Introduction
Osteoarthritis (OA) is a substantial global economic
and familial burden (1). The
epidemiology of OA is complex and multifaceted and includes a
variety of genetic, biological, and biomechanical factors. Clinical
manifestations of OA primarily include recurrent joint pain,
limited mobility, and progressive joint deformity (2–4). As
the hallmarks of OA, abnormal chondrocyte death, cartilage
extracellular matrix (ECM) destruction and abnormal homeostasis are
gaining more attention as therapeutic targets for cartilage
regeneration (5,6). Moreover, during the progression of
OA, the cartilage ECM undergoes substantial pathological
alterations, which can act as promising biomarkers in identifying
the pathological stages of OA (7).
Evidence has suggested that chondrocytes undergo diverse forms of
cell death, including necroptosis, apoptosis and ferroptosis, in OA
(8,9). A recent study demonstrated that
knockdown of the ferritin regulator NCOA4 can suppress
IL-1β-induced ferroptosis and ECM degradation in chondrocytes
(10). This indicates that
ferroptosis acts on not only chondrocytes themselves but also on
the chondrocyte ECM.
Ferroptosis, a form of programmed cell death
involving iron and lipid peroxidation metabolism, is distinct from
apoptosis and necrosis in terms of cell morphology, protein
expression and genetic mechanisms (11–14).
It has been reported that ferroptosis is widespread in chondrocytes
in OA, and the role of glutathione peroxidase 4 (GPX4), a key
regulator of ferroptosis, has also been extensively studied
(15–17). Downregulation of GPX4 can increase
the sensitivity of chondrocytes to oxidative stress and intensify
degradation of the ECM through the MAPK/NF-κB pathway, thereby
exacerbating the OA process (18).
Additionally, osteophyte formation has been shown to be
significantly increased in a mouse model of OA, and this effect was
revealed to be further enhanced by the knockdown of GPX4 (18). Recently, several chemicals, such as
curcumin and quercetin, have been reported to protect chondrocytes
from pathological ferroptosis and thus to ameliorate the
progression of OA (19–21). Therefore, the development of
ferroptosis inhibitors holds potential for augmenting the efficacy
of existing treatment modalities and addressing the shortcomings of
drug-based interventions.
Nuclear factor erythroid 2-related factor 2 (Nrf2)
serves a crucial role in maintaining cellular homeostasis under
oxidative stress conditions (22),
as well as a key role in mediating iron/heme metabolism (23). Ferroptosis is triggered by the
iron-dependent oxidation of polyunsaturated fatty acids, and Nrf2
specifically inhibits ferroptosis by reducing lipid peroxidation
(24). Ferritin and iron
transporters are controlled by Nrf2 (25,26).
Numerous studies have shown that antioxidants affect ferroptosis
via Nrf2 activation. For example, Guo et al (27) reported that deferoxamine alleviated
OA by inhibiting chondrocyte ferroptosis and activating the Nrf2
pathway. Moreover, biochanin A can protect against iron overload
associated with knee OA by regulating iron levels and the
Nrf2/system xc−/GPX4 axis (19,28).
Its pivotal role makes it an important candidate for mediating
protective or deleterious effects through ferroptosis. Previous
studies have also reported that heme oxygenase-1 (HO-1), a classic
downstream antioxidant protein of Nrf2, is widely involved in
ferroptosis (29,30). Furthermore, several lines of
evidence have highlighted the role of HO-1 in the progression of
OA, and its expression and activity are associated with cartilage
growth, apoptosis, and ferroptosis (31–35).
These results provide a potential treatment for OA in the form of a
compound targeting the Nrf2/HO-1 pathway.
Curcuma longa L. is a traditional Chinese
herb that is widely used in the treatment of rheumatoid arthritis,
stroke, Alzheimer's disease, and even cancer; curcumin is the best
studied active ingredient of C. longa due to its
antioxidative and anti-inflammatory properties (36,37).
However, its low solubility and low absorption in the human gut
limit its application. Acetyl zingerone (AZ), a stable and more
powerful derivative of curcumin, has been shown to protect skin
cells from DNA damage (38),
inhibit metalloproteinases (39),
and stabilize other reducing agents to exert high-efficiency
antioxidative effects and increase collagen levels (40). The main principle of the Fenton
reaction is the addition of an H2O2 oxidizing
agent and a Fe2+ catalyst, both of which react under
acidic conditions (generally pH <4) to produce hydroxyl
radicals. Compared with curcumin, AZ has higher solubility, better
photostability, and a much higher absorption rate, and AZ can
inhibit the Fenton reaction (41).
The latest research has shown that hyperoside, a flavonoid compound
similar to AZ, exerts anti-OA effects through its anti-apoptosis
and anti-inflammatory effects in vitro and in vivo
(42); therefore, it was
hypothesized that AZ would have a positive effect on OA. The
present study aimed to investigate whether AZ can alleviate
ferroptosis in OA by mediating Nrf2/HO-1 signaling; the data may
provide new insights and targets for the treatment of OA.
Materials and methods
Reagents
AZ was synthesized by Dr Miao's laboratory at
Changzhou University (Changzhou, China). Recombinant rat IL-1β
(cat. no. M21094) and ML385 (Nrf2 inhibitor) (cat. no. M8692) were
purchased from Abmole Bioscience, Inc. Ferrostatin-1 (Fer-1), a
potent and selective ferroptosis inhibitor and the HO-1 inhibitor
tin protoporphyrin IX (cat. no. 14325-05-4) were purchased from
MedChemExpress. BeyoClick™ 5-ethynyl-2′-deoxyuridine (EdU)-488 Cell
Proliferation Assay Kit (cat. no. C0071S), Cell Counting Kit-8
(CCK-8; cat. no. C0037), a reactive oxygen species (ROS) detection
kit (cat. no. S0033), Calcein/PI Cell Viability/Cytotoxicity Assay
Kit (cat. no. C2015M) and the Annexin V-FITC/PI Cell Apoptosis
Detection kit (cat. no. C1062S) were purchased from Beyotime
Institute of Biotechnology. Type II collagenase (cat. no. LS004176)
was purchased from Worthington Biochemical Corporation. Super
sensitive ECL luminescence substrate (cat. no. U10010A) was
supplied by Yuyou Biotechnology Co., Ltd. The ATDC5 mouse
chondrogenic cell line (cat. no. ZQ0938) was purchased from
Shanghai Zhongqiao Xinzhou Biotechnology Co., Ltd.
Nuclear magnetic resonance (NMR)
experiments and analysis
NMR spectra were run on a 500 MHz Bruker AVANCE DRX
instrument using a broadband probe equipped with a z-gradient coil
(Bruker). For NMR, a 600-µg sample of AZ was run in standard 5-mm
NMR tubes at 25°C. Pulse programs used were standard sequences
taken from the Bruker XWINNMR pulse sequence library. NMR
experiments were set up and processed generally using the
parameters according to the manufacturer's protocol. The pulse
programs selected for the 2D homonuclear chemical shift,
heteronuclear multiple quantum coherence), and 1H detected
heteronuclear multiple bond correlation experiments employed
z-gradients for coherence pathway selection. 1H and
13C chemical shifts were calibrated relative to the
solvents and tetramethyl silane. To determine the content of AZ in
the sample, measurement analysis was performed. To calculate AZ
content, the absorption peak area (A1/n1) caused by a proton on AZ
was compared with the absorption peak area (A2/n2) caused by a
proton on the sample, and the content of AZ was calculated
according to the following formula. Relative percentage of the
AZ={(A1/n1)/(A2/n2)} ×100.
Isolation and culture of primary rat
chondrocytes
A total of 10 8-week-old male Sprague-Dawley rats
(weight, 220 g) were purchased from Changzhou Cavens Experimental
Animal Co., Ltd. and were maintained under the following
conditions: 20–25°C, 50–60% humidity, free access to pure water and
special feed, under a 12-h light/dark cycle. The rats were
sacrificed within a week of purchase. Chondrocytes were isolated
from the articular cartilage of 8-week-old male Sprague-Dawley
rats. The present study was approved by the Jiangsu Science
Standard Medical Testing Committee (approval no. IACUC22-0097),
which is a regional committee that sets standards for scientific
research in Jiangsu province. For the extraction of chondrocytes,
rats were sacrificed the day after arrival by CO2
asphyxiation (fill rate, 60% chamber volume/min), and death was
confirmed by observing a lack of respiration and faded eye color
(42,43). The method of sacrifice used is able
to fulfill the objective of rapid unconsciousness with minimal
distress to the animals (44).
After the rats were sacrificed, they were sterilized with 75%
ethanol and iodophor, and the cartilage tissues of the rat knee
joints were collected in a biological safety cabinet hood and
placed into PBS (cat. no. P1010-2L; Beijing Solarbio Science &
Technology Co., Ltd.) containing 20 ml penicillin-streptomycin. The
joints were then washed twice with PBS and placed in Dulbecco's
modified Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific,
Inc.) containing 2 mg/ml type II collagenase for ~8 h at 37°C. The
next day, chondrocytes were harvested by filtering through a 70-µm
cell strainer, washed twice with DMEM, and seeded into a 10-cm cell
culture dish (Labserv; Thermo Fisher Scientific, Inc.) with DMEM
containing 10% FBS (Gibco; Thermo Fisher Scientific, Inc.), 1%
insulin-transferrin-selenium (cat. no. ITSS-10201; Cyagen
Biosciences, Inc.) and 1% penicillin-streptomycin, and placed in a
37°C incubator containing 5% CO2. The culture medium was
replaced every 2 days. All cells were mycoplasma free. Chondrocytes
at passages 2–4 were used for the subsequent experiments.
Evaluation of cell viability
The effect of AZ on the viability of chondrocytes
was evaluated by CCK-8 assay. mouse ATDC5 cells and rat
chondrocytes were used for double validation in this experiment,
and they were treated using the same conditions. Briefly,
chondrocytes were seeded in 96-well plates at a density of
3×103/well. To examine the rescue effect of AZ on
chondrocytes in the inflammatory environment, chondrocytes were
pretreated with 20 ng/ml rat IL-1β for 24 h, and were then treated
with AZ (25, 50 and 100 µM) for 24 or 48 h at 37°C. The cells were
then incubated with 10% CCK-8 reagent for 2 h at 37°C. In the
control group, chondrocytes were treated with 0.1% DMSO (cat. no.
D8371; Beijing Solarbio Science & Technology Co., Ltd.), as AZ
was dissolved in DMSO. The absorbance at 450 nm was measured
immediately using an Epoch microplate reader (BioTek Instruments,
Inc.).
EdU staining
To directly evaluate chondrocyte proliferation, EdU
staining was performed. After the cells were seeded for 8 h on
coverslips in a 12-well plate (6×103 cells/well), they
were pretreated with IL-1β (20 ng/ml) for 24 h and were then
treated with different concentrations of AZ (25, 50 and 100 µM) for
another 24 h at 37°C. For EdU analysis, the 10-mM stock solution
was diluted to an appropriate working concentration (1X) with
complete medium before starting the experiment. An EdU working
solution was added to the 6-well plate, and the cells were
incubated for an additional 2 h at 37°C. After EdU labeling of the
cells, the culture medium was removed, 1 ml EdU working solution
was added, and the cells were fixed at room temperature for 15 min.
The fixative solution was removed, and the cells were washed with
PBS three times. Subsequently, the washing solution was removed,
and the cells were incubated at room temperature for 10–15 min in 1
ml permeabilization solution (PBS containing 0.3% Triton X-100) per
well. The cells were washed with PBS, and 0.5 ml Click reaction
solution (Beyotime Institute of Biotechnology) was added to each
well and incubated at room temperature for 30 min in the dark.
After washing with PBS three times, 1 ml 1X Hoechst 33342 solution
was added to each well, and the cells were incubated at room
temperature for 10 min in the dark. The cells were washed with PBS
a further three times (3–5 min/wash) and fluorescence detection was
then performed. Hoechst 33342 is a blue fluorescent dye. EdU has a
maximum excitation wavelength of 346 nm and a maximum emission
wavelength of 460 nm. Images were captured using a CX41-32RFL
inverted fluorescence microscope (Olympus Corporation).
Crystal violet staining
To measure the number of chondrocytes, cells were
seeded in 6-well plates, pretreated with IL-1β (20 ng/ml) for 24 h,
and then treated with different concentrations of AZ (25, 50 and
100 µM) for another 24 h at 37°C. The culture medium was discarded,
the cells were carefully washed twice with PBS, and 0.5 ml 4%
paraformaldehyde solution was added to each well to fix the cells
for 30 min at 20°C. The paraformaldehyde solution was aspirated,
0.5 ml 50% crystal violet staining solution was added to each well,
and the plate was incubated at room temperature for 20 min. The
staining solution was gently removed by shaking, each well was
washed with distilled water, and the culture plate was placed
upside down on absorbent paper to absorb the water. The plate was
then dried at room temperature for 20 min. The 6-well plate was
then scanned using an Epson Perfection V370 photograph scanner
(Epson).
Toluidine blue staining
To measure the ECM content in chondrocytes, cells
were seeded in 6-well plates, pretreated with IL-1β (20 ng/ml) for
24 h at 37°C, and then treated with different concentrations of AZ
(0, 50 or 100 µM) for another 24 h at 37°C. The culture medium was
then removed, and cells were carefully washed twice with cold PBS
and fixed with 0.5 ml 4% paraformaldehyde solution for 30 min at
20°C. Paraformaldehyde was aspirated and 0.5 ml crystal violet
staining solution was added to each well, before the cells were
incubated for 20 min at 20°C. After removing the staining solution,
each well was washed with distilled water, and the culture plate
was placed upside down on absorbent paper to absorb the water And
was dried naturally. The 6-well plate was then scanned using an
Epson Perfection V370 photograph scanner (Epson).
Calcein/PI cell viability and
cytotoxicity detection
Rat chondrocytes were seeded in 6-well plates
(3×104 cells/well), and the cell status was observed
after the cells had adhered to the wall, after which the cells were
pretreated with IL-1β (20 ng/ml) or Fer-1 (60 nmol/ml) for 24 h,
and then treated with or without AZ (100 µM) for 24 h at 37°C.
After treatment, the culture medium was aspirated, and the cells
were washed 1–2 times with PBS. After washing, Calcein AM/PI
working solution (1 ml/well) was added, and the cells were
incubated at 37°C for 30–60 min in the dark. Subsequently, staining
was observed under a fluorescence microscope (Calcein AM emits
green fluorescence, whereas PI emits red fluorescence). Images of
the cells were captured under a fluorescence microscope
(CX41-32RFL; Olympus Corporation).
Flow cytometry
Flow cytometry was performed to analyze the
apoptotic rate of mouse ATDC5 cells and rat chondrocytes. Cells
were seeded in 6-well plates at a density of 1×105
cells/well, stimulated with or without IL-1β (20 ng/ml) for 24 h,
and then incubated with AZ (100 µM) for another 24 h at 37°C. The
culture medium was aspirated, the cells were rinsed with 2 ml PBS,
and EDTA-free trypsin was added to digest the cells. The cells were
gently transferred to 15-ml centrifuge tubes and centrifuged at
1,000 × g for 5 min at 20°C. The supernatant was discarded and the
cells were gently resuspended in 195 µl binding solution.
Subsequently, 5 µl Annexin V-FITC and 10 µl PI staining solution
was added and mixed gently. The samples were incubated at room
temperature in the dark for 20 min. Finally, the samples were
immediately analyzed with a BD FACSCalibur flow cytometer (BD
Biosciences). Data were analyzed using FlowJo 10 for Mac v10.4
(FlowJo, LLC).
Measurement of ROS levels
Cells were first treated as described for crystal
violet staining, and a commercially available ROS detection kit was
used to measure intracellular ROS levels in the chondrocytes. The
DCFH-DA probe was diluted in serum-free medium (1:1,000) to a final
concentration of 10 µM. The culture medium was removed, and 1 ml
diluted DCFH-DA was added to each well and the cells were incubated
for 20 min at 37°C. The cells were rinsed three times with
serum-free culture medium to fully remove the excess DCFH-DA, and
were then immediately observed and photographed under a CX41-32RFL
fluorescence microscope (Olympus Corporation).
Malondialdehyde (MDA) assay
MDA concentration was evaluated using an MDA assay
kit (cat. no. MAK085; MilliporeSigma) according to the
manufacturer's instructions. Rat and mouse cells were used for
double validation in this experiment, and they were treated using
the same conditions. Cells were seeded in 6-well plates at a
density of 1×105 cells/well, stimulated with or without
IL-1β (20 ng/ml) for 24 h, and then incubated with AZ (100 µM) or
Fer-1 (60 nmol/ml) for another 24 h at 37°C. Cells
(1×106) were homogenized on ice in 300 µl MDA lysis
buffer containing 3 µl 2-methyl-2-propenoic acid (100X). The
samples were centrifuged at 13,000 × g for 10 min at 20°C to remove
any insoluble material, after which 600 µl thiobarbituric acid
solution was added to each sample. The samples were incubated at
95°C for 60 min and cooled to room temperature in an ice bath for
10 min. Finally, 200 µl reaction mixture was pipetted into a
96-well plate and the absorbance was measured at 532 nm.
Glutathione (GSH) assay
GSH concentration was measured using a total GSH
assay kit (cat. no. BC1175; Beijing Solarbio Science &
Technology Co., Ltd.) according to the manufacturer's protocol. Rat
and mouse cells were used for double validation in this experiment,
and they were treated using the same conditions. Cells were seeded
in 6-well plates at a density of 1×105 cells/well,
stimulated with or without IL-1β (20 ng/ml) for 24 h, and then
incubated with AZ (100 µM) or Fer-1 (60 nmol/ml) for another 24 h
at 37°C. A total of 1×107 cells were washed with PBS and
then centrifuged at 600 × g for 5 min at 20°C to obtain a compact
cell pellet. The supernatant was removed, and three volumes of a 5%
sodium thiosulfate solution were added to the cell pellet and
vortexed. The suspension was freeze-thawed twice (liquid nitrogen
was used for freezing and a 37°C water bath was used for thawing)
and left for 5 min at 2–8°C. The extract was then centrifuged at
10,000 × g for 10 min at 20°C and 200 µl was added to a 96-well
plate to measure the absorbance at 412 nm.
Western blot analysis
Cells were processed using a method similar to that
used for EdU staining. Subsequently, the cells were washed three
times with cold PBS and lysed for 20 min on ice with RIPA buffer
(Beyotime Institute of Biotechnology) containing a 10% phosphatase
and protease inhibitor cocktail (complete, EDTA-free; Roche Applied
Science) and PMSF (Beyotime Institute of Biotechnology). The lysate
and loading buffer (Beyotime Institute of Biotechnology) were mixed
in a 1X ratio, heated in a 100°C water bath for 5 min, and then
maintained at −80°C until further use. The proteins (20 µg) were
separated by SDS-PAGE on 8–12% gels and transferred to
polyvinylidene fluoride (PVDF) membranes, which were blocked in 5%
skim milk at room temperature for 1 h. The PVDF membranes were then
incubated with primary antibodies for 10 h at 4°C and were then
incubated with 1:8,000 secondary antibodies at room temperature for
1 h. ECL chemiluminescent imaging was performed, and the proteins
were immediately visualized with an Amersham 600 chemiluminescence
system (Cytiva) and analyzed by ImageJ V 1.8.0 (National Institutes
of Health). The following primary and secondary antibodies were
used in the present study: Nrf2 (cat. no. 16396-1-AP; 1:2,000),
HO-1 (cat. no. 10701-1-AP; 1:1,000), GPX4 (cat. no. 67763-1-lg;
1:2,000), ADAMTS-4 (cat. no. 11865-1-AP; 1:500), inducible nitric
oxide synthase (iNOS; cat. no. 18985-1-AP; 1:1,000), MMP13 (cat.
no. 18165-1-AP; 1:1,000), cyclooxygenase 2 (COX2; cat. no.
27308-1-AP; 1:1,000), aggrecan (cat. no. 13880-1-AP; 1:1,000),
collagen type II α1 (COL2A1; cat. no. 28459-1-AP; 1:1,000), Notch1
(cat. no. 20687-1-AP; 1:1,000), suppressor of cytokine signaling 3
(SOCS3; cat. no. 14025-1-AP; 1:1,000) and β-actin (cat. no.
66009-1-lg; 1:4,000) (all from Proteintech Group Inc.), and caspase
3 (cat. no. A0214; 1:1,000) and cleaved caspase 3 (cat. no. A19654;
1:1,000) (both from ABclonal Biotech Co., Ltd.) primary antibodies;
HRP-conjugated AffiniPure goat anti-rabbit IgG (H+L) (cat. no.
SA00001-2; 1:8,000) and HRP-conjugated AffiniPure goat anti-mouse
IgG (H+L) (cat. no. SA00001-1; 1:8,000) secondary antibodies (both
from Proteintech Group Inc.).
Transmission electron microscopy
(TEM)
Cells were seeded in 6-well plates at a density of
1×105 cells/well, stimulated with or without IL-1β (20
ng/ml) for 24 h, and then incubated with AZ (100 µM) or Fer-1 (60
nmol/ml) for another 24 h at 37°C. Chondrocytes were fixed in 2.5%
glutaraldehyde in 0.05 M sodium cacodylate buffer (pH 7.2) for 2 h
at 25°C, followed by 2 h in 2% OsO4 in 0.1 M cacodylic
acid sodium buffer, and incubation with 1% uranyl acetate aqueous
solution for 18 h at 25°C. After dehydration through an ethanol
series, the specimens were embedded in Epon 812, and 5 µm sections
were collected on copper plates. After staining with 50% uranyl
acetate and 30~40 g/l lead citrate for 2 h at 25°C, the sections
were examined using an electron microscope (HT7800; Hitachi,
Ltd.).
Induction of a knee OA model by
destabilization of the medial meniscus (DMM) surgery in mice
A total of 20 2-month-old male C57BL/6J mice
(weight, 20 g) were purchased from Changzhou Laboratory Animal
Company and were fed commercial food and water under specific
pathogen-free conditions. The mice were maintained at 20–25°C and
50–60% humidity, had free access to pure water and special feed,
and were kept under a 12-h light/dark cycle. The present study was
approved by the Animal Ethical Committee of Nanjing Medical
University (Changzhou, China); all animal experiments complied with
the National Institutes of Health Guide for the Care and Use of
Laboratory Animals (45). Mice
were sacrificed by CO2 asphyxiation (fill rate, 60%
chamber volume/min), and death was confirmed by observing a lack of
respiration and faded eye color (46,47).
The method of sacrifice used is able to fulfill the objective of
rapid unconsciousness with minimal distress to the animals
(47). The mice were anesthetized
by intraperitoneal injection of pentobarbital sodium (50 mg/kg)
before surgery. DMM surgery was performed on the right knee of the
mice to surgically induce an OA model. DMM-induced abnormal
mechanical loading-associated OA of the right knee was established
according to a previous study (35). The 20 mice were randomly divided
into four groups (n=5/group) and all of the mice were maintained
for 4 weeks after surgery. The groups were as follows: i) The
control group, in which the joint capsule was opened, and the
incision was sutured; ii) the DMM group, in which the mice were
injected with PBS in the joints once every other day for 4 weeks
after DMM surgery; iii) 0.25 and iv) 1 mg/kg body weight AZ groups,
in which the mice were injected with AZ by intra-articular
injection once every other day for 4 weeks.
Micro-CT scanning and analysis
After the mice were sacrificed, the whole hind limbs
were collected and fixed in 10% neutral buffered formalin for 24 h
at 20°C, and the excess soft tissue was removed. The whole hind
limbs were scanned using a Skyscan 1276 micro-CT instrument (Bruker
Belgium SA) using the following settings: Source voltage, 55 kV;
source current, 200 µA; 0.25-mm filter; pixel size, 6 µm; and
rotation step, 0.3 degrees. The images were then reconstructed with
NRecon V 1742 software (Bruker Belgium SA); the target area was the
knee joint. The three-dimensional structural parameters that were
analyzed were bone volume (BV), total volume (TV), their ratio
(BV/TV), trabecular number (Tb. N), and trabecular thickness (Tb.
Th).
Immunohistochemical staining
After all mice were sacrificed, the whole hind limbs
were decalcified in 10% EDTA/PBS and then embedded in paraffin. All
samples were fixed in 4% paraformaldehyde for 24–48 h. Sagittal
sections of the knee joint medial compartment were cut to a 4-µm
thickness and were stained with hematoxylin & eosin (H&E)
and Safranin O/Fast Green (S&F) for 24 h at 20°C. Staining was
evaluated using the Osteoarthritis Research Society International
(OARSI) method and OARSI 2019 guidelines (48). Immunohistochemical staining was
performed with antibodies against Nrf2, GPX4, and aggrecan (1:200;
Proteintech Group, Inc.) for 2 h at 4°C. Tissues (4 µm) were fixed
in 4% paraformaldehyde for 24 h at 20°C, then dehydrated in a
descending ethanol series and permeabilized in xylene before being
embedded in paraffin at 20°C. Samples were also stained with
diaminobenzidine (Dako; Agilent Technologies, Inc.) and
counterstained with hematoxylin (MilliporeSigma) for 24 h at 20°C.
Images were captured using a Zeiss Axio Imager light microscope
(Zeiss AG).
Statistical analysis
All quantitative data are presented as the mean ±
standard deviation or as median and IQR. The in vitro
experiments were repeated three times. Statistical analysis was
performed using GraphPad Prism 7 software (Dotmatics). One-way
ANOVA and Tukey's multiple comparisons test was used to analyze the
significance of differences among the groups. Kruskal-Wallis test
and Dunn's post hoc test was used to analyze the significant
differences in OARSI score. P<0.05 was considered to indicate a
statistically significant difference.
Results
AZ relieves osteophyte formation in
mice following DMM surgery
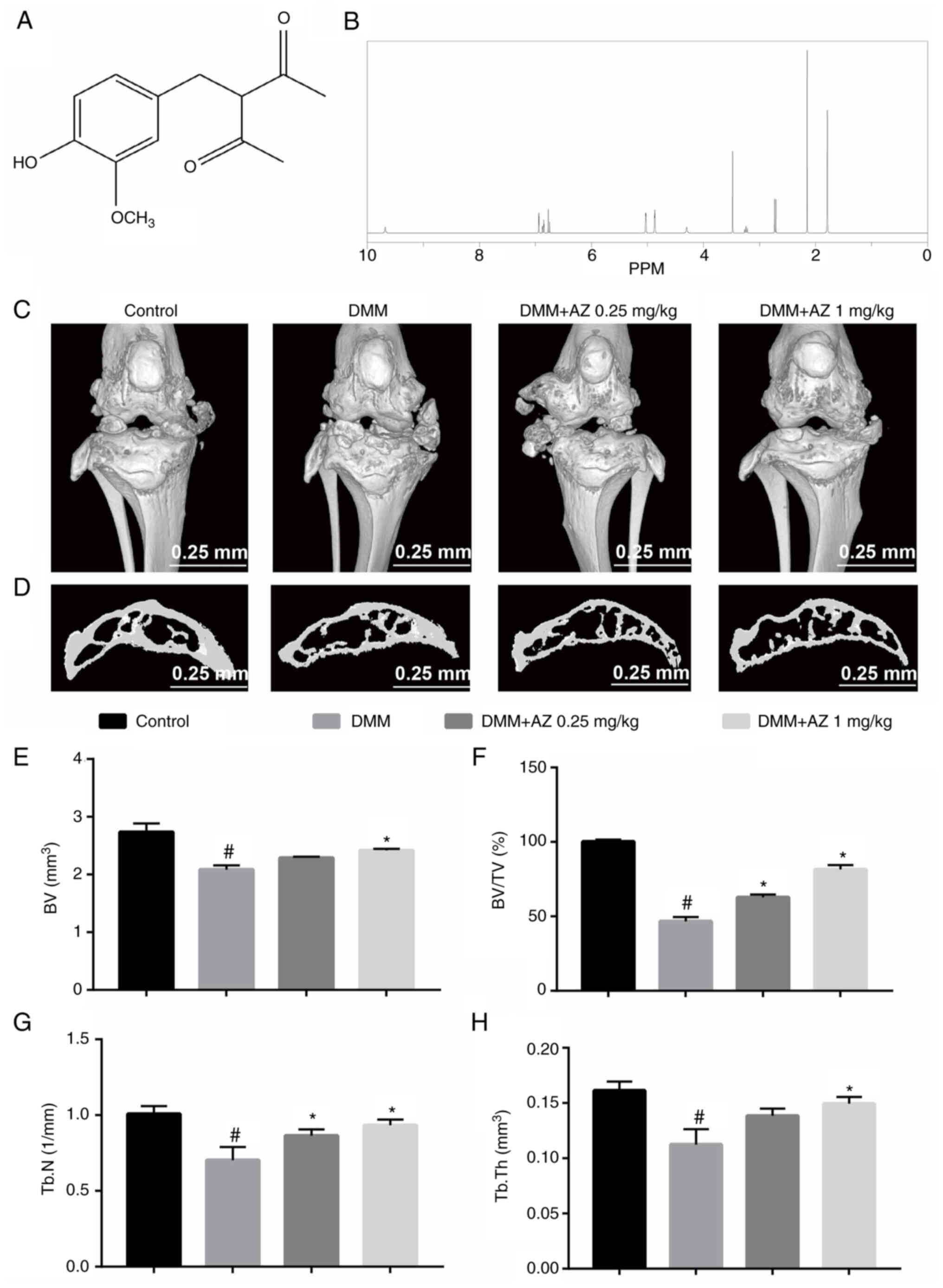
The chemical structure of AZ is shown in Fig. 1A. To exclude false positives caused
by impurities, the NMR spectrum of AZ was obtained to verify the
purity of the compound that was used. The NMR results showed that
the purity of AZ was >98% (Fig.
1B). To investigate the effects of AZ on cartilage destruction
and osteophyte formation in OA, mice were intra-articularly
injected with AZ after DMM surgery. The results showed that DMM
surgery caused bone destruction; however, AZ treatment
dose-dependently reduced cartilage destruction, increasing the
integrity of the kneecap and cartilage mass in the subchondral
cartilage (Fig. 1C and D). The BV
of the tibial plateau subchondral cartilage in the DMM group was
2.14±0.06 mm3, which was significantly lower than that
in the control group (2.60±0.08 mm3). In mice treated
with 0.25 and 1 mg/kg AZ, the BV was 2.27±0.07 and 2.39±0.05
mm3, respectively, but there were no significant
differences between the two groups (Fig. 1E). It can be seen from the results
that after DMM surgery, bone destruction increased, BV decreased,
and the BV/TV (Fig. 1F), Tb. N
(Fig. 1G) and Tb. Th (Fig. 1H) were decreased. In the AZ
treatment groups, osteophytes decreased, bone volume increased, and
Tb. N and Tb. Th increased accordingly. There was no significant
difference in bone volume and trabecular thickness in the AZ
low-dose group compared with in the DMM group. The BV and other
parameters, including BV/TV, Tb. N and Tb. Th, indicated that AZ
inhibited the increase in osteophytes induced by DMM surgery.
AZ promotes chondrocyte proliferation
and maintains ECM homeostasis in vitro
The results of a CCK-8 assay showed that AZ at
concentrations of 25, 50 and 100 µM had no cytotoxic effects and
promoted the viability of chondrocytes after 24 and 48 h compared
with the IL-1β-treated group (Fig.
2A). In addition, similar results were obtained in the mouse
cell line ATDC5 (Fig. S1A).
Furthermore, EdU staining showed that the decrease in the
proliferation of chondrocytes following IL-1β treatment was
reversed by AZ in a dose-dependent manner (Fig. 2B). Subsequently, the effect of AZ
on the expression levels of cartilage ECM proteins were determined.
After IL-1β treatment, aggrecan and COL2A1 were significantly
reduced compared with in the control group, whereas AZ
significantly promoted aggrecan and COL2A1 expression compared with
that in the IL-1β group, which was beneficial for maintaining the
integrity of the articular cartilage (Fig. 2C and E). In addition, western
blotting showed that AZ inhibited the expression levels of
inflammatory factors (iNOS and COX2) and inhibited the expression
of matrix-degrading enzymes (ADAMTS-4 and MMP13) in IL-1β-treated
chondrocytes (Fig. 2D and E).
These data suggested that AZ may inhibit inflammatory
factor-induced degradation of ECM proteins to maintain cartilage
integrity.
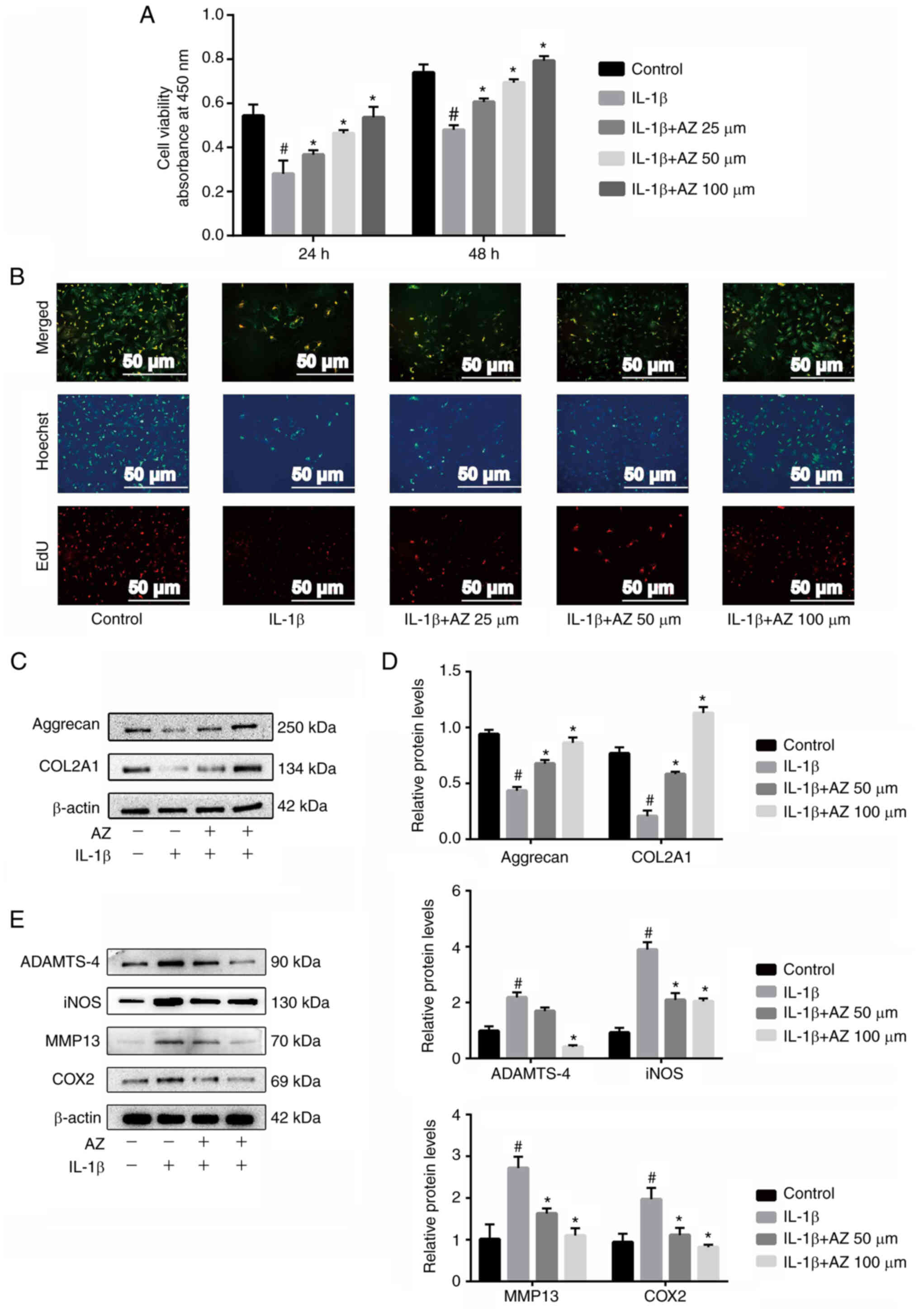 | Figure 2.AZ promotes chondrocyte
proliferation, and inhibits the expression of inflammatory factors
and matrix-degrading enzymes. Chondrocytes were pretreated with
DMSO and IL-1β (20 ng/ml) for 24 h, and were then treated with AZ
(25, 50 and 100 µM) for another 24 or 48 h. (A) Cell viability was
assessed using the Cell Counting Kit-8 assay. (B) EdU staining (red
fluorescence) was used to assess cell proliferation. (C) Expression
levels of aggrecan and COL2A1. (D) ImageJ was used to analyze the
relative protein expression levels of aggrecan, COL2A1, ADAMTS-4,
iNOS, MMP13 and COX2. (E) ADAMTS-4, iNOS, MMP13 and COX2 were
detected by western blotting. #P<0.05 vs. control;
*P<0.05 vs. IL-1β. AZ, acetyl zingerone; COL2A1, collagen type
II α1; COX2, cyclooxygenase 2; EdU, 5-ethynyl-2′-deoxyuridine;
iNOS, inducible nitric oxide synthase. |
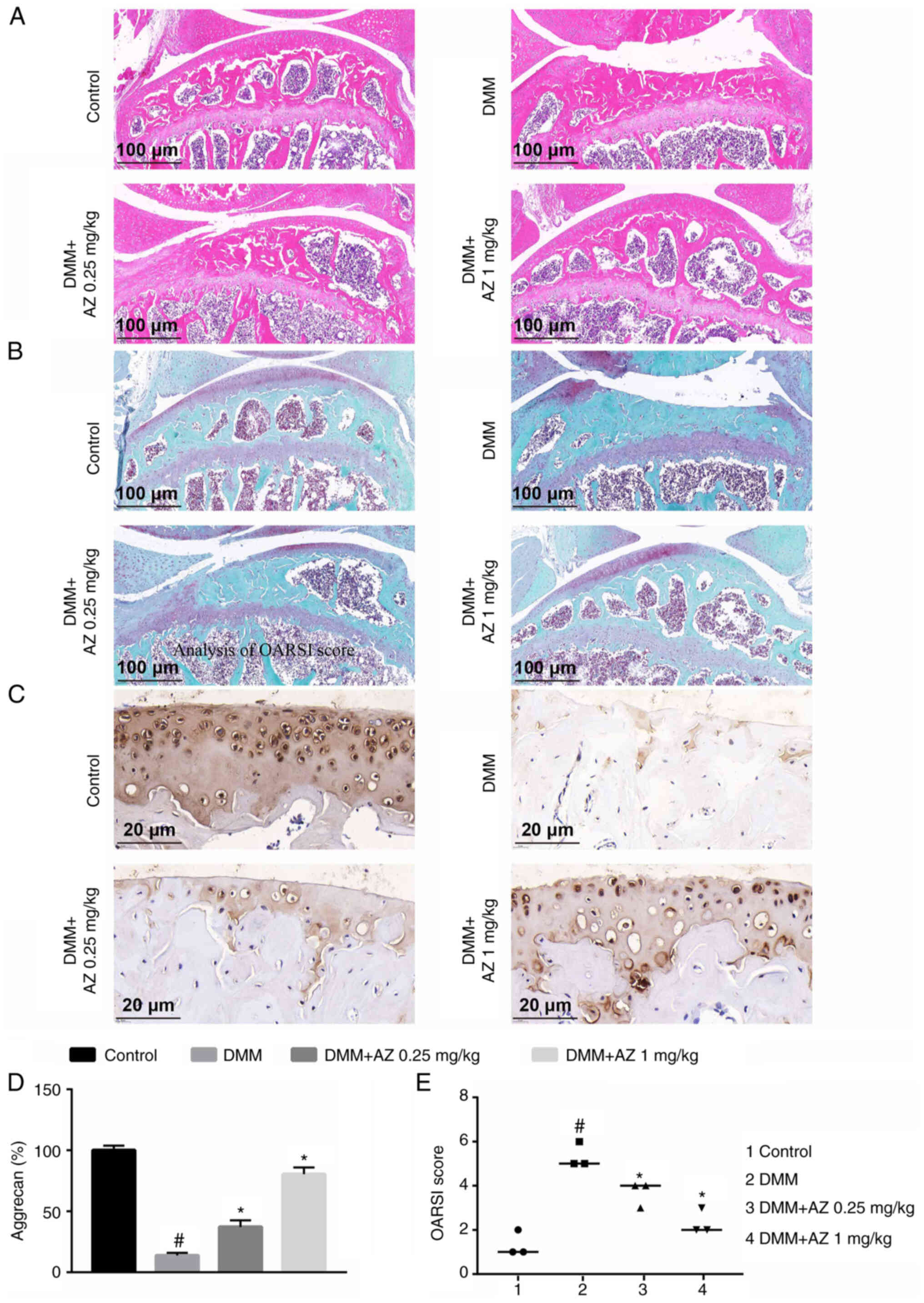
AZ maintains ECM homeostasis in
vivo
Following treatment with AZ, both H&E (Fig. 3A) and S&F staining (Fig. 3B) showed that AZ could promote
cartilage repair and reduce bone loss in the DMM group. H&E and
S&F staining demonstrated the loss of proteoglycans and
decreased thickness of the articular cartilage caused by DMM
surgery (Fig. 3A and B). From the
results, it can be concluded that the content of aggrecan in the
DMM group was significantly lower than that in the control group.
Compared with in the DMM group, there was a significant increase in
aggrecan in the AZ group (Fig. 3C and
D). Moreover, OARSI was significantly increased in the DMM
group compared with that in the control group. Compared with in the
DMM group, there was a significant decrease in OARSI in the AZ
group (Fig. 3E).
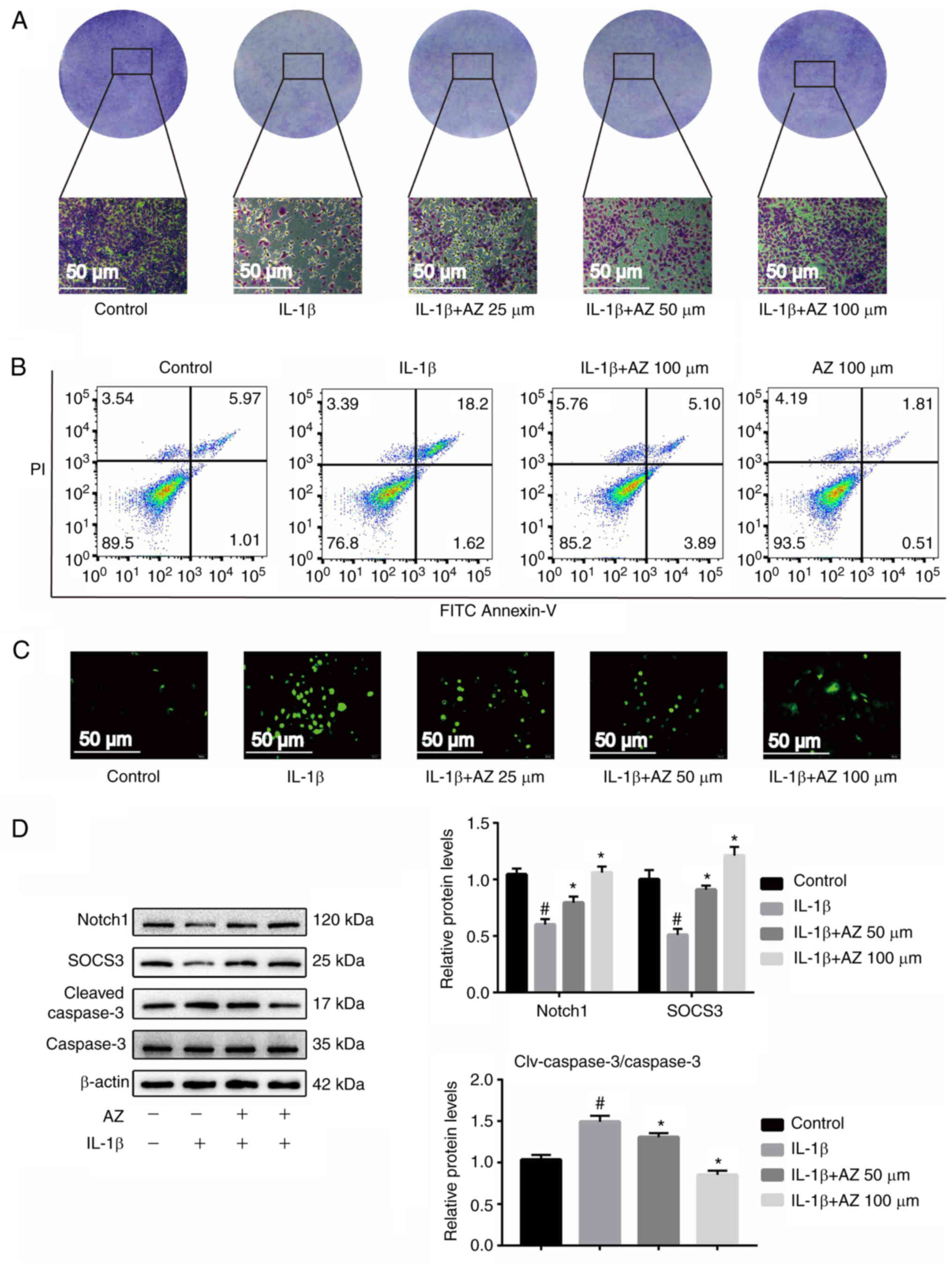
AZ inhibits chondrocyte apoptosis and
promotes Notch1 expression
Chondrocyte apoptosis caused by chronic inflammation
in OA is an important factor in the progression of the disease
(42). The results of crystal
violet staining showed that the number of chondrocytes in the IL-1β
group was reduced compared with the control group, whereas AZ
treatment inhibited this trend, and the number of chondrocytes was
increased compared with the IL-1β group (Fig. 4A). Similar results were obtained
from the mouse cell experiments. From the results of toluidine blue
staining, after treatment with IL-1β, the content of the ECM was
markedly reduced, whereas it was increased after AZ compared with
that in the IL-1β group (Fig.
S1C). As determined by flow cytometry, the proportion of early
+ late apoptotic cells in the IL-1β-induced group was markedly
increased compared with that in the control group. By contrast,
compared with in the IL-1β group, the AZ treatment groups exhibited
reduced apoptosis, thus indicating that AZ may have a marked effect
on inhibiting the apoptosis of chondrocytes. (Fig. 4B). In addition, similar results
were obtained in the mouse cell line ATDC5 (Fig. S1B). These findings indicated that
AZ could reverse the increase in apoptosis induced by IL-1β. The
results of ROS analysis revealed that in the IL-1β group, a marked
accumulation of ROS was detected compared with that in the control
group; however, compared with in the IL-1β group, AZ markedly
reduced the accumulation of ROS (Fig.
4C).
It has previously been reported that Notch signaling
activation contributes to the synthesis of the chondrocyte matrix
and promotes joint repair (49,50).
In addition, the inhibition of Notch signaling can significantly
reduce the proliferation of OA chondrocytes (51). Therefore, normal Notch expression
is important for chondrocytes. Notably, SOCS3 has been identified
as a pivotal regulator of the Notch pathway, which is involved in
multiple physiological processes (52). SOCS3 is a downstream protein of the
Notch pathway, which can reflect the activation of Notch. Notch1 is
the active state of Notch, which indicates activation of the Notch
pathway. The present results showed that the expression levels of
the key factor Notch1 (cleaved Notch) and SOCS3 in the Notch
pathway were significantly decreased (Figs. 4D and S1D), and the cleaved caspase-3/caspase-3
ratio was increased following treatment with IL-1β, whereas AZ
significantly increased Notch1 and SOCS3 expression, and decreased
the cleaved caspase-3/caspase-3 ratio (Fig. 4D). In addition, similar results
were determined in the mouse cell line ATDC5 (Fig. S1D). Therefore, AZ may inhibit
chondrocyte apoptosis through the Notch pathway.
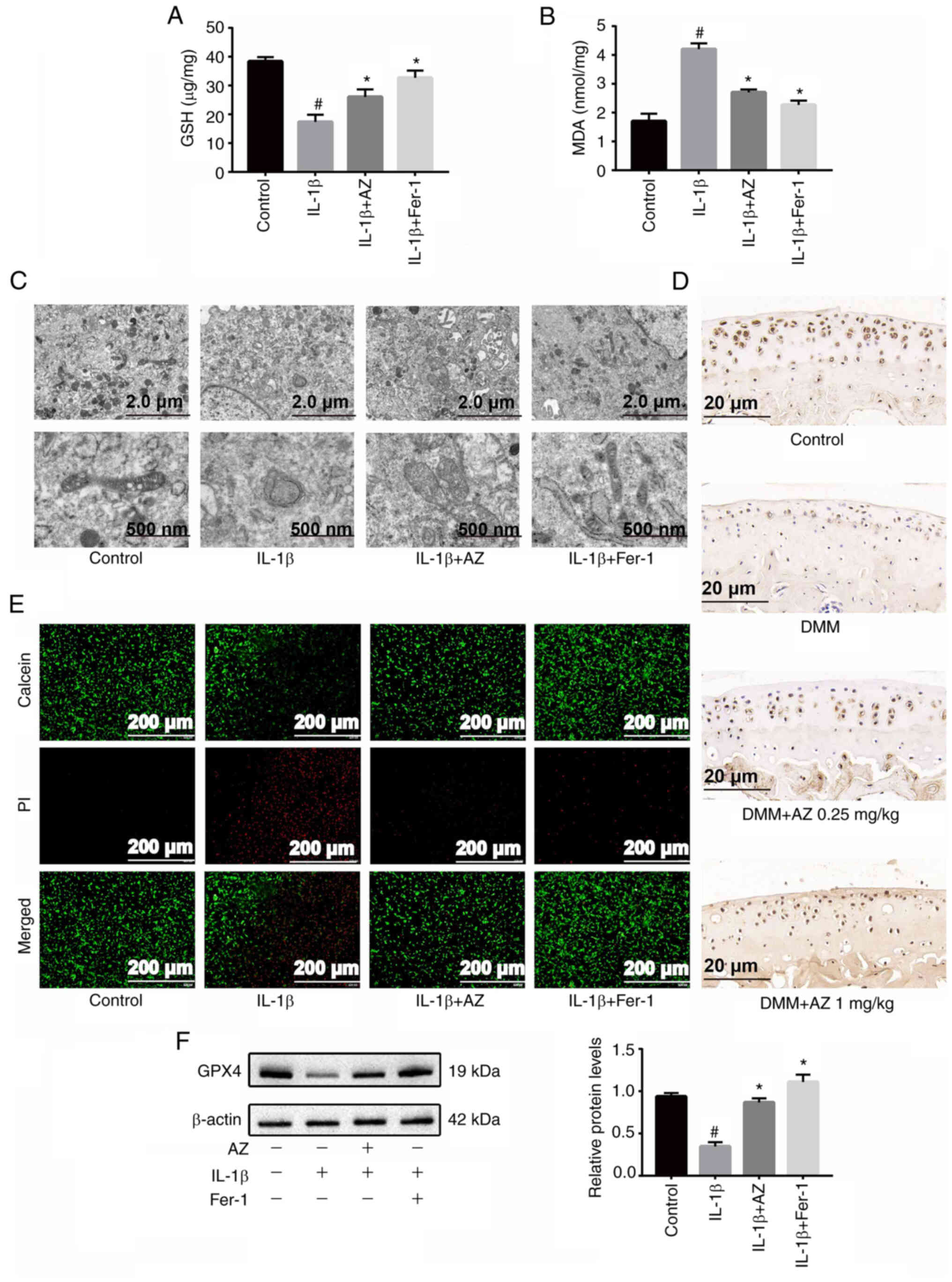
AZ inhibits chondrocyte
ferroptosis
MDA and GSH are considered key effective metabolic
indicators involved in the regulation of ferroptosis (53). IL-1β is used as a classic inducer
of OA in vitro, and recent studies have demonstrated that it
also induces ferroptosis in chondrocytes (24–26).
Fer-1 is a specific inhibitor of ferroptosis, which was used as a
control to confirm the effect of AZ. The present results showed
that IL-1β induced a decrease in GSH levels and an increase in MDA
levels; however, these effects were alleviated by the addition of
AZ and Fer-1 (60 nmol/ml) (Fig. 5A and
B); AZ significantly increased the levels of GSH in
chondrocytes and reduced the accumulation of MDA. In addition,
similar results were obtained in the mouse cell line ATDC5
(Fig. S2A and B). A reduction in
or the disappearance of mitochondrial cristae is a characteristic
indicator of ferroptosis (54).
TEM was used to examine the morphological changes characteristic of
ferroptosis. In the IL-1β group, the mitochondrial morphology was
markedly altered, the size of the mitochondria decreased, the
density of the double membrane increased, the outer membrane of the
mitochondria was broken and the mitochondrial ridge disappeared,
which were all signs of ferroptosis. By contrast, AZ or Fer-1 (60
nmol/ml) markedly inhibited these morphological changes, and
stabilized the structure and shape of mitochondria (Fig. 5C). Calcein/PI cell viability and
cytotoxicity assay results showed that after IL-1β induction, the
proportion of dead cells was increased and the live number of rat
chondrocytes was decreased. Treatment with AZ or Fer-1 (60 nmol/ml)
inhibited the ferroptosis of chondrocytes (Fig. 5D). Subsequently, western blotting
showed that the expression levels of GPX4, a key protein involved
in ferroptosis, were significantly decreased in the IL-1β group,
whereas AZ and Fer-1 (60 nmol/ml) promoted the expression of GPX4,
which in turn may protect chondrocytes from ferroptosis (Fig. 5E); similar results were determined
in the mouse cell line ATDC5 (Fig.
S2C). Furthermore, immunohistochemical staining confirmed that
the expression of GPX4 was reduced in the DMM mice, whereas AZ
promoted its expression (Fig. 5F).
Taken together, these findings suggested that AZ may inhibit
ferroptosis.
AZ inhibits ferroptosis in OA through
the Nrf2/HO-1 pathway
The results of western blotting showed that both
Nrf2 and HO-1 were markedly increased in the IL-1β group,
indicating that ferroptosis occurred in chondrocytes, and Nrf2 and
HO-1 responded to this change. By contrast, AZ and Fer-1 (60
nmol/ml) markedly promoted the expression levels of Nrf2 and HO-1
compared with those in the IL-1β group (Fig. 6A and B). To explore this finding
further, inhibitors of Nrf2 (ML385) and HO-1 (tin protoporphyrin
IX) were used. IL-1β-suppressed the expression levels of GPX4. The
application of these inhibitors exacerbated the IL-1β-induced
suppression of GPX4. Moreover, the anti-ferroptosis effect of AZ
was also abolished. GPX4 is a downstream protein of Nrf2 and HO-1.
When Nrf2 and HO-1 are inhibited, intracellular antioxidant
substances will decrease, and the expression of GPX4 will increase
accordingly (55). However, due to
the increased levels of oxidative stress substances, such as ROS,
GPX4 will reduce. From the results, it can be seen that the
expression of GPX4 was decreased following treatment with the Nrf2
inhibitor. Since HO-1 is regulated by Nrf2, the expression of HO-1
was also reduced after Nrf2 inhibitor treatment (Fig. 6A). In addition, the expression of
GPX4 was decreased following treatment with the HO-1 inhibitor. The
result of Nrf2 immunohistochemical staining showed that the levels
of Nrf2, which is in the ferroptosis pathway (56), were increased in the DMM group,
whereas AZ treatment further promoted Nrf2 expression (Fig. 6B). Since Nrf2 is not regulated by
HO-1, the expression of Nrf2 was not affected by HO-1 inhibitor
treatment (Fig. 6C). The potential
mechanism by which AZ inhibits chondrocyte ferroptosis is shown in
Fig. 6D. Taken together, these
data suggested that AZ may inhibit ferroptosis in OA through the
Nrf2/HO-1 pathway.
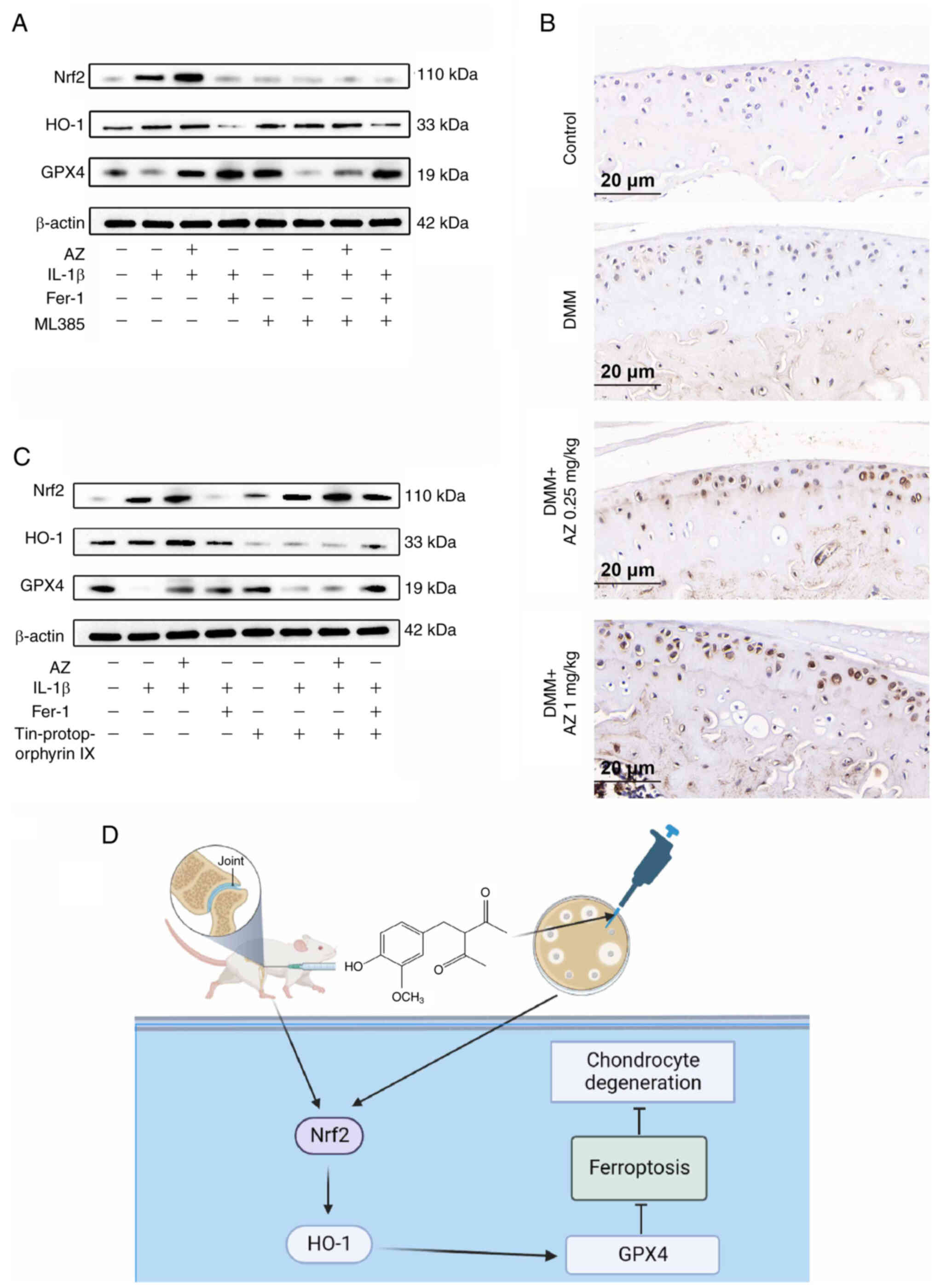 | Figure 6.AZ inhibits ferroptosis in
osteoarthritis through the Nrf2/HO-1 pathway. Chondrocytes were
pretreated with IL-1β (20 ng/ml) for 24 h, with or without ML385
(Nrf2 inhibitors) or Fer-1 (60 nmol/ml) for 12 h, and then treated
with or without AZ (100 µM) for an additional 24 h. (A) Protein
expression levels of Nrf2, HO-1 and GPX4 was determined by western
blotting. (B) Nrf2 staining of ferroptosis in chondrocytes.
Chondrocytes were pretreated with IL-1β (20 ng/ml) for 24 h, with
or without tin protoporphyrin IX (HO-1 inhibitors) or Fer-1 (60
nmol/ml) for 12 h, and then treated with or without AZ (100 µM) for
an additional 24 h. (C) Protein expression levels of Nrf2, HO-1 and
GPX4 was determined by western blotting. (D) Schematic diagram
showing how AZ affects ferroptosis through the Nrf2/HO-1 signaling
pathway. AZ, acetyl zingerone; DMM, destabilization of the medial
meniscus; Fer-1, ferrostatin-1; GPX, glutathione peroxidase 4;
HO-1, heme oxygenase-1; Nrf2, nuclear factor erythroid 2-related
factor 2. |
Discussion
OA is a dynamic process caused by the repair and
damage of joint tissues (2). The
pathogenesis of this disease is complicated, and it involves
factors such as inflammation, aging and metabolism, which
eventually lead to the destruction of the joint structure (57). An abnormal chondrocyte programmed
cell death rate is a major trigger of OA. Results from the present
study revealed that AZ inhibited the expression of proinflammatory
factors in chondrocytes, thereby reducing the accumulation of ROS.
Notch signaling is also closely related to the pathogenesis of OA
(58). Long-term inhibition of
Notch signal transduction leads to an imbalance in cartilage
homeostasis and the growth of osteophytes (59). The present study found that AZ may
promote chondrocyte proliferation by activating the Notch
pathway.
Ferroptosis is iron-dependent regulated cell death
that is caused by an accumulation of lipid peroxidation products
and is prevented by GPX4, a key antioxidant enzyme (60). In previous years, chondrocyte
ferroptosis has attracted attention, and effective inhibition of
this type of cell death may be a new target in the treatment of OA
(61). It has been reported that
mechanical overload induces GPX4-regulated chondrocyte ferroptosis
in OA through Piezo1 channel-facilitated calcium influx (62). In the present study, AZ was shown
to upregulate the expression of GPX4, thus potentially inhibiting
ferroptosis. The present study simulated the microenvironment of OA
by treating chondrocytes with IL-1β. In this model, the expression
of GPX4 was significantly decreased, and the chondrocyte
mitochondria were markedly deformed or even disappeared. These
morphological and protein expression change are consistent with the
occurrence of ferroptosis (63).
Further study revealed that the features of IL-1β-induced
ferroptosis could be reversed by AZ. Furthermore, in the DMM rat
model, AZ successfully alleviated joint degeneration and osteophyte
formation, and promoted the expression of GPX4. Taken together, the
present results suggested that AZ may attenuate the degeneration of
cartilage in OA through the inhibition of chondrocyte
ferroptosis.
Both Nrf2 and HO-1 are important elements in the
antioxidant response to stress (64). When cells are under oxidative
stress, corresponding increases in Nrf2 and HO-1 levels induce the
expression of downstream genes, thereby enhancing the resistance of
cells to oxidative stress or programmed cell death, and maintaining
cell stability (65). Previous
reports have demonstrated that the Nrf2/HO-1 pathway protects cells
from ferroptosis. Notably, increased expression of Nrf2/HO-1 can
protect against ferroptosis (66,67).
Furthermore, Nrf2/HO-1 serves an important role in regulating the
expression of GPX4 in various inflammatory diseases (68), and GPX4 is a downstream molecule of
Nrf2 (69). The present study
aimed to examine whether Nrf2/HO-1 is essential for the mechanism
by which AZ suppressed IL-1β-induced ferroptosis in chondrocytes;
therefore, Nrf2 and HO-1 expression levels were assessed in
vitro. AZ treatment significantly suppressed IL-1β cytotoxicity
in osteoarthritic chondrocytes and inhibited IL-1β-induced
ferroptosis by promoting Nrf2/HO-1 expression. Moreover, the levels
of GPX4, a key inhibitory factor of ferroptosis, were evaluated
after the application of Nrf2 or HO-1 inhibitors. The results were
consistent with our hypothesis that these inhibitors aggravated the
decrease in GPX4 induced by IL-1β and abolished the rescue effect
of AZ. These data demonstrated that AZ may inhibit chondrocyte
ferroptosis through Nrf2/HO-1. Ferroptosis has broad clinical
application prospects in the treatment of OA. The present study
demonstrated that AZ treatment may be effective in preventing the
death of osteoarthritic chondrocytes. Future preclinical studies
are required to assess whether AZ can inhibit the progression of
OA.
Considering the consistency of in vivo and
in vitro experiments, mouse ATDC5 cells should preferably
have been chosen for in vitro experiments; however, rat
cells extracted from 8-week-old rats were considered more
appropriate for the following reasons (61). First, fully mature and
differentiated chondrocytes were required to mimic the in
vivo OA environment. Second, the number of rat chondrocytes
isolated according to the literature is abundant, and the cell
viability is excellent (70).
According to our observation, signs of aging only appear after
>8 passages in vitro. Third, it is much easier to
separate the articular cartilage with a scalpel from the tibia of
an 8-week-old rat than in a mouse, without contaminating other
tissues. Finally, 8-week-old rat chondrocytes were used for
vitro experiments. Regarding cell selection for in
vitro models, three types of cells were considered when
designing experiments: ATDC5 cells, mouse primary chondrocytes, and
rat primary chondrocytes. We attempted to develop mouse
chondrocytes based on the study by Haseeb and Lefebvre (71). Unfortunately, we failed twice due
to limited chondrocyte yield and poor proliferation. In addition,
commercial mouse chondrocytes were considered too expensive.
Therefore, the experiments were conducted in ATDC5 cells and rat
chondrocytes. Both cell types showed similar responses to IL-1β and
AZ. Notably, the ATDC5 cell line is derived from mouse
teratocarcinoma cells, which are characterized as chondrogenic cell
lines, undergoing a continuous process similar to chondrocyte
differentiation; however, primary cultured chondrocytes were
considered a better model and were thus selected as they appeared
to be more suitable.
In conclusion, in vitro and in vivo
studies demonstrated the positive effect of AZ on OA. AZ inhibited
ferroptosis in chondrocytes, and mechanistically targeted the
Nrf2/HO-1 pathway to inhibit the accumulation of harmful substances
in chondrocyte. Furthermore, AZ attenuated articular cartilage
degeneration, suggesting that targeting ferroptosis in chondrocytes
may be an effective strategy for the treatment of OA. The results
of the present study suggested that AZ may be an effective
therapeutic candidate for OA treatment.
Supplementary Material
Supporting Data
Acknowledgments
The authors would like to thank the Institute of
Changzhou Second People's Hospital Affiliated with Nanjing Medical
University (Changzhou, China) for providing the experimental
site.
Funding
This work was supported by the Top Talent of Changzhou ‘The 14th
Five-Year Plan’ High-Level Health Talents Training Project (grant
no. 2022CZBJ078 to SN) and the Major Research Project of Changzhou
Commission of Health (grant no. ZD202218 to SN).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XC, JC and SN performed experiments, obtained data,
performed formal analysis and designed the study. ZZ performed
experiments and analysis. XC, JC, GY and SN were involved in the
methodology, conception and analysis of the study, and
interpretation of data. RS and CM was involved in project
administration, acquisition of data and technical assistance
(synthesis of AZ). All authors read and approved the final
manuscript. XC, JC, CM, GY, ZZ, RS and SN confirm the authenticity
of all the raw data.
Ethics approval and consent to
participate
The present study was approved by the Jiangsu
Science Standard Medical Testing Committee. Code: IACUC22-0097.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mahmoudian A, Lohmander LS, Mobasheri A,
Englund M and Luyten FP: Early-stage symptomatic osteoarthritis of
the knee-time for action. Nat Rev Rheumatol. 17:621–632. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Glyn-Jones S, Palmer AJR, Agricola R,
Price AJ, Vincent TL, Weinans H and Carr AJ: Osteoarthritis.
Lancet. 386:376–387. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Abramoff B and Caldera FE: Osteoarthritis.
Med Clinics North Am. 104:293–311. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bijlsma JWJ, Berenbaum F and Lafeber FPJG:
Osteoarthritis: An update with relevance for clinical practice.
Lancet. 377:2115–2126. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Peng Z, Sun H, Bunpetch V, Koh Y, Wen Y,
Wu D and Ouyang H: The regulation of cartilage extracellular matrix
homeostasis in joint cartilage degeneration and regeneration.
Biomaterials. 268:1205552021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Musumeci G, Castrogiovanni P, Trovato FM,
Weinberg AM, Al-Wasiyah MK, Alqahtani MH and Mobasheri A:
Biomarkers of chondrocyte apoptosis and autophagy in
osteoarthritis. Int J Mol Sci. 16:20560–20575. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yin H, Wang Y, Sun X, Cui G, Sun Z, Chen
P, Xu Y, Yuan X, Meng H, Xu W, et al: Functional tissue-engineered
microtissue derived from cartilage extracellular matrix for
articular cartilage regeneration. Acta Biomater. 77:127–141. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang J, Hu S, Bian Y, Yao J, Wang D, Liu
X, Guo Z, Zhang S and Peng L: Targeting cell death: Pyroptosis,
ferroptosis, apoptosis and necroptosis in osteoarthritis. Front
Cell Dev Biol. 9:7899482022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bertheloot D, Latz E and Franklin BS:
Necroptosis, pyroptosis and apoptosis: An intricate game of cell
death. Cell Mol Immunol. 18:1106–1121. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sun K, Hou L, Guo Z, Wang G, Guo J, Xu J,
Zhang X and Guo F: JNK-JUN-NCOA4 axis contributes to chondrocyte
ferroptosis and aggravates osteoarthritis via ferritinophagy. Free
Radical Biol Med. 200:87–101. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen X, Kang R, Kroemer G and Tang D:
Ferroptosis in infection, inflammation, and immunity. J Exp Med.
18:e202105182021. View Article : Google Scholar
|
|
12
|
Jiang X, Stockwell BR and Conrad M:
Ferroptosis: Mechanisms, biology and role in disease. Nat Rev Mol
Cell Biol. 22:266–282. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhou B, Liu J, Kang R, Klionsky DJ,
Kroemer G and Tang D: Ferroptosis is a type of autophagy-dependent
cell death. Semin Cancer Biol. 66:89–100. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tang D, Chen X, Kang R and Kroemer G:
Ferroptosis: Molecular mechanisms and health implications. Cell
Res. 31:107–125. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang S, Li W, Zhang P, Wang Z, Ma X, Liu
C, Vasilev K, Zhang L, Zhou X, Liu L, et al: Mechanical overloading
induces GPX4-regulated chondrocyte ferroptosis in osteoarthritis
via Piezo1 channel facilitated calcium influx. J Adv Res. 41:63–75.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Friedmann Angeli JP, Schneider M, Proneth
B, Tyurina YY, Tyurin VA, Hammond VJ, Herbach N, Aichler M, Walch
A, Eggenhofer E, et al: Inactivation of the ferroptosis regulator
Gpx4 triggers acute renal failure in mice. Nat Cell Biol.
16:1180–1191. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang Y, Zheng L, Shang W, Yang Z, Li T,
Liu F, Shao W, Lv L, Chai L, Qu L, et al: Wnt/beta-catenin
signaling confers ferroptosis resistance by targeting GPX4 in
gastric cancer. Cell Death Differ. 29:2190–2202. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Miao Y, Chen Y, Xue F, Liu K, Zhu B, Gao
J, Yin J, Zhang C and Li G: Contribution of ferroptosis and GPX4′s
dual functions to osteoarthritis progression. EBioMedicine.
76:1038472022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wan Y, Shen K, Yu H and Fan W: Baicalein
limits osteoarthritis development by inhibiting chondrocyte
ferroptosis. Free Radic Biol Med. 196:108–120. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
He Q, Yang J, Pan Z, Zhang G, Chen B, Li
S, Xiao J, Tan F, Wang Z, Chen P and Wang H: Biochanin A protects
against iron overload associated knee osteoarthritis via regulating
iron levels and NRF2/System xc-/GPX4 axis. Biomed Pharmacother.
157:1139152023. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gong Z, Wang Y, Li L, Li X, Qiu B and Hu
Y: Cardamonin alleviates chondrocytes inflammation and cartilage
degradation of osteoarthritis by inhibiting ferroptosis via p53
pathway. Food Chem Toxicol. 174:1136442023. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ma Q: Role of nrf2 in oxidative stress and
toxicity. Annu Rev Pharmacol Toxicol. 53:401–426. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kerins MJ and Ooi A: The roles of NRF2 in
modulating cellular iron homeostasis. Antioxid Redox Signal.
29:1756–1773. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dodson M, Castro-Portuguez R and Zhang DD:
NRF2 plays a critical role in mitigating lipid peroxidation and
ferroptosis. Redox Biol. 23:1011072019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Agyeman AS, Chaerkady R, Shaw PG, Davidson
NE, Visvanathan K, Pandey A and Kensler TW: Transcriptomic and
proteomic profiling of KEAP1 disrupted and sulforaphane-treated
human breast epithelial cells reveals common expression profiles.
Breast Cancer Res Treat. 132:175–187. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Harada N, Kanayama M, Maruyama A, Yoshida
A, Tazumi K, Hosoya T, Mimura J, Toki T, Maher JM, Yamamoto M and
Itoh K: Nrf2 regulates ferroportin 1-mediated iron efflux and
counteracts lipopolysaccharide-induced ferroportin 1 mRNA
suppression in macrophages. Arch Biochem Biophys. 508:101–109.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Guo Z, Lin J, Sun K, Guo J, Yao X, Wang G,
Hou L, Xu J, Guo J and Guo F: Deferoxamine alleviates
osteoarthritis by inhibiting chondrocyte ferroptosis and activating
the Nrf2 pathway. Front Pharmacol. 13:7913762022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mo Z, Xu P and Li H: Stigmasterol
alleviates interleukin-1beta-induced chondrocyte injury by
down-regulatingsterol regulatory element binding transcription
factor 2 to regulateferroptosis. Bioengineered. 12:9332–9340. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang J, Mo J, Dai J, Ye C, Cen W, Zheng X,
Jiang L and Ye L: Cetuximab promotes RSL3-induced ferroptosis by
suppressing the Nrf2/HO-1 signalling pathway in KRAS mutant
colorectal cancer. Cell Death Dis. 12:10792021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cai X, Hua S, Deng J, Du Z, Zhang D, Liu
Z, Khan NU, Zhou M and Chen Z: Astaxanthin activated the Nrf2/HO-1
pathway to enhance autophagy and inhibit ferroptosis, ameliorating
acetaminophen-induced liver injury. ACS Appl Mater Interfaces.
14:42887–42903. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhou X, Zhang Y, Hou M, Liu H, Yang H,
Chen X, Liu T, He F and Zhu X: Melatonin prevents cartilage
degradation in Early-stage osteoarthritis through activation of
miR-146a/NRF2/HO-1 axis. J Bone Miner Res. 37:1056–1072. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen M, Wen H, Zhou S, Yan X and Li H:
Patchouli alcohol inhibits D-Gal induced oxidative stress and
ameliorates the quality of aging cartilage via activating the
Nrf2/HO-1 pathway in mice. Oxid Med Cell Longev.
2022:68211702022.PubMed/NCBI
|
|
33
|
Xiong L, Bao H, Li S, Gu D, Li Y, Yin Q,
Li W, Miao L and Liu C: Cerium oxide nanoparticles protect against
chondrocytes and cartilage explants from oxidative stress via
Nrf2/HO-1 pathway in temporomandibular joint osteoarthritis. Front
Bioeng Biotechnol. 11:10762402023. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang Y, Han S, Kong M, Tu Q, Zhang L and
Ma X: Single-cell RNA-seq analysis identifies unique chondrocyte
subsets and reveals involvement of ferroptosis in human
intervertebral disc degeneration. Osteoarthritis Cartilage.
29:1324–1334. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Luo P, Huang Q, Chen S, Wang Y and Dou H:
Asiaticoside ameliorates osteoarthritis progression through
activation of Nrf2/HO-1 and inhibition of the NF-κB pathway. Int
Immunopharmacol. 108:1088642022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Witkin JM and Li X: Curcumin, an active
constiuent of the ancient medicinal herb Curcuma longa L.:
Some uses and the establishment and biological basis of medical
efficacy. CNS Neurol Disord Drug Targets. 12:487–497. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Menon VP and Sudheer AR: Antioxidant and
anti-inflammatory properties of curcumin. Adv Exp Med Biol.
595:105–125. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chaudhuri RK, Meyer T, Premi S and Brash
D: Acetyl zingerone: An efficacious multifunctional ingredient for
continued protection against ongoing DNA damage in melanocytes
after sun exposure ends. Int J Cosmetic Sci. 42:36–45. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Swindell WR, Bojanowski K and Chaudhuri
RK: A Zingerone analog, acetyl zingerone, bolsters matrisome
synthesis, inhibits matrix metallopeptidases, and represses IL-17A
target gene expression. J Invest Dermatol. 140:602–614.e15. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Swindell WR, Randhawa M, Quijas G,
Bojanowski K and Chaudhuri RK: Tetrahexyldecyl ascorbate (THDC)
degrades rapidly under oxidative stress but can be stabilized by
Acetyl zingerone to enhance collagen production and antioxidant
effects. Int J Mol Sci. 22:87562021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tang Z, Zhao P, Wang H, Liu Y and Bu W:
Biomedicine meets fenton chemistry. Chem Rev. 121:1981–2019. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sun K, Luo J, Jing X, Xiang W, Guo J, Yao
X, Liang S, Guo F and Xu T: Hyperoside ameliorates the progression
of osteoarthritis: An in vitro and in vivo study. Phytomedicine.
80:1533872021. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Conlee KM, Stephens ML, Rowan AN and King
LA: Carbon dioxide for euthanasia: Concerns regarding pain and
distress, with special reference to mice and rats. Lab Anim.
39:137–161. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Valentim AM, Guedes SR, Pereira AM and
Antunes LM: Euthanasia using gaseous agents in laboratory rodents.
Lab Anim. 50:241–253. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ahmadi-Noorbakhsh S, Mirabzadeh Ardakani
E, Sadighi J, Aldavood SJ, Farajli Abbasi M, Farzad-Mohajeri S,
Ghasemi A, Sharif-Paghaleh E, Hatami Z, Nikravanfard N and Shamsi
Gooshki E: Guideline for the care and use of laboratory animals in
iran. Lab Anim (NY). 50:303–305. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Danneman PJ, Stein S and Walshaw SO:
Humane and practical implications of using carbon dioxide mixed
with oxygen for anesthesia or euthanasia of rats. Lab Anim Sci.
47:376–385. 1997.PubMed/NCBI
|
|
47
|
Tavallaee G, Lively S, Rockel JS, Ali SA,
Im M, Sarda C, Mitchell GM, Rossomacha E, Nakamura S, Potla P, et
al: Contribution of MicroRNA-27b-3p to synovial fibrotic responses
in knee osteoarthritis. Arthritis Rheumatol. 74:1928–1942. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Arden NK, Perry TA, Bannuru RR, Bruyère O,
Cooper C, Haugen IK, Hochberg MC, McAlindon TE, Mobasheri A and
Reginster JY: Non-surgical management of knee osteoarthritis:
Comparison of ESCEO and OARSI 2019 guidelines. Nat Rev Rheumatol.
17:59–66. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Hwang HS and Kim HA: Chondrocyte apoptosis
in the pathogenesis of osteoarthritis. Int J Mol Sci.
16:26035–26054. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Liu Z, Chen J, Mirando AJ, Wang C, Zuscik
MJ, O'Keefe RJ and Hilton MJ: A dual role for NOTCH signaling in
joint cartilage maintenance and osteoarthritis. Sci Signal.
8:ra712015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lin NY, Distler A, Beyer C,
Philipi-Schöbinger A, Breda S, Dees C, Stock M, Tomcik M, Niemeier
A, Dell'Accio F, et al: Inhibition of Notch1 promotes hedgehog
signalling in a HES1-dependent manner in chondrocytes and
exacerbates experimental osteoarthritis. Ann Rheum Dis.
75:2037–2044. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Narayana Y and Balaji KN: NOTCH1
up-regulation and signaling involved in Mycobacterium bovis
BCG-induced SOCS3 expression in macrophages. J Biol Chem.
283:12501–12511. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Linkermann A, Skouta R, Himmerkus N, Mulay
SR, Dewitz C, De Zen F, Prokai A, Zuchtriegel G, Krombach F, Welz
PS, et al: Synchronized renal tubular cell death involves
ferroptosis. Proc Natl Acad Sci USA. 111:16836–16841. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Li Y, Feng D, Wang Z, Zhao Y, Sun R, Tian
D, Liu D, Zhang F, Ning S, Yao J and Tian X: Ischemia-induced ACSL4
activation contributes to ferroptosis-mediated tissue injury in
intestinal ischemia/reperfusion. Cell Death Differ. 26:2284–2299.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Wang Y, Yan S, Liu X, Deng F, Wang P, Yang
L, Hu L, Huang K and He J: PRMT4 promotes ferroptosis to aggravate
doxorubicin-induced cardiomyopathy via inhibition of the Nrf2/GPX4
pathway. Cell Death Differ. 29:1982–1995. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Zi Y, Wang X, Zi Y, Yu H, Lan Y, Fan Y,
Ren C, Liao K and Chen H: Cigarette smoke induces the ROS
accumulation and iNOS activation through deactivation of
Nrf-2/SIRT3 axis to mediate the human bronchial epithelium
ferroptosis. Free Radic Biol Med. 200:73–86. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Kuang L, Wu J, Su N, Qi H, Chen H, Zhou S,
Xiong Y, Du X, Tan Q, Yang J, et al: FGFR3 deficiency enhances
CXCL12-dependent chemotaxis of macrophages via upregulating CXCR7
and aggravates joint destruction in mice. Ann Rheum Dis.
79:112–122. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Hosaka Y, Saito T, Sugita S, Hikata T,
Kobayashi H, Fukai A, Taniguchi Y, Hirata M, Akiyama H, Chung UI
and Kawaguchi H: Notch signaling in chondrocytes modulates
endochondral ossification and osteoarthritis development. Proc Natl
Acad Sci USA. 110:1875–1880. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Artavanis-Tsakonas S, Rand MD and Lake RJ:
Notch signaling: Cell fate control and signal integration in
development. Science. 284:770–776. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta
R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS,
et al: Ferroptosis: An iron-dependent form of nonapoptotic cell
death. Cell. 149:1060–1072. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Wang W, Jing X, Du T, Ren J, Liu X, Chen
F, Shao Y, Sun S, Yang G and Cui X: Iron overload promotes
intervertebral disc degeneration via inducing oxidative stress and
ferroptosis in endplate chondrocytes. Free Radical Biol Med.
190:234–246. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Xiang Q, Zhao Y, Lin J, Jiang S and Li W:
The Nrf2 antioxidant defense system in intervertebral disc
degeneration: Molecular insights. Exp Mol Med. 54:1067–1075. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Torrente L and DeNicola GM: Targeting NRF2
and its downstream processes: Opportunities and challenges. Ann Rev
Pharmacol Toxicol. 62:279–300. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Li J, Lu K, Sun F, Tan S, Zhang X, Sheng
W, Hao W, Liu M, Lv W and Han W: Panaxydol attenuates ferroptosis
against LPS-induced acute lung injury in mice by Keap1-Nrf2/HO-1
pathway. J Transl Med. 19:962021. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Ma H, Wang X, Zhang W, Li H, Zhao W, Sun J
and Yang M: Melatonin suppresses ferroptosis induced by high
glucose via activation of the Nrf2/HO-1 signaling pathway in type 2
diabetic osteoporosis. Oxid Med Cell Longev. 2020:90676102020.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Wu S, Zhu J, Wu G, Hu Z, Ying P, Bao Z,
Ding Z and Tan X: 6-Gingerol alleviates ferroptosis and
inflammation of diabetic cardiomyopathy via the Nrf2/HO-1 pathway.
Oxid Med Cell Longev. 2022:30275142022. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Li S, Zhou C, Zhu Y, Chao Z, Sheng Z,
Zhang Y and Zhao Y: Ferrostatin-1 alleviates angiotensin II (Ang
II)-induced inflammation and ferroptosis in astrocytes. Int
Immunopharmacol. 90:1071792021. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Yang Y, Cai X, Yang J, Sun X, Hu C, Yan Z,
Xu X, Lu W, Wang X and Cao P: Chemoprevention of dietary
digitoflavone on colitis-associated colon tumorigenesis through
inducing Nrf2 signaling pathway and inhibition of inflammation. Mol
Cancer. 13:482014. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Hourihan JM, Moronetti Mazzeo LE,
Fernández-Cárdenas LP and Blackwell TK: Cysteine sulfenylation
directs IRE-1 to activate the SKN-1/Nrf2 antioxidant response. Mol
Cell. 63:553–566. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Liu Z, Lang Y, Li L, Liang Z, Deng Y, Fang
R and Meng Q: Effect of emodin on chondrocyte viability in an in
vitro model of osteoarthritis. Exp Ther Med. 16:5384–5389.
2018.PubMed/NCBI
|
|
71
|
Haseeb A and Lefebvre V: Isolation of
Mouse Growth Plate and Articular Chondrocytes for Primary Cultures.
Methods Mol Biol. 2245:39–51. 2021. View Article : Google Scholar : PubMed/NCBI
|