Introduction
Targeted therapies have gained interest as a way for
safe and selective delivery of cytotoxic agents, such as
radionuclides, toxins, and drugs, to malignant cells (1). The use of high affinity small
engineered scaffold proteins (ESPs) as targeting agents enables the
efficient delivery of payloads to targets such as cancer-specific
markers on tumor cells. Compared with antibodies, ESPs have a more
rapid clearance from blood and non-targeted tissues (2–4).
Radiolabeled affibody molecules and Albumin binding domain
(ABD)-Derived Affinity ProTeins (ADAPTs) are ESPs that can provide
high contrast visualization of human epidermal growth factor
receptor 2 (HER2)-expressing tumors in breast cancer patients
(5–7). During the past years, several other
promising ESPs, such as anticalins, cystine-knot peptides, and
designed ankyrin repeat proteins (DARPins), have been developed and
investigated in preclinical or early clinical studies (8–12).
ADAPTs are small (5 kDa) engineered
non-immunoglobulin proteins, with the selected variant against HER2
named ADAPT6 (13). In previous
studies, we have investigated different aspects of the molecular
design of radiolabeled ADAPTs to increase the sensitivity for
imaging of disseminated cancers (14–19).
For stratification of cancer patients for HER2 targeting therapy,
[99mTc]Tc-ADAPT6 has demonstrated excellent sensitivity
and specificity as an imaging agent using single-photon emission
computed tomography (SPECT). Moreover, no signs of acute toxicity
in patients at doses up to 1 mg were observed (6). ADAPTs have also shown promising
potential as therapeutic agents. ABD-fused ADAPT6 labeled with
177Lu has shown promising results in radionuclide
therapy of HER2-expressing SKOV-3 ×enografts in mice. It was
demonstrated that the median survival in mice was increased more
than two-fold after a single injection of 18 MBq of
[177Lu]Lu-2,2′,2”,2”'-(1,4,7,10-tetraazacyclododecane-1,4,7,10-tetrayl)tetraacetic
acid (DOTA)-ADAPT6-ABD035 (20).
The kidneys are the main excretion pathway of
peptides and proteins below 60 kDa and therefore, a high renal
uptake is common for ESP-based radiopharmaceuticals. High renal
uptake leads to reduced sensitivity for detection of abdominal
lesions close to the kidneys and it also decreases the maximum
tolerated therapeutic dose. Radiopharmaceuticals excreted via the
glomeruli undergo reabsorption and internalization in proximal
tubular cells. Internalized radiopharmaceuticals are degraded by
lysosomal enzymes leading to the formation of radiometabolites. The
main reason for the high retention of radioactivity in kidneys is
due to the degradation of proteins labeled with residualizing
radiometals (e.g. 177Lu) leading to the formation of
hydrophilic radiometabolites that cannot cross the cellular
membrane and will therefore be trapped inside the cells. High renal
retention has been observed for ADAPTs labeled with radiometals
regardless of the label position, at both N- and C-terminus
(13,16,17).
Several strategies have been developed to reduce the
renal retention of ESP-based radiopharmaceuticals, and one strategy
is to prevent glomerular filtration by fusing the
radiopharmaceutical to an ABD. The ABD will bind to albumin in the
blood which will increase the total size of the construct above the
kidney filtration barrier (60 kDa). This strategy has been
successfully applied to both 177Lu-labeled anti-HER2
affibody molecules and [177Lu]Lu-ADAPT6, where the
kidney radioactivity retention was reduced 25- and 14-fold compared
with the native constructs, respectively (20,21).
However, the increased circulatory half-life by ABD-fusion will
also increase the risk of undesired bone marrow exposure.
Therefore, exploring alternative strategies to reduce the kidney
accumulation of ADAPTs is of high interest for radionuclide
therapy. Pretargeting is a possible approach to reduce the renal
activity uptake using a non-labeled primary agent coupled to a
recognition tag injected prior to the radiolabeled probe (22–24).
A study using affibody-based peptide nucleic acid (PNA)-mediated
pretargeting in mice demonstrated a 20-fold lower uptake of
radioactivity in kidneys compared with the regular targeting
strategy (24).
Another method that does not require modification of
the ESP is the use of a pharmacological agent as a competitor or
inhibitor of the reabsorption process in kidneys. These agents may
act on transporters (e.g. saturation by lysine, arginine,
gelofusine, or blocking by probenecid) or by decreasing the level
of energy-mediated endocytosis (e.g. pre-injection of sodium
maleate and fructose) (25). We
have recently investigated these pharmacological approaches for
renal uptake reduction of DARPins, affibodies, and ADAPT6. It was
shown that sodium maleate and fructose could reduce the
accumulation of activity in kidneys, however, both agents are toxic
at the required doses (26–28).
An alternative approach for reducing the activity
uptake in the kidneys is the use of a non-residualizing label (e.g.
radioiodine). Radiometabolites of a non-residualizing label are
lipophilic which enables diffusion through the cell membrane out
into the extracellular space. In that way, the radiometabolites can
return to the blood circulation and be excreted from the body.
Reduction of the renal activity retention using a non-residualizing
label has been demonstrated for [125I]I-HPEM-ADAPT6 in
mice bearing SKOV-3 ×enografts (17). This approach is particularly useful
for radionuclide therapy with the β-emitter 131I.
131I has suitable physicochemical properties and the
emitted 365 keV gamma-rays (81% abundance) could be used for
monitoring therapy. On the other hand, it has been demonstrated
that ADAPT6 labeled with radiometals provided higher radioactivity
retention in the tumor com-pared to a non-residualizing
radiohalogen label (14). Longer
retention of radioactivity at the target location reduces the
frequency of injections for radionuclide therapy. Therefore, it is
of high interest to find a strategy to reduce the reabsorption in
the kidneys of ADAPTs labeled with radiometals (e.g. beta-emitting
177Lu).
The introduction of an enzyme-cleavable peptide
linker between the radiometal-chelator complex and the scaffold
protein could be another method for reducing the renal reabsorption
of radiopharmaceuticals. Once the peptide linker has been cleaved
by the enzyme located in the kidneys, the radiometal-chelator
complex will be separated from the scaffold protein and excreted
with the urine. It is crucial that the peptide linker has high
selectivity to the selected enzyme and not to other enzymes (e.g.
in the blood). The proximal tubular brush border enzyme is a
potential target enzyme for this approach. Previous studies have
shown that the glycine-lysine (GK) sequence is a substrate for
carboxypeptidase M located at the renal brush border membrane
(29–31). Uehara et al also
demonstrated that the construct
188Re-tricarbonyl-(cyclopentadienyl)-glycyl-lysine-Fab
was recognized and cleaved by the brush border enzymes, resulting
in a significant decrease of renal activity uptake 6 h after
injection without influencing the tumor uptake (32). Another study demonstrated the same
activity uptake in the tumor but a lower renal activity uptake due
to an efficient cleavage of 66/67Ga-NOTA-Met from a
66/67Ga-labeled Fab fragment (33,34).
In this study, we investigated if the introduction
of a cleavable glycine-leucine-glycine-lysine (GLGK) peptide linker
between the [177Lu]Lu-DOTA-complex and ADAPT6 would
reduce the renal activity retention due to cleavage by the brush
border enzymes. This strategy was also compared to the use of a
non-residualizing [125I]I-HPEM label.
Materials and methods
Preparation of radiolabeled ADAPT6
constructs
Three constructs (1–3) of
ADAPT6 (Fig. 1A), containing
cysteine either at the N- or C-terminus, were recombinantly
produced in E. coli BL21*(DE3) cells and purified as described
earlier (13,14,17).
The molecular mass was confirmed by MALDI.
DOTA-GLGK(maleimide)-COOH was synthesized in two steps (Data S1). First, DOTA-GLGK-COOH (4) (Fig.
1B) was synthesized by Fmoc solid phase peptide synthesis on
2-CTC resin using Oxyma Pure and N,N'-diisopropylcarbodiimide (DIC)
as coupling reagents and the cleaved peptide was purified by
RP-HPLC. In a second step, N-(methoxycarbonyl) maleimide was
coupled to the free amine in 4 to generate
DOTA-GLGK(maleimide)-COOH (5)
(Fig. 1B) which was purified by
RP-HPLC. The identity and purity of the compound were determined by
LC-MS. Through a Michael addition, constructs 1 and 2 were
conjugated with compound 5 and DOTA-maleimide separately followed
by purification by RP-HPLC. 177Lu-labeling was performed
according to a method optimized by Altai et al (35). Construct 3 was labeled with
[125I]I-HPEM according to the protocol optimized by
Tolmachev et al for indirect iodination of affibody
molecules (36).
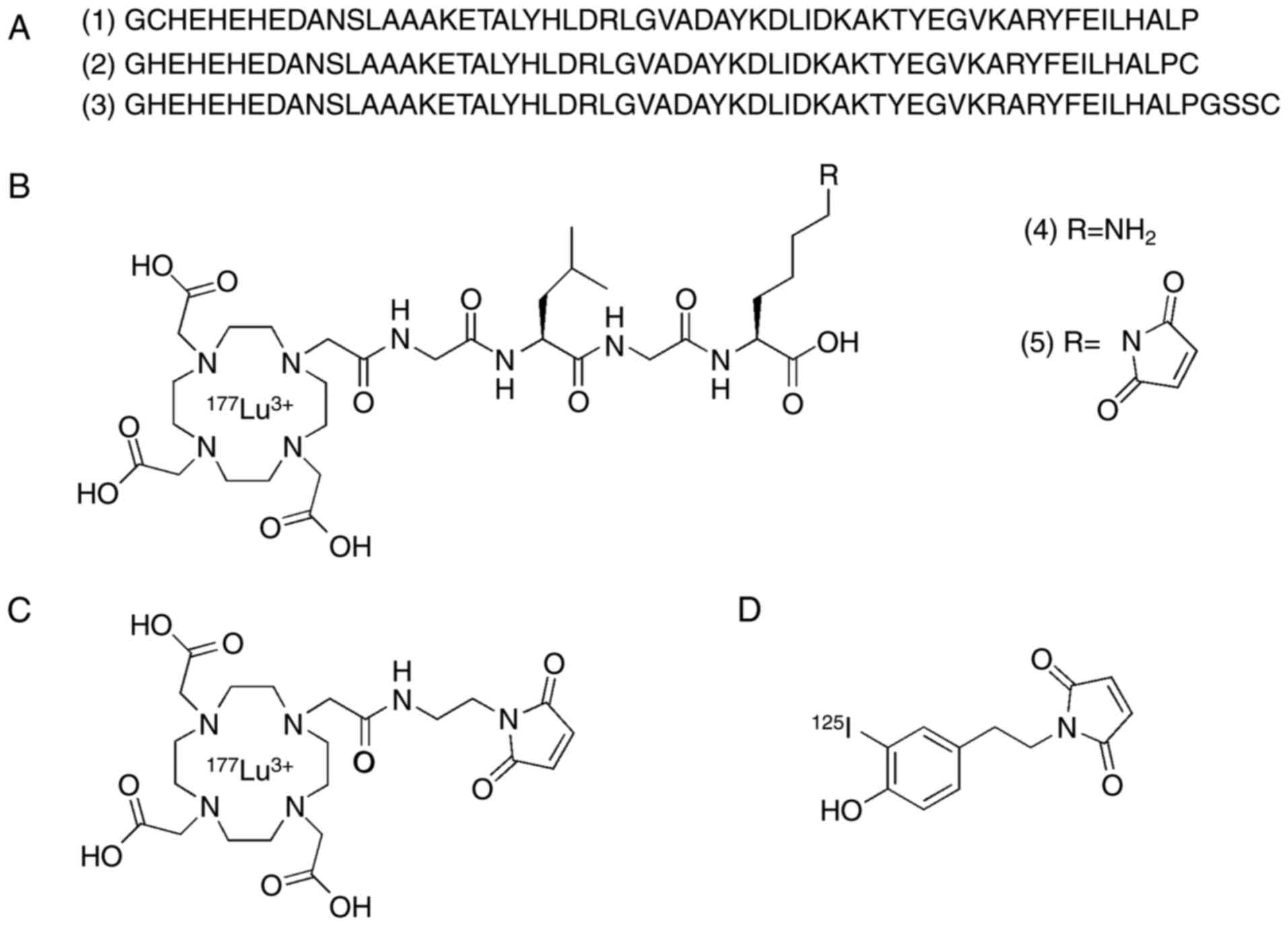 | Figure 1.Schematic overview of the structures
of ADAPT6 and radiolabeling methods used in the study. (A) Amino
acid sequences of ADAPT6 constructs (1–3)
containing cysteine (red) either at the N- or C-terminus. (B)
Structure of the cleavable peptide linker (4) [177Lu]Lu-DOTA-GLGK-COOH and
(5)
[177Lu]Lu-DOTA-GLGK-(maleimide)-COOH. (C) Structure of
[177Lu]Lu-DOTA-maleimide. (D) Structure of
[125I]I-HPEM. ADAPT, albumin-binding domain-derived
affinity protein; 177Lu, lutetium-177; DOTA,
2,2′,2”,2”'-(1,4,7,10-tetraazacyclododecane-1,4,7,10-tetrayl)tetraacetic
acid; GLGK, glycine-leucine-glycine-lysine;
[125I]I-HPEM,
[125I]I-[(4-hydroxyphenyl)ethyl]maleimide. |
The specificity of in vitro cell
binding of radiolabeled ADAPT6
The specificity of binding of ADAPT6 constructs
labeled with 177Lu or 125I was tested using
the HER2-expressing SKOV3 ovarian cancer cell line
(1.6×106 receptors/cell) as described by Wållberg and
Orlova (37). Briefly, 50 nM of
the labeled compound was added to two sets of Petri dishes in
triplicates (1 million cells/dish). HER2 receptors in one set of
the dishes were saturated by the addition of non-labeled ADAPT6
construct (1000-fold molar excess) 15 min prior to the addition of
the labeled compound. The dishes were incubated at 37°C for 1 h in
a humidified incubator. The media were aspirated, the cells were
harvested by trypsinisation and radioactivity content in the
samples was measured. The percent of cell-associated radioactivity
was calculated and the data were normalized to the highest value of
average cell-associated radioactivity.
In vivo studies
The animal experiments were planned in agreement
with the Swedish laws on laboratory animal welfare and were
approved by the Ethics Committee for Animal Research in Uppsala
(Permit Number C4/2016). The biodistribution properties of ADAPTs
labeled with 177Lu or 125I were evaluated in
non-tumor bearing NMRI mice, randomized into five groups with four
animals per group. Mice were injected with a total dose of 3 µg per
mouse (40 kBq/15 kBq of 177Lu/125I,
correspondingly, and the dose was adjusted by the addition of
non-labeled compound) in 100 µl PBS. At 4 h after injection,
animals were euthanized by intraperitoneal injection of xylazine
and ketamine. The dose of xylazine was 20 mg/kg, the dose of
ketamine was 200 mg/kg. After the injection of anesthesia, its
effectiveness was ensured by the absence of a paw withdrawal reflex
in mice. The sedated mice were euthanized by exsanguination via
cardiac puncture and collection of blood. Tissue samples and organs
of interest were collected, weighed, and the uptake of activity was
measured using an automated γ-spectrometer. The data were corrected
for dead time, spillover, background, and decay.
Statistical analysis
GraphPad Prism (version 8.00 for Windows; GraphPad
Software, San Diego, CA, USA) was used for statistical analysis. To
determine significant differences (P<0.05) between two groups
the obtained data were analyzed by an unpaired two-tailed t-test.
Data analysis for more than two groups was performed using one-way
ANOVA with Bonferroni's multiple comparisons test.
Results
Synthesis of DOTA-GLGK(maleimide)-COOH
(5)
DOTA-GLGK(maleimide)-COOH was successfully
synthesized and purified. The purity was >98 % (Fig. S1) and the identity of the product
was confirmed (calculated m/z 840.4, found m/z 840.1) by LC-MS
(Fig. S2).
Preparation of radiolabeled ADAPT6
constructs
ADAPT6 constructs were successfully produced and
purified. MALDI-TOF analysis confirmed the expected mass of the
constructs (6447 Da), which was in good agreement with the
theoretical value (6445.2 Da). The difference was within the
accuracy of the method. The constructs intended for labeling with
177Lu were successfully conjugated with construct 5 or
DOTA-maleimide, and the purity and identity of the constructs were
confirmed by mass spectrometry (Fig.
S3). All ADAPT6 conjugates were successfully labeled with
177Lu or [125I]I-HPEM, and the radiochemical
yields are presented in Table I.
The purity after size-exclusion chromatography was over 99% for all
constructs. No release of the radiolabels could be detected after
incubation with an excess of EDTA or KI over 3 h (Table I). The radiolabeling of construct 3
(Fig. 1A) with
[125I]I-HPEM was in good agreement with the previously
reported data for indirect radioiodination of ADAPT6 and affibody
molecules (17,37). The maximum achieved effective
specific radioactivity was 1 MBq/µg (7 GBq/µmol) and 0.3 MBq/µg
(1.96 GBq/µmol) for 177Lu and 125I,
respectively.
 | Table I.Radiochemical yields of radiolabeled
ADAPT6 constructs. |
Table I.
Radiochemical yields of radiolabeled
ADAPT6 constructs.
| ADAPT6
construct | Radiochemical yield
(%) | Radiochemical
purity (%) | Radiochemical
purity after challenge (%) |
|---|
|
[177Lu]Lu-DOTA-GLGK-1 | 82±2 | >99 | >99a |
|
[177Lu]Lu-DOTA-1 | 90±4 | >99 | >99a |
|
[177Lu]Lu-DOTA-GLGK-2 | 87±3 | >99 | >99a |
|
[177Lu]Lu-DOTA-2 | 95±2 | >99 | >99a |
|
[125I]I-HPEM-3 | 60±6 | >99 | >99b |
Specificity of in vitro cell binding
of radiolabeled ADAPT6 constructs
The in vitro specificity test demonstrated
HER2-mediated binding to HER2-expressing SKOV-3 cells for all
radiolabeled ADAPT6 constructs (Fig.
2). Presaturation of HER2 receptors by non-labeled ADAPT6
resulted in a significant decrease of cell-associated radioactivity
(P<0.001).
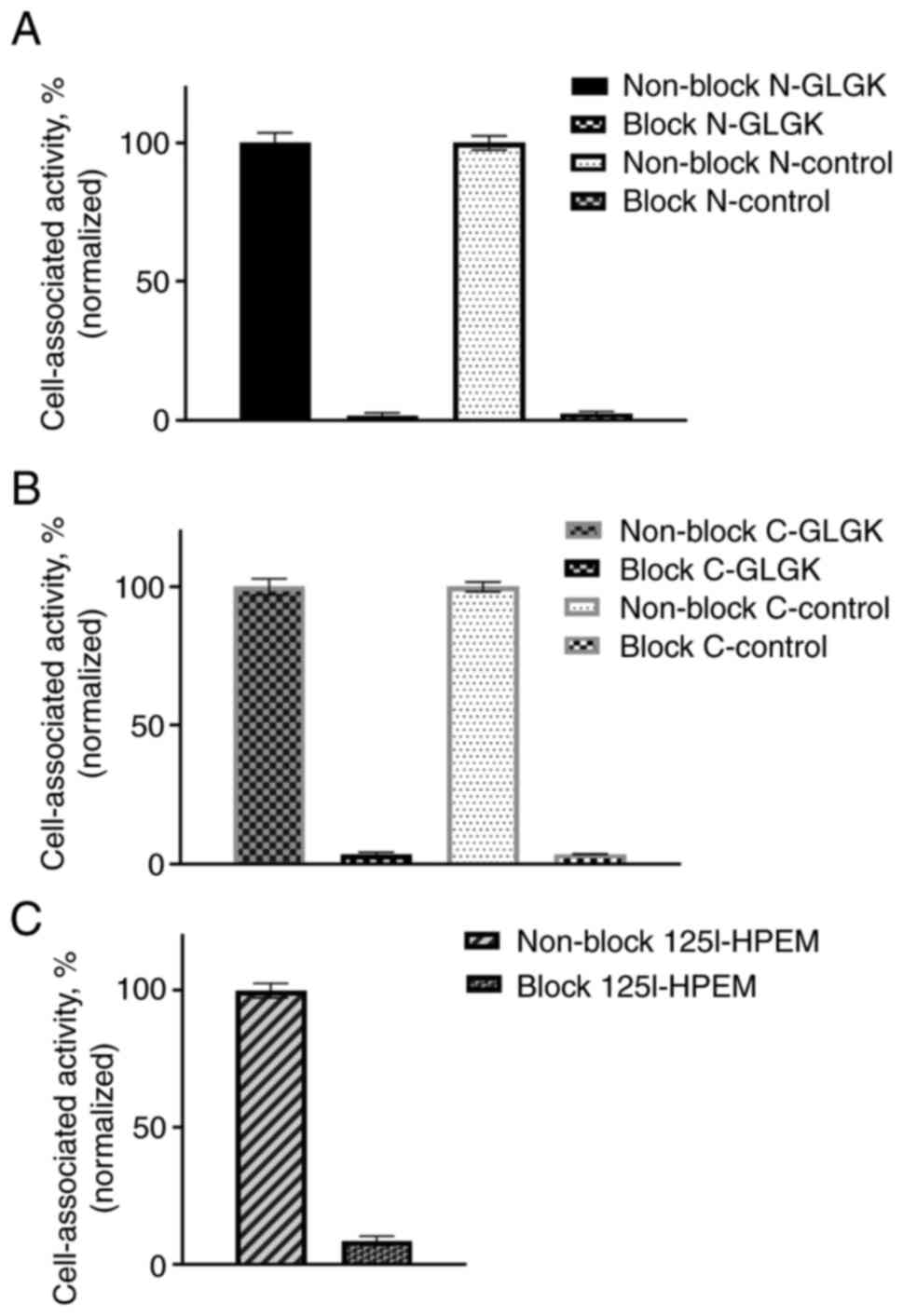 | Figure 2.Binding specificity of radiolabeled
ADAPT6 constructs to HER2-expressing SKOV3 cells. (A)
[177Lu]Lu-DOTA-GLGK-1 and [177Lu]Lu-DOTA-1;
(B) [177Lu]Lu-DOTA-GLGK-2 and
[177Lu]Lu-DOTA-2; (C) [125I]I-HPEM-3. In the
blocked groups, HER2 receptors were presaturated by an excess of
non-labeled ADAPT6. ADAPT, albumin-binding domain-derived affinity
protein; 177Lu, lutetium-177; DOTA,
2,2′,2”,2”'-(1,4,7,10-tetraazacyclododecane-1,4,7,10-tetrayl)tetraacetic
acid; GLGK, glycine-leucine-glycine-lysine;
[125I]I-HPEM,
[125I]I-[(4-hydroxyphenyl)ethyl]maleimide. |
In vivo studies
The biodistribution study in NMRI mice 4 h after
injection demonstrated a fast excretion and an activity in blood
below 0.2% ID/g for all radiolabeled ADAPT6 constructs (Fig. 3). Constructs labeled with
177Lu at the C-terminus had a significantly (P<0.001,
one-way ANOVA test) lower activity uptake in blood compared to the
N-terminus constructs. The liver uptake of
[125I]I-HPEM-ADAPT6 was significantly higher
(P<0.001, one-way ANOVA test) compared with the
177Lu-labeled constructs. For all constructs, the uptake
of activity in non-targeted organs and tissues was lower than 1%
ID/g (except in the kidneys for the 177Lu-labeled
constructs). There was no significant difference (P>0.05,
one-way ANOVA test) in the radioactivity uptake in the kidneys
between the 177Lu-labeled ADAPT6 constructs containing
the cleavable peptide linker and the controls, regardless of the
label position. The renal radioactivity uptake of
[125I]I-HPEM-ADAPT6 was significantly lower than that of
all other constructs. For example, the renal activity of
[125I]-HPEM-ADAPT6 was 507-fold lower than the activity
of [177Lu]Lu-DOTA-GLGK-2 (0.59±0.21% ID/g vs. 299±21%
ID/g, respectively). Numerical biodistribution data is presented in
Table SI.
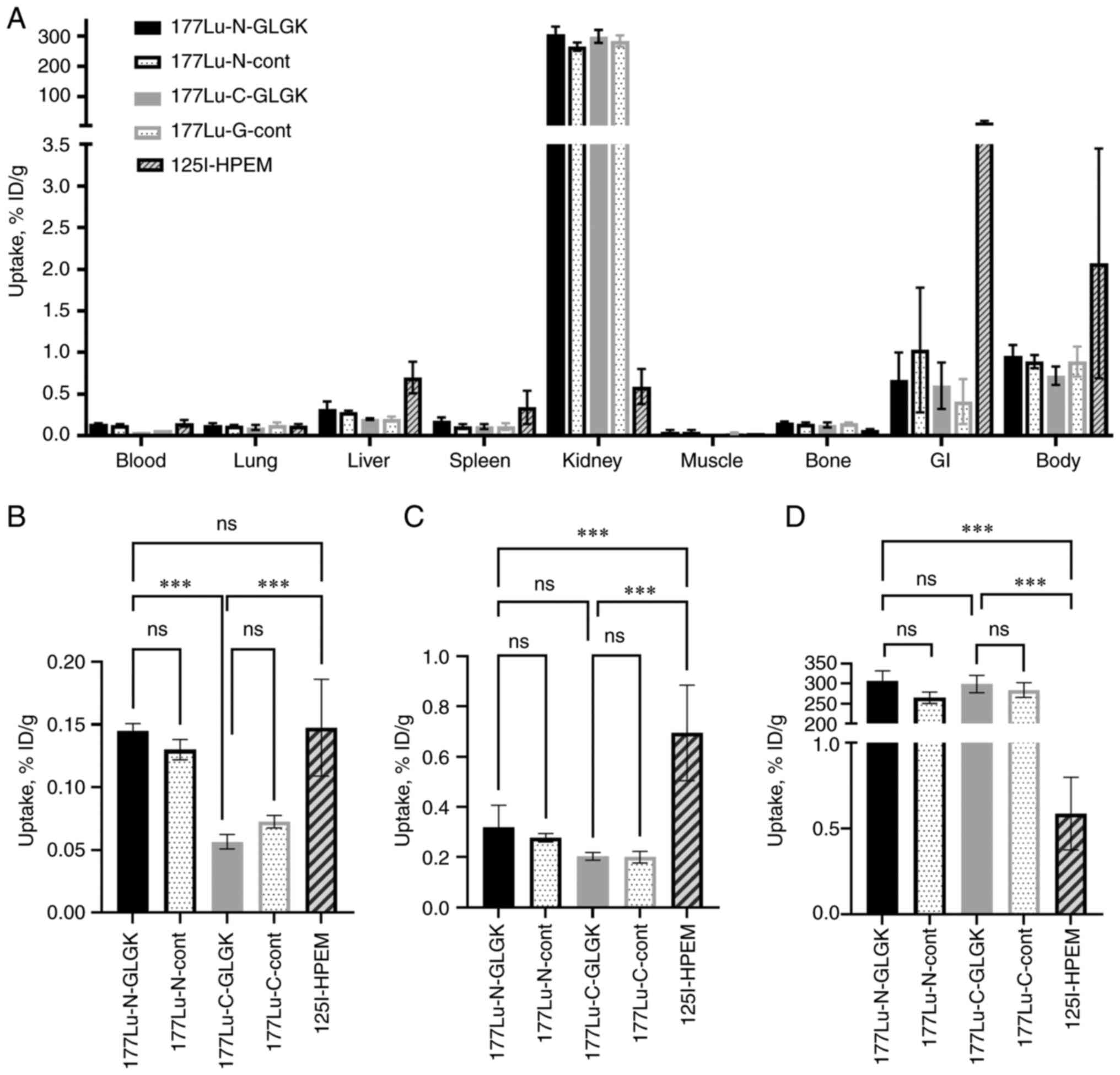 | Figure 3.Biodistribution profile of ADAPT6
constructs labeled with 177Lu or [125I]I-HPEM
in NMRI mice at 4 h after injection: (A) Whole biodistribution; (B)
blood uptake; (C) liver uptake; and (D) kidney uptake. Results are
presented as an average % ID/g and SD of four animals.
***P<0.001. ns, not significant; ADAPT, albumin-binding
domain-derived affinity protein; 177Lu, lutetium-177;
GI, gastrointestinal; DOTA,
2,2′,2”,2”'-(1,4,7,10-tetraazacyclododecane-1,4,7,10-tetrayl)tetraacetic
acid; GLGK, glycine-leucine-glycine-lysine;
[125I]I-HPEM,
[125I]I-[(4-hydroxyphenyl)ethyl]maleimide. |
Discussion
ESPs, such as ADAPTs, have a high affinity to their
targets and possess suitable pharmacokinetics for selective
delivery of radioactivity for imaging and therapy. However, due to
their small size (<60 kDa), their biodistribution is associated
with high renal uptake as a result of reabsorption in proximal
tubular cells (5–7,13).
After reabsorption, degradation of the radiopharmaceuticals occurs
leading to the formation of radiometabolites. Radiometabolites of
proteins labeled with radiometals (177Lu,
111In) usually have better residualizing properties than
proteins labeled with radiohalogens (125I,
131I). The two most common ways to lower renal
radioactivity exposure are to re-duce the uptake of the
radiopharmaceutical in the kidneys or to reduce the renal retention
of the radiometabolites. We have already demonstrated two different
methods for the reduction of kidney uptake of radiolabeled ADAPTs.
However, these methods are associated with toxicity in some organs
and tissues such as the bone marrow (20–28).
Therefore, it is desirable to investigate other approaches for this
purpose.
In this study, we have investigated if the strategy
of incorporating a cleavable linker between the radiometal-chelator
complex and the targeting agent would result in a reduction of
renal activity retention, which has been demonstrated for
Fab-fragments earlier (32–34).
The cleavable linker used in this study was GLGK, a substrate for
the brush border enzymes in kidneys, which upon cleavage is
suggested to lead to excretion of the radiometal-chelator complex
with the urine. We also compared the use of a cleavable linker with
another strategy based on the use of a non-residualizing label via
indirect radiohalogenation. 177Lu and 125I (a
surrogate of 131I used for therapy) were used as
radionuclides to evaluate and compare the two strategies. Their
preferable characteristics, such as a physical half-life between 6
and 7 days, stable daughter products, well-developed chemistry for
radiolabeling with high stability, and association of an additional
low energy gamma emission for diagnostic purposes make them
suitable for radionuclide therapy in clinics (38).
After successful preparation of all ADAPT6
constructs, the two labeling approaches provided stable labels. All
radiolabeled ADAPT6 constructs demonstrated specific binding to the
HER2-expressing cells in vitro.
Surprisingly, no significant difference in renal
activity retention could be demonstrated between the constructs
containing the cleavable linkers and the controls. Despite the same
structure of the cleavable linker as in the construct
[111In]In-DOTA-GLGK-Cys-diabody used by Li and
co-workers, there was no reduction in renal activity retention in
our study (39). An explanation
for this could be the differences in the type and size of the
targeting agent, which could have negatively affected the cleavage
of the linker. The different types of radionuclides used
(177Lu vs. 111In) is also a factor that can
influence the results. Arano et al demonstrated that the
efficiency of this approach is influenced in different ways by the
size and composition of the linker for different radionuclides and
chelators (40). Based on this,
the application of in vitro system using brush border membrane
enzymes could be helpful for the selection of a suitable peptide
linker for future studies (34,41).
While the presence and position of the cleavable
linker did not affect the kidney uptake, results from the
biodistribution study demonstrated that the uptake of radioactivity
in blood was significantly lower for the C-terminus constructs than
for the N-terminus constructs. An explanation for this could be
that the placement of DOTA-GLGK(maleimide)-COOH at the C-terminus
increases the local hydrophilicity at this position, which would
decrease the interactions with blood proteins and thereby result in
a lower blood uptake. In that way, these constructs would have a
local hydrophilicity at both termini: the HEHEHE-tag at the
N-terminus and the cleavable peptide linker at the C-terminus. The
N-terminus construct will only have a local hydrophilicity at one
terminus due to the placement of the HEHEHE-tag and the cleavable
peptide linker at the same position. Higher local hydrophobicity at
the C-terminus of these constructs could also be a possible
explanation for the slightly, but not significantly, higher uptake
in the liver.
When comparing the cleavable linker strategy with
the use of a non-residualizing label, the renal retention of
[125I]I-HPEM-ADAPT6 was significantly lower than for all
the 177Lu-labeled constructs. This was an expected
possibility as a non-residualizing iodine label is not trapped
inside the cell after internalization to the same extent as a
residualizing radiometal (17).
Amongst the constructs studied herein,
[125I]I-HPEM-ADAPT6 was deemed optimal due to its lower
uptake in the kidneys. The use of a non-residualizing label
resulted in significantly lower renal activity retention of
radiolabeled ADAPT6 compared with the use of a residualizing
radiometal in combination with the cleavable GLGK-linker.
It was previously observed that an elevated
lipophilicity was associated with elevated hepatic uptake for
affibody molecules and DARPins (42–44).
The addition of hydrophilic chelators such as DOTA increases the
hydrophilicity of radiolabeled ADAPTs, while the addition of the
HPEM group increases their lipophilicity. This might explain the
observed phenomenon that the liver uptake of
[125I]I-HPEM-ADAPT6 (0.70±0.19% ID/g) was ca. two to
three times higher than of the 177Lu-labeled constructs
(ca. 0.2–0.4% ID/g). It should be noted that the tumor uptake of
ADAPT6 is typically much higher (ca. 10–20% ID/g; 17) and an
elevated hepatic uptake would not be a limitation for radionuclide
therapy
In conclusion, to prevent the kidneys from high
radiation exposure in the course of targeted therapy, molecular
design can be used to reduce renal activity retention. Earlier
studies showed that the incorporation of a cleavable linker between
the targeting agent and the radiometal chelator reduced renal
retention of radiolabeled Fab fragments (32–34).
In the present study, we investigated if the same strategy could be
applied to reduce the renal retention of radiolabeled ADATPs.
Surprisingly, incorporation of the cleavable GLGK-linker did not
result in lower renal retention of 177Lu-labeled ADAPT6.
This study emphasizes that when aiming to reduce the renal
retention of ESPs labeled with radiometals, the molecular design of
each construct is crucial. However, further investigations into
suitable methods for the reduction of renal activity uptake of
radiolabeled ADAPTs during radionuclide therapy are needed.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
The authors would like to thank Professor Anna
Orlova (Uppsala University, Uppsala, Sweden) and Mr. Jesper Borin
(Royal Institute of Technology, Stockholm, Sweden) for their
technical assistance.
Funding
This research was funded by the grants from the Swedish Cancer
Society (Cancerfonden; grant nos. 20 0181 P, 20 0893 Pj and 23 0650
JIA).
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JG designed, coordinated and supervised the study.
FL, AV, YL, SL, TX, MO, SSR and JG performed the experiments. JG
analyzed the data. FL, SL and JG performed production, purification
and analysis of compounds. UR participated in the molecular design
and supervised the production, purification and analysis. FL, AV
and JG wrote the first version of the manuscript. All authors read
and approved the manuscript. FL and JG confirm the authenticity of
all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Authors' information
Fanny Lundmark, ORCID 0000-0002-9153-2832; Anzhelika
Vorobyeva, ORCID 0000-0002-4778-3909; Yongsheng Liu, ORCID
0000-0001-5871-5779; Sarah Lindbo, ORCID 0000-0001-5908-4315;
Tianqi Xu, ORCID 0000-0002-1826-4093; Maryam Oroujeni, ORCID
0000-0003-2660-9837; Sara S. Rinne, ORCID 0000-0003-2141-3982;
Ulrika Rosenström, ORCID 0000-0002-0817-8140; Javad Garousi, ORCID
0000-0002-7224-6304.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ADAPT
|
albumin-binding domain-derived
affinity protein
|
|
ABD
|
albumin binding domain
|
|
DARPin
|
designed ankyrin repeat protein
|
|
DOTA
|
2,2′,2”,2”'-(1,4,7,10-tetraazacyclododecane-1,4,7,10-tetrayl)tetraacetic
acid
|
|
EDTA
|
ethylenediaminetetraacetic acid
|
|
ESP
|
engineered scaffold protein
|
|
HER2
|
human epidermal growth factor receptor
2
|
|
HPEM
|
[(4-hydroxyphenyl)ethyl]maleimide
|
|
SPECT
|
single-photon emission computed
tomography
|
References
|
1
|
Tolmachev VM, Chernov VI and Deyev SM:
Targeted nuclear medicine. Seek and destroy. Russ Chem Rev.
91:RCR50342022. View
Article : Google Scholar
|
|
2
|
Krasniqi A, D'Huyvetter M, Devoogdt N,
Frejd FY, Sörensen J, Orlova A, Keyaerts M and Tolmachev V:
Same-Day imaging using small proteins: Clinical experience and
translational prospects in oncology. J Nucl Med. 59:885–891. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bragina OD, Deyev SM, Chernov VI and
Tolmachev VM: The evolution of targeted radionuclide diagnosis of
HER2-Positive breast cancer. Acta Naturae. 14:4–15. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stern LA, Case BA and Hackel BJ:
Alternative Non-Antibody protein scaffolds for molecular imaging of
cancer. Curr Opin Chem Eng. 2:10.1016/j.coche.2013.08.009. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sörensen J, Velikyan I, Sandberg D,
Wennborg A, Feldwisch J, Tolmachev V, Orlova A, Sandström M,
Lubberink M, Olofsson H, et al: Measuring HER2-Receptor expression
in metastatic breast cancer using [68Ga]ABY-025 affibody
PET/CT. Theranostics. 6:262–271. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bragina O, von Witting E, Garousi J,
Zelchan R, Sandström M, Medvedeva A, Orlova A, Doroshenko A,
Vorobyeva A, Lindbo S, et al: Phase I Study of
99mTc-ADAPT6, a scaffold protein-based probe for
visualization of HER2 expression in breast cancer. J Nucl Med.
62:493–499. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bragina O, Chernov V, Schulga A,
Konovalova E, Hober S, Deyev S, Sörensen J and Tolmachev V: Direct
intra-patient comparison of scaffold protein-based tracers,
[99mTc]Tc-ADAPT6 and
[99mTc]Tc-(HE)3-G3, for imaging of
HER2-Positive breast cancer. Cancers (Basel). 15:31492023.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rothe C and Skerra A:
Anticalin® proteins as therapeutic agents in human
diseases. BioDrugs. 32:233–243. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ackerman SE, Currier NV, Bergen JM and
Cochran JR: Cystine-knot peptides: Emerging tools for cancer
imaging and therapy. Expert Rev Proteomics. 11:561–572. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vorobyeva A, Schulga A, Konovalova E,
Güler R, Löfblom J, Sandström M, Garousi J, Chernov V, Bragina O,
Orlova A, et al: Optimal composition and position of
histidine-containing tags improves biodistribution of
99mTc-labeled DARPin G3. Sci Rep. 9:94052019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Deyev SM, Vorobyeva A, Schulga A,
Abouzayed A, Günther T, Garousi J, Konovalova E, Ding H, Gräslund
T, Orlova A, et al: Effect of a radiolabel biochemical nature on
tumor-targeting properties of EpCAM-binding engineered scaffold
protein DARPin Ec1. Int J Biol Macromolecules. 145:216–225. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bragina O, Chernov V, Schulga A,
Konovalova E, Garbukov E, Vorobyeva A, Orlova A, Tashireva L,
Sörensen J, Zelchan R, et al: Phase I Trial of
99mTc-(HE)3-G3, a DARPin-Based probe for
imaging of HER2 Expression in Breast Cancer. J Nucl Med.
63:528–535. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Garousi J, Lindbo S, Nilvebrant J, Åstrand
M, Buijs J, Sandström M, Honarvar H, Orlova A, Tolmachev V and
Hober S: ADAPT, a novel scaffold protein-based probe for
radionuclide imaging of molecular targets that are expressed in
disseminated cancers. Cancer Res. 75:4364–4371. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Garousi J, Lindbo S, Borin J, von Witting
E, Vorobyeva A, Oroujeni M, Mitran B, Orlova A, Buijs J, Tolmachev
V, et al: Comparative evaluation of dimeric and monomeric forms of
ADAPT scaffold protein for targeting of HER2-expressing tumours.
Eur J Pharm Biopharm. 134:37–48. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Garousi J, Lindbo S, Mitran B, Buijs J,
Vorobyeva A, Orlova A, Tolmachev V and Hober S: Comparative
evaluation of tumor targeting using the anti-HER2 ADAPT scaffold
protein labeled at the C-terminus with indium-111 or
technetium-99m. Sci Rep. 7:147802017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lindbo S, Garousi J, Mitran B, Vorobyeva
A, Oroujeni M, Orlova A, Hober S and Tolmachev V: Optimized
molecular design of ADAPT-Based HER2-Imaging probes labeled with
111In and 68Ga. Mol Pharm. 15:2674–2683.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lindbo S, Garousi J, Mitran B, Altai M,
Buijs J, Orlova A, Hober S and Tolmachev V: Radionuclide tumor
targeting using ADAPT scaffold proteins: Aspects of label
positioning and residualizing properties of the label. J Nucl Med.
59:93–99. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
von Witting E, Garousi J, Lindbo S,
Vorobyeva A, Altai M, Oroujeni M, Mitran B, Orlova A, Hober S and
Tolmachev V: Selection of the optimal macrocyclic chelators for
labeling with 111In and 68Ga improves
contrast of HER2 imaging using engineered scaffold protein ADAPT6.
Eur J Pharm Biopharm. 140:109–120. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lindbo S, Garousi J, Åstrand M, Honarvar
H, Orlova A, Hober S and Tolmachev V: Influence of
Histidine-Containing tags on the biodistribution of ADAPT scaffold
proteins. Bioconjug Chem. 27:716–726. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Garousi J, von Witting E, Borin J,
Vorobyeva A, Altai M, Vorontsova O, Konijnenberg MW, Oroujeni M,
Orlova A, Tolmachev V, et al: Radionuclide therapy using ABD-fused
ADAPT scaffold protein: Proof of Principle. Biomaterials.
266:1203812021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tolmachev V, Orlova A, Pehrson R, Galli J,
Baastrup B, Andersson K, Sandström M, Rosik D, Carlsson J,
Lundqvist H, et al: Radionuclide therapy of HER2-positive
microxenografts Using a 177Lu-Labeled HER2-Specific
affibody molecule. Cancer Res. 67:2773–2782. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Goldenberg DM, Chatal JF, Barbet J,
Boerman O and Sharkey RM: Cancer imaging and therapy with
bispecific antibody pretargeting. Update Cancer Ther. 2:19–31.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Honarvar H, Westerlund K, Altai M,
Sandström M, Orlova A, Tolmachev V and Karlström AE: Feasibility of
affibody Molecule-Based PNA-Mediated radionuclide pretargeting of
malignant tumors. Theranostics. 6:93–103. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Westerlund K, Altai M, Mitran B,
Konijnenberg M, Oroujeni M, Atterby C, de Jong M, Orlova A,
Mattsson J, Micke P, et al: Radionuclide therapy of HER2-Expressing
human xenografts using Affibody-Based Peptide nucleic acid-mediated
pretargeting: In Vivo proof of principle. J Nucl Med. 59:1092–1098.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vegt E, de Jong M, Wetzels JFM, Masereeuw
R, Melis M, Oyen WJG, Gotthardt M and Boerman OC: Renal toxicity of
radiolabeled peptides and antibody fragments: Mechanisms, impact on
radionuclide therapy, and strategies for prevention. J Nucl Med.
51:1049–1058. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Altai M, Garousi J, Rinne SS, Schulga A,
Deyev S and Vorobyeva A: On the prevention of kidney uptake of
radiolabeled DARPins. EJNMMI Res. 10:72020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Garousi J, Vorobyeva A and Altai M:
Influence of several compounds and drugs on the renal uptake of
radiolabeled affibody molecules. Molecules. 25:26732020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Vorobyeva A, Oroujeni M, Lindbo S, Hober
S, Xu T, Liu Y, Rinne SS and Garousi J: Investigation of a
pharmacological approach for reduction of renal uptake of
radiolabeled ADAPT scaffold protein. Molecules. 25:44482020.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Arano Y: Strategies to reduce renal
radioactivity levels of antibody fragments. Q J Nucl Med.
42:262–270. 1998.PubMed/NCBI
|
|
30
|
Arano Y, Fujioka Y, Akizawa H, Ono M,
Uehara T, Wakisaka K, Nakayama M, Sakahara H, Konishi J and Saji H:
Chemical design of radiolabeled antibody fragments for low renal
radioactivity levels. Cancer Res. 59:128–134. 1999.PubMed/NCBI
|
|
31
|
Fujioka Y, Arano Y, Ono M, Uehara T, Ogawa
K, Namba S, Saga T, Nakamoto Y, Mukai T, Konishi J, et al: Renal
metabolism of 3′-iodohippuryl N(epsilon)-maleoyl-L-lysine
(HML)-conjugated Fab fragments. Bioconjug Chem. 12:178–185. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Uehara T, Koike M, Nakata H, Hanaoka H,
Iida Y, Hashimoto K, Akizawa H, Endo K and Arano Y: Design,
synthesis, and evaluation of [188Re]organorhenium-labeled antibody
fragments with renal enzyme-cleavable linkage for low renal
radioactivity levels. Bioconjug Chem. 18:190–198. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Uehara T, Yokoyama M, Suzuki H, Hanaoka H
and Arano Y: A Gallium-67/68-Labeled antibody fragment for
Immuno-SPECT/PET shows low renal radioactivity without loss of
tumor uptake. Clin Cancer Res. 24:3309–3316. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bendre S, Zhang Z, Kuo HT, Rousseau J,
Zhang C, Merkens H, Roxin Á, Bénard F and Lin KS: Evaluation of
Met-Val-Lys as a renal brush border Enzyme-Cleavable linker to
reduce kidney uptake of 68Ga-Labeled DOTA-Conjugated peptides and
peptidomimetics. Molecules. 25:38542020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Altai M, Westerlund K, Velletta J, Mitran
B, Honarvar H and Karlström AE: Evaluation of affibody
molecule-based PNA-mediated radionuclide pretargeting: Development
of an optimized conjugation protocol and 177Lu labeling.
Nucl Med Biol. 54:1–9. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tolmachev V, Mume E, Sjöberg S, Frejd FY
and Orlova A: Influence of valency and labelling chemistry on in
vivo targeting using radioiodinated HER2-binding Affibody
molecules. Eur J Nucl Med Mol Imaging. 36:692–701. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wållberg H and Orlova A: Slow
internalization of anti-HER2 synthetic affibody monomer
111In-DOTA-ZHER2:342-pep2: Implications for development of labeled
tracers. Cancer Biother Radiopharm. 23:435–442. 2008.PubMed/NCBI
|
|
38
|
Yeong CH, Cheng M and Ng KH: Therapeutic
radionuclides in nuclear medicine: current and future prospects. J
Zhejiang Univ Sci B. 15:845–863. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li L, Olafsen T, Anderson AL, Wu A,
Raubitschek AA and Shively JE: Reduction of kidney uptake in
radiometal labeled peptide linkers conjugated to recombinant
antibody fragments. Site-specific conjugation of DOTA-peptides to a
Cys-diabody. Bioconjug Chem. 13:985–995. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Arano Y: Renal brush border strategy: A
developing procedure to reduce renal radioactivity levels of
radiolabeled polypeptides. Nucl Med Biol. 92:149–155. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Biber J, Stieger B, Stange G and Murer H:
Isolation of renal proximal tubular brush-border membranes. Nat
Protoc. 2:1356–1359. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hosseinimehr SJ, Tolmachev V and Orlova A:
Liver uptake of radiolabeled targeting proteins and peptides:
Considerations for targeting peptide conjugate design. Drug Discov
Today. 17:1224–1232. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hofstrom C, Orlova A, Altai M, Wangsell F,
Graslund T and Tolmachev V: Use of a HEHEHE purification tag
instead of a hexahistidine tag improves biodistribution of affibody
molecules site-specifically labeled with (99m)Tc, (111)In, and
(125)I. J Med Chem. 54:3817–3826. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Vorobyeva A, Sсhulga A, Konovalova E,
Güler R, Mitran B, Garousi J, Rinne S, Löfblom J, Orlova A, Deyev S
and Tolmachev V: Comparison of tumor-targeting properties of
directly and indirectly radioiodinated designed ankyrin repeat
protein (DARPin) G3 variants for molecular imaging of HER2. Int J
Oncol. 54:1209–1220. 2019.PubMed/NCBI
|

















