Introduction
Bone lesions due to trauma, inflammation and cancer
are common in orthopedics (1,2).
Currently, there are numerous methods for repairing bone defects,
including bone grafting, membrane guided tissue regeneration and
gene therapy. However, the efficacy of these methods for repairing
bone defects is not satisfactory and the clinical outcomes when
these methods are used, are not adequate to solve these problems in
the clinic (3–7). Tissue engineering is a process based
on principles and technologies from cell biology and material
science that allows scientists to fabricate a biomaterial complex,
which is used to repair a specific tissue or organ (8,9). It
was reported that biological materials could be integrated with
target cells or growth factors in the lab or clinic with the
potential to induce bone growth (10). Thus, this process makes it possible
to provide an effective approach for the treatment of large bone
lesion in orthopedics.
Porous Tantalum (pTa) is a promising material for
bone regeneration due to its excellent biocompatibility,
osteoconductive and osseointegration properties (11,12).
It has been reported that pTa has the potential to promote
osteogenic differentiation of human bone marrow mesenchymal stem
cells (BMSCs) and is beneficial for the attachment, growth and
differentiation of human osteoblasts (13). Moreover, pTa has been reported to
promote revascularization of areas of the femoral head which
contain avascular necrosis, promoting cell proliferation and
improving the osteogenic ability of osteoblasts (14).
BMSCs are known as seed cells in bone tissue
engineering because of their excellent self-renewal and
differentiation potential, as well as their ability to induce
osteogenesis in in vitro settings (15). However, risks such as immune
rejection, thrombosis, tumor formation and excessive proliferation,
limits their clinical application (16–18).
Exosomes are endosome-derived membrane nanovesicles with a diameter
of 40–100 nm and have recently been reported to serve crucial roles
in mediating intercellular communication with proteins, lipids, and
genetic material such as mRNA and microRNA (miRNA) (19,20).
Furthermore, exosomes are characterized by low immunogenicity, and
excellent biocompatibility and biodegradability. It was previously
reported that exosomes have become the preferred activator for bone
repair and reconstruction (21).
Critical-size bone defects are characterized as bone injuries or
defects that exceed a particular size threshold, impeding the
body's natural healing mechanisms and necessitating external
intervention for proper bone regeneration, previous studies have
demonstrated that exosomes secreted by BMSCs promote bone
regeneration in a model of critical-size bone defects (22–25).
The aim of the present study was to evaluate the
effects of exosomes for improving osteogenic differentiation and
cell proliferation. Moreover, in vivo pTa scaffolds combined
with exosomes extracted from BMSCs were implanted into the defected
regions of distal full thickness femurs in rats to assess the
effect of exosomes in bone defect repair in combination with
pTa.
Materials and methods
Ethics and animals
A total of 54 healthy male, specific pathogen-free
(SPF) Sprague-Dawley (SD) rats (age, 6 weeks; weight 400–450 g)
were used in the present study. They were purchased from the Animal
Experiment Center at Dalian Medical University. The conditions
under which the rats were housed during the experiment were as
follows: temperature of 18–26°C, relative humidity maintained at
40–70%, 12 h light/dark cycle, a feeding regimen with adherence to
standardized formula feed, and provision of sterile and clean feed
and drinking water. The animal experiments were all performed under
the standards of the Animal Ethics Committee of The Affiliated
Zhongshan Hospital of Dalian University (approval no.
201612009).
Isolation and culture of BMSCs
A total of 6 of the aforementioned rats were
anesthetized with 1% pentobarbital sodium injected
intraperitoneally (40 mg/kg) and the rats were then euthanized by
dislocation of the spine. Bone marrow was subsequently harvested
via gentle puncture to the tibia and femur. The total bone marrow
samples were combined (~8.0 ml) and resuspended in H-DMEM
(Invitrogen; Thermo Fisher Scientific, Inc.) and supplemented with
10% fetal bovine serum (FBS; Invitrogen; Thermo Fisher Scientific,
Inc.), 100 UI/ml penicillin (Invitrogen; Thermo Fisher Scientific,
Inc.) and 100 µg/ml streptomycin (Invitrogen; Thermo Fisher
Scientific, Inc.). Cells were subsequently plated on cell culture
plates for use in the studies. The cells were kept in humidified
incubators at 37°C and 5% CO2. Cultures were rinsed with
PBS (Biological Industries) after 2 days to remove nonadherent
cells and fresh growth media was added. After 10–12 days, the cells
reached ~80% confluence and were used in subsequent experimental
studies. Growth and proliferation of BMSCs were observed using a
CKX53 light microscope (Olympus Corporation).
Flow cytometry analysis of BMSCs
Flow cytometry was performed on BMSCs collected from
the third passage of cell culture. Cells were washed with PBS at
room temperature and subsequently suspended in 4% paraformaldehyde
(Sigma-Aldrich; Merck KGaA) for 15 min. The supernatant was divided
equally into five test tubes then incubated with cluster of
differentiation (CD)29-FITC (1:100; cat. no. 561796; BD Pharmingen;
BD Biosciences), CD34-FITC (1:200; cat. no. ab78165; Abcam),
CD44-FITC (1:100; cat. no. 203906; BioLegend, Inc.), CD45-FITC
(1:200; cat. no. ab33916; Abcam) or CD90-FITC (1:100; cat. no.
206105; BioLegend, Inc.) antibodies overnight at 4°C. After cells
were washed and resuspended in PBS, cell fluorescence was analyzed
using a FACS Calibur flow cytometer (Becton, Dickinson and
Company), and the data acquisition and analysis was performed with
CytExpert (v2.4; Beckman Coulter, Inc.).
Assessment of the induction of
differentiation ability of BMSCs
BMSCs (1×108/ml) from the fourth cell
passage were collected and seeded in two 6-well plates. Adipogenic
differentiation was induced using H-DMEN containing adipogenic
inducer [20% FBS (Invitrogen; Thermo Fisher Scientific, Inc.), 5
µg/ml insulin (Sigma-Aldrich; Merck KGaA), 50 µM indomethacin
(Sigma-Aldrich; Merck KGaA), 1 µM dexamethasone, 0.5 µM glutamine,
100 U/ml penicillin (Beyotime Institute of Biotechnology) and 100
µg/ml streptomycin (Beyotime Institute of Biotechnology)]. Cells
were cultured at 37°C and 5% CO2, culture medium was
changed every 2 or 3 days, and adipogenic induction was performed
for 21 days in total. Then the cells were fixed in 10% formalin at
room temperature for 20 min before oil red O staining to assess
lipid deposition using modified oil red O staining kit (no. C0158S,
Beyotime Institute of Biotechnology) for 15 min at room
temperature. Osteogenic differentiation was induced using H-DMEN
supplemented with osteogenic inducer [20% FBS (Invitrogen; Thermo
Fisher Scientific, Inc.), 20 mM β-phosphate glycerol
(Sigma-Aldrich; Merck KGaA), 1 nM dexamethasone, 50 ng/ml Thyroxine
(Sigma-Aldrich; Merck KGaA), 0.5 µM Ascorbate 2-phosphate
(Sigma-Aldrich; Merck KGaA), 12 mM glutamine, 100 U/ml penicillin
and 100 µg/ml streptomycin]. Cells were cultured at 37°C and 5%
CO2, culture medium was changed every 2 or 3 days, and
osteogenic induction was performed for 21 days in total. To
qualitatively evaluate intracellular alkaline phosphatase (ALP)
activity, cells were fixed with 10% formalin for 20 min at room
temperature, and stained with BCIP/NBT ALP color development kit
(no. C3206, Beyotime Institute of Biotechnology) and incubated in
the dark for 20 min at room temperature. The BCIP/NBT staining
working solution was prepared by adding 3 ml ALP chromogenic
buffer, 10 µl BCIP solution, 20 µl NBT solution according to the
manufacturer's instructions.
Isolation and purification of
exosomes
The present study utilized size exclusion
chromatography (SEC) to isolate and concentrate exosomes, which is
considered one of the optimal methods for separating and purifying
exosomes from samples (26). The
following procedures were performed according to the manufacturer's
instructions for the SuperEV 1.0 kit (Runji Biotechnology Co.,
Ltd.). Briefly, BMSCs were cultured and the supernatant (1 ml) was
centrifuged at 3,000 × g at 4°C for 10 min to remove cells or cell
debris. The resulting supernatant was transferred to the top of a
sieve plate and a 15 ml centrifuge tube was used to collect the
filtrate. After the sample was completely transferred to the sieve
plate, 7 ml of PBS was added. The first fraction (~8 ml), which did
not contain extracellular vesicles, was collected. The washing step
was repeated with 1 ml of PBS each time until no liquid flowed out
of the outlet, and each fraction was collected in 1 ml volumes. The
extracellular vesicles were mainly concentrated in fractions 2–4,
with a total volume of ~3 ml. The purification process of
extracellular vesicles was performed as follows: the aforementioned
fractions (~3 ml) were mixed with 0.3 ml binding buffer in a
centrifuge tube. After thorough mixing by inversion, 200 µl of
binding resin was added, and the mixture was inverted and mixed for
15 min at room temperature, followed by centrifugation at 1,500 × g
at room temperature for 3 min. Then, 0.5 ml of the supernatant was
aspirated with a pipette, the resin was gently blown up, and the
entire supernatant was transferred to a purification column in an
equipped collection tube. The column was allowed to stand for 2 min
and then centrifuged at 3,000 × g at room temperature for 2 min.
The filtrate and collection tube were discarded, and the
purification column was placed in a 1.5 ml centrifuge tube. A total
of 200 µl of elution buffer was added to the column, and it was
allowed to stand for 5 min. The column was then centrifuged at 500
× g at room temperature for 2 min, and the filtrate was added back
to the column. The column was allowed to stand for 2 min, and then
centrifuged at 500 × g at room temperature for 2 min. Finally, the
column was centrifuged at 3,000 × g at room temperature for 2 min,
and the resulting filtrate contained the concentrated exosomes. The
extracted exosomes were directly used in subsequent experiments or
stored at 2–8°C for one week.
Characterization of exosomes
Using a micropipette, 20 µl of the prepared exosome
suspension was deposited onto a copper grid, allowing for
spontaneous adsorption over a duration of 10 min. Subsequently,
surplus liquid droplets were removed using filter paper. Next, 20
µl of 2% phosphotungstic acid solution (Structure Probe, Inc.) was
deposited onto the copper grid, and allowed to rest for 5 min.
After which excess liquid droplets were removed using filter paper
and grids were left to air dry thoroughly. Transmission electron
microscopy (TEM; H7650; Hitachi, Ltd) was then performed at 80 kV
to capture images.
Diluted samples (1,000 fold) in PBS were analyzed to
assess the size of the exosomes using zeta view nanoparticle
tracking analyzer (version, PMX110; Particle Metrix GmbH), which
was calibrated using polystyrene microspheres.
Extracted exosomes were lysed in RIRF-PMSF buffer
(Beyotime Institute of Biotechnology) and quantified using a BCA
protein kit. Western blot was performed on these lysed extracts.
After the 10% SDS-PAGE separation gel was prepared, a total of 20
µl of sample (1 mg/µl) was loaded into each well of the gel, and
the gel was electrophoresed at 60 v for 30 min for the upper layer
and 110 v for 120 min for the lower layer. Bands were transferred
to a PVDF membrane. Next the membrane was cut and blocked with 5%
skimmed milk (0.75 g milk powder + 15 ml PBS) at 37°C for 1 h. The
blocked membrane was washed with PBS-T (1,000 ml 1×PBS + 1 ml
Tween-20), and then incubated with primary antibodies against
TSG101 (1:1,000; cat. no. 28283-1-AP; Proteintech Group, Inc.), CD9
(1:1,000; cat. no. 20597-1-AP; Proteintech Group, Inc.), HSP70
(1:3,000; cat. no. 10995-1-AP; Proteintech Group, Inc.), and GAPDH
(1:10,000; cat. no. 60004-1-Ig; Proteintech Group, Inc) overnight
at 4°C. The membrane was washed with PBST and then incubated with
Goat anti-rabbit IgG (H+L)-HRP secondary antibodies (1:5,000; cat.
no. 111-035-003; Jackson ImmunoResearch Laboratories, Inc.) at 37°C
for 1 h. The control group received the same treatment. The protein
band images were obtained by exposing the membrane to ECL
ultra-sensitive luminescence reagent (Beyotime Institute of
Biotechnology) and imaged using a gel imaging system.
Differentiation and proliferation of
BMSCs in vitro
BMSCs (1×108/l) at cell passages 4 and 5
were collected and inoculated on 6-well cell culture plates. Each
group of cells was treated as indicated in Table I. The concentration gradient set
for exosomes in the partial group and total exosome group was 50
and 100%, respectively, based on previous reports (22,23),
in order to evaluate whether exosome activity was related to the
concentration. A total of 2.5 ml of osteogenesis inducer (prepared
according to the aforementioned method) was added to each group
once every 3 days over a 21 day period. Culture fluid was discarded
from each sample and samples were fixed in 10% paraformaldehyde for
20 min at room temperature and stained for 20 min at room
temperature using an alizarin red S staining kit for osteogenesis
(no. C0148S, Beyotime Institute of Biotechnology) and BCIP/NBT ALP
color development kit. Samples were washed with PBS and examined
under a light microscope for cell staining. Induced osteoblasts
were cultured in L-DMEM complete medium at 37°C and 5%
CO2 (Gibco; Thermo Fisher Scientific, Inc.) and the cell
culture media was changed once every 3 days. Osteoblasts in passage
4 were seeded into 96-well plates (200 µl/well) at a concentration
of 1×104/ml. Each sample was divided into 3 groups as
follows: i) PBS (100 µl); ii) PBS (75 µl) + BMSCs-exosome
suspension (25 µl); and iii) PBS (50 µl) + BMSCs-exosome suspension
(50 µl), and incubated overnight with 5% CO2 at 37°C.
Microscopy was used to assess cell adherence after which 20 µl of
CCK-8 reagent (Corning, Inc.) was added to cells. After 4 h, the
absorbance value of each well was measured at a wavelength of 450
nm using a Multiskan SkyHigh microplate analyzer (Thermo Fisher
Scientific, Inc.). This experiment was repeated in triplicate and
the average value including standard error were recorded as the
final result. Furthermore, the column diagram of the cell
proliferation rates was drawn as an abscissa using proliferation
rates as vertical coordinates.
 | Table I.Experimental grouping for assessment
of the promotion of osteogenesis by exosomes in vitro. |
Table I.
Experimental grouping for assessment
of the promotion of osteogenesis by exosomes in vitro.
| Group | PBS (µl) | Exosomes suspension
(µl) |
|---|
| Control | 40 | 0 |
| Partial
exosomes | 20 | 20 |
| Total exosomes | 0 | 40 |
Generation of a three-dimensional pTa
scaffold
pTa materials were provided by the Orthopedics
Laboratory at The Affiliated Zhongshan Hospital of Dalian
University (China). pTa metal was machine-cut into flat cylinders
with a diameter of 3 mm and a height of 2 mm, based on the intended
shape and size of the bone defect area in the animal model.
Ultrasonic cleaning of the modules was performed twice in anhydrous
acetone, 70% ethanol and distilled water for 20 min each.
Subsequently, they were sterilized using high pressure steam and
dried before further use. Scanning electron microscopy (SEM) was
used to view the pTa modules after being treated with ion
sputtering using a gold spray apparatus.
Surgical procedure for in vivo
studies
A total of 48 of the aforementioned healthy male SPF
SD rats were randomly divided into 4 groups (n=12), the age of
which were the same as those in the aforementioned in vitro
studies section. The treatment of each group was presented in
Table II. Anesthesia was
delivered via intraperitoneal injection with 1% pentobarbital
sodium at 40 mg/kg. The dorsal surgical sites of each rat were
disinfected with iodophor solution after administration of
anesthesia. A longitudinal incision ~1.5 cm in length was made in
the anterior midline of the right knee joint. The joint was flexed
and the patella was laterally distended to expose the inclined
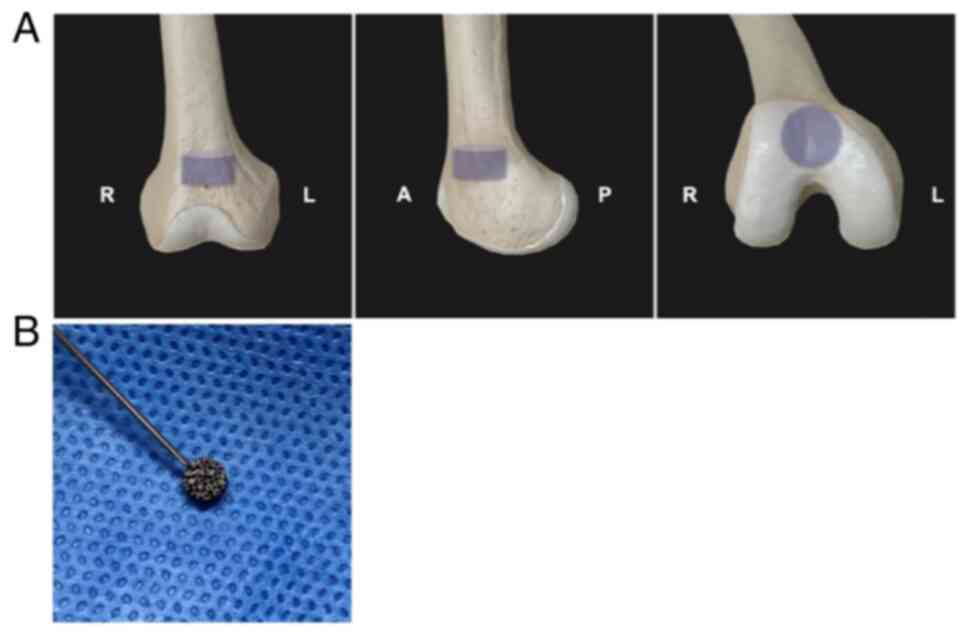
surface of the femoral condyle metaphyseal. A drill with diameter
of 2 mm was used to drill vertically 3 mm above femoral condyle to
reach the medullary bone and moved horizontally 3 mm leaving a
cavity 2 mm wide, 3 mm in length and 2 mm in height, as indicated
in Fig. 1A. Then, appropriate
modifications were made at the site according to the experiment
scheme and treatment group (Table
II). Negative control (NC) group rats contained surgically
drilled cavities without implantation of material. After bones
cavities of the exosome group of rats were rinsed and cleaned, 0.02
ml of BMSCs-exosome suspension was dropped into the cavities of the
exosomes (Exo) group. A pTa metal module was implanted in the bone
cavities of rats in the pTa (PT) group. A total of 0.02 ml of
BMSCs-exosome suspension was dropped onto the tantalum block using
a sterile injection syringe (Fig.
1B). After the exosome suspension completely penetrated the pTa
pores by standing for 20 min after dropping, they were implanted
into the bone cavities of the pTa integrated with exosomes (PE)
group. The incisions were sutured and the start date of the
experiment was noted. In order to protect the experimental animals
from unnecessary pain, humane endpoints were included in the study
design, briefly: The experiment must be stopped immediately if the
animals experienced unbearable pain or suffering, were subjected to
inappropriate housing conditions, if the scientific purpose of the
experiment was not fulfilled, if the animals were not adequately
protected and cared for, or if the experiment posed serious ethical
issues such as lack of sufficient approval from an animal ethics
committee.
 | Table II.Experimental grouping of the in
vivo experiment. |
Table II.
Experimental grouping of the in
vivo experiment.
| Group | Abbreviation | Treatments | Quantity of
exosomes (µl) |
|---|
| Control | NC | Full-thickness bone
defect | 0 |
| pTa | PT | Full-thickness bone
defect with pTa implantation | 0 |
| Exosomes | Exo | Full-thickness bone
defect with exosomes | 20 |
| pTa integrated with
exosomes | PE | Full-thickness bone
defect with pTa integrated with exosomes | 20 |
Imaging evaluation
Rats were sacrificed via cervical dislocation at 4,
8 and 12 weeks post-operation after intraperitoneal injection of 1%
pentobarbital sodium at a dose of 40 mg/kg, 4 rats in each group
were sacraficed at each time point. Right femur samples were
collected and fixed using 10% paraformaldehyde for 48 h at room
temperature. micro-CT scans (Siemens AG) were performed and visual
analysis. The scanning scheme was 80 kV and 500 A with a pixel size
set at 28.21 µm. Images and CT scan gray values were analyzed to
evaluate bone healing for each group. Visual data were collected
and reconstructed using Inveon Acquisition Workplace (v4.7, Siemens
AG) and Inveon Research Workplace software (v4.1, Siemens AG).
Histomorphology
Specimens scanned via micro-CT were removed from the
10% paraformaldehyde solution and dehydrated using an increasing
concentration alcohol series. Specimens were embedded in methyl
methacrylate resin for tissue slicing. Sections (10 µm) were
prepared from different sites in the bone for analysis using Gieson
staining, which was performed as follows: Tissue sections were
immersed in formic acid for 3 min and subsequently methanol for 2 h
at room temperature. Tissues were washed with deionized water and
incubated with methylene blue (Sigma-Aldrich; Merck KGaA) at 60°C
for 5 min. Tissue slices were washed with deionized water 3 times
and subsequently stained with picric acid-magenta solution
(Sigma-Aldrich; Merck KGaA) for 15 min at room temperature. Slides
were washed with anhydrous ethanol and observed under ×4 and ×10
magnifications using a CKX53 inverted microscope.
Statistical analysis
All data were statistically analyzed using SPSS 22.0
software (IBM Corp), with quantitative data presented as mean ±
standard deviation. Multiple comparisons were performed using
one-way ANOVA followed by LSD/SNK analyses to determine the
statistical significance between each group. P<0.05 was
considered to indicate a statistically significant difference.
Results
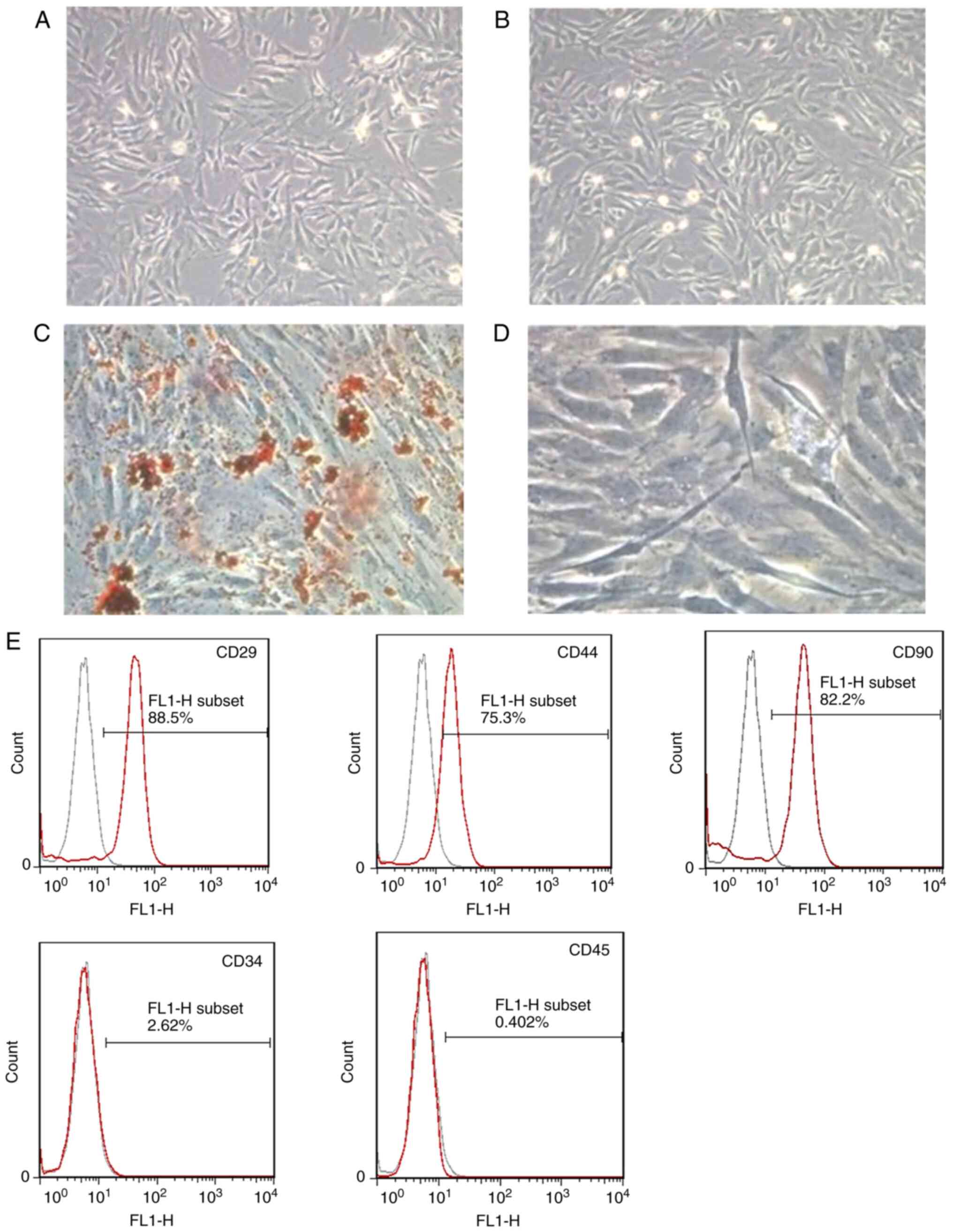
Characteristics of BMSCs
BMSCs seeded in fresh, complete culture medium were
observed using an inverted microscope. Representative histological
images of day 7 and day 14 were presented in Fig. 2A and B, respectively. After 7 days
of cell culture, cells could be seen adhering to the walls of the
culture dish and growing with varying rod-like or spindle-shaped
appearances. After 14 days of cell culture, cells could be seen
growing well with a uniform distribution and a single spindle or
spindle-like shape. Flow cytometry was used to assess antigen
surface markers and BMSCs. The cells isolated from bone marrow were
demonstrated to express CD29, CD44 and CD90 at high levels, whereas
minimal expression was demonstrated for CD34 and CD45 markers
(Fig. 2E). These results suggested
that cells extracted from bone marrow consisted of primarily BMSCs
(27,28). The multipotent differentiation
capacity of the extracted BMSCs was assessed by monitoring the
lipid droplets and ALP deposition after adipogenic and osteogenic
induction, without conducting baseline assessments. After 21 days
of adipogenesis induction, red-stained lipid droplets were observed
deposited inside the cells following Oil Red O staining (Fig. 2C). Furthermore, it was observed
that cells extracted from bone marrow expressed ALP after
osteogenic induction for 21 days (Fig.
2D). The staining results indicated that the cells isolated
during the bone marrow studies potentially had the capacity for
differentiation.
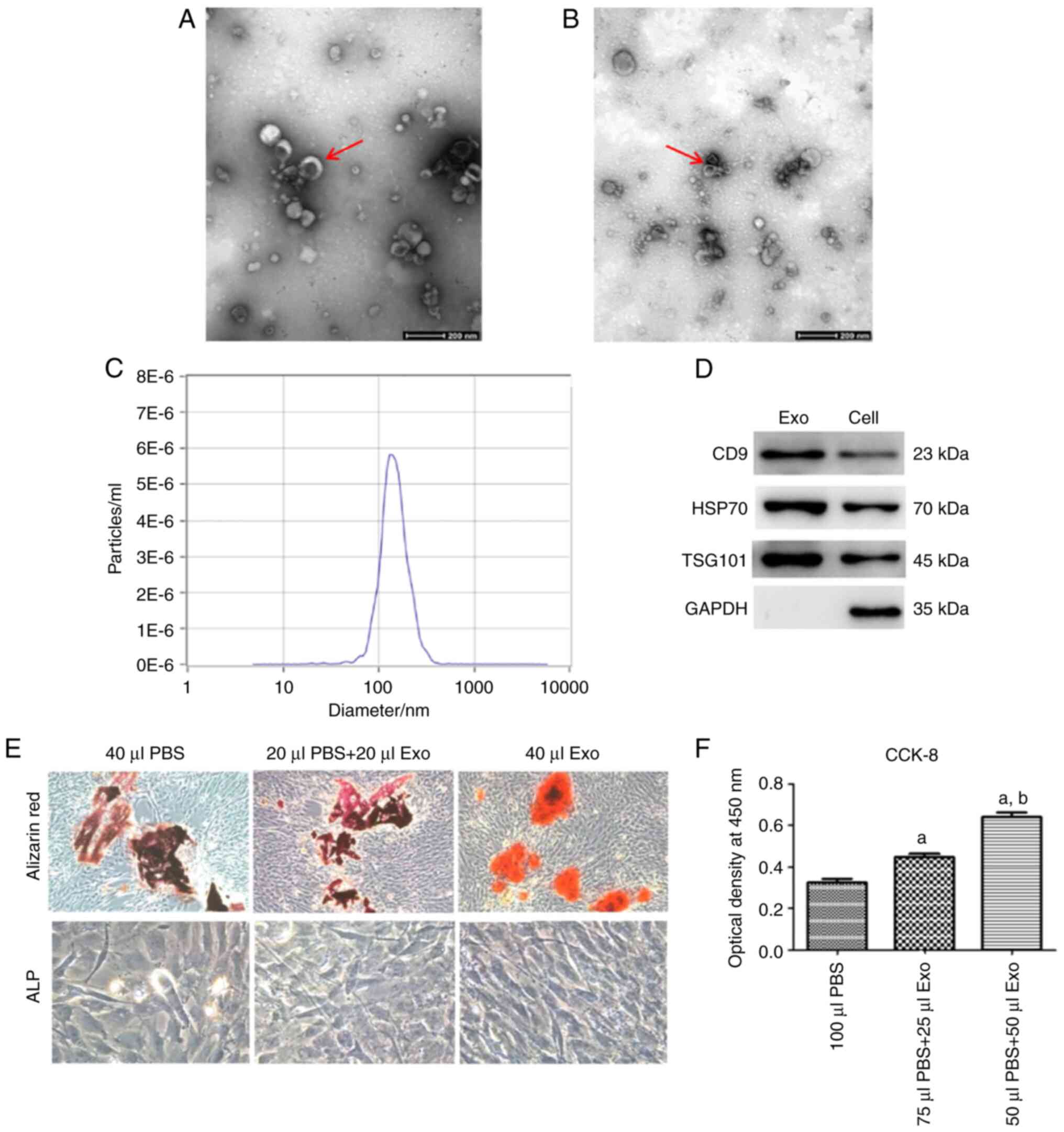
Identification of BSMC-exosomes
Exosomes were characterized using TEM to determine
size and structure, which indicated that their structure was a
cup-shaped phospholipid bilayer 40–100 nm in diameter (Fig. 3A and B). Size distribution of the
exosomes was determined using nanoparticle tracking analysis (NTA).
The concentration of the exosomes was assessed to be
6.0×1010/ml, with a mode size of 142.2 nm (Fig. 3C). Western blotting results
indicated that proteins extracted from these exosomes expressed
traditional exosome markers, such as CD9, HSP70 and TSG101
(Fig. 3D). Taken collectively,
these data suggested successful isolation of BMSCs-exosomes from
the bone marrow cells.
Promotion of BMSCs-exosomes in
differentiation and proliferation
Primary BMSCs were cultured with osteogenic inducer
for 21 days, after which Alizarin red and ALP staining of samples
was used to determine if exosome treatment could promote BMSCs
differentiation (Fig. 3E). Cells
cultured with 40 µl of BMSCs-exosomes demonstrated robust staining,
which suggested calcium nodule formation enhancement. Samples
cultured with exosomes demonstrated an overall higher degree of
staining compared with the control group. Cells cultured with
exosomes demonstrated a significantly higher-level of ALP activity
compared with the control group. Moreover, it was demonstrated that
cells treated with BMSCs-exosomes (40 µl) demonstrated
significantly higher levels of cell death compared with the cells
treated with the PBS (20 µl) and BMSCs-exosome (20 µl) mixture.
These data further indicated an association between the amount of
exosomes and ALP activity in BMSCs after osteogenic induction.
Calcium nodule formation and high ALP activity in cells treated
with high levels of exosomes further indicated their role in
osteogenic differentiation.
To evaluate if BMSCs-exosomes had the potential to
enhance BMSCs proliferation, the CCK-8 assay was used to evaluate
the OD value for each cell culture sample after passage 4, which
reflected indirectly, the extent of cell proliferation. A positive
association between the proliferation of BMSCs and concentration of
exosomes was demonstrated (Fig.
3F). Cell cultures containing exosomes promoted higher
proliferation of BMSCs than controls containing no exosomes.
Furthermore, the OD value for each cell culture increased as the
number of exosomes added to the medium increased. The data
presented were representative of triplicate experiments for all
groups. These results indicated that exosome treatment not only
promoted BMSCs proliferation, but also had the potential to
accelerate this process at higher doses.
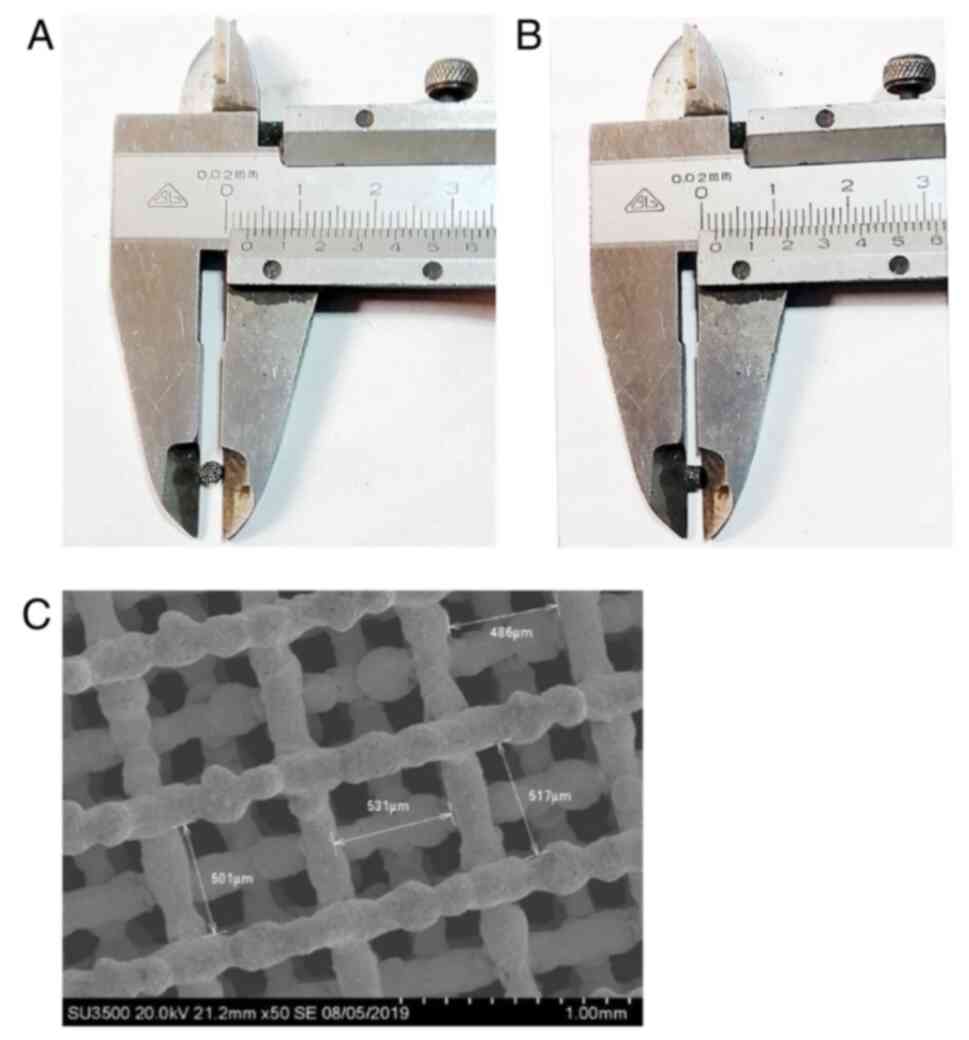
Characteristics of pTa
The pTa module used in the present study was flat
and cylinder-shaped, approximately 3 mm in diameter (Fig. 4A) and 2 mm in height (Fig. 4B). The surface was comprised of
interlinked three-dimensional honeycomb pores. The surface
structures of pTa were imaged using SEM (Fig. 4C). SEM results showed that the
internal micropores of pTa were cross-linked with each other, with
pore gaps of ~500 µm and a pore size of ~80%.
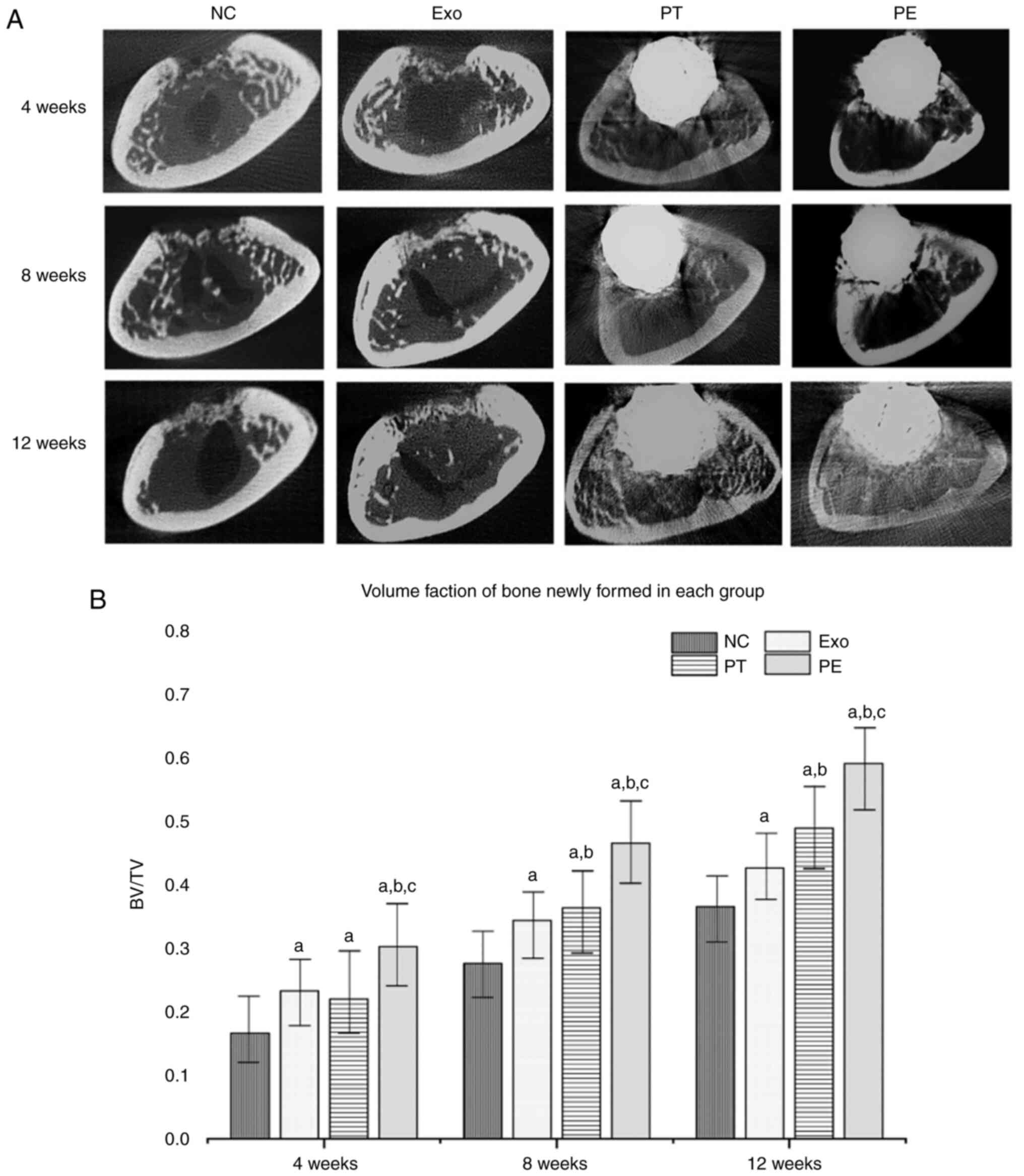
Bone cavity repair assessment
Micro-CT scans were used to observe the osteogenic
effect associated with the scaffold at 4, 8 and 12 weeks
post-surgery (Fig. 5A). At 4
weeks, no marked increase in osteogenic activity was demonstrated
in the NC group, with a small amount of bone callus formed;
however, the bone cavity was still clearly visible. It should be
noted that markedly more regenerative growth was demonstrated in
the PE group compared with NC, Exo groups. At 8 weeks, new bone was
beginning to form in all samples compared with week 4. Only minimal
cell growth was observed in the NC and Exo groups, whereas markedly
more growth was observed in the PT group containing scaffolds;
however, this growth was markedly less than that of the PE treated
group. At 12 weeks, the bone cavity in the NC group was filled;
however, the micro-CT demonstrated low bone density compared with
the control group, as well as a lack of contact with the scaffold.
There was no marked difference demonstrated between the NC and Exo
groups. The bone density of new bone around the implant material in
the PT group was close to the control group; however, there was
still as small gap between the new bone and the implant. In the PE
treated group, the newly formed bone around the implant was similar
to natural bone, which suggested that bone growth was stimulated
with the pTa scaffold and exosomes compared with the other groups.
Based on the micro-CT scans, a column diagram of the volume
fraction of new bone at different time points was constructed
(Fig. 5B). The PE group had a
better osteogenic value at 4, 8 and 12 weeks compared with the
other groups during the same period, which indicated better bone
repair due to the combined pTa scaffold and exosome treatment.
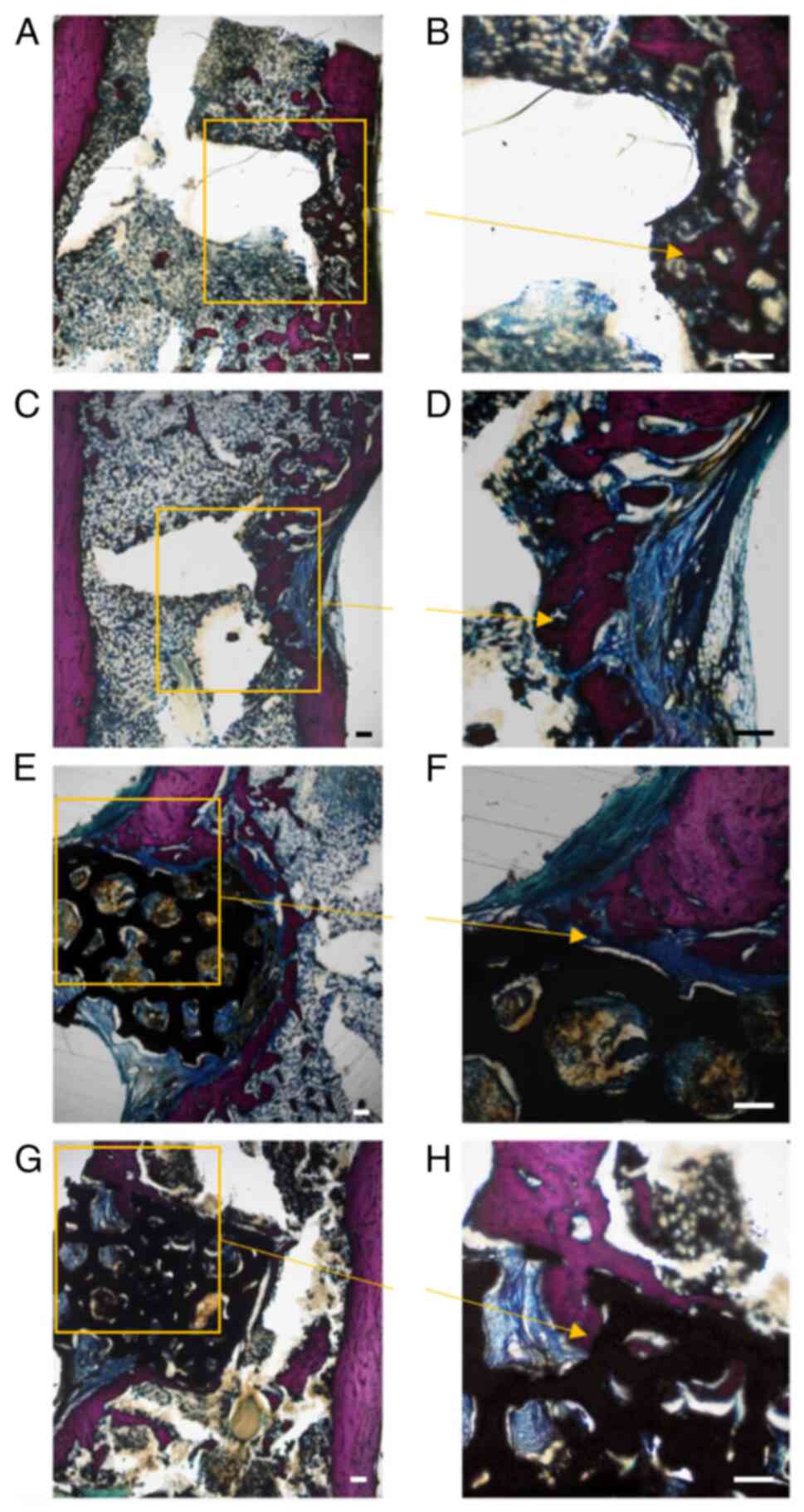
Histological images of Gieson staining of each bone
cavity at 12 weeks post-surgery presented bones from the NC group
which contained few and scattered new bone trabeculae surrounding
blue-stained fibrous tissues at the edge of each cavity (Fig. 6A and B). In contrast, the amount of
newly formed bone was evident in bone treated with exosomes
(Fig. 6C and D). These bones
contained a more compact fiber structure than the other groups.
However, it should be noted that in both the NC and Exo groups,
there were large areas containing fibrous connective tissue.
Implants in the PT and PE groups were observed to be compatible
with bone tissue and remained in appropriate positions (Fig. 6E and G). Most importantly, the PT
and PE groups contained a higher amount of calcium salt deposition,
high levels of cartilage formation and high levels of osteoblasts
(Fig. 6F and H). New bone mass and
maturity gradually increased at the interface and inside the inner
pores of the scaffold in the PT and PE groups, which indicated the
biocompatibility and osteogenic ability of pTa. Notably, there was
a visible seam between the scaffold and adjacent bone in PT group
while the interface between the bone tissue and pTa was more
uniform and smooth in the PE group, which indicated that the bone
integration of pTa was further enhanced after being combined with
exosomes (Fig. 6E-H). These
results demonstrated that pTa combined with exosomes had the
potential to accelerate the formation of new bone at 12 weeks
post-implantation.
Discussion
Challenges to large bone defect repair include high
incidence rate, requirement for a long course of treatment and
difficulties, such as physical disability, promoted by deep lesions
(29). Exosomes have great
potential in the field of bone defect repair due to properties,
such as promotion of tissue repair and angiogenesis, improvement of
the local cell microenvironment and low immunogenicity (3,25,30).
Wang et al (31) recently
reported that BMSCs-exosomes immobilized on a titanium (Ti) surface
had the potential to enhance BMSCs adhesion and proliferation, and
upregulate stromal cell-derived factor gene expression. Exosomes
have the potential to be designed for drug delivery based to their
properties in order to increase efficacy. Liu et al
(32) recently reported that
exosomes derived from bacteria have the potential to be bioactive
nanocarriers for drug delivery to damaged bones. BMSCs-exosomes
have been reported to serve a role in BMSCs paracrine signaling,
specifically in the bone tissue repair process, which indicated a
protective effect (25). However,
the mechanism of how exosomes influence BMSCs is still unclear. A
recent study reported that exosomes secreted by BMSCs contain
miRNAs related to osteogenesis, which may be partly responsible for
inducing osteogenic differentiation of receptor BMSCs (33). Zhang et al (28) reported that BMSCs-exosomes have the
potential to promote osteogenic differentiation through the
activation of the BMP-2/Smad1/RUNX2 signaling pathway, which may be
one of the underlying mechanisms in bone fracture healing. The
present study demonstrated that as the concentration of the
BMSCs-exosomes increased, so did calcium nodule deposition as shown
through Alizarin red and ALP staining (Fig. 3E). Furthermore, alkaline
phosphatase activity was demonstrated to be increased and cell
proliferation to be accelerated. Thus, it could be hypothesized
that BMSCs-exosomes serve an important role in improving the
proliferation and differentiation of BMSCs. Researchers have
previously generated certain engineered exosomes through
modification, to obtain more efficient and accurate therapeutic
effects (34). Hu et al
(35) constructed engineered
exosomes from NIH-3T3 cells that highly expressed a C-X-C motif
chemokine receptor 4 and combined them with liposomes carrying
antiagmir-188, which would allow for targeted drug released and
promotion of the osteogenesis of bone marrow stromal cells, as well
as inhibition of fat production. Lin et al (36) constructed a specific gene-activated
exosome that could effectively modulate the gene release of
vascular endothelial growth factor 165, as well as serve a role in
enhancing therapy for vascular bone regeneration.
Bone tissue engineering typically requires bioactive
materials for mechanical support and loading of bioactive factors.
Swanson et al recently reported the loading of exosomes on
poly(lactic-co-glycolic acid) and poly(ethylene glycol) triblock
copolymer microspheres and incorporated them into a nanofiber
poly(l-lactic acid) scaffold, which allowed for the local and
controlled release of exosomes into the tissue, which protected
their bioactivity (37).
Furthermore, Ma et al (38)
reported that bioactive factors loaded on pTa had slow-release
properties that promote osteogenesis. In the present study,
exosomes were loaded on pTa scaffolds and implanted into the bone
defect to evaluate if they served a synergistic role in the
promotion of bone regeneration. The data indicated that from 4 to 8
weeks post-surgery the rate of new bone growth was much lower in
the EXO group compared with PT and PE groups, this could be because
the BMSCs-exosomes were rendered inoperable once applied to bone
defects, which lead to ineffective drug delivery to the area.
Furthermore, the volume fraction of new bone in the PE group was
significantly higher than in the other groups, with bone growth
higher than that of the PT group 4–8 weeks post-surgery. This
suggested that pTa was able to effectively prevent degradation or
inactivation of exosomes resulting in more efficient bone
regeneration. Nevertheless, the release curve of the exosomes was
not assessed in the present study and remains to be elucidated.
Many materials have been reported to show promise in
the promotion of bone regeneration in animal models (39,40).
For example, titanium (Ti) is a common metal implant material,
which was has been reported to promote cell adhesion, proliferation
and bone tissue regeneration, and is widely used in clinic practice
(41). However, the intrinsic
properties of Ta affords greater biocompatibility for BMSCs and
improves overall osteogenesis compared with Ti (42–44).
Thus, the present study used Ti as the carrier of choice to repair
bone defects in the models used. Previous studies have reported
excellent biocompatibility, bone growth and sufficient mechanical
strength using pTa (45,46). Zhao et al (14,47)
used pTa manufactured via 3-dimensional printing and chemical
deposition technology to treat femoral head necrosis in the clinic.
This indicated that pTa not only had the ability to act as an
excellent scaffold, but also to act as a major mediator of
osteoblast proliferation and growth for bone defects. The results
of the CT scans in the present study (Fig. 5A) demonstrated mature osteocytes
around the pTa material resembling normal bone tissue 12 weeks
post-operation in the pTa-exosome treated group. Notably, a large
amount of the new bone adhered tightly to the scaffold without
obvious gaps. The results of previous reports and the present study
both suggest superior biocompatibility can be achieved with
pTa.
pTa has previously been reported to be beneficial
for bone growth and bone-implant fixation in in vivo models
(43). Images of Gieson staining
of bone tissue samples at 12 weeks post-surgery were captured for
evaluation and analysis (Fig. 6).
These data demonstrated sparse and scattered nascent trabeculae
formation in the NC group, while the exosome and PT groups
expressed tightly integrated trabeculae, further illustrating the
osteoinductive properties of BMSCs-exosomes and pTa. Interestingly,
the thickness and regularity of nascent bone trabeculae was
significantly better in the PE group compared with the others,
which suggested a trend of bone tissue regeneration. In the PE
group the new bone tissue not only tightly wrapped around the
surface of the scaffold but also grew into the internal pores,
while the rest of the pores were occupied by fibrous connective
tissue, which further improved the stability of the implant. The
microscopy results were consistent with the micro-CT scans. Based
on the above histological and imaging results, it is possible to
attribute the excellent bone integration and regeneration of the PE
group to the synergistic effects of exosome therapy and pTa.
In summary, all of our studies have demonstrated
that exosomal therapy combined with pTa can improve bone
regeneration of femur defects in rats by promoting osteogenesis.
However, the relationship between the quantity and concentration of
exosomes and the amount of newly formed bone needs to be
investigated more thoroughly in future studies.
In conclusion it is known that long-term preclinical
testing for decades is required to create engineering scaffolds for
use in clinical practice. One of the most important factors in
implantation and cellular therapy is patient safety. Using the
approach reported in the present study, pTa combined with
BMSCs-exosomes had no inhibitory effect on the proliferation of
BMSCs in vitro and also promoted the adhesion and
differentiation of autologous cells. Furthermore, the in
vivo studies demonstrated that exosomes and pTa could be
leveraged for bone regeneration with a high degree of safety,
effectiveness and simplicity. This work demonstrated the potential
for significant clinical advancement for the repair of large bone
defects in the future.
Acknowledgements
Not applicable.
Funding
This work was supported by The China Postdoctoral Science
Foundation (grant no. 285395), The Dalian Medical Science Research
Project (grant no. 2111038), Dalian Key Medical Specialties ‘Peak
Climbing’ Program 2021 (grant no. 243) and The National Natural
Science Foundation of China (grant no. 82172398).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
BL and DZ conceived and designed the experiments.
HC, SM, JL, YL, CL and JY performed the experiments. FY and MW
analyzed the data. FY and BL confirm the authenticity of all the
raw data. FY and MW were major contributors in writing the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The animal experiments (approval no. 201612009) were
all performed according to the standards of The Animal Ethics
Committee of The Affiliated Zhongshan Hospital of Dalian
University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jeon YS, Jeong HY, Lee DK and Rhee YG:
Borderline glenoid bone defect in anterior shoulder instability:
Latarjet procedure versus bankart repair. Am J Sports Med.
46:2170–2176. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Inglis S, Schneider KH, Kanczler JM, Redl
H and Oreffo ROC: Harnessing human decellularized blood vessel
matrices and cellular construct implants to promote bone healing in
an ex vivo organotypic bone defect model. Adv Healthc Mater.
8:e18000882019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Iordachescu A, Hulley P and Grover LM: A
novel method for the collection of nanoscopic vesicles from an
organotypic culture model. RSC Adv. 8:7622–7632. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gleeson JP, Plunkett NA and O'Brien FJ:
Addition of Hydroxyapatite Improves Stiffness, Interconnectivity
And Osteogenic Potential Of A Highly Porous Collagen-Based Scaffold
For Bone Tissue Regeneration. Eur Cell Mater. 20:218–230. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ghanaati S, Barbeck M, Booms P, Lorenz J,
Kirkpatrick CJ and Sader RA: Potential lack of ‘standardized’
processing techniques for production of allogeneic and xenogeneic
bone blocks for application in humans. Acta Biomater. 10:3557–3562.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Andrzejowski P, Masquelet A and Giannoudis
PV: Induced Membrane Technique (Masquelet) for bone defects in the
distal tibia, foot, and ankle: systematic review, case
presentations, tips, and techniques. Foot Ankle Clin. 25:537–586.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bouguennec N and Colombet P: Iterative
rupture of the patellar tendon: A case report of an original
technique for revision reconstruction using an adjustable loop and
an artificial ligament. Case Rep Orthop.
2018:61072872018.PubMed/NCBI
|
|
8
|
Henkel J, Woodruff MA, Epari DR, Steck R,
Glatt V, Dickinson IC, Choong PF, Schuetz MA and Hutmacher DW: Bone
regeneration based on tissue engineering conceptions-A 21st century
perspective. Bone Res. 1:216–248. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Langer R and Vacanti JP: Tissue
engineering. Science. 260:920–926. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu S, Liu X, Yeung KWK, Liu C and Yang X:
Biomimetic porous scaffolds for bone tissue engineering. Mater Sci
Eng R-Rep. 80:1–36. 2014. View Article : Google Scholar
|
|
11
|
Stiehler M, Lind M, Mygind T, Baatrup A,
Dolatshahi-Pirouz A, Li H, Foss M, Besenbacher F, Kassem M and
Bünger C: Morphology, proliferation, and osteogenic differentiation
of mesenchymal stem cells cultured on titanium, tantalum, and
chromium surfaces. J Biomed Mater Res A. 86:448–458. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Levine BR, Sporer S, Poggie RA, Della
Valle CJ and Jacobs JJ: Experimental and clinical performance of
porous tantalum in orthopedic surgery. Biomaterials. 27:4671–4681.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Findlay DM, Welldon K, Atkins GJ, Howie
DW, Zannettino AC and Bobyn D: The proliferation and phenotypic
expression of human osteoblasts on tantalum metal. Biomaterials.
25:2215–2227. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhao D, Zhang Y, Wang W, Liu Y, Li Z, Wang
B and Yu X: Tantalum rod implantation and vascularized iliac
grafting for osteonecrosis of the femoral head. Orthopedics.
36:789–795. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao D, Cui D, Wang B, Tian F, Guo L, Yang
L, Liu B and Yu X: Treatment of early stage osteonecrosis of the
femoral head with autologous implantation of bone marrow-derived
and cultured mesenchymal stem cells. Bone. 50:325–330. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Herberts CA, Kwa MS and Hermsen HP: Risk
factors in the development of stem cell therapy. J Transl Med.
9:292011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Amariglio N, Hirshberg A, Scheithauer BW,
Cohen Y, Loewenthal R, Trakhtenbrot L, Paz N, Koren-Michowitz M,
Waldman D, Leider-Trejo L, et al: Donor-derived brain tumor
following neural stem cell transplantation in an ataxia
telangiectasia patient. PLoS Med. 6:e10000292009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kansu E: Thrombosis in stem cell
transplantation. Hematology. 17 (Suppl 1):S159–S162. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Valadi H, Ekstrom K, Bossios A, Sjostrand
M, Lee JJ and Lotvall JO: Exosome-mediated transfer of mRNAs and
microRNAs is a novel mechanism of genetic exchange between cells.
Nat Cell Biol. 9:654–659. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Braicu C, Tomuleasa C, Monroig P, Cucuianu
A, Berindan-Neagoe I and Calin GA: Exosomes as divine messengers:
Are they the Hermes of modern molecular oncology? Cell Death
Differ. 22:34–45. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li J, Liu K, Liu Y, Xu Y, Zhang F, Yang H,
Liu J, Pan T, Chen J, Wu M, et al: Exosomes mediate the
cell-to-cell transmission of IFN-α-induced antiviral activity. Nat
Immunol. 14:793–803. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Qi X, Zhang J, Yuan H, Xu Z, Li Q, Niu X,
Hu B, Wang Y and Li X: Exosomes secreted by human-induced
pluripotent stem cell-derived mesenchymal stem cells repair
critical-sized bone defects through enhanced angiogenesis and
osteogenesis in osteoporotic rats. Int J Biol Sci. 12:836–849.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang J, Liu X, Li H, Chen C, Hu B, Niu X,
Li Q, Zhao B, Xie Z and Wang Y: Exosomes/tricalcium phosphate
combination scaffolds can enhance bone regeneration by activating
the PI3K/Akt signaling pathway. Stem Cell Res Ther. 7:1362016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang S, Chu WC, Lai RC, Lim SK, Hui JH
and Toh WS: Exosomes derived from human embryonic mesenchymal stem
cells promote osteochondral regeneration. Osteoarthritis Cartilage.
24:2135–2140. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Furuta T, Miyaki S, Ishitobi H, Ogura T,
Kato Y, Kamei N, Miyado K, Higashi Y and Ochi M: Mesenchymal stem
cell-derived exosomes promote fracture healing in a mouse model.
Stem Cells Transl Med. 5:1620–1630. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Guan S, Yu H, Yan G, Gao M, Sun W and
Zhang X: Characterization of urinary exosomes purified with size
exclusion chromatography and ultracentrifugation. J Proteome Res.
19:2217–2225. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gao J, Zhang G, Xu K, Ma D, Ren L, Fan J,
Hou J, Han J and Zhang L: Bone marrow mesenchymal stem cells
improve bone erosion in collagen-induced arthritis by inhibiting
osteoclasia-related factors and differentiating into chondrocytes.
Stem Cell Res Ther. 11:1712020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang L, Jiao G, Ren S, Zhang X, Li C, Wu
W, Wang H, Liu H, Zhou H and Chen Y: Exosomes from bone marrow
mesenchymal stem cells enhance fracture healing through the
promotion of osteogenesis and angiogenesis in a rat model of
nonunion. Stem Cell Res Ther. 11:382020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Faldini C, Traina F, Perna F, Borghi R,
Nanni M and Chehrassan M: Surgical treatment of aseptic forearm
nonunion with plate and opposite bone graft strut. Autograft or
allograft? Int Orthop. 39:1343–1349. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Qin Y, Sun R, Wu C, Wang L and Zhang C:
Exosome: A novel approach to stimulate bone regeneration through
regulation of osteogenesis and angiogenesis. Int J Mol Sci.
17:7122016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang X, Shah FA, Vazirisani F, Johansson
A, Palmquist A, Omar O, Ekström K and Thomsen P: Exosomes influence
the behavior of human mesenchymal stem cells on titanium surfaces.
Biomaterials. 230:1195712020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu H, Zhang Q, Wang S, Weng W, Jing Y and
Su J: Bacterial extracellular vesicles as bioactive nanocarriers
for drug delivery: Advances and perspectives. Bioact Mater.
14:169–181. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang XQ, Omar O, Vazirisani F, Thomsen P
and Ekstrom K: Mesenchymal stem cell-derived exosomes have altered
microRNA profiles and induce osteogenic differentiation depending
on the stage of differentiation. PLoS One. 13:e01930592018.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ma S, Zhang Y, Li S, Li A, Li Y and Pei D:
Engineering exosomes for bone defect repair. Front Bioeng
Biotechnol. 10:10913602022. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hu Y, Li X, Zhang Q, Gu Z, Luo Y, Guo J,
Wang X, Jing Y, Chen X and Su J: Exosome-guided bone targeted
delivery of Antagomir-188 as an anabolic therapy for bone loss.
Bioact Mater. 6:2905–2913. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lin T, Zha Y, Zhang X, Chen J, Li Y, Wang
Z, Zhang S, Wang J and Li Z: Gene-activated engineered exosome
directs osteoblastic differentiation of progenitor cells and
induces vascularized osteogenesis in situ. Chem Eng J.
400:1259392020. View Article : Google Scholar
|
|
37
|
Swanson WB, Zhang Z, Xiu K, Gong T, Eberle
M, Wang Z and Ma PX: Scaffolds with controlled release of
pro-mineralization exosomes to promote craniofacial bone healing
without cell transplantation. Acta Biomater. 118:215–232. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ma L, Cheng S, Ji X, Zhou Y, Zhang Y, Li
Q, Tan C, Peng F, Zhang Y and Huang W: Immobilizing magnesium ions
on 3D printed porous tantalum scaffolds with polydopamine for
improved vascularization and osteogenesis. Mater Sci Eng C Mater
Biol Appl. 117:1113032020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shi Y, He R, Deng X, Shao Z, Deganello D,
Yan C and Xia Z: Three-dimensional biofabrication of an
aragonite-enriched self-hardening bone graft substitute and
assessment of its osteogenicity in vitro and in vivo. Biomater
Transl. 1:69–81. 2020.PubMed/NCBI
|
|
40
|
Jing X, Ding Q, Wu Q, Su W, Yu K, Su Y, Ye
B, Gao Q, Sun T and Guo X: Magnesium-based materials in
orthopaedics: Material properties and animal models. Biomater
Transl. 2:197–213. 2021.PubMed/NCBI
|
|
41
|
Liu B, Ma Z, Li J, Xie H, Wei X, Wang B,
Tian S, Yang J, Yang L, Cheng L, et al: Experimental study of a 3D
printed permanent implantable porous Ta-coated bone plate for
fracture fixation. Bioact Mater. 10:269–280. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lu MM, Wu PS, Guo XJ, Yin LL, Cao HL and
Zou D: Osteoinductive effects of tantalum and titanium on bone
mesenchymal stromal cells and bone formation in ovariectomized
rats. Eur Rev Med Pharmacol Sci. 22:7087–7104. 2018.PubMed/NCBI
|
|
43
|
Wang H, Su K, Su L, Liang P, Ji P and Wang
C: Comparison of 3D-printed porous tantalum and titanium scaffolds
on osteointegration and osteogenesis. Mater Sci Eng C Mater Biol
Appl. 104:1099082019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lu M, Zhuang X, Tang K, Wu P, Guo X, Yin
L, Cao H and Zou D: Intrinsic surface effects of tantalum and
titanium on integrin α5β1/ERK1/2 pathway-mediated osteogenic
differentiation in rat bone mesenchymal stromal cells. Cell Physiol
Biochem. 51:589–609. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Dou X, Wei X, Liu G, Wang S, Lv Y, Li J,
Ma Z, Zheng G, Wang Y, Hu M, et al: Effect of porous tantalum on
promoting the osteogenic differentiation of bone marrow mesenchymal
stem cells in vitro through the MAPK/ERK signal pathway. J Orthop
Transl. 19:81–93. 2019.
|
|
46
|
Wei X, Liu B, Liu G, Yang F, Cao F, Dou X,
Yu W, Wang B, Zheng G, Cheng L, et al: Mesenchymal stem cell-loaded
porous tantalum integrated with biomimetic 3D collagen-based
scaffold to repair large osteochondral defects in goats. Stem Cell
Res Ther. 10:722019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhao D, Liu B, Wang B, Yang L, Xie H,
Huang S, Zhang Y and Wei X: Autologous bone marrow mesenchymal stem
cells associated with tantalum rod implantation and vascularized
iliac grafting for the treatment of end-stage osteonecrosis of the
femoral head. Biomed Res Int. 2015:2405062015.PubMed/NCBI
|