Introduction
Stroke is one of the leading causes of death from
diseases. According to the Heart Disease and Stroke Statistics
report from the American Heart Association (2019 updated), more
than 10 million people are affected by stroke every year (1). A stroke occurs due to
cerebrovascular abnormalities, of which 87% are ischemic stroke
(2). When the blood supply to the
brain tissue is interrupted, neuronal core death occurs due to the
lack of glucose and oxygen in min (3).
Thrombolytic therapy is a standard strategy for
clinical treatment for ischemic stroke (4). However, brain damage is always
inevitable, even if the blood flow is restored, and it may leave a
long-term disability (5).
Previous studies revealed that the severity of cerebral edema
predicted long-term functional outcomes (6,7).
Cerebral edema occurs immediately after ischemia, reaches its peak
at 15 min and continues with the vascular obstruction (8). With prolonged ischemia status, the
blood-brain barrier (BBB) will collapse, and the fluid from the
blood will enter the cerebral interstitial space, forming a later
phase of edema that is well known as vasogenic edema. Cerebral
edema cannot be relieved or even worsened after blood flow recovery
(9). Therefore, controlling the
sudden increase in cerebral blood flow may relieve cerebral edema
in addition to reperfusion-induced hemorrhage (10). Since systemic blood pressure
regulates blood flow distribution in the body, it is also important
to control systemic blood pressure in the treatment of cerebral
ischemia. A recent meta-analysis has shown that receiving blood
pressure medicine, such as angiotensin-converting enzyme inhibitors
or diuretics, will reduce the risk of recurrent stroke from
transient ischemic attack (11).
An animal study also proved that blood pressure lowering after
reperfusion in acute ischemia may protect against neurovascular
damage (12).
Astragaloside IV is a pure small molecular compound
isolated from Radix Astragali, and it is well documented
that astragaloside IV has neuroprotective effect on cerebral
ischemia reperfusion (CIR) injury through numerous mechanisms,
including antioxidant, anti-inflammatory and anti-apoptotic
(13). Moreover, the
antihypertension effect of astragaloside IV has been proven in
different hypertension animal models (14,15). However, such an effect during CIR
remains unclear. Thus, the aim of the present study was to
investigate the effect of astragaloside IV on systemic blood
pressure during CIR in a middle cerebral artery occlusion (MCAO)
animal model.
Materials and methods
Animals and group assignments
The experimental procedures in the present study
were in accordance with the ARRIVE (Animals in Research: Reporting
In Vivo Experiments) guidelines and were approved (approval
no. 20210304037) by the Animal Care and Use Committee of Guangzhou
University of Traditional Chinese Medicine (Guangzhou, China). Male
Sprague-Dawley rats (8–10 weeks old; 250–300 g; Experimental Animal
Center affiliated with Guangzhou University of Traditional Chinese
Medicine, Guangzhou, China) were kept in a clean animal room, with
free access to food and water (12 h:12 h light/dark cycle, 22±1°C,
50% humidity). The rats were randomly assigned to 5 groups: Sham,
the group of rats with sham operation; CIR, the group of rats with
MCAO; Saline, the group of rats with MCAO and saline pretreatment;
Ast, the group of rats with MCAO and astragaloside IV pretreatment;
Bum, the group of rats with MCAO and bumetanide pretreatment. The
animals with tissue sampling were anesthetized by sodium
pentobarbital (20 mg/ml, i.p.), and the rest were sacrificed by
carbon dioxide asphyxiation (flow rate=70% of the cage volume per
min). Finally, the valid data was produced from a total of 86 rats.
All possible efforts were made to minimize the number of animals
and their suffering.
CIR modelling
Rats were first subjected to 5% isoflurane (RWD Life
Science) in 30% O2 balanced with N2O, then
with 1.5% isoflurane for anesthesia maintenance. Cerebral blood
flow was monitored in the territory of the middle cerebral artery
using a laser doppler flowmetry (RWD Life Science). Thereafter, the
right common carotid artery, internal carotid artery and external
carotid artery of individual rats were exposed. Focal ischemia was
induced by inserting a monofilament nylon suture with a round tip
(Yuyan Instruments Co., Ltd.) inserted into the internal carotid
artery through the external carotid artery stump and gently
advanced to the middle cerebral artery. After 30 min of ischemia,
the filament was removed to restore blood flow (reperfusion) and
the skin incision was sutured. Sham-operated control rats received
the same surgical procedure without insertion of a filament. Rats
with intracranial hemorrhage and those that did not show a
reduction in cerebral blood flow >80% during MCAO were
excluded.
Drug administration
Astragaloside IV (Shanghai Yuanye Bio-Technology
Co., Ltd.) was diluted in corn oil. Rats were treated with
astragaloside IV (7.5 mg/ml) or saline via intraperitoneal
injection 15 min before surgery. Bumetanide (0.18 mg/ml, 2 µl, 0.25
µl/min; cat. no. B3023; MilliporeSigma) was microinjected into
bilateral paraventricular [AP 1.5 mm, ML 0.4 mm, DV 7.7 mm
(16)] with a microinjection pump
(Yuyan Instruments Co. Ltd.).
Mean arterial pressure (MAP)
monitoring
Tail cuff blood pressure system (IITC Life Science
Inc.) was used for the measurement of MAP. First, the rats were
sufficiently acclimated to the restraint holder, and each rat was
separated by an opaque partition. Then the tail was warmed with a
warming pad for 15–20 min (until the rat was no longer irritable)
before each cycle of blood pressure measurements. The final data
was obtained from the average of 10 valid data. The data of animals
those could not cooperate well in the experiment were
eliminated.
Neurologic score
To estimate the degree of neurological impairment, a
48-point scoring system was used. This scoring system composes of
general status (spontaneous activity, body symmetry, gait; 0–12),
simple motor deficit (forelimb asymmetry, circling, hind-limb
placement; 0–14), complex motor deficit (vertical screen climbing,
beam walking; 0–8), and sensory deficit (hind limb, trunk,
vibrissae and face touch; 0–14). The total score was the sum of the
4 individual scores, with 0=no deficit and 48=maximal deficit.
2,3,5-triphenyltetrazolium chloride
(TTC) staining
Rats were euthanized on indicated time point. The
brain sections were placed in 1% TTC (cat. no. 298-96-4;
MilliporeSigma) at 37°C for 30 min. The slices were flipped once
every 5 min and then washed 3 times with ddH2O. Images
of the sections were captured with a digital camera. The area of
infarct of each section (1 mm) was measured by subtracting the
non-infarcted area in the ipsilateral hemisphere from the total
area of the contralateral hemisphere, and then the final infarct
volume was calculated by summing the infarct areas in all sections
and multiplying by the section thickness (ImageJ v1.53e; National
Institutes of Health).
Enzyme-linked immuno-sorbent assay
(ELISA)
To detect the level of arginine vasopressin (AVP),
rat pituitary tissue was isolated, and dissolved with homogenizing
medium (1:9). The sample was centrifuged at 3,500 × g for 10 min,
and the supernatant was harvested. Diluted supernatant was added to
the AVP ELISA kit (Rat) (cat. no. OKCD08532; Aviva Systems Biology,
Corp.). Then, the plate was sealed with sealing film and incubated
at 37°C for 30 min. The sealing plate film was carefully removed
and the liquid was discarded. Τhe plate was washed for 5 times.
Then, labeling reagents (50 µl) were added into each well (except
blank). The incubation and washing process were repeated.
Chromogenic agent A and B (50 µl) was added into each well in
sequence. After 10 min coloration at 37°C, 50 µl termination
solution was added to each well. The absorbance of each well was
measured at the wavelength of 450 nm.
Western blotting
The paraventricular nucleus (PVN) tissue was
homogenized in the lysis buffer (10 mM Tris-HCl and 320 mM sucrose)
containing 1% protease inhibitor mixture (cat. no. 36978; Thermo
Fisher Scientific) and then centrifuged (Eppendorf 5810) at 8,000 ×
g for 5 min. Then the supernatant was centrifuged (Eppendorf 5810)
at 40,000 × g for 30 min to obtain crude membrane in the pellet.
The cellular membranes were resuspended in lysis buffer with
protease inhibitor mixture. Protein concentrations were determined
using Bio-Rad protein reagent (Bio-Rad Laboratories, Inc.). Samples
were heated at 95°C for 10 min in loading buffer before 20 µg
protein was loaded for 10% sodium dodecyl sulfate polyacrylamide
gel electrophoresis, electrophoretically separated, and then
transferred to polyvinylidene difluoride membranes at 4°C. After
blocking with 5% non-fat milk for 2 h at room temperature,
membranes were incubated overnight at 4°C with a primary antibody
[rabbit anti-Na+-K+−2Cl−
cotransporter isoform 1 (NKCC1) (1:1,000; cat. no. 4828S; Cell
Signaling Technology, Inc.)]. Membranes were washed with PBS
buffer, incubated with horseradish peroxidase-conjugated secondary
anti-rabbit IgG (1:2,000; cat. no. ab6721; Abcam) for 2 h at room
temperature, and the bands were visualized using Immobilon Western
Chemiluminescent HRP Substrate (cat. no. WBKLS0050;
MilliporeSigma). The relative levels of the target protein were
determined by performing a densitometry analysis using ImageJ
v1.53e software (National Institutes of Health).
Statistical analysis
SigmaStat 3.5 (Jandel Scientific Software) was used
to perform the statistical analyses. The data were expressed as the
mean ± SD. Student's t-test (independent t-test) was used when two
groups were compared. The MAP data were analyzed by using one-way
repeated-measures ANOVA or two-way repeated-measures ANOVA followed
by the Bonferroni post hoc test. P≤0.05 was considered to indicate
a statistically significant difference.
Results
CIR induces brain damage and MAP
elevation in rats
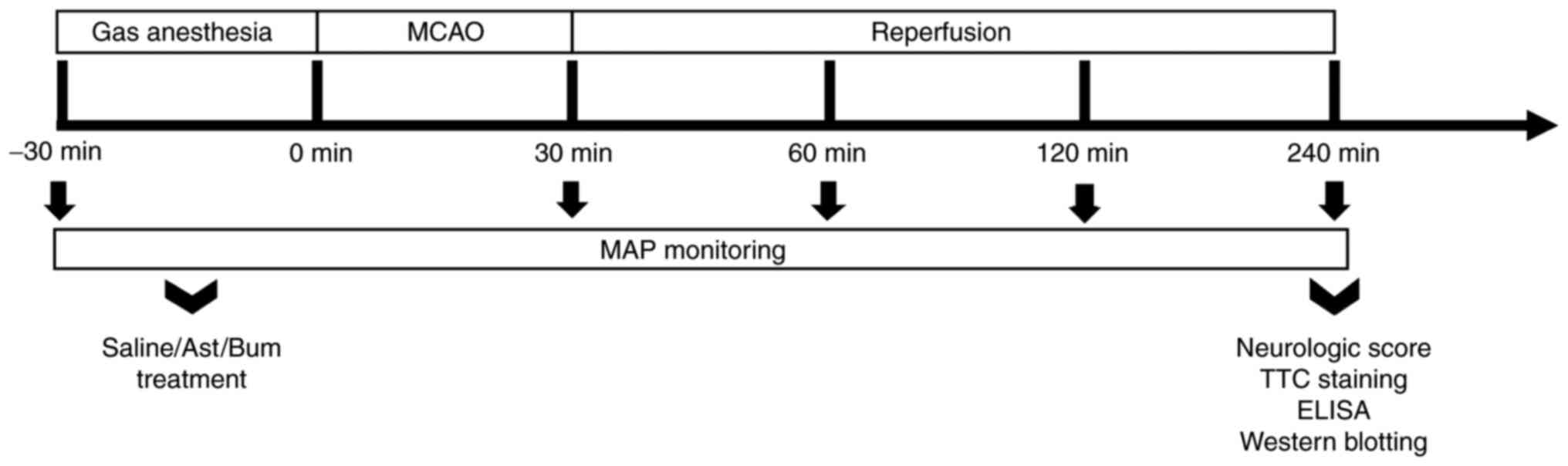
To reduce the mortality of the animals, the middle
cerebral artery of the rat was occluded for only 30 min, and the
subsequent reperfusion lasted 210 min in the present study
(Fig. 1). The results showed
significant neurologic dysfunction (Sham vs. CIR=0 vs. 22.60±5.07,
n=5 per group, Student's t-test, P<0.001, Fig. 2A) and increased infarct volume
(Sham vs. CIR=0 mm3 vs. 130.13±27.08 mm3, n=5
per group, Student's t-test, P<0.001, Fig. 2B and C) after CIR. Meanwhile, the
MAP significantly elevated, and prolonged at least for 210 min
(n=10 per group, two-way repeated-measures ANOVA followed by the
Bonferroni post hoc test, P<0.001, Fig. 2D, Table I). These results indicated that
CIR induces a long-term MAP elevation.
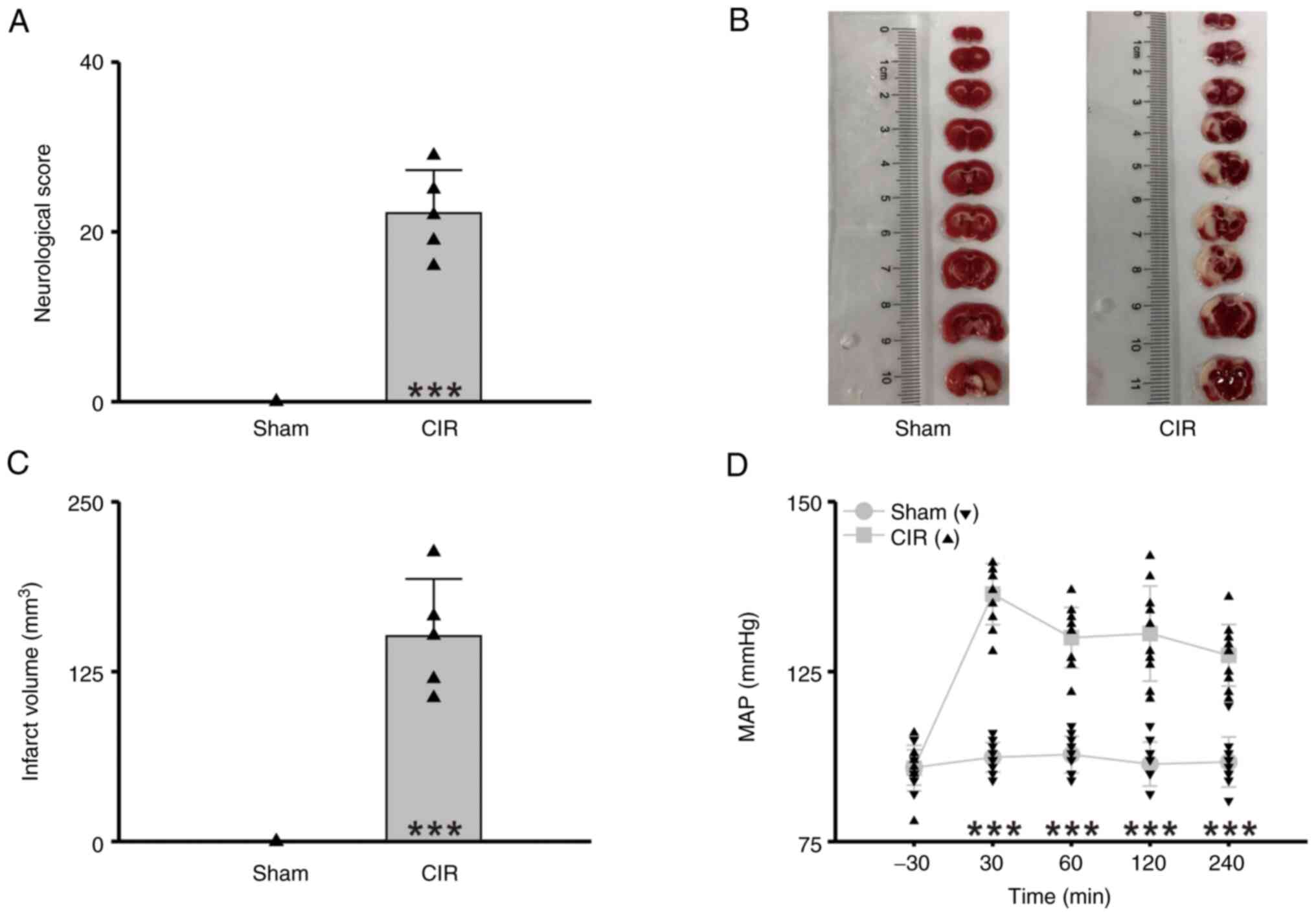 | Figure 2.CIR-induced brain damage and
hypertension. (A) Statistical chart of neurologic score (n=5 per
group, Student's t-test, ***P<0.001). (B)
2,3,5-triphenyltetrazolium chloride-stained brain tissue specimens.
(C) Statistical chart of infarct volume (n=5 per group, Student's
t-test, ***P<0.001). (D) Statistical chart of MAP (n=10 per
group, two-way repeated-measures ANOVA followed by the Bonferroni
post hoc test, ***P<0.001, Sham vs. CIR at the same time point).
Sham, the group of rats with sham operation; CIR, the group of rats
with CIR. Triangles indicate the individual data obtained from each
group. CIR, cerebral ischemia reperfusion; MAP, mean arterial
pressure. |
 | Table I.Mean arterial pressure (Sham vs.
CIR). |
Table I.
Mean arterial pressure (Sham vs.
CIR).
| Group | −30 min | 30 min | 60 min | 120 min | 240 min |
|---|
| Sham (mmHg) | 85.90±2.60 | 87.40±2.17 | 87.80±2.70 | 85.80±3.36 | 86.70±3.68 |
| CIR (mmHg) | 86.40±3.24 | 111.40±4.50 | 105.00±4.45 | 105.60±7.00 | 102.40±4.53 |
| Ast (mmHg) | 86.80±3.08 | 102.10±6.23 | 88.60±4.60 | 87.80±4.59 | 89.30±3.40 |
| Bum (mmHg) | 86.10±3.48 | 98.50±6.29 | 88.50±4.12 | 85.70±4.50 | 87.30±6.00 |
Astragaloside IV relieves CIR-induced
brain damage and MAP elevation
It is consistent with most of previous findings that
astragaloside IV pretreatment relieved the neurologic dysfunction
of rats induced by CIR (Saline vs. Ast=23.67±4.55 vs. 14.33±4.46,
n=6 per group, Student's t-test, P=0.005, Fig. 3A) and reduced infarct volume
(Saline vs. Ast=173.46±60.97 mm3 vs. 74.97±35.05
mm3, n=6 per group, Student's t-test, P=0.006, Fig. 3B and C). Moreover, the rats
treated with astragaloside IV showed a milder fluctuation of MAP
(n=10, one-way repeated-measures ANOVA followed by the Holm-Sidak
method, P<0.001 compared with the baseline before modeling,
Fig. 3D, Table I). These results indicated that
astragaloside IV relieves CIR-induced MAP elevation.
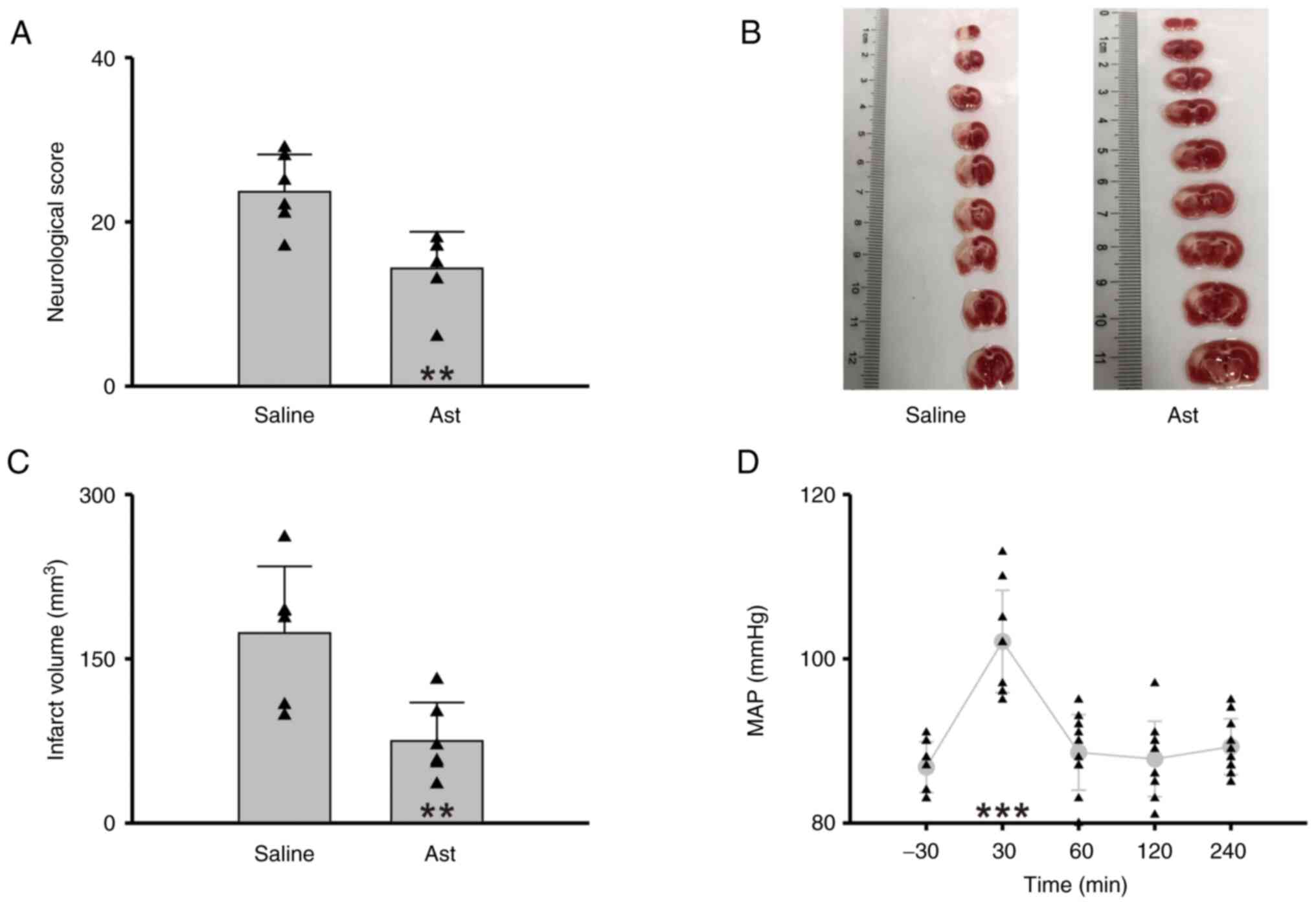 | Figure 3.Astragaloside IV relieves CIR-induced
brain damage and hypertension. (A) Statistical chart of neurologic
score (n=6 per group, Student's t-test, **P<0.01). (B)
2,3,5-triphenyltetrazolium chloride-stained brain tissue specimens.
(C) Statistical chart of infarct volume (n=6 per group, Student's
t-test, **P<0.01). (D) Statistical chart of MAP (n=10, one-way
repeated-measures ANOVA followed by the Holm-Sidak method,
***P<0.001, compared with the baseline before modeling). Saline,
the group of rats with CIR and saline pretreatment; Ast, the group
of rats with CIR and Astragaloside IV pretreatment. Triangles
indicate the individual data obtained from each group. CIR,
cerebral ischemia reperfusion; MAP, mean arterial pressure. |
Upregulation of NKCC1 in
hypothalamus-mediated MAP elevation during CIR
AVP, also called antidiuretic hormone, is
synthesized in the PVN of the hypothalamus and stored in the
pituitary gland. It is released into the blood circulatory system
to regulate systemic blood pressure when needed (17). It has been demonstrated that the
upregulation of NKCC1 in AVP neurons contributes to the generation
of sodium-dependent hypertension by increasing AVP release
(18). One of the main functions
of NKCC1 is to transport Cl− into cells, increasing the
concentration of intracellular Cl−, thereby weakening
GABAergic inhibition and even turning it into depolarization
(19,20). Therefore, the upregulation of
NKCC1 in hypothalamus may promote the excitability of hypothalamic
neurons, thus increasing the synthesis and secretion of AVP.
The present results showed that CIR significantly
increased the AVP level (ng) in the pituitary gland (g) (Sham vs.
CIR=18.12±2.25 ng/g vs. 48.64±9.06 ng/g, n=6 per group, Student's
t-test, P<0.001, Fig. 4A) and
the expression level of NKCC1 in the PVN (Sham vs. CIR=1.00±0.19
vs. 3.61±1.30, n=6 per group, Student's t-test, P<0.001,
Fig. 4B). To investigate the
contribution of NKCC1 to generating MAP elevation, bumetanide, an
NKCC1 inhibitor, was delivered by paraventricular microinjection.
It was found that bumetanide pretreatment significantly reduced the
MAP during CIR (n=10, one-way repeated-measures ANOVA followed by
the Holm-Sidak method, P<0.001 compared with the baseline before
modeling, Fig. 4C, Table I). These results indicated that
upregulation of NKCC1 in hypothalamus mediates MAP elevation during
CIR.
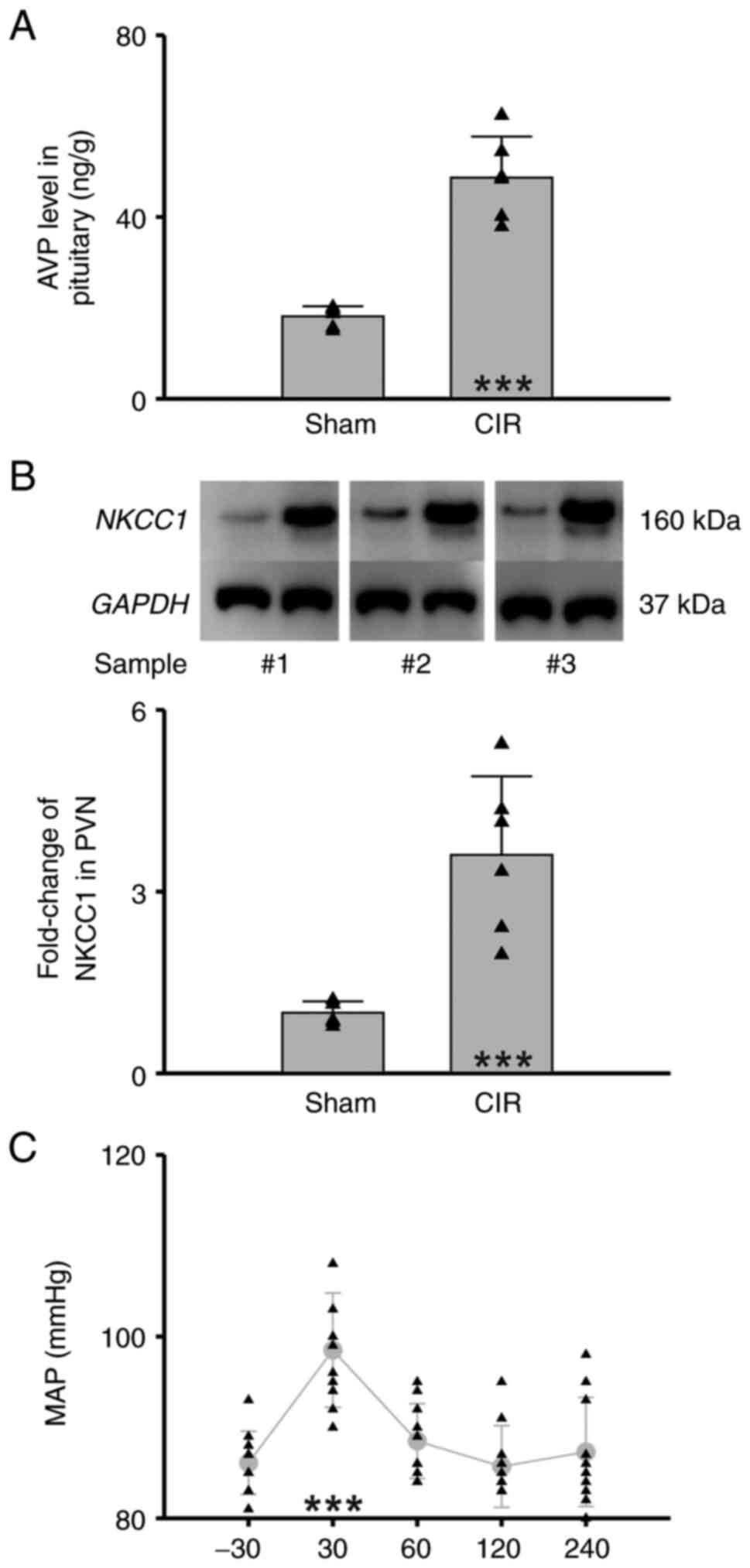 | Figure 4.Upregulation of NKCC1 in
hypothalamus-mediated hypertension during CIR. (A) Statistical
chart of AVP level in pituitary (n=6 per group, Student's t-test,
***P<0.001). (B) Statistical chart of NKCC1 level in
hypothalamus. Upper panel represents three pairs of western blot
samples (n=6 per group, Student's t-test, ***P<0.001). (C)
Statistical chart of MAP with bumetanide pretreatment (n=10,
one-way repeated-measures ANOVA followed by the Holm-Sidak method,
***P<0.001, compared with the baseline before modeling). Sham,
the group of rats with sham operation; CIR, the group of rats with
CIR. Triangles indicate the individual data obtained from each
group. NKCC1, Na+-K+−2Cl−
cotransporter isoform 1; CIR, cerebral ischemia reperfusion; AVP,
arginine vasopressin; MAP, mean arterial pressure. |
Astragaloside IV inhibits NKCC1
expression in hypothalamus of MCAO rats
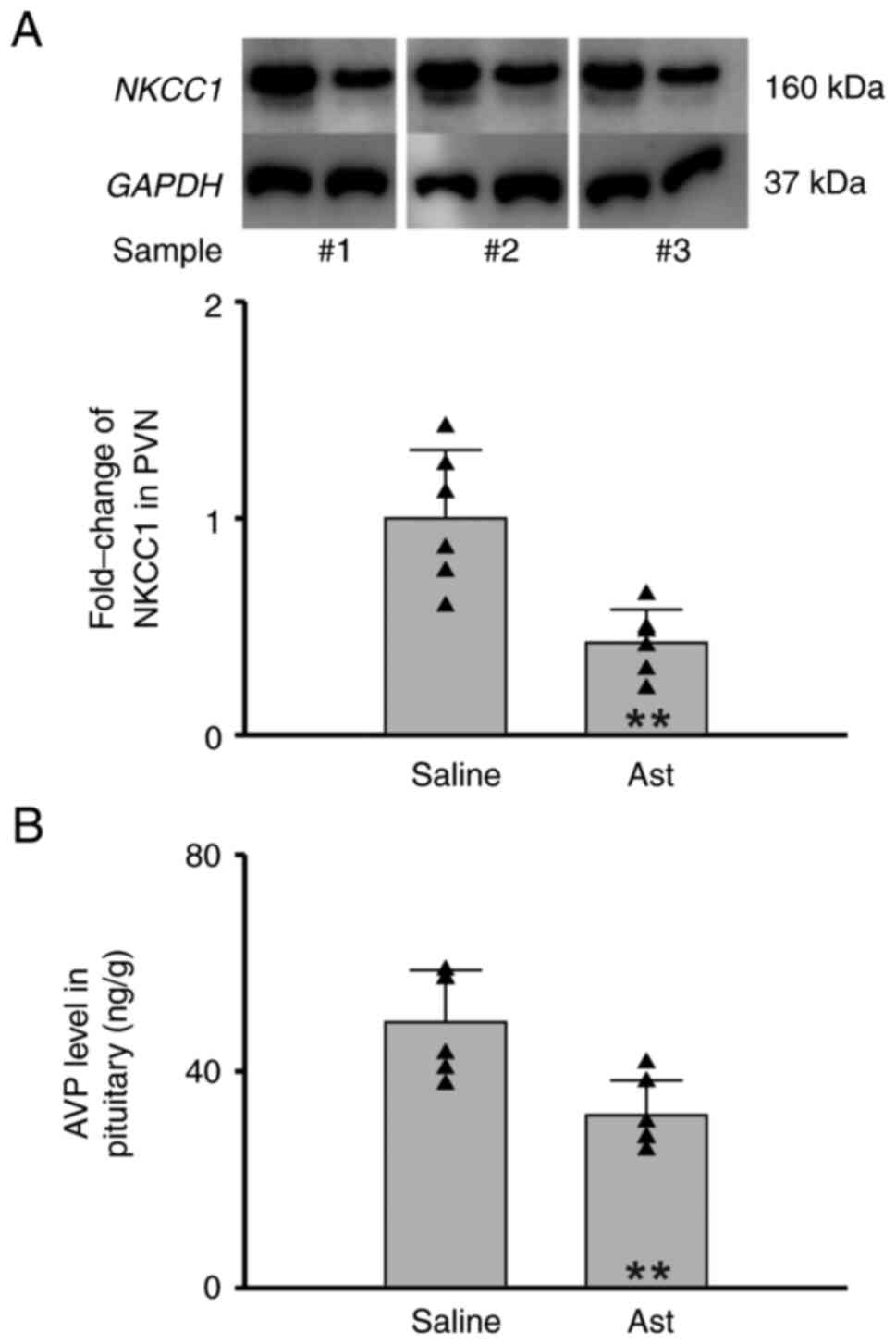
The results of the present study showed that
astragaloside IV intraperitoneal injection significantly inhibited
the NKCC1 expression in PVN of MCAO rats (Saline vs. Ast=1.00±0.32
vs. 0.43±0.15, n=6 per group, Student's t-test, P=0.002, Fig. 5A). Moreover, the AVP level in
pituitary gland was also reduced. (Saline vs. Ast=49.05±9.59 ng/g
vs. 31.89±6.45 ng/g, n=6 per group, Student's t-test, P=0.005,
Fig. 5B). These results indicated
that astragaloside IV inhibits NKCC1 expression in hypothalamus of
MCAO rats.
Discussion
The human brain accounts for 15–20% of the cardiac
output and is extremely sensitive to ischemia. Blood pressure is
one of the important vital signs of the human body. As a driving
force to promote the blood flow, blood pressure ensures the blood
supply of important organs. When ischemia occurs in tissues or
organs, the human body may activate the autoregulation system
(heart rate, cardiac output, blood pressure) to maintain normal
hemodynamics (21). Thus, the
elevation of blood pressure after cerebral ischemia is very common,
and blood pressure lowering is an important strategy to improve
functional outcomes after ischemic stroke (22,23). It has been reported that the MAP
increased immediately upon MCAO and remained elevated after 24 h of
reperfusion (12). Prolonged
elevation of MAP in the MCAO model was also revealed in the present
study. Such prolonged hypertensive state may worsen the cerebral
edema, thereby aggravating the reperfusion injury (24).
AVP elevates blood pressure mainly by promoting
water reabsorption, blood volume and the contraction of vascular
smooth muscle (25,26). It has been reported that cerebral
ischemia may increase the AVP level of plasma (27–29). The current study showed that
ischemia reperfusion led to the increase of AVP in rat pituitary.
Meanwhile, the expression of NKCC1 was upregulated in PVN. The
results of the present study also showed that the increase of MAP
during CIR could be significantly reduced when NKCC1 is interfered
by pretreatment of bumetanide. These results indicated that CIR
drives the occurrence of central hypertension.
In traditional Chinese medicine, the stroke is
described as a series of symptoms (abrupt coma, paraesthesia,
hemiplegia, facial paralysis, speech disorder) induced by
obstruction of cerebral meridians (ischemic stroke) or blood spill
over from cerebral meridians (hemorrhagic stroke). The most
important pathogenesis of ischemic stroke is the decline of vital
Qi (energy) with aging or prolonged illness. Radix Astragali
(Huang Qi) is a commonly used herbal medicine in China, that
enriches the primordial Qi, nourishes the heart and dredges the
pulse, fortifies the spleen and disinhibits the dampness according
to the theory of traditional Chinese medicine. The main active
components of Radix Astragali are polysaccharides and
astragalosides (30).
Astragaloside contains four subtypes (I–IV), among which
astragaloside IV has the best biological activity (31,32). Due to the multiple target
advantages of astragaloside IV, its preparation is widely used in
the adjuvant treatment of cardiovascular and cerebrovascular
diseases (33,34). It has the effects of improving
immunity, reducing stress reaction, antivirus, anti-inflammation,
analgesia, organ protection and promoting metabolism (31,32). In addition, astragaloside IV has
the effect of regulating blood pressure (35,36).
It is consistent with previous findings that
astragaloside IV had an obvious preventive effect on CIR injury.
Meanwhile, the rats treated with astragaloside IV showed a milder
fluctuation of MAP. Those results indicated that the inhibitory
effect of astragaloside IV on blood pressure may contribute to
alleviate ischemia reperfusion injury to a certain extent. In
addition, pretreatment with astragaloside IV inhibited the
expression of NKCC1 in PVN nuclei and the level of AVP in pituitary
of CIR rats. However, in the present study, it was not clear
whether the antihypertensive effect of astragaloside IV directly
acts on the central system or peripheral.
It was considered that there are two possible
mechanisms for astragaloside IV regulating blood pressure during
CIR. According to the data provided by Traditional Chinese Medicine
Systems Pharmacology database and analysis platform, astragaloside
IV is BBB non-penetrable, which may have limited effect on the
central nervous system. It has been reported that astragaloside IV
relieved hypertension via improving inflammation, pulmonary artery
remodelling and oxidative stress (35,36). Then the decrease of NKCC1
expression may be due to the stable blood pressure after CIR that
led to the reduction of AVP demand. Another possibility is that the
permeability of BBB will increase since its structure is damaged
after ischemic stroke (37,38). Then astragaloside IV is likely to
have opportunity to act on the central nervous system. However, in
any case, it will not affect the contribution of antihypertension
effect to the alleviation of CIR by astragaloside IV. Therefore, a
possible mechanism for astragaloside IV to reduce CIR injury was
proposed in the present study. Further investigation is needed to
explore the integrated mechanism of CIR-induced central
hypertension and the specific target of astragaloside IV.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Foundation of Shanghai
Municipal Science and Technology Commission (grant no. 19ZR1407500)
and the Shenzhen Traditional Chinese Medicine Hospital ‘3030
Program’ Chinese Medicine Clinical Research Project (grant no.
3030202108).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
FS, JH and LW designed the research. FS, YM and YH
conducted the experiments. FS, YM and BH analyzed the data. JH and
LW confirm the authenticity of all the raw data. FS, JH and LW
prepared the manuscript. All authors read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
The experimental procedures in the present study
were in accordance with the ARRIVE (Animals in Research: Reporting
In Vivo Experiments) guidelines and were approved (approval
no. 20210304037) by the Animal Care and Use Committee of Guangzhou
University of Traditional Chinese Medicine (Guangzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Benjamin EJ, Muntner P, Alonso A,
Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR,
Cheng S, Das SR, et al: Heart disease and stroke statistics-2019
update: A report from the American heart association. Circulation.
139:e56–e528. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stubblefield JJ and Lechleiter JD: Time to
target stroke: Examining the circadian system in stroke. Yale J
Biol Med. 92:349–357. 2019.PubMed/NCBI
|
|
3
|
Puig B, Brenna S and Magnus T: Molecular
communication of a dying neuron in stroke. Int J Mol Sci.
19:28342018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Feske SK: Ischemic stroke. Am J Med.
134:1457–1464. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wu MY, Yiang GT, Liao WT, Tsai AP, Cheng
YL, Cheng PW, Li CY and Li CJ: Current mechanistic concepts in
ischemia and reperfusion injury. Cell Physiol Biochem.
46:1650–1667. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liang D, Bhatta S, Gerzanich V and Simard
JM: Cytotoxic edema: Mechanisms of pathological cell swelling.
Neurosurg Focus. 22:E22007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stokum JA, Gerzanich V and Simard JM:
Molecular pathophysiology of cerebral edema. J Cereb Blood Flow
Metab. 36:513–538. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mestre H, Du T, Sweeney AM, Liu G, Samson
AJ, Peng W, Mortensen KN, Stæger FF, Bork PAR, Bashford L, et al:
Cerebrospinal fluid influx drives acute ischemic tissue swelling.
Science. 367:eaax71712020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Han D, Sun M, He PP, Wen LL, Zhang H and
Feng J: Ischemic postconditioning alleviates brain edema after
focal cerebral ischemia reperfusion in rats through down-regulation
of aquaporin-4. J Mol Neurosci. 56:722–729. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Simao F, Ustunkaya T, Clermont AC and
Feener EP: Plasma kallikrein mediates brain hemorrhage and edema
caused by tissue plasminogen activator therapy in mice after
stroke. Blood. 129:2280–2290. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zonneveld TP, Richard E, Vergouwen MD,
Nederkoorn PJ, de Haan R, Roos YB and Kruyt ND: Blood
pressure-lowering treatment for preventing recurrent stroke, major
vascular events, and dementia in patients with a history of stroke
or transient ischaemic attack. Cochrane Database Syst Rev.
7:CD0078582018.PubMed/NCBI
|
|
12
|
Elewa HF, Kozak A, Johnson MH, Ergul A and
Fagan SC: Blood pressure lowering after experimental cerebral
ischemia provides neurovascular protection. J Hypertens.
25:855–859. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang HL, Zhou QH, Xu MB, Zhou XL and Zheng
GQ: Astragaloside IV for experimental focal cerebral ischemia:
Preclinical evidence and possible mechanisms. Oxid Med Cell Longev.
2017:84243262017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jiang P, Ma D, Wang X, Wang Y, Bi Y, Yang
J, Wang X and Li X: Astragaloside IV prevents obesity-associated
hypertension by improving pro-inflammatory reaction and leptin
resistance. Mol Cells. 41:244–255. 2018.PubMed/NCBI
|
|
15
|
Zhang X, Chen J, Xu P and Tian X:
Protective effects of Astragaloside IV against hypoxic pulmonary
hypertension. Medchemcomm. 9:1715–1721. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Su Z, Miao B, Xu MQ, Yang MJ, Fei SJ and
Zhang JF: Protective effect of microinjection of glutamate into
hypothalamus paraventricular nucleus on chronic visceral
hypersensitivity in rats. Brain Res. 1747:1470482020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Savić B, Murphy D and Japundžić-Žigon N:
The Paraventricular Nucleus of the Hypothalamus in Control of Blood
Pressure and Blood Pressure Variability. Front Physiol.
13:8589412022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim YB, Kim YS, Kim WB, Shen FY, Lee SW,
Chung HJ, Kim JS, Han HC, Colwell CS and Kim YI: GABAergic
excitation of vasopressin neurons: Possible mechanism underlying
sodium-dependent hypertension. Circ Res. 113:1296–1307. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kaila K, Price TJ, Payne JA, Puskarjov M
and Voipio J: Cation-chloride cotransporters in neuronal
development, plasticity and disease. Nat Rev Neurosci. 15:637–654.
2014. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Virtanen MA, Uvarov P, Hubner CA and Kaila
K: NKCC1, an elusive molecular target in brain development: Making
sense of the existing data. Cells. 9:26072020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gorelick PB and Ruland S: Cerebral
vascular disease. Dis Mon. 56:39–100. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Spengos K, Tsivgoulis G and Zakopoulos N:
Blood pressure management in acute stroke: A long-standing debate.
Eur Neurol. 55:123–135. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Willmot M, Leonardi-Bee J and Bath PM:
High blood pressure in acute stroke and subsequent outcome: A
systematic review. Hypertension. 43:18–24. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gorelick PB and Aiyagari V: The management
of hypertension for an acute stroke: What is the blood pressure
goal? Curr Cardiol Rep. 15:3662013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Aoyagi T, Koshimizu TA and Tanoue A:
Vasopressin regulation of blood pressure and volume: Findings from
V1a receptor-deficient mice. Kidney Int. 76:1035–1039. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bankir L, Bichet DG and Morgenthaler NG:
Vasopressin: Physiology, assessment and osmosensation. J Intern
Med. 282:284–297. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Barreca T, Gandolfo C, Corsini G, Del
Sette M, Cataldi A, Rolandi E and Franceschini R: Evaluation of the
secretory pattern of plasma arginine vasopressin in stroke
patients. Cerebrovasc Dis. 11:113–118. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu X, Jin Y, Zheng H, Chen G, Tan B and
Wu B: Arginine vasopressin gene expression in supraoptic nucleus
and paraventricular nucleus of hypothalamous following cerebral
ischemia and reperfusion. Chin Med Sci J. 15:157–161.
2000.PubMed/NCBI
|
|
29
|
Vakili A, Kataoka H and Plesnila N: Role
of arginine vasopressin V1 and V2 receptors for brain damage after
transient focal cerebral ischemia. J Cereb Blood Flow Metab.
25:1012–1019. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shahzad M, Shabbir A, Wojcikowski K,
Wohlmuth H and Gobe GC: The antioxidant effects of Radix Astragali
(Astragalus membranaceus and related species) in protecting tissues
from injury and disease. Curr Drug Targets. 17:1331–1340. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li L, Hou X, Xu R, Liu C and Tu M:
Research review on the pharmacological effects of Astragaloside IV.
Fundam Clin Pharmacol. 31:17–36. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang J, Wu C, Gao L, Du G and Qin X:
Astragaloside IV derived from Astragalus membranaceus: A research
review on the pharmacological effects. Adv Pharmacol. 87:89–112.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kang X, Su S, Hong W, Geng W and Tang H:
Research progress on the ability of Astragaloside IV to protect the
brain against ischemia-reperfusion injury. Front Neurosci.
15:7559022021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tan YQ, Chen HW and Li J: Astragaloside
IV: An effective drug for the treatment of cardiovascular diseases.
Drug Des Devel Ther. 14:3731–3746. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jin H, Jiao Y, Guo L, Ma Y, Zhao R, Li X,
Shen L, Zhou Z, Kim SC and Liu J: Astragaloside IV blocks
monocrotaline-induced pulmonary arterial hypertension by improving
inflammation and pulmonary artery remodeling. Int J Mol Med.
47:595–606. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jing H, Xie R, Bai Y, Duan Y, Sun C, Wang
Y, Cao R, Ling Z and Qu X: The mechanism actions of Astragaloside
IV prevents the progression of hypertensive heart disease based on
network pharmacology and experimental pharmacology. Front
Pharmacol. 12:7556532021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Candelario-Jalil E, Dijkhuizen RM and
Magnus T: Neuro-inflammation, stroke, blood-brain barrier
dysfunction, and imaging modalities. Stroke. 53:1473–1486. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yang C, Hawkins KE, Doré S and
Candelario-Jalil E: Neuroinflammatory mechanisms of blood-brain
barrier damage in ischemic stroke. Am J Physiol Cell Physiol.
316:C135–C153. 2019. View Article : Google Scholar : PubMed/NCBI
|