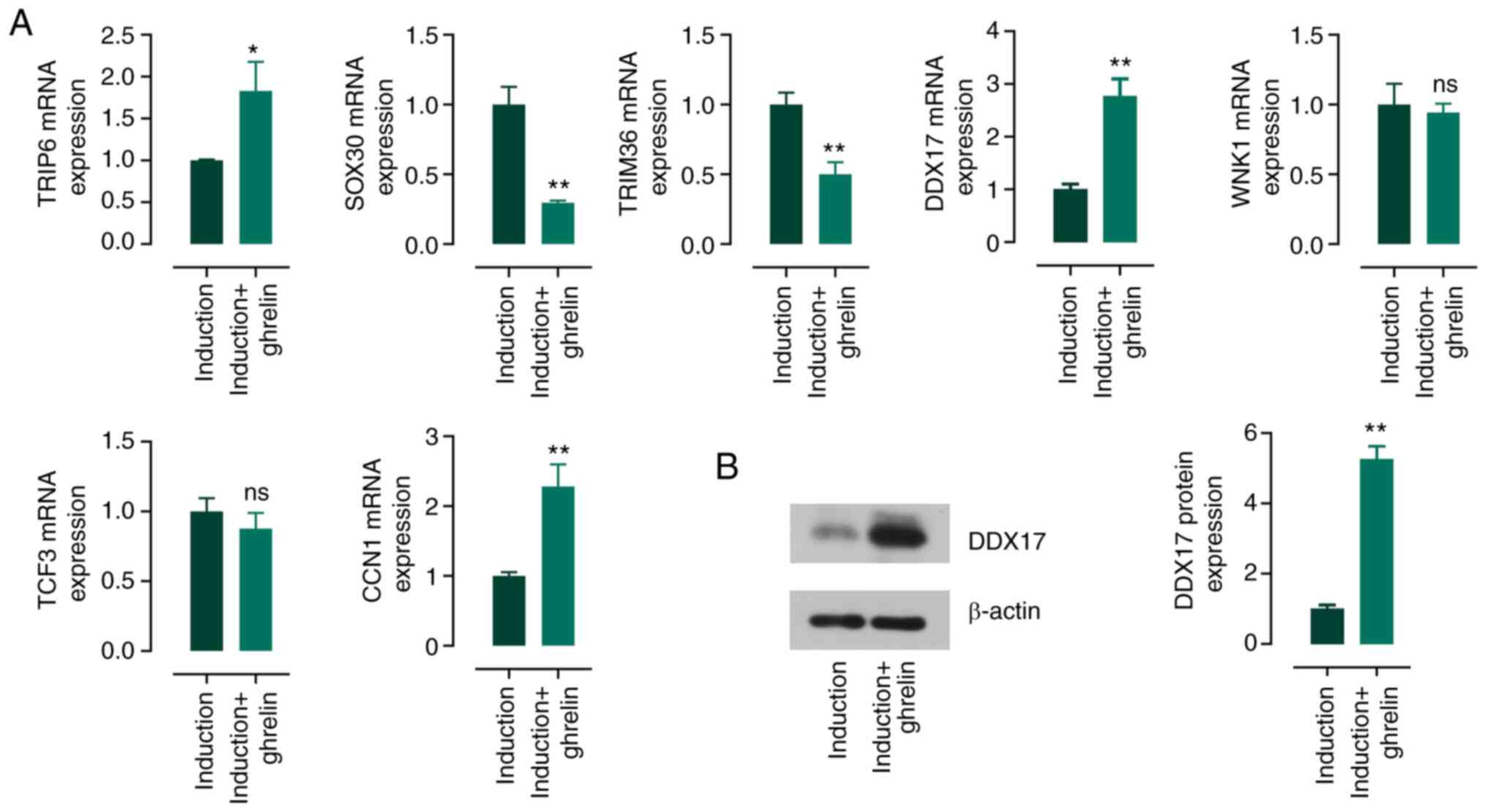
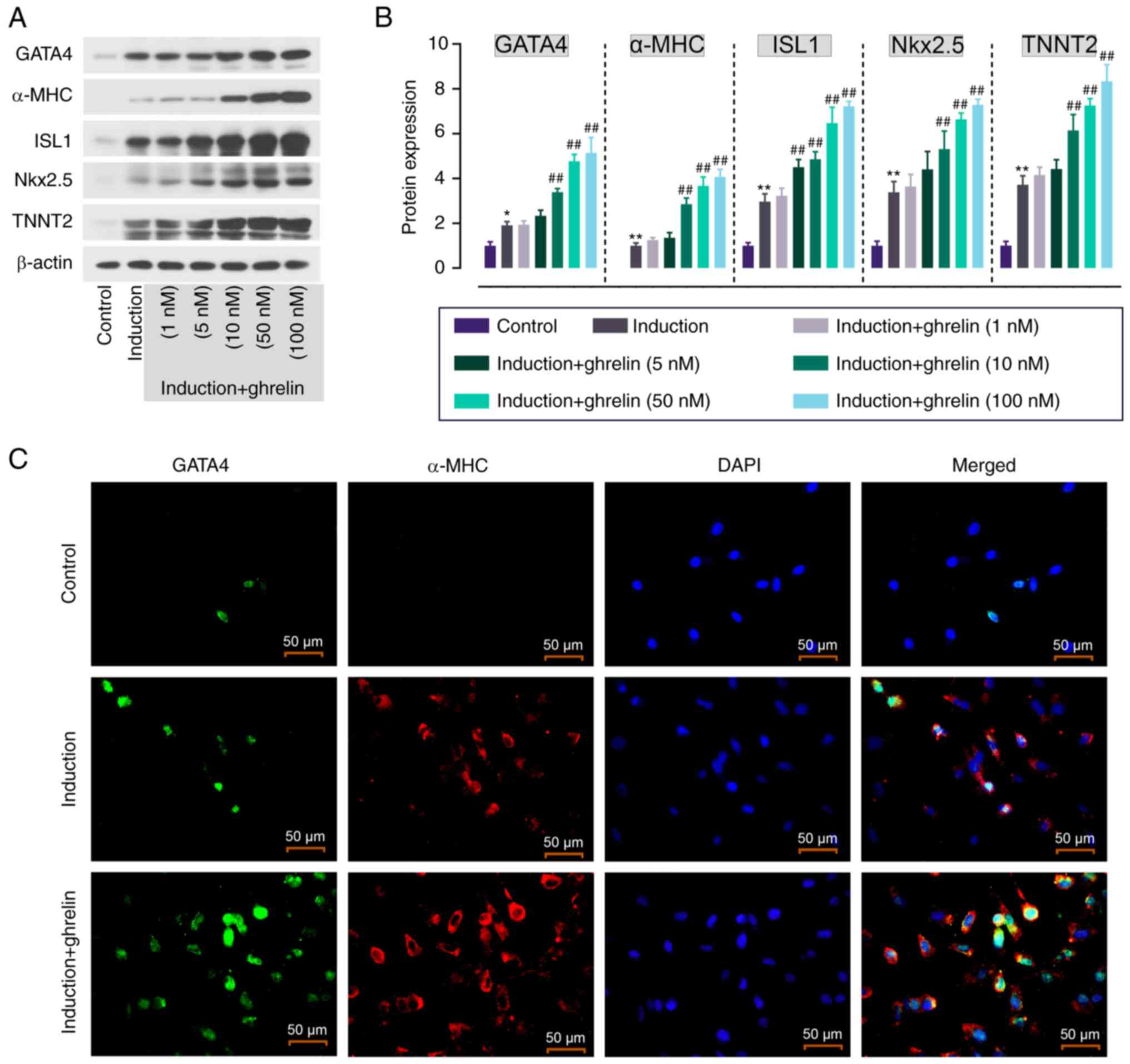
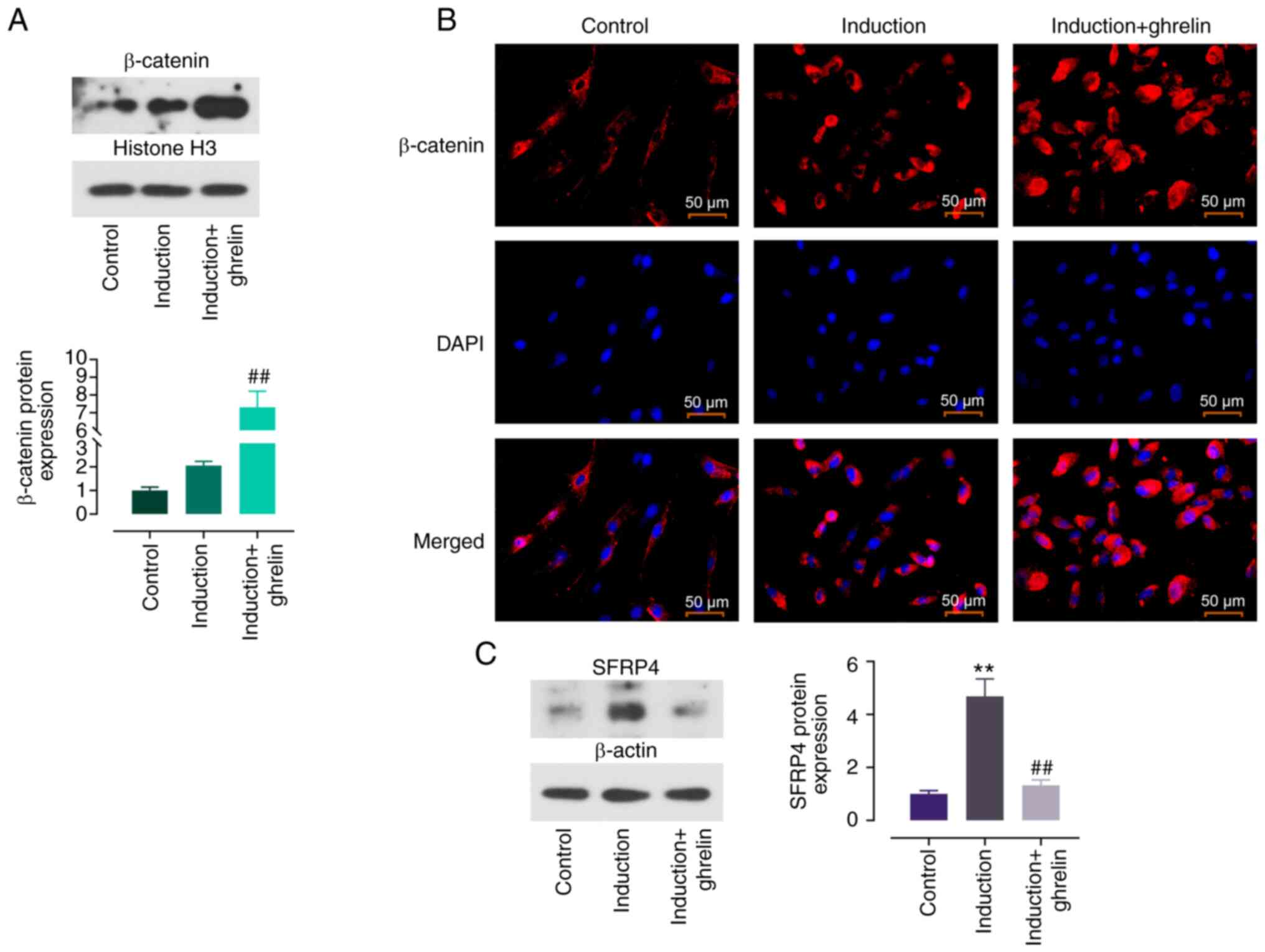
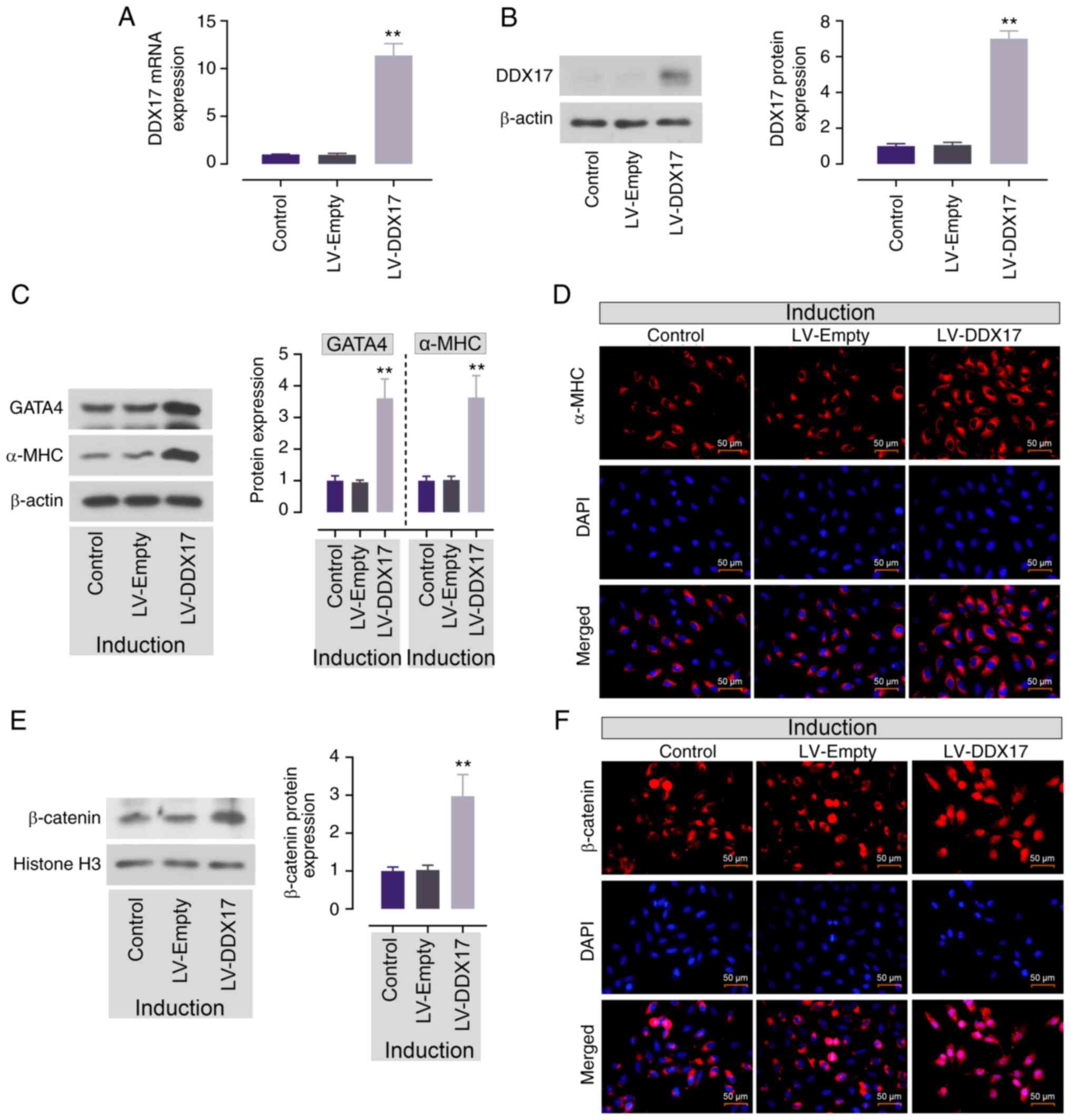
|
1
|
Zhou Z, Chen Y, Zhang H, Min S, Yu B, He B
and Jin A: Comparison of mesenchymal stromal cells from human bone
marrow and adipose tissue for the treatment of spinal cord injury.
Cytotherapy. 15:434–448. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Joo HJ, Kim JH and Hong SJ: Adipose
tissue-derived stem cells for myocardial regeneration. Korean Circ
J. 47:151–159. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Miyahara Y, Nagaya N, Kataoka M, Yanagawa
B, Tanaka K, Hao H, Ishino K, Ishida H, Shimizu T, Kangawa K, et
al: Monolayered mesenchymal stem cells repair scarred myocardium
after myocardial infarction. Nat Med. 12:459–465. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kojima M, Hosoda H, Date Y, Nakazato M,
Matsuo H and Kangawa K: Ghrelin is a growth-hormone-releasing
acylated peptide from stomach. Nature. 402:656–660. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yuan MJ, Li W and Zhong P: Research
progress of ghrelin on cardiovascular disease. Biosci Rep.
41:BSR202033872021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Khatib MN, Shankar A, Kirubakaran R, Agho
K, Simkhada P, Gaidhane S, Saxena D B U, Gode D, Gaidhane A and
Zahiruddin SQ: Effect of ghrelin on mortality and cardiovascular
outcomes in experimental rat and mice models of heart failure: A
systematic review and meta-analysis. PLoS One. 10:e01266972015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tokudome T, Otani K, Miyazato M and
Kangawa K: Ghrelin and the heart. Peptides. 111:42–46. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tokudome T and Kangawa K: Physiological
significance of ghrelin in the cardiovascular system. Proc Jpn Acad
Ser B Phys Biol Sci. 95:459–467. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu GB, Pan YM, Liu YS, Hu JH, Zhang XD,
Zhang DW, Wang Y, Feng YK, Yu JB and Cheng YX: Ghrelin promotes
neural differentiation of adipose tissue-derived mesenchymal stem
cell via AKT/mTOR and β-catenin signaling pathways. Kaohsiung J Med
Sci. 36:405–416. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Eid RA, Alkhateeb MA, Al-Shraim M, Eleawa
SM, Shatoor AS, El-Kott AF, Zaki MSA, Shatoor KA, Bin-Jaliah I and
Al-Hashem FH: Ghrelin prevents cardiac cell apoptosis during
cardiac remodelling post experimentally induced myocardial
infarction in rats via activation of Raf-MEK1/2-ERK1/2 signalling.
Arch Physiol Biochem. 125:93–103. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Eid RA, Alkhateeb MA, Eleawa S, Al-Hashem
FH, Al-Shraim M, El-Kott AF, Zaki MSA, Dallak MA and Aldera H:
Cardioprotective effect of ghrelin against myocardial
infarction-induced left ventricular injury via inhibition of SOCS3
and activation of JAK2/STAT3 signaling. Basic Res Cardiol.
113:132018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sun L and Zhang W: Preconditioning of
mesenchymal stem cells with ghrelin exerts superior
cardioprotection in aged heart through boosting mitochondrial
function and autophagy flux. Eur J Pharmacol. 903:1741422021.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gao M, Yang J, Wei R, Liu G, Zhang L, Wang
H, Wang G, Gao H, Chen G and Hong T: Ghrelin induces cardiac
lineage differentiation of human embryonic stem cells through
ERK1/2 pathway. Int J Cardiol. 167:2724–2733. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang J, Liu GQ, Wei R, Hou WF, Gao MJ, Zhu
MX, Wang HN, Chen GA and Hong TP: Ghrelin promotes differentiation
of human embryonic stem cells into cardiomyocytes. Acta Pharmacol
Sin. 32:1239–1245. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jiang M, Liu T, Zhang J, Gao S, Tao B, Cao
R, Qiu Y, Liu J, Li Y, Wang Y and Cao F: Rapamycin promotes
cardiomyocyte differentiation of human induced pluripotent stem
cells in a stage-dependent manner. Stem Cells Dev. 29:1229–1239.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ueno S, Weidinger G, Osugi T, Kohn AD,
Golob JL, Pabon L, Reinecke H, Moon RT and Murry CE: Biphasic role
for Wnt/beta-catenin signaling in cardiac specification in
zebrafish and embryonic stem cells. Proc Natl Acad Sci USA.
104:9685–9690. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li B, Zeng M, He W, Huang X, Luo L, Zhang
H and Deng DY: Ghrelin protects alveolar macrophages against
lipopolysaccharide-induced apoptosis through growth hormone
secretagogue receptor 1a-dependent c-Jun N-terminal kinase and
Wnt/β-catenin signaling and suppresses lung inflammation.
Endocrinology. 156:203–217. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gay D, Ghinatti G, Guerrero-Juarez CF,
Ferrer RA, Ferri F, Lim CH, Murakami S, Gault N, Barroca V, Rombeau
I, et al: Phagocytosis of Wnt inhibitor SFRP4 by late wound
macrophages drives chronic Wnt activity for fibrotic skin healing.
Sci Adv. 6:eaay37042020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang Y, Guan H, Fu Y, Wang X, Bai L, Zhao
S and Liu E: Effects of SFRP4 overexpression on the production of
adipokines in transgenic mice. Adipocyte. 9:374–383. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Visweswaran M, Schiefer L, Arfuso F,
Dilley RJ, Newsholme P and Dharmarajan A: Wnt antagonist secreted
frizzled-related protein 4 upregulates adipogenic differentiation
in human adipose tissue-derived mesenchymal stem cells. PLoS One.
10:e01180052015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yamada A, Iwata T, Yamato M, Okano T and
Izumi Y: Diverse functions of secreted frizzled-related proteins in
the osteoblastogenesis of human multipotent mesenchymal stromal
cells. Biomaterials. 34:3270–3278. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tian Y, Wang W, Lu Q, Chen P, Ma K, Jia Z
and Zhou C: Cross-talk of SFRP4, integrin α1β1, and Notch1 inhibits
cardiac differentiation of P19CL6 cells. Cell Signal. 28:1806–1815.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Qazi RE, Naeem N, Khan I, Qadeer Q,
Shaheen F and Salim A: Effect of a dianthin G analogue in the
differentiation of rat bone marrow mesenchymal stem cells into
cardiomyocytes. Mol Cell Biochem. 475:27–39. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhao X, Jiang C, Xu R, Liu Q, Liu G and
Zhang Y: TRIP6 enhances stemness property of breast cancer cells
through activation of Wnt/β-catenin. Cancer Cell Int. 20:512020.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gou H, Liang JQ, Zhang L, Chen H, Zhang Y,
Li R, Wang X, Ji J, Tong JH, To KF, et al: TTPAL promotes
colorectal tumorigenesis by stabilizing TRIP6 to activate
Wnt/β-catenin signaling. Cancer Res. 79:3332–3346. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Han F, Liu WB, Shi XY, Yang JT, Zhang X,
Li ZM, Jiang X, Yin L, Li JJ, Huang CS, et al: SOX30 inhibits tumor
metastasis through attenuating Wnt-signaling via transcriptional
and posttranslational regulation of β-catenin in lung cancer.
EBioMedicine. 31:253–266. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fu Q, Sun Z, Yang F, Mao T, Gao Y and Wang
H: SOX30, a target gene of miR-653-5p, represses the proliferation
and invasion of prostate cancer cells through inhibition of
Wnt/β-catenin signaling. Cell Mol Biol Lett. 24:712019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tong Q, Yi M, Kong P, Xu L, Huang W, Niu
Y, Gan X, Zhan H, Tian R and Yan D: TRIM36 inhibits tumorigenesis
through the Wnt/β-catenin pathway and promotes caspase-dependent
apoptosis in hepatocellular carcinoma. Cancer Cell Int. 22:2782022.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhao B, Qiao G, Li J, Wang Y, Li X, Zhang
H and Zhang L: TRIM36 suppresses cell growth and promotes apoptosis
in human esophageal squamous cell carcinoma cells by inhibiting
Wnt/β-catenin signaling pathway. Hum Cell. 35:1487–1498. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li K, Mo C, Gong D, Chen Y, Huang Z, Li Y,
Zhang J, Huang L, Li Y, Fuller-Pace FV, et al: DDX17
nucleocytoplasmic shuttling promotes acquired gefitinib resistance
in non-small cell lung cancer cells via activation of β-catenin.
Cancer Lett. 400:194–202. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shin S, Rossow KL, Grande JP and Janknecht
R: Involvement of RNA helicases p68 and p72 in colon cancer. Cancer
Res. 67:7572–7578. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Germann S, Gratadou L, Zonta E, Dardenne
E, Gaudineau B, Fougère M, Samaan S, Dutertre M, Jauliac S and
Auboeuf D: Dual role of the ddx5/ddx17 RNA helicases in the control
of the pro-migratory NFAT5 transcription factor. Oncogene.
31:4536–4549. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Serysheva E, Berhane H, Grumolato L, Demir
K, Balmer S, Bodak M, Boutros M, Aaronson S, Mlodzik M and Jenny A:
Wnk kinases are positive regulators of canonical Wnt/β-catenin
signalling. EMBO Rep. 14:718–725. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sato A, Shimizu M, Goto T, Masuno H,
Kagechika H, Tanaka N and Shibuya H: WNK regulates Wnt signalling
and β-Catenin levels by interfering with the interaction between
β-Catenin and GID. Commun Biol. 3:6662020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kuwahara A, Sakai H, Xu Y, Itoh Y,
Hirabayashi Y and Gotoh Y: Tcf3 represses Wnt-β-catenin signaling
and maintains neural stem cell population during neocortical
development. PLoS One. 9:e944082014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yang Y, Qi Q, Wang Y, Shi Y, Yang W, Cen
Y, Zhu E, Li X, Chen D and Wang B: Cysteine-rich protein 61
regulates adipocyte differentiation from mesenchymal stem cells
through mammalian target of rapamycin complex 1 and canonical Wnt
signaling. FASEB J. 32:3096–3107. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Caretti G, Schiltz RL, Dilworth FJ, Di
Padova M, Zhao P, Ogryzko V, Fuller-Pace FV, Hoffman EP, Tapscott
SJ and Sartorelli V: The RNA helicases p68/p72 and the noncoding
RNA SRA are coregulators of MyoD and skeletal muscle
differentiation. Dev Cell. 11:547–560. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Choi YH, Kurtz A and Stamm C: Mesenchymal
stem cells for cardiac cell therapy. Hum Gene Ther. 22:3–17. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gupta S, Sharma A S A and Verma RS:
Mesenchymal stem cells for cardiac regeneration: From
differentiation to cell delivery. Stem Cell Rev Rep. 17:1666–1694.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Neshati V, Mollazadeh S, Fazly Bazzaz BS,
de Vries AA, Mojarrad M, Naderi-Meshkin H, Neshati Z and Kerachian
MA: Cardiomyogenic differentiation of human adipose-derived
mesenchymal stem cells transduced with Tbx20-encoding lentiviral
vectors. J Cell Biochem. 119:6146–6153. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Shafei AE, Ali MA, Ghanem HG, Shehata AI,
Abdelgawad AA, Handal HR, Talaat KA, Ashaal AE and El-Shal AS:
Mesenchymal stem cell therapy: A promising cell-based therapy for
treatment of myocardial infarction. J Gene Med. 19:2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Casteilla L, Planat-Benard V, Laharrague P
and Cousin B: Adipose-derived stromal cells: Their identity and
uses in clinical trials, an update. World J Stem Cells. 3:25–33.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Fraser JK, Wulur I, Alfonso Z and Hedrick
MH: Fat tissue: An underappreciated source of stem cells for
biotechnology. Trends Biotechnol. 24:150–154. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Otto Beitnes J, Oie E, Shahdadfar A,
Karlsen T, Müller RM, Aakhus S, Reinholt FP and Brinchmann JE:
Intramyocardial injections of human mesenchymal stem cells
following acute myocardial infarction modulate scar formation and
improve left ventricular function. Cell Transplant. 21:1697–1709.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Irion CI, Martins EL, Christie MLA, de
Andrade CBV, de Moraes ACN, Ferreira RP, Pimentel CF, Suhett GD, de
Carvalho ACC, Lindoso RS, et al: Acute myocardial infarction
reduces respiration in rat cardiac fibers, despite adipose tissue
mesenchymal stromal cell transplant. Stem Cells Int.
2020:43279652020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Davy PM, Lye KD, Mathews J, Owens JB, Chow
AY, Wong L, Moisyadi S and Allsopp RC: Human adipose stem cell and
ASC-derived cardiac progenitor cellular therapy improves outcomes
in a murine model of myocardial infarction. Stem Cells Cloning.
8:135–148. 2015.PubMed/NCBI
|
|
48
|
Cai L, Johnstone BH, Cook TG, Tan J,
Fishbein MC, Chen PS and March KL: IFATS collection: Human adipose
tissue-derived stem cells induce angiogenesis and nerve sprouting
following myocardial infarction, in conjunction with potent
preservation of cardiac function. Stem Cells. 27:230–237. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Cho JW, Seo MS, Kang KK and Sung SE:
Effect of human thymus adipose tissue-derived mesenchymal stem
cells on myocardial infarction in rat model. Regen Ther.
11:192–198. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kuraitis D, Ruel M and Suuronen EJ:
Mesenchymal stem cells for cardiovascular regeneration. Cardiovasc
Drugs Ther. 25:349–362. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Penicka M, Widimsky P, Kobylka P, Kozak T
and Lang O: Images in cardiovascular medicine. Early tissue
distribution of bone marrow mononuclear cells after transcoronary
transplantation in a patient with acute myocardial infarction.
Circulation. 112:e63–e65. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Müller-Ehmsen J, Krausgrill B, Burst V,
Schenk K, Neisen UC, Fries JW, Fleischmann BK, Hescheler J and
Schwinger RH: Effective engraftment but poor mid-term persistence
of mononuclear and mesenchymal bone marrow cells in acute and
chronic rat myocardial infarction. J Mol Cell Cardiol. 41:876–884.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Russo C, Mannino G, Patanè M, Parrinello
NL, Pellitteri R, Stanzani S, Giuffrida R, Lo Furno D and Russo A:
Ghrelin peptide improves glial conditioned medium effects on
neuronal differentiation of human adipose mesenchymal stem cells.
Histochem Cell Biol. 156:35–46. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Li K, Fan L, Lin J, Heng BC, Deng Z, Zheng
Q, Zhang J, Jiang Y and Ge Z: Nanosecond pulsed electric fields
prime mesenchymal stem cells to peptide ghrelin and enhance
chondrogenesis and osteochondral defect repair in vivo. Sci China
Life Sci. 65:927–939. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Ge S, He W, Zhang L, Lin S, Luo Y, Chen Q
and Zeng M: Ghrelin pretreatment enhanced the protective effect of
bone marrow-derived mesenchymal stem cell-conditioned medium on
lipopolysaccharide-induced endothelial cell injury. Mol Cell
Endocrinol. 548:1116122022. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Han D, Huang W, Ma S, Chen J, Gao L, Liu
T, Zhang R, Li X, Li C, Fan M, et al: Ghrelin improves functional
survival of engrafted adipose-derived mesenchymal stem cells in
ischemic heart through PI3K/Akt signaling pathway. Biomed Res Int.
2015:8583492015. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Lian X, Zhang J, Azarin SM, Zhu K,
Hazeltine LB, Bao X, Hsiao C, Kamp TJ and Palecek SP: Directed
cardiomyocyte differentiation from human pluripotent stem cells by
modulating Wnt/β-catenin signaling under fully defined conditions.
Nat Protoc. 8:162–175. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Lian X, Hsiao C, Wilson G, Zhu K,
Hazeltine LB, Azarin SM, Raval KK, Zhang J, Kamp TJ and Palecek SP:
Robust cardiomyocyte differentiation from human pluripotent stem
cells via temporal modulation of canonical Wnt signaling. Proc Natl
Acad Sci USA. 109:E1848–E1857. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Klaus A, Müller M, Schulz H, Saga Y,
Martin JF and Birchmeier W: Wnt/β-catenin and Bmp signals control
distinct sets of transcription factors in cardiac progenitor cells.
Proc Natl Acad Sci USA. 109:10921–10926. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Gong B, Jiao L, Du X, Li Y, Bi M, Jiao Q
and Jiang H: Ghrelin promotes midbrain neural stem cells
differentiation to dopaminergic neurons through Wnt/β-catenin
pathway. J Cell Physiol. 235:8558–8570. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Liu X, Chen D, Wu Z, Li J, Li J, Zhao H
and Liu T: Ghrelin inhibits high glucose-induced 16HBE cells
apoptosis by regulating Wnt/β-catenin pathway. Biochem Biophys Res
Commun. 477:902–907. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Qu R, Chen X, Yuan Y, Wang W, Qiu C, Liu
L, Li P, Zhang Z, Vasilev K, Liu L, et al: Ghrelin fights against
Titanium particle-induced inflammatory osteolysis through
activation of β-catenin signaling pathway. Inflammation.
42:1652–1665. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Zeng W, Cao Y, Jiang W, Kang G, Huang J
and Xie S: Knockdown of Sfrp4 attenuates apoptosis to protect
against myocardial ischemia/reperfusion injury. J Pharmacol Sci.
140:14–19. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Lin B, Wang F, Wang J, Xu C, Zhao H, Cheng
Z and Feng D: The protective role of p72 in doxorubicin-induced
cardiomyocytes injury in vitro. Mol Med Rep. 14:3376–3380.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Wang MK, Sun HQ, Xiang YC, Jiang F, Su YP
and Zou ZM: Different roles of TGF-β in the multi-lineage
differentiation of stem cells. World J Stem Cells. 4:28–34. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
MacGrogan D, Münch J and de la Pompa JL:
Notch and interacting signalling pathways in cardiac development,
disease, and regeneration. Nat Rev Cardiol. 15:685–704. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Fang X, Miao S, Yu Y, Ding F, Han X, Wu H,
Zhao ZA, Wang Y, Hu S and Lei W: MIR148A family regulates
cardiomyocyte differentiation of human embryonic stem cells by
inhibiting the DLL1-mediated NOTCH signaling pathway. J Mol Cell
Cardiol. 134:1–12. 2019. View Article : Google Scholar : PubMed/NCBI
|