Introduction
The chili pepper Capsicum annuum L., which
belongs to the family Solanaceae in the class
Magnoliopsida, is an annual or limited perennial herb widely
used gobally as a medicinal and edible plant. The fruit is used in
the traditional medicines of China and other countries for warming
the body, ‘dispelling cold’ and promoting digestion. The fruit
contains various active components, including capsaicin, which is
the most abundant pungent compound; capsaicinoids and carotenoids
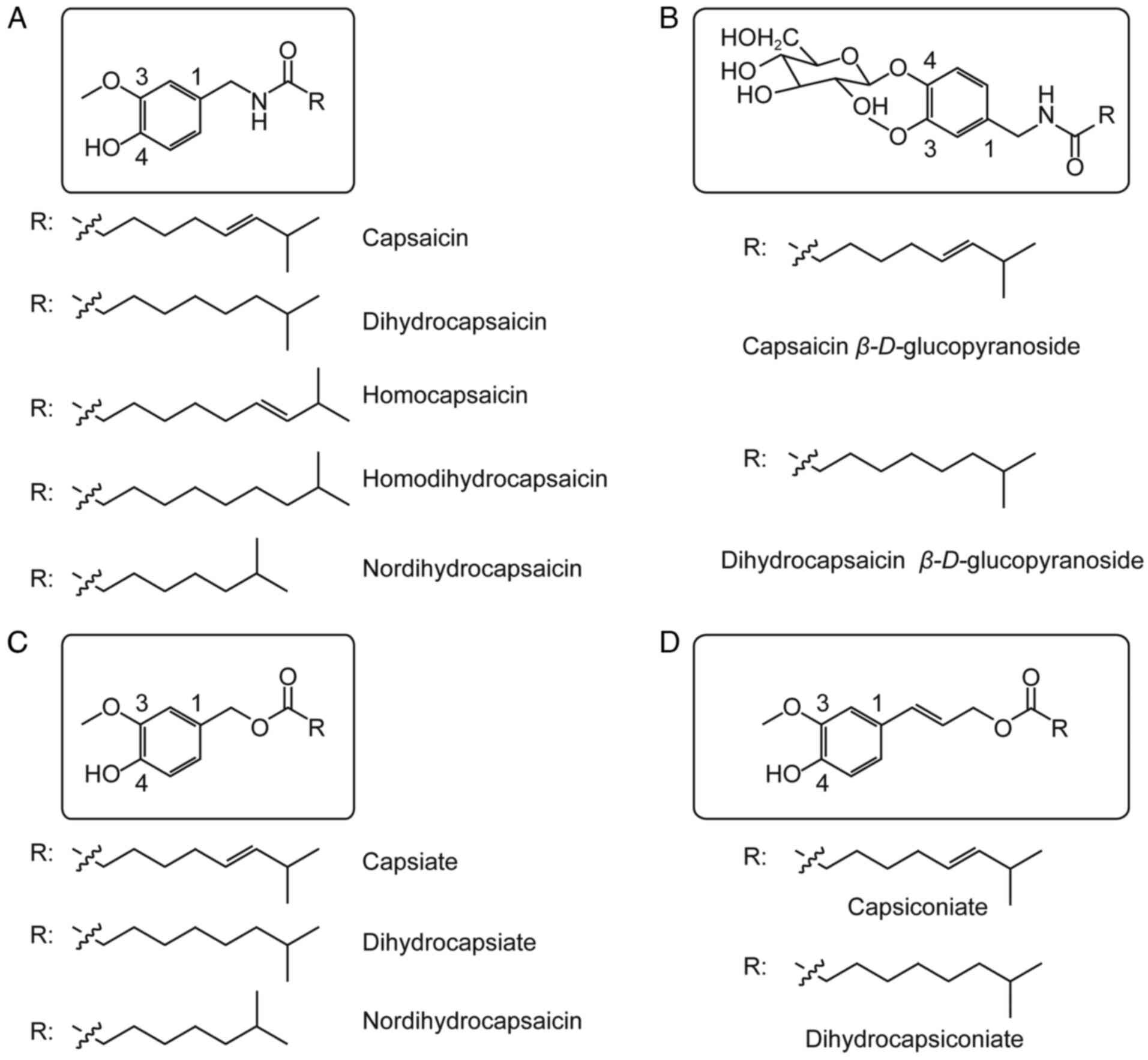
(1). Capsaicin, in turn, exists as
a family of compounds including capsaicin, dihydrocapsaicin,
homocapsaicin, homodihydrocapsaicin, nordihydrocapsaicin, capsaicin
esters, dihydrocapsaicin esters, nordihydrocapsaicin esters,
capsanthin-β-d-glucoside and dihydrocapsanthin-β-d-glucoside
(2) (Fig. 1). Capsaicin exerts analgesic,
antioxidant, cardioprotective, anticancer and thermogenic effects,
and it can promote weight loss (3). Some of these effects are mediated by
the receptor called ‘transient receptor potential cation channel
subfamily V member 1’ (TRPV1), to which capsaicin binds
specifically. Some evidence suggests that capsaicin may inhibit
signal transducer and activator of transcription 3 (STAT3), but the
minimal concentration needed to inhibit STAT3 (50 M) is
substantially higher than the concentration required to stimulate
TRPV1 (1–5 M) (4,5).
Structure and physicochemical properties of
capsaicin
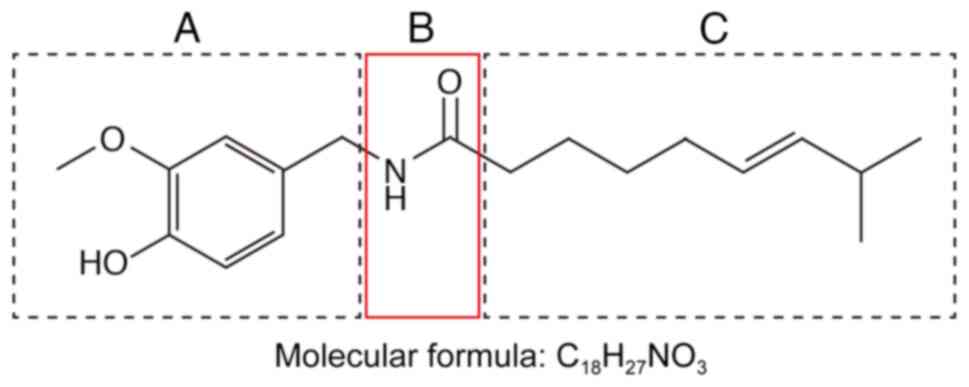
Capsaicin
(trans−8-methyl-N-vanillyl-6-nonenamide,
C18H27NO3) is a colorless
lipophilic crystalline substance (Fig.
2). It is an amide that forms through condensation of
vanillylamine and caprylic acid. It has a melting point of 65°C and
a boiling point of 210–220°C and it is highly soluble in ethanol,
ether, benzene and chloroform, but only slightly soluble in carbon
disulfide. The capsaicin structure can be divided into an aromatic
ring (Fig. 2A), amide bond
(Fig. 2B) and hydrophobic side
chain (Fig. 2C). The various
members of the capsaicin family differ from capsaicin mainly in the
substitutions on the aromatic ring and hydrophobic side chain
(Fig. 1) (6,7). The
substituents at positions 3 and 4 on the aromatic ring (Fig. 1) are important active groups. The
phenolic hydroxyl group at position 4, for example, acts as a
hydrogen bond donor or acceptor in capsaicin agonists; substitution
of this hydroxyl group for a hydrophobic group can increase
capsaicin activity. Whether the hydrophobic side chain is a
saturated or unsaturated alkyl chain, substituted naphthyl group,
or something else can influence the activity of capsaicin (8,9).
Pharmacological effects of capsaicin
Antioxidant effects
Capsaicin has been revealed to inhibit lipid
peroxidation in red blood cell membranes as well as in the liver
and mitochondria of mice, and it can block the peroxidation of
low-density lipoproteins in humans (10,11).
In fact, the antioxidant activity of capsaicin exceeds that of
vitamin E in some cases (12). The
levels of capsaicin in food can alleviate oxidative stress and
increase cellular antioxidant capacity by preventing reactive
oxygen species from oxidizing glutathione (13). Capsaicin can reverse the ability of
high blood cholesterol levels to inhibit the antioxidant enzymes
glutathione reductase, glutathione transferase and superoxide
dismutase (14,15). Capsaicin can also scavenge free
radicals such as 1,1′-diphenyl-2-picrylhydrazyl (DPPH) (13). Other members of the capsaicin
family, such as dihydrocapsaicin and 9-hydroxycapsaicin, appear to
have antioxidant activity similar to that of capsaicin (16).
In capsaicin and other members of the family, the
benzene ring and the substituents of the benzene ring appear to be
important for antioxidant activity. The benzene ring of capsaicin
may interact with the benzene ring of DPPH, while the methoxy and
hydroxy substituents at the ortho position of the benzene ring can
strongly influence antioxidant activity (13).
Adults who received capsaicin for 4 weeks
demonstrated lower levels of oxidation of serum lipoproteins
(17). In mitochondria, capsaicin
can reduce lipid peroxidation and, more generally, oxidative
stress. It can alleviate ischemia-reperfusion injury in myocardium
and kidney. Most of these antioxidant effects appear to be mediated
by TRPV1 (18). The search
continues for capsaicin analogues with even stronger antioxidant
activity.
Analgesic effects
TRPV1 is a Ca2+-selective member of the
family of transient release potential ion channels, which sense
heat. TRPV1 in the prelimbic and infralimbic cortex has also been
revealed to mediate neuropathic pain. TRPV1 is broadly distributed
in tissues of the brain, bladder, kidneys, intestines, epidermal
keratinocytes, glial cells, liver, polymorphonuclear granulocytes,
mast cells and macrophages (19).
Capsaicin is an agonist of TRPV1 that reduces its
activation threshold. Uniquely, after TRPV1 has been activated by
capsaicin, the receptor enters a long-lasting refractory state, in
which it does not respond to mechanical pressure, pain or
inflammatory agents (20). This
so-called ‘defunctionalization’ results from the closing of the
channel pore due to conformational changes that depend on
extracellular Ca2+. To what extent this transient
‘defunctionalization’ explains the observed analgesic effects of
capsaicin remains unclear (21).
When activated by capsaicin, TRPV1 mediates
Ca2+ influx and glutamate release, which may damage
cutaneous autonomic nerve fibers and sensory nerve endings,
decreasing pain sensation. In adult rats, the capsaicin analogue
resiniferatoxin damages TRPV1-expressing myelinated nerve fibers
and eliminates TRPV1-expressing unmyelinated nerve fibers, reducing
perception of thermal pain.
The US Food and Drug Administration has approved
capsaicin as an 8% dermal analgesic patch (640 mcg/cm2,
total dose 179 mg), while lower doses do not relieve pain
effectively (22). The 8% patch
has proven safe and effective in controlling neuropathic pain
resulting from post-herpetic neuralgia, post-surgical neuralgia,
post-traumatic neuropathy, polyneuropathy and mixed pain syndrome
(23). In a previous trial
(24), capsaicin markedly reduced
pain attacks, prolonged sleep duration and improved sleep quality,
while reducing dependence on opioids and antiepileptics. ~10% of
patients in that trial reported adverse drug reactions, the most
frequent of which were erythema and pain at the application site.
In a different study including two clinical trials, it has been
suggested that the 8% patch is effective against HIV-associated
distal sensory polyneuropathy (25).
Adlea, a highly purified form of capsaicin, has
exhibited analgesic efficacy in clinical trials involving patients
with intermetatarsal neuromas, lateral epicondylitis or end-stage
osteoarthritis. A trial is ongoing to assess the safety and
efficacy of the drug for patients undergoing total knee
arthroplasty (23,26).
N-palmitoyl-vanillamide, also called palvanil, is
also present in Capsicum but at markedly lower levels than
capsaicin. Palvanil has demonstrated analgesic potential while
inducing smaller fluctuations in body temperature and
bronchoconstriction than capsaicin. It also exerts the analgesic
effects through TRPV1, activating the receptor more slowly and
defunctionalizing it more completely than capsaicin does (27).
Antitumor effects
Similar to numerous other dietary phytochemicals,
capsaicin shows antitumor activity. It alters the expression of
several genes that arrest the cell cycle in tumor cells and
promotes apoptosis. These effects have been demonstrated in colon
adenocarcinoma, pancreatic cancer, hepatocellular carcinoma,
prostate cancer, breast cancer and numerous other types of cancer
(Table I), without damage to
normal cells. The way capsaicin exerts these effects is only
beginning to emerge and the mechanisms appear to involve
accumulation of intracellular Ca2+, generation of
reactive oxygen species, disruption of mitochondrial membrane
potential and upregulation of the transcription factors NF-κB and
STATS. Capsaicin has been revealed to act through TRPV1 to promote
apoptosis of numerous types of cancers. Whether it also acts
through other TRPVs, such as TRPV6 in prostate cancer, remains to
be clarified (28,29).
 | Table I.Reported antitumor effects of
capsaicin in animal models. |
Table I.
Reported antitumor effects of
capsaicin in animal models.
|
| Capsaicin
treatment |
|
|---|
|
|
|
|
|---|
| Animal model | Dose | Regime | Results |
|---|
| BALB/cJ and BALB/cJ
nu/nu mice injected with CT26 tumor cells | 100-200 µg | Intratumoral on
days 5, 10 and 15 | Reduced tumor
growth |
| BNX nu/nu male mice
injected with PC-3 cells | 5 mg/kg | Gavage 3 days per
week for 4 weeks | Reduced tumor
growth |
| Athymic nude mice
injected with PC-3 cells | 5 mg/kg | Subcutaneous
injection every two days for 14 days | Reduced tumor
growth and induced apoptosis |
| Female athymic nude
mice injected subcutaneously with AsPC-1 tumor | 2.5 mg/kg | Five times a
week | Reduced tumor
growth without adverse effects |
| cells | 5 mg/kg | Three times a
week |
|
| Male athymic nu/nu
mice injected with U266 cells | 1 mg/kg | Twice a week for 3
weeks | Reduced tumor
growth |
| Male athymic nude
mice injected subcutaneously with T24 cells | 5 mg/kg | Subcutaneous
injection every 3 days for 4 weeks | Reduced tumor
growth |
| Female triple
deficient beige/nude/xid mice injected with MDA-MB231 cells | 5 mg/kg | Oral gavage 3 days
per week for 4 weeks | Reduced tumor
growth by 50% |
| Male nude mice
injected subcutaneously with H69 cells | 10 mg/kg | Solid diet until
tumors of control group reached 2,000 mm3 | Reduced tumor
growth |
| Female BALB/c
athymic nude mice injected subcutaneously with Colo 205 cells | 1 or 3 mg/kg | Intraperitoneal
injection once daily for 4 weeks | Reduced tumor
growth |
| Female athymic nude
mice injected subcutaneously with AsPC-1 tumor cells | 2.5 mg/kg | Orally fed 5 days a
week for 6 weeks | Reduced superoxide
dismutase activity in tumors by 60%, while increasing the ratio of
oxidized to reduced glutathione |
| Male BALB/c (nu/nu)
athymic nude mice injected subcutaneously with PANC-1 cells | 5 mg/kg | Gavage 3 days per
week for 4 weeks | Reduced tumor
growth |
Weight-lowering effects
Capsaicin causes TRPV1 to stimulate the release of
catecholamine from catecholaminergic neurons in the rostral
ventrolateral medulla of the brain, thereby promoting weight loss
(30). It upregulates adiponectin
and other adipokines to reduce fat accumulation in obese mice.
Capsaicin has been shown to decrease appetite (31). When delivered as part of a high-fat
diet, it increases thermogenesis and lipid oxidation, while also
reducing levels of fasting glucose and plasma triglycerides, which
suggests therapeutic potential for obesity-related diseases such as
insulin resistance and type 2 diabetes mellitus (32,33).
Indeed, studies of various capsaicin doses in obese mice have
suggested that it can partially reverse obesity-induced glucose
intolerance by suppressing inflammatory responses and enhancing
fatty acid oxidation in adipose tissue and liver (34).
On the other hand, certain studies (35,36)
have failed to detect any effect of capsaicin on energy expenditure
or lipid oxidation. While the absence of these effects may be real,
it may also be an artifact of administering too little capsaicin
for a short period of time, or the thermogenic effects may be too
subtle to detect in the relatively small animal groups and short
measurement periods in those studies.
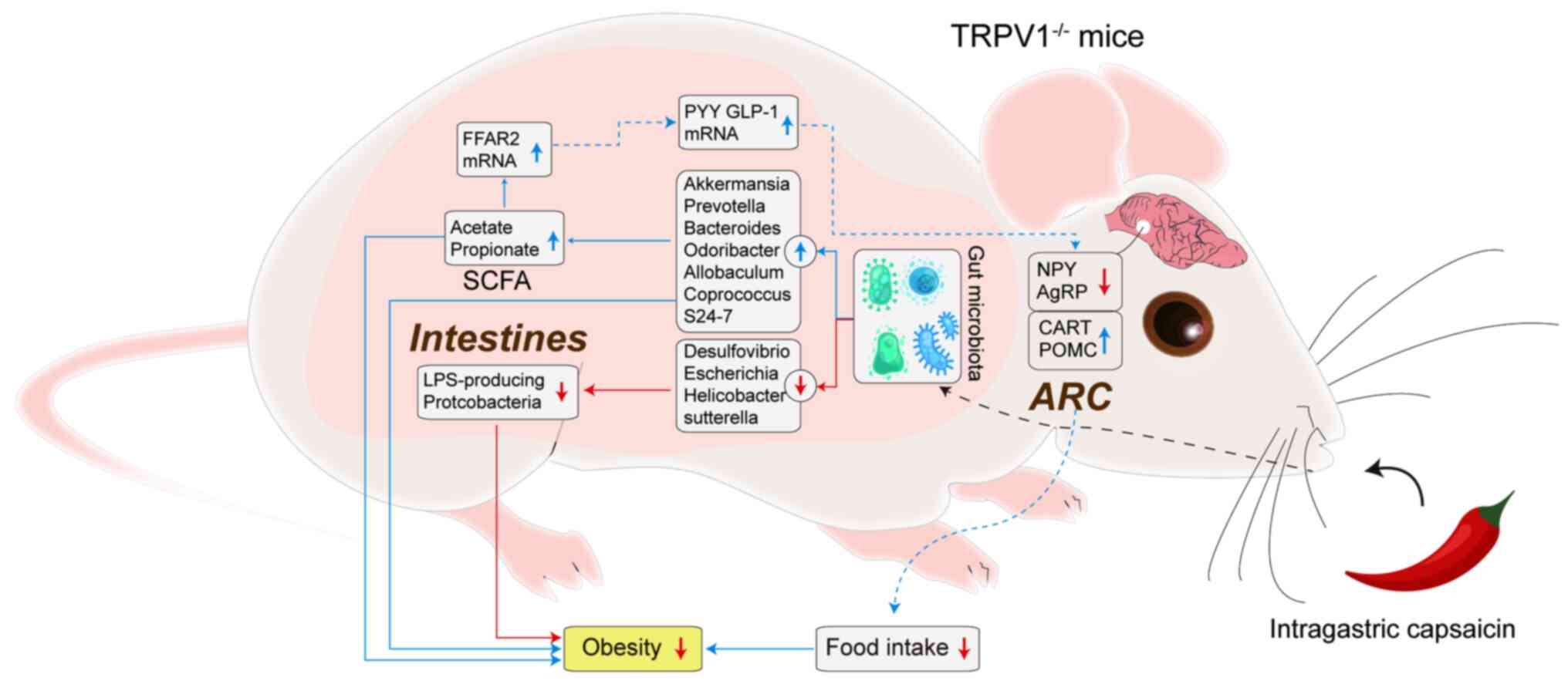
The available evidence suggests that capsaicin
lowers lipid levels by altering intestinal permeability and the gut
microbiome, in turn influencing the gut-brain axis (37) (Fig.
3). Future studies are needed to verify and elucidate the
molecular pathways involved.
Gastrointestinal effects
In rats and guinea pigs, TRPV1 is expressed and
active within the myenteric ganglia and inter-ganglionic fiber
tracts that extend throughout the gastrointestinal tract, including
the muscle layers, blood vessels and mucosa within the tract
(38). TRPV1 is also expressed
outside the gastrointestinal nervous system, such as in gastric
epithelial cells, in which it stimulates the secretion of gastrin
(39).
Previous studies have attributed several positive
gastrointestinal effects to capsaicin: It induces the release of
calcitonin gene-related peptide, activates gastroprotective
cyclooxygenase-1 and increases the absorptive surface of the small
intestine by lengthening and thickening microvilli and by altering
the permeability of the brush border membrane, in turn increasing
zinc absorption (40–42). In non-alcoholic fatty liver
disease, dietary capsaicin has been revealed to promote hepatic
phosphorylated hormone-sensitive lipase, carnitine
palmitoyltransferase 1 and peroxisome proliferator-activated
receptor δ (43).
On the other hand, a number of studies have
suggested that prolonged exposure to high doses of capsaicin can
harm the gastrointestinal tract. Thus, exploring the minimum
effective doses required to achieve the desired therapeutic effects
and minimizing the potential side effects are quite necessary for
enhancing the clinical utility of capsaicin-based treatments. TRPV1
activation induces release of substance P, which can drive
gastrointestinal inflammation (44). In addition, TRPV1 is upregulated in
irritable bowel syndrome and appears to contribute to the
gastrointestinal hypersensitivity and pain associated with the
condition (22,45).
Anti-neurodegenerative effects
Capsaicin has demonstrated therapeutic potential in
several animal models of Alzheimer's disease (AD). It can partially
reverse streptozotocin-induced biochemical and behavioral changes
that mimic AD (46). In the
APP/PS1 mouse model, capsaicin reduced the formation of amyloid
fibrils from amyloid precursor protein. In a third AD model
(47), capsaicin substantially
ameliorated synaptic damage and tau hyperphosphorylation induced by
cold water stress. Further studies should explore the therapeutic
potential of dietary capsaicin for treating and possibly even
preventing AD (48).
In an animal model of Parkinson's disease based on
lipopolysaccharide-induced inflammation, capsaicin appeared to
activate TRPV1 in M1/M2 dopaminergic neurons, which may alleviate
neuro-inflammation and oxidative stress from activated glia
(49). The beneficial effects of
capsaicin and TRPV1 were confirmed in these studies using
appropriate antagonists (50).
Future studies should continue to explore the potential of
capsaicin for treating Parkinson's disease and should elucidate the
mechanisms involved.
Dermatological effects
TRPV1 is expressed in human keratinocytes. Although
activation of epidermal TRPV1 induces the release of inflammatory
factors, capsaicin downregulates hypoxia-inducible factor-1α in
psoriatic epidermis, slowing epidermal proliferation (51). It also mitigates itching mediated
by histamine, substance P and proteinase activated receptor-2. On
the other hand, previous studies have failed to detect therapeutic
effects of capsaicin against hemodialysis-induced pruritis,
idiopathic intractable pruritis and notalgia paresthetica (52,53).
In fact, an animal study linked capsaicin to the development of
chronically relapsing pruritic dermatitis, which was associated
with an elevated number of mast cells and hyperproduction of
immunoglobulin E (54,55).
Cardiovascular effects
TRPV1 is expressed in the sensory nerves in
cardiovascular structures, near the epicardium and in vascular
endothelial cells (56). When
blood flow to myocardium is reduced, such as during myocardial
infarction, free oxygen radicals are produced, which activate TRPV1
(57). Myocardial injury also
upregulates 12-hydroperoxyeicosatetraenoic acid, a metabolite of
12-lipooxygenase arachidonic acid that may bind to TRPV1 (58). Activation of the receptor may exert
cardio-protective effects, leading to smaller infarcts and milder
ischemic/reperfusion injury (59).
TRPV1 in the vasculature can promote
vasoconstriction or vasodilation, depending on the situation. In
the case of vasoconstriction, TRPV1 activation results in the
release of substance P, which binds to neurokinin 1 (60,61).
In the case of vasodilation, TRPV1 activation results in the
release of calcitonin gene-related peptide or of protein kinase A
and nitric oxide synthase (62,63).
In both cases, TRPV1 activation leads to an increase in
intracellular Ca2+ (64,65).
Capsaicin inhibits platelet aggregation, potentially
by altering the fluidity of the platelet membrane. This mechanism
appears to be independent of TRPV1 because the effects are not
inhibited by a competitive TRPV1 inhibitor. On the other hand,
capsaicin has also been shown to promote platelet aggregation
through a mechanism dependent on TRPV1 (66–69).
In that mechanism, TRPV1 may induce release of serotonin to drive
platelet activation in response to adenosine diphosphate and
thrombin (70–74).
Pharmacokinetics of capsaicin
Numerous studies have indicated a relatively short
half-life of capsaicin in various parts of the organism, including
liver, kidney, intestine, lung and blood, restricting its clinical
use (75). Within the organism,
most capsaicin is metabolized in the liver, where it appears to be
metabolized faster in microsomes than in the S9 fraction (76,77).
The most abundant metabolites produced in liver microsomes are
16-hydroxycapsaicin, followed by 16,17-dehydrocapsaicin; the most
abundant metabolites produced in the S9 fraction are different but
have not been definitively identified (78). A number of experiments suggested
that P450 enzymes can oxidize capsaicin to generate free radical
intermediates (79–81). Further studies should clarify the
metabolites of capsaicin in the liver, since some of the
discrepancies reported so far may reflect different dosing
conditions (82,83).
Capsaicin in the body diffuses into intestinal
tissues, the jejunum and serosal fluid (84). A previous study has suggested that
capsaicin and dihydrocapsaicin are absorbed to a greater extent by
the jejunum and ileum than by the stomach (85).
Capsaicin is metabolized only slowly on the skin,
where the main metabolites are vanillylamine and vanillic acid. It
penetrates the skin with first-order kinetics. These
characteristics make topical administration of capsaicin effective
(86,87).
Capsaicin is widely used worldwide, but there is
ongoing debate about the safety. For example, epidemiological and
laboratory data have suggested that capsaicin can act as a
carcinogen or anticarcinogen (88–90).
Capsaicin appears to interact with xenobiotic-metabolizing enzymes,
particularly microsomal cytochrome P450-dependent monooxygenases,
which are involved in activation as well as detoxification of
various chemical carcinogens and mutagens (1,91,92).
The Indian population consumes several-fold more chili than
populations in other countries, yet this does not appear to
adversely affect growth, organ weight, nitrogen balance or blood
chemistry (93). Previous studies
in animals and mammalian cell lines have not suggested any
mutagenic effects of capsaicin in somatic cells or the germline
(94). Capsaicin cream has been
used in the clinic for numerous years to relieve various types of
pain, and long-term local application of capsaicin can be effective
for treating skin cancer in mice (28,50,95–98).
Although animal studies have indicated few or no
side effects of capsaicin, it can irritate the skin of humans and
excessive ingestion can cause nausea, vomiting, abdominal pain and
burning diarrhea (99). Contact of
capsaicin with eyes can cause severe tearing, pain, conjunctivitis,
eyelid spasms and it can trigger mucosal irritation leading to
serious gastritis and diarrhea. Loading capsaicin into
nanoparticles may reduce these adverse effects while improving its
efficacy by counteracting its hydrophobicity and prolonging its
half-life in the circulation (100,101). Combining capsaicin with other
phytochemicals may also mitigate the side effects (102–104); these compounds may include
vanilloids, flavonoids, alkaloids, terpenoids, terpenyl phenols,
fatty acids, cannabinoids and sulfur-containing compounds.
Furthermore, different individuals have different intolerance to
capsaicin, personalized treatment programs are needed. Therefore,
capsaicin products should be used carefully in light of the range
of pharmacological activities and potential for adverse effects;
meanwhile, further research is needed to assess the safety of
prolonged capsaicin exposure (105).
Conclusion
In addition to being widely used as a local
analgesic, capsaicin has also demonstrated antioxidant, anticancer,
antiobesity and gastroprotective activities. The longer half-life
of capsaicin in the lungs and skin implies that it may have
stronger effects in these tissues. Systemic administration of
capsaicin seems unlikely to be effective because of its metabolic
instability and short half-life in the circulation. Further efforts
to develop capsaicin analogues and nanoparticles delivery system
may succeed in prolonging half-life while also increasing efficacy,
making it an effective analgesic against numerous diseases.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Natural Science
Foundation of China (grant no. U1804179).
Availability of data and materials
Not applicable.
Authors' contributions
XS and WZ conceived and designed the study. YZ, JF
and ZF analyzed the data. XS wrote the manuscript. Data
authentication is not applicable. All authors read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Surh YJ and Lee SS: Capsaicin, a
double-edged sword: Toxicity, metabolism, and chemopreventive
potential. Life Sci. 56:1845–1855. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Higashiguchi F, Nakamura H, Hayashi H and
Kometani T: Purification and structure determination of glucosides
of capsaicin and dihydrocapsaicin from various Capsicum
fruits. J Agric Food Chem. 54:5948–5953. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cunha MR, Tavares MT, Fernandes TB and
Parise-Filho R: Peppers: A ‘hot’ natural source for antitumor
compounds. Molecules. 26:15212021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang S, Wang D, Huang J, Hu Y and Xu Y:
Application of capsaicin as a potential new therapeutic drug in
human cancers. J Clin Pharm Ther. 45:16–28. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Popescu GDA, Scheau C, Badarau IA,
Dumitrache MD, Caruntu A, Scheau AE, Costache DO, Costache RS,
Constantin C, Neagu M and Caruntu C: The effects of capsaicin on
gastrointestinal cancers. Molecules. 26:942020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nanok K and Sansenya S: α-Glucosidase,
α-amylase, and tyrosinase inhibitory potential of capsaicin and
dihydrocapsaicin. J Food Biochem. 44:e130992020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Katritzky AR, Xu YJ, Vakulenko AV, Wilcox
AL and Bley KR: Model compounds of caged capsaicin: Design,
synthesis, and photoreactivity. J Org Chem. 68:9100–9104. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Basith S, Cui M, Hong S and Choi S:
Harnessing the therapeutic potential of capsaicin and its analogues
in pain and other diseases. Molecules. 21:9662016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Walpole CS, Bevan S, Bloomfield G,
Breckenridge R, James IF, Ritchie T, Szallasi A, Winter J and
Wrigglesworth R: Similarities and differences in the
structure-activity relationships of capsaicin and resiniferatoxin
analogues. J Med Chem. 39:2939–2952. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Srinivasan K: Antioxidant potential of
spices and their active constituents. Crit Rev Food Sci Nutr.
54:352–372. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Naidu KA and Thippeswamy NB: Inhibition of
human low density lipoprotein oxidation by active principles from
spices. Mol Cell Biochem. 229:19–23. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kursunluoglu G, Taskiran D and Kayali HA:
The investigation of the antitumor agent toxicity and capsaicin
effect on the electron transport chain enzymes, catalase activities
and lipid peroxidation levels in lung, heart and brain tissues of
rats. Molecules. 23:32672018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kogure K, Goto S, Nishimura M, Yasumoto M,
Abe K, Ohiwa C, Sassa H, Kusumi T and Terada H: Mechanism of potent
antiperoxidative effect of capsaicin. Biochim Biophys Acta.
1573:84–92. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ochi T, Takaishi Y, Kogure K and Yamauti
I: Antioxidant activity of a new capsaicin derivative from
Capsicum annuum. J Nat Prod. 66:1094–1096. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kempaiah RK and Srinivasan K: Influence of
dietary curcumin, capsaicin and garlic on the antioxidant status of
red blood cells and the liver in high-fat-fed rats. Ann Nutr Metab.
48:314–320. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kempaiah RK and Srinivasan K: Antioxidant
status of red blood cells and liver in hypercholesterolemic rats
fed hypolipidemic spices. Int J Vitam Nutr Res. 74:199–208. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Qin Y, Ran L, Wang J, Yu L, Lang HD, Wang
XL, Mi MT and Zhu JD: Capsaicin supplementation improved risk
factors of coronary heart disease in individuals with low HDL-C
levels. Nutrients. 9:10372017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nakagawa H and Hiura A: Capsaicin,
transient receptor potential (TRP) protein subfamilies and the
particular relationship between capsaicin receptors and small
primary sensory neurons. Anat Sci Int. 81:135–155. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ramsey IS, Delling M and Clapham DE: An
introduction to TRP channels. Annu Rev Physiol. 68:619–647. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Knotkova H, Pappagallo M and Szallasi A:
Capsaicin (TRPV1 Agonist) therapy for pain relief: Farewell or
revival? Clin J Pain. 24:142–154. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Aiello F, Badolato M, Pessina F, Sticozzi
C, Maestrini V, Aldinucci C, Luongo L, Guida F, Ligresti A, Artese
A, et al: Design and synthesis of new transient receptor potential
vanilloid type-1 (TRPV1) channel modulators: Identification,
molecular modeling analysis, and pharmacological characterization
of the N-(4-Hydroxy-3-methoxybenzyl)-4-(thiophen-2-yl)butanamide, a
small molecule endowed with agonist TRPV1 Activity and protective
effects against oxidative stress. ACS Chem Neurosci. 7:737–748.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sharma SK, Vij AS and Sharma M: Mechanisms
and clinical uses of capsaicin. Eur J Pharmacol. 720:55–62. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lo Vecchio S, Andersen HH, Elberling J and
Arendt-Nielsen L: Sensory defunctionalization induced by 8% topical
capsaicin treatment in a model of ultraviolet-B-induced cutaneous
hyperalgesia. Exp Brain Res. 239:2873–2886. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gašparini D, Ljubičić R and Mršić-Pelčić
J: Capsaicin-potential solution for chronic pain treatment.
Psychiatr Danub. 32 (Suppl 4):S420–S428. 2020.PubMed/NCBI
|
|
25
|
Brown S, Simpson DM, Moyle G, Brew BJ,
Schifitto G, Larbalestier N, Orkin C, Fisher M, Vanhove GF and
Tobias JK: NGX-4010, a capsaicin 8% patch, for the treatment of
painful HIV-associated distal sensory polyneuropathy: Integrated
analysis of two phase III, randomized, controlled trials. AIDS Res
Ther. 10:52013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Anand P and Bley K: Topical capsaicin for
pain management: Therapeutic potential and mechanisms of action of
the new high-concentration capsaicin 8% patch. Br J Anaesth.
107:490–502. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Luongo L, Costa B, D'Agostino B, Guida F,
Comelli F, Gatta L, Matteis M, Sullo N, De Petrocellis L, de
Novellis V, et al: Palvanil, a non-pungent capsaicin analogue,
inhibits inflammatory and neuropathic pain with little effects on
bronchopulmonary function and body temperature. Pharmacol Res.
66:243–250. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chapa-Oliver AM and Mejía-Teniente L:
Capsaicin: From plants to a cancer-suppressing agent. Molecules.
21:9312016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Merritt JC, Richbart SD, Moles EG, Cox AJ,
Brown KC, Miles SL, Finch PT, Hess JA, Tirona MT, Valentovic MA and
Dasgupta P: Anti-cancer activity of sustained release capsaicin
formulations. Pharmacol Ther. 238:1081772022. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Akabori H, Yamamoto H, Tsuchihashi H, Mori
T, Fujino K, Shimizu T, Endo Y and Tani T: Transient receptor
potential vanilloid 1 antagonist, capsazepine, improves survival in
a rat hemorrhagic shock model. Ann Surg. 245:964–970. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yoshioka M, St-Pierre S, Suzuki M and
Tremblay A: Effects of red pepper added to high-fat and
high-carbohydrate meals on energy metabolism and substrate
utilization in Japanese women. Br J Nutr. 80:503–510. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kang JH, Tsuyoshi G, Le Ngoc H, Kim HM, Tu
TH, Noh HJ, Kim CS, Choe SY, Kawada T, Yoo H and Yu R: Dietary
capsaicin attenuates metabolic dysregulation in genetically obese
diabetic mice. J Med Food. 14:310–315. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Josse AR, Sherriffs SS, Holwerda AM,
Andrews R, Staples AW and Phillips SM: Effects of capsinoid
ingestion on energy expenditure and lipid oxidation at rest and
during exercise. Nutr Metab (Lond). 7:652010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lejeune MPGM, Kovacs EMR and
Westerterp-Plantenga MS: Effect of capsaicin on substrate oxidation
and weight maintenance after modest body-weight loss in human
subjects. Br J Nutr. 90:651–659. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lee GR, Shin MK, Yoon DJ, Kim AR, Yu R,
Park NH and Han IS: Topical application of capsaicin reduces
visceral adipose fat by affecting adipokine levels in high-fat
diet-induced obese mice. Obesity (Silver Spring). 21:115–122. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Okumura T, Tsukui T, Hosokawa M and
Miyashita K: Effect of caffeine and capsaicin on the blood glucose
levels of obese/diabetic KK-A(y) mice. J Oleo Sci. 61:515–523.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang Y, Zhou Y and Fu J: Advances in
antiobesity mechanisms of capsaicin. Curr Opin Pharmacol. 61:1–5.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ward SM, Bayguinov J, Won KJ, Grundy D and
Berthoud HR: Distribution of the vanilloid receptor (VR1) in the
gastrointestinal tract. J Comp Neurol. 465:121–135. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ericson A, Nur EM, Petersson F and
Kechagias S: The effects of capsaicin on gastrin secretion in
isolated human antral glands: Before and after ingestion of red
chilli. Dig Dis Sci. 54:491–498. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ohno T, Hattori Y, Komine R, Ae T,
Mizuguchi S, Arai K, Saeki T, Suzuki T, Hosono K, Hayashi I, et al:
Roles of calcitonin gene-related peptide in maintenance of gastric
mucosal integrity and in enhancement of ulcer healing and
angiogenesis. Gastroenterology. 134:215–225. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Prakash UNS and Srinivasan K: Beneficial
influence of dietary spices on the ultrastructure and fluidity of
the intestinal brush border in rats. Br J Nutr. 104:31–39. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Prakash UNS and Srinivasan K: Enhanced
intestinal uptake of iron, zinc and calcium in rats fed pungent
spice principles-piperine, capsaicin and ginger (Zingiber
officinale). J Trace Elem Med Biol. 27:184–190. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Li Q, Li L, Wang F, Chen J, Zhao Y, Wang
P, Nilius B, Liu D and Zhu Z: Dietary capsaicin prevents
nonalcoholic fatty liver disease through transient receptor
potential vanilloid 1-mediated peroxisome proliferator-activated
receptor δ activation. Pflugers Arch. 465:1303–1316. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang L, Hu CP, Deng PY, Shen SS, Zhu HQ,
Ding JS, Tan GS and Li YJ: The protective effects of rutaecarpine
on gastric mucosa injury in rats. Planta Med. 71:416–419. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Akbar A, Yiangou Y, Facer P, Walters JR,
Anand P and Ghosh S: Increased capsaicin receptor TRPV1-expressing
sensory fibres in irritable bowel syndrome and their correlation
with abdominal pain. Gut. 57:923–929. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hardy J and Selkoe DJ: The amyloid
hypothesis of Alzheimer's disease: Progress and problems on the
road to therapeutics. Science. 297:353–356. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Postina R, Schroeder A, Dewachter I, Bohl
J, Schmitt U, Kojro E, Prinzen C, Endres K, Hiemke C, Blessing M,
et al: A disintegrin-metalloproteinase prevents amyloid plaque
formation and hippocampal defects in an Alzheimer disease mouse
model. J Clin Invest. 113:1456–1464. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wang J, Sun BL, Xiang Y, Tian DY, Zhu C,
Li WW, Liu YH, Bu XL, Shen LL, Jin WS, et al: Capsaicin consumption
reduces brain amyloid-beta generation and attenuates Alzheimer's
disease-type pathology and cognitive deficits in APP/PS1 mice.
Transl Psychiatry. 10:2302020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Shi Z, El-Obeid T, Riley M, Li M, Page A
and Liu J: High chili intake and cognitive function among 4582
adults: An open cohort study over 15 years. Nutrients. 11:11832019.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Tyagi S, Shekhar N and Thakur AK:
Protective role of capsaicin in neurological disorders: An
overview. Neurochem Res. 47:1513–1531. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Li WH, Lee YM, Kim JY, Kang S, Kim S, Kim
KH, Park CH and Chung JH: Transient receptor potential vanilloid-1
mediates heat-shock-induced matrix metalloproteinase-1 expression
in human epidermal keratinocytes. J Invest Dermatol. 127:2328–2335.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Yu CS: Study on HIF-1α gene translation in
psoriatic epidermis with the topical treatment of capsaicin
ointment. ISRN Pharm. 2011:8218742011.PubMed/NCBI
|
|
53
|
Sekine R, Satoh T, Takaoka A, Saeki K and
Yokozeki H: Anti pruritic effects of topical crotamiton, capsaicin,
and a corticosteroid on pruritogen-induced scratching behavior. Exp
Dermatol. 21:201–204. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Gooding SM, Canter PH, Coelho HF, Boddy K
and Ernst E: Systematic review of topical capsaicin in the
treatment of pruritus. Int J Dermatol. 49:858–865. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Back SK, Jeong KY, Li C, Lee J, Lee SB and
Na HS: Chronically relapsing pruritic dermatitis in the rats
treated as neonate with capsaicin; a potential rat model of human
atopic dermatitis. J Dermatol Sci. 67:111–119. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Zahner MR, Li DP, Chen SR and Pan HL:
Cardiac vanilloid receptor 1-expressing afferent nerves and their
role in the cardiogenic sympathetic reflex in rats. J Physiol.
551:515–523. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Poblete IM, Orliac ML, Briones R,
Adler-Graschinsky E and Huidobro-Toro JP: Anandamide elicits an
acute release of nitric oxide through endothelial TRPV1 receptor
activation in the rat arterial mesenteric bed. J Physiol.
568:539–551. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Huang HS, Pan HL, Stahl GL and Longhurst
JC: Ischemia- and reperfusion-sensitive cardiac sympathetic
afferents: Influence of H2O2 and hydroxyl radicals. Am J Physiol.
269:H888–H901. 1995.PubMed/NCBI
|
|
59
|
Schultz HD and Ustinova EE: Capsaicin
receptors mediate free radical-induced activation of cardiac
afferent endings. Cardiovasc Res. 38:348–355. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Pan HL and Chen SR: Sensing tissue
ischemia: Another new function for capsaicin receptors?
Circulation. 110:1826–1831. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Steagall RJ, Sipe AL, Williams CA, Joyner
WL and Singh K: Substance P release in response to cardiac ischemia
from rat thoracic spinal dorsal horn is mediated by TRPV1.
Neuroscience. 214:106–119. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Ide R, Saiki C, Makino M and Matsumoto S:
TRPV1 receptor expression in cardiac vagal afferent neurons of
infant rats. Neurosci Lett. 507:67–71. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Jones WK, Fan GC, Liao S, Zhang JM, Wang
Y, Weintraub NL, Kranias EG, Schultz JE, Lorenz J and Ren X:
Peripheral nociception associated with surgical incision elicits
remote nonischemic cardioprotection via neurogenic activation of
protein kinase C signaling. Circulation. 120 (11 Suppl):S1–S9.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Wang L and Wang DH: TRPV1 gene knockout
impairs postischemic recovery in isolated perfused heart in mice.
Circulation. 112:3617–3623. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Sexton A, McDonald M, Cayla C, Thiemermann
C and Ahluwalia A: 12-Lipoxygenase-derived eicosanoids protect
against myocardial ischemia/reperfusion injury via activation of
neuronal TRPV1. FASEB J. 21:2695–2703. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Yang D, Luo Z, Ma S, Wong WT, Ma L, Zhong
J, He H, Zhao Z, Cao T, Yan Z, et al: Activation of TRPV1 by
dietary capsaicin improves endothelium-dependent vasorelaxation and
prevents hypertension. Cell Metab. 12:130–141. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Chen Q, Zhu H, Zhang Y, Zhang Y, Wang L
and Zheng L: Vasodilating effect of capsaicin on rat mesenteric
artery and its mechanism. Zhejiang Da Xue Xue Bao Yi Xue Ban.
42:177–183. 2013.(In Chinese). PubMed/NCBI
|
|
68
|
Adams MJ, Ahuja KD and Geraghty DP: Effect
of capsaicin and dihydrocapsaicin on in vitro blood coagulation and
platelet aggregation. Thromb Res. 124:721–723. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Mittelstadt SW, Nelson RA, Daanen JF, King
AJ, Kort ME, Kym PR, Lubbers NL, Cox BF and Lynch JJ III:
Capsaicin-induced inhibition of platelet aggregation is not
mediated by transient receptor potential vanilloid type 1. Blood
Coagul Fibrinolysis. 23:94–97. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Raghavendra RH and Naidu KA: Spice active
principles as the inhibitors of human platelet aggregation and
thromboxane biosynthesis. Prostaglandins Leukot Essent Fatty Acids.
81:73–78. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Sylvester DM and LaHann TR: Effects of
capsaicinoids on platelet aggregation. Proc West Pharmacol Soc.
32:95–100. 1989.PubMed/NCBI
|
|
72
|
Meddings JB, Hogaboam CM, Tran K, Reynolds
JD and Wallace JL: Capsaicin effects on non-neuronal plasma
membranes. Biochim Biophys Acta. 1070:43–50. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Aranda FJ, Villalaín J and Gómez-Fernández
JC: Capsaicin affects the structure and phase organization of
phospholipid membranes. Biochim Biophys Acta. 1234:225–234. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Harper AG, Brownlow SL and Sage SO: A role
for TRPV1 in agonist-evoked activation of human platelets. J Thromb
Haemost. 7:330–338. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Batiha GE, Alqahtani A, Ojo OA, Shaheen
HM, Wasef L, Elzeiny M, Ismail M, Shalaby M, Murata T,
Zaragoza-Bastida A, et al: Biological properties, bioactive
constituents, and pharmacokinetics of some Capsicum spp. and
capsaicinoids. Int J Mol Sci. 21:51792020. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Jung SH, Kim HJ, Oh GS, Shen A, Lee S,
Choe SK, Park R and So HS: Capsaicin ameliorates cisplatin-induced
renal injury through induction of heme oxygenase-1. Mol Cells.
37:234–240. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Valentovic MA, Ball JG, Brown JM, Terneus
MV, McQuade E, Van Meter S, Hedrick HM, Roy AA and Williams T:
Resveratrol attenuates cisplatin renal cortical cytotoxicity by
modifying oxidative stress. Toxicol In Vitro. 28:248–257. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Ito K, Nakazato T, Yamato K, Miyakawa Y,
Yamada T, Hozumi N, Segawa K, Ikeda Y and Kizaki M: Induction of
apoptosis in leukemic cells by homovanillic acid derivative,
capsaicin, through oxidative stress: Implication of phosphorylation
of p53 at Ser-15 residue by reactive oxygen species. Cancer Res.
64:1071–1078. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Mózsik G, Past T, Abdel Salam OM, Kuzma M
and Perjési P: Interdisciplinary review for correlation between the
plant origin capsaicinoids, non-steroidal antiinflammatory drugs,
gastrointestinal mucosal damage and prevention in animals and human
beings. Inflammopharmacology. 17:113–150. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Luo XJ, Peng J and Li YJ: Recent advances
in the study on capsaicinoids and capsinoids. Eur J Pharmacol.
650:1–7. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Kang JY, Yeoh KG, Chia HP, Lee HP, Chia
YW, Guan R and Yap I: Chili-protective factor against peptic ulcer?
Dig Dis Sci. 40:576–579. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Chanda S, Bashir M, Babbar S, Koganti A
and Bley K: In vitro hepatic and skin metabolism of capsaicin. Drug
Metab Dispos. 36:670–675. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Reilly CA, Ehlhardt WJ, Jackson DA,
Kulanthaivel P, Mutlib AE, Espina RJ, Moody DE, Crouch DJ and Yost
GS: Metabolism of capsaicin by cytochrome P450 produces novel
dehydrogenated metabolites and decreases cytotoxicity to lung and
liver cells. Chem Res Toxicol. 16:336–349. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Kawada T, Suzuki T, Takahashi M and Iwai
K: Gastrointestinal absorption and metabolism of capsaicin and
dihydrocapsaicin in rats. Toxicol Appl Pharmacol. 72:449–456. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Wang YY, Hong CT, Chiu WT and Fang JY: In
vitro and in vivo evaluations of topically applied capsaicin and
nonivamide from hydrogels. Int J Pharm. 224:89–104. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
O'Neill J, Brock C, Olesen AE, Andresen T,
Nilsson M and Dickenson AH: Unravelling the mystery of capsaicin: A
tool to understand and treat pain. Pharmacol Rev. 64:939–971. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Suresh D and Srinivasan K: Tissue
distribution & elimination of capsaicin, piperine &
curcumin following oral intake in rats. Indian J Med Res.
131:682–691. 2010.PubMed/NCBI
|
|
88
|
Rollyson WD, Stover CA, Brown KC, Perry
HE, Stevenson CD, McNees CA, Ball JG, Valentovic MA and Dasgupta P:
Bioavailability of capsaicin and its implications for drug
delivery. J Control Release. 196:96–105. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Thornton T, Mills D and Bliss E:
Capsaicin: A potential treatment to improve cerebrovascular
function and cognition in obesity and ageing. Nutrients.
15:15372023. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Petroianu GA, Aloum L and Adem A:
Neuropathic pain: Mechanisms and therapeutic strategies. Front Cell
Dev Biol. 11:10726292023. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Erin N and Szallasi A: Carcinogenesis and
metastasis: Focus on TRPV1-positive neurons and immune cells.
Biomolecules. 13:9832023. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Fernández-Carvajal A, Fernández-Ballester
G and Ferrer-Montiel A: TRPV1 in chronic pruritus and pain: Soft
modulation as a therapeutic strategy. Front Mol Neurosci.
15:9309642022. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Zhang L, Angst E, Park JL, Moro A, Dawson
DW, Reber HA, Eibl G, Hines OJ, Go VL and Lu QY: Quercetin aglycone
is bioavailable in murine pancreas and pancreatic xenografts. J
Agric Food Chem. 58:7252–7257. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Santos VAM, Bressiani PA, Zanotto AW,
Almeida IV, Berti AP, Lunkes AM, Vicentini VEP and Düsman E:
Cytotoxicity of capsaicin and its analogs in vitro. Braz J Biol.
83:e2689412023. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Chaiyasit K, Khovidhunkit W and
Wittayalertpanya S: Pharmacokinetic and the effect of capsaicin in
Capsicum frutescens on decreasing plasma glucose level. J
Med Assoc Thai. 92:108–113. 2009.PubMed/NCBI
|
|
96
|
Braga Ferreira LG, Faria JV, Dos Santos
JPS and Faria RX: Capsaicin: TRPV1-independent mechanisms and novel
therapeutic possibilities. Eur J Pharmacol. 887:1733562020.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Liu T, Wan Y, Meng Y, Zhou Q, Li B, Chen Y
and Wang L: Capsaicin: A novel approach to the treatment of
functional dyspepsia. Mol Nutr Food Res. 10:e22007932023.
View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Szallasi A: Capsaicin for weight control:
‘Exercise in a pill’ (or just another fad)? Pharmaceuticals
(Basel). 15:8512022. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Huang Z, Sharma M, Dave A, Yang Y, Chen ZS
and Radhakrishnan R: The antifibrotic and the anticarcinogenic
activity of capsaicin in hot chili pepper in relation to oral
submucous fibrosis. Front Pharmacol. 13:8882802022. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Malewicz NM, Rattray Z, Oeck S, Jung S,
Escamilla-Rivera V, Chen Z, Tang X, Zhou J and LaMotte RH: Topical
capsaicin in Poly(lactic-co-glycolic)acid (PLGA) nanoparticles
decreases acute itch and heat pain. Int J Mol Sci. 23:52752022.
View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Yue WWS, Yuan L, Braz JM, Basbaum AI and
Julius D: TRPV1 drugs alter core body temperature via central
projections of primary afferent sensory neurons. Elife.
11:e801392022. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Abbas MA: Modulation of TRPV1 channel
function by natural products in the treatment of pain. Chem Biol
Interact. 330:1091782020. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Yeon KY, Kim SA, Kim YH, Lee MK, Ahn DK,
Kim HJ, Kim JS, Jung SJ and Oh SB: Curcumin produces an
antihyperalgesic effect via antagonism of TRPV1. J Dent Res.
89:170–174. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Sui F, Zhang CB, Yang N, Li LF, Guo SY,
Huo HR and Jiang TL: Anti-nociceptive mechanism of baicalin
involved in intervention of TRPV1 in DRG neurons in vitro. J
Ethnopharmacol. 129:361–366. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Dludla PV, Nkambule BB, Cirilli I,
Marcheggiani F, Mabhida SE, Ziqubu K, Ntamo Y, Jack B, Nyambuya TM,
Hanser S and Mazibuko-Mbeje SE: Capsaicin, its clinical
significance in patients with painful diabetic neuropathy. Biomed
Pharmacother. 153:1134392022. View Article : Google Scholar : PubMed/NCBI
|