Introduction
Acute myeloid leukemia (AML) is a clonal malignant
proliferative disease that is caused by the blocking of
hematopoietic stem cells at a specific stage of directed
differentiation and the accumulation of immature blood cells. The
current standard treatment regimen can achieve complete remission
(CR) in 50–70% of patients with AML, but ~76% of patients relapse
or die due to the development of resistance (1,2). In
recent years, with the use of all-trans retinoic acid to induce the
differentiation of cancer cells in acute promyelocytic leukemia,
with its advantages of high efficacy and low side effects, tumor
differentiation therapy has become a research hotspot; however, the
mechanism of its action remains unclear (1). Therefore, clarifying the mechanisms
of differentiation in the treatment of leukemia may assist in
identifying low toxicity and efficacious treatments, to eventually
reduce the recurrence of AML in patients (2).
Nod-like receptor (NLR) family pyridine domain
containing 3 (NLRP3) is an important pattern recognition receptor
(PRR) in the cytoplasm, and its tripartite domain organization
consists of a carboxyterminal leucine-rich repeat (LRR) domain with
self-inhibitory function and signal recognition ability, a central
nucleotide-binding domain (NACHT) with ATPase activity and
mediating self-oligomerization, and an amino-terminal pyridines
(PYD) domain recruiting apoptosis-associated dot-like proteins
containing CARD (ASC) (3). For
innate immune defense and maintenance of intracellular
environmental homeostasis in the face of microbial infection,
endogenous danger signals, and environmental stimuli including
antitumor drugs, NLRP3 acts as a sensor that recruits ASC and
caspase-1 to form a cytoplasmic multiprotein complex, known as the
NLRP3 inflammasome. The NLRP3 inflammasome can activate protease
caspase-1 to promote the release of active IL-1β and IL-18 and
participate in the body's immune response (4). Recent studies have shown that NLRP3
inflammasome activation leads not only to pyroptosis, but also to
other types of cell death, including apoptosis, necrosis, and
ferroptosis. In addition, various cell death effectors have been
reported to regulate NLRP3 inflammasome activation, suggesting that
cell death is closely related to NLRP3 inflammasome activation
(5–7).
Aberrant activation of the NLRP3 inflammasome has
been implicated in the pathogenesis of various inflammatory
diseases, such as diabetes, cancer, and Alzheimer's disease. There
is evidence that NLRP3 has protective anti-tumor effects as well as
pro-tumor effects in different types of tumors. In leukemia, the
NLRP3 inflammasome can cause bone marrow hyperplasia, cytopenia,
and splenomegaly amongst other diseases (8). However, whether the activation of the
NLRP3 inflammasome plays a malignant role in the progression of
leukemia remains contested. It has been found that bone marrow
dendritic cells (DC) activate NLRP3, playing an anti-leukemic role
in AML through the IL-1β/Th1/IFN-γ pathway (9). In addition, the activation of the
NLRP3 inflammasomes by cancer chemotherapy drugs has been confirmed
in the treatment of malignant mesothelioma, hematological, and
other solid tumors. The mechanism primarily involves the activation
of IL-1β by NLRP3 inflammasomes to induce a burst of inflammation
(10,11). However, to the best of our
knowledge, whether NLRP3 activation is involved in the apoptosis of
leukemic cells induced by chemotherapeutic drugs has not been
reported.
In the present study, it was shown that NLRP3
activation played an anti-cancer role in the treatment of AML
through in vitro experiments; NLRP3 was involved in the
differentiation and apoptosis of cells in the differentiation
treatment of leukemia.
Materials and methods
Cell culture
THP-1 Human Monocyte Leukemia Cells (promonocytic
cell line) were cultured in RPMI-1640 medium (cat. no. 11875176;
Invitrogen; Thermo Fisher Scientific, Inc.) with 10% FBS (cat. no.
10100147C; Invitrogen; Thermo Fisher Scientific, Inc.), 0.05 mM
β-mercaptoethanol (cat. no. 21985023; Invitrogen; Thermo Fisher
Scientific, Inc.) and 1% penicillin-streptomycin (cat. no. P1400;
Beijing Solarbio Science & Technology Co., Ltd.) at 37°C in a
humidified incubator supplied with 5% CO2.
Phorbol 12-Myristate 13-Acetate (PMA)
treatment
Different concentrations (0, 50, 100, 200 and 400
ng/ml) of PMA (cat. no. 16561-29-8; MilliporeSigma) were added to
1×105/ml cells and cells were incubated as above for 24
or 48 h prior to the following assays.
Giemsa staining
A Giemsa Stain kit (cat. no. G8220) was purchased
from Beijing Solarbio Science & Technology Co., Ltd. THP-1 cell
slides were prepared and fixed with methanol for 2 min at room
temperature according to the manufacturer's protocol. The cell
slides were stained using the Giemsa staining solution at room
temperature for 40-60 min, after which they were washed three times
with PBS buffer. Once they had dried, they were examined by light
microscopy (Leica Microsystems GmbH) at a magnification of ×20, ×40
and ×100.
Cell Counting Kit (CCK)-8 assay
Cells were seeded into 96 well plates according to
the instructions of the CCK-8 assay (cat. no. G4103, Wuhan
ServiceBio Technology Co., Ltd.). After the addition of the CCK-8
solution, cells were incubated for a further 4 h. Subsequently, the
absorbance was measured at 450 nm using a microplate reader
(Multiskan sky; Thermo Fisher Scientific, Inc.).
Apoptosis analysis
An Annexin V-FITC/7-AAD (cat. no. MA0428, Meilune)
was used followed by flow cytometry to assess apoptosis. The cells
were centrifuged at 200 × g for 5 min at room temperature,
harvested, resuspended in precooled 1× PBS, and centrifuged at 200
× g for 5 min at room temperature, after which 300 µl 1× Binding
Buffer was added. After the addition of 5 µl Annexin V-FITC to the
mixture, the mixture was incubated for 15 min in the dark at room
temperature. An additional 5 µl PI was added and cells were stained
for 5 min at room temperature, after which 200 µl 1× Binding Buffer
was added, and loaded onto the flow cytometer (NovoCyte, Agilent
Technologies, Inc.). Apoptosis was analyzed using FlowJo 10.8.1
software (Becton, Dickinson and Company).
Detection of CD11b and CD14 by flow
cytometry
After collecting the cells, the concentration was
adjusted to 1×106 cells/ml. Precooled 100 µl PBS was
added to each flow tube and the cells were resuspended, followed by
the addition of 5 µl PE-CD11b fluorescent antibody (cat. no.
301306, BioLegend, Inc.) and FITC-CD14 fluorescent antibody (cat.
no. 301804, BioLegend, Inc.), respectively, and incubated at 4°C in
the dark for 30 min. Cells were subsequently resuspended in 200 µl
PBS at 4°C by centrifugation at 200 × g for 4 min and washed twice
with PBS. Isotype rat IgG was used as the negative control.
Western blotting
Total cell protein was extracted using RIPA lysis
buffer (cat. no. P0013B, Beyotime Institute of Biotechnology),
loaded (30 µg/lane) on a 10% SDS-gel, resolved using SDS-PAGE and
transferred to PVDF membranes (MilliporeSigma). The primary
antibodies against NLRP3 (1:1,000; cat. no. 13158, Cell Signaling
Technology, Inc.) or GAPDH (1:2,000; cat. no. TA-0A, Beijing
Zhongshan Golden Bridge Biotechnology Co., Ltd.) were added to
membranes and incubated overnight at 4°C. The secondary antibodies
used were HRP-conjugated Affinipure Goat Anti-Rabbit IgG (H+L)
(1:3,000; cat. no. SA00001-2, ProteinTech Group, Inc.) and
HRP-conjugated Affinipure Goat Anti-Mouse IgG (H+L) (1:4,000; cat.
no. SA00001-1, ProteinTech Group, Inc.). The bands were visualized
using a Tanon 5200 using an ECL assay and assessed using ImageJ
version 1.53 (National Institutes of Health). β-actin was used as
the loading control.
RNA interference
shRNAs targeting NLRP3 (sh-NLRP3) and the negative
control were obtained from Shanghai GenePharma Co., Ltd. The
element sequence of the vector GV493 (Shanghai GenePharma Co.,
Ltd.) was hU6-MCS-CBh-gcGFP-IRES-puromycin and the shRNA sequence
was inserted into MCS. The DNA sequence for shRNA-NLRP3 was
5′-CCGGCCGTAAGAAGTACAGAAAGTACTCGAGTACTTTCTGTACTTCTTACGGTTTTTG-3′;
and the sequence of the negative control was
5′-CCGGCCTTCTCCGAACGTGTCACGTCTCGAGTACTTTCTGTACTTCTTACGGTTTTTG-3′.
THP-1 cells were cultured in six-well plates (5×105
cells/well) and transfected with 1.5 µg shRNA-NLRP3 using 10 µl
Lipofectamine™ 2000 (Thermo Fisher Scientific, Inc.) according to
the manufacturer's instructions.
Reverse transcription-quantitative
(RT-q) PCR
Total RNA was extracted from cells using a GoldHi
Plasmid Mini Kit (cat. no. CW0581, Jiangsu Cowin Biotech Co., Ltd)
according to the manufacturer's instructions. The mRNA levels of
target genes were analyzed by qPCR using a Bio-Rad iCycler system
(Bio-Rad Laboratories, Inc.). cDNA was synthesized from 0.5 ng
total RNA using an Evo M-MLV RT MasterMix (cat. no. AG11728,
Accurate biology) in a 10 µl reaction volume according to the
manufacturer's protocol. qPCR was performed with 2 µl cDNA and
gene-specific primers in a final reaction volume of 25 µl.
Amplification was performed using a SYBR® Green Pro Taq
HS Premix (cat. no. AG11740, Accurate biology), and the
thermocycling conditions were as follows: Initial denaturation at
95°C for 30 sec; followed by 40 cycles of 95°C for 5 sec and 60°C
for 30 sec. The relative mRNA expression was assessed using the
2−∆∆Cq method (12).
The sequences of the primers used were: NLRP3 forward,
5′-GCGCCTCAGTTAGAGGATGT-3′ and reverse,
5′-ACCAGCTACAAAAAGCATGGA-3′; and GAPDH forward
5′-AGAAGGCTGGGGCTCATTTG-3′ and reverse,
5′-AGGGGCCATCCACAGTCTTC-3′.
Statistical analysis
All the experiments were performed in triplicate;
all data were analyzed using GraphPad Prism version 9 (GraphPad
Software, Inc.) and are presented as the mean ± standard error of
the mean. Differences in mean values between groups were examined
using a one-way ANOVA followed by a Tukey's post hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
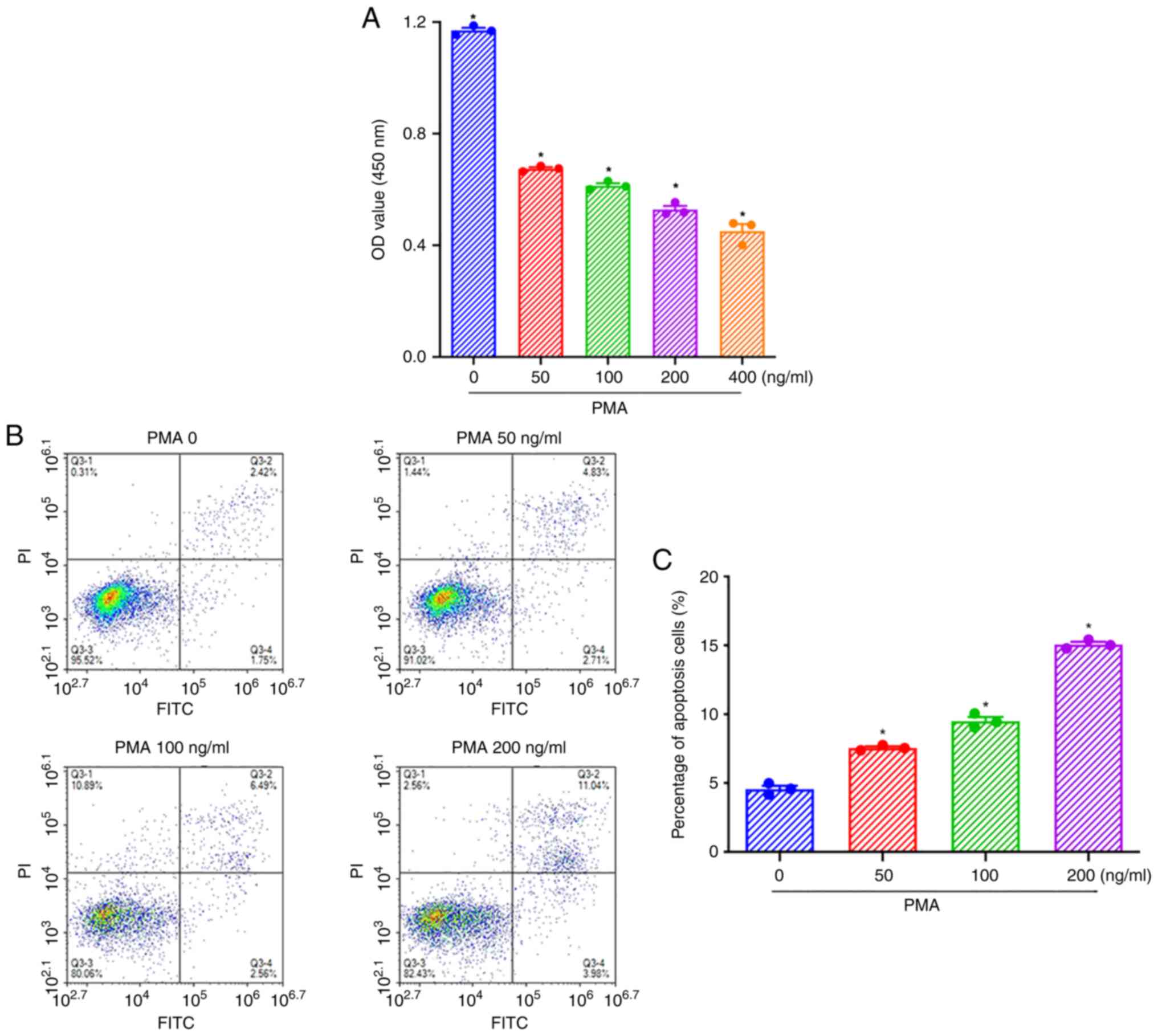
PMA at 100 ng/ml is the optimal
concentration to stimulate the apoptosis of THP-1 cells
To determine the optimal concentration of PMA, THP-1
cells were stimulated with 50, 100, 200, or 400 ng/ml PMA for 48 h.
The results of the CCK-8 assays showed that all concentrations of
PMA could significantly inhibit the proliferation of THP-1 cells,
and the degree of inhibition of cell proliferation increased in a
concentration-dependent manner (Fig.
1A). The results of flow cytometry showed that treatment with
50, 100, and 200 ng/ml PMA for 48 h significantly induces apoptosis
of THP-1 cells (Fig. 1B).
Statistical analysis showed that the apoptosis of THP-1 cells
increased significantly in a dose-dependent manner (Fig. 1C). Thus, PMA at 100 ng/ml was
chosen as the optimal concentration, as this concentration was also
used in previous studies (13,14).
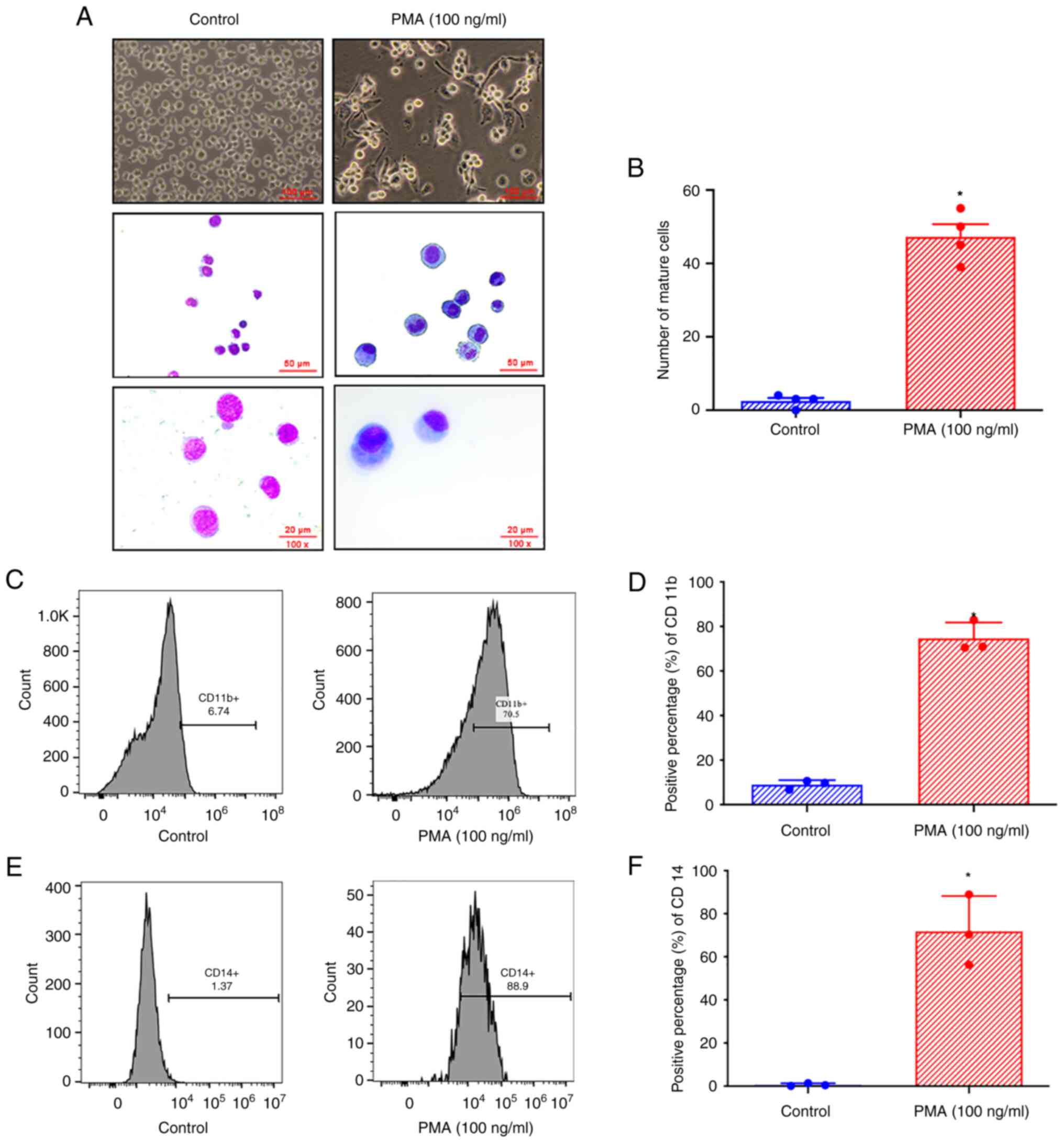
PMA promotes the maturation and
differentiation of THP-1 cells into monocytes/macrophages
Giemsa staining showed that after 48 h of treatment
with 100 ng/ml PMA, the morphology of THP-1 cells was notably
altered; the cells became significantly larger, accompanied by an
increase in the volume of cytoplasm, had a decreased
nucleus-cytoplasm ratio, and exhibited a decrease in the size of
the nucleus (Fig. 2A). Cell
enlargement was accompanied by increased cytoplasmic volume, a
decreased nucleus-cytoplasm ratio, and a decrease in the size of
the nucleus. Compared with the control group, THP-1 cells treated
with 100 ng/ml PMA for 48 h had a statistically significant
increase in the number of mature cells per high-power field of view
(Fig. 2B), indicating that PMA
promoted the maturation of THP-1 cells. Flow cytometry was used to
determine the expression of CD11b in THP-1 cells after 48 h of
treatment with 100 ng/ml PMA (Fig.
2C). Analysis showed that compared with the control group, the
expression of CD11b in THP-1 cells treated with 100 ng/ml PMA for
48 h was significantly increased (Fig.
2D). Flow cytometry was used to determine the expression of
CD14 in THP-1 cells treated with 100 ng/ml PMA for 48 h (Fig. 2E). Analysis showed that compared
with the control group, the expression of CD14 in THP-1 cells
treated with 100 ng/ml PMA for 48 h was significantly increased
(Fig. 2F), which indicated that
PMA promoted the differentiation of THP-1 cells into
monocytes/macrophages.
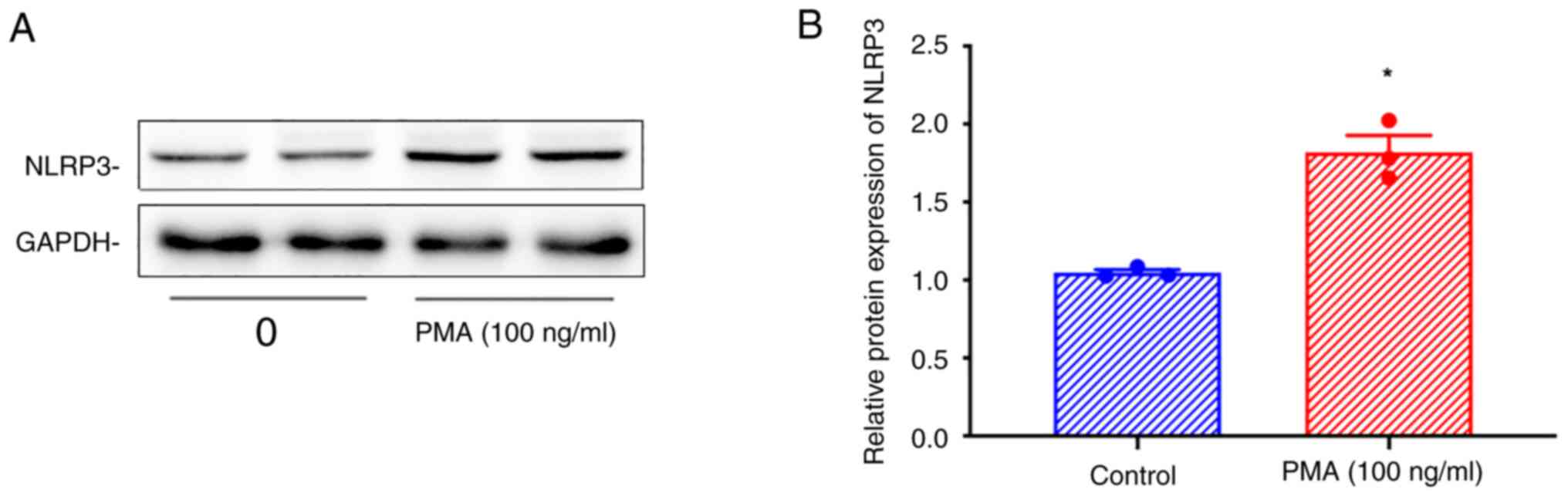
PMA treatment increases NLRP3
expression in THP-1 cells
Western blotting showed that the expression of NLRP3
was increased in THP-1 cells treated with 100 ng/ml PMA for 48 h
(Fig. 3A and B).
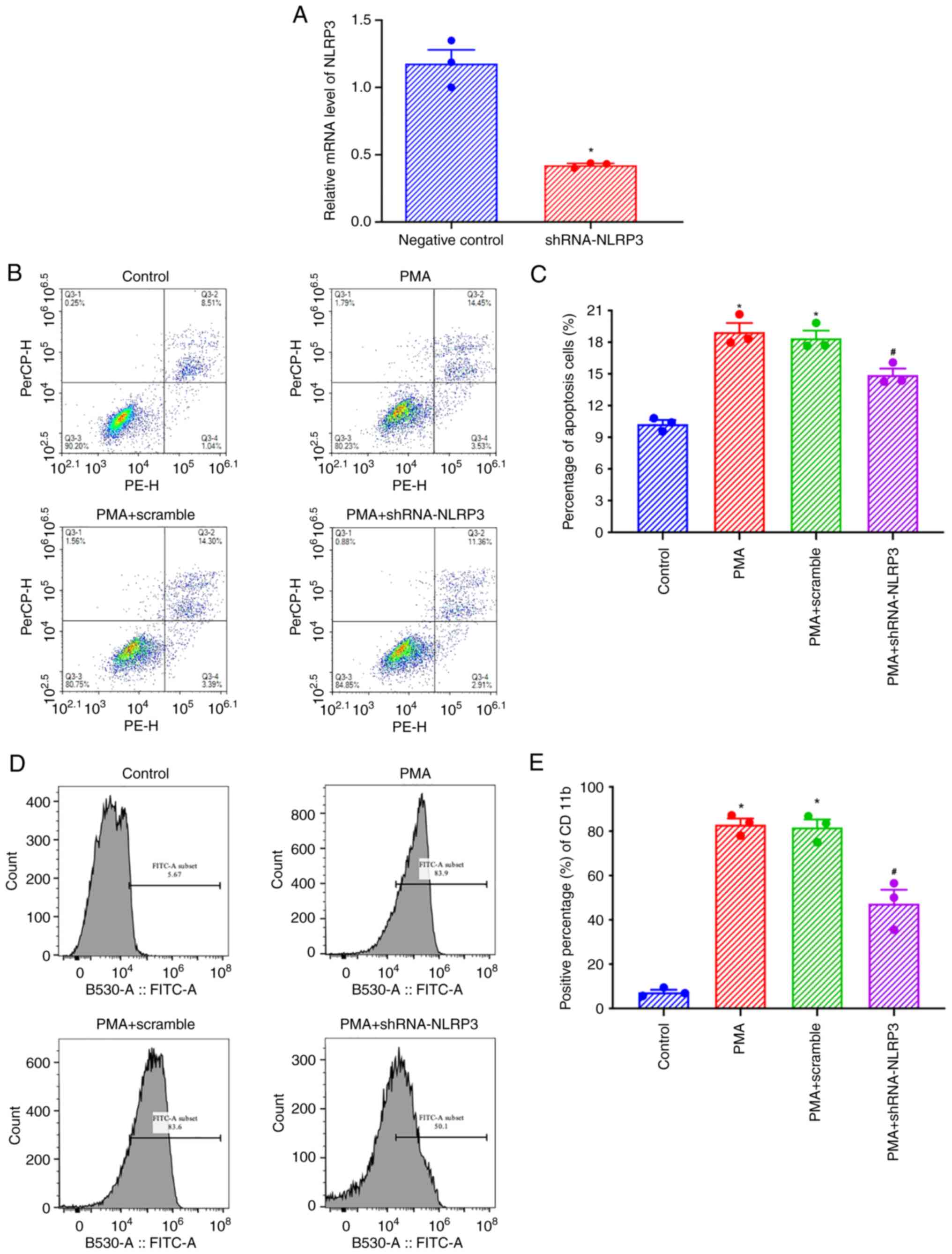
NLRP3 knockdown inhibits the
pro-apoptotic effects of PMA on THP-1 cells and the increased
expression of CD11b
After shRNA-NLRP3 transfection, the expression of
NLRP3 in THP-1 cells was significantly decreased (Fig. 4A). Flow cytometry showed that the
levels of apoptosis of THP-1 cells increased after 48 h of
treatment with 100 ng/ml PMA, and this apoptosis decreased in
shNLRP3 transfected cells (Fig.
4B). Statistical analysis showed that compared with the control
group, THP-1 cells treated with 100 ng/ml PMA for 48 h exhibited
increased apoptosis, and shNLRP3 transfection reduced apoptosis
significantly, indicating that NLRP3 was involved in the regulation
of PMA-induced THP-1 cell apoptosis (Fig. 4C). Flow cytometry showed that the
expression of CD11b in THP-1 cells increased after treatment with
100 ng/ml PMA for 48 h, and the expression of CD11b decreased after
transfection with shNLRP3 (Fig.
4B). Statistical analysis found that compared with the control
group, the apoptosis of THP-1 cells treated with 100 ng/ml PMA for
48 h increased, and the expression of CD11b in cells transfected
with shNLRP3 decreased significantly, indicating that NLRP3 was
involved in the regulation of PMA-induced expression of CD11b in
THP-1 cells (Fig. 4C).
Discussion
AML is a differentiating system (15). For >40 years, although
combination therapy with cytarabine and anthracyclines, such as
daunorubicin, has been the mainstay of AML treatment, the 5-year
survival rate for adult AML patients is <30% (16). The overall long-term survival rate
of the combined treatment with all-trans retinoic acid and arsenic
trioxide for acute promyelocytic leukemia (APL) is >90%,
suggesting that treatment based on differentiation of AML may have
a promising future (17).
Therefore, exploring the molecular mechanism of differentiation
therapy and identifying novel targets for differentiation therapy
has become a topic of increased interest in the treatment of AML.
However, due to the high heterogeneity of AML, relevant targets and
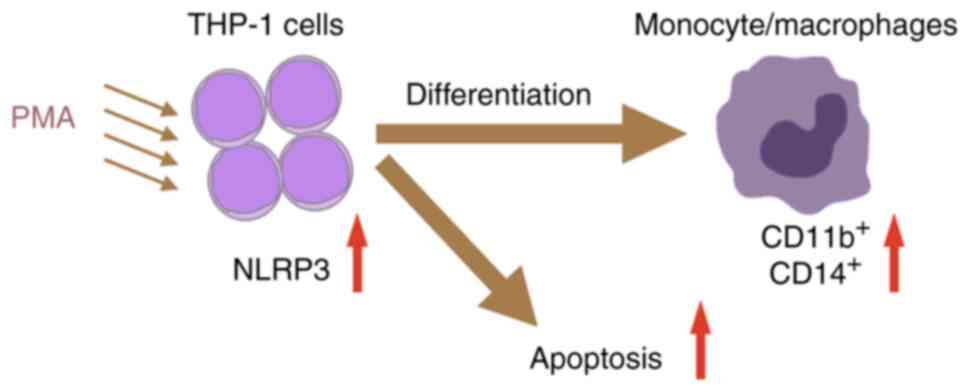
drugs have remained elusive. In the present study, the molecular
mechanisms identified showed that NLRP3 promoted the apoptosis and
differentiation of leukemia cells, through the upregulation of
CD11b+ and CD14+ cells in the process of
THP-1 differentiation following PMA treatment (Fig. 5).
The inflammasome is a polymeric protein complex
sensitive to stimuli such as microbial motifs, endogenous danger
signals and environmental irritants, and this is part of the innate
immune response (18). NLRP3 is
the most extensively studied and characterized inflammatory body,
and present as a nucleotide-binding and oligomerization (NACHT)
domain and C-terminal leucine-rich repeat (LRR), and N-terminal
pyrin domain (PYD). When NLRP3 detects a danger signal, the NACHT
domains homo-oligomerize. Subsequently, the PYD domain of NLRP3
recruits the adaptor apoptosis speckprotein (ASC). Subsequently,
ASC recruits the CARD of pro-caspase-1, promotes its self-cleavage
and formation of active caspase-1, and finally cleaves pro-IL-1β
and pro-IL-18 into active IL-1β and IL-18 (4). In short, activation of the NLRP3
inflammasome has two primary roles, one is a pro-inflammatory
response to the release of the inflammatory cytokines IL-1β and
IL-18, and the other is the induction of programmed cell death
(pyroptosis) (19). ROS generation
is involved in this NLRP3 activation process (20,21).
The NLRP3 inflammasome is involved in a variety of
inflammation-related diseases, including cancer, and is a very
attractive potential target for the study of novel therapeutic
agents. At present, several small molecule compounds, such as
MCC950, are hypothesized to possess specific inhibitory effects on
NLRP3 activation (22).
Activation of the NLRP3 inflammasome is a
double-edged sword in tumor therapy, with evidence of protective
anti-tumor and pro-tumor effects in different types of cancer
(23). NLRP3 inflammatory bodies
and IL-1β production promote the infiltration of bone marrow cells,
such as myeloid-derived suppressor cells (MDSCs) and
tumor-associated macrophages (TAMs), providing an inflammatory
microenvironment that contributes to the partial protection of
breast cancer progression and skin cancer development (24,25).
In addition, the role of inflammatory bodies in the pathogenesis of
melanoma has also been confirmed (26). NLRP3 signaling may drive
immunosuppression in pancreatic cancer by promoting tolerant T-cell
differentiation and adaptive immunosuppression through IL-10
(27). Conversely, the NLRP3
inflammasome has also been shown to possess antitumor effects.
Tumor cell initiation by dendritic cell-mediated IFN-γ-producing T
lymphocytes requires NLRP3 inflammasomes (28). Inflammasome-related molecules play
a positive protective anti-tumor role in colorectal cancer
metastasis and intestinal inflammatory injury (29,30).
NLRP3 plays an antitumor role in melanoma by promoting the
migration of myeloid-derived suppressor cells (MDSCs) (31). NLRP3 also plays a protective
anti-tumor role in liver cancer (29).
Whether the NLRP3 inflammasome exerts disease
progression or protective anti-tumor effects in hematological
malignancies remains contested. In chronic myelomonocytic leukemia,
juvenile myelomonocytic leukemia, and other rare types of AML,
NLRP3 inflammasome activation that harbor KRAS mutations play a key
role in the characteristic symptoms of leukemia, such as cell
reduction, splenomegalysis, and bone marrow hyperplasia (32). Conversely, NLRP3-activated bone
marrow dendritic cells have been found to play an anti-leukemic
role in AML through an IL-1β/Th1/IFN-γ axis. Activation of NLRP3
inflammasomes affects Th cells in AML. They induce IL-1β-dependent
immunity and promote the transformation of CD4+ T cells into Th1
cells that produce tumor-specific IFN-γ allowing for recognition of
leukemia cells. In recent years, a considerable number of DC
treatments have achieved non-specific and antigen-specific immune
effects on AML (9). However,
whether each component of the NLRP3 inflammasome is involved in the
process of leukemia cell differentiation, and whether it plays a
protective anti-tumor or a pro-tumor role has not been determined,
to the best of our knowledge. This study focused on the role of
NLRP3, the core component of the NLRP3 inflammasome, in the process
of THP-1 differentiation. It was found that NLRP3 expression was
increased during THP-1 differentiation, and its deletion inhibited
the differentiation of leukemia cells, suggesting that the changes
in NLRP3 expression levels were directly involved in the
differentiation of leukemia cells, which plays a protective
anti-tumor role in the differentiation and maturation of leukemia
cells.
PMA can promote the apoptosis of tumor cells in the
process of promoting the differentiation and maturation of leukemia
cells (33). In the present study,
different concentrations and durations of PMA treatment were used.
The CCK-8 assay showed that >50 ng/ml PMA significantly
inhibited the proliferation of THP-1 cells, and the flow cytometry
results also showed that >50 ng/ml PMA significantly induced the
apoptosis of THP-1 cells. In a preliminary experiment (data not
shown), our group also used 72 h of PMA induction. There was no
significant difference between cells that underwent PMA induction
for 72 h and cells that underwent PMA induction for 48 h in flow
cytometry assays, and as the cell growth was fast, the state of
cells after 72 h was not as good as that after 48 h of PMA
induction. Meanwhile, the concentrations of PMA in other studies
ranged from 100-200 nM (61.6-132 ng/ml) (13,14,34–36).
Based on previous studies and the results of experiments, 100 ng/ml
PMA treatment for 48 h was chosen. In addition, it was found that
the effect of PMA on THP-1 cell differentiation and maturation was
also affected by cell culture conditions (37). Cells were more sensitive to PMA and
CD14+ cells were increased in high-density culture,
while cells were less responsive to PMA under low-density culture
conditions (37). The present
study mainly examined the effect of PMA on NLRP3 in the process of
differentiation and maturation of THP-1 cells in high-density cell
cultures, while the changes and effects of PMA on NLRP3 in THP-1
cells in low-density cell cultures were not investigated.
Therefore, culture conditions will also be considered in future
experiments.
NLRP3 knockdown inhibited the proapoptotic effects
of PMA on leukemia cells, suggesting that NLRP3 was directly
involved in the proapoptotic effects of PMA. Numerous studies have
shown that NLRP3 is involved in inflammatory pyroptosis during
tumor progression (38,39). Pyroptosis, as a type of apoptosis,
plays a prominent role in tumor development and metastasis,
although the inflammatory microenvironment of pyroptosis can
promote tumor development and metastasis during tumor development
(40). As the knockdown of NLRP3
can partially reverse the differentiation and apoptosis of THP-1
cells, NLRP3 was hypothesized to participate in the differentiation
of THP-1 cells but was not solely responsible.
The present study is only a preliminary exploration
of the role of NLRP3 in the differentiation of acute leukemia
cells, so there were some limitations. These included the fact that
the effect of NLRP3 knockout on CD14 expression and the changes of
cell cycle-related proteins in leukemia cells were not assessed.
Additionally, PMA was used to induce differentiation of leukemia
cells towards a monocyte/macrophage-like lineage via the JNK/c-JUN
pathway, which may involve inflammation. Therefore, the role played
by NLRP3 in this induced monocytic lineage differentiation system
may not be applicable to other models of leukemia cell
differentiation induced by other inducers, such as APL NB4 cells
induced by all-trans retinoic acid, which will be assessed in a
future study. Additionally, a series of experiments to further
confirm the role of NLRP3 in THP-1 cells and K256 cells induced by
PMA, and the effect of knockdown and overexpression of NLRP3 using
vectors or ATP+LPS priming in differentiation and cell cycle
progression and the role of NK cell interactions with leukemia
cells together will be performed.
In conclusion, in the present study, the protective
anti-tumor role of NLRP3 in the differentiation and maturation of
leukemia cells was investigated. It was found that the upregulation
of NLRP3 expression induced by PMA played a role in promoting the
differentiation and maturation of leukemia cells, and it was
directly involved in the apoptosis of leukemia cells and the
differentiation and maturation into monocyte/macrophages. The
results of the present study provide a novel theoretical basis for
exploring the mechanism of differentiation therapy in leukemia and
improves our understanding of the role of NLRP3 in hematologic
tumors.
Acknowledgements
Not applicable.
Funding
This study was funded by the Independent Innovation Foundation
of Binzhou Medical University (grant no. BY2020KJ46).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YW and CX designed the study, collected the data,
performed the statistical analysis, and drafted the manuscript. XL
assisted in data collection and statistical analysis. XC and RJ
designed the study and wrote the manuscript. YW and CX confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Stahl M and Tallman MS: Acute
promyelocytic leukemia (APL): Remaining challenges towards a cure
for all. Leuk Lymphoma. 60:3107–3115. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Su R, Qing Y and Chen J: Targeting
differentiation blockade in AML: New hope from cell-surface-based
CRISPR screens. Cell Stem Cell. 28:585–587. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
He Y, Hara H and Núñez G: Mechanism and
regulation of NLRP3 inflammasome activation. Trends Biochem Sci.
41:1012–1021. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Martinon F, Burns K and Tschopp J: The
inflammasome: A molecular platform triggering activation of
inflammatory caspases and processing of proIL-beta. Mol Cell.
10:417–426. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vince JE and Silke J: The intersection of
cell death and inflammasome activation. Cell Mol Life Sci.
73:2349–2367. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gaidt MM and Hornung V: The NLRP3
inflammasome renders cell death Pro-inflammatory. J Mol Biol.
430:133–141. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zheng M, Williams EP, Malireddi RKS, Karki
R, Banoth B, Burton A, Webby R, Channappanavar R, Jonsson CB and
Kanneganti TD: Impaired NLRP3 inflammasome activation/pyroptosis
leads to robust inflammatory cell death via caspase-8/RIPK3 during
coronavirus infection. J Biol Chem. 295:14040–14052. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hamarsheh S, Osswald L, Saller BS, Unger
S, De Feo D, Vinnakota JM, Konantz M, Uhl FM, Becker H, Lübbert M,
et al: Oncogenic KrasG12D causes myeloproliferation via
NLRP3 inflammasome activation. Nat Commun. 11:16592020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu Q, Hua M, Zhang C, Wang R, Liu J, Yang
X, Han F, Hou M and Ma D: NLRP3-activated bone marrow dendritic
cells play antileukemic roles via IL-1β/Th1/IFN-γ in acute myeloid
leukemia. Cancer Lett. 520:109–120. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sauter KA, Wood LJ, Wong J, Iordanov M and
Magun BE: Doxorubicin and daunorubicin induce processing and
release of interleukin-1β through activation of the NLRP3
inflammasome. Cancer Biol Ther. 11:1008–1016. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Westbom C, Thompson JK, Leggett A,
MacPherson M, Beuschel S, Pass H, Vacek P and Shukla A:
Inflammasome modulation by chemotherapeutics in malignant
mesothelioma. PLoS One. 10:e01454042015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Myou S, Zhu X, Boetticher E, Qin Y, Myo S,
Meliton A, Lambertino A, Munoz NM, Hamann KJ and Leff AR:
Regulation of adhesion of AML14.3D10 cells by surface clustering of
beta2-integrin caused by ERK-independent activation of cPLA2.
Immunology. 107:77–85. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lam CF, Yeung HT, Lam YM and Ng RK:
Reactive oxygen species activate differentiation gene transcription
of acute myeloid leukemia cells via the JNK/c-JUN signaling
pathway. Leuk Res. 68:112–119. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bonnet D and Dick JE: Human acute myeloid
leukemia is organized as a hierarchy that originates from a
primitive hematopoietic cell. Nat Med. 3:730–737. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yates JW, Wallace HJ Jr, Ellison RR and
Holland JF: Cytosine arabinoside (NSC-63878) and daunorubicin
(NSC-83142) therapy in acute nonlymphocytic leukemia. Cancer
Chemother Rep. 57:485–488. 1973.PubMed/NCBI
|
|
17
|
Lo-Coco F, Avvisati G, Vignetti M, Thiede
C, Orlando SM, Iacobelli S, Ferrara F, Fazi P, Cicconi L, Di Bona
E, et al: Retinoic acid and arsenic trioxide for acute
promyelocytic leukemia. N Engl J Med. 369:111–121. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Swanson KV, Deng M and Ting JP: The NLRP3
inflammasome: Molecular activation and regulation to therapeutics.
Nat Rev Immunol. 19:477–489. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang Y, Xu W and Zhou R: NLRP3
inflammasome activation and cell death. Cell Mol Immunol.
18:2114–2127. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Heid ME, Keyel PA, Kamga C, Shiva S,
Watkins SC and Salter RD: Mitochondrial reactive oxygen species
induces NLRP3-dependent lysosomal damage and inflammasome
activation. J Immunol. 191:5230–5238. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sorbara MT and Girardin SE: Mitochondrial
ROS fuel the inflammasome. Cell Res. 21:558–560. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Coll RC, Robertson AA, Chae JJ, Higgins
SC, Muñoz-Planillo R, Inserra MC, Vetter I, Dungan LS, Monks BG,
Stutz A, et al: A small-molecule inhibitor of the NLRP3
inflammasome for the treatment of inflammatory diseases. Nat Med.
21:248–255. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sharma BR and Kanneganti TD: NLRP3
inflammasome in cancer and metabolic diseases. Nat Immunol.
22:550–559. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Guo B, Fu S, Zhang J, Liu B and Li Z:
Targeting inflammasome/IL-1 pathways for cancer immunotherapy. Sci
Rep. 6:361072016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Drexler SK, Bonsignore L, Masin M,
Tardivel A, Jackstadt R, Hermeking H, Schneider P, Gross O, Tschopp
J and Yazdi AS: Tissue-specific opposing functions of the
inflammasome adaptor ASC in the regulation of epithelial skin
carcinogenesis. Proc Natl Acad Sci USA. 109:18384–18389. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dunn JH, Ellis LZ and Fujita M:
Inflammasomes as molecular mediators of inflammation and cancer:
potential role in melanoma. Cancer Lett. 314:24–33. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Daley D, Mani VR, Mohan N, Akkad N,
Pandian GSDB, Savadkar S, Lee KB, Torres-Hernandez A, Aykut B,
Diskin B, et al: NLRP3 signaling drives macrophage-induced adaptive
immune suppression in pancreatic carcinoma. J Exp Med.
214:1711–1724. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ghiringhelli F, Apetoh L, Tesniere A,
Aymeric L, Ma Y, Ortiz C, Vermaelen K, Panaretakis T, Mignot G,
Ullrich E, et al: Activation of the NLRP3 inflammasome in dendritic
cells induces IL-1beta-dependent adaptive immunity against tumors.
Nat Med. 15:1170–1178. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dupaul-Chicoine J, Arabzadeh A, Dagenais
M, Douglas T, Champagne C, Morizot A, Rodrigue-Gervais IG, Breton
V, Colpitts SL, Beauchemin N and Saleh M: The Nlrp3 inflammasome
suppresses colorectal cancer metastatic growth in the liver by
promoting natural killer cell tumoricidal activity. Immunity.
43:751–763. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Huber S, Gagliani N, Zenewicz LA, Huber
FJ, Bosurgi L, Hu B, Hedl M, Zhang W, O'Connor W Jr, Murphy AJ, et
al: IL-22BP is regulated by the inflammasome and modulates
tumorigenesis in the intestine. Nature. 491:259–263. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
van Deventer HW, Burgents JE, Wu QP,
Woodford RM, Brickey WJ, Allen IC, McElvania-Tekippe E, Serody JS
and Ting JP: The inflammasome component NLRP3 impairs antitumor
vaccine by enhancing the accumulation of tumor-associated
myeloid-derived suppressor cells. Cancer Res. 70:10161–10169. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Karki R and Kanneganti TD: Diverging
inflammasome signals in tumorigenesis and potential targeting. Nat
Rev Cancer. 19:197–214. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang J, Liu L, Xie L, Xiang G and Zhou Y:
Induction of differentiation-specific miRNAs in TPA-induced myeloid
leukemia cells through MEK/ERK activation. Int J Mol Med. 31:59–66.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Daigneault M, Preston JA, Marriott HM,
Whyte MK and Dockrell DH: The identification of markers of
macrophage differentiation in PMA-stimulated THP-1 cells and
monocyte-derived macrophages. PLoS One. 5:e86682010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Maeß MB, Wittig B, Cignarella A and
Lorkowski S: Reduced PMA enhances the responsiveness of transfected
THP-1 macrophages to polarizing stimuli. J Immunol Methods.
402:76–81. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Park EK, Jung HS, Yang HI, Yoo MC, Kim C
and Kim KS: Correction to: Optimized THP-1 differentiation is
required for the detection of responses to weak stimuli. Inflamm
Res. 69:11572020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Aldo PB, Craveiro V, Guller S and Mor G:
Effect of culture conditions on the phenotype of THP-1 monocyte
cell line. Am J Reprod Immunol. 70:80–86. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Faria SS, Costantini S, de Lima VCC, de
Andrade VP, Rialland M, Cedric R, Budillon A and Magalhães KG:
NLRP3 inflammasome-mediated cytokine production and pyroptosis cell
death in breast cancer. J Biomed Sci. 28:262021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Teng JF, Mei QB, Zhou XG, Tang Y, Xiong R,
Qiu WQ, Pan R, Law BY, Wong VK, Yu CL, et al: Polyphyllin VI
induces Caspase-1-mediated pyroptosis via the induction of
ROS/NF-κB/NLRP3/GSDMD signal axis in non-small cell lung cancer.
Cancers (Basel). 12:1932020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xia X, Wang X, Cheng Z, Qin W, Lei L,
Jiang J and Hu J: The role of pyroptosis in cancer: pro-cancer or
pro-‘host’ ? Cell Death Dis. 10:6502019. View Article : Google Scholar : PubMed/NCBI
|