Introduction
Retinal ganglion cells (RGCs) are the only neurons
that transmit the visual information from the eye to brain
retinorecipient areas via the optic nerves (1). The bodies of RGCs reside in the inner
retina, while the long axons are located in retinal nerve fiber
layer, forming the optic nerve head (2). Several pathological factors can cause
RGC degeneration, such as compression and transection of optic
nerve, retinal ischemia, intracranial hypertension, as well as
photic and thermal damage (3). The
degeneration of RGCs can lead to several irreversible blindness,
including glaucoma (4), traumatic
optic neuropathy (5) and optic
neuritis (6). The mechanisms
causing RGC degeneration include neurotrophic factor deprivation,
axonal transport failure, mitochondrial dysfunction, excitotoxic
damage, activation of apoptotic signals, oxidative stress,
misbehaving reactive glia and loss of synaptic connectivity
(7). In addition, RGC degeneration
is associated with abnormal proteostasis, including dysregulation
of protein translation, chaperone-assisted protein folding and
protein degradation (8,9). Protein imbalance can lead to the
activation of inflammatory pathways, excitotoxic lesions and
oxidative stress. A variety of neuroprotective methods have been
developed to reduce RGC degeneration, such as neurotrophic agents,
steroid hormones, glutamate receptor antagonists, antioxidants and
electrical stimulation (10).
However, similar to other parts of central nervous system (CNS) in
adult mammals, the degeneration of RGCs and axonal nerve fibers are
difficult to regenerate (11).
Currently, it is still a great challenge for restoring RGC function
following RGC degeneration. Further study is still required to
clarify the potential mechanism of RGC degeneration.
The optic nerve transection (ONT) model has been
widely used to study the regeneration of RGCs and axonal
regeneration (12). Approximately
80% of RGCs die within 2 weeks following RGC axon injury (13). A number of biomarkers have been
found to be associated with RGC degeneration, ranging from nucleic
acids to proteins, such as microRNAs (14), long noncoding RNAs (15) and circular RNAs (16). However, the sensitivity,
specificity and reliability of these biomarkers remain elusive and
limit their applications in clinical practices.
As the ultimate products of the gene, mRNA and
protein activity, the metabolites represent the most downstream
stages of biological physiological and pathological processes
(17). Metabolomics has been used
for studying the dynamic changes of metabolites and metabolic
pathways in response to external and internal stimuli (18) and identifying the biomarkers for
disease prediction and therapeutic intervention. A growing number
of metabolomics studies have been conducted in various types of
cancer (19), neurological
diseases (20) and cardiovascular
diseases (21). Recent studies
have revealed the obvious changes of metabolites in
neurodegenerative diseases through the metabolomics analysis of
cerebrospinal fluid, plasma, urine, saliva, and brain tissue from
clinical samples or animal models. For example, Mapstone et
al (22) reported that the
changes in lipids and particularly phospholipids in plasma can
identify amnestic mild cognitive impairment or Alzheimer's disease.
The pathway enrichment data from the brain suggests that the
dysfunction of taurine and hypotaurine metabolism, bile acid
biosynthesis, serine and threonine metabolism or tricarboxylic acid
cycle is tightly related to the onset and progression of
Parkinson's disease (23). Lipid
and sugar metabolism dysfunction is involved in the pathogenesis of
neurodegenerative diseases (24).
The metabolomics analysis of ocular
neurodegenerative diseases has also been conducted. The levels of
several amino acids, acetoacetate, and citrate increase in the
aqueous humor of chronic glaucoma rat model (25). Mitochondrial dysfunction,
senescence and polyamines deficiency have been detected in the
pathogenesis of glaucoma (26).
Thus, altered retinal metabolites can lead to abnormal nutrient
availability and impaired visual function.
At present, to the best of our knowledge, there is
still no metabolomics study for the prediction of RGC degeneration
based on retinal tissues, which can directly reflect the
pathological condition of retinal neurodegeneration. The present
study used untargeted metabolomics to investigate the changes of
retinal metabolomic profiles following ONT injury in a mouse
model.
Materials and methods
Animal experiment and ethical
statement
C57BL/6J mice (age, 8 weeks old; male; weight, 22–25
g) were obtained from the Animal Core Facility of Nanjing Medical
University (Nanjing, China). All experiments were approved by the
Animal Ethics and Experimentation Committee of Nanjing Medical
University (approval no. 2103027). The animals were treated
according to the ARVO Statement for the Use of Animals in
Ophthalmic and Vision Research and housed with a 12 h light/12 h
dark cycle with standard chow and water ad libitum under the
controlled environment (temperature, 25°C; humidity, 50%). A total
of 80 animals were used in this study.
Optic nerve transection
The mice were anesthetized with an intraperitoneal
injection of the mixture of ketamine (100 mg/kg) and xylazine (10
mg/kg) and the eyes were topically anesthetized with 0.5%
proparacaine hydrochloride. The optic nerve was accessed within the
ocular orbit via an incision in the tissue covering the superior
border of orbital bone. The eye muscle and orbital fat was bluntly
dissected above the eyeball. The optic nerve was disclosed through
the orbital muscle cone and transected 2 mm posterior to the
eyeball with a pair of jeweler forceps. The cut site was thoroughly
examined to ensure that the optic nerve was completely cut. The
fundus was examined fundoscopically to confirm the absence of
injuries to retinal vascular supply. After the surgery,
erythromycin eye ointment was applied to prevent further
infection.
Retinal whole-mount
immunofluorescence
At 7 days following building ONT model, the mice
were anesthetized with an intraperitoneal injection of the mixture
of ketamine (100 mg/kg) and xylazine (10 mg/kg). Euthanasia was
performed by cervical dislocation when the animals were under deep
anesthesia. Then, the eyes were fixed in 4% paraformaldehyde (PFA;
cat. no. BL539A; Biosharp Life Sciences) for 30 min at room
temperature and dissected into the petal shape as a whole-mount.
Following incubation in 0.2% Triton-X-100 at 4°C overnight and 5%
BSA for 1 h, the intact retina was incubated with anti-β-III
tubulin (Tuj1) antibody (1:400; cat. no. 801201; BioLegend, Inc.)
overnight at 4°C. Following washing with PBS buffer, the retinas
were incubated with Alexa Fluor 594 goat anti-mouse IgG (1:500;
cat. no. A11005; Invitrogen; Thermo Fisher Scientific, Inc.)
antibody for 2 h at room temperature. The representative images
were captured from the peripheral areas of the retinas using a
fluorescence microscope (×400 magnification; Olympus IX-73; Olympus
Corporation). To evaluate RGC survival, four regions were randomly
selected for each retina. The number of Tuj1+ cells was
counted using ImageJ software (version 1.8.0; National Institutes
of Health) and averaged. The survival rate of RGCs was presented as
the percentage of the number of Tuj1+ cells in the
injured retina compared with that in the uninjured retina.
Hematoxylin and eosin (HE)
staining
At day 7 following building ONT model, euthanasia
was performed by cervical dislocation when the animals were under
deep anesthesia with the mixture of ketamine (100 mg/kg) and
xylazine (10 mg/kg). The eyeballs were immediately enucleated and
fixed in the Fekete's solution for 3 h at room temperature and the
optic nerves were fixed in 4% PFA at 4°C overnight. Then, the optic
nerves were dehydrated using gradient ethanol, embedded in paraffin
and sliced into 5 µm thickness. To observe the structural changes
of axons, the longitudinal sections were deparaffinized with
xylene, rehydrated through a graded ethanol series, and stained
with hematoxylin and eosin (H&E; BP-DL001; Nanjing SenBeiJia
Biological Technology Co., Ltd.). The sections were observed using
a light microscope (×400 magnification; Olympus IX-73; Olympus
Corporation) equipped with a DP80 camera.
Sample collection and preparation
ONT model was induced in the left optic nerve and
the contralateral eye served as the control. On the day 7 following
ONT injury, the mice were euthanized and the retinas were
harvested. The pooled retinas from the ipsilateral eyes of five
mice was taken as a sample. Approximately 7 mg of retinal sample
was subsequently vortexed in 200 µl methanol/H2O (4/1,
vol/vol) cold solvent, containing 10 µl of 2-chlorophenylalanine
(0.06 mg/ml) as the internal standard. After grinding at 60 Hz and
−20°C for 2 min, each retinal sample was sonicated at 40 KHz in an
ice-water bath for 10 min at 0°C and then centrifuged at 4°C
(15,620 × g) for 10 min. The supernatant of each sample was dried
with a freeze-concentration centrifugal dryer and dissolved in 190
µl of methanol/H2O (4/1, vol/vol) solvent at 4°C for 30
sec. After that, the samples were sonicated at 40KHz in an
ice-water bath at 0°C for 3 min and centrifuged at 4°C (15,620 × g)
for 10 min, followed by the collection through a crystal syringe
and filtration through a 0.22 µm microfilter into LC vials. The
vials were stored at −80°C before analysis. The quality control
(QC) samples were prepared by mixing the aliquots from all retinal
samples.
Liquid-chromatography tandem mass
spectrometry (LC-MS/MS) analysis
Metabolic profiling was performed using the ACQUITY
UPLC I-Class plus system (Waters Corporation) coupled with
Q-Exactive plus quadrupole-Orbitrap mass spectrometer (Thermo
Fisher Scientific, Inc.) in both positive ion and negative ion
modes of electrospray ionization (ESI). In brief, ultra-performance
liquid chromatography separation was performed on an ACQUITY UPLC
HSS T3 column (100×2.1 mm; 1.8 µm) at 45°C. Gradient elution was
performed using (A) water (0.1% formic acid) and (B) acetonitrile
(0.1% formic acid) as the mobile phase with an injection volume of
5 µl and a flow rate of 0.35 ml/min, starting at 95% A for 2 min
and decreasing to 20% within 8 min. Next, the gradient decreased to
0 within 4 min and kept for 1 min, and then increased to 95% within
0.1 min and kept for 1 min.
The sample mass spectrum signals were collected in
the positive and negative ion scanning modes. The scanning range
was from 100 to 1,200 mass-to-charge ratio (m/z). The resolution
was set at 70,000 in the full scan mode and 17,500 in the HCD MS/MS
scan model. The aux gas and sheath flow rates were 10 and 40
arbitrary units, respectively. The nitrogen gas temperature was
350°C. The spray voltages were set to 3800 V(+) and 3200 V(−). QCs
were added periodically to assess the repeatability.
Metabolomics data analysis
Software Progenesis QI V2.3 (Nonlinear Dynamics) was
used to handle raw peak exaction, baseline filtering, retention
time correction, peak alignment, peak identification and
normalization. The compounds were identified based on m/z,
secondary fragments and isotopic distribution. They were then
characterized using the Human Metabolome Database (HMDB; http://hmdb.ca/), Metlin (https://metlin.scripps.edu), Lipidmaps (V2.3)
(https://www.lipidmaps.org/) and EMDB2.0
(27). EMDB2.0 database is a local
mass spectrometry database established by Lu-ming Biotechnology
through standardized methods and standards, covering over 2,000
common metabolites. The extracted data were then processed by
removing the peaks with a missing value (ion intensity=0) in
>50% in groups, replacing 0 value by half of the minimum value,
and screening according to the qualitative results of the compound.
Compounds with the resulting scores below 36 (out of 60) points
were also deemed to be inaccurate and removed. A data matrix was
combined from the positive and negative ion data. Principal
component analysis (PCA) was performed to reduce data
dimensionality and visualize the relationship among samples.
Orthogonal Partial Least-Squares-Discriminant Analysis (OPLS-DA)
was applied for multivariate statistical analysis to identify the
differential metabolites. To assess the quality of a model, 7-fold
cross-validation and 200 Response Permutation Testing were
conducted to prevent overfitting. A two-sided unpaired Student's
t-test was used to calculate the statistical significance and
fold-change of the metabolites. The relative importance of each
metabolite to the OPLS-DA model was evaluated by the parameter,
variable importance in projection (VIP). The metabolites with
P<0.05 and VIP ≥1 were considered as the differential
metabolites for group discrimination.
The impact pathway was determined using the Pathway
Analysis Module of MetaboAnalyst 5.0 (http://www.metaboanalyst.ca/) based on the Kyoto
Encyclopedia of Genes and Genomes (KEGG) pathway library
(https://www.kegg.jp/), with the criteria as
P<0.05, false discovery rate (FDR) <0.05 and impact >0.05.
Before MetaboAnalyst analysis, the sample was normalized by the
median and the data was log10-transformed for comparison. The
parameters were as follows: ‘Scatter plot’ visualization method,
‘global test’ enrichment method, ‘relative-betweeness centrality’
topology analysis and reference metabolome of all compounds in the
selected pathway library of ‘Mus musculus (KEGG)’.
The areas under the receiver operating
characteristic (ROC) curves (AUC) analysis was performed using the
Biomarker Analysis Module of MetaboAnalyst 5.0 to assess the
predictive capacities of the potential biomarkers with an AUC of
>0.8 as the potential markers. Before MetaboAnalyst analysis,
the sample was normalized by the median. The data was
log10-transformed for comparison and auto-scaled (mean-centered and
divided by the standard deviation of each variable). The 95%
confidence intervals were calculated using 500 bootstrappings. For
multivariate ROC analysis, the linear support vector machine
algorithm was used to evaluate the combined biomarker model of the
selected metabolites via ROC curve based model evaluation (Tester)
section. 100 cross-validation were performed and the results were
averaged to generate the plot.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism 8 software (Dotmatics). All data were expressed as the mean ±
SD. For normally distributed data, an unpaired Student's t-test was
used for pairwise comparisons. For non-normally distributed data,
Mann-Whitney U test was performed for pairwise comparisons.
P<0.05 was considered to indicate a statistically significant
difference.
Results
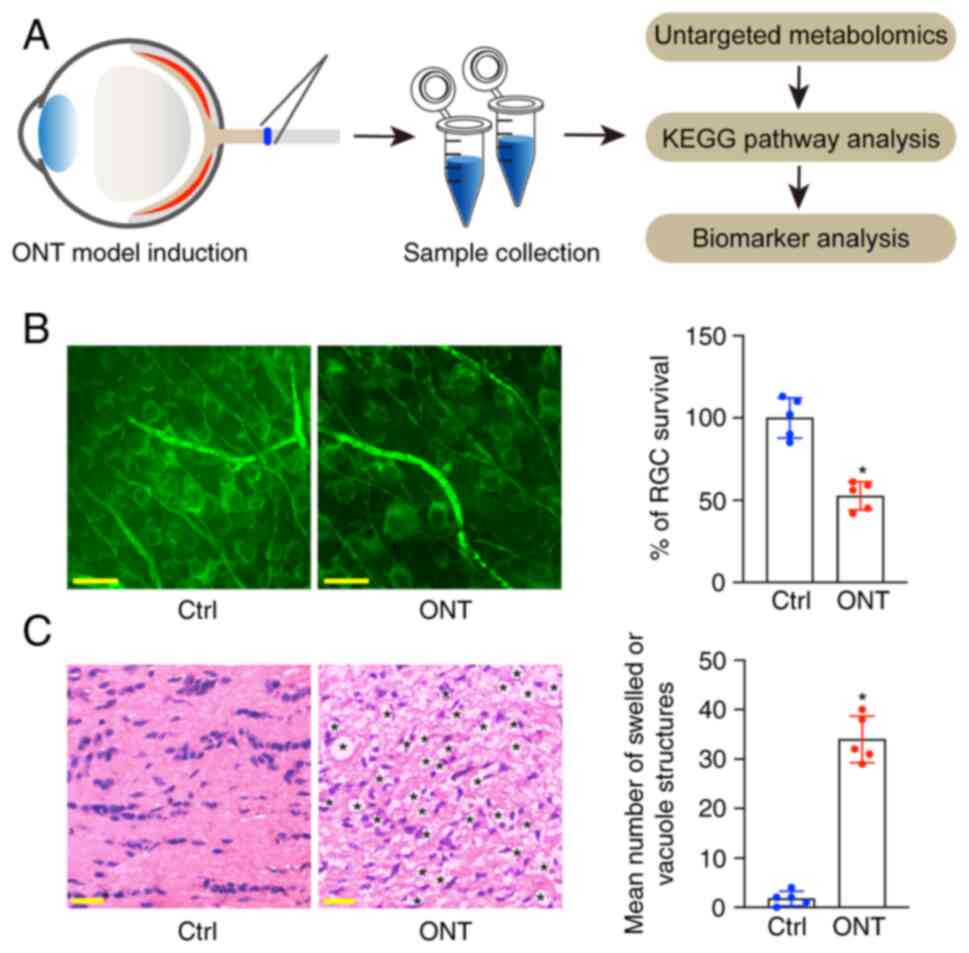
Establishment of murine ONT model
The untargeted metabolomics was used to detect the
changes of retinal metabolomic profile in the ONT model. The
experimental flow of metabolomics analysis is presented in Fig. 1A. To confirm the successful
establishment of the ONT model, the whole-mounted retinas were
stained with Tuj1 to detect the survival of RGCs at day 7 following
ONT injury. The results showed that ONT injury led to a ~50%
decrease in the number of survival RGCs (Fig. 1B). H&E staining of the
longitudinal sections revealed that the axons in the normal group
were well organized and the optic nerve fibers were intact. By
contrast, the axons in ONT injured group were swollen and contained
vacuole structures (Fig. 1C). The
aforementioned results indicated that the building of ONT model was
successful.
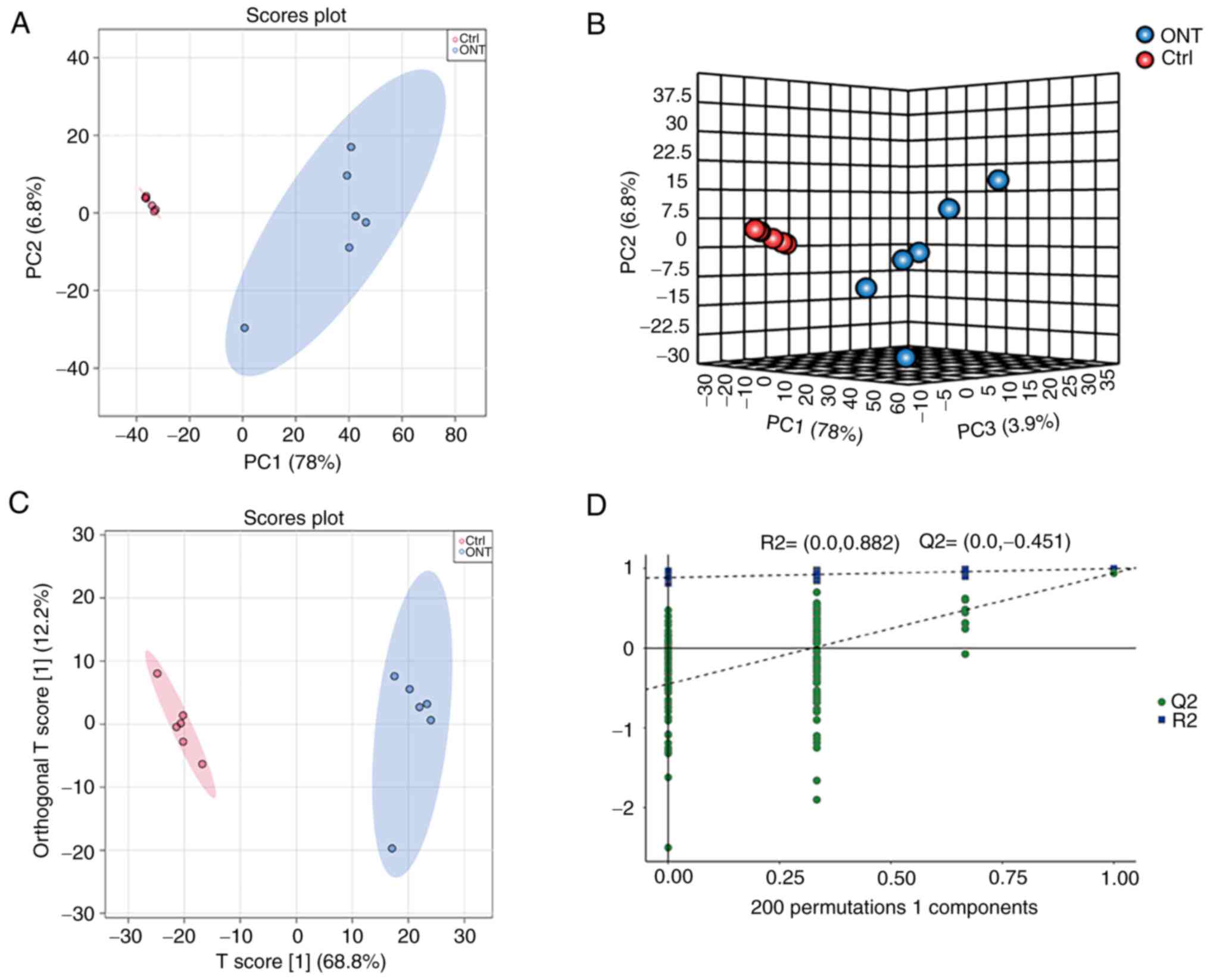
Untargeted metabolomic profile of
retinal tissues of ONT mice and non-ONT controls
To reveal the global metabolomic profile change
following ONT injury, a total of 12 retinal samples were collected
from six ONT retinas and six non-ONT retinas. A total of 2,928
anionic peaks and 3,745 cationic peaks of metabolites were
recorded. Two-dimensional and three-dimensional PCA models with the
score plots indicated that ONT samples clustered together, while
the non-ONT Ctrl samples clustered together, indicating that the
experiments displayed good repeatability (Fig. 2A and B). Next, OPLS-DA model with
the supervised method was constructed to determine the metabolomic
profile differences for the separation of two groups. As shown in
Fig. 2C, separations of ONT group
and non-ONT control group were observed, with the model values of
R2X (cum)=0.688, R2Y (cum)=0.939, and Q2 (cum)=0.93. In addition,
the permutation analysis showed that the R2 value was
lower compared with the original value. The Q2 intercept
was negative, indicating that there was no sign of overfitting,
which ensured the validity and stability of the OPLS-DA model
fitting (Fig. 2D).
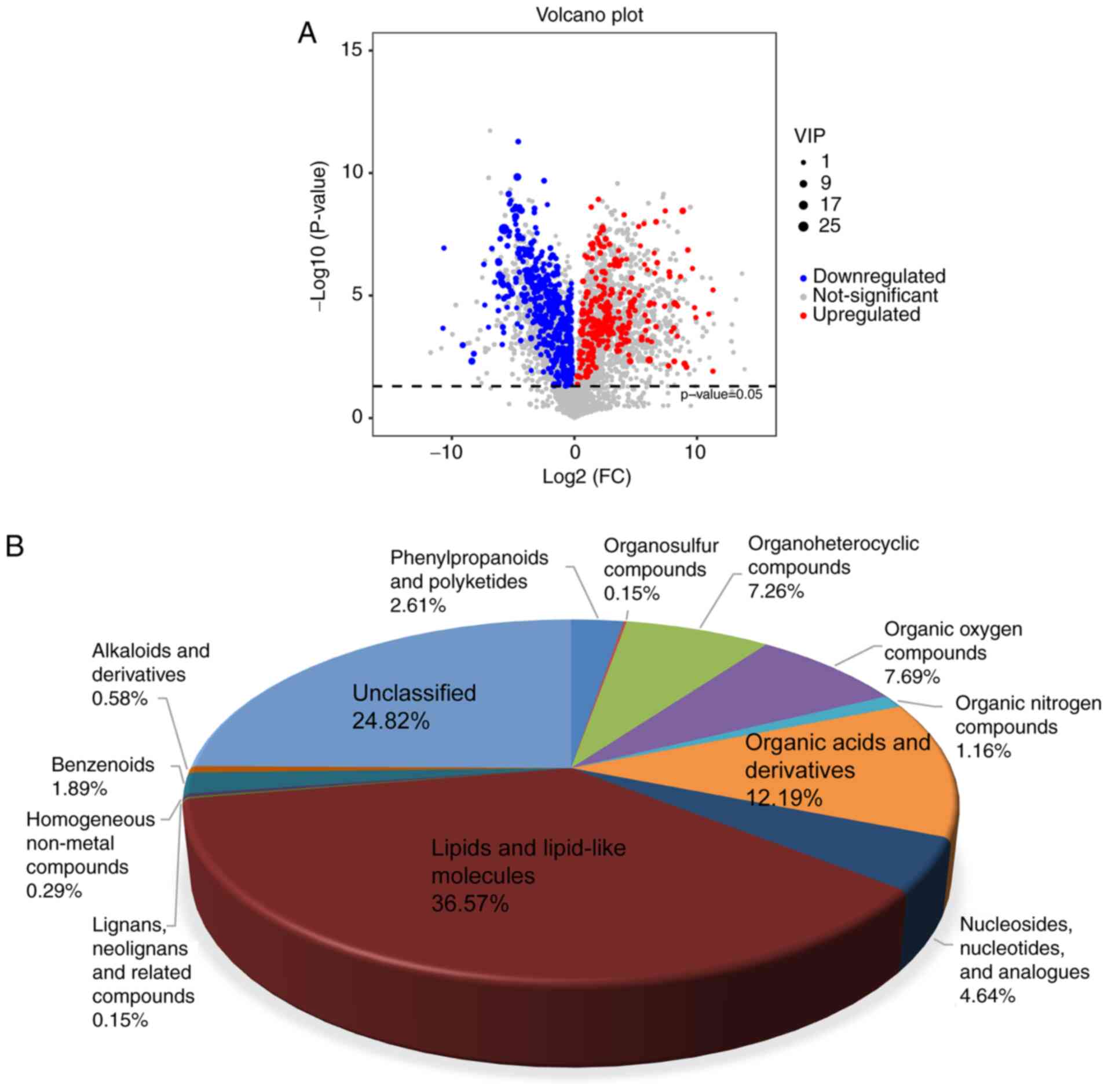
Identification of altered metabolites
and metabolic pathways following ONT injury
A volcano plots was used to calculate the
fold-change and P-value of all metabolites for the identification
of altered metabolites following ONT injury. VIP was used to
measure the effects of metabolite expression patterns on the
classification and discrimination of the samples in each group. The
results showed 689 differential metabolites were identified
according to the threshold of P<0.05 and VIP >1 (Fig. 3A), including lipids and lipid-like
molecules (36.57%), organic acids and derivatives (12.19%), organic
oxygen compounds (7.69%), organoheterocyclic compounds (7.26%),
nucleosides, nucleotides, and analogues (4.64%), phenylpropanoids
and polyketides (2.61%), benzenoids (1.89%), organic nitrogen
compounds (1.16%), alkaloids and derivatives (0.58%), homogeneous
non-metal compounds (0.29%), lignans, neolignans and related
compounds (0.15%), organosulfur compounds (0.15%), and unclassified
(24.82%) (Fig. 3B).
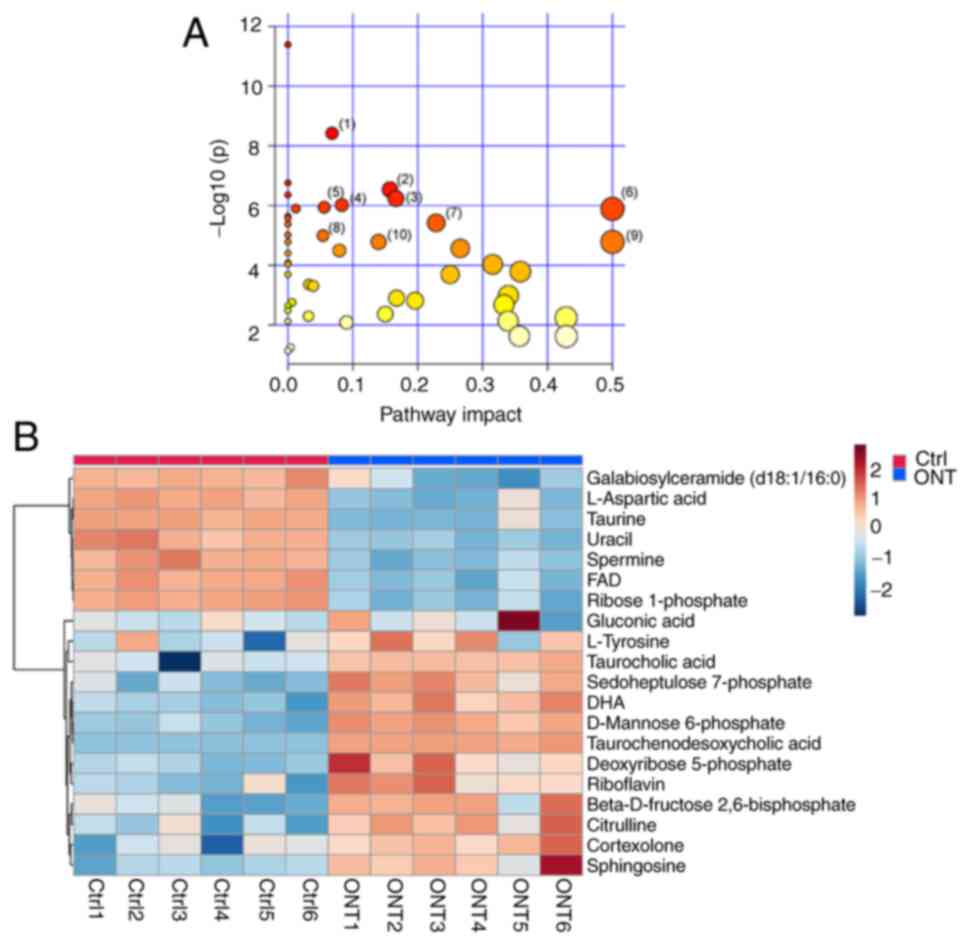
KEGG analyses were then carried out to determine the
involvement of the signaling pathways of altered metabolites
following ONT injury. The results revealed that 122 metabolites
were annotated and enriched in 50 KEGG pathway databases (Table SI). A total of 25 metabolic
pathways were identified with P<0.05 and impact >0.05. As
demonstrated in Fig. 4A, the top
10 enriched pathways were: i) ‘Primary bile acid biosynthesis’; ii)
‘Fructose and mannose metabolism’; iii) ‘Pentose phosphate
pathway’; iv) ‘Sphingolipid metabolism’; v) ‘β-Alanine metabolism’;
vi) ‘Riboflavin metabolism’; vii) ‘Arginine biosynthesis’; viii)
‘Steroid hormone biosynthesis’; ix) ‘Phenylalanine, tyrosine and
tryptophan biosynthesis’; and x) ‘Tyrosine metabolism’. The impact
factors were 0.07, 0.16, 0.17, 0.08, 0.06, 0.5, 0.23, 0.05, 0.5 and
0.14, respectively. As demonstrated in Fig. 4B, 20 altered metabolites were
identified in the aforementioned 10 metabolic pathways, including
13 upregulated metabolites and 7 downregulated metabolites in ONT
retinal tissues (Table SII).
Potential biomarkers for ONT
injury
Lipids are the major macromolecule in the brain,
accounting for 50% of dry brain weight (28). The lipid content of CNS is only
lower compared with that in adipose tissue. Moreover, altered lipid
metabolism has been proven to be involved in the pathogenesis of
neurodegenerative diseases (29).
Among the altered metabolic pathways following ONT
injury, the primary bile acid biosynthesis and sphingolipid
metabolism are the key pathways affecting lipid metabolism. To
screen for the metabolites as the candidate markers for ONT injury,
the present study investigated the altered metabolites enriched in
these two pathways. As demonstrated in Fig. 5A, three altered metabolites were
chosen as the candidate biomarkers in the primary bile acid
biosynthesis pathway, including taurine, taurochenodesoxycholic
acid and taurocholic acid (TCA). The P-values were
7.16×10−6, 4.79×10−4, and 0.0083,
respectively. AUC-ROC was used to detect the possibility of these
metabolites as the candidate biomarkers. The results showed the AUC
values of these biomarkers were 1 (Fig. 5B-D). The levels of
taurochenodesoxycholic acid and TCA were significantly increased
following ONT injury, while the level of taurine was significantly
decreased following ONT injury. Next, the multivariate biomarker
model was used by applying these three metabolites for ROC analysis
to test whether the primary bile acid biosynthesis was sufficient
to distinguish ONT group from non-ONT control group. The result
also showed that the value of AUC was 1 (Fig. 5E).
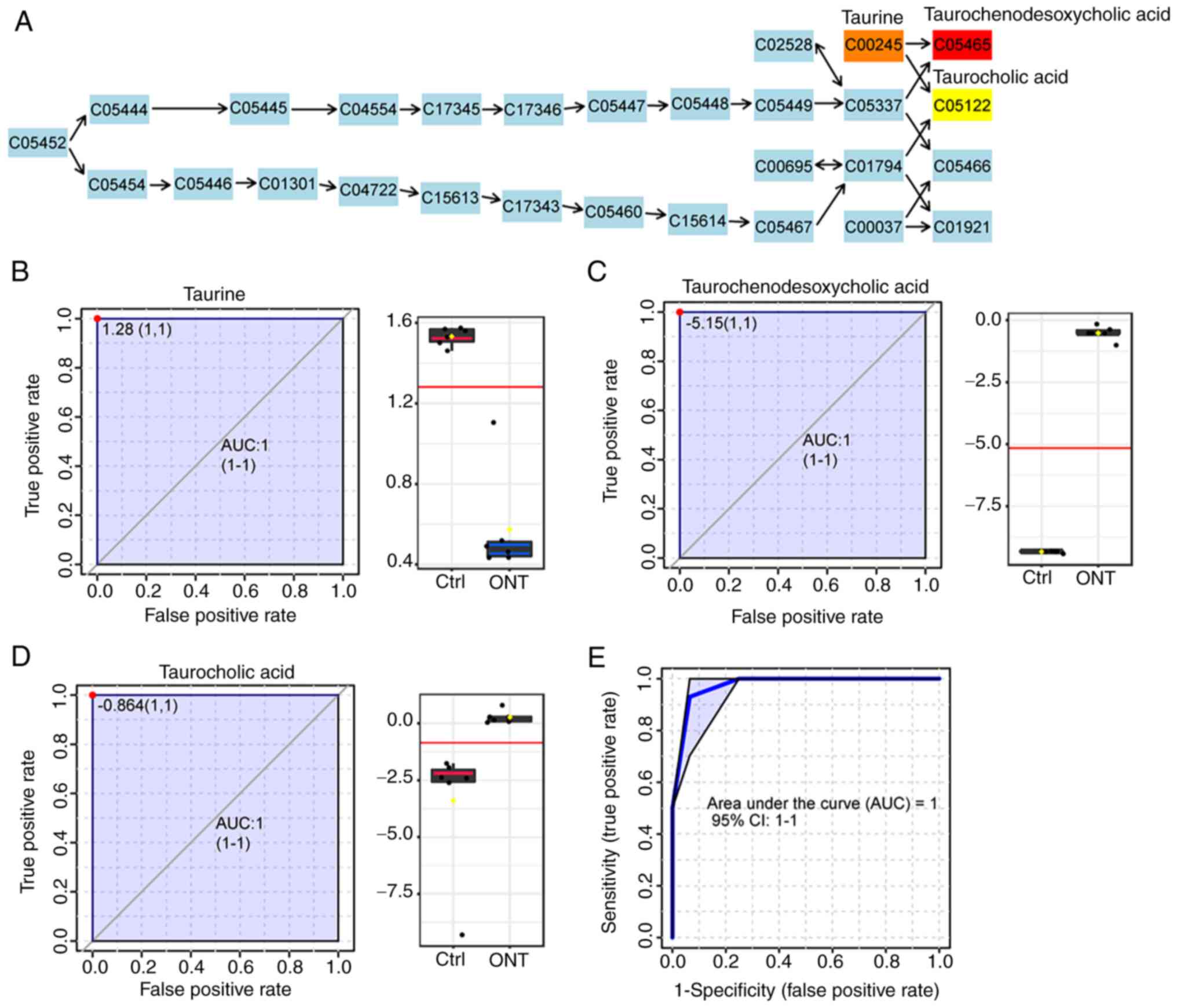 | Figure 5.Multivariate analysis of primary bile
acid biosynthesis. (A) Graphical presentation of KEGG signaling map
of primary bile acid biosynthesis. Blue indicates the metabolites
used as background; red, orange, and yellow indicate the
metabolites with different levels of significant variability
between ONT group and non-ONT ctrl group. ROC curve of the
metabolites from primary bile acid biosynthesis: (B) Taurine, (C)
Taurochenodesoxycholic acid and (D) TCA. (E) ROC curve of primary
bile biosynthesis (combination of taurine, taurochenodesoxycholic
acid and TCA) following ONT injury. ONT, optic nerve transection;
KEGG, Kyoto Encyclopedia of Genes and Genomes; ROC, receiver
operating characteristic; TCA, taurocholic acid; Ctrl, control;
AUC, area under the curve. |
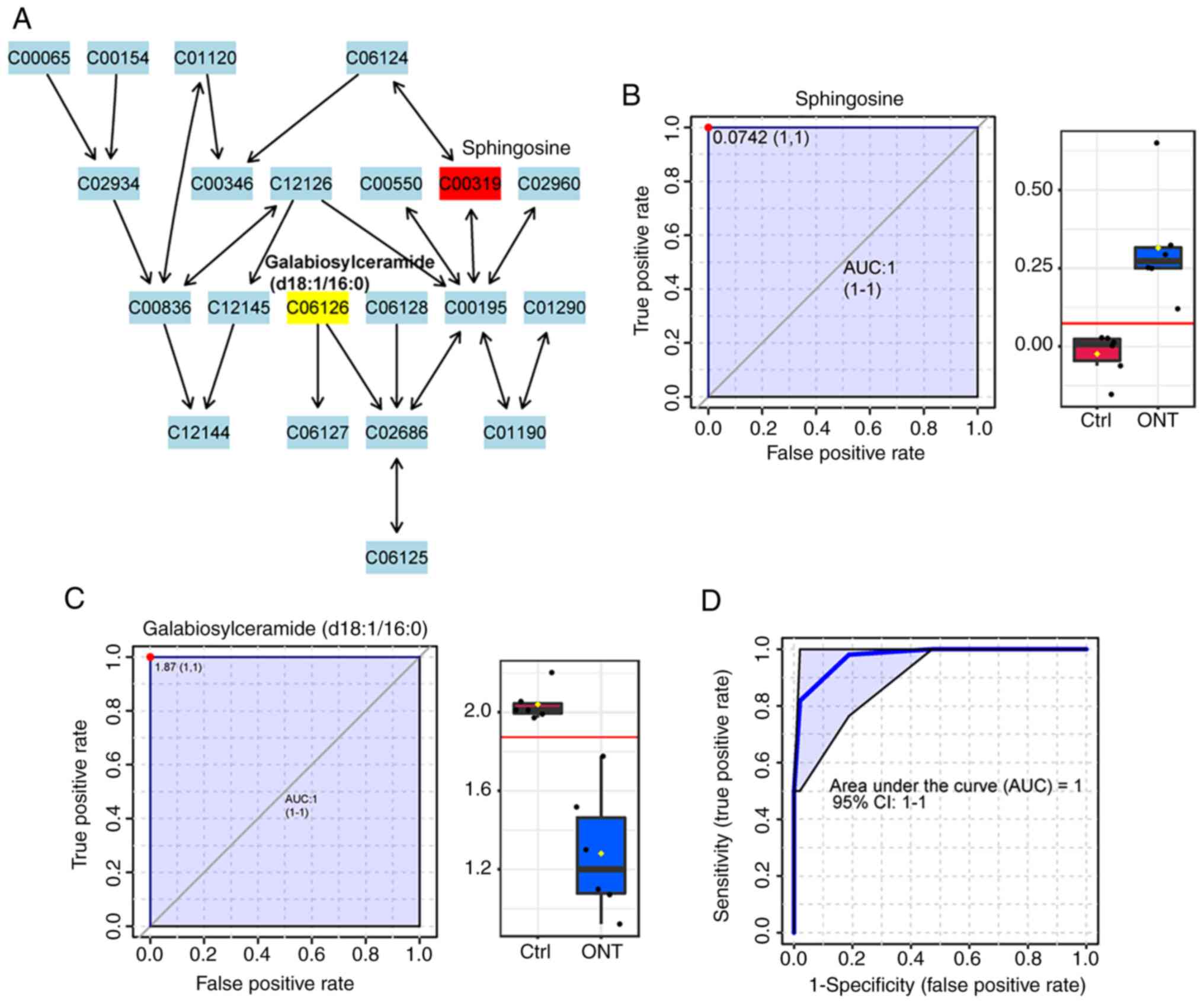
As shown in Fig.
6A, two metabolites, sphingosine and galabiosylceramide
(d18:1/16:0), were selected as the candidate biomarkers of
sphingolipid metabolism pathway, with P-values of 0.0010 and
0.0026, respectively. The level of sphingosine was significantly
increased following ONT injury. By contrast, the level of
galabiosylceramide (d18:1/16:0) was decreased following ONT injury.
ROC analysis showed that the AUC values of these two metabolites
were 1 (Fig. 6B and C). The
multivariate biomarker model also showed the value of AUC was 1
(Fig. 6D).
Discussion
RGCs serve as the connecting ring between the
neuroretina and the sensory retina. Their axons can form optic
nerve and bring the visual input to the brain (4). Once the axons pass through the lamina
cribrosa, they are surrounded by the myelin, thereby accelerating
the transmission speed of neurons. However, due to the limitation
in the length, trajectory and space, the axons are highly
susceptible to external damage (30). RGC loss has been recognized as the
hallmark of neurodegenerative diseases. Several animal models of
RGC degeneration have been established, which can be induced by
ocular hypertension (injection of saline/silicon oil/hyaluronic
acid into the eyes, episcleral vein occlusion, pressure-induced
retinal ischemia/reperfusion, and inbred DBA/2J mice) (31), mechanical stress (optic nerve
compression/transection and ocular blast) (12,14),
and glutamate neurotoxicity (intravitreal injection of
N-methyl-D-aspartate, glutamate transporter or specific NMDAR
deficit mice) (1). However, the
injury sites in these models are not identical.
The optic nerve is composed of fibers projecting to
the brain from the neuronal RGCs, whose cell bodies are located in
the retina. Ocular hypertension and glutamate neurotoxicity
primarily leads to an obvious injury to RGC axons and somas, which
is intraretinal (1,32). However, optic nerve injury in the
models of mechanical stress is extraretinal. Following optic nerve
injury, the injury signals travel retrogradely to RGC somata
located in the retina, eventually causing RGC degeneration
(33). Thus, mechanical stress
models are important models for studying the mechanism of RGC
degeneration following extraretinal injury.
Notably, the animal models mimicking mechanical
injury mainly include optic nerve crush (ONC) and ONT models
(34). ONC is generally less
severe compared with ONT and can be used to determine axon
regeneration across the lesion sites. However, the differences in
crush force and duration can affect tissue responses and lesions,
resulting in the difficulty in replication and bias in the
identification of potential biomarkers. ONT is a model of RGC
degeneration through direct injury of RGC fibers, ensuring that the
severity of injury in the experimental individuals is consistent
(35).
The present study thus selected an ONT model for
studying retinal neurodegeneration. ONT can cause rapid
degeneration of RGCs due to the loss of trophic support. In an ONT
model, axonal injury directly damages the axolemma, immediately
exposing the injured axonal cylinder to extracellular environment
and causing the influx of sodium and calcium (36). The resultant calcium-dependent
cysteine protease called calpain is activated in the later stage of
axonal degeneration, which degrades the axons and causes neuronal
death (37). Similarly to other
CNS in the higher vertebrates, optic nerve axon damage can cause
the loss of RGCs, resulting in irreversible vision impairment.
Hence, functional recovery of vision requires restoring the
functions of injured RGCs (38).
However, the exact mechanism of RGC degeneration remains unclear.
Metabolomics has been widely used to investigate the mechanism and
identify the metabolic signatures of disease progression (39). The untargeted metabolomics can
obtains the comprehensive reads of the detailed analyses of
detected metabolites (40). Thus,
identifying the metabolic signature of RGC degeneration can provide
novel insights into the identification of potential drug targets
for treating retinal neurodegeneration.
LC-MS/MS technology was used to screen for the
altered metabolites following ONT injury. The metabolomics profiles
were conducted between ONT retinas and non-ONT retinas and 689
differential metabolites were identified. The metabolite pathways,
including primary bile acid biosynthesis and sphingolipid
metabolism related to lipid metabolism, were altered following ONT
injury. A total of five metabolites were identified as the
candidate biomarkers for retinal neurodegeneration. In addition,
altered amino acids and glucose metabolism were observed in the
retinas following ONT injury.
The present study also observed that lipids and
lipid-like molecules were mainly involved in the altered metabolite
pathways following ONT injury, suggesting that lipids exert
imperative effects on the progression of ONT injury, which
confirmed the association between lipid metabolism and
neurodegeneration (28). As the
major components of the bilayer membrane, lipids exert imperative
effects in maintaining normal physiological functions of CNS,
including energy production and cell-to-cell signal transduction
(41). Damaged neurons require
large amounts of lipids to form the membranes during regeneration
(42). A previous study has
revealed that lipin1, a critical regulator of glycerolipid
metabolism in neurons, can enhance axon regeneration after optic
nerve injury (43).
Subsequently, the altered metabolites following ONT
injury were annotated using KEGG, HMDB, Lipidmaps (V2.3), Metlin
and EMDB2.0. These altered metabolites following ONT injury were
highly enriched in 50 metabolite pathways. Among the top 10
metabolic pathways, ‘fructose and mannose metabolism’ (44), ‘pentose phosphate’ (45), ‘β-Alanine metabolism’ (46), ‘arginine biosynthesis’ (47), ‘sphingolipid metabolism’ (48) and ‘steroid hormone biosynthesis’
(49) are involved in the
pathogenesis of diabetic retinopathy. Altered ‘sphingolipid
metabolism’ (50), ‘tyrosine
metabolism’ (51) and ‘steroid
hormone biosynthesis’ (52) have
been found in the pathogenesis of glaucoma. Altered ‘arginine
metabolism’ has been detected between Vogt-Koyanagi-Harada and
healthy controls (53). The
metabolites of primary bile acid biosynthesis are tightly
associated with the pathogenesis of age-related macular
degeneration (54). Riboflavin
deficiency can cause severe decreases in retinal function
accompanied by structural changes in the neural retina and retinal
pigment epithelium (55). Among
these altered pathways, ‘primary bile acid biosynthesis’ and
‘sphingolipid metabolism’ are tightly associated with lipid
metabolism and involve several pathological processes, such as
inflammation, neovascularization, survival and death of neurons and
migration of endothelial cells (54,56).
Primary bile acid biosynthesis is a crucial pathway
for cholesterol catabolism and homeostasis maintenance (57). Primary bile acid is synthesized
from cholesterol in the liver and converted to secondary bile acid
by anaerobic bacteria in the gut. Finally, bile acid is reabsorbed
by enterocytes and transited back to the liver via the portal vein
to produce glycine and taurine (58). Dysfunction of bile acid
biosynthesis is involved in the onset and progression of
Parkinson's disease (23). In
addition, a previous study has revealed that patients with
Alzheimer's disease have lower levels of serum primary bile acids
compared with the normal healthy controls (58). The present study detected the
differential expression of taurine, taurochenodesoxycholic acid and
TCA following ONT injury. ROC curve analysis indicated that these
altered metabolites could be selected as the candidate biomarkers
for detecting retinal neurodegeneration. Taurine, a
sulfur-containing amino acid, is reduced by 76.29% in the ONT group
(59,60). Taurochenodesoxycholic acid and TCA
are increased in concentrations following optic nerve injury.
Taurine is highly expressed in the mammalian brain, heart and
leukocytes, and is one of the most abundant amino acids (61). Taurine plays protective roles in
diverse CNS disorders by regulating intracellular calcium
transport, affecting antioxidation and acting as a neuroprotector
against L-glutamate-induced neurotoxicity (62,63).
Additionally, treatment with taurine can suppress NMDA-induced
retinal cell apoptosis by reducing oxidative stress (64).
Taurochenodesoxycholic acid and TCA have been
reported to be the highest bile acid in the serum of the patients
with cirrhosis (65).
Taurochenodesoxycholic acid, the conjugate of taurine and bile
acid, exerts its anti-inflammatory and immunomodulatory functions
and enhances apoptosis via the activation of PKC-c-JNK/P38
signaling (66). The significant
upregulation of taurochenodesoxycholic acid in ONT group may be
associated with the activation of inflammatory response and
apoptosis of RGCs following ONT injury. TCA (a major
12α-hydroxylated bile acid), composed of taurine and chlolic acid,
is significantly increased in the ONT group (67). Even though its roles in
neurodegeneration have not yet been elucidated, previous studies
reported the altered plasma levels of bile acids in patients with
AMD, demonstrating a protective effect of TCA on both degeneration
and neovascular AMD (54).
Moreover, plasma levels of TCA are tightly related to inflammatory
biomarkers in patients coinfected with human immunodeficiency
virus/hepatitis C virus (68). As
an upstream compound of taurochenodesoxycholic acid and TCA,
taurine is becoming a promising target for reversing the disorders
caused by axonal injury.
Sphingolipid is highly expressed in myelin sheaths
and plays important roles in maintaining neuronal survival, signal
transduction and synaptic stability (69). Abnormal sphingolipid metabolism
occurs in several CNS diseases (70). Altered sphingolipid metabolism due
to Golgi-associated retrograde protein (GARP) mutations contributes
to neurodegeneration, and inhibitor of sphingolipid synthesis can
greatly improve the outcomes and survival in GARP mutant wobbler
mice (a model of motor neuron degeneration) (71). Sphingolipid metabolites,
particularly sphingosine-1-phosphate and ceramide, are crucial for
the proliferation, survival and activation of astrocytes, microglia
and neurons (72,73). The present study observed higher
levels of sphingosine and lower levels of galabiosylceramide
(d18:1/16:0) in the ONT group compared with those in the control
group. Furthermore, the combination of these two metabolites showed
good sensitivity and specificity. Altered patterns of lipid
metabolism and apoptosis through ceramide pathways have been
reported in another metabolomics study targeting 195 metabolites
(74).
Sphingosine consists of hydrophobic sphingoid
long-chain bases and can be converted to sphingosine-1-phosphate by
sphingosine kinase (75).
Increased levels of sphingosine have also been detected in the
patients with primary open-angle glaucoma based on an untargeted
plasma metabolomic study (76).
This study suggests that axonal injury-induced excessive
sphingosine may be involved RGC degeneration through ceramide
apoptosis signaling. However, no difference was detected in
sphingosine-1-phosphate level in the ONT group in the present
study, which differed from another study on Parkinson's disease
(77). The study consistency and
differences present the opportunities for further metabolic in
retinal neurodegenerative diseases. Galabiosylceramide
(d18:1/16:0), the last unit of glycosphingolipids, decreased by
62.5% after axonal injury in the present study. Although the
mechanism of galabiosylceramide (d18:1/16:0) remains unknown, it
may serve as a potential biomarker of retinal neurodegeneration to
some extent.
There are still some limitations in the present
study. A relatively typical sampling time was selected. The
potential impacts of sampling time points on retinal metabolic
profile should be further considered. Although the present study
predicted the involvement of altered metabolite pathways following
ONT injury, the potential mechanism associated with the altered
pathways and metabolites in ONT and retinal neurodegeneration
remains unclear and requires further study. In addition, the key
metabolic enzymes, including bile acid-CoA:amino acid
N-acyltransferase (78,79), Acyl-coenzyme A amino acid
N-acyltransferase 1 (80), acid
ceramidase, neutral ceramidase, alkaline ceramidase (81), sphingosine-1-phosphate phosphatase
(82) and phospholipid
phosphatase, which are in charge of the production of these altered
metabolites, should be analyzed in the further research (83). To identify the reliable biomarkers
for the diagnosis of retinal neurodegeneration, the targeted
metabolomics should also be conducted on the clinical samples.
In conclusion, the present study identified the
changes of metabolomic profile in retinal tissues of ONT mice via
LC-MS/MS analysis. A total of 689 differential metabolites and 50
altered metabolic pathways were identified following ONT injury.
The major types of altered metabolites were lipids and lipid-like
molecules, suggesting that lipid-related metabolism was tightly
associated with the process of retinal neurodegeneration. Primary
bile acid biosynthesis pathway, sphingolipid metabolism pathway and
five altered metabolites with high sensitivity and specificity
(AUC>0.8) were identified following ONT injury. The levels of
taurochenodesoxycholic acid, TCA and sphingosine increased, while
the levels of taurine and galabiosylceramide decreased in the ONT
model, implying that these altered metabolites are the potential
therapeutic target for ONT injury. The present study may provide
novel insights into the pathogenesis of RGC degeneration and
provide the candidate metabolic targets for the diagnosis and
treatment of retinal neurodegenerative diseases.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural Science
Foundation of China (grant nos. 82225013, 82171074 and 81970809)
and the Shanghai Youth Talent Support Program (grant no. 2016).
Availability of data and materials
Raw datasets for metabolic profiling have been
deposited to the MetaboLights Repository (www.ebi.ac.uk/metabolights/MTBLS7911) with the
Study Identifier MTBLS7911. Other datasets used and/or analyzed
during the current study available from the corresponding author on
reasonable request.
Authors' contributions
BY and JY designed this study. JZ, XN, SL and YJ
performed the experiments, figure preparation and manuscript draft.
JZ, XN and XH developed the methods and performed data analysis. JZ
and XN acquired and interpreted the data. JZ and BY wrote and
revised the paper. JZ and JY confirm the authenticity of all the
raw data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All experiments were approved by the Animal Ethics
and Experimentation Committee of Nanjing Medical University
(approval no. 2103027).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Guo X, Zhou J, Starr C, Mohns EJ, Li Y,
Chen EP, Yoon Y, Kellner CP, Tanaka K, Wang H, et al: Preservation
of vision after CaMKII-mediated protection of retinal ganglion
cells. Cell. 184:4299–4314.e12. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Parisi V, Oddone F, Ziccardi L, Roberti G,
Coppola G and Manni G: Citicoline and retinal ganglion cells:
Effects on morphology and function. Curr Neuropharmacol.
16:919–932. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Levin LA and Gordon LK: Retinal ganglion
cell disorders: Types and treatments. Prog Retin Eye Res.
21:465–484. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fry LE, Fahy E, Chrysostomou V, Hui F,
Tang J, van Wijngaarden P, Petrou S and Crowston JG: The coma in
glaucoma: Retinal ganglion cell dysfunction and recovery. Prog
Retin Eye Res. 65:77–92. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jiang S, Kametani M and Chen DF: Adaptive
immunity: New aspects of pathogenesis underlying neurodegeneration
in glaucoma and optic neuropathy. Front Immunol. 11:652020.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bojcevski J, Stojic A, Hoffmann DB,
Williams SK, Muller A, Diem R and Fairless R: Influence of retinal
NMDA receptor activity during autoimmune optic neuritis. J
Neurochem. 153:693–709. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Almasieh M, Wilson AM, Morquette B, Cueva
Vargas JL and Di Polo A: The molecular basis of retinal ganglion
cell death in glaucoma. Prog Retin Eye Res. 31:152–181. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wert KJ, Velez G, Kanchustambham VL,
Shankar V, Evans LP, Sengillo JD, Zare RN, Bassuk AG, Tsang SH and
Mahajan VB: Metabolite therapy guided by liquid biopsy proteomics
delays retinal neurodegeneration. EBioMedicine. 52:1026362020.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lehtonen Š, Sonninen TM, Wojciechowski S,
Goldsteins G and Koistinaho J: Dysfunction of cellular proteostasis
in Parkinson's disease. Front Neurosci. 13:4572019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pardue MT and Allen RS: Neuroprotective
strategies for retinal disease. Prog Retin Eye Res. 65:50–76. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tran AP, Warren PM and Silver J: The
biology of regeneration failure and success after spinal cord
injury. Physiol Rev. 98:881–917. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fung JCL and Cho EYP: Methylene blue
promotes survival and GAP-43 expression of retinal ganglion cells
after optic nerve transection. Life Sci. 262:1184622020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tran NM, Shekhar K, Whitney IE, Jacobi A,
Benhar I, Hong G, Yan W, Adiconis X, Arnold ME, Lee JM, et al:
Single-cell profiles of retinal ganglion cells differing in
resilience to injury reveal neuroprotective genes. Neuron.
104:1039–1055.e12. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mead B, Kerr A, Nakaya N and Tomarev SI:
miRNA changes in retinal ganglion cells after optic nerve crush and
glaucomatous damage. Cells. 10:15642021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ayupe AC, Beckedorff F, Levay K, Yon B,
Salgueiro Y, Shiekhattar R and Park KK: Identification of long
noncoding RNAs in injury-resilient and injury-susceptible mouse
retinal ganglion cells. BMC Genomics. 22:7412021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang JJ, Liu C, Shan K, Liu BH, Li XM,
Zhang SJ, Zhou RM, Dong R, Yan B and Sun XH: Circular RNA-ZNF609
regulates retinal neurodegeneration by acting as miR-615 sponge.
Theranostics. 8:3408–3415. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rinschen MM, Ivanisevic J, Giera M and
Siuzdak G: Identification of bioactive metabolites using activity
metabolomics. Nat Rev Mol Cell Biol. 20:353–367. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li Q, Wei S, Wu D, Wen C and Zhou J:
Urinary metabolomics study of patients with gout using gas
chromatography-mass spectrometry. Biomed Res Int. 2018:34615722018.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang QJ, Zhao JR, Hao J, Li B, Huo Y, Han
YL, Wan LL, Li J, Huang J, Lu J, et al: Serum and urine
metabolomics study reveals a distinct diagnostic model for cancer
cachexia. J Cachexia Sarcopenia Muscle. 9:71–85. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shao Y and Le W: Recent advances and
perspectives of metabolomics-based investigations in Parkinson's
disease. Mol Neurodegener. 14:32019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
McGarrah RW, Crown SB, Zhang GF, Shah SH
and Newgard CB: Cardiovascular metabolomics. Circ Res.
122:1238–1258. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mapstone M, Cheema AK, Fiandaca MS, Zhong
X, Mhyre TR, MacArthur LH, Hall WJ, Fisher SG, Peterson DR, Haley
JM, et al: Plasma phospholipids identify antecedent memory
impairment in older adults. Nat Med. 20:415–418. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Graham SF, Rey NL, Yilmaz A, Kumar P,
Madaj Z, Maddens M, Bahado-Singh RO, Becker K, Schulz E, Meyerdirk
LK, et al: Biochemical profiling of the brain and blood metabolome
in a mouse model of prodromal Parkinson's disease reveals distinct
metabolic profiles. J Proteome Res. 17:2460–2469. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Botas A, Campbell HM, Han X and
Maletic-Savatic M: Metabolomics of neurodegenerative diseases. Int
Rev Neurobiol. 122:53–80. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mayordomo-Febrer A, López-Murcia M,
Morales-Tatay JM, Monleón-Salvado D and Pinazo-Durán MD:
Metabolomics of the aqueous humor in the rat glaucoma model induced
by a series of intracamerular sodium hyaluronate injection. Exp Eye
Res. 131:84–92. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Leruez S, Marill A, Bresson T, de Saint
Martin G, Buisset A, Muller J, Tessier L, Gadras C, Verny C, Gohier
P, et al: A metabolomics profiling of glaucoma points to
mitochondrial dysfunction, senescence, and polyamines deficiency.
Invest Ophthalmol Vis Sci. 59:4355–4361. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu J, Cao C, Jin Y, Wang Y, Ma X, Li J,
Guo S, Yang J, Niu J and Liang X: Induced neural stem cells
suppressed neuroinflammation by inhibiting the microglial
pyroptotic pathway in intracerebral hemorrhage rats. iScience.
26:1070222023. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kao YC, Ho PC, Tu YK, Jou IM and Tsai KJ:
Lipids and Alzheimer's disease. Int J Mol Sci. 21:15052020.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sastry PS: Lipids of nervous tissue:
Composition and metabolism. Prog Lipid Res. 24:69–176. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Smith JA, Nicaise AM, Ionescu RB, Hamel R,
Peruzzotti-Jametti L and Pluchino S: Stem cell therapies for
progressive multiple sclerosis. Front Cell Dev Biol. 9:6964342021.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kang EY, Liu PK, Wen YT, Quinn PMJ, Levi
SR, Wang NK and Tsai RK: Role of oxidative stress in ocular
diseases associated with retinal ganglion cells degeneration.
Antioxidants (Basel). 10:19482021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Belforte N, Agostinone J, Alarcon-Martinez
L, Villafranca-Baughman D, Dotigny F, Cueva Vargas JL and Di Polo
A: AMPK hyperactivation promotes dendrite retraction, synaptic
loss, and neuronal dysfunction in glaucoma. Mol Neurodegener.
16:432021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Galan A, Dergham P, Escoll P, de-la-Hera
A, D'Onofrio PM, Magharious MM, Koeberle PD, Frade JM and Saragovi
HU: Neuronal injury external to the retina rapidly activates
retinal glia, followed by elevation of markers for cell cycle
re-entry and death in retinal ganglion cells. PLoS One.
9:e1013492014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Syc-Mazurek SB, Fernandes KA and Libby RT:
JUN is important for ocular hypertension-induced retinal ganglion
cell degeneration. Cell Death Dis. 8:e29452017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Do JL, Allahwerdy S, David RC, Weinreb RN
and Welsbie DS: Sheath-preserving optic nerve transection in rats
to assess axon regeneration and interventions targeting the retinal
ganglion cell axon. J Vis Exp. 6:3791/61748. 2020.
|
|
36
|
Rosenberg LJ, Emery DG and Lucas JH:
Effects of sodium and chloride on neuronal survival after neurite
transection. J Neuropathol Exp Neurol. 60:33–48. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gerdts J, Summers DW, Milbrandt J and
DiAntonio A: Axon self-destruction: New links among SARM1, MAPKs,
and NAD+ metabolism. Neuron. 89:449–460. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Krishnan A, Kocab AJ, Zacks DN,
Marshak-Rothstein A and Gregory-Ksander M: A small peptide
antagonist of the Fas receptor inhibits neuroinflammation and
prevents axon degeneration and retinal ganglion cell death in an
inducible mouse model of glaucoma. J Neuroinflammation. 16:1842019.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gertsman I and Barshop BA: Promises and
pitfalls of untargeted metabolomics. J Inherit Metab Dis.
41:355–366. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Di Minno A, Gelzo M, Stornaiuolo M,
Ruoppolo M and Castaldo G: The evolving landscape of untargeted
metabolomics. Nutr Metab Cardiovasc Dis. 31:1645–1652. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hallett PJ, Engelender S and Isacson O:
Lipid and immune abnormalities causing age-dependent
neurodegeneration and Parkinson's disease. J Neuroinflammation.
16:1532019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bradke F, Fawcett JW and Spira ME:
Assembly of a new growth cone after axotomy: The precursor to axon
regeneration. Nat Rev Neurosci. 13:183–193. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yang C, Wang X, Wang J, Wang X, Chen W, Lu
N, Siniossoglou S, Yao Z and Liu K: Rewiring neuronal glycerolipid
metabolism determines the extent of axon regeneration. Neuron.
105:276–292.e5. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang J, Wang Z, Zhang Y and Li J:
Proteomic analysis of vitreal exosomes in patients with
proliferative diabetic retinopathy. Eye (Lond). 37:2061–2068. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Haines NR, Manoharan N, Olson JL,
D'Alessandro A and Reisz JA: Metabolomics analysis of human
vitreous in diabetic retinopathy and rhegmatogenous retinal
detachment. J Proteome Res. 17:2421–2427. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Li L, Yang K, Li C, Zhang H, Yu H, Chen K,
Yang X and Liu L: Metagenomic shotgun sequencing and metabolomic
profiling identify specific human gut microbiota associated with
diabetic retinopathy in patients with type 2 diabetes. Front
Immunol. 13:9433252022. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Paris LP, Johnson CH, Aguilar E, Usui Y,
Cho K, Hoang LT, Feitelberg D, Benton HP, Westenskow PD, Kurihara
T, et al: Global metabolomics reveals metabolic dysregulation in
ischemic retinopathy. Metabolomics. 12:152016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ensari Delioğlu EN, Uğurlu N, Erdal E,
Malekghasemi S and Çağıl N: Evaluation of sphingolipid metabolism
on diabetic retinopathy. Indian J Ophthalmol. 69:3376–3380. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chaurasia RK, Singh R, Agrawal JK and
Maurya OP: Sex hormones and diabetic retinopathy. Ann Ophthalmol.
25:227–230. 1993.PubMed/NCBI
|
|
50
|
Aljohani AJ, Edwards G, Guerra Y, Dubovy
S, Miller D, Lee RK and Bhattacharya SK: Human trabecular meshwork
sphingolipid and ceramide profiles and potential latent fungal
commensalism. Invest Ophthalmol Vis Sci. 55:3413–3422. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kouassi Nzoughet J, Guehlouz K, Leruez S,
Gohier P, Bocca C, Muller J, Blanchet O, Bonneau D, Simard G, Milea
D, et al: A data mining metabolomics exploration of glaucoma.
Metabolites. 10:492020. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Qiu Y, Yu J, Tang L, Ren J, Shao M, Li S,
Song Y, Cao W and Sun X: Association between sex hormones and
visual field progression in women with primary open angle glaucoma:
A cross-sectional and prospective cohort study. Front Aging
Neurosci. 13:7561862021. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Chang R, Zhu Y, Xu J, Chen L, Su G,
Kijlstra A and Yang P: Identification of urine metabolic biomarkers
for Vogt-Koyanagi-Harada disease. Front Cell Dev Biol.
9:6374892021. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Warden C, Barnett JM and Brantley MA Jr:
Taurocholic acid inhibits features of age-related macular
degeneration in vitro. Exp Eye Res. 193:1079742020. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Sinha T, Ikelle L, Makia MS, Crane R, Zhao
X, Kakakhel M, Al-Ubaidi MR and Naash MI: Riboflavin deficiency
leads to irreversible cellular changes in the RPE and disrupts
retinal function through alterations in cellular metabolic
homeostasis. Redox Biol. 54:1023752022. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Chen H, Chan AY, Stone DU and Mandal NA:
Beyond the cherry-red spot: Ocular manifestations of
sphingolipid-mediated neurodegenerative and inflammatory disorders.
Surv Ophthalmol. 59:64–76. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Chiang JYL and Ferrell JM: Bile acid
receptors FXR and TGR5 signaling in fatty liver diseases and
therapy. Am J Physiol Gastrointest Liver Physiol. 318:G554–G573.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
MahmoudianDehkordi S, Arnold M, Nho K,
Ahmad S, Jia W, Xie G, Louie G, Kueider-Paisley A, Moseley MA,
Thompson JW, et al: Altered bile acid profile associates with
cognitive impairment in Alzheimer's disease-An emerging role for
gut microbiome. Alzheimers Dement. 15:76–92. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Rafiee Z, Garcia-Serrano AM and Duarte
JMN: Taurine supplementation as a neuroprotective strategy upon
brain dysfunction in metabolic syndrome and diabetes. Nutrients.
14:12922022. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Bocca C, Le Paih V, Chao de la Barca JM,
Kouassy Nzoughet J, Amati-Bonneau P, Blanchet O, Védie B, Géromin
D, Simard G, Procaccio V, et al: A plasma metabolomic signature of
Leber hereditary optic neuropathy showing taurine and nicotinamide
deficiencies. Hum Mol Genet. 30:21–29. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Seol SI, Kim HJ, Choi EB, Kang IS, Lee HK,
Lee JK and Kim C: Taurine protects against postischemic brain
injury via the antioxidant activity of taurine chloramine.
Antioxidants (Basel). 10:3722021. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Wu JY and Prentice H: Role of taurine in
the central nervous system. J Biomed Sci. 17 (Suppl 1):S12010.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Menzie J, Prentice H and Wu JY:
Neuroprotective mechanisms of taurine against ischemic stroke.
Brain Sci. 3:877–907. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Jafri AJA, Agarwal R, Iezhitsa I, Agarwal
P and Ismail NM: Taurine protects against NMDA-induced retinal
damage by reducing retinal oxidative stress. Amino Acids.
51:641–646. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Wang X, Xie G, Zhao A, Zheng X, Huang F,
Wang Y, Yao C, Jia W and Liu P: Serum bile acids are associated
with pathological progression of hepatitis B-induced cirrhosis. J
Proteome Res. 15:1126–1134. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Qi Y, Shi L, Duan G, Ma Y and Li P:
Taurochenodeoxycholic acid increases cAMP content via specially
interacting with bile acid receptor TGR5. Molecules. 26:70662021.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Mahalak KK, Bobokalonov J, Firrman J,
Williams R, Evans B, Fanelli B, Soares JW, Kobori M and Liu L:
Analysis of the ability of capsaicin to modulate the human gut
microbiota in vitro. Nutrients. 14:12832022. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Virseda-Berdices A, Rojo D, Martínez I,
Berenguer J, González-García J, Brochado-Kith O,
Fernández-Rodríguez A, Díez C, Hontañon V, Pérez-Latorre L, et al:
Metabolomic changes after DAAs therapy are related to the
improvement of cirrhosis and inflammation in HIV/HCV-coinfected
patients. Biomed Pharmacother. 147:1126232022. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Abou-Ghali M and Stiban J: Regulation of
ceramide channel formation and disassembly: Insights on the
initiation of apoptosis. Saudi J Biol Sci. 22:760–772. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Alaamery M, Albesher N, Aljawini N,
Alsuwailm M, Massadeh S, Wheeler MA, Chao CC and Quintana FJ: Role
of sphingolipid metabolism in neurodegeneration. J Neurochem.
158:25–35. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Petit CS, Lee JJ, Boland S, Swarup S,
Christiano R, Lai ZW, Mejhert N, Elliott SD, McFall D, Haque S, et
al: Inhibition of sphingolipid synthesis improves outcomes and
survival in GARP mutant wobbler mice, a model of motor neuron
degeneration. Proc Natl Acad Sci USA. 117:10565–10574. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Tham CS, Lin FF, Rao TS, Yu N and Webb M:
Microglial activation state and lysophospholipid acid receptor
expression. Int J Dev Neurosci. 21:431–443. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Scheiblich H, Schlütter A, Golenbock DT,
Latz E, Martinez-Martinez P and Heneka MT: Activation of the NLRP3
inflammasome in microglia: the role of ceramide. J Neurochem.
143:534–550. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Agudo-Barriuso M, Lahoz A, Nadal-Nicolás
FM, Sobrado-Calvo P, Piquer-Gil M, Diaz-Llopis M, Vidal-Sanz M and
Mullor JL: Metabolomic changes in the rat retina after optic nerve
crush. Invest Ophthalmol Vis Sci. 54:4249–4259. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Woodcock J: Sphingosine and ceramide
signalling in apoptosis. IUBMB Life. 58:462–466. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Burgess LG, Uppal K, Walker DI, Roberson
RM, Tran V, Parks MB, Wade EA, May AT, Umfress AC, Jarrell KL, et
al: Metabolome-wide association study of primary open angle
glaucoma. Invest Ophthalmol Vis Sci. 56:5020–5028. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Schwedhelm E, Englisch C, Niemann L,
Lezius S, von Lucadou M, Marmann K, Böger R, Peine S, Daum G,
Gerloff C and Choe CU: Sphingosine-1-phosphate, motor severity, and
progression in Parkinson's disease (MARK-PD). Mov Disord.
36:2178–2182. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Garcia CJ, Kosek V, Beltrán D,
Tomás-Barberán FA and Hajslova J: Production of new microbially
conjugated bile acids by human gut microbiota. Biomolecules.
12:6872022. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Styles NA, Shonsey EM, Falany JL, Guidry
AL, Barnes S and Falany CN: Carboxy-terminal mutations of bile acid
CoA:N-acyltransferase alter activity and substrate specificity. J
Lipid Res. 57:1133–1143. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Reilly SJ, O'Shea EM, Andersson U, O'Byrne
J, Alexson SE and Hunt MC: A peroxisomal acyltransferase in mouse
identifies a novel pathway for taurine conjugation of fatty acids.
FASEB J. 21:99–107. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Lin CL, Xu R, Yi JK, Li F, Chen J, Jones
EC, Slutsky JB, Huang L, Rigas B, Cao J, et al: Alkaline ceramidase
1 protects mice from premature hair loss by maintaining the
homeostasis of hair follicle stem cells. Stem Cell Reports.
9:1488–1500. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Hernández-Corbacho MJ, Salama MF, Canals
D, Senkal CE and Obeid LM: Sphingolipids in mitochondria. Biochim
Biophys Acta Mol Cell Biol Lipids. 1862:56–68. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Dalto DB, Tsoi S, Dyck MK and Matte JJ:
Gene ontology analysis of expanded porcine blastocysts from gilts
fed organic or inorganic selenium combined with pyridoxine. BMC
Genomics. 19:8362018. View Article : Google Scholar : PubMed/NCBI
|