Introduction
Small interfering RNA (siRNA) therapeutics are a
novel class of drugs that inhibit gene expression by cleaving mRNA
with a sequence complementary to the siRNA (1). However, a major limitation of siRNA
therapeutics is the delivery of siRNAs to the target cells. siRNA
is not readily taken up by cells because of membrane impermeability
due to the hydrophilicity of negatively charged siRNA and the
enzymatic degradation of siRNA by serum endonucleases (2). Therefore, an siRNA delivery system is
essential to protect the siRNA molecules until they reach the
target cell and efficiently introduce them into the cells through
the cell membrane (2–5).
Among the available delivery systems, siRNA/cationic
liposome complexes (siRNA lipoplexes) are attractive carriers
(6,7). Cationic lipids and phospholipids are
commonly used as components of cationic liposomal formulations to
control transfection efficiency and stability (8,9). In
particular, cationic lipids in liposomes are essential for
interaction with negatively charged siRNAs (10). In addition, the structures of
cationic lipids and phospholipids, such as the head group, alkyl or
acyl chain length and saturation and the linker between the head
group and the lipid anchor, affect the transfection efficiency of
siRNA lipoplexes (8,9,11).
Therefore, for achieving efficient siRNA transfection using siRNA
lipoplexes, the optimal combination of cationic lipids and
phospholipids in liposomal formulations must be determined.
Previously, three types of cationic lipid,
1,2-dioleoyl-3-trimethylammonium-propane (DOTAP),
dimethyldioctadecylammonium bromide (DDAB) and
11-[{1,3-bis(dodecanoyloxy)-2-((dodecanoyloxy)methyl)propan-2-yl}amino]-N,N,N-trimethyl-11-oxoundecan-1-aminium
bromide (TC-1-12) were selected, and the effect of phospholipids in
cationic liposome formulations on the gene-knockdown efficacy of
siRNA lipoplexes was examined (9).
DOTAP and DDAB are cationic diacyl (C18) and dialkyl (C18) lipids,
respectively, and TC-1-12 is a cationic triacyl (C12) lipid. Among
them, siRNA lipoplexes composed of
DDAB/1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE),
TC-1-12/DOPE and
TC-1-12/1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine
(POPE) result in significant suppression of targeted mRNA both
in vitro and in vivo, indicating that the optimal
phospholipids in cationic liposomes for siRNA transfection are
notably affected by the type of cationic lipid used. In the present
study, to examine whether alkyl or acyl chain length and saturation
and the linker between the head group and lipid anchor in cationic
lipids affect optimal combination with phospholipid for
gene-knockdown by siRNA lipoplexes, six other types of cationic
lipid, namely
N,N-dimethyl-N-tetradecyltetradecan-1-aminium
bromide (DC-1-14),
N-hexadecyl-N,N-dimethylhexadecan-1-aminium
bromide (DC-1-16),
2-[bis{2-(tetradecanoyloxy)ethyl}amino]-N,N,N-trimethyl-2-oxoethan-1-aminium
chloride (DC-6-14), 1,2-di-O-octadecenyl-3-trimethylammonium
propane chloride (DOTMA),
1,2-distearoyl-3-trimethylammonium-propane chloride (DSTAP) and
1,2-dioleoyl-3-dimethylammonium-propane (DODAP) were selected, and
the effect of cationic lipids and phospholipids in liposomal
formulations on in vitro and in vivo gene-knockdown
after transfection with siRNA lipoplexes were evaluated.
Materials and methods
Materials
N,N-Dimethyl-N-tetradecyltetradecan-1-aminium
bromide (cat. no. DC-1-14),
N-hexadecyl-N,N-dimethylhexadecan-1-aminium
bromide (cat. no. DC-1-16) and
2-[bis{2-(tetradecanoyloxy)ethyl}amino]-N,N,N-trimethyl-2-oxoethan-1-aminium
chloride (cat. no. DC-6-14) were obtained from Sogo Pharmaceutical
Co., Ltd. 1,2-Di-O-octadecenyl-3-trimethylammonium propane
chloride (DOTMA; cat. no. 14476) and
1,2-distearoyl-3-trimethylammonium-propane chloride (DSTAP; cat.
no. 14486) were obtained from Polysciences, Inc.
1,2-Dioleoyl-3-dimethylammonium-propane (DODAP; cat. no.
TRC-D483000) was obtained from Toronto Research Chemicals, Inc (LGC
Group). 1,2-Distearoyl-sn-glycero-3-phosphoethanolamine
(DSPE; cat. no. ME-8080),
1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine (DPPE; cat.
no. ME-6060),
1,2-dimyristoyl-sn-glycero-3-phosphoethanolamine (DMPE; cat.
no. ME-4040), 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine
(DOPE; cat. no. ME-8181) and
1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine (POPE;
cat. no. ME-6081) were obtained from NOF Co., Ltd. All other
chemicals used were of the highest grade available.
siRNAs
Firefly luciferase siRNA (Luc siRNA), non-silencing
siRNA (Control #1 siRNA) and cyanine 5-conjugated Control #1 siRNA
(Cy5-siRNA) were designed as previously reported (hLuc sequence:
GenBank accession no. AY535007.1) (9) and synthesized by Sigma-Aldrich Japan
K.K. (Merck KGaA). siRNA sequences were as follows: Luc siRNA
passenger strand, 5′-CCGUGGUGUUCGUGUCUAAGA-3′, and guide strand,
5′-UUAGACACGAACACCACGGUA-3′; Control #1 siRNA passenger strand,
5′-GUACCGCACGUCAUUCGUAUC-3′, and guide strand,
5′-UACGAAUGACGUGCGGUACGU-3′. For Cy5-siRNA, Cy5 dye was conjugated
at the 5′-end of the passenger strand of Control #1 siRNA. siRNAs
for mouse Tie2 and firefly Luc (Control #2 siRNA) were designed as
previously reported (pGL2 Luc sequence: GenBank accession no.
X65323.2; Tie2 sequence: GenBank accession no. NM_013690.3)
(9) and synthesized by Japan Bio
Services Co., Ltd. The siRNA sequences were as follows: Tie2
passenger strand, 5′-CcAuCaUuUgCcCaGaUaU-3′, and guide strand,
5′-aUaUcUgGgCaAaUgAuGg-3′; and Control #2 siRNA passenger strand,
5′-AuCaCgUaCgCgGaAuAcUuCgA-3′, and guide strand,
5′-uCgAaGuAuUcCgCgUaCgUgAu-3′. Lowercase letters represent
2′-O-methyl-modified nucleotides. Control #2 siRNA was used
as a negative control for Tie2 siRNA.
Preparation of cationic liposomes and
siRNA lipoplexes
Cationic liposomes were prepared using cationic
lipids and phospholipids at a molar ratio of 1:1 as described
previously (9). To prepare siRNA
lipoplexes, a cationic liposome suspension was added to 50 pmol
siRNA at a charge ratio (+:-) of 4:1, vortexed for 10 sec and left
at room temperature for 15 min, as previously described (9).
Particle size and distribution [polydispersity index
(PDI)] of cationic liposomes and siRNA lipoplexes were measured
using a light-scattering photometer (cat. no. ELS-Z2; Otsuka
Electronics Co., Ltd.), as previously described (9).
Cell culture
Human breast cancer MCF-7 cells stably expressing
firefly Luc (MCF-7-Luc) were donated by Dr. Kenji Yamato
(University of Tsukuba, Tsukuba, Japan) (12). Using STR DNA profile analysis, it
was confirmed that the MCF-7-Luc cells used in the present study
were identical to the MCF-7 cells registered in the database of
Japanese Collection of Research Bioresources Cell Bank (JCRB,
National Institute of Biomedical Innovation). MCF-7-Luc cells were
cultured in RPMI-1640 medium (FUJIFILM Wako Pure Chemical
Corporation) supplemented with 10% heat-inactivated fetal bovine
serum (FBS; Thermo Fisher Scientific) and 1.2 mg/ml G418 sulfate
(geneticin; FUJIFILM Wako Pure Chemical Corporation) at 37°C in a
humidified atmosphere with 5% CO2.
Gene-knockdown in MCF-7 cells using
siRNA lipoplexes
MCF-7-Luc cells were seeded in six-well culture
plates at a density of 3×105 cells/well at 37°C. After
24 h, each siRNA lipoplex containing 50 pmol Control #1 siRNA or
Luc siRNA was diluted in 1 ml of RPMI-1640 medium supplemented with
10% FBS (final concentration, 50 nM siRNA) and added to the cells.
MCF-7-Luc cells without the addition of cationic liposomes or siRNA
lipoplexes were used as untreated cells. Following 48 h of
incubation at 37°C, the cells were lysed by the addition of 250 µl
of cell lysis buffer (Pierce™ Luciferase Cell Lysis
Buffer; Pierce; Thermo Fisher Scientific, Inc.) after washing with
PBS, and subjected to one cycle of freezing (−80°C) and thawing at
37°C, followed by centrifugation at 13,000 × g for 10 sec at 4°C.
Aliquots of 10 µl of the supernatants of cell lysates were mixed
with 50 µl of PicaGene MelioraStar-LT Luminescence Reagent (Toyo
Ink Mfg. Co., Ltd.) and the luminescence was measured as counts per
sec (cps) with a chemoluminometer (ARVO X2; PerkinElmer, Inc.).
Protein concentrations of the supernatants were determined with
bicinchoninic acid (BCA) reagent (Pierce™ BCA Protein
Assay Kit; Pierce; Thermo Fisher Scientific, Inc.), using bovine
serum albumin (Thermo Fisher Scientific, Inc.) as a standard, and
the luciferase activity (cps/µg protein) was calculated. Luc
activity (%) was calculated relative to that of untreated cells
according to the formula below. Luc activity (%)=(Luc activity
(cps/µg protein) in siRNA transfected cells/Luc activity (cps/µg
protein) in untreated cells) ×100.
Cytotoxicity of siRNA lipoplexes
Each siRNA lipoplex sample with 5 pmol Control #1
siRNA was diluted in 100 µl of RPMI-1640 medium supplemented with
10% FBS (final concentration, 50 nM siRNA) and added to MCF-7-Luc
cells at 50% confluency in 96-well plates. MCF-7-Luc cells without
the addition of cationic liposomes or siRNA lipoplexes were used as
untreated cells. At 24 h post-transfection, cell viability was
measured using a Cell Counting Kit-8 (Dojindo Laboratories, Inc.),
as previously reported (9). WST-8
substrate was incubated with cells at 37°C for 60 min. The cell
viability (%) was calculated relative to that of untreated cells
according to the formula below. Cell viability (%)=(absorbance at
450 nm in siRNA transfected cells/absorbance at 450 nm in untreated
cells) ×100.
Biodistribution of siRNA following
intravenous injection of siRNA lipoplexes into mice
Ethical approval was obtained from the Institutional
Animal Care and Use Committee of Hoshi University (approval no.
P22-016). A total of six female BALB/c mice (weight, 18–20
g; age, 8 weeks; Sankyo Labo Service Corporation, Inc.) were housed
at 24°C and 55% humidity under a 12/12-h light/dark cycle (lights
on at 8:00 a.m.) with ad libitum access to food and water.
siRNA lipoplexes with 20 µg of Cy5-siRNA were systemically
administered to mice via the lateral tail vein (n=1/siRNA
lipoplex). At 1 h after injecting the siRNA lipoplexes, the mice
were euthanized by cervical dislocation, and death was confirmed by
cessation of the heartbeat. Tissues (lungs, heart, liver, spleen
and kidneys) were analyzed through Cy5 fluorescence imaging using
the NightOWL LB981 NC100 system (Berthold Technologies GmbH &
Co. KG) as previously described (9,13).
Thereafter, the tissue samples were frozen on dry ice and sliced
into 16-µm sections. The localization of the Cy5-siRNA was examined
using a fluorescence microscope (Eclipse TS100-F; Nikon
Corporation).
Hemolysis and agglutination assay
Blood (0.2 ml) was collected from the jugular vein
of one female BALB/c mouse (age, 8 weeks; Sankyo Labo Service
Corp., Inc.) under anesthesia induced by isoflurane inhalation
(1.5% gas-air mixture; FUJIFILM Wako Pure Chemical Corporation).
After the blood collection, the mice were euthanized by cervical
dislocation. Erythrocytes were collected from the blood at 4°C by
centrifugation at 300 × g for 3 min and resuspended in PBS as a 2%
(v/v) suspension of erythrocytes. siRNA lipoplexes with 2 µg of
siRNA were added to 100 µl of a 2% (v/v) erythrocyte suspension.
Following incubation for 15 min at 37°C, the sample was placed on a
glass plate and agglutination was observed using a light microscope
(Eclipse TS100-F; Nikon Corporation). For hemolysis of
erythrocytes, 100 µl of the 2% (v/v) erythrocyte suspension was
centrifugated at 200 × g for 3 min after incubation with 2 µg of
siRNA lipoplexes for 15 min at 37°C and hemolysis of erythrocytes
was observed. Hemolysis (%) was calculated as described previously
(14).
Expression of Tie2 mRNA in the lung
following systemic injection of siRNA lipoplexes
siRNA lipoplexes with 20 µg of Control #2 or Tie2
siRNA were administered systemically to 8-week-old female BALB/c
mice via the lateral tail veins (n=3-4/siRNA lipoplex). All mice
were euthanized by cervical dislocation 48 h after injection of
siRNA lipoplexes and then the lungs were excised. The lungs from
mouse without the injection of siRNA lipoplexes were used as
untreated lungs (n=4). Total RNA was isolated from the lungs (a
total of 31 mice) using Isogen II (Nippon Gene Co., Ltd.). cDNA was
synthesized from total RNA using PrimeScript™ RT Master
Mix (Takara Bio, Inc.) according to the manufacturer's protocol and
quantitative PCR was performed using a Roche Light Cycler 96 system
with FastStart Essential DNA Probes Master (Roche Diagnostics GmbH)
and TaqMan™ Gene expression assays (cat. no. 4331182;
Applied Biosystems; Thermo Fisher Scientific, Inc.)
[FAM™ dye-labeled TaqMan® MGB probe and PCR
primers: Tie2, cat. no. Mm00443243_m1; phosphatase and tensin
homolog (PTEN); cat. no. Mm00477208_m1; Applied Biosystems; Thermo
Fisher Scientific, Inc.; primer sequences not available]. The
thermocycling conditions were as follows: Initial denaturation at
95°C for 600 sec, followed by 45 cycles of denaturation at 95°C for
10 sec and primer annealing and extension at 60°C for 30 sec
(two-step amplification). Tie2 and PTEN are expressed in normal
endothelial cells (15,16), and PTEN has been used as a
reference gene of endothelial cells (9,17,18).
Therefore, the expression levels of Tie2 mRNA (threshold cycle (Cq)
value, ~23-24) were normalized to those of PTEN mRNA (Cq value,
~22-23) in each sample and analyzed using the comparative Cq
(2−ΔΔCq) method (19).
Statistical analysis
Data are presented as the mean ± SD of triplicate
measurements. Statistical significance was determined using an
unpaired Student's t-test or one-way ANOVA followed by Tukey's
post-hoc test using GraphPad Prism (version 4.0; GraphPad Software,
Inc.). P<0.05 was considered to indicate a statistically
significant difference.
Results
Size of cationic liposomes and siRNA
lipoplexes
To examine the effects of cationic lipids and
phospholipids in liposomal formulations on gene-knockdown efficacy
after treatment with siRNA lipoplexes, DC-1-14, DC-1-16, DC-6-14,
DOTMA, DSTAP and DODAP were used as cationic lipids for the
preparation of cationic liposomes (Fig. 1). In our previous study on cationic
liposomes containing cationic lipids DDAB and DOTAP, compared with
phosphatidylcholines, the inclusion of phosphatidylethanolamines in
liposomal formulations results in high gene-knockdown activity in
cells (9); therefore, in the
present study, DSPE, DPPE, DMPE, POPE and DOPE were used as
phospholipids (Fig. 1). Cationic
liposomes were prepared from cationic lipid and
phosphatidylethanolamine at a molar ratio of 1:1 (Table I).
 | Figure 1.Structure of cationic lipids and
phospholipids. DC-1-14,
N,N-dimethyl-N-tetradecyltetradecan-1-aminium
bromide; DC-1-16,
N-hexadecyl-N,N-dimethylhexadecan-1-aminium
bromide; DC-6-14,
2-[bis{2-(tetradecanoyloxy)ethyl}amino]-N,N,N-trimethyl-2-oxoethan-1-aminium
chloride; DSTAP, 1,2-distearoyl-3-trimethylammonium-propane
chloride; DOTMA, 1,2-di-O-octadecenyl-3-trimethylammonium
propane chloride; DODAP, 1,2-dioleoyl-3-dimethylammonium-propane;
DSPE, 1,2-distearoyl-sn-glycero-3-phosphoethanolamine; DPPE,
1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine; DMPE,
1,2-dimyristoyl-sn-glycero-3-phosphoethanolamine; DOPE,
1,2-dioleoyl-sn-glycero-3-phosphoethanolamine; POPE,
1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine. |
 | Table I.Particle size of cationic liposomes
and siRNA lipoplexes. |
Table I.
Particle size of cationic liposomes
and siRNA lipoplexes.
|
|
| Liposomes |
Lipoplexa |
|---|
|
|
|
|
|
|---|
| Liposome | Formulation (mol
ratio) | Sizeb (nm) | PDI | Sizeb (nm) | PDI |
|---|
|
LP-DC-1-14/DSPE | DC-1-14:DSPE
(1:1) | Aggregation | - | N.D. | N.D. |
|
LP-DC-1-14/DPPE | DC-1-14:DPPE
(1:1) | 123.4±1.5 | 0.20±0.02 | 428.1±86.6 | 0.20±0.03 |
|
LP-DC-1-14/DMPE | DC-1-14:DMPE
(1:1) | 104.5±6.6 | 0.25±0.00 | 240.1±14.2 | 0.16±0.06 |
|
LP-DC-1-14/DOPE | DC-1-14:DOPE
(1:1) | 102.4±2.6 | 0.23±0.01 | 290.2±18.2 | 0.25±0.04 |
|
LP-DC-1-14/POPE | DC-1-14:POPE
(1:1) | 80.4±2.3 | 0.24±0.01 | 540.9±87.8 | 0.24±0.03 |
|
LP-DC-1-16/DSPE | DC-1-16:DSPE
(1:1) | 134.8±4.7 | 0.21±0.01 | Aggregation | - |
|
LP-DC-1-16/DPPE | DC-1-16:DPPE
(1:1) | 195.7±6.7 | 0.29±0.00 | 579.2±85.0 | 0.25±0.04 |
|
LP-DC-1-16/DMPE | DC-1-16:DMPE
(1:1) | 99.3±1.0 | 0.22±0.01 | 207.6±38.7 | 0.15±0.07 |
|
LP-DC-1-16/DOPE | DC-1-16:DOPE
(1:1) | 107.1±1.9 | 0.22±0.01 | 180.8±7.3 | 0.19±0.04 |
|
LP-DC-1-16/POPE | DC-1-16:POPE
(1:1) | 116.9±1.4 | 0.26±0.00 | 517.6±91.8 | 0.23±0.03 |
|
LP-DC-6-14/DSPE | DC-6-14:DSPE
(1:1) | 107.6±1.7 | 0.25±0.00 | Aggregation | - |
|
LP-DC-6-14/DPPE | DC-6-14:DPPE
(1:1) | 157.7±2.3 | 0.26±0.02 | 593.1±18.4 | 0.26±0.01 |
|
LP-DC-6-14/DMPE | DC-6-14:DMPE
(1:1) | 92.3±0.5 | 0.22±0.01 | 195.1±14.1 | 0.20±0.03 |
|
LP-DC-6-14/DOPE | DC-6-14:DOPE
(1:1) | 113.4±2.6 | 0.26±0.01 | 260.3±27.6 | 0.19±0.06 |
|
LP-DC-6-14/POPE | DC-6-14:POPE
(1:1) | 109.3±0.5 | 0.23±0.01 | 223.0±22.1 | 0.16±0.08 |
| LP-DOTMA/DSPE | DOTMA:DSPE
(1:1) | 124.3±1.1 | 0.25±0.00 | 937.3±89.8 | 0.39±0.04 |
| LP-DOTMA/DPPE | DOTMA:DPPE
(1:1) | 232.3±10.8 | 0.29±0.01 | 650.8±51.2 | 0.28±0.02 |
| LP-DOTMA/DMPE | DOTMA:DMPE
(1:1) | 202.6±9.3 | 0.29±0.01 | 922.4±96.1 | 0.37±0.03 |
| LP-DOTMA/DOPE | DOTMA:DOPE
(1:1) | 199.0±1.6 | 0.29±0.01 | 338.7±16.8 | 0.15±0.01 |
| LP-DOTMA/POPE | DOTMA:POPE
(1:1) | 292.5±4.9 | 0.31±0.00 | 631.2±28.4 | 0.27±0.01 |
| LP-DSTAP/DSPE | DSTAP:DSPE
(1:1) | 160.6±38.1 | 0.19±0.01 | Aggregation | - |
| LP-DSTAP/DPPE | DSTAP:DPPE
(1:1) | 133.7±1.5 | 0.24±0.01 | 794.0±110.0 | 0.33±0.04 |
| LP-DSTAP/DMPE | DSTAP:DMPE
(1:1) | 116.1±1.0 | 0.23±0.01 | 261.0±17.9 | 0.16±0.03 |
| LP-DSTAP/DOPE | DSTAP:DOPE
(1:1) | 150.8±1.8 | 0.25±0.01 | 335.1±11.6 | 0.15±0.00 |
| LP-DSTAP/POPE | DSTAP:POPE
(1:1) | 148.1±2.8 | 0.20±0.01 | 206.8±0.2 | 0.22±0.00 |
| LP-DODAP/DSPE | DODAP:DSPE
(1:1) | Aggregation | - | N.D. | N.D. |
| LP-DODAP/DPPE | DODAP:DPPE
(1:1) | 182.1±2.2 | 0.26±0.00 | Aggregation | - |
| LP-DODAP/DMPE | DODAP:DMPE
(1:1) | 177.8±2.0 | 0.22±0.01 | Aggregation | - |
| LP-DODAP/DOPE | DODAP:DOPE
(1:1) | 121.1±0.8 | 0.22±0.01 | 255.7±31.5 | 0.23±0.03 |
| LP-DODAP/POPE | DODAP:POPE
(1:1) | 149.6±0.6 | 0.21±0.00 | 528.2±121.7 | 0.23±0.05 |
The size of cationic liposomes was 80–293 nm (PDI,
0.19–0.31) (Table I) although both
LP-DC-1-14/DSPE and LP-DODAP/DSPE aggregated during preparation
(>1 µm in size) (Table I). Our
previous study determined that the optimal charge ratio (+:-) for
the preparation of siRNA lipoplexes was 4:1 for LP-DC-1-14/DOPE,
LP-DC-1-16/DOPE and LP-DC-6-14/DOPE (8,11).
Therefore, the present study employed a charge ratio (+:-) of 4:1
to prepare the siRNA lipoplexes. LP-DC-1-16/DSPE, LP-DC-6-14/DSPE,
LP-DSTAP/DSPE, LP-DODAP/DPPE and LP-DODAP/DMPE lipoplexes
aggregated (>1 µm in size) when the liposomes were mixed with
the siRNA solution (Table I). The
size of LP-DC-1-14/DPPE, LP-DC-1-14/POPE, LP-DC-1-16/DPPE,
LP-DC-1-16/POPE, LP-DC-6-14/DPPE, LP-DOTMA/DSPE, LP-DOTMA/DPPE,
LP-DOTMA/DMPE, LP-DOTMA/POPE, LP-DSTAP/DPPE and LP-DODAP/POPE
lipoplexes increased to 428–937 nm (PDI, 0.20–0.39). However, the
sizes of the other lipoplexes were 181–339 nm (PDI, 0.15–0.22).
Overall, the inclusion of DSPE or DPPE in the liposomal formulation
increased the size of the siRNA lipoplexes after preparation.
Effect of phosphatidylethanolamines in
cationic liposomes on gene-knockdown efficacy in MCF-7 cells
To determine the effect of phosphatidylethanolamine
in liposomal formulations on gene-knockdown efficacy using siRNA
lipoplexes, each Luc siRNA lipoplex was transfected into MCF-7-Luc
cells and gene-knockdown efficacy was evaluated by measuring Luc
activity. LP-DC-1-16/DOPE, LP-DC-6-14/DOPE, LP-DOTMA/DPPE,
LP-DOTMA/DMPE, LP-DOTMA/DOPE, LP-DOTMA/ POPE, LP-DSTAP/DOPE and
LP-DODAP/DOPE lipoplexes with Luc siRNA strongly suppressed Luc
activity (>70% knockdown) compared with Control #1 siRNA
(Fig. 2 and Table II). LP-DC-1-14/DOPE,
LP-DC-1-16/DSPE, LP-DOTMA/DSPE, LP-DODAP/DPPE and LP-DODAP/POPE
lipoplexes with Luc siRNA moderately suppressed Luc activity
(50–70% knockdown) compared with Control #1 siRNA (Fig. 2 and Table II). Furthermore, LP-DC-1-14/DMPE,
LP-DC-1-14/POPE, LP-DC-1-16/DPPE, LP-DC-1-16/POPE and LP-DODAP/DMPE
lipoplexes with Luc siRNA slightly suppressed Luc activity (30–50%
knockdown) compared with Control #1 siRNA (Fig. 2 and Table II).
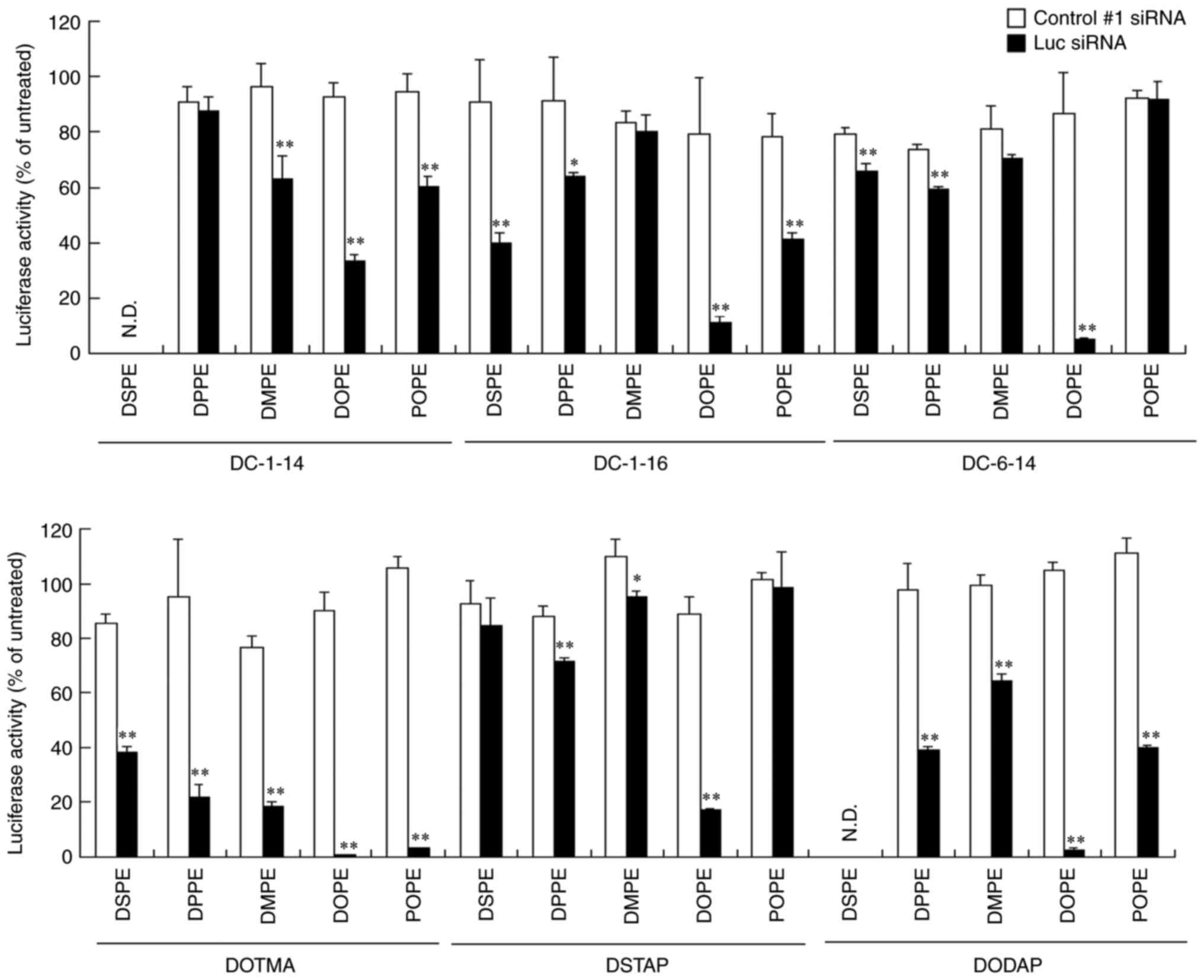 | Figure 2.Effect of cationic lipids and
phosphatidylethanolamines in liposomal formulations on the efficacy
of gene-knockdown after transfection of siRNA lipoplexes into
MCF-7-Luc cells. siRNA lipoplexes with Control #1 or Luc siRNA were
added to MCF-7-Luc cells at a concentration of 50 nM and Luc assay
was performed 48 h post-transfection. (n=3). *P<0.05,
**P<0.01 vs. Control #1 siRNA. Luc, luciferase; si, small
interfering; DC-1-14,
N,N-dimethyl-N-tetradecyltetradecan-1-aminium
bromide; DC-1-16,
N-hexadecyl-N,N-dimethylhexadecan-1-aminium
bromide; DC-6-14,
2-[bis{2-(tetradecanoyloxy)ethyl}amino]-N,N,N-trimethyl-2-oxoethan-1-aminium
chloride; DSTAP, 1,2-distearoyl-3-trimethylammonium-propane
chloride; DOTMA, 1,2-di-O-octadecenyl-3-trimethylammonium
propane chloride; DODAP, 1,2-dioleoyl-3-dimethylammonium-propane;
DSPE, 1,2-distearoyl-sn-glycero-3-phosphoethanolamine; DPPE,
1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine; DMPE,
1,2-dimyristoyl-sn-glycero-3-phosphoethanolamine; DOPE,
1,2-dioleoyl-sn-glycero-3-phosphoethanolamine; POPE,
1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine. |
 | Table II.Summary of in vitro gene
knockdown efficacy after treatment with siRNA lipoplexes. |
Table II.
Summary of in vitro gene
knockdown efficacy after treatment with siRNA lipoplexes.
|
| Cationic lipid |
|---|
|
|
|
|---|
| Phospholipid | DC-1-14 | DC-1-16 | DC-6-14 | DOTMA | DSTAP | DODAP |
|---|
| DSPE | N.D. | ++ | - | ++ | - | N.D. |
| DPPE | - | + | - | +++ | - | ++ |
| DMPE | + | - | - | +++ | - | + |
| DOPE | ++ | +++ | +++ | +++ | +++ | +++ |
| POPE | + | + | - | +++ | - | ++ |
Cytotoxicity after treatment with
siRNA lipoplexes
The toxicity of phosphatidylethanolamines in the
liposomal formulations on MCF-7-Luc cells was evaluated 24 h
post-transfection with siRNA lipoplexes. LP-DC-1-14/DPPE,
LP-DC-1-14/POPE, LP-DC-1-16/POPE, LP-DOTMA/DSPE, LP-DSTAP/DMPE and
LP-DODAP/DPPE lipoplexes showed slight cytotoxicity (70–80% cell
viability), whereas the other lipoplexes did not exhibit
cytotoxicity (>87% cell viability compared to untreated cells;
Fig. 3). These results suggest
that cationic liposomes composed of DOPE can be used for efficient
gene-knockdown using siRNA lipoplexes with minimal toxicity.
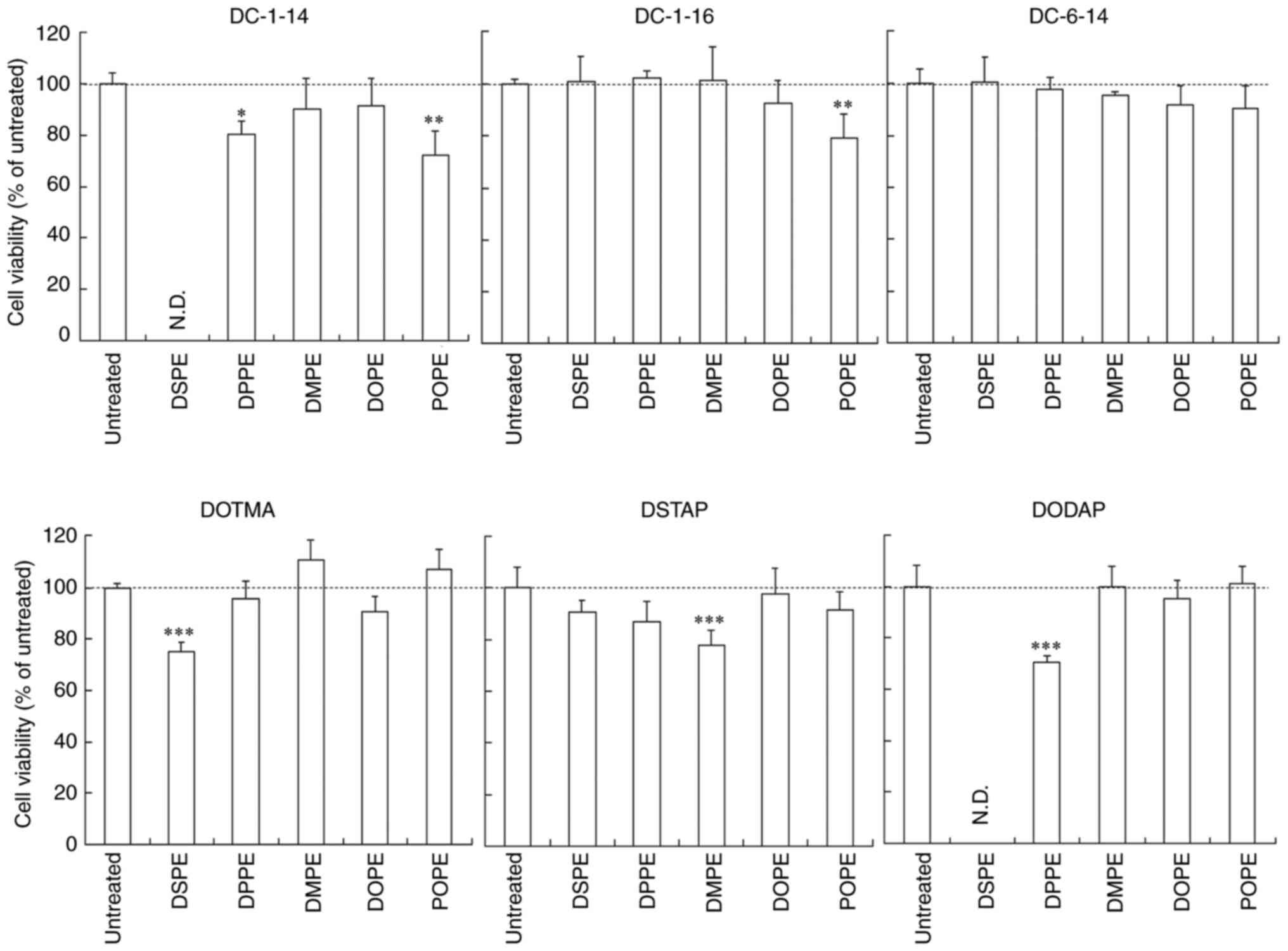 | Figure 3.Effect of cationic lipids and
phosphatidylethanolamines in liposomal formulations on cell
viability at 24 h after transfection of siRNA lipoplexes into
MCF-7-Luc cells. siRNA lipoplexes were transfected into MCF-7-Luc
cells at a concentration of 50 nM. (n=4-6). *P<0.05,
**P<0.01, ***P<0.001 vs. untreated. si, small interfering;
DC-1-14,
N,N-dimethyl-N-tetradecyltetradecan-1-aminium
bromide; DC-1-16,
N-hexadecyl-N,N-dimethylhexadecan-1-aminium
bromide; DC-6-14,
2-[bis{2-(tetradecanoyloxy)ethyl}amino]-N,N,N-trimethyl-2-oxoethan-1-aminium
chloride; DSTAP, 1,2-distearoyl-3-trimethylammonium-propane
chloride; DOTMA, 1,2-di-O-octadecenyl-3-trimethylammonium
propane chloride; DODAP, 1,2-dioleoyl-3-dimethylammonium-propane;
DSPE, 1,2-distearoyl-sn-glycero-3-phosphoethanolamine; DPPE,
1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine; DMPE,
1,2-dimyristoyl-sn-glycero-3-phosphoethanolamine; DOPE,
1,2-dioleoyl-sn-glycero-3-phosphoethanolamine; POPE,
1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine. |
Biodistribution of siRNA after
intravenous injection of siRNA lipoplexes
The present study investigated the effect of
cationic lipids in liposomal formulations on the biodistribution of
siRNA 1 h after the systemic injection of lipoplexes with
Cy5-siRNA. LP-DC-1-14/DOPE, LP-DC-1-16/DOPE, LP-DC-6-14/DOPE,
LP-DOTMA/DOPE, LP-DSTAP/DOPE and LP-DODAP/DOPE were selected
because their lipoplexes showed high gene-knockdown efficacy in
cells (Table II) and were
relatively small (<340 nm). The LP-DC-1-14/DOPE and
LP-DODAP/DOPE lipoplexes caused siRNA accumulation in the liver
(Figs. 4 and 5). The LP-DC-1-16/DOPE and
LP-DC-6-14/DOPE lipoplexes caused siRNA accumulation in the lungs
and liver. In particular, the LP-DOTMA/DOPE and LP-DSTAP/DOPE
lipoplexes led to high siRNA accumulation in the lungs. For all
lipoplexes, siRNAs were weakly detected in the spleen but not in
the heart. All siRNA lipoplexes led to a relatively high
accumulation of siRNA in the kidneys.
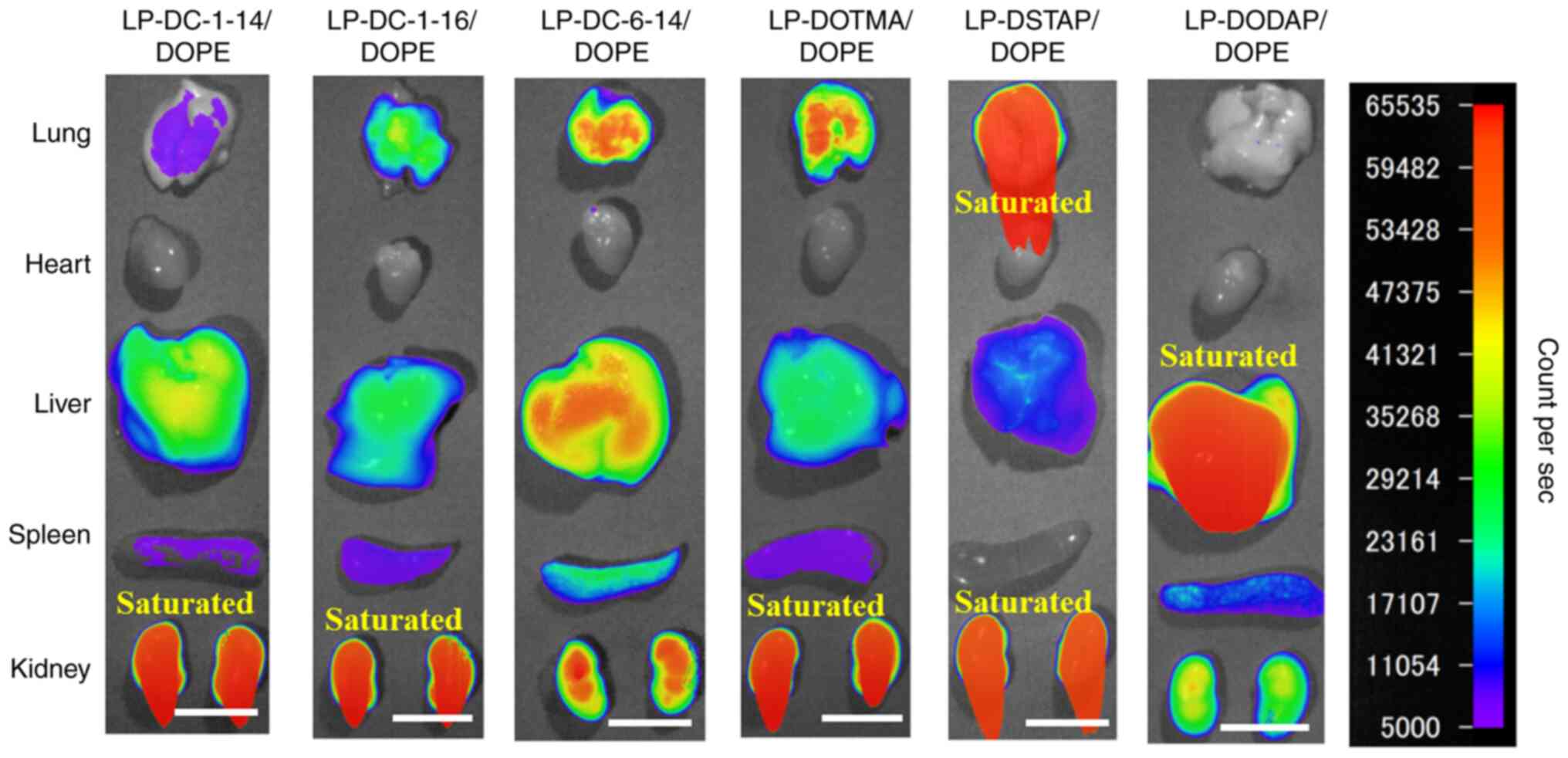 | Figure 4.Effect of cationic lipids in
liposomal formulations on the biodistribution of siRNA in mice at 1
h after systemic injection of siRNA lipoplexes. siRNA lipoplexes
with 20 µg of Cy5-siRNA were administered systemically to mice. Cy5
fluorescence imaging of tissue was performed 1 h post-injection.
Fluorescence intensity is illustrated using a color-coded scale
(red, maximum; purple, minimum). Scale bar, 1 cm. si, small
interfering; Cy5, cyanine 5; DC-1-14,
N,N-dimethyl-N-tetradecyltetradecan-1-aminium
bromide; DC-1-16,
N-hexadecyl-N,N-dimethylhexadecan-1-aminium
bromide; DC-6-14,
2-[bis{2-(tetradecanoyloxy)ethyl}amino]-N,N,N-trimethyl-2-oxoethan-1-aminium
chloride; DSTAP, 1,2-distearoyl-3-trimethylammonium-propane
chloride; DOTMA, 1,2-di-O-octadecenyl-3-trimethylammonium
propane chloride; DODAP, 1,2-dioleoyl-3-dimethylammonium-propane;
DOPE, 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine. |
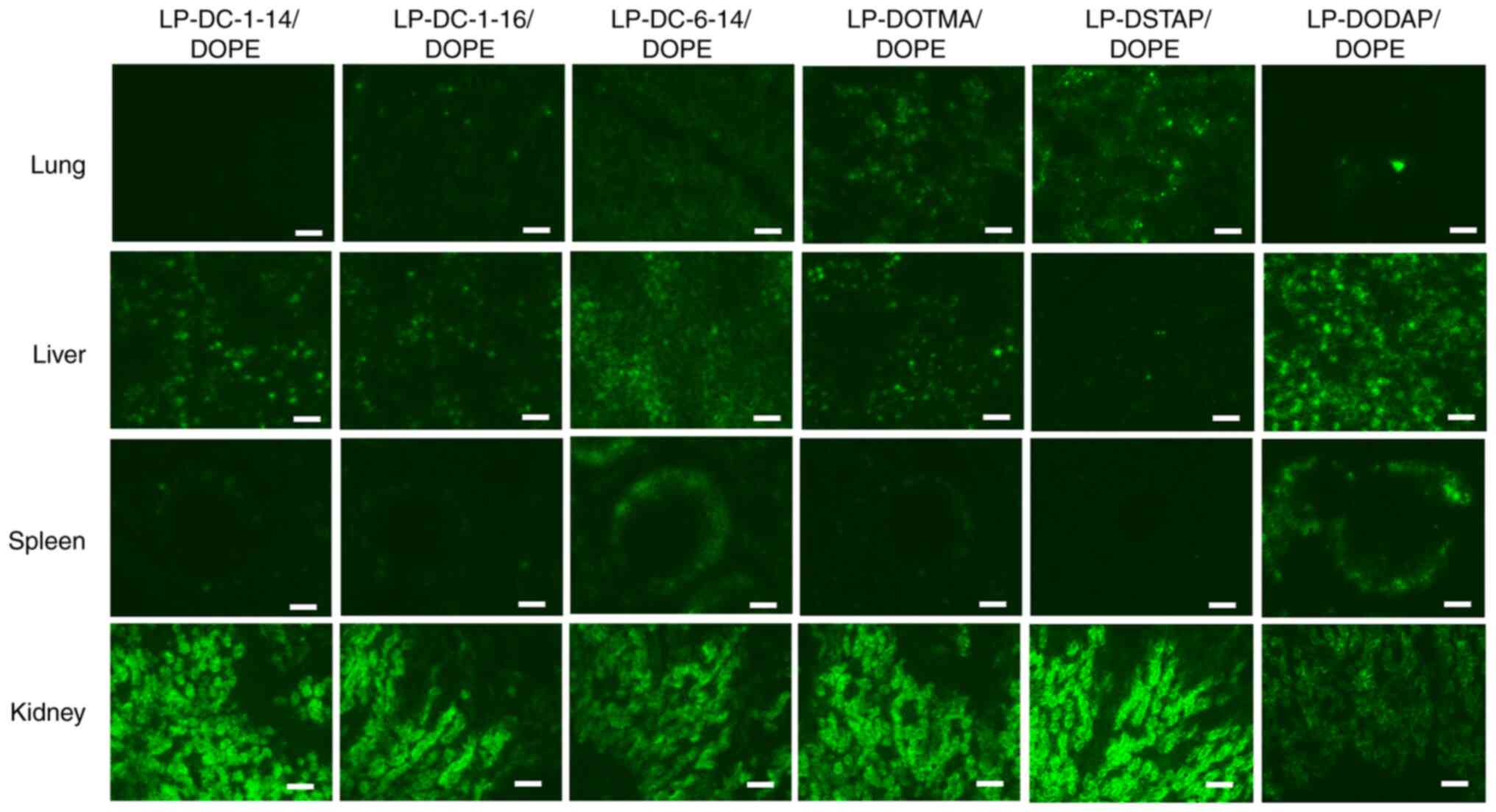 | Figure 5.Effect of cationic lipids in
liposomal formulation on the biodistribution of siRNA in mice 1 h
following systemic injection of siRNA lipoplexes. siRNA lipoplexes
with 20 µg of Cy5-siRNA were administered intravenously to mice. At
1 h post-injection, tissues were collected, frozen, and sliced to
observe the localization of Cy5-siRNA (green) using a fluorescent
microscope. Scale bar, 100 µm. si, small interfering; Cy5, cyanine
5; DC-1-14,
N,N-dimethyl-N-tetradecyltetradecan-1-aminium
bromide; DC-1-16,
N-hexadecyl-N,N-dimethylhexadecan-1-aminium
bromide; DC-6-14,
2-[bis{2-(tetradecanoyloxy)ethyl}amino]-N,N,N-trimethyl-2-oxoethan-1-aminium
chloride; DSTAP, 1,2-distearoyl-3-trimethylammonium-propane
chloride; DOTMA, 1,2-di-O-octadecenyl-3-trimethylammonium
propane chloride; DODAP, 1,2-dioleoyl-3-dimethylammonium-propane;
DOPE, 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine. |
Aggregation and hemolysis after mixing
siRNA lipoplexes with erythrocytes
The effect of cationic lipids in cationic liposomes
on aggregation and hemolysis was evaluated by mixing siRNA
lipoplexes with an erythrocyte suspension. The LP-DC-1-14/DOPE,
LP-DC-1-16/DOPE, LP-DC-6-14/DOPE, LP-DOTMA/DOPE and LP-DSTAP/DOPE
lipoplexes exhibited agglutination after mixing with the
erythrocyte suspension (Fig. 6A).
In particular, the LP-DC-1-16/DOPE, LP-DC-6-14/DOPE and
LP-DOTMA/DOPE lipoplexes formed large aggregates after mixing with
the erythrocyte suspension. By contrast, the LP-DODAP/DOPE
lipoplexes did not agglutinate erythrocytes. The LP-DC-1-14/DOPE
and LP-DOTMA/DOPE lipoplexes exhibited moderate levels of hemolysis
(28.6 and 24.1%, respectively) after mixing with the erythrocyte
suspension. However, other lipoplexes exhibited low levels of
hemolysis (<14%).
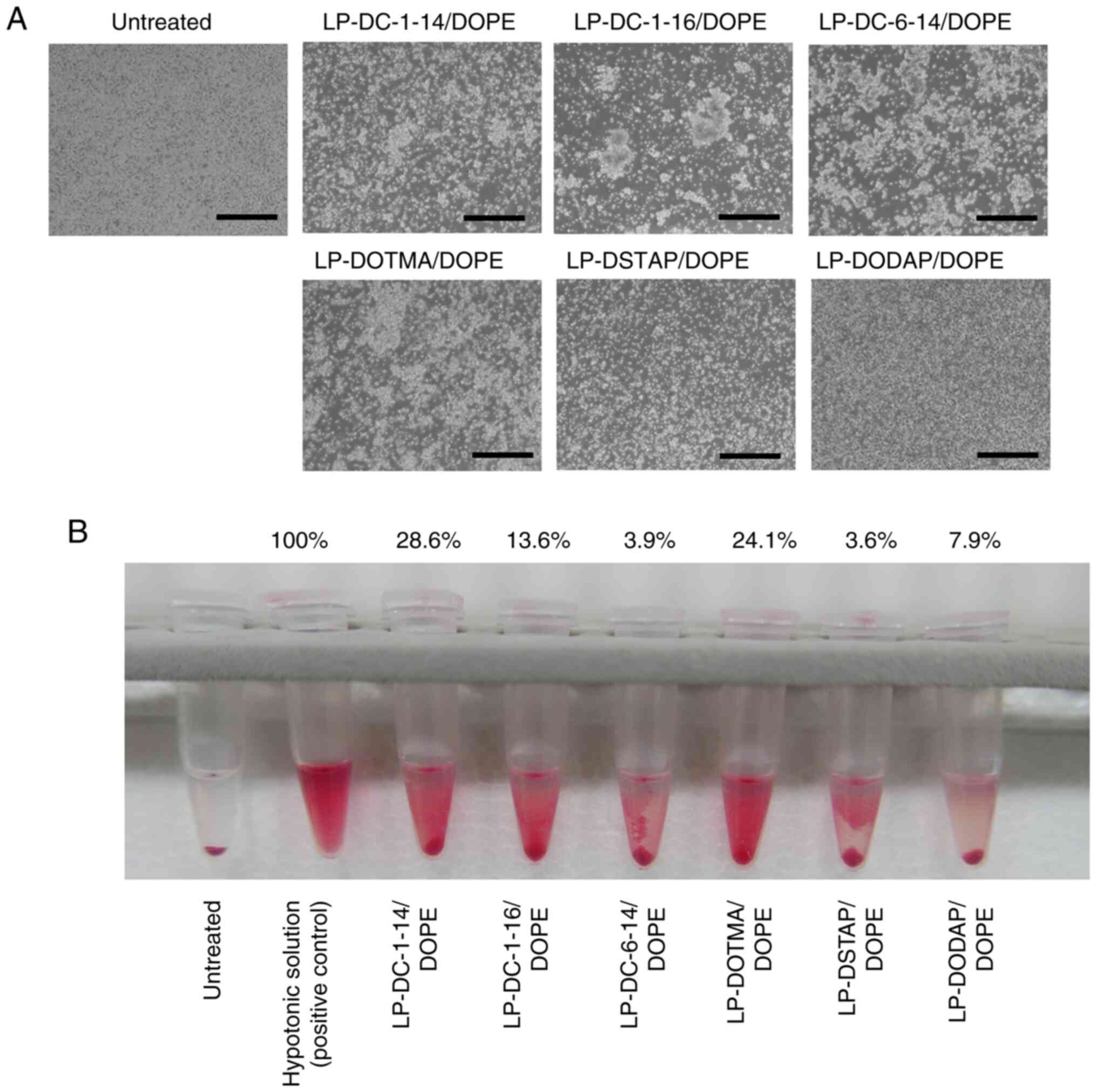 | Figure 6.Effect of cationic lipids in
liposomal formulations on the agglutination and hemolysis of
erythrocytes after treatment with siRNA lipoplexes. Lipoplexes with
2 µg of siRNA were incubated with erythrocyte suspension. (A)
Agglutination was observed under a light microscope (scale bar, 200
µm). (B) Hemolysis of erythrocytes after treatment with siRNA
lipoplexes was observed. As a positive control for hemolysis (100%
hemolysis), erythrocytes were suspended in hypotonic solution
(water). Hemolysis (%) was calculated relative to the absorbance of
treatment with hypotonic solution, and it is presented as mean
(n=4). DC-1-14,
N,N-dimethyl-N-tetradecyltetradecan-1-aminium
bromide; DC-1-16,
N-hexadecyl-N,N-dimethylhexadecan-1-aminium
bromide; DC-6-14,
2-[bis{2-(tetradecanoyloxy)ethyl}amino]-N,N,N-trimethyl-2-oxoethan-1-aminium
chloride; DSTAP, 1,2-distearoyl-3-trimethylammonium-propane
chloride; DOTMA, 1,2-di-O-octadecenyl-3-trimethylammonium
propane chloride; DODAP, 1,2-dioleoyl-3-dimethylammonium-propane;
DOPE, 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine. |
Gene-knockdown in the lungs after
systemic injection of siRNA lipoplexes
Tie2 has previously been used to evaluate the
gene-knockdown efficacy of siRNA lipoplexes in the vascular
endothelium of the lungs (17).
The present study evaluated the effect of liposomal formulations on
Tie2 mRNA knockdown in the pulmonary vascular endothelium 48 h
after a single systemic injection of Tie2 siRNA lipoplexes into
mice (Fig. 7). LP-DC-1-16/DOPE,
LP-DC-6-14/DOPE, LP-DOTMA/DOPE and LP-DSTAP/DOPE were selected to
evaluate the knockdown efficacy, as their lipoplexes exhibited
siRNA accumulation in the lungs (Figs.
4 and 5). LP-DC-1-16/DOPE
lipoplexes with Tie2 siRNA highly suppressed Tie2 mRNA expression
(37.4% knockdown) compared with Control #2 siRNA. However,
LP-DOTMA/DOPE lipoplexes with Tie2 siRNA slightly suppressed Tie2
mRNA expression in the lungs (14.8% knockdown) compared with
Control #2 siRNA and LP-DC-6-14/DOPE, and LP-DSTAP/DOPE lipoplexes
with Tie2 siRNA did not significantly suppress Tie2 mRNA
expression. None of the siRNA lipoplexes caused mice mortality
after systemic injection.
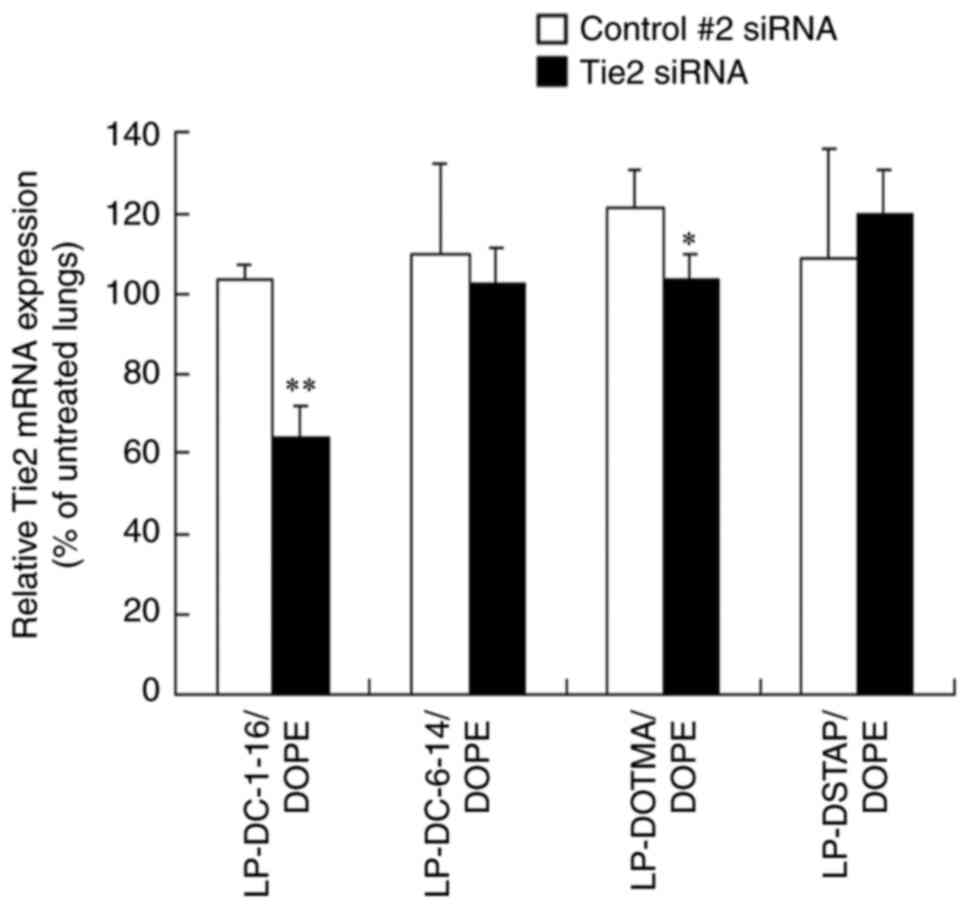 | Figure 7.Effect of cationic lipids in
liposomal formulations on the knockdown of Tie2 mRNA in the lung
following systemic injection of Tie2 siRNA lipoplexes into mice.
Tie2 mRNA level in the lung was normalized to PTEN mRNA level at 48
h after systemic administration of siRNA lipoplex with 20 µg of
Control #2 or Tie2 siRNA (n=3-4). Tie2 expression (%) was
calculated relative to that in untreated lungs. *P<0.05,
**P<0.01 vs. Control #2 siRNA. si, small interfering; PTEN,
phosphatase and tensin homolog; DC-1-16,
N-hexadecyl-N,N-dimethylhexadecan-1-aminium
bromide; DC-6-14,
2-[bis{2-(tetradecanoyloxy)ethyl}amino]-N,N,N-trimethyl-2-oxoethan-1-aminium
chloride; DSTAP, 1,2-distearoyl-3-trimethylammonium-propane
chloride; DOTMA, 1,2-di-O-octadecenyl-3-trimethylammonium propane
chloride; DOPE,
1,2-dioleoyl-sn-glycero-3-phosphoethanolamine. |
Discussion
Generally, cationic liposomes are prepared by
combining cationic lipids with neutral helper lipids. However, to
achieve efficient siRNA transfection, identifying the optimal
combination of cationic and neutral helper lipids in the liposomal
formulations is important. In the present study, the effect of
phosphatidylethanolamines in cationic liposomes on the
gene-knockdown efficacy of siRNA lipoplexes was determined using
six types of cationic lipids. In in vitro transfection,
LP-DC-1-16/DOPE, LP-DC-6-14/DOPE, LP-DOTMA/DPPE, LP-DOTMA/DMPE,
LP-DOTMA/DOPE, LP-DOTMA/POPE, LP-DSTAP/DOPE and LP-DODAP/DOPE
lipoplexes containing Luc siRNA strongly suppressed Luc activity
(>70% knockdown) compared with Control #1 siRNA. Regardless of
the type of cationic lipid, the inclusion of DOPE induced strong
Luc knockdown in MCF-7-Luc cells. DOPE contains two unsaturated
acyl chains and a relatively small head group, which forms a
hexagonal phase at acidic pH (20)
and destabilizes the liposomal structure (21). Therefore, DOPE may improve
gene-knockdown efficiency by destabilizing the endosomal membrane
after cellular uptake of siRNA lipoplexes via endocytosis, thereby
resulting in the release of siRNA into the cytoplasm. However, in
DOTMA-based cationic liposomes, the inclusion of DMPE, DPPE and
POPE resulted in high gene-knockdown activity in the present study.
DOTMA is a stable diether analog of DOTAP which has hydrolysable
ester bonds as a linker between a quaternary ammonium head group
and acyl chains (22). In our
recent study, siRNA lipoplexes composed of DOTAP and DOPE or DOTAP
and POPE demonstrated strong gene-knockdown efficacy, whereas siRNA
lipoplexes composed of DOTAP and DPPE or DOTAP and DMPE did not
(9). This finding suggests that
differences in the linkers in cationic lipids may affect the
transfection efficacy of siRNA lipoplexes in combination with DMPE
and DPPE.
DOTMA and DSTAP contain a quaternary ammonium head
group with alkyl and acyl chains (C18), respectively, and are
positively charged irrespective of pH (23). Regarding siRNA biodistribution,
systemic injection of LP-DOTMA/DOPE and LP-DSTAP/DOPE lipoplexes
resulted in high siRNA accumulation in the lungs. Generally, in
systemic circulation, positively charged siRNA lipoplexes form
agglutinates with negatively charged erythrocytes (24), which are entrapped by pulmonary
capillaries soon after systemic injection (25). These findings indicate that
LP-DOTMA/DOPE and LP-DSTAP/DOPE lipoplexes may form stable
agglutinations with blood components, resulting in efficient
entrapment in lung capillaries. However, systemic injection of
LP-DODAP/DOPE lipoplexes resulted in high siRNA accumulation in the
liver in the present study. DODAP is an ionizable cationic lipid
with a pKa of 6.6 (26) and
contains a ternary ammonium head group with acyl chains (C18). The
LP-DODAP/DOPE lipoplexes did not agglutinate erythrocytes. Dilliard
et al (27) reported that
the inclusion of DODAP in lipid nanoparticles (LNPs) increases
apolipoprotein E (ApoE)-binding to LNPs in the plasma, and then
enhances the uptake by hepatocytes via the low density lipoprotein
(LDL) receptor. This finding suggests that LP-DODAP/DOPE lipoplexes
might interact with ApoE, but not with erythrocytes in the systemic
circulation, resulting in accumulation in the liver via the LDL
receptor. By contrast, the systemic injection of siRNA lipoplexes
containing cationic lipids with shorter dialkyl chains (C14 or C16)
tend to increase siRNA accumulation in the liver. The systemic
injection of LP-DC-1-16/DOPE and LP-DC-6-14/DOPE lipoplexes in the
present study caused siRNA accumulation in both the lungs and
liver, whereas LP-DC-1-14/DOPE lipoplexes caused siRNA accumulation
in the liver. Generally, liposomes composed of lipids with short
dialkyl chains are unstable because lipids with short dialkyl
chains weaken the hydrophobic interactions between the alkyl chains
by decreasing the hydrophobic area in the liposomal membrane
(28). Therefore, the
LP-DC-1-14/DOPE lipoplexes might be lysed soon after systemic
injection, followed by their capture by Kupffer cells in the
liver.
Regarding in vivo gene-knockdown, in the
present study, LP-DC-1-16/DOPE lipoplexes with Tie2 siRNA induced a
significant knockdown of Tie2 mRNA (37% knockdown) compared with
Control #2 siRNA. By contrast, LP-DOTMA/DOPE lipoplexes exhibited a
slight gene-knockdown, whereas LP-DC-6-14/DOPE and LP-DSTAP/DOPE
lipoplexes did not show knockdown efficacy. Tagami et al
(29) reported that some genes are
upregulated or downregulated by treatment with non-specific siRNA
lipoplexes, suggesting that the expression in some genes might be
affected by inflammatory cytokines induced by a non-specific
cellular immune response for siRNA lipoplexes via toll-like
receptors (30). It has been
reported that Tie2 expression in endothelial cells is stimulated by
inflammatory cytokines (31).
Therefore, the LP-DOTMA/DOPE lipoplexes with Control #2 siRNA
lipoplexes might increase slightly Tie2 expression in the lungs
(120%, compared with untreated lungs). However, further studies are
required to investigate whether the injection of siRNA lipoplexes
induce immune reactions such as inflammation and complement
activation in mice. In our recent study, siRNA lipoplexes composed
of DDAB and DOPE containing Tie2 siRNA induced significant
knockdown of Tie2 mRNA (50% knockdown) compared with Control #2
siRNA, but those of DOTAP and DOPE did not (9). It is unclear why cationic liposomes
composed of DC-1-16/DOPE exhibit high gene-knockdown efficiency
in vivo, but the linker between the hydrophilic and
hydrophobic lipid moieties in cationic lipid might be an important
factor in transfecting siRNA lipoplexes into the lungs (32–34).
In our previous study, inclusion of dialkyl cationic
lipid (DDAB) with a non-biodegradable linker into liposomal
formulation exhibited high in vivo gene-knockdown by siRNA
lipoplexes compared with that of diacyl cationic lipid (DOTAP) with
a biodegradable linker (9).
However, it was not clear the effect of alkyl or acyl chain length
and saturation in cationic lipid on a gene-knockdown by siRNA
lipoplexes. The present study revealed that cationic lipids with a
non-biodegradable linker and dialkyl saturated chains (C16-C18) are
potential lipids for in vivo gene-knockdown by cationic
liposomes.
In conclusion, the present study determined the
effect of phosphatidylethanolamines in liposomal formulations on
gene-knockdown efficacy in breast tumor cells and mouse lungs using
siRNA lipoplexes. Differences in the structures of alkyl or acyl
chains, head groups and linkers in cationic lipids may affect the
optimal combination of phospholipids for gene-knockdown using siRNA
lipoplexes. Overall, the present study provides insights onto the
optimal liposomal formulation for siRNA delivery using cationic
liposomes.
Acknowledgements
The authors would like to thank Ms Kumi Kawano
(Department of Molecular Pharmaceutics, Hoshi University, Tokyo,
Japan) for assistance with the animal experiments.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed in the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
YH conceived and designed the study, and wrote the
manuscript. MT, AA, ME, HS and KO performed the experiments. YH and
MT confirm the authenticity of all the raw data. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Institutional Animal
Care and Use Committee of Hoshi University (approval no.
P22-016).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zimmermann TS, Lee AC, Akinc A, Bramlage
B, Bumcrot D, Fedoruk MN, Harborth J, Heyes JA, Jeffs LB, John M,
et al: RNAi-mediated gene silencing in non-human primates. Nature.
441:111–114. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang J, Lu Z, Wientjes MG and Au JLS:
Delivery of siRNA therapeutics: Barriers and carriers. AAPS J.
12:492–503. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Barba AA, Cascone S, Caccavo D, Lamberti
G, Chiarappa G, Abrami M, Grassi G, Grassi M, Tomaiuolo G, Guido S,
et al: Engineering approaches in siRNA delivery. Int J Pharm.
525:343–358. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Khalil IA, Yamada Y and Harashima H:
Optimization of siRNA delivery to target sites: Issues and future
directions. Expert Opin Drug Deliv. 15:1053–1065. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yan Y, Liu XY, Lu A, Wang XY, Jiang LX and
Wang JC: Non-viral vectors for RNA delivery. J Control Release.
342:241–279. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang Y, Satterlee A and Huang L: In vivo
gene delivery by nonviral vectors: Overcoming hurdles? Mol Ther.
20:1298–1304. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xue HY, Guo P, Wen WC and Wong HL:
Lipid-based nanocarriers for RNA delivery. Curr Pharm Des.
21:3140–3147. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hattori Y, Tamaki K, Ozaki KI, Kawano K
and Onishi H: Optimized combination of cationic lipids and neutral
helper lipids in cationic liposomes for siRNA delivery into the
lung by intravenous injection of siRNA lipoplexes. J Drug Deliv Sci
Technol. 52:1042–1050. 2019. View Article : Google Scholar
|
|
9
|
Hattori Y, Tang M, Torii S, Tomita K,
Sagawa A, Inoue N, Yamagishi R and Ozaki KI: Optimal combination of
cationic lipid and phospholipid in cationic liposomes for gene
knockdown in breast cancer cells and mouse lung using siRNA
lipoplexes. Mol Med Rep. 26:2532022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xia Y, Tian J and Chen X: Effect of
surface properties on liposomal siRNA delivery. Biomaterials.
79:56–68. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hattori Y, Nakamura M, Takeuchi N, Tamaki
K, Shimizu S, Yoshiike Y, Taguchi M, Ohno H, Ozaki KI and Onishi H:
Effect of cationic lipid in cationic liposomes on siRNA delivery
into the lung by intravenous injection of cationic lipoplex. J Drug
Target. 27:217–227. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yamato K, Yamada T, Kizaki M, Ui-Tei K,
Natori Y, Fujino M, Nishihara T, Ikeda Y, Nasu Y, Saigo K and
Yoshinouchi M: New highly potent and specific E6 and E7 siRNAs for
treatment of HPV16 positive cervical cancer. Cancer Gene Ther.
15:140–153. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hattori Y, Tamaki K, Sakasai S, Ozaki KI
and Onishi H: Effects of PEG anchors in PEGylated siRNA lipoplexes
on in vitro gene-silencing effects and siRNA biodistribution
in mice. Mol Med Rep. 22:4183–4196. 2020.PubMed/NCBI
|
|
14
|
Tang M, Sakasai S, Onishi H, Kawano K and
Hattori Y: Effect of PEG anchor in PEGylation of folate-modified
cationic liposomes with PEG-derivatives on systemic siRNA delivery
into the tumor. J Drug Target. 31:74–88. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Suzuki A, Hamada K, Sasaki T, Mak TW and
Nakano T: Role of PTEN/PI3K pathway in endothelial cells. Biochem
Soc Trans. 35:172–176. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dumont DJ, Gradwohl GJ, Fong GH, Auerbach
R and Breitman ML: The endothelial-specific receptor tyrosine
kinase, tek, is a member of a new subfamily of receptors. Oncogene.
8:1293–1301. 1993.PubMed/NCBI
|
|
17
|
Fehring V, Schaeper U, Ahrens K, Santel A,
Keil O, Eisermann M, Giese K and Kaufmann J: Delivery of
therapeutic siRNA to the lung endothelium via novel lipoplex
formulation DACC. Mol Ther. 22:811–820. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gutbier B, Kube SM, Reppe K, Santel A,
Lange C, Kaufmann J, Suttorp N and Witzenrath M: RNAi-mediated
suppression of constitutive pulmonary gene expression by small
interfering RNA in mice. Pulm Pharmacol Ther. 23:334–344. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cheng X and Lee RJ: The role of helper
lipids in lipid nanoparticles (LNPs) designed for oligonucleotide
delivery. Adv Drug Deliv Rev. 99:129–137. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Paliwal SR, Paliwal R and Vyas SP: A
review of mechanistic insight and application of pH-sensitive
liposomes in drug delivery. Drug Deliv. 22:231–242. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sun D and Lu ZR: Structure and function of
cationic and ionizable lipids for nucleic acid delivery. Pharm Res.
40:27–46. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen H, Ren X, Xu S, Zhang D and Han T:
Optimization of lipid nanoformulations for effective mRNA delivery.
Int J Nanomedicine. 17:2893–2905. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Eliyahu H, Servel N, Domb AJ and Barenholz
Y: Lipoplex-induced hemagglutination: Potential involvement in
intravenous gene delivery. Gene Ther. 9:850–858. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hattori Y, Nakamura A, Hanaya S, Miyanabe
Y, Yoshiike Y, Kikuchi T, Ozaki K and Onishi H: Effect of
chondroitin sulfate on siRNA biodistribution and gene silencing
effect in mice after injection of siRNA lipoplexes. J Drug Deliv
Sci Technol. 41:401–409. 2017. View Article : Google Scholar
|
|
26
|
Thi TTH, Suys EJA, Lee JS, Nguyen DH, Park
KD and Truong NP: Lipid-based nanoparticles in the clinic and
clinical trials: From cancer nanomedicine to COVID-19 vaccines.
Vaccines (Basel). 9:3592021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dilliard SA, Cheng Q and Siegwart DJ: On
the mechanism of tissue-specific mRNA delivery by selective organ
targeting nanoparticles. Proc Natl Acad Sci USA.
118:e21092561182021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Funakoshi Y, Iwao Y, Noguchi S and Itai S:
Effect of alkyl chain length and unsaturation of the phospholipid
on the physicochemical properties of lipid nanoparticles. Chem
Pharm Bull (Tokyo). 63:731–736. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tagami T, Hirose K, Barichello JM, Ishida
T and Kiwada H: Global gene expression profiling in cultured cells
is strongly influenced by treatment with siRNA-cationic liposome
complexes. Pharm Res. 25:2497–2504. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ma Z, Li J, He F, Wilson A, Pitt B and Li
S: Cationic lipids enhance siRNA-mediated interferon response in
mice. Biochem Biophys Res Commun. 330:755–759. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Willam C, Koehne P, Jürgensen JS, Gräfe M,
Wagner KD, Bachmann S, Frei U and Eckardt KU: Tie2 receptor
expression is stimulated by hypoxia and proinflammatory cytokines
in human endothelial cells. Circ Res. 87:370–377. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rajesh M, Sen J, Srujan M, Mukherjee K,
Sreedhar B and Chaudhuri A: Dramatic influence of the orientation
of linker between hydrophilic and hydrophobic lipid moiety in
liposomal gene delivery. J Am Chem Soc. 129:11408–11420. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Srujan M, Chandrashekhar V, Reddy RC,
Prabhakar R, Sreedhar B and Chaudhuri A: The influence of the
structural orientation of amide linkers on the serum compatibility
and lung transfection properties of cationic amphiphiles.
Biomaterials. 32:5231–5240. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhi D, Bai Y, Yang J, Cui S, Zhao Y, Chen
H and Zhang S: A review on cationic lipids with different linkers
for gene delivery. Adv Colloid Interface Sci. 253:117–140. 2018.
View Article : Google Scholar : PubMed/NCBI
|





















