Introduction
Endotracheal intubation during general anaesthesia
may cause haemodynamic changes, which can be life threatening in
elderly patients with diseases (1). Throughout the operation,
epipharyngeal and parapharyngeal areas can be stimulated, leading
to sympathoadrenal incitement and, consequently, significant rises
in serum levels of catecholamine, blood pressure (BP) and heart
rate. These increases may result in myocardial infarctions and
arrhythmias in patients (2,3). The
therapeutic effects of medications on attenuating haemodynamic
reactions during intubation of laryngoscopy and avoiding a
significant increase in BP have been studied (4–6).
Electroacupuncture (EA) is based on traditional
acupuncture and involves placing needles onto acupoints to generate
stimulation as well as nervous system actions in the human body.
Acupoints from the ‘governor vessel’ are used as a remedy method by
acupuncture in human beings and in animals following the onset of
spinal cord injury (SCI) (7,8). EA
at the Zusanli point (ST-36) and Neiting point (ST-44) boosts
microcirculation and cerebral integrity in mature rats (9).
MicroRNAs (miRNAs/miRs) are a category of noncoding
RNAs that control posttranscriptional gene expression by repressing
or enhancing RNA degradation. miRNAs are crucial regulators of
different biological and behavioral procedures, such as cell
regeneration, apoptosis, differentiation and carcinogenesis
(10). Evidence indicates that
~30% of individual genes are modulated by miRNAs (11). A previous analysis detected miRNA
levels after EA therapy and found that the expression of a group of
miRNAs, including miR-214, was changed following the EA procedure;
hence, miR-214 was further investigated by predicting the putative
binding sites of miR-214 (12).
The endogenous generation of nitric oxide (NO),
especially in cardiovascular disease, is mainly related to binding
to its receptor, endothelial NO synthase (eNOS). Lower NO
generation enhances cardiovascular ailments, which are responsible
for a number of fatalities throughout the world (13). Under physiological states, eNOS is
responsible for the generation of the majority of
endothelium-derived NO. eNOS serves a critical role in
cardiovascular homeostasis (14,15).
Inhibition of eNOS causes important alterations as demonstrated by
increased blood pressure in eNOS knockout mice (15). These findings suggest a protective
role for eNOS against heart failure. In bronchial cells, the
localization of both Arg1 and Arg2 compared with eNOS is not
important compared with the severity of L-arginine depletion and
inhibition of NO generation (16).
The role of LPS-induced NOS3 has been verified in mouse bone
marrow-derived macrophages in vitro and in vivo
(17). The involvement of Fc
receptors triggers NOS-1 (along with eNOS) activation with minimal
NO generation, which suppresses autocrine and paracrine
phagocytosis (18).
EA may exert an inhibitory effect on the expression
of miR-155, miR-335 and miR-383 and eNOS may be a shared target
gene of these three miRNAs (19–21).
Furthermore, previous reports have shown that acupuncture may have
a vasodilative effect during intubation for general anaesthesia
(22). The present study enrolled
subjects who received intubation for general anaesthesia and
treated them with EA to study its effect on the expression of
miR-155, miR-335, miR-383 and NO/eNOS.
Materials and methods
Human subject sample collection
This was a prospective study conducted between
January 2019 and August 2020. Patients who had received elective
abdominal operations with a physical status of class 1 or 2 based
on the American Society of Anesthesiologists physical status
classification system were enrolled in this study at the First
Affiliated Hospital of Jinan University. The exclusion criteria
were as follows: Coagulation ailments; present or past histories of
drug misuse; alcoholism; and childbirth and pregnancy issues.
All subjects were randomly divided into two groups.
The patients in the control group (n=36) received EA therapy at 1
cm below the acupoints and the patients in the treatment group
(n=36) received EA at the exact acupoints. The acupoints were
located as the Taichong (LR3) site at the distal end of the first
metatarsal space on the dorsum of the foot and the Hegu (LI4) site
at the radial side of the middle of the second metacarpal bone of
the hand. To avoid over-stimulation, first the Hegu site and then
the Taichong site were stimulated instead of stimulating them at
the same time. The time lapse between the stimulation of the Hegu
site and Taichong site was 15 min per session. The acupoints of
both groups were ~6.4-12.8 mm deep and the acupuncture apparatus
was an Acuhealth Guru 900 (Eastern Currents, Ltd.). For the
acupuncture treatment, the acupuncture needles were inserted either
at 1 cm below the acupuncture point or at the exact acupuncture
point. Following needling, fentanyl (0.001 mg/kg) or midazolam
(0.04 mg/kg) was administered orally to reduce pain. After
acupuncture, an anesthesiologist intubated the individuals
receiving the treatment and a monitoring device was inserted
immediately to measure the indices for 5 min. The demographics and
features of all subjects were collected and compared between the
two groups. In addition, the systolic blood pressure (SBP),
diastolic blood pressure (DBP), mean arterial pressure (MAP) and
heart rate (HR) of patients in the two groups were measured before
and after needling and the collected data was presented in Table I. Furthermore, peripheral blood
monocytes (PBMCs) and peripheral blood samples were collected from
all patients for later analyses. The Ethics Committee of the First
Affiliated Hospital of Jinan University approved the protocol of
the present study (approval no. JNDX007546228FSY). Written informed
consent was obtained from all participants before the initiation of
the present study.
 | Table I.Demographic and clinicopathological
features of subjects. |
Table I.
Demographic and clinicopathological
features of subjects.
|
Characteristics | Control group
(n=36) | Treatment group
(n=36) | P-value |
|---|
| Age, years | 47.2±6.8 | 5.8±5.6 | 0.428 |
| Sex |
|
| 0.407 |
| Male
(%) | 25 (69.4) | 28 (77.8) |
|
| Female
(%) | 11 (30.6) | 8 (22.2) |
|
RNA isolation and reverse
transcription-quantitative (RT-q) PCR
Complete RNA was isolated from the human and cell
samples (1×105 cells) with TRIzol® (Thermo
Fisher Scientific, Inc.) according to the manufacturer's
guidelines. RNA concentration and purity were tested by UV
spectrophotometer. RNA (300 µl) extracted from each sample was
precipitated with ethanol, washed, resuspended and converted to
cDNA using reverse transcription following manufacturer's
instructions. cDNA was then subjected to RT-qPCR using the
SYBR-green method (MilliporeSigma) and a 7900HT real-time PCR
cycler (Applied Biosystems; Thermo Fisher Scientific, Inc.). An
annealing temperature of ~62°C for 15 min was used. Subsequently,
the relative expression of miR-155 (Forward:
5′-TGCTAATCGTGATAGGGG-3′; Reverse: 5′-GAACATGTCTGCGTATCTC-3′),
miR-335 (Forward: 5′-AAGAGCAATAACGAAAAATG-3′; Reverse:
5′-GAACATGTCTGCGTATCTC-3′), miR-383 (Forward:
5′-TCAGAAGGTGATTGTGGC-3′; Reverse: 5′-GAACATGTCTGCGTATCTC-3′) and
eNOS mRNA (Forward: 5′-GAAGGCGACAATCCTGTATGGC-3′; Reverse:
5′-TGTTCGAGGGACACCACGTCAT-3′) was determined through the
2−ΔΔCq method (23).
The thermocycling protocol for RT-PCR was 10 min at 95°C, followed
by 40 cycles of 30 sec at 95°C, 30 sec at 60°C and 30 sec at 72°C.
U6 (Forward: 5′-CTCGCTTCGGCAGCACA-3′; Reverse:
5′-AACGCTTCACGAATTTGCGT-3′) and β-actin mRNA (Forward:
5′-CACCATTGGCAATGAGCGGTTC-3′; Reverse:
5′-AGGTCTTTGCGGATGTCCACGT-3′) were used as the internal controls
for miRNAs and mRNA respectively. Each experiment was repeated 3
times.
Cell culture and transfection
THP-1 cells and human umbilical vein endothelial
cells (HUVECs) from ATCC were cultured in Dulbecco's modified
Eagle's medium (Gibco; Thermo Fisher Scientific, Inc.) containing
100 µg/ml streptomycin, 100 U/ml penicillin (Thermo Fisher
Scientific, Inc.) and 10% inactivated fetal bovine serum (Harlan
Sera-Lab, Ltd.). Cells were cultured in 24-well plates at a density
of 5×105 cells/well for the establishment of two
different sets of experiments. In one set, cells were divided into
the following three groups: NC group in which cells were
transfected with 50 nM negative controls (NC;
5′-UUCUCCGAACGUGUCACGUTT-3′ for miR-155 precursors and
5′-CAGUACUUUUGUGUAGUACAA-3′ for anti-miR-155), miR-155 precursors
group in which cells were transfected with miR-155 precursors
(5′-CTGTTAATGCTAATCGTGATAGGGGTTTTTGCCTCCAACTGACTCCTACATATTAGCATTAACAG-3′)
and anti-miR-155 group in which cells were transfected with
anti-miR-155 (5′-ACCCCUAUCACGAUUAGCAUUAA-3′). In the other set,
cells were divided into the following three groups: NC group in
which cells were transfected with NC, miR-383 precursors group in
which cells were transfected with miR-383 precursors and
anti-miR-383 group in which cells were transfected with
anti-miR-383. All cells were transfected with the corresponding
miRNA precursors or antagomirs with TransIT-LT1 (Mirus Bio, LLC)
4°C overnight according to the manufacturer's instructions. The
cells were collected via trypsinization and assayed for the
expression of the corresponding genes of interest 24 h
post-transfection.
Vector construction, mutagenesis and
luciferase assay
The sequence analysis results by bioinformatic tool
TargetScan (Relase 8.0, http://www.targetscan.org/vert_80/) indicated that
eNOS may be a potential target gene shared by miR-155, miR-335 and
miR-383. To further explore the inhibitory role of miR-155, miR-335
and miR-383 in the expression of eNOS, i.e., the regulatory
relationship of miR-155/eNOS, miR-335/eNOS and miR-383/eNOS,
luciferase vectors containing wild-type and mutant eNOS 3′ UTRs
were established. In brief, the eNOS 3′ UTRs containing the binding
sites for miR-155, miR-335 and miR-383 were amplified by PCR and
cloned into pmirGLO dual-luciferase miRNA target expression vectors
(cat. no. E1330; Promega Corporation) downstream of the reporter
gene to generate wild-type eNOS vectors which contained according
binding sites for miR-155, miR-335 and miR-383. In addition,
site-directed mutagenesis was performed using a Stratagene Quick
Change II mutagenesis assay kit (Stratagene; Agilent Sumitomo
Dainippon Pharma Co., Ltd.) following the manufacturer's guidelines
to generate mutant eNOS 3′ UTR sequences which contained according
binding sites for miR-155, miR-335 and miR-383. Finally, both the
mutant and wild-type eNOS vectors were transfected into the THP-1
cells and HUVECs with along with the mimics and antagomirs of
miR-155, miR-335 and miR-383 using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's guidelines. At 24 h after transfection, a Bright Glo
luciferase assay kit (Promega Corporation) was used to analyze the
luciferase activity of the transfected cells which were normalized
to Renilla luciferase activity following the manufacturer's
guidelines.
Western blot analysis
Western blots were used in conjunction with an
Odyssey Infrared Imaging system (LI-COR Biosciences) using
conventional western blotting procedures. In brief, collected PBMCs
and cultured cells were subjected to RIPA lysis treatment to
collect the proteins, the concentrations of which were determined
using a BCA assay kit (Bio-Rad Laboratories, Inc.) following the
manufacture's guidelines. Protein samples (50 µg) were then
resolved by 12% SDS-PAGE and blotted onto NC membranes (Thermo
Fisher Scientific, Inc.). Subsequently, the membranes were blocked
with 5% non-fat milk for 60 min at room temperature, followed by
overnight incubation at 4°C with primary anti-eNOS antibodies
(1:1,000; cat. no. ab76198; Abcam). GAPDH (1:1,000; cat. no.
ab8245; Abcam) was used as the loading control. Furthermore, the
membranes were incubated with anti-rabbit IgG secondary antibodies
conjugated to horseradish peroxidase (HRP) (1:1,000; cat. no. 7074;
Cell Signaling Technology, Inc.) for 2 h at room temperature and
then developed using an ECL reagent (Thermo Fisher Scientific,
Inc.). The relative protein expression of eNOS was calculated using
Quantity One software (v4.6.7; Bio-Rad Laboratories, Inc.).
ELISA
The supernatants of peripheral blood samples
collected from different patient groups were retrieved by
centrifugation (250 × g) at 4°C for 5 min, and the content of NO in
each sample was assayed by a sandwich ELISA kit (cat. no. ab233628;
Abcam) following the manufacturer's protocol.
Statistical analysis
All statistical analyses were conducted with SPSS
16.0 statistical computer software (SPSS, Inc.). Non-parametric
parameters were compared using the χ2 test. Wilcoxon sum
rank test or Student's t test were used to analyze differences
between two groups, while one-way analysis of variance (ANOVA) in
conjunction with Tukey's post hoc test were used to analyze
differences among multiple groups. Pearson correlations were used
to determine the values of correlation coefficients. All
statistical values are presented as the mean ± standard deviation.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Demographic and clinicopathological
characteristics of the participants recruited in the present
study
The participants of this study were divided into two
groups. One group of patients (n=36) received EA therapy 1 cm below
the acupoints and the other group (n=36) received EA at the exact
acupoints. The average age for the control group was 47.2±6.8 years
old, while the average age for the treatment group was 51.8±5.6
years old. There were 25 male participants in the control group and
28 male participants in the treatment group. There were no obvious
differences in these indicators between the two groups.
EA decreases the SBP, DBP and MAP but
increases the HR of patients
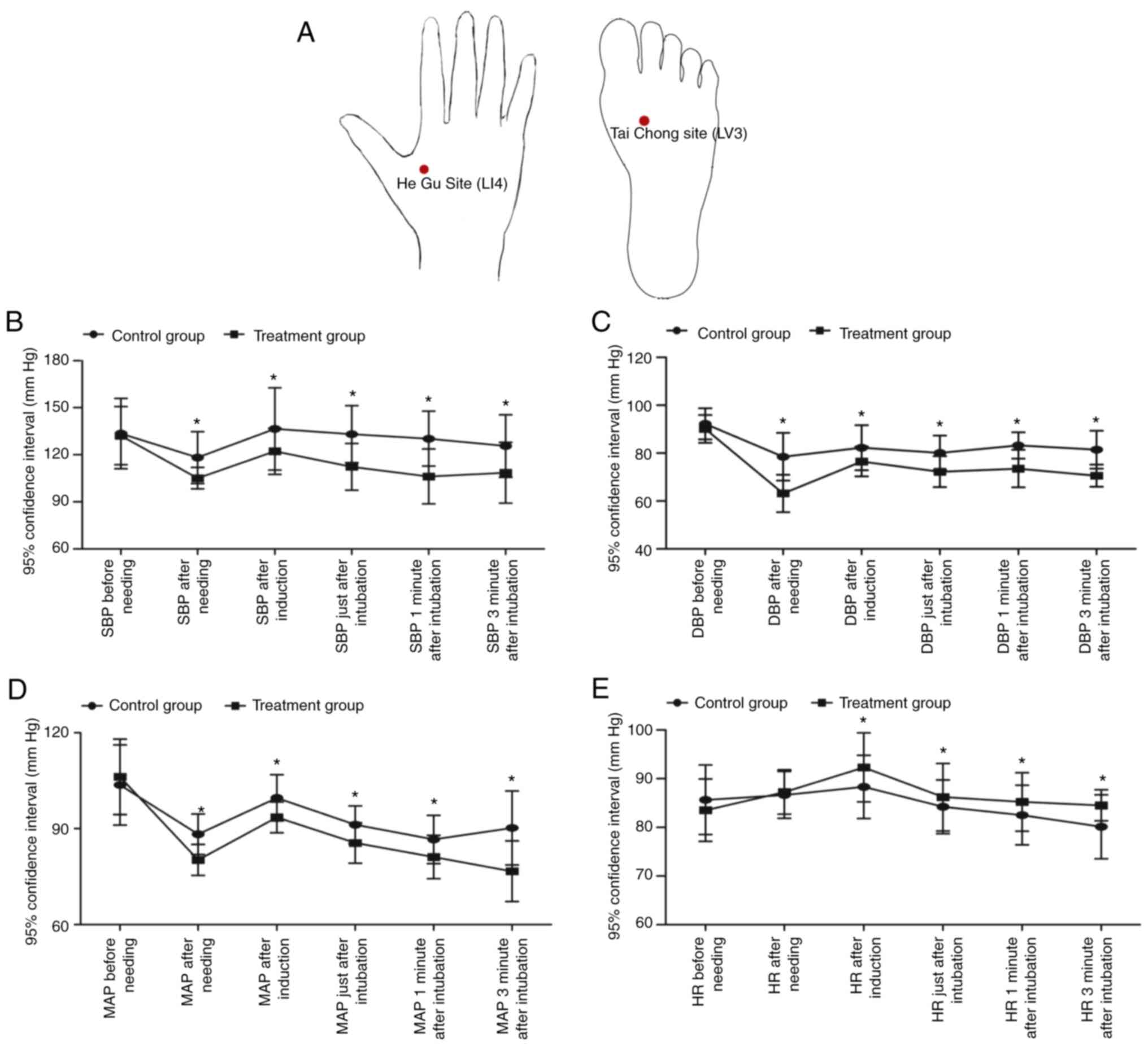
The diagrams which indicated the locations of the
acupuncture points, Taichong site and Hegu site, are shown in
Fig. 1A. The SBP, DBP, MAP and HR
of patients in the above two groups were measured before and after
needling and 3 min of intubation. It was found that the SBP, DBP,
MAP and HR were changed following needling in both groups, whereas
no obvious difference was observed for the SBP, DBP, MAP and HR
before needling. The SBP (Fig.
1B), DBP (Fig. 1C) and MAP
(Fig. 1D) in patients receiving EA
therapy at 1 cm below the acupoints were significantly elevated
compared with those in patients receiving EA at the exact acupoints
following the time-point of needling. However, the HR (Fig. 1E) in patients receiving EA therapy
at 1 cm below the acupoints was markedly reduced compared with that
in patients receiving EA at the exact acupoints following the
time-point of induction.
EA inhibits the expression of miRNAs
and increases the expression of NO in the peripheral blood and
PBMCs of patients
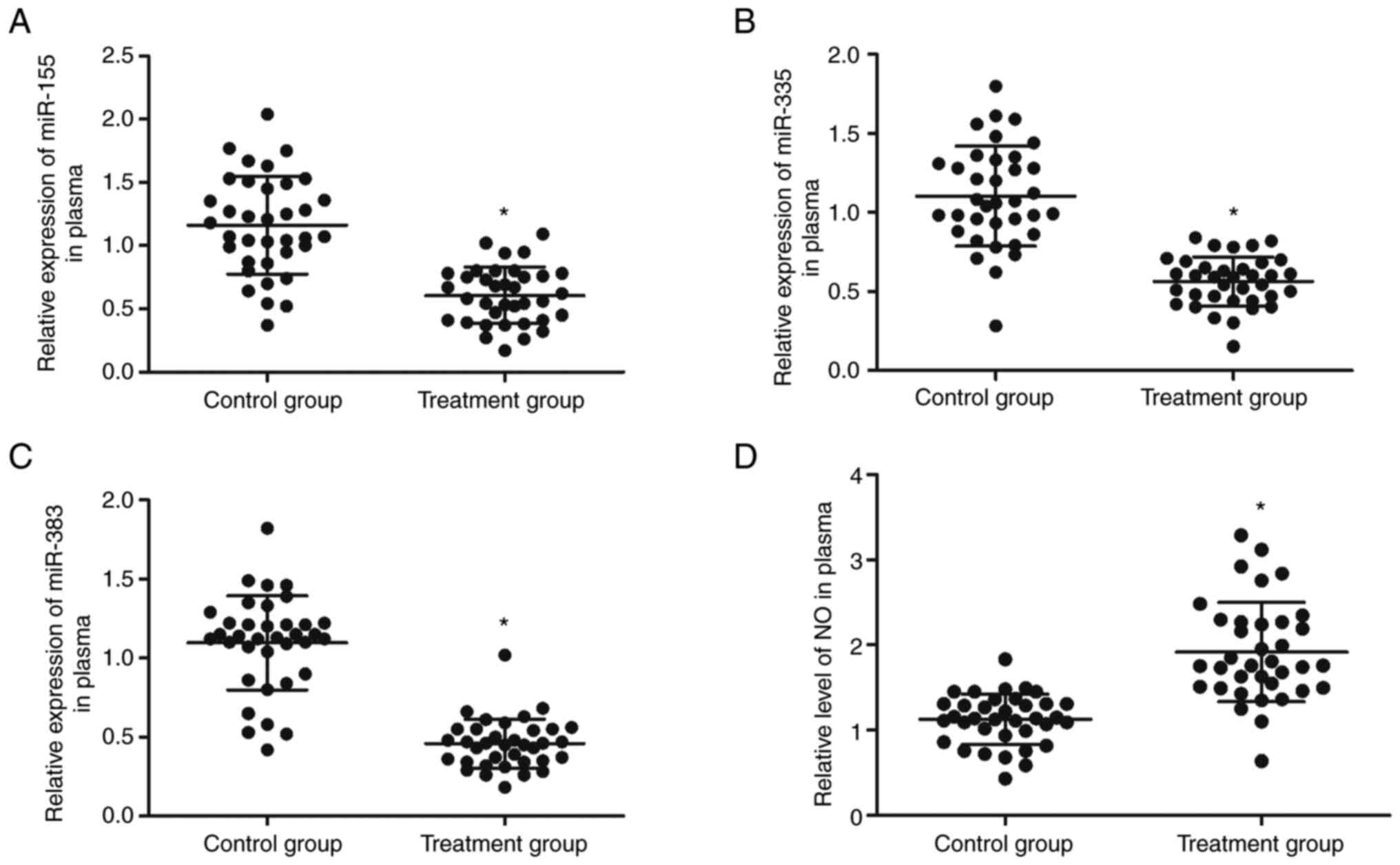
The expression of miR-155, miR-335, miR-383 and NO
was examined in the peripheral blood collected from patients
receiving EA therapy at 1 cm below the acupoints or on the exact
acupoints. The expression of miR-155 (Fig. 2A), miR-335 (Fig. 2B) and miR-383 (Fig. 2C) was significantly inhibited in
peripheral blood samples from patients receiving EA therapy at the
acupoints compared with that in patients receiving EA therapy at 1
cm below the acupoints. In addition, ELISA was performed to
evaluate the production of NO in the peripheral blood of patients
receiving EA therapy at 1 cm below the acupoints or on the exact
acupoints. The production of NO (Fig.
2D) in the peripheral blood of patients receiving EA therapy at
the acupoints was increased compared with those receiving EA
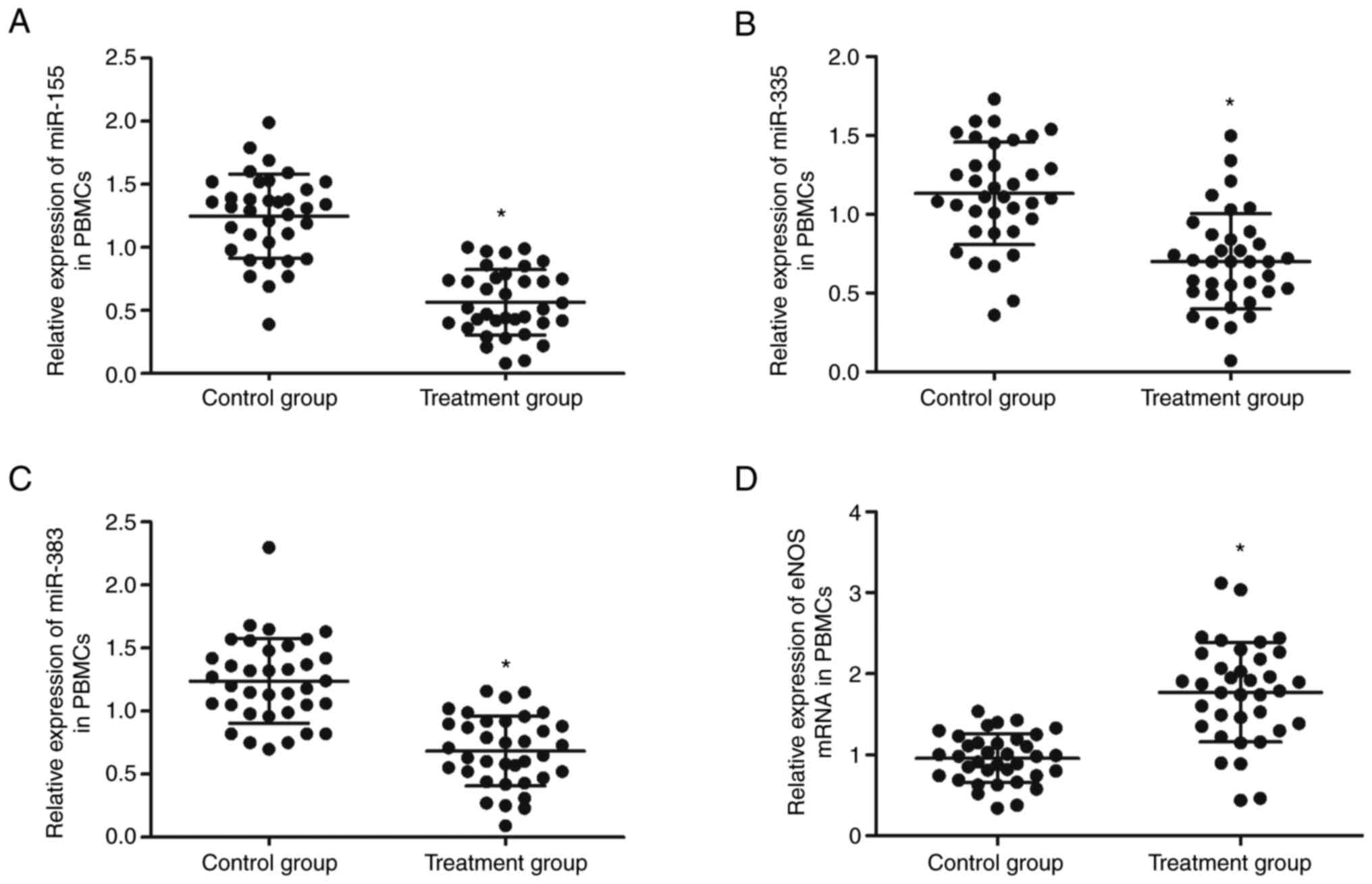
therapy at 1 cm below the acupoints. Furthermore, PBMCs were
isolated from patients receiving EA therapy at 1 cm below the
acupoints or on the exact acupoints and the expression of miR-155,
miR-335, miR-383 and eNOS analyzed using qPCR. The expression of
miR-155 (Fig. 3A), miR-335
(Fig. 3B) and miR-383 (Fig. 3C) was significantly reduced in the
PBMCs from patients receiving EA therapy at the exact acupoints
compared with those receiving EA therapy at 1 cm below the
acupoints, while the expression of eNOS mRNA (Fig. 3D) was significantly enhanced in the
PBMCs of patients receiving EA therapy at the exact acupoints.
miR-155, miR-335 and miR-383 inhibit
the expression of eNOS by binding to the 3′ UTR
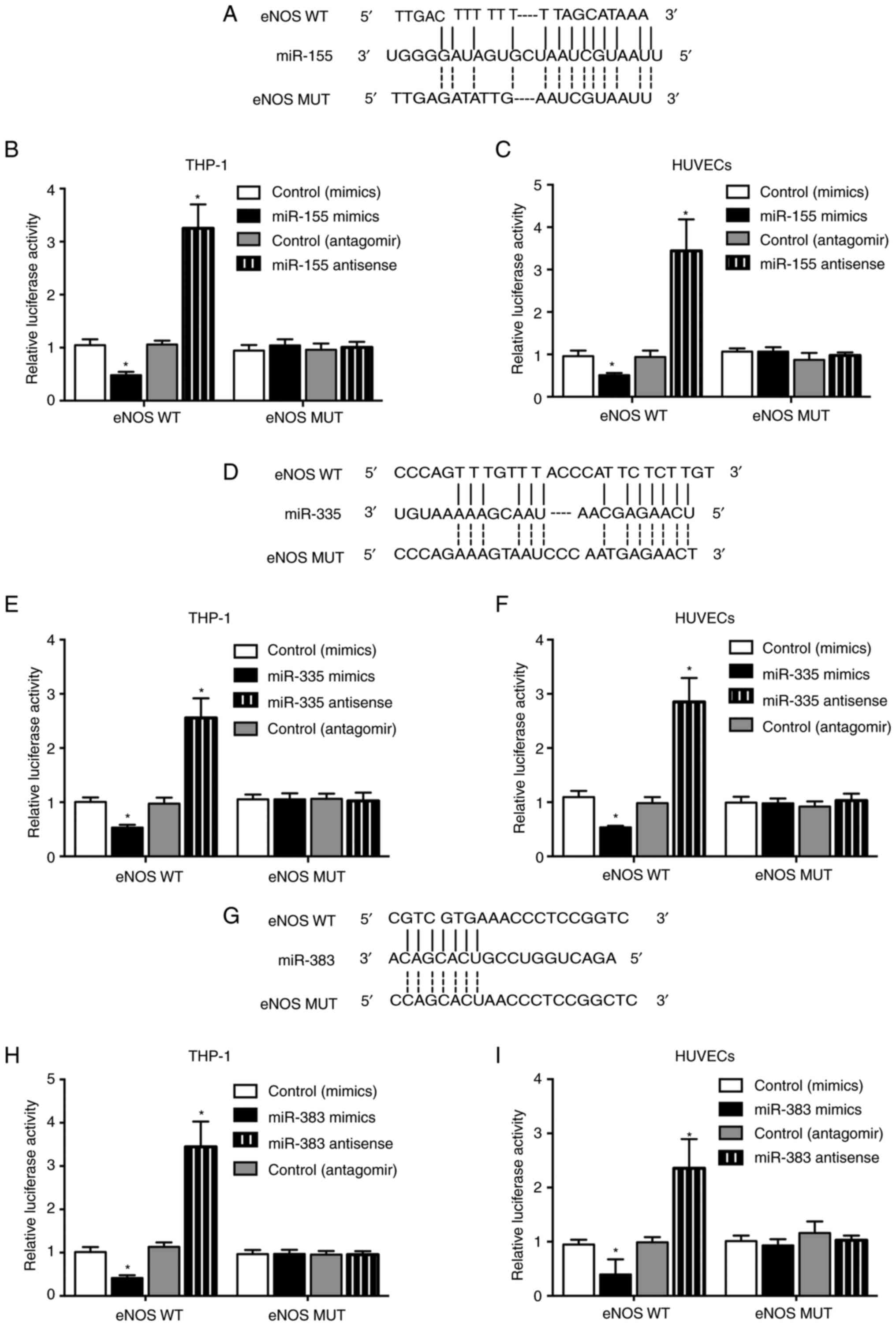
Sequence analysis indicated that eNOS was a
potential binding target of miR-155 (Fig. 4A), miR-335 (Fig. 4D) and miR-383 (Fig. 4G). Luciferase vectors containing
wild-type and mutant eNOS 3′ UTR were generated and cotransfected
into THP-1 cells and HUVECs with miR-155, miR-335 and miR-383
mimics and antagomirs. The luciferase activity of wild-type eNOS
was efficiently inhibited by miR-155 (Fig. 4B and C), miR-335 (Fig. 4E and F) and miR-383 (Fig. 4H and I) mimics in THP-1 cells and
HUVECs, whereas miR-155, miR-335 and miR-383 antagomirs
significantly enhanced the luciferase activity of wild-type eNOS
vectors. No apparent changes were observed for the luciferase
activity of mutant eNOS.
miR-155, miR-335 and miR-383
precursors suppress the expression of eNOS mRNA and protein, while
miR-155, miR-335 and miR-383 antagomirs activate the expression of
eNOS mRNA and protein in THP-1 cells and HUVECs
To further explore the inhibitory role of miR-155,
miR-335 and miR-383 in the expression of eNOS, miR-155, miR-335 and
miR-383 precursors and antagomirs were transfected into THP-1 cells
and HUVECs. The successful transfection of miR-155, miR-335 and
miR-383 precursors and antagomirs were confirmed by observing the
changes of miR-155 (Supplementary Fig. S1A and B), miR-335 (Supplementary
Fig. S1C and D) and miR-383
(Supplementary Fig. S1E and F).
Subsequently, the expression of eNOS mRNA and protein was analyzed
using qPCR and western blot analysis, respectively. The expression
of eNOS mRNA and protein was significantly suppressed by miR-155
(Figs. 5A and B; 6A and B), miR-335 (Figs. 5C and D; 6C and D) and miR-383 (Figs. 5E and F; 6E and F) precursors in THP-1 cells and
HUVECs, while the expression of eNOS mRNA and protein was activated
by miR-155 (Figs. 5A and B;
6A and B), miR-335 and miR-383
antagomirs in THP-1 cells and HUVECs.
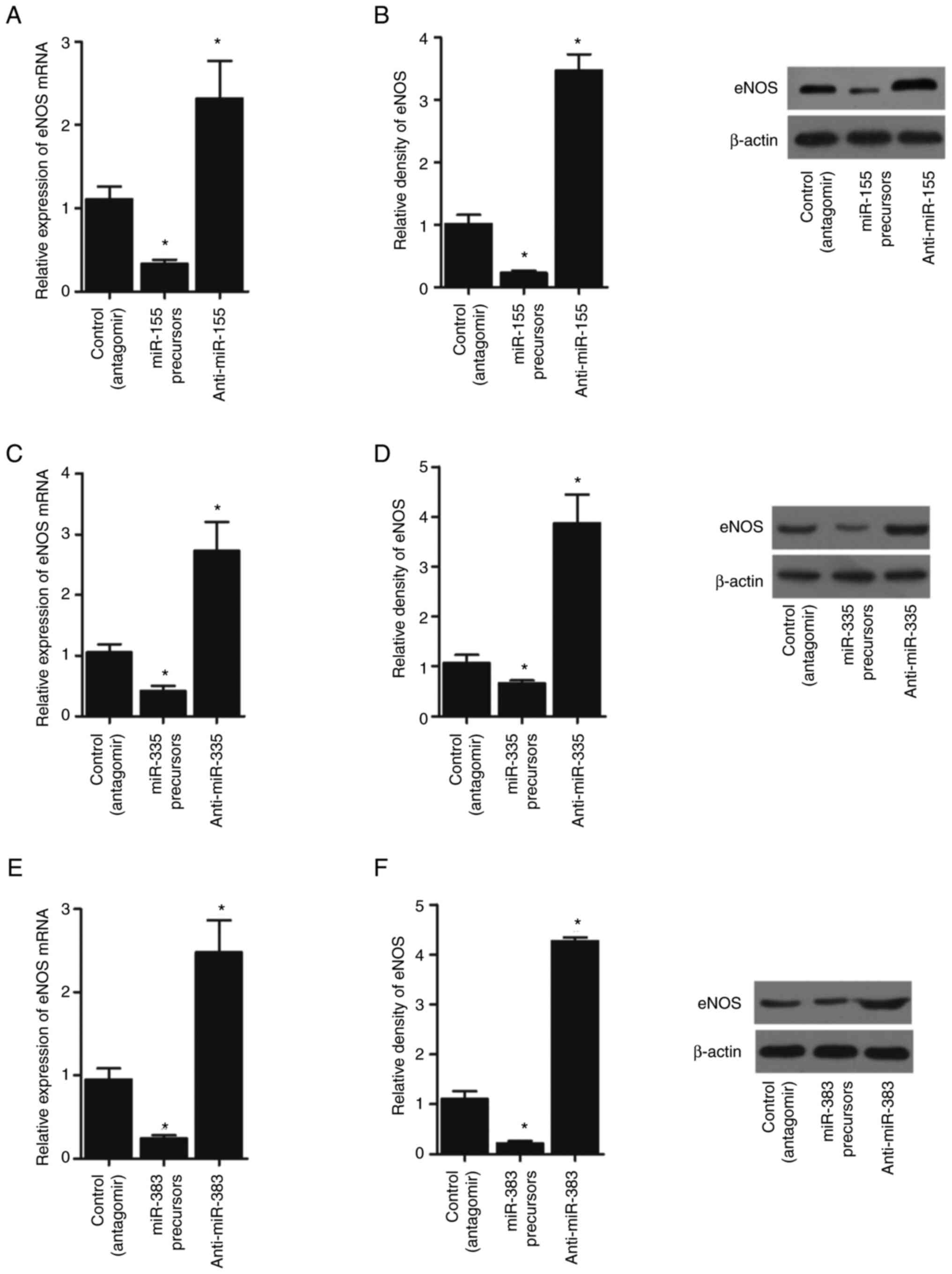 | Figure 5.miR-155, miR-335 and miR-383
precursors suppress the expression of eNOS, while miR-155, miR-335
and miR-383 antagomirs activate the expression of eNOS in THP-1
cells (*P<0.05 vs. control (antagomir) group; one-way ANOVA).
(A) miR-155 precursors suppressed the expression of eNOS mRNA,
while miR-155 antagomirs enhanced the expression of eNOS mRNA in
THP-1 cells. (B) miR-155 precursors suppressed the expression of
eNOS protein, while miR-155 antagomirs enhanced the expression of
eNOS protein in THP-1 cells. (C) miR-335 precursors suppressed the
expression of eNOS mRNA, while miR-335 antagomirs enhanced the
expression of eNOS mRNA in THP-1 cells. (D) miR-335 precursors
suppressed the expression of eNOS protein, while miR-335 antagomirs
enhanced the expression of eNOS protein in THP-1 cells. (E) miR-383
precursors suppressed the expression of eNOS mRNA, while miR-383
antagomirs enhanced the expression of eNOS mRNA in THP-1 cells. (F)
miR-383 precursors suppressed the expression of eNOS protein, while
miR-383 antagomirs enhanced the expression of eNOS protein in THP-1
cells. miRNA/miR, microRNA; eNOS, endothelial NO synthase. |
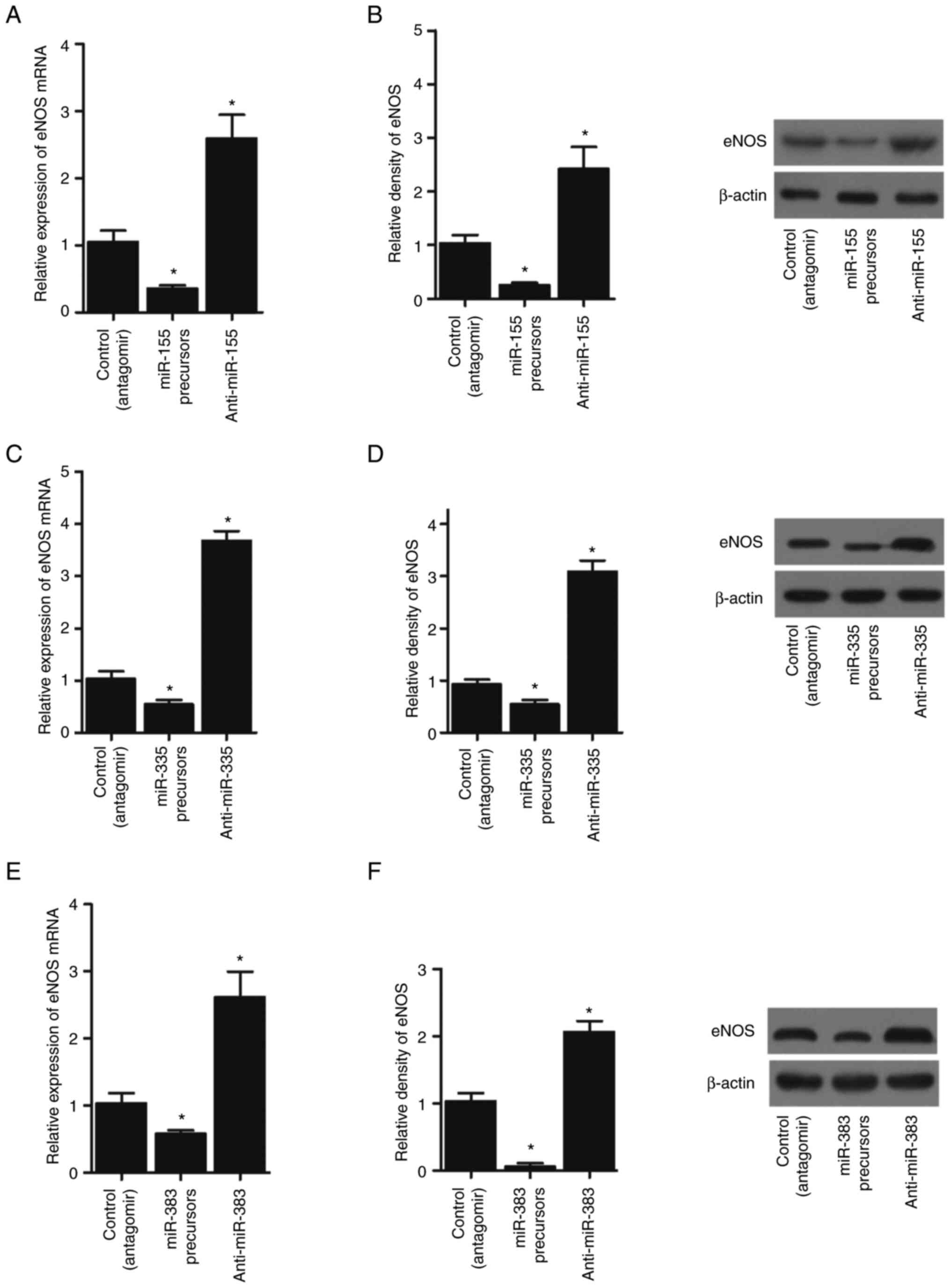 | Figure 6.miR-155, miR-335 and miR-383
precursors suppressed the expression of eNOS, while miR-155,
miR-335 and miR-383 antagomirs activated the expression of eNOS in
HUVECs (*P<0.05 vs. control (antagomir) group; one-way ANOVA).
(A) miR-155 precursors suppressed the expression of eNOS mRNA,
while miR-155 antagomirs enhanced the expression of eNOS mRNA in
HUVEC cells. (B) miR-155 precursors suppressed the expression of
eNOS protein, while miR-155 antagomirs enhanced the expression of
eNOS protein in HUVEC cells. (C) miR-335 precursors suppressed the
expression of eNOS mRNA, while miR-335 antagomirs enhanced the
expression of eNOS mRNA in HUVEC cells. (D) miR-335 precursors
suppressed the expression of eNOS protein, while miR-335 antagomirs
enhanced the expression of eNOS protein in HUVEC cells. (E) miR-383
precursors suppressed the expression of eNOS mRNA, while miR-383
antagomirs enhanced the expression of eNOS mRNA in HUVEC cells. (F)
miR-383 precursors suppressed the expression of eNOS protein, while
miR-383 antagomirs enhanced the expression of eNOS protein in HUVEC
cells. miRNA/miR, microRNA; eNOS, endothelial NO synthase; HUVEC,
human umbilical vein endothelial cell. |
Discussion
The present study found that EA decreased the levels
of SBP, DBP and MAP and increased the production of peripheral
blood NO and the level of HR in the recruited patients. Luciferase
assays confirmed that the overexpression of miR-155, miR-335 and
miR-383 inhibited the luciferase activity of eNOS. Therefore, EA
may exert a vasodilative effect during intubation for general
anaesthesia by promoting NO production and upregulating eNOS
expression. As reported by Dashti et al (24), haemodynamic responses to tracheal
intubation are caused by laryngoscopy and stimulation of the
structures of both the larynx and trachea (24), and the upward lifting force needed
to expose the glottis is less with a GlideScope than the
laryngoscope, resulting in less traction applied to soft tissues,
which may be correlated with significantly less sympathetic
stimulation. Nevertheless, fluctuations in the concentration of
anesthetic agents in blood and effector sites occur with regard to
the onset and offset stimulation. Moreover, cardiovascular symptoms
leading to hypotension and bradycardia might be depressed by
pharmacologic interventions, such as opioids. However, opioids can
have deleterious respiratory effects on the fetus (24). Another strategy to reduce the
cardiovascular response to tracheal intubation is to alter the
method of tracheal intubation (25).
EA (EA) is an essential sensory stimulation that is
accompanied by a range of responses from the nervous system. The
responses to EA treatment have been demonstrated to be suppressed
using anaesthesia and these modifications are manifested by somatic
afferent and adrenal efferent nerves (26–28).
The present study recruited patients to receive EA treatment. The
SBP, DBP, MAP and HR of the patients was measured to show that EA
effectively decreased the SBP, DBP and MAP but increased the HR.
The interaction of certain methods with acupuncture was studied. In
a crossover study, treatment with anesthetic at acupuncture site
PC6 helps to avoid an antiemetic response (29). In a controlled study on the
ramifications of acupuncture under anaesthesia, no differences were
found in volunteers awarded acupuncture or placebo (30). Responses to EA have been shown in
animals, demonstrating the consequences of reduced use of
anaesthesia following EA (31).
Saleh (32) conducted a randomized
clinical analysis on more than 200 surgical samples to evaluate the
consequence of EA at the Neiguan (P6) and Quchi (Li 11) sites in
the prevention of endotracheal intubation-induced strain, reporting
that the DP, SP and MAP in the control group is increased but that
the increase in the experimental group is much higher.
Microarrays using total RNA have been used to
measure the expression of miRNAs at medullas immediately following
acupuncture remedy at the Taichong site to show the reaction of
miRNA profiling to acupuncture treatment of SHRs. Preliminary
evaluation of miRNA expression statistics shows that >200 miRNAs
have considerable fluctuations with regard to their expression
inside the body, including 23 miRNAs with a ≥1.5-fold shift under
acupuncture therapy. Compared with healthy SD rats, miR-155,
miR-335 and miR-383 are significantly downregulated in the
experimental group compared with the control group (21). The present study collected
peripheral blood and PBMCs from patients receiving EA. The
expression of miR-155, miR-335 and miR-383 was reduced the
peripheral blood and PBMCs. Furthermore, luciferase assays were
performed to explore the regulatory role of miRNAs in eNOS
expression. miR-155, miR-335 and miR-383 inhibited the luciferase
activity of eNOS by binding to its 3′ UTR. Moreover, THP-1 cells
and HUVECs were transfected with miR-155, miR-335 and miR-383
precursors and antagomirs. The expression of eNOS was significantly
inhibited by miR-155, miR-335 and miR-383 precursors but enhanced
by miR-155, miR-335 and miR-383 antagomirs.
NO is a soluble compound synthesized by endothelial
cells using L-arginine amino acid via the constitutively active
calcium calmodulin-dependent enzyme, nitric oxide synthase (NOS)
(33). A five-electron oxidation
reaction in L-arginine is catalyzed by NOS along with several
cofactors (34). The first
evidence that NO acts as a calming substance for the relaxation of
vascular tissue was found by Devlin et al (35), who reveal that endothelial cells
release NO if they are subjected to bradykinin treatment according
to a chemiluminescence assay. As eNOS and neuronal NOS (nNOS) are
constitutively expressed, they are termed calcium-dependent enzymes
(although eNOS might be activated in a calcium-independent manner)
(35). The mRNA level of the eNOS
enhancer is reduced in a reaction to hypoxia in HUVECs (36). However, from the molecular point of
view, eNOS stimulation and eNOS expression are more complex and
rely on PI3K/Akt- or AC/PKA signaling at the posttranscriptional,
transcriptional and posttranslational levels (37,38).
As a major part of traditional Chinese medicine, EA
is widely used in China. Although some tragedies have been reported
in the past years in western countries (39,40),
from our perspective, this was mainly caused by the mis-location of
the pressor points (although few details are known). Similar to
other part of the traditional medicine, the factor that prevents
widespread use of EA in clinical practice is the lack of
comprehensive understanding of the underlying molecular mechanism
and the risk of EA is primarily caused by unexperienced
practitioner. Therefore, it will take a long time for the widely
use of EA in clinical practice outside China.
In conclusion, the findings of the present study
suggested that EA may exert a vasodilative effect during intubation
for general anaesthesia, which may be due to the ability of EA to
promote the production of NO by upregulating eNOS expression.
Furthermore, the effect of EA on eNOS may be mediated by its
inhibitory effect on the expression of miR-155, miR-335 and
miR-383.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by Shandong Traditional Chinese
Medicine Science and Technology Development Project (grant no.
2017-469; 2019-0851; 2019-0861) and the Key Research Plan of
Shandong Province (grant no. 2019GSF108253).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XZ and WW planned the study, WW and KW searched the
literature, WW, KW and XZ collected and analyzed the data. WW and
XZ confirm the authenticity of all raw data. XZ and WW wrote the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The Ethics Committee of the First Affiliated
Hospital of Jinan University approved the protocol of the present
study (Approval no. JNDX007546228FSY). Written informed consent was
obtained from all participants before the initiation of the present
study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dix P and Howell S: Survey of cancellation
rate of hypertensive patients undergoing anaesthesia and elective
surgery. Br J Anaesth. 86:789–793. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kayhan Z, Aldemir D, Mutlu H and Ogus E:
Which is responsible for the haemodynamic response due to
laryngoscopy and endotracheal intubation? Catecholamines,
vasopressin or angiotensin? Eur J Anaesthesiol. 22:780–785. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Russell WJ, Morris RG, Frewin DB and Drew
SE: Changes in plasma catecholamine concentrations during
endotracheal intubation. Br J Anaesth. 53:837–839. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Beissner F, Meissner K, Bar KJ and Napadow
V: The autonomic brain: An activation likelihood estimation
meta-analysis for central processing of autonomic function. J
Neurosci. 33:10503–10511. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mendonca FT, de Queiroz LM, Guimaraes CC
and Xavier AC: Effects of lidocaine and magnesium sulfate in
attenuating hemodynamic response to tracheal intubation:
Single-center, prospective, double-blind, randomized study. Braz J
Anesthesiol. 67:50–56. 2017.PubMed/NCBI
|
|
6
|
Nazir M, Salim B and Khan FA:
Pharmacological agents for reducing the haemodynamic response to
tracheal intubation in paediatric patients: A systematic review.
Anaesth Intensive Care. 44:681–691. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ma R, Liu X, Clark J, Williams GM and Doi
SA: The impact of acupuncture on neurological recovery in spinal
cord injury: A systematic review and meta-analysis. J Neurotrauma.
32:1943–1957. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Meng Q, Liu X, Shan Q, Yu P, Mao Z, Zhang
F, Li J and Zhao T: Acupuncture for treatment of secondary
osteoporosis in patients with spinal cord injury: A controlled
study. Acupunct Med. 32:381–386. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jiang DX, Lu ZS, Li GB, Sun SY, Mu X, Lee
P and Chen W: Electroacupuncture improves microcirculation and
neuronal morphology in the spinal cord of a rat model of
intervertebral disc extrusion. Neural Regen Res. 10:237–243. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lumbers ER, Delforce SJ, Arthurs AL and
Pringle KG: Causes and consequences of the dysregulated maternal
renin-angiotensin system in preeclampsia. Front Endocrinol
(Lausanne). 10:5632019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Krichevsky AM: MicroRNA profiling: From
dark matter to white matter, or identifying new players in
neurobiology. ScientificWorldJournal. 7:155–166. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu J and Wu Y:
Electro-acupuncture-modulated miR-214 prevents neuronal apoptosis
by targeting Bax and inhibits sodium channel Nav1.3 expression in
rats after spinal cord injury. Biomed Pharmacother. 89:1125–1135.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pagidipati NJ and Gaziano TA: Estimating
deaths from cardiovascular disease: A review of global
methodologies of mortality measurement. Circulation. 127:749–756.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fish JE and Marsden PA: Endothelial nitric
oxide synthase: Insight into cell-specific gene regulation in the
vascular endothelium. Cell Mol Life Sci. 63:144–162. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Albrecht EW, Stegeman CA, Heeringa P,
Henning RH and van Goor H: Protective role of endothelial nitric
oxide synthase. J Pathol. 199:8–17. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Elms S, Chen F, Wang Y, Qian J, Askari B,
Yu Y, Pandey D, Iddings J, Caldwell RB and Fulton DJ: Insights into
the arginine paradox: Evidence against the importance of
subcellular location of arginase and eNOS. Am J Physiol Heart Circ
Physiol. 305:H651–H666. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Connelly L, Madhani M and Hobbs AJ:
Resistance to endotoxic shock in endothelial nitric-oxide synthase
(eNOS) knock-out mice: A pro-inflammatory role for eNOS-derived no
in vivo. J Biol Chem. 280:10040–10046. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang Z, Hoffmann FW, Fay JD, Hashimoto
AC, Chapagain ML, Kaufusi PH and Hoffmann PR: Stimulation of
unprimed macrophages with immune complexes triggers a low output of
nitric oxide by calcium-dependent neuronal nitric-oxide synthase. J
Biol Chem. 287:4492–4502. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zheng HZ, Jiang W, Zhao XF, Du J, Liu PG,
Chang LD, Li WB, Hu HT and Shi XM: Electroacupuncture induces acute
changes in cerebral cortical miRNA profile, improves cerebral blood
flow and alleviates neurological deficits in a rat model of stroke.
Neural Regen Res. 11:1940–1950. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Duan DM, Dong X, Tu Y and Liu P: A
microarray study of chronic unpredictable mild stress rat blood
serum with electro-acupuncture intervention. Neurosci Lett.
627:160–167. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang JY, Li H, Ma CM, Wang JL, Lai XS and
Zhou SF: MicroRNA profiling response to acupuncture therapy in
spontaneously hypertensive rats. Evid Based Complement Alternat
Med. 2015:2043672015.PubMed/NCBI
|
|
22
|
Rahimi M, Farhanchi A, Taheri M and Samadi
S: The effects of acupuncture on hemodynamic changes during
endotracheal intubation for general anesthesia. Med Acupunct.
31:123–129. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dashti M, Amini S, Azarfarin R, Totonchi Z
and Hatami M: Hemodynamic changes following endotracheal intubation
with glidescope(®) video-laryngoscope in patients with
untreated hypertension. Res Cardiovasc Med. 3:e175982014.PubMed/NCBI
|
|
25
|
Amini S and Shakib M: Hemodynamic changes
following endotracheal intubation in patients undergoing cesarean
section with general anesthesia: Application of glidescope(R)
videolaryngoscope versus direct laryngoscope. Anesth Pain Med.
5:e218362015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Stener-Victorin E, Kobayashi R and
Kurosawa M: Ovarian blood flow responses to electro-acupuncture
stimulation at different frequencies and intensities in
anaesthetized rats. Auton Neurosci. 108:50–56. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Noguchi E, Ohsawa H, Kobayashi S, Shimura
M, Uchida S and Sato Y: The effect of electro-acupuncture
stimulation on the muscle blood flow of the hindlimb in
anesthetized rats. J Auton Nerv Syst. 75:78–86. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ohsawa H, Yamaguchi S, Ishimaru H, Shimura
M and Sato Y: Neural mechanism of pupillary dilation elicited by
electro-acupuncture stimulation in anesthetized rats. J Auton Nerv
Syst. 64:101–106. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dundee JW and Ghaly G: Local anesthesia
blocks the antiemetic action of P6 acupuncture. Clin Pharmacol
Ther. 50:78–80. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Morioka N, Akca O, Doufas AG, Chernyak G
and Sessler DI: Electro-acupuncture at the Zusanli, Yanglingquan,
and Kunlun points does not reduce anesthetic requirement. Anesth
Analg. 95:98–102. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kvorning N, Christiansson C, Beskow A,
Bratt O and Akeson J: Acupuncture fails to reduce but increases
anaesthetic gas required to prevent movement in response to
surgical incision. Acta Anaesthesiol Scand. 47:818–822. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Saleh RH: Efficacy of laser acupuncture in
attenuating hemodynamic response to orotracheal intubation and
postoperative nausea and vomiting in children undergoing strabismus
surgery. Egypt J Anaesth. 30:411–416. 2019. View Article : Google Scholar
|
|
33
|
Palmer RM, Ashton DS and Moncada S:
Vascular endothelial cells synthesize nitric oxide from L-arginine.
Nature. 333:664–666. 1988. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Woodward JJ, Nejatyjahromy Y, Britt RD and
Marletta MA: Pterin-centered radical as a mechanistic probe of the
second step of nitric oxide synthase. J Am Chem Soc. 132:5105–5113.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Devlin J, Palmer RM, Gonde CE, O'Grady J,
Heaton N, Tan KC, Martin JF, Moncada S and Williams R: Nitric oxide
generation. A predictive parameter of acute allograft rejection.
Transplantation. 58:592–595. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Casanello P, Krause B, Torres E, Gallardo
V, Gonzalez M, Prieto C, Escudero C, Farias M and Sobrevia L:
Reduced l-arginine transport and nitric oxide synthesis in human
umbilical vein endothelial cells from intrauterine growth
restriction pregnancies is not further altered by hypoxia.
Placenta. 30:625–633. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fulton D, Gratton JP, McCabe TJ, Fontana
J, Fujio Y, Walsh K, Franke TF, Papapetropoulos A and Sessa WC:
Regulation of endothelium-derived nitric oxide production by the
protein kinase Akt. Nature. 399:597–601. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wu KK: Regulation of endothelial nitric
oxide synthase activity and gene expression. Ann N Y Acad Sci.
962:122–130. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fu QN, Shi GX, Li QQ, He T, Liu BZ, Sun
SF, Wang J, Tan C, Yang BF and Liu CZ: Acupuncture at local and
distal points for chronic shoulder pain: Study protocol for a
randomized controlled trial. Trials. 15:1302014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lathia AT, Jung SM and Chen LX: Efficacy
of acupuncture as a treatment for chronic shoulder pain. J Altern
Complement Med. 15:613–618. 2009. View Article : Google Scholar : PubMed/NCBI
|