|
1
|
Saunders PTK and Horne AW: Endometriosis:
Etiology, pathobiology, and therapeutic prospects. Cell.
184:2807–2824. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zondervan KT, Becker CM and Missmer SA:
Endometriosis. N Engl J Med. 382:1244–1256. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Taylor HS, Kotlyar AM and Flores VA:
Endometriosis is a chronic systemic disease: Clinical challenges
and novel innovations. Lancet. 397:839–852. 2021. View Article : Google Scholar : PubMed/NCBI
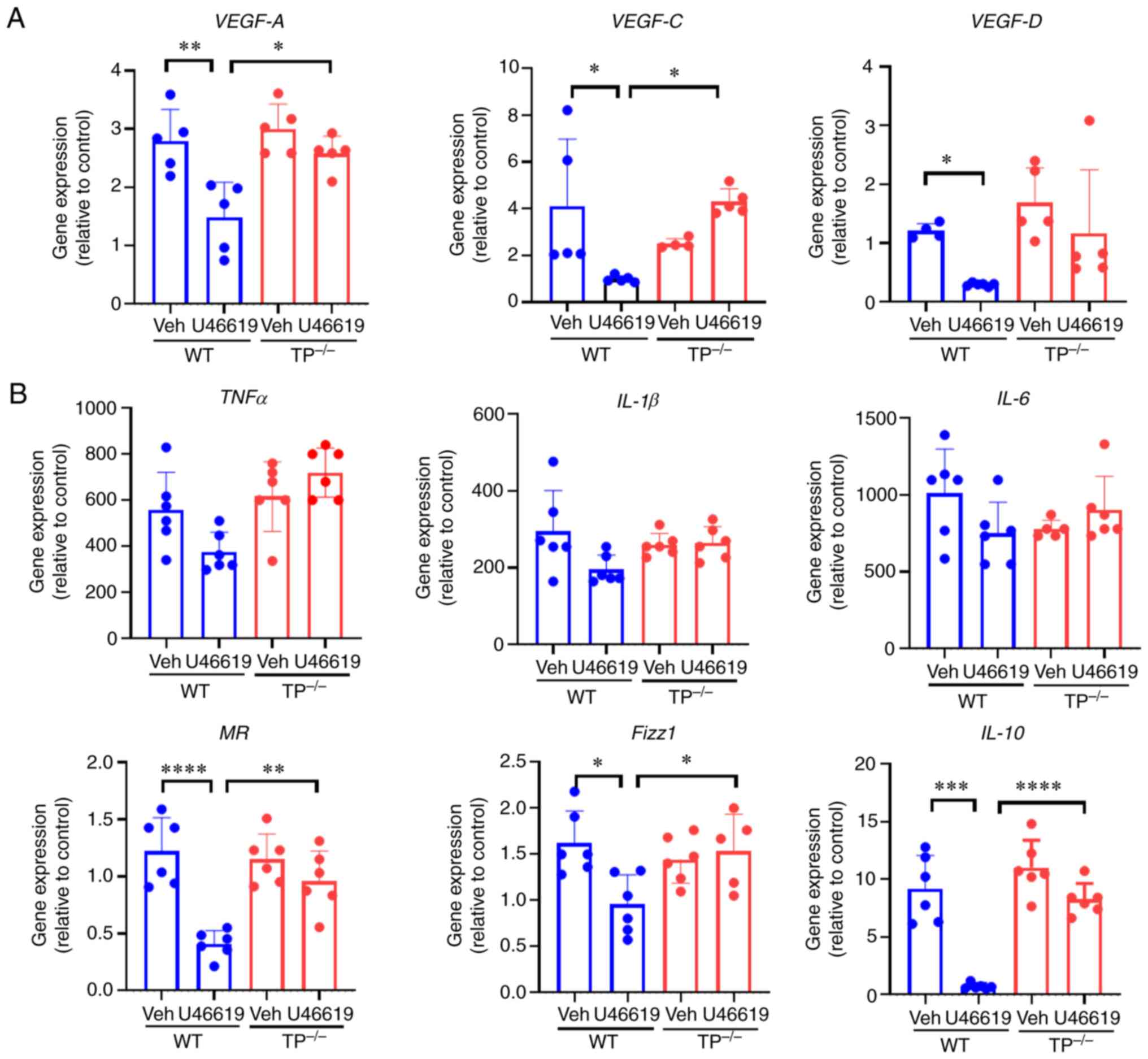
|
|
4
|
Hey-Cunningham AJ, Peters KM, Zevallos HB,
Berbic M, Markham R and Fraser IS: Angiogenesis, lymphangiogenesis
and neurogenesis in endometriosis. Front Biosci (Elite Ed).
5:1033–1056. 2013. View
Article : Google Scholar : PubMed/NCBI
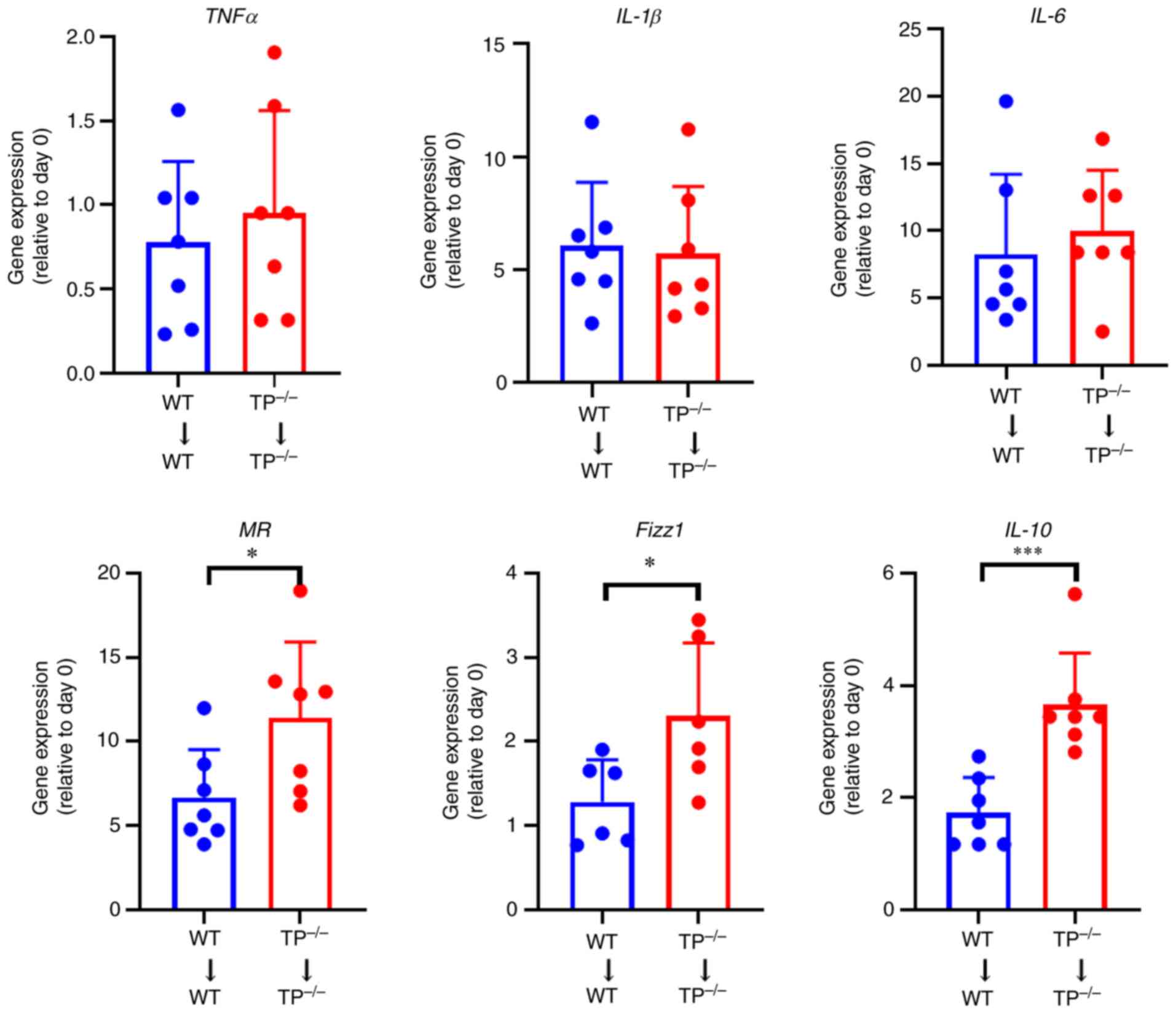
|
|
5
|
Chung MS and Han SJ:
Endometriosis-associated angiogenesis and anti-angiogenic therapy
for endometriosis. Front Glob Womens Health. 3:8563162022.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sekiguchi K, Ito Y, Hattori K, Inoue T,
Hosono K, Honda M, Numao A, Amano H, Shibuya M, Unno N and Majima
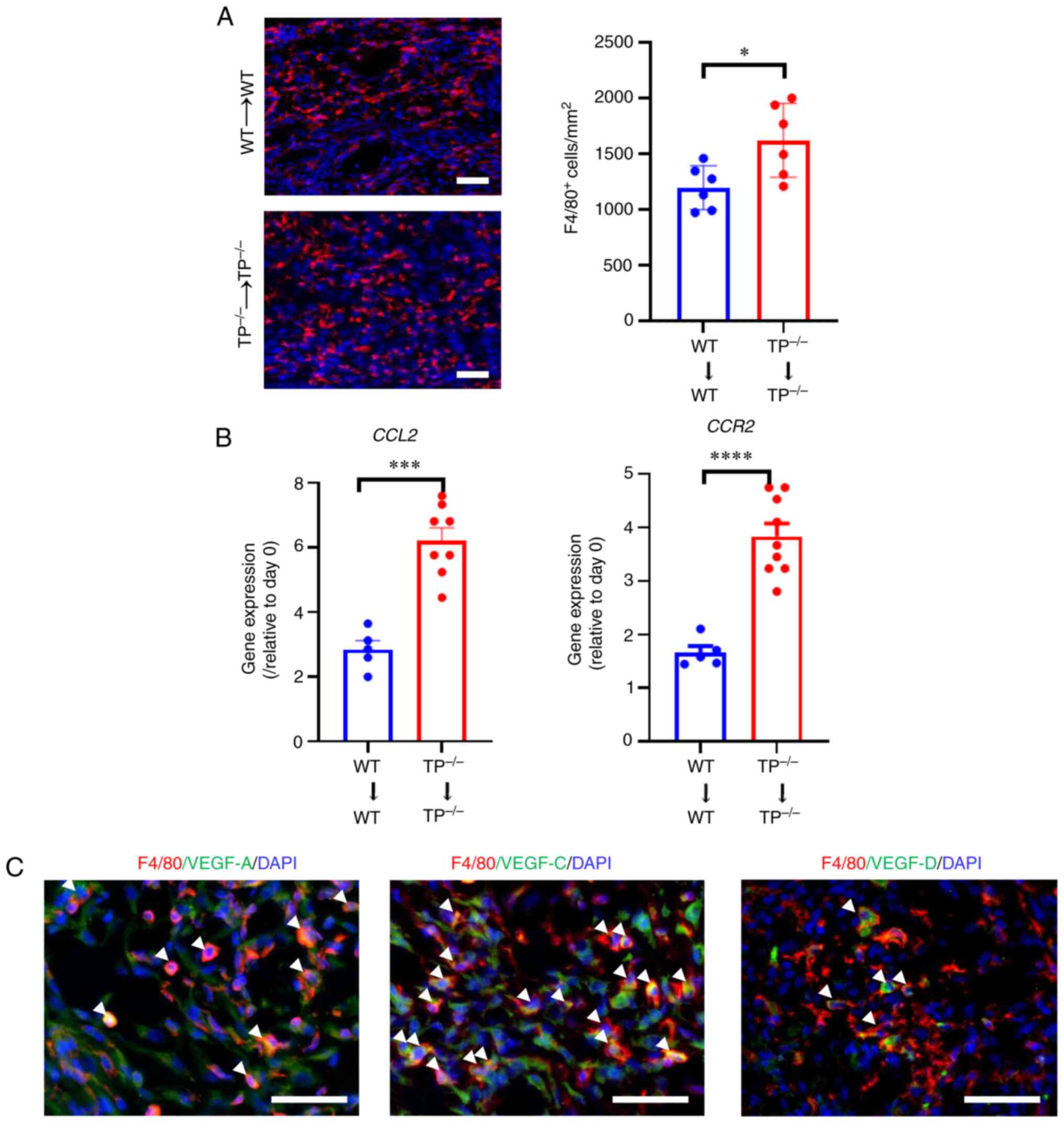
M: VEGF receptor 1-expressing macrophages recruited from bone
marrow enhances angiogenesis in endometrial tissues. Sci Rep.
9:70372019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Numao A, Hosono K, Suzuki T, Hayashi I,
Uematsu S, Akira S, Ogino Y, Kawauchi H, Unno N and Majima M: The
inducible prostaglandin E synthase mPGES-1 regulates growth of
endometrial tissues and angiogenesis in a mouse implantation model.
Biomed Pharmacother. 65:77–84. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hattori K, Ito Y, Honda M, Sekiguchi K,
Hosono K, Shibuya M, Unno N and Majima M: Lymphangiogenesis induced
by vascular endothelial growth factor receptor 1 signaling
contributes to the progression of endometriosis in mice. J
Pharmacol Sci. 143:255–263. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Honda M, Ito Y, Hattori K, Hosono K,
Sekiguchi K, Tsujikawa K, Unno N and Majima M: Inhibition of
receptor activity-modifying protein 1 suppresses the development of
endometriosis and the formation of blood and lymphatic vessels. J
Cell Mol Med. 24:11984–11997. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Motohashi E, Kawauchi H, Endo H, Kondo H,
Kitasato H, Kuramoto H, Majima M, Unno N and Hayashi I: Regulatory
expression of lipoxin A4 receptor in physiologically estrus cycle
and pathologically endometriosis. Biomed Pharmacother. 59:330–338.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lai ZZ, Yang HL, Ha SY, Chang KK, Mei J,
Zhou WJ, Qiu XM, Wang XQ, Zhu R, Li DJ and Li MQ: Cyclooxygenase-2
in endometriosis. Int J Biol Sci. 15:2783–2797. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lousse JC, Defrere S, Colette S, Van
Langendonckt A and Donnez J: Expression of eicosanoid biosynthetic
and catabolic enzymes in peritoneal endometriosis. Hum Reprod.
25:734–741. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hosono K, Suzuki T, Tamaki H, Sakagami H,
Hayashi I, Narumiya S, Alitalo K and Majima M: Roles of
prostaglandin E2-EP3/EP4 receptor signaling in the enhancement of
lymphangiogenesis during fibroblast growth factor-2-induced
granulation formation. Arterioscler Thromb Vasc Biol. 31:1049–1058.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kashiwagi S, Hosono K, Suzuki T, Takeda A,
Uchinuma E and Majima M: Role of COX-2 in lymphangiogenesis and
restoration of lymphatic flow in secondary lymphedema. Lab Invest.
91:1314–1325. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ae T, Ohno T, Hattori Y, Suzuki T, Hosono
K, Minamino T, Sato T, Uematsu S, Akira S, Koizumi W and Majima M:
Role of microsomal prostaglandin E synthase-1 in the facilitation
of angiogenesis and the healing of gastric ulcers. Am J Physiol
Gastrointest Liver Physiol. 299:G1139–G1146. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hosono K, Isonaka R, Kawakami T, Narumiya
S and Majima M: Signaling of prostaglandin E receptors, EP3 and EP4
facilitates wound healing and lymphangiogenesis with enhanced
recruitment of M2 macrophages in mice. PLoS One. 11:e01625322016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Narumiya S, Sugimoto Y and Ushikubi F:
Prostanoid receptors: Structures, properties, and functions.
Physiol Rev. 79:1193–1226. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Szczuko M, Koziol I, Kotlega D, Brodowski
J and Drozd A: The role of thromboxane in the course and treatment
of ischemic stroke: Review. Int J Mol Sci. 22:116442021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Amano H, Ito Y, Eshima K, Kato S, Ogawa F,
Hosono K, Oba K, Tamaki H, Sakagami H, Shibuya M, et al:
Thromboxane A2 induces blood flow recovery via platelet adhesion to
ischaemic regions. Cardiovasc Res. 107:509–521. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Matsuda H, Ito Y, Hosono K, Tsuru S, Inoue
T, Nakamoto S, Kurashige C, Hirashima M, Narumiya S, Okamoto H and
Majima M: Roles of thromboxane receptor signaling in enhancement of
lipopolysaccharide-induced lymphangiogenesis and lymphatic drainage
function in diaphragm. Arterioscler Thromb Vasc Biol. 41:1390–1407.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Takayama K, Yuhki K, Ono K, Fujino T, Hara
A, Yamada T, Kuriyama S, Karibe H, Okada Y, Takahata O, et al:
Thromboxane A2 and prostaglandin F2alpha mediate inflammatory
tachycardia. Nat Med. 11:562–566. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Navarro KL, Huss M, Smith JC, Sharp P,
Marx JO and Pacharinsak C: Mouse anesthesia: The art and science.
ILAR J. 62:238–273. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wigle JT and Oliver G: Prox1 function is
required for the development of the murine lymphatic system. Cell.
98:769–778. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Majima M, Hosono K, Ito Y and Amano H:
Biologically active lipids in the regulation of lymphangiogenesis
in disease states. Pharmacol Ther. 232:1080112022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wei J, Yan W, Li X, Ding Y and Tai HH:
Thromboxane receptor alpha mediates tumor growth and angiogenesis
via induction of vascular endothelial growth factor expression in
human lung cancer cells. Lung Cancer. 69:26–32. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fonseca MAS, Haro M, Wright KN, Lin X,
Abbasi F, Sun J, Hernandez L, Orr NL, Hong J, Choi-Kuaea Y, et al:
Single-cell transcriptomic analysis of endometriosis. Nat Genet.
55:255–267. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hogg C, Horne AW and Greaves E:
Endometriosis-associated macrophages: Origin, phenotype, and
function. Front Endocrinol (Lausanne). 11:72020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bacci M, Capobianco A, Monno A, Cottone L,
Di Puppo F, Camisa B, Mariani M, Brignole C, Ponzoni M, Ferrari S,
et al: Macrophages are alternatively activated in patients with
endometriosis and required for growth and vascularization of
lesions in a mouse model of disease. Am J Pathol. 175:547–556.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ono Y, Yoshino O, Hiraoka T, Akiyama I,
Sato E, Ito M, Kobayashi M, Nakashima A, Wada S, Onda T, et al:
IL-33 exacerbates endometriotic lesions via polarizing peritoneal
macrophages to M2 subtype. Reprod Sci. 27:869–876. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Johan MZ, Ingman WV, Robertson SA and Hull
ML: Macrophages infiltrating endometriosis-like lesions exhibit
progressive phenotype changes in a heterologous mouse model. J
Reprod Immunol. 132:1–8. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tan Y, Flynn WF, Sivajothi S, Luo D, Bozal
SB, Davé M, Luciano AA, Robson P, Luciano DE and Courtois ET:
Single-cell analysis of endometriosis reveals a coordinated
transcriptional programme driving immunotolerance and angiogenesis
across eutopic and ectopic tissues. Nat Cell Biol. 24:1306–1318.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Machado DE, Berardo PT, Landgraf RG,
Fernandes PD, Palmero C, Alves LM, Abrao MS and Nasciutti LE: A
selective cyclooxygenase-2 inhibitor suppresses the growth of
endometriosis with an antiangiogenic effect in a rat model. Fertil
Steril. 93:2674–2679. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Olivares C, Ricci A, Bilotas M, Baranao RI
and Meresman G: The inhibitory effect of celecoxib and
rosiglitazone on experimental endometriosis. Fertil Steril.
96:428–433. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ashton AW, Zhang Y, Cazzolli R and Honn
KV: The role and regulation of thromboxane A2 signaling
in cancer-trojan horses and misdirection. Molecules. 27:62342022.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ashton AW, Yokota R, John G, Zhao S,
Suadicani SO, Spray DC and Ware JA: Inhibition of endothelial cell
migration, intercellular communication, and vascular tube formation
by thromboxane A(2). J Biol Chem. 274:35562–35570. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tsou PS, Amin MA, Campbell PL, Zakhem G,
Balogh B, Edhayan G, Ohara RA, Schiopu E, Khanna D, Koch AE and Fox
DA: Activation of the thromboxane A2 receptor by 8-isoprostane
inhibits the pro-angiogenic effect of vascular endothelial growth
factor in scleroderma. J Invest Dermatol. 135:3153–3162. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Eckenstaler R, Ripperger A, Hauke M,
Petermann M, Hemkemeyer SA, Schwedhelm E, Ergün S, Frye M, Werz O,
Koeberle A, et al: A thromboxane A2 receptor-driven
COX-2-dependent feedback loop that affects endothelial homeostasis
and angiogenesis. Arterioscler Thromb Vasc Biol. 42:444–461. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Matsuda H, Hosono K, Tsuru S, Kurashige C,
Sekiguchi K, Akira S, Uematsu S, Okamoto H and Majima M: Roles of
mPGES-1, an inducible prostaglandin E synthase, in enhancement of
LPS-induced lymphangiogenesis in a mouse peritonitis model. Life
Sci. 142:1–7. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mishima T, Ito Y, Nishizawa N, Amano H,
Tsujikawa K, Miyaji K, Watanabe M and Majima M: RAMP1 signaling
improves lymphedema and promotes lymphangiogenesis in mice. J Surg
Res. 219:50–60. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Nishizawa N, Ito Y, Eshima K, Ohkubo H,
Kojo K, Inoue T, Raouf J, Jakobsson PJ, Uematsu S, Akira S, et al:
Inhibition of microsomal prostaglandin E synthase-1 facilitates
liver repair after hepatic injury in mice. J Hepatol. 69:110–120.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Jolicoeur C, Boutouil M, Drouin R, Paradis
I, Lemay A and Akoum A: Increased expression of monocyte
chemotactic protein-1 in the endometrium of women with
endometriosis. Am J Pathol. 152:125–133. 1998.PubMed/NCBI
|
|
43
|
Gou Y, Li X, Li P, Zhang H, Xu T, Wang H,
Wang B, Ma X, Jiang X and Zhang Z: Estrogen receptor β upregulates
CCL2 via NF-κB signaling in endometriotic stromal cells and
recruits macrophages to promote the pathogenesis of endometriosis.
Hum Reprod. 34:646–658. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Minamino T, Ito Y, Ohkubo H, Hosono K,
Suzuki T, Sato T, Ae T, Shibuya A, Sakagami H, Narumiya S, et al:
Thromboxane A(2) receptor signaling promotes liver tissue repair
after toxic injury through the enhancement of macrophage
recruitment. Toxicol Appl Pharmacol. 259:104–114. 2012. View Article : Google Scholar : PubMed/NCBI
|