Introduction
Glioma is the commonest primary malignancy of the
central nervous system. Despite the current combination of surgical
and pharmacological treatment options for glioma, the global
incidence of glioma remains high and most patients are already at a
very malignant stage at the time of presentation, with a 5-year
survival rate of <10% and some patients have a survival period
of <2 years (1) therefore, it
is crucial to explore the pathological mechanisms of glioma to find
more effective treatments for the disease.
The malignant proliferation of glioma cells is often
accompanied by a hypoxic growth environment due to inadequate blood
vessel formation and a severe lack of oxygen supply within the
tissue (2). Hypoxia affects
several aspects of glioma cells, including invasion, proliferation,
heterogeneity and drug resistance (2–4).
Hypoxia-inducible factor-1α (HIF-1α) is a molecule closely related
to hypoxia that can accumulate in the cytoplasm under hypoxia and
subsequently enter the nucleus to regulate the expression of
molecules closely related to the hypoxic response through the
hypoxia response element, thus affecting the formation of
neovascularization, cell metabolism and apoptosis in tumors under
hypoxia (2–5). Since the growth environment of glioma
is in hypoxia, exploring the growth characteristics of glioma under
hypoxia cannot be separated from the role of hypoxia-related
molecules (e.g., HIF-1α). Studying the relationship between them
may provide a possible way for early diagnosis and treatment of
glioma cells.
In addition, a number of lipids microvesicles of
30–100 nm in diameter, called exosomes, have been found in the
blood of some glioma patients (2–6).
These exosomes can isolate and extract a variety of small RNAs,
proteins, lipids and other substances as signaling molecules for
molecular transduction and cross-linking between tumor cells
(7). It has been shown that
microRNAs (miRNAs) isolated from exosomes are involved in the
regulation of tumor cell growth, metabolism and apoptosis through
various pathways and have potential value for early tumor diagnosis
(8). miRNAs are a class of RNA
molecules 18–22 bases in length that do not have a coding function
but can affect protein expression by inhibiting the transcription
and translation process of proteins. miR-29a-3p is a molecule that
is abnormally expressed in a variety of tumor cells and can
regulate oncogenes in recent years (9,10).
In the present study, the expression level of miR-29a-3p in plasma
exosomes of patients with glioma in The First People's Hospital of
Jiashan (China) was examined and the effects of miR-29a-3p on the
proliferation and apoptosis of glioma cells under hypoxia were
verified by cell experiments. Then, the mechanism of the effect of
exosomes and their included miRNAs on glioma was explored. The
present study may provide a possible theoretical basis for the
early diagnosis and targeted therapy of glioma.
Materials and methods
Sample collection
Peripheral blood (~10 ml) was collected from 20
patients with glioma who underwent routine surgery in the
Department of Neurosurgery and 20 healthy controls in the Health
Management Centre between January 2019 and December 2020 at The
First People's Hospital of Jiashan. The patients and healthy
controls consisted of 8 males and 12 females, respectively, aged
42–68 years, the clinicopathological features of patients are
presented in Table I.
 | Table I.Clinical characteristics of glioma
patients. |
Table I.
Clinical characteristics of glioma
patients.
| Grouping
information | Characteristic |
|---|
| Sample size | n=20 |
| Sex | 8 males and 12
females |
| Age (years) | 55.85±10.16 |
| Tumor
classification | WHO I–II grade
(n=9) |
|
| WHO III–IV grade
(n=11) |
The inclusion criteria were i) Glioma confirmed by
postoperative pathology, ii) no preoperative radiotherapy or
chemotherapy was given, iii) first time of brain tumor resection
and iv) patients with complete clinical records. Exclusion criteria
were i) previous history of brain surgery, ii) combination with
other tumor diseases, mental diseases and other encephalopathy
affecting neurological function and iii) complications with serious
heart, liver and kidney diseases. All patients had pathological
confirmation of glioma and had not been previously treated with
chemotherapy. All subjects were formally informed of the purpose of
sample use and signed the informed consent form. The present study
was approved by the Ethics Committee of Jiashan First People's
Hospital (approval no. KY2022-030).
Reagents
Human glioma U251 cells were purchased from Shanghai
CAS Cell Bank, DMEM was purchased from Gibco (Thermo Fisher
Scientific, Inc.), fetal bovine serum, TRIzol®, One Step
PrimeScript cDNA Synthesis kit, Lipofectamine® 2000 were
purchased from Thermo Fisher Scientific, Inc., trypsin-EDTA
solutions (0.25%) were purchased from MilliporeSigma, a plasma
exosome extraction kit was purchased from Invitrogen (Thermo Fisher
Scientific, Inc.), a qPCR kit was purchased from Takara Bio USA,
Inc. The miR-29-3p-5p mimic/inhibitor was synthesized by Shanghai
Jima Co., Ltd., the double luciferase gene reporter assay was
commissioned from Tianjin Sheweisi Biotechnology Co., Ltd., the
CCK-8 cell viability assay kit was purchased from Bestbio, Annexin
V-phycoerythrin (PE) and 7-AAD were purchased from Becton,
Dickinson and Company, rabbit anti-human PI3K (cat. no. 4249), AKT
(cat. no. 4685) and phosphorylated (p-)AKT (cat. no. 4060)
antibodies were purchased from Cell Signaling Technology, Inc.,
rabbit anti-human HIF-1α (cat. no. 20960-1-AP) antibodies were
purchased from Proteintech Group, Inc. and rabbit anti-human Bax
(cat. no. 41162) antibody was purchased from Cell Signaling
Technology, Inc., rabbit anti-human Bcl-2 (cat. no. ab32124),
pyruvate dehydrogenase kinase (PDK)-1 (cat. no. ab202468), PDK2
(cat. no. ab154549) from Abcam, β-actin (cat. no. TA-09), goat
anti-rabbit secondary antibody (cat. no. ZB-5301) was purchased
from Beijing Zhongshan Jinqiao Biotechnology Co., Ltd., the PVDF
membrane was purchased from MilliporeSigma (cat. no. ISEQ00010),
the ECL luminescent solution was purchased from Jiangsu KGI
Biotechnology Co., Ltd. (cat. no. KGP1127) and the glycolysis assay
kit was purchased from Agilent (cat. no. 103020-100).
Isolation and identification of
exosomes
The plasma sample was centrifuged at room
temperature (25°C) for 20 min at 2,000 × g; the supernatant was
transferred to a new centrifuge tube and centrifuged at 4°C for 30
min at 2,000 × g; the supernatant was transferred to a new
precooled centrifuge tube and centrifuged again at 4°C for 45 min
at 10,000 × g to remove the larger vesicles. The supernatant was
collected by filtration through a 0.45 µm membrane and centrifuged
at 4°C for 70 min at 100,000 × g. The supernatant was removed,
resuspended in 10 ml of precooled 1× PBS, and centrifuged again at
4°C for 70 min at 100,000 × g. The supernatant was removed and the
resulting precipitate was the exosome. In addition, the exosome
information was analyzed using the ExoCarta database (http://www.exocarta.org; version 3.1.4).
Transmission electron microscopy
(TEM)
For TEM 10 µl exosomes were placed onto
copper-coated grids and precipitated for 10 min and the excess
liquid was absorbed by filter paper. The grids were transferred to
a solution containing 2.5% glutaraldehyde (diluted with PBS) at
4°C, suspended for 5 min and dried with filter paper before being
washed with PBS for four times. The grids were transferred to 40
g/l uranyl acetate (MilliporeSigma) for 10 min at 25°C and the
excess liquid was absorbed by filter paper. The grids were
transferred to 10 g/l methyl-cellulose (MilliporeSigma) for 5 min
at 25°C and dried with filter paper. After natural drying at room
temperature (25°C; ~30 min), the grids were analyzed on a HT-7700
transmission electron microscope (Hitachi, Ltd.) and images were
analyzed by Digital Micrograph (version 3.51, Gatan, Inc.).
Cell culture and drug treatment
The human glioma cell line U251 was cultured using
DMEM complete medium containing 10% fetal bovine serum. The normal
culture conditions were 5% CO2 + 95% air in a constant
temperature incubator at 37°C; the hypoxic culture conditions were
5% CO2 + 94% N2 + 1% O2 and the
cells were placed in a hypoxic incubator at 37°C. Recilisib
complete medium with a masterbatch concentration of 100 mM was
prepared and cells were transfected for 24 h and incubated at 37°C,
then added to the cell supernatant at 1:1,000 and mixed as the
miR-29a-3p + Recilisib group. The miR-NC + DMOG group was used as
the control group to start the experiment 48 h after
transfection.
Transfection of miR-29a-3p mimics and
inhibitor
Glioma cells in the log growth phase were selected
and inoculated in 6-well plates at a rate of 2×105
cells/well and the transfection experiment was started when the
cell confluence reached 60%. During transfection, 12 µl
Lipofectamine® 2000 was added to 150 µl serum-free
medium and mixed with 10 µl miR-29a-3p mimics/inhibitors, then
incubated at room temperature (25°C). The final concentration of
miR-29a-3p mimics was 50 nM and the final concentration of
miR-29a-3p inhibitor was 100 nM. After incubation for 5 min, the
mixed solution was added to the 6-well plates to complete the
transfection. The miR-29a-3p control group (miR-NC) received the
same processing. After 48 h, the transfection efficiency of each
well was measured by reverse transcription-quantitative (RT-q) PCR.
The sequences of the miR-29a-3p mimics/inhibitor/control are given
in Table II.
 | Table II.miR-29a-3p mimics/inhibitor/control
and PI3K/β-actin primer sequences. |
Table II.
miR-29a-3p mimics/inhibitor/control
and PI3K/β-actin primer sequences.
| Gene | Primer
sequence |
|---|
| miR-29a-3p
mimics | F:
5′-UAGCACCAUCUGAAAUCGGUUA-3′ |
|
| R:
5′-ACCGAUUUCAGAUGGUGCUAUU-3′ |
| Mimics control | F:
5′-UUCUCCGAACGUGUCACGUTT-3′ |
|
| R:
5′-ACGUGACACGUUCGGAGAATT-3′ |
| miR-29a-3p
inhibitor | F:
5′-UAACCGAUUUCAGAUGGUGCUA-3′ |
| Inhibitor
control | R:
5′-CAGUACUUUUGUGUAGUACAA-3′ |
| PI3K | F:
5′-GGACCCGATGCGGTTAGAG-3′ |
|
| R:
5′-ATCAAGTGGATGCCCCACAG-3′ |
| β-actin | F:
5′-GTGGATCAGCAAGCAGGAGT-3′′ |
|
| R:
5′-CTCGGCCACATTGTGAACTT-3′ |
RT-qPCR
The cells were spread into 6-well plates at
2×105 cells/well, 1 ml TRIzol® (Thermo Fisher
Scientific, Inc.) was added when the convergence degree of the
cells was 80–90% and total RNA was extracted by the
TRIzol® method. For mRNA, cDNA was formed by reverse
transcription according to the instructions of the One Step
PrimeScript cDNA Synthesis kit (Thermo Fisher Scientific, Inc.) and
mir-X miRNA first-strand synthesis kit (Takara Biotechnology Co.,
Ltd.) was used for miRNA. RT-qPCR R was performed using TB
Green® Advantage® qPCR premix regent (Takara
Biotechnology Co., Ltd.) and the results were calculated by the
2−ΔΔCq method to detect the relative expression of the
genes (11), using U6 as the
internal reference for miRNA and β-actin as the internal reference
for mRNA. The reaction conditions were as follows: 95°C, 2 min,
followed by 40 cycles of 10 sec at 95°C, 30 sec at 55°C and 30 sec
at 72°C. The primer sequences (purchased from Sangon Biotech,
Shanghai) are given in Table
II.
Cell proliferation assay
Cell proliferation was detected by the CCK-8 assay.
Cells were seeded into 96-well plates at 2×104
cells/well and complete medium without cells was used as a blank
control. After the cells were grown for 24 h, 10 µl of CCK-8
solution was added to each well and ~3–5 replicate wells were set
up in each well. The cells were cultured in an incubator for 2.5 h
at 37°C and the D450 value was measured at 450 nm with a microplate
reader. Cell proliferation level=(experimental group-blank
group)/(untreated group-blank group) ×100%.
Apoptosis detected by flow
cytometry
Cells (2.5×106 cells/tube) were
collected, centrifuged at 4°C for 5 min at 210 × g and washed 3
times with precooled PBS. Then cells were collected again,
centrifuged at 4°C for 5 min at 160 × g and suspended with 100 µl
1X binding buffer. Annexin V-PE (5 µl) was added and cells were
incubated at 4°C for 15 min containing after which 7-AAD was added
and incubated for 5 min at 4°C. Annexin V-PE was used to detect
early apoptotic cells, and Annexin V-PE and 7-AAD were used to
detect necrotic cells or/and late apoptotic cells. The percentage
of early apoptotic cells was used to calculate the apoptotic rate
and the cell apoptosis level was detected by a BD Accuri C6 Flow
cytometer and analyzed by BD Accuri C6 Software (version 1.0; BD
Biosciences).
Dual-Luciferase reporter assay
The binding site of miR-29-3p-5p to PI3K was
predicted by the online database Targets can (https://www.targetscan.org/vert_80/; version
8.0). Wild-type PI3K 3′-UTR (WT-PI3K) or mutant PI3K 3′-UTR
(MUT-PI3K) was inserted into the XhoI and NotI sites
of the pSI-Check2 vector (Sangon Biotech Co., Ltd.), respectively.
Plasmid transfection was performed when the cell density reached
~50–70%. Briefly, 5 pmol miR-29 mimics or miR-29 control was mixed
with 0.16 µg PI3K 3′-UTR (WT-UTR) plasmid or PI3K 3′-UTR (MUT-PI3K)
plasmid, respectively, then above solution were mixed with 10 µl
DMEM containing 0.3 µl Lipofectamine® 2000 (Thermo
Fisher Scientific, Inc.) and placed at room temperature (25°C) for
20 min before transfection. Following transfection, the transfected
cells were incubated in the incubator for 48 h at 37°C, then cells
were collected. Passive Lysis Buffer was added at 100 µl/well at
room temperature (25°C) and slowly shaken for 15 min, then the cell
lysate was aspirated into a 1.5 ml centrifuge tube, centrifuged at
4°C at 13,000 × g for 10 min and the supernatant was taken and
transferred into a new tube. Luciferase Assay Agent II (LAR II; 100
µl; Promega Corporation) and 20 µl cell lysate were added. The
firefly luciferase value (R1) was measured by Promega Dual
Luciferase system (Promega Corporation) after 2 sec. Then, 100 µl
Stop & Glo reagent (Luciferase Assay Reagent; Promega
Corporation) was added and mixed 2–3 times and the Renilla
luciferase value of each well (R2) was detected. Finally, the ratio
value (R1/R2) was recorded as the relative luciferase activity.
Western blotting
The cells were lysed in RIPA buffer: 50 mM Tris-HCl
(MilliporeSigma), 150 mM NaCl (MilliporeSigma), 1% Triton
X(MilliporeSigma), 1% Sodium deoxycholate (MilliporeSigma), 0.1%
SDS (MilliporeSigma), 5 mM EDTA (MilliporeSigma), with 2 µg/ml
aprotinin (MilliporeSigma), 1 mmol/l PMSF (Beijing Solarbio Science
& Technology Co., Ltd.), and the protein concentration was
measured by the BCA method. After protein extraction, 50 µg of
proteins were loaded per lane and 10% of SDS-PAGE gels were
prepared to separate the proteins, which were then transferred to
PVDF membranes. Skimmed milk powder (5%) was used for blocking for
2 h at room temperature and the membranes incubated with primary
antibodies against PI3K (1:2,000), AKT (1:1,000), p-AKT (1:1,000),
Bax (1:1,000), Bcl-2 (1:1,000), HIF-1α (1:2,000), PDK-1 (1:1,000),
PDK-2 (1:1,000) and β-actin (1:4,000) overnight at 4°C. This was
followed by incubation with a secondary antibody (1:5,000) for 2 h
at room temperature, color development with ECL luminescent
solution and the protein levels were measured using a ChemiDoc MP
Imaging System (Bio-Rad Laboratories, Inc.). Image Lab 5.1 (Bio-Rad
Laboratories, Inc.) was used to analyze the gray values.
Glycolytic capacity was examined with
a Seahorse system
The cells were inoculated at 1.5×105
cells/well into energy metabolism assay plates. A total of five
secondary wells were set up for each strain of cells and the plates
were soaked with activation solution and placed in a
CO2-free incubator at 37°C overnight. On the second day,
1 ml of sodium pyruvate was added to 100 ml of XF Base medium and
the pH was adjusted to 7.35-7.45. The prepared XF base medium was
used to wash the cells twice and 500 µl of XF base medium was added
and placed in the cell culture box for 1 h. Oligomycin, 2-DG and
glucose working solutions were prepared and added to the dosing
wells of the hydration plate. The hydration plate was moved to the
machine and programmed according to the Energy Metabolizer manual
XFe24 Training Manual (Seahorse Bioscience; Agilent Technologies,
Inc.). After 1 h of running the program, the cell culture plate was
placed on the machine and after 2 h, the glycolysis level of the
cells was analyzed.
Statistical analysis
All data are expressed as the mean ± standard
deviation. A t-test was used for intragroup comparisons and one-way
ANOVA was used for comparisons between multiple groups. P<0.05
was considered to indicate a statistically significant
difference.
Results
miR-29a-3p expression levels in plasma
exosomes of patients with glioma
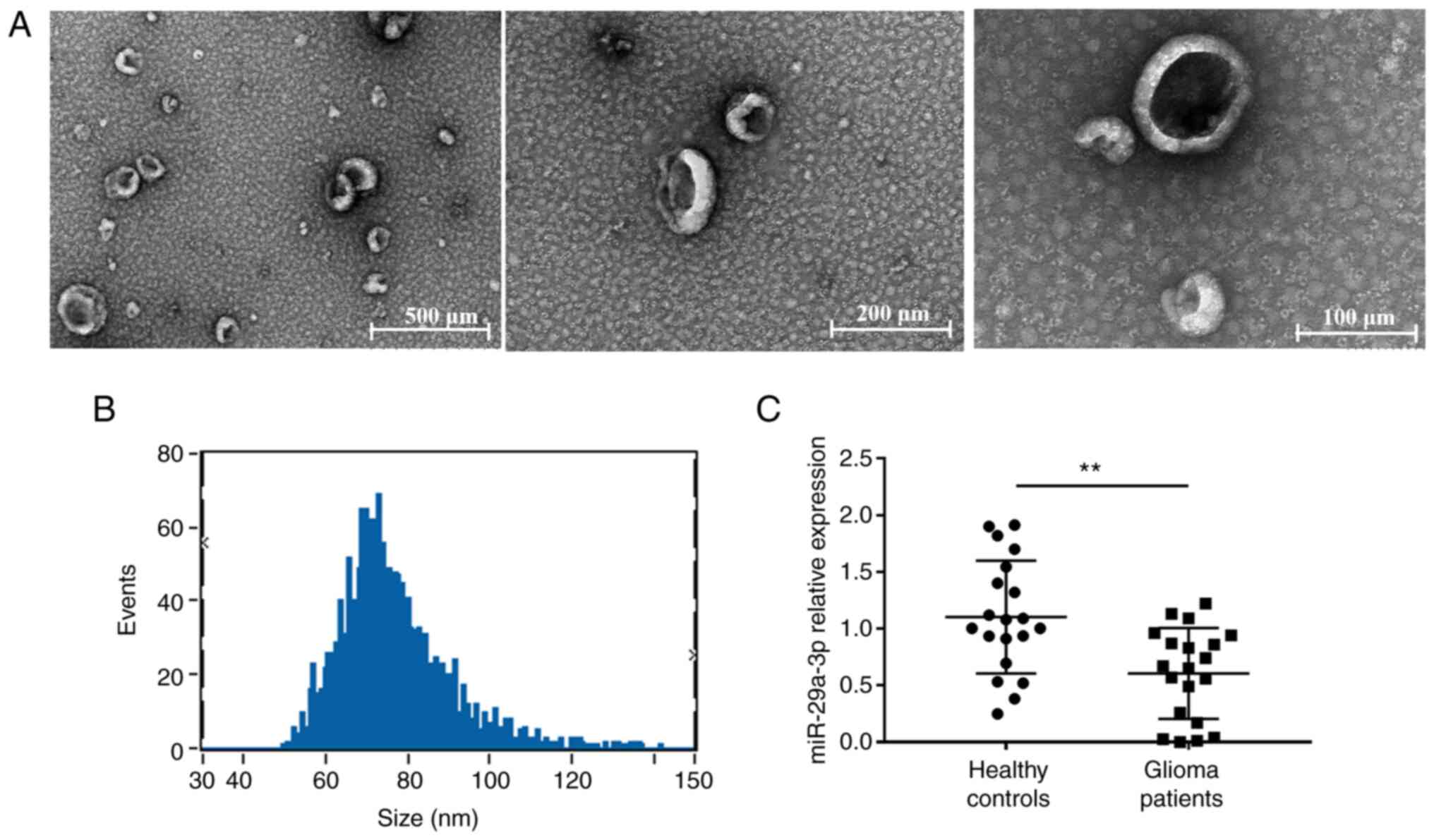
The purified exosomes were examined using TEM, as
shown in Fig. 1A, where unevenly
distributed vesicle-like structures <200 nm in diameter with a
lipid bilayer structure can be seen against a dark background and
were more typical. The particle size analysis revealed that the
particle size value was 76.64±13.25 nm and diameters between 30–150
nm accounted for >90% of all vesicles (Fig. 1B), indicating that the vesicles
obtained were of uniform diameter and high purity. Then the
expression of miR-29a-3p was detected. In plasma exosomes of
patients with glioma, the miRNA-29a-3p levels were evaluated by
RT-qPCR. The results showed a significant decrease in miR-29a-3p
expression levels in plasma exosomes from glioma patients compared
with those from healthy controls (Fig.
1C).
Effect of miR-29a-3p on proliferation
and apoptosis levels in gliomas under normoxic and hypoxic
conditions
The expression levels of miR-29a-3p in the glioma
cell line U251 were examined by RT-qPCR. The results showed that
the expression levels of miR-29a-3p in glioma cells were decreased
under hypoxic conditions (Fig. 2A)
compared with normoxia. In addition, it was found that hypoxia
increased the proliferation level of glioma cells, but
overexpression of miR-29a-3p significantly inhibited their
proliferation (Fig. 2B). The
results of flow cytometry showed that overexpression of miR-29a-3p
significantly increased apoptosis in glioma cells under normoxic
and hypoxic conditions (Fig. 2C).
Western blotting was applied to examine apoptosis-related proteins.
Overexpression of miR-29a-3p increased the pro-apoptotic marker Bax
while decreasing the anti-apoptotic marker Bcl-2. The role of
miR-29a-3p in the above processes was more prominent under normoxic
conditions. In addition, glioma cells under normoxic conditions
expressed a certain level of hypoxia-Inducible Factor (HIF)-1 and
hypoxia increased its expression, whereas the overexpression of
miR-29-3p inhibited its expression (Fig. 2D).
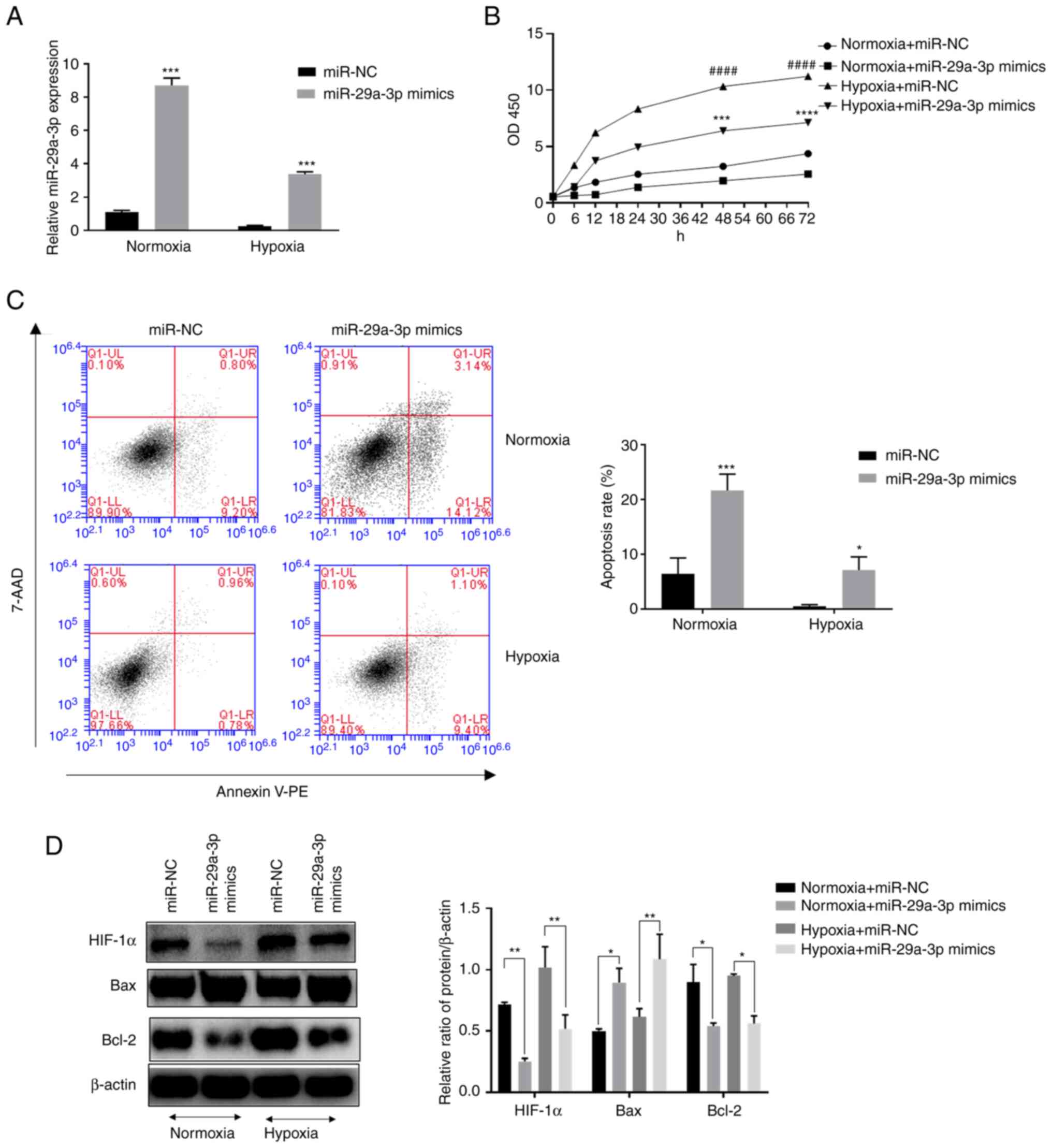 | Figure 2.Effect of miR-29a-3p on the
proliferation and apoptosis levels of glioma cells under normoxic
and hypoxic conditions. (A) The expression level of miR-29a-3p by
reverse transcription-quantitative PCR, ***P<0.001 vs. miR-NC
under hypoxia. (B) The effect of miR-29a-3p on the proliferation
level of glioma cells U251, ####P<0.0001 vs. miR-NC
under normoxia; ***P<0.001, ****P<0.0001 vs. miR-NC under
hypoxia. (C) Effect of miR-29a-3p on apoptotic levels in glioma
cells U251, *P<0.05 vs. miR-NC under hypoxia, ***P<0.001 vs.
miR-NC under normoxia. (D) Effect of miR-29a-3p on the expression
levels of Bax, Bcl-2 and HIF-1α. *P<0.05, **P<0.01. All data
were expressed as the mean ± standard deviation (n=3). miR,
microRNA; NC, negative control; HIF-1α, hypoxia-inducible factor
1. |
miR-29a-3p inhibits HIF-1α expression
by targeting the PI3K/AKT pathway
TargetScan was used to predict the presence of
miR-29a-3p binding sites in the PI3K gene. Then to further
understand the target role of miR-29a-3p on PI3K, dual-luciferase
gene reporter assay was performed. Results showed that in Wt-PI3K
groups, compared with miR-NC, miR-29a-3p mimics could decrease the
luciferase activity. However, when the binding site of PI3K to
miR-29a-3p was mutated (MUT-PI3K), miR-29a-3p lost its effect on
the luciferase activity, the above results indicated the target
regulation of miR-29a-3p on PI3K gene (Fig. 3A and B). qPCR and western blotting
results showed that miR-29a-3p could inhibit PI3K protein
expression, resulting in a reduction in AKT phosphorylation levels
and HIF-1α protein expression levels, as shown in Fig. 3C-E.
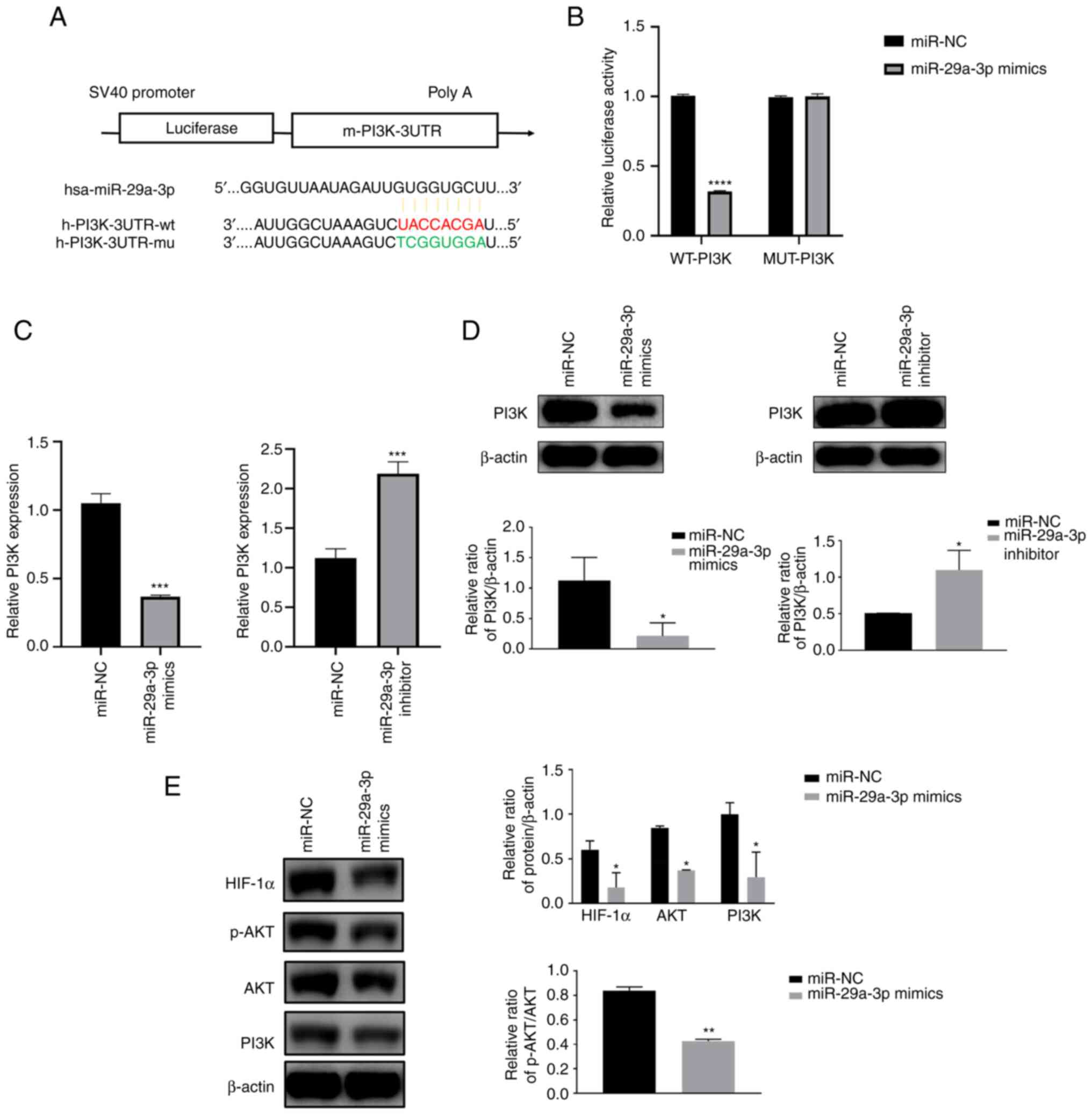 | Figure 3.miR-29a-3p inhibits HIF-1α expression
by targeting the PI3K/AKT pathway. (A) A schematic diagram of the
binding site of miR-29a-3p to the PI3K gene and the PI3K mutation
site was predicted by TargetScan. (B) Dual luciferase gene reporter
assay was used to verify the targeting negative regulatory effect
of miR-29a-3p on PI3K, ****P<0.0001. The expression level of
PI3K was analyzed by (C) quantitative PCR and (D) western blotting
after U251 cells were transfected with miR-29a-3p mimics and
inhibitor, *P<0.05, ***P<0.001. (E) Effect of miR-29a-3p on
the expression levels of the PI3K downstream molecules AKT, p-AKT
and HIF-1α, *P<0.05, **P<0.01. All data were expressed as the
mean ± standard deviation (n=3). miR, microRNA; HIF-1α,
hypoxia-inducible factor 1; NC, negative control; UTR, 3′
untranslated region; wt, wild-type; mu, mutant; p-,
phosphorylated. |
Effect of miR-29a-3p on glioma
proliferation and apoptosis levels through targeting the regulation
of the PI3K/AKT/HIF-1α pathway
The present study investigated the effect of
miR-29a-3p on the proliferation and apoptosis of glioma cells via
the PI3K/AKT/HIF-1α regulatory axis under normoxia because HIF-1α
exhibited a certain expression level under normoxia and previous
experiments demonstrated that overexpression of miR-29a-3p could
have a more pronounced effect on apoptosis under normoxic
conditions. The results showed that overexpression of miR-29a-3p
significantly reduced cell proliferation, increased apoptosis and
the expression of the pro-apoptotic molecule Bax. In addition, it
reduced the expression of the anti-apoptotic molecule Bcl-2 and
HIF-1α, p-AKT, AKT and PI3K compared with the control group
(miR-NC) after 24 h of normoxic culture. By contrast, when the PI3K
agonist Recilisib was used, the inhibitory effect of miR-29a-3p on
cell proliferation and apoptosis, the reduction in PI3K, AKT,
p-AKT, Bcl-2 and HIF-1α expression and the increase in Bax
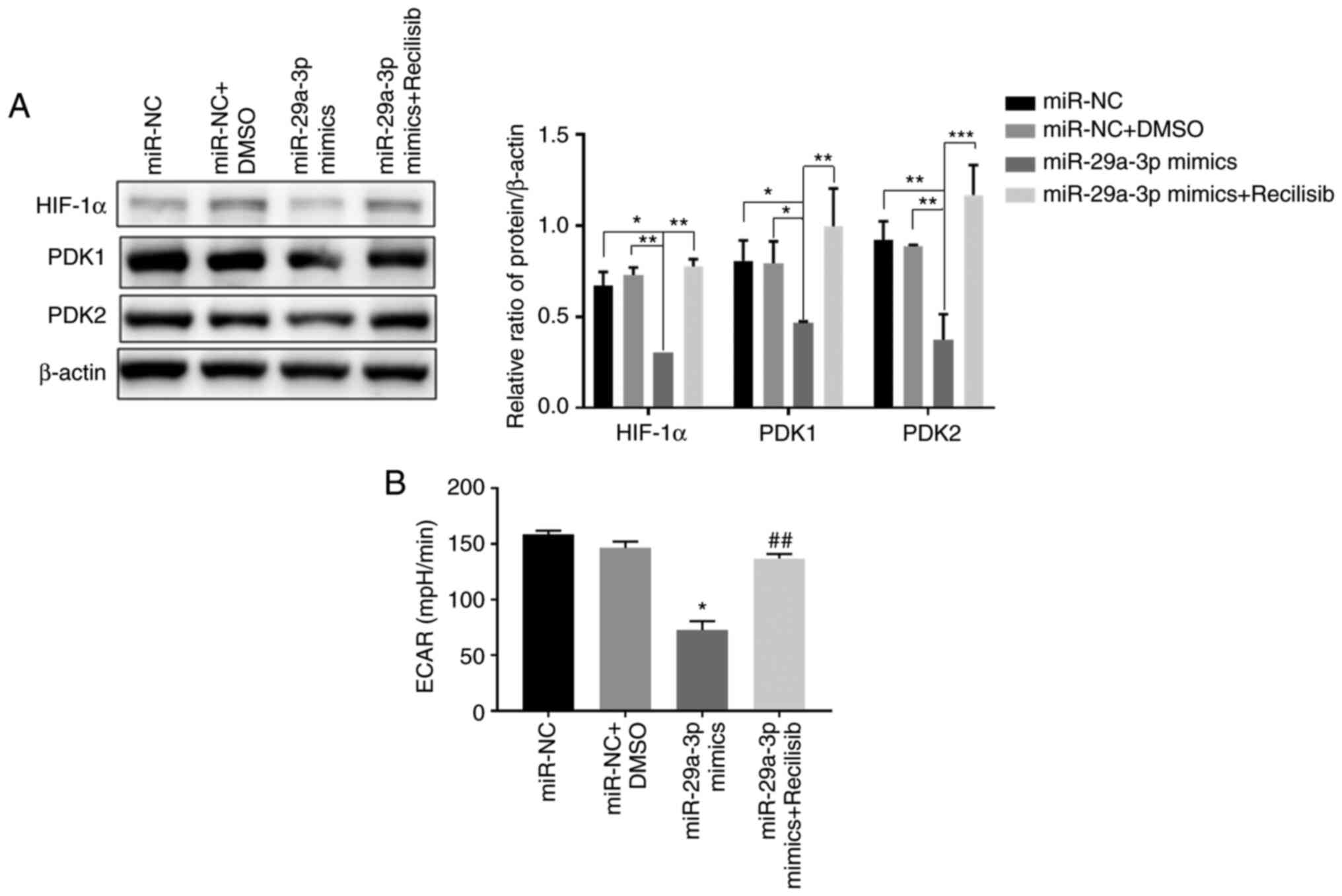
expression levels were all reversed, as shown in Fig. 4.
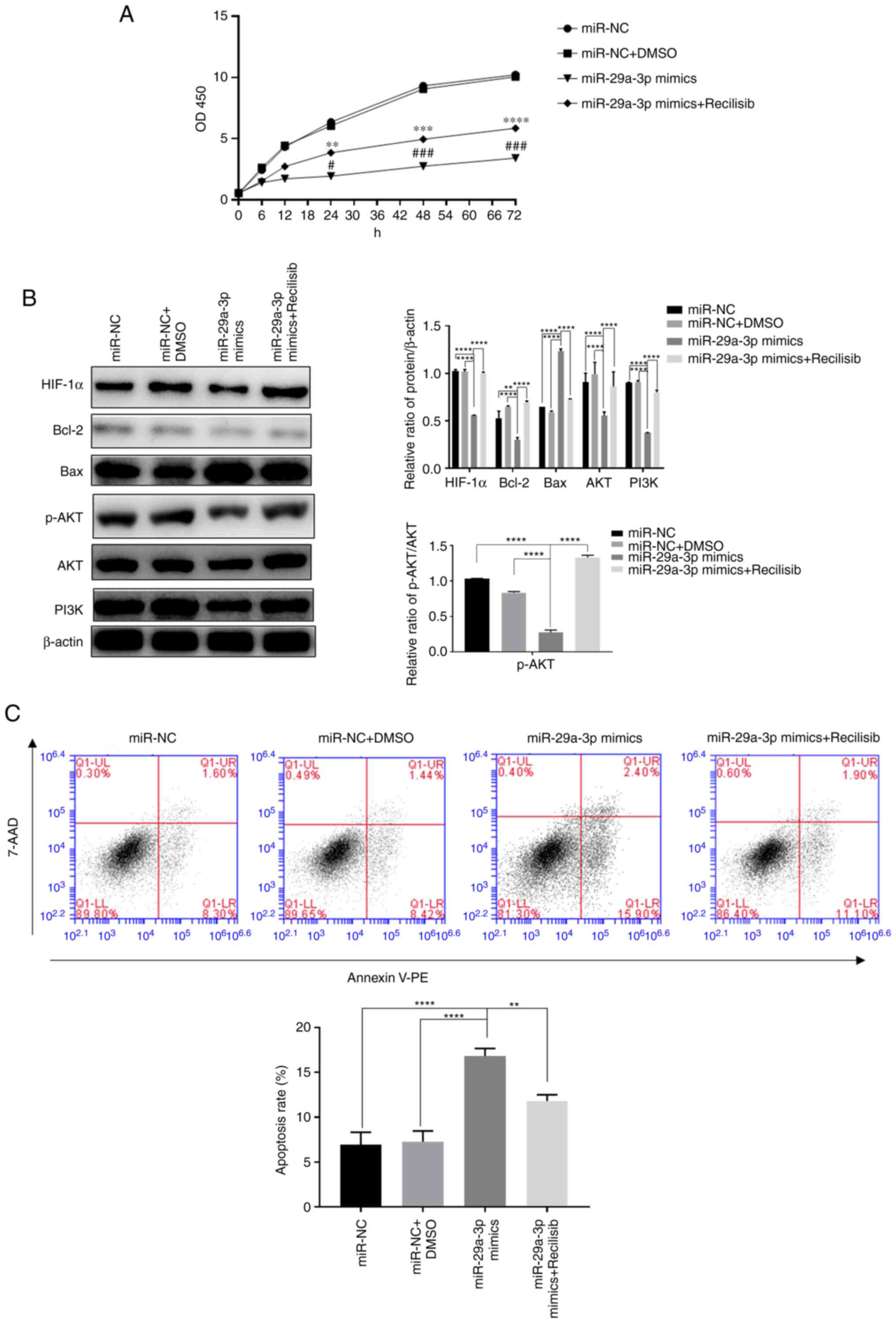 | Figure 4.The effect of miR-29a-3p via the
PI3K/AKT/HIF-1α pathway on glioma cells' proliferation and
apoptosis levels. (A) The proliferation level was detected by CCK-8
assay, **P<0.01, ***P<0.001, ****P<0.0001 vs. miR-NC +
DMSO; #P<0.05, ###P<0.001 vs. miR-NC.
(B) The expression levels of HIF-1α, Bcl-2, Bax, AKT, PI3K and the
ratio of p-AKT/AKT, were detected by western blotting, **P<0.01,
****P<0.0001. (C) The levels of apoptosis in each group were
detected by flow cytometry, **P<0.01, ****P<0.0001. All data
were expressed as the mean ± standard deviation (n=3). miR,
microRNA; HIF-1α, hypoxia-inducible factor 1; NC, negative control;
HIF-1α, hypoxia-inducible factor 1; p-, phosphorylated; PE,
phycoerythrin. |
miR-29a-3p inhibits glycolysis levels
in glioma cells by targeted regulation of the PI3K/AKT/HIF-1α
pathway
The results showed that compared with the control
group (miR-NC, miR-NC + DMSO), overexpression of miR-29a-3p
significantly inhibited the expression of HIF-1α, PDK1 and PDK2,
thereby decreasing the ECAR level in glioma cells. However, when
Recilisib was used (miR-29a-3p + Recilisib group), the expression
levels of HIF-1α and PDK1 and PDK2 were increased and the ECAR
level was enhanced compared with the miR-29a-3p overexpression
group, as shown in Fig. 5A and
B.
Discussion
Normal brain cells are supplied with normal oxygen
concentrations (~20%), while the oxygen concentrations in glioma
cells are between 0.1-2.5% (12).
Glioma cells are in a state of chronic hypoxia but do not inhibit
the proliferation of tumor cells. This growth characteristic
stimulates more abnormal gene expression, making them more adapted
to hypoxia. Therefore, hypoxia is one of the key features of the
glioma growth microenvironment (13). The search for mechanisms of
malignant transformation of glioma cells under hypoxic conditions
may provide alternative directions to resolve these problems.
Studies have demonstrated that hypoxia can increase
the secretion of exosomes in tumor cells or cause the loss of the
transportation of more molecules (14–16).
Exosomes act as carriers of intercellular molecular signals and can
carry a variety of oncogenes or inhibitory factors that affect the
different stages of tumor growth (17,18).
Research on the composition and molecular function of exosomes is
of great diagnostic and clinical importance. In addition, according
to the ExoCarta database, exosomes are known to contain 9,769
proteins, 3,408 mRNAs and 1,116 lipids, among which there are 2,838
types of miRNAs, accounting for the highest proportion of noncoding
RNAs in exosomes (19,20). Exosomes deliver these miRNAs to
other target cells and are extensively involved in tumor
proliferation, apoptosis, cycling, invasion and adhesion-related
processes by regulating the 3′ untranslated region (UTR) of target
molecules and degrading mRNA directly or indirectly (21,22).
Therefore, the detection of exosomes may be of great value to the
diagnosis of diseases and other research.
The exosomes derived from peripheral blood may
originate from some peripheral blood cells, such as lymphocytes,
monocytes and/or from tumors and other normal cells. Based on the
above, the substance in exosomes such as miR-29a-3p may have
different significance and effects. Reports have shown that the
detection of miR-29a-3p in glioma can be used as a malignant marker
after making a definite diagnosis (23–25),
then, if possible, the glioma can be staged according to the
expression of miR-29a-3p and clinical data. As miR-29a-3p expressed
in peripheral blood can be used as a biomarker for the preliminary
diagnosis of disease, and may be used as an early marker for
disease screening, this highlights the significance of detecting it
in peripheral blood (26–29).
The PI3K-AKT pathway is involved in the regulation
of cell proliferation, differentiation, apoptosis and glucose
transport (47–50). The main biological functions of
miR-29a target genes are also focused on these aspects, so there
may be an association between the two. PI3K/AKT is an important
antiapoptotic pathway and several molecules affected by this
pathway, such as VEGF and endothelial NOS in angiogenesis, Bad,
Bcl-2 and BNIP3 in apoptosis and the glioma cell invasion molecule
PAI-1, are associated with the expression of HIF-1α, which is an
important molecule regulated by the PI3K/AKT pathway upon
activation (51,52). Therefore, it is reasonable to
hypothesize that the process of HIF-1α inhibition by miR-29a-3p may
occur via the PI3K/AKT pathway and this phenomenon was confirmed in
the present experiments. In addition, the Warburg effect is the use
of glycolysis by tumor cells under normoxia as their main metabolic
mode of energy production (53,54).
By contrast, HIF-1α can regulate the level of glycolysis by
promoting the transcriptional activation of pyruvate dehydrogenase
kinase (PDK), a key rate-limiting enzyme of glycolysis, thereby
inhibiting pyruvate dehydrogenase activity and thus preventing
pyruvate from participating in the tricarboxylic acid cycle instead
of participating in the glycolytic pathway (55). This change in the energy metabolism
of glioma cells is an important reason for tumor heterogeneity,
aggressiveness and increased drug resistance. The results of the
present study showed that the overexpression of miR-29a-3p could
significantly inhibit the expression of PDK1 and PDK2, which in
turn caused a decrease in the glycolysis level of glioma cells. By
contrast, the activation of the PI3K pathway using Recilisib based
on overexpression of miR-29a-3p could partially restore the
glycolysis level of tumor cells. The above results revealed that
miR-29a-3p inhibited glycolysis in tumor cells through the
PI3K/AKT/HIF-1α pathway, thus affecting the proliferation and
apoptosis levels of glioma cells.
The present study had several limitations. First,
the experiments were performed in only one glioma cell line, more
glioma cell lines should be applied to validate the experimental
consequence in future research. Second, cell proliferation and
apoptosis should need more methods based on molecular biology
approaches to further verify the experimental results.
Currently, the main treatment option for glioma
patients is surgical resection combined with radiotherapy. However,
the limitations of surgical resection, drug resistance and
resistance to radiotherapy have resulted in short survival cycles,
high mortality and recurrence rates. As a result, a number of
researchers are focusing on early diagnosis and molecularly
targeted therapy for patients with glioma. The results of the
present study revealed that miR-29a-3p is an important suppressor
molecule in glioma and this molecule can inhibit the growth and
proliferation of glioma cells by targeting the negative regulation
of the PI3K/AKT/HIF-1α pathway and reducing glycolytic energy
supply, thus increasing the apoptosis of glioma cells. The present
study reveals the potential value of miR-29a-3p in tumor diagnosis
and treatment and provides an additional theoretical basis for the
screening of novel molecular markers for glioma and possible
therapeutic pathways.
However, the specific mechanism of miR-29a-3p
transmitted by peripheral blood cells for glioma remains to be
elucidated. In addition, how miR-29a-3p affects the glioma cells
needs to be experimentally verified, which is also the purpose of
the present study. The present study found that miR-29a-3p was
significantly lower in plasma exosomes of glioma patients compared
with normal subjects and that its expression increased apoptosis
and inhibited proliferation levels of glioma cells, initially
showing the cancer marker effect of miR-29a-3p.
The core response event of hypoxia is the reduction
of oxygen concentration. Therefore, exploring the effects of the
hypoxic microenvironment on tumor cells can be studied using
molecules closely related to oxygen metabolism. In-depth studies
reveal that miR-29a significantly inhibits the expression of
HIF-1α, a key regulatory molecule closely associated with hypoxia
(31). HIF-1α is an oxygen stress
molecule that is degraded by the ubiquitin-proteasome pathway when
oxygen is available. It accumulates stably in the cytoplasm when
the oxygen concentration is reduced and then enters the nucleus to
serve a transcriptional regulatory role. HIF-1α expressed in the
nucleus can form a dimer with HIF-1β, that is, the complete HIF-1
protein, which can affect the survival of tumor cells in a hypoxic
environment through a variety of pathways. U251 is a glioblastoma
cell line with a high degree of malignancy that is in a serious
hypoxic state inside the tumor tissue. To adapt to hypoxia, genes
inside the tumor cells are abnormally expressed so that the
expression of HIF-1α is not dependent on reduced oxygen levels
(32,33), which explains why this tumor cell
also has a certain level of HIF-1α protein under normoxic
conditions. In addition, the present study showed that hypoxia
could reduce the apoptosis level and increase the proliferation of
U251 cells. Harmful metabolic intermediates in normal cells can be
removed in time, while in malignant tumors with rapid growth, the
metabolism of tumor cells is relatively vigorous and abnormally
vigorous metabolic processes will cause cells to accumulate more
products that cannot be removed promptly (34–36).
A number of studies have found that the number of mitochondria in
malignant tumors is less than that in surrounding normal cells;
their shapes are different and they are more prone to degeneration
and change (37–39). Abnormal mitochondrial function is
not only deprived of the efficient use of oxygen for energy supply
but also increases the production of reactive oxygen species (ROS)
and harmful intermediate metabolites, the incomplete elimination of
which, especially those derived from biological processes related
to oxygen, may accumulate more into the cell, have adverse effects
on nucleic acid, protein structure, amino acid modification, energy
metabolism and other molecular biological activities and ultimately
cause the onset of cell apoptosis and death (40–42).
Nevertheless, for some malignancies, glycolysis has become their
major source of energy and hypoxia could initially reduce the
oxygen intake of cells, which can lead to lower levels of tumor
oxidative phosphorylation and then reduce the accumulation of
metabolic hazards related to oxygen, such as ROS, which explains
why cells had improved survival under hypoxia in the present study.
In addition, studies have shown that HIF-1α can inhibit the
accumulation of harmful metabolites such as lactic acid and ROS
through various pathways and then enhance tumor cell survival in
other ways, such as increasing the expression of VEGF to promote
neovascularization, increasing the expression of the antiapoptotic
molecule Bcl-2 and decreasing the expression of the proapoptotic
molecule Bax to inhibit tumor cell apoptosis or increasing the
level of cellular glycolysis through glucose transporter/PDK to
maintain tumor cell growth (43–46).
Therefore, the presence of HIF-1α under normoxia can avoid the
apoptosis and death of tumor cells to a certain extent, but once
the expression of HIF-1α is inhibited, the tumor cells without
HIF-1α compensation cannot effectively inhibit the harmful
metabolites and their apoptosis will also increase, which explains
why the inhibition of HIF-1α expression in glioma cells by
miR-29a-3p caused more apoptosis production compared with hypoxic
conditions.
Acknowledgements
Not applicable.
Funding
The present study was partially supported by ‘Research on
comprehensive prevention and control technology, accurate risk
assessment and application demonstration of individualized
prevention and control measures for senile prostate hyperplasia’,
National Key R&D projects (grant no. 2021YFC2009300).
Availability of data and materials
The datasets generated and/or analyzed during the
current study are not publicly available due to information that
could compromise the privacy of research participants but are
available from the corresponding author upon reasonable
request.
Authors' contributions
ZL was responsible for data acquisition,
experimental design, analysis and interpretation and drafting of
the article, including primer design, result statistics, charting.
LH conceptualized and co-designed the study, critically screened
the revised article for important intellectual content and provided
final approval of the submitted manuscript. ZY was responsible for
sample acquisition. ZL and ZY confirm the authenticity of all the
raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The protocol for this assay was approved by the
Ethics Committee of Jiashan First People's Hospital (approval no.
KY2022-030). Subjects provided written informed consent to
participate in this study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Touati S, Djekkoun R, El-Okki ME and Satta
D: Epidemiology and survival analyses of 333 adult glioma patients
from Eastern Algeria (2008–2016). Afr Health Sci. 20:1250–1258.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Domènech M, Hernández A, Plaja A,
Martínez-Balibrea E and Balañà C: Hypoxia: The cornerstone of
glioblastoma. Int J Mol Sc. 22:126082021. View Article : Google Scholar
|
|
3
|
Mohlin S, Wigerup C, Jögi A and Påhlman S:
Hypoxia, pseudohypoxia and cellular differentiation. Exp Cell Res.
356:192–196. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tamai S, Ichinose T, Tsutsui T, Tanaka S,
Garaeva F, Sabit H and Nakada M: Tumor microenvironment in glioma
invasion. Brain Sci. 12:5052022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ke Q and Costa M: Hypoxia-inducible
factor-1 (HIF-1). Mol Pharmacol. 70:1469–1480. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Saadatpour L, Fadaee E, Fadaei S, Nassiri
Mansour R, Mohammadi M, Mousavi SM, Goodarzi M, Verdi J and Mirzaei
H: Glioblastoma: Exosome and microRNA as novel diagnosis
biomarkers. Cancer Gene Ther. 23:415–418. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Whiteside TL: Tumor-derived exosomes and
their role in cancer progression. Adv Clin Chem. 74:103–141. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yu X, Odenthal M and Fries JWU: Exosomes
as miRNA carriers: Formation-function-future. Int J Mol Sci.
17:20282016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang JY, Zhang Q, Wang DD, Yan W, Sha HH,
Zhao JH, Yang SJ, Zhang HD, Hou JC, Xu HZ, et al: MiR-29a: A
potential therapeutic target and promising biomarker in tumors.
Biosci Rep. 38:BSR201712652018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yan B, Guo Q, Fu FJ, Wang Z, Yin Z, Wei YB
and Yang JR: The role of miR-29b in cancer: Regulation, function,
and signaling. Onco Targets Ther. 8:539–548. 2015.PubMed/NCBI
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yahara K, Ohguri T, Udono H, Yamamoto J,
Tomura K, Onoda T, Imada H, Nishizawa S and Korogi Y: Radiotherapy
using IMRT boosts after hyperbaric oxygen therapy with chemotherapy
for glioblastoma. J Radiat Res. 58:351–356. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang S, Luo X, Wan F and Lei T: The roles
of hypoxia-inducible factors in regulating neural stem cells
migration to glioma stem cells and determinating their fates.
Neurochem Res. 37:2659–2666. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cheng H, Chang S, Xu R, Chen L, Song X, Wu
J, Qian J, Zou Y and Ma J: Hypoxia-challenged MSC-derived exosomes
deliver miR-210 to attenuate post-infarction cardiac apoptosis.
Stem Cell Res Ther. 11:2242020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jiang H, Zhao H, Zhang M, He Y, Li X, Xu Y
and Liu X: Hypoxia induced changes of exosome cargo and subsequent
biological effects. Front Immunol. 13:8241882022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sun H, Meng Q, Shi C, Yang H, Li X, Wu S,
Familiari G, Relucenti M, Aschner M, Wang X and Chen R:
Hypoxia-inducible exosomes facilitate liver-tropic premetastatic
niche in colorectal cancer. Hepatology. 74:2633–2651. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kumar A and Deep G: Exosomes in
hypoxia-induced remodeling of the tumor microenvironment. Cancer
Lett. 488:1–8. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yaghoubi S, Najminejad H, Dabaghian M,
Karimi MH, Abdollahpour-Alitappeh M, Rad F, Mahi-Birjand M,
Mohammadi S, Mohseni F, Sobhani Lari M, et al: How hypoxia regulate
exosomes in ischemic diseases and cancer microenvironment? IUBMB
Life. 72:1286–1305. 2020. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sheehan C and D'Souza-Schorey C:
Tumor-derived extracellular vesicles: Molecular parcels that enable
regulation of the immune response in cancer. J Cell Sci.
132:jcs2350852019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Indira Chandran V, Welinder C, Gonçalves
de Oliveira K, Cerezo-Magaña M, Månsson AS, Johansson MC,
Marko-Varga G and Belting M: Global extracellular vesicle proteomic
signature defines U87-MG glioma cell hypoxic status with potential
implications for non-invasive diagnostics. J Neurooncol.
144:477–488. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
van der Vos KE, Abels ER, Zhang X, Lai C,
Carrizosa E, Oakley D, Prabhakar S, Mardini O, Crommentuijn MH,
Skog J, et al: Directly visualized glioblastoma-derived
extracellular vesicles transfer RNA to microglia/macrophages in the
brain. Neuro Oncol. 18:58–69. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cai Q, Zhu A and Gong L: Exosomes of
glioma cells deliver miR-148a to promote proliferation and
metastasis of glioblastoma via targeting CADM1. Bull Cancer.
105:643–651. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
He PY, Yip WK, Chai BL, Chai BY, Jabar MF,
Dusa N, Mohtarrudin N and Seow HF: Inhibition of cell migration and
invasion by miR-29a-3p in a colorectal cancer cell line through
suppression of CDC42BPA mRNA expression. Oncol Rep. 38:3554–3566.
2017.PubMed/NCBI
|
|
24
|
Wang J, Chen X, Xie C, Sun M, Hu C, Zhang
Z, Luan L, Zhou J, Zhou J, Zhu X, et al: MicroRNA miR-29a inhibits
colon cancer progression by downregulating B7-H3 expression:
Potential molecular targets for colon cancer therapy. Mol
Biotechnol. 63:849–861. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kuo TY, Hsi E, Yang IP, Tsai PC, Wang JY
and Juo SHH: Computational analysis of mRNA expression profiles
identifies microRNA-29a/c as predictor of colorectal cancer early
recurrence. PLoS One. 7:e315872012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yamada A, Horimatsu T, Okugawa Y, Nishida
N, Honjo H, Ida H, Kou T, Kusaka T, Sasaki Y, Yagi M, et al: Serum
miR-21, miR-29a, and miR-125b are promising biomarkers for the
early detection of colorectal neoplasia. Clin Cancer Res.
21:4234–4242. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Uratani R, Toiyama Y, Kitajima T, Kawamura
M, Hiro J, Kobayashi M, Tanaka K, Inoue Y, Mohri Y, Mori T, et al:
Diagnostic potential of cell-free and exosomal MicroRNAs in the
identification of patients with high-risk colorectal adenomas. PLoS
One. 11:e01607222016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Marcuello M, Duran-Sanchon S, Moreno L,
Lozano JJ, Bujanda L, Castells A and Gironella M: Analysis of A
6-Mirna signature in serum from colorectal cancer screening
participants as non-invasive biomarkers for advanced adenoma and
colorectal cancer detection. Cancers (Basel). 11:15422019.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Huang Z, Huang D, Ni S, Peng Z, Sheng W
and Du X: Plasma microRNAs are promising novel biomarkers for early
detection of colorectal cancer. Int J Cancer. 127:118–126. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Guo X, Qiu W, Wang J, Liu Q, Qian M, Wang
S, Zhang Z, Gao X, Chen Z, Guo Q, et al: Glioma exosomes mediate
the expansion and function of myeloid-derived suppressor cells
through microRNA-29a/Hbp1 and microRNA-92a/Prkar1a pathways. Int J
Cancer. 144:3111–3126. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Huang YH, Lian WS, Wang FS, Wang PW, Lin
HY, Tsai MC and Yang YL: MiR-29a curbs hepatocellular carcinoma
incidence via targeting of HIF-1α and ANGPT2. Int J Mol Sci.
23:16362022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Agani F and Jiang BH: Oxygen-independent
regulation of HIF-1: Novel involvement of PI3K/AKT/mTOR pathway in
cancer. Curr Cancer Drug Targets. 13:245–251. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang J, Chen X, Hu H, Yao M, Song Y, Yang
A, Xu X, Zhang N, Gao J and Liu B: PCAT-1 facilitates breast cancer
progression via binding to RACK1 and enhancing oxygen-independent
stability of HIF-1α. Mol Ther Nucleic Acids. 24:310–324. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Currie E, Schulze A, Zechner R, Walther TC
and Farese RV Jr: Cellular fatty acid metabolism and cancer. Cell
Metab. 18:153–161. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tang Y, Xiao G, Shen Z, Zhuang C, Xie Y,
Zhang X, Yang Z, Guan J, Shen Y, Chen Y, et al: Noninvasive
detection of extracellular pH in human benign and malignant liver
tumors using CEST MRI. Front Oncol. 10:5789852020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xu L, Wang L, Zhou L, Dorfman RG, Pan Y,
Tang D, Wang Y, Yin Y, Jiang C, Zou X, et al: The SIRT2/cMYC
pathway inhibits peroxidation-related apoptosis in
cholangiocarcinoma through metabolic reprogramming. Neoplasia.
21:429–441. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zong WX, Rabinowitz JD and White E:
Mitochondria and cancer. Mol Cell. 61:667–676. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Guerra F, Arbini AA and Moro L:
Mitochondria and cancer chemoresistance. Biochim Biophys Acta
Bioenerg. 1858:686–699. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Porporato PE, Filigheddu N, Pedro JMB,
Kroemer G and Galluzzi L: Mitochondrial metabolism and cancer. Cell
Res. 28:265–280. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Księżakowska-Łakoma K, Żyła M and
Wilczyński JR: Mitochondrial dysfunction in cancer. Prz
Menopauzalny. 13:136–144. 2014.PubMed/NCBI
|
|
41
|
Beltrà M, Pin F, Ballarò R, Costelli P and
Penna F: Mitochondrial dysfunction in cancer cachexia: Impact on
muscle health and regeneration. Cells. 10:31502021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hsu CC, Tseng LM and Lee HC: Role of
mitochondrial dysfunction in cancer progression. Exp Biol Med
(Maywood). 241:1281–1295. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Liu ZZ, Tian YF, Wu H, Ouyang SY and Kuang
WL: LncRNA H19 promotes glioma angiogenesis through
miR-138/HIF-1α/VEGF axis. Neoplasma. 67:111–118. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Miska J, Lee-Chang C, Rashidi A, Muroski
ME, Chang AL, Lopez-Rosas A, Zhang P, Panek WK, Cordero A, Han Y,
et al: HIF-1α Is a metabolic switch between glycolytic-driven
migration and oxidative phosphorylation-driven immunosuppression of
tregs in glioblastoma. Cell Rep. 27:226–237.e4. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wang Z, Li Q, Xia L, Li X, Sun C, Wang Q,
Cai X and Yang G: Borneol promotes apoptosis of human glioma cells
through regulating HIF-1a expression via mTORC1/eIF4E pathway. J
Cancer. 11:4810–4822. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Gabriely G, Wheeler MA, Takenaka MC and
Quintana FJ: Role of AHR and HIF-1α in glioblastoma metabolism.
Trends Endocrinol Metab. 28:428–436. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yang D, Fan L, Song Z, Fang S, Huang M and
Chen P: The KMT1A/TIMP3/PI3K/AKT circuit regulates tumor growth in
cervical cancer. Reprod Biol. 22:1006442022. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ma Y, Ran D, Zhao H, Song R, Zou H, Gu J,
Yuan Y, Bian J, Zhu J and Liu Z: Cadmium exposure triggers
osteoporosis in duck via P2X7/PI3K/AKT-mediated osteoblast and
osteoclast differentiation. Sci Total Environ. 750:1416382021.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhang W, Hou J, Yan X, Leng J, Li R, Zhang
J, Xing J, Chen C, Wang Z and Li W: Platycodon grandiflorum
saponins ameliorate cisplatin-induced acute nephrotoxicity through
the NF-κB-mediated inflammation and PI3K/Akt/apoptosis signaling
pathways. Nutrients. 10:13282018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Iheagwam FN, Iheagwam OT, Onuoha MK,
Ogunlana OO and Chinedu SN: Terminalia catappa aqueous leaf extract
reverses insulin resistance, improves glucose transport and
activates PI3K/AKT signalling in high fat/streptozotocin-induced
diabetic rats. Sci Rep. 12:107112022. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhang Z, Yao L, Yang J, Wang Z and Du G:
PI3K/Akt and HIF-1 signaling pathway in hypoxia-ischemia (review).
Mol Med Rep. 18:3547–3554. 2018.PubMed/NCBI
|
|
52
|
Zheng HL, Wang LH, Sun BS, Li Y, Yang JY
and Wu CF: Oligomer procyanidins (F2) repress HIF-1α expression in
human U87 glioma cells by inhibiting the EGFR/AKT/mTOR and
MAPK/ERK1/2 signaling pathways in vitro and in vivo. Oncotarget.
8:85252–85262. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
D'Alessandro G, Quaglio D, Monaco L, Lauro
C, Ghirga F, Ingallina C, De Martino M, Fucile S, Porzia A, Di
Castro MA, et al: 1H-NMR metabolomics reveals the
glabrescione B exacerbation of glycolytic metabolism beside the
cell growth inhibitory effect in glioma. Cell Commun Signal.
17:1082019. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Mao XG, Xue XY, Wang L, Wang L, Li L and
Zhang X: Hypoxia regulated gene network in glioblastoma has special
algebraic topology structures and revealed communications involving
Warburg effect and immune regulation. Cell Mol Neurobiol.
39:1093–1114. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Kim JW, Tchernyshyov I, Semenza GL and
Dang CV: HIF-1-mediated expression of pyruvate dehydrogenase
kinase: A metabolic switch required for cellular adaptation to
hypoxia. Cell Metab. 3:177–185. 2006. View Article : Google Scholar : PubMed/NCBI
|