Introduction
Endometriosis is a serious gynecological pathology
characterized by the ectopic implantation of endometrial stroma and
epithelium outside the uterus (1).
This disease affects ~10% of women of reproductive age and leads to
numerous consequences. Despite its prevalence, patients often
experience severe dysmenorrhea (painful menstruation), chronic
pelvic pain, and infertility, and current therapeutic regimens
frequently fail to alleviate these symptoms (2,3).
Furthermore, its exact pathophysiology remains unknown.
Endometriosis is highly dependent on angiogenesis,
which involves the formation of new blood vessels from pre-existing
ones (4,5). In women with endometriosis, the
potent pro-angiogenic stimulating factor vascular endothelial
growth factor (VEGF)-A is elevated in peritoneal fluids and
endometriosis lesions (4). In a
model of ectopic endometriosis established by the transplantation
of endometrial fragments from donor mice into the peritoneal wall
of host mice, the implant area exhibited enhanced angiogenesis, as
evidenced by the upregulation of pro-angiogenic factors, including
VEGF-A, and endothelial cell markers, including cluster of
differentiation 31 (CD31) and VEGF receptor 2 (VEGFR2), in the
implant (6). Inhibition of VEGF-A
suppressed the growth of endometriosis and vascular density in
endometrial implants in experimental models (5,7).
Recent studies have revealed that lymphangiogenesis,
the formation of new lymphatic vessels, is enhanced in
endometriotic lesions (8,9). In humans, endometriosis is associated
with an increased expression of pro-lymphangiogenic stimulating
factors and lymphatic endothelial cell markers (4). In an ectopic endometrial
transplantation model, implant growth and lymphangiogenesis were
associated with increased mRNA expression of the two
pro-lymphangiogenic factors VEGF-C and VEGF-D
(8,9). VEGFR3, a receptor for VEGF-C and
VEGF-D, kinase inhibitor reduced implant growth and
lymphangiogenesis, implying that lymphangiogenesis is involved in
endometriosis progression (8).
However, the mechanisms underlying angiogenesis and
lymphangiogenesis in endometriosis remain unclear.
Prostanoids, including prostaglandin (PG) and
thromboxane (TX), are arachidonic acid metabolites. PGs have been
linked to the pathogenesis of endometriosis (7,10),
particularly via the cyclooxygenase-2 (COX-2)/PGE2
pathway. COX-2 participates in the onset and progression of
endometriosis (11), and enhanced
expression of COX-2 has been observed in endometriotic lesions
(12). In addition, VEGF-A induced
by microsomal PGE synthase-1 (mPGES-1), an essential enzyme in
PGE2 synthesis, promoted endometriosis and angiogenesis
of endometriotic lesions in mice (7). In terms of PGE2-mediated
lymphangiogenesis, COX-2-derived PGE2 was found to
enhance lymphangiogenesis through upregulation of
pro-lymphangiogenic stimulating factors including VEGF-C and
VEGF-D, in chronic inflammation (13,14),
and during the healing process of gastric ulcers (15) and wounds (16).
In addition to PGE2, TXA2 is
another representative prostanoid produced by COX and TX synthase
(TXS), which exerts its activity via the TXA2 receptor,
also known as the thromboxane prostanoid (TP) receptor (17). TP plays a key role in the
aggregation of platelets and in the constriction of vascular smooth
muscle cells, thus contributing to cardiovascular and
cerebrovascular diseases, particularly ischemic stroke, which is
associated with high expression of TXA2 (18). In mice, TP signaling facilitated
blood flow recovery from hind limb ischemia via angiogenesis in
ischemic tissues (19).
Furthermore, TP signaling in macrophages promoted lymphangiogenesis
in the diaphragm during endotoxin-induced peritonitis (20). The above findings imply that TP
signaling is involved in angiogenesis and lymphangiogenesis in
endometriotic lesions and promotes endometriotic growth. Thus, the
present study aimed to examine the effect of inhibition of TP
signaling on the development of endometriosis and the formation of
new blood and lymphatic vessels.
Materials and methods
Animals
Female TP-deficient (TP−/−) mice (8 weeks
old) were generated as previously described (21). Female C57BL/6 WT mice (8 weeks old)
were obtained from CLEA Japan, Inc. All experimental studies were
approved by the Dean of Kitasato University School of Medicine
after review by the Institutional Animal Care and Use Committee
(approval no. 2022-060). The experiments were performed in
accordance with the guidelines of the Science Council of Japan for
animal experiments. In the present study, 75 mice were subjected to
endometrial transplantation (n=39), pharmacological intervention
(n=12), and cell culture (n=24).
Experimental model of
endometriosis
An endometrial transplantation model was established
as previously described (9).
Briefly, mice were anesthetized by intraperitoneal (i.p.) injection
of mixed anesthetic agents containing 4.0 mg/kg of midazolam (cat.
no. 614243022; Sandoz; Novartis), 0.75 mg/kg medetomidine
hydrochloride (cat. no. 14111; Nippon Zenyaku Kogyo), and 5.0 mg/kg
butorphanol (cat. no. 219711; Meiji Seika Pharma). The anesthetic
mixture of medetomidine, midazolam, and butorphanol has been
recently used as a substitute for ketamine or pentobarbital sodium
(22). The combination of
anesthetics was approved by the Dean of Kitasato University School
of Medicine after the review by the Institutional Animal Care and
Use Committee. To rule out endogenous estrogen effects and
menstrual cycle influences in mice, the bilateral ovaries were
removed through paravertebral incisions. The effects of
medetomidine were reversed by an i.p. injection of 0.75 mg/kg
atipamezole (Nippon Zenyaku Kogyo). All donor and recipient mice
were subcutaneously administered estradiol valerate (100 mg/kg)
(Pelanin Depot; cat. no. 4987224136400; Mochida Seiyaku) weekly
from the day of ovariectomy (6).
Uterine tissues from donor mice were harvested 7 days after
ovariectomy. A circular uterine fragment with a diameter of 3 mm
was implanted into each side of the peritoneal wall of recipient
mice and secured with 7-0 polypropylene sutures (cat. no. C0024501;
B-Brown Ace Scrap, Inc.). Recipient WT or TP−/− mice
received an implant from donor WT or TP−/− mice
(Henceforth referred to as WT−/−→WT−/− and
TP−/−→TP−/−). The day of implantation was
defined as day 0. Six animals received daily oral administration of
ozagrel (30 mg/kg; cat. no. 87449; Kissei Pharmaceutical Co.,
Ltd.), a selective TXS inhibitor (20). Humane endpoints during the
experiment were defined as food-water intake difficulties, weight
loss ≥20% of the body weight, and reduced activities and
movement.
On day 14 after endometrial transplantation, the
animals were euthanized using 5% isoflurane (cat. no.
4987114133403; Pfizer) for 5 min followed by cervical dislocation,
and the death was confirmed by a lack of heartbeat and respiration.
Then, each side of the implanted tissue was excised. Day 0 samples
were collected immediately after implantation. The excised implants
were digitally photographed, and the implant area (mm2)
was determined using ImageJ version 1.53e (National Institutes of
Health). The results are presented as the implant area per
mm2. One implant sample was prepared for reverse
transcription-quantitative PCR (RT-qPCR), and the other for
histological analysis.
Immunofluorescence analysis
Fixed endometrial samples were embedded in
Tissue-Tek O.C.T. Compound (Sakura Finetek USA, Inc.), frozen at
−80°C, and cut into 8-µm thick sections. The sections were
incubated overnight at 4°C with one of the following primary
antibodies: Rabbit anti-mouse TP (1:100; cat. no. APR-069; Almone
Labs, Jerusalem, Israel), rabbit anti-mouse TXS (1:100; cat. no.
bs-4019R; Bioss Antibodies Inc, Woburn, MA, USA), rat anti-mouse
CD31 monoclonal antibody (1:200; cat. no. 553370; BD Biosciences),
rabbit anti-mouse lymphatic vessel endothelial hyaluronan receptor
1 (LYVE-1) (1:100; cat. no. ab14917; Abcam), goat anti-mouse LYVE-1
(1:100; cat. no. AF2125; R&D Systems Inc.), goat anti-mouse
VEGF-A (1:100; cat. no. AF-493-NA; R&D Systems Inc.), rabbit
anti-VEGF-C (1:100; cat. no. ab9546; Abcam), goat anti-VEGF-D
(1:50; cat. no. sc6313; Santa Cruz Biotechnology, Inc.), rabbit
anti-mouse CD41, a marker for platelets (1:100; cat. no. MCA2245GA;
Bio-Rad Laboratories, Inc.), and rat anti-mouse F4/80, a marker for
macrophages (1:100; cat. no. MCA497G; Bio-Rad Laboratories, Inc.).
The sections were incubated for 1 h at room temperature with one of
the following species-appropriate secondary antibodies: Alexa Fluor
488-conjugated donkey anti-rabbit IgG (cat. no. A21206), Alexa
Fluor 488-conjugated donkey anti-goat IgG (cat. no. A11055), Alexa
Fluor 594-conjugated donkey anti-rat IgG (cat. no. A21209) or Alexa
Fluor 594-conjugated donkey anti-goat IgG (cat. no. A11058) (1:200;
Molecular Probes; Thermo Fisher Scientific, Inc.). Images of
stained sections were captured using a fluorescence microscope
(Biozero BZ-700; Keyence Corporation). The number of
F4/80+ cells was counted in five fields of view (×400
magnification) of the endometrial tissue.
Determination of vessel density
Microvessel density (MVD) and lymphatic vessel
density (LVD) within endometrial tissue implants have been used to
assess angiogenesis and lymphangiogenesis, respectively (9). The number of CD31+ blood
vessels and LYVE-1+ lymphatic vessels in four fields of
view were counted using a fluorescence microscope (Biozero BZ-700;
Keyence Corporation) (×200 magnification). The results are
presented as the number of CD31+ blood vessels or
LYVE-1+ lymphatic vessels per square millimeter
(MVD/mm2 or LVD/mm2). In addition, the area
covered by blood and lymphatic vessels was determined using ImageJ
and presented as the percentage of the total area observed
[microvessel area (MVA)% or lymphatic vessel area (LVA)%].
RT-qPCR analysis
Total RNA was extracted from endometriotic tissues
and homogenized in TRIzol® reagent (cat. no. 15596018;
Thermo Fisher Scientific, Inc.). Total RNA (1 µg) was transcribed
into single-stranded cDNA using ReverTra Ace qPCR RT Kit (cat. no.
FSQ-201; TOYOBO Co., Ltd.) according to the manufacturer's
protocol. qPCR was performed using TB Green Premix Ex Taq II (Tli
RNase H Plus; cat. no. RR820A; Takara Bio, Inc.). PCR amplification
was performed under the following thermocycling conditions:
Pre-denaturation at 95°C for 10 sec, followed by a two-step PCR
program consisting of 40 cycles at 95°C for 3 sec and 60°C for 20
sec. mRNA expression levels were calculated using the comparative
threshold cycle method (2-∆∆Cq) (23) and normalized to GAPDH expression in
each sample. The sequences of the primers used are listed in
Table I.
 | Table I.Sequences of the primers used for
PCR. |
Table I.
Sequences of the primers used for
PCR.
| Gene | Forward primer
sequence (5′-3′) | Reverse primer
sequence (5′-3′) |
|---|
| TP |
CCTCCTGCTCAACACCGTTAG |
CTGAACCATCATCTCCACCTC |
| TXS |
GGATTCTGCCCAATAAGAACC |
GAAGTCTCTCCGCCTCTCTTC |
| VEGF-A |
ACGACAGAAGGAGAGCAGAAG |
ATGTCCACCAGGGTCTCAATC |
| VEGF-C |
TCTGTGTCCAGCGTAGATGAG |
GTCCCCTGTCCTGGTATTGAG |
| VEGF-D |
CCTATTGACATGCTGTGGGAT |
GTGGGTTCCTGGAGGTAAGAG |
| CD31 |
CAGAGCCAGCAGTATGAGGAC |
GCAACTATTAAGGTGGCGATG |
| VEGFR2 |
CTGCCTACCTCACCTGTTTCC |
CGGCTCTTTCGCTTACTGTTC |
| VEGFR3 |
GGAAGGCTCTGAAGATAAAGG |
ACAGAAGATGAGCAGGAGGAG |
| LYVE-1 |
GCTCTCCTCTTCTTTGGTGCT |
TGACGTCATCAGCCTTCTCTT |
| Prox1 |
GTTCTTTTACACCCGCTACCC |
ACTCACGGAAATTGCTGAACC |
| CCL2 |
CGGAACCAAATGAGATCAGAA |
TTGTGGAAAAGGTAGTGGATG |
| CCR2 |
TTACCTCAGTTCATCCACGGC |
CAAGGCTCACCATCATCGTAG |
| TNFα |
TCTTCTCATTCCTGCTTGTGG |
GATCTGAGTGTGAGGGTCTGG |
| IL-1β |
TACATCAGCACCTCACAAGCA |
CCAGCCCATACTTTAGGAAGA |
| IL-6 |
CAAAGCCAGAGTCCTTCAGAG |
TAGGAGAGCATTGGAAATTGG |
| MR |
TTTGTCCATTGCACTTTGAGG |
TGCCAGGTTAAAGCAGACTTG |
| Fizz1 |
CAAGGAACTTCTTGCCAATCCAG |
CCAAGATCCACAGGCAAAGCCA |
| IL-10 |
CGGAAATGATCCAGTTTTACC |
TGAGGGTCTTCAGCTTCTCAC |
| GAPDH |
ACATCAAGAAGGTGGTGAAGC |
AAGGTGGAAGAGTGGGAGTTG |
Cell preparation and culture
Bone marrow (BM) cells were isolated from the femur
and tibia of 8-week-old female WT and TP−/− mice. BM
cells were cultured in 6-well plates (1.0×106
cells/well) and maintained in RPMI-1640 medium (cat. no. 11875-093;
Gibco; Thermo Scientific) supplemented with 10% fetal calf serum
and 20 ng/ml macrophage colony-stimulating factor (cat. no. 576406;
BioLegend, Inc.), as previously described (9). On day 7, the cells stimulated with
the TP receptor agonist U46619 (100 nM; catalog no. 16450; Cayman
Chemical) were either left untreated or treated with
lipopolysaccharide (10 ng/ml; cat. no. L3012; MilliporeSigma) in
RPMI 1640 medium for 3 h. Cultured BM-derived macrophages were
harvested and homogenized in TRIzol® reagent (cat. no.
15596018; Thermo Fisher Scientific, Inc.), and mRNA levels were
determined using RT-qPCR.
Statistical analysis
All results are presented as the mean and SD. All
statistical analyses were performed using GraphPad Prism version 9
(GraphPad Software, Inc.). Data between two groups were compared
using an unpaired two-tailed Student's t-test, whereas comparisons
between multiple groups were conducted using a one-way ANOVA
followed by a Tukey's post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Inhibition of TP signaling in the host
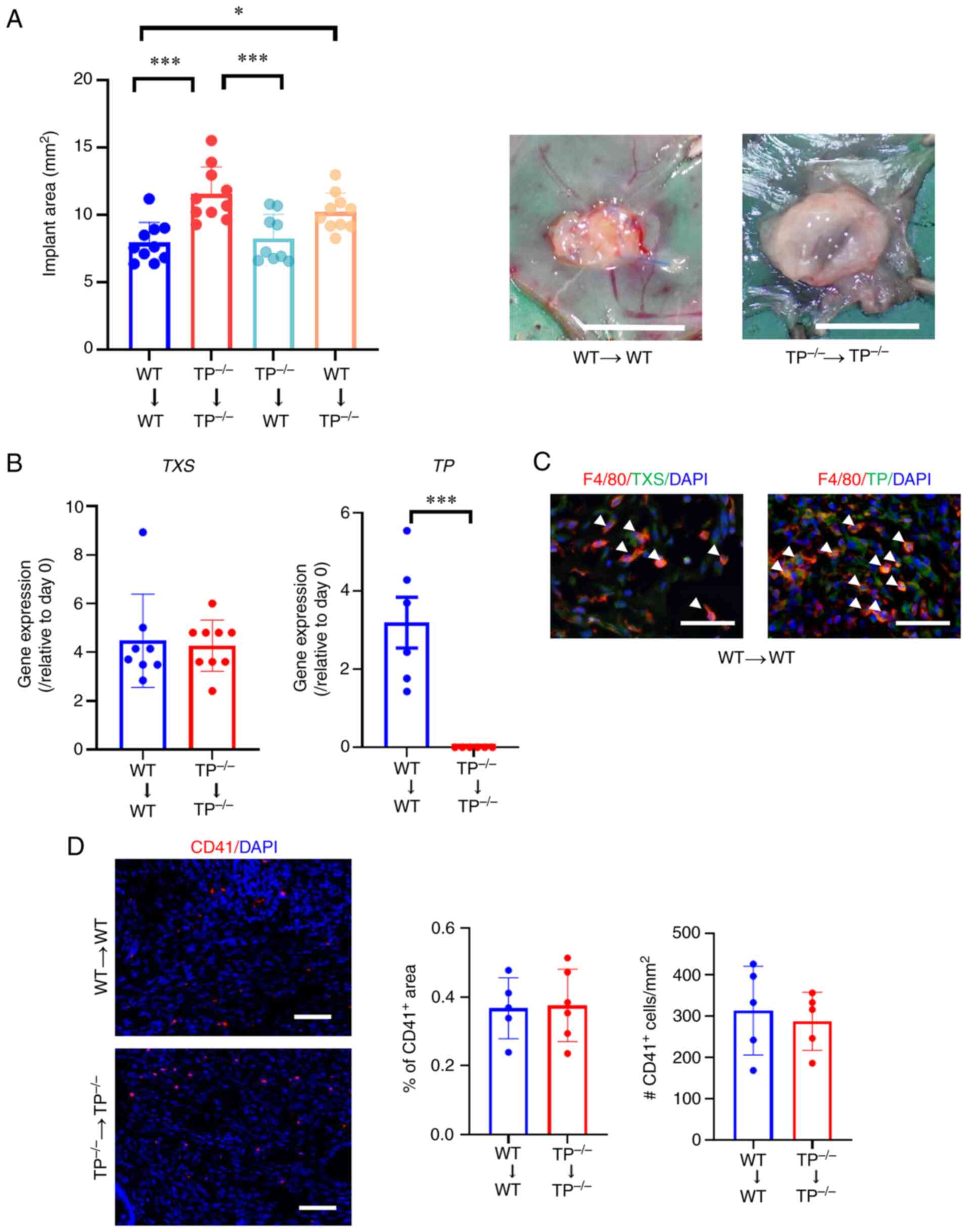
enhances the development of endometriosis lesions
To investigate the involvement of TP signaling in
implant growth, WT or TP−/− endometrial tissues were
implanted into either WT or TP−/− recipient mice
(Fig. 1). As previous data have
shown that the area of endometrial implants reached a maximum on
day 14 (6,9), the endometrial implant area was
assessed on day 14. When TP−/− endometrial fragments
were implanted into TP−/− mice
(TP−/−→TP−/− mice), implant growth was
greater than that in WT→WT mice (Fig.
1A). Similarly, implant growth was enhanced in the
WT→TP−/− mice; however, no statistically significant
difference in implant growth was observed between
TP−/−→WT and WT→WT mice. These findings suggested that
TP signaling acquired from recipients suppressed the development of
transplanted endometrial fragments. Accordingly, the growth of
implants as well as blood and lymphatic vessels in WT→WT and
TP−/−→TP−/− mice was assessed in the
subsequent experiments.
On day 14, the mRNA expression levels of TXS and TP
in WT→WT mouse implants increased. However, TXS mRNA expression
levels in TP−/−→TP−/− mouse implants did not
differ from those in the WT→WT mouse implants. In addition, TP mRNA
expression levels were reduced in TP−/−→TP−/−
mouse implants (Fig. 1B). On day
14, double immunofluorescence analysis demonstrated that TXS and TP
expression in endometrial implants from WT→WT mice co-localized
with those of F4/80 in stromal tissues, indicating macrophages as a
source of TXS and TP in the implants (Fig. 1C).
Since TP is expressed in platelets, platelets were
also assessed in endometrial tissues. Immunofluorescence analysis
revealed that the distribution of CD41, a platelet marker, did not
differ between WT→WT and TP−/−→TP−/− mice
(Fig. 1D).
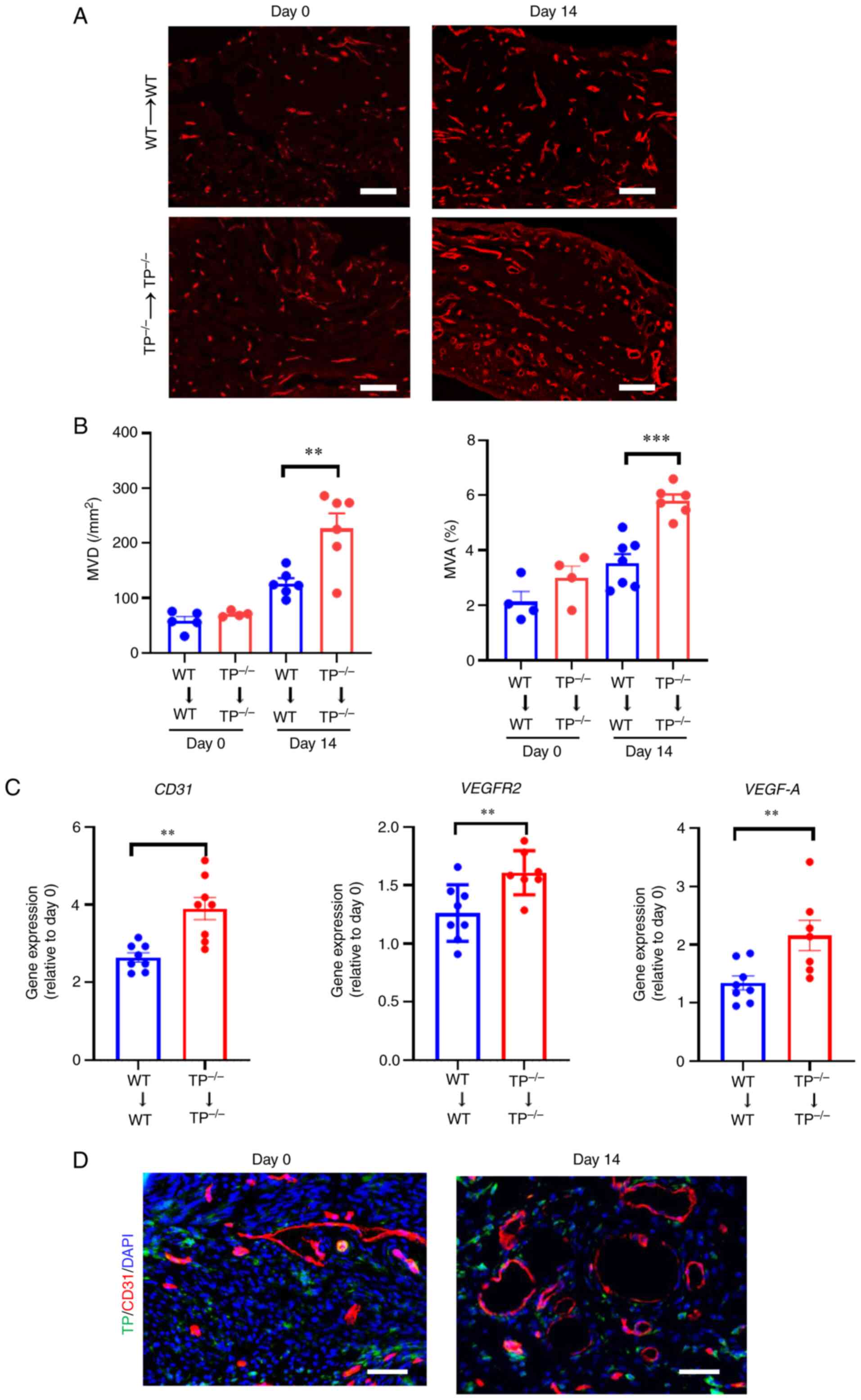
Angiogenesis in endometrial implants
is enhanced in TP−/−→TP−/− mice
As endometrial implant growth is attributed to
angiogenesis (6,9), the involvement of TP signaling in
angiogenesis was investigated by counting the number of
CD31+ blood vessels in the WT→WT and
TP−/−→TP−/− mouse implants. Fig. 2A shows that the
TP−/−→TP−/− mouse implants displayed a
greater number of newly formed CD31+ blood vessels
compared with WT→WT mouse implants. The MVD and MVA% in the
implants of TP−/−→TP−/− mice were greater
than those in the WT→WT mouse implants (Fig. 2B). Additionally, mRNA expression
levels of CD31, VEGFR2, and pro-angiogenic factor VEGF-A were
higher in the TP−/−→TP−/− mouse implants than
those in the WT→WT mouse implants (Fig. 2C). Dual immunofluorescence staining
demonstrated a lack of co-localization of TP with CD31 in implant
tissues (Fig. 2D), suggesting that
TP was not expressed in newly formed blood vessels. These findings
suggest that inhibition of TP signaling does not directly
facilitate angiogenesis in endometrial implants.
Lymphangiogenesis in endometrial
implants is enhanced in TP−/−→TP−/− mice
The role of TP signaling in lymphangiogenesis was
investigated by determining LVD and LVA% (Fig. 3A and B). LVD and LVA% in
TP−/−→TP−/− mouse implants were higher than
those in WT→WT mouse implants. The mRNA expression levels of
lymphatic endothelial cell markers, including LYVE-1, VEGFR3, and
Prospero-related homeobox 1 (Prox1), a marker for lymphangiogenesis
(24), in
TP−/−→TP−/− mouse implants were higher than
those in WT→WT mouse implants (Fig.
3C). The mRNA expression levels of VEGF-C and VEGF-D were also
higher in TP−/−→TP−/− mouse implants. These
results indicate that inhibition of TP signaling stimulates
lymphangiogenesis through upregulation of VEGF-C and VEGF-D.
Accordingly, the localization of TP in lymphatic endothelial cells
(ECs) was investigated (Fig. 3D).
Dual immunofluorescence staining for TP and LYVE-1 revealed the
absence of colocalization, suggesting that TP was not expressed in
newly formed lymphatic vessels. Therefore, these results suggested
that inhibition of TP signaling did not directly facilitate
lymphangiogenesis in endometrial implants.
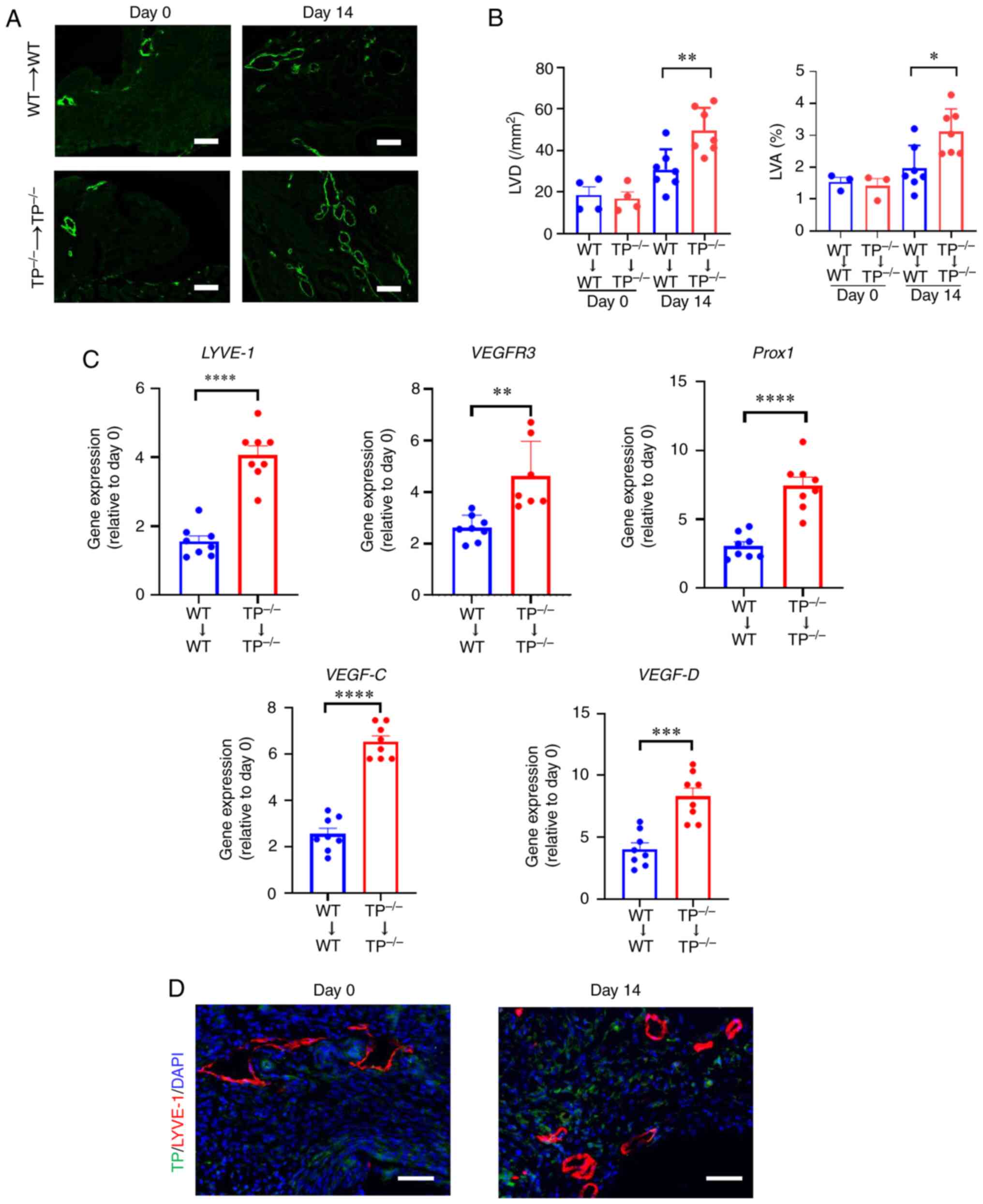 | Figure 3.TP signaling inhibition promotes
lymphangiogenesis in endometrial implants. (A) LYVE-1
immunofluorescence in WT→WT and TP−/−→TP−/−
mouse implants on day 14 Scale bar, 100 µm. (B) LVD and LVA in
WT→WT and TP−/−→TP−/− mouse implants on days
0 and 14. (C) mRNA expression levels of LYVE-1, VEGFR3, Prox1,
VEGF-C, and VEGF-D in WT→WT and TP−/−→TP−/−
mouse implants on day 14. (D) Double immunostaining of TP (green)
and LYVE-1 (red) in WT→WT mouse implants on day 14. Scale bar, 50
µm. Data are presented as the mean ± SD. *P<0.05, **P<0.01,
***P<0.001, ****P<0.0001. TP, thromboxane prostanoid; LYVE-1,
lymphatic vessel endothelial hyaluronan receptor 1; LVD, lymphatic
vessel density; LVA, lymphatic vessel area; VEGFR, vascular
endothelial growth factor receptor; Prox1, prospero-related
homeobox 1. |
TXS inhibition promotes implant growth
and the formation of new blood and lymphatic vessels
To confirm the role of TP signaling in
endometriosis, the effects of ozagrel, a TXS inhibitor, on implant
growth, angiogenesis, and lymphangiogenesis were investigated
(Fig. 4). Ozagrel increased the
implant area in WT→WT mice (Fig.
4A), enhanced angiogenesis and lymphangiogenesis in the
implant, as evidenced by the increased MVD and MVA% (Fig. 4B), and increased LVD and LVA%,
respectively (Fig. 4C).
Furthermore, it enhanced the mRNA expression levels of
pro-angiogenesis-related genes, including CD31, VEGFR2, and VEGF-A
(Fig. 4B), and
pro-lymphangiogenesis-related genes, including LYVE-1, VEGFR3,
Prox1, VEGF-C, and VEGF-D in the implants (Fig. 4C).
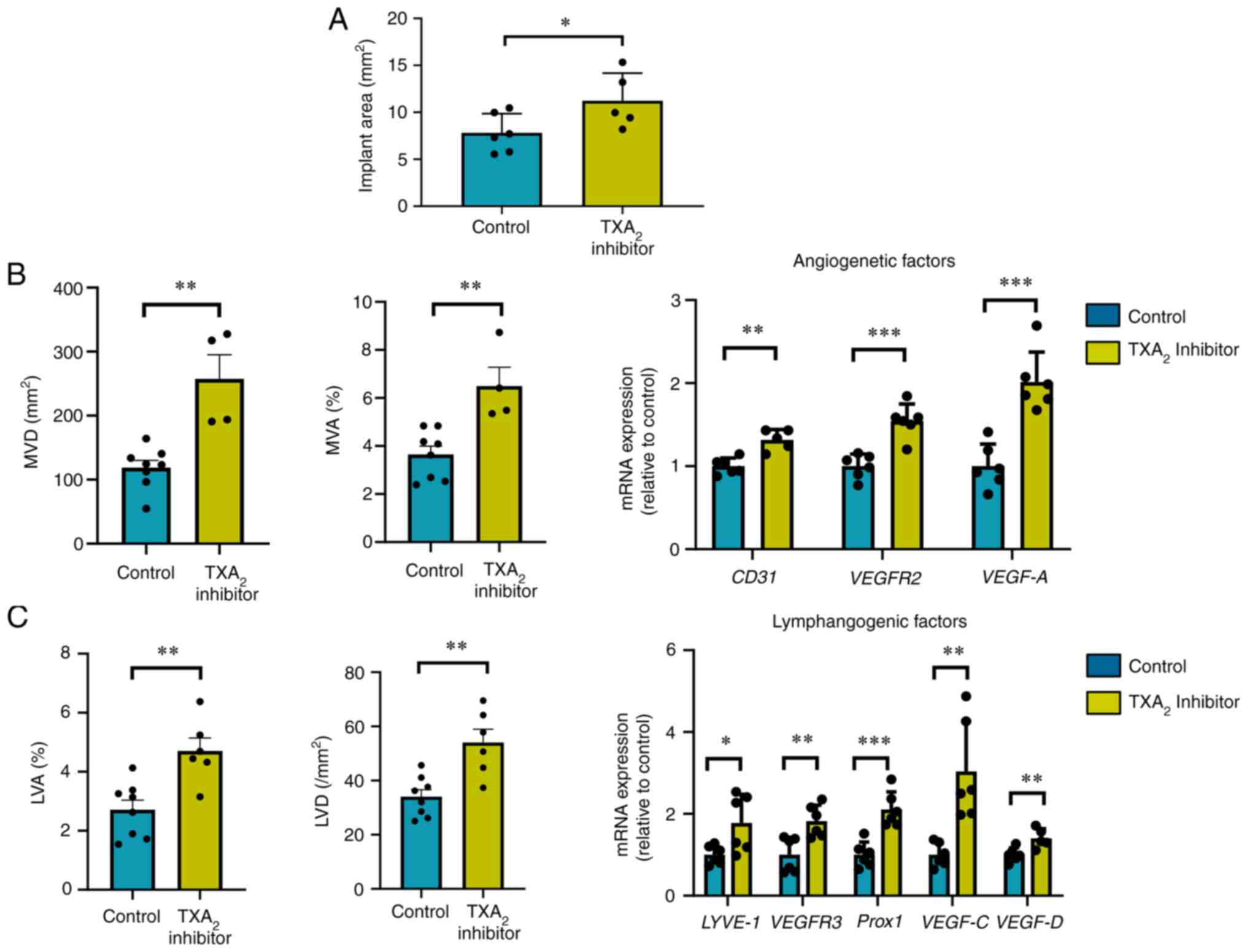 | Figure 4.Effects of TXA2 synthase
inhibitor on endometrial tissue implant growth, angiogenesis, and
lymphangiogenesis. (A) Implant area in TXA2 inhibitor-
or control-treated WT→WT mice. (B) MVD, MVA, and angiogenic factors
in TXA2 inhibitor- or control-treated WT→WT mouse
implants. mRNA expression levels of pro-angiogenic genes CD31,
VEGFR2, and VEGF-A. (C) LVD, LVA, and lymphangiogenic factors in
TXA2 inhibitor- or control-treated WT→WT mouse implants.
mRNA expression levels of pro-lymphangiogenic genes LYVE-1, VEGFR3,
Prox1, VEGF-C, and VEGF-D. Data are presented as the mean ± SD.
*P<0.05, **P<0.01, ***P<0.001. TXS, thromboxane synthase;
MVD, microvessel density; MVA, microvessel area; VEGFR, vascular
endothelial growth factor receptor; LVD, lymphatic vessel density;
LVA, lymphatic vessel area; LYVE-1, lymphatic vessel endothelial
hyaluronan receptor 1; Prox1, prospero-related homeobox 1. |
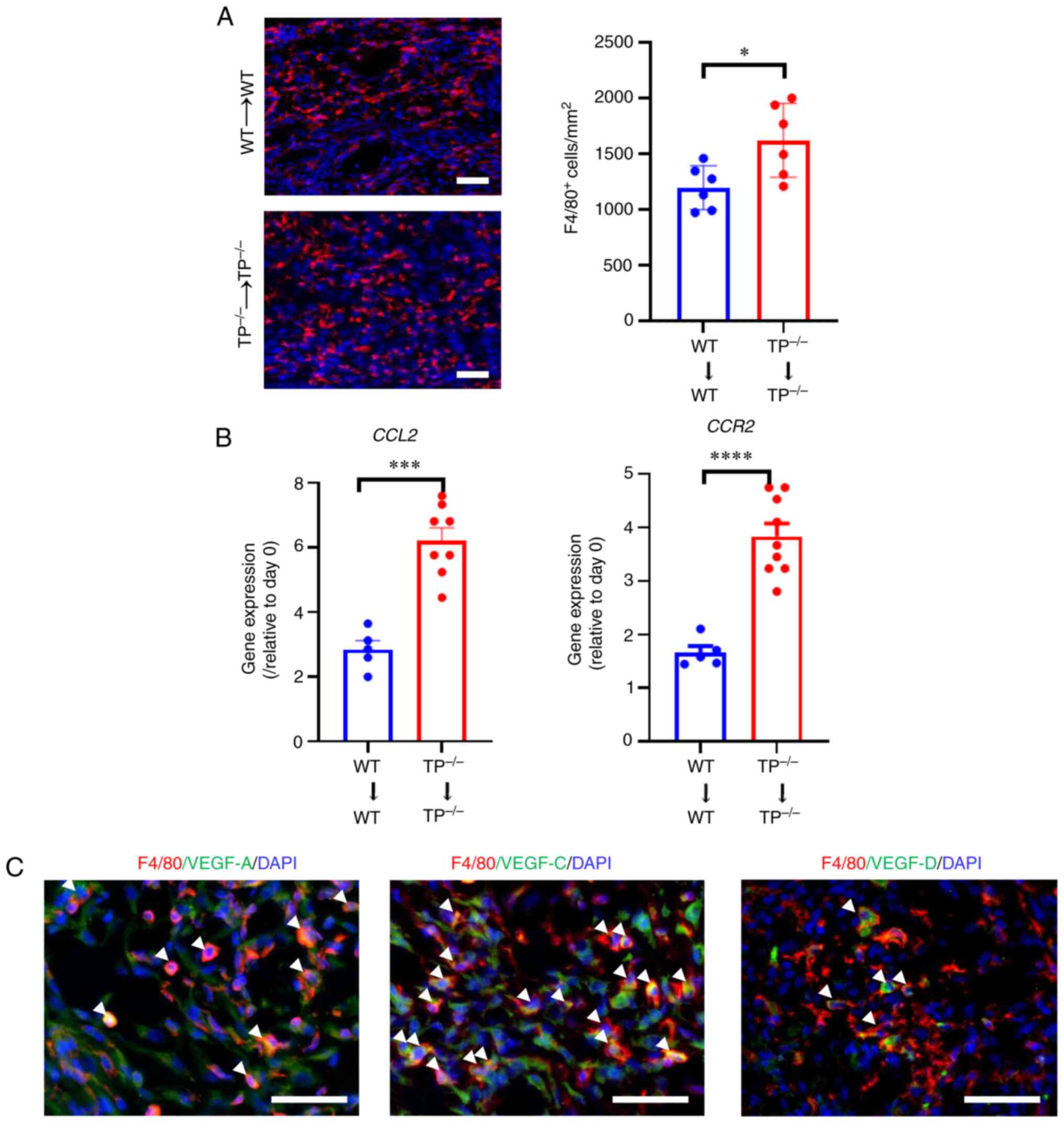
Macrophages recruited in implants
express pro-angiogenic or lymphangiogenic cytokines
Since macrophages contribute to angiogenesis and
lymphangiogenesis during endometriosis development in mice
(9), the number of
F4/80+ cells within endometrial implants was counted
(Fig. 5A). The number of
F4/80+ cells was higher in
TP−/−→TP−/− mouse implants than that in WT→WT
mouse implants, indicating that F4/80+ cell accumulation
is linked to angiogenesis and lymphangiogenesis in the implants. In
addition, enhanced F4/80+ cell accumulation in implants
was associated with upregulation of the macrophage chemoattractant
C-C motif chemokine 2 (CCL2) and its receptor C-C motif
chemokine 2 (CCR2) (Fig.
5B).
Macrophages play a pivotal role in synthesizing the
VEGF protein family, which is responsible for angiogenesis (VEGF-A)
and lymphangiogenesis (VEGF-C and VEGF-D). Accordingly, whether
macrophages recruited in implants expressed VEGF-A, VEGF-C, or
VEGF-D was determined (Fig. 5C).
Double immunofluorescence analyses demonstrated that F4/80+ cells
co-localized with VEGF-A as well as VEGF-C/D expression in WT→WT
mouse implants on day 14 (Fig.
5C). These findings suggest that macrophages participate in
angiogenesis and lymphangiogenesis in endometrial implants through
upregulation of VEGF-A and VEGF-C/D, respectively.
The expression levels of the
anti-inflammatory factors are increased in TP-/-→TP-/- mouse
implants
The mRNA expression levels of tumor necrosis
factor-α (TNF-α), interleukin (IL)-1β, and IL-6 in pro-inflammatory
macrophages, and those of mannose receptor (MR), Fizz1, and IL-10
in anti-inflammatory macrophages were investigated (Fig. 6). The mRNA expression levels
related to pro-inflammatory macrophage phenotypes did not differ
substantially between the WT→WT and
TP−/−→TP−/− mice, whereas those related to
anti-inflammatory macrophage phenotypes were higher in
TP−/−→TP−/− mice than in WT→WT mice,
suggesting the role of anti-inflammatory macrophages in
endometriosis growth.
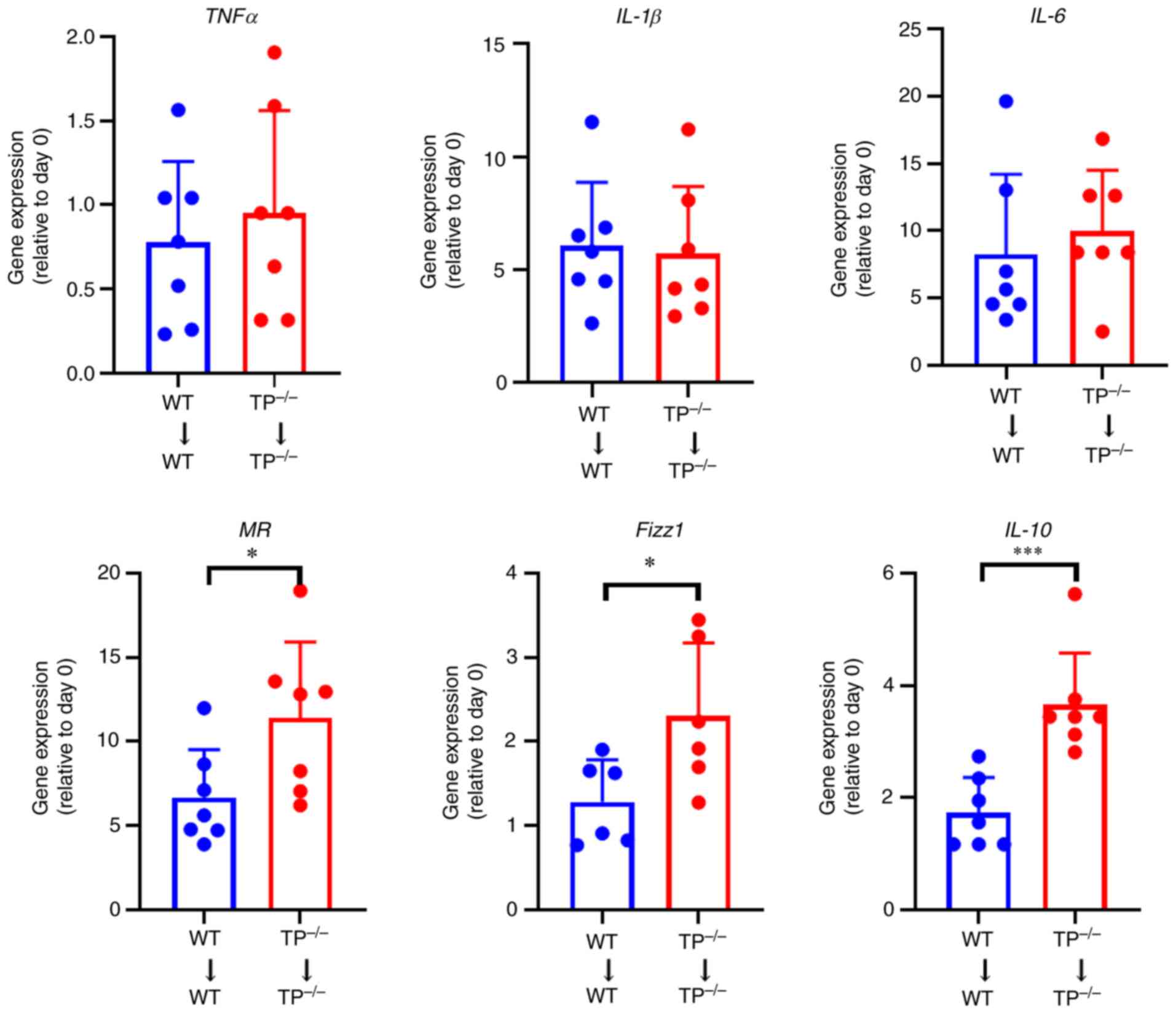 | Figure 6.The mRNA expression levels of
pro-inflammatory and anti-inflammatory macrophage phenotype-related
genes in endometrial implant tissues. mRNA expression levels of
pro-inflammatory macrophage phenotype-related genes, including
TNF-α, IL-1β, and IL-6, and anti-inflammatory macrophage
phenotype-related genes, including MR, Fizz1, and IL-10, in WT→WT
and TP−/−→TP−/− mouse implants on day 14.
Data are presented as the mean ± SD. *P<0.05, ***P<0.001. TP,
thromboxane prostanoid; TNF, tumor necrosis factor; IL,
interleukin; MR, mannose receptor. |
TP stimulation reduces the expression
of vascular growth factors and anti-inflammatory markers in
cultured macrophages
Finally, whether inhibition of TP signaling in
macrophages regulates pro-angiogenic or pro-lymphangiogenic factors
in vitro was investigated. When cultured BM-macrophages were
stimulated with U46619, a TXA2 analog, the mRNA
expression levels of VEGF-A, VEGF-C, and VEGF-D were downregulated
in BM-macrophages from WT mice, but were not altered in those from
TP−/− mice (Fig. 7A).
These findings indicated that VEGF-A, VEGF-C, and VEGF-D levels
were reduced in cultured macrophages via TP signaling.
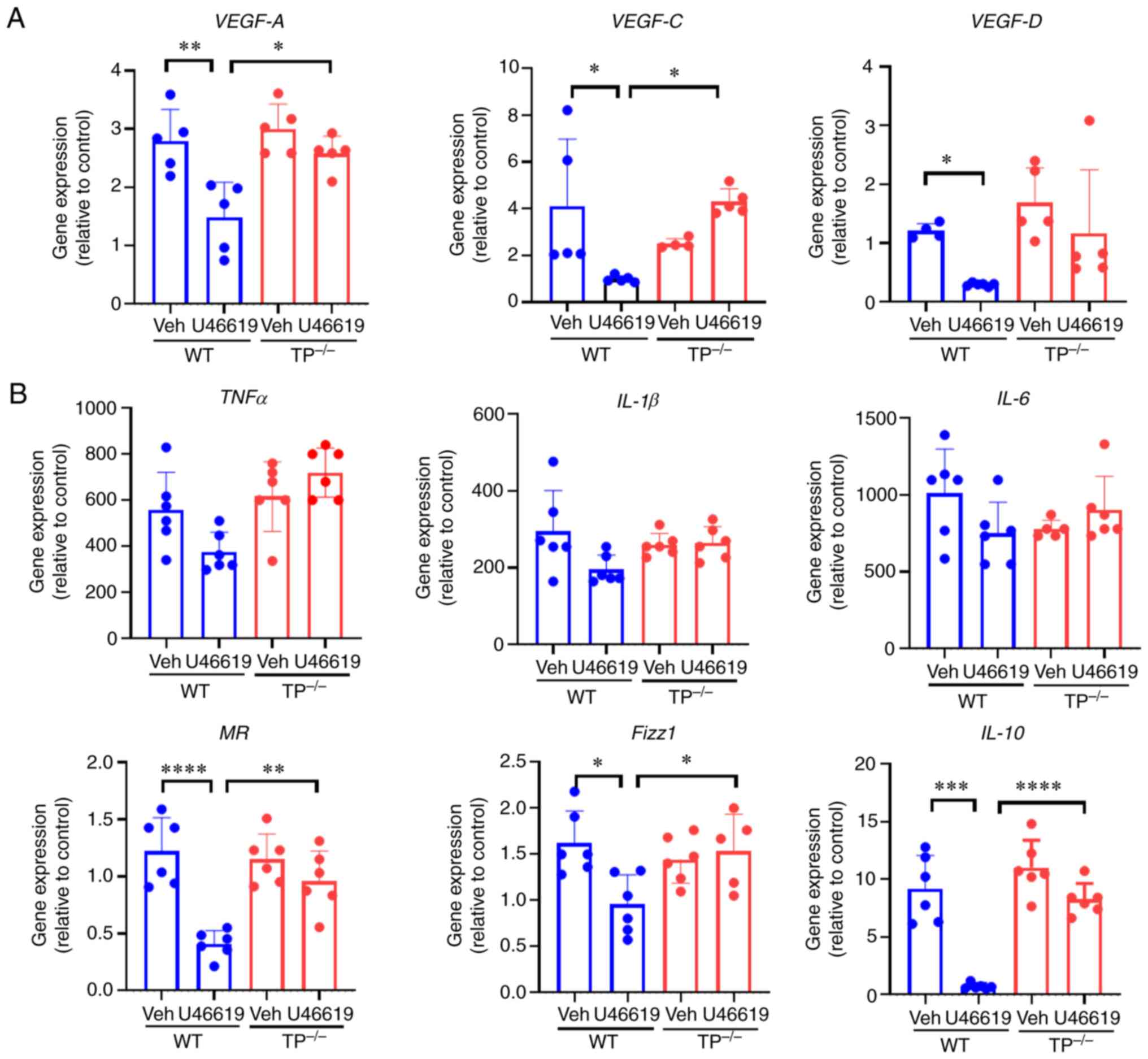 | Figure 7.Vascular growth factors and pro- and
anti-inflammatory macrophage phenotype-related genes in cultured
macrophages. (A) Expression levels of VEGF-A, VEGF-C, and VEGF-D in
cultured BM-derived macrophages stimulated with U46619 (100 nM).
(B) Effects of U46619 (100 nM) on the expression levels of
pro-inflammatory macrophage-related genes, including TNF-α, IL-1β,
and IL-6, and anti-inflammatory macrophage-related genes, including
mannose receptor (MR), Fizz1, and IL-10, in cultured BM-derived
macrophages from WT and TP−/− mice. Data are presented
as the mean ± SD. *P<0.05, **P<0.01, ***P<0.001,
****P<0.0001. TP, thromboxane prostanoid; VEGF, vascular
endothelial growth factor; TNF, tumor necrosis factor; IL,
interleukin; MR, mannose receptor. |
Additionally, the mRNA expression levels of pro- and
anti-inflammatory factors in macrophages were determined in
vitro. U46619 stimulation had no effect on the expression
levels of pro-inflammatory factors, including TNFα, IL-1β, and
IL-6, in WT and TP−/− mouse macrophages. In contrast,
U46619 reduced the expression levels of anti-inflammatory factors,
such as MR, Fizz1, and IL-10, in WT mouse macrophages, but had no
effect on TP−/− mouse macrophages (Fig. 7B).
Discussion
Endometriosis is associated with both angiogenesis
and lymphangiogenesis. Recent studies have demonstrated that TP
signaling enhances pro-angiogenic activities under pathological
conditions, including inflammation and tumor growth (19,25,26).
The present study was the first to demonstrate that TP signaling
facilitated lymphangiogenesis in inflamed diaphragms in mice
(20). Additionally, using an
endometrial transplantation model, the present study revealed that
inhibiting endogenous TP signaling contributed to the enhancement
of angiogenesis and lymphangiogenesis in endometrial lesions in
addition to endometriotic growth. TXS inhibitors increased
endometrial growth and formation of new blood and lymphatic
vessels. Anti-inflammatory macrophages lacking TP signaling in
endometrial lesions enhanced the levels of angiogenesis and
lymphangiogenesis-related cytokines. These findings suggest that
activation of TP signaling mitigates endometrial growth and
neovascularization.
Although the underlying mechanisms of endometriosis
are unknown, angiogenesis and lymphangiogenesis in endometrial
lesions contribute to its development. The pro-angiogenic factor
VEGF-A is upregulated in patients and mice with endometriosis.
Inhibition of VEGF-A and VEGFR1 suppresses endometriosis growth and
the formation of new blood vessels in endometrial lesions (6). In addition, the pro-lymphangiogenic
factors VEGF-C and VEGF-D were increased during the development of
endometriosis in humans and mice (9), and VEGFR3 inhibition suppressed
endometriosis growth and the formation of new lymphatic vessels in
endometrial lesions (8).
Endometriosis was associated with overexpression of pro-angiogenic
and pro-lymphangiogenic genes (27). Consistent with this, our findings
revealed that the promotion of angiogenesis and lymphangiogenesis
in endometrial tissue was associated with endometriotic growth.
Endometrial stromal cells have been implicated in
angiogenesis and lymphangiogenesis. Macrophage accumulation in
endometrial lesions was shown to be associated with
neovascularization (6,8,9).
BM-derived macrophages were found to be involved in endometriosis
and angiogenesis (6). By contrast,
large peritoneal macrophages were implicated in endometriosis
(28). The accumulation of
macrophages in endometrial tissues warrants further investigation.
Furthermore, the specific macrophage phenotype is critical for
endometriosis development (28).
The results of the present study suggested that the
anti-inflammatory macrophage phenotype (alternatively activated
macrophages) contributed to endometriosis progression. In a murine
endometriosis model, anti-inflammatory macrophages promoted the
growth of endometriotic lesions, whereas pro-inflammatory
macrophages protected against the disease (29). Endometriotic lesions were reduced
when the anti-inflammatory macrophage phenotype expressing CD206
was deleted (30). In addition,
the macrophage phenotype changes as endometriosis progresses.
Pro-inflammatory macrophages appeared in the peritoneal cavity
within the first 14 days after endometrial tissue implantation, and
the macrophage phenotype switched to the anti-inflammatory
phenotype 14 days after endometriosis induction (31). However, the oversimplification of
macrophage polarization makes it difficult to understand the
macrophage phenotype complex in tissues. Recent technological
advances in single-cell transcript analyses have revealed five
distinct subpopulations of macrophages in human endometrial
lesions, suggesting the heterogeneity of macrophages during
endometriosis development (32).
Further studies are required to determine the macrophage
subpopulations involved in the progression of endometriosis.
COX-2 is involved in the development of
endometriosis (11). In patients
with endometriosis, its expression is upregulated in the
endometrial tissue (12). COX-2
inhibitors reduced endometrial growth, vascular density, and VEGF-A
expression in rodent endometrial implants (7,33,34).
Additionally, mPGES-1 deficient mice showed attenuated
endometriosis growth and angiogenesis through the downregulation of
VEGF-A in endometrial implants (7). These findings indicate that
COX-2/mPGES-1-derived PGE2 contributes to the
development of endometriosis by enhancing angiogenesis. However,
the role of TXA2 in endometriosis pathology remains to
be elucidated. The findings of the present study revealed that
inhibition of TP signaling or TXS stimulated endometrial growth,
angiogenesis, and lymphangiogenesis in endometrial lesions.
Under pathological conditions, TP signaling has both
pro- and anti-angiogenic properties (35). In addition, TP signaling promotes
tumor growth and angiogenesis. TXA2 stimulates the
secretion of VEGF-A from tumor cells (26). Platelet-derived TXA2
promotes angiogenesis and blood flow recovery after hindlimb
ischemia (19) and gastric ulcer
(15). In contrast, TP activation
of endothelial cells (ECs) reduces cell migration and proliferation
(36). The activation of TP
signaling inhibits the pro-angiogenic capacity of human umbilical
vein endothelial cells (HUVECs). Deletion of TP signaling in ECs
enhances VEGF-induced angiogenesis in vivo. In addition, TP
activation reduces VEGFR2 expression in HUVECs, whereas TP
knockdown increases its expression (37,38).
The present study showed that ablation of TP signaling enhanced
angiogenesis via the production of VEGF-A from macrophages
accumulated in the stroma of endometrial implants. In cultured
BM-macrophages, VEGF-A expression was reduced via TP signaling. As
TP was not expressed in endometrial implant microvessels,
activating TP signaling did not directly induce pro-angiogenic
functions in the endothelium but indirectly induced angiogenesis by
releasing pro-angiogenic factors from macrophages accumulated in
endometrial implants.
COX-2 inhibition prolonged inflammation-induced
lymphedema in mouse tails and ears (13,14),
and COX-2-derived PGE2 was responsible for
lymphangiogenesis (39). In terms
of endogenous TXA2, recent studies have shown that TP
signaling in macrophages facilitated lymphangiogenesis in the
diaphragm during endotoxin-induced peritonitis (20). In contrast, ablation of TP
signaling enhanced lymphangiogenesis in this murine model of
endometriosis, and decreased VEGF-C and VEGF-D levels via TP
signaling in cultured BM-derived macrophages. TP expression was
observed in endometrial stromal macrophages rather than in
lymphatic vessels. These findings imply that TP signaling in
macrophages suppresses lymphangiogenesis during endometriosis.
However, studies on the role of TP signaling in lymphangiogenesis
have yielded contradictory results, possibly because of the
differences in the experimental systems used and the sex of the
mice. Recruitment of pro-inflammatory macrophages to endometrial
implants can also impair lymphangiogenesis, as pro-inflammatory
macrophages can disrupt the formation of new lymphatic vessels at
the site of injury (40).
Stimulation of macrophages with PGE2 has
been reported to change their phenotype from a pro-inflammatory to
an anti-inflammatory state (16,41);
however, little is known regarding the effect of TP signaling on
macrophage polarization. The results of the current study suggested
that ablation of TP signaling increased anti-inflammatory
macrophage phenotype-related gene expression during endometriosis,
including upregulation of CCL2 expression. Increased CCL2
expression has been observed in the endometrium of women with
endometriosis (42) and
endometrial stromal tissues from women as well as in mice with
endometriosis (43), suggesting
endometrial gland and stromal cells in the implant tissues as a
source of CCL2. In addition, the recruitment of anti-inflammatory
macrophages was associated with the upregulation of CCL2 expression
in endometrial lesions, and the CCR2 inhibitor RS102895 reduced
endometrial growth in endometriotic mice (43). These data suggest that the
accumulation of anti-inflammatory macrophages in endometrial
lesions via the CCL2/CCR2 pathway promotes endometriosis
development. Further studies are required to elucidate the role of
the CCL2/CCR2 axis in the recruitment of anti-inflammatory
macrophages to endometrial implant tissues from TP−/−
mice. The application of a TP agonist (U46619) reduced
anti-inflammatory macrophage phenotype-related gene expression in
cultured BM-derived macrophages, suggesting that TP signaling
switches the macrophage phenotype, resulting in anti-inflammatory
macrophages. In contrast, a previous study found that TP
stimulation increased pro-inflammatory macrophage phenotype-related
gene expression in isolated peritoneal thioglycolate-induced
macrophages from male mice (44).
The discrepancies in the results could be attributed to differences
in sex and origin of macrophages and stimulation prior to TP
agonist application.
In conclusion, the results of the present study
showed that inhibition of TP signaling promoted endometriosis
development by enhancing neovascularization in endometrial lesions.
Inhibition of TP signaling did not act on blood and lymphatic ECs,
but rather on the accumulation of anti-inflammatory macrophages
into the endometrial lesions. TP receptor agonist decreased
anti-inflammatory macrophage phenotype-related and
neovascularization-related gene expression in cultured macrophages.
Therefore, the promotion of endometriosis and neovascularization
are likely driven by the inhibition of TP signaling in
anti-inflammatory macrophages. However, further studies are
necessary to confirm this possibility. Given that selective TP
receptor activation with an agonist may reduce endometrial growth,
targeting TP signaling may be a viable option for treating
endometriosis; however, further research is needed.
Acknowledgments
The authors would like to thank Ms. Michiko Ogino
and Ms. Kyoko Yoshikawa (both Department of Pharmacology, Kitasato
University School of Medicine, Sagamihara, Japan) for their
technical assistance.
Funding
This work was supported by grants from the Japanese Ministry of
Education, Culture, Sports, Science and Technology (MEXT) (grant
nos. 21K16821 and 21K09502), the Integrative Research Program of
the Graduate School of Medical Science at Kitasato University
(grant no. 2022-B26) and the Parents' Association Grant of the
Kitasato University School of Medicine.
Availability of data and materials
The datasets used and/or analyzed during the
current study are available from the corresponding author upon
reasonable request.
Authors' contributions
AF conceived and designed the study, performed the
experiments and wrote the manuscript. KHa, ES, MT and KHo performed
the experiments. MH, KS and YI performed the data analysis and
interpretation, and provided technical support. MM and SN were
involved in the breeding process of the TP−/− mice and
helped to design the animal experiments. KK and HA designed the
study, analyzed the data and revised the manuscript. AF, YI and HA
confirm the authenticity of all the raw data. All authors have read
and approved the final manuscript.
Ethics approval and consent to
participate
The animal experiments were approved by the Animal
Experimentation and Ethics Committee of Kitasato University School
of Medicine (approval no. 2022-060).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
BM
|
bone marrow
|
|
CCL2
|
C-C motif chemokine 2
|
|
CCR2
|
C-C motif chemokine receptor 2
|
|
COX
|
cyclooxygenase
|
|
IL
|
interleukin
|
|
i.p.
|
intraperitoneal
|
|
LVA
|
lymphatic vessel area
|
|
LVD
|
lymphatic vessel density
|
|
LYVE-1
|
lymphatic vessel endothelial
hyaluronan receptor 1
|
|
mPGES-1
|
microsomal prostaglandin E
synthase-1
|
|
MR
|
mannose receptor
|
|
MVA
|
microvessel area
|
|
MVD
|
microvessel density
|
|
PG
|
prostaglandin
|
|
Prox1
|
prospero-related homeobox 1
|
|
TP
|
thromboxane prostanoid
|
|
TNF-α
|
tumor necrosis factor-α
|
|
TXA2
|
thromboxane A2
|
|
TXS
|
thromboxane synthase
|
|
VEGF
|
vascular endothelial growth
factor
|
|
VEGFR
|
VEGF receptor
|
References
|
1
|
Saunders PTK and Horne AW: Endometriosis:
Etiology, pathobiology, and therapeutic prospects. Cell.
184:2807–2824. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zondervan KT, Becker CM and Missmer SA:
Endometriosis. N Engl J Med. 382:1244–1256. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Taylor HS, Kotlyar AM and Flores VA:
Endometriosis is a chronic systemic disease: Clinical challenges
and novel innovations. Lancet. 397:839–852. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hey-Cunningham AJ, Peters KM, Zevallos HB,
Berbic M, Markham R and Fraser IS: Angiogenesis, lymphangiogenesis
and neurogenesis in endometriosis. Front Biosci (Elite Ed).
5:1033–1056. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chung MS and Han SJ:
Endometriosis-associated angiogenesis and anti-angiogenic therapy
for endometriosis. Front Glob Womens Health. 3:8563162022.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sekiguchi K, Ito Y, Hattori K, Inoue T,
Hosono K, Honda M, Numao A, Amano H, Shibuya M, Unno N and Majima
M: VEGF receptor 1-expressing macrophages recruited from bone
marrow enhances angiogenesis in endometrial tissues. Sci Rep.
9:70372019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Numao A, Hosono K, Suzuki T, Hayashi I,
Uematsu S, Akira S, Ogino Y, Kawauchi H, Unno N and Majima M: The
inducible prostaglandin E synthase mPGES-1 regulates growth of
endometrial tissues and angiogenesis in a mouse implantation model.
Biomed Pharmacother. 65:77–84. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hattori K, Ito Y, Honda M, Sekiguchi K,
Hosono K, Shibuya M, Unno N and Majima M: Lymphangiogenesis induced
by vascular endothelial growth factor receptor 1 signaling
contributes to the progression of endometriosis in mice. J
Pharmacol Sci. 143:255–263. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Honda M, Ito Y, Hattori K, Hosono K,
Sekiguchi K, Tsujikawa K, Unno N and Majima M: Inhibition of
receptor activity-modifying protein 1 suppresses the development of
endometriosis and the formation of blood and lymphatic vessels. J
Cell Mol Med. 24:11984–11997. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Motohashi E, Kawauchi H, Endo H, Kondo H,
Kitasato H, Kuramoto H, Majima M, Unno N and Hayashi I: Regulatory
expression of lipoxin A4 receptor in physiologically estrus cycle
and pathologically endometriosis. Biomed Pharmacother. 59:330–338.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lai ZZ, Yang HL, Ha SY, Chang KK, Mei J,
Zhou WJ, Qiu XM, Wang XQ, Zhu R, Li DJ and Li MQ: Cyclooxygenase-2
in endometriosis. Int J Biol Sci. 15:2783–2797. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lousse JC, Defrere S, Colette S, Van
Langendonckt A and Donnez J: Expression of eicosanoid biosynthetic
and catabolic enzymes in peritoneal endometriosis. Hum Reprod.
25:734–741. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hosono K, Suzuki T, Tamaki H, Sakagami H,
Hayashi I, Narumiya S, Alitalo K and Majima M: Roles of
prostaglandin E2-EP3/EP4 receptor signaling in the enhancement of
lymphangiogenesis during fibroblast growth factor-2-induced
granulation formation. Arterioscler Thromb Vasc Biol. 31:1049–1058.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kashiwagi S, Hosono K, Suzuki T, Takeda A,
Uchinuma E and Majima M: Role of COX-2 in lymphangiogenesis and
restoration of lymphatic flow in secondary lymphedema. Lab Invest.
91:1314–1325. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ae T, Ohno T, Hattori Y, Suzuki T, Hosono
K, Minamino T, Sato T, Uematsu S, Akira S, Koizumi W and Majima M:
Role of microsomal prostaglandin E synthase-1 in the facilitation
of angiogenesis and the healing of gastric ulcers. Am J Physiol
Gastrointest Liver Physiol. 299:G1139–G1146. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hosono K, Isonaka R, Kawakami T, Narumiya
S and Majima M: Signaling of prostaglandin E receptors, EP3 and EP4
facilitates wound healing and lymphangiogenesis with enhanced
recruitment of M2 macrophages in mice. PLoS One. 11:e01625322016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Narumiya S, Sugimoto Y and Ushikubi F:
Prostanoid receptors: Structures, properties, and functions.
Physiol Rev. 79:1193–1226. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Szczuko M, Koziol I, Kotlega D, Brodowski
J and Drozd A: The role of thromboxane in the course and treatment
of ischemic stroke: Review. Int J Mol Sci. 22:116442021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Amano H, Ito Y, Eshima K, Kato S, Ogawa F,
Hosono K, Oba K, Tamaki H, Sakagami H, Shibuya M, et al:
Thromboxane A2 induces blood flow recovery via platelet adhesion to
ischaemic regions. Cardiovasc Res. 107:509–521. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Matsuda H, Ito Y, Hosono K, Tsuru S, Inoue
T, Nakamoto S, Kurashige C, Hirashima M, Narumiya S, Okamoto H and
Majima M: Roles of thromboxane receptor signaling in enhancement of
lipopolysaccharide-induced lymphangiogenesis and lymphatic drainage
function in diaphragm. Arterioscler Thromb Vasc Biol. 41:1390–1407.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Takayama K, Yuhki K, Ono K, Fujino T, Hara
A, Yamada T, Kuriyama S, Karibe H, Okada Y, Takahata O, et al:
Thromboxane A2 and prostaglandin F2alpha mediate inflammatory
tachycardia. Nat Med. 11:562–566. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Navarro KL, Huss M, Smith JC, Sharp P,
Marx JO and Pacharinsak C: Mouse anesthesia: The art and science.
ILAR J. 62:238–273. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wigle JT and Oliver G: Prox1 function is
required for the development of the murine lymphatic system. Cell.
98:769–778. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Majima M, Hosono K, Ito Y and Amano H:
Biologically active lipids in the regulation of lymphangiogenesis
in disease states. Pharmacol Ther. 232:1080112022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wei J, Yan W, Li X, Ding Y and Tai HH:
Thromboxane receptor alpha mediates tumor growth and angiogenesis
via induction of vascular endothelial growth factor expression in
human lung cancer cells. Lung Cancer. 69:26–32. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fonseca MAS, Haro M, Wright KN, Lin X,
Abbasi F, Sun J, Hernandez L, Orr NL, Hong J, Choi-Kuaea Y, et al:
Single-cell transcriptomic analysis of endometriosis. Nat Genet.
55:255–267. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hogg C, Horne AW and Greaves E:
Endometriosis-associated macrophages: Origin, phenotype, and
function. Front Endocrinol (Lausanne). 11:72020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bacci M, Capobianco A, Monno A, Cottone L,
Di Puppo F, Camisa B, Mariani M, Brignole C, Ponzoni M, Ferrari S,
et al: Macrophages are alternatively activated in patients with
endometriosis and required for growth and vascularization of
lesions in a mouse model of disease. Am J Pathol. 175:547–556.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ono Y, Yoshino O, Hiraoka T, Akiyama I,
Sato E, Ito M, Kobayashi M, Nakashima A, Wada S, Onda T, et al:
IL-33 exacerbates endometriotic lesions via polarizing peritoneal
macrophages to M2 subtype. Reprod Sci. 27:869–876. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Johan MZ, Ingman WV, Robertson SA and Hull
ML: Macrophages infiltrating endometriosis-like lesions exhibit
progressive phenotype changes in a heterologous mouse model. J
Reprod Immunol. 132:1–8. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tan Y, Flynn WF, Sivajothi S, Luo D, Bozal
SB, Davé M, Luciano AA, Robson P, Luciano DE and Courtois ET:
Single-cell analysis of endometriosis reveals a coordinated
transcriptional programme driving immunotolerance and angiogenesis
across eutopic and ectopic tissues. Nat Cell Biol. 24:1306–1318.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Machado DE, Berardo PT, Landgraf RG,
Fernandes PD, Palmero C, Alves LM, Abrao MS and Nasciutti LE: A
selective cyclooxygenase-2 inhibitor suppresses the growth of
endometriosis with an antiangiogenic effect in a rat model. Fertil
Steril. 93:2674–2679. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Olivares C, Ricci A, Bilotas M, Baranao RI
and Meresman G: The inhibitory effect of celecoxib and
rosiglitazone on experimental endometriosis. Fertil Steril.
96:428–433. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ashton AW, Zhang Y, Cazzolli R and Honn
KV: The role and regulation of thromboxane A2 signaling
in cancer-trojan horses and misdirection. Molecules. 27:62342022.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ashton AW, Yokota R, John G, Zhao S,
Suadicani SO, Spray DC and Ware JA: Inhibition of endothelial cell
migration, intercellular communication, and vascular tube formation
by thromboxane A(2). J Biol Chem. 274:35562–35570. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tsou PS, Amin MA, Campbell PL, Zakhem G,
Balogh B, Edhayan G, Ohara RA, Schiopu E, Khanna D, Koch AE and Fox
DA: Activation of the thromboxane A2 receptor by 8-isoprostane
inhibits the pro-angiogenic effect of vascular endothelial growth
factor in scleroderma. J Invest Dermatol. 135:3153–3162. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Eckenstaler R, Ripperger A, Hauke M,
Petermann M, Hemkemeyer SA, Schwedhelm E, Ergün S, Frye M, Werz O,
Koeberle A, et al: A thromboxane A2 receptor-driven
COX-2-dependent feedback loop that affects endothelial homeostasis
and angiogenesis. Arterioscler Thromb Vasc Biol. 42:444–461. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Matsuda H, Hosono K, Tsuru S, Kurashige C,
Sekiguchi K, Akira S, Uematsu S, Okamoto H and Majima M: Roles of
mPGES-1, an inducible prostaglandin E synthase, in enhancement of
LPS-induced lymphangiogenesis in a mouse peritonitis model. Life
Sci. 142:1–7. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mishima T, Ito Y, Nishizawa N, Amano H,
Tsujikawa K, Miyaji K, Watanabe M and Majima M: RAMP1 signaling
improves lymphedema and promotes lymphangiogenesis in mice. J Surg
Res. 219:50–60. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Nishizawa N, Ito Y, Eshima K, Ohkubo H,
Kojo K, Inoue T, Raouf J, Jakobsson PJ, Uematsu S, Akira S, et al:
Inhibition of microsomal prostaglandin E synthase-1 facilitates
liver repair after hepatic injury in mice. J Hepatol. 69:110–120.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Jolicoeur C, Boutouil M, Drouin R, Paradis
I, Lemay A and Akoum A: Increased expression of monocyte
chemotactic protein-1 in the endometrium of women with
endometriosis. Am J Pathol. 152:125–133. 1998.PubMed/NCBI
|
|
43
|
Gou Y, Li X, Li P, Zhang H, Xu T, Wang H,
Wang B, Ma X, Jiang X and Zhang Z: Estrogen receptor β upregulates
CCL2 via NF-κB signaling in endometriotic stromal cells and
recruits macrophages to promote the pathogenesis of endometriosis.
Hum Reprod. 34:646–658. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Minamino T, Ito Y, Ohkubo H, Hosono K,
Suzuki T, Sato T, Ae T, Shibuya A, Sakagami H, Narumiya S, et al:
Thromboxane A(2) receptor signaling promotes liver tissue repair
after toxic injury through the enhancement of macrophage
recruitment. Toxicol Appl Pharmacol. 259:104–114. 2012. View Article : Google Scholar : PubMed/NCBI
|