Introduction
Nasopharyngeal carcinoma (NPC) is a rare type of
primary malignancy that originates from the nasopharyngeal region
(1) and is predominant in
southeast Asia and southern China (2,3). It
is estimated that there were 129,079 new cases of NPC and 72,987
cancer mortalities caused by NPC worldwide in 2018 (4). In spite of the great progress that
has been made in treating NPC with radiation therapy and combined
chemotherapy, the prognosis of patients with NPC remains
unsatisfactory, especially in patients at an advanced stage of the
disease (5); ~30% of patients with
NPC develop distant metastasis and/or recurrence (1,3), as
a result of which the 5-year overall survival rate is reduced from
~90–70% (4). Owing to the annual
increase of the NPC incidence rate, it is necessary to investigate
the molecular mechanisms underlying the progression of NPC and to
develop novel therapeutic strategies for NPC treatment.
Cell division cycle gene 25 (CDC25) family members
have been demonstrated to function in several steps in multiple
physiological processes, including cell cycle and mitosis (6). CDC25A is a core regulator of the cell
cycle that has a dual-specific phosphatase activity. CDC25s cause
the dephosphorylation of cyclin-dependent kinase (CDK)4, CDK6 and
CDK2, stimulating G1 progression and accelerating into
the S phase (6). CDC25A has been
reported to be a promising biomarker for the diagnosis of
non-small-cell lung cancer (NSCLC) and its upregulation has been
shown to predict an unfavorable prognosis for patients with NSCLC
(7). Moreover, CDC25A upregulation
promotes the radioresistance of cervical cancer cells, whereas
CDC25A knockdown reduces the cell survival rate and promotes
apoptosis (8). A previous study
demonstrated that reduced expression levels of CDC25A caused by the
silencing of fibroblast growth factor receptor 2 contributed to the
efficacy of cisplatin treatment, including attenuating cell
viability and stimulating cell cycle arrest (9). Notably, it has been reported that
radiation treatment can decrease CDC25A expression in human NPC
cells, leading to cell cycle arrest and cell apoptosis (10). However, the specific mechanism
underlying the role of CDC25A in NPC progression has yet to be
fully elucidated. E2F transcription factor 1 (E2F1) belongs to the
E2F family of transcription factors, participating in cellular
differentiation and tissue development to maintain body metabolism
and homeostasis as a metabolic regulator (11). An number of studies have
highlighted the crucial role of E2F1 in various types of
malignancies (12), including
ovarian cancer (13), prostate
cancer (14) and glioblastoma
(15). It is noteworthy that the
importance of E2F1 in NPC inflammation and tumorigenesis has also
been identified in previously published studies (16,17).
Nevertheless, whether CDC25A functions in NPC via transcriptional
regulation of E2F1 remains unclear.
Therefore, the aim of the present study was to
investigate the role of CDC25A in cell proliferation and cell cycle
in NPC and to explore the potential underlying mechanism. The
findings showed how E2F1 suppression-mediated CDC25A silencing
attenuated cell proliferation and induced cell cycle arrest in NPC,
thereby raising the possibility that CDC25A may be a promising
therapeutic target for NPC treatment.
Materials and methods
Bioinformatic analysis
The Coexpedia database (http://www.coexpedia.org/) was used to analyze the
co-expression between CDC25A and E2F1 (‘CDC25A’ entered in the
Search panel and E2F1 search for in the Co-expressed Genes panel).
The Cyclebase database (https://cyclebase.org/) was searched to identify E2F1
as a transcription factor that could regulate G1/S phase
transition (‘E2F1’ entered in the in Search panel). In addition,
the predicted binding sequences between E2F1 and the CDC25A
promoter was analyzed using the JASPAR database (https://jaspar.genereg.net; relative profile score
threshold: 80%).
Cell culture and transfection
A human nasopharyngeal epithelial cell line (NP69)
and four NPC cell lines (C666-1, HNE-3, NPC-039 and HK1) were
obtained from the Cell Bank of Type Culture Collection of Chinese
Academy of Sciences. The cells were cultured in RPMI-1640 medium
(Thermo Fisher Scientific, Inc.). The NP69 cell line was used as
the control group. Cells were supplied with 10% HyClone fetal
bovine serum (HyClone; Cytiva) and cultured at 37°C with 5%
CO2. Cells (5×103/well) at 80% confluence
were transfected with 100 nM empty vector siRNA-negative control
(NC; sequence, 5′-UUCUCCGAACGUGUCACGU-3′) or short interfering
(si)RNA-CDC25A (siRNA-CDC25A-1, 5′-GCUUAGCUAGCAUUACUAACC-3′; and
siRNA-CDC25A-2, 5′-GCGUGUCAUUGUUGUGUUUCA-3′) or siRNA-E2F1
(siRNA-E2F1-1, 5′-GAUGGUUAUGGUGAUCAAAGC-3′; and siRNA-E2F1-2,
5′-AGAUGGUUAUGGUGAUCAAAG-3′), all synthesized by Guangzhou RiboBio
Co., Ltd. using Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manufacturer's
instructions. In addition, the full-length sequences of E2F1 were
inserted into pcDNA3.1 vector (Invitrogen; Thermo Fisher
Scientific, Inc.) to construct E2F1 overexpression plasmid
(OV-E2F1). The plasmids for pcDNA3.1 (400 ng/µl; OV-NC and OV-E2F1)
were respectively transfected into HK1 cells using Lipofectamine
2000 for 48 h at 37°C according to the manufacturer's instructions.
After 6 h, the medium was replaced with fresh DMEM (Thermo Fisher
Scientific, Inc.) containing 10% FBS and maintained in a 5%
CO2 incubator for a further 48 h.
Reverse transcription-quantitative
(RT-q)PCR analysis
Total RNA was isolated from NPC cells
(1×106 cells) using an RNA isolation kit (Tiangen
Biotech Co., Ltd.), following the manufacturer's instructions. The
RNA quality was examined using a NanoDrop™ 2000 spectrophotometer
(NanoDrop Technologies; Thermo Fisher Scientific, Inc.). A RT kit
(Takara Bio, Inc.) was used for RT to obtain the first-chain cDNAs,
according to the manufacturer's protocol. A standard
SYBR® Green PCR kit (Takara Bio, Inc.) was used to
amplify the target fragments, according to the manufacturer's
protocol. qPCR was performed at 50°C for 2 min and 95°C for 2 min,
followed by 40 cycles at 95°C for 15 sec, 60°C for 1 min and
extension at 72°C for 1 min, followed by a final extension step at
72°C for 10 min. The relative gene expression levels were
calculated using the 2−ΔΔCq method (18). The cDNA fragment of the GAPDH gene
was used as an internal control for analysis of the results. The
primer sequences used were: CDC25A forward,
5′-TTCCTCTTTTTACACCCCAGTCA-3′ and reverse,
5′-TCGGTTGTCAAGGTTTGTAGTTC-3′; E2F1 forward,
5′-TGAGGGCATCCAGCTCATTG-3′ and reverse, 5′-AAACATCGATCGGGCCTTGT-3′;
Cyclin D1 forward, 5′-CTGATTGGACAGGCATGGGT-3′ and reverse,
5′-GTGCCTGGAAGTCAACGGTA-3′; CDK4 forward,
5′-GTGTATGGGGCCGTAGGAAC-3′ and reverse, 5′-CAGTCGCCTCAGTAAAGCCA-3′;
CDC6 forward, 5′-GCGAGGCCTGAGCTGTG-3′ and reverse,
5′-GCTGAGAGGCAGGGCTTTTA-3′; and GAPDH forward,
5′-TGACTTCAACAGCGACACCCA-3′ and reverse,
5′-CACCCTGTTGCTGTAGCCAAA-3′. This experiment was repeated three
times.
Western blot analysis
Following transfection, total proteins were
extracted with RIPA lysis buffer (Wuhan Servicebio Technology Co.,
Ltd.) containing protease/phosphatase inhibitor cocktail. The total
protein concentration was measured with a BCA protein assay kit
(Beijing Solarbio Science and Technology Co., Ltd.). Equal amounts
of protein per lane (25 µg) were separated by SDS-PAGE on 10% gels
and were transferred onto PVDF membranes. Membranes were blocked
with 5% skimmed milk for 1 h at room temperature and probed with
primary antibodies at 4°C overnight. The primary antibodies
included anti-CDC25A (cat. no. ab989; 1:1,000 dilution; Abcam),
anti-Ki67 (cat. no. ab16667; 1:1,000; Abcam), anti-PCNA (cat. no.
ab92552; 1:1,000; Abcam) and anti-E2F1 (cat. no. ab288369; 1:1,000;
Abcam) antibodies. Subsequently, the membranes were incubated with
HRP-conjugated goat anti-rabbit IgG (cat. no. ab6721; 1:2,000;
Abcam) at room temperature for 2 h and images were acquired using a
Tanon-5200 Chemiluminescence Imager (Tanon Science and Technology
Co., Ltd.). Finally, the band density was analyzed using ImageJ
software v1.8.0 (National Institutes of Health).
Cell counting kit-8 (CCK-8) assay
Following transfection, CCK-8 assay was performed to
determine the cell viability. After incubating the HK1 cells for 0,
24, 48 and 72 h, CCK-8 solution (10 µl/well; Dojindo Laboratories,
Inc.) was added and incubated with the cells for a further 2 h.
Finally, the absorbance at 450 nm was measured using a microplate
reader (Bio-Rad Laboratories, Inc.).
Flow cytometry
HK1 cells were planted in 6-well plates
(1×105 cells/well) and transfected with or without
OV-E2F1 and siRNA-CDC25A. At 24 h after transfection, the HK1 cells
were collected. A Cell Cycle Assay kit (BD Biosciences) was used to
analyze the cell cycle following the manufacturer's instructions.
Briefly, HK1 cells were incubated with 200 µl liquid A for 10 min
and 150 µl liquid B at 4°C for a further 10 min. Subsequently, HK1
cells were incubated with 120 µl liquid C in the dark at 4°C for 10
min prior to flow cytometry analysis on a BD FACSort system (BD
Biosciences). Finally, the results were analyzed using ModFit
software, version 3.2 (Verity Software House, Inc.).
Luciferase reporter gene assay
The full length of CDC25A promoter (FL group) and
the full length of CDC25A promoter including mutational site 1
(CGTAATAT) (Site 1 group) and the full length of CDC25A promoter
including mutational site 2 (GGCTATAT) (Site 2 group) were
respectively cloned into the firefly luciferase reporter plasmid,
pGL3-basic vector (Addgene, Inc.). HK1 cells were seeded in 24-well
plates and cultured at 37°C. Subsequently, the OV-NC and OV-E2F1
overexpression plasmids were co-transfected into HK1 cells using
Lipofectamine 2000 for 48 h. The cells were then lysed and the
firefly luciferase activity was measured and normalized against
Renilla luciferase activity using a Dual-Luciferase Reporter
Assay System (Promega Corporation) according to the manufacturer's
instructions. The relative activity of luciferase was determined
through measuring the ratio of the two identified activities. The
sequences of full length of CDC25A promoter are shown in Table SI.
Chromatin immunoprecipitation (ChIP)
assay
ChIP assay was performed as previously described by
using a Magna ChIP™ kit (MilliporeSigma). In brief, HK1 cells
(1×106) were transfected with E2F1 overexpression
plasmids for 48 h and subsequently DNA associated with specific
immunoprecipitates or with mouse immunoglobulin G as a negative
control was isolated and used as a template for PCR, amplifying the
CDC25A promoter sequence containing the E2F1-binding site. qPCR was
performed at 50°C for 2 min and 95°C for 2 min, followed by 40
cycles at 95°C for 15 sec and 60°C for 1 min as aforementioned
using the SYBR Green PCR kit. The relative gene expression levels
were calculated using the 2−ΔΔCq method (18). The primer sequence used in this
study were as follows: CDC25A forward, 5′-CTTCTGAGAGCCGATGACCT-3′
and reverse, 5′-CACCTCTTACCCAGGCTGTC-3′ (19).
Statistical analysis
The statistical analysis was conducted using SPSS
22.0 software (IBM Corp.) and GraphPad Prism 6 software (GraphPad
Software; Dotmatics). Data are expressed as the mean ± standard
error of the mean of results derived from three independent
experiments performed in triplicate. Statistical analysis was
performed using one-way ANOVA followed by Tukey or Bonferroni
post-hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
CDC25A is highly expressed in NPC cell
lines
To explore the role of CDC25A in the progression of
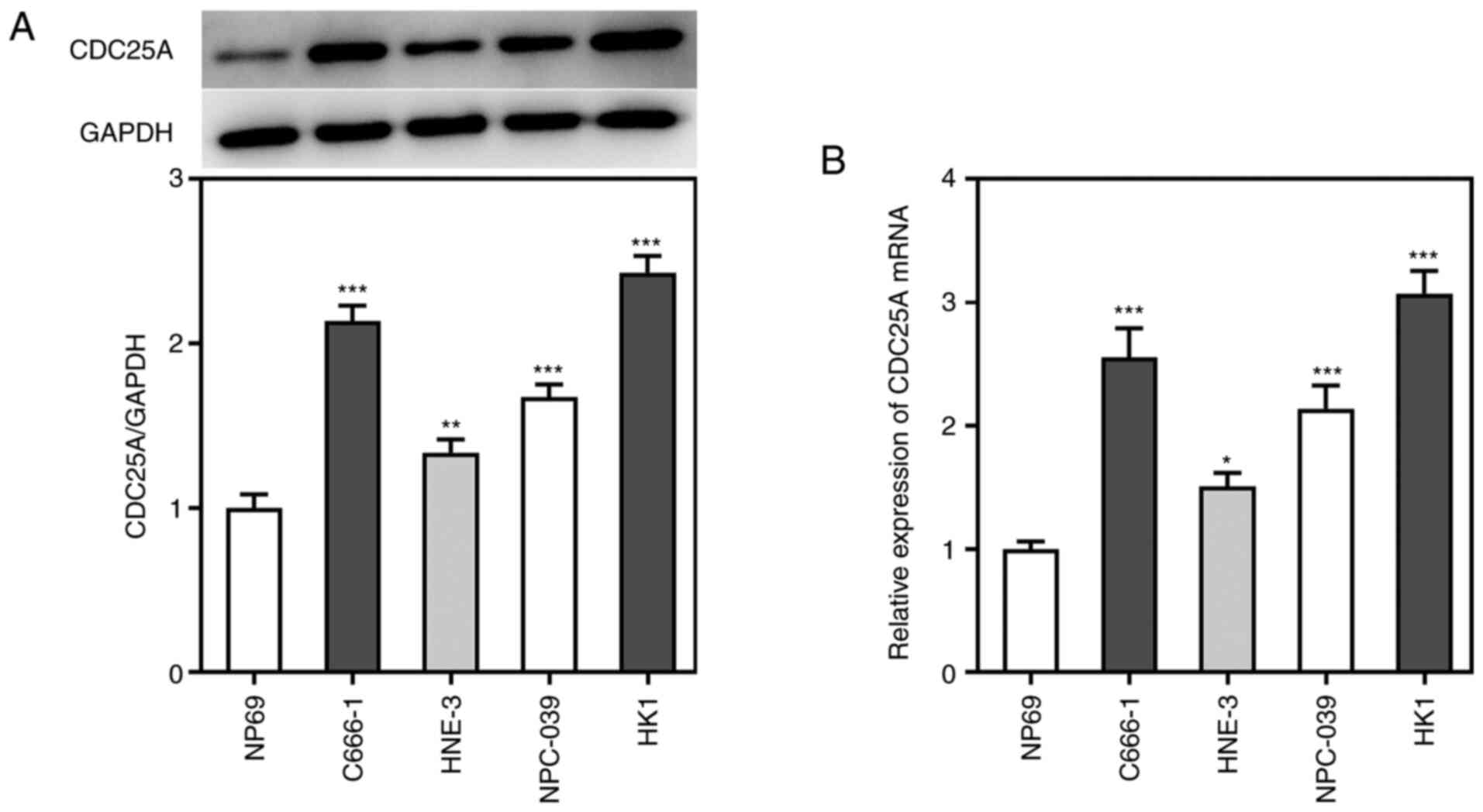
NPC, its expression levels were detected by western blot and
RT-qPCR analyses. As shown in Fig. 1A
and B, the results from the western blotting and RT-qPCR
experiments showed that, compared with NP69 cells, the expression
of CDC25A was significantly upregulated in the NPC cell lines. HK1
cells showed the highest mRNA and protein levels of CDC25A,
therefore HK1 cells were selected as the cell line of choice in
subsequent experiments. These results also suggested that CDC25A
may be involved in NPC progression.
CDC25A silencing inhibits cell
proliferation and induces G1 arrest in NPC
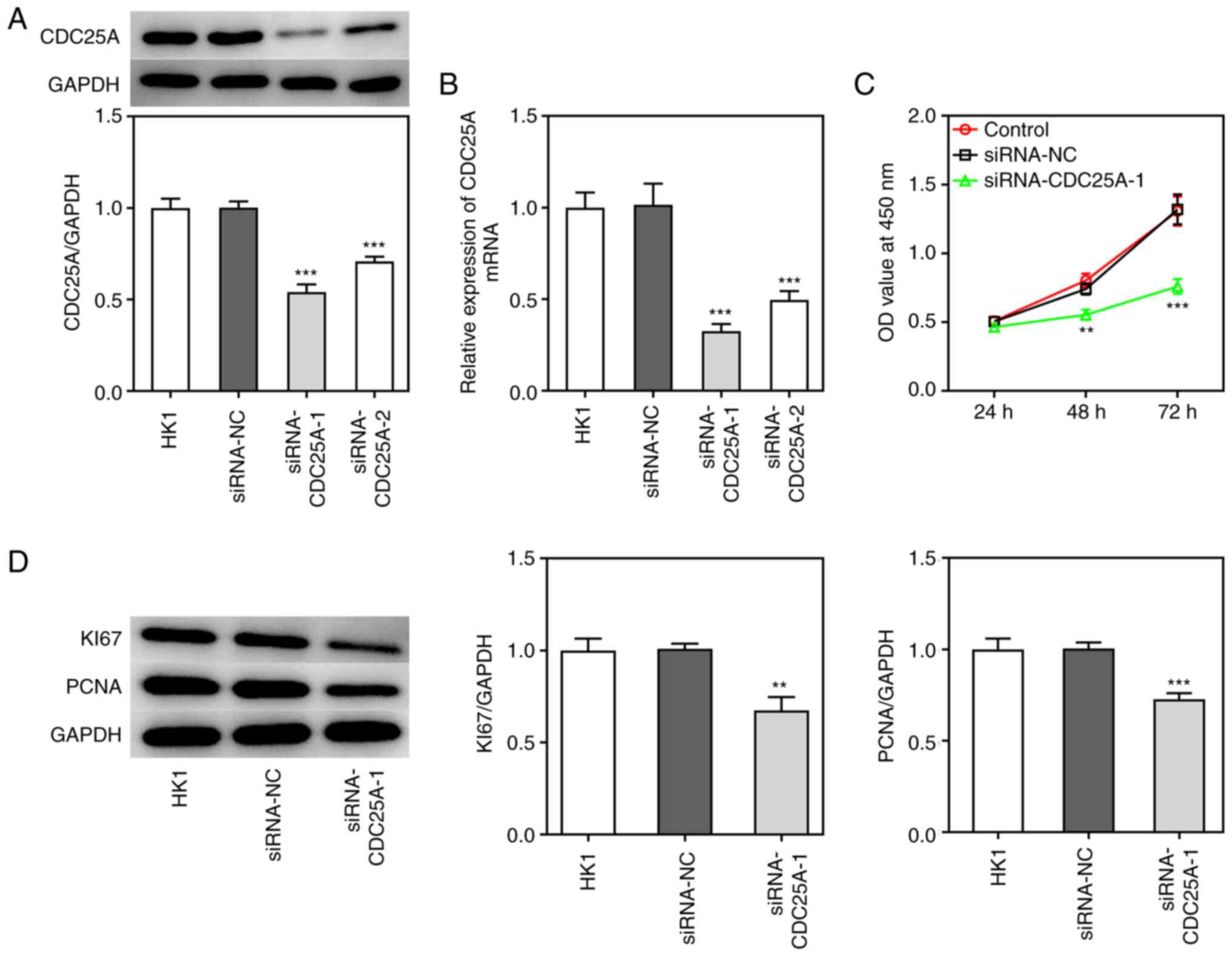
To investigate the role of CDC25A in NPC
progression, the transfection of siRNA-CDC25A-1 and siRNA-CDC25A-2
was performed, first to achieve CDC25A silencing, as demonstrated
by the results from western blotting and RT-qPCR analyses (Fig. 2A and B). Due to the lower
expression level of CDC25A in the siRNA-CDC25A-1 group compared
with that in the siRNA-CDC25A-2 group, siRNA-CDC25A-1 was used for
further studies. Subsequently, the proliferation and cell cycle of
HK1 cells were analyzed. The cell viability of HK1 cells
transfected with siRNA-CDC25A was appraised using a CCK-8 assay. As
shown in Fig. 2C, CDC25A silencing
suppressed HK1 cell viability. Furthermore, CDC25A knockdown
suppressed the expression of proliferation markers, including Ki67
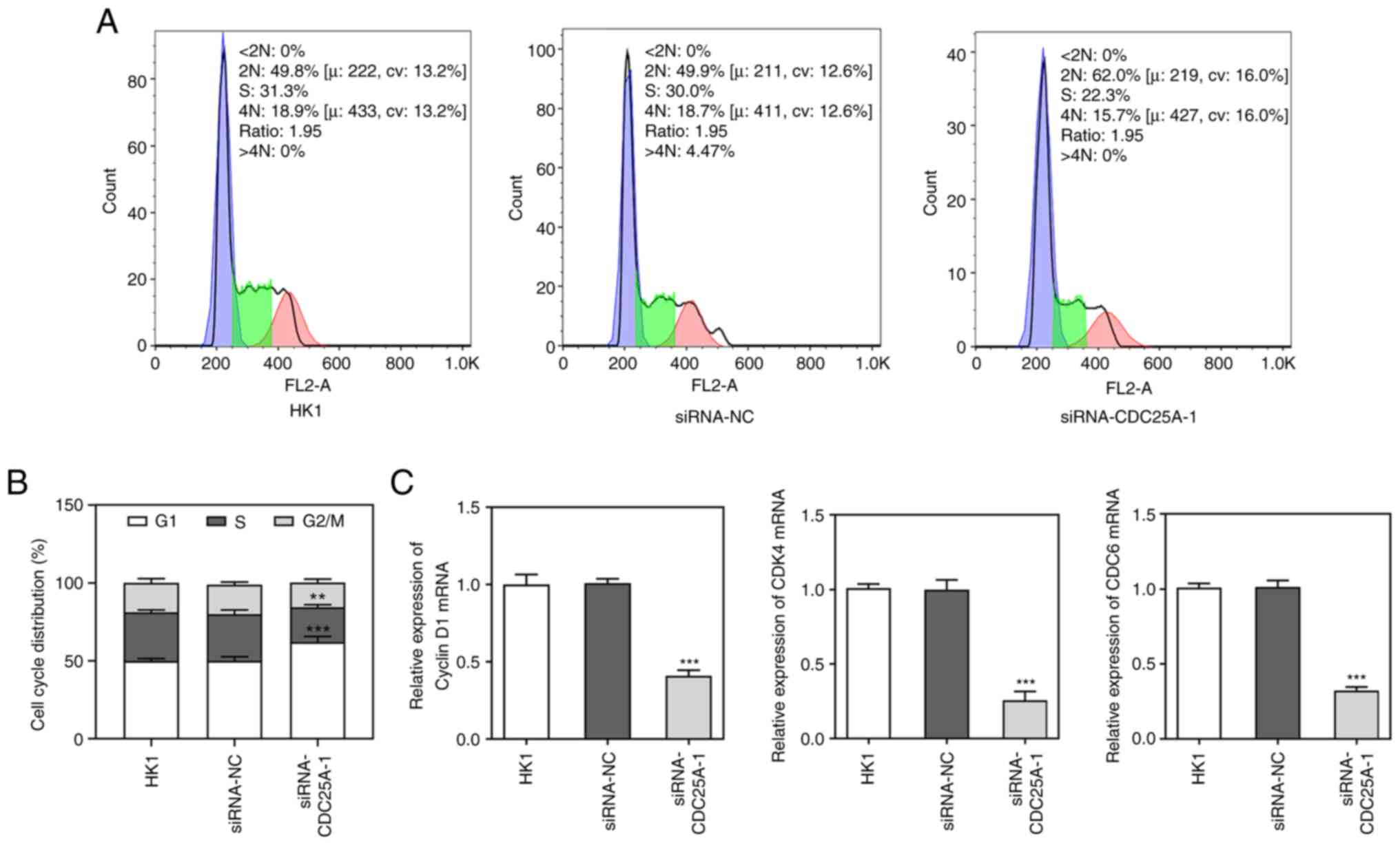
and PCNA (Fig. 2D). Cell cycle was
then analyzed by flow cytometry. As shown in Fig. 3A and B, it was observed that the
numbers of HK1 cells transfected with siRNA-CDC25A in
G1-phase were significantly larger compared with those
of the siRNA-NC group. In addition, CDC25A knockdown reduced the
levels of Cyclin D1, CDK4 and CDK6 (Fig. 3C). Therefore, it was possible to
conclude that CDC25A silencing inhibited the proliferation and
induced G1 arrest of HK1 cells.
CDC25A is transcriptionally regulated
by E2F1
To investigate the potential mechanism underlying
the role of CDC25A in NPC, co-expression between CDC25A and E2F1
was screened using the Coexpedia database (http://www.coexpedia.org/; Fig. 4A) and E2F1 was identified as a
transcription factor that could regulate G1/S-phase
transition using the Cyclebase database (https://cyclebase.org/; Fig. 4B), suggesting that E2F1 may exert a
regulatory effect on CDC25A expression. The predicted binding
sequences between E2F1 and the CDC25A promoter are shown in
Fig. 4C. Subsequently, the OV-E2F1
plasmid was constructed to achieve E2F1 overexpression and the
transfection efficacy was demonstrated through RT-qPCR and western
blot analyses (Fig. 4D and E).
Moreover, CDC25A upregulation resulted in E2F1 overexpression
(Fig. 4F and G). The si-E2F1
plasmid was also constructed to achieve E2F1 knockdown. Due to the
lower expression level of E2F1 that was observed, si-E2F1-1 was
selected in subsequent experiments (Fig. 4H and I). Notably, E2F1 knockdown
also caused downregulation of CDC25A expression (Fig. 4J and K). Furthermore, the results
from the luciferase reporter assay showed that luciferase activity
were reduced in Site 1 and Site 2 groups compared with the FL group
(Fig. 4L). Moreover, ChIP assays
corroborated that CDC25A could interact with E2F1 (Fig. 4M). These results indicated that
CDC25A could both interact with E2F1 and was transcriptionally
regulated by E2F1.
CDC25A silencing abolished the effect
of E2F1 overexpression on cell proliferation and cell cycle in
NPC
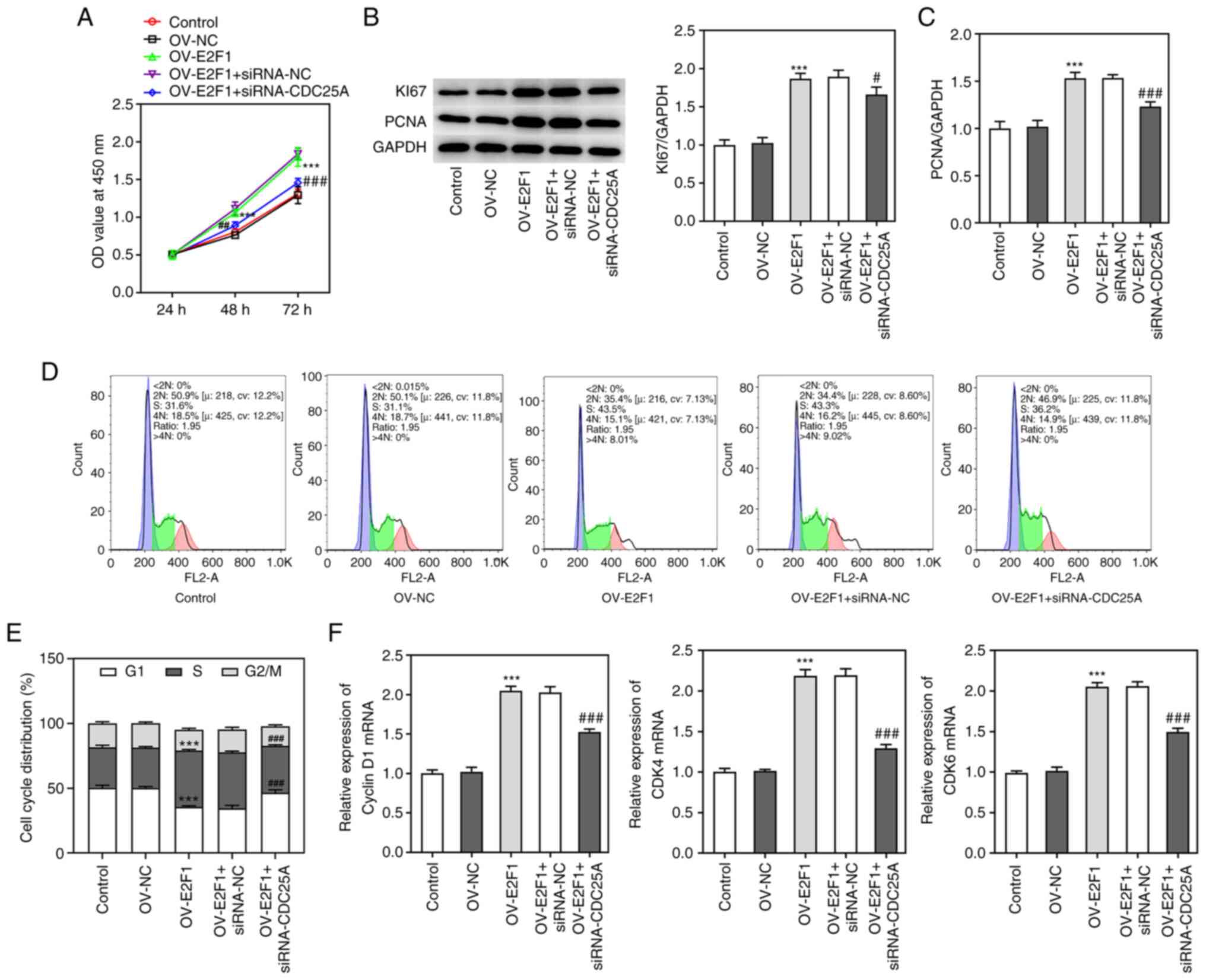
To study whether E2F1 could mediate the role of
CDC25A in NPC progression, the proliferation of HK1 cells
transfected with E2F1 overexpression plasmids and the cell cycle
were both analyzed, as described above. As shown in Fig. 5A, E2F1 overexpression enhanced cell
viability compared with the control group, whereas CDC25A silencing
suppressed the viability of HK1 cells transfected with OV-E2F1.
Additionally, the expression levels of Ki67 and PCNA were increased
by E2F1 overexpression, whereas they were decreased through CDC25A
silencing (Fig. 5B and C). More
significantly, E2F1 overexpression promoted the transition of the
cell cycle from G1-phase to S-phase in HK1 cells and
this effect was partially reversed by CDC25A silencing (Fig. 5D and E). E2F1 overexpression
increased the levels of Cyclin D1, CDK4 and CDK6 while CDC25A
silencing reversed the effects of E2F1 overexpression on the levels
of Cyclin D1, CDK4 and CDK6 (Fig.
5F). Taken together, these results implied that CDC25A
silencing abolished the effect of E2F1 overexpression on the
proliferation and cell cycle of HK1 cells.
Discussion
Nasopharyngeal carcinoma (NPC) is a distinct
head-and-neck cancer with high incidence of locoregional recurrence
or metastasis (20). Broadly
speaking, at present NPC is associated with an increasing number of
new cases and mortality and it remains a major public health
concern. However, the mechanisms underpinning the pathogenesis of
NPC have yet to be fully elucidated. In the present study, the
expression levels of CDC25A were found to be markedly elevated in
all the NPC cell lines tested, including C666-1, HNE-3, NPC-039 and
HK1 cells, compared with the normal nasopharyngeal epithelial cell
line (NP69), suggesting that CDC25A may have an oncogenic role in
NPC progression.
Accumulating evidence has identified CDC25A as an
oncogene in multiple types of cancer, including ovarian cancer,
liver cancer and breast cancer and it has been shown to be
associated with the induction of chemoresistance (21,22).
It has been reported that CDC25A knockdown inhibits cell
proliferation, migration and invasion in colorectal cancer
(23). CDC25A targeted by
miR-34a-5p exacerbates cervical cancer growth and migration
(24). Liu et al (25) also propose that CDC25A aggravates
cell proliferation and impedes cell cycle in hepatocellular
carcinoma. As far as is known at present, the latent mechanism
underlying the carcinogenic role of CDC25A is complicated,
primarily involving cell proliferation, apoptosis and the cell
cycle (26,27). Collectively, the findings in the
present study demonstrated that CDC25A silencing suppressed the
viability of HK1 cells. Ki67 and PCNA are regarded as proliferation
markers: Ki67, mainly expressed in the nucleus of proliferating
cells, is closely associated with mitosis (28), whereas PCNA has also been
documented to mediate cell proliferation via lipid phosphatase
activity, protein phosphatase activity and various signaling
pathways (29). The experimental
data presented in this study have also shown that the expression
levels of Ki67 and PCNA were suppressed by CDC25A knockdown, which
further suggested that CDC25A interference could apparently
suppress the proliferation of NPC cells. The accurate transition of
the cell cycle from G1-phase to S-phase fulfills an
important role in controlling cell proliferation and its
dysregulation may contribute to oncogenesis (30). As a member of the CDC25 family of
cell division cycle proteins, CDC25A promotes the transition of
cells from G1-phase to S-phase by targeting cyclin
A/CDK2 and cyclin E/CDK2 (26,31).
For example, a previous study demonstrated that CDC25A has an
important role in regulating cell cycle-mediated chemoresistance in
colorectal cancer (32). CDC25A
also aggravates the course of breast cancer via the cell-cycle
pathway (21). In the present
study, CDC25A silencing induced G1 arrest of HK1 cells,
a finding that was consistent with the previous study. Hence,
CDC25A may serve as an oncogene in NPC progression.
To investigate the potential mechanism underlying
the role of CDC25A in NPC progression, additional studies were
performed. E2F1 has been shown to function chiefly through
transcriptional activation events in the E2F family of
transcription factors. Forkhead box protein O1 halts cell
proliferation by downregulating the E2F1-activated expression of
NOD-, LRR- and pyrin domain-containing protein 3 (NLRP3) in
prostate cancer (33).
E2F1-mediated SEC61G promotes breast cancer tumor growth and
metastasis (34). In a similar
way, the co-expression of CDC25A and E2F1 was screened using the
Coexpedia database and E2F1 was identified as a transcription
factor that could regulate G1/S-phase transition through
the Cyclebase database. E2F1 has been proposed to participate in
myriad cell biological processes, including cell cycle, DNA repair,
apoptosis, development, differentiation and metabolism (35,36).
A previous study reported that the overexpression of E2F1 led to a
decrease in the radiosensitivity of NPC cells (37). The findings from the present study
suggested that E2F1, as a transcription factor, could bind to the
promoter of CDC25A and thereby positively regulate its expression.
Luciferase reporter assay showed that the luciferase activity were
reduced in cells with mutational sites compared with the cells with
the normal sequences of CDC25A promoter, which may be because the
promoter of CDC25A with mutational sequences is harder to combined
with E2F1. Notably, CDC25A silencing abolished the effect of E2F1
overexpression on the proliferation of HK1 cells and on the
G1/S-phase cell cycle, indicating that E2F1 could
mediate the role of CDC25A in NPC progression.
Taken together, the results of the present study
have elucidated the specific role of CDC25A in cell proliferation
and the cell cycle in NPC, increasing our understanding of the
underlying regulatory mechanism by revealing the association
between CDC25A and E2F1 transcription factor. Nevertheless, there
are several limitations in the present study. It did not examine
the expression of CDC25A in nasopharyngeal carcinoma patients or
animals, so animal experiments in vivo and clinical trials
in humans are required to augment the findings of the present
study. Additionally, whether signaling pathways have any functional
roles downstream of CDC25A also requires further investigation. The
present study proved E2F1 targets to CDC25A in its transcription,
but did not explore the mechanism the E2F1-CDC2A interaction in the
cell cycle regulation and nor did it explore CyclinD, CyclinE,
CDK2/CDC1 and other specific markers for cell cycle; these will be
investigated in a future study. In addition, the present study only
used the HK1 cell line to explore the functional role of CDC25A in
NPC and the biological effects of CDC25A in other NPC cell lines
will be explored in a future study. Moreover, the present study
aimed to explore the effects of CDC25A on cell proliferation and
cell cycle in nasopharyngeal carcinoma, thus it did not perform
wound healing and invasion assays; the effects of CDC25A on cell
migration and invasion will be explored in a future study.
In conclusion, the present study demonstrated for
the first time, to the best of the authors' knowledge, that CDC25A
silencing could attenuate cell proliferation and induce cell cycle
arrest in NPC and the regulation of CDC25A were transcriptionally
modulated by the E2F1 transcription factor. Therefore, the present
study has provided valuable information that improves our
understanding of the pathogenesis of NPC, also indicating that
CDC25A may be a promising therapeutic target for NPC treatment.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by Medical Health Science and
Technology Project of Zhejiang Provincial Health Commission (grant
no. 2020KY695), and The Construction Fund of Key Medical
Disciplines of Hangzhou (grant no. OO20200123).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
BW and QG designed the study, drafted and revised
the manuscript. BW and FC analyzed the data and searched the
literature. BW, QG and FC performed the experiments. BW and QG
confirm the authenticity of all the raw data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhang P, He Q, Lei Y, Li Y, Wen X, Hong M,
Zhang J, Ren X, Wang Y, Yang X, et al: m6A-mediated
ZNF750 repression facilitates nasopharyngeal carcinoma progression.
Cell Death Dis. 9:11692018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chow JC, Ngan RK, Cheung KM and Cho WC:
Immunotherapeutic approaches in nasopharyngeal carcinoma. Expert
Opin Biol Ther. 19:1165–1172. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zheng ZQ, Li ZX, Zhou GQ, Lin L, Zhang LL,
Lv JW, Huang XD, Liu RQ, Chen F, He XJ, et al: Long noncoding RNA
FAM225A promotes nasopharyngeal carcinoma tumorigenesis and
metastasis by acting as ceRNA to sponge miR-590-3p/miR-1275 and
upregulate ITGB3. Cancer Res. 79:4612–4626. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee HM, Okuda KS, González FE and Patel V:
Current perspectives on nasopharyngeal carcinoma. Adv Exp Med Biol.
1164:11–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Guo Y, Zhai J, Zhang J, Ni C and Zhou H:
Improved radiotherapy sensitivity of nasopharyngeal carcinoma cells
by miR-29-3p targeting COL1A1 3′-UTR. Med Sci Monit. 25:3161–3169.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Brenner AK, Reikvam H, Lavecchia A and
Bruserud Ø: Therapeutic targeting the cell division cycle 25
(CDC25) phosphatases in human acute myeloid leukemia-the
possibility to target several kinases through inhibition of the
various CDC25 isoforms. Molecules. 19:18414–18447. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lin TC, Lin PL, Cheng YW, Wu TC, Chou MC,
Chen CY and Lee H: MicroRNA-184 deregulated by the MicroRNA-21
promotes tumor malignancy and poor outcomes in non-small cell lung
cancer via targeting CDC25A and c-Myc. Ann Surg Oncol. 22 (Suppl
3):S1532–S1539. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ding FN, Gao BH, Wu X, Gong CW, Wang WQ
and Zhang SM: miR-122-5p modulates the radiosensitivity of cervical
cancer cells by regulating cell division cycle 25A (CDC25A). FEBS
Open Bio. 9:1869–1879. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pu L, Su L and Kang X: The efficacy of
cisplatin on nasopharyngeal carcinoma cells may be increased via
the downregulation of fibroblast growth factor receptor 2. Int J
Mol Med. 44:57–66. 2019.PubMed/NCBI
|
|
10
|
Li MY, Liu JQ, Chen DP, Li ZY, Qi B, He L,
Yu Y, Yin WJ, Wang MY and Lin L: Radiotherapy induces cell cycle
arrest and cell apoptosis in nasopharyngeal carcinoma via the ATM
and Smad pathways. Cancer Biol Ther. 18:681–693. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Denechaud PD, Fajas L and Giralt A: E2F1,
a novel regulator of metabolism. Front Endocrinol (Lausanne).
8:3112017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shen C, Li J, Chang S and Che G:
Advancement of E2F1 in common tumors. Zhongguo Fei Ai Za Zhi.
23:921–926. 2020.(In Chinese). PubMed/NCBI
|
|
13
|
Farra R, Dapas B, Grassi M, Benedetti F
and Grassi G: E2F1 as a molecular drug target in ovarian cancer.
Expert Opin Ther Targets. 23:161–164. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bi XC, Pu XY, Liu JM and Huang S: Effect
of transcription factor E2F1 expression on the invasion of prostate
cancer. Zhonghua Yi Xue Za Zhi. 97:2856–2859. 2017.(In Chinese).
PubMed/NCBI
|
|
15
|
Godoy PRDV, Donaires FS, Montaldi APL and
Sakamoto-Hojo ET: Anti-proliferative effects of E2F1 suppression in
glioblastoma cells. Cytogenet Genome Res. 161:372–381. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu P, Zhang X, Li Z, Wei L, Peng Q, Liu
C, Wu Y, Yan Q and Ma J: A significant role of transcription
factors E2F in inflammation and tumorigenesis of nasopharyngeal
carcinoma. Biochem Biophys Res Commun. 524:816–824. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang X, Nie P and Zhu D: LncRNA HOXA10-AS
activated by E2F1 facilitates proliferation and migration of
nasopharyngeal carcinoma cells through sponging miR-582-3p to
upregulate RAB31. Am J Rhinol Allergy. 36:348–359. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ru Lee W, Chen CC, Liu S and Safe S:
17beta-estradiol (E2) induces cdc25A gene expression in breast
cancer cells by genomic and non-genomic pathways. J Cell Biochem.
99:209–220. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xiao Z and Chen Z: Deciphering
nasopharyngeal carcinoma pathogenesis via proteomics. Expert Rev
Proteomics. 16:475–485. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Qin H and Liu W: MicroRNA-99a-5p
suppresses breast cancer progression and cell-cycle pathway through
downregulating CDC25A. J Cell Physiol. 234:3526–3537. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sun Y, Li S, Yang L, Zhang D, Zhao Z, Gao
J and Liu L: CDC25A facilitates chemo-resistance in ovarian cancer
multicellular spheroids by promoting E-cadherin expression and
arresting cell cycles. J Cancer. 10:2874–2884. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yin W, Xu J, Li C, Dai X, Wu T and Wen J:
Circular RNA circ_0007142 facilitates colorectal cancer progression
by modulating CDC25A expression via miR-122-5p. Onco Targets Ther.
13:3689–3701. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jiang T and Cheng H: miR-34a-5p blocks
cervical cancer growth and migration by downregulating CDC25A. J
BUON. 26:1768–1774. 2021.PubMed/NCBI
|
|
25
|
Liu P, Xia P, Fu Q, Liu C, Luo Q, Cheng L,
Yu P, Qin T and Zhang H: miR-199a-5p inhibits the proliferation of
hepatocellular carcinoma cells by regulating CDC25A to induce cell
cycle arrest. Biochem Biophys Res Commun. 571:96–103. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sadeghi H, Golalipour M, Yamchi A,
Farazmandfar T and Shahbazi M: CDC25A pathway toward tumorigenesis:
Molecular targets of CDC25A in cell-cycle regulation. J Cell
Biochem. 120:2919–2928. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shen T and Huang S: The role of Cdc25A in
the regulation of cell proliferation and apoptosis. Anticancer
Agents Med Chem. 12:631–639. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sun X and Kaufman PD: Ki-67: More than a
proliferation marker. Chromosoma. 127:175–186. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Worby CA and Dixon JE: PTEN. Annu Rev
Biochem. 83:641–669. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang Z, Wang Y, Wang S, Meng X, Song F,
Huo W, Zhang S, Chang J, Li J, Zheng B, et al: Coxsackievirus A6
induces cell cycle arrest in G0/G1 phase for viral production.
Front Cell Infect Microbiol. 8:2792018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ditano JP, Sakurikar N and Eastman A:
Activation of CDC25A phosphatase is limited by CDK2/cyclin
A-mediated feedback inhibition. Cell Cycle. 20:1308–1319. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ma Y, Wang R, Lu H, Li X, Zhang G, Fu F,
Cao L, Zhan S, Wang Z, Deng Z, et al: B7-H3 promotes the cell
cycle-mediated chemoresistance of colorectal cancer cells by
regulating CDC25A. J Cancer. 11:2158–2170. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tang Y, Jiang L, Zhao X, Hu D, Zhao G, Luo
S, Du X and Tang W: FOXO1 inhibits prostate cancer cell
proliferation via suppressing E2F1 activated NPRL2 expression. Cell
Biol Int. 45:2510–2520. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ma J, He Z, Zhang H, Zhang W, Gao S and Ni
X: SEC61G promotes breast cancer development and metastasis via
modulating glycolysis and is transcriptionally regulated by E2F1.
Cell Death Dis. 12:5502021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bramis J, Zacharatos P, Papaconstantinou
I, Kotsinas A, Sigala F, Korkolis DP, Nikiteas N, Pazaiti A, Kittas
C, Bastounis E and Gorgoulis VG: E2F-1 transcription factor
immunoexpression is inversely associated with tumor growth in colon
adenocarcinomas. Anticancer Res. 24:3041–3047. 2004.PubMed/NCBI
|
|
36
|
Chan AB, Huber AL and Lamia KA:
Cryptochromes modulate E2F family transcription factors. Sci Rep.
10:40772020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tan Y, Wei X, Zhang W, Wang X, Wang K, Du
B and Xiao J: Resveratrol enhances the radiosensitivity of
nasopharyngeal carcinoma cells by downregulating E2F1. Oncol Rep.
37:1833–1841. 2017. View Article : Google Scholar : PubMed/NCBI
|