Introduction
Epithelial-mesenchymal transition (EMT) is an
important process of embryonic development and a normal
physiological phenomenon associated with tissue repair (1). However, pathological EMT has been
shown to exert a key role in the occurrence and development of
numerous lung diseases, including asthma, acute respiratory
distress syndrome and chronic obstructive pulmonary disease
(2–4).
It has previously been demonstrated that the
inflammatory microenvironment is a decisive factor in inducing
pathological EMT (5). The activity
of lipopolysaccharide (LPS) is significantly affected by changes in
sugar composition, the length and arrangement (as well as the
modification) of the lipid A, core oligosaccharides and the
glycochain structure of O antigen (6), among which the number of lipid A acyl
chains present is an important determinant of immune activation
(7). A previous study showed that
hexacylated (proacylated) LPS (P-LPS) was one of the most effective
Toll-like receptor 4 (TLR4) agonists. By contrast, the pentacylated
and tetracylated lipopolysaccharide (A-LPS) exerted a competitive
antagonistic effect on the pro-inflammatory activity of P-LPS
(8).
Intestinal flora fulfill a crucial role in
maintaining host intestinal homeostasis. Under normal
circumstances, host intestinal inflammation does not occur, which
is due to the optimal control of P-LPS activity via the dynamic
balance of various intestinal flora, which enable the host to
maintain intestinal homeostasis. Among the human intestinal flora,
the phylum Proteobacteria is a major contributor to P-LPS
synthesis, whereas the phylum Bacteroidetes is a major contributor
to A-LPS synthesis, producing A-LPS that antagonizes the
pro-inflammatory activity of P-LPS, thereby driving the process of
immunosilencing throughout the intestinal environment. In the case
of colitis regulated by A-LPS, mice with low abundance of
Proteobacteria and high abundance of Bacteroidetes show low levels
of intestinal endotoxin and maintained mucosal immune homeostasis
(9). Conversely, mice with high
abundance of Proteobacteria and low abundance of Bacteroidetes are
more likely to develop colitis (9). Similar to the situation in the gut,
LPS in the lung is mainly derived from the intestinal flora and
TLR4 is widely distributed in lung tissue (10,11).
However, to the best of the authors' knowledge,
whether A-LPS can effectively prevent P-LPS-induced pulmonary
inflammation and pathological EMT has yet to be reported.
Therefore, in the present study, lung epithelial cells and mouse
lung tissues were induced by A-LPS and P-LPS either alone or in
combination to explore whether A-LPS is able to effectively prevent
the P-LPS-induced inflammatory response and pathological EMT.
Material and methods
Bacterial strains and LPS
For the present study, B. vulgatus was
purchased from the China Typical Culture Preservation Center
(strain preservation number. CCTCC AB 2015378). BV-LPS purification
was performed according to the hot phenol-water method (12), whereas EC-LPS extracted from the
membrane of E. coli 055:B5 was purchased from
MilliporeSigma.
Cell culture and viability
examination
Human type II alveolar epithelial A549 cells were
purchased from the Type Culture Collection of the Chinese Academy
of Sciences and cultured in DMEM medium supplemented with 10% fetal
bovine serum (cat. no. S9030; Beijing Solarbio Technology Co.,
Ltd.), 100 U/ml penicillin and 100 ng/ml streptomycin at 37°C in a
humidified atmosphere comprising 5% CO2/95% air. Cell
viability was assessed using the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay (cat. no. M6494; Thermo Fisher Scientific, Inc.). After
seeding the A549 cells (2×104 cells/ml) into the wells
of a 96-well plate, the cells were treated with BV-LPS (0, 2.5, 5,
10, 20, 40, 80, 160 or 320 µg/ml) or EC-LPS (0, 1, 2, 5, 10, 15 or
20 µg/ml) for 72 h. After 72 h, the medium was removed and the
cells were washed three times with phosphate-buffered saline (PBS).
Subsequently, 20 µl MTT (final concentration, 5 mg/ml) was added to
each well and the plates were incubated at 37°C for 4 h. The MTT
solution was then removed and 100 µl dimethyl sulfoxide was added
to dissolve the colored formazan crystals. Finally, the optical
density was measured at a wavelength of 490 nm using a microplate
reader (Thermo Fisher Scientific, Inc.) and cell viability was
calculated as the percentage of the absorbance of untreated control
cells.
Induction of A549 cells by drug
administration
The cells were divided into the following four
experimental groups: Control group (Control), EC-LPS group (EC),
BV-LPS group (BV) and combined group (EC-BV). To observe the effect
of BV-LPS on the prevention of EMT induced by EC-LPS in A549 cells,
after the cells had reached 80% confluence they were treated with
EC-LPS (10 µg/ml) and BV-LPS (60 µg/ml) (13,14)
either alone or in combination for up to 48 h. The morphological
changes of cells were subsequently observed and recorded using an
inverted phase contrast microscope (DP73; Olympus Corporation). The
extent of inflammation and EMT were then detected by reverse
transcription-quantitative (RT-q)PCR, immunofluorescence staining
and western blotting analyses, as detailed below.
Animal feeding and experimental
protocols
A total of 40 nine-week old male C57BL/6 mice (18–20
g) were obtained from the Institute of Laboratory Animals, Guizhou
University of Traditional Chinese Medicine. The mice were housed
under conditions of a 12-h light/dark cycle with free access to tap
water and standard chow (cat. no. D12450B; Research Diets, Inc.) at
a temperature of 22±1°C and a relative humidity of 50–70% and
received one daily health observation. The present study was
approved by the Experimental Animal Ethics Committee of Guizhou
University of Traditional Chinese Medicine (approval number:
20220039).
Induction of mouse lung tissue by drug
administration
The mice were randomly divided into the Control, EC,
BV and EC-BV groups as mentioned above, with 10 mice in each group.
To observe the effect of BV-LPS on preventing EMT induced by EC-LPS
in lung tissue of mice, after following a protocol of adaptive
feeding for 1 week, the mice were anesthetized with 1% (v/v) sodium
pentobarbital injected intraperitoneally (40 mg/kg) and the mice
were subsequently administered EC-LPS (5 mg/kg) and BV-LPS (30
mg/kg) (13,15) either alone or in combination, with
pulmonary administration via airway intubation (administration
volume; 2.5 ml/kg) (16,17). After 28 days of normal feeding,
none of the mice in the study reached the humane endpoints
(including labored breathing, nasal discharge, lethargy or
persistent recumbency, difficulty with ambulation or an inability
to obtain food or water). Mice were anesthetized with 20% urethane
injected intraperitoneally (2,000 mg/kg) prior to sacrifice of the
mice by cervical dislocation of the spine. Subsequently, the mice
were weighed, dissected and the lung tissue was collected for
weighing. The left-lobe lung was then fixed in 4% paraformaldehyde
solution (Beijing Solarbio Technology Co., Ltd.) at room
temperature for 48 h, whereas the right-lobe lung was directly
frozen in liquid nitrogen and later transferred to the refrigerator
at −80°C for storage. The percentages of lung mass/body mass were
used for the lung index.
Cell morphological observation
To obtain the morphological images, A549 cells were
visualized and images captured using a phase-contrast microscope
equipped with a digital camera (Leica DM IL; Leica Microsystems
GmbH).
Immunofluorescence staining
A549 cells were seeded into 24-well plates with
adhesive slides at the density of 1×103 cells/ml and the
cells were divided into the Control, EC, BV and EC-BV groups, with
3 wells in each group. After the A549 cells had been attached to
the plates (for 6 h), with the exception of the Control group, the
EC-LPS group, BV-LPS group and EC-BV combined group were
respectively induced by the addition of EC-LPS (10 µg/ml) alone,
BV-LPS (60 µg/ml) alone, or a combination of EC-LPS and BV-LPS.
After 48 h induction, the medium was discarded, 1 ml 4%
paraformaldehyde was added and fixed at room temperature for 30
min. At room temperature, 0.5% Triton X-100 (Beijing Solarbio
Technology, Co., Ltd.) was added and allowed to permeate the cells
for 20 min, followed by washing of the cells thrice, for 5 min each
time, with phosphate-buffered normal saline (PBS; Beijing Solarbio
Technology Co., Ltd.) and sealing with immunochromic sealing
solution (Beyotime Institute of Biotechnology) for 60 min. The
immunochromic sealing solution was subsequently removed and rabbit
polyclonal antibodies against epithelial cadherin (E-cadherin;
1:200; cat. no. AF0131; Affinity Biosciences, Ltd.), α-smooth
muscle actin (α-SMA; 1:200; cat. no. AF1032; Affinity Biosciences,
Ltd.) and vimentin (1:200; cat. no. AF7013; Affinity Biosciences,
Ltd.) were then added overnight at 4°C. The cells were subsequently
washed five times (5 min each time) with cold PBS. FITC-labeled
goat anti-rabbit IgG secondary antibody (1:200; cat. no. S0008;
Affinity Biosciences, Ltd.) was incubated with the cells at room
temperature and the cells were kept in the dark for 2 h. The nuclei
were then stained with DAPI (Beyotime Institute of Biotechnology)
for 5 min at room temperature, prior to removal of the DAPI
staining solution and washing the cells three times with PBS. The
cell slides were removed from the plate hole and the side
containing the cells was then placed on a slide containing an
anti-fluorescence quenching agent (cat. no. P0126; Beyotime
Institute of Biotechnology). Finally, images were captured and
analyzed using a fluorescence microscope (Olympus Corporation) and
ImageJ software vij149 (National Institutes of Health).
RT-qPCR
Lung tissues or A549 cells were treated with
TriQuick Reagent total RNA extraction reagent (Beijing Solarbio
Technology Co., Ltd.) and lung tissues were crushed by passing
through a high-throughput tissue grinder (Ningbo Xinzhi
Biotechnology Co., Ltd.) at 4°C. Subsequently, the RNA was
extracted according to the instructions provided in the kit. The
first-strand cDNA was then synthesized using a HiFiScript gDNA
Removal cDNA Synthesis Kit (Beijing ComWin Biotech Co., Ltd.)
according to the instructions described by the manufacturer.
RT-qPCR was performed with UltraSYBR Mixture (Beijing ComWin
Biotech Co., Ltd.) and a CFX96 quantitative PCR instrument (Bio-Rad
Laboratories, Inc.), according to the manufacturer's instructions.
The qPCR was performed at 95°C for 10 min, followed by 40 cycles at
95°C for 15 sec and 60°C for 1 min. After having performed the
amplification reaction, the average threshold cycle number was used
as the Ct value of each sample. GAPDH was used as the reference
gene for normalization of the mRNA levels and the relative
expression levels of the target genes were calculated using the
2−ΔΔCq method (18).
The RT-qPCR experiments were repeated for three times. Primer
synthesis was carried out by Shanghai Shengong Bioengineering Co.,
Ltd. and the primer sequences for the human and mice genes are
shown in Table I.
 | Table I.Primer sequences used in the present
study. |
Table I.
Primer sequences used in the present
study.
| Gene name | Forward, 5′→3′ | Reverse, 5′→3′ |
|---|
| IL-1β (h) |
ACGATGCACCTGTACGATCA |
TCTTTCAACACGCAGGACAG |
| IL-6 (h) |
GATGAGTACAAAAGTCCTGATCCA |
CTGCAGCCACTGGTTCTGT |
| TNF-α (h) |
CTCCTCACCCACACCATCAGCCGCA |
ATAGATGGGCTCATACCAGGGCTTG |
| GAPDH (h) |
AACAGCGACACCCACTCCTC |
GGAGGGGAGATTCAGTGTG |
| IL-1β (m) |
CAAGGAGAACCAAGCAACGA |
TTTCATTACACAGGACAGGTATAGA |
| IL-6 (m) |
ACTTCCATCCAGTTGCCTTCTTGG |
TTAAGCCTCCGATTGTGAAGTG |
| TNF-α (m) |
AGGTTCTCTTCAAGGGACAA |
GACTTTCTCCTGGTATGAGATAG |
| TLR4 (m) |
CGCTTTCACCTCTGCCTTCAC |
TTGCCGTTTCTTGTTCTTCTTC |
| BAMBI (m) |
CTCAAATTCCCCACTCACCCA |
GCTGATACCTGTTTCCTTGTCCTG |
| Snail (m) |
TTTACCTTCCAGCAGCCCTA |
CCCACTGTCCTCATCTGACA |
| GAPDH (m) |
CCTCGTCCCGTAGACAAAATG |
TCTCCACTTTGCCACTGCAA |
Western blot analysis
Total protein was extracted from lung tissue or A549
cells and the protein concentration was determined by following the
instructions in the protein extraction kit (Beijing Solarbio
Technology Co., Ltd.) and the BCA protein quantification kit
(Beijing Solarbio Technology Co., Ltd.), respectively. According to
the determined protein concentration, 1,000 µg sample was diluted
to 200 µl with PBS solution and then 50 µl 5X protein loading
buffer (Beijing Solarbio Technology Co., Ltd.) was added, mixed and
boiled in boiling water for 5 min, prior to storage at −20°C for
later use. The samples (10 µl/40 µg)were then subjected to SDS-PAGE
(8%) before being transferred on to a PVDF membrane and blocking
with 5% skimmed milk powder for 2 h at room temperature.
Subsequently, the protein samples were incubated with rabbit
polyclonal antibodies against E-cadherin (1:1,000; cat. no. AF0131;
Affinity Biosciences, Ltd.), α-SMA (1:1,000; cat. no. AF1032;
Affinity Biosciences, Ltd.), vimentin (1:1,000; cat. no. AF7013;
Affinity Biosciences, Ltd.) and GAPDH (1:5,000; cat. no. AP0063;
Biogot Technology, Co, Ltd) overnight at 4°C. The membranes were
then washed 3 times with TBST (1X) containing 0.05% Tween20 (10 min
each wash) and incubated with goat anti-rabbit IgG (H+L)-HRP
secondary antibody (1:5,000; cat. no. BS13278; Biogot Technology,
co, Ltd, USA) for 2 h at room temperature. Exposure development was
performed using ECL chemiluminescent color developer (Biogot
Technology, Co, Ltd.) and ChemicDoc XRS+ imager (Bio-Rad
Laboratories, Inc.). After scanning the exposed protein bands,
ImageJ software vij149 (National Institutes of Health) was used to
calculate the optical density of the protein bands and the levels
of the target proteins of interest were normalized against that of
GAPDH as the control protein.
Histological and immunohistochemical
analysis
Lung tissue fixed at room temperature for 48 h with
4% paraformaldehyde, followed by dehydration (slides washed in 100,
95, 80 and 75% alcohol for 2 min each), embedding and sectioning (5
µm-thick sections). To observe the histomorphology of lung injury,
hematoxylin and eosin (H&E) (cat. no. G1003; Wuhan Servicebio
Technology Co., Ltd.) stained for 5 min at room temperature,
following the manufacturer's protocol. The lung sections were also
immunostained with rabbit polyclonal antibodies raised against
IL-1β (cat. no. P420B; Thermo Fisher Scientific, Inc.), IL-6 (cat.
no. GB11117; Wuhan Servicebio Technology Co., Ltd.), TNF-α (cat.
no. GB11188; Wuhan Servicebio Technology Co., Ltd.), TLR4 (cat. no.
GB11519; Wuhan Servicebio Technology Co., Ltd.), bone morphogenic
protein and activin membrane-bound inhibitor (BAMBI) (cat. no.
PA5-109443; Thermo Fisher Scientific, Inc.) and Snail (cat. no.
GB11132; Wuhan Servicebio Technology Co., Ltd.) overnight at 4°C,
all antibodies at a dilution of 1:100. Subsequently, the lung
sections were incubated at room temperature with biotinylated
secondary antibodies (cat. no. GB23303; Wuhan Servicebio Technology
Co., Ltd.) for 50 min, followed by staining with
3,3′-diaminobenzidine (DAB) solution (cat. no. G1212; Wuhan
Servicebio Technology Co., Ltd.) to detect the avidin-biotin
complex signal. Finally, images were captured and analyzed using a
microscope (E100; Nikon Corporation) equipped with a digital camera
and ImageJ software vij149 (National Institutes of Health).
Statistical analysis
All data are shown as the mean ± standard deviation
and statistical analyses were performed using statistical software
(SPSS version 26.0; IBM Corp.). Statistically significant
differences among the results were analyzed by one-way analysis of
variance (ANOVA) with Tukey's post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
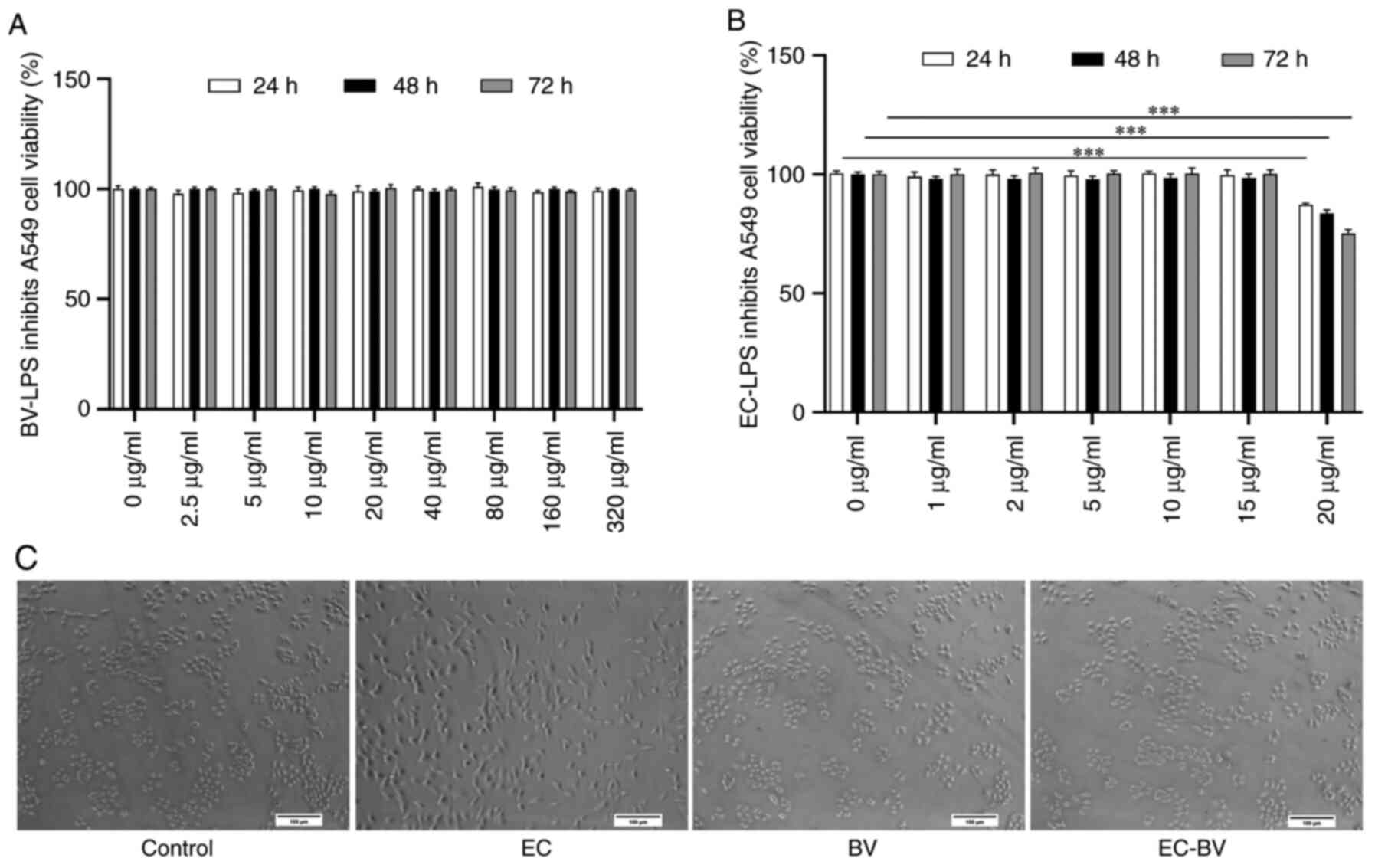
BV-LPS abolishes EC-LPS-induced
morphological changes in A549 cells
EC-LPS has been demonstrated to be an effective
inducer of EMT in epithelial cells (16,19,20).
In the present series of experiments, A549 cells were cultured in
different concentrations of EC-LPS and BV-LPS for 72 h in order to
determine the appropriate dose required for the EMT-induction model
of EC-LPS and the appropriate dose for administration of BV-LPS. As
shown in Fig. 1A and B, the
results of MTT assay showed that 0–15 µg/ml EC-LPS and 0–320 µg/ml
BV-LPS had no effect on the viability of A549 cells. However, it
was also noted that incubation of A549 cells with 10 µg/ml EC-LPS
for 48 h resulted in a more stretched, or elongated, spindle-shaped
morphology of the cells (Fig. 1C),
which is a typical feature of EMT (21). However, this morphological change
was effectively abolished by treatment with BV-LPS.
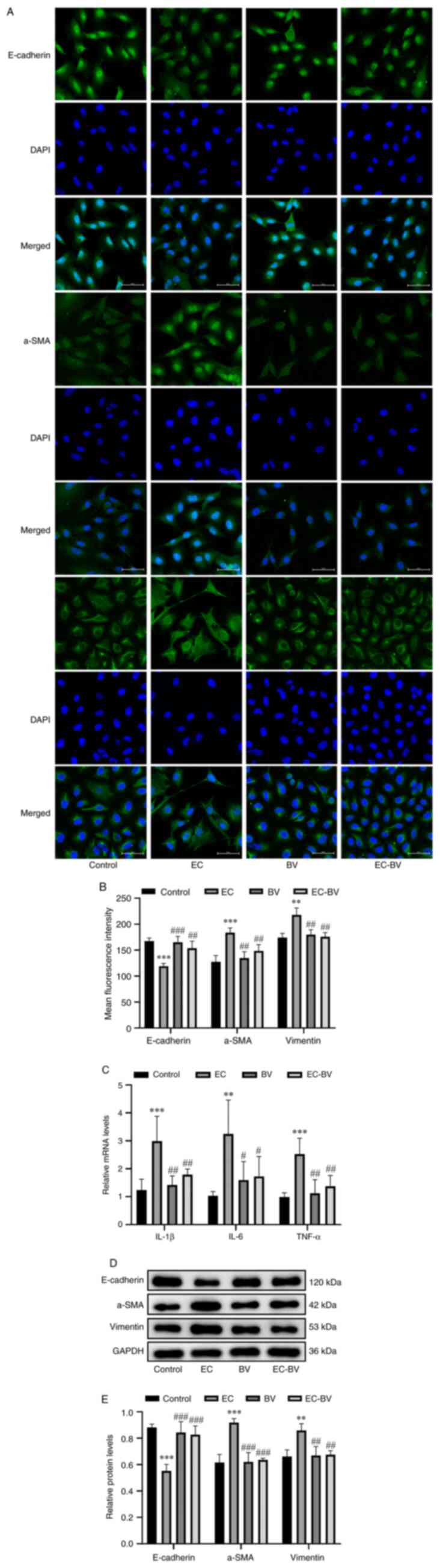
BV-LPS abolishes EC-LPS-induced
inflammation and EMT in A549 cells
A549 cells were subsequently treated with EC-LPS (10
µg/ml) and BV-LPS (60 µg/ml) alone or in combination for up to 48
h. Immunofluorescence staining and western blot analysis revealed
that, consistent with the morphological changes, EC-LPS treatment
led to a significant upregulation of the mesenchymal marker
proteins α-SMA and vimentin, whereas the epithelial cell marker
protein E-cadherin was markedly downregulated, although treatment
with BV-LPS abolished these changes (Fig. 2A, B, D and E). In a subsequent
series of experiments, the mRNA expression levels of the
inflammatory cytokines IL-1β, IL-6 and TNF-α were also examined by
RT-qPCR. As shown in Fig. 2C,
EC-LPS treatment led to a significant upregulation of these
inflammatory cytokines, whereas treatment with BV-LPS almost
completely abolished the effects of EC-LPS. Taken together, these
results suggested that BV-LPS was able to counteract the
EC-LPS-induced inflammation and EMT observed in A549 cells.
BV-LPS abolishes EC-LPS-induced lung
injury and inflammation in vivo
At 4 weeks following drug administration, the
protective effect of BV-LPS was further examined in a mouse EMT
model. The body weight and wet lung weight of mice were counted and
there was no significant difference in body weight before
administration. EC-LPS treatment resulted in a significant decrease
in body weight and a significant increase in wet lung weight, while
BV-LPS treatment abolished these changes (Fig. 3A-C). Using the percentage of lung
wet weight/body weight as the lung index, the calculated results
showed that BV-LPS treatment led to a canceling-out of the increase
in lung index induced by EC-LPS in mice (Fig. 3D). The extent of lung injury was
evaluated using H&E staining. As shown in Fig. 3E, EC-LPS induced a thickening of
the alveolar wall and the collapse of alveoli, with an unclear
structure of lung tissue in mice, although these changes were
greatly ameliorated by treatment with BV-LPS. The mRNA and protein
expression levels of the inflammatory cytokines (IL-1β, IL-6 and
TNFα) were also detected and a notable reduction in the release of
IL-1β, IL-6 and TNF-α was observed following BV-LPS therapy
(Fig. 3F and G). Collectively,
these results suggested that BV-LPS could abolish EC-LPS-induced
lung injury and inflammation in mice lung tissue.
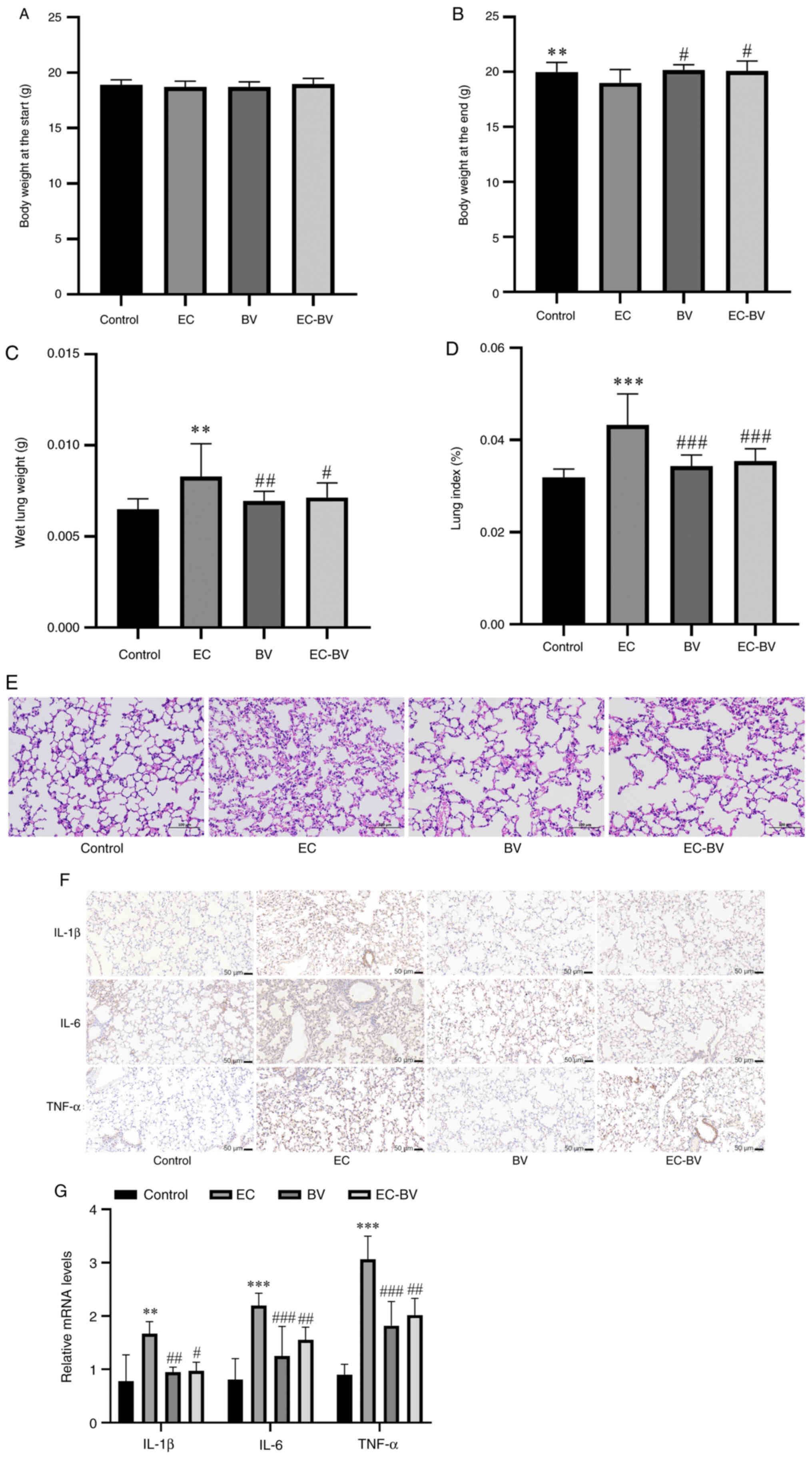 | Figure 3.BV-LPS abolishes EC-LPS-induced lung
injury and inflammation in mice. EC-LPS treatment resulted in (A
and B) a significant decrease in body weight and (C) a significant
increase in wet lung weight, while BV-LPS treatment abolished these
changes. (D) BV-LPS was found to abolish the increase in the lung
index induced by EC-LPS. (E) Hematoxylin and eosin staining of lung
tissue, showing that BV-LPS could abolish EC-LPS-induced lung
injury in mice (magnification, ×200). (F) Immunohistochemical
(scale bar, 50 µm) and (G) reverse transcription-quantitative PCR,
showing that BV-LPS could abolish EC-LPS-induced lung inflammation
in mice. **P<0.01 and ***P<0.001 vs. the control group;
#P<0.05, ##P<0.01 and
###P<0.001 vs. the EC-LPS group. BV-LPS, LPS
extracted from Bacteroides vulgatus; EC-LPS, LPS extracted
from Escherichia coli; Control, control group; EC, EC-LPS
group; BV, BV-LPS group; EC-BV, co-treated with EC-LPS and BV-LPS
group. |
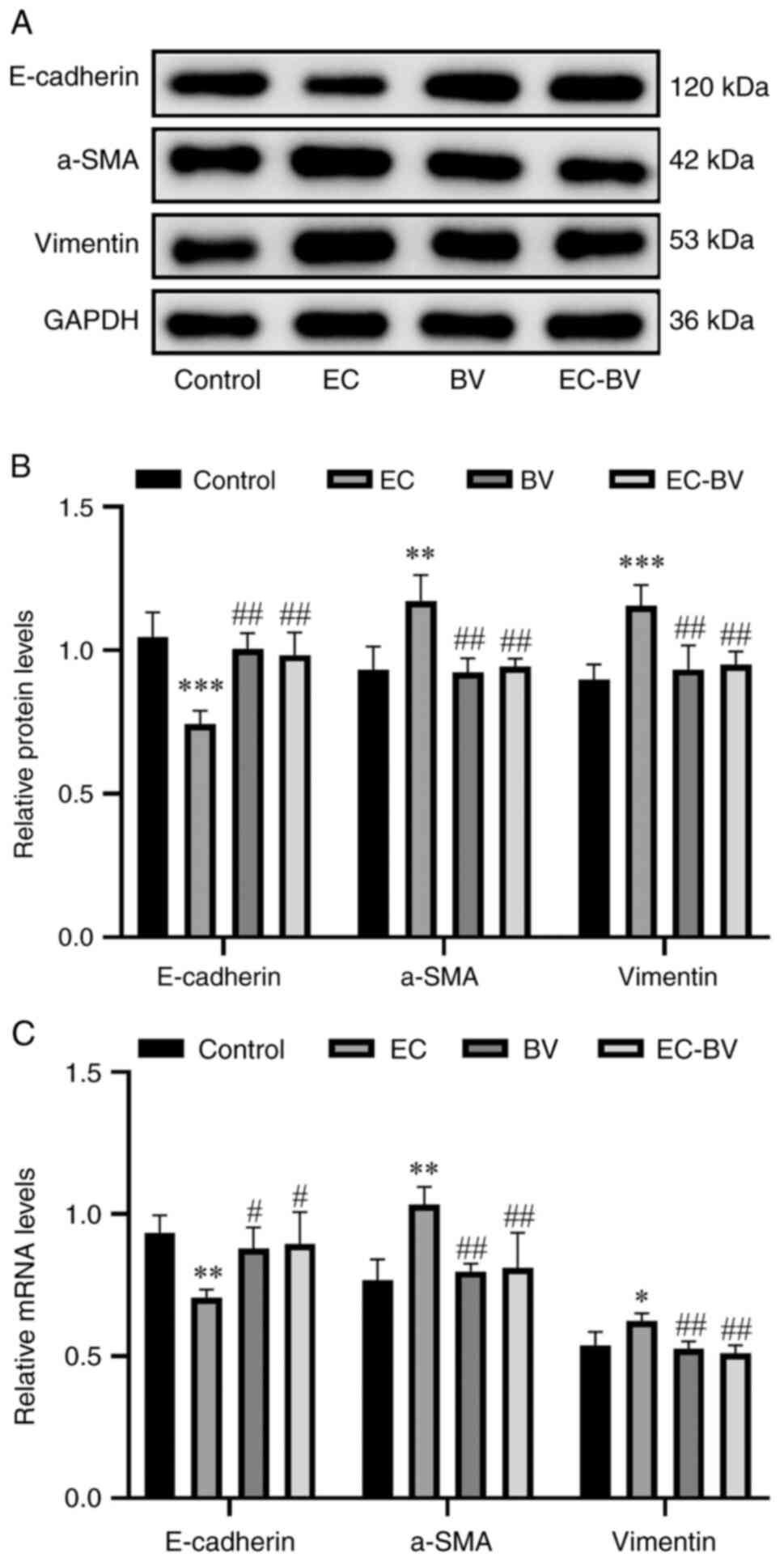
BV-LPS abolishes EC-LPS induced EMT in
vivo
In a subsequent set of experiments, western blotting
and RT-qPCR were employed to detect the levels of mesenchymal
marker proteins and epithelial marker proteins. The results were
found to be consistent with those obtained with the A549 cell
experiments; namely, α-SMA and vimentin were significantly
upregulated and E-cadherin was markedly downregulated, upon
treatment with EC-LPS, whereas BV-LPS therapy led to the reversal
of these changes (Fig. 4A-C).
Taken together, these results suggested that BV-LPS was also able
to abolish EC-LPS-induced EMT in mice lung tissue.
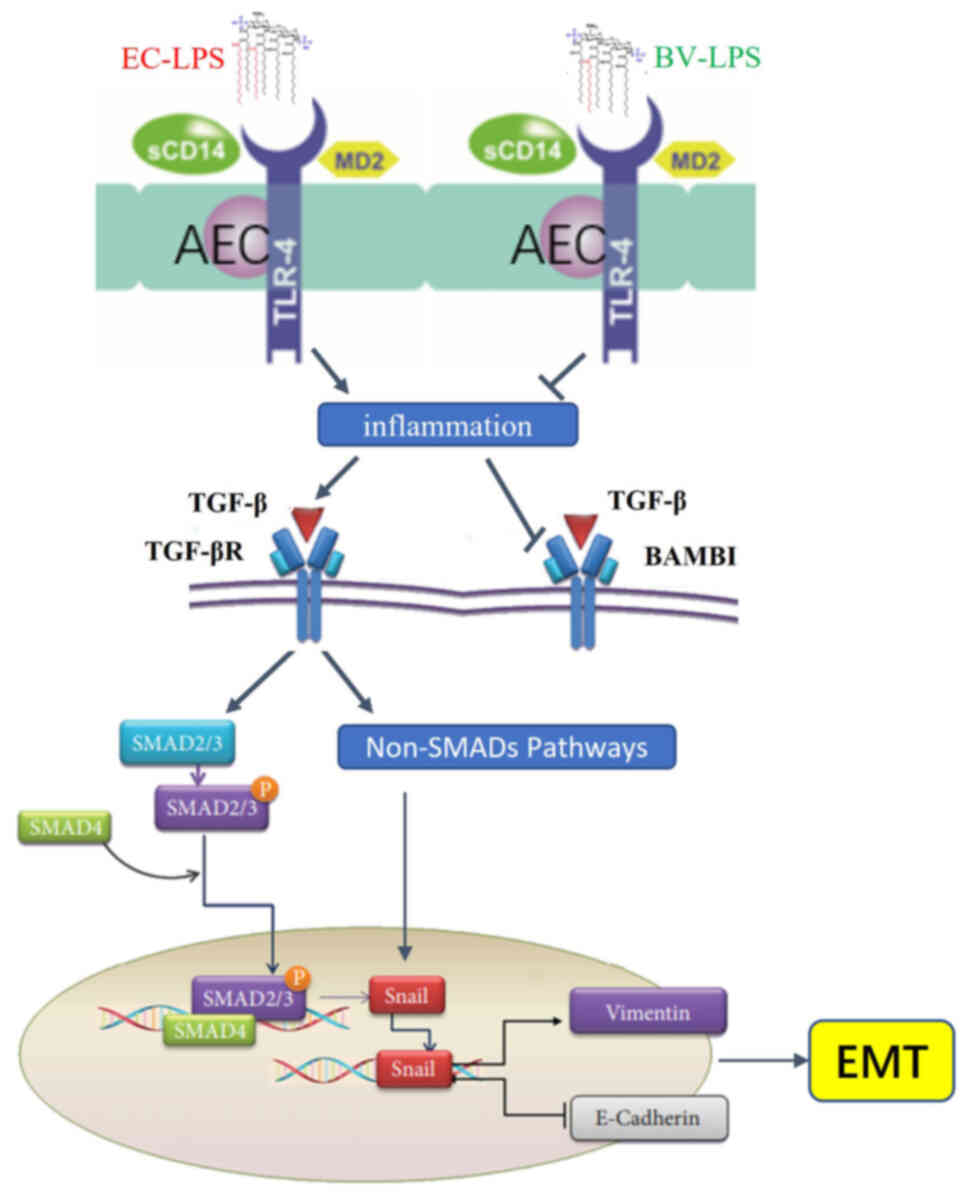
The TLR4/BAMBI/Snail pathway may be
involved in the mechanism governing how BV-LPS abolishes
EC-LPS-induced EMT
The TLR4/BAMBI/Snail pathway is involved in the
direct induction of EMT (10).
TLR4, a receptor for LPS, is one of the strongest inducers of
inflammation (22). The
pseudoreceptor BAMBI acts as a regulator between LPS/TLR4 signaling
and TGF-β-induced EMT and it also functions as a negative regulator
of the TGF-β signaling pathway (23). However, Snail proteins have been
shown to induce EMT and are promoted by the TGF-β signaling pathway
(24). The results of the RT-qPCR
and western blot analyses, with the goal of detecting the levels of
TLR4, BAMBI and Snail, revealed that EC-LPS treatment caused a
significant upregulation of TLR4 and Snail, whereas BAMBI was
markedly downregulated. Notably, BV-LPS treatment was able to
reverse these changes (Fig. 5A and
B). Taken together, these results suggested that the
TLR4/BAMBI/Snail pathway may be involved in the mechanism via which
BV-LPS could abolish EC-LPS-induced EMT.
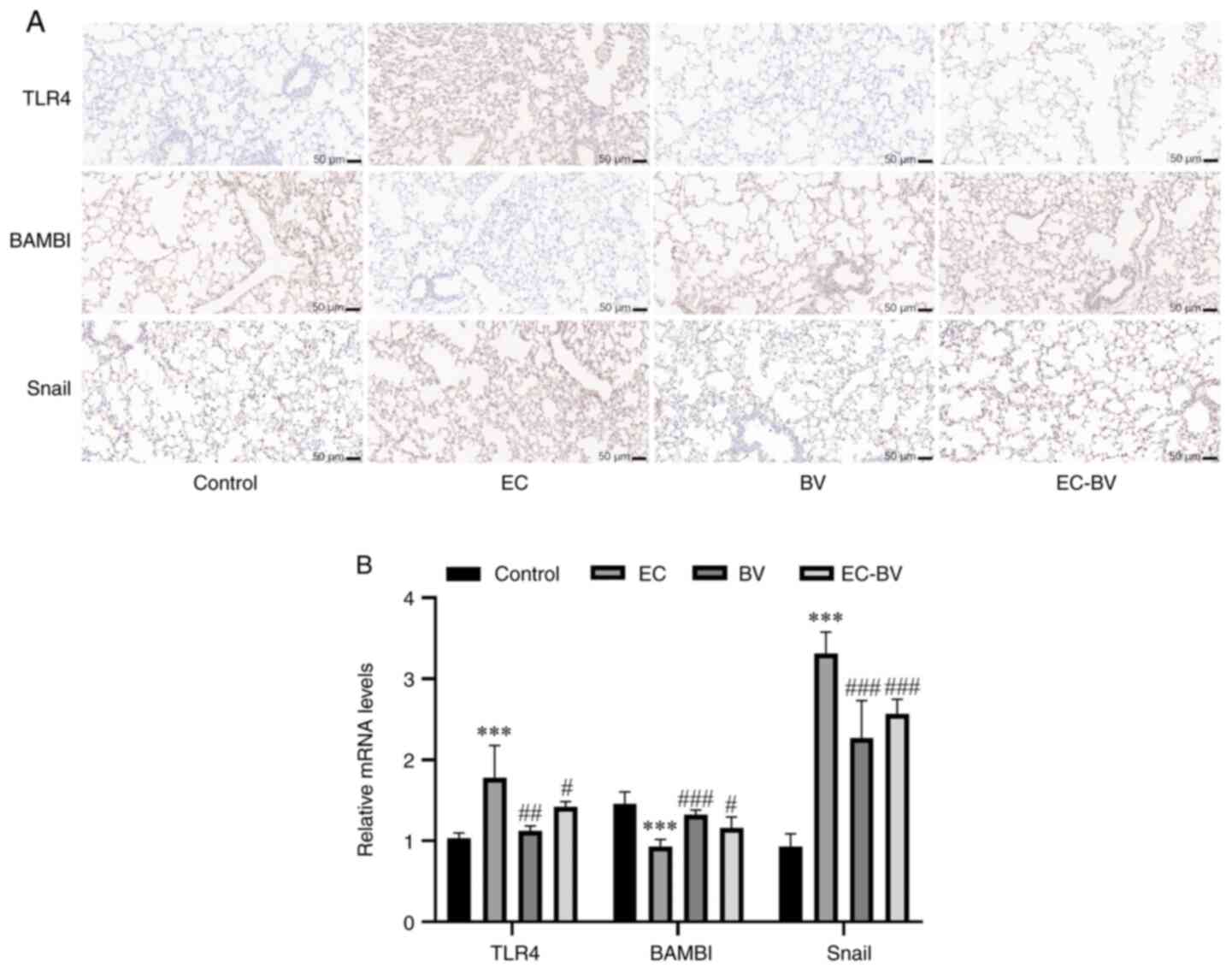 | Figure 5.The TLR4/BAMBI/Snail pathway may be
involved in BV-LPS abolished EC-LPS induced EMT. The (A)
immunohistochemical and (B) reverse transcription-quantitative PCR
showed that BV-LPS could reverse the downregulation of E-cadherin
and upregulation of α-SMA and vimentin induced by EC-LPS (scale
bar, 50 µm). ***P<0.001 vs. the control group;
#P<0.05, ##P<0.01 and
###P<0.001 vs. the EC-LPS group. TLR4, Toll-like
receptor 4; BAMBI, bone morphogenic protein and activin
membrane-bound inhibitor; BV-LPS, LPS extracted from Bacteroides
vulgatus; EC-LPS, LPS extracted from Escherichia coli;
EMT, epithelial-mesenchymal transition; α-SMA, α-smooth muscle
actin; Control, control group; EC, EC-LPS group; BV, BV-LPS group;
EC-BV, co-treated with EC-LPS and BV-LPS group. |
Discussion
The immunoinhibitory structure of low-acylated lipid
A is conserved in Bacteroides and there are many
Bacteroides species that produce lipopolysaccharides in the
intestinal flora, among which B. ovatus, B. uniformis and
B. vulgatus are the most dominant (13). The BV-LPS is a pentacylated A-LPS
that has been shown to antagonize P-LPS-induced inflammation
(25,26); therefore, BV-LPS was selected for
the A-LPS in the present study.
Previous studies have shown that EC-LPS, as an
independent factor triggering EMT in epithelial cells (27), can be used to induce the EMT
process in epithelial cells and lung tissues (16,19,20).
Proteobacteria are a major contributor to P-LPS synthesis, whereas
Bacteroidetes are a major contributor to A-LPS synthesis. It has
been reported that Bacteroides contribute a mean of 79% of
the total LPS in the intestinal flora of healthy subjects (13), so A-LPS is >4 times higher than
P-LPS in the intestinal flora of healthy subjects. Therefore, in
the present study chose BV-LPS with a concentration 6 times higher
than EC-LPS for the experiment. Inflammation is a key factor in
pathological EMT (5) and
EC-LPS-induced EMT models have been used both in vivo and
in vitro (16,19,20).
During EMT, the level of E-cadherin, a marker protein in epithelial
cells, is decreased, while those of mesenchymal marker proteins,
such as vimentin and α-SMA, are increased (14,15).
In the present study, it was found that the morphology of A549
alveolar epithelial cells exhibited clearly depolarized, mutual
dispersion and fibrous growth under conditions of EC-LPS induction,
whereas the treatment comprising a combination of BV-LPS with
EC-LPS failed to induce the morphological changes of the A549
cells. The expression levels of the pro-inflammatory cytokines
IL-1β, IL-6, TNF-α and the marker proteins vimentin and α-SMA in
mesenchymal cells were upregulated in A549 cells induced by EC-LPS,
whereas the expression level of the marker protein E-cadherin in
epithelial cells was downregulated. By contrast, treatment with
BV-LPS combined with EC-LPS failed to induce these changes.
Following EC-LPS induction, the lung index of mice was increased,
the alveolar wall was found to have thickened, the alveolar
structure collapsed and the structure was unclear; moreover, the
expression levels of the pro-inflammatory cytokines IL-1β, IL-6 and
TNF-α, and the marker proteins vimentin and α-SMA of mesenchymal
cells, were upregulated, whereas the expression level of the marker
protein E-cadherin in epithelial cells was downregulated. The
addition of BV-LPS in combination with EC-LPS failed to induce
these changes. The results of the in vitro cell experiments
and in vivo experiments were therefore found to corroborate
each other, both supporting that BV-LPS could effectively
counteract EC-LPS-induced inflammation, thereby preventing
pathological EMT induced by an inflammatory microenvironment.
The pseudoreceptor BAMBI is expressed in numerous
organs, including lung tissue and is involved in the development of
fibrosis and cancer in these organs (28–30).
TLR4 is highly expressed in diseased skin and lung tissues and is
associated with extracellular matrix remodeling and fiber formation
(29). BAMBI is structurally
homologous with TGF-β receptor I (TGF-β RI) and is a member of the
TGF-β type I receptor family, although it lacks an intracellular
kinase domain and is a negative regulator of the TGF-β signaling
pathway (31,32). The LPS/TLR4 signaling pathway has
been shown to reduce TGF-β binding to BAMBI via inhibiting BAMBI
expression, which has the effect of inducing the TGF-β response to
EMT, ultimately leading to progressive fiber formation (22,30).
TGF-β is able to trigger the EMT through both the SMAD and non-SMAD
signaling pathways (33). In the
SMAD pathway, EMT is activated by the TGF-β1/SMAD/Snail signaling
axis, leading to the induction of EMT-dependent fibrosis (34–36).
TGF-β receptor consists of three receptor types, RI, RII and RIII
(37). TGF-β binds to RIII and is
subsequently recruited to cell membrane RII/RI, which is
phosphorylated to form an activated heterotetramer serine/threonine
kinase complex (38).
Phosphorylation activates the SMAD2/3 protein, leading to the
subsequent formation of a phosphorylated SMAD2/3-SMAD4 complex with
SMAD4 (39). The SMAD complex
migrates to the nucleus and induces Snail via activating
transcription factors (40,41).
Snail induces the EMT process, thereby leading to the
downregulation of epithelial markers such as E-cadherin and the
upregulation of mesenchymal markers such as vimentin and α-SMA
(24,42). TGF-β1 has also been shown to induce
EMT by activating Snail via the non-SMAD-dependent PI3K/AKT and
MAPK/ERK1/2 signaling pathways (43). In the present study, the expression
levels of TLR4 and Snail were found to be significantly upregulated
and BAMBI was significantly downregulated, in lung tissue induced
by EC-LPS. However, these changes were reversed when the tissue
sections were treated with a combination of BV-LPS and EC-LPS.
Taken together, these results suggested that BV-LPS may antagonize
EC-LPS-induced EMT through inhibiting the TLR4/BAMBI/Snail pathway,
as shown in Fig. 6.
However, the present study did have certain
limitations. First, the concentration ratio of BV-LPS which
competitively inhibits EC-LPS activity was not explored. Second,
only preliminary observations have been made of the mechanism
underlying how BV-LPS may prevent EC-LPS-induced EMT and further
studies are required to fully elucidate the mechanism. Subsequent
experiments will determine the concentration ratio of BV-LPS to
competitively inhibit EC-LPS activity and identify the mechanism of
BV-LPS preventing EMT induced by EC-LPS via the TLR4/BAMBI/Snail
pathway with inhibitors or additional evidence when further funding
is obtained. In conclusion, the present study demonstrated that
BV-LPS can effectively prevent EC-LPS-induced EMT in mouse lung
tissue and A549 cells and the underlying mechanism may be
associated with inhibition of TLR4/BAMBI/Snail pathway.
Acknowledgements
Not applicable.
Funding
The present study was supported by the NHC Key Laboratory of
Pulmonary Immune-related Diseases (grant no. 2019PT320003), the
Guizhou Province Department of Education Youth Science and
Technology Talent Growth Project [Guizhou Education KY word; grant
no. (2022)266], the National Natural Science Foundation of China
(grant no. 82260872) and the Program of Innovative Scientific and
Technological Talent Team of Guizhou Province (grant no.
2020-5010).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HZ and CY designed the study. YL, MX, ZK, QX, JY and
ZS performed the experiments. YL, WC, JO and HZ analyzed the data.
YL and HZ drafted and revised the manuscript. YL and CY confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The animal study protocol was reviewed and approved
by the Experimental Animal Ethics Committee of Guizhou University
of Traditional Chinese Medicine (approval no. 20220039).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rout-Pitt N, Farrow N, Parsons D and
Donnelley M: Epithelial mesenchymal transition (EMT): A universal
process in lung diseases with implications for cystic fibrosis
pathophysiology. Respir Res. 19:1362018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yang Y, Hu L, Xia H, Chen L, Cui S, Wang
Y, Zhou T, Xiong W, Song L, Li S, et al: Resolvin D1 attenuates
mechanical stretch-induced pulmonary fibrosis via
epithelial-mesenchymal transition. Am J Physiol Lung Cell Mol
Physiol. 316:L1013–L1024. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang ZC, Qu ZH, Yi MJ, Shan YC, Ran N, Xu
L and Liu XJ: MiR-448-5p inhibits TGF-β1-induced
epithelial-mesenchymal transition and pulmonary fibrosis by
targeting Six1 in asthma. J Cell Physiol. 234:8804–8814. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nieto MA, Huang RY, Jackson RA and Thiery
JP: EMT: 2016. Cell. 166:21–45. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
López-Novoa JM and Nieto MA: Inflammation
and EMT: An alliance towards organ fibrosis and cancer progression.
EMBO Mol Med. 1:303–314. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fedele G, Nasso M, Spensieri F, Palazzo R,
Frasca L, Watanabe M and Ausiello CM: Lipopolysaccharides from
Bordetella pertussis and Bordetella parapertussis differently
modulate human dendritic cell functions resulting in divergent
prevalence of Th17-polarized responses. J Immunol. 181:208–216.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Park BS, Song DH, Kim HM, Choi BS, Lee H
and Lee JO: The structural basis of lipopolysaccharide recognition
by the TLR4-MD-2 complex. Nature. 458:1191–1195. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lin TL, Shu CC, Chen YM, Lu JJ, Wu TS, Lai
WF, Tzeng CM, Lai HC and Lu CC: Like Cures Like: Pharmacological
activity of anti-inflammatory lipopolysaccharides from gut
microbiome. Front Pharmacol. 11:5542020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cardinelli CS, Sala PC, Alves CC,
Torrinhas RS and Waitzberg DL: Influence of intestinal microbiota
on body weight gain: A narrative review of the literature. Obes
Surg. 25:346–353. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Brown GC: The endotoxin hypothesis of
neurodegeneration. J Neuroinflammation. 16:1802019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
He Z, Zhu Y and Jiang H: Inhibiting
toll-like receptor 4 signaling ameliorates pulmonary fibrosis
during acute lung injury induced by lipopolysaccharide: An
experimental study. Respir Res. 10:1262009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hirschfeld M, Ma Y, Weis JH, Vogel SN and
Weis JJ: Cutting edge: Repurification of lipopolysaccharide
eliminates signaling through both human and murine toll-like
receptor 2. J Immunol. 165:618–622. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
d'Hennezel E, Abubucker S, Murphy LO and
Cullen TW: Total lipopolysaccharide from the human gut microbiome
silences toll-like receptor signaling. mSystems. 2:e00046–17.
2017.PubMed/NCBI
|
|
14
|
Ding Z, Wu X, Wang Y, Ji S, Zhang W, Kang
J, Li J and Fei G: Melatonin prevents LPS-induced
epithelial-mesenchymal transition in human alveolar epithelial
cells via the GSK-3β/Nrf2 pathway. Biomed Pharmacother.
132:1108272020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen D, Qiu YB, Gao ZQ, Wu YX, Wan BB, Liu
G, Chen JL, Zhou Q, Yu RQ and Pang QF: Sodium propionate attenuates
the lipopolysaccharide-induced epithelial-mesenchymal transition
via the PI3K/Akt/mTOR signaling pathway. J Agric Food Chem.
68:6554–6563. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang YQ, Liu YJ, Mao YF, Dong WW, Zhu XY
and Jiang L: Resveratrol ameliorates lipopolysaccharide-induced
epithelial mesenchymal transition and pulmonary fibrosis through
suppression of oxidative stress and transforming growth factor-β1
signaling. Clin Nutr. 34:752–760. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tang S, Jiang X, Wu L, Chen S, Chen L,
Jiang J, Yan P, Wang F, Tu K, Wang D, et al: Toll-like receptor 4
shRNA attenuates lipopolysaccharide-induced epithelial-mesenchymal
transition of intrahepatic biliary epithelial cells in rats. Biomed
Pharmacother. 107:1210–1217. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta DeltaC(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu W, Sun T and Wang Y: Integrin αvβ6
mediates epithelial-mesenchymal transition in human bronchial
epithelial cells induced by lipopolysaccharides of Pseudomonas
aeruginosa via TGF-β1-Smad2/3 signaling pathway. Folia Microbiol
(Praha). 65:329–338. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gong JH, Cho IH, Shin D, Han SY, Park SH
and Kang YH: Inhibition of airway epithelial-to-mesenchymal
transition and fibrosis by kaempferol in endotoxin-induced
epithelial cells and ovalbumin-sensitized mice. Lab Invest.
94:297–308. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li H, Li J, Zhang G, Da Q, Chen L, Yu S,
Zhou Q, Weng Z, Xin Z, Shi L, et al: HMGB1-Induced p62
overexpression promotes snail-mediated epithelial-mesenchymal
transition in glioblastoma cells via the degradation of GSK-3β.
Theranostics. 9:1909–1922. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lim KH and Staudt LM: Toll-like receptor
signaling. Cold Spring Harb Perspect Biol. 5:a0112472013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
He Y, Ou Z, Chen X, Zu X, Liu L, Li Y, Cao
Z, Chen M, Chen Z, Chen H, et al: LPS/TLR4 signaling enhances TGF-β
response through downregulating BAMBI during prostatic hyperplasia.
Sci Rep. 6:270512016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kaufhold S and Bonavida B: Central role of
Snail1 in the regulation of EMT and resistance in cancer: A target
for therapeutic intervention. J Exp Clin Cancer Res. 33:622014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yoshida N, Emoto T, Yamashita T, Watanabe
H, Hayashi T, Tabata T, Hoshi N, Hatano N, Ozawa G, Sasaki N, et
al: Bacteroides vulgatus and Bacteroides dorei Reduce gut microbial
lipopolysaccharide production and inhibit atherosclerosis.
Circulation. 138:2486–2498. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Steimle A, Michaelis L, Di Lorenzo F,
Kliem T, Münzner T, Maerz JK, Schäfer A, Lange A, Parusel R,
Gronbach K, et al: Weak agonistic LPS restores intestinal immune
homeostasis. Mol Ther. 27:1974–1991. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhao L, Yang R, Cheng L, Wang M, Jiang Y
and Wang S: LPS-induced epithelial-mesenchymal transition of
intrahepatic biliary epithelial cells. J Surg Res. 171:819–825.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pulskens WP, Rampanelli E, Teske GJ,
Butter LM, Claessen N, Luirink IK, van der Poll T, Florquin S and
Leemans JC: TLR4 promotes fibrosis but attenuates tubular damage in
progressive renal injury. J Am Soc Nephrol. 21:1299–1308. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bhattacharyya S, Kelley K, Melichian DS,
Tamaki Z, Fang F, Su Y, Feng G, Pope RM, Budinger GR, Mutlu GM, et
al: Toll-like receptor 4 signaling augments transforming growth
factor-β responses: A novel mechanism for maintaining and
amplifying fibrosis in scleroderma. Am J Pathol. 182:192–205. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Seki E, De Minicis S, Osterreicher CH,
Kluwe J, Osawa Y, Brenner DA and Schwabe RF: TLR4 enhances TGF-beta
signaling and hepatic fibrosis. Nat Med. 13:1324–1332. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lutz M and Knaus P: Integration of the
TGF-beta pathway into the cellular signalling network. Cell Signal.
14:977–988. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen J, Bush JO, Ovitt CE, Lan Y and Jiang
R: The TGF-beta pseudoreceptor gene Bambi is dispensable for mouse
embryonic development and postnatal survival. Genesis. 45:482–486.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang YE: Non-Smad pathways in TGF-beta
signaling. Cell Res. 19:128–139. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sisto M, Lorusso L, Ingravallo G, Ribatti
D and Lisi S: TGFβ1-Smad canonical and -Erk noncanonical pathways
participate in interleukin-17-induced epithelial-mesenchymal
transition in Sjögren's syndrome. Lab Invest. 100:824–836. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fabregat I, Moreno-Càceres J, Sánchez A,
Dooley S, Dewidar B, Giannelli G and Ten Dijke P; IT-LIVER
Consortium, : TGF-β signalling and liver disease. FEBS J.
283:2219–2232. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Willis BC and Borok Z: TGF-beta-induced
EMT: Mechanisms and implications for fibrotic lung disease. Am J
Physiol Lung Cell Mol Physiol. 293:L525–L534. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Prud'homme GJ: Pathobiology of
transforming growth factor beta in cancer, fibrosis and immunologic
disease and therapeutic considerations. Lab Invest. 87:1077–1091.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Heldin CH and Moustakas A: Signaling
receptors for TGF-β family members. Cold Spring Harb Perspect Biol.
8:a0220532016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wrighton KH, Lin X and Feng XH:
Phospho-control of TGF-beta superfamily signaling. Cell Res.
19:8–20. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hill CS: Transcriptional control by the
SMADs. Cold Spring Harb Perspect Biol. 8:a0220792016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Brandl M, Seidler B, Haller F, Adamski J,
Schmid RM, Saur D and Schneider G: IKK(α) controls canonical
TGF(ß)-SMAD signaling to regulate genes expressing SNAIL and SLUG
during EMT in panc1 cells. J Cell Sci. 123((Pt 24)): 4231–4239.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sisto M, Lorusso L, Ingravallo G, Tamma R,
Ribatti D and Lisi S: The TGF-1 signaling pathway as an attractive
target in the fibrosis pathogenesis of Sjögren's Syndrome.
Mediators Inflamm. 2018:19659352018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chen XF, Zhang HJ, Wang HB, Zhu J, Zhou
WY, Zhang H, Zhao MC, Su JM, Gao W, Zhang L, et al: Transforming
growth factor-β1 induces epithelial-to-mesenchymal transition in
human lung cancer cells via PI3K/Akt and MEK/Erk1/2 signaling
pathways. Mol Biol Rep. 39:3549–3556. 2012. View Article : Google Scholar : PubMed/NCBI
|