Sepsis is a syndrome that occurs when microorganisms
invade and cause systemic disease, which can be life-threatening
(1,2). The third international consensus
definition of septic shock and sepsis was published in 2016 and
defined septic shock as organ dysfunction caused by systemic
infection (3). The immune system
is suppressed during sepsis, which leads to increased inflammatory
response (4,5). The immune cells are triggered by
bacteria, toxins and other factors that result in infection,
releasing large quantities of inflammatory mediators (6,7). The
release of inflammatory mediators without an appropriate
anti-inflammatory response destroys the immune system, resulting in
unrestrained inflammatory state and a decreased ability to
neutralize pathogens (8–10).
Septic cardiomyopathy (SC), or septic shock, is a
condition defined by cardiac dysfunction caused by sepsis. SC is
clinically characterized by defective left ventricular systolic
function and ventricular hypertrophy. According to statistics from
the beginning of 2018, up to two-thirds of patients with septic
shock experience SC, which has become one of the most common fatal
diseases (11). Therefore, novel
pathogenic mechanisms of SC must be researched. The present review
aimed to summarize the pathophysiology of SC, focusing on
mitochondrial dysfunction, metabolic alterations, signaling
pathways and other mechanisms. These mechanisms of pathogenesis may
be used to validate discovery of novel ways to treat sepsis
contribute to decreased mortality in patients with SC.
Before the onset of SC, pathogenic bacteria that
infect the body and their endotoxins enter the bloodstream,
stimulating the immune system and producing large amounts of
inflammatory factors, leading to cytokine storm (12). Myocardial dysfunction may be caused
by chronic inflammation with prolonged lasting effects. During
sepsis, the inflammatory response contributes to an overproduction
of catecholamines, which impairs myocardial function and myocardial
contractility. Cardiac output is affected when tachycardia leads to
reduced coronary perfusion and cardiac output (13). In addition, mitochondria in septic
cardiomyocytes undergo structural changes, DNA damage, elevated
permeability and activation of apoptotic pathways, which decrease
metabolism, to accommodate inadequate ATP production caused by
mitochondrial dysfunction (14).
SC can be characterized by elevated cardiac enzymes, characteristic
changes in the electrocardiogram, hemodynamic changes, decreased
left ventricular ejection fraction and systolic dysfunction
(15). Clinical treatment is
mainly divided into two aspects: Treatment of sepsis
characteristics using antibiotics, vasoactive drugs, dopamine,
glucocorticoids and antibacterial peptides. Traditional Chinese
Medicine (TCM) treats septic cardiomyopathy through
anti-inflammatory and anti-viral effects, and inhibition of
apoptosis. Currently, TCM injections used clinically include
Xuebijing injection and Shenfu injection.
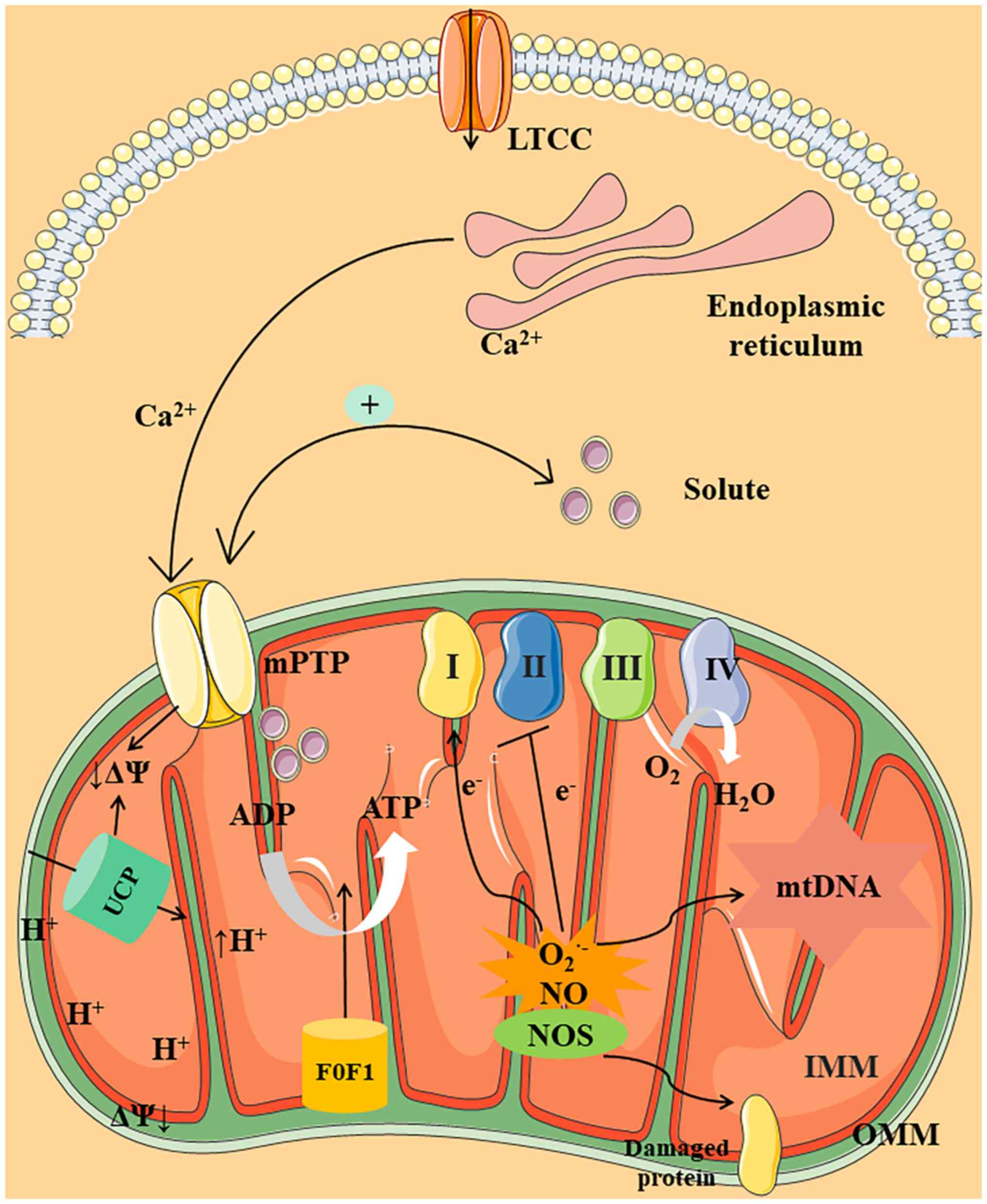
ATP is a compound synthesized in mitochondria and
the cytosol during glycolysis. Mitochondria are abundant in the
heart and are responsible for a significant amount of ATP
production (16). The primary
products following substrate oxidation, nicotinamide adenine
dinucleotide and flavin adenine dinucleotide, provide electrons for
complexes I and II. Under physiological conditions, electrons move
from complex I to complex II, then from complex III to complex IV
by oxidative phosphorylation (OXPHOS) (17). Complexes I–IV are involved in
transferring electrons from the tricarboxylic acid cycle to
mitochondria (18). During this
process, a proton can be transferred from the mitochondrial matrix
to the inner mitochondrial membrane (IMM) and O2 is
reduced to H2O in the mitochondria (19). Between the IMM space and the
mitochondrial matrix, protons accumulate, causing a proton motive
force (ΔΨ). ATP regeneration via F0F1-ATPase
(ATP synthase) is activated by ΔΨ, which transfers the proton from
the mitochondrial matrix to the IMM (20–22).
Therefore, F0F1-ATPase activity is associated
with respiratory chain activity and ATP formation.
Mitochondria also produce NO via the activity of
mitochondrial NO synthase (mtNOS), which physiologically regulates
mitochondrial respiration by inhibition of cytochrome C oxidase
(37,38). Under physiological conditions,
abundant O2− and NO react to form peroxynitrite
(ONOO−), which acts as a strong oxidizing agent. The
enhanced expression of mtNOS is induced in CLP-induced mouse
models, which contributes to increased mitochondrial
ONOO− levels (39,40).
The critical causative factors responsible for mitochondrial
dysfunction include inducible NOS (iNOS) synthase and mtNOS. It has
been reported that mitochondrial dysfunction is not observed in the
iNOS knockout mouse model (41).
Pharmacological inhibition or genetic deletion of iNOS improves
heart function in mouse models (41,42).
Moreover, studies have found that the activities of complexes I and
IV on the IMM are decreased by significantly boosting NO for a long
time (43). Therefore,
mitochondrial disorders attributed to NO may be primarily caused by
abnormal iNOS expression. At present, most of the aforementioned
studies have been conducted on iNOS-induced NO production, which
may have some limitations, such as focus only on iNOS induced NO
production, with a lack of mtNOS studies. However, NO is produced
by multiple NOS isoforms (not only mitochondria) in different
intracellular locations and cell types. In summary, one of the
major causes of mitochondrial dysfunction involves ROS and NO,
which may be key mechanisms of action in sepsis (Table I).
Mitochondrial membrane permeability occurs within
the mitochondrial membrane via the Ca2+ transport
channel mPTP (44), the primary
components of which are ATP synthase dimers and mitochondrial
phosphate transporters (45). The
three key processes involved in calcium transport are as follows:
Firstly, cyclophilin D activates the pores in response to changes
in mitochondrial calcium levels (46). Secondly, mPTP activation
facilitates release of calcium from the mitochondria into the
cytosol, where it activates calcium-dependent pathways (47,48).
Ca2+ overload triggers the mPTP to open and release
cytochrome C into the cytoplasm and the cytoplasm is released
(45). In addition, the
Ca2+-dependent state of the mPTP is influenced by the
calcium concentration within the cell (49). Generally, calcium transport causes
mitochondrial swelling and dysfunction as a result of calcium
transport. A study has shown mitochondrial vacuolation and damaged
mitochondrial cristae in cardiomyocytes of septic rats with
increased cytochrome C in the cytoplasm (50). At the same time, the amount of
Ca2+ able to enter the cytoplasm is determined by the
number of membrane L-type calcium channels and the amount of
Ca2+ stored in the sarcoplasmic reticulum (51). Additionally, dantrolene prevents
mitochondrial Ca2+ overload, which improves
mitochondrial dysfunction, by inhibiting sarcoplasmic reticulum
Ca2+ leaks (52). Taken
together, these conditions may result in cytoplasmic calcium
overload, leading to mitochondrial deterioration and contractile
dysfunction due to mPTP opening.
Triphenylphosphonium (TPP), covalent quinone (MitoQ)
and vitamin E have been prescribed as medications for treating SC.
A powerful antioxidant targeting mitochondria, MitoQ binds coenzyme
Q10 via triphenylphosphine, used for improving mitochondrial
function in SC (53). In a rat
model of sepsis, treatment with vitamin E conjugated to TPP
decreased ROS-related damage (54). Additionally, as an antioxidant,
lipoic acid might improve mitochondrial performance and alleviate
septic shock (55). To determine
whether the treatment modality used to treat mitochondrial
dysfunction could be a viable therapeutic option in the future,
researchers need to use drugs to prevent or reverse specific
mitochondrial functions.
There is evidence that metabolic dysregulation
occurs in SC, suggesting that targeting metabolic pathways may
offer notable benefits in SC treatment (56). During sepsis, hypermetabolism is
characterized by a catabolic state that depletes carbohydrate,
lipid and protein stores (57).
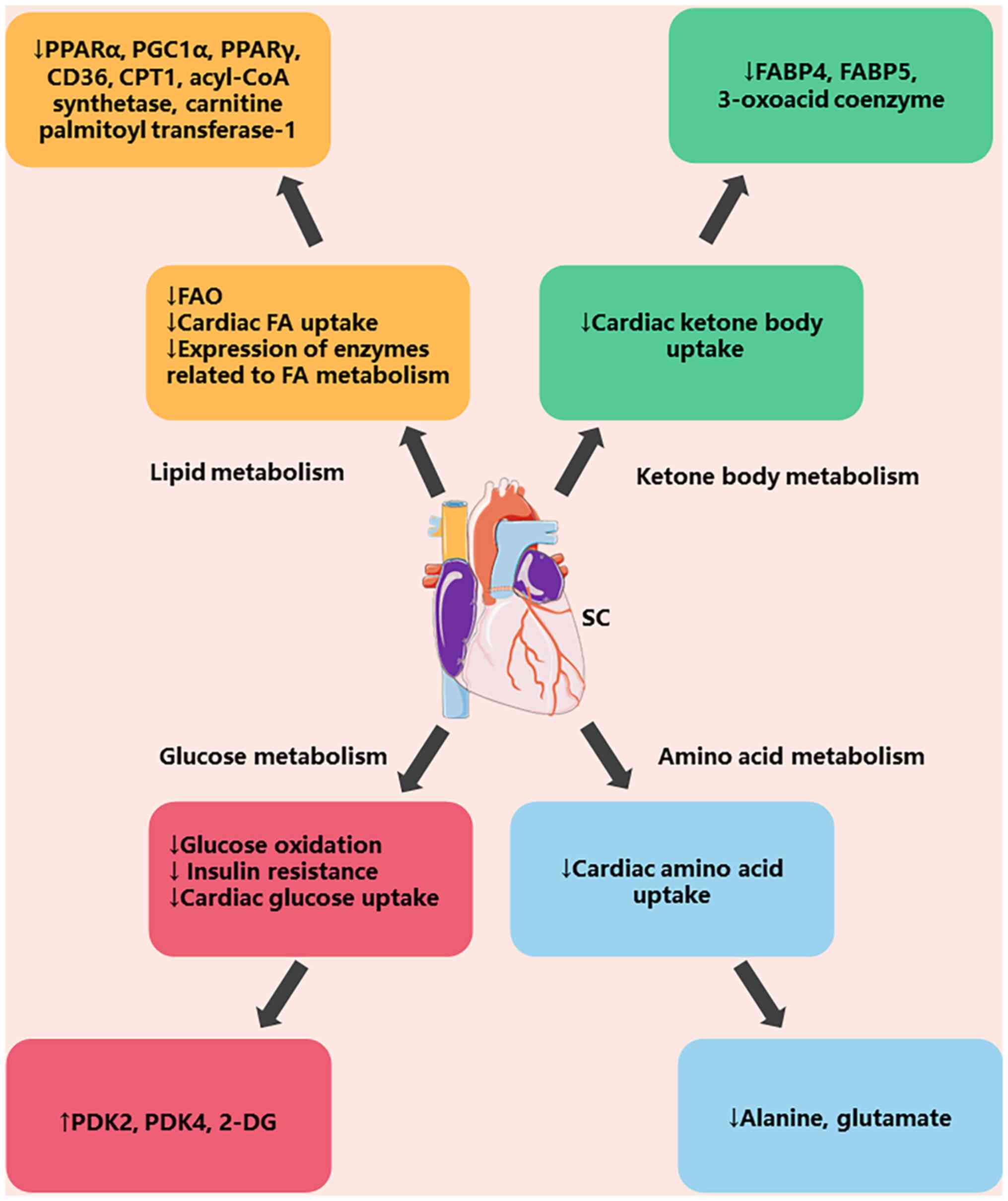
The primary metabolic processes during sepsis involve lipid,
ketone, glucose and amino acid metabolism (Fig. 2). The physiological indices of
lipid metabolism, such as fatty acid oxidation, are reduced during
sepsis and the expression of both cardiac fatty acids and lipid
metabolizing enzymes is reduced. When glucose is metabolized,
glucose oxidation, insulin resistance and cardiac glucose uptake
are decreased. Additionally, there is a reduction in the absorption
of ketone bodies and amino acids during ketone and amino acid
metabolism (58).
During sepsis, there is a significant demand for
energy, which is met by lipid mobilization (59). To make up for energy loss, adipose
tissue undergoes increased lipolysis to release fatty acid and
glycerol into the bloodstream (60). Sepsis is characterized by notable
deregulation of genes typically involved in lipid metabolism due to
the inflammatory response, such as PPARα, PGC1α and PPARγ. At the
same time, FA metabolism stops when carnitine palmitoyltransferase
1, acyl coenzyme a synthetase and carnitine palmitoyltransferase-1
expression is impaired. Studies have shown that LPS reduces PPARα
and PGC1α expression in LPS-induced rats, thereby regulating the
β-oxidation pathway (61,62). The inhibition of PPARγ activation
protects mice from sepsis-related cardiac dysfunction (63). In addition, studies have found that
defects in the enzymes carnitine palmitoyl transferase 1 and CD36
cause inefficient fatty acid transport, which contributes to fatty
acid oxidation (64). Finally,
studies have found that LPS reduces enzymes activity related to FA
metabolism, such as acyl-CoA synthetase and carnitine palmitoyl
transferase-1 (65,66). Imbalance of FA demand and supply
between the cytoplasm and mitochondria may cause lipid accumulation
in the cytoplasm (67). Moreover,
patients with sepsis exhibit fat buildup in cardiac muscle, kidney
and liver (68). Taken together,
lipid metabolism and associated enzyme transport are notable energy
providers in sepsis (Table
II).
Sepsis may lead to a high metabolic state throughout
the body, which increases ketone body production and lipid
breakdown (69). During prolonged
fasting, hypoglycemia occurs, resulting in promotion of ketogenesis
in hepatocyte mitochondria. The ketogenic effect may serve a
valuable role in biodefense as ketone bodies confer resistance to
ROS (70). Ketone body metabolism
may increase ATP production or contribute to systemic
hypercatabolism associated with calorie restriction (71,72).
Ketone metabolism is a method to maintain cardiac energy
homeostasis. Studies have found that LPS injection in mice lacking
fatty acid binding protein 4 (FABP4) and FABP5 inhibits hepatic and
cardiac ketogenesis, as FABP4 serves an active role in FA transport
(73,74). At the same time, gene expression
associated with 3-oxoacid coenzyme was significantly reduced in
both DoubleClick and wild-type mice (73,74).
The aforementioned studies suggest that ketone
bodies may represent a pathogenic mechanism in sepsis (Table II).
During SC, glucose oxidation does not increase to
compensate for the decrease in FAO caused by insulin resistance and
glucose metabolism inhibition (75,76).
In mice models of endotoxic shock, there is a rapid drop in
myocardial glucose levels compared with hemorrhagic shock (77,78)
Increased levels of pyruvate dehydrogenase kinase 2 (PDK2) and PDK4
protein inhibit glucose oxidation (79). Moreover, 2-deoxy-D-glucose (2-DG)
also improves cardiac function and survival outcomes in a mouse
model of sepsis (80). The
aforementioned findings indicate that increased glycolytic
metabolism contributes to cardiac dysfunction in sepsis and that
modulating metabolism following sepsis would be an appropriate
strategy (Table II).
Amino acids play crucial roles in both the synthesis
and breakdown of proteins, which is vital for maintaining cellular
homeostasis. Sepsis activates proteolysis, which splits proteins
into smaller polypeptides and amino acids, allowing them to rebuild
energy-rich molecules (81–83).
It is reported that amino acid uptake by the heart is 90% lower
compared with other organs in CLP-induced mouse models (84,85).
Moreover, studies have demonstrated that decreases in alanine and
glutamate lead to changes in cardiac metabolism in rats (86,87).
Collectively, amino acids may be required for the liver to maintain
protein hydrolysis in sepsis (Table
II).
Nuclear receptor transcription factors regulate
metabolic homeostasis, inflammatory response and cell death through
nuclear receptors (76,77). Studies have found that PPARα is
present in the liver, PPARβ is highly active in skeletal muscle and
PPARγ is associated with the control of the inflammatory reaction,
apoptosis and sepsis (88,89). PPARγ suppresses expression of
pro-inflammatory genes, mainly by scavenging transcription factors
and their cofactors, thus preventing binding to their cognate
binding sites in the promoters of target genes (90,91).
In addition, immune cells can produce large amounts of
pro-inflammatory mediators in the early stages of sepsis, and PPARγ
regulates sepsis by promoting apoptosis (92,93).
In a mouse model of CLP-induced inflammation, inhibition of NF-κB
p65 phosphorylation and activation via upregulation of PPARγ
attenuates inflammation (94).
Studies have found that total protein concentration, neutrophils
and macrophages are reduced in LPS-induced mice (95,96)
Decreased inflammatory factor release is attributed to the
conversion of macrophages from type M1 to M2. Moreover, the M1
macrophage increases chemokine ligand production in a CLP-induced
mouse model by increasing endothelial cell hyperpermeability and
phosphorylation of p38 by inhibiting PPARγ (97). In conclusion, activation of PPARγ
may contribute to reduction of pro-inflammatory properties during
SC (Table III).
When the NF-κB signaling pathway is engaged,
phosphorylation of NF-κB pathway factors such as p65 and inhibitor
κBα occurs (104,105). Moreover, studies have shown that
decreasing TNF-α and IL-6 secretion, cytokines that promote
inflammation in a LPS-induced rat model, stimulates the NF-κB
signaling pathway (106,107). In summary, NF-κB suppresses
inflammatory factors and proinflammatory genes that might be
involved in sepsis symptoms (Table
III).
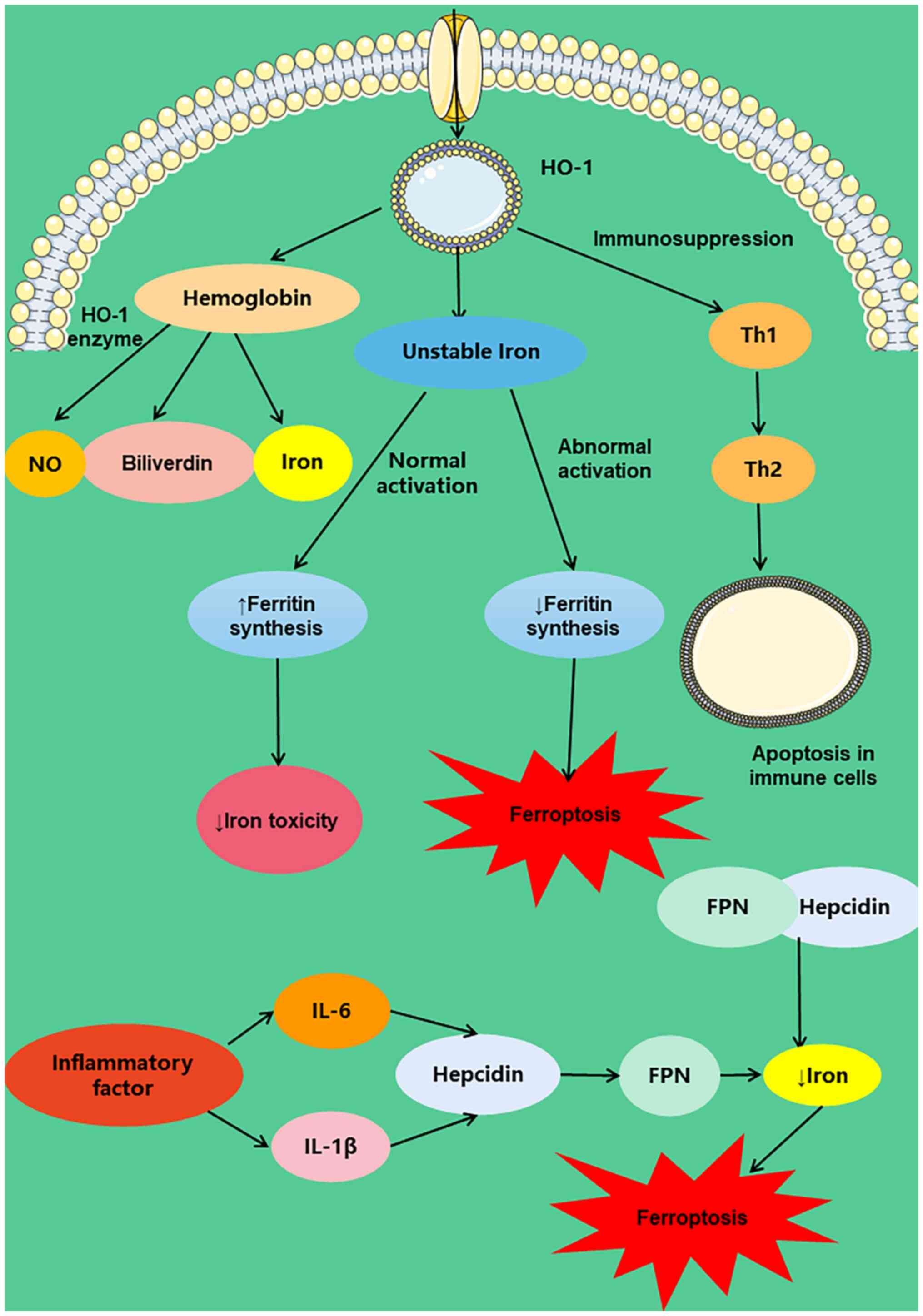
Ferroptosis pathways are iron-dependent,
non-apoptotic and characterized by specific biochemical and
morphological changes (108). The
majority of iron in the body is bound to hemoglobin and myoglobin,
with the rest primarily bound to ferritin and transferrin (109). In some cases, the cellular
defense mechanism limits the cell iron export system, leading to an
overload of cellular iron. Peroxylated lipids are produced as a
result of the Fenton reaction, resulting in the damage of
organelles (110). The
bloodstream contains tetrapyrrole hemoglobin containing iron during
SC. Under the action of heme oxygenase 1 (HO-1) enzyme, stable heme
is degraded to biliverdin, carbon monoxide and iron in vivo
(111,112). Additionally, HO-1 induces
immunosuppression during sepsis, promotes helper T cell 1(Th1) to
Th2 cytokine transfer and induces apoptosis in immune cells
(113). The product of the HO-1
reaction is unstable iron, which promotes synthesis of ferritin and
reduces the toxic effects of iron. The abnormal activation of HO-1
may result in the loss of the antioxidant action, increase levels
of the labile iron pool (LIP) and eventually cause iron deficiency
(114). In a mouse model of
sepsis, the expression of HO-1 leads to altered iron metabolism
protein levels and ferroptosis (115). Taken together, HO-1 causes
ferroptosis by degrading heme and elevating LIP to release ferritin
into the body.
Hepcidin, a liver-derived peptide hormone, maintains
iron homeostasis in the body. Studies have shown that hepcidin
ubiquitinates ferroportin (FPN) and reduces its activity, thus
lowering iron concentrations (116,117) Patients with sepsis have
significantly higher concentrations of hepcidin. Expression of
hepcidin is induced by IL-6 and IL-1β when inflammation takes place
(118). The serum iron levels are
effectively regulated by hepcidin in a mouse model induced by LPS
(119). Furthermore, studies have
shown that high hepcidin expression decreases FPN activity, which
decreases iron levels in plasma (120,121) Hence, hepcidin protein expression
inhibits iron transport, causing an imbalance in iron homeostasis,
which results in death from iron deficiency (Fig. 3).
Under physiological conditions, pyroptosis is
mediated by inflammasome-activated caspases and gasdermin D
(GSDMD), final effectors of the GSDM protein family, leading to
pore formation in the plasma membrane and leakage of inflammatory
mediators (122,123). Under pathogenic conditions, LPS
from Gram-negative bacteria directly activates caspase 4/5/11, in
the inflammasome pathway, without the need for the inflammasome or
caspase-1. GSDMD can be cleaved to produce N-GSDMD by activated
caspase 4/5/11. N-GSDMD indirectly promotes NLRP3 inflammasome
assembly via K+ efflux, which aggravates pyroptosis
(124,125). Studies have found that
doxorubicin upregulates NADPH oxidase 1 and NADPH oxidase 4
expression, thereby activating dynamin-related protein-1and
promoting mitochondrial fission, leading to excessive accumulation
of ROS in mitochondria and activation of the NLRP3 inflammasome and
caspase-1dependent apoptosis (126,127) Certain studies have found that
GSDMD knockout significantly decreases NLRP3 and caspase-1
expression, increases survival and improves cardiac dysfunction in
mice (128,129). Moreover, LPS directly affects
nuclear localization of sting and interferon regulatory
factor-3-activated sting then activates NLRP3, leading to cardiac
dysfunction as well as pyroptosis (130). Collectively, pyroptosis is
induced in most forms of cardiomyopathy and blocking pyroptosis by
direct or indirect approaches that target the pyroptotic machinery
or upstream regulators may exert a protective effect.
Myocardial dysfunction caused by sepsis is one of
the main reasons for the high mortality rate of sepsis and it is
crucial to investigate the pathogenesis of sepsis-induced cardiac
dysfunction and find treatment methods. The present study
summarized the primary factors that contribute to the pathogenesis
of SC, such as mitochondrial dysfunction, metabolic changes, cell
death and signaling pathways. Mitochondrial dysfunction, primarily
due to increased ROS and no steady-state concentrations inside
mitochondria, increases various reversible and irreversible toxic
modifications on biomolecules, such as protein carbonylation and
lipid peroxidation (28,131). Meanwhile, excessive ROS and NO
damage the mitochondrial respiratory chain structure and aggravate
the biosynthesis of ROS (132,133). Metabolism in sepsis requires the
adjustment of immune function via metabolism of fat, the metabolism
of amino acids, metabolism of glucose and the absorption of a large
amount of energy from cell's own metabolism (134,135). In addition, ferroptosis and
pyroptosis contribute to pathogenesis of SC. Iron molecules
contribute to the aggregation of ferritin at the cell membrane
through the HO-1 reaction; however, activation of iron molecules by
ferritin leads to an increase in iron output, leading to iron
enrichment (136,137). By regulating the key molecule
GSDMD, pyroptosis activates NLRP 3 inflammatory bodies and caspase
1-dependent apoptosis, leading to myocardial dysfunction in sepsis
(138,139).
According to previous treatment methods, the
relevant methods for the treatment of sepsis were divided into two
main categories, the first category was the basic treatment methods
for the characteristics of sepsis. Antibiotics decrease the release
of inflammatory factors and mediators by regulating pathogenic
microorganisms and the immune system to improve shock relieve
clinical symptoms and signs of sepsis (140,141). Dopamine, a vasoactive drug,
maintains a steady state of cardiac function by regulating the mean
arterial tone (142,143). Glucocorticoids are effective in
decreasing the duration of vasopressor use and maintaining
haemodynamic balance and improve the clinical symptoms of patients
with sepsis within a short period of time (144,145). The second type of treatment is
herbal injections, whose mechanism of action is to attenuate the
release of inflammatory factors and increase body immunity.
Xuebijing injection inhibits release of high mobility group protein
B1 in the serum of patients and decreases release of inflammatory
factor mediators, thus treating sepsis (146). Effective interventions to control
the way sepsis develops are necessary to translate basic research
into clinical practice. Knowledge of sepsis and heart failure may
lead to better treatment of myocardial infarction in future.
To the best of our knowledge, the metabolism of
cells has not been investigated in SC. Secondly, although metabolic
changes during sepsis have been reported, there is a lack of
information on specific mechanism of action studies. Lastly, it is
unclear how ferroptosis and pyroptosis occur during SC. Therefore,
researchers should investigate the pathogenesis of SC using new
methods and tools, such as network pharmacology, proteomics,
metabolomics and gut microbiota analysis.
The present study reviewed the pathogenesis of SC
with the goal of providing new ideas for the prevention and
treatment of SC. In conclusion, this review summarizes the
mitochondrial dysfunction (including reactive oxygen species,
nitric oxide and calcium ion transport), metabolic changes
(including lipid metabolism, ketone body metabolism, glucose
metabolism and amino acid metabolism) and cell death modes
(including iron death and cellular pyroptosis) associated with
septic cardiomyopathy during sepsis. SC was not caused by all
pathogenic mechanisms, but only a few that were relatively
important were discussed in the review.
Not applicable.
The present study was supported by the Natural Science
Foundation of Jilin Province (grant no. YDZJ202201ZYTS199) and the
National College Students Innovation and Entrepreneurship Project
Training Program (grant no. 202210199020).
Not applicable.
JS and XF conceived the subject of the review,
performed the investigation, and wrote and edited the original
draft. KZ, HB and LL wrote, reviewed, and edited the manuscript.
All authors have read and approved the final manuscript. Data
authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Hammond N, Kumar A, Kaur P, Tirupakuzhi
Vijayaraghavan BK, Ghosh A, Grattan S, Jha V, Mathai D and
Venkatesh B; Sepsis in India Prevalence Study (SIPS) Investigator
Network, : Estimates of sepsis prevalence and outcomes in adult
patients in the ICU in India: A cross-sectional Study. Chest.
161:1543–1554. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Salomão R, Ferreira BL, Salomão MC, Santos
SS, Azevedo LCP and Brunialti MKC: Sepsis: Evolving concepts and
challenges. Braz J Med Biol Res. 52:e85952019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shankar-Hari M, Phillips G, Levy ML,
Seymour CW, Liu VX, Deutschman CS, Angus DC, Rubenfeld GD and
Singer M; Sepsis Definitions Task Force, : Developing a new
definition and assessing new clinical criteria for septic shock:
For the third international consensus definitions for sepsis and
septic shock (sepsis-3). JAMA. 315:775–787. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bone RC, Balk RA, Cerra FB, Dellinger RP,
Fein AM, Knaus WA, Schein RM and Sibbald WJ: Definitions for sepsis
and organ failure and guidelines for the use of innovative
therapies in sepsis. The ACCP/SCCM consensus conference committee.
American college of chest physicians/society of critical care
medicine. Chest. 101:1644–1655. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Makic MBF and Bridges E: CE: Managing
sepsis and septic shock: Current guidelines and definitions. Am J
Nurs. 118:34–39. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Delano MJ and Ward PA: The immune system's
role in sepsis progression, resolution, and long-term outcome.
Immunol Rev. 274:330–353. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Antonucci E, Fiaccadori E, Donadello K,
Taccone FS, Franchi F and Scolletta S: Myocardial depression in
sepsis: From pathogenesis to clinical manifestations and treatment.
J Crit Care. 29:500–511. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rello J, Valenzuela-Sánchez F,
Ruiz-Rodriguez M and Moyano S: Sepsis: A review of advances in
management. Adv Ther. 34:2393–2411. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Skirecki T and Cavaillon JM: Inner sensors
of endotoxin-implications for sepsis research and therapy. FEMS
Microbiol Rev. 43:239–256. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Torres L, Pickkers P and van der Poll T:
Sepsis-induced immunosuppression. Annu Rev Physiol. 84:157–181.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ehrman RR, Sullivan AN, Favot MJ, Sherwin
RL, Reynolds CA, Abidov A and Levy PD: Pathophysiology,
echocardiographic evaluation, biomarker findings, and prognostic
implications of septic cardiomyopathy: A review of the literature.
Crit Care. 22:1122018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Purcarea A and Sovaila S: Sepsis, a 2020
review for the internist. Rom J Intern Med. 58:129–137.
2020.PubMed/NCBI
|
|
13
|
Gotts JE and Matthay MA: Sepsis:
Pathophysiology and clinical management. BMJ. 353:i15852016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang M, Cai S and Su J: The pathogenesis
of sepsis and potential therapeutic targets. Int J Mol Sci.
20:53762019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ackerman MH, Ahrens T, Kelly J and
Pontillo A: Sepsis. Crit Care Nurs Clin North Am. 33:407–418. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang H, Feng YW and Yao YM: Potential
therapy strategy: Targeting mitochondrial dysfunction in sepsis.
Mil Med Res. 5:412018.PubMed/NCBI
|
|
17
|
Cheung R, Pizza G, Chabosseau P, Rolando
D, Tomas A, Burgoyne T, Wu Z, Salowka A, Thapa A, Macklin A, et al:
Glucose-dependent miR-125b is a negative regulator of β-cell
function. Diabetes. 71:1525–1545. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Doke T and Susztak K: The multifaceted
role of kidney tubule mitochondrial dysfunction in kidney disease
development. Trends Cell Biol. 32:841–853. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Park K and Lee MS: Essential role of
lysosomal Ca2+-mediated TFEB activation in mitophagy and functional
adaptation of pancreatic β-cells to metabolic stress. Autophagy.
18:3043–3045. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Eldeeb MA, Thomas RA, Ragheb MA, Fallahi A
and Fon EA: Mitochondrial quality control in health and in
Parkinson's disease. Physiol Rev. 102:1721–1755. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hocaoglu H and Sieber M: Mitochondrial
respiratory quiescence: A new model for examining the role of
mitochondrial metabolism in development. Semin Cell Dev Biol.
138:94–103. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Subramanian GN, Yeo AJ, Gatei MH, Coman DJ
and Lavin MF: Metabolic stress and mitochondrial dysfunction in
ataxia-telangiectasia. Antioxidants (Basel). 11:6532022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Joffre J and Hellman J: Oxidative stress
and endothelial dysfunction in sepsis and acute inflammation.
Antioxid Redox Signal. 35:1291–1307. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Doi K, Leelahavanichkul A, Yuen PST and
Star RA: Animal models of sepsis and sepsis-induced kidney injury.
J Clin Invest. 119:2868–2878. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Salari S, Ghorbanpour A, Marefati N,
Baluchnejadmojarad T and Roghani M: Therapeutic effect of lycopene
in lipopolysaccharide nephrotoxicity through alleviation of
mitochondrial dysfunction, inflammation, and oxidative stress. Mol
Biol Rep. 49:8429–8438. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
de Souza Stork S, Hübner M, Biehl E,
Danielski LG, Bonfante S, Joaquim L, Denicol T, Cidreira T, Pacheco
A, Bagio E, et al: Diabetes exacerbates sepsis-induced
neuroinflammation and brain mitochondrial dysfunction.
Inflammation. 45:2352–2367. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Soriano FG, Nogueira AC, Caldini EG, Lins
MH, Teixeira AC, Cappi SB, Lotufo PA, Bernik MM, Zsengellér Z, Chen
M and Szabó C: Potential role of poly(adenosine
5′-diphosphate-ribose) polymerase activation in the pathogenesis of
myocardial contractile dysfunction associated with human septic
shock. Crit Care Med. 34:1073–1079. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Galley HF: Oxidative stress and
mitochondrial dysfunction in sepsis. Br J Anaesth. 107:57–64. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cimolai MC, Alvarez S, Bode C and Bugger
H: Mitochondrial mechanisms in septic cardiomyopathy. Int J Mol
Sci. 16:17763–17778. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee S, Xu H, Van Vleck A, Mawla AM, Li AM,
Ye J, Huising MO and Annes JP: β-Cell succinate dehydrogenase
deficiency triggers metabolic dysfunction and insulinopenic
diabetes. Diabetes. 71:1439–1453. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hu J, Cheng Y, Chen P, Huang Z and Yang L:
Caffeine citrate protects against sepsis-associated encephalopathy
and inhibits the UCP2/NLRP3 axis in astrocytes. J Interferon
Cytokine Res. 42:267–278. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Huang Q, Ding Y, Fang C, Wang H and Kong
L: The emerging role of ferroptosis in sepsis, opportunity or
challenge? Infect Drug Resist. 16:5551–5562. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ji L, He Q, Liu Y, Deng Y, Xie M, Luo K,
Cai X, Zuo Y, Wu W, Li Q, et al: Ketone body β-hydroxybutyrate
prevents myocardial oxidative stress in septic cardiomyopathy. Oxid
Med Cell Longev. 2022:25138372022. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhao H, Lin X, Chen Q, Wang X, Wu Y and
Zhao X: Quercetin inhibits the NOX2/ROS-mediated NF-κB/TXNIP
signaling pathway to ameliorate pyroptosis of cardiomyocytes to
relieve sepsis-induced cardiomyopathy. Toxicol Appl Pharmacol.
477:1166722023. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu Z, Pan H, Zhang Y, Zheng Z, Xiao W,
Hong X, Chen F, Peng X, Pei Y, Rong J, et al: Ginsenoside-Rg1
attenuates sepsis-induced cardiac dysfunction by modulating
mitochondrial damage via the P2X7 receptor-mediated Akt/GSK-3β
signaling pathway. J Biochem Mol Toxicol. 36:e228852022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang J, Yang S, Jing G, Wang Q, Zeng C,
Song X and Li X: Inhibition of ferroptosis protects
sepsis-associated encephalopathy. Cytokine. 161:1560782023.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Vanasco V, Saez T, Magnani ND, Pereyra L,
Marchini T, Corach A, Vaccaro MI, Corach D, Evelson P and Alvarez
S: Cardiac mitochondrial biogenesis in endotoxemia is not
accompanied by mitochondrial function recovery. Free Radic Biol
Med. 77:1–9. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Burgoyne J, Rudyk O, Mayr M and Eaton P:
Nitrosative protein oxidation is modulated during early
endotoxemia. Nitric Oxide. 25:118–124. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Boveris A, Alvarez S and Navarro A: The
role of mitochondrial nitric oxide synthase in inflammation and
septic shock. Free Radic Biol Med. 33:1186–1193. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Escames G, López L, Ortiz F, López A,
García JA, Ros E and Acuña-Castroviejo D: Attenuation of cardiac
mitochondrial dysfunction by melatonin in septic mice. FEBS J.
274:2135–2147. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
van de Sandt AM, Windler R, Gödecke A,
Ohlig J, Zander S, Reinartz M, Graf J, van Faassen EE, Rassaf T,
Schrader J, et al: Endothelial NOS (NOS3) impairs myocardial
function in developing sepsis. Basic Res Cardiol. 108:3302013.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
McCall CE, Zhu X, Zabalawi M, Long D,
Quinn MA, Yoza BK, Stacpoole PW and Vachharajani V: Sepsis,
pyruvate, and mitochondria energy supply chain shortage. J Leukoc
Biol. 112:1509–1514. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Joshi MS, Julian MW, Huff JE, Bauer JA,
Xia Y and Crouser ED: Calcineurin regulates myocardial function
during acute endotoxemia. Am J Respir Crit Care Med. 173:999–1007.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Giorgio V, von Stockum S, Antoniel M,
Fabbro A, Fogolari F, Forte M, Glick GD, Petronilli V, Zoratti M,
Szabó I, et al: Dimers of mitochondrial ATP synthase form the
permeability transition pore. Proc Natl Acad Sci USA.
110:5887–5892. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Giorgio V, Guo L, Bassot C, Petronilli V
and Bernardi P: Calcium and regulation of the mitochondrial
permeability transition. Cell Calcium. 70:56–63. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Bernardi P: The mitochondrial permeability
transition pore: A mystery solved? Front Physiol. 4:952013.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Rasola A and Bernardi P: Mitochondrial
permeability transition in Ca(2+)-dependent apoptosis and necrosis.
Cell Calcium. 50:222–233. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Takeuchi A, Kim B and Matsuoka S: The
destiny of Ca(2+) released by mitochondria. J Physiol Sci.
65:11–24. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Halestrap AP: Calcium, mitochondria and
reperfusion injury: A pore way to die. Biochem Soc Trans.
34:232–237. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Bernardi P and Di Lisa F: The
mitochondrial permeability transition pore: Molecular nature and
role as a target in cardioprotection. J Mol Cell Cardiol.
78:100–106. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ballard-Croft C, Maass DL, Sikes PJ and
Horton JW: Sepsis and burn complicated by sepsis alter cardiac
transporter expression. Burns. 33:72–80. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Hassoun SM, Marechal X, Montaigne D,
Bouazza Y, Decoster B, Lancel S and Neviere R: Prevention of
endotoxin-induced sarcoplasmic reticulum calcium leak improves
mitochondrial and myocardial dysfunction. Crit Care Med.
36:2590–2596. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Supinski GS, Murphy MP and Callahan LA:
MitoQ administration prevents endotoxin-induced cardiac
dysfunction. Am J Physiol Regul Integr Comp Physiol.
297:R1095–R1102. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Zang QS, Sadek H, Maass DL, Martinez B, Ma
L, Kilgore JA, Williams NS, Frantz DE, Wigginton JG, Nwariaku FE,
et al: Specific inhibition of mitochondrial oxidative stress
suppresses inflammation and improves cardiac function in a rat
pneumonia-related sepsis model. Am J Physiol Heart Circ Physiol.
302:H1847–H1859. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Vanasco V, Cimolai MC, Evelson P and
Alvarez S: The oxidative stress and the mitochondrial dysfunction
caused by endotoxemia are prevented by alpha-lipoic acid. Free
Radic Res. 42:815–823. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Vandewalle J and Libert C: Sepsis: A
failing starvation response. Trends Endocrinol Metab. 33:292–304.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Lelubre C and Vincent JL: Mechanisms and
treatment of organ failure in sepsis. Nat Rev Nephrol. 14:417–427.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Collins K and Huen SC: Metabolism and
nutrition in sepsis: In need of a paradigm shift. Nephron. Sep
13–2023.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Wolowczuk I, Verwaerde C, Viltart O,
Delanoye A, Delacre M, Pot B and Grangette C: Feeding our immune
system: Impact on metabolism. Clin Dev Immunol. 2008:6398032008.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Rittig N, Bach E, Thomsen HH, Pedersen SB,
Nielsen TS, Jørgensen JO, Jessen N and Møller N: Regulation of
lipolysis and adipose tissue signaling during acute
endotoxin-induced inflammation: A human randomized crossover trial.
PLoS One. 11:e01621672016. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Drosatos K, Drosatos-Tampakaki Z, Khan R,
Homma S, Schulze PC, Zannis VI and Goldberg IJ: Inhibition of
c-Jun-N-terminal kinase increases cardiac peroxisome
proliferator-activated receptor alpha expression and fatty acid
oxidation and prevents lipopolysaccharide-induced heart
dysfunction. J Biol Chem. 286:36331–36339. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Wang W, Xu RL, He P and Chen R: MAR1
suppresses inflammatory response in LPS-induced RAW 264.7
macrophages and human primary peripheral blood mononuclear cells
via the SIRT1/PGC-1α/PPAR-γ pathway. J Inflamm (Lond). 18:82021.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Drosatos K, Khan RS, Trent CM, Jiang H,
Son NH, Blaner WS, Homma S, Schulze PC and Goldberg IJ: Peroxisome
proliferator-activated receptor-γ activation prevents
sepsis-related cardiac dysfunction and mortality in mice. Circ
Heart Fail. 6:550–562. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Sharma S, Adrogue JV, Golfman L, Uray I,
Lemm J, Youker K, Noon GP, Frazier OH and Taegtmeyer H:
Intramyocardial lipid accumulation in the failing human heart
resembles the lipotoxic rat heart. FASEB J. 18:1692–1700. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Memon RA, Fuller J, Moser AH, Smith PJ,
Feingold KR and Grunfeld C: In vivo regulation of acyl-CoA
synthetase mRNA and activity by endotoxin and cytokines. Am J
Physiol. 275:E64–E72. 1998.PubMed/NCBI
|
|
66
|
Feingold K, Kim M, Shigenaga J, Moser A
and Grunfeld C: Altered expression of nuclear hormone receptors and
coactivators in mouse heart during the acute-phase response. Am J
Physiol Endocrinol Metab. 286:E201–E207. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Rossi MA, Celes MRN, Prado CM and Saggioro
FP: Myocardial structural changes in long-term human severe
sepsis/septic shock may be responsible for cardiac dysfunction.
Shock. 27:10–18. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Koskinas J, Gomatos IP, Tiniakos DG, Memos
N, Boutsikou M, Garatzioti A, Archimandritis A and Betrosian A:
Liver histology in ICU patients dying from sepsis: A
clinico-pathological study. World J Gastroenterol. 14:1389–1393.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Shimazu T, Hirschey MD, Newman J, He W,
Shirakawa K, Le Moan N, Grueter CA, Lim H, Saunders LR, Stevens RD,
et al: Suppression of oxidative stress by β-hydroxybutyrate, an
endogenous histone deacetylase inhibitor. Science. 339:211–214.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Aubert G, Martin OJ, Horton JL, Lai L,
Vega RB, Leone TC, Koves T, Gardell SJ, Krüger M, Hoppel CL, et al:
The failing heart relies on ketone bodies as a fuel. Circulation.
133:698–705. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Wang A, Huen SC, Luan HH, Yu S, Zhang C,
Gallezot JD, Booth CJ and Medzhitov R: Opposing effects of fasting
metabolism on tissue tolerance in bacterial and viral inflammation.
Cell. 166:1512–1525.e12. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Umbarawan Y, Syamsunarno MRAA, Obinata H,
Yamaguchi A, Sunaga H, Matsui H, Hishiki T, Matsuura T, Koitabashi
N, Obokata M, et al: Robust suppression of cardiac energy
catabolism with marked accumulation of energy substrates during
lipopolysaccharide-induced cardiac dysfunction in mice. Metabolism.
77:47–57. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Soni S, Martens MD, Takahara S, Silver HL,
Maayah ZH, Ussher JR, Ferdaoussi M and Dyck JRB: Exogenous ketone
ester administration attenuates systemic inflammation and reduces
organ damage in a lipopolysaccharide model of sepsis. Biochim
Biophys Acta Mol Basis Dis. 1868:1665072022. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Dhainaut JF, Huyghebaert MF, Monsallier
JF, Lefevre G, Dall'Ava-Santucci J, Brunet F, Villemant D, Carli A
and Raichvarg D: Coronary hemodynamics and myocardial metabolism of
lactate, free fatty acids, glucose, and ketones in patients with
septic shock. Circulation. 75:533–541. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Chew MS, Shekar K, Brand BA, Norin C and
Barnett AG: Depletion of myocardial glucose is observed during
endotoxemic but not hemorrhagic shock in a porcine model. Crit
Care. 17:R1642013. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Liu T, Wen Z, Shao L, Cui Y, Tang X, Miao
H, Shi J, Jiang L, Feng S, Zhao Y, et al: ATF4 knockdown in
macrophage impairs glycolysis and mediates immune tolerance by
targeting HK2 and HIF-1α ubiquitination in sepsis. Clin Immunol.
254:1096982023. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Standage SW, Bennion BG, Knowles TO, Ledee
DR, Portman MA, McGuire JK, Liles WC and Olson AK: PPARα augments
heart function and cardiac fatty acid oxidation in early
experimental polymicrobial sepsis. Am J Physiol Heart Circ Physiol.
312:H239–H249. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Zheng Z, Ma H, Zhang X, Tu F, Wang X, Ha
T, Fan M, Liu L, Xu J, Yu K, et al: Enhanced glycolytic metabolism
contributes to cardiac dysfunction in polymicrobial sepsis. J
Infect Dis. 215:1396–1406. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Lang CH, Frost RA, Jefferson LS, Kimball
SR and Vary TC: Endotoxin-induced decrease in muscle protein
synthesis is associated with changes in eIF2B, eIF4E, and IGF-I. Am
J Physiol Endocrinol Metab. 278:E1133–E1143. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Lang CH, Frost RA, Nairn AC, MacLean DA
and Vary TC: TNF-alpha impairs heart and skeletal muscle protein
synthesis by altering translation initiation. Am J Physiol
Endocrinol Metab. 282:E336–E347. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Plank LD and Hill GL: Sequential metabolic
changes following induction of systemic inflammatory response in
patients with severe sepsis or major blunt trauma. World J Surg.
24:630–638. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Warner BW, Hummel RP III, Hasselgren PO,
James JH and Fischer JE: Inhibited amino acid uptake in skeletal
muscle during starvation. JPEN J Parenter Enteral Nutr. 13:344–348.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Zhang Q, Bao X, Cui M, Wang C, Ji J, Jing
J, Zhou X, Chen K and Tang L: Identification and validation of key
biomarkers based on RNA methylation genes in sepsis. Front Immunol.
14:12318982023. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Hotchkiss RS, Song SK, Neil JJ, Chen RD,
Manchester JK, Karl IE, Lowry OH and Ackerman JJ: Sepsis does not
impair tricarboxylic acid cycle in the heart. Am J Physiol.
260:C50–C57. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Sun S, Wang D, Dong D, Xu L, Xie M, Wang
Y, Ni T, Jiang W, Zhu X, Ning N, et al: Altered intestinal
microbiome and metabolome correspond to the clinical outcome of
sepsis. Crit Care. 27:1272023. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Chang WH and Lai AG: The pan-cancer
mutational landscape of the PPAR pathway reveals universal patterns
of dysregulated metabolism and interactions with tumor immunity and
hypoxia. Ann N Y Acad Sci. 1448:65–82. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Anghel SI and Wahli W: Fat poetry: A
kingdom for PPAR gamma. Cell Res. 17:486–511. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Christodoulides C and Vidal-Puig A: PPARs
and adipocyte function. Mol Cell Endocrinol. 318:61–68. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Villarroel-Vicente C, Gutiérrez-Palomo S,
Ferri J, Cortes D and Cabedo N: Natural products and analogs as
preventive agents for metabolic syndrome via peroxisome
proliferator-activated receptors: An overview. Eur J Med Chem.
221:1135352021. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
von Knethen A, Soller M and Brüne B:
Peroxisome proliferator-activated receptor gamma (PPAR gamma) and
sepsis. Arch Immunol Ther Exp (Warsz). 55:19–25. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Li Z, Jia Y, Feng Y, Cui R, Wang Z, Qu K,
Liu C and Zhang J: Methane-rich saline protects against
sepsis-induced liver damage by regulating the PPAR-γ/NF-κB
signaling pathway. Shock. 52:e163–e172. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Gong W, Zhu H, Lu L, Hou Y and Dou H: A
benzenediamine analog FC-99 drives M2 macrophage polarization and
alleviates lipopolysaccharide-(LPS-) induced liver injury.
Mediators Inflamm. 2019:78230692019. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Wen Q, Miao J, Lau N, Zhang C, Ye P, Du S,
Mei L, Weng H, Xu Q, Liu X, et al: Rhein attenuates
lipopolysaccharide-primed inflammation through NF-κB inhibition in
RAW264.7 cells: targeting the PPAR-γ signal pathway. Can J Physiol
Pharmacol. 98:357–365. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Xia H, Ge Y, Wang F, Ming Y, Wu Z, Wang J,
Sun S, Huang S, Chen M, Xiao W and Yao S: Protectin DX ameliorates
inflammation in sepsis-induced acute lung injury through mediating
PPARγ/NF-κB pathway. Immunol Res. 68:280–288. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Chen Q, Shao X, He Y, Lu E, Zhu L and Tang
W: Norisoboldine attenuates sepsis-induced acute lung injury by
modulating macrophage polarization via PKM2/HIF-1α/PGC-1α pathway.
Biol Pharm Bull. 44:1536–1547. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Zhu XX, Wang X, Jiao SY, Liu Y, Shi L, Xu
Q, Wang JJ, Chen YE, Zhang Q, Song YT, et al: Cardiomyocyte
peroxisome proliferator-activated receptor α prevents septic
cardiomyopathy via improving mitochondrial function. Acta Pharmacol
Sin. Jun 16–2023.(Epub ahead of print).
|
|
97
|
Chen W, Wang Y, Zhou Y, Xu Y, Bo X and Wu
J: M1 macrophages increase endothelial permeability and enhance p38
phosphorylation via PPAR-γ/CXCL13-CXCR5 in sepsis. Int Arch Allergy
Immunol. 183:997–1006. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Mitchell S, Vargas J and Hoffmann A:
Signaling via the NFκB system. Wiley Interdiscip Rev Syst Biol Med.
8:227–241. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Somensi N, Rabelo TK, Guimarães AG,
Quintans-Junior LJ, de Souza Araújo AA, Moreira JCF and Gelain DP:
Carvacrol suppresses LPS-induced pro-inflammatory activation in RAW
264.7 macrophages through ERK1/2 and NF-kB pathway. Int
Immunopharmacol. 75:1057432019. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Liu B, Wu Y, Wang Y, Cheng Y, Yao L, Liu
Y, Qian H, Yang H and Shen F: NF-κB p65 Knock-down inhibits TF,
PAI-1 and promotes activated protein C production in
lipopolysaccharide-stimulated alveolar epithelial cells type II.
Exp Lung Res. 44:241–251. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Wu Z, Chen J, Zhao W, Zhuo CH and Chen Q:
Inhibition of miR-181a attenuates sepsis-induced inflammation and
apoptosis by activating Nrf2 and inhibiting NF-κB pathways via
targeting SIRT1. Kaohsiung J Med Sci. 37:200–207. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Liu SF and Malik AB: NF-kappa B activation
as a pathological mechanism of septic shock and inflammation. Am J
Physiol Lung Cell Mol Physiol. 290:L622–L645. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Zang B and Wang L: Synthesis and
protective effect of pyrazole conjugated imidazo[1,2-a]pyrazine
derivatives against acute lung injury in sepsis rats via
attenuation of NF-κB, oxidative stress, and apoptosis. Acta Pharm.
73:341–362. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Cao L and Yang K: Paeoniflorin attenuated
TREM-1-mediated inflammation in THP-1 cells. J Healthc Eng.
2022:70516432022. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Wang X, Xu T, Jin J, Ting Gao MM, Wan B,
Gong M, Bai L, Lv T and Song Y: Topotecan reduces sepsis-induced
acute lung injury and decreases the inflammatory response via the
inhibition of the NF-κB signaling pathway. Pulm Circ.
12:e120702022. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Franco JH, Chen X and Pan ZK: Novel
treatments targeting the dysregulated cell signaling pathway during
sepsis. J Cell Signal. 2:228–234. 2021.PubMed/NCBI
|
|
107
|
Ruan W, Ji X, Qin Y, Zhang X, Wan X, Zhu
C, Lv C, Hu C, Zhou J, Lu L and Guo X: Harmine alleviated
sepsis-induced cardiac dysfunction by modulating macrophage
polarization via the STAT/MAPK/NF-κB pathway. Front Cell Dev Biol.
9:7922572022. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Dang X, Huan X, Du X, Chen X, Bi M, Yan C,
Jiao Q and Jiang H: Correlation of ferroptosis and other types of
cell death in neurodegenerative diseases. Neurosci Bull.
38:938–952. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Kim J and Wessling-Resnick M: The role of
iron metabolism in lung inflammation and injury. J Allergy Ther. 3
(Suppl 4):S0042012.
|
|
110
|
de Lima VM, Batista BB and da Silva Neto
JF: The regulatory protein ChuP connects heme and
siderophore-mediated iron acquisition systems required for
chromobacterium violaceum virulence. Front Cell Infect Microbiol.
12:8735362022. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Englert FA, Seidel RA, Galler K, Gouveia
Z, Soares MP, Neugebauer U, Clemens MG, Sponholz C, Heinemann SH,
Pohnert G, et al: Labile heme impairs hepatic microcirculation and
promotes hepatic injury. Arch Biochem Biophys. 672:1080752019.
View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Stefanson AL and Bakovic M: Falcarinol Is
a potent inducer of heme oxygenase-1 and was more effective than
sulforaphane in attenuating intestinal inflammation at
diet-achievable doses. Oxid Med Cell Longev. 2018:31535272018.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Yoon SJ, Kim SJ and Lee SM: Overexpression
of HO-1 contributes to sepsis-induced immunosuppression by
modulating the Th1/Th2 balance and regulatory T-cell function. J
Infect Dis. 215:1608–1618. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Puentes-Pardo JD, Moreno-SanJuan S, Carazo
Á and León J: Heme oxygenase-1 in gastrointestinal tract health and
disease. Antioxidants (Basel). 9:12142020. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Fernández-Mendívil C, Luengo E,
Trigo-Alonso P, García-Magro N, Negredo P and López MG: Protective
role of microglial HO-1 blockade in aging: Implication of iron
metabolism. Redox Biol. 38:1017892021. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Qiao B, Sugianto P, Fung E,
Del-Castillo-Rueda A, Moran-Jimenez MJ, Ganz T and Nemeth E:
Hepcidin-induced endocytosis of ferroportin is dependent on
ferroportin ubiquitination. Cell Metab. 15:918–924. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Cross JH, Jarjou O, Mohammed NI, Gomez SR,
Touray BJB, Kessler NJ, Prentice AM and Cerami C: Iron homeostasis
in full-term, normal birthweight Gambian neonates over the first
week of life. Sci Rep. 13:103492023. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Drakesmith H and Prentice AM: Hepcidin and
the iron-infection axis. Science. 338:768–772. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Scindia Y, Wlazlo E, Leeds J, Loi V,
Ledesma J, Cechova S, Ghias E and Swaminathan S: Protective role of
hepcidin in polymicrobial sepsis and acute kidney injury. Front
Pharmacol. 10:6152019. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Deng Q, Yang S, Sun L, Dong K, Li Y, Wu S
and Huang R: Salmonella effector SpvB aggravates dysregulation of
systemic iron metabolism via modulating the hepcidin-ferroportin
axis. Gut Microbes. 13:1–18. 2021. View Article : Google Scholar
|
|
121
|
Czempik PF and Wiórek A: Iron deficiency
in sepsis patients based on reticulocyte hemoglobin and hepcidin
concentration: A prospective cohort study. Arch Med Sci.
19:805–809. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Martinon F, Burns K and Tschopp J: The
inflammasome: A molecular platform triggering activation of
inflammatory caspases and processing of proIL-beta. Mol Cell.
10:417–426. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Shi J, Zhao Y, Wang K, Shi X, Wang Y,
Huang H, Zhuang Y, Cai T, Wang F and Shao F: Cleavage of GSDMD by
inflammatory caspases determines pyroptotic cell death. Nature.
526:660–665. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Feng Y, Li M, Yangzhong X, Zhang X, Zu A,
Hou Y, Li L and Sun S: Pyroptosis in inflammation-related
respiratory disease. J Physiol Biochem. 78:721–737. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Man SM, Karki R and Kanneganti TD:
Molecular mechanisms and functions of pyroptosis, inflammatory
caspases and inflammasomes in infectious diseases. Immunol Rev.
277:61–75. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Zeng C, Duan F, Hu J, Luo B, Huang B, Lou
X, Sun X, Li H, Zhang X, Yin S and Tan H: NLRP3
inflammasome-mediated pyroptosis contributes to the pathogenesis of
non-ischemic dilated cardiomyopathy. Redox Biol. 34:1015232020.
View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Wu S, Liao J, Hu G, Yan L, Su X, Ye J,
Zhang C, Tian T, Wang H and Wang Y: Corilagin alleviates
LPS-induced sepsis through inhibiting pyroptosis via targeting TIR
domain of MyD88 and binding CARD of ASC in macrophages. Biochem
Pharmacol. 1158062023.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Dai S, Ye B, Zhong L, Chen Y, Hong G, Zhao
G, Su L and Lu Z: GSDMD mediates LPS-induced septic myocardial
dysfunction by regulating ROS-dependent NLRP3 inflammasome
activation. Front Cell Dev Biol. 9:7794322021. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Meng L, Gu T, Wang J, Zhang H and Nan C:
Knockdown of PHLDA1 alleviates sepsis-induced acute lung injury by
downregulating NLRP3 inflammasome activation. Allergol Immunopathol
(Madr). 51:41–47. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Li W, Shen X, Feng S, Liu Y, Zhao H, Zhou
G, Sang M, Sun X, Jiao R and Liu F: BRD4 inhibition by JQ1 protects
against LPS-induced cardiac dysfunction by inhibiting activation of
NLRP3 inflammasomes. Mol Biol Rep. 49:8197–8207. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Zhao M, Zheng Z, Zhang P, Xu Y, Zhang J,
Peng S, Liu J, Pan W, Yin Z, Xu S, et al: IL-30 protects against
sepsis-induced myocardial dysfunction by inhibiting
pro-inflammatory macrophage polarization and pyroptosis. iScience.
26:1075442023. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Nong Y, Wei X and Yu D: Inflammatory
mechanisms and intervention strategies for sepsis-induced
myocardial dysfunction. Immun Inflamm Dis. 11:e8602023. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Lima MR and Silva D: Septic
cardiomyopathy: A narrative review. Rev Port Cardiol. 42:471–481.
2023.(In English, Portuguese). View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Nadamuni M, Venable AH and Huen SC: When a
calorie isn't just a calorie: A revised look at nutrition in
critically ill patients with sepsis and acute kidney injury. Curr
Opin Nephrol Hypertens. 31:358–366. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Costa NA, Pereira AG, Sugizaki CSA, Vieira
NM, Garcia LR, de Paiva SAR, Zornoff LAM, Azevedo PS, Polegato BF
and Minicucci MF: Insights into thiamine supplementation in
patients with septic shock. Front Med (Lausanne). 8:8051992022.
View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Huo L, Liu C, Yuan Y, Liu X and Cao Q:
Pharmacological inhibition of ferroptosis as a therapeutic target
for sepsis-associated organ damage. Eur J Med Chem. 257:1154382023.
View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Zhou P, Zhang S, Wang M and Zhou J: The
induction mechanism of ferroptosis, necroptosis, and pyroptosis in
inflammatory bowel disease, colorectal cancer, and intestinal
injury. Biomolecules. 13:8202023. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Wu J, Lan Y, Wu J and Zhu K:
Sepsis-induced acute lung injury is alleviated by small molecules
from dietary plants via pyroptosis modulation. J Agric Food Chem.
71:12153–12166. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Perveen I, Bukhari B, Najeeb M, Nazir S,
Faridi TA, Farooq M, Ahmad QU, Abusalah MAHA, ALjaraedah TY, Alraei
WY, et al: Hydrogen therapy and its future prospects for
ameliorating COVID-19: Clinical applications, efficacy, and
modality. Biomedicines. 11:18922023. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Expert Panel on Urological Imaging, .
Smith AD, Nikolaidis P, Khatri G, Chong ST, De Leon AD, Ganeshan D,
Gore JL, Gupta RT, Kwun R, et al: ACR appropriateness
criteria® acute pyelonephritis: 2022 Update. J Am Coll
Radiol. 19((11S)): S224–S239. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Diaconescu B, Uranues S, Fingerhut A,
Vartic M, Zago M, Kurihara H, Latifi R, Popa D, Leppäniemi A,
Tilsed J, et al: The bucharest ESTES consensus statement on
peritonitis. Eur J Trauma Emerg Surg. 46:1005–1023. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Evans L, Rhodes A, Alhazzani W, Antonelli
M, Coopersmith CM, French C, Machado FR, Mcintyre L, Ostermann M,
Prescott HC, et al: Surviving sepsis campaign: International
guidelines for management of sepsis and septic shock 2021.
Intensive Care Med. 47:1181–1247. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Annane D, Pastores SM, Rochwerg B, Arlt W,
Balk RA, Beishuizen A, Briegel J, Carcillo J, Christ-Crain M,
Cooper MS, et al: Guidelines for the diagnosis and management of
critical illness-related corticosteroid insufficiency (CIRCI) in
critically ill patients (Part I): Society of critical care medicine
(SCCM) and European society of intensive care medicine (ESICM)
2017. Intensive Care Med. 43:1751–1763. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Marik PE, Pastores SM, Annane D, Meduri
GU, Sprung CL, Arlt W, Keh D, Briegel J, Beishuizen A, Dimopoulou
I, et al: Recommendations for the diagnosis and management of
corticosteroid insufficiency in critically ill adult patients:
Consensus statements from an international task force by the
American college of critical care medicine. Crit Care Med.
36:1937–1949. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Zhong G, Han Y, Zhu Q, Xu M, Chang X, Chen
M, Men L, Zhang Q and Wang L: The effects of Xuebijing injection
combined with ulinastatin as adjunctive therapy on sepsis: An
overview of systematic review and meta-analysis. Medicine
(Baltimore). 101:e311962022. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Xiaoxia Q, Cheng C, Minjian W, Huilin C,
Zhen L, Yuedong Y and Xingyu Z: Effect of integrative medicines on
28-day mortality from sepsis: A systematic review and network
meta-analysis. Eur Rev Med Pharmacol Sci. 26:664–677.
2022.PubMed/NCBI
|