Introduction
Bradycardia, including pathological sinus syndrome
and atrioventricular block, is a serious threat to human life.
Compared with electronic pacemakers, which have numerous defects,
biological pacemakers have a promising application in the treatment
of bradyarrhythmia (1). However,
it is inefficient to induce stem cells to differentiate into
pacemaker cells in vitro; therefore, the mechanism of
pacemaker cell differentiation needs to be elucidated (2).
The T-box (Tbx) gene family, including Tbx3 and
Tbx18, serves an important role in the formation of the sinoatrial
node. Tbx18-mediated phenotypic transformation of bone marrow
mesenchymal stem cells (BMSCs) into pacemaker-like cells has been
reported at both the mRNA and protein levels (3–5).
In vivo, it has been reported that Tbx18 can directly
reprogram cardiomyocytes into sinoatrial node-like cells, increase
the expression of hyperpolarized-activated cyclic nucleotide-gated
cation channel (HCN)4 on the membrane and thus improve the beating
frequency of the cell (6,7). However, the differentiation
efficiency of pacemaker-like cells is still not high and only ~7%
of BMSC-Tbx18 cells were reported to beat on day 10 after
transfection with a Tbx18 overexpression vector (8). Therefore, the present study assessed
a potential new method to improve the efficiency of differentiation
of BMSCs into pacemaker-like cells.
Previous studies have reported that histone H3 at
lysine 9 dimethylation (H3K9me2) is closely related to cell
differentiation, proliferation and reprogramming. It has been
reported that regulation of the expression of H3K9me2 during
cardiomyocyte maturation can affect the expression of cardiac
development-related genes (9).
Furthermore, the euchromatic histone lysine methyltransferase 2
(G9a) inhibitor BIX01294 can increase the number of cardiac
progenitor cells without impairing their differentiation ability,
which suggests that the drug may have the effect of producing a
large number of cardiac progenitor cells for cardiac repair
(10). Moreover, after BIX01294
pretreatment, the levels of cardiomyocyte markers GATA4, Nkx2.5 and
myocardin produced by Wnt11 factor-induced mesenchymal stem cells
were reported to be 2.6-5.6× higher than those in the untreated
group (9,11). These studies indicated that G9a
may participate in the development of cardiomyocytes.
In the present study, to increase the efficiency of
pacemaker-like cell formation, BMSCs treated with BIX01294 were
induced to form pacemaker-like cells by overexpression of Tbx18.
The present study optimized the efficiency of pacemaker-like cell
induction in vitro, which may lay the foundation for
clinical application.
Materials and methods
BMSCs isolation and cell culture
A total of 20 healthy male and 20 healthy female
Wistar rats (weight, 50–200 g; age, 3–8 weeks) were provided by
Yangzhou University (Yangzhou, China) and three female or male rats
were used in each of three individual experiments of BMSCs
isolation and cell culture. Rats received standard care under a
12-h dark/light cycle (25°C with humidity of 60%), and were given
free access to food and water. Before the experiment, adaptive
feeding was performed for 1 week. The health and behavior of the
animals were monitored daily until death. All procedures involving
the care and use of animals conformed to the Animal Research:
Reporting of In Vivo Experiments (ARRIVE guidelines 2.0;
http://arriveguidelines.org/arrive-guidelines) and
were approved by the Laboratory Animal Management and Experimental
Animal Ethics Committee of Yangzhou University (approval no.
202003545). All rats were anesthetized using 20% sodium urethane
(0.2 g/ml; 1.0 g/kg; intraperitoneal injection) and were
subsequently sacrificed by cervical dislocation. Animal death was
verified by ascertaining cardiac and respiratory arrest. Bone
marrow cells were extracted from the tibia and femur. The bone
marrow liquid was centrifuged at 1,100 × g for 10 min at 37°C and
the supernatant was discarded. The cell suspension was transferred
to a 15 ml centrifuge tube containing 5 ml Percoll (1.073 g/ml;
Sigma-Aldrich; Merck KGaA). Cells were dispersed by pipetting again
and centrifuged at 1,500 × g for 30 min at 4°C. The mononuclear
cells in the middle layer were obtained. After washing with
phosphate-buffered saline (PBS), the cells were resuspended in
low-glucose DMEM (Thermo Fisher Scientific, Inc.) containing 20%
FBS (Biowest) and 100 U/ml penicillin/streptomycin, and then
inoculated in the culture plate. The cells were cultured in a 5%
CO2 incubator at 37°C for 72 h and then half the
solution replaced. During the expansion and proliferation of MSCs,
the culture medium was replaced every three days and cells were
passaged once they reached 80% confluency. After that, the culture
medium was passed every ~3 days and third-generation cells were
collected for subsequent experimental use. Bone marrow mesenchymal
stem cells grow adherent to the wall, and cellular morphological
feature exhibiting a spindle shape.
Flow cytometry
For cell phenotypic characterization, BMSCs from the
third passage were stained using mouse anti-CD29 (1:100; cat. no.
sc-9970; Santa Cruz Biotechnology, Inc.), anti-CD34 (1:100; cat.
no. sc-7324; Santa Cruz Biotechnology, Inc.), anti-CD44 (1:100;
cat. no. sc-7297; Santa Cruz Biotechnology, Inc.) and anti-CD45
(1:100; cat. no. sc-1178; Santa Cruz Biotechnology, Inc.)
antibodies for 1 h at room temperature. Phycoerythrin A-labeled
anti-mouse IgG (1:100; cat. no. sc-516141; Santa Cruz
Biotechnology, Inc.) was used as the secondary antibody for 1 h at
room temperature. After washing with PBS, flow cytometry was
performed using a BD FACSCalibur (BD Biosciences). The results were
analyzed and processed by FlowJo version 10.0 (FlowJo LLC).
Construction of the Tbx18 lentiviral
vector and cell transduction
According to the Tbx18 sequence accessed from the
National Center for Biotechnology Information, primers for
full-length expression of the CDS region were designed as follows:
Forward, 5′-TATAGGGAGACCCAAGCTGGATGGCGGAGAAGCGGAGG-3′ and reverse
5′-CGGGCCCTCTAGACTCGAGCTCAGACCATATGCGCAGACAC-3′. T4 ligase was used
to connect the CDS with the pcDH-CMV-MCS-EF1-TAGFP + Puro vector
from our laboratory, which was digested using NotI and
NheI to construct the Tbx18 overexpression plasmid
(OE-Tbx18). The plasmid was sent to Shanghai TsingKE Biologicals
for Sanger sequencing for verification of the construct. After
sequencing, OE-Tbx18 was sent to Jiman Biotechnology (Shanghai)
Co., Ltd. for lentiviral packaging (titer: 2.5×108
TU/ml).
BMSCs were plated in 24-well plates, grown to 70–80%
confluence and transfected using 1 µg/plate OE-Tbx18 and 6 µg/ml
polybrene. Cells not transfected with plasmids were used as
controls. BMSCs transfected, according to the same protocol as
OE-Tbx18, with a no-load vector were the negative control. After
incubation at 37°C for 24 h, the virus-containing medium was
replaced with fresh medium. Continue incubation for 48 h, the cells
were observed daily using a TE2000 fluorescence microscope
(magnification, ×100, ×200 and ×400; Nikon Corporation). The
beating rate of 100 cells was assessed three times each day during
3 consecutive days after 10 days.
Immunofluorescence and
immunohistochemistry (IHC)
BMSCs were fixed using 4% paraformaldehyde at 4°C
for 30 min. Fixed cells were permeabilized using 0.1% Triton X-100
(Beijing Solarbio Science & Technology Co., Ltd.) and blocked
using 2% FBS (Biowest) and 0.1% Tween-20 (Beijing Solarbio Science
& Technology Co., Ltd.) blocking solution at 4°C for 2 h. The
cells were then incubated overnight at 4°C with primary antibody
against rat cardiac troponin I (cTnI; 1:200; cat. no. 21652-1-AP;
ProteinTech Group, Inc.). Cells stained with diluent only served as
the negative control. After overnight incubation, cells were washed
with PBS three times (5 min/wash), incubated with Cy3-conjugated
secondary antibody (1:500; cat. no. A0516; Beyotime Institute of
Biotechnology) for 1 h at room temperature and washed five times
with PBS. Nuclei were stained using 4′,6-diamidino-2-phenylindole
(Beijing Solarbio Science & Technology Co., Ltd.) for 10 min at
room temperature. Slides were mounted and assessed using a TE2000
fluorescence microscope.
The rat heart tissue were collected for IHC
staining. The tissue sections were dewaxed, hydrated, incubated in
citrate buffer for antigen retrieval and fixed, then blocked in
TBS-0.05% Tween 20 containing 5% BSA. Tissue sections were
incubated with anti-G9a (1:300; cat. no. sc-515726; Santa Cruz
Biotechnology, Inc.), anti-HCN4 (1:200; cat. no. ab289962; Abcam,
Inc.) at 4°C overnight, washed in TBST three times for 10 min and
then incubated with secondary antibodies; anti-rabbit (1:400, cat.
no. ab150081; Abcam, Inc.) and biotinylated anti-mouse (1:400, cat.
no. B7401; Sigma-Aldrich; Merck KGaA) for 2 h at room temperature
and tertiary antibodies (streptavidin-HRP; cat. no. 18-152;
Sigma-Aldrich; Merck KGaA) to label targets with HRP, incubated for
30 sec-1 min with DAB (cat. no. 11718096001; Sigma-Aldrich; Merck
KGaA) substrate. Slides were counterstained with hematoxylin for 2
min at room temperature and coverslips were added before
imaging.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from the BMSCs of different
treatment groups and cardiomyocytes (CM) using TRIzol®
(Invitrogen; Thermo Fisher Scientific, Inc.) and cDNA was
synthesized from sample RNA using FastKing One Step RT-qPCR kit
Qiagen GmbH. The amplification steps included 50°C 10 min, 95°C 3
min, 95°C 15 sec and 60°C 30 sec for 40 cycles. cDNA was amplified
by qPCR using a Qiagen PCR kit (Qiagen GmbH) according to the
manufacturer's protocols at 50°C for 30 min, 95°C for 3 min, 95°C
for 15 sec and 60°C for 30 sec. The mRNA expression levels of
Tbx18, α-1 antitrypsin (α-SA), cTnI, HCN4, and the pathological
hypertrophy marker genes natriuretic peptide A (Nppa), natriuretic
peptide B (Nppb) and myosin heavy chain 7 (Myh7) were assessed
using qPCR. The 20 µl PCR amplification reaction included 2 µl
cDNA, 10 µl SYBR Taq, 0.8 µl forward primer, 0.8 µl reverse primer,
0.4 µl RoxII and 6 µl double-distilled water. PCR was performed on
the basis of the two-step procedure (95°C for 15 min; 95°C for 10
sec and 60°C for 32 sec) repeated 40 times. The primer sequences
used are presented in Table I.
The PCR instrument used for qPCR was an ABIPRISM 7500 (Applied
Biosystems; Thermo Fisher Scientific, Inc.). Each experimental
condition was repeated in triplicate. Each experiment was repeated
three times. The relative mRNA expression levels were quantified
using the 2−ΔΔCq method (9,12),
and normalized to GAPDH.
 | Table I.Sequences of primers used for reverse
transcription-quantitative PCR. |
Table I.
Sequences of primers used for reverse
transcription-quantitative PCR.
| Gene | Sequence (5′-3′) | Accession number |
|---|
| cTnI | F:
GCCGGAAGTGTAGGAAGA | NM_012676.1 |
|
| R:
GGGGAGCAGATGATGGT |
|
| Nppa |
F:TGCCGGTAGAAGATGAGGT | NM_012612.2 |
|
| R:
GTTGACTTCCCCAGTCCAG |
|
| Nppb | F:
ATTCTGCTCCTGCTTTTCC | NM_031545.1 |
|
| R:
GCTTCTGCATCGTGGATT |
|
| Myh7 | F:
ATTGCCGAGTCCCAGGT | NM_017240.2 |
|
| R:
TCCAGGTCTCAGGGCTTC |
|
| HCN4 | F:
CAGCCAGAAAGCAGTGGA | NM_021658.1 |
|
| R:
ATCAGCAACAGCATCGTCA |
|
| Tbx18 |
F:CCCGTGGACAACAAAAGA | NM_001108173.1 |
|
| R:
CGGTGAGTCTGGATGAATG |
|
| α-SA |
F:TGGCATCTGAATGGGTCT | NR_045097.1 |
|
| R:
CAGTGGGTTCTTGGCTTC |
|
| GAPDH | F:
GGAAAGCTGTGGCGTGATGG | NM_017008.4 |
|
| R:
GTAGGCCATGAGGTCCACCA |
|
Western blotting
Total protein was extracted from BMSCs and
myocardial tissue using RIPA lysis buffer (cat. no. R0010; Beijing
Solarbio Science & Technology Co., Ltd.) on ice for 30 min.
Total protein concentrations were evaluated using a protein assay
kit (Beyotime Institute of Biotechnology). The protein samples (10
µg/lane) were then separated using 10% SDS-PAGE and
electrophoresed. The proteins were electro-transferred onto PVDF
membranes. Subsequently, the membranes were blocked for 2 h at room
temperature in TBS-0.05% Tween 20 containing 5% nonfat milk.
Primary antibodies against H3K9me2 (1:1,000; cat. no. 39239; Active
Motif, Inc.), H3 (1:3,000; cat. no. H0164; Sigma-Aldrich; Merck
KGaA), G9a (1:1,000; cat. no. sc-515726; Santa Cruz Biotechnology,
Inc.), Nappa (1:1,000; cat. no. 13299-1-AP; ProteinTech Group,
Inc.), Nappb (1:1,000; cat. no. 27426-1-AP; ProteinTech Group,
Inc.), Myh7 (1:1,000; cat. no. 22280-1-AP; ProteinTech Group,
Inc.), β-actin (1:1,000; cat. no. SAB3500350; Sigma-Aldrich; Merck
KGaA), HCN4 (1:1,000; cat. no. 55224-1-AP; ProteinTech Group,
Inc.), cTnI (1:1,000; cat. no. 21652-1-AP; ProteinTech Group,
Inc.), α-SA (1:1,000; cat. no. 23660-1-AP; ProteinTech Group, Inc.)
and Tbx18 (1:1,000; cat. no. 23237-1-AP; ProteinTech Group, Inc.)
were incubated with the membranes overnight at 4°C. Finally, the
membranes were incubated with the corresponding secondary
antibodies (1:3,000, cat. no. A9169; 1:5,000, cat. no. AP160P; both
Sigma-Aldrich; Merck KGaA). for 2 h at room temperature and
assessed using an ECL Chemiluminescent Substrate Reagent Kit
(Thermo Fisher Scientific, Inc.). H3 is used as loading control to
detect H3K9me2. Densiometric analysis was performed using Image Lab
software (version 3.0, Bio-Rad Laboratories, Inc.).
Chromatin immunoprecipitation
(ChIP)-qPCR analysis
BMSCs treated with BIX01294 at day 10 were collected
and crosslinked using 1% formaldehyde at 37°C for 10 min followed
by glycine un-crosslinking. SDS lysis solution (Applygen
Technologies, Inc.) was used to lyse the cells and the DNA was
sonicated on ice to fragmentation for 5 sec with 10 sec intervals
in a cycle lasting 5 min followed by centrifugation at 12,000 × g
for 10 min at 4°C, The supernatant was collected and incubated
overnight at 4°C on a shaker with 60 µl of Protein A/G Agarose in
equal volumes and anti-H3K9me2 (cat. no. 39239; Active Motif,
Inc.). The immunoprecipitation complexes are washed sequentially as
follows: Low salt wash buffer (one wash), high salt wash buffer
(one wash), LiCl wash buffer (one wash) and TE buffer (two washes).
Elution buffer (120 µl) was added to each group to elute DNA,
incubated for 15 min and centrifuged at 2,000 × g for 1 min at room
temperature to collect the supernatant. NaCl (20 µl of 5 M; final
NaCl concentration of 0.2 M) was added to each group, mixed well
and uncrosslinked at 65°C overnight. Tthe DNA was purified and
recovered, and RT-qPCR was performed according to the
aforementioned method. Specific steps were performed according to
the manufacturer's protocol for the Chromatin Immunoprecipitation
(ChIP) Assay kit (MilliporeSigma). In these experiments, ‘input’
was used to indicate the total protein, the percentage of its
protein content bound by the IgG antibody (1:1,000; cat. no.
HA1001; HUABIO, Inc.) was used as the ‘control’ and the percentage
of protein bound by the target antibody was used as the
‘experimental group’.
Animal experiment
A total of 20 Wistar rats (10 male and 10 female;
age, 3–4 weeks; weight, 70±10 g) were randomly divided into two
groups as follows: Treatment group and control group. The housing,
handling and euthanasia procedures were the same as the
aforementioned methods for BMSCs isolation. The tails of all rats
were washed with warm water and wiped with 75% alcohol cotton ball
for disinfection. Rats in the treatment group were injected with
BIX01294 (10 mg/kg/day; MedChemExpress, Inc.) through the tail vein
from day 1 to day 10 and rats in the control group were injected
with normal saline. All of the rats were sacrificed for cardiac
tissue collection at day 50.
Hematoxylin and eosin (H&E)
staining
The sinus nodal region tissue was fixed in 4%
paraformaldehyde for 24 h at room temperature. After dehydration
using an ascending ethanol series, xylene was used as a clearing
agent, and the tissues were embedded in paraffin and sectioned (5–6
µm). The slices were dewaxed using xylene, hydrated using a
descending ethanol series at room temperature, then stained with
hematoxylin (0.4%) and eosin (0.1%) (H&E) solution at 37°C for
5 min, dehydrated using a rising ethanol series and neutral resin
sealed at room temperature. The sections were assessed using a
light microscope (Leica Microsystems GmbH).
Statistical analysis
The results are presented as the mean ± standard
error of the mean. Data were assessed using one-way ANOVA and
Tukey's post hoc test using SPSS 23 (IBM Corp). GraphPad Prism 7.0
GraphPad Software, Inc. was used for graph generation. Each test
was evaluated at the 0.05 alpha level and P<0.05 was considered
to indicate a statistically significant difference.
Results
Characteristics of BMSCs and
transfection efficiency
To obtain rat BMSCs, the cells collected by density
gradient centrifugation were cultured for 48 h. Certain adherent
cells that grew in a spindle shape could be seen under the
microscope and a few cells were polygonal. After 7–10 days, the
fusion rate of cells, which were arranged in a swirling pattern,
grew to 80–90% (Fig. 1B). The
expression of surface markers of BMSCs were assessed and the
proportion of cells positive for CD29 and CD44 expression were
96.34±3.15 and 81.64±3.26%, respectively, whereas the proportion of
cells positive for CD34 and CD45 were only 0.28±0.12 and
1.28±0.43%, respectively (Fig.
1A), which was consistent with previously reported results
(13). These results indicated
that BMSCs were successfully isolated. When the cell fusion rate
reached 80%, cells were transfected with the target vector and
empty vector. Red fluorescence was assessed 24 h after transfection
(Fig. 1C). After 72 h of
transfection, western blotting and RT-qPCR revealed that the
protein and mRNA expression levels of Tbx18 were significantly
increased compared with those in the control group (Fig. 1E and F), which indicated that the
OE-Tbx18 vector was successfully transfected into BMSCs
(BMSCs-Tbx18).
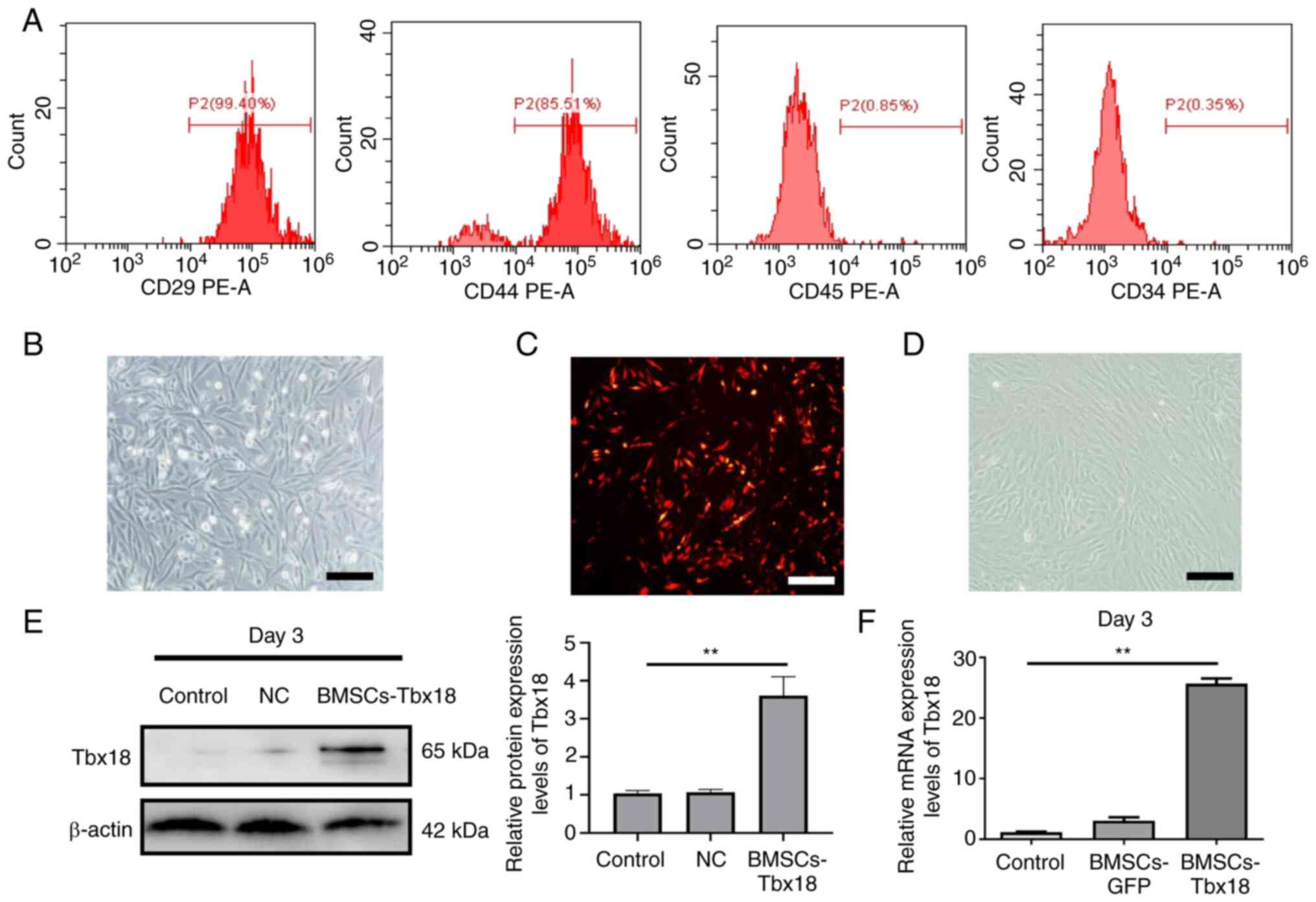 | Figure 1.Characteristics of BMSCs and
transfection efficiency. (A) Fluorescence-activated cell sorting
analysis of CD29, CD44, CD34 and CD45 protein expression levels in
BMSCs. (B) Cellular morphological features of BMSCs, which
exhibited a spindle shape. Scale bar, 50 µm. (C) Red fluorescence
of BMSCs after transfection with OE-Tbx18 for 48 h. Scale bar, 50
µm. (D) The microscopic morphology of BMSCs gradually evolved into
strip-like features after 10 days of transduction by OE-Tbx18.
Scale bar, 50 µm. (E) Western blotting of Tbx18 protein expression
levels after transfection for 72 h. BMSCs without transfection were
used as the control, BMSCs transfected with the empty vector were
used as the NC. (F) Reverse transcription-quantitative PCR analysis
of Tbx18 mRNA expression levels after transfection for 72 h.
**P<0.01. BMSCs, bone marrow mesenchymal stem cells; OE-Tbx18,
T-box 18 overexpression plasmid; NC, negative control. |
Tbx18 overexpression in BMSCs promotes
BMSCs differentiation into pacemaker-like cells
To evaluate whether overexpression of Tbx18 could
enable BMSCs to differentiate into pacemaker-like cells, the cells
were assessed using a microscope, which demonstrated morphological
changes in the BMSCs 10 days after transfection (Fig. 1D). The spindle-shaped BMSCs
transformed into strips, which was a typical feature of sinoatrial
node cells. Furthermore, ~5% of BMSCs-Tbx18 were observed to be
beating at day 10. Immunofluorescence demonstrated that certain
cells expressed cTnI (Fig. 2A).
Cardiomyocyte was used as a positive control, the mRNA and protein
expression levels of α-SA, cTnI and HCN4 were significantly
increased in BSMCs-Tbx18 compared with in the control group, as
determined using RT-qPCR and western blotting (Fig. 2B and C). These results
demonstrated that overexpression of Tbx18 promoted BMSCs
differentiation into pacemaker-like cells.
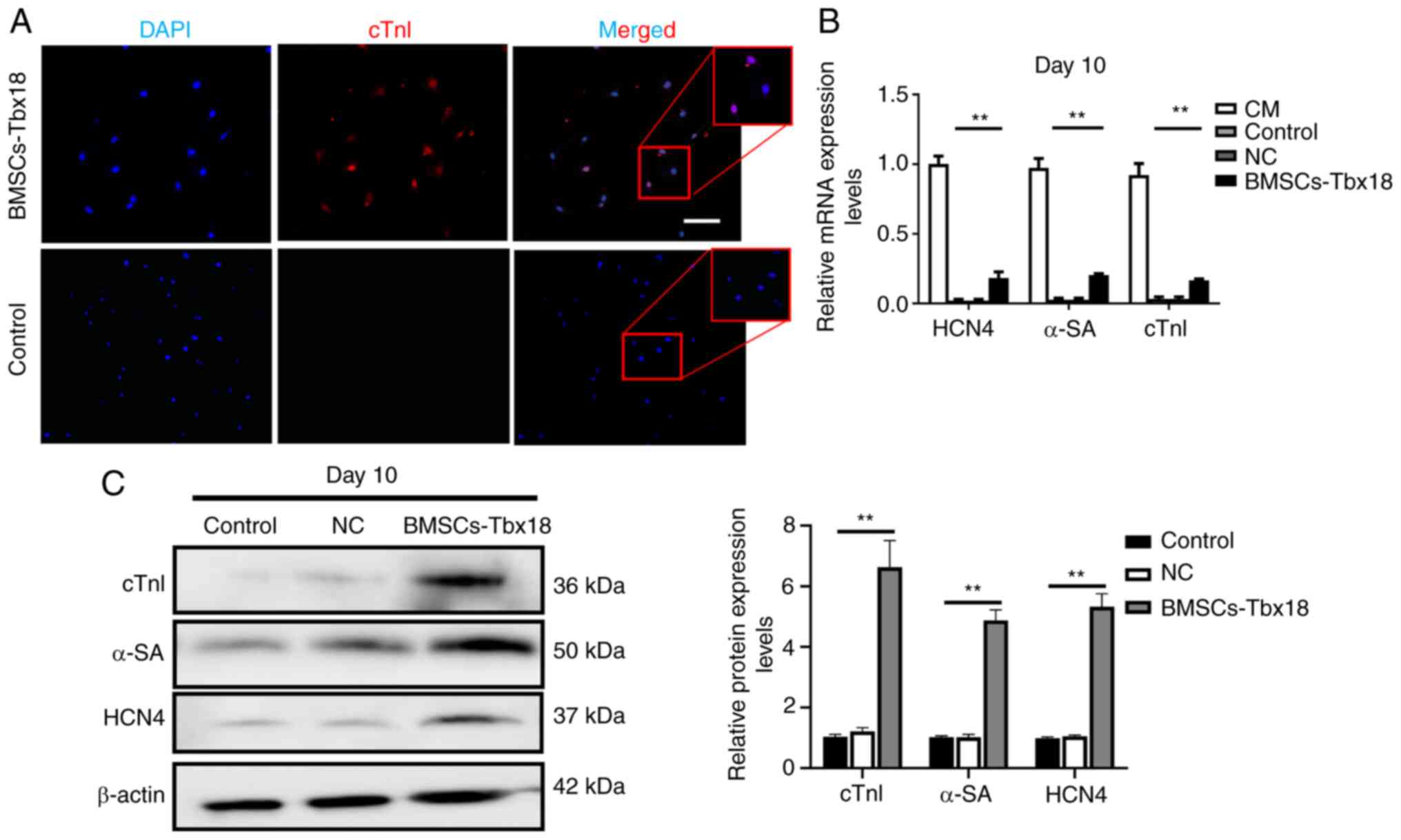 | Figure 2.Immunostaining, western blotting and
reverse transcription-quantitative PCR analysis of target protein
and mRNA expression levels after transfection. (A) Protein
expression levels of cTnI in BMSCs were assessed using
immunostaining with cTnI-specific primary antibodies and a Cy3
secondary antibody. Scale bar, 50 µm. (B) Quantitative analysis of
the relative mRNA expression levels of HCN4, α-SA and cTnI. (C)
Western blotting demonstrated increased HCN4, α-SA and cTnI protein
expression levels. BMSCs without transfection were used as the
control and BMSCs transfected with the empty vector were used as
the NC. **P<0.01. cTnI, cardiac troponin I; HCN4,
hyperpolarization-activated cyclic nucleotide-gated channel 4;
α-SA, α-striated actin; NC, negative control; CM, cardiomyocyte;
BMSCs, bone marrow mesenchymal stem cells. |
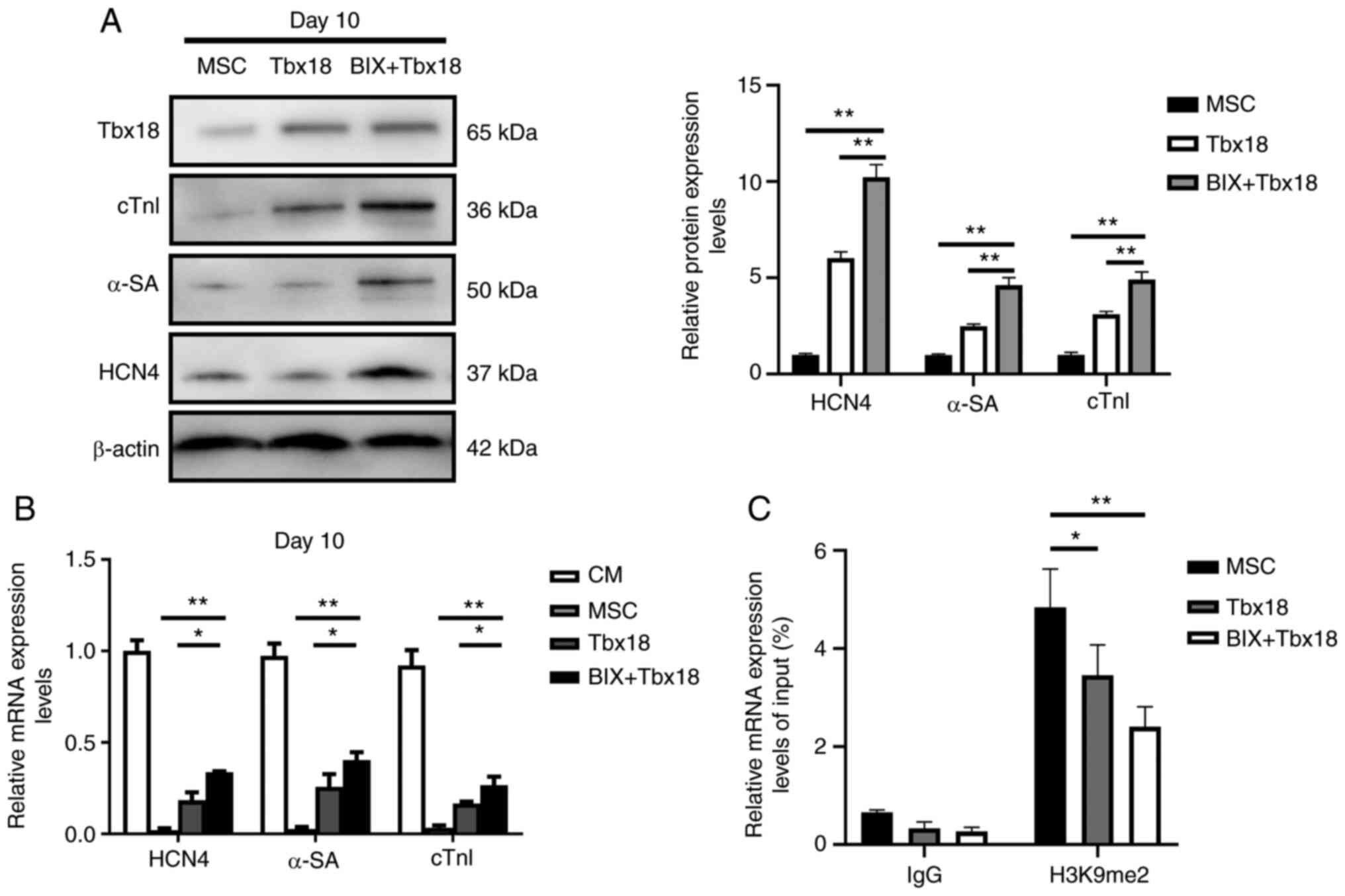
G9a inhibition stimulates the
expression of HCN4 sequences in BMSCs
To evaluate the effect of G9a on the differentiation
of BMSCs into pacemaker-like cells, the OE-Tbx18 was transfected
into BMSCs after treatment with 1 µM BIX01294 for 12 h. Western
blotting demonstrated that the protein expression levels of α-SA,
cTnI and HCN4 in BMSCs treated with BIX01294 (BIX-BMSCs) were
significantly higher compared with those in the untreated group
(Fig. 3A). The results of RT-qPCR
were similar to those in of western blotting (Fig. 3B). Cell beating was observed in
~15% BMSCs-Tbx18 at day 10 under a light microscope (magnification,
×100, ×200 and ×400; Leica Microsystems GmbH). To further evaluate
how G9a affected HCN4 expression, the enrichment of H3K9me2 in the
HCN4 promoter was assessed. Compared with in the control group,
H3K9me2 was significantly downregulated in the HCN4 promoter region
following interference with G9a (Fig.
3C). These results demonstrated that G9a changed the enrichment
level of H3K9me2 in the HCN4 promoter region, which suggested that
H3K9me2 could regulate the expression of HCN4 and affect the
formation of pacemaker-like cells through differential enrichment
in the HCN4 promoter region.
Knocking down G9a promotes myocardial
hypertrophy
The results of the in vivo study demonstrated
that the heart volume and weight of rats were significantly
increased following injection of BIX01294 compared with in the
control group (P<0.05; Fig.
4A). H&E staining demonstrated that cardiomyocytes were
hypertrophic and the nuclei were malformed in the treatment group
(Fig. 4B). Immunohistochemical
staining demonstrated that BIX01294 inhibited G9a, increased the
protein expression levels of HCN4 in the sinoatrial node (Fig. 4B). Western blotting demonstrated
that at day 50, the protein expression levels of H3K9me2 normalized
to H3 were significantly decreased, whereas the protein expression
levels of Nppa, Nppb and Myh7, indicators of cardiac hypertrophy,
were significantly increased in the BIX01294 group compared with in
the control group (Fig. 4D),
which was consistent with their mRNA expression levels (P<0.05;
Fig. 4C). These results
demonstrated that interference with G9a in vivo increased
proliferation of cardiomyocytes including the sinoatrial node
cells.
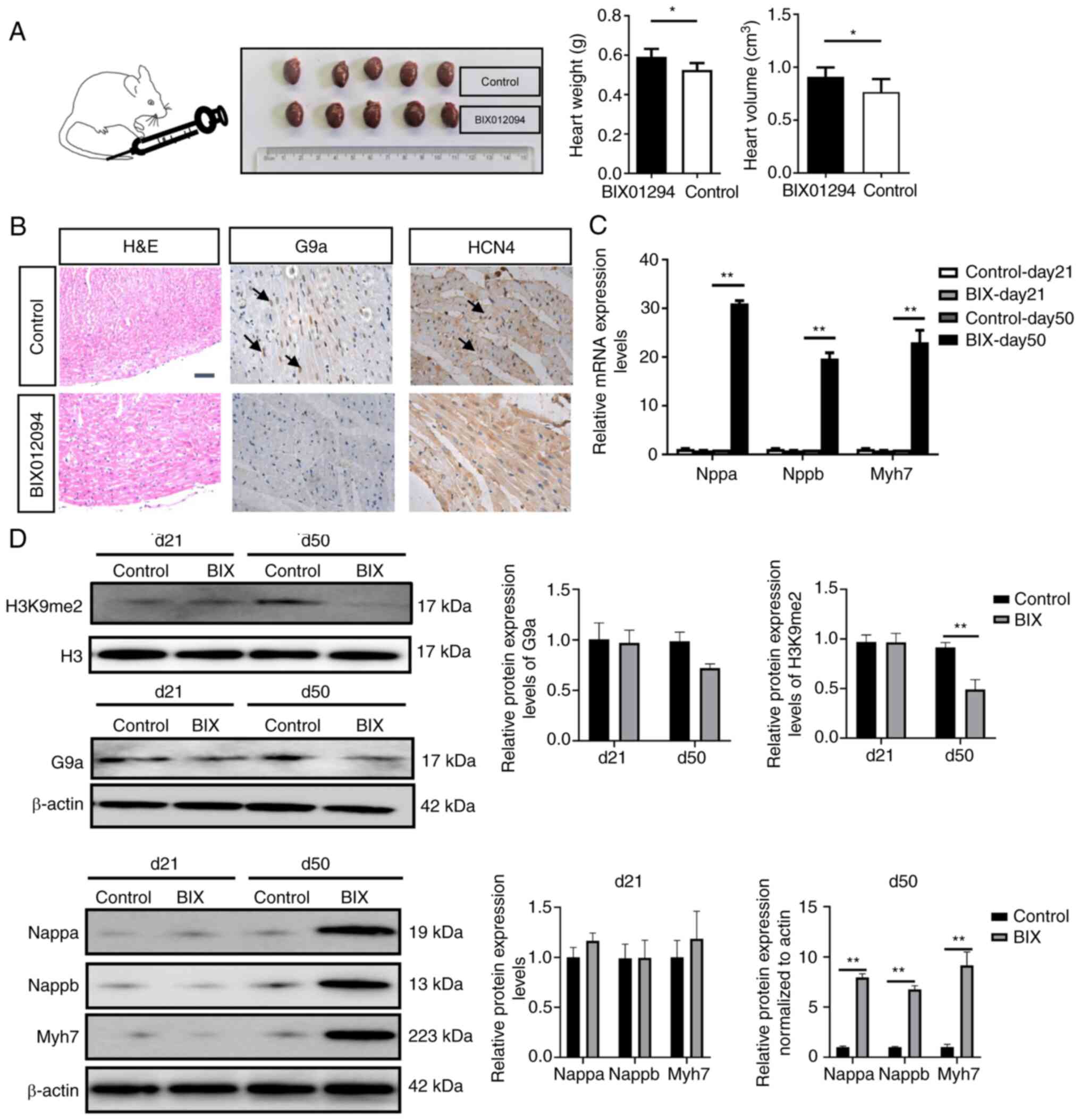 | Figure 4.BIX01294 promotes cardiac hypertrophy.
(A) BIX01294 was injected through the caudal vein, and heart volume
and weight were assessed at day 50. (B) H&E staining of the
heart and immunohistochemistry of sinoatrial node tissue. The arrow
indicated positive cells. Scale bar, 50 µm. (C) Quantitative
analysis of the mRNA expression levels of Nppa, Nppb and Myh7. (D)
Western blotting was used to assess the protein expression levels
of H3K9me2, G9a, Nppa, Nppb and Myh7. *P<0.05 and **P<0.01.
H3K9me2, histone H3 at lysine 9 dimethylation; G9a, euchromatic
histone lysine methyltransferase 2; H&E, haematoxylin and
eosin; Nppa, natriuretic peptide A; Nppb, natriuretic peptide B;
Myh7, myosin heavy chain 7. |
Discussion
An ideal bio-pacemaker needs to have the same
sustained, robust pacing ability as sinoatrial node cells. The
generation methods of biological pacemakers in current studies
mainly rely on making non-pacemaker cells possess the
characteristics and functions of pacemaker cells while retaining
their phenotypes. These methods can generate pacing current by
regulating membrane potential or depolarization of cells; however,
they have drawbacks, such as an unstable current, conduction block
or lack of autonomous regulation. It was previously reported that
in vitro and in vivo, both fibroblasts and quiescent
cardiomyocytes transfected with the Tbx18 gene differentiated into
sinoatrial node-like cells in phenotype and function (14). Moreover, HCN4, a key pacemaker
gene, the expression levels of which were reported to be
significantly upregulated after Tbx18 transduction, may be related
to the formation of pacemaker-like cells (15,16). In the present study, through the
overexpression of Tbx18, the mRNA and protein expression levels of
HCN4 and the surface markers of cardiomyocytes were increased,
which was consistent with previous reports (8,17,18). Moreover, it has been reported that
Tbx18 transfection of neonatal rat myocardium not only increased
the expression levels of HCN4, but also increased the frequency of
spontaneous beating (5). These
effects were reported to occur in cells at hyperpolarization due to
the pacemaker current, which is driven by HCN protein (19). However, in the present study,
beating was only demonstrated in ~5% of BMSCs-Tbx18 at day 10. The
low efficiency on inducing cardiac differentiation of BMSCs was
possibly due to the reduced response to other factors produced in
the microenvironment that could be involved in the process of
cardiac differentiation (20).
Therefore, a method to improve the differentiation efficiency is
required. It is well known that H3K9 is widely involved in cell
proliferation and differentiation. Our previous study reported that
knockdown of the histone methyltransferase G9a increased expression
of early transcription factors during the differentiation of MSCs
into cardiomyocytes (21).
BIX01294 was originally reported to be a G9a inhibitor during a
chemical library screen of small molecules and has previously been
used in the generation of induced pluripotent stem cells. It is
reported to upregulate precardiac markers and allow bone marrow
cells to respond to cardiogenic signals (22). Therefore, in the present study,
H3K9me2 was significantly downregulated in the HCN4 promoter region
following interference with G9a, which promoted HCN4 expression,
thereby increasing the number of pacemaker-like cells and the
beating of cells. In vivo, the reduction of microRNA-217
levels has been reported to reduce the expression of G9a, thereby
promoting the reduction of H3K9me2 expression in the promoter
region of Myh7, which resulted in pathological hypertrophy
(9). In the present study, after
BIX01294 injection, the cardiac volume and weight of rats
significantly increased, as did certain indicators related to
cardiac hypertrophy. The present study also demonstrated that the
expression levels of HCN4 in the sinoatrial node increased after
inhibition of G9a, which indicated that G9a was involved in the
process of proliferation and the development of sinoatrial node
cells.
The mechanism by which H3K9 is involved in the
regulation of the differentiation of MSCs into cardiomyocytes is
still unclear; however, it may be related to gene silencing by H3K9
methylation in the promoter region of genes (23,24). As the methylation modification
enzyme of H3K9, G9a can catalyze single, double and trimethylation
at H3K9 sites (25,26). The results of the present study
demonstrated that interference with G9a could significantly reduce
H3K9me2 protein expression levels. G9a can modify the H3K9
methylation of histones in certain gene promoter regions through
its histone methyltransferases activity, thereby regulating gene
transcriptional silencing (27).
Therefore, as a substrate competition inhibitor, BIX01294 can be
used to inhibit G9a to reverse transcriptional suppression of stem
cell genes (28–30). Furthermore, it has previously been
reported that BIX01294-pretreated human BMSCs can effectively
differentiate into neuron-like cells by inducing the expression of
neuronal-specific genes containing RE-1 sequences (31). The present study provided further
functional verification, which demonstrated that interference with
G9a could significantly downregulate the H3K9me2 enrichment level
in the HCN4 promoter region. It may be hypothesized that the
reduced enrichment of H3K9me2 in the HCN4 promoter region led to
changes in chromosome state, promoted the binding of Tbx18 to the
HCN4 promoter region and increased HCN4 transcription, which
promoted cell differentiation to pacemaker-like cells. Furthermore,
considering the complexity of the pacemaker-like cell formation
process, further studies are needed, including to evaluate the
effect of histone methylation modification on transcription factor
formation in early cardiomyocytes to clarify the role of epigenetic
modification in the formation of pacemaker-like cells.
In conclusion, the present study demonstrated that
BIX01294 increased the differentiation efficiency of pacemaker-like
cells by inhibiting the enrichment level of H3K9me2 in the HCN4
promoter region in vitro and in vivo. Therefore,
using a G9a inhibitor such as BIX01294 may provide a better
strategy for promoting stem cell differentiation into
pacemaker-like cells. These results have important implications for
regenerative medicine related to cellular therapy of chronic
arrhythmias.
Acknowledgements
The authors would like to thank Professor Lei Sun
(Cardiology Department, Northern Jiangsu People's Hospital,
Yangzhou, China) for help with the experimental design.
Funding
This work was supported by a grant from Taizhou People's
hospital (grant no. ZL202035).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
PX, KJ, JZ, LD,JG,XG and XS were involved in the
conception, design and implementation of the experimental plan,
drafting the article and statistical analysis. PX and XS confirm
the authenticity of all the raw data. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
All procedures involving the care and use of animals
conformed to the Animal Research: Reporting of In Vivo
Experiments guidelines and were approved by the Laboratory Animal
Management and Experimental Animal Ethics Committee of Yangzhou
University (approval no. 202003545).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sun X, Li H, Zhu Y, Xu P, Zuo Q, Li B and
Gu X: 5-Azacytidine-induced cardiomyocyte differentiation of very
small embryonic-like stem cells. Stem Cells Int. 2020:51623502020.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Farraha M, Kumar S, Chong J, Cho HC and
Kizana E: Gene therapy approaches to biological pacemakers. J
Cardiovasc Dev Dis. 5:502018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li Y, Yang M, Zhang G, Li L, Ye B, Huang C
and Tang Y: Transcription factor TBX18 promotes adult rat bone
mesenchymal stem cell differentiation to biological pacemaker
cells. Int J Mol Med. 41:845–851. 2018.PubMed/NCBI
|
|
4
|
Xiao H, Yang YJ, Lin YZ, Peng S, Lin S and
Song ZY: Transcription factor Tbx18 induces the differentiation of
c-kit+ canine mesenchymal stem cells (cMSCs) into
SAN-like pacemaker cells in a co-culture model in vitro. Am J
Transl Res. 10:2511–2528. 2018.PubMed/NCBI
|
|
5
|
Gorabi AM, Hajighasemi S, Tafti HA, Atashi
A, Soleimani M, Aghdami N, Saeid AK, Khori V, Panahi Y and Sahebkar
A: TBX18 transcription factor overexpression in human-induced
pluripotent stem cells increases their differentiation into
pacemaker-like cells. J Cell Physiol. 234:1534–1546. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wiese C, Grieskamp T, Airik R, Mommersteeg
MT, Gardiwal A, de Gier-de Vries C, Schuster-Gossler K, Moorman AF,
Kispert A and Christoffels VM: Formation of the sinus node head and
differentiation of sinus node myocardium are independently
regulated by Tbx18 and Tbx3. Circ Res. 104:388–397. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Szabo E, Rampalli S, Risueño RM, Schnerch
A, Mitchell R, Fiebig-Comyn A, Levadoux-Martin M and Bhatia M:
Direct conversion of human fibroblasts to multilineage blood
progenitors. Nature. 468:521–526. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hu Y, Li N, Liu L, Zhang H, Xue X, Shao X,
Zhang Y and Lang X: Genetically modified porcine mesenchymal stem
cells by lentiviral Tbx18 create a biological pacemaker. Stem Cells
Int. 2019:36213142019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Thienpont B, Aronsen JM, Robinson EL,
Okkenhaug H, Loche E, Ferrini A, Brien P, Alkass K, Tomasso A,
Agrawal A, et al: The H3K9 dimethyltransferases EHMT1/2 protect
against pathological cardiac hypertrophy. J Clin Invest.
127:335–348. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kaur K, Yang J, Edwards JG, Eisenberg CA
and Eisenberg LM: G9a histone methyltransferase inhibitor BIX01294
promotes expansion of adult cardiac progenitor cells without
changing their phenotype or differentiation potential. Cell Prolif.
49:373–385. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang J, Kaur K, Edwards JG, Eisenberg CA
and Eisenberg LM: Inhibition of histone methyltransferase, histone
deacetylase, and β-catenin synergistically enhance the cardiac
potential of bone marrow cells. Stem Cells Int. 2017:34649532017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang YL, Qiu RF, Mai WY, Kuang J, Cai XY,
Dong YG, Hu YZ, Song YB, Cai AP and Jiang ZG: Effects of
insulin-like growth factor-1 on the properties of mesenchymal stem
cells in vitro. J Zhejiang Univ Sci B. 13:20–28. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kapoor N, Liang W, Marbán E and Cho HC:
Direct conversion of quiescent cardiomyocytes to pacemaker cells by
expression of Tbx18. Nat Biotechnol. 31:54–62. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schweizer PA, Darche FF, Ullrich ND,
Geschwill P, Greber B, Rivinius R, Seyler C, Müller-Decker K,
Draguhn A, Utikal J, et al: Subtype-specific differentiation of
cardiac pacemaker cell clusters from human induced pluripotent stem
cells. Stem Cell Res Ther. 8:2292017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen L, Deng ZJ, Zhou JS, Ji RJ, Zhang X,
Zhang CS, Li YQ and Yang XQ: Tbx18-dependent differentiation of
brown adipose tissue-derived stem cells toward cardiac pacemaker
cells. Mol Cell Biochem. 433:61–77. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang F, Zhao H, Yin L, Tang Y, Wang X,
Zhao Q, Wang T and Huang C: Transcription factor TBX18 reprograms
vascular smooth muscle cells of ascending aorta to pacemaker-like
cells. DNA Cell Biol. 38:1470–1479. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu L, Du J, Jing X, Yan Y, Deng S and Hao
Z: Bone morphogenetic protein 4 promotes the differentiation of
Tbx18-positive epicardial progenitor cells to pacemaker-like cells.
Exp Ther Med. 17:2648–2656. 2019.PubMed/NCBI
|
|
19
|
DiFrancesco D: The role of the funny
current in pacemaker activity. Circ Res. 106:434–446. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang G, Tian J, Feng C, Zhao LL, Liu Z and
Zhu J: Trichostatin a promotes cardiomyocyte differentiation of rat
mesenchymal stem cells after 5-azacytidine induction or during
coculture with neonatal cardiomyocytes via a mechanism independent
of histone deacetylase inhibition. Cell Transplant. 21:985–996.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sun X, Gu X, Li H, Xu P, Li M, Zhu Y, Zuo
Q and Li B: H3K9me2 regulates early transcription factors to
promote mesenchymal stem-cell differentiation into cardiomyocytes.
Mol Med Rep. 24:6162021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hayes M and Zavazava N: Strategies to
generate induced pluripotent stem cells. Methods Mol Biol.
1029:77–92. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Snowden AW, Gregory PD, Case CC and Pabo
CO: Gene-specific targeting of H3K9 methylation is sufficient for
initiating repression in vivo. Curr Biol. 12:2159–2166. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sasidharan Nair V, El Salhat H, Taha RZ,
John A, Ali BR and Elkord E: DNA methylation and repressive H3K9
and H3K27 trimethylation in the promoter regions of PD-1, CTLA-4,
TIM-3, LAG-3, TIGIT, and PD-L1 genes in human primary breast
cancer. Clin Epigenetics. 10:782018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Collins RE, Tachibana M, Tamaru H, Smith
KM, Jia D, Zhang X, Selker EU, Shinkai Y and Cheng X: In vitro and
in vivo analyses of a Phe/Tyr switch controlling product
specificity of histone lysine methyltransferases. J Biol Chem.
280:5563–5570. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kubicek S, O'Sullivan RJ, August EM,
Hickey ER, Zhang Q, Teodoro ML, Rea S, Mechtler K, Kowalski JA,
Homon CA, et al: Reversal of H3K9me2 by a small-molecule inhibitor
for the G9a histone methyltransferase. Mol Cell. 25:473–481. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Son HJ, Kim JY, Hahn Y and Seo SB:
Negative regulation of JAK2 by H3K9 methyltransferase G9a in
leukemia. Mol Cell Biol. 32:3681–3694. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang J, Kaur K, Ong LL, Eisenberg CA and
Eisenberg LM: Inhibition of G9a histone methyltransferase converts
bone marrow mesenchymal stem cells to cardiac competent
progenitors. Stem Cells Int. 2015:2704282015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hou P, Li Y, Zhang X, Liu C, Guan J, Li H,
Zhao T, Ye J, Yang W, Liu K, et al: Pluripotent stem cells induced
from mouse somatic cells by small-molecule compounds. Science.
341:651–654. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim HT, Jeong SG and Cho GW: G9a
inhibition promotes neuronal differentiation of human bone marrow
mesenchymal stem cells through the transcriptional induction of
RE-1 containing neuronal specific genes. Neurochem Int. 96:77–83.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mezentseva NV, Yang J, Kaur K, Iaffaldano
G, Rémond MC, Eisenberg CA and Eisenberg LM: The histone
methyltransferase inhibitor BIX01294 enhances the cardiac potential
of bone marrow cells. Stem Cells Dev. 22:654–667. 2013. View Article : Google Scholar : PubMed/NCBI
|