Introduction
Human parvovirus B19 (B19V), discovered in 1975, is
known as a non-enveloped single-stranded DNA virus that spreads
through the respiratory tract (1,2).
Epidemiological surveys of England and Japan have shown that the
prevalence of individuals with antibodies against B19V is 2–15% for
children aged <5 years, 15–60% for adolescents aged 6–19 years,
30–60% for adults aged 16–40 years and >85% for the elderly
population aged >71 years (3,4).
B19V infection has been associated with numerous pathological
conditions, such as fifth disease, persistent anemia, myocarditis,
hydrops fetalis, arthropathy and autoimmune disorders (2,5–9). The
genome of B19V encodes two viral structural proteins [viral
envelope protein (VP) 1 and VP2] and three non-structural proteins
[non-structural protein 1 (NS1), 7.5-kDa and 11-kDa] that are
required for the regulation of viral capsid packaging and DNA
replication (10). VP2 protein
comprises 95% of the B19V capsid. B19V VP1 is identical to VP2
except for an additional 227 amino acids at the N-terminal, which
is known as the VP1 unique region and contributes to B19V infection
(11–14). Although the precise mechanism of
B19V infection is still unclear, the receptor-binding domain and
phospholipase A2 activity within B19V VP1u play important roles in
viral tropism, uptake and subcellular trafficking (11–14).
B19V NS1 is known as a transcriptional activator for
DNA replication by binding to the P6 promoter (15). Evidence has also indicated that
B19V NS1 can bind TATA box and GC-rich elements, and interact with
various DNA-binding proteins, such as the activating transcription
factor/cAMP response element binding protein, NF-κB/c-Rel and
GC-box binding factors, such as specificity protein 1 (16). In recent decades, B19V NS1 has been
demonstrated to induce various cytokines, such as IL-2, IL-6, IL-9,
IL-17A, IL-21, IL-22, interferon γ and TNF-α (17,18).
Additionally, B19V NS1 has been shown to cause cytotoxic activity
by inducing the caspase-3-dependent apoptotic pathway and various
inflammatory cytokines, such as IL-1β, IL-6 and IL-18 (19,20),
in both parvovirus permissive and nonpermissive cells (21,22),
such as nonpermissive THP-1 and nonpermissive U937 monocytic cell
lines (19,23). These findings suggested that both
B19V NS1 and macrophages play essential roles in inflammatory
processes.
Celastrol is a quinone methide from the roots of
Tripterygium wilfordii and has been demonstrated to exert a
protective effect against a variety of disorders, such as psoriasis
and RA (24). Substantial evidence
has indicated the suppressive effects of celastrol on
hepatocellular carcinoma through inhibition of CXCR4-related
signaling (25). The beneficial
effects of celastrol against neurodegenerative diseases and
cardiovascular disorders have also been reported (26,27).
Additionally, celastrol is known to inhibit replication of the
hepatitis C virus through the JNK MAPK/nuclear factor erythroid
2-related factor 2 pathway (28).
Increasing attention has focused on the therapeutic properties of
celastrol in inflammatory diseases, such as allergy, rheumatoid
arthritis, inflammatory bowel diseases, diabetes and osteoarthritis
(24). However, very little is
known about the effects and underlying mechanisms of celastrol on
B19V NS1-related inflammatory disorders. The present study
investigated the ameliorating effects of celastrol on B19V
NS1-induced inflammatory responses in U937 and THP-1 human acute
monocytic leukemia cell-derived macrophages, as well as its
underlying signaling.
Materials and methods
Chemicals and cell culture
All other chemicals used in this study for which no
manufacturer was identified were purchased from MilliporeSigma.
Celastrol was purchased from ChemFaces. A total of two human acute
monocytic leukemia cell lines, U937 [Bioresource Collection and
Research Centre (BCRC) 60435] and THP-1 (BCRC 60430), were
purchased from the BCRC (Food Industry Research and Development
Institute). The cell lines were subjected to short tandem repeat
profiling through the National Cheng Kung University Center for
Genomic Medicine (Tainan, Taiwan) to confirm their authenticity
(report nos. 23070813 and 23070814). The cells were cultured in
complete RPMI 1640 medium (Thermo Fisher Scientific, Inc.)
supplemented with 10% (v/v) FBS (Gibco; Thermo Fisher Scientific,
Inc.) and 100 U/ml penicillin at 37°C in a humidified atmosphere of
95% air and 5% CO2. To differentiate monocytes into
adherent macrophages, the cells were seeded at a density of
1×105 cells/well in 24-well plates and incubated at 37°C
for 2 days in the presence of 100 nM phorbol 12-myristate
13-acetate (MilliporeSigma). The cells were then washed and
incubated in normal growth medium for another 24 h prior to
treatment with purified B19V NS1 (1 µg/ml) as described in our
previous study (29).
Cell viability
To assess the survival of cells, MTT assay was
performed. A total of 2×105 cells were cultured
overnight at 37°C in each well of a 24-well plate. After treatment
with different concentrations of celastrol (0, 0.5, 1 and 2 µM) or
combinational treatment of 1 µg/ml B19V NS1 and celastrol (0, 0.5,
1 and 2 µM) for another 24 h, the culture medium was removed and
MTT reagent (0.5 mg/ml) was added and incubated for another 4 h. To
measure the absorbance of the culture medium, 0.3 ml dimethyl
sulfoxide was added to each well of the plate and the absorbance of
the medium was detected at 570 nm with a microplate reader
(SpectraMax M5®; Molecular Devices, LLC).
Migration assay
Millicell Hanging Cell Culture inserts (pore size, 8
µm; MilliporeSigma) were used to detect the effect of B19V NS1 on
cell motility. Briefly, the upper chamber containing cells in
serum-free RPMI 1640 medium (5×105 cells/well) treated
with different concentrations of celastrol (0, 0.5, 1 and 2 µM) or
combinational treatment of 1 µg/ml B19V NS1 and celastrol (0, 0.5,
1 and 2 µM) was merged with the bottom chamber containing standard
medium (RPMI 1640 with 10% FBS) and then incubated at 37°C for 24
h. The migrating cells were fixed with neutral-buffered formalin
(10%) at 25°C for 2 h and subsequently stained with 0.05% Giemsa
stain at 25°C for 2 h. A total of 10 random fields from each
experiment were observed for counting the number of migrated cells
under a light microscope (Zeiss Axioskop 2; Zeiss AG) at a
magnification of ×200 per filter.
Assessment of phagocytosis
Latex beads were used to assess the phagocytosis of
macrophages. A total of 2×105 U937 or THP-1 cells were
cultured overnight in each well of a 16-well Lab-Tek®II
Chamber Slide™ (Thermo Fisher Scientific, Inc.) and then stimulated
with 1 µg/ml B19V NS1 recombinant proteins or different
concentrations of celastrol (0, 0.5, 1 and 2 µM) for another 16 h
at 37°C before incubation with FITC-labeled latex beads
(MilliporeSigma) for 24 h at 37°C in a cell culture incubator. A
total of 100 macrophages in five random fields were counted under a
light microscope (Zeiss Axioskop 2; Zeiss AG) at a magnification of
×200. The phagocytic index was the number of phagocytosed particles
divided by the total number of macrophages and was expressed as a
percentage. The phagocytic ratio was the number of cells that
swallowed beads divided by the total number of macrophages and was
expressed as a percentage.
Zymography assay
U937 and THP-1 cells were stimulated with 1 µg/ml
B19V NS1 recombinant proteins or different concentrations of
celastrol (0, 0.5, 1 and 2 µM) for 24 h at 37°C and the activity of
MMP-9 in the medium was measured by gelatin-zymography assays. A
total of 10 µl culture medium from each treatment was separated by
SDS-PAGE on 8% gels containing 0.1% gelatin. After soaking in 2.5%
Triton X-100 to remove the SDS at 37°C for 30 min, the gels were
then washed in reaction buffer [40 mM Tris-HCl (pH 8.0), 10 mM
CaCl2, 0.02% NaN3] at 37°C for another 30
min. The gelatinolytic activity was visualized by staining the gels
with 0.5% Coomassie brilliant blue R-250 after being de-stained
with a methanol-acetic acid solution. Relative MMP levels were
semi-quantified using a gel documentation and analysis system
(AlphaImager HP 2200; ProteinSimple, Inc.).
ELISA
The measurement of cytokine levels in cell culture
media were performed using ELISA kits for human IL-1β (cat. no.
88-7261-88), IL-6 (cat. no. 88-7066-88) and TNF-α (cat. no.
88-7346-88) according to the manufacturer's instructions
(Invitrogen; Thermo Fisher Scientific, Inc.).
Immunoblotting
The cell pellets were collected by centrifugation at
800 × g for 5 min at 4°C and suspended in 600 µl PRO-PREP™ buffer
(iNtRON Biotechnology, Inc.) for lysis. The supernatant was then
obtained by centrifugation at 16,600 × g for 5 min at 4°C. The
concentrations of protein were measured using a modified Bradford's
assay with a spectrophotometer (Hitachi U 3000; HITACHI) at 595 nm,
with BSA (Merck KGaA) as the standard. Protein lysates (25 µg/lane)
were separated by SDS-PAGE using 10% gels and were
electrophoretically transferred to a polyvinylidene fluoride
membrane (Immobilon-E, 0.45 µM; MilliporeSigma). The membrane was
blocked with 5% non-fat dry milk in PBS for 2 h at 25°C with gentle
agitation, and then incubated with antibodies against NLR family
pyrin domain-containing 3 (NLRP3; 1:2,000; cat. no. A12694;
ABclonal), apoptosis-associated speck-like protein (ASC; 1:2,000;
cat. no. A1170; ABclonal), caspase-1 (1:2,000; cat. no. A0964;
ABclonal Biotech Co., Ltd.), IL-18 (1:1,000; cat. no. 061115;
Abclonal MilliporeSigma) and GAPDH (1:1,000; cat. no. NB300221;
Novus Biologicals, LLC) for 3 h at 25°C. Subsequently, secondary
antibodies conjugated with horseradish peroxidase (HRP) (1:5,000;
cat. no. sc-2005; Santa Cruz Biotechnology, Inc.) were added and
the membranes were incubated for 1 h. Finally, antigen-antibody
complexes were visualized using an Immobilon Western HRP
Chemiluminescent Substrate kit (MilliporeSigma) and semi-quantified
by densitometry (GE ImageQuant TL 8.1; Cytiva).
Statistical analysis
For in vitro assays, GraphPad Prism 5
software (GraphPad Software; Dotmatics) was used to calculate the
significant differences among groups by one-way analysis of
variance followed by Tukey's test. All data are presented as the
mean ± standard error of mean of at least three independent
experiments. P<0.05 was considered to indicate a statistically
significant difference.
Results
Effects of celastrol on B19V
NS1-induced human macrophage functions
To evaluate the cytotoxicity of celastrol on human
acute monocytic leukemia U937 and THP-1 derived macrophages, the
viability of U937 and THP-1 macrophages treated with different
concentrations of celastrol was determined using an MTT assay.
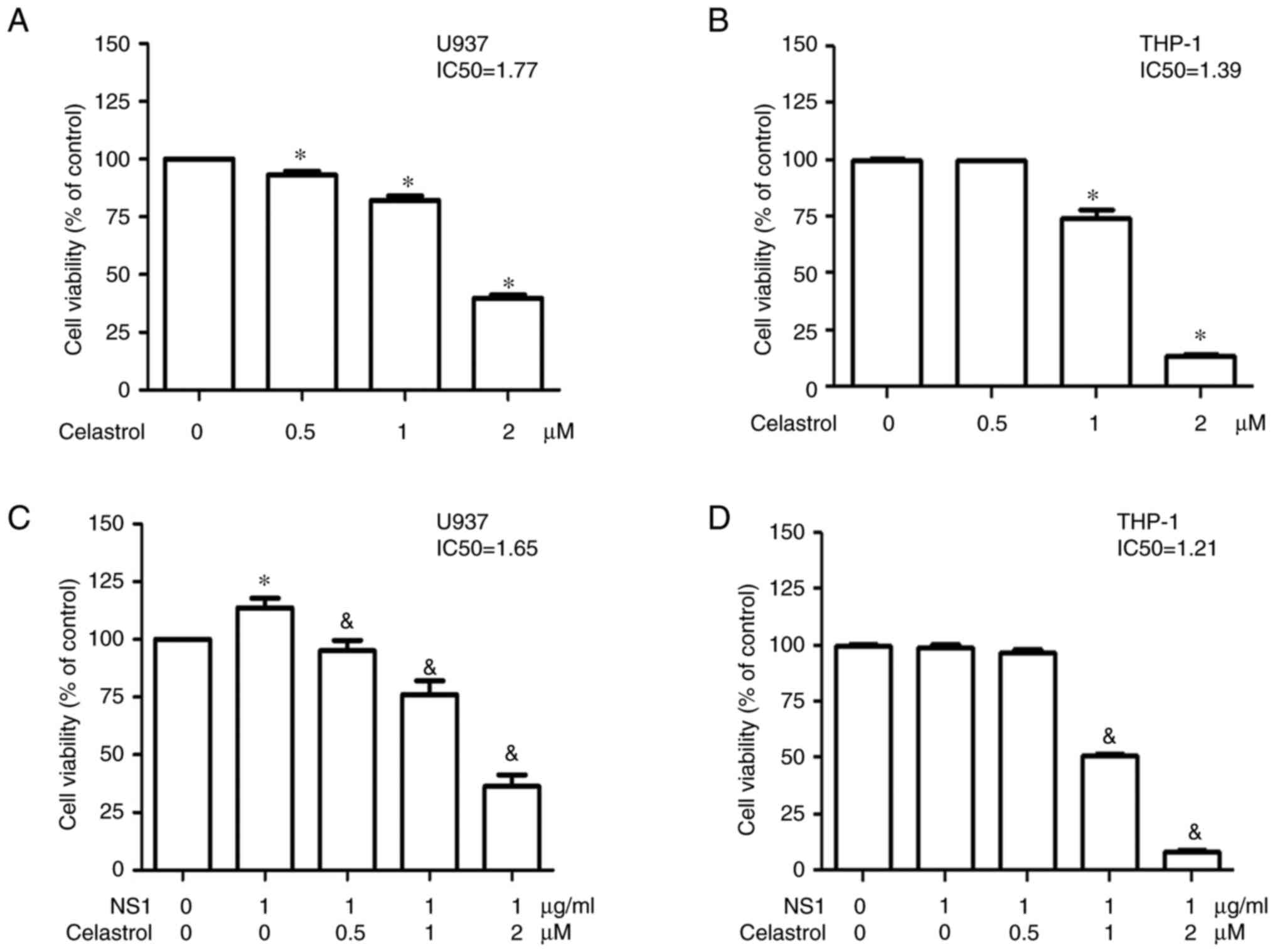
Significantly decreased viability of both U937 and THP-1
macrophages was detected in the presence of celastrol in a
dose-dependent manner with an IC50 value of 1.77 and
1.39, respectively (Fig. 1A and
B). Similar results were observed in both U937 and THP-1
macrophages in the presence of celastrol and 1 µg/ml B19V NS1 with
an IC50 value of 1.65 and 1.21, respectively, (Fig. 1C and D). In the presence of 1 µg/ml
B19V NS1 with no celastrol, no difference in THP-1 macrophage
viability was observed, whereas increased viability of U937
macrophages was detected in the presence of 1 µg/ml B19V NS1, thus
indicating no significant cytotoxicity of 1 µg/ml B19V NS1 in both
cells (Fig. 1C and D).
To further evaluate the effects of celastrol on B19V
NS1-induced macrophage functions, cell migration and phagocytosis
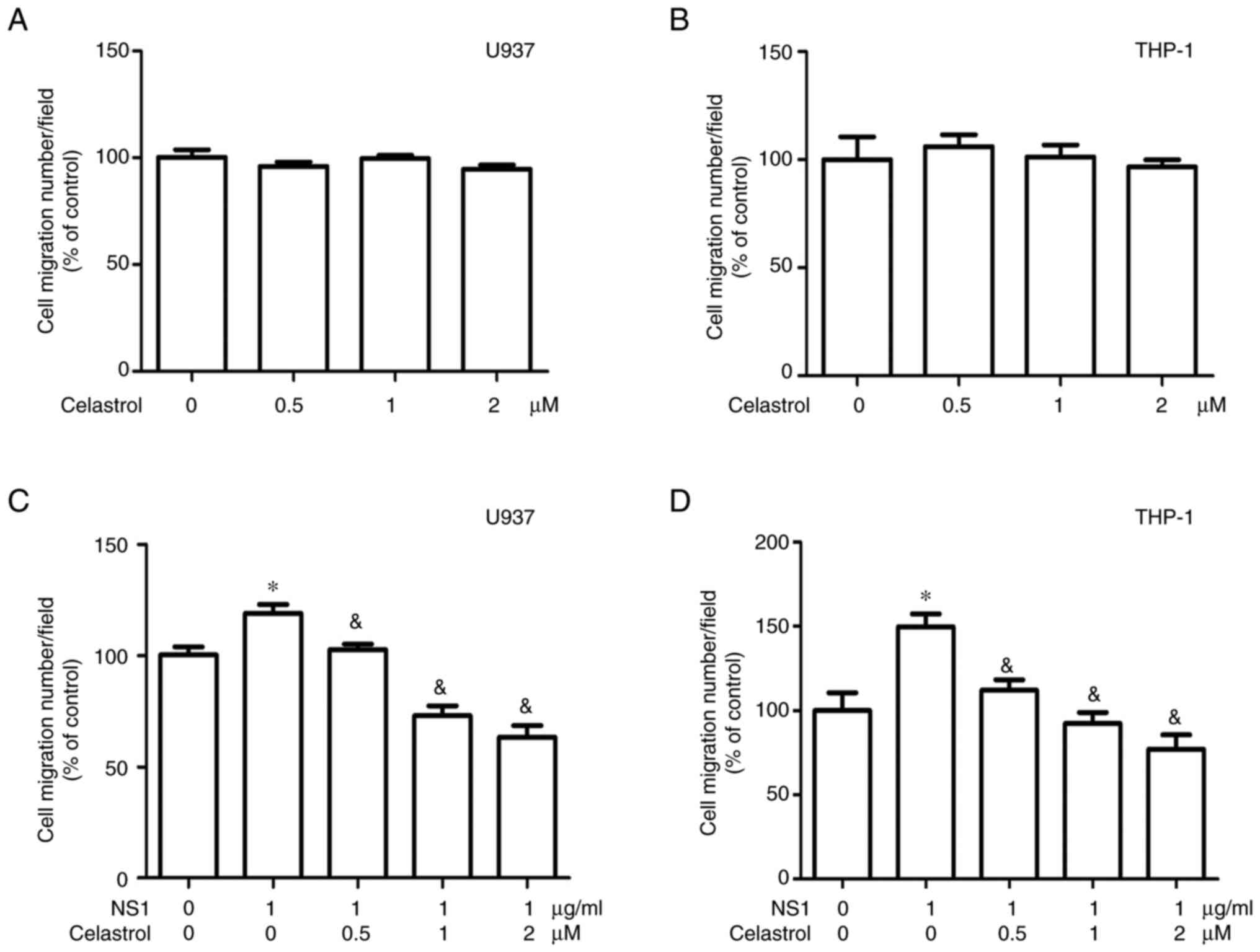
assays were performed. No significant cell migration was observed
in both U937 and THP-1 macrophages in the presence of different
concentrations of celastrol alone (Fig. 2A and B). Significant inhibitory
effects of celastrol on cell migration were detected in both U937
and THP-1 macrophages treated with 1 µg/ml B19V NS1 (Fig. 2C and D). Additionally, celastrol
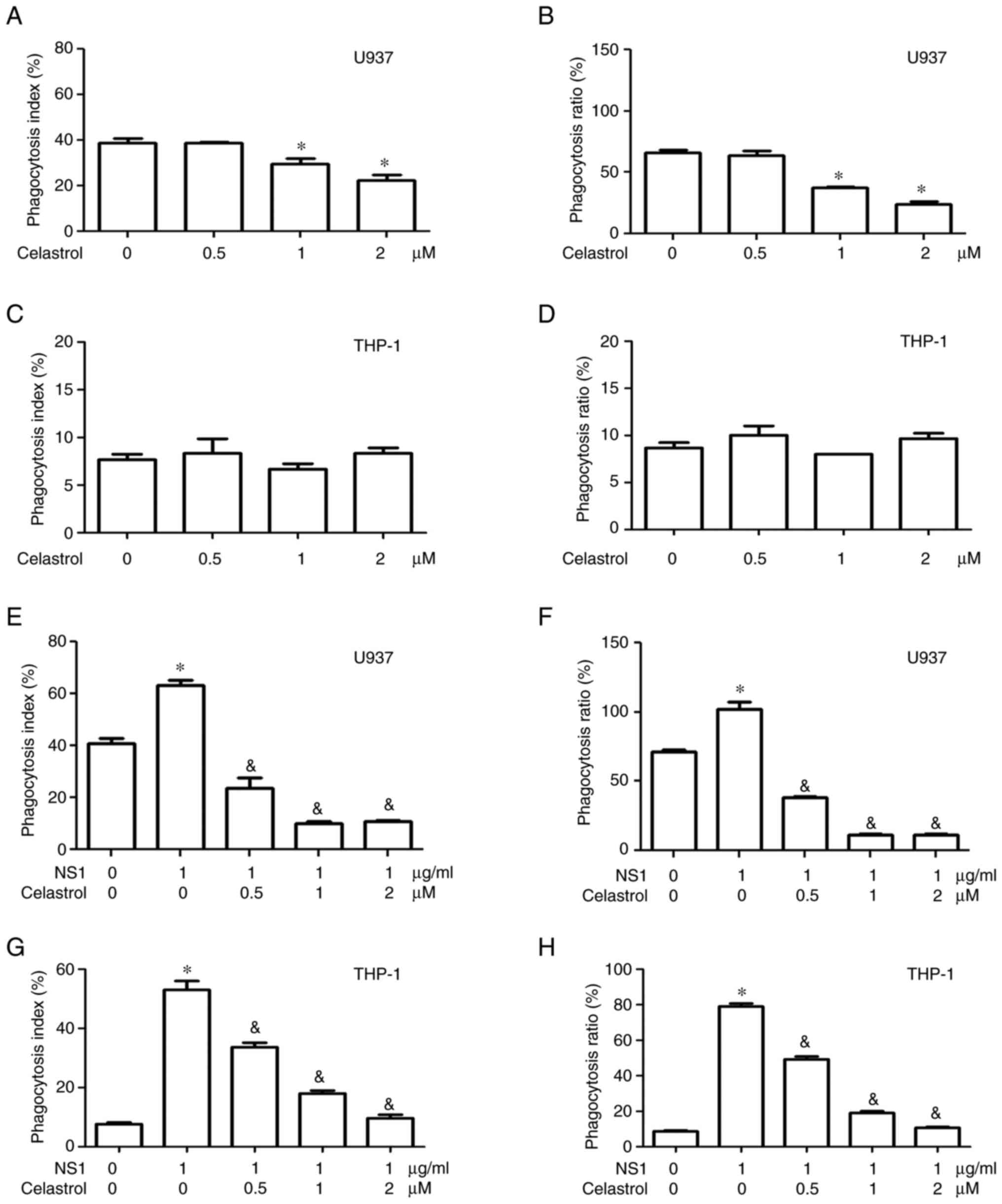
attenuated the phagocytosis index and ratio in U937 macrophages in
a dose-dependent manner but had no significant influence on the
phagocytosis index and ratio in THP-1 macrophages (Fig. 3A-D). Notably, celastrol
significantly decreased the phagocytosis index and ratio in both
U937 and THP-1 macrophages that were activated by 1 µg/ml B19V NS1
(Fig. 3E-H). The representative
images of Figs. 2 and 3 are shown in Figs. S1 and S2.
Effects of celastrol on B19V
NS1-induced inflammatory responses in human macrophages
To evaluate the effects of celastrol on inflammatory
responses in B19V NS1-activated macrophages, MMP-9 activity and
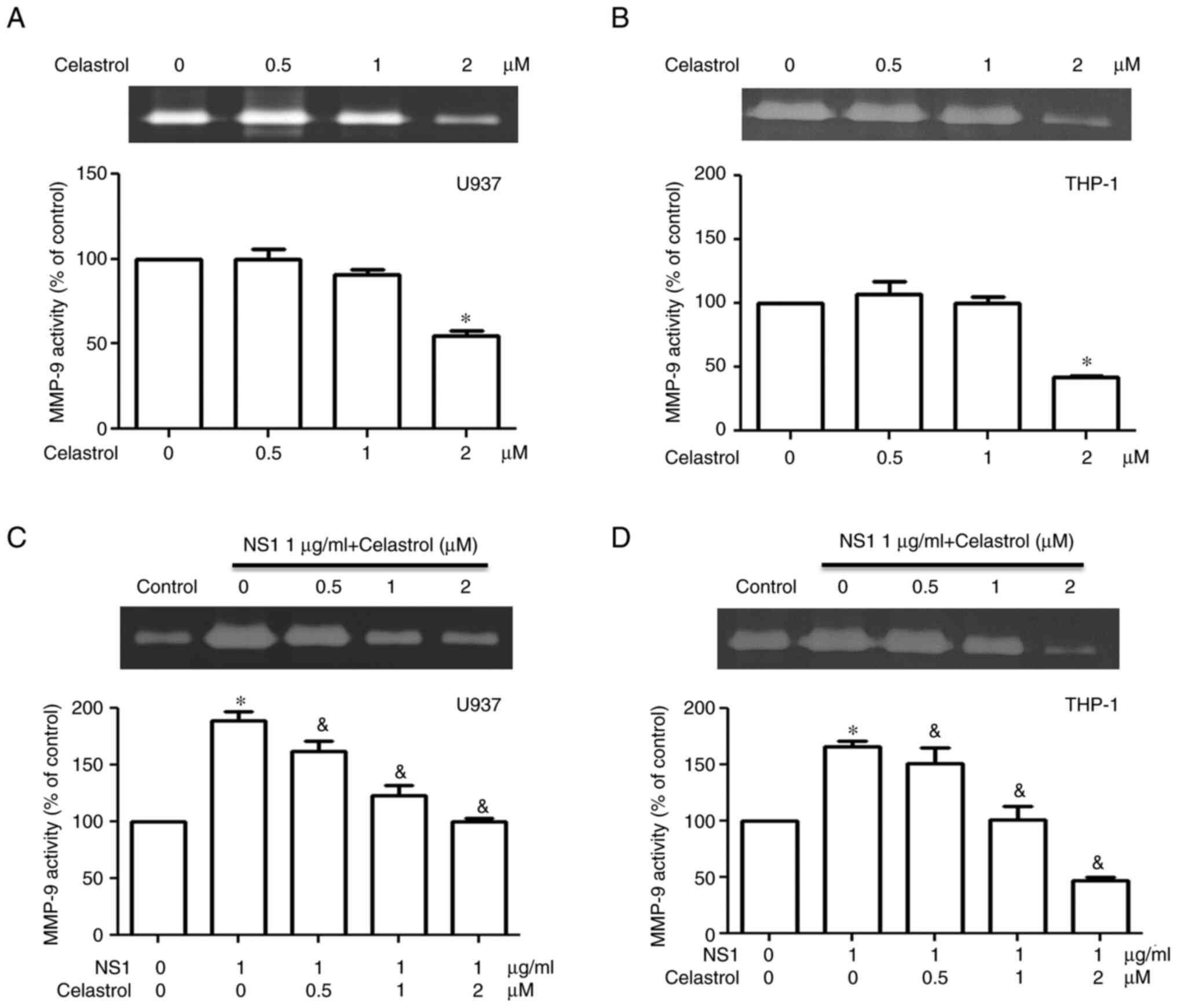
inflammatory cytokine levels were measured. Significantly decreased
MMP-9 activity was detected only in U937 and THP-1 macrophages
treated with 2 µM celastrol, but not in those treated with lower
concentrations of celastrol (Fig. 4A
and B). Significantly decreased MMP-9 activity was observed in
B19V NS1-activated U937 and THP-1 macrophages treated with
celastrol in a dose-dependent manner (Fig. 4C and D). High cytotoxicity on both
U937 and THP-1 macrophages was exhibited with 2 µM celastrol;
therefore, the subsequent experiments in the present study only
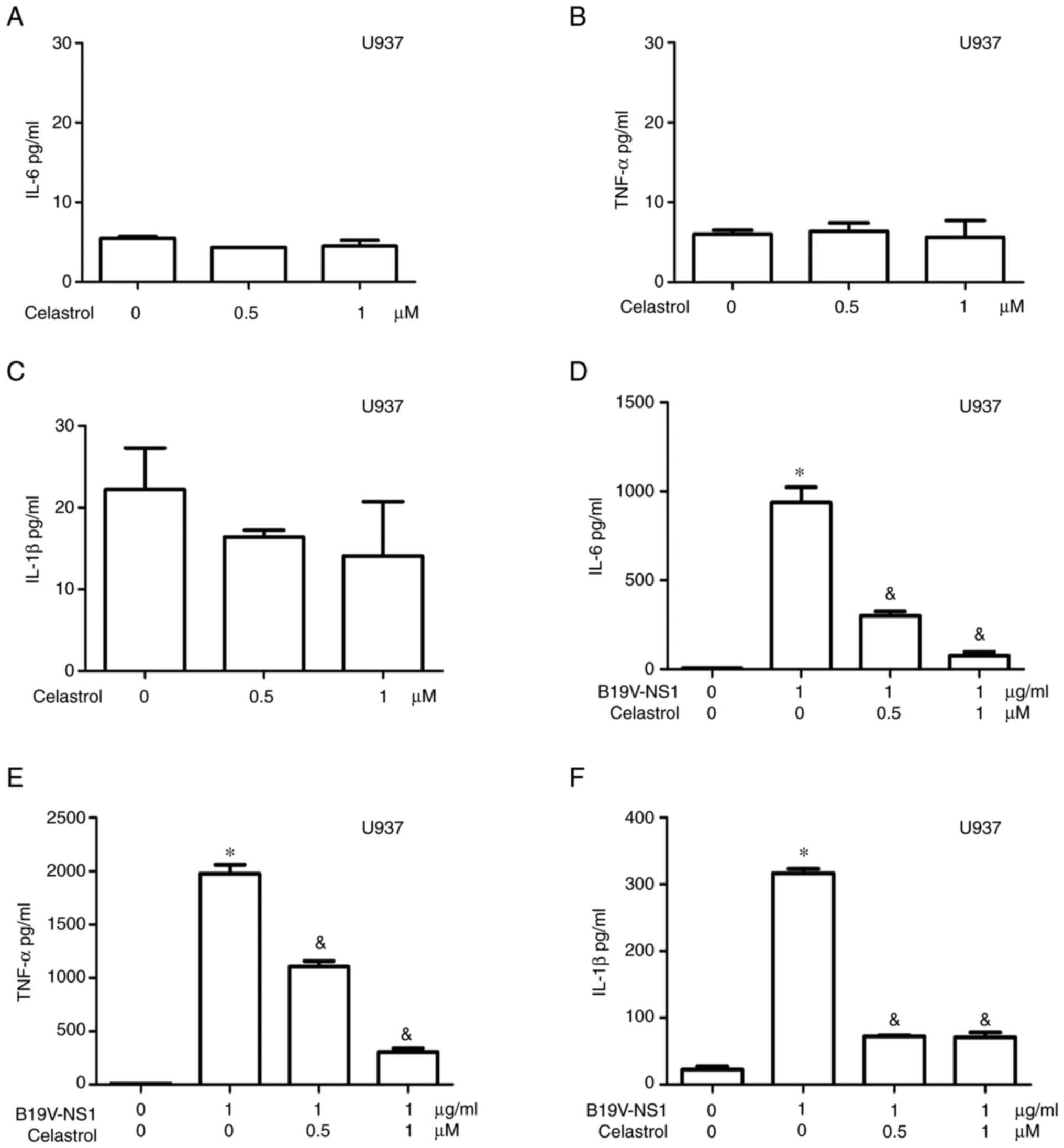
adopted 0.5 and 1 µM celastrol. No statistical differences in IL-6,
TNF-α and IL-1β levels were observed in the medium of U937
macrophages treated with different concentrations of celastrol
(Fig. 5A-C). Significantly
decreased IL-6 and TNF-α levels were detected in the medium of B19V
NS1-activated U937 macrophages in a dose-dependent manner (Fig. 5D and E). Significantly decreased
IL-1β level was detected in the medium of B19V NS1-activated U937
macrophages but it was not dose-dependent (Fig. 5F). Significantly increased IL-6 and
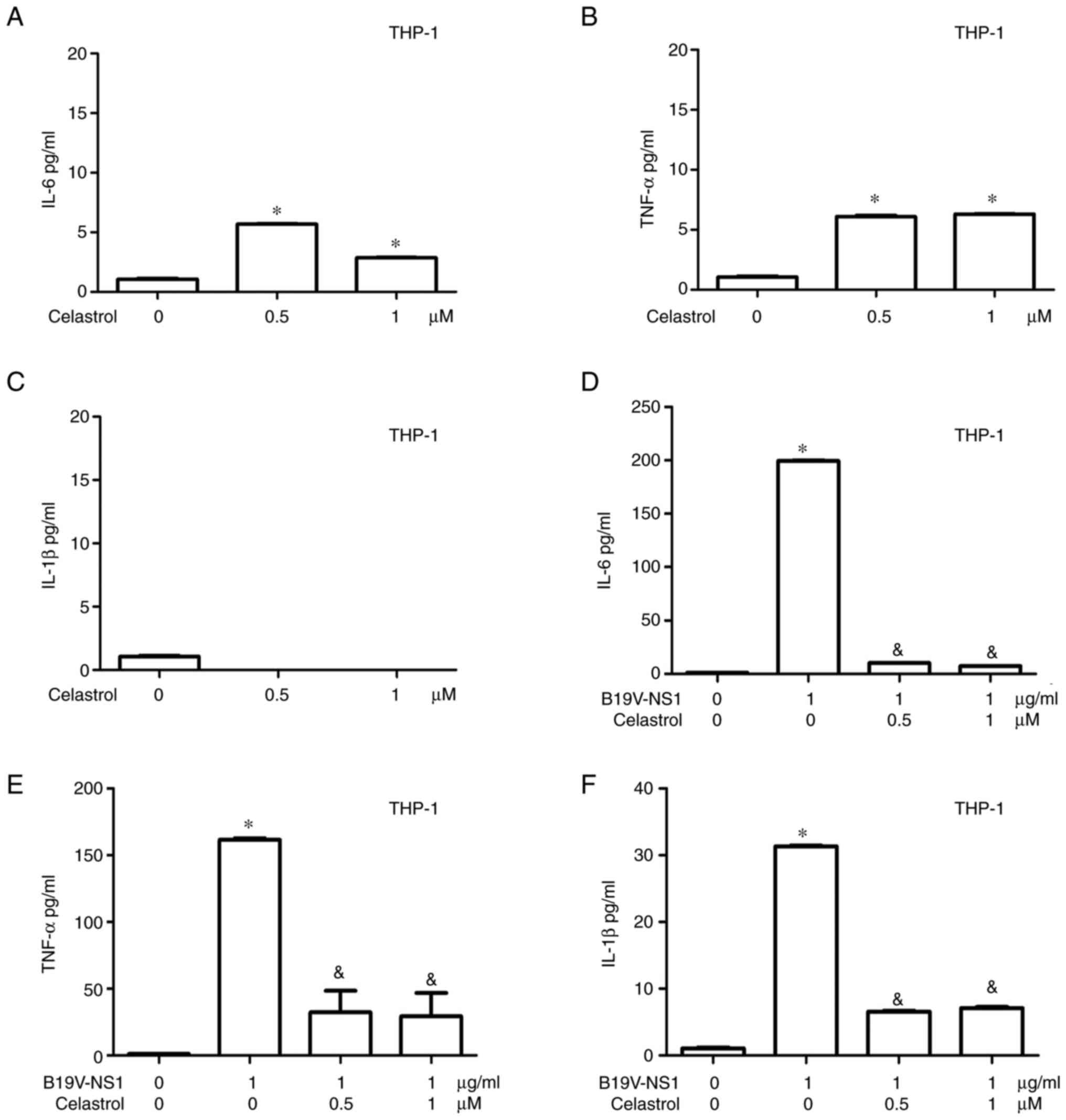
TNF-α levels, but not IL-1β levels, were observed in the medium of
THP-1 macrophages treated with different concentrations of
celastrol (Fig. 6A-C). Conversely,
significantly decreased IL-6, TNF-α and IL-1β levels were detected
in the medium of B19V NS1-activated THP-1 macrophages, but it was
not dose-dependent (Fig.
6D-F).
Effects of celastrol on B19V
NS1-induced inflammasome signaling in human macrophages
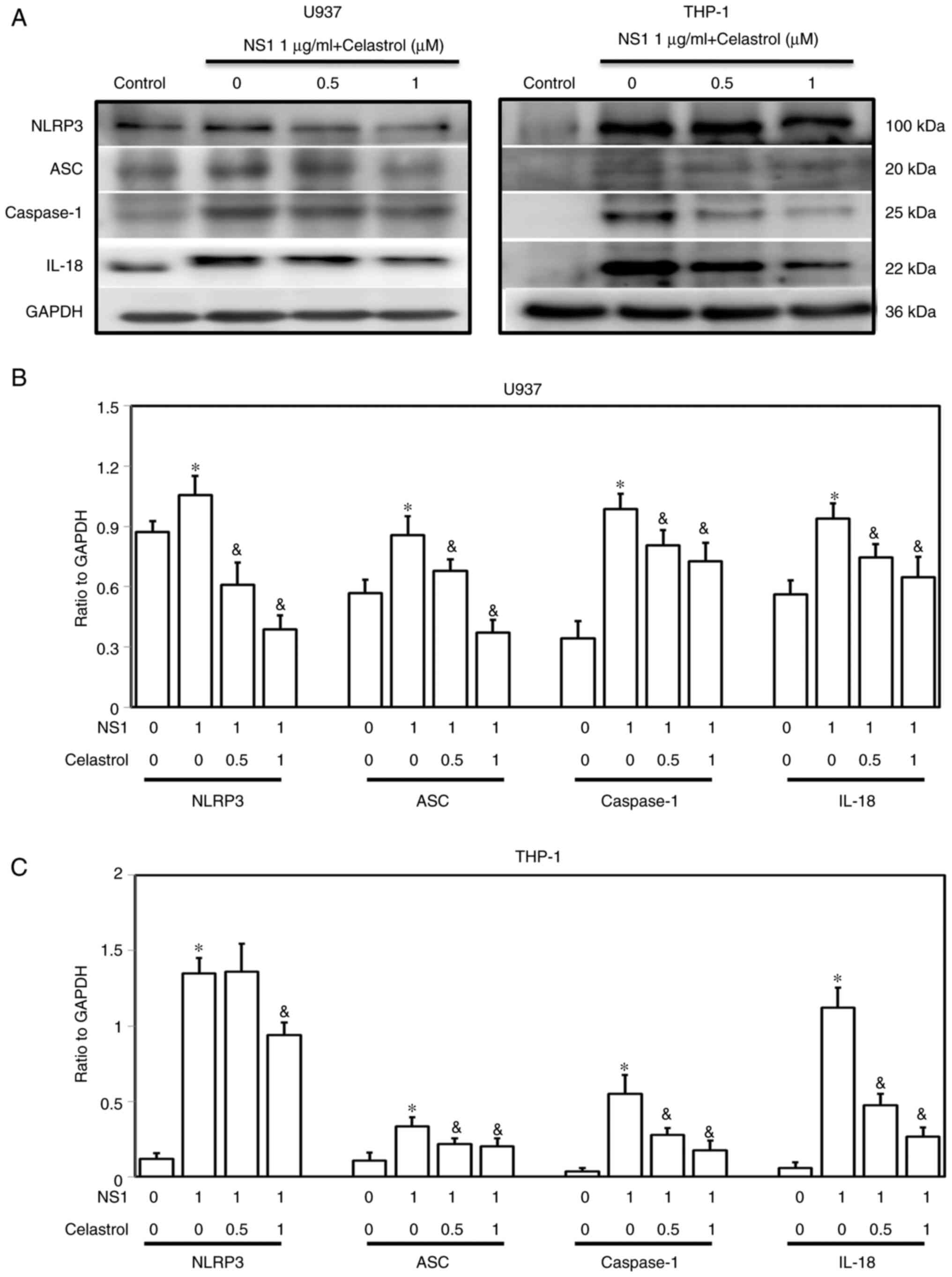
To evaluate the effects of celastrol on B19V
NS1-induced inflammasome signaling, the expression levels of NLRP3,
ASC, caspase-1 and IL-18 proteins were detected. Significantly
increased amounts of NLRP3, ASC, caspase-1 and IL-18 proteins were
observed in both U937 and THP-1 macrophages treated with 1 µg/ml
B19V NS1 as compared with those in the untreated control group
(Fig. 7). Notably, celastrol
significantly decreased the expression levels of NLRP3, ASC,
caspase-1 and IL-18 proteins in both B19V NS1-activated U937 and
THP-1 macrophages in a dose-dependent manner (Fig. 7).
Discussion
Although B19V NS1 is known to evade host innate
immunity by inhibiting the exogenous type I IFN signaling at
interferon-sensitive response element, interferon-stimulated gene,
and signal transducer and activator of transcription 1 (STAT1)
(30), mounting evidence has
demonstrated the pivotal roles of B19V NS1 in various diseases,
especially inflammatory and autoimmune disorders (7,18).
In in vivo studies, B19V NS1 transgenic mice have been
demonstrated to exhibit susceptibility to polyarthritis (31) and are considered a model for
non-immune hydrops fetalis (32).
Additionally, B19V NS1 transgenic mice develop vascular damage in
the heart and have been recognized as a mouse model for myocarditis
associated with B19V infection (33).
Previous studies have reported that B19V NS1 can
induce various inflammatory cytokines in monocytes (17,18,23)
with B19V NS1 associated with elevated Th-17-related cytokines,
such as IL-17, IL-6, IL-1β and TNF-α in patients with systemic
lupus erythematosus presenting with dilated cardiomyopathy
(34). Additionally, B19V NS1 has
been reported to elevate IL-1β and IL-18 levels in adult-onset
Still's disease through activating NLRP3 inflammasome signaling
(29). These findings indicated
that B19V NS1 can induce inflammatory cytokines and inflammasome
signaling in monocytes. Accordingly, the present study reported
that B19V NS1 significantly activated human macrophages by
increasing migration, phagocytosis, inflammatory cytokines and
inflammasome signaling. Notably, celastrol significantly
ameliorated the B19V NS1-induced inflammatory responses in human
U937 and THP-1 macrophages, including decreased cell migration,
MMP-9 activity, phagocytosis, inflammatory cytokines and
inflammasome signaling; therefore, this suggested the therapeutic
potential of celastrol in B19V NS1-related inflammatory
disorders.
Celastrol, a compound from traditional Chinese
herbs, has long been used in the treatment of a number of diseases
due to its significant anti-inflammatory and antioxidant properties
(24). Celastrol is recognized as
a therapeutic agent for numerous pathological diseases, including
arthritis, asthma and autoimmune disorders, through inhibition of
NF-κB (35). A previous study
reported that celastrol inhibits the induction of inducible nitric
oxide synthase by reducing the binding activity of NF-κB in
lipopolysaccharide-treated macrophages (36,37).
Another study also indicated that celastrol can attenuate the
migration and invasion of MCF-7 cells by downregulating
NF-κB-mediated MMP-9 expression (38). Apart from NF-κB signaling, various
signaling pathways, such as MAPK signaling, JAK/STAT signaling and
receptor activator of NF-κB/osteoprotegerin signaling were recently
reported as specific targets for celastrol (39). Accordingly, a new molecular target
for celastrol was reported in a recent study where celastrol
ameliorated type 2 diabetes by blocking carbohydrate response
element-binding protein and inhibiting its nuclear translocation
(40). These findings provide
evidence that support the idea that the anti-inflammatory effects
of celastrol in B19V NS1-activated macrophages are due to the
diverse influences of celastrol through multiple pathways. However,
further investigations are merited to verify the precise mechanisms
of celastrol in ameliorating B19V NS1-induced inflammation.
There were some limitations in the present study.
Firstly, no significant migration in both U937 and THP-1
macrophages was detected in the presence of celastrol alone.
However, significantly decreased migration in B19 NS1-treated U937
and THP-1 macrophages was observed in the presence of celastrol.
Although this finding reveals that celastrol significantly
attenuates the cell migration of both U937 and THP-1 macrophages
activated by 1 µg/ml B19V NS1, further investigations are required
to verify the precise mechanisms of action of celastrol in B19
NS1-induced signaling in U937 and THP-1 macrophages. Additionally,
in vivo studies involving celastrol require further
investigation. To further explore the possibility of the clinical
applications of celastrol, it is important to understand the
toxicity of celastrol, which can be achieved through animal
testing, such as use of collagen-induced arthritis mice. A previous
study has reported that oral administration of 2.5 µg/g/day
celastrol is non-toxic and the lowest effective dosage of celastrol
for rats with adjuvant-induced arthritis. Conversely, a dose of 7.5
µg/g/day can induce severe toxicity, such as thymic and liver
lesions (41). Another report also
indicated that administration of celastrol by intradermal injection
significantly attenuated paw swelling, arthritic scores,
pro-inflammatory cytokines and oxidative stresses in rats with
collagen-induced arthritis (42).
These findings provide insight into the effective and safe dosage
of celastrol for future animal experiments on B19 infection while
avoiding possible complications or adverse events.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was supported by Chung Shan Medical University and
Changhua Christian Hospital cooperative project (grant no.
CSMU-CCH-110-07) and in part by The Ministry of Science and
Technology, Taiwan (grant nos. MOST 108-2314-B-040-018 and
109-2314-B040-021, and NSTC 112-2314-B040-015).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CLH and DYC conceived, reviewed and edited the
manuscript. CCT and JWL performed experiments and analysis of data.
TCH and BST were involved in the study conception and design,
drafting and revising of the manuscript and analysis of data. TCH
and BST confirm the authenticity of all the raw data. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cossart Y: Parvovirus B19 finds a disease.
Lancet. 2:988–989. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Qiu J, Söderlund-Venermo M and Young NS:
Human parvoviruses. Clin Microbiol Rev. 30:43–113. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cohen BJ and Buckley MM: The prevalence of
antibody to human parvovirus B19 in England and Wales. J Med
Microbiol. 25:151–153. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tsujimura M, Matsushita K, Shiraki H, Sato
H, Okochi K and Maeda Y: Human parvovirus B19 infection in blood
donors. Vox Sang. 69:206–212. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brown KE and Young NS: Parvovirus B19 in
human disease. Annu Rev Med. 48:59–67. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Heegaard ED and Brown KE: Human parvovirus
B19. Clin Microbiol Rev. 15:485–505. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lehmann HW, von Landenberg P and Modrow S:
Parvovirus B19 infection and autoimmune disease. Autoimmun Rev.
2:218–223. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Young NS and Brown KE: Parvovirus B19. N
Engl J Med. 350:586–597. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Page C, François C, Goëb V and Duverlie G:
Human parvovirus B19 and autoimmune diseases. Review of the
literature and pathophysiological hypotheses. J Clin Virol.
72:69–74. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ros C, Bieri J and Leisi R: The VP1u of
human parvovirus B19: A multifunctional capsid protein with
biotechnological applications. Viruses. 12:14632020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cotmore SF, McKie VC, Anderson LJ, Astell
CR and Tattersall P: Identification of the major structural and
nonstructural proteins encoded by human parvovirus B19 and mapping
of their genes by procaryotic expression of isolated genomic
fragments. J Virol. 60:548–557. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ozawa K and Young N: Characterization of
capsid and noncapsid proteins of B19 parvovirus propagated in human
erythroid bone marrow cell cultures. J Virol. 61:2627–2630. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kawase M, Momoeda M, Young NS and Kajigaya
S: Most of the VP1 unique region of B19 parvovirus is on the capsid
surface. Virology. 211:359–366. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tzang BS, Tsay GJ, Lee YJ, Li C, Sun YS
and Hsu TC: The association of VP1 unique region protein in acute
parvovirus B19 infection and anti-phospholipid antibody production.
Clin Chim Acta. 378:59–65. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Astell CR, Luo W, Brunstein J and St Amand
J: B19 parvovirus: biochemical and molecular features. Human
parvovirus B19. Anderson LJ and Young NS: Karger Publishers; Basel,
Switzerland: pp. 16–41. 1997, View Article : Google Scholar
|
|
16
|
Gareus R, Gigler A, Hemauer A,
Leruez-Ville M, Morinet F, Wolf H and Modrow S: Characterization of
cis-acting and NS1 protein responsive elements in the P6 promoter
of parvovirus B19. J Virol. 72:609–616. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mitchell LA: Parvovirus B19 nonstructural
(NS1) protein as a transactivator of interleukin-6 synthesis:
Common pathway in inflammatory sequelae of human parvovirus
infections? J Med Virol. 67:267–274. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jalali S, Farhadi A, Rafiei Dehbidi G,
Farjadian S, Sharifzadeh S, Ranjbaran R, Seyyedi N, Namdari S and
Behzad-Behbahani A: The pathogenic aspects of human parvovirus B19
NS1 protein in chronic and inflammatory diseases. Interdiscip
Perspect Infect Dis. 2022:16399902022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Moffatt S, Tanaka N, Tada K, Nose M,
Nakamura M, Muraoka O, Hirano T and Sugamura K: A cytotoxic
nonstructural protein, NS1, of human parvovirus B19 induces
activation of interleukin-6 gene expression. J Virol. 70:8485–8491.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Moffatt S, Yaegashi N, Tada K, Tanaka N
and Sugamura K: Human parvovirus B19 nonstructural (NS1) protein
induces apoptosis in erythroid lineage cells. J Virol.
72:3018–3028. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hsu TC, Wu WJ, Chen MC and Tsay GJ: Human
parvovirus B19 non-structural protein (NS1) induces apoptosis
through mitochondria cell death pathway in COS-7 cells. Scand J
Infect Dis. 36:570–577. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Poole BD, Kivovich V, Gilbert L and Naides
SJ: Parvovirus B19 nonstructural protein-induced damage of cellular
DNA and resultant apoptosis. Int J Med Sci. 8:88–96. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fu Y, Ishii KK, Munakata Y, Saitoh T, Kaku
M and Sasaki T: Regulation of Tumor necrosis factor alpha promoter
by human parvovirus B19 NS1 through activation of AP-1 and AP-2. J
Virol. 76:5395–5403. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cascão R, Fonseca JE and Moita LF:
Celastrol: A spectrum of treatment opportunities in chronic
diseases. Front Med (Lausanne). 4:692017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kun-Ming C, Chih-Hsien C, Chen-Fang L,
Ting-Jung W, Hong-Shiue C and Wei-Chen L: Potential anticancer
effect of celastrol on hepatocellular carcinoma by suppressing
CXCR4-related signal and impeding tumor growth in vivo. Arch Med
Res. 51:297–302. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cui Y, Jiang X and Feng J: The therapeutic
potential of triptolide and celastrol in neurological diseases.
Front Pharmacol. 13:10249552022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li Z, Zhang J, Duan X, Zhao G and Zhang M:
Celastrol: A promising agent fighting against cardiovascular
diseases. Antioxidants (Basel). 11:15972022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tseng CK, Hsu SP, Lin CK, Wu YH, Lee JC
and Young KC: Celastrol inhibits hepatitis C virus replication by
upregulating heme oxygenase-1 via the JNK MAPK/Nrf2 pathway in
human hepatoma cells. Antiviral Res. 146:191–200. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen DY, Chen YM, Chen HH, Hsieh CW, Gung
NR, Hung WT, Tzang BS and Hsu TC: Human parvovirus B19
nonstructural protein NS1 activates NLRP3 inflammasome signaling in
adult-onset Still's disease. Mol Med Rep. 17:3364–3371.
2018.PubMed/NCBI
|
|
30
|
Wu J, Chen X, Ye H, Yao M, Li S and Chen
L: Nonstructural protein (NS1) of human parvovirus B19 stimulates
host innate immunity and blunts the exogenous type I interferon
signaling in vitro. Virus Res. 222:48–52. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Takasawa N, Munakata Y, Ishii KK,
Takahashi Y, Takahashi M, Fu Y, Ishii T, Fujii H, Saito T, Takano
H, et al: Human parvovirus B19 transgenic mice become susceptible
to polyarthritis. J Immunol. 173:4675–4683. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chisaka H, Morita E, Murata K, Ishii N,
Yaegashi N, Okamura K and Sugamura K: A transgenic mouse model for
non-immune hydrops fetalis induced by the NS1 gene of human
parvovirus B19. J Gen Virol. 83((Pt 2)): 273–281. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bachelier K, Biehl S, Schwarz V,
Kindermann I, Kandolf R, Sauter M, Ukena C, Yilmaz A, Sliwa K, Bock
CT, et al: Parvovirus B19-induced vascular damage in the heart is
associated with elevated circulating endothelial microparticles.
PLoS One. 12:e01763112017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen DY, Chen YM, Tzang BS, Lan JL and Hsu
TC: Th17-related cytokines in systemic lupus erythematosus patients
with dilated cardiomyopathies: A possible linkage to parvovirus B19
infection. PLoS One. 9:e1138892014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nam NH: Naturally occurring NF-kappaB
inhibitors. Mini Rev Med Chem. 6:945–951. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dirsch VM, Kiemer AK, Wagner H and Vollmar
AM: The triterpenoid quinonemethide pristimerin inhibits induction
of inducible nitric oxide synthase in murine macrophages. Eur J
Pharmacol. 336:211–217. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jin HZ, Hwang BY, Kim HS, Lee JH, Kim YH
and Lee JJ: Antiinflammatory constituents of Celastrus orbiculatus
inhibit the NF-kappaB activation and NO production. J Nat Prod.
65:89–91. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kim Y, Kang H, Jang SW and Ko J: Celastrol
inhibits breast cancer cell invasion via suppression of
NF-ĸB-mediated matrix metalloproteinase-9 expression. Cell Physiol
Biochem. 28:175–184. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Venkatesha SH, Dudics S, Astry B and
Moudgil KD: Control of autoimmune inflammation by celastrol, a
natural triterpenoid. Pathog Dis. 74:ftw0592016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhou D, Li X, Xiao X, Wang G, Chen B, Song
Y, Liu X, He Q, Zhang H, Wu Q, et al: Celastrol targets the
ChREBP-TXNIP axis to ameliorates type 2 diabetes mellitus.
Phytomedicine. 110:1546342023. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Cascão R, Vidal B, Carvalho T, Lopes IP,
Romão VC, Goncalves J, Moita LF and Fonseca JE: Celastrol efficacy
by oral administration in the adjuvant-induced arthritis model.
Front Med. (Lausanne). 7:4552020.
|
|
42
|
Gao Q, Qin H, Zhu L, Li D and Hao X:
Celastrol attenuates collagen-induced arthritis via inhibiting
oxidative stress in rats. Int Immunopharmacol. 84:1065272020.
View Article : Google Scholar : PubMed/NCBI
|