Introduction
Periodontitis is a chronic inflammatory disease
occurring in periodontal supporting tissue, mainly manifested as
the destruction of local soft tissue and the absorption of bone
tissue. It is a common disease with high incidence that seriously
endangers human health and is a major public health problem
affecting more than half of adults in the world (1). Severe periodontitis can cause
excessive absorption of alveolar bone and tooth loss, which
seriously affects daily activities such as chewing, swallowing and
speaking, as well as physical health (2). A large number of relevant studies
have shown that periodontitis not only seriously affects oral
health, but also is a risk factor for the occurrence and
development of numerous systemic diseases such as diabetes and
these systemic diseases also increase the risk rate of
periodontitis and aggravated periodontal tissue destruction.
Diabetes mellitus is a group of clinical syndromes characterized by
hyperglycemia and caused by genetic and environmental factors. With
the change of life style and the acceleration of aging process, the
prevalence of diabetes in China is also showing a rapidly rising
trend. Periodontitis is the sixth most common complication of
diabetes and diabetic periodontitis (DP) is particularly severe in
numerous cases (3,4). Local periodontal inflammation leads
to poor blood glucose control and aggravates the condition of
diabetes (3,5). Periodontitis is mainly caused by a
specific periodontopathic bacterium, Porphyromonas
gingivalis (6).
Lipopolysaccharide (LPS) is an important component of the outer
membrane of gram-negative bacteria and can induce inflammation
(7). In vivo, LPS enhances
the activity of osteoclasts and induces the differentiation of
osteoclasts, which is associated with the occurrence of
periodontitis (8). Based on the
above, anti-inflammatory and inhibition of osteoclast
differentiation are important research directions in the treatment
of DP.
TIFA [tumor necrosis factor receptor-associated
factor (TRAF)-interacting protein with forkhead-associated domain]
is a protein that contains a forkhead-associated (FHA) domain and a
TRAF6 binding motif. It is well known that the FHA can directly
bind to phosphothreonine and phosphoserine (9). Some studies have demonstrated that
TIFA as a key regulator of inflammation affects tumor progression
(10,11) and various fungal inflammatory
lesions (12,13). TIFA expression is suppressed in
hepatocellular carcinoma. In addition, it promotes cell apoptosis
and suppresses cell proliferation via p53-dependent and
-independent mechanisms (10). In
vascular endothelial cells, TIFA is a regulator of Nod-like
receptor family pyrin domain-containing protein 3 (NLRP3)
inflammasome initiation and activation signals (14). However, the role of TIFA in DP
remains to be elucidated.
NF-κB as a protein complex is involved in the
regulation of gene transcription. P65 (also known as RelA) is one
of the transcription factors that constitutes the NF-κB complex
(15,16). Normally, NF-κB complex remains in
an inactive state in the cytoplasm. After the stimulation by
inflammatory cytokines, the NF-κB is activated and translocated
into nucleus (17). Previous
reports have shown that the NF-κB signaling pathway mediated the
treatment of periodontitis and participated in the suppression of
osteoclast differentiation (18,19).
In addition, TIFA as a key factor activates the NF-κB signaling
pathway in Helicobacter pylori (12). During Shigella flexneri
infection, the oligomerization of TIFA is a crucial process of the
subsequent oligomerization of TRAF6 and the activation of NF-κB
pathway (13). Therefore, the
present study aimed to investigate whether TIFA plays a role in DP
through the NF-κB pathway.
The present study investigated the role of TIFA in
the periodontal tissues of a DP mouse model and RAW264.7 cells.
TIFA was highly expressed in periodontal tissues of the DP model.
Downregulation of TIFA suppressed inflammatory reaction induced by
high glucose (HG) and lipopolysaccharide (LPS) from
Porphvromonas ginaivalis (LPS-PG) via the NF-κB signaling
pathway. Additionally, osteoclast differentiation induced by (RANK)
ligand (RANKL) was inhibited by knockdown of TIFA. Hence, the
present study might provide a potential molecular target for the
treatment of DP.
Materials and methods
Data acquisition
The data of GSE156993 downloaded from the Gene
Expression Omnibus (GEO) database were obtained from expression
profiling by array of peripheral blood mononuclear cells of
patients with DP. There were six healthy subjects, including two
males and four females (average age: 42±3.2 years, average fasting
blood glucose: 87±8.8 mg/dl) and five patients with DP, including
one male and four females (average age: 48±9.7 years, average
fasting blood glucose: 274±48.4 mg/dl).
Establishment of diabetic
periodontitis (DP) mouse model
According to previous studies (20,21),
12 male C57BL/6 mice (20–22 g) aged 6–7 weeks were used to
establish the DP model. The mice were purchased from Liaoning
Changsheng Biological Technology Co., Ltd. They were randomly
divided into two groups, including the control group and DP group
(n=6 in each group). The mice were housed at a temperature of
22±1°C, a humidity of 45–55%, and 12 h light/dark cycle. Food and
water were available ad libitum. After mice were adaptively
fed for one week, 55 mg/kg streptozotocin (STZ; cat. no. S110910;
Shanghai Aladdin Biochemical Technology Co., Ltd.) was used to
induce diabetes through intraperitoneal injection once a day for 4
consecutive days. Control mice were injected with the equal volume
of normal saline. Blood glucose was detected on 3 days after the
last injection of STZ and the mice whose random blood glucose
concentration reached 300 mg/dl were considered as diabetic models.
After 4 weeks, 1 µl (20 µg/µl) LPS-PG was injected into bases of
the four proximal palatal gingival papillae between left and right
maxillary molars, twice a week for 2 consecutive weeks. Control
group was injected with equal volume of PBS. The mice were
sacrificed with carbon dioxide (CO2) before tissue
collection. The experimental animals were placed in a euthanasia
chamber and CO2 was perfused into the chamber at a
volume replacement rate of 40% per minute. After confirming that
the animal maintained motionlessness, no breathing and had dilated
pupils, CO2 perfusion was stopped. The animals were
observed for an additional 3 min to confirm their demise.
Subsequently, gingiva and periodontal tissue were collected for
follow-up examinations. All animal experiments were conducted in
accordance with the Guide for the Care and Use of Laboratory
Animals (22) and the procedures
were approved by the Medical Research Ethics Review Committee of
General Hospital of Ningxia Medical University (approval no.
KYLL-2023-0399).
Hematoxylin-eosin (HE) staining
Briefly, the fixed tissues were rinsed with running
water for 4 h and then placed in a gradient series of alcohol from
low to high concentrations. The tissues were placed in xylene for
30 min. Subsequently, the tissue blocks were placed in the mixture
of xylene and paraffin for 2 h at 60°C. The treated tissues were
placed in dissolved paraffin, and then cut into 5 µm slices after
freezing. Tissue sections expanded in warm water were transferred
to slides and dried at 60°C for 2 h. Then, sections were
respectively immersed in xylene and anhydrous ethanol for dewaxing.
After 5 min of soaking in hematoxylin (cat. no. H8070; Beijing
Solarbio Science & Technology Co., Ltd.) and 3 min of soaking
in eosin (cat. no. A600190; Sangon, Shanghai, China) at room
temperature, sections were dehydrated and sealed. The images were
captured using a BX53 light microscope (Olympus Corporation).
Reverse transcription-quantitative
(RT-q) PCR
According to the manufacturer's protocols, total RNA
of samples was isolated by TRIpure (BioTeke Corporation) and
concentration was examined by ultraviolet spectrophotometer NANO
2000 (Thermo Fisher Scientific, Inc.). The cDNA was synthesized
from total RNA using the BeyoRT II M-MLV reverse transcriptase
(RNase H-; cat. no. D7160L; Beyotime Institute of Biotechnology)
according to the protocol from the manufacturer. RT-qPCR was
performed using SYBR Green I (cat. no. SY1020; Beijing Solarbio
Science & Technology Co., Ltd.) and 2X Taq PCR MasterMix (cat.
no. PC1150; Beijing Solarbio Science & Technology Co., Ltd.)
according to the manufacturer's instructions. The forward (F) and
reverse (R) primer sequences were as follow: TIFA-F,
5′-GTTCAACAGCTCCGTTCT-3′, TIFA-R, 5-GTAAGGCAGGTCCATTTT-3′,
β-actin-F, 5′-CTGTGCCCATCTACGAGGGCTAT-3′, β-actin-R,
5′-TTTGATGTCACGCACGATTTCC-3′. The optimal PCR amplification
procedure was as follows: Pre-denaturation at 94°C for 5 min, 40
cycles at 94°C for 10 sec, 60°C for 20 sec and extension at 72°C
for 30 sec, followed by incubation at 72°C for 2 min 30 sec, 40°C
for 1 min 30 sec, melting at 60°C to 94°C, every 1°C for 1 sec and
a final incubation at 25°C for 1–2 min. Relative mRNA expression
was calculated based on β-actin with 2−ΔΔCq method
(23).
Western blotting
Total protein was extracted by cell lysis buffer for
western and immunoprecipitation (cat. no. P0013; Beyotime Institute
of Biotechnology). Nuclear and cytoplasmic protein extraction kit
(cat. no. P0027, Beyotime Institute of Biotechnology) was used to
separate and extract protein of nucleus and cytoplasm. The
concentration of extracted protein was determined using the BCA
protein assay kit (cat. no. P0011; Beyotime Institute of
Biotechnology). Different masses of proteins were separated by
sodium dodecyl sulfate polyacrylamide gel electrophoresis
(SDS-PAGE). Then, 40 µg of NF-κB p65 protein or 20 µg of other
proteins was separately loaded per lane. A 5% polyacrylamide gel
was prepared to concentrate the proteins, and the 8, 10, 12 and 15%
gels were prepared for separating different proteins. Then,
proteins were transferred to PVDF membrane (MilliporeSigma). After
blocking with 5% skimmed milk for 1 h at room temperature,
membranes with proteins were incubated with primary antibodies at
4°C overnight. Next, membranes were washed by TBST (1.5‰ Tween 20)
and probed with secondary antibodies at 37°C for 45 min. Finally,
ECL chemiluminescence reagent (cat. no. P0018; Beyotime Institute
of Biotechnology) interacted with membranes, which were visualized
and captured by gel imaging system (cat. no. WD-9413B; Beijing
Liuyi Biotechnology Co., Ltd.). The densitometry of blots was
analyzed using Gel-Pro Analyzer 4.0 (Media Cybernetics, Inc.). The
details of antibodies are shown in Table I.
 | Table I.Antibodies employed. |
Table I.
Antibodies employed.
| Type | Name | Dilution | Catalogue
number | Supplier |
|---|
| Primary
antibody | TIFA | 1:1,000 | K108843P | Beijing Solarbio
Science & Technology Co., Ltd. |
|
| NF-κB p65 | 1:1,000 | AF1234 | Beyotime Institute
of Biotechnology |
|
| NLRP3 | 1:1,000 | DF7438 | Affinity
Biosciences, Ltd. |
|
| ASC | 1:1,000 | AF6234 | Beyotime Institute
of Biotechnology |
|
| Cathepsin K | 1:1,000 | AF6597 | Beyotime Institute
of Biotechnology |
|
| MMP9 | 1:1,000 | AF5228 | Affinity
Biosciences, Ltd. |
|
| NFATc1 | 1:1,000 | DF6446 | Affinity
Biosciences, Ltd. |
|
| Histone H3 | 1:5,000 | AF0009 | Beyotime Institute
of Biotechnology |
|
| β-actin | 1:5,000 | AF5001 | Beyotime Institute
of Biotechnology |
| Secondary
antibody | Goat anti-rabbit
IgG | 1:5,000 | A0208 | Beyotime Institute
of Biotechnology |
|
| Goat anti-mouse
IgG | 1:5,000 | A0216 | Beyotime Institute
of Biotechnology |
Cell culture and treatments
i) RAW264.7 cells were cultured in normal glucose
(NG) DMEM (Procell Life Science & Technology Co., Ltd.) and HG
DMEM (Wuhan Servicebio Technology Co., Ltd.) respectively.
Meanwhile, 1 ng/ml LPS-PG (cat. no. tlrl-pglps; Invitrogen; Thermo
Fisher Scientific, Inc.) was added in the cells cultured with HG
DMEM for 12, 24, 48, 72 h, following the determination of TIFA mRNA
and protein expression.
ii) RAW264.7 cells were infected with
1×108 TU/ml lentivirus-carried short hairpin RNA (shRNA)
targeting TIFA (shTIFA) or negative control (shNC) and cultured in
an incubator with 5% CO2 at 37°C for 48 h. Subsequently,
the mRNA and protein levels of TIFA was examined.
iii) RAW264.7 cells were infected by shTIFA and shNC
for 48 h, following the culture with HG medium and 1 ng/ml LPS-PG
for 24 h. Subsequently, cells were collected for the follow-up
experiments, including ELISA, western blotting and
immunofluorescence (IF).
iv) To induce osteoclast differentiation, RAW264.7
cells were treated with different concentrations (0, 25, 50, 75,
100 ng/ml) of RANKL (cat. no. R0525; MilliporeSigma) and then
cultured in an incubator with 5% CO2 at 37°C for 5 days.
Then, RT-qPCR and western blotting were performed to evaluate TIFA
expression levels and the tartrate-resistant acid phosphatase
(TRAP) staining was used for detecting osteoclast differentiation
of cells.
v) After the infection of shTIFA and shNC, RAW264.7
cells were treated with 100 ng/ml RANKL and cultured in the
incubator at 37°C for 5 days to induce osteoclast differentiation.
Since osteoclast differentiation was significantly induced at 100
ng/ml RANKL, this concentration was chosen for the following
experiments, including RT-qPCR, western blotting, TRAP staining and
IF, to investigate the effect of TIFA on osteoclast
differentiation.
Enzyme-linked immunosorbent assay
(ELISA)
The cell supernatant was collected to detect
different inflammatory factors. The content of TNF-α, IL-6, IL-1β
and methyl-accepting chemotaxis protein 1 (MCP-1) was examined by
ELISA kits (Multisciences (Lianke) Biotech Co., Ltd.).
TRAP
When RAW264.7 cells were treated with different
concentrations of RANKL for 5 days, or when cells infected by
lentivirus were treated by 100 ng/ml RANKL for 5 days, TRAP
solution in TRAP stain kit (cat. no. G1492; Beijing Solarbio
Science & Technology Co., Ltd.) was added into cells to fix
then at 4°C for 1 min. After washing with PBS, cells were incubated
with TRAP incubated buffer at 37°C for 60 min in the dark. RAW264.7
cells were stained by hematoxylin for 5 min at room temperature
followed by image capture.
IF
Cells induced by 100 ng/ml RANKL for 5 days were
fixed with 4% paraformaldehyde for 15 min at room temperature. Then
they were incubated with 0.1% TritonX-100 (cat. no. ST795; Beyotime
Institute of Biotechnology) at room temperature for 30 min. Then,
1% BSA (cat. no. A602440-0050; Sangon Biotech Co., Ltd.) was added
in cells at room temperature for 15 min. Next, cells were incubated
with NF-κB p65 antibody (1:200; cat. no. 8242; Cell Signaling
Technology, Inc.) at 4°C overnight. The cells were then incubated
with Cy3 labeled Goat anti-rabbit IgG (1:200; cat. no. A27039;
Invitrogen; Thermo Fisher Scientific, Inc.) at room temperature for
1 h and stained with DAPI (cat. no. D106471-5 mg; Shanghai Aladdin
Biochemical Technology Co., Ltd.) in the dark. Images of p65
nuclear translocation were captured by an immunofluorescence
microscope (Olympus Corporation).
Statistical analysis
Data shown are represented as the mean ± standard
deviation. Statistical analysis was performed using GraphPad Prism
8.0 (GraphPad Software; Dotmatics). The data from cell experiments
are collected from three independent experiments consisting of
three replicates per experiment. Statistical significances between
two groups were analyzed by unpaired Student's t-test.
Significances among three or more groups were tested by one-way
analysis of variance (ANOVA), followed by Tukey's multiple
comparisons test. P<0.05 was considered to indicate a
statistically significant difference.
Results
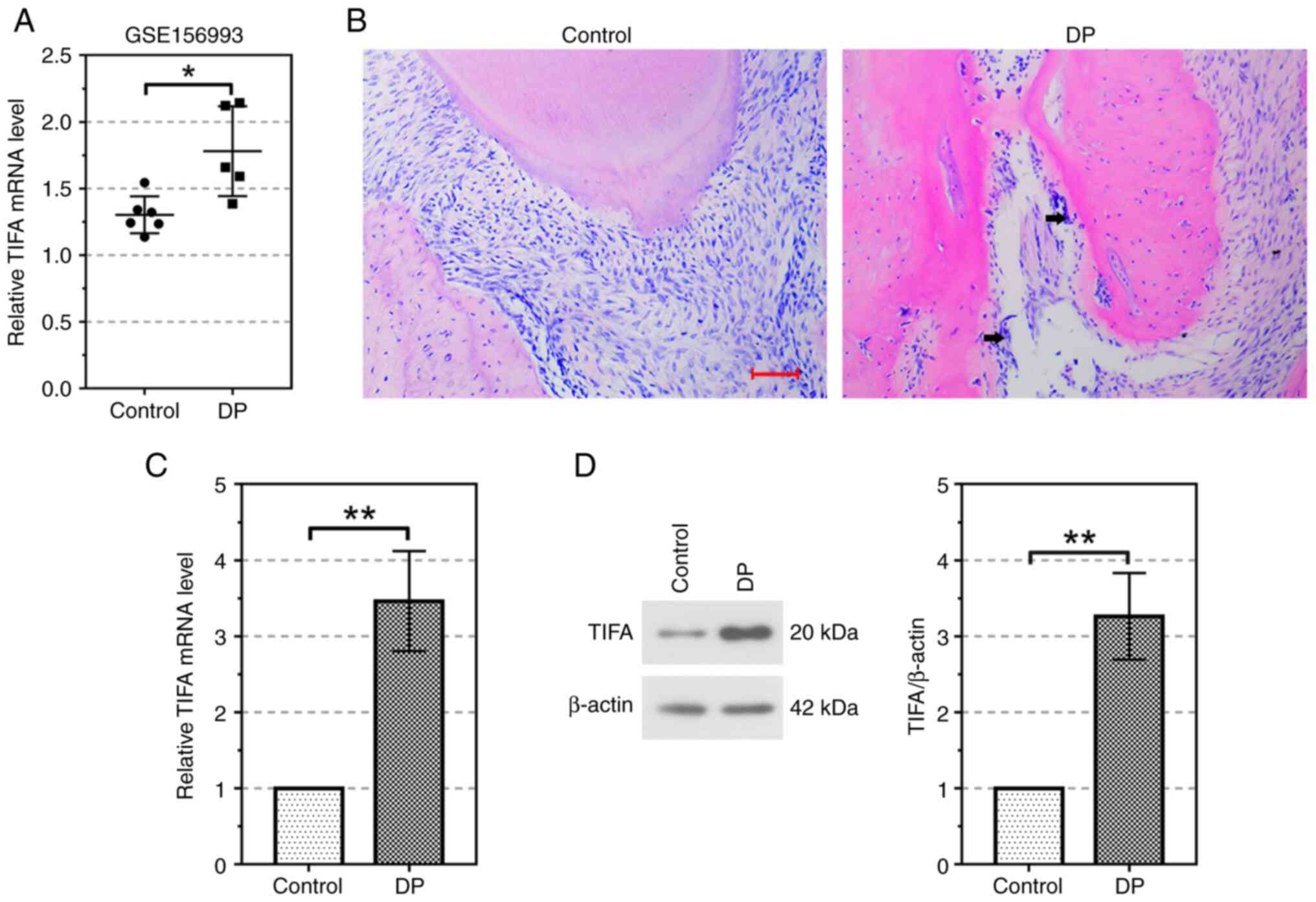
TIFA is highly expressed in the
periodontium of the DP mouse model
To determine whether TIFA is involved in the
regulation of the periodontitis in diabetic patients, the relative
mRNA expression of TIFA was analyzed using the data from GEO
dataset GSE156993. Compared with the healthy controls, relative
mRNA expression of TIFA was significantly increased in the DP group
(P<0.05; Fig. 1A). To further
explore the effect of TIFA on DP, we established the DP mouse model
using STZ and LPS. As shown in Fig.
1B, inflammatory infiltration marked by black arrows and bone
resorption suggested that the DP mouse model was successfully
established. In addition, the mRNA level of TIFA was significantly
increased in the periodontium of DP mouse model (P<0.01;
Fig. 1C). Meanwhile, the TIFA
protein expression was also induced by periodontitis. (P<0.01;
Fig. 1D).
Downregulation of TIFA attenuates
inflammatory response induced by HG and LPS in RAW264.7 cells
To examine whether the expression of TIFA was
affected by DP conditions in vitro, RAW264.7 cells were
stimulated with HG and LPS for different time points and the
expression of TIFA was detected. As depicted in Fig. 2A, the relative mRNA and protein
levels of TIFA were upregulated in RAW264.7 cells after the
treatment of HG and LPS in a time-depend manner. TIFA mRNA and
protein expressions were significantly knocked down by shTIFA
(P<0.01; Fig. 2B).
Additionally, compared with NG treatment, the levels of crucial
inflammatory cytokines (TNF-α, IL-6, IL-1β, MCP-1) were
significantly upregulated in the RAW264.7 cells treated by HG and
LPS (P<0.01). Meanwhile, knockdown of TIFA markedly decreased
the contents of TNF-α, IL-6, IL-1β, MCP-1 in HG and LPS-induced
RAW264.7 cells (P<0.01; Fig.
2C). These results demonstrated that downregulation of TIFA
alleviated HG and LPS-induced inflammatory response in
vitro.
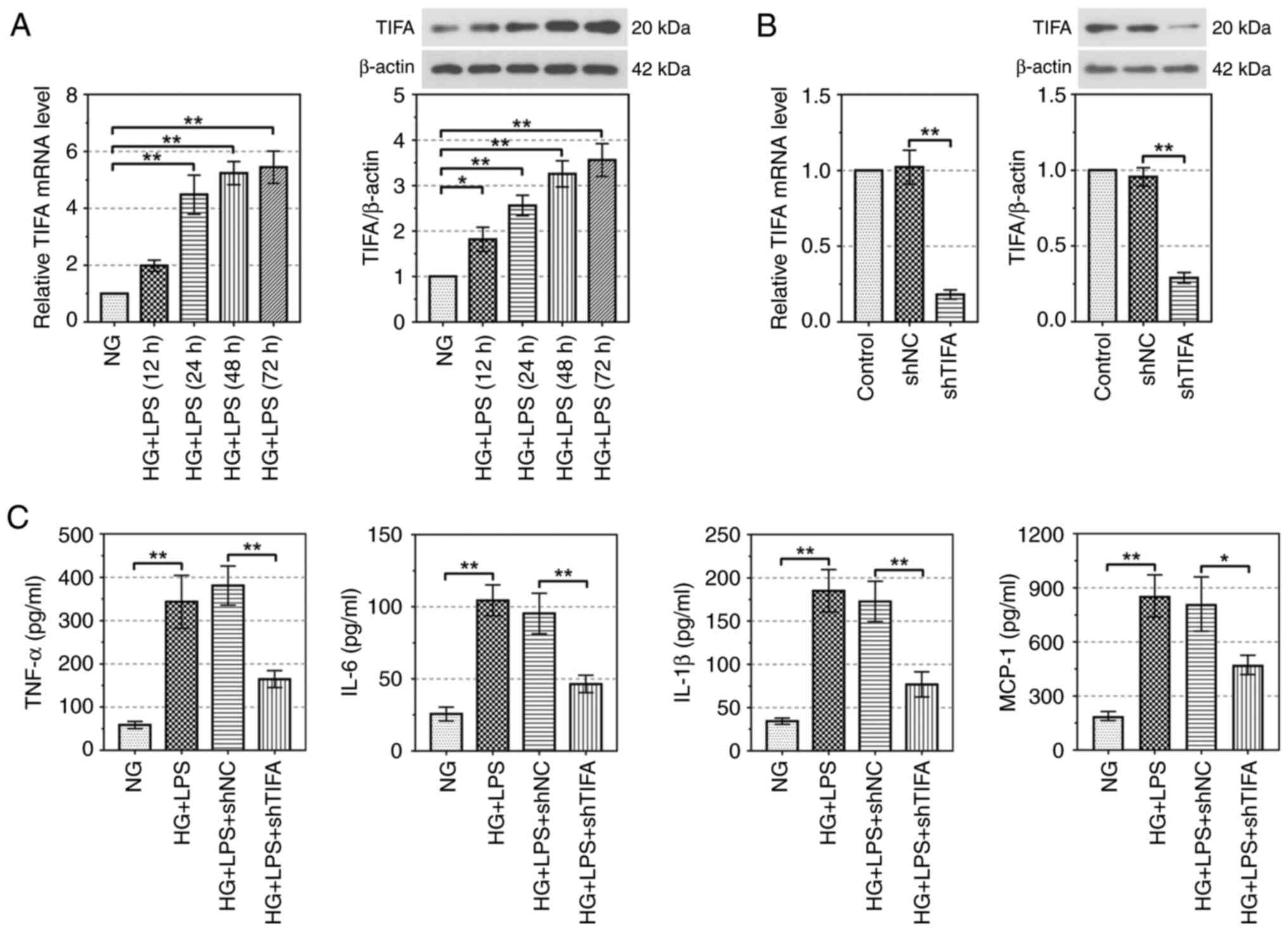 | Figure 2.TIFA promotes the release of
inflammatory factors. (A) The expression of TIFA in the RAW264.7
cells treated by HG and LPS-PG was evaluated by RT-qPCR and western
blotting. (B) The inhibiting efficiency of shTIFA in RAW264.7 cells
was detected by RT-qPCR and western blotting. (C) The concentration
of TNF-α, IL-6, IL-1β and MCP-1 was examined by ELISA. Data are
represented as the mean ± standard deviation, *P<0.05,
**P<0.01. TIFA, tumor necrosis factor receptor-associated
factor-interacting protein with forkhead-associated domain; HG,
high glucose; LPS-PG, lipopolysaccharide (LPS) from
Porphvromonas ginaivalis; sh, short hairpin; RT-qPCR,
reverse transcription-quantitative PCR; MCP-1, methyl-accepting
chemotaxis protein 1; NG, normal glucose. |
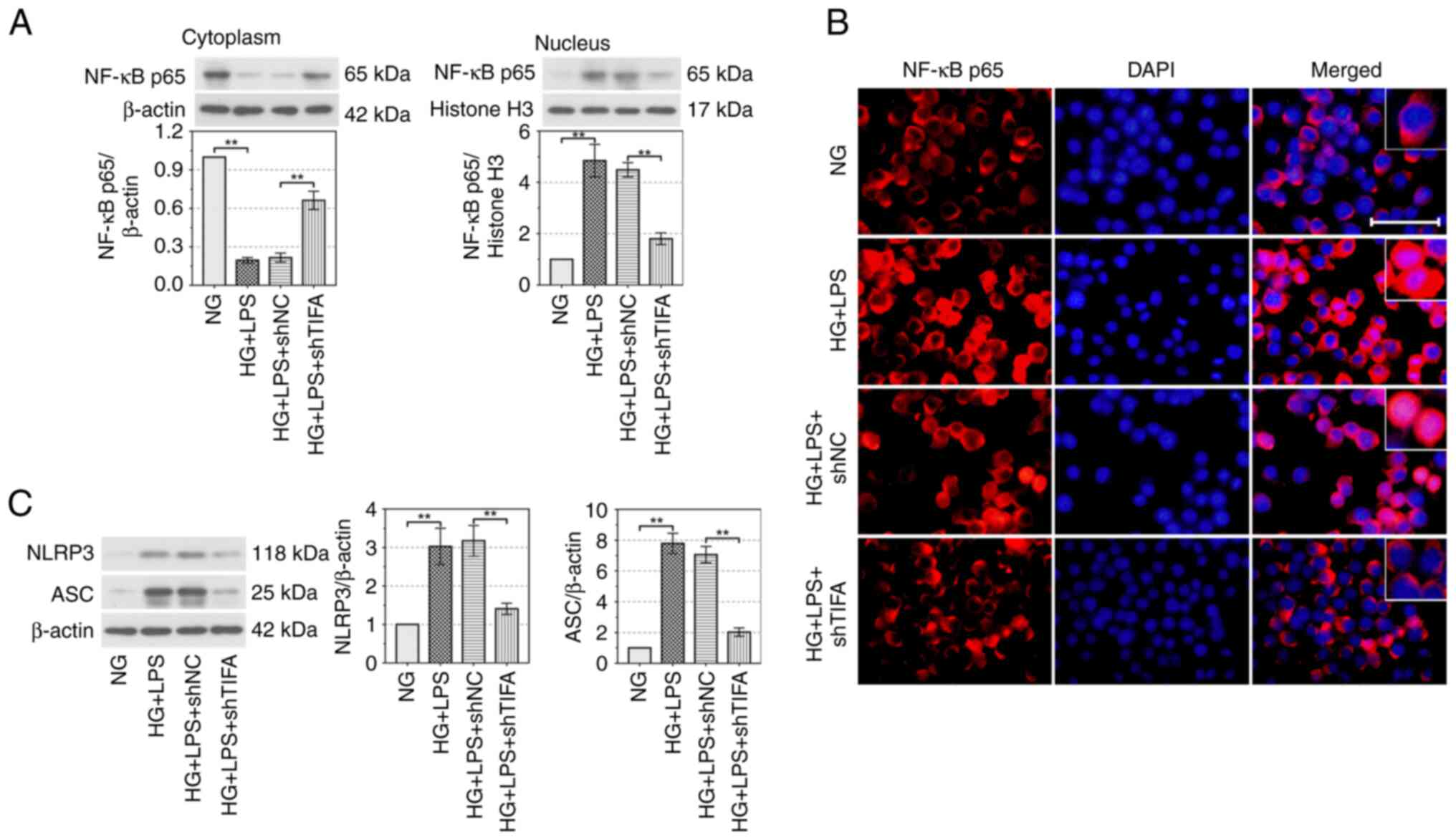
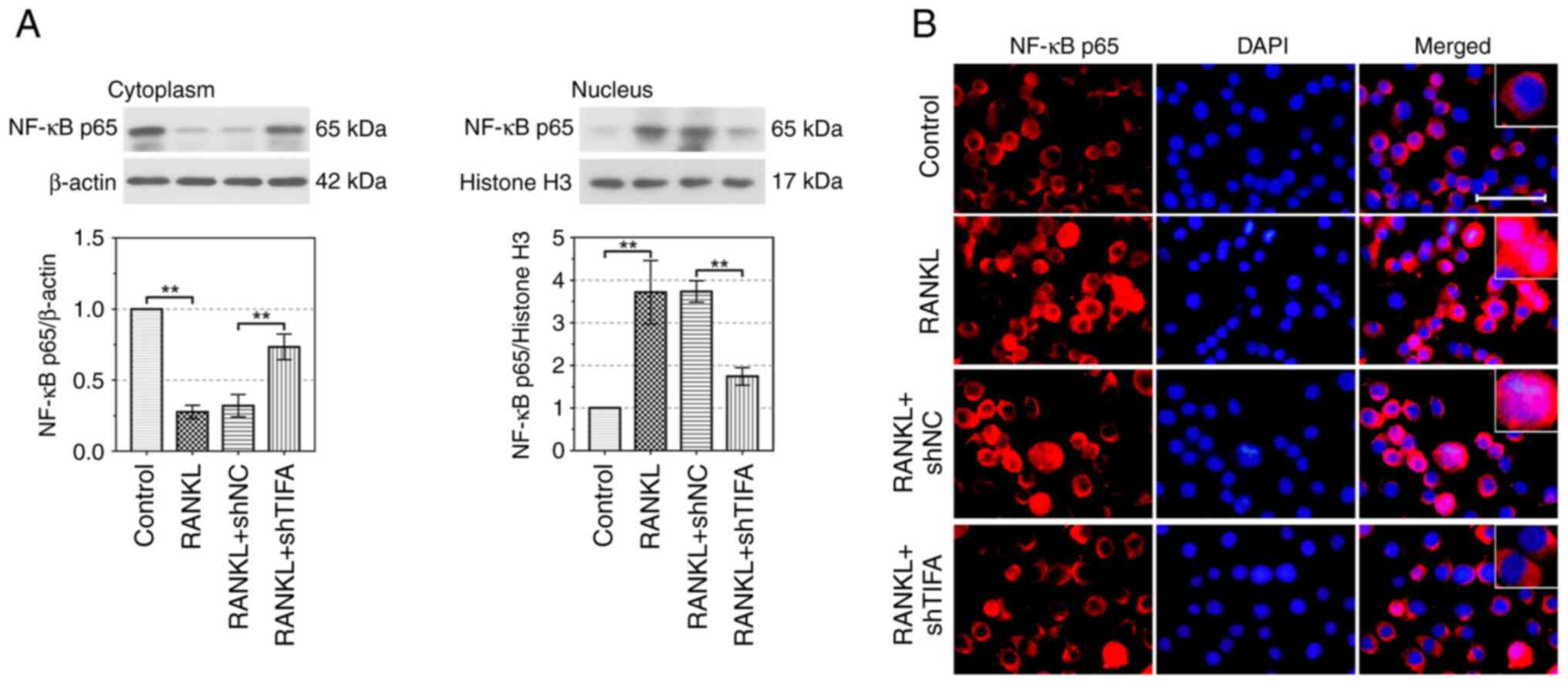
TIFA promotes cell inflammation
through activating the NF-κB signaling pathway
A previous study reported that TIFA is a crucial
upstream factor activating the NF-κB signaling pathway (24). The protein expression of NF-κB p65
was reduced in the cytoplasm (P<0.01) and was increased in the
nucleus (P<0.01) by the treatment of HG and LPS and these
changes were clearly reversed by the downregulation of TIFA
(P<0.01; Fig. 3A). The results
of IF revealed that p65 located in nucleus was enhanced by HG and
LPS treatment. Absence of TIFA blocked the HG and LPS-induced
activation of NF-κB signaling pathway. (Fig. 3B). The protein expression levels of
NLRP3 and apoptosis-associated speck-like protein (ASC) were
upregulated by HG and LPS in RAW264.7 cells (P<0.01) and was
clearly suppressed by knockdown of TIFA (P<0.01; Fig. 3C). In summary, these findings
indicated that TIFA activated the NF-κB signaling pathway and
upregulated the expression of NLRP3 and ASC in DP conditions in
vitro.
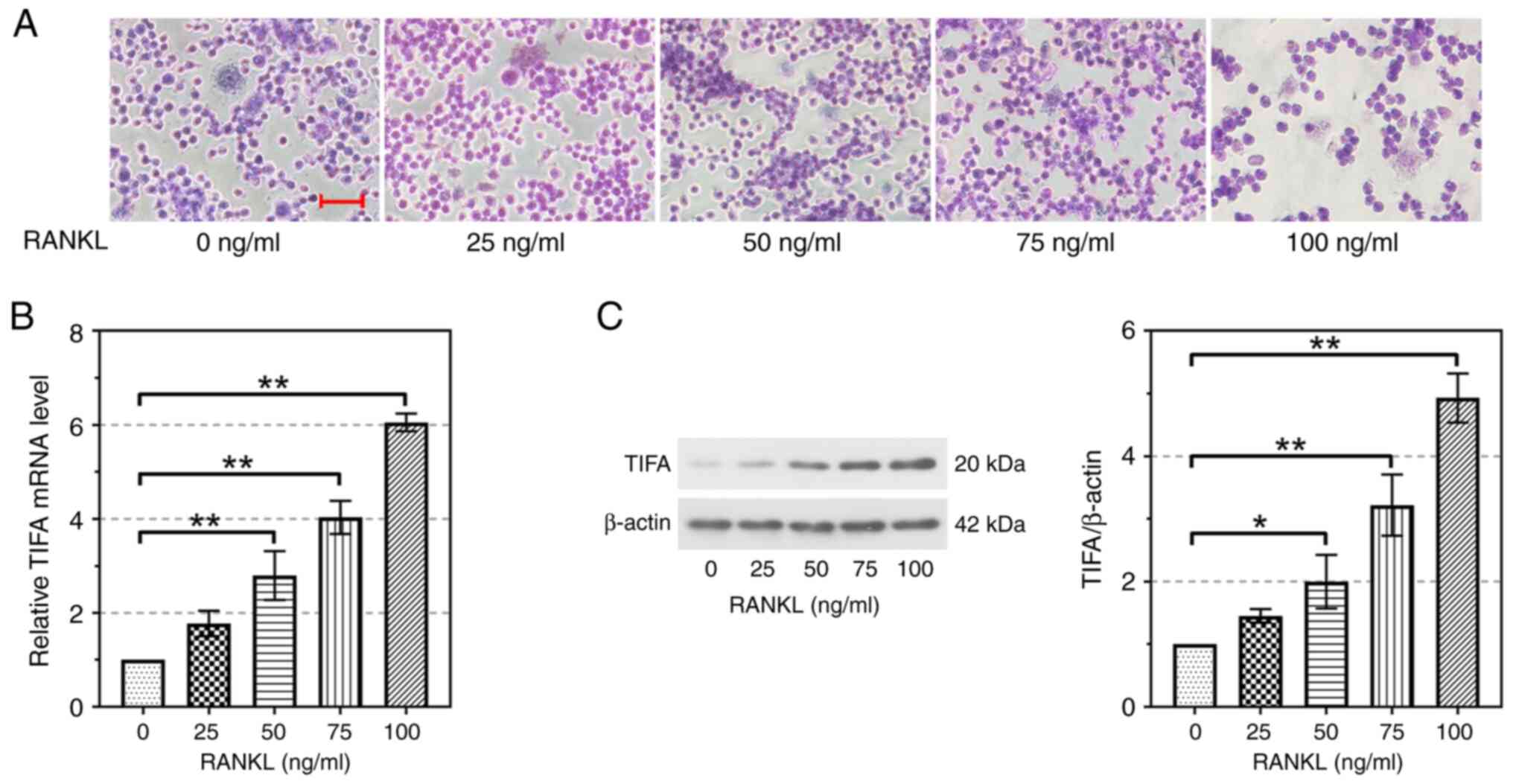
TIFA expression is upregulated in
RAW264.7 cells induced by different concentrations of RANKL
Different concentrations of RANKL were used to treat
osteoclastic cell line RAW264.7 to induce osteoclast
differentiation. Results of TRAP staining suggested that osteoclast
differentiation was clearly induced by 100 ng/ml of RANKL (Fig. 4A). Therefore, 100 ng/ml of RANKL
was determined to induce osteoclast differentiation. As shown in
Fig. 4B, the relative mRNA level
of TIFA upregulated gradually with the increase of RANKL
concentration. When RANKL induced RAW264.7 cells at 50, 75 and 100
ng/ml, TIFA expression was significantly increased compared with
the control group (P<0.01; Fig.
4B). The protein expression of TIFA was consistent with the
mRNA expression changes (Fig. 4C).
Collectively, the aforementioned findings confirmed that the
expression of TIFA was upregulated alongside the differentiation of
osteoclastic cells.
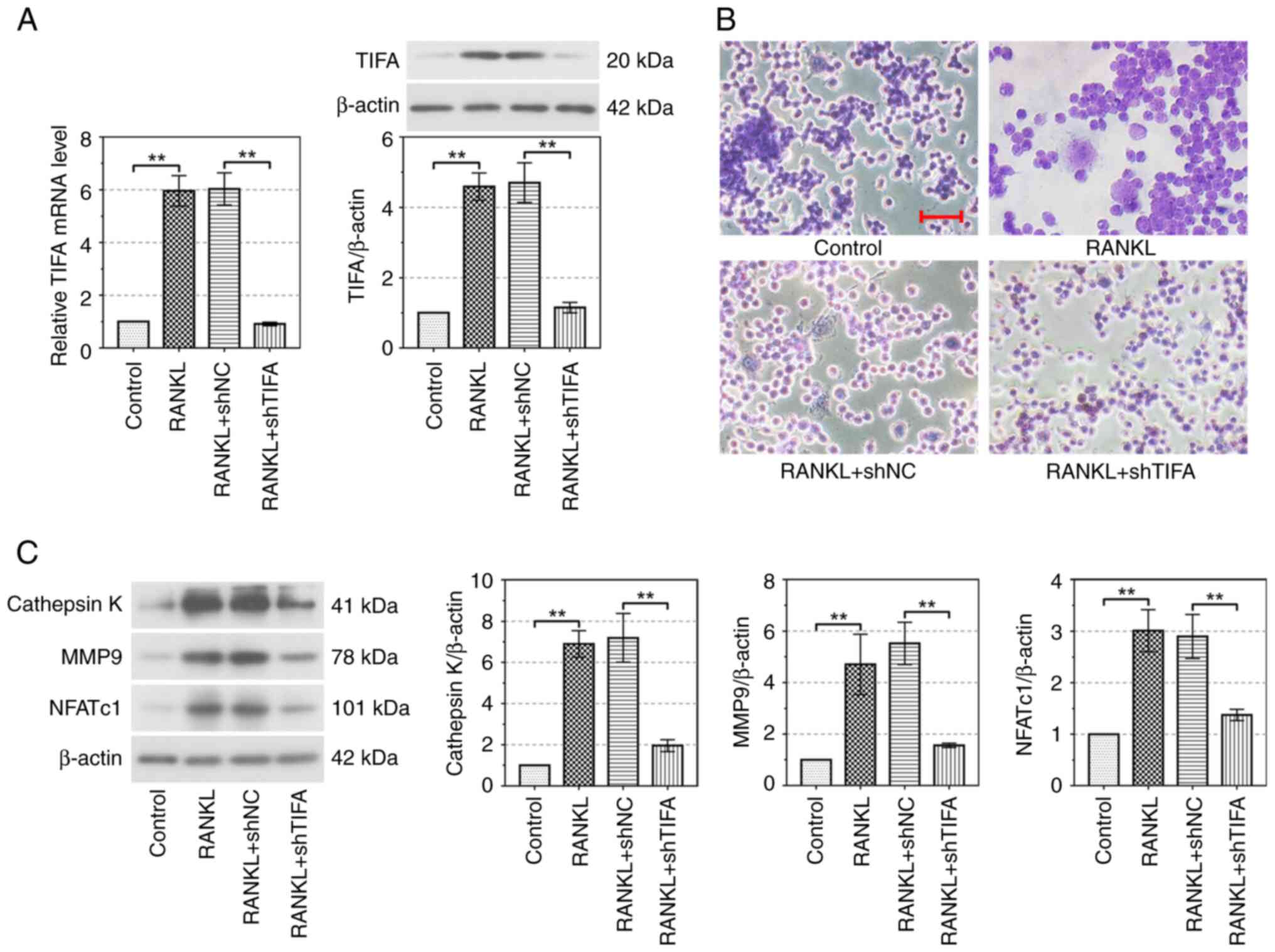
Knockdown of TIFA suppresses
RANKL-induced osteoclast differentiation
After the infection of shNC and shTIFA in RAW26.7
cells, the mRNA and protein expression levels of TIFA significantly
reversed the osteoclast differentiation induced by RANKL
(P<0.01; Fig. 5A). In contrast
to cells in the control group, the number of multinucleated
osteoclasts was upregulated in cells treated with RANKL. A decrease
of TIFA reduced the number of multinucleated osteoclasts,
indicating that TIFA promoted osteoclast differentiation (Fig. 5B). Cathepsin K, an enzyme that
breaks down other proteins, plays an important role in the
breakdown of bone matrix and is an important marker of bone
resorption (25). MMP9, which
belongs to the MMP family, is involved in extracellular matrix
degradation (26). These two
proteins are downstream of nuclear factor of activated T cells
cytoplasmic 1 (NFATc1) and regulated by the NF-κB signaling
pathway. The protein expressions of cathepsin K, MMP9 and NFATc1
were significantly increased by RANKL in RAW264.7 cells
(P<0.01). In addition, the silencing of TIFA markedly reduced
cathepsin K, MMP9 and NFATc1 protein expression in RAW264.7 cells
(P<0.01; Fig. 5C). Therefore,
silencing of TIFA promotes the RAW264.7 cells from osteoclast
differentiation induced by RANKL.
TIFA facilitates osteoclast
differentiation through activating the NF-κB signaling pathway
As shown in Fig.
6A, the protein expression of NF-κB p65 in cytoplasm was
significantly suppressed by RANKL, while it was increased following
the infection of shTIFA in RAW264.7 cells (P<0.01). In addition,
p65 expression in the nucleus was upregulated by RANKL and reversed
by knockdown of TIFA (P<0.01), suggesting that the activation of
NF-κB signaling pathway induced by RANKL was blocked by absence of
TIFA. Results of IF verified that the expression of p65 in nucleus
was clearly upregulated following RANKL treatment in RAW264.7 cells
and was significantly reduced by downregulation of TIFA. (Fig. 6B). In summary, these findings
verified that TIFA promoted the osteoclast differentiation by
activating the NF-κB signaling pathway.
Discussion
The present study determined the expression of TIFA
in the periodontal tissues in a DP model and explored the effect of
TIFA on inflammatory response and osteoclast differentiation in
RAW264.7 cells. The mRNA and protein expression levels of TIFA were
found to be upregulated in the periodontal tissues of the DP mouse
model, as well as in RAW264.7 cells stimulated by HG and LPS.
Meanwhile, the activation of NF-κB signaling pathway was induced by
HS and LPS, following the upregulation of NLRP3 and ASC expression.
The inflammatory cytokines (TNF-α, IL-6, IL-1β, MCP-1) were
promoted by HS and LPS in the RAW264.7 cells. In addition, the
expression of TIFA was increased when the osteoclast
differentiation of RAW264.7 cells was induced by RANKL. The NF-κB
signaling pathway was activated by RANKL in the RAW264.7 cells,
along with the upregulation of cathepsin K and MMP9 (Fig. 7). Therefore, TIFA was proved to
promote the cell inflammation and osteoclast differentiation of
RAW264.7 cells through activating the NF-κB signaling pathway.
Based on these findings, the construction of molecules targeting
TIFA partially is a novel and effective approach to meliorate the
symptoms of DP in clinic. However, there remains numerous trials
and more detailed and in-depth exploration before the findings of
the present study can be truly applied in clinical practice.
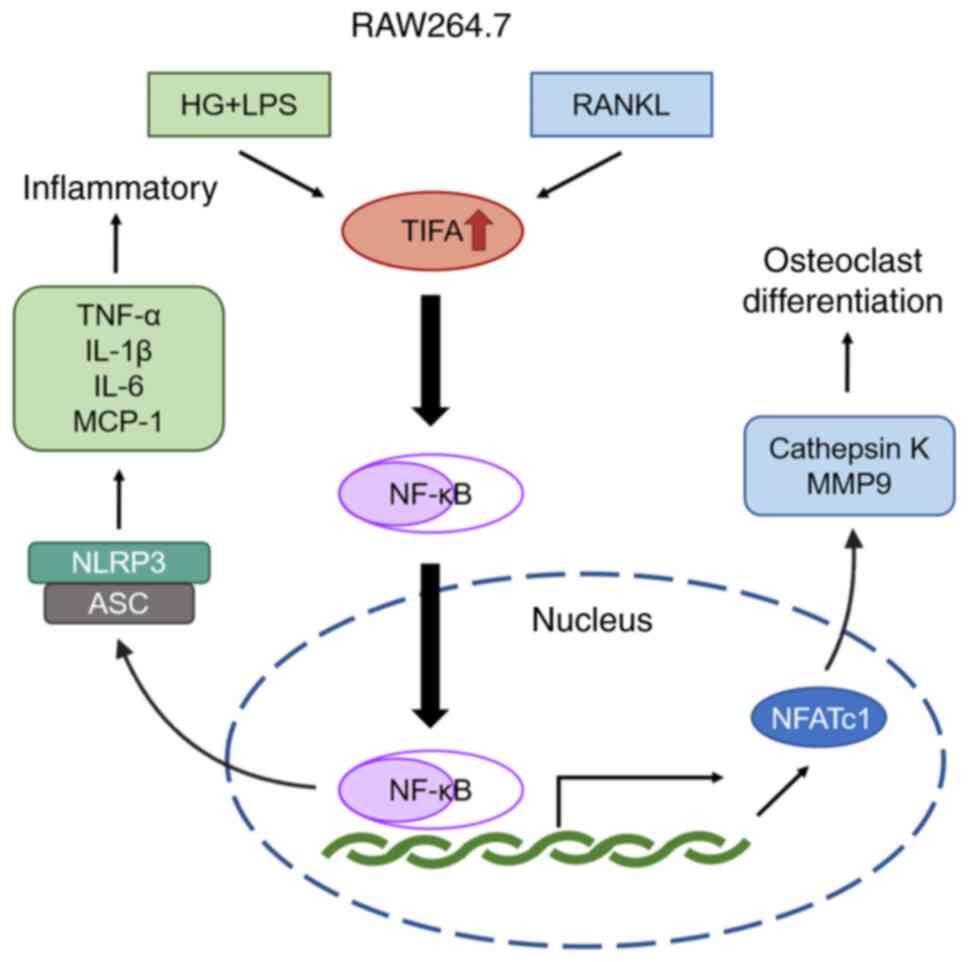 | Figure 7.Schematic diagram of TIFA regulating
the inflammatory and osteoclast differentiation in the RAW264.7
cells. The upregulation of TIFA, which is induced by the treatment
of HG and LPS or RANKL, promotes the cell inflammation and
osteoclast differentiation in the RAW264.7 cells through the
activation of the NF-κB signaling pathway. TIFA, tumor necrosis
factor receptor-associated factor-interacting protein with
forkhead-associated domain; HG, high glucose; LPS,
lipopolysaccharide from Porphvromonas ginaivalis; RANKL,
receptor activator of NF-κB ligand; MCP-1, methyl-accepting
chemotaxis protein 1; NLRP3, Nod-like receptor family pyrin
domain-containing protein 3; ASC, apoptosis-associated speck-like
protein; NFATc1, nuclear factor of activated T cells cytoplasmic 1;
HG, high glucose. |
Diabetes is a chronic disease with higher risk and
severity of periodontal disease than non-diabetic patients
(3,27). Periodontal disease begins with
inflammation of the gingiva and leads to the destruction of
periodontal support tissues (28).
Most bone diseases are caused by overactivity of osteoclasts,
resulting in a greater osteoclast resorption than osteoblast
construction (29). A previous
study demonstrated that controlling the inflammatory response is a
useful treatment for periodontal disease (30). In 2015, resveratrol was found to
serve as a potential therapeutic factor to treat DP. It reduced
patients' blood glucose levels and alveolar bone loss through the
TLR4 signaling pathway. The expression of inflammatory factors
IL-1β, IL-6, IL-8 and TNF-α and the activation of NF-κB p65 were
also suppressed at the same time (31). In addition, cynaropicrin was found
to decrease the expression of inflammatory cytokines induced by
P. gingivalis LPS and suppress the osteoclast
differentiation of RAW264.7 cells induced by RANKL (32). These previous studies provide
approaches for the treatment of DP. The current study focused on
the expression of the endogenous molecule TIFA under DP conditions
and the effect of TIFA on the inflammation and osteoclast
differentiation in RAW264.7 cells.
TIFA as an inflammatory signaling adaptor is
involved in numerous signaling pathways such as the NF-κB signaling
pathway (9,10). It directly interacts with TRAF. A
previous study revealed that DNA damage induced by the activation
of NF-κB is affected by the changes of TIFA expression. TIFA, as an
activator of the NF-κB pathway in the cytosol, plays a crucial role
in carcinogenesis and cellular senescence (33). In addition, TIFA is found to
involved in the regulation of liver cancer (10) and pulmonary arterial hypertension
(34). However, the expression of
TIFA on the inflammation of gingival and periodontal tissues
remains to be elucidated. The current study confirmed the
upregulation of TIFA expression in the periodontal tissues of a DP
mouse model and in peripheral blood monocytes of patients with
DP.
P. gingivalis LPS is the leading cause of
periodontal cell inflammation. A previous study showed that LPS
significantly induces the expression of proinflammatory cytokines
such as TNF-α, IL-1β and IL-6 in RAW264.7 cells (35). In the present study, an LPS-induced
mice model was used to simulate the clinical conditions of
periodontitis to investigate and determine whether TIFA is
differentially expressed in periodontal tissues of patients with
DP. This modeling method is simple to operate and can efficiently
induce the occurrence of periodontitis. The establishment of cell
model enables the further exploration of the effect and mechanism
of TIFA on cellular inflammatory response. Clinically, the symptoms
and causes of DP are various. It is difficult to regulate TIFA
expression in macrophages of gingival tissues specifically. An
effective way to precisely knock down TIFA expression in
macrophages of periodontal tissue has not yet been found. The
present study found that TIFA expression level was significantly
upregulated in RAW264.7 cells treated by HG and LPS. With the
increase of TIFA expression, the NF-κB signaling pathway was
activated and the inflammatory cytokines were upregulated in the
RAW264.7 cells. The results illustrated that TIFA was involved in
the inflammation of RAW264.7 cells. It is well known that the
inflammatory cytokines such as TNF-α, IL-1β and IL-6 are the
downstream of the NF-κB signaling pathway. Studies have shown that
TIFA acts as an important intermediate of inflammatory response
(36,37). In 2016, TIFA was found to activate
the signals of NLRP3 inflammasome in the vascular endothelial cells
(14). ASC provides a scaffold for
NLRP3 to form the structure of the inflammasome, which depends on
the interaction of the pyrin domain (PYD) and the caspase
activation and recruitment domain (CARD) in ASC, NLRP3 and
procaspase-1 (38,39). TIFA, which does not have PYD or
CARD, can directly bind to NLRP3 and ASC to promote the formation
of the NLRP3 inflammasome (14).
The expression of inflammasome NLRP3 and ASC was both enhanced by
the upregulation of TIFA. These provide evidence for the promotion
effect of TIFA on the inflammatory response of RAW264.7 cells via
the NF-κB signaling pathway.
RANKL is an essential cytokine for osteoclast
progression (40,41). RANKL interacts with its homologous
receptor RANK. The interaction of RANKL/RANK adsorbs TRAF6 near the
cell membrane and activates a cascade of downstream signaling
pathways, such as the NF-κB and MAPK pathways (42). RANKL can independently induce the
osteoclast differentiation of RAW264.7 cells (43), which is consistent with the
findings of the present study. To verify whether osteoclast
differentiation was affected by changes in TIFA expression, the
inflammatory infiltration of cells and the expression of cathepsin
K, MMP9 and NFATc1 were examined. Previous studies have
demonstrated that cathepsin K, MMP9 and NFATc1 are osteoclastic
markers, which increase with the osteoclast differentiation induced
by RANKL (44–46). Cathepsin K participates in the
proteolytic processing of TRAP (47). Cathepsin K and MMP-9 have both been
found to be involved in matrix protein degradation during bone
resorption (26). NFATc1 is known
as a major transcription factor in osteoclast differentiation
(48). These findings also suggest
that TIFA promotes the expression of cathepsin K, MMP9 and NFATc1
through activating the NF-κB pathway to facilitate the osteoclast
differentiation.
The present study mainly focused on basic research
and investigated the role and regulatory mechanism of TIFA in the
periodontitis. One of the main symptoms of periodontitis is the
local infiltration of macrophages in gingival tissues. However, it
is difficult to regulate TIFA expression in macrophages of gingival
tissues specifically. It is proposed to find an effective way to
specifically knock down TIFA expression in macrophages of
periodontal tissue in the future. The present study verified that
TIFA was highly expressed in the periodontal tissues of patients
with DP and DP mice, and promoted inflammatory reaction and
osteoclast differentiation. Therefore, designing and synthesizing
the molecules specifically knocking down TIFA might be an effective
therapeutic method for diabetic periodontitis. The clinical
application of the study results will be further investigated.
In summary, the present study verified that TIFA was
highly expressed in the periodontal tissues of a DP mouse model and
promoted the HG- and LPS-induced inflammatory reaction and
RANKL-induced osteoclast differentiation by activating the NF-κB
signaling pathway.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Natural Science
Foundation of Ningxia (grant no. 2020AAC03355), the School Level
Scientific Research Project of Ningxia Medical University (grant
no. XM2016086) and Ningxia Medical University Scientific Research
Project (grant no. XZ2023020).
Availability of data and materials
The datasets generated during and/or analyzed during
the current study are available from the corresponding author on
reasonable request.
Authors' contributions
XG, GQ and YW designed the experiments and
contributed to the analysis plan. XG, GQ, JW, CY, MZ and QZ
performed the experiments and analyzed data. XG and GQ prepared the
manuscript with contributions from all co-authors. XG and YW
confirm the authenticity of all the raw data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
All animal experiments were conducted in accordance
with the Guide for the Care and Use of Laboratory Animals and the
procedures were approved by the Medical Research Ethics Review
Committee of General Hospital of Ningxia Medical University
(approval no. KYLL-2023-0399).
Patient consent for publication
Not applicable.
Conflict of interest
The authors declare that they have no competing
interests.
References
|
1
|
Pihlstrom BL, Michalowicz BS and Johnson
NW: Periodontal diseases. Lancet. 366:1809–1820. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Eke PI, Dye BA, Wei L, Thornton-Evans GO
and Genco RJ; CDC Periodontal Disease Surveillance workgroup, :
James Beck (University of North Carolina, Chapel Hill, USA), Gordon
Douglass (Past President, American Academy of Periodontology), Roy
Page (University of Washin: Prevalence of periodontitis in adults
in the United States: 2009 and 2010. J Dent Res. 91:914–920. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Preshaw PM, Alba AL, Herrera D, Jepsen S,
Konstantinidis A, Makrilakis K and Taylor R: Periodontitis and
diabetes: A two-way relationship. Diabetologia. 55:21–31. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sanz M, Ceriello A, Buysschaert M, Chapple
I, Demmer RT, Graziani F, Herrera D, Jepsen S, Lione L, Madianos P,
et al: Scientific evidence on the links between periodontal
diseases and diabetes: Consensus report and guidelines of the joint
workshop on periodontal diseases and diabetes by the International
Diabetes Federation and the European Federation of Periodontology.
J Clin Periodontol. 45:138–149. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Polak D and Shapira L: An update on the
evidence for pathogenic mechanisms that may link periodontitis and
diabetes. J Clin Periodontol. 45:150–166. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hajishengallis G: Periodontitis: From
microbial immune subversion to systemic inflammation. Nat Rev
Immunol. 15:30–44. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bohannon JK, Hernandez A, Enkhbaatar P,
Adams WL and Sherwood ER: The immunobiology of toll-like receptor 4
agonists: From endotoxin tolerance to immunoadjuvants. Shock.
40:451–462. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Suda K, Udagawa N, Sato N, Takami M, Itoh
K, Woo JT, Takahashi N and Nagai K: Suppression of osteoprotegerin
expression by prostaglandin E2 is crucially involved in
lipopolysaccharide-induced osteoclast formation. J Immunol.
172:2504–2510. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Takatsuna H, Kato H, Gohda J, Akiyama T,
Moriya A, Okamoto Y, Yamagata Y, Otsuka M, Umezawa K, Semba K and
Inoue J: Identification of TIFA as an adapter protein that links
tumor necrosis factor receptor-associated factor 6 (TRAF6) to
interleukin-1 (IL-1) receptor-associated kinase-1 (IRAK-1) in IL-1
receptor signaling. J Biol Chem. 278:12144–12150. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shen W, Chang A, Wang J, Zhou W, Gao R, Li
J, Xu Y, Luo X, Xiang R, Luo N and Stupack DG: TIFA, an
inflammatory signaling adaptor, is tumor suppressive for liver
cancer. Oncogenesis. 4:e1732015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shen W, Du R, Li J, Luo X, Zhao S, Chang
A, Zhou W, Gao R, Luo D, Wang J, et al: TIFA suppresses
hepatocellular carcinoma progression via MALT1-dependent and
-independent signaling pathways. Signal Transduct Target Ther.
1:160132016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zimmermann S, Pfannkuch L, Al-Zeer MA,
Bartfeld S, Koch M, Liu J, Rechner C, Soerensen M, Sokolova O,
Zamyatina A, et al: ALPK1- and TIFA-Dependent Innate immune
response triggered by the helicobacter pylori type IV secretion
system. Cell Rep. 20:2384–2395. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Milivojevic M, Dangeard AS, Kasper CA,
Tschon T, Emmenlauer M, Pique C, Schnupf P, Guignot J and
Arrieumerlou C: ALPK1 controls TIFA/TRAF6-dependent innate immunity
against heptose-1,7-bisphosphate of gram-negative bacteria. PLoS
Pathog. 13:e10062242017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lin TY, Wei TW, Li S, Wang SC, He M,
Martin M, Zhang J, Shentu TP, Xiao H, Kang J, et al: TIFA as a
crucial mediator for NLRP3 inflammasome. Proc Natl Acad Sci USA.
113:15078–15083. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jimi E and Ghosh S: Role of nuclear
factor-kappaB in the immune system and bone. Immunol Rev.
208:80–87. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hayden MS and Ghosh S: Regulation of NF-κB
by TNF family cytokines. Semin Immunol. 26:253–266. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Guan X, He Y, Wei Z, Shi C, Li Y, Zhao R,
Pan L, Han Y, Hou T and Yang J: Crosstalk between Wnt/β-catenin
signaling and NF-κB signaling contributes to apical periodontitis.
Int Immunopharmacol. 98:1078432021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tian Y, Li Y, Liu J, Lin Y, Jiao J, Chen
B, Wang W, Wu S and Li C: Photothermal therapy with regulated
Nrf2/NF-κB signaling pathway for treating bacteria-induced
periodontitis. Bioact Mater. 9:428–445. 2021.PubMed/NCBI
|
|
19
|
Odes-Barth S, Khanin M, Linnewiel-Hermoni
K, Miller Y, Abramov K, Levy J and Sharoni Y: Inhibition of
osteoclast differentiation by carotenoid derivatives through
inhibition of the NF-ƙB pathway. Antioxidants (Basel). 9:11672020.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lalla E, Lamster IB, Feit M, Huang L,
Spessot A, Qu W, Kislinger T, Lu Y, Stern DM and Schmidt AM:
Blockade of RAGE suppresses periodontitis-associated bone loss in
diabetic mice. J Clin Invest. 105:1117–1124. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Krongbaramee T, Zhu M, Qian Q, Zhang Z,
Eliason S, Shu Y, Qian F, Akkouch A, Su D, Amendt BA, et al:
Plasmid encoding microRNA-200c ameliorates periodontitis and
systemic inflammation in obese mice. Mol Ther Nucleic Acids.
23:1204–1216. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals: Guide for the Care and Use of Laboratory Animals. 8th
edition. National Academies Press (US); Washington, DC: 2011
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bauer M, Nascakova Z, Mihai AI, Cheng PF,
Levesque MP, Lampart S, Hurwitz R, Pfannkuch L, Dobrovolna J,
Jacobs M, et al: The ALPK1/TIFA/NF-κB axis links a bacterial
carcinogen to R-loop-induced replication stress. Nat Commun.
11:51172020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fuller K, Lawrence KM, Ross JL, Grabowska
UB, Shiroo M, Samuelsson B and Chambers TJ: Erratum to ‘Cathepsin K
inhibitors prevent matrix-derived growth factor degradation by
human osteoclasts’. Bone. 145:1153542021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sundaram K, Nishimura R, Senn J, Youssef
RF, London SD and Reddy SV: RANK ligand signaling modulates the
matrix metalloproteinase-9 gene expression during osteoclast
differentiation. Exp Cell Res. 313:168–178. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Preshaw PM: Periodontal disease and
diabetes. J Dent. 37:S575–S577. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kinane DF: Causation and pathogenesis of
periodontal disease. Periodontol 2000. 25:8–20. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Boyle WJ, Simonet WS and Lacey DL:
Osteoclast differentiation and activation. Nature. 423:337–342.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Van Dyke TE and Serhan CN: Resolution of
inflammation: A new paradigm for the pathogenesis of periodontal
diseases. J Dent Res. 82:82–90. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhen L, Fan DS, Zhang Y, Cao XM and Wang
LM: Resveratrol ameliorates experimental periodontitis in diabetic
mice through negative regulation of TLR4 signaling. Acta Pharmacol
Sin. 36:221–228. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hayata M, Watanabe N, Kamio N, Tamura M,
Nodomi K, Tanaka K, Iddamalgoda A, Tsuda H, Ogata Y, Sato S, et al:
Cynaropicrin from Cynara scolymus L. suppresses Porphyromonas
gingivalis LPS-induced production of inflammatory cytokines in
human gingival fibroblasts and RANKL-induced osteoclast
differentiation in RAW264.7 cells. J Nat Med. 73:114–123. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fu J, Huang D, Yuan F, Xie N, Li Q, Sun X,
Zhou X, Li G, Tong T and Zhang Y: TRAF-interacting protein with
forkhead-associated domain (TIFA) transduces DNA damage-induced
activation of NF-κB. J Biol Chem. 293:7268–7280. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chang HC, Wei TW, Wu PY, Tsai MD, Yu WC,
Chen CH and Sung SH: TIFA protein expression is associated with
pulmonary arterial hypertension. Sci Rep. 11:141402021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Müller AK, Albrecht F, Rohrer C, Koeberle
A, Werz O, Schlörmann W, Glei M, Lorkowski S and Wallert M: Olive
Oil extracts and oleic acid attenuate the LPS-Induced inflammatory
response in murine RAW264.7 macrophages but induce the release of
prostaglandin E2. Nutrients. 13:44372021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li JY, Zheng ZX, Liu L, Du O, Yu NW, Zou
Y, Seong SY and Du JR: Neuroprotective effect of alpha-kinase 1
knockdown against cerebral ischemia through inhibition of the NF-κB
pathway and neuroinflammation. Int Immunopharmacol. 113((Pt A)):
1093302022. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Snelling T, Shpiro N, Gourlay R,
Lamoliatte F and Cohen P: Co-ordinated control of the
ADP-heptose/ALPK1 signalling network by the E3 ligases TRAF6,
TRAF2/c-IAP1 and LUBAC. Biochem J. 479:2195–2216. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yu JW, Wu J, Zhang Z, Datta P, Ibrahimi I,
Taniguchi S, Sagara J, Fernandes-Alnemri T and Alnemri ES:
Cryopyrin and pyrin activate caspase-1, but not NF-kappaB, via ASC
oligomerization. Cell Death Differ. 13:236–249. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fernandes-Alnemri T, Wu J, Yu JW, Datta P,
Miller B, Jankowski W, Rosenberg S, Zhang J and Alnemri ES: The
pyroptosome: A supramolecular assembly of ASC dimers mediating
inflammatory cell death via caspase-1 activation. Cell Death
Differ. 14:1590–1604. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Roodman GD: Cell biology of the
osteoclast. Exp Hematol. 27:1229–1241. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Teitelbaum SL: Bone resorption by
osteoclasts. Science. 289:1504–1508. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
He Y, Zhang Q, Shen Y, Chen X, Zhou F and
Peng D: Schisantherin A suppresses osteoclast formation and wear
particle-induced osteolysis via modulating RANKL signaling
pathways. Biochem Biophys Res Commun. 449:344–350. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Song C, Yang X, Lei Y, Zhang Z, Smith W,
Yan J and Kong L: Evaluation of efficacy on RANKL induced
osteoclast from RAW264.7 cells. J Cell Physiol. 234:11969–11975.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kang MR, Jo SA, Yoon YD, Park KH, Oh SJ,
Yun J, Lee CW, Nam KH, Kim Y, Han SB, et al: Agelasine D suppresses
RANKL-induced osteoclastogenesis via down-regulation of c-Fos,
NFATc1 and NF-κB. Mar Drugs. 12:5643–5656. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Li X, Jiang J, Yang Z, Jin S, Lu X and
Qian Y: Galangin suppresses RANKL-induced osteoclastogenesis via
inhibiting MAPK and NF-κB signalling pathways. J Cell Mol Med.
25:4988–5000. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wu L, Luo Z and Liu Y, Jia L, Jiang Y, Du
J, Guo L, Bai Y and Liu Y: Aspirin inhibits RANKL-induced
osteoclast differentiation in dendritic cells by suppressing NF-κB
and NFATc1 activation. Stem Cell Res Ther. 10:3752019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zenger S, Hollberg K, Ljusberg J, Norgård
M, Ek-Rylander B, Kiviranta R and Andersson G: Proteolytic
processing and polarized secretion of tartrate-resistant acid
phosphatase is altered in a subpopulation of metaphyseal
osteoclasts in cathepsin K-deficient mice. Bone. 41:820–832. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Takayanagi H: The role of NFAT in
osteoclast formation. Ann N Y Acad Sci. 1116:227–237. 2007.
View Article : Google Scholar : PubMed/NCBI
|