Introduction
Glucocorticoids (GCs) are drugs that are widely used
in the treatment of local or systemic chronic inflammatory or
autoimmune diseases (1,2), and are also frequently used in the
enhanced recovery protocol following certain types of surgery such
as total knee arthroplasty (3–5).
However, the prolonged use of GCs sometimes results in side effects
related to the skeletal system and cartilage (1,6).
Previous studies reported that GCs can induce chondrocyte autophagy
and the increased level of autophagy is associated with a reduction
in cell viability (7–9). Abnormal levels of chondrocyte
autophagy are closely related to the occurrence and development of
certain osteoarticular diseases, such as Kashin-Beck disease and
osteoarthritis (10,11).
Autophagy is a process through which eukaryotic
lysosomes provide nutrients to cells by degrading damaged
organelles, misfolded proteins and intracellular pathogens
(12). The PI3K/AKT/mTOR signaling
pathway is one of the classic autophagy regulatory signaling
pathways, and it is currently the only known inhibitory pathway of
autophagy (13). The activation of
the PI3K/AKT/mTOR signaling pathway can inhibit autophagy, while
the inhibition of this pathway induces autophagy (13).
Lithium is a classical regulator of autophagy
(14). Previous studies reported
that lithium can inhibit the apoptosis of bone marrow-derived
mesenchymal stem cells (BMSCs) induced by serum deprivation or
maintain the proliferative ability of liver cells by activating
autophagy (15,16). Lithium also exerts neuroprotective
effects by regulating the levels of autophagy in mouse models of
hypoxic-ischemic encephalopathy and Alzheimer's disease (17,18).
However, to the best of our knowledge, no studies available to date
have examined the effects of lithium on chondrocyte autophagy.
Thus, the present study aimed to determine whether lithium can
prevent GC-induced chondrocyte autophagy by regulating the
PI3K/AKT/mTOR signaling pathway.
Materials and methods
Ethics approval
The present study was approved to use commercially
available primary cells by the Clinical Trials and Biomedical
Ethics Committee of Sichuan University West China Hospital
(approval no. 2021-102).
Cells and cell culture
All chondrocytes used in the present study were
primary rat (cat. no. RAT-iCell-s003; each vial containing
>5×105 cells in 1 ml volume) or human chondrocytes
(cat. no. HUM-iCell-s018; each vial containing >5×105
cells in 1 ml volume), and were provided by iCell Bioscience Inc.
The cells were cultured in a specialized primary chondrocyte
culture system (cat. no. PriMed-iCell-020; iCell Bioscience
Inc.).
CYTO-ID® autophagy
fluorescence staining
The CYTO-ID Autophagy Detection kit (Enzo Life
Sciences, Inc.) detects autophagy vesicles using novel dyes that
selectively label the accumulation of autophagy vesicles so that
they exhibit bright fluorescence. Primary rat and human
chondrocytes at the logarithmic growth phase were prepared into a
single-cell suspension. The cell density was adjusted to
1×105 cells/ml and 1 ml cell suspension was transferred
in each well of 12-well plate. The cells were either left untreated
(blank control), or treated with dexamethasone (200 µM; Beijing
Solarbio Science & Technology Co., Ltd.), or dexamethasone (200
µM) combined with lithium chloride (MilliporeSigma) at various
concentrations (0.01, 0.1, 1 or 10 mM), with four replicates in
each group. The concentration of dexamethasone was determined
according to a previous study (7)
and our preliminary experiments. A previous study showed that 200
µM dexamethasone could increase the autophagy level of chondrocytes
and significantly reduce cell viability (7). Similar results have been obtained
from our preliminary experiments (unpublished data). After the
cells adhered to the walls, each well was added with the drugs
corresponding to the aforementioned grouping, followed by
incubation for 24 or 48 h at 37°C in an incubator with 5%
CO2. Subsequently, 300 µl pre-configured CYTO-ID
staining solution was added to each well, and the cells were
stained for 20 min at 37°C in an incubator with 5% CO2.
The cells were then observed and photographed under an inverted
fluorescence microscope (Zeiss AG). Under an ×20 magnification
field of view, five fields of view were randomly selected and the
average fluorescence intensity was semi-quantitatively calculated
using ImageJ software (version 1.8.0; US National Institutes of
Health).
After screening for the suitable lithium chloride
concentration (10 mM) and the duration of action (48 h) (under
these conditions, the autophagy level of chondrocytes was changed
most significantly), the following experiments were performed under
these conditions.
Transmission electron microscopy
(TEM)
Primary chondrocytes at the logarithmic growth phase
were prepared into a single-cell suspension. The cell density was
adjusted to 1×105 cells/ml and 2 ml of cell suspension
was transferred into each well of three 6-well plates. The cells
were either left untreated, or treated with dexamethasone (200 µM),
or dexamethasone (200 µM) combined with lithium chloride (10 mM)
for 48 h at 37°C in an incubator with 5% CO2, with four
replicates in each group. The cells were washed with PBS twice,
digested and collected using trypsin. Subsequently, the cell
suspensions were centrifuged at 250 × g for 5 min at room
temperature, the supernatants were discarded and 0.5%
glutaraldehyde fixation solution was slowly added along the tube
wall using a dropper. Following resuspension, the cell suspension
was placed in an environment at 4°C for 10 min for fixation, and
the cell suspension was then transferred into a 1.5-ml tip bottom
EP tube and centrifuged at 12,000 rpm for 10 min at 4°C. The
supernatant was gently discarded, whereas the precipitate was
retained and 3% glutaraldehyde fixing solution was slowly added
using a dropper. The samples were then observed under a
transmission electron microscope (JEM-1400FLASH, JEOL, Ltd.). A
total of five normal cells were randomly selected from each sample
and autophagosomes in the cytoplasm were counted. The average
number of autophagosomes was compared between groups.
RNA isolation and reverse
transcription-quantitative PCR (RT-qPCR)
The cell density was adjusted to 1×105
cells/ml and 2 ml of cell suspension was transferred into each well
of three 6-well plates. The cells were either left untreated, or
treated with dexamethasone (200 µM), or dexamethasone (200 µM)
combined with lithium chloride (10 mM) for 48 h at 37°C in an
incubator with 5% CO2, with four replicates in each
group to extract RNA. To evaluate the expression levels of
autophagy-related genes, including LC3B, AKT and mTOR, total RNA
was extracted from the chondrocytes using TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to
the manufacturer's instructions. Total RNA was then
reverse-transcribed into cDNA using the PrimeScript™ RT reagent kit
(Promega Corporation). Reactions were performed using a 20 µl final
volume with 2 µl cDNA and 10 µl SYBR™-Green PCR Master Mix (Promega
Corporation). The nucleotide sequences of the PCR primers are
presented in Table I.
 | Table I.Nucleotide sequences of PCR
primers. |
Table I.
Nucleotide sequences of PCR
primers.
|
| Primers for rat
chondrocytes | Primers of human
chondrocytes |
|---|
|
|
|
|
|---|
| Gene IDs | Forward | Reverse | Forward | Reverse |
|---|
| ACTB |
5′-CATCACTATCGGCA |
5′-ACGCAGCTCAGT | 5′-CATGTACGTTG | 5′-CTCCTTAATGT |
|
|
ATGAGCGGTTCC-3′ |
AACAGTCCGCCTA-3′ | CTATCCAGGC-3′ | CACGCACGAT-3′ |
| AKT |
5′-AACGGCAGGAGGA |
5′-CTCGTTCATGGTC | 5′-TGACCATGAAC | 5′-GAGGATCTTCA |
|
|
GGAGACGATGGA-3′ |
ACACGGTGCTTGG-3′ | GAGTTTGAGTA-3′ | TGGCGTAGTAG-3′ |
| LC3B |
5′-CCGTCCTGGACAA |
5′-ACACTCACCATGC | 5′-GCCGTCGGAGA | 5′-TGGTTGGATGC |
|
|
GACCAAGTTCCT-3′ |
TGTGCCCATTCA-3′ | AGACCTTCAAG-3′ | TCTCGAATAAG-3′ |
| m-TOR |
5′-AGAGGACCAGCAG |
5′-GCAGTGGTGGTGG | 5′-GAGATACGCTG |
5′-CTGTATTATTGA |
|
|
CACAAGCAGGAG-3′ |
CATTGGTGATGTT-3′ | TCATCCCTTTA-3′ | CGGCATGCTC-3′ |
Cycle threshold (Ct) values were obtained using
Thermo Scientific PikoReal software (version 2.2; Thermo Fisher
Scientific, Inc.). Relative expression was calculated using the
2−ΔΔCq method and normalized to the internal reference
gene β-actin (19).
Cell protein extraction and western
blot analysis
The cell density was adjusted to 1×105
cells/ml and 2 ml of cell suspension was transferred into each well
of three 6-well plates. The cells were either left untreated, or
treated with dexamethasone (200 µM), or dexamethasone (200 µM)
combined with lithium chloride (10 mM) for 48 h at 37°C in an
incubator with 5% CO2, with four replicates in each
group to extract the protein. RIPA buffer (Wuhan Servicebio
Technology Co., Ltd.) was used to lyse the rat and human
chondrocytes from on ice for 10 min. The lysate mixture was then
centrifuged at 12,000 rpm for 10 min at 4°C. The BCA Protein Assay
kit (Beyotime Institute of Biotechnology) was used to measure the
total protein concentration and 20 µg protein/lane was separated by
SDS-PAGE on a 10% or 12% gel. The separated proteins were
subsequently transferred onto a PVDF membrane and blocked for 2 h
at 25°C in a PBS solution containing 5% skim milk powder.
Subsequently, the membranes were incubated overnight at 4°C with
primary antibodies against LC3B (rabbit; 1:2,000; cat. no.
ab192890; Abcam), phosphorylated (p-)AKT (rabbit; 1:1,000; cat. no.
310021; Chengdu Zen Bioscience Co., Ltd.), AKT (rabbit; 1:1,000;
cat. no. 382804; Chengdu Zen Bioscience Co., Ltd.), phosphorylated
mTOR (mouse; 1:1,000; cat. no. sc-293133; Santa Cruz Biotechnology,
Inc.), mTOR (mouse; 1:1,000; cat. no. sc-517464; Santa Cruz
Biotechnology, Inc.), and β-tubulin (rabbit; 1:2,000; cat. no.
AF7011; Affinity Biosciences). After washing with PBST, the
membranes were incubated with the secondary polyclonal goat
anti-rabbit/mouse HRP-conjugated antibodies (cat. no. ZDR-5306 and
ZDR-5307; both 1:5,000; OriGene Technologies, Inc.) at room
temperature for 2 h. Finally, after washing the membranes with
PBST, the protein bands were visualized using the ECL method
(Torchlight Hypersensitive ECL Western HRP Substrate; cat. no.
17046; Chengdu Zen Bioscience Co., Ltd.) and the Quantity One
software (version 4.6.6; Bio-Rad Laboratories, Inc.) was used to
analyze the results with β-tubulin as the loading control.
Cell Counting Kit-8 (CCK-8) assay
To measure the cell viability, the cell density was
adjusted to 3×104 cells/ml and 100 µl of cell suspension
was transferred into each well of a 96-well plate. The cells were
either left untreated, or treated with dexamethasone (200 µM), or
dexamethasone (200 µM) combined with lithium chloride (10 mM) for
48 h at 37°C in an incubator with 5% CO2, with four
replicates in each group. The CCK-8 reagent (Apexbio Technology
LLC) was diluted 1:10 and 110 µl diluted CCK-8 solution was added
into each well. After 2 h, the optical density (OD) value of each
well was examined at 450 nm according to the manufacturer's
instructions. Cell viability was calculated as follows: Cell
viability (%)=(OD value of observation group-OD value of zero
adjustment group)/(OD value of control group-OD value of zero
adjustment group) ×100%.
Statistical analysis
Statistical analysis was performed using SPSS 26.0
software (IBM Corp.). For continuous data, one-way analysis of
variance (ANOVA) was used and Tukey's test was used as the post hoc
test. Continuous data are presented as the mean ± standard
deviation unless otherwise indicated. P<0.05 was considered to
indicate a statistically significant difference.
Results
Effects of GC and lithium on the
chondrocyte autophagy level
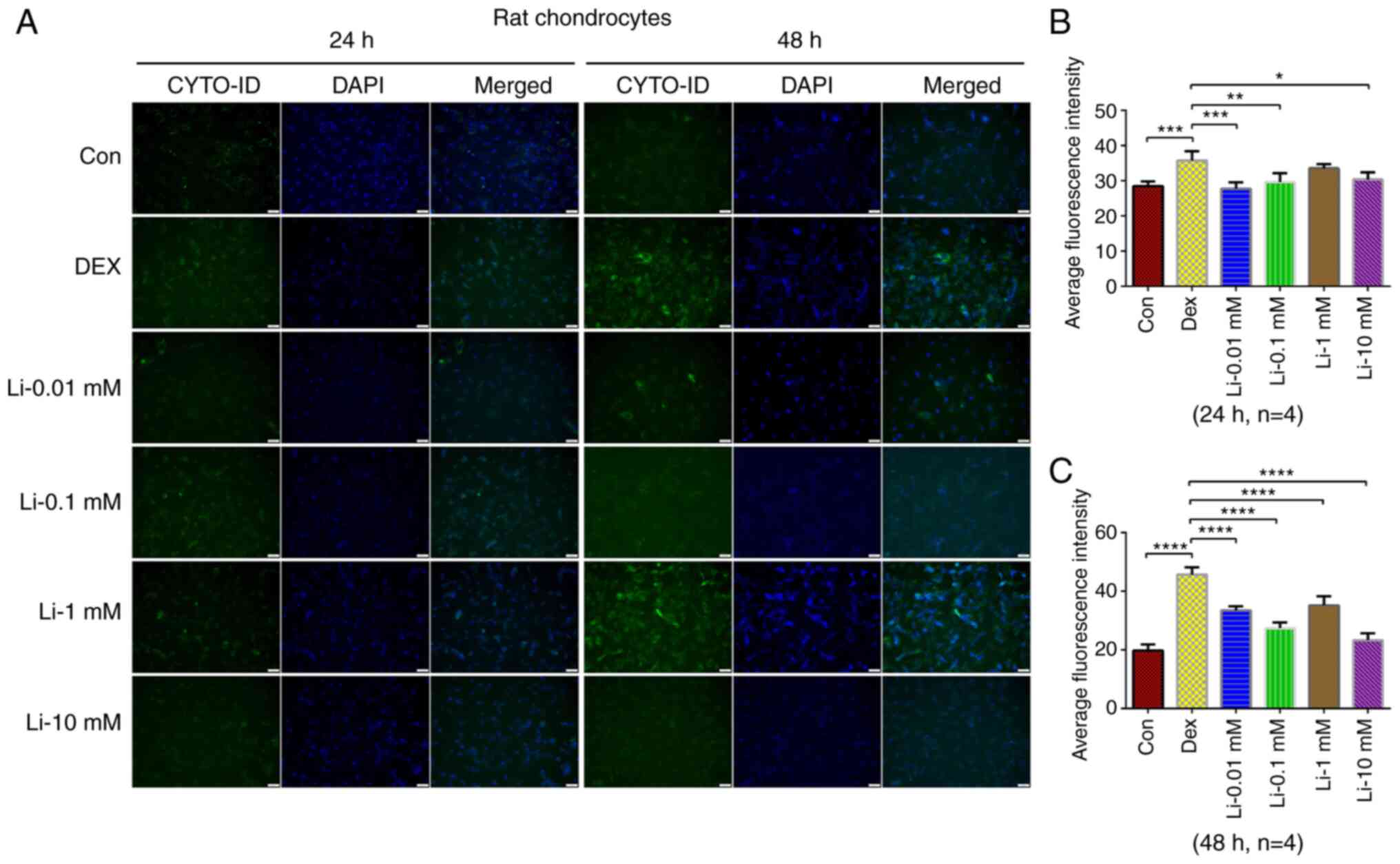
The results of CYTO-ID autophagy fluorescence
staining revealed that the autophagy level of rat chondrocytes
increased significantly following treatment with dexamethasone for
24 and 48 h (Fig. 1). Following
treatment with dexamethasone combined with various concentrations
of lithium chloride for 24 and 48 h, it was found that 10 mM
lithium chloride for 48 h significantly reduced the autophagy level
overactivated by dexamethasone in rat chondrocytes.
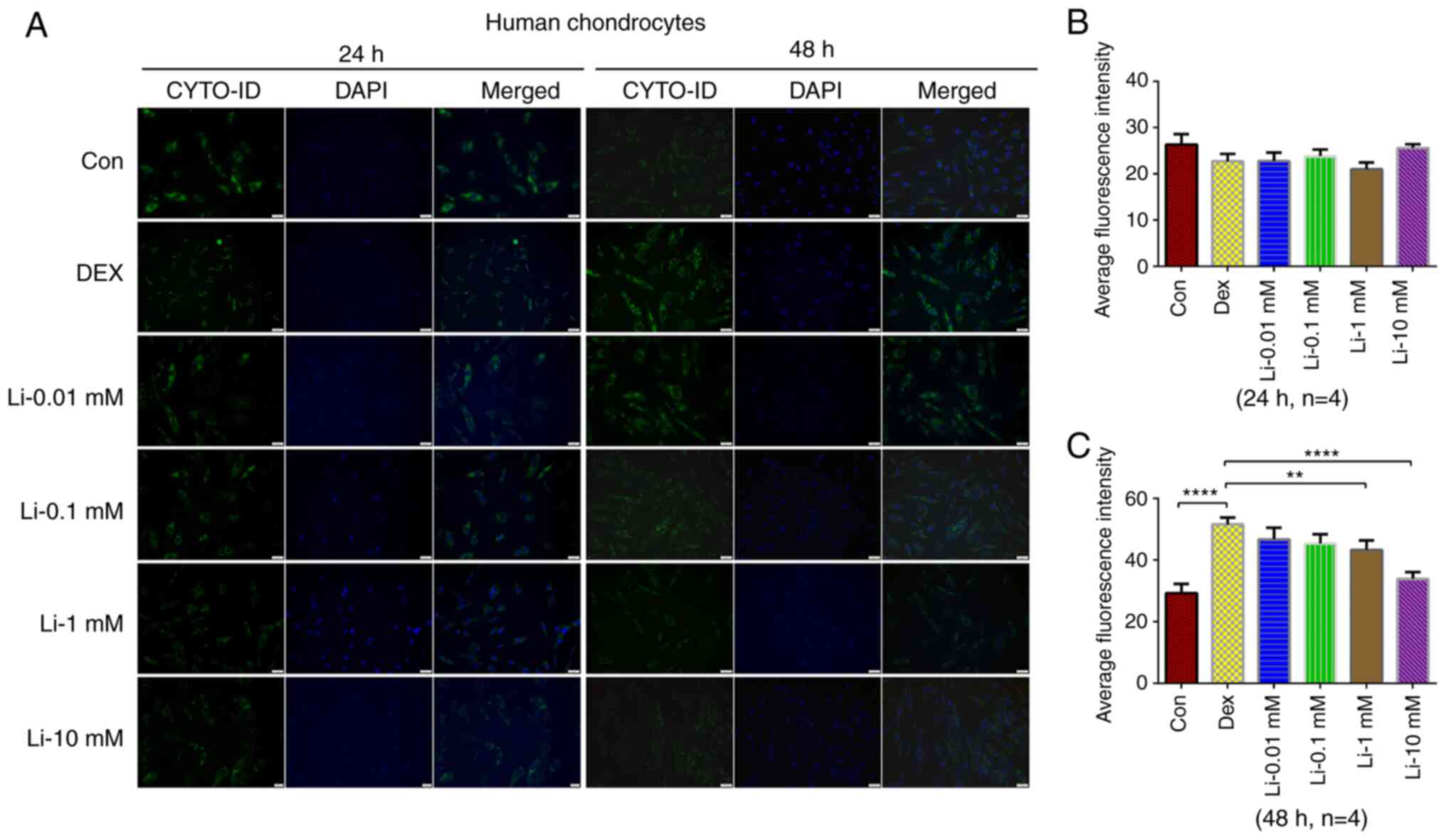
The results of CYTO-ID autophagy fluorescence
staining revealed that the autophagy level of human chondrocytes
did not change significantly following treatment with dexamethasone
for 24 h and the autophagy level of human chondrocytes increased
significantly following treatment with dexamethasone for 48 h
(Fig. 2). Following treatment with
dexamethasone combined with various concentrations of lithium
chloride for 24 and 48 h, it was found that 10 mM lithium chloride
for 48 h significantly reduced the autophagy level overactivated by
dexamethasone in human chondrocytes. Therefore, the rat and human
chondrocytes were divided into three groups in subsequent
experiments: The control group was composed of cells untreated; the
dexamethasone group was composed of cells treated with 200 µM
dexamethasone for 48 h; the lithium group was composed of cells
treated with 200 µM dexamethasone and 10 mM lithium chloride for 48
h.
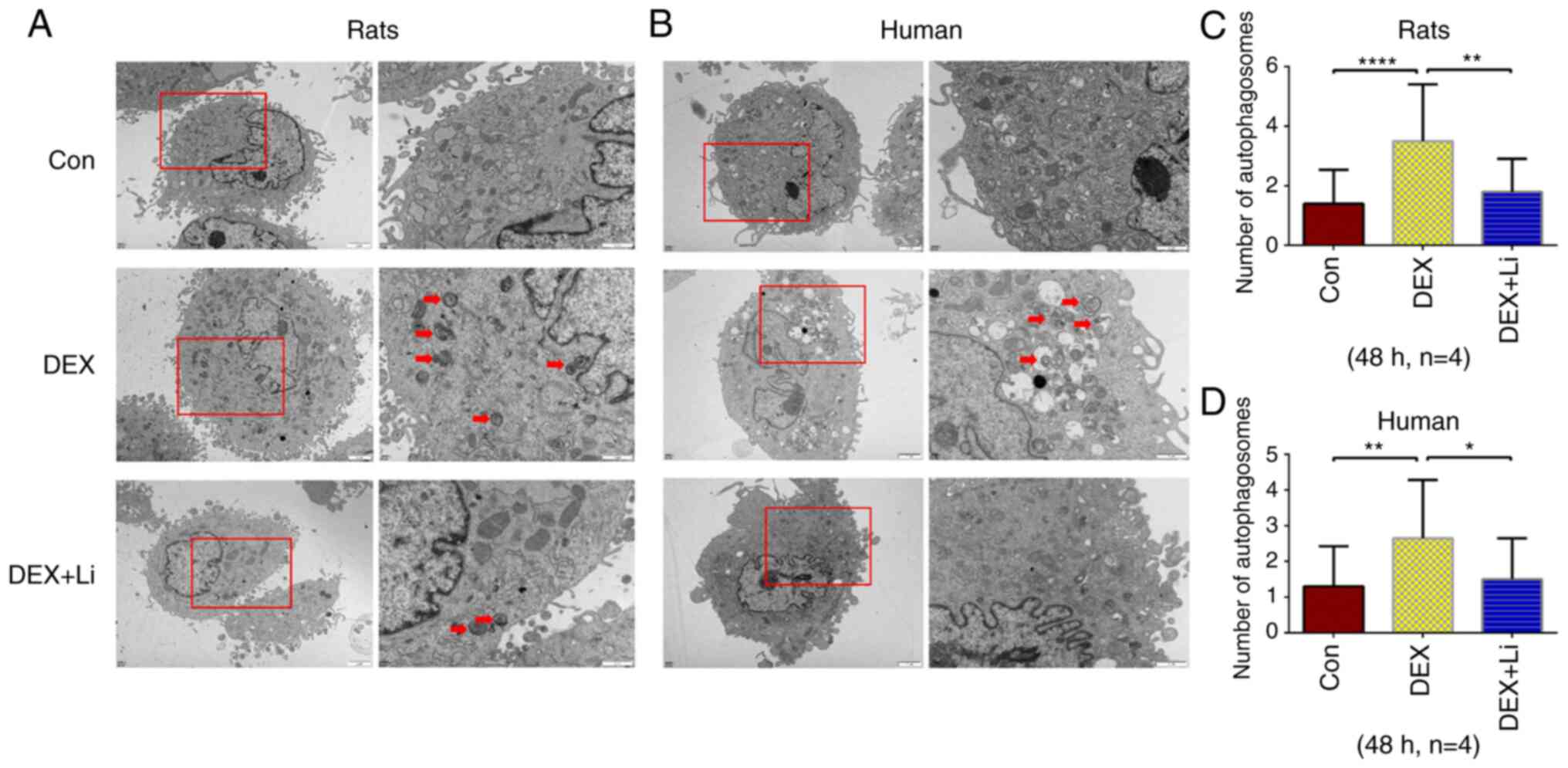
Compared with the control and lithium groups, a
significantly greater number of autophagosomes was observed by TEM
in the dexamethasone group of rat chondrocytes (Fig. 3A and C). Compared with the control
and lithium groups, a significantly greater number of
autophagosomes was observed by TEM in the dexamethasone group of
human chondrocytes (Fig. 3B and
D).
Effects of GC and lithium on cell
viability
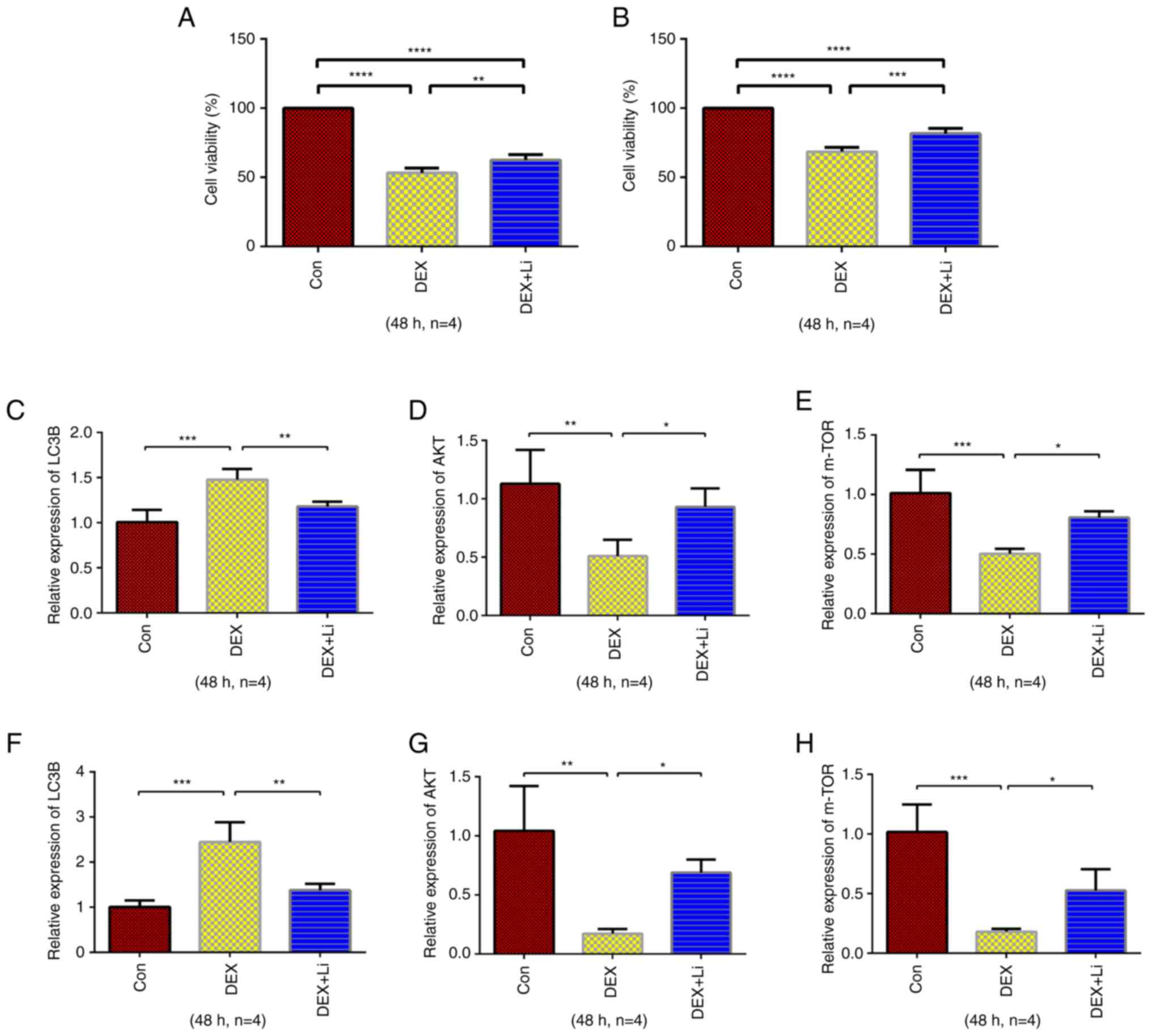
The results of the CCK-8 assay of rat chondrocytes
revealed that the average cell viability of the dexamethasone group
was significantly lower than that of the control and lithium
groups, and the average cell viability of the lithium group was
significantly lower than that of the control group (Fig. 4A). The results of the CCK-8 assay
of human chondrocytes revealed that the average cell viability of
the dexamethasone group was significantly lower than that of the
control and lithium groups, and the average cell viability of the
lithium group was significantly lower than that of the control
group (Fig. 4B).
Expression of genes related to
autophagy in chondrocytes
The results of RT-qPCR of rat chondrocytes revealed
that the relative expression of LC3B in the dexamethasone group was
significantly higher than that of the control and lithium groups,
while the relative expression of AKT and mTOR in the dexamethasone
group was significantly lower than that of the control and lithium
groups (Fig. 4C-E). The results of
RT-qPCR of human chondrocytes revealed that the relative expression
of LC3B in the dexamethasone group was significantly higher than
that of the control and lithium groups, while the relative
expression of AKT and mTOR in the dexamethasone group was
significantly lower than that of the control and lithium groups
(Fig. 4F-H).
The results of western blot analysis of rat
chondrocytes revealed that the relative expression of LC3II/I in
the dexamethasone group was significantly higher than that of the
control and lithium groups, while the relative expression of
phosphorylated AKT/AKT and phosphorylated mTOR/mTOR in the
dexamethasone group was significantly lower than that of the
control and lithium groups (Fig.
5A-D). The results of western blot analysis of human
chondrocytes revealed that the relative expression of LC3II/I in
the dexamethasone group was significantly higher than that of the
control and lithium groups, while the relative expression of
phosphorylated AKT/AKT and phosphorylated mTOR/mTOR in the
dexamethasone group was significantly lower than that of the
control and lithium groups (Fig.
5E-H).
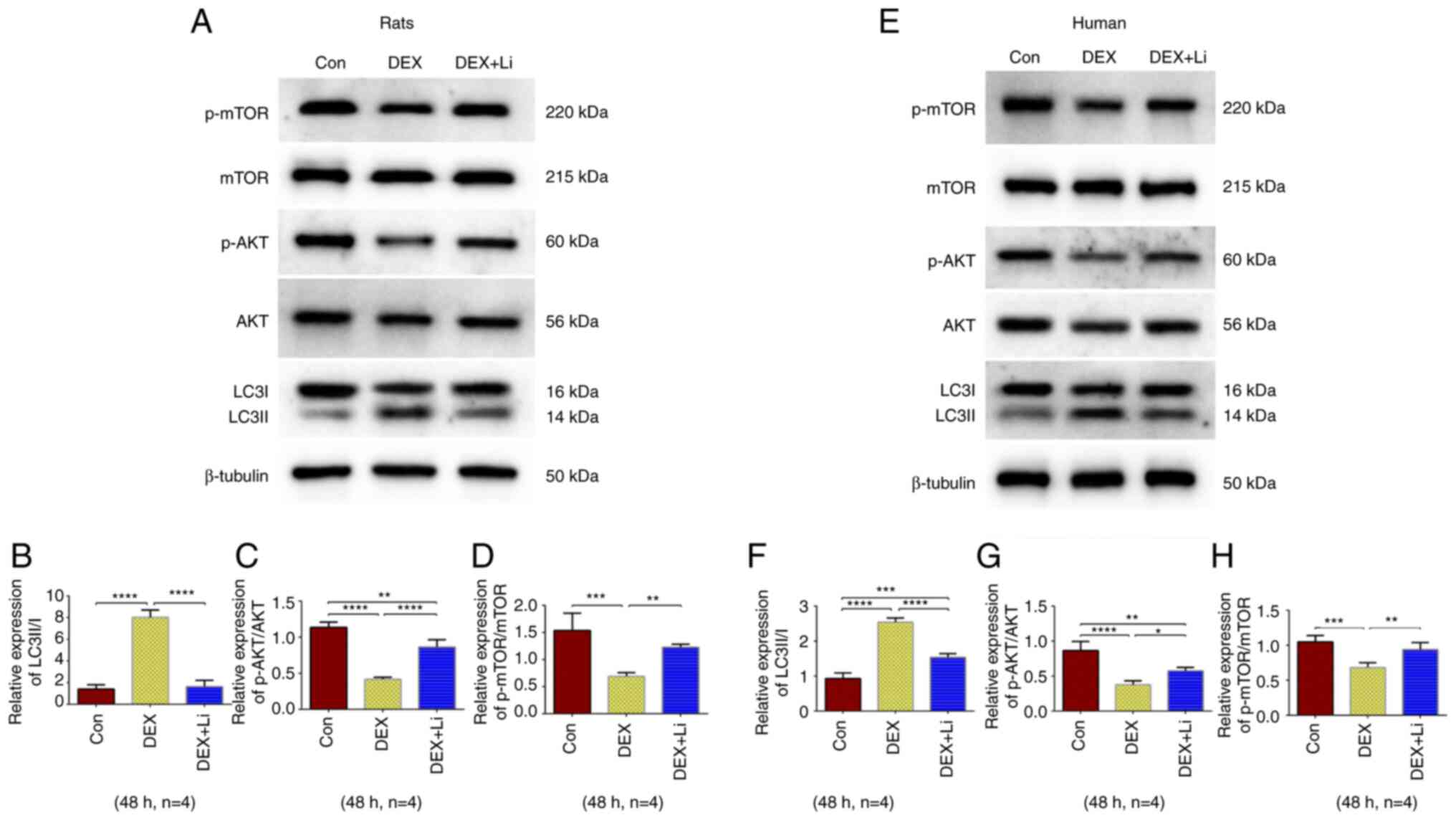 | Figure 5.Results of western blot analysis. (A)
Representative western blots of rat chondrocytes. The average
relative expression of (B) LC3II/I, (C) p-AKT/AKT and (D)
p-mTOR/mTOR from rat chondrocytes. (E) Representative western blots
of human chondrocytes. The average relative expression of (F)
LC3II/I, (G) p-AKT/AKT, and (H) p-mTOR/mTOR from human
chondrocytes. *P<0.05, **P<0.01, ***P<0.001 and
****P<0.0001. Con, control group; DEX, dexamethasone group (200
µM); DEX + Li, dexamethasone (200 µM) combined with lithium
chloride (10 mM) group; p-AKT, phosphorylated AKT; p-mTOR,
phosphorylated mTOR; p-, phosphorylated. |
Discussion
To the best of our knowledge, the present study is
the first to identify that lithium can prevent GC-induced autophagy
by activating the PI3K/AKT/mTOR signaling pathway, as well as
prevent the GC-induced decrease in the viability of
chondrocytes.
GCs can affect the normal physiological function of
chondrocytes in some diseases of the skeletal system, such as
osteoarthritis and skeletal dysplasia (6,20–24).
For example, GCs can promote chondrocyte apoptosis and inhibit
chondrocyte viability (22,23).
Apoptosis may reduce the number of chondrocytes and decrease the
extracellular matrix components, including proteoglycan and
collagen type II, contributing to osteoarthritis (24). In addition, the decreased
proliferation of chondrocytes has been linked to the thinning of
the growth plate, thus leading to an impairment in skeletal
development and growth (2,25).
It was previously found that GCs can induce
chondrocyte autophagy and this is associated with a reduction in
cell viability (7–9). A recent study reported that
GC-induced osteonecrosis of the femoral head was associated with
abnormal chondrocyte hyperplasia in articular surface cartilage,
which may be related to the GC-induced overactivation of autophagy
in chondrocytes (26). The change
in the level of autophagy of chondrocytes is also related to the
pathogenesis of osteoarthritis (27–29).
At present, it is generally considered that autophagy, as an
adaptive response, can reduce chondrocyte death in the early stages
of osteoarthritis; however, with the development of osteoarthritis,
excessive autophagy may also cause chondrocyte death (27); therefore, GC-induced chondrocyte
autophagy may cause certain types of pathological changes.
Lithium, a common drug used in the treatment of
psychosis, is also an autophagy regulator (14). Recently, lithium has been found to
regulate autophagy levels in cells of the skeletal system (30). The same study reported that lithium
chloride reversed the effects of ovariectomy on BMSCs extracted
from ovariectomized mice, promoting osteogenesis and suppressing
apoptosis by regulating autophagy (30). In addition, lithium chloride was
found to regulate autophagy, decrease apoptosis and promote bone
formation, thus protecting tooth movement in osteoporotic mice
(30). In the present study, it
was first found that lithium chloride maintained the viability of
chondrocytes by regulating autophagy. Lithium may thus have immense
potential for use in the field of diseases related to abnormal
chondrocyte autophagy levels, such as osteoarthritis and
Kashin-Beck disease (10,11,27–29).
A previous study indicated that lithium-containing scaffolds are
effective in promoting cartilage regeneration (31). However, the underlying mechanisms
of cartilage regeneration mediated by lithium-containing
biomaterials remain unclear.
A recent study reported that the treatment of
BMSC-derived exosomes (Li-BGC-Exo) with lithium chloride markedly
facilitated cartilage regeneration in vivo (32). In that previous study, the
researchers selected a lithium-substituted bioglass ceramic
(Li-BGC) model and systematically evaluated the regulatory role of
Li-BGC-Exo following Li-BGC treatment. The results revealed that
Li-BGC-Exo significantly promoted chondrogenesis, which was
attributed to the upregulation of exosomal miR-455-3p transfer,
resulting in the inhibition of histone deacetylase 2 and the
enhanced acetylation of histone H3 in chondrocytes (32). These findings suggest that lithium
may promote cartilage repair through other mechanisms in addition
to the regulation of autophagy.
Several signaling pathways were reported to be
associated with the level of autophagy in cells and several key
molecules can regulate the autophagy pathway (27). The PI3K/AKT/mTOR signaling pathway
is one of the classic autophagy regulatory signaling pathways and
this pathway is currently the only known inhibitory pathway of
autophagy (13). A previous study
explored the association between the levels of autophagy of
articular chondrocytes in rats with osteoarthritis and the
PI3K/AKT/mTOR signaling pathway (28). That previous study found that
inflammation inhibited the proliferation of rat chondrocytes and
reduced the rate of autophagy. The inhibition of the PI3K/AKT/mTOR
signaling pathway promoted chondrocyte autophagy and reduced
inflammation (28). The findings
of the present study suggested that lithium could also prevent
GC-induced autophagy by activating the classic PI3K/AKT/mTOR
signaling pathway. However, the PI3K/AKT/mTOR signaling pathway is
a complex signaling pathway with multiple regulators and effectors.
Most importantly, this signaling pathway is essential for the
normal physiological function of chondrocytes. It was previously
suggested that targeting this pathway may be a viable treatment
option for osteoarthritis (33).
However, merely activating or inhibiting the PI3K/AKT/mTOR
signaling pathway to prevent or treat osteoarthritis can be a
double-edged sword, as the side effects of this approach appear
inevitable (33). For example, the
PI3K/AKT/mTOR signaling-mediated synovial inflammation, subchondral
bone sclerosis, extracellular matrix homeostasis, chondrocyte
proliferation, apoptosis, autophagy and inflammation greatly affect
cell fate and OA pathophysiology. There will be an imbalance among
these cell processes if simply activating or inhibiting
PI3K/AKT/mTOR signaling. Therefore, future studies are required to
further elucidate the role of the PI3K/AKT/mTOR signaling pathway
in the different pathophysiological stages of osteoarthritis and
clarify the specific molecular mechanisms, such as how this
signaling pathway interacts with other signaling pathways. In
addition, it needs to be determined how to target this pathway in
osteoarthritis without affecting the regulatory processes of other
critical physiological signaling pathways. If these issues are
addressed, PI3K/AKT/mTOR-based osteoarthritis treatments may become
effective, safe and promising.
Previous studies reported that the induction of
autophagy by dexamethasone can be inhibited by 3-methyladenine, an
autophagy inhibitor, and RU486, a GC antagonist, and the inhibitory
effects are concentration-dependent in a certain range (100–400 µM
for 3-methyladenine, and 10–300 µM for RU486) (7,8). In
the present study, it was found that treatment with 10 mM lithium
chloride for 48 h significantly reduced the autophagy level
overactivated by dexamethasone. Lithium chloride functioned as an
autophagy regulator and played a role by activating the
PI3K/AKT/mTOR signaling pathway. Herein, autophagy inhibitors were
not used to compare the ability of lithium to inhibit autophagy
directly.
Previous studies on chondrocyte autophagy mostly
used rat and mouse chondrocytes (7–9,28).
In the present study, both rat and human chondrocytes were used,
and consistent results were obtained. Species-dependent effects
were not observed.
The present study has two limitations. First, the
results from cell studies were not verified or extended on animal
models. It is important to further apply these findings and results
using animal models, as it is well known that results can vary
between in vivo and in vitro experiments. Second,
autophagy inhibitors were not used to compare the ability of
lithium to inhibit autophagy. Further studies are thus required to
address these limitations.
In conclusion, under the conditions of the present
study, lithium was shown to prevent GC-induced autophagy by
activating the PI3K/AKT/mTOR signaling pathway, and it was also
found to prevent the GC-induced decrease in chondrocyte viability.
Lithium may thus have immense potential for use in the field of
diseases related to abnormal chondrocyte autophagy levels, such as
osteoarthritis and Kashin-Beck disease.
Acknowledgements
Not applicable.
Funding
The present study was funded by the 1.3.5 Project of Sichuan
University West China Hospital (grant no. ZYJC18040).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QW and WZ conducted the experiments and wrote the
manuscript. JH, CZ and LC analyzed the data and assisted in the
writing of the manuscript. PK oversaw the study and made important
intellectual contributions to the manuscript. PK was involved in
the conception and design of the study. QW and PK confirm the
authenticity of all the raw data. All authors read and approved the
final version of the manuscript. The authors clarify that no
artificial intelligence (AI) tools were used in this study or when
writing this manuscript.
Ethics approval and consent to
participate
This study was approved to use commercially
available primary cells by the Clinical Trials and Biomedical
Ethics Committee of Sichuan University West China Hospital
(approval no. 2021-102).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Huscher D, Thiele K, Gromnica-Ihle E, Hein
G, Demary W, Dreher R, Zink A and Buttgereit F: Dose-related
patterns of glucocorticoid-induced side effects. Ann Rheum Dis.
68:1119–1124. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Canalis E and Delany AM: Mechanisms of
glucocorticoid action in bone. Ann N Y Acad Sci. 966:73–81. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xu H, Zhang S, Xie J, Lei Y, Cao G and Pei
F: Multiple doses of perioperative dexamethasone further improve
clinical outcomes after total knee arthroplasty: A prospective,
randomized, controlled study. J Arthroplasty. 33:3448–3454. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xu B, Ma J, Huang Q, Huang ZY, Zhang SY
and Pei FX: Two doses of low-dose perioperative dexamethasone
improve the clinical outcome after total knee arthroplasty: A
randomized controlled study. Knee Surg Sports Traumatol Arthrosc.
26:1549–1556. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tammachote N and Kanitnate S: Intravenous
dexamethasone injection reduces pain from 12 to 21 hours after
total knee arthroplasty: A double-blind, randomized,
placebo-controlled trial. J Arthroplasty. 35:394–400. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Annefeld M: Changes in rat epiphyseal
cartilage after treatment with dexamethasone and
glycosaminoglycan-peptide complex. Pathol Res Pract. 188:649–652.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhao Y, Zuo Y, Huo HJ, Xiao YL, Yang XJ
and Xin DQ: Glucocorticoid induced autophagy in N1511 chondrocyte
cells. Eur Rev Med Pharmacol Sci. 18:3573–3579. 2014.PubMed/NCBI
|
|
8
|
Zhao Y, Zuo Y, Huo H, Xiao Y, Yang X and
Xin D: Dexamethasone reduces ATDC5 chondrocyte cell viability by
inducing autophagy. Mol Med Rep. 9:923–927. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xue E, Zhang Y, Song B, Xiao J and Shi Z:
Effect of autophagy induced by dexamethasone on senescence in
chondrocytes. Mol Med Rep. 14:3037–3044. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu C, Zheng J, Yao X, Shan H, Li Y, Xu P
and Guo X: Defective autophagy in chondrocytes with Kashin-Beck
disease but higher than osteoarthritis. Osteoarthritis Cartilage.
22:1936–1946. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huang Z, He J and Hong Z: Advancement of
research on the regulation of PI3K/Akt/mTOR signaling pathway for
prevention and treatment of osteoarthritis. J Chin Orthop Trauma.
31:40–44. 2019.
|
|
12
|
Li M, Gao P and Zhang J: Crosstalk between
autophagy and apoptosis: Potential and emerging therapeutic targets
for cardiac diseases. Int J Mol Sci. 17:3322016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Heras-Sandoval D, Pérez-Rojas JM,
Hernández-Damián J and Pedraza-Chaverri J: The role of
PI3K/AKT/mTOR pathway in the modulation of autophagy and the
clearance of protein aggregates in neurodegeneration. Cell Signal.
26:2694–2701. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Motoi Y, Shimada K, Ishiguro K and Hattori
N: Lithium and autophagy. ACS Chem Neurosci. 5:434–442. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kazemi H, Noori-Zadeh A, Darabi S and
Rajaei F: Lithium prevents cell apoptosis through autophagy
induction. Bratisl Lek Listy. 119:234–239. 2018.PubMed/NCBI
|
|
16
|
Dossymbekova R, Bgatova N, Tungushbayeva
Z, Sharipov K, Taneyeva G, Kydyrbaeva A and Solovieva A: Effect of
lithium carbonate on autophagy and proliferative activity of
isolated hepatocytes. Biochem Biophys Res Commun. 528:343–346.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li Q, Li H, Roughton K, Wang X, Kroemer G,
Blomgren K and Zhu C: Lithium reduces apoptosis and autophagy after
neonatal hypoxia-ischemia. Cell Death Dis. 1:e562010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang X, Heng X, Li T, Li L, Yang D, Zhang
X, Du Y, Doody RS and Le W: Long-term treatment with lithium
alleviates memory deficits and reduces amyloid-β production in an
aged Alzheimer's disease transgenic mouse model. J Alzheimers Dis.
24:739–749. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Baron J, Klein KO, Colli MJ, Yanovski JA,
Novosad JA, Bacher JD and Cutler GB Jr: Catch-up growth after
glucocorticoid excess: A mechanism intrinsic to the growth plate.
Endocrinology. 135:1367–1371. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kember NF and Walker KV: Control of bone
growth in rats. Nature. 229:428–429. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zaman F, Chrysis D, Huntjens K, Chagin A,
Takigawa M, Fadeel B and Sävendahl L: Dexamethasone differentially
regulates Bcl-2 family proteins in human proliferative
chondrocytes: Role of pro-apoptotic Bid. Toxicol Lett. 224:196–200.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hong D, Chen HX, Yu HQ, Wang C, Deng HT,
Lian QQ and Ge RS: Quantitative proteomic analysis of
dexamethasone-induced effects on osteoblast differentiation,
proliferation, and apoptosis in MC3T3-E1 cells using SILAC.
Osteoporos Int. 22:2175–2186. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Aigner T, Hemmel M, Neureiter D, Gebhard
PM, Zeiler G, Kirchner T and McKenna L: Apoptotic cell death is not
a widespread phenomenon in normal aging and osteoarthritis human
articular knee cartilage: A study of proliferation, programmed cell
death (apoptosis), and viability of chondrocytes in normal and
osteoarthritic human knee cartilage. Arthritis Rheum. 44:1304–1312.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Altman A, Hochberg Z and Silbermann M:
Interactions between growth hormone and dexamethasone in skeletal
growth and bone structure of the young mouse. Calcif Tissue Int.
51:298–304. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang QR, Yang ZY, Zhang WL, Li QH and Kang
PD: Abnormal hyperplasia of chondrocytes in a rat model of
glucocorticoid-induced osteonecrosis of the femoral head. Eur Rev
Med Pharmacol Sci. 26:6536–6549. 2022.PubMed/NCBI
|
|
27
|
Duan R, Xie H and Liu ZZ: The role of
autophagy in osteoarthritis. Front Cell Dev Biol. 8:6083882020.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xue JF, Shi ZM, Zou J and Li XL:
Inhibition of PI3K/AKT/mTOR signaling pathway promotes autophagy of
articular chondrocytes and attenuates inflammatory response in rats
with osteoarthritis. Biomed Pharmacother. 89:1252–1261. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen X, Gong W, Shao X, Shi T, Zhang L,
Dong J, Shi Y, Shen S, Qin J, Jiang Q and Guo B: METTL3-mediated
m6A modification of ATG7 regulates autophagy-GATA4 axis to promote
cellular senescence and osteoarthritis progression. Ann Rheum Dis.
81:87–99. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Huang L, Yin X, Chen J, Liu R, Xiao X, Hu
Z, He Y and Zou S: Lithium chloride promotes osteogenesis and
suppresses apoptosis during orthodontic tooth movement in
osteoporotic model via regulating autophagy. Bioact Mater.
6:3074–3084. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen L, Deng C, Li J, Yao Q, Chang J, Wang
L and Wu C: 3D printing of a lithium-calcium-silicate crystal
bioscaffold with dual bioactivities for osteochondral interface
reconstruction. Biomaterials. 196:138–150. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu L, Yu F, Chen L, Xia L, Wu C and Fang
B: Lithium-containing biomaterials stimulate cartilage repair
through bone marrow stromal cells-derived exosomal miR-455-3p and
histone H3 acetylation. Adv Healthc Mater. 12:e22023902023.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sun K, Luo J, Guo J, Yao X, Jing X and Guo
F: The PI3K/AKT/mTOR signaling pathway in osteoarthritis: A
narrative review. Osteoarthritis Cartilage. 28:400–409. 2020.
View Article : Google Scholar : PubMed/NCBI
|