.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bellaye PS, Yanagihara T, Granton E, Sato
S, Shimbori C, Upagupta C, Imani J, Hambly N, Ask K, Gauldie J, et
al: Macitentan reduces progression of TGF-β1-induced pulmonary
fibrosis and pulmonary hypertension. Eur Respir J. 52:17018572018.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Choi YH, Yoo DS, Choi CW, Cha MR, Kim YS,
Lee HS, Lee KR and Ryu SY: Platyconic acid A, a genuine
triterpenoid saponin from the roots of Platycodon
grandiflorum. Molecules. 13:2871–2879. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Choi JH, Kim SM, Lee GH, Jin SW, Lee HS,
Chung YC and Jeong HG: Platyconic acid A, Platycodi
radix-derived saponin, suppresses TGF-1-induced activation of
hepatic stellate cells via blocking SMAD and activating the PPAR
signaling pathway. Cells. 8:15442019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Biernacka A, Dobaczewski M and
Frangogiannis NG: TGF-β signaling in fibrosis. Growth Factors.
29:196–202. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yingling JM, Blanchard KL and Sawyer JS:
Development of TGF-beta signalling inhibitors for cancer therapy.
Nat Rev Drug Discov. 3:1011–1022. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yoshida K and Matsuzaki K: Differential
regulation of TGF-β/Smad signaling in hepatic stellate cells
between acute and chronic liver injuries. Front Physiol. 3:532012.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lee KJ, Kim JY, Jung KS, Choi CY, Chung
YC, Kim DH and Jeong HG: Suppressive effects of Platycodon
grandiflorum on the progress of carbon tetrachloride-induced
hepatic fibrosis. Arch Pharm Res. 27:1238–1244. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Choi JH, Jin SW, Kim HG, Khanal T, Hwang
YP, Lee KJ, Choi CY, Chung YC, Lee YC and Jeong HG: Platycodi
radix attenuates dimethylnitrosamine-induced liver fibrosis in
rats by inducing Nrf2-mediated antioxidant enzymes. Food Chem
Toxicol. 56:231–239. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Choi JH, Jin SW, Choi CY, Kim HG, Kim SJ,
Lee HS, Chung YC, Kim EJ, Lee YC and Jeong HG: Saponins from the
roots of Platycodon grandiflorum ameliorate high fat
diet-induced non-alcoholic steatohepatitis. Biomed Pharmacother.
86:205–212. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Katzenstein AL and Myers JL: Idiopathic
pulmonary fibrosis: Clinical relevance of pathologic
classification. Am J Respir Crit Care Med. 157:1301–1315. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Thannickal VJ, Toews GB, White ES, Lynch
JP III and Martinez FJ: Mechanisms of pulmonary fibrosis. Annu Rev
Med. 55:395–417. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li Y, Wu Y, Xia Q, Zhao Y, Zhao R and Deng
S: Platycodon grandiflorus enhances the effect of DDP
against lung cancer by down regulating PI3K/Akt signaling pathway.
Biomed Pharmacother. 120:1094962019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lawrence T, Gilroy DW, Colville-Nash PR
and Willoughby DA: Possible new role for NF-kappaB in the
resolution of inflammation. Nat Med. 7:1291–1297. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lawrence T: The nuclear factor NF-kappaB
pathway in inflammation. Cold Spring Harb Perspect Biol.
1:a0016512009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Jin H: Imrecoxib inhibits paraquat-induced
pulmonary fibrosis through the NF-κB/Snail signaling pathway.
Comput Math Methods Med. 2020:63740142020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Tian Y, Li H, Qiu T, Dai J, Zhang Y, Chen
J and Cai H: Loss of PTEN induces lung fibrosis via alveolar
epithelial cell senescence depending on NF-κB activation. Aging
Cell. 18:e128582019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Park EJ and Lee HJ: Immunomodulatory
effects of fermented Platycodon grandiflorum extract through
NF-κB signaling in RAW 264.7 cells. Nutr Res Pract. 14:453–462.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yim NH, Hwang YH, Liang C and Ma JY: A
platycoside-rich fraction from the root of Platycodon
grandiflorum enhances cell death in A549 human lung carcinoma
cells via mainly AMPK/mTOR/AKT signal-mediated autophagy induction.
J Ethnopharmacol. 194:1060–1068. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Hwang YP, Choi JH, Kim HG, Lee HS, Chung
YC and Jeong HG: Saponins from Platycodon grandiflorum
inhibit hepatic lipogenesis through induction of SIRT1 and
activation of AMP-activated protein kinase in high-glucose-induced
HepG2 cells. Food Chem. 140:115–123. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Xiang HC, Lin LX, Hu XF, Zhu H, Li HP,
Zhang RY, Hu L, Liu WT, Zhao YL, Shu Y, et al: AMPK activation
attenuates inflammatory pain through inhibiting NF-κB activation
and IL-1β expression. J Neuroinflammation. 16:342019. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Jiang X, Ru Q, Li P, Ge X, Shao K, Xi L,
Xu B, Wang Q and Huang S: LncRNA SNHG16 induces proliferation and
fibrogenesis via modulating miR-141-3p and CCND1 in diabetic
nephropathy. Gene Ther. 27:557–566. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zhou Z, Ni J, Li J, Huo C, Miao N, Yin F,
Cheng Q, Xu D, Xie H, Chen P, et al: RIG-I aggravates interstitial
fibrosis via c-Myc-mediated fibroblast activation in UUO mice. J
Mol Med (Berl). 98:527–540. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Dai F, Duan X, Liang YY, Lin X and Feng
XH: Coupling of dephosphorylation and nuclear export of Smads in
TGF-beta signaling. Methods Mol Biol. 647:125–137. 2010. View Article : Google Scholar : PubMed/NCBI
|