Introduction
In recent decades, due to social and economic
factors, an increasing number of women have postponed pregnancy
until an advanced age (1), and an
increased maternal age has been shown to be associated with
infertility and a diminished ovarian reserve (DOR) (2). Ovarian aging has an important impact
on reproductive capacity, as it alters the hormonal equilibrium,
decreases the quality of oocytes and worsens reproductive outcomes
(1). Hypotheses for the
age-related decline in reproductive capacity and ovarian reserve
involve oocyte aneuploidy (3),
spindle defects (1), oxidative
stress response (4), mitochondrial
dysfunction (5), autophagy
deficiency (6) and altered
epigenetic patterns (1). Telomere
attrition has also been shown to play a critical role in the
reproductive aging process of humans (7). Telomere shortening has been proposed
as a possible mechanism leading to a reduction in the quality of
oocytes as it may affect chromosome segregation and genome
stability (1).
Telomeres are highly preserved nucleoprotein
structures comprising 5–15 kb-long tandem repeat hexanucleotide
sequence (TTAGGG) repeats that form protective caps at the ends of
eukaryotic chromosomes (8,9). Telomeres play a vital role in the
sequential cycles of cell division and maintenance of chromosomal
stability (10). The progressive
shortening of telomeres within granulosa cells (GCs) due to
inadequate DNA repair and long-term oxidative stress has been
suggested to be the main driving factor underlying reproductive
aging (11).
Telomere length (TL) is typically regulated by
telomerase, a reverse transcriptase enzyme that adds the TTAGGG
sequence to telomeres, thus protecting them from progressive
erosion during cell division. One of the key roles of telomerase is
to delay programmed telomere shortening for the purpose of ensuring
the accurate synthesis of DNA during replication (12). Telomerase consists of two
fundamental elements, namely telomerase reverse transcriptase
(TERT) and telomerase RNA component (TERC). The former is the main
component of telomerase and the latter serves as template for
telomeric DNA synthesis. As the main component of telomerase, TERT
plays an important role in the synthesis of the hexameric sequence
at the end of chromosomes. The regulation of TERT expression in
cells is tissue-dependent (13).
TERT activity prevents telomere shortening with cell division,
thereby preventing cell aging and allowing extensive self-renewal
(8).
A previous study suggested that decreased expression
of TERT and the telomere-binding proteins telomeric repeat-binding
factor 1 (TRF1), TRF2 and protection of telomeres 1 may cause the
age-related shortening of ovarian telomeres, which may be
associated with the decline of female fertility (14). A study of mouse models showed that
the telomere shortening of ovaries leads to elevated embryo
fragmentation, increased levels of cellular arrest, degeneration
and chromosomal abnormalities (15). In a study of humans in which in
vitro matured oocytes from the germinal vesicle stage were used
as experimental materials, TL was found to be a negative predictor
of fragmentation in day-3 preimplantation embryos (16). Also, certain studies demonstrated
that telomeres in polar bodies from women in whom a pregnancy was
established after in vitro fertilization (IVF)-embryo
transfer were longer than those from women who did not conceive
(17). However, another study
found no significant associations between the relative TL (RTL) of
GCs and IVF outcomes (11). The
exact association between TL/telomerase and IVF outcomes remains
unclear and requires further investigation. Notably, the GCs
surrounding the oocytes are considered markers for oocyte quality
and competence (18) and play a
role at the early stage of embryonic development (19).
The aim of the present study was to investigate
alterations in RTL and relative TERT expression in GCs during
aging. The association between RTL/TERT and ovarian/embryonic
performance, and the probability of clinical pregnancy in infertile
women undergoing IVF or intracytoplasmic sperm injection (ICSI)
treatment was also assessed.
Materials and methods
Retrospective analysis
Clinical data from patients that had undergone
frozen-thawed embryo transfer cycles and were divided into
>35-year (n=4,068) and ≤35-year (n=17,505) age groups at the
Reproductive Medical Center of the First Affiliated Hospital of
Anhui Medical University (Hefei, China) between 2018 and 2020 were
retrospectively analyzed. The age range of these patients was 20–50
years (median age, 31.00 years). Preimplantation genetic testing
(PGT) of chromosome aneuploidies (PGT-A; n=53) in the older age
group during the same period was also performed and the data were
analyzed.
Study subjects
A total of 160 women aged between 23 and 45 years
(median age, 33.50 years) who underwent their first fresh cycle of
IVF or ICSI between March 2019 and December 2020 were recruited at
the Reproductive Medical Center of the First Affiliated Hospital of
Anhui Medical University. These included 100 women who enrolled for
RTL measurement and 60 women who enrolled for TERT measurement. All
160 women underwent follicular fluid (FF) anti-Mullerian hormone
(AMH) measurements.
The enrolled women met the following criteria: i)
Age, ≤35 years (young age group) or >35 years (older age group);
and ii) had undergone their first fresh cycle of IVF or ICSI. The
exclusion criteria were as follows: i) Patients with chromosomal
abnormalities; ii) patients with gynecological, endocrine or
autoimmune diseases, such as polycystic ovary syndrome (PCOS),
hyperprolactinemia, endometriosis, adenomyosis, hyperthyroidism and
systemic lupus erythematosus; iii) patients with a history of
radiotherapy, chemotherapy or ovarian surgery; iv) patients
diagnosed with premature ovarian failure. GCs and FF were collected
from all participants. The clinical pregnancy rate was determined
based on the first frozen-thawed blastocyst-stage embryo transfer
cycle.
The present study was reviewed and approved by the
Ethics Committee of Anhui Medical University (approval no.
20190228) and written informed consent was obtained from all
participants.
Clinical sample collection
A pooled collection of FF was obtained from each
patient on the day of oocyte retrieval. The first and last tubes of
FF collected from each patient were not included because they
contained follicle flushing fluid (cat. no. 511119; Vitrolife,
Inc.). A tube containing 10–15 ml FF was obtained from each
enrolled patient and then centrifuged for 15 min at 458 × g at room
temperature. The supernatants were collected and stored at −80°C
for future AMH measurement. Excess GCs mechanically separated from
the cumulus oocyte complexes (COCs) using a 1-ml syringe needle
(cat. no. 300841; Becton, Dickinson and Company) were used as
experimental materials. The separated cells were washed and
resuspended in PBS (cat. no. 8122153; Gibco; Thermo Fisher
Scientific, Inc.) and then prepared for culture or storing at −20°C
for subsequent experiments.
FF AMH level measurement
The FF AMH concentration was measured by ELISA using
a sensitive diagnostic kit (cat. no. K-1401-100N; Guangzhou Kangrun
Biotechnology Co., Ltd.) for the quantitative detection of AMH,
according to the manufacturer's protocol (20). Standards covered a range of
0.06–18.0 ng/ml and the coefficient of variation was <10%. The
intra- and inter-assay coefficients of variation were <10 and
<15%, respectively.
RTL measurement of GCs by quantitative
PCR (qPCR)
Genomic DNA was extracted from GCs using a DNeasy
Tissue Kit (cat. no. 69504; Qiagen, Inc.). The RTL was determined
by a modified qPCR method as described previously using a
LightCycler® 480 platform (Roche Diagnostics GmbH)
(21–23). The RTL was calculated as the
telomere/single copy gene (T/S) ratio.
The primer sequences used for qPCR are shown in
Table I. The samples were analyzed
in triplicate. Each reaction mixture contained 10 µl 2X SYBR Green
qPCR Master Mix (cat. no. 43513; Jiangsu RepoDx Biotechnology Co.,
Ltd.), 1.0 µl each primer (5 µmol/l) and 1.0 µl genomic DNA (~200
ng/µl), and was adjusted to 20 µl using double distilled
H2O. For telomere and single copy gene (36B4 gene) PCR,
pre-denaturation was performed at 94°C for 4 min followed by 40
cycles of 95°C for 15 sec and 60°C for 30 sec.
 | Table I.Primer sequences used for
quantitative PCR. |
Table I.
Primer sequences used for
quantitative PCR.
| Gene | OMIM accession
no. | Primer (5′ to
3′) | Amplicon length
(bp) |
|---|
| Telomere | - | F: GGTTTTTG
GGGTGAGGGTGAGGGTGAGGGTGAGGGT | - |
|
|
| R:
TCCCGACTATCCCTATCCCTATCCCTATCCCTATCCCTA |
|
| 36B4 | 180510 | F:
CAGCAAGTGGGAAGGTG TAATCC | 75 |
|
|
| R:
CCCATTCTATCATCAACGGGTACAA |
|
| TERT | 187270 | F: CTCCC
ATTTCATCAGCAA GTTT | 96 |
|
|
| R:
CTTGGCTTTCAGGATGGAGTAG |
|
| GAPDH | 138400 | F:
GGAAGCTTGTCATCAATGGAAATC | 167 |
|
|
| R:
TGATGACCCTTTTGGCTCCC |
|
TL was determined by calculating the T/S ratio using
ΔCq (Cq telomere/Cq single copy gene). The T/S ratio of each sample
was normalized to the mean T/S ratio of the reference sample
[2−(ΔCqx-ΔCqr)=2−ΔΔCq; where × is the
experimental group and r is the control group] (21).
TERT expression measurement of GCs by
reverse transcription-qPCR
Data on the chromosomal location and protein
structure of the TERT gene were searched in the University of
California Santa Cruz (UCSC) website (http://genome.ucsc.edu; comparative 3D structure was
predicted by ModBase: http://modbase.compbio.ucsf.edu/) (24). For TERT gene measurement, RNA was
extracted from GCs using an RNeasy Mini kit (cat. no. 74134; Qiagen
AB). The ratio of the absorbance at 260 nm to that at 280 nm was
used to assess the purity of the extracted RNA. The quality and
concentration of the extracted RNA met the standards for subsequent
experiments. Then, 2 µg RNA was reverse transcribed into cDNA (42°C
for 60 min, 70°C for 5 min and 4°C hold; RevertAid RT Kit, Thermo
Fisher Scientific, Inc.) followed by quantification using qPCR as
described for RTL measurement of GCs by qPCR. SYBR Green Master Mix
(cat. no. 55499820; Roche Diagnostics GmbH) was used to detect the
expression level of TERT. Gene expression was quantified relative
to that of the reference gene GAPDH using the 2−ΔΔCq
method (25). The primer sequences
are listed in Table I.
Cell culture
The fresh GCs were digested and dispersed in
hyaluronidase solution (cat. no. 90101; FUJIFILM Irvine Scientific,
Inc.). The digestion was then terminated with DMEM (cat. no.
SH30022.01; HyClone; Cytiva) supplemented with 10% FBS (cat. no.
16000-044; Gibco; Thermo Fisher Scientific, Inc.). The cells were
cultured in DMEM supplemented with 10% FBS and 1%
penicillin/streptomycin in a sterile cell culture dish (cat. no.
353001; Falcon®; Corning Life Sciences) at 37°C with 5%
CO2 for 24 h.
Immunofluorescence (IF)
Glass bottom dishes were first pre-treated with
poly-lysine solution for ~40 min in a humidified 37°C incubator to
coat the bottom of the dishes and then washed three times with PBS,
which was removed after washing. The cells were seeded in the
pre-treated glass bottom dishes as described in the cell culture
section.
IF was performed to identify ovarian GCs and the
intracellular localization of TERT in the GCs. The cultured cells
were fixed with 4% paraformaldehyde for 20 min at room temperature
followed by three washes with PBS, permeabilized for 20 min with
0.5% Triton X-100 following by rinsing with PBS three times, and
then blocked for 30 min with 1% bovine serum albumin (cat. no.
V900933; Sigma-Aldrich; Merck KGaA) at room temperature. The fixed
samples were incubated overnight at 4°C with anti-follicle
stimulating hormone (FSH) receptor (FSHR; cat. no. ab75200;
dilution, 1:100; Abcam) or anti-TERT antibodies (dilution, 1:50;
cat. no. ab230527; Abcam). After washing three times with PBS for 3
min per time, the samples were incubated with a goat anti-rabbit
IgG secondary antibody (cat. no. A23220; dilution, 1:400; Abbkine
Scientific Co., Ltd.) for 2 h at room temperature and then
counterstained with DAPI (cat. no. 28718-90-3; Beyotime Institute
of Biotechnology) for 15 min at room temperature followed by
washing three times with PBS. Fluorescent images were captured
using a laser scanning confocal microscope (Zeiss AG). GCs
incubated with only a secondary antibody were used as a negative
control. The mean fluorescence intensity was measured using ImageJ
version 1.8.0 software (National Institutes of Health), which
included the extraction of image-color-split channels with
automatic image threshold adjustment and analysis of the
measurement data.
Western blotting (WB)
Proteins of the GCs were extracted using RIPA buffer
(Wuhan Servicebio Technology Co., Ltd.). Protease and phosphatase
inhibitors (protease and phosphatase inhibitor cocktail for general
use; 50X; Beyotime Institute of Biotechnology) were added (the
final concentration of protease and phosphatase inhibitors was 2%)
into the RIPA buffer to prevent protein degradation. The protein
concentration was determined using bicinchoninic acid (Wuhan
Servicebio Technology Co., Ltd.). Proteins (10 µg/lane) were
separated by SDS-PAGE (8% separation gel) and then transferred onto
0.45-µm PVDF membranes (MilliporeSigma). The membranes were
incubated with rabbit anti-TERT (dilution, 1:1,000; Abcam) and
anti-β-actin (dilution, 1:1,000; cat. no. ab179467; Abcam) primary
antibodies overnight at 4°C. The latter were used to ensure equal
protein loading. The membranes were then incubated with a
horseradish peroxidase-conjugated secondary antibody (cat. no.
E-AB-1041; Elabscience, Inc.) at room temperature for 90 min. The
immunoreactive protein bands were then developed using Pierce™ ECL
Plus Western Blotting Substrate (Thermo Fisher Scientific, Inc.)
and AlphaEaseFC 4.0 software (Alpha Innotech Corporation) was used
to quantify band intensity.
Statistical analysis
The normality of data was assessed using the
Kolmogorov-Smirnov test. The RTL was log10
(LG)-transformed due to a skewed distribution. Kruskal-Wallis test
with Bonferroni correction was performed for the comparison of RTL
and TERT data in multiple age groups as several groups of data did
not conform to the normal distribution. A binary logistic
regression model was used to determine the pregnancy probability.
The predictive values of TERT and RTL for clinical pregnancy were
determined using receiver operating characteristic curves and the
area under the curves. Mann-Whitney U tests were performed in the
comparison of clinical parameters including age, body mass index,
day 3 serum FSH, gonadotropin (Gn) dose and IVF outcome measures
between the RTL and TERT groups; the comparison of FF AMH levels,
RTL and relative TERT mRNA expression levels between the
<35-year and ≥35-year age groups; and the comparison of RTL and
relative TERT mRNA expression levels between pregnant and
non-pregnant patients. Independent sample t-tests were used for
comparison of TERT protein expression between the <35-year and
≥35-year age groups. A Chi-square test was performed to analyze the
counts of different ovulation induction protocols in the RTL and
TERT groups. Pearson's correlation test was used to analyze the
correlation of normally distributed data and Spearman's rank
correlation test was used for skewed data. A two-sided P<0.05
was considered to indicate a statistically significant difference.
Linear regression models were established when a correlation
analysis had P<0.05. Data are presented as the mean ± SD.
Statistical analysis was performed using SPSS 16.0 (SPSS, Inc.) and
GraphPad Prism 5.0 (GraphPad Software; Dotmatics).
Results
Clinical data analysis
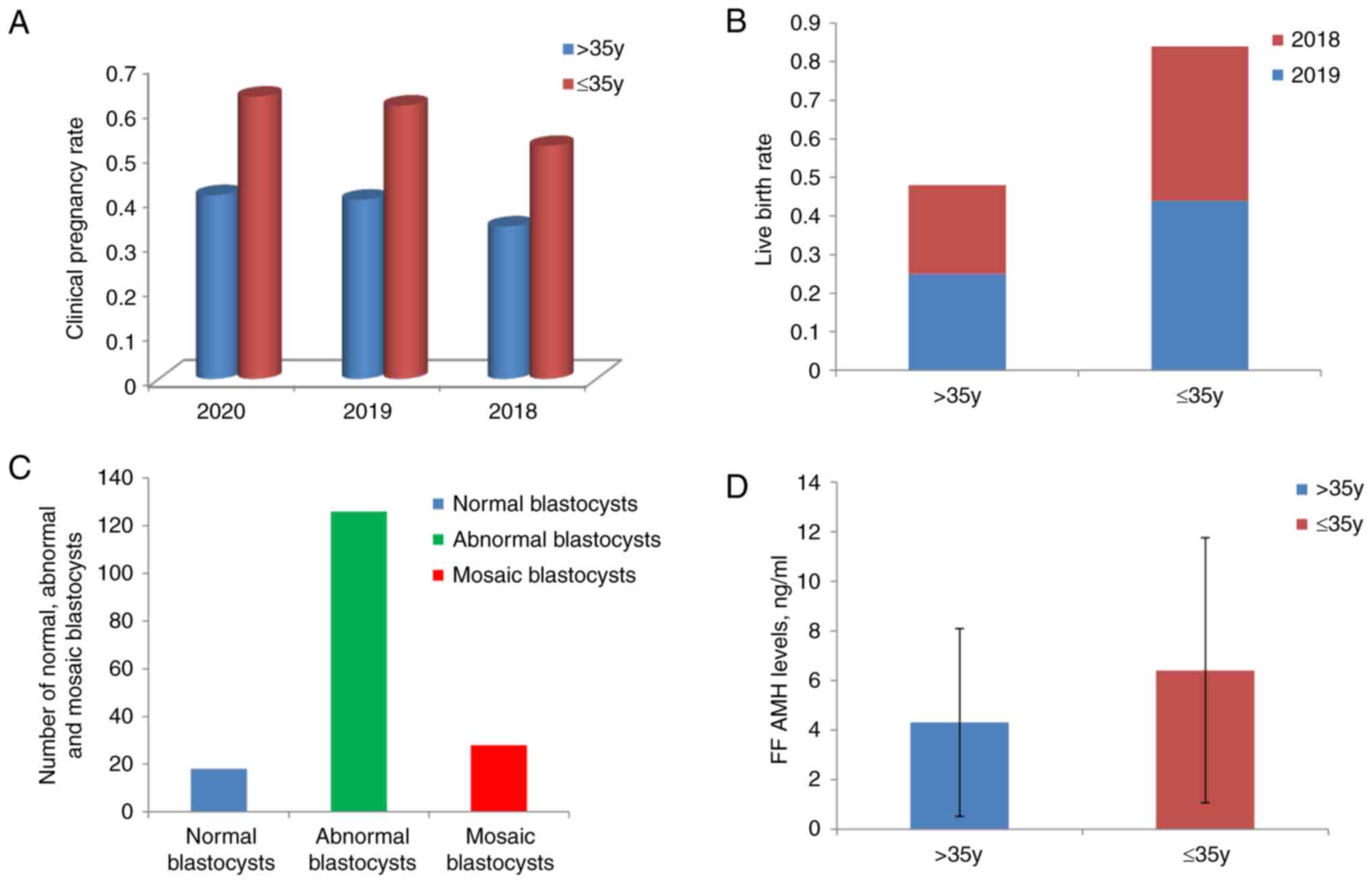
In the retrospective analysis, clinical pregnancy
rates of 41, 40 and 34% were reported in 2020, 2019 and 2018,
respectively, in the >35-year age group. The respective rates in
the ≤35-year age group were 63, 61 and 52%. In 2019 and 2018, the
live birth rates of the >35-year age group were 25 and 23%,
respectively, compared with 44 and 40%, respectively, in the
≤35-year age group. With regard to PGT-A cycles, a total of 172
blastocysts from 53 patients were analyzed, of which 18 blastocysts
(10.47%) were euploid, 126 blastocysts (73.26%) were aneuploid and
28 blastocysts (9.30%) were mosaic. The pregnancy and live birth
rates for women aged >35 years were ~20% lower than those of
women aged ≤35 years, and in the older women the number of embryos
with aneuploidy was 7-fold higher that of those without euploidy
(Fig. 1A-C; Table SI).
General characteristics of the study
subjects
A total of 160 women aged 23–45 years met the
inclusion criteria for the present study, of which 100 were
enrolled for RTL measurement and 60 were enrolled for TERT
measurement. All 160 women underwent FF AMH measurement. No
significant difference in clinical characteristics, including age,
body mass index, day-3 serum FSH, total Gn dose and IVF outcome
measures was detected between the RTL and TERT groups (P>0.05;
Table II). Antagonist and long
Gn-releasing hormone-agonist (GnRH-a) protocols were the main
ovulation induction protocols in both the RTL and TERT groups,
accounting for >80% of the ovulation induction protocols. In the
RTL group, the percentages of antagonist, long GnRH-a and other
ovulation induction protocols (milder stimulation protocols) were
used in 61 (61.0%), 32 (32.0%) and 7 (7.0%) patients, respectively.
In the TERT group, these protocols were used in 32 (53.3%), 21
(35.0%) and 7 (11.7%) patients, respectively. No significant
difference in the frequencies of the various ovulation induction
protocols was found between the RTL and TERT groups (Table III).
 | Table II.Characteristics and in vitro
fertilization outcomes of patients from whom the RTL and TERT data
of granulosa cells were collected. |
Table II.
Characteristics and in vitro
fertilization outcomes of patients from whom the RTL and TERT data
of granulosa cells were collected.
| Patient
characteristics | RTL group
(n=100) | TERT group
(n=60) |
|---|
| Age, years | 33.40±6.30 | 32.70±6.70 |
| BMI,
kg/m2 | 22.87±3.36 | 23.25±3.22 |
| D3 serum FSH,
mIU/ml | 8.10±3.55 | 8.09±2.87 |
| Total Gn dose,
IU |
2,268.20±784.02 |
2,234.8.30±752.59 |
| Oocytes retrieved,
n | 11.78±7.15 | 13.38±10.05 |
| MII oocytes, n | 9.22±5.66 | 9.87±7.06 |
| Fertilized oocytes,
n | 8.35±5.17 | 9.05±6.65 |
| Cleaved embryos,
n | 8.11±4.96 | 8.80±6.56 |
| Blastocysts, n | 4.72±3.80 | 4.98±4.20 |
 | Table III.Ovulation induction protocols in the
RTL and TERT groups. |
Table III.
Ovulation induction protocols in the
RTL and TERT groups.
| Ovulation induction
protocols | RTL group, n
(%) | TERT group, n
(%) |
|---|
| Antagonist | 61 (61.0) | 32 (53.3) |
| Long GnRH-a | 32 (32.0) | 21 (35.0) |
| Other | 7 (7.0) | 7 (11.7) |
Measurement of FF AMH levels between
the older and younger age groups
FF samples were obtained from 71 patients aged ≥35
years and 89 patients aged <35 years. A lower FF AMH level was
observed in the older age group compared with the younger age group
(4.31±3.79 vs. 6.41±5.35 ng/ml; P<0.01; Fig. 1D).
Biological characteristics of
senescent GCs
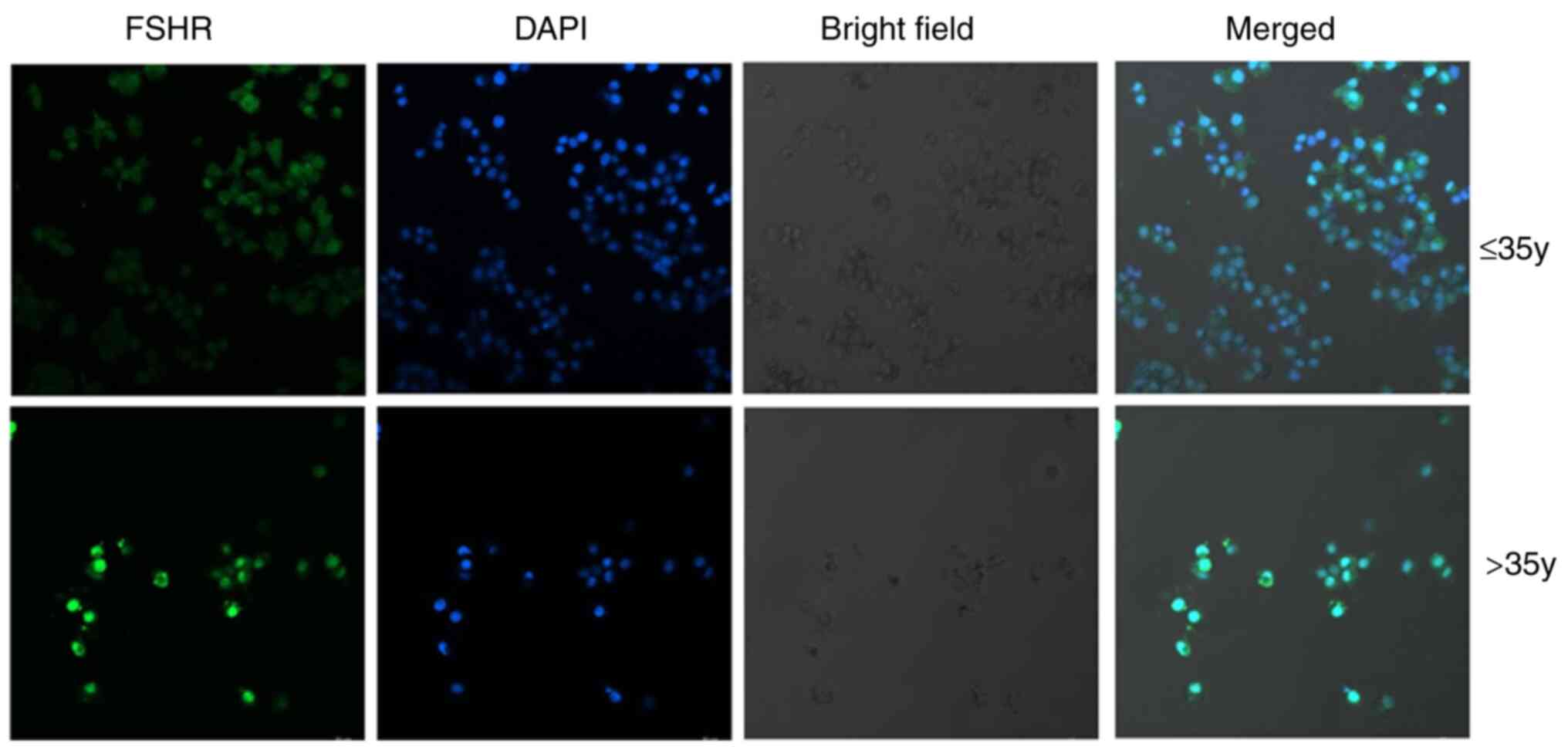
Following overnight culture for 24 h, the cells grew
well and were adherent. FSHR is a marker of human ovarian GCs
(26). As shown by
immunofluorescence staining (Fig.
2), FSHR was expressed in the GCs of women from both age
groups. Although the expression of FSHR appeared to be stronger in
the older age group, the number of COCs retrieved from the older
age group was lower than that of the younger age group and the cell
density of the COCs from the older age group was lower.
RTL association with age and
ovarian/embryonic outcome
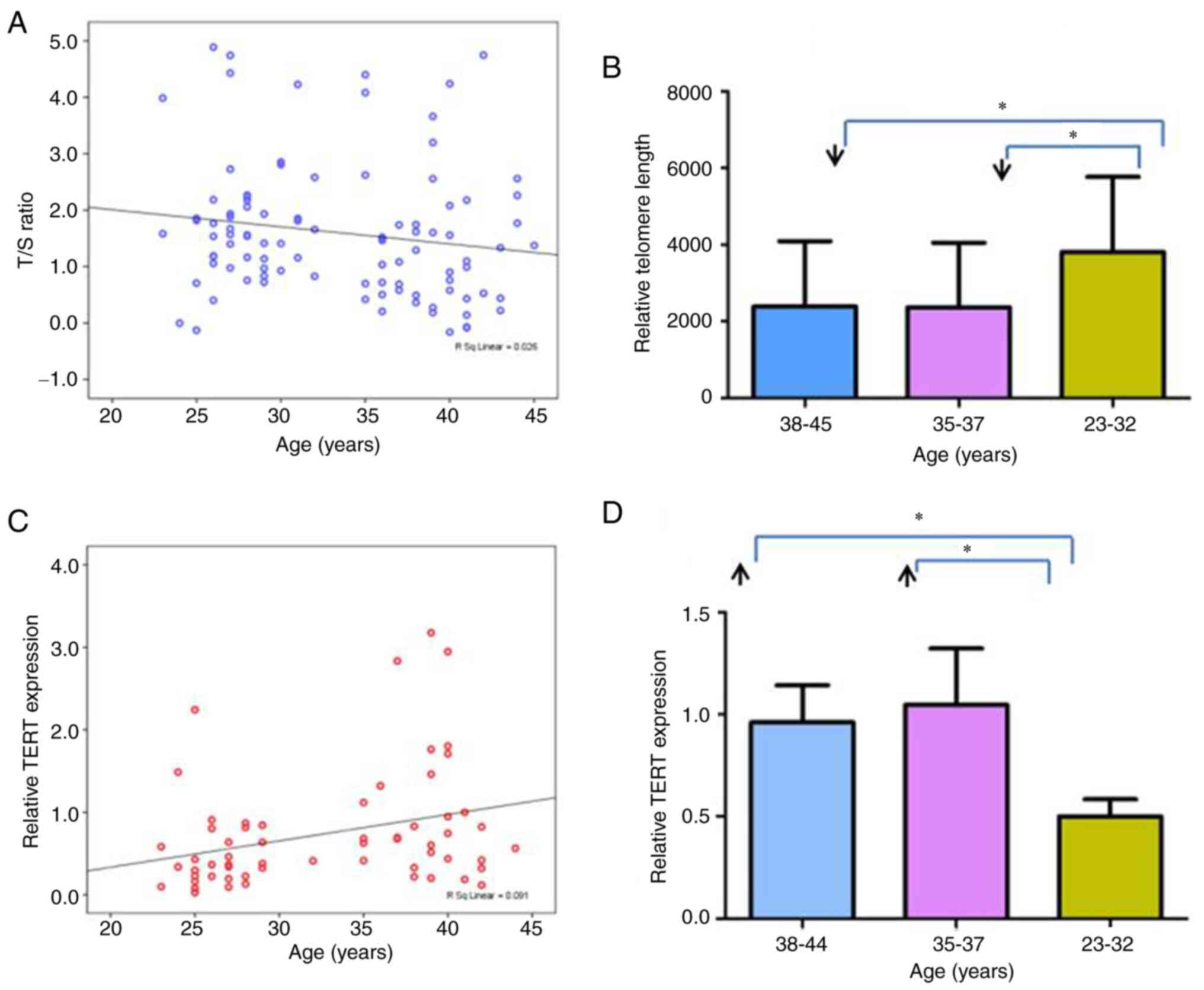
A total of 100 women were enrolled for RTL
measurement, and the results are shown in Table IV (1.29±1.12 and 1.86±1.18 in the
old and young group, respectively). Linear regression analysis
revealed a statistically significant negative association between
the RTL of GC samples and patient age [LG RTL=−0.03 × year + 2.62;
R2=0.03; correlation coefficient (r)=−0.20, P=0.04;
Fig. 3A]. To better understand the
association of RTL with age, the 100 samples were divided into
three subgroups (38–45, 35–37 and 23–32 years). These groupings
were chosen because although women >35 years old were considered
as older in the present study, an age of 35–37 years may be
considered as transitional. Notably, there is a discontinuity
between the two lower age groups as no GCs were obtained from
patients aged 33 or 34 years. The RTL was significantly higher in
patients aged 23–32 years compared with the two older age groups
(P<0.05). However, no significant difference was detected
between the patients aged 38–45 years and those aged 35–37 years
(Fig. 3B).
 | Table IV.RTL and relative TERT expression
measurements by quantitative PCR in patients according to age. |
Table IV.
RTL and relative TERT expression
measurements by quantitative PCR in patients according to age.
|
| >35 years | ≤35 years |
|
|---|
|
|
|
|
|
|---|
| Variable | n | Mean ± SD | n | Mean ± SD | P-value |
|---|
| RTL, LG T/S | 45 | 1.29±1.12 | 55 | 1.86±1.18 | 0.004 |
| Relative TERT
expression | 26 | 1.02±0.87 | 34 | 0.52±0.45 | 0.013 |
No significant correlations were found for GC RTL
with day-3 serum FSH level (P=0.11), the number of oocytes
retrieved (P=0.07), the number of mature (MII) oocytes retrieved
(P=0.17) and blastocyst formation rate (P=0.99) (Table V).
 | Table V.Correlations of RTL and relative TERT
expression in granulosa cells with ovarian/embryonic outcomes. |
Table V.
Correlations of RTL and relative TERT
expression in granulosa cells with ovarian/embryonic outcomes.
|
| RTL | Relative TERT
expression |
|---|
|
|
|
|
|---|
| Ovarian/embryonic
outcome | P-value | Correlation
coefficient | P-value | Correlation
coefficient |
|---|
| Number of oocytes
retrieveda | 0.07 | −0.18 | <0.001 | −0.48 |
| Mature (MII)
oocytes retrieveda | 0.17 | −0.14 | 0.001 | −0.41 |
| Blastocyt formation
rateb | 0.99 | 0.002 | 0.10 | 0.22 |
| Day-3 serum
FSHa | 0.11 | 0.16 | 0. 23 | 0.16 |
Relative TERT expression in relation
to age and ovarian/embryonic outcome, as determined by qPCR
The 3D structure of the TERT gene located on
chromosome 5p15.33 was found on the UCSC website (Fig. 4). In order to investigate the
relative expression of TERT in GCs according to age, GCs from 26
older patients (36–44 years old) and 34 younger patients (23–35
years old) were obtained. Higher relative TERT expression was
observed in the older age group compared with the younger age group
(1.02±0.87 vs. 0.52±0.45; P<0.05; Table IV). Linear regression analysis
revealed a significant positive association between the TERT
expression of GC samples and patient age (P<0.05; Fig. 3C). On average, relative TERT
expression increased by 0.03 every year from the age of 23 years to
the age of 44 years (relative TERT gene expression=0.03 × year
−0.31; R2=0.09; r=0.30, P=0.02). To better understand
its association with age, the 60 samples were further divided into
three subgroups by age (38–44, 35–37 and 23–32 years). The level of
TERT expression was lower in the 23–32-year age group than in the
two older age groups (P<0.05). No significant difference was
detected between the patients aged 38–44 years and those aged 35–37
years (Fig. 3D).
When evaluating the correlations between the
relative TERT gene expression of GC samples and ovarian/embryonic
outcome, a statistically significant inverse correlation was
observed between relative TERT gene expression and the number of
oocytes (r=−0.48, P<0.001) and MII oocytes (r=−0.41, P<0.001)
retrieved. However, no statistically significant correlation was
observed between relative TERT gene expression in GC samples and
the serum FSH level (P=0.23) and blastocyst formation rate
(P=0.10). The results of the correlation analysis between the TERT
gene expression of GC samples and ovarian/embryonic outcomes are
shown in Table V.
Associations between RTL, relative
TERT expression and the probability of clinical pregnancy
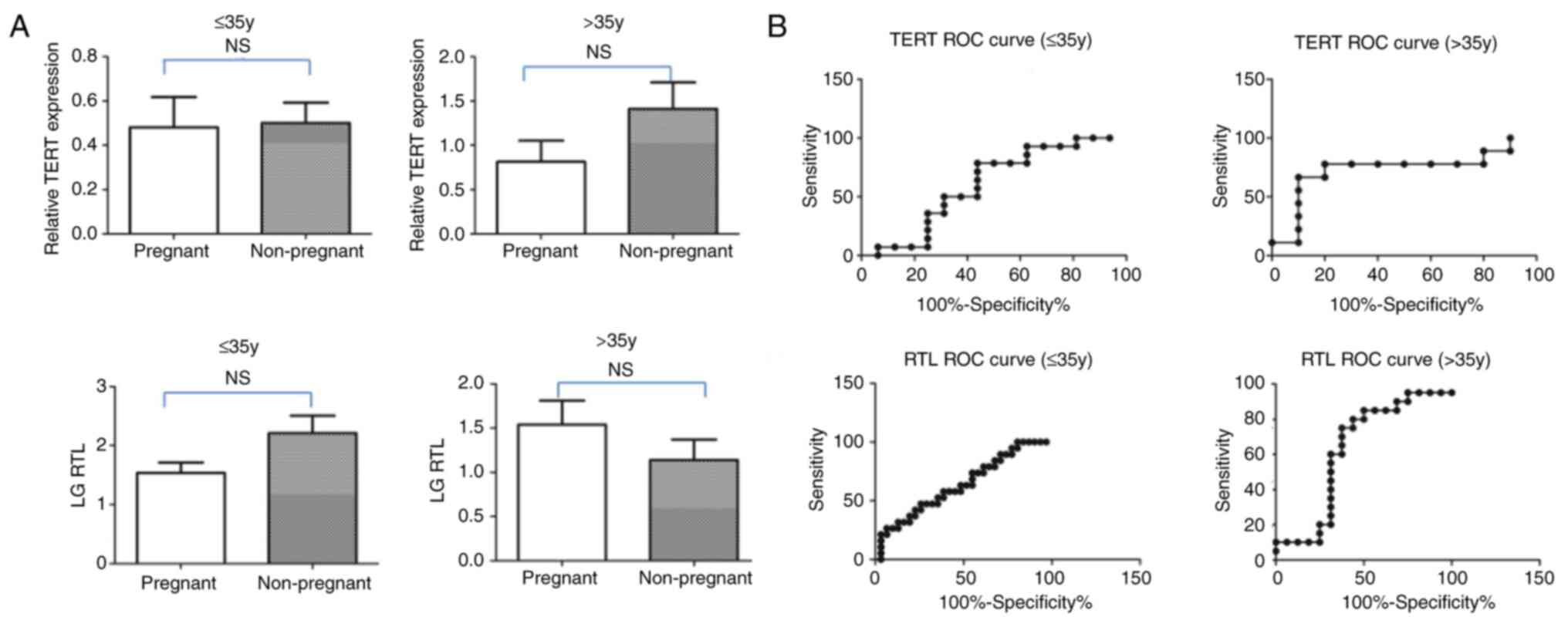
Based on the clinical pregnancy outcome, all
patients (>35 and ≤35 years) were divided into two groups,
namely pregnant and non-pregnant groups. The RTL and relative TERT
expression at the mRNA level were analyzed in both groups. The
associations of RTL and relative TERT expression with the
probability of clinical pregnancy were analyzed by binary logistic
regression and the results were assessed using odds ratios and 95%
confidence intervals. As age is an important factor for clinical
pregnancy rate, the logistic regression was performed separately in
the older and younger age groups. No significant associations of
RTL or relative TERT expression with the probability of clinical
pregnancy were found, regardless of patient age (Table VI; Fig. 5A). The predictive values of TERT
and RTL for clinical pregnancy were determined using receiver
operating characteristic curves and are shown in Fig. 5B. Although the area under the curve
value of 0.630 for RTL for the >35-year age group was high, TERT
and RTL were determined to have no predictive value for clinical
pregnancy owing to the lack of significance being found (Fig. 5B; Table VI).
 | Table VI.Associations of RTL and relative TERT
expression in granulosa cells with the probability of clinical
pregnancy in patients according to age. |
Table VI.
Associations of RTL and relative TERT
expression in granulosa cells with the probability of clinical
pregnancy in patients according to age.
|
| >35 years | ≤35 years |
|---|
|
|
|
|
|---|
| Statistical
measure | RTL | TERT | RTL | TERT |
|---|
| OR | 1.458 | 0.364 | 0.580 | 0.908 |
| 95% CI | 0.748–2.842 | 0.093–1.427 | 0.333–1.012 | 0.184–4.487 |
| P-value | 0.268 | 0.147 | 0.055 | 0.906 |
| AUC | 0.630 | 0.267 | 0.360 | 0.390 |
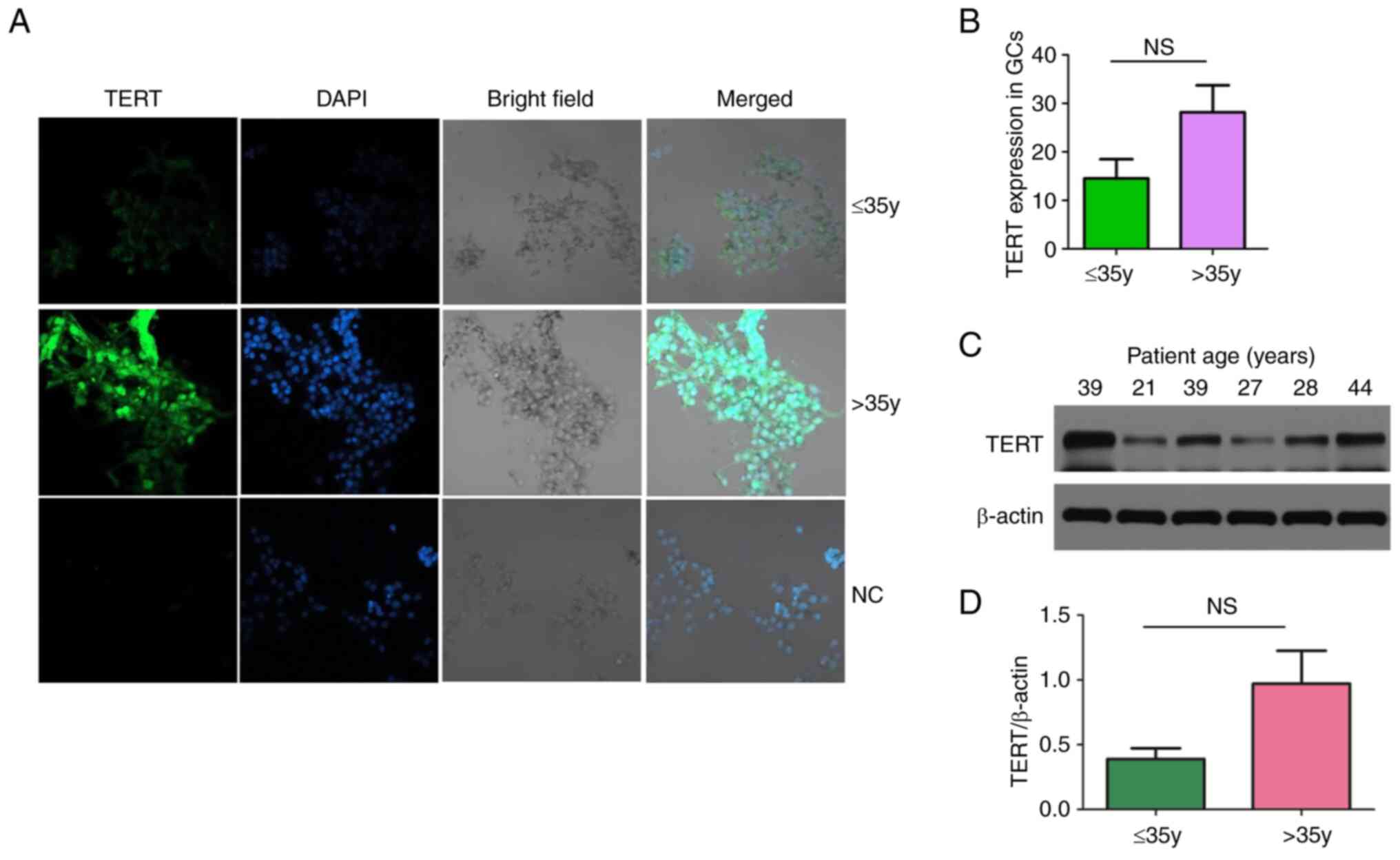
Verification of TERT expression in
GCs
IF and WB experiments were further performed to
verify the expression of TERT in the younger and older patient age
groups. The results of these assays showed that TERT was highly
expressed in the patients ages >35 years, but the difference in
TERT expression compared with the patients aged ≤35 years was not
significant (P>0.05; Fig.
6).
Discussion
It is well established that a woman's fertility
declines with age, leading to a lower chance of pregnancy. Both the
quantity and quality of oocytes decline during aging. The clinical
data in the present study were consistent with this, as they showed
that the pregnancy and live birth rates for women aged >35 years
were ~20% lower than those aged <35 years, and the number of
embryos with aneuploidy was 7-fold that of women with euploidy in
older women. Although the mechanism underlying the loss of
fertility and higher risk of aneuploidies induced by aging has been
widely considered, the specific mechanism is not fully
understood.
Since GCs support oocytes during follicular growth
and maturation, exploration of the mechanism of GC aging provides
important insights into ovarian aging. For that reason, ovarian GCs
from infertile women undergoing IVF were selected as ovarian aging
research models in the present study, and GCs from older patients
were found to have higher FSHR expression and lower FF AMH levels
than those from younger patients. Telomeres themselves do not
encode any specific products (27). Telomere dysfunction is associated
with increased aneuploidy, impaired mitochondrial function,
decreased gluconeogenesis and increased cellular reactive oxygen
species levels (28). A previous
study suggested that age-associated DOR may result from telomere
attrition which is usually regulated by telomerase (13). Telomerase activity in GCs is
important for their proliferation and differentiation capacity
(29), as it plays versatile roles
in various reproduction pathways. TERT encodes the catalytic
subunit of telomerase, which counters telomere shortening during
cell division (30). The TERT mRNA
is accompanied by another enzyme component TERC, which is an RNA
molecule that encodes the catalytic component of telomerase
(31). The TERT and TERC together
components constitute active telomerase, which counteracts the
gradual shortening of telomeres with each round of DNA replication
by maintaining the telomere sequences and conferring a sustained
proliferation capacity to growing cells (32).
In the present study, changes to telomeres and TERT
during the natural aging of GCs were explored. Patients with
endocrine diseases, such as PCOS, which may affect TL (33,34),
thus confounding the results, were excluded from the study. To
further explore the role of telomeres and the TERT gene in
reproduction, the associations of RTL/relative TERT expression with
ovarian/embryonic outcome as well as the probability of clinical
pregnancy were assessed. Most studies on ovarian aging are
conducted by calculating the number of non-growing follicles (NGF),
which ultimately determines the reserve and function of the
ovaries. The reduction in NGF counts accelerates in women aged ≥38
years (35). The data in the
present study also showed that the number of oocytes retrieved by
women aged ≥38 years declined sharply (data not shown), which is
the theoretical basis for the allocation of older patients into two
subgroups for the analysis of relative TERT expression and RTL. The
results showed that the RTL of GCs gradually decreased with age,
which conflicts with a previous study that found no significant
change in the RTL of GCs with aging (11). However, the results of the present
study also indicated that RTL was not associated with any measure
of ovarian/embryonic performance or clinical pregnancy. This
finding contrasts with those of previous studies which have
demonstrated that variations in embryonic outcomes are associated
with the shortening or elongation of TL in GCs (36,37).
In the present study, an increased TERT expression
at the transcriptional level was detected in the GCs of older
women. Increased TERT expression in GCs may affect telomerase
activity, resulting in a shortening of TL that is associated with
chromosomal and genomic instability during aging. In addition, it
is worth noting that although TERT appears to be a more notable
biomarker than RTL for the prediction ovarian response among
infertile women, it plays no role in the prediction of embryonic
development potential and clinical pregnancy, as no statistically
significant correlation was identified between TERT and blastocyst
formation rate. The findings of previous studies support the
decline in TERT expression and activity in mouse oocytes with
reproductive aging (32) and its
association with telomere shortening and decline in oocyte quality
(16). The data in the present
study also indicate that TERT expression alters in GCs with aging,
but RNA-sequencing data (data not shown) did not detect any
significant changes in TERT expression between old and young human
oocytes. Kosebent and Ozturk (38)
suggested that TERT expression was differentially expressed at
different developmental follicular stages in both young and old
mice. These seemingly discordant results indicate that the
regulation of TERT expression is tissue- and stage-dependent.
In addition to its established role in the
opposition of cell replication-dependent telomere shortening, TERT
exhibits multiple physiological activities beyond its canonical
action. For instance, TERT has been shown to modulate mitochondrial
and ubiquitin proteasomal function or act as a transcription
co-factor (39), and thereby
contribute to the regulation of gene expression, promotion of cell
survival and growth and protection of cells from apoptosis
(30). It has also been shown to
display RNA-dependent RNA polymerase activity (39). Previous studies have also reported
a pleiotropic role for TERT in the regulation of the epigenetic
clock (30) and intrinsic DNA
methylation age during the proliferation of growing cells (40). In combination, all these effects of
TERT are actively involved in its biological activity, which
ultimately affects the aging process. The activities possessed by
TERT, whether or not they are associated with telomere maintenance,
may contribute to the aging process. Notably, the difference of
TERT expression in GCs at the protein level between the younger and
older age groups was not found to be significant. The reason for
this result may be the small sample size and high heterogeneity of
human samples, or other telomere regulatory mechanisms may exist at
the protein level.
Hormonal regulation of telomere maintenance by
telomerase in the ovary affects cell proliferation and ovarian
aging. Specifically, telomerase activity in GCs is controlled by
growth factors and female steroid hormones (29). The TERT gene promoter contains an
estrogen response element (38).
The microenvironment of GCs contains estrogens that are reported to
influence telomerase activity and length (41). Mordechai et al (29) suggested that Gn from the serum of
pregnant mares increased TERT expression at the transcriptional and
translational levels in rat ovarian GCs. The present study also
revealed a reduction in the AMH in the FF in the old age group
compared with the young age group. Overall, these studies indicate
that the changes of TERT gene expression in GCs that occur during
aging may be due to changes in female hormone levels. Although the
telomere theory of reproductive aging has mostly focused on the
effect of shorter telomeres on meiotic errors and the elevated risk
of aneuploidies (40,41), it is also possible that TL or TERT
interaction with endocrine homeostasis in the ovarian
microenvironment may play a role in ovarian aging. The GC and FF
surrounding the oocyte together constitute the local ovarian
follicle microenvironment. Reproductive aging decreases
endocrinological output and compromises the homeostasis of the FF
(42), with effects such as
lowering the activity of antioxidant enzymes and increasing
oxidative protein damage (43).
Furthermore, TL is associated with the adverse effects of oxidative
stress and homeostasis impairment. The TLs of GCs have been found
to be shorter in patients with PCOS (34) or biochemical primary ovarian
insufficiency (44) than in
relevant control, and a high estradiol-17 concentration has been
shown to increase the TL of GCs (45). Moreover, it has been shown that FF
AMH levels can be used to determine the apoptosis rate of GCs in
assisted reproductive technology cycles (46). These findings suggest an
association between telomeres and the endocrine system in the
ovarian microenvironment (45).
Additionally, the microenvironment in ovarian follicles differs
with regard to telomere biology when compared with that of certain
other somatic tissues, as it has been observed that the telomeres
of GCs are longer than those of leukocytes, which suggests a
different mechanism of telomere maintenance in the ovarian follicle
microenvironment (47). The
molecular mechanism requires investigation in future studies to
explore the intrinsic association between these indicators.
The present study was not without limitations. Its
primary limitation is the limited sample size, which creates a
certain bias and decreases the strength of the conclusions.
Secondly, it would be more statistically valuable to analyze RTL
and TERT in the same GC sample to assess their associations;
however, it was not possible to do that due to the small number of
ovarian GCs obtained from older patients. Finally, individual
differences in human samples may reduce the credibility of the
conclusions. Other methods, such as telomerase activity
measurements, are necessary to verify the results.
In conclusion, the findings of the present
demonstrate that RTL and TERT alterations in GCs may be
determinants of ovarian aging. In addition, TERT appears to be a
potential biomarker for the prediction ovarian response among
infertile women during IVF/ICSI treatment. The measurement of TERT
in GCs provides a novel strategy for female fertility assessment
and useful guidance for the research and clinical treatment of
reproductive aging. Well-designed larger-scale studies are required
to confirm the results of the present study and shed light on the
mechanism underlying the effects of telomeres and TERT in
reproductive aging.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by National Natural Science
Foundation of China (grant no. 82001631), Major Science and
Technology Projects in Anhui Province (grant no. 202003a07020012)
and Research Funding for Doctoral Talents of the First Affiliated
Hospital of Anhui Medical University (grant no. 1465).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YXC and ZGZ designed the experiments and revised the
manuscript. YH was responsible for the measurement of RTL by qPCR
and relative TERT expression by RT-qPCR. YH also drafted the
manuscript. MRL and JP analyzed the clinical data and verified TERT
expression by IF and western blotting. ZHZ contributed to data
analysis. ZZ, TTW and BY contributed to the measurement of FF AMH
levels. DK was responsible for sample collection and IF to
determine the biological characteristic of senescent GCs. PZ and
ZLW helped to enroll the patients and designed the study. YXC and
ZGZ confirm the authenticity of all the raw data. All authors read
and approved the final version of the manuscript.
Ethics approval and consent to
participate
The study was reviewed and approved by the Ethics
Committee of Anhui Medical University (approval no. 20190228), and
written informed consent was obtained from all participants.
Patient consent for publication
The patients consented to the publication of their
data.
Competing interests
The authors declare they have no competing
interests.
References
|
1
|
Chico-Sordo L, Córdova-Oriz I, Polonio AM,
S-Mellado LS, Medrano M, García-Velasco JA and Varela E:
Reproductive aging and telomeres: Are women and men equally
affected? Mech Ageing Dev. 198:1115412021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Armstrong DT: Effects of maternal age on
oocyte developmental competence. Theriogenology. 55:1303–1322.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mikwar M, MacFarlane AJ and Marchetti F:
Mechanisms of oocyte aneuploidy associated with advanced maternal
age. Mutat Res Rev Mutat Res. 785:1083202020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Agarwal A, Gupta S and Sharma RK: Role of
oxidative stress in female reproduction. Reprod Biol Endocrinol.
3:282005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kasapoğlu I and Seli E: Mitochondrial
dysfunction and ovarian aging. Endocrinology. 161:bqaa0012020.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Peters AE, Mihalas BP, Bromfield EG, Roman
SD, Nixon B and Sutherland JM: Autophagy in female fertility: A
role in oxidative stress and aging. Antioxid Redox Signal.
32:550–568. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kordowitzki P, López de Silanes I,
Guío-Carrión A and Blasco MA: Dynamics of telomeric
repeat-containing RNA expression in early embryonic cleavage stages
with regards to maternal age. Aging (Albany NY). 12:15906–15917.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Thilagavathi J, Venkatesh S and Dada R:
Telomere length in reproduction. Andrologia. 45:289–304. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fattet AJ, Toupance S, Thornton SN, Monnin
N, Guéant JL, Benetos A and Koscinski I: Telomere length in
granulosa cells and leukocytes: A potential marker of female
fertility? A systematic review of the literature. J Ovarian Res.
13:962020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang W, Chen H, Li R, Ouyang N, Chen J,
Huang L, Mai M, Zhang N, Zhang Q and Yang D: Telomerase activity is
more significant for predicting the outcome of IVF treatment than
telomere length in granulosa cells. Reproduction. 147:649–657.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hanson BM, Tao X, Zhan Y, Kim JG, Klimczak
AM, Herlihy NS, Scott RT Jr and Seli E: Shorter telomere length of
white blood cells is associated with higher rates of aneuploidy
among infertile women undergoing in vitro fertilization. Fertil
Steril. 115:957–965. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lingner J, Cooper JP and Cech TR:
Telomerase and DNA end replication: No longer a lagging strand
problem? Science. 269:1533–1534. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rocca MS, Foresta C and Ferlin A: Telomere
length: Lights and shadows on their role in human reproduction.
Biol Reprod. 100:305–317. 2019.PubMed/NCBI
|
|
14
|
Uysal F, Kosebent EG, Toru HS and Ozturk
S: Decreased expression of TERT and telomeric proteins as human
ovaries age may cause telomere shortening. J Assist Reprod Genet.
38:429–441. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kalmbach KH, Fontes Antunes DM, Dracxler
RC, Knier TW, Seth-Smith ML, Wang F, Liu L and Keefe DL: Telomeres
and human reproduction. Fertil Steril. 99:23–29. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Keefe DL, Franco S, Liu L, Trimarchi J,
Cao B, Weitzen S, Agarwal S and Blasco MA: Telomere length predicts
embryo fragmentation after in vitro fertilization in women-toward a
telomere theory of reproductive aging in women. Am J Obstet
Gynecol. 192:1256–1260; discussion 1260-1. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Keefe DL, Liu L and Marquard K: Telomeres
and aging-related meiotic dysfunction in women. Cell Mol Life Sci.
64:139–143. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hamel M, Dufort I, Robert C, Gravel C,
Leveille MC, Leader A and Sirard MA: Identification of
differentially expressed markers in human follicular cells
associated with competent oocytes. Hum Reprod. 23:1118–1127. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
van Montfoort AP, Geraedts JP, Dumoulin
JC, Stassen AP, Evers JL and Ayoubi TA: Differential gene
expression in cumulus cells as a prognostic indicator of embryo
viability: A microarray analysis. Mol Hum Reprod. 14:157–168. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pacheco A, Cruz M, Iglesias C and
García-Velasco JA: Very low anti-müllerian hormone concentrations
are not an independent predictor of embryo quality and pregnancy
rate. Reprod Biomed Online. 37:113–119. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pedroso DCC, Santana VP, Donaires FS,
Picinato MC, Giorgenon RC, Santana BA, Pimentel RN, Keefe DL,
Calado RT, Ferriani RA, et al: Telomere length and telomerase
activity in immature oocytes and cumulus cells of women with
polycystic ovary syndrome. Reprod Sci. 27:1293–1303. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cawthon RM: Telomere length measurement by
a novel monochrome multiplex quantitative PCR method. Nucleic Acids
Res. 37:e212009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cawthon RM: Telomere measurement by
quantitative PCR. Nucleic Acids Res. 30:e472002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kent WJ, Sugnet CW, Furey TS, Roskin KM,
Pringle TH, Zahler AM and Haussler D: The human genome browser at
UCSC. Genome Res. 12:996–1006. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang N, Si C, Xia L, Wu X, Zhao S, Xu H,
Ding Z and Niu Z: TRIB3 regulates FSHR expression in human
granulosa cells under high levels of free fatty acids. Reprod Biol
Endocrinol. 19:1392021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Blumenstiel J: Telomeres: A new means to
an end. Curr Biol. 21:R32–R34. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sahin E, Colla S, Liesa M, Moslehi J,
Müller FL, Guo M, Cooper M, Kotton D, Fabian AJ, Walkey C, et al:
Telomere dysfunction induces metabolic and mitochondrial
compromise. Nature. 470:359–365. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mordechai A, Wasserman M, Abramov M,
Ben-Menahem D, Har-Vardi I, Levitas E and Priel E: Increasing
telomerase enhanced steroidogenic genes expression and steroid
hormones production in rat and human granulosa cells and in mouse
ovary. J Steroid Biochem Mol Biol. 197:1055512020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lu AT, Xue L, Salfati EL, Chen BH,
Ferrucci L, Levy D, Joehanes R, Murabito JM, Kiel DP, Tsai PC, et
al: GWAS of epigenetic aging rates in blood reveals a critical role
for TERT. Nat Commun. 9:3872018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Blackburn EH: Switching and signaling at
the telomere. Cell. 106:661–673. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yamada-Fukunaga T, Yamada M, Hamatani T,
Chikazawa N, Ogawa S, Akutsu H, Miura T, Miyado K, Tarín JJ, Kuji
N, et al: Age-associated telomere shortening in mouse oocytes.
Reprod Biol Endocrinol. 11:1082013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Velazquez ME, Millan AL, Rojo M, Abruzzese
GA, Cocucci SE, Iglesias Molli AE, Frechtel GD, Motta AB and
Cerrone GE: Telomere length differently associated to obesity and
hyperandrogenism in women with polycystic ovary syndrome. Front
Endocrinol (Lausanne). 12:6042152021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wei D, Xie J, Yin B, Hao H, Song X, Liu Q,
Zhang C and Sun Y: Significantly lengthened telomere in granulosa
cells from women with polycystic ovarian syndrome (PCOS). J Assist
Reprod Genet. 34:861–866. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bukulmez O: Diminished Ovarian Reserve and
Assisted Reproductive Technologies Current Research and Clinical
Management. Springer International Publishing; Berlin: 2020
|
|
36
|
Cheng EH, Chen SU, Lee TH, Pai YP, Huang
LS, Huang CC and Lee MS: Evaluation of telomere length in cumulus
cells as a potential biomarker of oocyte and embryo quality. Hum
Reprod. 28:929–936. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cimadomo D, Fabozzi G, Vaiarelli A, Ubaldi
N, Ubaldi FM and Rienzi L: Impact of maternal age on oocyte and
embryo competence. Front Endocrinol (Lausanne). 9:3272018.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kosebent EG and Ozturk S: Telomere
associated gene expression as well as TERT protein level and
telomerase activity are altered in the ovarian follicles of aged
mice. Sci Rep. 11:155692021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yuan X and Xu D: Telomerase reverse
transcriptase (TERT) in Action: Cross-talking with epigenetics. Int
J Mol Sci. 20:33382019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Morin SJ, Tao X, Marin D, Zhan Y, Landis
J, Bedard J, Scott RT and Seli E: DNA methylation-based age
prediction and telomere length in white blood cells and cumulus
cells of infertile women with normal or poor response to ovarian
stimulation. Aging (Albany NY). 10:3761–3773. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kosebent EG, Uysal F and Ozturk S:
Telomere length and telomerase activity during folliculogenesis in
mammals. J Reprod Dev. 64:477–484. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Babayev E and Duncan FE: Age-associated
changes in cumulus cells and follicular fluid: The local oocyte
microenvironment as a determinant of gamete quality. Biol Reprod.
106:351–365. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ferreira AF, Soares M, Almeida-Santos T,
Ramalho-Santos J and Sousa AP: Aging and oocyte competence: A
molecular cell perspective. WIREs Mech Dis. May 29–2023.(Epub ahead
of print). View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Xu X, Chen X, Zhang X, Liu Y, Wang Z, Wang
P, Du Y, Qin Y and Chen ZJ: Impaired telomere length and telomerase
activity in peripheral blood leukocytes and granulosa cells in
patients with biochemical primary ovarian insufficiency. Hum
Reprod. 32:201–207. 2017.PubMed/NCBI
|
|
45
|
Endo M, Kimura K, Kuwayama T, Monji Y and
Iwata H: Effect of estradiol during culture of bovine
oocyte-granulosa cell complexes on the mitochondrial DNA copies of
oocytes and telomere length of granulosa cells. Zygote. 22:431–439.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Esencan E, Beroukhim G and Seifer DB:
Age-related changes in Folliculogenesis and potential modifiers to
improve fertility outcomes-A narrative review. Reprod Biol
Endocrinol. 20:1562022. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lara-Molina EE, Franasiak JM, Marin D, Tao
X, Díaz-Gimeno P, Florensa M, Martin M, Seli E and Pellicer A:
Cumulus cells have longer telomeres than leukocytes in
reproductive-age women. Fertil Steril. 113:217–223. 2020.
View Article : Google Scholar : PubMed/NCBI
|