Introduction
Polycystic ovarian syndrome (PCOS) is a
heterogeneous disease that often occurs during the childbearing
years; its etiology includes reproductive, endocrine and metabolic
aspects (1). Although the specific
pathophysiological mechanism of PCOS remains unclear, dysfunction
of the hypothalamic-pituitary-gonadal (HPG) axis is considered to
be an important component (2,3). The
increased secretion of total gonadotropin-releasing hormone (GnRH)
is a very important feature of the abnormal endocrine function of
the HPG axis in PCOS (3). The
hypothalamus is not only a secretory organ of GnRH, but also a
regulatory center, which triggers tissue inflammation and metabolic
abnormalities, and its dysfunction can lead to a series of
metabolic abnormalities and organ tissue dysfunction (4). Hypothalamic function is an important
pathophysiological mechanism of metabolic diseases; therefore,
changes in the hypothalamic microenvironment may be closely
associated with the occurrence and development of metabolic
diseases, especially the inflammatory microenvironment.
Previous studies have reported that hypothalamic
inflammation may be an underlying pathophysiological mechanism of
PCOS heterogeneity (4,5). Currently, microglia-mediated
hypothalamic inflammation is a well-recognized marker of central
inflammatory responses and is a key process in the pathogenesis of
chronic metabolic diseases (6–8).
Microglia, as a macrophage of the central nervous system, serve a
dual role in various pathophysiological states, mainly due to its
two polarized states (pro-inflammatory M1 polarization and
neurotrophic-protective M2 polarization) (9). Under normal physiological conditions,
microglia can be found in a resting state (M0) which serves a role
in immune surveillance. However, microglia are rapidly activated in
pathological conditions, which is accompanied by adaptive
polarization changes in transcriptional functions, resulting in M1
and/or M2 polarization (6–9). M1-polarized microglia induce an
inflammatory response and release a large number of inflammatory
factors, such as nitric oxide, IL-6, TNF-α and reactive oxygen
species (6–7), which are closely associated with
insulin resistance, obesity and the HPG axis in PCOS.
In recent years, aerobic exercise has been reported
to be one of the effective treatments for PCOS (10), but its mechanism remains to be
elucidated. Regular aerobic exercise not only improves PCOS
endocrine disorders, but also helps to reduce the risk of
developing metabolic complications (10,11).
Obese PCOS patients can restore normal menstrual cycle and
pregnancy through diet and exercise intervention (12). Moreover, if aerobic exercise
intervention is performed prior to employing assisted reproductive
technology, the pregnancy rate can be significantly increased
(12). The improved effect of
aerobic exercise on hypothalamic inflammation has attracted
increasing attention from many scholars. Aerobic exercise can
improve whole-body metabolic health and also reduce hypothalamic
inflammatory factors in obese mice (13); however, there is little evidence
that exercise affects the hypothalamus.
However, few study has reported the relation of
microglia and the GnRH/GnRH receptor (GnRHR) system, as well as its
roles in the pathophysiology of PCOS. Therefore, we hypothesized
that the GnRHR may be expressed in rat hypothalamic microglia, and
that excessive GnRH secretion may increase the amount of M1
polarized microglia in the hypothalamus of PCOS, which could lead
to the occurrence of inflammation. The present study evaluated the
effects of microglia polarization on hypothalamic dysfunction in
PCOS, which could provide a new direction for the treatment of
hypothalamic inflammation.
Materials and methods
Cell culture and treatment
Hypothalamic microglial cells were isolated from
newborn female rats (age, <24 h postpartum) as previously
described (14). Briefly, each
newborn female animal was injected subcutaneously with 300 mg/kg
pentobarbital sodium (15),
cardiac arrest was used to confirm death, and then the hypothalamus
tissue was excised and digested into a single-cell suspension using
DMEM/F12 (Hyclone; Cytiva) containing 0.25% trypsin (Hyclone;
Cytiva) for 30 min at 37°C in a 5% carbon dioxide cell incubator.
The hypothalamic cell suspension was then filtrated and gradient
centrifugation at 300 × g for 10 min at 37°C was performed to
obtain presumptive microglia. Finally, microglial cells were sorted
for anti-CD11b-FITC (1:100; cat. no. FITC-65229; Proteintech Group,
Inc.) using a FACScan Flow Cytometer (BD Biosciences). The cells
were divided into five groups and treated with 0, 10−12,
10−10, 10−8 and 10−6 mol/l
leuprolide acetate (LA, MedChemExpress) at 37°C in a 5% carbon
dioxide cell incubator for 6, 12 and 24 h.
Animals
Thirty-two Sprague-Dawley rats were purchased from
Wushi Experimental Animal Supply Co. Ltd. The animals were
maintained under a 14 h light/10 h dark schedule at 24±2°C with
humidity of 50±10% and free access to chow and water. The
experimental protocol was in accordance with the Guide for the Care
and Use of Laboratory Animals, by the Institutional Animal Care and
Use Committee, Fujian Normal University (Fuzhou, China; approval
no. IACUC-20180011). Each animal was anesthetized using 0.05 mg/kg
atropine (Sigma-Aldrich; Merck KGaA) administered subcutaneously
and 2.5 mg/kg diazepam (Sigma-Aldrich; Merck KGaA) administered
intraperitoneally for deep anesthesia. The abdomen was opened, then
the ovaries were removed for subsequent analysis and blood was
drawn for the assessment of serum hormone concentrations. All
animals were then sacrificed by cervical dislocation while still
anesthetized. All efforts were made to minimize animal discomfort
and to reduce the number of animals used.
Letrozole-induced PCOS rats
Six-week-old female rats with two consecutive 4-day
estrous cycles were randomly divided into 2 groups as follows: The
carboxymethyl cellulose (CMC) group as a vehicle group (n=16) and a
PCOS group (n=16). PCOS was induced in the PCOS group by the
intragastric administration of 1.0 mg/kg/day letrozole dissolved in
1.0% CMC (2.0 ml/kg) for 21 days, while the CMC group was injected
with equal volume of CMC, as previously described (16). The estrous cycles of all rats were
assessed using a vaginal smear method as previously reported
(1). PCOS rats were diagnosed
using the Rotterdam diagnostic criteria (1), which are based on the exclusion other
diseases that cause hyperandrogenism and the meeting of two of the
following three points: i) Oligo-ovulation or anovulation, ii)
clinical hyperandrogenism and/or hyperandrogenism, and iii)
polycystic ovary.
Treadmill running intervention
Following modeling, the CMC and PCOS groups were
further divided into CMC + quiet (CQ, n=8), CMC + exercise (CE,
n=8), PCOS + quiet (PQ, n=8) and PCOS + exercise (PE, n=8) groups.
After 1 week of acclimatization, all rats in the CE and PE groups
were trained on the treadmill at 75% VO2max for 60 min
per day, 6 days a week for 4 consecutive weeks; during that time,
all the rats in the CQ and PQ groups were allowed free movement
around the cage. The treadmill training was performed between 8:00
and 10:00 daily.
Immunocytochemistry analysis for
GnRHR
The microglial cells attached to the cell slides at
37°C in a 5% carbon dioxide cell incubator for 2 days. Following
washing, all slides were fixed in 4% paraformaldehyde at room
temperature for 10 min followed by incubation with 0.3%
H2O2 at room temperature for 15 min and
blocked using 10% bovine serum albumin (Sigma-Aldrich; Merck KGaA)
at room temperature for 45 min. The slides were incubated with
anti-transmembrane protein (TMEM)119 (1:200; cat. no. NBP2-30551;
Novus Biologicals, LLC), anti-ionized calcium binding adaptor
molecule 1 (Iba1; 1:200; cat. no. ab178846; Abcam) and anti-GnRHR
(1:50; cat. no. 19950-1-AP; Proteintech Group, Inc.) primary
antibodies separately, overnight at 4°C. Following washing with
PBS, the slides were incubated with CoraLite 594-conjugated goat
anti-rabbit IgG secondary antibody (1:200; cat. no. SA00013-4,
Proteintech Group, Inc.) at room temperature for 1 h. The nuclei
were stained using DAPI at room temperature for 10 min. All slides
were imaged using a fluorescence microscope.
RNA extraction and agarose gel
electrophoresis analysis for GnRHR
Total mRNA was extracted from the primary cultured
rat microglia using TRIzol® solution (Life Technologies;
Thermo Fisher Scientific. Inc.). The extracted mRNA samples were
then reverse-transcribed using an iScript™ Select cDNA synthesis
kit (Bio-Rad Laboratories, Inc.). The reverse-transcribed products
were amplified using a FasQuant RT Kit (Tiangen Biotech Co., Ltd.)
and the mRNA expression levels of GnRHR were assessed using 1%
agarose gel electrophoresis, which was stained with ethidium
bromide at room temperature for 30 min and imaged using an
ultraviolet lamp. The primer sequences used were as follows: GnRHR
forward (F), 5′-AGGACCCACGCAAACTACAG-3′ and reverse (R),
5′-TCCAGCAGATGACAAAGGAG-3′; and β-actin F,
5′-CGTAAAGACCTCTATGCCAACA-3′ and R, 5′-AGCCACCAATCCACACAGAG-3′.
β-actin was used as a loading control. All procedures were
performed according to the manufacturer's protocols.
Ovarian histology
The aforementioned rat ovaries were fixed in 4%
paraformaldehyde at 4°C for 48 h and embedded using paraffin.
Sections (5 µm) were cut and mounted on slides. The sections were
stained at room temperature with hematoxylin for 8 min and eosin
for 10 sec, and then imaged using a BX51 light microscope (Olympus
Corporation).
ELISA analysis
Blood was centrifuged at 1,000 × g for 20 min to
obtain serum at 4°C. The serum concentrations of testosterone (T),
luteinizing hormone (LH), follicle-stimulating hormone (FSH) and
GnRH were assessed using T ELISA kit (H090-1-1, Nanjing Jiancheng
Bioengineering Institute), LH ELISA kit (H206-1-2, Nanjing
Jiancheng Bioengineering Institute), FSH ELISA kit (H101-1-2,
Nanjing Jiancheng Bioengineering Institute) and GnRH ELISA kit
(H297, Nanjing Jiancheng Bioengineering Institute) according to the
manufacturer's protocols.
Western blot analysis
Total protein from cells and hypothalamic tissue was
extracted using RIPA lysis buffer (Beyotime Institute of
Biotechnology). A total of 20 µg of protein samples were subjected
to 10% SDS-PAGE gel electrophoresis and then transferred onto a
PVDF membrane. These membranes were washed with TBST (0.1%
Tween-20) and probed with primary antibodies (Table SI) overnight at 4°C. Following
washing with TBST (0.1% Tween-20), the membrane was incubated with
the corresponding secondary antibodies at 37°C for 1 h. The
immunoblotting signals were assessed using enhanced
chemiluminescence BeyoECL Plus buffer (P0018S, Beyotime Institute
of Biotechnology) and semi-quantified by densitometry using ImageJ
(version 1.44p; National Institutes of Health).
Statistical analysis
Data were presented as the mean ± SE. Differences in
mean values among multiple groups were evaluated using one-way
ANOVA, followed by Tukey's post-hoc test. P<0.05 was considered
to indicate a statistically significant difference.
Results
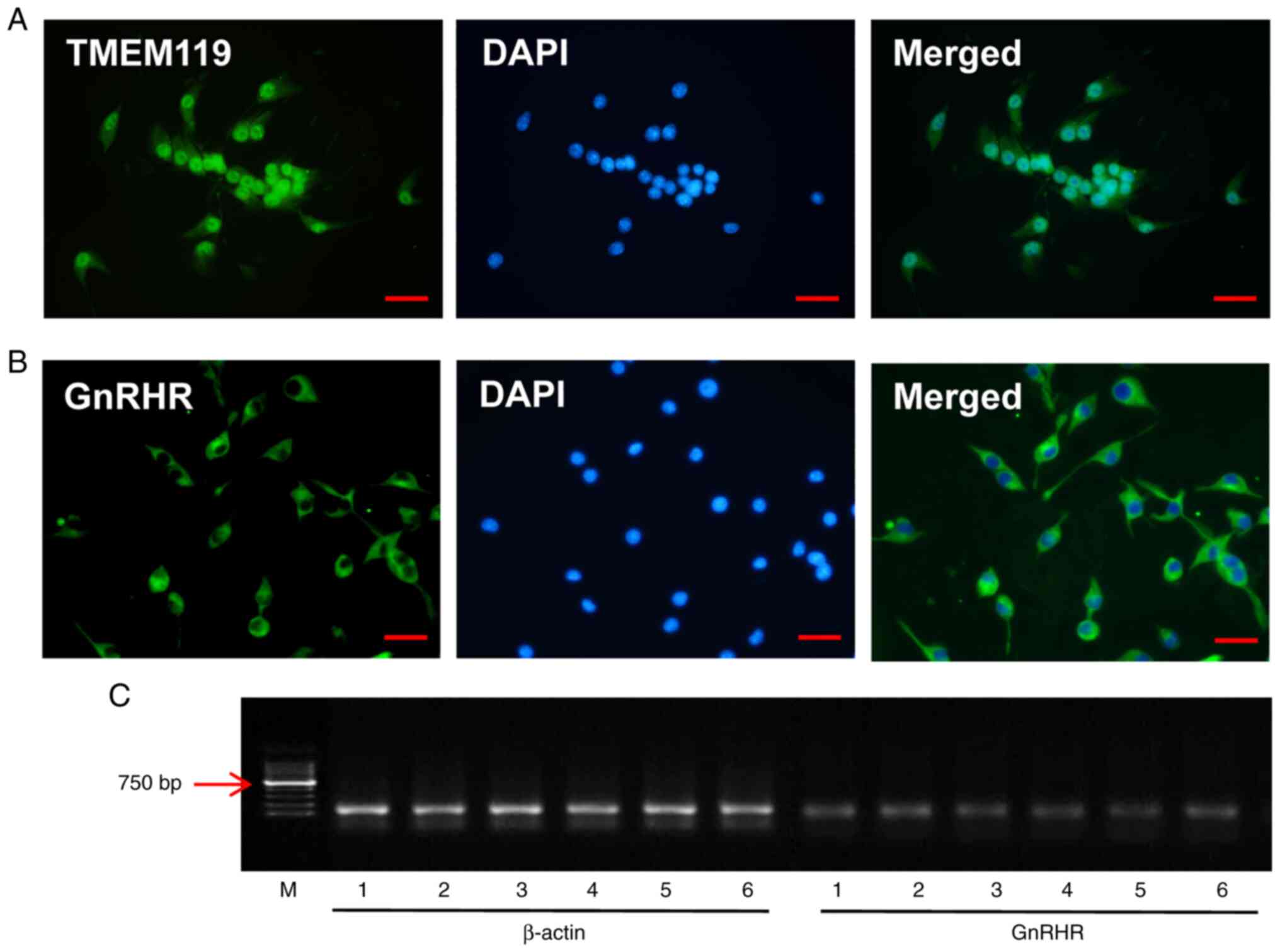
GnRHR expression in rat microglia
In vitro, rat microglia were identified using
the marker of microglia, TMEM119 (Fig.
1A). The expression of GnRHR in microglia was demonstrated
using immunocytochemistry (Fig.
1B) and reverse transcription PCR (Fig. 1C), which indicated the expression
of GnRHR in rat microglia.
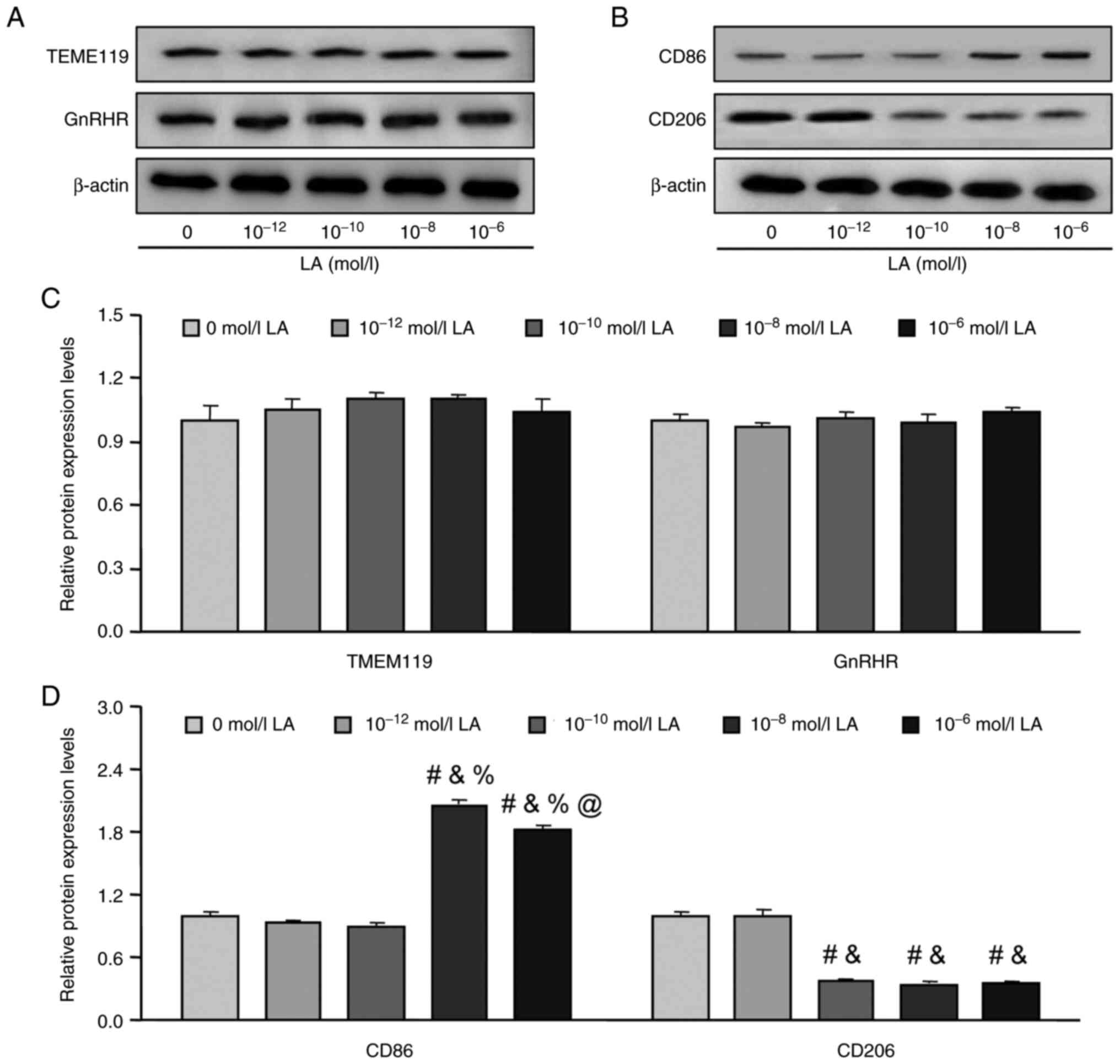
Effects of GnRH agonist LA on the
polarization of rat microglia
To the best of our knowledge there are no previous
reports of the effect of GnRH agonist LA on rat hypothalamic
microglia. Furthermore, due to the different concentrations of
certain factors, LA may have different effects in different
microglial polarization states (17); therefore, different concentrations
of LA were used to treat the rat primary cultured microglia to
evaluate the effects of GnRH on hypothalamic microglia. The results
demonstrated that there were no significant differences in the
protein expression levels of TMEM119 and GnRHR among all 5 groups
following 6 h of treatment (Fig. 2A
and C). The markers of microglia polarization were assessed and
a significant increase in CD86 protein expression levels was
demonstrated in the 10−8 and 10−6 mol/l
groups compared with the 0, 10−12, 10−10
mol/l groups (P<0.05, Fig. 2B and
D) and a significant decrease in CD206 expression was
identified in the 10−10, 10−8 and
10−6 mol/l groups compared with the control (0 mol/l)
group (P<0.05, Fig. 2B and D)
following 6 h of treatment. Moreover, no significant differences in
the protein expression levels of TMEM119 and GnRHR were
demonstrated among groups following 12 (Fig. S1A and C) and 24 (Fig. S2A and C) h of treatment, which was
consistent with the results shown by each group when treated for 6
h. Further analysis also demonstrated that the CD86 and CD206
protein expression level changes among the groups when treated for
12 h (Fig. S1B and D) and 24 h
treatment (Fig. S2B and D) were
similar to those demonstrated following treatment for 6 h.
Effects of aerobic exercise on ovarian
histology and endocrine hormone levels in PCOS rats
A PCOS rat model was diagnosed based on the
Rotterdam diagnostic criteria (1).
The ovarian histology results demonstrated a normal structure
follicle with multi-layered (mostly 8–9 layers) granulosa cells, a
normal sized follicular antrum and oocyte-corona cumulus complex in
the CQ, CE and PE groups (Fig.
3A), the PQ group presented cystic follicles with fewer layers
(mostly 2–4 layers) of granulosa cells, a bigger follicular antrum
and a lack of oocyte-corona cumulus complex in the ovaries
(Fig. 3A). Further analysis
demonstrated that, compared with the CQ group, the levels of serum
T (P<0.05, Fig. 3B), LH
(P<0.05, Fig. 3C) and GnRH
(P<0.05, Fig. 3E) were
significantly higher in the PQ group, while the level of FSH
(P<0.05, Fig. 3D) was
significantly lower. Moreover aerobic exercise significantly
decreased the levels of serum T, LH and GnRH, and significantly
increased the serum FSH level in the PE group compared with PCOS
rats without the aerobic exercise intervention (PQ, P<0.05;
Fig. 3).
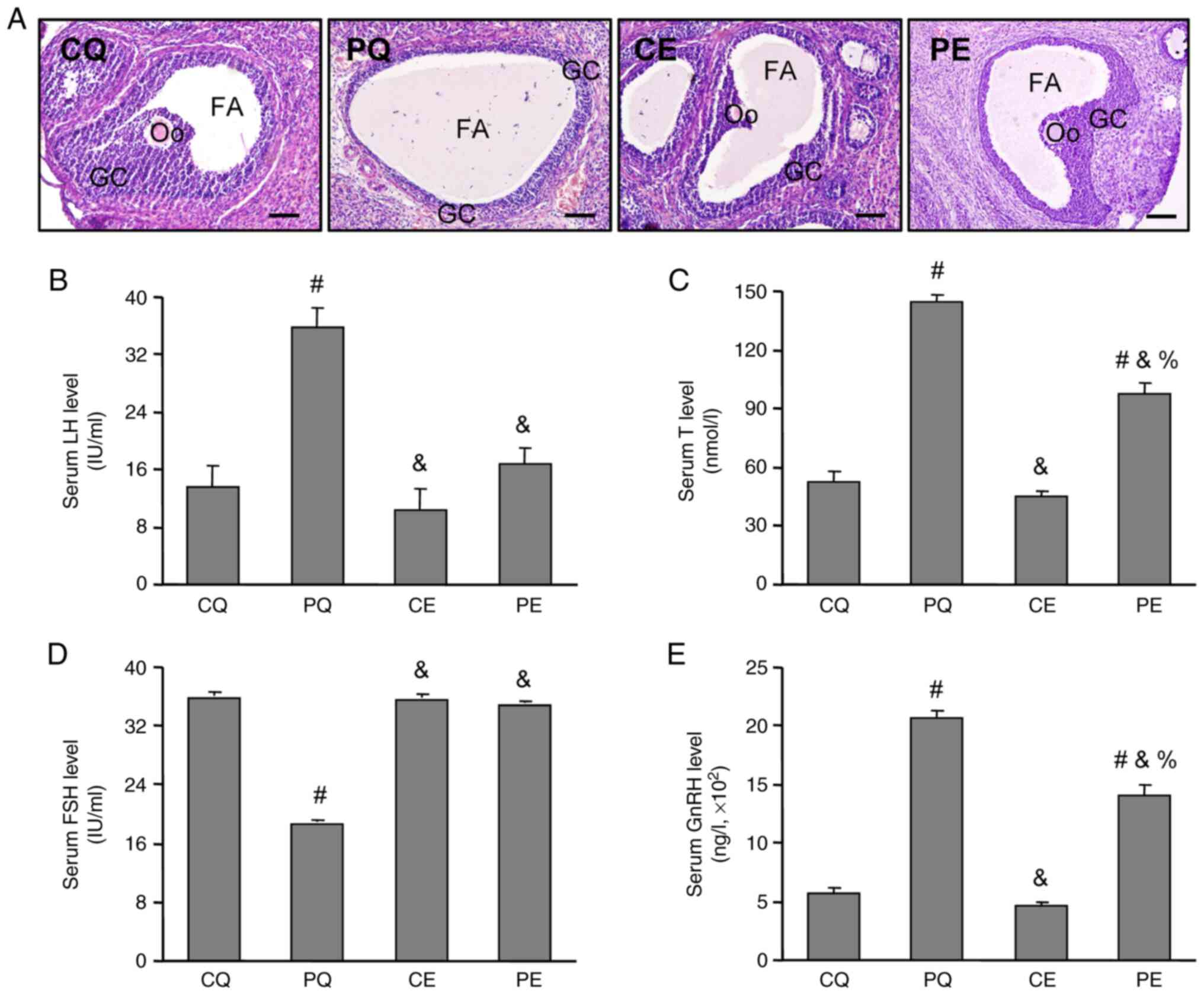 | Figure 3.Effects of aerobic exercise on
endocrine hormone levels and the ovarian histology of PCOS rats.
(A) Effects of aerobic exercise on ovarian histology in PCOS rats.
The ovarian sections were stained using hematoxylin and eosin and
imaged using a light microscope. Effects of aerobic exercise on
serum (B) LH, (C) T, (D) FSH and (E) GnRH levels in PCOS rats. The
levels of serum hormones were examined using ELISA kits. Data are
presented as the mean ± SE. Scale bar=100 µm. #P<0.05
vs. CQ, &P<0.05 vs. PQ, %P<0.05 vs.
CE. PCOS, polycystic ovarian syndrome; GC, granulosa cell; Oo,
oocyte; FA, follicular autrum; CMC, carboxymethyl cellulose; CQ,
CMC + quiet; CE, CMC + exercise; PQ, PCOS + quiet; PE, PCOS +
exercise; LH, luteinizing hormone; T, testosterone; FSH, follicle
stimulating hormone; GnRH, gonadotropin-releasing hormone. |
Effects of aerobic exercise on the
hypothalamic inflammation of PCOS rats
As the level of serum C reactive protein (CRP) is
one of the most important properties of chronic low-grade
inflammation (1,5), the present study assessed CRP protein
expression levels and demonstrated it was significantly increased
in the hypothalamic tissue of PQ rats compared with the CQ group
(P<0.05; Fig. 4A and C).
Furthermore, it was significantly decreased following aerobic
exercise (PE) compared with the PQ group (P<0.05; Fig. 4A and C). Moreover, the protein
expression levels of Iba1, a marker of microglia activity, was also
assessed, which demonstrated that the changes in Iba1 protein
expression levels were similar to the changes in the protein
expression levels of CRP (Fig. 4A and
C), which indicated that microglia activity was increased on
the hypothalamic inflammation of PCOS rats.
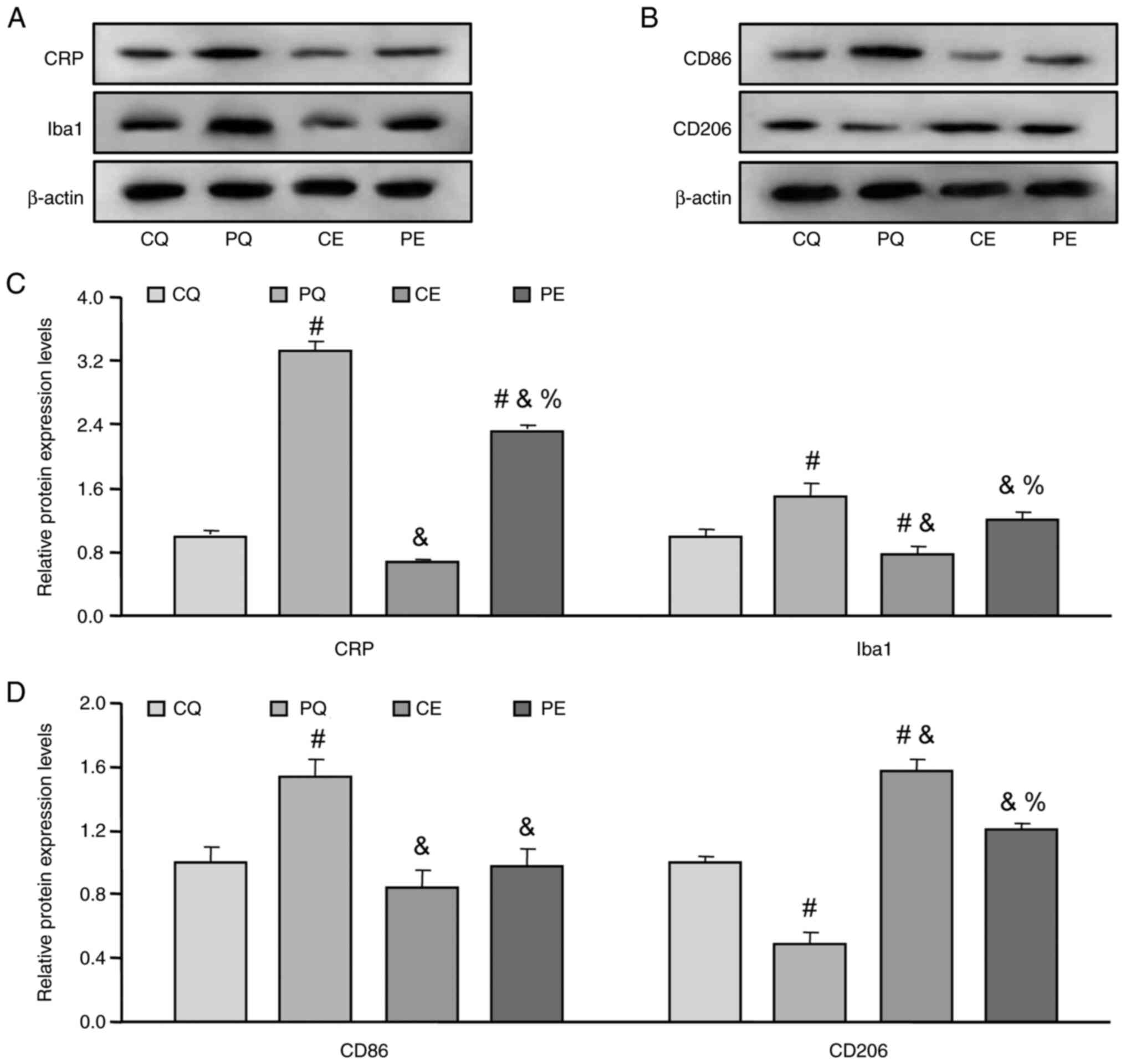 | Figure 4.Effects of aerobic exercise on the
hypothalamic inflammation and the microglial polarization of PCOS
rats. Representative western blots of the protein expression levels
of (A) CRP and Iba1 and (B) CD86 and CD206. Protein expression
levels of (C) CRP and Iba1 and (D) CD86 and CD206 blots normalized
to CQ. Data are presented as the mean ± SE. #P<0.05
vs. CQ, &P<0.05 vs. PQ, %P<0.05 vs.
CE. CRP, C reactive protein; PCOS, polycystic ovarian syndrome; CQ,
CMC + quiet; CE, CMC + exercise; PQ, PCOS + quiet; PE, PCOS +
exercise; CRP, C reactive protein; Iba1, ionized calcium binding
adaptor molecule 1. |
Effects of aerobic exercise on the
microglia polarization of PCOS rats
To further evaluate the involvement of
microglia-mediated inflammation in the hypothalamic inflammation of
PCOS rats, the polarization of hypothalamic microglia was also
assessed. The present study demonstrated that CD86 protein
expression levels, as a marker of M1 polarized microglia, was
significantly increased in the hypothalamic tissue of PQ rats
compared with the CQ group (P<0.05; Fig. 4B and D), and then significantly
decreased following aerobic exercise (PE) compared with the PQ
group (P<0.05; Fig. 4A and C).
Furthermore, the protein expression levels of CD206, a marker of M2
polarized microglia, were also examined, which demonstrated that
CD206 expression was significantly decreased in the hypothalamic
tissue of PQ rats compared with the CQ) group (P<0.05; Fig. 4B and D) and that CD206 protein
expression levels were significantly increased following aerobic
exercise (PE) compared with the PQ group (P<0.05; Fig. 4B and D). These results demonstrated
that microglia polarization was involved in the hypothalamic
inflammation of PCOS rats, while aerobic exercise may promote
microglia to transform from M1 to M2 polarization.
Discussion
To the best of our knowledge, the present study is
the first to have demonstrated the expression of GnRHR in
hypothalamus microglia and the effect of GnRH signaling on
microglia polarization; it also demonstrated that aerobic exercise
improved hypothalamic inflammation by promoting the transformation
of microglia from M1 to M2 polarization in PCOS rats.
Due to the heterogeneous clinical characteristics of
PCOS, its exact etiology is still to be determined; however, the
dysfunction of the HPG axis is an important component of the
pathophysiology of PCOS (3,4). In
PCOS, GnRH pulse frequency is increased to 50–60 min per pulse,
which is similar to the menopausal pulse frequency (3). This pattern of GnRH secretion results
in the synthesis and secretion of predominantly LH rather than FSH,
which causes further excess androgen secretion (3). Furthermore, aerobic exercise,
especially moderate intensity exercise, has been reported to be
effective in improving the symptoms of PCOS, including endocrine
dysfunction and polycystic ovaries (18,19).
Early intervention with aerobic exercise can reduce androgen levels
in PCOS rats, promoting ovulation and preventing the development of
PCOS (17), which is consistent
with the results of the present study. In the present study, it was
demonstrated that aerobic exercise could significantly reduce serum
T, LH and GnRH levels, and significantly increase the serum FSH
level in PCOS rats.
Previous studies have reported that hypothalamic
inflammation is involved in the pathophysiology of PCOS (2,5,20).
Microglia-mediated inflammatory reaction serves an important role
in the hypothalamic inflammation (8). Moreover, a recent study focused on
the role of microglia in PCOS-like brains, reported that microglia
may serve an important role in driving the abnormal neuronal wiring
that leads to PCOS-like features in prenatally androgenized mice
(21). Furthermore, a main
characteristic of endocrine dysfunction in the PCOS hypothalamus is
the increased pulsatile secretion of GnRH (2,3). Gao
et al (22) reported that
certain hormones, such as leptin, affected the presence and
activity levels of hypothalamic microglia in obesity. Therefore,
whether the GnRH/GnRHR system affects microglia activation and/or
polarization was evaluated. In vitro experiments
demonstrated the expression of GnRHR in hypothalamic microglia. LA
as a GnRH agonist can bind to GnRHR to activate a series of
signaling pathways (4). In the
present study, LA did not affect the expression of GnRHR and
TMEM119 in rat microglia, which suggested that GnRH was not
involved in the regulation of microglia proliferation.
CD86 and CD206 are the markers of M1 and M2
polarized microglia, respectively (23). In the present study, low protein
expression levels of CD86 and high protein expression levels of
CD206 were demonstrated in microglia treated with 0 and
10−12 mol/l LA, which suggested that low LA
concentration may contribute to protective M2 microglia
polarization. However, the protein expression levels of CD86 were
significantly higher under 10−8 and 10−6
mol/l LA, which indicated that the high LA concentration may cause
pro-inflammatory M1 polarization. In the 10−10 mol/l LA
group, the protein expression level of CD86 was markedly lower
compared with the 10−8 and 10−6 mol/l LA
groups, whereas the protein expression level of CD206 in the
10−10 mol/l LA group was not significantly different
compared with the 0 and 10−12 mol/l LA groups. These
results suggested that 10−10 mol/l LA may contribute to
the restoration of the resting state of microglia. These results
indicated that the high concentration of LA may contribute to M1
polarization; it was therefore hypothesized that the increased
pulsatile release of GnRH may promote M1 polarization to induce
hypothalamic inflammation in PCOS.
Studies on local inflammation in PCOS have mainly
focused on the surrounding tissues, such as serum, adipose tissue
and ovarian tissue, with only a few reports on hypothalamic tissue.
Lian et al (5) reported a
chronic low-grade inflammatory state in the hypothalamus of PCOS
rats, but the reasons remain unexplained. A recent study reported
that diet-induced hypothalamic inflammation may contribute to the
endocrine pathogenesis of PCOS, and modulation of GnRH secretion
could be a potential target of hypothalamic inflammation (24). Based on the present in vitro
results, a letrozole-induced PCOS rat model was used and an aerobic
exercise intervention that can effectively reduce GnRH levels was
used to explore whether the increased GnRH secretion affected the
hypothalamic microenvironment of PCOS. The results demonstrated
that hypothalamic inflammation and overactivated microglia existed
in PCOS rats, and that aerobic exercise could markedly decrease the
degree of inflammation and the number of activated microglia, which
suggested that aerobic exercise may improve microglia-mediated
inflammation in PCOS, possibly through the reduction of excessive
GnRH secretion.
Furthermore, the present results demonstrated that
the amount of M1 polarized microglia was increased and the amount
of M2 polarized microglia was decreased in PCOS rats, and that
aerobic exercise could reverse this phenomenon. This suggested that
aerobic exercise could reduce overactivated M1 polarized microglia
to release a mass of pro-inflammatory factors, in turn, serving a
protective role by promoting M2 polarized transformation through
inhibition of the effects of GnRH on microglia polarization. Lee
et al (23) reported that
excessive pro-inflammatory factors released by M1 polarized
microglia may activate inflammatory signaling in adjacent neurons,
further leading to hypothalamic dysfunctions. Clinical studies have
also reported that hyperactivated M1 polarized microglia can cause
neuronal incapacitation, damage and degeneration (25). To the best of our knowledge, there
are no studies on the effect of aerobic exercise on microglia
polarization, but in adipose tissue, aerobic exercise inhibits
inflammation through the acceleration of phenotypic switching from
M1 to M2 macrophages in high-fat-diet-induced obese mice (26). A limitation of the present study
was that the molecular mechanism of the GnRH signaling pathway was
not elucidated using transgenic mice.
In conclusion, the present study demonstrated the
expression of GnRHR in rat hypothalamic microglia, and that high
concentrations of GnRH agonist LA may promote microglia to M1
polarization in vitro, which suggested that GnRH signaling
may be involved in the polarization of hypothalamic microglia.
Moreover, the results of the in vivo experiments
demonstrated that aerobic exercise not only decreased the amount of
overactivated microglia, but also promoted the switch from M1 to M2
polarization by reducing the increased secretion of GnRH to
alleviate hypothalamic inflammation in PCOS rats. These findings
provided a new direction for the evaluation of the pathophysiology
and therapeutic mechanisms of PCOS.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
This research was funded by the Key Projects of Scientific and
Technological Innovation in Fujian Province (grant nos. 2021G02003,
2022G023 and 2022G028), Special Funds of the Central Government
Guiding Local Science and Technology Development (grant no.
2020L3008), Fujian Provincial Natural Science Foundation (grant
nos. 2019R1011-3, 2020J01176 and 2022J01172), Scientific Research
Talent Project of Fujian Provincial Health Commission (grant no.
2018-ZQN-20), and the Innovation and Entrepreneurship Project of
Fujian Normal University (grant nos. I202003009 and
I202102008).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
FW, ZZ and ZW conceived and designed the study. FW,
ZZ, JH, JZ, XW and ZW performed the experiments. FW, ZZ, JH, JZ and
XW analyzed the data. FW and ZZ drafted the first version of the
manuscript. ZW critically revised the manuscript. FW and ZW confirm
the authenticity of all the raw data. All authors read and approved
the final version of the manuscript.
Ethics approval and consent to
participate
The animal study protocol was approved by the Ethics
Committee of Fujian Normal University (Fuzhou, China; approval no.
IACUC-20180011).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wang F, Wang SB, Zhang ZH, Lin Q, Liu Y,
Xiao Y, Xiao K and Wang ZC: Activation of NLRP3 inflammasome in the
ovaries during the development and treatment of polycystic ovary
syndrome. Int J Clin Exp Patho. 10:5022–5030. 2017.
|
|
2
|
da Costa CS, Oliveira TF, Freitas-Lima LC,
Padilha AS, Krause M, Carneiro MTWD, Salgado BS and Graceli JB:
Subacute cadmium exposure disrupts the
hypothalamic-pituitary-gonadal axis, leading to polycystic ovarian
syndrome and premature ovarian failure features in female rats.
Environ Pollut. 269:1161542021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang F, Zhang ZH, Xiao KZ and Wang ZC:
Roles of hypothalamic-pituitary-adrenal axis and
hypothalamus-pituitary-ovary axis in the abnormal endocrine
functions in patients with polycystic ovary syndrome. Zhongguo Yi
Xue Ke Xue Yuan Xue Bao. 39:699–704. 2017.PubMed/NCBI
|
|
4
|
Barlampa D, Bompoula MS, Bargiota A,
Kalantaridou S, Mastorakos G and Valsamakis G: Hypothalamic
inflammation as a potential pathophysiologic basis for the
heterogeneity of clinical, hormonal, and metabolic presentation in
PCOS. Nutrients. 13:5202021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lian Y, Zhao F and Wang W: Central leptin
resistance and hypothalamic inflammation are involved in
letrozole-induced polycystic ovary syndrome rats. Biochem Biophys
Res Commun. 476:306–312. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ávalos Y, Kerr B, Maliqueo M and Dorfman
M: Cell and molecular mechanisms behind diet-induced hypothalamic
inflammation and obesity. J Neuroendocrino. 30:e125982018.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
André C, Guzman-Quevedo O, Rey C,
Rémus-Borel J, Clark S, Castellanos-Jankiewicz A, Ladeveze E,
Leste-Lasserre T, Nadjar A, Abrous DN, et al: Inhibiting microglia
expansion prevents diet-induced hypothalamic and peripheral
inflammation. Diabetes. 66:908–919. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tang Y, Purkayastha S and Cai D:
Hypothalamic microinflammation: A common basis of metabolic
syndrome and aging. Trends Neurosci. 38:36–44. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Deczkowska A, Amit I and Schwartz M:
Microglial immune checkpoint mechanisms. Nat Neurosci. 21:779–786.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kite C, Lahart IM, Afzal I, Broom DR,
Randeva H, Kyrou I and Brown JE: Exercise, or exercise and diet for
the management of polycystic ovary syndrome: A systematic review
and meta-analysis. Syst Rev. 8:512019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim CH, Chon SJ and Lee SH: Effects of
lifestyle modification in polycystic ovary syndrome compared to
metformin only or metformin addition: A systematic review and
meta-analysis. Sci Rep. 10:78022020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lim SS, Hutchison SK, Van Ryswyk E, Norman
RJ, Teede HJ and Moran LJ: Lifestyle changes in women with
polycystic ovary syndrome. Cochrane Database Syst Rev.
3:CD0075062019.PubMed/NCBI
|
|
13
|
Park SY, Jeon YK and Choi J: Effects of
calorie restriction and aerobic exercise on hypothalamic
inflammation factors, appetite suppression neuropeptide and daily
feed intake in obese mouse. Korean J Sports Sci. 26:935–945. 2017.
View Article : Google Scholar
|
|
14
|
Dorfman MD, Krull JE, Douglass JD,
Fasnacht R, Lara-Lince F, Meek TH, Shi X, Damian V, Nguyen HT,
Matsen ME, et al: Sex differences in microglial CX3CR1 signalling
determine obesity susceptibility in mice. Nat Commun. 8:145562017.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Maslyukov PM, Emanuilov AI and Korzina MB:
Development of neurotransmitter specificity in sympathetic
ganglionic neurons. Auton Neurosci. 135:66–67. 2007. View Article : Google Scholar
|
|
16
|
Kafali H, Iriadam M, Ozardali I and Demir
N: Letrozole-induced polycystic ovaries in the rat: A new model for
cystic ovarian disease. Arch Med Res. 35:103–108. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jie W, Xiang JY, Wang Y, Hui-Min L, Zhou
S, Hou JP and Wang B: Effects of baicalin and geniposide on
polarization of BV2 microglia. Chin J Pharmacol Toxicol.
33:911–912. 2019.
|
|
18
|
Brunk D: Moderate exercise helps insulin
sensitivity in PCOS. Fam Prac. 35:22. 2005.
|
|
19
|
Cao SF, Hu WL, Wu MM and Jiang LY: Effects
of exercise intervention on preventing letrozole-exposed rats from
polycystic ovary syndrome. Reprod Sci. 24:456–462. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nehir Aytan A, Bastu E, Demiral I, Bulut
H, Dogan M and Buyru F: Relationship between hyperandrogenism,
obesity, inflammation and polycystic ovary syndrome. Gynecol
Endocrinol. 32:709–713. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sati A, Prescott M, Holland S, Jasoni CL,
Desroziers E and Campbell RE: Morphological evidence indicates a
role for microglia in shaping the PCOS-like brain. J
Neuroendocrinol. 33:e129992021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gao Y, Ottaway N, Schriever SC, Legutko B,
García-Cáceres C, de la Fuente E, Mergen C, Bour S, Thaler JP,
Seeley RJ, et al: Hormones and diet, but not body weight, control
hypothalamic microglial activity. Glia. 62:17–25. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee CH, Suk K, Yu R and Kim MS: Cellular
contributors to hypothalamic inflammation in obesity. Mol Cells.
43:431–437. 2020.PubMed/NCBI
|
|
24
|
Zeng F, Wu Y, Li X, Ge X, Guo Q, Lou X,
Cao Z, Hu B, Long NJ, Mao Y and Li C: Custom-made ceria
nanoparticles show a neuroprotective effect by modulating
phenotypic polarization of the microglia. Angew Chem Int Ed Eng.
l57:5805–5812. 2018.
|
|
25
|
Zhao YN, Wang F, Fan YX, Ping GF, Yang JY
and Wu CF: Activated microglia are implicated in cognitive
deficits, neuronal death, and successful recovery following
intermittent ethanol exposure. Behav Brain Res. 236:270–282. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kawanishi N, Yano H, Yokogawa Y and Suzuki
K: Exercise training inhibits inflammation in adipose tissue via
both suppression of macrophage infiltration and acceleration of
phenotypic switching from M1 to M2 macrophages in high-fat-diet
induced obese mice. Exerc Immunol Rev. 16:105–118. 2010.PubMed/NCBI
|